Abstract
The COVID-19 vaccination campaign is an ongoing worldwide effort to vaccinate large numbers of people against COVID-19 in order to ensure protection from the disease, control the rate of infection, reduce severe outcomes, and get back to normal life. Most African countries had a delay in the initiation of their COVID-19 vaccine national rollout compared to other regions in the world, and the goal of the immunization exercise in the continent is to vaccinate over 60% of the African population to attain herd immunity. Over the years, vaccination programmes are usually faced with challenges in Africa because of numerous factors. So far, some of the major challenges threatening the success of the COVID-19 vaccination rollout in most African countries includes the slow onset of the vaccination exercise, limited funds, concerns around vaccine safety and uncertainties, storage requirements and regulatory hurdles for vaccines, limited shelf life of COVID-19 vaccines, inability to access vulnerable communities in a timely fashion, problems around the use of different vaccines, and wars and conflicts. The solutions and other imperative recommendations to these challenges were provided so as to optimize the vaccination programme and to achieve an appreciable success in the COVID-19 vaccination programme on the continent. In conclusion, a holistic and timely planning, fast execution of plans, rigorous community involvement, and a robust multi-sectoral partnership will ensure a successful COVID-19 vaccination campaign in Africa.
Keywords: COVID-19, vaccination, Africa, challenges, solutions
Introduction
The first case of coronavirus disease-19 (COVID-19), which is causing a global health crisis, was confirmed on the continent of Africa on February 14th, 2020 in Egypt, while the very first case of the disease in a sub-Saharan African country was reported in Nigeria at the end of February, 2020.1 Due to suboptimal testing capacity, and non-adherence to preventive measures, the disease swiftly spread across Africa within months, and towards the end of May 2020, community transmission of the virus was evident in most countries in the continent.1 Although the novel disease was first reported in China, the majority of the confirmed infections in Africa during the early periods of the pandemic were imported from European countries and the United States (US) due to unrestricted travelling.2
Importantly, some other virulent strains of severe acute respiratory syndrome-2 (SARS-CoV-2) for example the alpha, beta and delta strains had been reported in over 38, 35 and 26 countries in Africa, respectively, while a general resurgence of COVID-19 infection cases have been evident in over 22 countries on the continent as of 8th August 2021.3 Globally, the alpha, beta, gamma, and the delta strains are classified as variants of concerns, while the epsilon, zeta, eta, theta, iota, kappa, and lambda strains are noted as variants of interests by health authorities and researchers.4
As the pandemic progresses, there were assiduous global efforts in search of viable vaccines to control the tides of alarming infection rates. Some countries in Europe started their COVID-19 vaccine rollout earlier on 21st December 2020, following the national and European Medicine Approval for Comirnaty urgent use, while on 31st December 2020, the permission and an urgent global use for a COVID-19 vaccine were granted by the World Health Organization (WHO), subsequently, such approvals for other numerous viable COVID-19 vaccines have also been granted.5,6 The equal accessibility to these harmless and safe vaccines for all countries is imperative to ending the pandemic.
Presently, there are massive vaccination programmes against COVID-19 ongoing in various continents and countries of the world. Despite the proven data for vaccine safety and efficacy, and coupled with the beneficial effects of vaccination for example, protection from severe COVID-19 disease outcomes, African countries remain the least vaccinated in the world owing to numerous challenges.6 The aims of this study are to provide extensive information on the vaccination programme for COVID-19, challenges, together with recommendations for mitigating these challenges, with focus on Africa because there is evidence of effectiveness of the COVID-19 vaccines that are widely used for the immunization exercise in Africa (Pfizer/BioNTech, Moderna and Oxford/AstraZeneca) from other parts and regions of the world such as in Israel, Scotland, US, and United Kingdom.7-10
Review criteria
The study employed the narrative review format to summarize the challenges confronting the ongoing COVID-19 vaccination programme in Africa, and suggestions were provided in order to control these challenges. Secondary data such as articles indexed in PubMed, Medline, Google, Scopus, Google scholar, and other various website pages which include the World Health Organization, Africa Center for Disease Control and Prevention, United Nations, US Food and Drug Administration, and International Committee of the Red Cross were searched to get viable literature for this study. The following keywords “COVID-19 in Africa”, “COVID-19 vaccination in Africa” and “Challenges to COVID-19 vaccination in Africa” were used as search terms between January 2021 - July 2021. Peer reviewed literatures and viable website documents were majorly limited to publications in the last two years, and included all types of studies if they were in line with the aims and objectives of this review, as stipulated in the title. In total, 54 articles were critically screened and deemed appropriate for discussion in this study, and are presented as follows.
Some of the major challenges to a successful COVID-19 vaccine rollout in Africa
On 13th January 2021, the African Union reported the procurement of nearly 300 million vaccine doses against COVID-19 for countries in the continent.11 This massive deal was a separate effort of the COVID-19 vaccine Global Access (COVAX) who also planned on getting millions of COVID-19 vaccines for underdeveloped and developing nations of the world.11 In spite of these vaccines procurement deals, and vaccines availability in Africa, the continent is the least vaccinated in the world despite having a combined COVID-19 case count of over 7 million as of 24th August, 2021.12
Over the years, various immunization programmes have been beneficial to public health in Africa.7 For example, the control, elimination, and eradication of some communicable disease such as diphtheria, pertussis, tetanus, measles, and polio have been achieved through different levels of immunization programmes in the continent.13-15
The current COVID-19 vaccination programme in Africa is the continent’s biggest-ever immunization campaign, and part of a worldwide effort to abate and get under control the rampaging COVID-19. So far, the majority of countries in Africa have begun the administration of COVID-19 vaccines for its populations and a combined figure of over 24 million shots have been administered in Africa, which is just 1.7% of the continent’s population having received their full doses as of 25th August, 2021.16
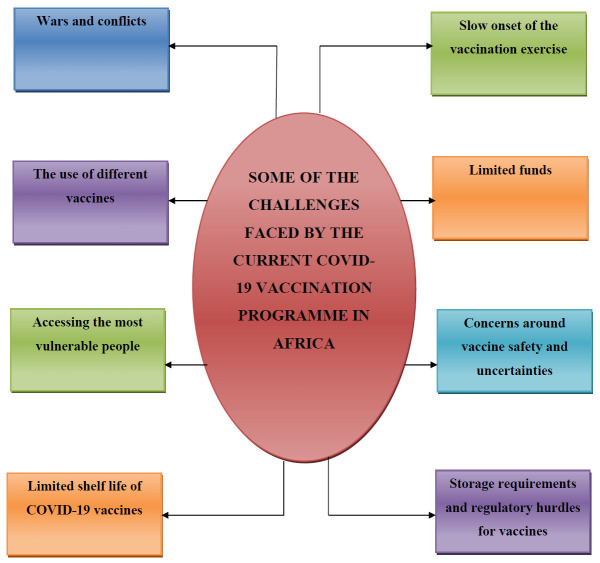
Despite the landmark achievements in the procurement of COVID-19 vaccines, and the initial progress made at the beginning of the vaccination exercise, the vaccination momentum is subsiding presently in Africa. For instance, because of numerous factors, less than 1% of the total COVID-19 shots administered in the world have been given in the continent.17 Highlighted below are some of the challenges hindering the successful implementation of the immunization programme against COVID-19 in Africa (Figure 1).
Figure 1.
Pictorial description of some of the challenges faced by the current COVID-19 vaccination programme in Africa
Slow onset of the vaccination exercise
During the early periods of December 2020, certain numbers of economically stable nations, including the United States of America, Canada, Britain, Switzerland, Bahrain, Malaysia, Mexico, China and Russia, began vaccinating their citizens against COVID-19 with the Pfizer, Moderna, and Sputnik V vaccines; African countries were still behind. In late January 2021 around 900 million COVID-19 vaccines doses were procured via various partnerships to be distributed across African countries, however hoarding by rich countries and other logistic problems such as funding and vaccines storage requirements led to slowed distributions of the vaccines to intending African countries.18
The COVID-19 vaccination programmes began in most African countries in March, 2021 after most countries received its first batches of the Oxford/AstraZeneca COVID-19 vaccines through the COVAX initiatives.17 There have been mixed outcomes from the ongoing COVID-19 vaccination exercise in Africa. According to the WHO, during the early periods of the vaccination exercise on the continent, only eight African countries exhausted their COVAX shots; nine nations administered less than a quarter of their shots, and fifteen nations administered less than half of the COVID-19 shots received.17 This majorly indicates low drive in the vaccination exercise on the continent, which is partly due to limited trained vaccination workers, inadequate funds, disorganized planning, inadequate medical infrastructures, hesitancy in the public against vaccine uptake, and specific countries or in-country regulatory hurdles.
In addition, countries like Nigeria, Ghana, and Kenya have been holding up for further supplies to their initial Oxford/AstraZeneca doses due to fears that they might be unable to administer a second shot to those who have gotten their first shots because of the shortages to the Oxford/AstraZeneca vaccines.17 Presently, Burundi and Eritrea are countries in the continent yet to receive any AstraZeneca COVID-19 vaccines, and have not started their COVID-19 vaccination campaign.16
Limited funds
Large amounts of funds are usually required before any immunization program can be implemented. For instance, the cost for a full immunization for just a child in Africa for the DTap vaccine (diphtheria, tetanus and pertussis) ranges between 25 to 45 US dollars, this excluding other immunization logistics requirements and costs.19 Most times, limited funds are allotted for immunization campaigns by African leaders in their respective countries despite the proven overall beneficial effects. For example, some percentages of the overall cost of the immunization programmes in Zambia in 2014 were provided by the Global Alliance for Vaccines and Immunization, while in many African countries, significant costs for immunization programmes are usually covered by external donors and bodies.20,21
Presently over $12 billion are needed by the continent for the COVID-19 vaccination exercise; this includes the funds needed to purchase over 1.4 billion doses to vaccinate 60% of the African population that is needed to attain herd immunity.17 The financial capabilities of most countries in the continent are seriously affected by the pandemic situation, and a significant portion of the funds needed for the vaccination exercise could not be met by individual countries.
COVAX is helping with the procurement of COVID-19 vaccines for underdeveloped and developing countries; however, 60% of the amount of money expended on vaccination represent operational costs such as storage facilities, movement of vaccines, training of vaccinators, protective equipment for vaccinators and other supporting staff, and vaccination materials.17 Eight African countries have finished their first batch of vaccines, but over twenty countries which include Mauritius, Rwanda, Egypt, Seychelles and Morocco have used less than 50% of their shots.22 In addition, the Governments of Uganda, Kenya, and Nigeria needed supplementary budgets to kick start their vaccination campaign, while countries like Benin republic, Botswana, Burundi, Cabo Verde, Central African Republic, Comoros, Chad, Djibouti Eritrea, Eswatini, Guinea-Bissau, Madagascar, Niger, Sao Tome and Principe, Somalia, South Sudan, and Sudan are having difficulties and struggling to raise funds to sustain their COVID-19 vaccination programs.
Concerns around vaccine safety and uncertainties
Malawi, Sierra Leone, Chad, Zimbabwe and some other African countries were skeptical about vaccinating their citizens with the Oxford/AstraZeneca COVID-19 vaccines due to safety concerns, which was fueled by reports of blood clots, and associated deaths among people in the United States and some European countries such as Denmark, Iceland, Norway, Estonia, Austria, Lithuania, Italy, Luxembourg, and Latvia.23 This also resulted in low uptake of the vaccines by medical workers and some educated vulnerable people in some African countries that initiated the vaccination programme despite the WHO advising its usage.24
In addition, numerous countries in Africa lacked the system and capabilities to track and monitor reports of unusual and rare side effects in people after being vaccinated, this is also raising the issues of safety concerns, and deterring people from getting the vaccines. For example, findings from a large study conducted by the Africa Center for Disease Control on COVID-19 vaccine perceptions in Burkina Faso, Côte d’Ivoire, Democratic Republic of the Congo, Ethiopia, Gabon, Kenya, Malawi, Morocco, Niger, Nigeria, Senegal, South Africa, Sudan, Tunisia, and Uganda revealed that the majority of the respondents had doubts about vaccine safety, while the majority of respondents from Ethiopia, Niger, Tunisia and the Democratic Republic of Congo outright rejected taking a vaccine.25
Also, issues regarding misinformation and superstitious beliefs that have emerged from the beginning of the pandemic in some African countries and the world generally, and unfounded theories surrounding the production of the COVID-19 vaccines, which are spreading via the social media, are threatening the uptake of the vaccines in the world and Africa, especially in rural communities.26,27
Storage requirements and regulatory hurdles for vaccines
Over the years, vaccination programmes in Africa have been met with the challenges of inadequate storage system due to limited funds to power, provide cold chain, and maintain a structured network to monitor the vaccine distributions, which usually results in expiration and inactivation of the products evident in most developing nations.28-30 These problems are mostly evident in rural communities and villages due to epileptic electricity and frequent power outage, limited cold chain equipment and facilities, bad road network which hinders movement of vaccines and vaccinators to intending communities, untrained vaccinators, and lack of basic social amenities such as clean running water and housing, which discourages the posting of health workers to villages.28,29,31
Importantly, most available vaccines and antibody products ought to be kept at a specific temperature (within the range of 36°F to 46°F (2°C to 8°C) to preserve their efficacy and safety.31,32 The available vaccines for COVID-19, for instance the ones produced by Pfizer and Moderna must be kept and transported to intending locations in an extremely cold temperature (-112°F (-80°C)).33 In addition, there are the logistical difficulties that require taking two doses of the Pfizer vaccine twenty-one days apart before it will offer protection, while two doses of the Moderna vaccine must be taken twenty-eight days apart to be fully effective.33 These logistical peculiarities made both vaccines inappropriate for mass usage in most African countries. The Oxford/AstraZeneca vaccine that is mostly distributed through the COVAX platforms only requires standard refrigeration temperatures; even at that some African countries have difficulty in keeping to these requirements, however cold chain freezers were made available to countries via the Africa Medical Supplies Platform.33
Limited shelf life of COVID-19 vaccines
The Oxford/AstraZeneca vaccine is confirmed to be safe and effective against COVID-19, and it is presently being used for mass vaccination exercise in most African countries. However, it only has six months shelf life after production when stored appropriately.34,35 The Oxford/AstraZeneca doses initially procured earlier in 2021 by the South African government had to be redistributed by the African Union to other countries like Madagascar, Togo and Ghana due to the 13th of April expiration date, and concerns regarding effectiveness against the beta variant rampant in the country.36
In addition, countries like South Sudan revealed they were unaware of the expiration date of the Oxford/AstraZeneca vaccine, while Nigeria and the Democratic Republic of Congo indicated they could not complete the usage of their acquired doses due to limited time.36 Furthermore, the health authorities in Malawi have discarded over 20,000 doses of the Oxford/AstraZeneca vaccine it got through the COVAX platform, while South Sudan opted to return over 72,000 doses to the African Union by the end May.37 The consequence is that large numbers of vaccines are being destroyed at a huge financial expense, and limited people are being vaccinated as well.
Accessing the most vulnerable people
Vaccinating the essential workforce, for example medical workers, security personnel, workers in elderly homes, transportation and food industry workers, and international students and personnel in the first phase of the immunization exercise is recommended to prevent economic shutdown and sustain important services to the public.38 The vulnerable population, for example, elderly people with or without medical conditions, should be vaccinated. Also, pregnant women and the general population should be vaccinated in subsequent phases in order to reduce infection rates.38 In addition, children should be prioritized for COVID-19 vaccination, and presently, the health authorities in the US have granted authorization for the administration of the Pfizer, Johnson & Johnson and Moderna vaccines for children of over 12 years of age, and individuals who are over the age of 18 respectively, while the health authorities in Europe have granted permission for the use of the Pfizer/BioNTech’s vaccine for use in children between the age of 12 to 16 in European countries. For now, no vaccine is authorized for use in children below 12 years of age.39,40
In most countries in Africa, the plan is to first vaccinate medical personnel, essential workers, elderly people with or without existing medical conditions, and the general population. Medical personnel are easily accessible in health facilities, and the administration of the vaccine would be easier on them compared to the elderly people with existing medical conditions whose identification would be hard to get, especially those living in rural villages and communities. The appropriate documentation and records of the individual’s health status in respective countries is essential for any successful immunization programmes.41 Nigeria, Angola and Ghana made use of ad hoc staff who visited the respective communities and villages asking for elderly individuals with existing medical conditions to ensure proper profiling and documentation prior to the start of the vaccination program.
Most countries in Africa lacked the access to updated basic medical information of their citizens such as socio-demographic characteristics, and medical conditions, while the resources needed to get this vital information for the immunization exercise in a limited time duration were inadequate. Because of these difficulties, significant numbers of people in most African countries who were planned for vaccination in the first phase have not been vaccinated. By comparison, 13% of elderly individuals have gotten a first shot of COVID-19 vaccine in the world, compared to just 1% in Africa.17 Zambia, Uganda, Gambia and Somalia are struggling in the vaccination of their elderly citizens due to inappropriate documentation and problems getting to the most vulnerable people.
The use of different vaccines
Presently, different COVID-19 vaccines such as the ones produced by Oxford/AstraZeneca, Johnson and Johnson, Moderna, Pfizer/BioNTech and Sinopharm are some of the vaccines that have been granted approval and certified for global usage by the WHO, and other different regulatory health authorities in the world. In Africa, Botswana, Rwanda and numerous other countries in Africa are using different types of vaccines in the ongoing COVID-19 vaccination exercise. For example, South Africa has vaccinated some medical personnel with the Johnson & Johnson vaccines in a phase 3B open label implementation study among 500,000 healthcare workers after it discontinued the use of the Oxford/AstraZeneca shots,42 while Egypt has been making use of both the Sinopharm and Oxford-AstraZeneca vaccines to vaccinate its citizens.17
There are some benefits with this method such as individuals getting protected quickly if managed well, but due to the particular problems of poor record keeping, which is evident in most African countries, it can cause challenges such as keeping track of the specific vaccine types used for different individuals, different logistics for vaccines and cold chain necessities and additional training of health personnel to give different vaccines. With diverse and different information on safety and efficacy for different vaccines, respective authorities might find it difficult to provide adequate information, and encourage the benefits of COVID-19 vaccination to their citizens. Hence, there is the need for pharmacovigilance post emergency use authorization (EUA) and the need to manage safety events during vaccines rollout, as well as the need to monitor field effectiveness during the vaccination exercise.
Wars and conflicts
Over two billion individuals, one in four of the world’s human inhabitants shelter in nations and places where economic growths are impacted and faced with fragility, conflict and violence (FCV).43 Some African countries such as Burkina Faso, Somalia, Cameroon, Nigeria, Central African Republic, Ethiopia, Mozambique, Sudan and the Democratic Republic of Congo are considered to be affected by FCV according to estimates from the World Bank.43
The access to basic medical services is usually difficult for individuals living in situations of wars and conflicts due to the devastating effects on medical infrastructures and supply chains, and infection tends to spread faster in this group compared to places with no conflicts or wars. Estimates from the International Committee of the Red Cross (ICRC) revealed that an estimated number of over 60 million individuals are living in places ruled by armed groups, fighters or insurgents who are not captured in the ongoing COVID-19 vaccination programmes in the respective African countries.44
Most countries in Africa are facing numerous internal conflicts and wars, which will affect the success of the current vaccination programmes by making it impossible to reach conflicts-torn areas. Some of these conflicts include the fighting in the Tigray region of Ethiopia, conflicts between armed groups in Sudan, insurgents fighting in Nigeria’s north east, the Lake Chad basin, as well as the Sahel region, unrests in Libya, fighting between government forces and insurgents in Mozambique’s northern Cabo Delgado region, Azawad fighting in northern and southern regions of Mali, and the Al-Shabaab group in Somalia has rejected the Oxford/AstraZeneca vaccine.44
Measures to mitigating these challenges
Continuous planning and timely execution of such plans is imperative to achieve a successful vaccination programme in Africa. Adequate planning for a vaccination programme incorporates a national strategy that enables an undisrupted vaccine procurement, storage, movement, distribution, and administration to intending populations.45 Before the commencement of the vaccination programme for COVID-19 in Africa, most countries on the continent revealed pre-planned outlines of how and when the vaccinations would take place; this was impressive and yielded positive results early in the vaccination exercise as evident in Ghana and Angola who vaccinated their targeted populations very early.
As the vaccination exercise progresses, planning ahead for the next phases is imperative to repeat the impressive results that were gained early in the first phase, and to correct the mistakes recorded in the ongoing first phase. Some of the major problems for the achievement of the set goals in an immunization exercise are inadequate vaccines at vaccination centers and the standard storage facilities for vaccines to ensure safety and to preserve efficacy.46 African countries should invest more in cold chain facilities that would preserve larger vaccines for the vaccination programme in the second phase in order to maintain the potency and safety of the vaccine doses before usage. The second phase of the vaccination exercise will involve larger populations compared to the first phase; there should be enough cold chain logistics in place that will preserve the vaccines that will serve the target and intended populations.
Countries and areas with interrupted electricity supply of <8 hours daily should make use of solar refrigerators to preserve the safety and efficacy of their vaccines.47 Solar refrigerators are effective ways for vaccines preservation, and may provide commensurable fund savings for vaccine doses administered over electricity powered cold chain equipment when electricity supply is inadequate.47 The procurement and movement of vaccines without disruption to the cold-chain storage is imperative and should be maintained, while vaccine doses should readily be available in vaccination centers, especially in the rural areas.
A robust and well-coordinated preparation was important to reaching individuals in rural areas in the ongoing first phase of the vaccination programme in Ghana, Rwanda and Mauritius. For instance, mobile vaccination personnel in conjunction with community volunteers were able to reach older people in remote villages and communities for the vaccination exercise, and this yielded positive results through high turnout. This method should be maintained in the second phase, while the numbers of community volunteers and mobilizers should be increased in order to ensure large coverage. In addition, pre-listing and early registration of target population for the next phase should be ongoing simultaneously, and when this is completed, routine mobile messages should be sent to each individual detailing their vaccination centers and time to be around for the vaccines so as to improve vaccination uptakes, reduce discontinuance rates, and prevent delays during the inoculation excercise.48
The ongoing COVID-19 immunization exercise of this large magnitude that needs all the general population to be vaccinated requires enormous funds. As stated earlier, over $12 billion are needed by African countries to purchase over 1.4 billion doses that will vaccinate 60% of the African population needed to attain herd immunity, while around $70 billion are required for the continent to fund the whole vaccination programme.17 The majority of African countries have struggled to raise these funds, while Uganda, Kenya, and Nigeria needed to pass series of supplementary budgets to get the funds needed for the commencement of the first phase of the vaccination exercise. Potential vaccination funds could be raised through internal revenues such as the passage of special intervention budgets and specific taxes. Also, funding for vaccination programmes could be sought by underdeveloped and developing countries through various external sources and platforms such as the World Bank International Development Association for eligible countries, International Monetary Fund, the Global Fund, and special grants and donations from non-governmental national and international organizations. Importantly, COVID-19 vaccination is a health intervention that will benefit all sectors, and effectively financing the vaccine exercise will indicate economy getting back-up globally.
It is evident that most African countries are faced with challenges of inadequate medical facilities and infrastructures.49 The use of conventional facilities has been employed in the past to cushion the effects of inadequate medical facilities in most African countries to battle various public health problem, for example the Ebola disease epidemics.50 As African countries prepare ahead for the second phase rollout of the vaccination exercise, which will involve a larger population, makeshift facilities such as large fields, open spaces with the provision of tents and seating equipment and various public venues, e.g. churches and mosques, could be used as vaccination centers. In addition, existing facilities and resources which have been used previously to fight numerous public health problems in the continent such as the Ebola outbreak in West Africa, yellow fever in Angola, and wild polio virus in Ghana and northern part of Nigeria could also be used in the current vaccination exercise, e.g. the existing storage facilities, vaccinators and other medical workers. These systems are readily available, faster to use, and with little financial commitments.
The safety of vaccinators and other medical workers is paramount and should be taken into consideration and planning before the second phase commences among countries in the continent. The availability of protective materials together with guidelines on usage is important to ensuring that vaccines are administered safely to people.51 Protective masks, medical gloves, and hygiene materials for example soap, detergent and clean running water should be made available in all the vaccination centers prior to the commencement of the second phase, while physical distancing between the vaccinators and the receivers should be maintained.
Myths and misinformation discouraging vaccine uptake are spreading very fast; dispelling these wrong information and misconceptions will enable efficient rollout to achieve massive uptake by the targeted population in Africa.52 While having in place mechanisms that ensure a rapid and unhindered allocation of COVID-19 vaccines the moment they are available is imperative, the role of a holistic community sensitization is paramount and should not be overlooked. African nations must design and implement methods on the most effective ways to improve community trust, understanding and to prevent wrong information about the COVID-19 vaccines.53
Early and continuous community engagements involving vaccine promotion, community sensitization and messages that dispel myths and misconceptions should be intensified in all communities and villages in order to increase vaccine uptake. Vaccination promoting messages could be relayed to the general public via the radio and television programs and routine announcements, daily newspapers, posters, pamphlets, short mobile messages, and various social media channels making use of all spoken languages in the respective countries. In rural and remote areas with limited access to internet and frequent electricity, messages promoting COVID-19 vaccine uptake should be relayed in churches, mosques, traditional functions, and large gatherings, while sensitization efforts making use of house-house methods with the help of trained community messengers and volunteered workers should be conducted as well.
In addition, making use of local champions, respected figures, and celebrities in the dissemination of vaccine promoting messages might result in higher vaccine acceptance rate in the public. Also, the provision and promotion of a robust intra- and inter-sectoral partnership would help to improve the acceptance and uptake of the COVID-19 vaccines via the provision of more human and material resources needed to tackle COVID-19 vaccine hesitancy in African countries.54 Likewise, the integration of the COVID-19 vaccine into the regular vaccination framework, and as a requirement, might help to fortify the public care system, promoting and increasing the acceptance of the COVID-19 vaccine, and improving the overall wellbeing of all individuals dwelling in Africa.54
Getting COVID-19 vaccines across to conflict and war-torn areas, and vaccinating the people in these areas is important to achieve herd immunity on the continent, and a moral obligation. Sub-standard health capacities because of the breakdown or destruction of health services, inadequate medical workers, depleted medical facilities, equipment and materials, and disputed borders might hamper vaccination programmes in conflict situations. Getting to areas not controlled by constituted authorities or the government brings problems such as logistic issues, the need to get a travel pass which is usually complicated and time consuming, and reduced access to electricity and standard storage facilities. Over 160 million individuals are faced with an increased risk of exclusion from the ongoing COVID-19 immunization exercise due to reasons of internal conflicts, unrests and wars in most African countries, including in Nigeria, South Sudan, Mali, Somalia, Chad, Libya, Ethiopia, and Mozambique.44
Brokering local ceasefires is needed and important to enable life-saving vaccination exercise to take place in these disturbed areas, as well as important to ensure the safety of courageous medical personnel and other charity workers working and providing care in a highly complex situations in conflict environments. In addition, members of the community, international humanitarian organizations such as the Red Cross, religious, traditional, and community or village figures should be drafted to assist in the planning and designing of vaccination frameworks in disturbed places or areas. Community partnership and providing communities with the right information and data would be crucial to achieve an impressive outcome or results during the COVID-19 immunization campaign, and to ensure the safety of medical care workers.
A big national intervention program such as the COVID-19 vaccination campaign needs a broader society partnership and approaches prior to, and during the rollout exercise. Strong leadership and coordination from the Health Ministry, partnerships across and beyond the government for example the private sectors will prove crucial for the success of the vaccination program. For example, a multi-sectoral partnership which involves the government and private organizations should be in place at the international, regional, national, district and community levels.
Due to Africa’s inability to produce vaccines for COVID-19 and other vaccination materials, the international and regional partnerships with COVAX, Coalition for Epidemic Preparedness Innovations, World Bank, Bill Gates foundation, and WHO is imperative to procure vaccines and other medical materials at lesser rates and with limited time. Once the vaccines are procured, national authorities with partnerships with private sectors will help with the plan layout and standard logistics for the safe, equal and timely distribution of vaccines into intending districts and communities.
Individuals in the community are the end receivers of vaccines in the vaccination chain. To ensure high uptake, communities need to understand the importance of the vaccines to them, their safety, and the method of administration, and that their social and traditional norms will be upheld and respected at all times during the vaccination process.
Other viable recommendations and suggestions
Regulatory challenges for EUA vaccines in Africa ought to be addressed in the ongoing COVID-19 vaccination drive to ensure unhindered and rapid availability of COVID-19 vaccines to the public. Authorities in the respective African countries should be proactive in their actions in order to expedite and fasten the approvals and registrations of COVID-19 vaccines and other immunization materials both at the international, regional, national and local levels once a EUA is issued. Also, sharing of vaccines or other immunization products regulatory information among other member countries in Africa is imperative to prevent delays in vaccines rollout that may ensue from vaccines manufacturers having to deal with numerous applications from individual countries.
The need for pharmacovigilance and management of rare side effects and other complications of vaccination in the continent and globally is important. Because most of the vaccines that are currently being used in the global COVID-19 vaccination efforts were produced at accelerated time, there might be some associated side effects that may results from their usage, hence a regularly and updated post-production surveillance and monitoring reports are warranted through pharmacovigilance systems in individual countries. Pharmacovigilance systems are defective in most African counties; however, this could be alleviated through the involvement of specific stakeholders in the health industry, the rapid training of medical care workers on methods to ensure intensive monitoring of individuals after immunization, and improving on existing pharmacovigilance frameworks which could be passive surveillance (continuous monitoring of individuals after immunization) or stimulated surveillance (in which medical personnel are motivated to report any adverse effects after immunization). The creation of frameworks and systems where constant data on associated adverse effects resulting from vaccinations could be reported and assessed by other countries may help strengthen the immunization drive and ensure the safety of the general public globally.
The use of electronic vaccine data systems for the ongoing COVID-19 immunization exercise would be important and could ensure rapid disease control and larger vaccination coverage in a timely fashion on the continent. Some African countries like Rwanda totally relied on manual methods such as paper and writing materials in collecting and storing information of its population during the vaccination exercises for measles, polio and meningitis, while most of the vaccination centers in Ethiopia still heavily relied on manual methods for data collection and storage in the past. Presently, in laudable efforts, South Africa has fashioned out an effective computer incorporated system for surveillance, evaluation and registration during the ongoing vaccination drive, while other countries in the continent such as Nigeria, Ethiopia, Tunisia, Egypt, Libya, Rwanda, and Ghana have set up online registration systems in their respective countries, and are also making use of internet powered platforms to promote the vaccination exercise to their populations.
Globally, many nations are contemplating making the COVID-19 vaccine passport a requirement for their populations to access public spaces. For example, countries such as the United Kingdom, France, Israel, China, and all countries in the European Union have set out measures that will integrate COVID-19 vaccine passports as requirements to access some specific large-public spaces. Presently, countries such as the US and Australia have ruled out enforcing a compulsory COVID-19 vaccine passport requirement for their citizens, although some states in the US have specific COVID-19 vaccine requirements. In addition, air movement rules are constantly being changed globally to incorporate the need for a vaccine passport for travelers, while in the capital city of Indonesia, monetary punishment are served to people who refuse to take the COVID-19 vaccine. In Africa, as of the time of writing this review, no country on the continent has mandated the need for a COVID-19 vaccine passport to access any public buildings or spaces.
The issue of mandatory vaccination in places of work is gaining momentum globally. In Africa, presently no countries have any law in place that expressly mandate or permit an employer to compel its workers to take the COVID-19 vaccine. Possibly, respective government authorities in individual African countries might decide to enact laws that would compel workers’ vaccination, a scenario which might likely be welcomed by employers, but faced with serious legal battles by employees. In order to prevent disruption and delay of the COVID-19 vaccination exercise due to protracted legal battles, respective government authorities globally should continue with the implementation of the vaccination exercise with strong advice, encouragement and guidance that people should get vaccinated.
Conclusions
This is the first time in history that the continent of Africa is witnessing a vaccination programme that is as large as the current COVID-19 vaccination exercise. Because of the particular problems that are evident in most countries in the continent, there are numerous challenges that could mare the success of the ongoing mass COVID-19 vaccination exercise. Summarily, deductions from this review indicate that, although there are many identified challenges to the current COVID-19 vaccination campaigns in respective African countries, a holistic and timely planning, and execution of such plans, rigorous community involvement, and a robust multi-sectoral partnership will help to yield beneficial results in the continent’s efforts to vaccinate its population against COVID-19.
Acknowledgement:
The contributors of this article appreciate all the editors and reviewers of this great journal for insightful corrections and suggestions.
Footnotes
Authors’ contributions: All authors contributed equally to this review. All authors read and approved the final version of the manuscript.
Conflicts of interest: none to declare.
Funding: None to declare.
References
- 1.Ayenigbara IO, Adeleke OR, Ayenigbara GO, Adegboro JS, Olofintuyi OO. COVID-19 (SARS-CoV-2) pandemic: fears, facts and preventive measures. Germs. 2020;10:218–28. doi: 10.18683/germs.2020.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayenigbara IO. COVID-19: an international public health concern. Cent Asian J Glob Health. 2020;9:e466. doi: 10.5195/cajgh.2020.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeniji G. UK, South Africa alpha, beta variants now in Nigeria, other African countries - WHO. 2021. [Accessed on: 25 August 2021]. Available at: https://punchng.com/uk-safrica-alpha-beta-variants-now-in-nigeria-other-african-countries-who/?utm_term=Autofeed&utm_medium=Social&utm_source=Facebook&fbclid=IwAR1r1gK7EtmcO7cEb1BdLDF-bx3DsbRXJOTp4QSkhXpt0yHk9evPX6Yvbqo#Echobox=1628396993.
- 4.Aleem A, Akbar Samad AB, Slenker AK. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19) StatPearls Treasure Island (FL): StatPearls Publishing; 2021. 2021. [Google Scholar]
- 5.European Medicine Agency. EMA recommends first COVID-19 vaccine for authorisation in the EU. 2020. [Accessed on: 25 August 2021]. Available at: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu.
- 6.World Health Organization. COVID-19 vaccines. 2021. [Accessed on: 25 August 2021]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines.
- 7.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–23. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–57. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlowski C, Lenehan P, Puranik A, et al. FDA-authorized COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y) 2021;2:979–92e8. doi: 10.1016/j.medj.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Africa News. Africa secures 300 million COVID-19 vaccine doses in deal with manufacturers. 2021. [Accessed on: 25 August 2021]. Available at: https://www.africanews.com/2021/01/13/africa-secures-300-million-covid-19-vaccine-doses-in-deal-with-manufacturers/
- 12.Worldometer. Reported cases and deaths by country or territory. 2021. [Accessed on: 25 August 2021]. Available at: https://www.worldometers.info/coronavirus/#countries.
- 13.Mihigo R, Okeibunor J, Anya B, Mkanda P, Zawaira F. Challenges of immunization in the African Region. Pan Afr Med J. 2017;27:12. doi: 10.11604/pamj.supp.2017.27.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moeti M. Winning the battle against the scourge of poliomyelitis in the African Region. Vaccine. 2016;34:S5142–3. doi: 10.1016/j.vaccine.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 16.Mwai P. COVID-19 Africa: what is happening with vaccine supplies? 2021. [Accessed on: 25 August 2021]. Available at: https://www.bbc.com/news/56100076.
- 17.World Health Organization. Risks and challenges in Africa’s COVID-19 vaccine rollout. 2021. [Accessed on: 25 August 2021]. Available at: https://www.afro.who.int/news/risks-and-challenges-africas-covid-19-vaccine-rollout.
- 18.Soy A. Africa’s long wait for the COVID-19 vaccine. 2021. [Accessed on: 25 August 2021]. Available at: https://www.bbc.com/news/world-africa-55751714.
- 19.Mihigo RM, Okeibunor JC, O’Malley H, Masresha B, Mkanda P, Zawaira F. Investing in life saving vaccines to guarantee life of future generations in Africa. Vaccine. 2016;34:5827–32. doi: 10.1016/j.vaccine.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths UK, Bozzani FM, Chansa C, et al. Costs of introducing pneumococcal, rotavirus and a second dose of measles vaccine into the Zambian immunization programme: are expansions sustainable? Vaccine. 2016;34:4213–20. doi: 10.1016/j.vaccine.2016.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Songane M. Challenges for nationwide vaccine delivery in African countries. Int J Health Econ Manag. 2018;18:197–219. doi: 10.1007/s10754-017-9229-5. [DOI] [PubMed] [Google Scholar]
- 22.Africa News. Coronavirus - Africa: supply bottleneck, financial challenges fuel delays in Africa’s COVID-19 roll out. [Accessed on: 25 August 2021]. Available at: https://www.africanews.com/2021/05/20/coronavirus-africa-supply-bottleneck-financial-challenges-fuel-delays-in-africa-s-covid-19-vaccine-rollout/
- 23.Wise J. COVID-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 24.European Medicine Agency. COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets. [Accessed on: 25 August 2021]. Available at: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots.
- 25.Africa CDC. Majority of Africans would take a safe and effective COVID-19 vaccine. 2021. 2021. [Accessed on: 25 August 2021]. Available at: https://africacdc.org/news-item/majority-of-africans-would-take-a-safe-and-effective-covid-19-vaccine/
- 26.Ullah I, Khan KS, Tahir MJ, Ahmed A, Harapan H. Myths and conspiracy theories on vaccines and COVID-19: potential effect on global vaccine refusals. Vacunas. 2021;22:93–7. doi: 10.1016/j.vacun.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerts D, Shelley CD, Parikh N, et al. “Thought I’d share first” and other conspiracy theory tweets from the COVID-19 infodemic: exploratory study. JMIR Public Health Surveill. 2021;7:e26527. doi: 10.2196/26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guichard S, Hymbaugh K, Burkholder B, et al. Vaccine wastage in Bangladesh. Vaccine. 2010;28:858–63. doi: 10.1016/j.vaccine.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Parmar D, Baruwa EM, Zuber P, Kone S. Impact of wastage on single and multi-dose vaccine vials: implications for introducing pneumococcal vaccines in developing countries. Hum Vaccin;2010;6:10397. doi: 10.4161/hv.6.3.10397. [DOI] [PubMed] [Google Scholar]
- 30.Karp CL, Lans D, Esparza J, et al. Evaluating the value proposition for improving vaccine thermostability to increase vaccine impact in low and middle-income countries. Vaccine. 2015;33:3471–9. doi: 10.1016/j.vaccine.2015.05.071. [DOI] [PubMed] [Google Scholar]
- 31.Zaffran M, Vandelaer J, Kristensen D, et al. The imperative for stronger vaccine supply and logistics systems. Vaccine. 2013;31(2):B73–80. doi: 10.1016/j.vaccine.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann JR, Miller R, Cheyne J. Vaccine supply chains need to be better funded and strengthened, or lives will be at risk. Health Affairs. 2011;30:1113–21. doi: 10.1377/hlthaff.2011.0368. [DOI] [PubMed] [Google Scholar]
- 33.Jerving S. Africa CDC outlines ultracold storage strategy for COVID-19 vaccines. 2021. [Accessed on: 5 August 2021]. Available at: https://www.devex.com/news/africa-cdc-outlines-ultracold-storage-strategy-for-covid-19-vaccines-98962.
- 34.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–93. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mwai P. COVID-19 vaccines: why some African states can’t use their vaccines. 2021. [Accessed on: 5 August 2021]. Available at: https://www.bbc.com/news/56940657.
- 37.Reliefwebpage. South Sudan - accountability to affected populations: community perceptions of COVID-19 and the COVID-19 vaccine (May 2021) 2021. [Accessed on: 25 August 2021]. Available at: https://reliefweb.int/report/south-sudan/south-sudan-accountability-affected-populations-community-perceptions-covid-19.
- 38.Yang J, Zheng W, Shi H, et al. Who should be prioritized for COVID-19 vaccination in China? A descriptive study. BMC Med. 2021;19:45. doi: 10.1186/s12916-021-01923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration. FDA approves first COVID-19 vaccine. 2021. [Accessed on: 25 August 2021]. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.
- 40.European Medicines Agency. First COVID-19 vaccine approved for children aged 12 to 15 in EU. 2021. [Accessed on: 25 August 2021]. Available at: https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu.
- 41.Mutshatshi TE, Mothiba TM, Mamogobo PM, Mbombi MO. Record-keeping: challenges experienced by nurses in selected public hospitals. Curationis. 2018;41:e1–6. doi: 10.4102/curationis.v41i1.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reuters. South Africa sells AstraZeneca COVID-19 vaccines to other African countries. 2021. [Accessed on: 25 August 2021]. Available at: https://www.reuters.com/article/us-health-coronavirus-safrica-vaccine-idUSKBN2BD0K4.
- 43.Bhalla N, Omer M. Insecurity, suspicion will mar vaccine rollout in Africa war zones. 2021. [Accessed on: 25 August 2021]. Available at: https://www.reuters.com/article/us-health-coronavirus-africa-vaccine-trf-idUSKBN2AJ160.
- 44.United Nations. Africa’s peace and security: the pressures of COVID-19. 2021. [Accessed on: 25 August 2021]. Available at: https://reliefweb.int/report/world/africa-s-peace-and-security-pressures-covid-19.
- 45.Williams SR, Driscoll AJ, LeBuhn HM, Chen WH, Neuzil KM, Ortiz JR. National routine adult immunization programmes among World Health Organization Member States: an assessment of health systems to deploy COVID-19 vaccines. Euro Surveill. 2021;26:2001195. doi: 10.2807/1560-7917.ES.2021.26.17.2001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwu CJ, Jaca A, Abdullahi LH, Ngcobo NJ, Wiysonge CS. A scoping review of interventions for vaccine stock management in primary health-care facilities. Hum Vaccin Immunother. 2019;15:2666–72. doi: 10.1080/21645515.2019.1607130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haidari LA, Brown ST, Wedlock P, Connor DL, Spiker M, Lee BY. When are solar refrigerators less costly than on-grid refrigerators: a simulation modeling study. Vaccine. 2017;35:2224–8. doi: 10.1016/j.vaccine.2016.11.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manakongtreecheep K. SMS-reminder for vaccination in Africa: research from published, unpublished and grey literature. Pan Afr Med J. 2017;27(3):23. doi: 10.11604/pamj.supp.2017.27.3.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dzinamarira T, Dzobo M, Chitungo I. COVID-19: a perspective on Africa’s capacity and response. J Med Virol. 2020;92:2465–72. doi: 10.1002/jmv.26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waya JLL, Ameh D, Mogga JLK, Wamala JF, Olu OO. COVID-19 case management strategies: what are the options for Africa? Infect Dis Poverty. 2021;10:30. doi: 10.1186/s40249-021-00795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chersich MF, Gray G, Fairlie L, et al. COVID-19 in Africa: care and protection for frontline healthcare workers. Global Health. 2020;16:46. doi: 10.1186/s12992-020-00574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wirsiy FS, Nkfusai CN, Ako-Arrey DE, Dongmo EK, Manjong FT, Cumber SN. Acceptability of COVID-19 vaccine in Africa. Int J MCH AIDS. 2021;10:134–8. doi: 10.21106/ijma.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dzinamarira T, Nachipo B, Phiri B, Musuka G. COVID-19 vaccine roll-out in South Africa and Zimbabwe: urgent need to address community preparedness, fears and hesitancy. Vaccines (Basel) 2021;9:250. doi: 10.3390/vaccines9030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afolabi AA, Ilesanmi OS. Dealing with vaccine hesitancy in Africa: the prospective COVID-19 vaccine context. Pan Afr Med J. 2021;38:3. doi: 10.11604/pamj.2021.38.3.27401. [DOI] [PMC free article] [PubMed] [Google Scholar]