Abstract
Purpose of Review
Pediatric obese asthma is a complex disease that remains poorly understood. The increasing worldwide incidence of both asthma and obesity over the last few decades, their current high prevalence and the challenges in treating obese asthmatic patients all highlight the importance of a better understanding of the pathophysiological mechanisms in obese asthma. While it is well established that patients with obesity are at an increased risk of developing asthma, the mechanisms by which obesity drives the onset of asthma, and modifies existing asthma, remain unclear. Here, we will focus on mechanisms by which obesity alters immune function in asthma.
Recent Findings
Lung parenchyma has an altered structure in some pediatric obese asthmatics, known as dysanapsis. Central adiposity is linked to reduced pulmonary function and a better predictor of asthma risk in children than body mass index. Obesity in young children is associated with an increased risk of developing asthma, as well as early puberty, and hormonal alterations are implicated in obese asthma. Obesity and asthma each yield immunometabolic dysregulation separately and we are learning more about alterations in these pathways in pediatric obese asthma and the potential impact of bariatric surgery on those processes.
Summary
The recent progress in clarifying the connections between childhood obesity and asthma and their combined impacts on immune function moves us closer to the goals of improved understanding of the pathophysiological mechanisms underpinning obese asthma and improved therapeutic target selection. However, this common inflammatory disease remains understudied, especially in children, and much remains to be learned.
Keywords: obese asthma, immune dysregulation, inflammation
Introduction
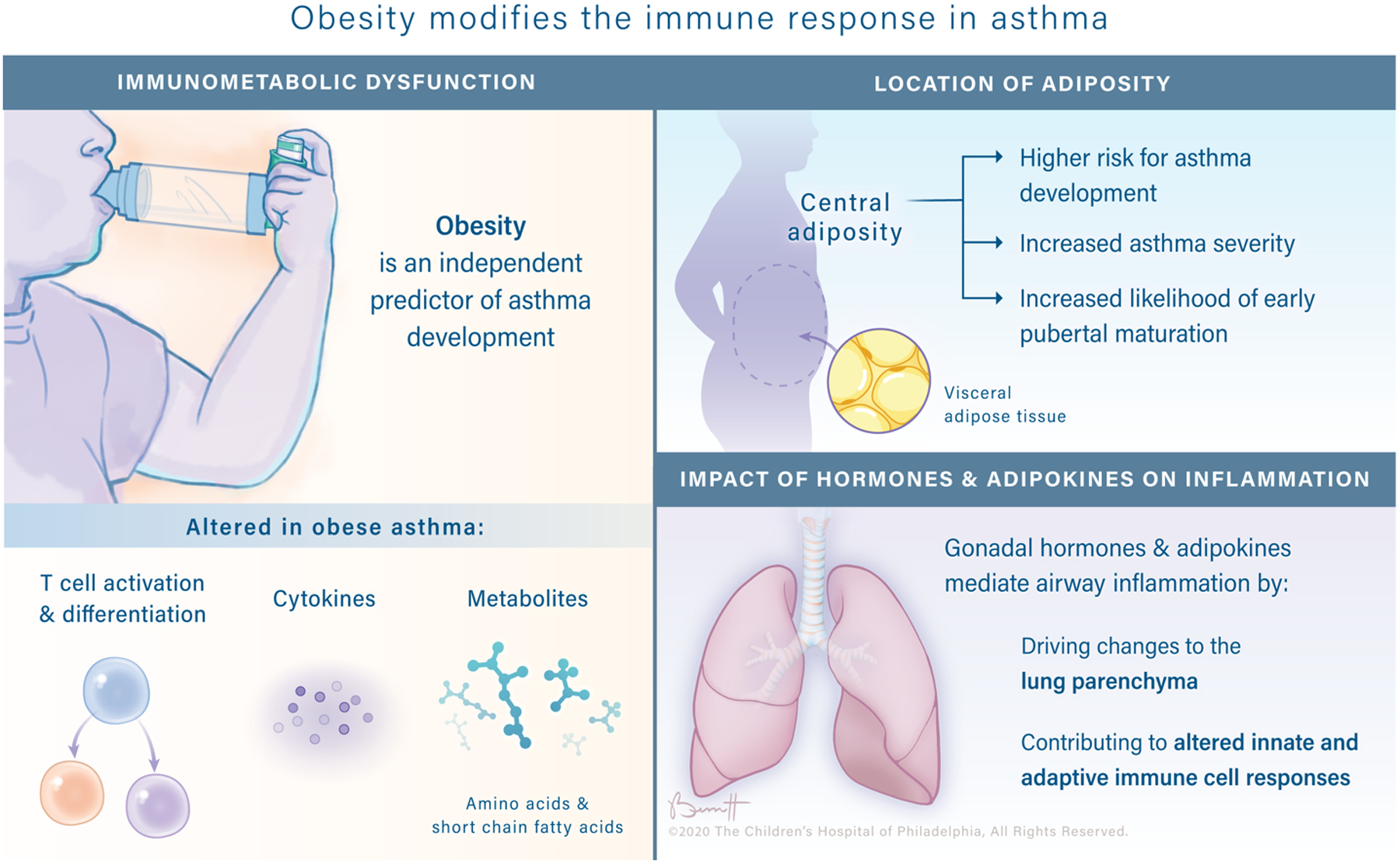
Obese asthma (OA) is the intersection of the two most commonly occurring chronic diseases of childhood (1). Over the last few decades, both asthma and obesity have increased significantly worldwide (2) and currently 42% of adults(3) and 18% of children(4) in the United States are obese and 8% of adults and children are asthmatic(5). Moreover, 11% of obese adults (6), including 14.6% of obese women(6), and 15.7% of obese children (7) also have asthma. To add complexity, asthma is not a single condition but a disease encompassing a complex set of overlapping clinical phenotypes, including atopy and obesity (8). Both asthma and obesity(9) independently impact the immune system in many complex ways (10). Finally, there is substantial evidence that obesity and asthma may be pathophysiologically linked. First, it is well established that asthma is more prevalent in obese children (13–24) and adults (24–26), compared to their healthy weight counterparts. Second, obesity is an independent risk factor for development of pediatric asthma (12–23), with approximately one quarter of new pediatric asthma cases reported as directly attributable to obesity(11). Third, weight loss in OA adults and children has been shown to improve control of asthma symptoms in some patients (27–41). Finally, obesity is associated with both an increased risk of asthma development and increased asthma severity (26,42–46), and there is evidence that asthma may be an independent predictor of obesity(47,48), suggesting the link between obesity and asthma may be bidirectional(46).
Adult (22,24) and pediatric (21,22,49–51) OA patients experience a treatment-refractory form of asthma that is less responsive to preventative regimens. OA patients generally have more significant asthma exacerbations (49,52) and typically experience increased health care utilization (49) and require greater health care expenditures to control their asthma symptoms. Taken together, these data suggest obesity is both a powerful predictor of asthma incidence and a clear modifiable factor influencing asthma control. However, the multifactorial nature of both asthma and obesity makes establishing mechanistic pathophysiological link(s) between the two chronic inflammatory disorders quite complex. Furthermore, just as asthma is considered to be an “umbrella” diagnosis that encompasses many different mechanistic endotypes and clinical phenotypes, OA is also likely to have a variety of relevant altered molecular pathways underlying clinical heterogeneity(53–55). For example, while the majority of pediatric asthma patients are atopic(56), including in pediatric OA(57), atopy is not a uniform finding in OA. In order to optimize medical care for this complex disease, it is crucial to better understand the impact of OA on key immune pathways and on the functioning of immune cells and apply those mechanistic insights to optimizing therapeutic strategies.
In this review, we summarize recent work elucidating the mechanisms by which obesity impacts inflammation and immune function in asthma and thus contributes to the severe symptomatology observed in OA (Figure 1). We will focus on pediatric OA, but given the limitations of the literature and the importance of long-term impacts, we will incorporate data from adult human subjects as well as mouse models. It is important to note that when engaging in mechanistic studies of asthma or obesity that mouse models can be quite helpful. However, only a minority of mouse models of OA have defined mechanistic links between obesity and asthma and here we will focus on the subset of studies that include evaluation of the impact of obesity and asthma on pulmonary function in OA, non-obese asthma, non-asthmatic obesity and control cohorts (58–69) (Table 1).
Figure 1: Proposed Mechanisms Mediating OA in Children.
Pediatric OA is a complex, heterogenous disease that is likely explained by multiple mechanisms. There is evidence to support roles for obesity-associated changes to lung structure, cytokine expression, metabolite production and hormone secretion in worsening asthma severity.
Table 1: Summary of results from mouse models of OA.
that interrogate lung function in OA cohorts compared to healthy control, asthma-alone and obesity-alone cohorts. Up arrows (↑) indicate enriched populations, down arrows (↓) indicate less prevalent populations, sideways arrows (→) indicate no significant difference measured and double hyphens (--) indicate no data was reported for the given variable. Each color-coded row represents the pairwise comparison specified in the table legend (dark gray= OA vs healthy control; white= OA vs asthma alone, light gray= OA vs obesity alone). Asthma: A= Allergen used to induce asthma, B= Immunization protocol, C= Challenge protocol. Within the immunization protocol (Asthma, B) and challenge protocol (Asthma, C) description, s.c. = subcutaneous, i.n. = intra-nasal, i.t.= intra-tracheal, (w/v) = weight per volume. Diet Induced obesity: D= High-fat diet (HFD) vendor, E= Catalog number of HFD, F= Percent of energy derived from HFD, G=Number of weeks on HFD. Pulmonary Function: AHR= Airway Hyperresponsiveness, AR= Airway Remodeling. Immune Response: Eos= Eosinophils, Neu= Neutrophils, MΦ= Macrophage.
| Publication | Mouse Model | Pulmonary Function | Immune Response | Adipokines | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author | Year | Sex | Strain | Asthma | Diet Induced Obesity | AHR | AR | T cells | Cytokines | Eos | Neu | MΦ | Leptin | Adiponectin |
| Lee [89] | 2019 | F | C57BL/6 |
A) OVA B) 25μg s.c. (2x) C) 20μg/50μl i.n. (4x) |
D) Harlan Laboratories E) D12492 F) 60% G) 12 |
↑ | ↑ | -- | Lung: ↑ IL-17 BALF: ↑ IL-4/5/13/17; ↓IFNγ Serum: ↑ IL-17 |
Lung: ↑1
BALF: ↑ |
Lung: ↑1 BALF: ↑ |
BALF: ↑ | BALF: ↑ Serum: ↑ |
BALF: → Serum: → |
| → | → | -- | Lung: → IL-17 BALF: →IL-4/13/17; ↓IFNγ; ↑ IL-5 Serum: ↑ IL-17 |
Lung: →1 BALF: → |
Lung: →1 BALF: → |
BALF: → | BALF: ↑ Serum: ↑ |
BALF: → Serum: → |
||||||
| -- | -- | -- | -- | -- | -- | -- | -- | -- | ||||||
| Schröder [90] | 2019 | F | C57BL/6JRj |
A) OVA B) 150μg/1mg i.p. (2x) C) 1.5% i.t.(4x) |
D) Ssniff EF R/M E) TD88137 F) 42% G) 12 |
↑ | ↑ | Lung: ↑ CD4+ BALF: ↑ T cells |
BALF: ↑ | BALF: ↑ | BALF: → | -- | -- | |
| ↓ | → | Lung: → CD4+ BALF: ↓ T cells |
BALF: → | BALF: ↓ | BALF: → | -- | -- | |||||||
| ↑ | ↑ | Lung: ↑ CD4+ BALF: → T cells |
BALF: ↑ | BALF: ↑ | BALF: → | -- | -- | |||||||
| Zeng [91] | 2019 | M | C57BL/6 |
A) OVA B) 10μg i.p. (2x) C) 1 mg/mL aerosol (30 min daily × 7d) |
D) MediScience Ltd. E) MD12032 F) 45% G) 16 |
↑ | ↑ | Lung: ↑ Th17 | Lung1: ↑ IL17A, RORγt Serum: ↑IL-1β/6/17A |
-- | -- | -- | -- | -- |
| ↑ | ↑ | Lung: → Th17 | Lung1: → IL17A, RORγt Serum: ↑IL-1β/6/17A |
-- | -- | -- | -- | -- | ||||||
| ↑ | ↑ | Lung: → Th17 | Lung1: → IL17A, RORγt Serum: ↑IL-1β/6/17A |
-- | -- | -- | -- | -- | ||||||
| *Chong [92] | 2018 | M/F | C57BL/6J |
A) OVA B) 0.01% OVA i.p. C) 1% OVA aerosol (30 min daily × 8d) |
D) MediScience Ltd. E) MD12032 F) 45% G) 12 |
↑ | ↑ | -- | -- | BALF: ↑ | BALF: ↑ | -- | -- | -- |
| ↑ | ↑ | -- | -- | -- | -- | -- | -- | -- | ||||||
| ↑ | ↑ | -- | -- | BALF: ↑ | BALF: ↓ | -- | -- | -- | ||||||
| Liang [93] | 2018 | F | C57BL/6 |
A) OVA B) 25μg i.p. (2x) C) 20μg/50μL i.n. (3x) |
D) Harlan Laboratories E) D12492 F) 60% G) 12 |
↑ | ↑ | -- | BALF: ↑ IL-4/5/13/17 | BALF: ↑ | BALF: → | BALF: ↑ | Serum: ↑ | Serum: ↓ |
| ↑ | → | -- | BALF: ↑ IL-4/5/17; → IL-13 | BALF: ↑ | BALF: → | BALF: ↑ | Serum: ↑ | Serum: ↓ | ||||||
| → | ↑ | -- | BALF: ↑ IL-4/5/13/17 | BALF: ↑ | BALF: → | BALF: ↑ | Serum: ↑ | Serum: ↑ | ||||||
| Zeng [94] | 2018 | M | C57BL/6 |
A) OVA B) 10μg i.p. (2x) C) 1 mg/mL aerosol (30 min daily × 7d) |
D) MediScience Ltd. E) MD12032 F) 45% G) 16 |
↑ | ↑ | Spleen: ↑Th17 | Lung1: ↑IL-17A Serum: ↑IL-17 |
-- | -- | -- | -- | -- |
| ↑ | ↑ | Spleen: ↑Th17 | Lung1: ↑IL-17A Serum: ↑IL-17 |
-- | -- | -- | -- | -- | ||||||
| ↑ | ↑ | Spleen: ↑Th17 | Lung1: ↑IL-17A Serum: ↑IL-17 |
-- | -- | -- | -- | -- | ||||||
| *Everaere [101] | 2016 | -- | C57BL/6J |
A) HDM B) 5 IR i.n. (1x) C) 5 IR i.n. (5x) |
D) Research Diet Inc. E) D12492 F) 60% G) 12 |
↑ | ↑ | Lung: ↑ CD4+, IL13+CD4+, IL-17A+CD4+ | Lung2: ↑IL-1β/4/13/17A/23/33 VAT2: → IL-1β/17A, IFNγ; ↓IL-5/33 |
BALF: ↑ | BALF: → | BALF: → | -- | -- |
| ↑ | ↑ | Lung: ↑ CD4+, IL13+CD4+, IL-17A+CD4+ | Lung2: ↑IL-1β/4/13/17A/23/33 VAT2: → IL-1β/17A, IFNγ; ↓IL-5/33 |
BALF: ↑ | BALF: → | BALF: → | -- | -- | ||||||
| ↑ | ↑ | Lung: → CD4+, IL13+CD4+, IL-17A+CD4+ | Lung2: ↑IL-4/13/17A/33; → IL-1β/23 VAT2: → IL-1β/17A, IFNγ; ↓IL-5/33 |
BALF: ↑ | BALF: → | BALF: → | -- | -- | ||||||
| Chen [95] | 2015 | -- | BALB/c |
A) OVA B) 25μg i.p. (3x) C) 6% (w/v) aerosol (daily × duration of diet > 12 weeks) |
D) Research Diet Inc. E) D12451 F) 45% G) 12 |
↑ | ↑ | -- | Serum: ↑IL-4; ↓IFNg | Lung: ↑ | -- | -- | -- | -- |
| ↑ | ↑ | -- | Serum: →IL-4; ↓IFNg | Lung: ↑ | -- | -- | -- | -- | ||||||
| ↑ | ↑ | -- | Serum: ↓IL-4; IFNg | Lung: ↑ | -- | -- | -- | -- | ||||||
| Kim [96] | 2015 | F | C57BL/6 |
A) OVA B) 100μg i.p. (2x) C) 10μg i.n. (3x) |
D) Research Diet Inc. E) D12492 F) 60% G) 16 |
↑ | ↑ | -- | BALF: ↑ TNFα Serum: ↑ TNFα |
BALF: ↑ | BALF: ↑ | -- | Lung: → Serum: → |
Lung: → Serum: → |
| ↑ | → | -- | Lung: ↑ TNFα BALF: ↑ TNFα Serum: ↑ TNFα |
BALF: → | BALF: → | -- | -- | -- | ||||||
| ↑ | ↑ | -- | BALF: ↑ TNFα Serum: ↑ TNFα |
BALF: ↑ | BALF: ↑ | -- | -- | -- | ||||||
| Jung [98] | 2013 | F | C57BL/6J |
A) OVA B) 25μg s.c. (5x) C) 20μg/50μL i.n. days 27, 29, 31 and then twice a week until week 18 |
D) Feedlab E) -- F) 45% G) 16 |
↑ | ↑ | -- | Lung2: ↑ VEGF, TNFα, TGFβ BALF: ↑ VEGF, TNFα, TGFβ |
BALF: ↑ | BALF: ↑ | BALF: ↑ | Lung: ↑ | Lung: ↓ |
| ↓ | ↑ | -- | Lung2: → VEGF, TGFβ; ↑ TNFα BALF: ↑ TNFα; → VEGF; ↓ TGFβ |
BALF: ↑ | BALF: → | BALF: → | Lung: ↑ | Lung: ↓ | ||||||
| ↑ | ↑ | -- | Lung2: → VEGF, TGFβ, ↑ TNFα BALF: ↑TNFα; →VEGF; ↓TGFβ |
BALF: ↑ | BALF: ↑ | BALF: ↑ | Lung: ↓ | Lung: ↑ | ||||||
| Ryu [99] | 2013 | F | C57BL/6J |
A) OVA B) 25μg s.c. (5x) C) 20μg/50μL i.n. days 27, 29, 31 and then twice a week until week 18 |
D) Feedlab E) -- F) 45% G) 16 |
↑ | -- | -- | Lung2: ↑ VEGF, TNFα, TGFβ BALF: ↑ VEGF, TGFβ |
BALF: ↑ | BALF: ↑ | BALF: ↑ | Lung2: ↑ | Lung2: → |
| ↓ | -- | -- | Lung2: ↑ VEGF, TNFα, TGFβ BALF: ↑ TGFβ; → VEGF |
BALF: → | BALF: → | BALF: → | Lung2: → | Lung2: → | ||||||
| ↓ | -- | -- | Lung2: ↑ TNFα, TGFβ; ↓ VEGF BALF: ↓TGFβ, VEGF |
BALF: ↑ | BALF: → | BALF: ↑ | Lung2: → | Lung2: → | ||||||
| Ge [97] | 2013 | M | C57BL/6J |
A) CRA B) 10μg s.c. + 10μg i.p (1x), 1μg s.c. (1x) (2x) C) 1μg i.n. (1x) then 4μg i.n. (1x) |
D) Bio-Serv E) F3282 E) 60% F) 16 |
↑ | ↑ | -- | Lung: ↑ IL-5, → IFNγ, IL-2/4/13, ↓ TNFα, | Lung: ↑ BALF: ↓ |
BALF: ↓ | BALF: ↑ | -- | -- |
| ↑ | ↓ | -- | Lung: → IL-4; ↓ IFNγ, TNFα, IL-2/5/13 | Lung: ↓ BALF: ↓ |
BALF: ↓ | BALF: ↑ | -- | -- | ||||||
| ↑ | ↑ | -- | Lung: ↑ IL-5; → IFNγ, TNFα, IL-2/4/13 | Lung: ↑ BALF: ↑ |
BALF: ↑ | BALF: ↑ | -- | -- | ||||||
| Table Legend |
| Obese Asthma vs Healthy Control |
| Obese Asthma vs Asthma Alone |
| Obese Asthma vs Obesity Alone |
Structural Alterations in Lung Parenchyma and Function
Obesity is linked to reductions in pulmonary function in both adult and pediatric populations which could be secondary to asthma or could, in part, be independent of asthma. While obese adults typically demonstrate a restrictive deficit in pulmonary function, characterized by a reduction in forced vital capacity (FVC) in the presence of a normal ratio between forced expiratory volume in 1 second (FEV1) and FVC (70,71), obese children typically demonstrate an obstructive pattern of pulmonary dysfunction characterized by increases in both FEV1 and FVC, but a lower FEV1/FVC ratio (72–74). Diminished pulmonary function in obese children may be partially a reflection of an altered relationship between lung parenchyma and airway caliber, known as dysanapsis. In the setting of dysanapsis, the growth of lung parenchyma is out of proportion to airway caliber, with normal measurements of FEV1 and FVC in the presence of an abnormal FEV1/FVC ratio(71). Children classified as overweight or obese were more likely to have airway dysanapsis, independent of asthma status (71). Importantly, in children with OA, dysanapsis was closely associated with increased severity of asthma symptoms and poor asthma control (71). Ekström et al. recently found the same pattern of airway resistance associated with persistent pediatric weight gain(75). To our knowledge, dysanapsis has not yet been evaluated in mice. A pathophysiological link between obesity and lung parenchymal growth (out of balance with airway caliber) has not been established and those mechanisms and potential connections to inflammation and immune dysregulation require further study.
Central Adiposity is Associated with Impaired Pulmonary Function and Increased Visceral Adipose Tissue
The quantification of extent and impact of obesity is challenging and age dependent. It is well-established that obesity, typically assessed in adults and children using body mass index (BMI), is an independent predictor of asthma risk (11,12,14–18,21,49,51,76). Children are considered overweight at BMI > 85th percentile and obese at BMI > 95th percentile for sex and age. However, in recent years the clinical relevance of BMI as a predictor of asthma risk has been called into question(77–79). BMI is a calculated assessment of the relationship of weight to height and therefore cannot not differentiate between muscle mass and adipose tissue, nor can it account for body fat distribution. This may explain why some authors have reported no association between obesity and asthma incidence(72,80–82). It is increasingly appreciated that specific patterns of body fat distribution, mainly abdominal obesity (also known as central obesity), may be a better predictor than BMI of pediatric asthma incidence (83–89). The mechanistic relationship between abdominal obesity and asthma remains unclear, especially in pediatric OA. However, there is evidence that poor lung function in asthmatic adults can be attributed to increases in visceral adipose tissue (VAT) (90). Furthermore, increases in VAT, as measured by magnetic resonance imaging or dual-energy x-ray absorptiometry, are associated with impaired pulmonary function and increased asthma risk in pediatric OA (83,89). Given the highly immunologically and metabolically active nature of VAT(9), and its role as a niche for key immune cells(91), it has also been proposed as an important mediator in OA (85). Moreover, several recent studies have reported that obesity-associated inflammatory signaling alters the inflammatory characteristics of VAT (9) in both mice(92–94) and humans (34,95,96). The potential roles of dysregulated VAT resident immune cells and peripheral immune cells impacted by the inflammatory environment in OA is discussed below (in “Immune Dysregulation in OA”).
The Role of Sex Hormones and Pubertal Timing
There are clear connections among sex hormones and asthma and obesity and pubertal timing. First, there is a well-known peripubertal shift in sex bias in asthma incidence(97). Specifically, in pre-pubescent populations asthma and other atopic diseases are more prevalent in males than females, but this trend is reversed post-puberty (97–100). This is seen in both more boys “growing out” of school-age asthma diagnoses, as well as late childhood (97,101) and adult-onset (102,103) asthma being more prevalent in females. Recent investigations have demonstrated potential roles for sex hormones in mediating asthma pathogenesis. For example, early pubertal onset is associated with increased asthma incidence (104,105). The mechanisms underlying this phenomenon remain unclear.
Obesity also has clear impacts on the hormonal state of the individual. Pre-pubescent rapid weight gain has been proposed to accelerate pubertal onset which may then hasten or otherwise encourage the development of asthma (106). A growing body of literature reports that early puberty appears to be correlated with the development of asthma in obese girls (107). A recent retrospective cohort study demonstrated pre-pubescent obesity conferred the highest risk of asthma development (108). Premenarchal females demonstrated an increased risk of developing obesity-related asthma compared to pre-pubertal males. In contrast, a separate study an increase in asthma risk in obese children of both sexes with early puberty (105). Recently, it was shown that in children in the Severe Asthma Research Program (SARP) increased serum androgen was correlated with improved lung function in boys, whereas increased serum estrogen was correlated with reduced lung function in girls (109). A separate population-based study of hormone levels in adult OA patients demonstrated that in obese women, but not non-obese women, increased serum levels of testosterone were associated with decreased asthma risk (110). This supports previous findings suggesting that sex hormones are potent modifiers of asthma and are influenced by obesity (111).
While female sex hormones are generally associated with increased airway inflammation and male sex hormones associated with decreased airway inflammation(112) in human studies, estrogen (113–117) and androgens, including testosterone (118–121), have each demonstrated protective (113–115,118,121) and pro-inflammatory (114–117,119) roles in mouse models of asthma. To our knowledge, only one study has been published that attempts to elucidate a mechanistic link between sex hormones and OA in mice, wherein estrogen mitigated airway inflammation by downregulating NLRP3 activation(122).
Overall, these data highlight the need for improved understanding of immunomodulatory capabilities of estrogen and testosterone, as well as other sex hormones, and the resulting mechanisms by which obesity may alter pubertal timing and affect both asthma onset and severity.
Immune Dysregulation in OA
Asthma and obesity are both chronic states of systemic, low-grade inflammation and have the ability to disrupt normal control points in a wide array of networks in our core physiological systems, ranging from the immune system to the cardiovascular and endocrine systems. In obesity(9,123) and asthma separately(124–129), and in their intersection in OA(34,130), there are alterations in adipokines (e.g. leptin)(124,130), chemokines(127), cytokines(34,125,128), and both innate(126,129) and adaptive cellular immune responses(34,123,129). In addition, there are well known alterations in the serum metabolites that bathe our circulating immune cells (e.g. glutamate(131) and short chain fatty acids(132)) as well as changes in the microbiome that are at least partially responsible for producing those altered metabolite levels(133)). All of these components exist in a complex network, which is carefully balanced. In obesity and asthma individually, there is evidence that this network has been disrupted and in OA we see evidence of immunometabolic dysfunction across human and mouse systems (Tables 1 and 2).
Table 2: Impact of OA on human immune cell subsets in adults and children.
Up arrows (↑) indicate enriched populations, down arrows (↓) indicate less prevalent populations, sideways arrows (→) indicate no significant difference measured for each comparison specified in the column header. Italicized entries indicate results from adult studies and non-italicized entries are from pediatric studies. OA = obese asthma, A= asthma (non-obese), O= obese (non-asthma), HC= healthy control (non-asthma, non-obese).
| Human Obese Asthma | |||||
|---|---|---|---|---|---|
| Results | OA vs HC | OA vs A | OA vs O | ||
| Immune Function | Innate Immune Cells | Eosinophils |
↑(143) submucosal
→(143) sputum and blood |
↑(161) | |
| Monocytes | CD14+CD16- ↓(141) CD14dimCD16+ ↓(141) |
||||
| Adaptive Immune Cells | CD4 T cells | →(128) | →(162) | →(162) | |
|
→(163)
|
→(155,163) | →(163) | |||
| Th1 | ↑(140,141) | ↑(140,141) | |||
| Th2 | ↓(140,141) | ||||
| Treg | ↑(163) | ↑(163) | ↑(163) | ||
| IgE | ↑(161) | ||||
| Type 2 cytokines (serum) | IL-4 | →(140) |
→(140,142,164) ↓(165) |
→(140,142,166) |
|
| IL-5 | →(140) | →(140,164) ↑(143) sputum |
→(140) | ||
| IL-13 | →(140) | ↓(140,164) | →(140) |
||
| Type 1 cytokines (serum) | IFN-g | ↑(165) →(140,141) ↓(142) |
↑(165) →(140–142,164) |
→(140–142) |
|
| TNF-α | ↑(140) →(127) |
→(140) | →(140) | ||
| IL-6 | ↑(140) |
→(140,164) | →(140) | ||
| IL-8 | ↑(164) | ||||
| Adipokines | Leptin | ↑(165) →(140,166) |
↑(140,141,165–167) ↑(166) |
→(140,166) | |
| Adiponectin | →(161) | →(161) | →(161) | ||
| Pulmonary Function | FEV 1 /FVC | →(161) | ↓(140) →(50,156,162,165) |
↓(140) →(161) |
|
Systemic inflammation is typically measured by acute-phase reactants (e.g. C-reactive protein (CRP), serum amyloid A (SAA), interleukin 6 (IL-6) and fibrinogen), which were recently reported to be significantly increased in the serum of OA and obese adults(134,135). Interestingly, SAA, but not CRP, has previously been linked to allergic airway inflammation in human adults (136). In addition, critical roles for SAA in mediating inflammatory responses have been shown in mouse models of asthma(137,138) and obesity(139,140). It is possible that these acute phase reactants, including SAA, may have direct mechanistic roles in OA and should be explored further.
Adipokine dysregulation, including altered leptin, is also a feature of OA. Obesity is associated with elevated serum leptin, which has many effects on immune function(141). Increases in serum leptin were associated with increased atopy in a cohort of children with allergic rhinitis, a related atopic condition (142). Leptin is known to impact immune function in pleotropic ways, including being a neutrophil chemoattractant(143), altering neutrophil chemotaxis and superoxide production in adult OA (144) and is connected to eosinophilic inflammation (145). Additionally, there is evidence that production of leptin by pulmonary tissue, as opposed to the systemic increases in leptin reported in obesity, enhances airway eosinophilia (146). Finally, leptin is known to have direct effects on immune cell function, particularly T cells(147–149), and the alterations in leptin level may have effects on this key anti-viral cell in OA as well(130,150).
Within the myriad of subsets of lymphocytes, CD4 T cells play an important role in guiding the character of the adaptive immune response and produce key cytokines driving that process. It is important to link our understanding of the more easily accessible peripheral blood immunophenotype(151,152) to the more challenging to access immunophenotype of the target tissues (e.g. adipose tissue)(95,153). In the peripheral blood, the inflammatory state triggered by obesity is classically thought to skew CD4+ T helper cells towards a T helper type 1 cell (Th1) phenotype(152,154,155). Allergic (or atopic) asthma, which accounts for more than half of all asthma cases in the United States(150), is classically associated with type 2 immune responses(156). Atopic asthma is linked to type 2 immunity, including alterations in the three main implicated cell types: eosinophils, type 2 Innate lymphoid cells (ILC2s) and CD4 T helper type 2 cells (Th2 cells). Here, we focus on the nature of the immune response in OA. Early studies showed that patients with OA and early onset asthma were more likely to be atopic (and thus likely have type 2 immune skewing) and more likely to have severe asthma(54). Using unsupervised clustering of asthmatic patients, two separate studies a cluster of older, obese, non-eosinophilic female asthmatics was identified (157,158). However, many of the OA patients were found outside this cluster, highlighting again that OA contains multiple endotypes (159). After those initial studies, with very limited immunophenotyping, peripheral blood studies in OA have provided a mixed picture, with evidence of increased (154,160), equivalent(160) and reduced(161) Th1-skewing, as well as evidence for decreased (160) and equivalent (161) Th2-skewing in OA patients compared to asthma alone. To add to the complexity in OA peripheral blood, sputum and endobronchial biopsies, in some settings, show increased eosinophil-relevant IL-5 and eosinophilia respectively in a subset of OA patients(162). Allergic asthma is the predominant form of asthma in children and may be underappreciated as a key endotype of OA, especially in pediatrics. Of note, there are racial and ethnic backgrounds wherein OA is known to be strongly associated with atopy, including in Puerto Rican children(20). Overall, there is not a monomorphic picture of CD4 T cell differentiation in OA, neither Th1 nor Th2, but the impression that there are multiple immune profiles possible in OA.
At the core of the symptomatology of OA is the severity of exacerbations, which are most often caused by viral respiratory infections. CD8 T cells play a key role in combating viral infections and there is recent evidence that obesity may alter their function. Specifically, there is an increase in inhibitory receptor expression (e.g. PD-1) in CD8 T cells from obese patients(163), suggestive of immune dysregulation in these patients. In the setting of persistent antigenic stimulation and chronic inflammation (e.g., chronic viral infection or malignancy) T cells can become exhausted, with altered expression of cell surface markers (including inhibitory receptors), transcriptional pathways and function(164). One of the recent advances in cancer therapy is the use of biologics targeting inhibitory receptors on immune cells to reinvigorate exhausted T cells(164) and improve immune targeting of tumors. Consistent with increased inhibitory receptor levels in obesity, obese patients with melanoma had better responses to these strategies (165,166). Further, should the immune dysregulation in CD8 T cells in obesity be similar to exhaustion, we could also expect concomitant altered mitochondrial function which has been shown in human T cell exhaustion(167).The impact of OA on CD8 T cell immunometabolic function and immunophenotype in the periphery has not yet been assessed in OA, though our group is studying these questions in humans and mice.
Metabolomics of obese and asthmatic patient samples, including from serum/plasma, breath condensate and urine(168–173), have identified altered water soluble and lipid metabolites. There are clear alterations in peripheral metabolites in obesity, including altered short chain fatty acids (SCFA) and water-soluble metabolites (e.g. elevated glutamate)(170–174). These metabolites may be altered in subject serum by diet directly or by alterations of the microbiome in content and/or function by inflammation and/or diet. In diet induced obesity in mice, high fat diet has been shown to yield higher acetate with blockade of allergic asthma and addition of propionate to mouse diet has been shown to impair Th2 differentiation and atopy(175). In addition, characterization of the adult respiratory metabolome delineated a metabolic phenotype in OA(176). This is relevant to immune function in OA because it is clear that SCFA and water soluble metabolites can directly skew T cell differentiation (e.g. elevated acetate and increased mouse Th17 differentiation(177)) and activation, including impacts on infection responses (e.g. acetate and influenza responses(178)). Thus, both water soluble and lipid metabolites may play important roles in the known increased severity of viral induced asthma exacerbations in OA via altered T cell function.
Beyond enumerating immune cell subsets and their individual functional states, transcriptional and epigenetic studies can guide our interpretation of immune profiling and immunometabolic dysregulation in OA. Work from Rastogi et al has shown altered methylation in both activation associated (PI3K pathway) and Th1 associated genes in pediatric OA(179). The former could possibly foster immune dysregulation via mimicry of persistent immune cell activation(164). Subsequent work in peripheral blood transcriptional studies in pediatric OA (both whole blood and CD4 T cells (155,180) and sputum cells from adult OA subjects(129)) has yielded evidence of complex immune dysregulation in these patients. Peripherally, there was evidence of altered CDC42 pathway signaling(152,155), involved in various aspects of CD4 T cell activation and differentiation(155), as well as altered NFκB, integrin and Hedgehog signaling in whole blood(180). In the target tissue, sputum cell RNA sequencing demonstrated a number of transcriptomic alterations, including in a gene module consistent with CTL function(181). This module was inversely correlated with BMI and included a number of markers of both CD8 T cell function (e.g. Granzyme B, IFN-γ, etc) and T cell exhaustion (e.g. TOX). Of note, many markers of CTL function are seen in exhaustion in a dysregulated pattern(167). However, it was unclear whether this suggests that CTL function was impaired in OA (given low gene module activity in OA) or whether an exhausted-like state was instead diminished in OA. Further studies of T cell signaling and activation pathways in OA are needed, including how they connect to clinical outcomes.
With regards to evidence of dysfunction, beyond alterations in cytokines, adipokines, metabolite and immune cell subset and function, we can look to infection responses as an evaluation of cellular function. Both H1N1 influenza(182) and COVID-19(183) have shown increased morbidity and mortality in obese adults and children, and COVID-19, unexpectedly, does not seem to show increased morbidity and mortality in atopic asthma(184). We currently lack information about the impact of OA on COVID-19 in humans and more broadly, additional insight will be gained by studies of baseline immune dysregulation and infection responses in mouse models of OA.
Altering weight and recovering immune function
What is the evidence that obesity is a modifiable factor in OA whose improvement can lead to clinical change? There is evidence that surgical weight loss interventions in adults (27,32,37–40) and non-surgical interventions in both adults (35,36) and children (29–31,33,34,41) lead to an improvement in asthma symptoms. While many of these studies do not report on pro-inflammatory biomarkers (27,29–32), there is evidence that weight loss may lead to a reduction in systemic inflammatory markers in children (34,41) and adults (35,40). However, the influence of bariatric surgery on inflammation and immune function is not well understood and much remains to be learned, especially in children. Some authors report improved asthma control in both children (33) and adults (36) without concomitant improvement in pro-inflammatory markers. Moreover, the efficacy of weight loss interventions in ameliorating asthma symptoms is reportedly influenced by atopy (37,39) and metabolic syndrome (38). To our knowledge, only two studies have investigated the effects of weight loss on systemic inflammation in pediatric OA (34,41). In these studies, non-surgical weight loss reduced systemic inflammatory markers in OA children compared to control groups. In a recent report on obese (non-asthmatic) children, weight loss following lifestyle intervention led to a reduction in serum markers of inflammation compared to baseline(185). The above data suggest the pro-inflammatory obesogenic environment worsens asthma and reductions in body weight can improve asthma control, but the underlying mechanisms warrant further investigation. Our group and others are learning more about alterations in pre/post bariatric surgery immune networks using studies of peripheral blood and adipose tissue.
CONCLUSION
Pediatric OA is a complex condition at the intersection of two chronic inflammatory diseases. Recent work has focused on structural alterations both in lung structure and location of adipose tissue, dysregulation of hormonal and immunometabolic function, with poorly characterized connections among these systems. Recent multi-modal studies in adult and pediatric subjects, with future work planned in human cells and mouse models, bring us closer to the goal of improved therapeutic targeting in these complex patients. However, there remains a dearth of immunometabolic data, especially in pediatric OA, and much remains to be done.
Key Points.
OA is a heterogenous disease comprising multiple clinical phenotypes that are not likely to be explained by a singular mechanism and may be affected by race, age, sex and atopic state, among other phenotypes.
There are significant immunometabolic and structural alterations in pediatric OA that remain incompletely understood.
Mechanistic investigations into pediatric OA, especially as it relates to immune dysfunction and response to viral respiratory infections, are of fundamental importance especially in the era of COVID-19 wherein obesity is a clear risk factor for morbidity and mortality.
1. Acknowledgments:
Thanks to the Henrickson lab for helpful discussions.
2. Financial support:
SEH: NIH K08AI135091 (SEH), the Burroughs Wellcome Fund Career Award for Medical Scientists, Chan Zuckerberg Initiative and CHOP Research Institute Developmental Awards.
Abbreviations:
- OA
Obese Asthma
- AHR
Airway Hyperresponsiveness
- ATM
Adipose Tissue Macrophages
- BMI
Body Mass Index
- FENO
Fractional exhaled nitric oxide
- SARP
Severe Asthma Research Program
- FVC
Forced Vital Capacity
- FEV1
Forced Expiratory Volume in 1 Second
3
Conflicts of interests: SEH has been on ad hoc advisory boards for Horizon Pharma, last 4/2019.
References
- 1.Skevaki C, Van den Berg J, Jones N, Garssen J, Vuillermin P, Levin M, et al. Immune biomarkers in the spectrum of childhood noncommunicable diseases. J Allergy Clin Immunol. 2016. May;137(5):1302–16. [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet Lond Engl. 2017. December 16;390(10113):2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Obesity is a Common, Serious, and Costly Disease [Internet]. Centers for Disease Control and Prevention. 2020. [cited 2020 Nov 28]. Available from: https://www.cdc.gov/obesity/data/adult.html
- 4.Childhood Obesity Facts | Overweight & Obesity | CDC [Internet]. 2019. [cited 2020 Oct 30]. Available from: https://www.cdc.gov/obesity/data/childhood.html
- 5.Most Recent National Asthma Data | CDC [Internet]. 2020. [cited 2020 Sep 18]. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm
- 6.Akinbami L, Fryar C. Current Asthma Prevalence by Weight Status Among Adults: United States, 2001–2014 [Internet]. dixo [cited 2020 Dec 6]. Available from: https://www.cdc.gov/nchs/products/databriefs/db239.htm [PubMed]
- 7.Akinbami LJ, Rossen LM, Fakhouri TH, Simon AE, Kit BK. Contribution of weight status to asthma prevalence racial disparities, 2–19 year olds, 1988–2014. Ann Epidemiol. 2017. August;27(8):472–478.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauthier M, Ray A, Wenzel SE. Evolving Concepts of Asthma. Am J Respir Crit Care Med. 2015. September 15;192(6):660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang X, Henao-Mejia J, Henrickson SE. Obesity and immune status in children. Curr Opin Pediatr [Internet]. 2020. October 21 [cited 2020 Nov 25];Publish Ahead of Print. Available from: https://journals.lww.com/10.1097/MOP.0000000000000953 [DOI] [PMC free article] [PubMed]
- 10.Peters U, Dixon A, Forno E. Obesity and Asthma. J Allergy Clin Immunol. 2018. April;141(4):1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang JE, Bunnell HT, Hossain MJ, Wysocki T, Lima JJ, Finkel TH, et al. Being Overweight or Obese and the Development of Asthma. Pediatrics [Internet]. 2018. December 1 [cited 2020 Sep 16];142(6). Available from: http://pediatrics.aappublications.org/content/142/6/e20182119 [DOI] [PubMed] [Google Scholar]
- 12.Black MH, Zhou H, Takayanagi M, Jacobsen SJ, Koebnick C. Increased Asthma Risk and Asthma-Related Health Care Complications Associated With Childhood Obesity. Am J Epidemiol. 2013. October 1;178(7):1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taveras EM, Rifas-Shiman SL, Camargo CA, Gold DR, Litonjua AA, Oken E, et al. Higher adiposity in infancy associated with recurrent wheeze in a prospective cohort of children. J Allergy Clin Immunol. 2008. May;121(5):1161–1166.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray CS, Canoy D, Buchan I, Woodcock A, Simpson A, Custovic A. Body mass index in young children and allergic disease: gender differences in a longitudinal study. Clin Exp Allergy. 2011;41(1):78–85. [DOI] [PubMed] [Google Scholar]
- 15.Mannino DM, Mott J, Ferdinands JM, Camargo CA, Friedman M, Greves HM, et al. Boys with high body masses have an increased risk of developing asthma: findings from the National Longitudinal Survey of Youth (NLSY). Int J Obes. 2006. January;30(1):6–13. [DOI] [PubMed] [Google Scholar]
- 16.Gold DR, Damokosh AI, Dockery DW, Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol. 2003;36(6):514–21. [DOI] [PubMed] [Google Scholar]
- 17.Castro-Rodríguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased Incidence of Asthmalike Symptoms in Girls Who Become Overweight or Obese during the School Years. Am J Respir Crit Care Med. 2001. May 1;163(6):1344–9. [DOI] [PubMed] [Google Scholar]
- 18.Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS, et al. Obesity and the Risk of Newly Diagnosed Asthma in School-age Children. Am J Epidemiol. 2003. September 1;158(5):406–15. [DOI] [PubMed] [Google Scholar]
- 19.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics [Internet]. 2018. March [cited 2020 Sep 20];141(3). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6109602/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forno E, Acosta-Pérez E, Brehm JM, Han Y-Y, Alvarez M, Colón-Semidey A, et al. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol. 2014. May;133(5):1308–1314.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinto KB, Zuraw BL, Poon K-YT, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol. 2011. November 1;128(5):964–9. [DOI] [PubMed] [Google Scholar]
- 22.Schatz M, Zeiger RS, Zhang F, Chen W, Yang S-J, Camargo CA. Overweight/Obesity and Risk of Seasonal Asthma Exacerbations. J Allergy Clin Immunol Pract. 2013. November 1;1(6):618–22. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Bever HPSV, Lim TK, Kuan WS, Goh DYT, Mahadevan M, et al. Obesity, asthma prevalence and IL-4: Roles of inflammatory cytokines, adiponectin and neuropeptide Y. Pediatr Allergy Immunol. 2015;26(6):530–6. [DOI] [PubMed] [Google Scholar]
- 24.Maalej S, Yaacoub Z, Fakhfekh R, Yaalaoui S, Kheder AB, Drira I. Association of Obesity with Asthma Severity, Control and Quality of Life. Tanaffos. 2012;11(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Dharmage SC, Perret JL, Custovic A. Epidemiology of Asthma in Children and Adults. Front Pediatr [Internet]. 2019. June 18 [cited 2020 Oct 31];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6591438/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barros R, Moreira P, Padrão P, Teixeira VH, Carvalho P, Delgado L, et al. Obesity increases the prevalence and the incidence of asthma and worsens asthma severity. Clin Nutr. 2017. August 1;36(4):1068–74. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa K, Tsugawa Y, Chang Y, Camargo CA. Risk of an asthma exacerbation after bariatric surgery in adults. J Allergy Clin Immunol. 2015. August 1;136(2):288–294.e8. [DOI] [PubMed] [Google Scholar]
- 28.Spriet SW, Davis KL. Diet-Induced Weight Loss in Obese Children With Asthma: A Randomized Controlled Trial. Pediatrics. 2014. November 1;134(Supplement 3):S170–S170. [DOI] [PubMed] [Google Scholar]
- 29.Leeuwen JC van, Hoogstrate M, Duiverman EJ, Thio BJ. Effects of dietary induced weight loss on exercise-induced bronchoconstriction in overweight and obese children. Pediatr Pulmonol. 2014;49(12):1155–61. [DOI] [PubMed] [Google Scholar]
- 30.Willeboordse M, van de Kant KDG, Tan FES, Mulkens S, Schellings J, Crijns Y, et al. A Multifactorial Weight Reduction Programme for Children with Overweight and Asthma: A Randomized Controlled Trial. PLoS ONE [Internet]. 2016. June 13 [cited 2020 Nov 22];11(6). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4905647/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luna-Pech JA, Torres-Mendoza BM, Luna-Pech JA, Garcia-Cobas CY, Navarrete-Navarro S, Elizalde-Lozano AM. Normocaloric Diet Improves Asthma-Related Quality of Life in Obese Pubertal Adolescents. Int Arch Allergy Immunol. 2014;163(4):252–8. [DOI] [PubMed] [Google Scholar]
- 32.Zerah-Lancner F, Boyer L, Rezaiguia-Delclaux S, D’Ortho M-P, Drouot X, Guilloteau-Schoennagel I, et al. Airway Responsiveness Measured by Forced Oscillation Technique in Severely Obese Patients, before and after Bariatric Surgery. J Asthma. 2011. October 1;48(8):818–23. [DOI] [PubMed] [Google Scholar]
- 33.Jensen ME, Gibson PG, Collins CE, Hilton JM, Wood LG. Diet-induced weight loss in obese children with asthma: a randomized controlled trial. Clin Exp Allergy. 2013;43(7):775–84. [DOI] [PubMed] [Google Scholar]
- 34.Al-Sharif FM, Abd El-Kader SM, Neamatallah ZA, AlKhateeb AM. Weight reduction improves immune system and inflammatory cytokines in obese asthmatic patients. Afr Health Sci. 2020. June;20(2):897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FLA, et al. The Role of Exercise in a Weight-Loss Program on Clinical Control in Obese Adults with Asthma. A Randomized Controlled Trial. Am J Respir Crit Care Med. 2016. July 19;195(1):32–42. [DOI] [PubMed] [Google Scholar]
- 36.Dias-Júnior SA, Reis M, Carvalho-Pinto RM de, Stelmach R, Halpern A, Cukier A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014. May 1;43(5):1368–77. [DOI] [PubMed] [Google Scholar]
- 37.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control and inflammation. J Allergy Clin Immunol. 2011. September;128(3):508–515.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forno E, Zhang P, Nouraie M, Courcoulas A, Mitchell JE, Wolfe BM, et al. The impact of bariatric surgery on asthma control differs among obese individuals with reported prior or current asthma, with or without metabolic syndrome. PLoS ONE [Internet]. 2019. April 9 [cited 2020 Dec 6];14(4). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6456172/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JHT, Dixon AE. The influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirol Carlton Vic. 2014. November;19(8):1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Huisstede A, Rudolphus A, Cabezas MC, Biter LU, van de Geijn G-J, Taube C, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015. July 1;70(7):659–67. [DOI] [PubMed] [Google Scholar]
- 41.Kader ME-, Al-Jiffri O, Ashmawy EM. Impact of weight loss on markers of systemic inflammation in obese Saudi children with asthma. Afr Health Sci. 2013. September 5;13(3):682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egan KB, Ettinger AS, Bracken MB. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. BMC Pediatr. 2013. August 13;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ekström S, Magnusson J, Kull I, Andersson N, Bottai M, Besharat Pour M, et al. Body Mass Index Development and Asthma Throughout Childhood. Am J Epidemiol. 2017. July 15;186(2):255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rzehak P, Wijga AH, Keil T, Eller E, Bindslev-Jensen C, Smit HA, et al. Body mass index trajectory classes and incident asthma in childhood: Results from 8 European Birth Cohorts—a Global Allergy and Asthma European Network initiative. J Allergy Clin Immunol. 2013. June 1;131(6):1528–1536.e13. [DOI] [PubMed] [Google Scholar]
- 45.Akinbami LJ, Rossen LM, Fakhouri THI, Fryar CD. Asthma prevalence trends by weight status among US children aged 2–19 years, 1988–2014. Pediatr Obes. 2018. June;13(6):393–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shan L-S, Zhou Q-L, Shang Y-X. Bidirectional Association Between Asthma and Obesity During Childhood and Adolescence: A Systematic Review and Meta-Analysis. Front Pediatr [Internet]. 2020. October 29 [cited 2020 Nov 18];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7658650/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, et al. Effects of Childhood Asthma on the Development of Obesity among School-aged Children. Am J Respir Crit Care Med. 2017. May 1;195(9):1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Contreras ZA, Chen Z, Roumeliotaki T, Annesi-Maesano I, Baïz N, von Berg A, et al. Does early-onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. Eur Respir J [Internet]. 2018. September [cited 2020 Sep 16];52(3). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6443037/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee DS, Gross E, Hotz A, Ngo KC, Rastogi D. Impact of Obesity on Asthma Morbidity During a Hospitalization. Hosp Pediatr. 2018. September;8(9):538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011. March;127(3):741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng X, Ma J, Yuan Y, Zhang Z, Niu W. Association between overweight or obesity and the risk for childhood asthma and wheeze: An updated meta-analysis on 18 articles and 73 252 children. Pediatr Obes. 2019;14(9):e12532. [DOI] [PubMed] [Google Scholar]
- 52.Ahmadizar F, Vijverberg SJH, Arets HGM, de Boer A, Lang JE, Kattan M, et al. Childhood obesity in relation to poor asthma control and exacerbation: a meta-analysis. Eur Respir J. 2016. October 1;48(4):1063–73. [DOI] [PubMed] [Google Scholar]
- 53.Dixon AE, Poynter ME. Mechanisms of Asthma in Obesity. Pleiotropic Aspects of Obesity Produce Distinct Asthma Phenotypes. Am J Respir Cell Mol Biol. 2016. May;54(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma, an association modified by age of asthma onset. J Allergy Clin Immunol. 2011. June;127(6):1486–1493.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Genova LD, Penta L, Biscarini A, Cara GD, Esposito S. Children with Obesity and Asthma: Which Are the Best Options for Their Management? Nutrients [Internet]. 2018. November [cited 2020 Sep 18];10(11). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6267365/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Comberiati P, Di Cicco ME, D’Elios S, Peroni DG. How Much Asthma Is Atopic in Children? Front Pediatr [Internet]. 2017. May 26 [cited 2020 Nov 29];5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5445121/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz A-M, et al. Association of Obesity with IgE and Allergy Symptoms in Children and Adolescents: Results from NHANES 2005–2006. J Allergy Clin Immunol. 2009. May;123(5):1163–1169.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee HY, Lee EG, Hur J, Rhee CK, Kim YK, Lee SY, et al. Pravastatin alleviates allergic airway inflammation in obesity-related asthma mouse model. Exp Lung Res. 2019. November 26;45(9–10):275–87. [DOI] [PubMed] [Google Scholar]
- 59.Schröder T, Wiese AV, Ender F, Quell KM, Vollbrandt T, Duhn J, et al. Short‐term high‐fat diet feeding protects from the development of experimental allergic asthma in mice. Clin Exp Allergy. 2019. September 1;49(9):1245–57. [DOI] [PubMed] [Google Scholar]
- 60.Zeng Z, Wang L, Ma W, Zheng R, Zhang H, Zeng X, et al. Inhibiting the Notch signaling pathway suppresses Th17-associated airway hyperresponsiveness in obese asthmatic mice. Lab Invest. 2019. December;99(12):1784–94. [DOI] [PubMed] [Google Scholar]
- 61.Chong L, Zhang W, Yu G, Zhang H, Zhu L, Li H, et al. High-fat-diet induces airway hyperresponsiveness partly through activating CD38 signaling pathway. Int Immunopharmacol. 2018. March 1;56:197–204. [DOI] [PubMed] [Google Scholar]
- 62.Liang L, Hur J, Kang JY, Rhee CK, Kim YK, Lee SY. Effect of the anti-IL-17 antibody on allergic inflammation in an obesity-related asthma model. Korean J Intern Med. 2018. November;33(6):1210–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng Z, Lin X, Zheng R, Zhang H, Zhang W. Celastrol Alleviates Airway Hyperresponsiveness and Inhibits Th17 Responses in Obese Asthmatic Mice. Front Pharmacol [Internet]. 2018. January 31 [cited 2020 Nov 2];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5797758/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y-P, Zhang J-H, Li C-Q, Sun Q-X, Jiang X-H. Obesity enhances Th2 inflammatory response via natural killer T cells in a murine model of allergic asthma. Int J Clin Exp Med. 2015. September 15;8(9):15403–12. [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JY, Sohn J-H, Lee J-H, Park J-W. Obesity Increases Airway Hyperresponsiveness via the TNF-α Pathway and Treating Obesity Induces Recovery. PLoS ONE [Internet]. 2015. February 6 [cited 2020 Oct 17];10(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4344461/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ge XN, Greenberg Y, Hosseinkhani MR, Long EK, Bahaie NS, Rao A, et al. High-fat diet promotes lung fibrosis and attenuates airway eosinophilia after exposure to cockroach allergen in mice. Exp Lung Res. 2013. November;39(9):365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung SH, Kwon J-M, Shim JW, Kim DS, Jung HL, Park MS, et al. Effects of Diet-Induced Mild Obesity on Airway Hyperreactivity and Lung Inflammation in Mice. Yonsei Med J. 2013. November 1;54(6):1430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryu SL, Shim JW, Kim DS, Jung HL, Park MS, Park S-H, et al. Expression of peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ in the lung tissue of obese mice and the effect of rosiglitazone on proinflammatory cytokine expressions in the lung tissue. Korean J Pediatr. 2013. April;56(4):151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Everaere L, Ait-Yahia S, Molendi-Coste O, Vorng H, Quemener S, LeVu P, et al. Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J Allergy Clin Immunol. 2016. November 1;138(5):1309–1318.e11. [DOI] [PubMed] [Google Scholar]
- 70.Pinto Pereira LM, Seemungal TAR, Teelucksingh S, Nayak BS. Restrictive pulmonary deficit is associated with inflammation in sub-optimally controlled obese diabetics. J Thorac Dis. 2013. June;5(3):289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forno E, Weiner DJ, Mullen J, Sawicki G, Kurland G, Han YY, et al. Obesity and Airway Dysanapsis in Children with and without Asthma. Am J Respir Crit Care Med. 2017. February 1;195(3):314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tantisira K, Litonjua A, Weiss S, Fuhlbrigge A. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP). Thorax. 2003. December;58(12):1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han Y-Y, Forno E, Celedón JC. Adiposity, Fractional Exhaled Nitric Oxide, and Asthma in U.S. Children. Am J Respir Crit Care Med. 2014. July 1;190(1):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weinmayr G, Forastiere F, Büchele G, Jaensch A, Strachan DP, Nagel G. Overweight/Obesity and Respiratory and Allergic Disease in Children: International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. PLoS ONE [Internet]. 2014. December 4 [cited 2020 Sep 16];9(12). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4256390/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ekström S, Hallberg J, Kull I, Protudjer JLP, Thunqvist P, Bottai M, et al. Body mass index status and peripheral airway obstruction in school-age children: a population-based cohort study. Thorax. 2018. June;73(6):538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho W-C, Lin Y-S, Caffrey JL, Lin M-H, Hsu H-T, Myers L, et al. Higher body mass index may induce asthma among adolescents with pre-asthmatic symptoms: a prospective cohort study. BMC Public Health. 2011. July 8;11:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forno E Childhood obesity and asthma –to BMI or not to BMI? J Allergy Clin Immunol. 2017. March;139(3):767–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shore SA. Obesity and asthma: location, location, location. Eur Respir J. 2013. February;41(2):253–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Forno E Moving Beyond the Confines of Body Mass Index in the Quest to Understand Obese Asthma. Am J Respir Crit Care Med. 2019. November 4;201(3):271–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scholtens S, Wijga AH, Seidell JC, Brunekreef B, de Jongste JC, Gehring U, et al. Overweight and changes in weight status during childhood in relation to asthma symptoms at 8 years of age. J Allergy Clin Immunol. 2009. June 1;123(6):1312–1318.e2. [DOI] [PubMed] [Google Scholar]
- 81.Eneli IU, Karmaus WK, Davis S, Kuehr J. Airway hyperresponsiveness and body mass index: The child health and environment cohort study in Hesse, Germany. Pediatr Pulmonol. 2006;41(6):530–7. [DOI] [PubMed] [Google Scholar]
- 82.Lu KD, Phipatanakul W, Perzanowski MS, Balcer-Whaley S, Matsui EC. Atopy, but Not Obesity is Associated with Asthma Severity Among Children with Persistent Asthma. J Asthma Off J Assoc Care Asthma. 2016. December;53(10):1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.den Dekker HT, Ros KPI, de Jongste JC, Reiss IK, Jaddoe VW, Duijts L. Body fat mass distribution and interrupter resistance, fractional exhaled nitric oxide, and asthma at school-age. J Allergy Clin Immunol. 2017. March 1;139(3):810–818.e6. [DOI] [PubMed] [Google Scholar]
- 84.Musaad SMA, Patterson T, Ericksen M, Lindsey M, Dietrich K, Succop P, et al. Comparison of Anthropometric Measures of Obesity in Childhood Allergic Asthma: Central Obesity is Most Relevant. J Allergy Clin Immunol. 2009. June;123(6):1321–7.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang D, Wang L, Bai C, Chen O. Association between abdominal obesity and asthma: a meta-analysis. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol [Internet]. 2019. March 22 [cited 2020 Oct 31];15. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6431003/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y-C, Tu Y-K, Huang K-C, Chen P-C, Chu D-C, Lee YL. Pathway from Central Obesity to Childhood Asthma. Physical Fitness and Sedentary Time Are Leading Factors. Am J Respir Crit Care Med. 2014. March 26;189(10):1194–203. [DOI] [PubMed] [Google Scholar]
- 87.Chih A-H, Chen Y-C, Tu Y-K, Huang K-C, Chiu T-Y, Lee YL. Mediating pathways from central obesity to childhood asthma: a population-based longitudinal study. Eur Respir J. 2016. September 1;48(3):748–57. [DOI] [PubMed] [Google Scholar]
- 88.Papoutsakis C, Chondronikola M, Antonogeorgos G, Papadakou E, Matziou V, Drakouli M, et al. Associations between central obesity and asthma in children and adolescents: a case–control study. J Asthma. 2015. February 7;52(2):128–34. [DOI] [PubMed] [Google Scholar]
- 89.Mensink-Bout SM, Santos S, van Meel ER, Oei EHG, de Jongste JC, Jaddoe VWV, et al. General and Organ Fat Assessed by Magnetic Resonance Imaging and Respiratory Outcomes in Childhood. Am J Respir Crit Care Med. 2019. October 9;201(3):348–55. [DOI] [PubMed] [Google Scholar]
- 90.de Oliveira PD, Wehrmeister FC, Horta BL, Pérez-Padilla R, de França GVA, Gigante DP, et al. Visceral and subcutaneous abdominal adiposity and pulmonary function in 30-year-old adults: a cross-sectional analysis nested in a birth cohort. BMC Pulm Med. 2017. November 28;17(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han S-J, Glatman Zaretsky A, Andrade-Oliveira V, Collins N, Dzutsev A, Shaik J, et al. White Adipose Tissue Is a Reservoir for Memory T Cells and Promotes Protective Memory Responses to Infection. Immunity. 2017. December;47(6):1154–1168.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vasanthakumar A, Chisanga D, Blume J, Gloury R, Britt K, Henstridge DC, et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature. 2020. March;579(7800):581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ishikawa A, Wada T, Nishimura S, Ito T, Okekawa A, Onogi Y, et al. Estrogen regulates sex-specific localization of regulatory T cells in adipose tissue of obese female mice. PLoS ONE [Internet]. 2020. April 2 [cited 2020 Dec 4];15(4). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7117686/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spallanzani RG, Zemmour D, Xiao T, Jayewickreme T, Li C, Bryce PJ, et al. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose-tissue immune and metabolic tenors. Sci Immunol [Internet]. 2019. May 3 [cited 2020 Dec 4];4(35). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6648660/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Periyalil HA, Wood LG, Wright TA, Karihaloo C, Starkey MR, Miu AS, et al. Obese asthmatics are characterized by altered adipose tissue macrophage activation. Clin Exp Allergy. 2018;48(6):641–9. [DOI] [PubMed] [Google Scholar]
- 96.Sideleva O, Suratt B, Black K, Tharp W, Pratley R, Forgione P, et al. Obesity and Asthma An Inflammatory Disease of Adipose Tissue Not the Airway. Am J Respir Crit Care Med. 2012. July 26;186:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fu L, Freishtat RJ, Gordish-Dressman H, Teach SJ, Resca L, Hoffman EP, et al. Natural Progression of Childhood Asthma Symptoms and Strong Influence of Sex and Puberty. Ann Am Thorac Soc. 2014. July;11(6):939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yung JA, Fuseini H, Newcomb DC. Sex hormones, gender and asthma. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2018. May;120(5):488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arathimos R, Granell R, Haycock P, Richmond RC, Yarmolinsky J, Relton CL, et al. Genetic and observational evidence supports a causal role of sex hormones on the development of asthma. Thorax. 2019. July;74(7):633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trivedi M, Denton E. Asthma in Children and Adults—What Are the Differences and What Can They Tell us About Asthma? Front Pediatr [Internet]. 2019. [cited 2020 Oct 5];7. Available from: https://www.frontiersin.org/articles/10.3389/fped.2019.00256/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vink NM, Postma DS, Schouten JP, Rosmalen JGM, Boezen HM. Gender differences in asthma development and remission during transition through puberty: The TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010. September 1;126(3):498–504.e6. [DOI] [PubMed] [Google Scholar]
- 102.de Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G. Incidence and remission of asthma: A retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002. August 1;110(2):228–35. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, Stewart P, Johansen H, McRae L, Taylor G. Sex difference in hospitalization due to asthma in relation to age. J Clin Epidemiol. 2003. February 1;56(2):180–7. [DOI] [PubMed] [Google Scholar]
- 104.Minelli C, van der Plaat DA, Leynaert B, Granell R, Amaral AFS, Pereira M, et al. Age at puberty and risk of asthma: A Mendelian randomisation study. PLoS Med [Internet]. 2018. August 7 [cited 2020 Nov 18];15(8). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6080744/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Y-C, Fan H-Y, Yang C, Lee. Early pubertal maturation and risk of childhood asthma: A Mendelian randomization and longitudinal study. Allergy. 2020;75(4):892–900. [DOI] [PubMed] [Google Scholar]
- 106.Chen Y-C, Lee YL. Relationship between early pubertal maturation and asthma: The role of adiposity rebound in early childhood. Allergy. 2020;75(4):999–1000. [DOI] [PubMed] [Google Scholar]
- 107.Castro-Rodriguez JA. A new childhood asthma phenotype: obese with early menarche. Paediatr Respir Rev. 2016. Mar 1;18:85–9. [DOI] [PubMed] [Google Scholar]
- 108.Lang JE, Bunnell HT, Lima JJ, Hossain MJ, Wysocki T, Bacharier L, et al. Effects of age, sex, race/ethnicity, and allergy status in obesity-related pediatric asthma. Pediatr Pulmonol. 2019;54(11):1684–93. [DOI] [PubMed] [Google Scholar]
- 109.DeBoer MD, Phillips BR, Mauger DT, Zein J, Erzurum SC, Fitzpatrick AM, et al. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm Med [Internet]. 2018. April 10 [cited 2020 Nov 18];18. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5891903/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Han Y-Y, Forno E, Celedón JC. Sex Steroid Hormones and Asthma in a Nationwide Study of U.S. Adults. Am J Respir Crit Care Med. 2019. September 16;201(2):158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scott HA, Gibson PG, Garg ML, Upham JW, Wood LG. Sex hormones and systemic inflammation are modulators of the obese-asthma phenotype. Allergy. 2016;71(7):1037–47. [DOI] [PubMed] [Google Scholar]
- 112.Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. 2017. March;17(3):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cai Y, Zhou J, Webb DC. Estrogen Stimulates Th2 Cytokine Production and Regulates the Compartmentalisation of Eosinophils during Allergen Challenge in a Mouse Model of Asthma. Int Arch Allergy Immunol. 2012;158(3):252–60. [DOI] [PubMed] [Google Scholar]
- 114.Kalidhindi RSR, Ambhore NS, Bhallamudi S, Loganathan J, Sathish V. Role of Estrogen Receptors α and β in a Murine Model of Asthma: Exacerbated Airway Hyperresponsiveness and Remodeling in ERβ Knockout Mice. Front Pharmacol [Internet]. 2020. February 4 [cited 2020 Nov 21];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7010956/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ambhore NS, Kalidhindi RSR, Loganathan J, Sathish V. Role of Differential Estrogen Receptor Activation in Airway Hyperreactivity and Remodeling in a Murine Model of Asthma. Am J Respir Cell Mol Biol. 2019. October;61(4):469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cephus J, Gandhi VD, Shah R, Brooke Davis J, Fuseini H, Yung JA, et al. Estrogen receptor‐α signaling increases allergen‐induced IL‐33 release and airway inflammation. Allergy. 2020. July 26;all.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fuseini H, Cephus J-Y, Wu P, Davis JB, Contreras DC, Gandhi VD, et al. ERα Signaling Increased IL-17A Production in Th17 Cells by Upregulating IL-23R Expression, Mitochondrial Respiration, and Proliferation. Front Immunol [Internet]. 2019. November 27 [cited 2020 Nov 30];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6892971/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laffont S, Blanquart E, Savignac M, Cénac C, Laverny G, Metzger D, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017. June 5;214(6):1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Becerra-Díaz M, Strickland AB, Keselman A, Heller NM. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J Immunol Baltim Md 1950. 2018. November 15;201(10):2923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fuseini H, Yung JA, Cephus JY, Zhang J, Goleniewka K, Poloshukin VV, et al. Testosterone decreases house dust mite-induced type 2 and IL-17A mediated airway inflammation. J Immunol Baltim Md 1950. 2018. October 1;201(7):1843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cephus J, Stier M, Fuseini H, Yung J, Toki S, Bloodworth M, et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017. November 28;21(9):2487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng C, Wu H, Wang M, Wang L, Zou H, Li S, et al. Estrogen ameliorates allergic airway inflammation by regulating activation of NLRP3 in mice. Biosci Rep [Internet]. 2019. January 8 [cited 2020 Nov 20];39(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6328879/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Song J, Deng T. The Adipocyte and Adaptive Immunity. Front Immunol [Internet]. 2020. November 27 [cited 2021 Jan 4];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7728694/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hao W, Wang J, Zhang Y, Wang Y, Sun L, Han W. Leptin positively regulates MUC5AC production and secretion induced by interleukin-13 in human bronchial epithelial cells. Biochem Biophys Res Commun. 2017. November 18;493(2):979–84. [DOI] [PubMed] [Google Scholar]
- 125.Liang Z, Liu L, Zhao H, Xia Y, Zhang W, Ye Y, et al. A Systemic Inflammatory Endotype of Asthma With More Severe Disease Identified by Unbiased Clustering of the Serum Cytokine Profile. Medicine (Baltimore) [Internet]. 2016. June 24 [cited 2021 Jan 4];95(25). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4998303/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Su M-W, Lin W-C, Tsai C-H, Chiang B-L, Yang Y-H, Lin Y-T, et al. Childhood asthma clusters reveal neutrophil-predominant phenotype with distinct gene expression. Allergy. 2018;73(10):2024–32. [DOI] [PubMed] [Google Scholar]
- 127.Godwin MS, Jones M, Blackburn JP, Yu Z, Matalon S, Hastie AT, et al. The chemokine CX3CL1/fractalkine regulates immunopathogenesis during fungal-associated allergic airway inflammation. Am J Physiol-Lung Cell Mol Physiol. 2020. December 16;ajplung.00376.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lambrecht BN, Hammad H, Fahy JV. The Cytokines of Asthma. Immunity. 2019. April 16;50(4):975–91. [DOI] [PubMed] [Google Scholar]
- 129.Peters MC, Ringel L, Dyjack N, Herrin R, Woodruff PG, Rios C, et al. A Transcriptomic Method to Determine Airway Immune Dysfunction in T2-High and T2-Low Asthma. Am J Respir Crit Care Med. 2019. February 15;199(4):465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Youssef DM, Elbehidy RM, Shokry DM, Elbehidy EM. The influence of leptin on Th1/Th2 balance in obese children with asthma. J Bras Pneumol Publicaçaäo Of Soc Bras Pneumol E Tisilogia. 2013. October;39(5):562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maltais-Payette I, Boulet M-M, Prehn C, Adamski J, Tchernof A. Circulating glutamate concentration as a biomarker of visceral obesity and associated metabolic alterations. Nutr Metab. 2018. December;15(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity. 2010. January;18(1):190–5. [DOI] [PubMed] [Google Scholar]
- 133.Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med. 2019. June 12;11(496):eaav1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Michalovich D, Rodriguez-Perez N, Smolinska S, Pirozynski M, Mayhew D, Uddin S, et al. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat Commun [Internet]. 2019. December 13 [cited 2020 Sep 30];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6911092/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma IL6 levels, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016. July;4(7):574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Büyüköztürk S, Gelincik AA, Genç S, Koçak H, Öneriyidogan Y, Erden S, et al. Acute Phase Reactants in Allergic Airway Disease. Tohoku J Exp Med. 2004;204(3):209–13. [DOI] [PubMed] [Google Scholar]
- 137.Smole U, Gour N, Phelan J, Hofer G, Köhler C, Kratzer B, et al. Serum amyloid A is a soluble pattern recognition receptor that drives type 2 immunity. Nat Immunol. 2020. July;21(7):756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ather JL, Fortner KA, Budd RC, Anathy V, Poynter ME. Serum amyloid A inhibits dendritic cell apoptosis to induce glucocorticoid resistance in CD4 + T cells. Cell Death Dis. 2013. September;4(9):e786–e786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ather JL, Poynter ME. Serum amyloid A3 is required for normal weight and immunometabolic function in mice. PLoS ONE [Internet]. 2018. February 1 [cited 2020 Sep 30];13(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5794179/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.den Hartigh LJ, Wang S, Goodspeed L, Ding Y, Averill M, Subramanian S, et al. Deletion of Serum Amyloid A3 Improves High Fat High Sucrose Diet-Induced Adipose Tissue Inflammation and Hyperlipidemia in Female Mice. PLoS ONE [Internet]. 2014. September 24 [cited 2020 Oct 12];9(9). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4177399/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heredia FP de, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012. May;71(2):332–8. [DOI] [PubMed] [Google Scholar]
- 142.Liu W, Zeng Q, Chen Y, Luo RZ. Role of Leptin/Osteopontin Axis in the Function of Eosinophils in Allergic Rhinitis with Obesity. Mediators Inflamm [Internet]. 2018. October 24 [cited 2020 Nov 23];2018. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6220382/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Montecucco F, Bianchi G, Gnerre P, Bertolotto M, Dallegri F, Ottonello L. Induction of Neutrophil Chemotaxis by Leptin. Ann N Y Acad Sci. 2006;1069(1):463–71. [DOI] [PubMed] [Google Scholar]
- 144.Brotfain E, Hadad N, Shapira Y, Avinoah E, Zlotnik A, Raichel L, et al. Neutrophil functions in morbidly obese subjects. Clin Exp Immunol. 2015. July;181(1):156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amorim NRT, Souza-Almeida G, Luna-Gomes T, Bozza PT, Canetti C, Diaz BL, et al. Leptin Elicits In Vivo Eosinophil Migration and Activation: Key Role of Mast Cell-Derived PGD2. Front Endocrinol [Internet]. 2020. September 29 [cited 2020 Nov 23];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7551309/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ubags ND, Vernooy JH, Burg E, Hayes C, Bement J, Dilli E, et al. The role of leptin in the development of pulmonary neutrophilia in infection and Acute Lung Injury. Crit Care Med. 2014. February;42(2):e143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rivadeneira DB, DePeaux K, Wang Y, Kulkarni A, Tabib T, Menk AV, et al. Oncolytic Viruses Engineered to Enforce Leptin Expression Reprogram Tumor-Infiltrating T Cell Metabolism and Promote Tumor Clearance. Immunity. 2019. September 17;51(3):548–560.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gerriets VA, Danzaki K, Kishton RJ, Eisner W, Nichols AG, Saucillo DC, et al. Leptin Directly Promotes T Cell Glycolytic Metabolism to Drive Effector T cell Differentiation in Autoimmunity. Eur J Immunol. 2016. August;46(8):1970–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moraes‐Vieira PMM, Larocca RA, Bassi EJ, Peron JPS, Andrade‐Oliveira V, Wasinski F, et al. Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur J Immunol. 2014;44(3):794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng H, Wu D, Wu X, Zhang X, Zhou Q, Luo Y, et al. Leptin Promotes Allergic Airway Inflammation through Targeting the Unfolded Protein Response Pathway. Sci Rep [Internet]. 2018. June 11 [cited 2020 Dec 2];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5995879/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nagakumar P, Puttur F, Gregory LG, Denney L, Fleming L, Bush A, et al. Pulmonary type-2 innate lymphoid cells in paediatric severe asthma: phenotype and response to steroids. Eur Respir J [Internet]. 2019. August 1 [cited 2020 Dec 5];54(2). Available from: https://erj.ersjournals.com/content/54/2/1801809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rastogi D, Johnston AD, Nico J, Loh LN, Jorge Y, Suzuki M, et al. Functional Genomics of the Pediatric Obese Asthma Phenotype Reveal Enrichment of Rho-GTPase Pathways. Am J Respir Crit Care Med. 2020. April 7;202(2):259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pachón-Peña G, Serena C, Ejarque M, Petriz J, Duran X, Oliva-Olivera W, et al. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells From Adipose Tissue. Stem Cells Transl Med. 2016. April;5(4):464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, et al. Inflammation, Metabolic Dysregulation, and Pulmonary Function among Obese Urban Adolescents with Asthma. Am J Respir Crit Care Med. 2015. January 15;191(2):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rastogi D, Nico J, Johnston AD, Tobias TAM, Jorge Y, Macian F, et al. CDC42-related genes are upregulated in T helper cells from obese asthmatic children. J Allergy Clin Immunol. 2018. February;141(2):539–548.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Caminati M, Pham DL, Bagnasco D, Canonica GW. Type 2 immunity in asthma. World Allergy Organ J [Internet]. 2018. June 26 [cited 2021 Jan 4];11(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6020328/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster Analysis and Clinical Asthma Phenotypes. Am J Respir Crit Care Med. 2008. August;178(3):218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of Asthma Phenotypes Using Cluster Analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010. February 15;181(4):315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Peters MC, Fahy JV. Type 2 Immune Responses in Obese Individuals with Asthma. Am J Respir Crit Care Med. 2013. September 15;188(6):633–4. [DOI] [PubMed] [Google Scholar]
- 160.Rastogi D, Canfield SM, Andrade A, Isasi CR, Hall CB, Rubinstein A, et al. Obesity-Associated Asthma in Children: A Distinct Entity. Chest. 2012. April 1;141(4):895–905. [DOI] [PubMed] [Google Scholar]
- 161.Sánchez-Zauco N, del Rio-Navarro B, Gallardo-Casas C, del Rio-Chivardi J, Muriel-Vizcaino R, Rivera-Pazos C, et al. High expression of Toll-like receptors 2 and 9 and Th1/Th2 cytokines profile in obese asthmatic children. Allergy Asthma Proc. 2014. May 1;35(3):e34–41. [DOI] [PubMed] [Google Scholar]
- 162.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, et al. Elevated Sputum Interleukin-5 and Submucosal Eosinophilia in Obese Individuals with Severe Asthma. Am J Respir Crit Care Med. 2013. September 15;188(6):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019. January;25(1):141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol. 2019. April 26;37(1):457–95. [DOI] [PubMed] [Google Scholar]
- 165.Woodall MJ, Neumann S, Campbell K, Pattison ST, Young SL. The Effects of Obesity on Anti-Cancer Immunity and Cancer Immunotherapy. Cancers. 2020. May 14;12(5):1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018. March;19(3):310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bengsch B, Ohtani T, Khan O, Setty M, Manne S, O’Brien S, et al. Epigenomic-Guided Mass Cytometry Profiling Reveals Disease-Specific Features of Exhausted CD8 T Cells. Immunity. 2018. May;48(5):1029–1045.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Adamko DJ. Urine metabolomic profiling of children with respiratory tract infections in the emergency department: a pilot study. 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Adamko DJ, Nair P, Mayers I, Tsuyuki RT, Regush S, Rowe BH. Metabolomic profiling of asthma and chronic obstructive pulmonary disease: A pilot study differentiating diseases. J ALLERGY CLIN IMMUNOL. 2015;136(3):13. [DOI] [PubMed] [Google Scholar]
- 170.Cirulli ET, Guo L, Leon Swisher C, Shah N, Huang L, Napier LA, et al. Profound Perturbation of the Metabolome in Obesity Is Associated with Health Risk. Cell Metab. 2019. February;29(2):488–500.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Petrus P, Lecoutre S, Dollet L, Wiel C, Sulen A, Gao H, et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020. February 4;31(2):375–390.e11. [DOI] [PubMed] [Google Scholar]
- 172.Yamakado M, Tanaka T, Nagao K, Ishizaka Y, Mitushima T, Tani M, et al. Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin Obes. 2012;2(1–2):29–40. [DOI] [PubMed] [Google Scholar]
- 173.Takashina C, Tsujino I, Watanabe T, Sakaue S, Ikeda D, Yamada A, et al. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr Metab. 2016. January 19;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Al-Sulaiti H, Diboun I, Agha MV, Mohamed FFS, Atkin S, Dömling AS, et al. Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J Transl Med. 2019. October 22;17(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014. February;20(2):159–66. [DOI] [PubMed] [Google Scholar]
- 176.Maniscalco M, Paris D, Melck DJ, D’Amato M, Zedda A, Sofia M, et al. Coexistence of obesity and asthma determines a distinct respiratory metabolic phenotype. J Allergy Clin Immunol. 2017. May 1;139(5):1536–1547.e5. [DOI] [PubMed] [Google Scholar]
- 177.Park J, Kim M, Kang S, Jannasch A, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. 2015;8(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Balmer ML, Ma EH, Bantug GR, Grählert J, Pfister S, Glatter T, et al. Memory CD8 + T Cells Require Increased Concentrations of Acetate Induced by Stress for Optimal Function. Immunity. 2016. June;44(6):1312–24. [DOI] [PubMed] [Google Scholar]
- 179.Rastogi D, Suzuki M, Greally JM. Differential epigenome-wide DNA methylation patterns in childhood obesity-associated asthma. Sci Rep [Internet]. 2013. July 16 [cited 2020 Sep 30];3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3712321/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Croteau‐Chonka DC, Chen Z, Barnes KC, Barraza‐Villarreal A, Celedón JC, Gauderman WJ, et al. Gene Coexpression Networks in Whole Blood Implicate Multiple Interrelated Molecular Pathways in Obesity in People with Asthma. Obesity. 2018;26(12):1938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fitzpatrick AM, Gillespie SE, Mauger DT, Phillips BR, Bleecker ER, Israel E, et al. Racial disparities in asthma-related healthcare utilization in the National Heart, Lung and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2019. June;143(6):2052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fezeu L, Julia C, Henegar A, Bitu J, Hu FB, Grobbee DE, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis: Influenza A and obesity comorbidities: meta-analysis. Obes Rev. 2011. August;12(8):653–9. [DOI] [PubMed] [Google Scholar]
- 183.Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr [Internet]. 2020. June 3 [cited 2020 Oct 31]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7270880/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020. August;146(2):327–329.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Marti A, Morell‐Azanza L, Rendo‐Urteaga T, García‐Calzón S, Ojeda‐Rodríguez A, Martín‐Calvo N, et al. Serum and gene expression levels of CT-1, IL-6, and TNF-α after a lifestyle intervention in obese children. Pediatr Diabetes. 2017;19(2):217–22. [DOI] [PubMed] [Google Scholar]


