Abstract
Infant autonomic reactivity to stress is a potential predictor of later life health complications, but research has not sufficiently examined sympathetic activity, controlled for effects of physical activity and respiration, or studied associations among autonomic adjustments, cardiac activity, and affect in infants. We studied 278 infants during the repeated Still-Face Paradigm, a standardized stressor, while monitoring cardiac activity (ECG) and respiratory pattern (respiratory inductance plethysmography). Video ratings of physical activity and affect were also performed. Respiratory sinus arrhythmia (RSA) and T-wave amplitude (TWA) served as noninvasive indicators of cardiac parasympathetic and sympathetic activity, respectively. Responses were compared between infants who completed two still-face exposures and those who terminated after one exposure due to visible distress. Findings, controlled for physical activity, showed robust reductions in respiration-adjusted RSA and TWA, with slower return to baseline for TWA. Infants completing only one still-face trial showed more pronounced autonomic changes and less recovery from stress. They also showed elevated minute ventilation, suggesting hyperventilation. Both reductions in adjusted RSA and TWA contributed equally to heart rate changes and were associated with higher negative and lower positive affect. These associations were more robust in the group of distressed infants unable to complete both still-face trials. Thus, cardiac sympathetic activation and parasympathetic withdrawal are part of the infant stress response, beyond associated physical activity and changes in respiration. Their association with cardiac chronotropy and affect increases as infants’ distress level increases. This excess reactivity to social stress should be examined as a predictor of future cardiovascular disease.
Keywords: Cardiac activity, infants, stress, respiratory sinus arrhythmia, T-wave amplitude, sympathetic activity, parasympathetic activity, physical activity, respiration, affect, Still-Face Paradigm
The coordination of autonomic outflow for regulation of cardiac activity during psychosocial stress has long been of interest. Extremes of cardiovascular stress reactivity compared to normative responding (i.e., higher or lower reactivity relative to a population average) have been considered as a cause or consequence of chronic illness (e.g., Obrist, 1982; Chida & Steptoe, 2010; Gianaros & Jennings, 2018; Lovallo, 2005; Ginty et al., 2017) and psychological disorders (e.g., Beauchaine et al., 2001; Bussher et al., 2010; Lang et al., 2000; Engel, 1979; Patrick, 2008; Phillips et al., 2011). Exaggerated cardiovascular stress responding may serve as an early marker of risk for later cardiovascular complications (Allen et al., 1997; Gerin et al., 2000; Treiber et al., 2003). However, the usefulness of cardiac stress reactivity as a risk or illness indicator is limited, given that cardiac end-organ activity remains ambiguous with respect to underlying autonomic regulation (Berntson et al., 1991; Koizumi & Kollai, 1981). Studying activity of the autonomic branches that regulate cardiac reactivity under psychological stress conditions may inform future behavioral and pharmacological intervention efforts.
Past research has made progress in elucidating cardiac stress dynamics in healthy adults (Berntson et al., 1996; Grossman et al., 1991; Stemmler, 1991). Both major autonomic branches, the sympathetic and parasympathetic systems, participate in cardiac regulation during psychological stress, with a typical pattern of reciprocal sympathetic activation and parasympathetic withdrawal. This pattern is particularly visible in challenging cognitive stressors such as mental arithmetic, spontaneous speech, or reaction time tasks. With respect to clinical relevance, particular attention was directed early on to the sympathetic system and its potentially detrimental role in mediating later cardiovascular complications, especially when activated chronically (Esler, 2017; Obrist, 1982).
Whereas progress has been made in elucidating autonomic nervous system (ANS) stress response profiles in childhood and adolescence (e.g., Beauchaine et al., 2013; Boyce et al., 2001; Gatzke-Kopp & Ram, 2018; Hinnant et al., 2011; Nederhof et al., 2015), ANS regulation of the heart in infants has been less well studied. An established paradigm for studying infant psychosocial stress responses is the Still-Face Paradigm (Weinberg & Tronick, 1996), during which the infant’s mother becomes non-responsive, disengaging from interacting and presenting a motionless neutral facial expression to the infant. Studies of autonomic adjustments mostly suggest that parasympathetic withdrawal is a typical feature of this response (Moore & Calkins, 2004; Ritz et al., 2012; Tibu et al, 2017; Weinberg & Tronick, 1996). However, other studies have reported only small effect sizes (Gray et al., 2017), did not find significant effects (Bazhenova et al., 2001), or found significant effects only in repeated testing (Bush et al., 2017). Cardiac sympathetic effects of stress have been studied more rarely in infants. Some of these studies used salivary alpha amylase measures as proxy indicators (Hill-Soderlund et al., 2008; Laurent et al., 2012; Rash et al., 2016), but this strategy is limited given a well-established directional fractionation of response systems depending on the nature of environmental challenge (Lacey, 1967) and the fact that the traditional notion that organ systems act in a uniform (“sympathetic”) fashion under stress is outdated (Berntson et al., 1991). One recent study measured sympathetic activity in infants during the Still-Face Paradigm, suggesting sympathetic activation and parasympathetic withdrawal, but no rigorous statistical analysis of the full time course was presented and physical activity was not controlled (Suurland et al., 2017). Earlier findings from our group with a smaller sample showed no general sympathetic effects across the Still-Face Paradigm, with only infants of insensitive mothers showing evidence for sympathetic stress activation (Bosquet Enlow et al., 2014). Thus, it remains unclear whether a more comprehensive and well-controlled analysis of ANS effects in an adequately powered sample would provide evidence for sympathetic stress effects in infants.
Research on infant ANS activity under stress has often neglected the additional influence of physical activity and respiratory pattern influences. Given the major role of the cardiovascular system in supporting metabolic rather than psychological functions of the organism, controlling for physical activity is desirable before any psychological interpretation of cardiac response profiles is attempted (Obrist, 1976; Turner & Carroll, 1985). This is particularly important in infant research, where cooperation of the participant with the requirement of (relative) physical immobility in a typical psychological laboratory paradigm cannot be expected. Cardiac acceleration by parasympathetic withdrawal to physical activity is immediate and pronounced (Berntson et al., 1991) and may be mistaken as psychologically induced. Earlier ambulatory monitoring research has shown that a disproportionally large amount of variance in cardiac activity can be attributed to physical activity compared to mood changes (Johnston & Anastasiades, 1990), necessitating a tight control of physical activity in research of affective determinants of heart rate (Myrtek & Brügner, 1996). In infant ANS stress research, accounting for variance due to observed physical activity has been emphasized (Ritz et al., 2012) but rarely implemented (Bazhenova et al., 2007). Of note, cardiac activation beyond levels needed to support physical activity might be particularly relevant to health, potentially indicating increased risk for negative long-term cardiovascular complications. In adults, there is evidence that the additional cardiac activation component beyond metabolic demand is more strongly related to sympathetic (beta-adrenergic) activity (Balanos et al., 2010), but for infants, it remains unclear whether excess cardiac reactivity to stress beyond physical demand is associated with sympathetic activity.
In the study of parasympathetic (vagal) dynamics of infant stress via noninvasive psychophysiological indices, such as respiratory sinus arrhythmia (RSA), or its frequency-domain equivalent, high-frequency heart rate variability, the consideration of respiratory pattern effects is of paramount importance. It has been demonstrated repeatedly that these measures are strongly dependent on respiration rate and volume (Bernardi et al., 2000; Brown et al., 1993; Eckberg, 2003; Grossman et al., 1991; Grossman & Taylor, 2007; Hirsch & Bischop, 1981; Ritz et al., 2001), with some estimations suggesting that more than 50% of the variance in RSA is attributable to respiratory pattern variations (Grossman et al., 1991; Ritz & Dahme, 2006). Although no ideal solution for considering these factors has been identified, consideration of variance proportions potentially attributable to respiration has been recommended by guidelines (Berntson et al., 1997), and methods for accounting for this variance have been suggested (Grossman et al., 1991; Saul et al., 1989; Wilhelm et al., 2004; Ritz & Dahme, 2006; Egizio et al., 2011). However, respiratory responses of infants to the still-face stressor have rarely been measured (Bosquet et al., 2009; Ritz et al., 2012), although the susceptibility of the respiratory pattern to stress and emotion is well documented (Boiten et al., 1994; Homma & Masaoka, 2008). Control of respiration rate and volume has also been shown to affect substantially findings in adult psychophysiology studies of cardiac vagal activity (Ritz, 2009; Overbeek et al., 2012; Simon et al., 2017). Moreover, using a small sample of infants administered the Still-Face Paradigm, we found considerable differences in RSA outcomes depending on the level of statistical control of respiratory pattern effects (Ritz et al., 2012).
There is also some uncertainty in the literature regarding the role of cardiac autonomic activity in affective behavior in infants. Although infant affect has been rated from infant behavior in a number of studies, there is no systematic test of its association with cardiac activity. Thus, it is unclear to what extent levels of affective expression and physiology are integrated in infants during stress. Using a between-subjects correlational approach during individual episodes of the Still-Face Paradigm, studies have found mostly weak or nonsignificant associations between negative affect and cardiac activity (Weinberg & Tronick, 1996; Moore, 2010). However, a perspective of within-individual changes across episodes is more informative for studying associations between observational levels of affect (i.e., is change of affect from one situation to another correlated with respective changes in cardiac activity within a particular individual?), compared to the between-subject perspective (i.e., do more affectively responsive infants show greater cardiac activity?). For an example of radically different findings emerging from between- versus within-subject correlational analysis, see the work of Cacioppo et al. (1992). This also applies to associations between physical activity and cardiac activity, which have, in the rare cases where physical activity was measured, been analyzed between-subjects (Conradt & Ablow, 2010). Thus, we elected to utilize multi-level modeling techniques, powerful methods for testing within-subject associations in repeated measurement designs (Kristjansson et al., 2007). Prior research has also not controlled for physical activity when studying expressive affect and autonomic responses in infants. To the extent that affective behaviors are associated with physical activity, any change in autonomic activity accompanying it may be attributable to the elevated metabolic demand and be less informative regarding psychobiological coupling.
To fill these gaps of knowledge about sympathetic and parasympathetic cardiac activity and affective behavior under stress in infants, we sought to examine:
the pattern of autonomic responding to the repeated Still-Face Paradigm while accounting for concurrent respiratory and physical activity,
the relative contribution of sympathetic excitation versus parasympathetic withdrawal to cardiac activity during stress exposure, and
the association of sympathetic and parasympathetic activation with negative and positive affective behavior under stress exposure and recovery conditions.
To study these open questions under conditions of repeated stress exposure in infants, we administered an extended stress protocol that included two still-face trials, each with a subsequent recovery period (Haley & Stansbury, 2003). Because a substantial number of infants typically show pronounced distress levels that prohibit administration of a second still-face trial (Bosquet Enlow et al., 2014), completion of the two-trial protocol may serve as a proxy for successful coping with stress and was therefore used as an additional independent variable in our analysis, allowing us to identify infants potentially at risk for exaggerated cardiac reactivity. Thus, we additionally hypothesized that infants unable to complete two trials would be more likely to show excessive cardiac parasympathetic withdrawal and sympathetic excitation during the first trial.
Methods
Participants included mother-infant pairs enrolled in the PRogramming of Intergenerational Stress Mechanisms (PRISM) study, a sociodemographically diverse pre-birth cohort that was designed to investigate the independent and joint effects of perinatal stress and other environmental influences on child development. Pregnant women were recruited from prenatal clinics in urban hospitals and community health centers in the northeastern United States. Eligibility criteria included: 1) English- or Spanish-speaking; 2) age ≥ 18 years at enrollment; and 3) single gestation birth. Exclusion criteria included 1) maternal endorsement of drinking ≥ 7 alcoholic drinks/week prior to pregnancy recognition or any alcohol following pregnancy recognition; 2) maternal positive HIV status, which would influence/confound biomarkers of interest. Based on screening data, there were no differences in race/ethnicity, education, or income between women who enrolled in PRISM and those who declined. Procedures were approved by the institutional review boards at the Brigham and Women’s Hospital and Boston Children’s Hospital; Beth Israel Deaconess Medical Center relied on Brigham & Women’s Hospital for review and oversight of the protocol. Written informed consent was obtained from mothers in their preferred language.
Stress paradigm
Mother-infant dyads participated in the repeated Still-Face Paradigm (Haley & Stansbury, 2003). The Still-Face Paradigm is a psychological stress challenge paradigm well-validated in sociodemographically diverse samples to assess infant affective, behavioral, and physiological reactivity to brief, moderate levels of induced stress (Weinberger & Tronick, 1996). The original paradigm involves three 2-minute episodes during which the infant is seated across from the mother, who is instructed to play with the infant for a two-minute baseline (“play”), followed by a still-face episode, during which the mother is instructed to maintain a neutral facial expression and avoid touching the infant or vocalizing (“still-face 1”). The assumed stressful nature of the still-face episode is thought to be due to the lack of behavioral cues from the mother that the infant uses to maintain an organized social and affective state (Weinberger & Tronick, 1996). During the third episode, the mother is instructed to resume playing with the infant (“reunion 1”). The repeated version of the paradigm includes a second trial of a still-face episode (“still-face 2”) and reunion episode (“reunion 2”). This second trial is administered immediately after cessation of reunion 1. The repeated version of the Still-Face Paradigm was designed to allow for an assessment of a more sustained challenge and an additional recovery period.
Measures
Infant respiration and cardiac activity
Infant respiration and cardiac activity were measured during the repeated Still-Face Paradigm using a non-invasive ambulatory AC-coupled biosignal recorder with respiratory inductance plethysmography capability (BioRadio, Great Lakes NeuroTechnologies) that continuously recorded and stored raw signals from two elastic inductance bands and a 3-lead ECG with a basic sampling rate of 960 Hz. The inductance bands were fitted to the infant’s thorax and abdomen, together with electrodes for a three-lead ECG attached to the sternum, the lower left rib, and the left clavicle. Raw signals from the two inductance bands and the ECG leads were amplified, A/D converted with a sampling rate of 1000Hz, and stored continuously on a laptop. Respiration and heart rate data were processed using VivoSense software (Vivonoetics, Newport Coast, CA), which was designed for analysis of complex waveforms, detection of artifacts, and calibration of respiratory parameters. The ECG was downsampled to 480 Hz and 0.05 Hz high-pass filtered. Ribcage and abdomen signals from the respiratory inductive plethysmograph were downsampled to 240 Hz, and a low-pass filter with a cut-off frequency of 2.5 Hz was applied.
Ratings of infant observed affect and maternal sensitivity.
Trained research assistants scored the infants’ affective states and maternal sensitivity throughout the Still-Face Paradigm from video recordings using the Parent-Child Interaction Rating Scales (PCIRS; Sosinsky, Carter, & Marakovitz, 2004). The PCIRS was developed for use in a mother-toddler sample and adapted in consultation with the PCIRS developers for use in the current study with mother-infant dyads. For each Still-Face Paradigm episode, infant positive affect and negative affect were scored on 7-point scales (scored 1 to 7), with 1=very low and 7=very high. The positive affect scale assessed the extent to which the infant appeared to be enjoying himself/herself, with indicators including smiles, laughter, cooing, squealing, body movements, or other expressions of enthusiasm. Lack of positive affect could be manifested by neutrality or negative affect. The negative affect scale assessed the extent to which the infant cried, whimpered, fussed, frowned, screamed, tensed their body while crying, or otherwise expressed discontentment, anger, or hostility. This scale also included any signs of anxiety, fear, wariness, or hypervigilance. Lack of negative affect could be manifested as positive affect, contentment, or neutrality. Ratings on both the positive and negative affect scales considered both the intensity of, and the amount of time in, each of these affective states. Higher scores indicate greater displays of that affective state. In addition, maternal sensitivity/responsivity ratings for the initial play episode were analyzed, which assessed the degree to which the mother responded appropriately to her infant’s activity and verbalizations; facilitated positive interactions with her infant that were appropriately timed and responsive to her infant’s cues/signals; provided an appropriate level of stimulation, changing the pace of interaction when her infant appeared under-stimulated, overexcited, or tired; and shared positive affect. Interrater reliability, conducted on 25% of the sample, was .91 for the infant positive affect scale and .92 for the infant negative affect scale across raters and episodes and was .94 for the maternal sensitivity/responsivity scale during the play episode across raters.
Infant physical activity level.
Infant movement was quantified by coding activity in 10-second intervals from videos of the repeated Still-Face Paradigm using a 4-point scale (0 = quiet motor: no movement other than slow moving of fingers, 1 = slow/mild movements: slow bending but not lifting of limbs, 2 = moderate movements: slow lifting of limbs, 3 = pronounced movements: forceful lifting of limbs), as done by others (scale modified from Bazhenova et al., 2007). Trained coders scored the videos; interrater reliability, conducted on 25% of the sample, was r=.83 across coders. All 10-second interval scores within an episode were averaged and then multiplied by 100 to create a possible activity score for each episode ranging from 0 to 300, with higher scores indicating more vigorous and/or continuous activity.
Infant temperament
Infant temperament was assessed via the Infant Behavior Questionnaire-Revised (IBQ-R; Gartstein & Rothbart, 2003), a caregiver-report instrument developed to assess temperament in infants from 3 to 12 months of age. The IBQ-R has demonstrated good internal consistency, reliability, and validity (Bosquet et al., 2016; Gartstein et al., 2005, 2009; Parade & Leerkes, 2008). It was administered as an interview to mothers during a home visit prior to the laboratory visit during which the SFP-R was administered. Mothers rated the frequency that their infant engaged in specific day-to-day behaviors in the prior 1–2 weeks using a 7-point scale, with responses ranging from 1 (never) to 7 (always). Item scores were summed according to the IBQ-R scoring rules to create scores on 14 scales, with higher scores indicating greater levels of that temperament dimension. The scales included the following: Approach, Vocal Reactivity, High Intensity Pleasure, Smiling and Laughter, Activity Level, Perceptual Sensitivity, Sadness, Distress to Limitations, Fear, Falling Reactivity/Rate of Recovery from Distress, Low Intensity Pleasure, Cuddliness, Duration of Orienting, and Soothability.
Procedure
Mother-infant dyads were invited to participate in a laboratory assessment when infants were approximately 6 months of age. Mothers were asked to ensure the infant was well-fed and rested prior to the visit. Approximately 45 minutes after arrival to the laboratory, to allow infants time to acclimate to the novelty of the laboratory setting, infants were fitted with the inductance bands and electrodes and placed in an infant seat secured to a table, where they remained for the duration of the procedure. Mothers were seated directly across from the infant, at infant eye-level, close enough to be able to physically engage the infant. Initial evaluation of the infant allowed us to determine whether the infant was tired, hungry, or otherwise uncooperative. Mothers were asked whether the child needed to be changed or fed, and feedings or changings were allowed prior to beginning the SFP-R if necessary. Napping was also allowed if the infant arrived at the visit asleep or fell asleep prior to beginning the assessment. If an infant became fussy during the play period of the SFP-R, the protocol was stopped and the infant was settled (e.g., mother fed the infant) prior to starting again. If the infant was unable to complete the play episode (e.g., fussy due to undetected illness, refusal to sit in car seat), then the SFP-R was not administered; this situation was rare. To prevent the infant from becoming excessively distressed, the still-face episode was curtailed per standard administration rules if the experimenter observed the infant fussing continuously for one minute or crying hard and continuously for 30 seconds. Additionally, the still-face 2 and reunion 2 episodes were only administered if the infant had returned to a non-distressed state by the end of the reunion 1 episode.
Data Reduction and Analysis
Respiratory pattern.
The proportionality constant between rib cage and abdominal amplifiers of the respiratory inductive plethysmograph was derived by the Qualitative Diagnostic Calibration procedure (Sackner et al., 1989). This procedure is based on equations of the isovolume maneuver calibration and is carried out during a 2- to 3-minute period of natural breathing. Following calibration, the data were edited for movement artifacts by exporting the data breath-by-breath and removing any segments that did not fall within upper and lower limits of total breath duration (TTOT, the inverse of respiration rate, fR), < 0.8 s or > 6 s, or inspiratory or expiratory volume, < 20 ml or > 400 ml; or where visual inspection of the raw waveform was suggestive of artifact (Dundas et al., 1998; Søvik et al., 2000). Tidal volume (VT,) as the average volume amplitude of a breath was calculated from the mean of inspiratory and expiratory volume. Integrity of questionable data episodes was determined by inspecting video recordings of the session for visible infant movement. Minute ventilation (V’E, the amount of volume inspired or expired in one minute) was calculated as the product of TTOT and VT.
Respiratory sinus arrhythmia (RSA).
ECG recordings were visually inspected for ectopic beats and sequences with excessive movement artifacts that visibly masked the R-wave and thus led to its misspecification or omission. Video recordings of the infant were used simultaneously where necessary (e.g., to confirm excessive motion), and affected sequences were deleted using the VivoSense biosignal analysis software (Vivonoetics, Newport Coast, CA). Mean distances between adjacent R-waves were calculated for each episode. RSA was extracted to estimate cardiac vagal activity as an indicator of parasympathetic influences on the heart. The time-domain peak-valley method (Grossman et al., 1991) was used by extracting modulation of the cardiac RR-interval (in ms) breath-by-breath with the customized rsaToolbox software (V.2.0.1, Schulz et al., 2009). This method subtracts the shortest RR-interval during inspiration from the longest RR-interval during expiration. High correlations (r=.92 and above) between the time-domain peak-valley index and frequency-domain high-frequency heart-rate variability index have been previously reported (Grossman et al., 1990). RSA was set to “missing” for breaths that were too short to accommodate at least two full RR-intervals (TTOT<RR-interval_t1+RR-interval_t2, or HR/2> fR, where t1 is the first RR-interval associated with the onset of inspiration, and t2 is the subsequent RR-interval) (Grossman & Taylor, 2007; Rother et al., 1989). The RSA of breaths that did not adhere to the basic peak-valley criterion (RR-interval minimum during inspiration preceding RR-interval maximum at expiration) were set to zero (Grossman, 1992). Raw RSA data were left-skewed and thus natural logarithm-transformed (ln) to improve distributional characteristics. An additional within-individual adjustment of RSA for respiratory pattern influence was employed (Ritz et al., 2012). RSA was normalized by VT (RSA/VT), ln-transformed because of its left-skewed distribution (lnRSA/VT), residualized for TTOT , and the grand mean of unadjusted lnRSA/VT was added to the residual to obtain the respiration-adjusted lnRSA/VT (lnRSA/VTc). Adjustments for both VT and TTOT have been shown to improve the estimation of cardiac vagal activity in adults (Grossman et al., 1991; Ritz & Dahme, 2006; Ritz, 2009; Wilhelm et al. 2004) and infants (Ritz et al., 2012). For comparison between unadjusted and adjusted RSA, major effects were also calculated for unadjusted ln-transformed RSA (lnRSA). With respiratory parameters accounted for, RSA decreases are suggestive of cardiac vagal withdrawal.
T-wave amplitude (TWA).
TWA of the ECG was extracted using the ECG boundary location function (Laguna et al., 1994) embedded in the AcqKnowledge software package (Version 4.1; Biopac Systems, Inc., Goleta, CA). TWA was used to estimate cardiac sympathetic activity noninvasively, as prior studies have suggested a sensitivity to beta-adrenergic blockade or stimulation (e.g., Contrada et al., 1989; Rau, 1991) and stressful laboratory challenges (e.g., Heslegrave & Furedy, 1979; Scher et al., 1984; Kline et al., 1998; Montoya et al., 1997), including the repeated Still-Face Paradigm in our earlier study from a different cohort (Bosquet Enlow et al., 2014). Noninvasive indices of impedance cardiography have alternatively been used for this purpose (e.g., Alkon et al., 2006; Quigley & Stifter, 2006), but this technique was not implemented to avoid possible interferences with the respiratory inductance plethysmography. A decrease (attenuation) in the TWA score suggests greater sympathetic activation.
Data Analysis
Differences between groups of infants in demographics and baseline physiology were examined with χ2–tests for frequency data and two-tailed t-tests for continuous data. Mixed effects models employing an unstructured repeated measures error covariance matrix were used to analyze the data. To investigate the pattern of autonomic responding to the repeated Still-Face Paradigm, we used a 2 × 5 incomplete factorial design: 2 infant groups (those completing only one still-face trial and those completing both still-face trials) and 5 episodes (play, still-face 1, reunion 1, still-face 2, reunion 2). The infants who terminated after reunion 1 only had the first three episodes. The Satterthwaite approximation was used to conservatively calculate the degrees of freedom for statistical tests.
To statistically compare the size of the effects of the procedure on different outcomes (e.g., respiration-adjusted RSA versus TWA), we used multivariate 2 × 5 factorial mixed effects models (Hox, 2010). The multiple dependent variables were z-scored and then dummy coded for comparison. A significant interaction between the dummy variable coding outcome and an independent variable (group and/or episode) indicates that the effect of the independent variable was different for the two outcome variables.
To examine the within-person associations (across episodes) between RSA or TWA and cardiac activity, and between affect and cardiac activity, we added the potential correlates of cardiac activity as time-varying predictors of cardiac activity in the 2 × 5 mixed effects models used in our first aim investigating the pattern of autonomic responding. Adding them to this model, rather than simply examining these relations on their own without covariates, controls for overall mean differences between groups and episodes and for the other covariates in the 2 × 5 model, thus preventing associations from occurring merely because both predictors and outcomes change between episodes. Further, research shows that time-varying predictors conflate between-subjects differences with within-subjects differences (e.g., a high score on RSA for an infant at a particular episode may reflect either that this infant has generally high RSA or had an increase in RSA above their own individual average). Thus, we disaggregated each time-varying predictor into two separate variables for each infant: the infant’s average score across all episodes on the time-varying predictor (i.e., the between-subjects difference on the time-varying predictor); and their deviation, at each episode, from their average score on the time-varying predictor (i.e., the within-subjects component of the time-varying predictor) (Wang & Maxwell, 2015). Note that these components are uncorrelated. A significant effect of the within-subjects component on the outcome indicates that the time-varying predictor and the outcome are associated within subjects, across episodes.
We also investigated whether the effects of some predictors on outcomes differed between different outcomes (e.g., the effect of the still-face challenge on RSA versus TWA). In these analyses, the different outcomes being compared were added as an additional within-subjects independent variable nested within participants, dummy coded (e.g., “0” to code RSA, “1” to code TWA). A significant interaction between the dummy variable that codes the different outcomes and a predictor indicates that the effect of the predictor is different for the different outcomes. To compare the effect of a predictor on outcomes in these analyses, the outcomes were z-scored to transform them onto the same scale (Hox, 2010).
All analyses included the following control variables: infant activity level, sex, age, race/ethnicity (White, Black, Hispanic, other), weight, and length, and mother’s highest level of education (coded 1=less than HS degree, 2=HS degree, 3=some college, 4=college degree, 5=graduate degree). The significance level was set to p<.05 (two-tailed). Cohen’s d was calculated as the measure of effect size.
Of the 294 dyads with usable physiological data, n=13 were excluded for lack of video recordings and n=3 for lack of maternal education data, resulting in a final analytical sample of N=278. Out of this final sample, n=51 completed only one still-face trial, and n=227 completed both still-face trials.
Results
Demographics and baseline physiology
The demographic characteristics of the final sample are presented in Table 1. Sex representation was relatively balanced, and the sample was sociodemographically diverse (“other” race category was mainly composed of Asian and mixed-race infants). Infants who completed one still-face trial did not differ from infants who completed both still-face trials on any of the assessed sociodemographic variables. There were no overall differences between groups in temperament, as indicated by a MANOVA of the 14 IBQ-R scales, F(14,256)=1.49, p=.113. All mean differences were nonsignificant (ps>.057 to .892), except for the Perceptual Sensitivity subscale, t(271)=3.02, p=.003, indicating that infants in the group with just one still-face trial had a greater tendency to detect slight, low intensity stimuli from the external environment, M1 =4.79 (SD=1.33), compared to the group with two still-face trials, M2 =4.23 (SD=1.15). Maternal sensitivity and physiological parameter mean scores did not differ between groups at play baseline (ps=.083 to .805), including baseline RSA (p=.236) and TWA (p=.805).
Table 1.
Demographics by total sample and by infant groups completing one versus two still-face trials and significance levels of tests for group differences (χ2 for frequency data and two-tailed t-tests for continuous data)
| Total sample (n=278) | Two SFP trials (n=227) | One SFP trial (n=51) | p | |
|---|---|---|---|---|
| Infant sex, female, n (%) | 133 (47.8) | 108(47.6) | 25 (49.0) | .852 |
| Infant age, weeks, M (Range) | 29.0 (25.9–35.2) | 29.2 (26.1–33.7) | 29.2 (25.9–35.2) | .388 |
| Infant race/ethnicity, n (%) | .393 | |||
| White | 85 (30.6) | 65 (28.6) | 20 (37.0) | |
| Black/Haitian | 70 (25.2) | 61 (26.9) | 12 (22.2) | |
| Hispanic | 97 (34.9) | 80 (35.2) | 17 (31.5) | |
| Other | 26 (9.4) | 21 (9.3) | 5 (9.8) | |
| Mother’s education level, n (%) | .955 | |||
| < high school | 61 (21.9) | 51 (22.5) | 10 (19.6) | |
| high school | 22 (7.9) | 17 (7.5) | 5 (9.8) | |
| some college | 60 (21.6) | 50 (22.0) | 10 (19.6) | |
| college degree | 65 (23.4) | 52 (22.9) | 13 (25.5) | |
| graduate level | 70 (25.2) | 57 (25.1) | 13 (25.5) | |
| Height, cm (M,SD) | 67.6 (2.9) | 67.6 (2.9) | 67.3 (3.2) | .453 |
| Weight, kg (M,SD) | 8.1 (0.9) | 8.1 (0.9) | 8.2 (1.0) | .883 |
| Gestational age, weeks (M,SD) | 39.1 (1.8) | 39.0 (1.8) | 39.2 (1.7) | .664 |
| Gestational weight, kg (M,SD) | 7.3 (1.2) | 7.3 (1.1) | 7.4 (1.4) | .987 |
Cardiac, respiratory, and behavioral responses to the still-face challenge
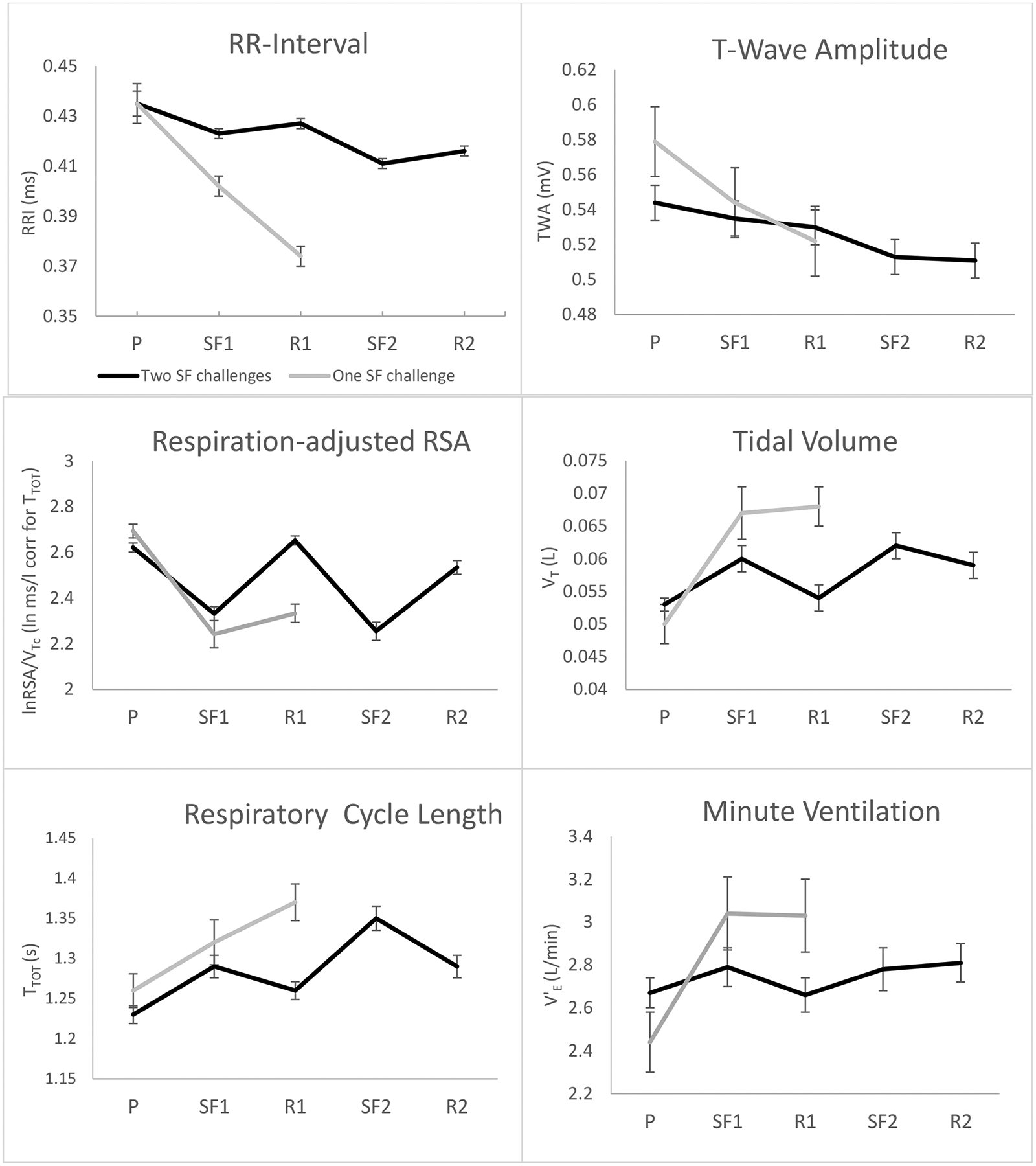
Analysis of main effects across both groups of infants (i.e., non-completers and completers) showed that RR-intervals significantly shortened during the still-face episodes relative to play baseline (Tables 2 and 3 and Figure 1). Similarly, both respiration-adjusted RSA and TWA were substantially reduced, suggesting cardiac vagal withdrawal and sympathetic activation. The change from play to still-face 1 for respiration-adjusted RSA compared to TWA showed that the effect was stronger for the former (when both are z-scored), b=.76, t(247)=9.49, p<.001, indicating a 0.76 SD stronger effect for RSA (equivalent to d=.76). VT, TTOT, physical activity, and negative affect also increased, and positive affect decreased, during still-face episodes compared to play. Reduction from play to still-face 1 was significantly stronger for respiration-adjusted RSA than for unadjusted RSA, b=.34, t(242)=9.44, p<.001, d=.34, for these z-scored measures.
Table 2.
Effects of the still-face challenge on cardiorespiratory parameters in infants who completed one versus two still-face trials
| Time | Group by Time | Sign. Covariates (p)a | |||||
|---|---|---|---|---|---|---|---|
| F | df | P | F | df | p | ||
| RR-interval | 80.68 | 4,381 | <.001 | 85.68 | 2,287 | <.001 | Physical activity(−) (<.001), Age (.006), Length (.013), Race, Black (.049) |
| TWA | 16.05 | 4,461 | <.001 | 7.72 | 2,283 | <.001 | |
| lnRSA | 6.10 | 4,368 | <.001 | 4.35 | 2,251 | .014 | Physical activity (<.001), Age (<.001) |
| lnRSA/VTc | 32.43 | 4,331 | <.001 | 17.84 | 2,246 | <.001 | Physical activity (<.001), Ethnicity, Hispanic (.002), Maternal Education (.022) |
| VT | 11.49 | 4,290 | <.001 | 12.45 | 2,250 | <.001 | Physical activity (<.001), Age (.030), Weight (<.001), Race, White (<.001) |
| TTOT | 15.06 | 4,372 | <.001 | 5.18 | 2,252 | .006 | Physical activity (<.001) |
| V’E | 4.06 | 4,290 | .003 | 6.91 | 2,248 | <.001 | Physical activity (<.001), Age (.040), Weight (<.001), Race, White (<.001) |
| Physical activity | 221.3 | 4,353 | <.001 | 28.66 | 2,278 | <.001 | Sex, Male(−) (.050) |
| Negative affect | 176.6 | 4,443 | <.001 | 107.0 | 2,288 | <.001 | Physical activity (<.001) |
| Positive affect | 141.1 | 4,452 | <.001 | 59.09 | 2,288 | <.001 | Physical activity (<.001) |
Covariates were maternal education level, infant age, sex, height, weight, race/ethnicity, physical activity; physical activity served as a within-individual covariate; all associations positive, except for (−)
Abbreviations: SFP, Still-Face Paradigm; RR-Interval, interval between adjacent R-waves of the ECG; TWA, T-wave amplitude; RSA, respiratory sinus arrhythmia; RSA/VTc, RSA adjusted for tidal volume (VT) and total breath duration (TTOT); V’E, minute ventilation
Table 3.
Significance (p) values for comparisons between play episode versus still-face and reunion episodes for infants completing one versus two still-face trials
| Still-face 1 (SF1) | Reunion 1 (R1) | Still-face 2 (SF2) | Reunion 2 (R2) | |
|---|---|---|---|---|
| RR-Interval | ||||
| Two SFP trials | <.001 | <.001 | <.001 | .020 |
| One SFP trial | <.001 | <.001 | ||
| TWA | ||||
| Two SFP trials | .137 | .003 | <.001 | <.001 |
| One SFP trial | <.001 | <.001 | ||
| lnRSA/VTc | ||||
| Two SFP trials | <.001 | .267 | <.001 | .025 |
| One SFP trial | <.001 | <.001 | ||
| lnRSA | ||||
| Two SFP trials | .005 | .014 | .006 | .661 |
| One SFP trial | .003 | .042 | ||
| TTOT | ||||
| Two SFP trials | <.001 | .007 | <.001 | <.001 |
| One SFP trial | .043 | <.001 | ||
| VT | ||||
| Two SFP trials | .005 | .500 | <.001 | .006 |
| One SFP trial | <.001 | <.001 | ||
| V’E | ||||
| Two SFP trials | .266 | .861 | .358 | .155 |
| One SFP trial | <.001 | <.001 | ||
| Physical activity | ||||
| Two SFP trials | <.001 | <.001 | <.001 | <.001 |
| One SFP trial | <.001 | <.001 | ||
| Negative affect | ||||
| Two SFP trials | <.001 | <.001 | <.001 | <.001 |
| One SFP trial | <.001 | <.001 | ||
| Positive affect | ||||
| Two SFP trials | <.001 | <.001 | <.001 | <.001 |
| One SFP trial | <.001 | <.001 |
Abbreviations: see Table 2.
Note: Entry “<.001” means p<.001. t-statistics ranged from t=0.18 to 26.25. Degrees of freedom for the t-statistics were calculated using the conservative Satterthwaite approximation, which estimates different dfs for every t-statistics. Those dfs ranged from df=192 to 518.
Figure 1.
Cardiac and respiratory responses to repeated Still-Face Paradigm episodes for infants completing one versus two still-face trials; P=play, SF1=still-face 1, R1=reunion 1, SF2=still-face 2, R2=reunion 2
Differential cardiac, respiratory, and behavioral responses in infants completing one versus two trials of the still-face challenge
Infants completing two still-face trials showed a different response pattern from those completing only one trial, with significant Group x Time interactions for all parameters (Table 2). Whereas RR-interval shortening during the still-face episodes was relatively small in those completing two challenges, those completing only one challenge showed a stronger reduction, which continued into the first reunion period (Figure 1). The RR-interval shortening was significantly stronger in the group with one episode, b=−.02, t(276)=−5.40, p<.001, as was the effect for respiration-adjusted RSA, b=−.16, t(242)=2.10, p=.036, and TWA, b=−.17, t(275)=−2.61, p=.010, but not for unadjusted RSA, b=−.06, t(243),=1.29, p=.199, although differences in the effect for the adjusted and unadjusted RSA were not significant, b=.20, t(2230)=.66, p=.511. It should be noted that, although visually suggestive (Figure 1), initial play values of TWA were not significantly different between groups (p=.158).
Whereas respiration-adjusted RSA showed full or partial recovery in the group with two still-face trials (p=.267 and p=.030 for difference between play and reunion 1 and play and reunion 2, respectively), it did not recover in the group with one still-face trial (p<.001) (Table 3). Of note, TWA showed no evidence of recovery during reunion in either group, with progressive attenuation across the protocol, and stronger attenuation in those completing only one challenge (all reunion values lower than play, p<.001).
Within the group completing only one challenge, the effect for the baseline to still-face 1 change for z-scored respiration-adjusted RSA was significantly greater compared to z-scored TWA, b=.98, t(937)=3.81, p<.001, d=.98, equivalent to a large effect difference. The same comparison for the group completing both still-face episodes was similar, b=.76, t(937)=6.21, p<.001, d=.76. For the group completing both still-face episodes, the effect for the baseline to still-face 2 change was also greater for z-scored respiration-adjusted RSA compared to z-scored TWA, b=.80, t(614)=5.96, p<.001.
Marked differences between groups were observed for the respiratory pattern (Figure 1 and Table 3). Whereas VT for the group undergoing two challenges returned to baseline after elevations during the still-face episode, the VT for the group with one challenge did not recover and remained elevated during the reunion 1 period. Similar differences were seen for TTOT during the reunion 1 period, where continued increases were observed for the group with one challenge, compared to partial recovery in the group with two challenges. As a result, V’E remained stable in the group with two challenges across all episodes, whereas it increased markedly in the group with only one challenge.
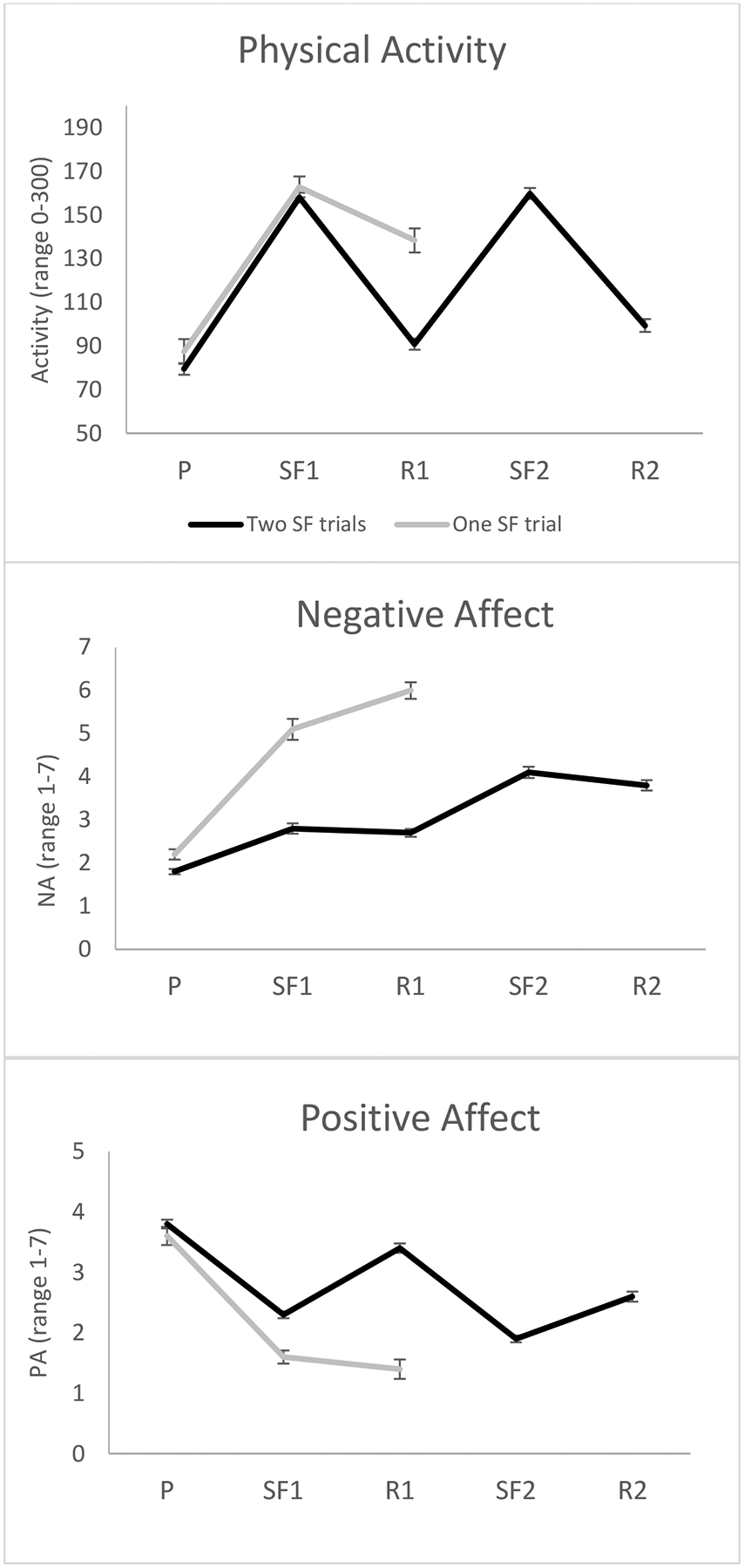
Observational ratings of affective behavior and physical activity confirmed the assignment to groups, in that infants in the group with one challenge showed more negative affect, less positive affect, and more physical activity during the reunion 1 episode than the infants who completed both challenges (Tables 2 and 3 and Figure 2).
Figure 2.
Observed physical activity, negative affect, and positive affect during repeated Still-Face Paradigm episodes for infants completing one versus two still-face trials; P=play, SF1=still-face 1, R1=reunion 1, SF2=still-face 2, R2=reunion 2
Associations of stress-induced RSA and TWA changes with chronotropic cardiac activity
Changes in RR-interval within-subjects across episodes were strongly associated with changes in z-scored respiration-adjusted RSA, b=.005, t(721)=8.41, p<.001, and z-scored TWA, b=.006, t(683)=9.13, p<.001 (z-scored for comparison). Unadjusted RSA was also strongly associated with RR-interval within-subjects, b=.008, t(657)=8.86, p<.001. The relation between z-scored respiration-adjusted RSA and z-scored TWA with RR-Interval was not different (p=.884). Similarly, the relation between unadjusted RSA and RR-interval was not different from the relation between adjusted RSA and RR-interval (p=.701). Entering both adjusted RSA and TWA as time-varying covariates into the model showed that both explained significant portions of the variance over the other, with b=.006, t(665)=7.95, p<.001 for respiration-adjusted RSA and b=.003, t(626)=5.63, p<.001 for TWA.
Differences between infants who completed one versus two still-face challenges in the association of stress-induced RSA and TWA changes with chronotropic cardiac activity
The strength of the association of stress-induced RR-interval changes on the one hand and RSA or TWA changes on the other hand also varied between groups, as suggested by significant interactions between group and the time-varying predictor for both respiration-adjusted RSA, b=−.002, t(474)=−2.24, p=.026, and for TWA, b=−.002, t(476)=−3.23, p=.001, but not for non-adjusted RSA, b= .002, t(254)=.36, p=.719 (calculated over the first 3 episodes for both groups). Associations were stronger for the group completing only one still-face challenge. The strength of the relation between respiration-adjusted RSA and RR-interval was not different from the strength of the relation between TWA and RR-interval for either group (ps>.70).
Associations of cardiac and respiratory responses with observed infant affect
Both negative and positive affect were associated with the RR-interval across episodes, in that RR-intervals were shorter when negative affect was higher, b=−.008, t(746)=16.98, p<.001, and longer when positive affect was higher, b=.009, t(712)=11.60, p<.001. TWA across episodes showed a negative association with negative affect, b=−.007, t(872)=−4.71, p<.001, and a positive association with positive affect, b=.005, t(837)=2.27, p=.023. Respiration-adjusted RSA and non-adjusted RSA were strongly and negatively associated with negative affect (b=−.06, t(847)=−6.05, p<.001 and b=−.02, t(818)=−3.60, p<.001, respectively) and positively associated with positive affect (b=.05, t(643)=3.68, p<.001 and b=.03, t(726)=3.57, p<.001, respectively). The association with positive affect was significantly weaker for TWA than for respiration-adjusted RSA, b=.05, t(643)=3.27, p=.001, d=.26.
Both negative and positive affect were also associated with VT and TTOT in general. Higher negative affect was linked to higher VT, b=5.81, t(796)=3.53, p<.001, and longer TTOT, b=1.51, t(827)=6.09, p<.001. Higher positive affect was linked to lower VT, b=−3.74, t(674)=−3.14, p<.001, and shorter TTOT, b=−.58, t(721)=−3.19, p<.001. V’E was lower for higher positive affect, b=−.06, t(667)=−2.16, p=.031, whereas no significant association was seen with negative affect, b=.05, t(777)=1.48, p=.140.
Differences between infants who completed one versus two still-face challenges in the association of cardiac and respiratory responses with observed affect
For the RR-interval, both Group x Negative Affect and Group x Positive Affect interactions were marginal, b=−.003, t(527)=−1.85,p=.065, and b=.004, t(471)=1.81, p=.071, respectively, suggesting that the association of RR-interval with both negative affect and positive affect did not vary greatly between infants who completed one versus two trials of the still-face challenge. Respiration-adjusted RSA showed differential associations with negative affect between groups, with the Group x Negative Affect interaction being significant, b=−.09, t(495)=−2.78, p=.006, indicating a stronger association with negative affect in the group that completed only one still-face trial. This group difference was only marginally significant when using the unadjusted RSA, b=−.04, t(487)=−1.86, p=.064.
None of the respiratory variables were differentially linked to affect in one versus the other group of infants.
Discussion
Our findings indicate that the stress of the still-face challenge elicits both cardiac sympathetic activation and parasympathetic withdrawal, as reflected in the ECG TWA and RSA, respectively, in infants. Most of the prior research on autonomic activity has focused on RSA and not considered cardiac sympathetic effects, which also are of potential relevance for long-term cardiovascular health. We found that, although effects sizes of RSA were greater than those of TWA, they contributed equally to changes in chronotropic cardiac activity. Moreover, whereas partial recovery following cessation of the still-face challenge was observed for RSA, TWA was progressively attenuated across the session, indicating differential time constants, with tonic sympathetic activation and more phasic parasympathetic withdrawal across stress episodes. Moreover, our study controlled for physical activity, an important confound when interpreting autonomic cardiac adjustments to stress as psychologically driven processes. We also considered respiration as an important confound in the interpretation of RSA, which has been largely ignored in prior infant stress research (Ritz et al., 2012). Our findings indicate that this strategy is significantly more sensitive for demonstrating parasympathetic withdrawal to stress. In addition, infants unable to complete both still-face trials showed stronger sympathetic activation and parasympathetic withdrawal, as well as elevated V’E. Moreover, their autonomic adjustments drove heart rate to a greater extent than in the infants who completed both trials. Although RR-interval, RSA, and TWA were all associated with observable infant negative and positive affective behavior, RSA was particularly tightly associated with negative affect in those who only completed the first still-face trial.
Control for physical activity is important when inferences are to be drawn to psychological processes driving autonomic adjustments. Infants cannot be expected to cooperate with standard laboratory assessment instructions to sit still, which is geared toward minimizing such effects in adult participants. Given the basic function of the cardiovascular and respiratory systems to meet metabolic demands, autonomic adjustments to the still-face stressor could simply reflect greater activity levels as infants become distressed by the unpleasantness of the challenge. It has been argued that the “additional heart rate” due to stress beyond basic coverage of metabolic demands is the part of stress-induced cardiac activity that is potentially detrimental for long-term health (Obrist, 1976). Our findings suggest that there is substantial sympathetic activity and parasympathetic withdrawal that is not explained by physical activity. Although the effect size for average reductions in RSA (parasympathetic withdrawal) was larger than for the TWA attenuation (sympathetic activation), associations with the RR-interval suggested that both were driving increases in the residual heart rate equally. These findings are in line with the reciprocity of cardiac sympathetic activation and parasympathetic withdrawal typically observed in adults during psychological stress challenges (e.g., Berntson et al., 1996), although increases in heart rate, when controlling for metabolic demand, seem to be driven more by sympathetic activity (Balanos et al., 2010). Our study goes beyond prior infant research that has relied on salivary alpha amylase as a sympathetic activity indicator (Hill-Soderlund et al., 2008; Laurent et al., 2012; Rash et al., 2016), which does not inform about cardiac autonomic activity, given that sympathetic outflow may be uncorrelated across organ systems and tissues (Morrison, 2001).
With potential relevance to long-term health, the autonomic adjustments were significantly stronger in the subgroup of infants who were not able to recover following cessation of the first still-face challenge (i.e., were behaviorally distressed at the end of reunion 1). Their physical activity-adjusted cardiac activity showed lack of recovery during the reunion 1 episode, potentially creating a state of more sustained additional cardiac activity due to stress. Such a state may put these infants at risk for later health complications. Recent research has progressed to identify molecular pathways of sympathetic effects, such as regional noradrenergic spill over (Esler et al., 2003) and genetic regulation of enzymatic activity (Wong et al., 2012), that are of potential significance for cardiovascular disease development and progression.
During the still-face episodes, infants were typically breathing slower and deeper compared to during the non-stressor episodes. These changes were not attributable to overt physical activity and could be due to crying. Strong elevations in V’E in the subgroup who completed only one still-face trial, compared to no changes in those who underwent two trials, likely amounted to hypocapnia during the still-face and reunion episodes. Hyperventilation is typically linked to states of overwhelming stress and panic (Boiten et al., 1994) and is common in adults with anxiety disorders in exposure to feared stimuli (e.g., Alpers et al., 2005; Ayala et al., 2010). Interestingly, one respiratory theory of panic disorder speculates that childhood separation anxiety is a precursor of panic (Klein, 1993). The fact that negative affective behavior was not significantly associated with V’E changes may limit this line of interpretation; however, panic attacks have often been described as spontaneous, “out of the blue,” without distinct experiential precursors, whereas cardiorespiratory perturbations can be observed in the hour leading up to an attack (Meuret et al, 2011). Future studies that monitor gas exchange are needed to consolidate these findings, and prospective studies could explore the usefulness of infant respiratory responses to separation paradigms as predictors of future psychopathology.
Of particular relevance for this study, associations of the respiratory pattern with RSA amplitude have long been observed and are known to complicate the interpretation of RSA as an index of tonic vagal control (for reviews, see Brown et al., 1993; Eckberg, 2003; Grossman & Taylor, 2007; Ritz & Dahme, 2006). In line with a previous study using a smaller sample of infants (Ritz et al., 2012), we adjusted RSA following earlier suggestions that the transfer function of RSA over VT at a particular respiration rate is a more valid estimator for tracking within-individual changes in vagal activity (Saul et al., 1989; Grossman et al., 1991; Ritz et al., 2001; Wilhelm et al., 2004). In the current study, we were able to show that RSA is substantially reduced by stress, and significantly more so in those infants who were too distressed to undergo a second challenge. RR-interval shortening and TWA attenuation also reflected this group distinction. Unadjusted RSA was less sensitive to it, demonstrating the importance of considering respiratory pattern in the interpretation of RSA in infant stress studies.
The observed affective behavior of infants was associated with changes in cardiac activity even when controlling for overt physical activity changes. Previous studies using the Still-Face Paradigm have not systematically studied affect in relation to autonomic responses or controlled for physical activity and respiration. We found that the cardiac parameters RR-interval, adjusted RSA, and TWA all reflected negative and positive affective behavior, although association of TWA with positive affect was weaker. Thus, vagal excitation appears to play a stronger role with respect to affective behavior, especially after controlling for physical activity and respiration. This is partially reflected in prior stress research with adult anxiety patients, where symptomatic and affective responses to fear exposure were associated with respiration-adjusted RSA, but not unadjusted RSA or TWA (Simon et al., 2017).
In an effort to remain parsimonious in our interpretation of autonomic adjustments to the still-face stressor, we have intentionally avoided assumptions involving “self-regulatory” behavior of the infant, common in the child developmental literature. From this perspective, autonomic recovery after the still-face exposure could be interpreted as a successful self-regulatory act directed at terminating an unpleasant state of arousal. It could also be speculated that the infant’s parasympathetic withdrawal (and sympathetic activation) during still-face exposure were reflections of an effort to re-engage the mother in communicative acts in order to terminate the unpleasant state of communicative breakdown. However, because central nervous system processes were not the subject of our study, such assumptions were not tested, and the observed autonomic adjustments would, at this stage, be more parsimoniously viewed as simple concomitants of experienced negative affect or distress. This does not exclude the possibility that adjustments could be explained as preparation for action (beyond the already observed physical activation), as the idea of a preparation for action or “felt action tendency” is often central to the definition of emotion (Frijda, 1986). Active imagery of exercise in the absence of actual action has been shown to trigger cardiac and respiratory adjustments (Oishi et al., 2000), and imagery of physiological concomitants of emotion has been shown to initiate consistent autonomic and somatic motor outflow (Lang, 1979; Van Diest et al., 2005). With the advance of neuroimaging techniques, future studies could expand our knowledge regarding brain processes that accompany mother-infant interaction. Such knowledge would allow for more conclusive evidence about the functionality or goal-directedness of infant autonomic adjustments in stressful situations.
Our study was limited in that RSA and the ECG TWA were measured by indirect, noninvasive indicators of cardiac autonomic activity. Whereas more direct techniques, such as sympathetic nerve activity recordings (Hart et al. 2017), might be desirable, their invasiveness would interfere with the study of more subtle emotional processes, in particular in infants and children. Also, it has become more common to use noninvasive indices from impedance cardiography (such as pre-ejection period) as noninvasive indicators of cardiac sympathetic activity in infant and childhood studies (Alkon et al., 2006; Quigley & Stifter, 2006; Suurland et al., 2017). However, given that it was necessary to monitor respiration by respiratory inductive plethysmography at the same time, we sought to avoid interference between these techniques and elected to extract TWA, which has been shown to be sensitive to beta-adrenergic blockade (Rau, 1991), mental stress (Heslegrave & Furedy, 1979; Scher et al., 1984; Kline et al., 1998; Montoya et al., 1997), and anger and fear (Kreibig, 2010). Our sample consisted primarily of healthy full-term infants; thus, the findings may not generalize to infants who experienced poor birth outcomes. The autonomic functions of infants born preterm may be less well integrated and, consequently, these infants may be at particular risk for long-term adverse developmental health outcomes through psychological stress (Neri et al., 2017). Additionally, our study strategy could be viewed as circular in that we divided infants into more or less distressed groups and subsequently showed that one group was more distressed than the other. However, we used behavioral observation for establishing the two groups and the analysis of negative affect served as a manipulation check for the success of this assignment. The actual focus of our study was on physiological responding (which was not a criterion for group assignment) and its association with observed variations in affect.
In conclusion, reciprocal cardiac sympathetic activation and parasympathetic withdrawal, estimated by TWA attenuation and RSA reduction, respectively, are part of the autonomic pattern of infants during challenge periods of a repeated still-face psychosocial stressor. Whereas parasympathetic withdrawal typically resolves within minutes after cessation of the stressor, sympathetic activity continues to increase. Of note, these autonomic adjustments are not explained by observable physical activity of the infant, suggesting additional ANS activation beyond metabolic requirements of activity. Both TWA and RSA are strongly associated with overt affective behavior of infants. Infants who cannot cope with a prolonged exposure to a psychosocial stressor show both stronger cardiac sympathetic activation and parasympathetic withdrawal, with stronger associations of TWA and RSA with cardiac activity and affect. Notably, adjusting RSA for respiratory pattern effects is necessary to show some of these associations. Additionally, infants who cannot complete a prolonged exposure also show signs of hyperventilation, a factor consistently linked to anxiety in adults. Longitudinal studies are needed to determine if stronger autonomic and respiratory responses beyond metabolic demand predispose infants for later health complications.
Acknowledgements
This work was supported by grants from the National Heart, Lung, & Blood Institute (R01HL095606; R01HL114396), the National Institute of Environmental Health Sciences (P30ES023515), and the Program for Behavioral Science in the Department of Psychiatry at Boston Children’s Hospital. None of the funding agencies had any role in the study design, the collection, analysis or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not represent the official views of any granting agency.
REFERENCES
- Alkon A, Lippert S, Vujan N, Rodriquez ME, Boyce WT, & Eskenazi B (2006). The ontogeny of autonomic measures in 6- and 12-month-old infants. Developmental Psychobiology, 48, 197–208. 10.1002/dev.20129. [DOI] [PubMed] [Google Scholar]
- Allen MT, Matthews KA, &Sherman FS (1997). Cardiovascular reactivity to stress and left ventricular mass in youth. Hypertension, 30, 782–787. 10.1161/01.HYP.30.4.782. [DOI] [PubMed] [Google Scholar]
- Alpers GW, Wilhelm FH, & Roth WT (2005). Psychophysiological assessment during exposure in driving phobic patients. J Abnormal Psychology, 114 (1), 126–139. 10.1037/0021-843X.114.1.126. [DOI] [PubMed] [Google Scholar]
- Ayala ES, Meuret AE, & Ritz T (2010). Confrontation with blood and disgust stimuliprecipitates respiratory dysregulation in blood-injection-injury phobia. Biological Psychology, 84, 88–97. 10.1016/j.biopsycho.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Balanos GM, Phillips AC, Frenneaux MP, McIntyre D, Lykidis C, Griffin HS, & Carroll D (2010). Metabolically exaggerated cardiac reactions to acute psychological stress: the effects of resting blood pressure status and possible underlying mechanisms. Biological Psychology, 85, 104–111. 10.1016/j.biopsycho.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Neuhaus E, Chipman J, & Reid MJ (2013). Sympathetic and parasympathetic- linked cardiac function and prediction of externalizing behavior, emotions regulation, and prosocial behavior among preschoolers treated for ADHD. Journal of Consulting and Clinical Psychology, 81(3), 481–493. 10.1037/a0032302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhenova OV, Plonskaia O, & Porges SW (2001). Vagal reactivity and affective adjustment in infants during interaction challenges. Child Development, 72, 1314–1326. 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Wdowczyk-Szulc J, Valenti C, Castoldi S, Passino C, Spadacini G, & Sleight P (2000). Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. Journal of the American College Cardiology, 35(6), 1462–1469. 10.1016/S0735-1097(00)00595-7. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT Jr., Eckberg DL, Grossman P, & Kaufmann PG (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology, 34, 623–648. 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Fieldstone A (1996). Illusions, arithmetic, and the bidirectional modulation of vagal control of the heart. Biological Psychology, 44(1), 1–17. 10.1016/S0301-0511(96)05197-6. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1991). Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review, 98(4), 459–487. 10.1037/0033-295X.98.4.459. [DOI] [PubMed] [Google Scholar]
- Boiten FA, Frijda NH, & Wientjes CJ (1994). Emotions and respiratory patterns: review and critical analysis. International Journal of Psychophysiology, 17(2), 103–128. 10.1016/0167-8760(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M, King L, Schreier H, Howard J, Schultz SM, Rosenfield D, Ritz T, & Wright RJ (2014). Maternal sensitivity and infant autonomic and endocrine stress responses. Early Human Development, 90, 377–385. 10.1016/j.earlhumdev.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Kullowatz A, Staudenmayer J, Spasojevic J, Ritz T, & Wright RJ (2009). Associations of maternal lifetime trauma and perinatal traumatic stress symptoms with infant cardiorespiratory reactivity to psychological challenge. Psychosomatic Medicine, 71(6), 607–614. 10.1097/PSY.0b013e3181ad1c8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, White MT, Hails K, Cabrera I, & Wright RJ (2016). The Infant Behavior Questionnaire-Revised: Factor structure in a culturally and sociodemographically diverse sample in the United States. Infant Behavior and Development, 43, 24–35. doi: 10.1016/j.infbeh.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, & Kupfer DJ (2001). Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry, 179(2), 144–150. 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Brown TE, Beightol LA, Koh J, & Eckberg DL (2003). Important influence of respiration on human R-R interval power spectra is largely ignored. Journal of Applied Physiology, 75, 2310–2317. 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- Bush NR, Jones-Mason K, Coccia M, Caron Z, Alkon A, Thomas M, Coleman-Phox K, Wadhwa PD, Laraia BA, Adler NE, & Epel ES (2017). Effects of pre- and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Development and Psychopathology, 29, 1553–1571. 10.1017/S0954579417001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busscher B, van Gerwen LJ, Spinhoven P, & de Geus EJ (2010). Physiological reactivity to phobic stimuli in people with fear of flying. Journal of Psychosomatic Research, 69(3), 309–317. 10.1016/j.jpsychores.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Crites SL, Snydersmith MA, Smith G, Berntson GG, & Lang PJ (1992). Relationship between facial expressiveness and sympathetic activation in emotion: a critical review, with emphasis on modeling underlying mechanisms and individual differences. Journal of Personality and Social Psychology, 62(1), 110–128. 10.1037/0022-3514.62.1.110. [DOI] [PubMed] [Google Scholar]
- Chida Y, & Steptoe A (2010). Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension, 55, 1026–1032. 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Conradt E, & Ablow J (2010). Infant physiological response to the still-face paradigm: contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior and Development, 33, 251–265. 10.1016/j.infbeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Contrada RJ, Krantz DS, Durel LA, Levy L, LaRiccia PJ, Anderson JR, & Weiss T (1989). Effects of beta-adrenergic activity on T-wave amplitude. Psychophysiology, 26(4), 488–492. [DOI] [PubMed] [Google Scholar]
- Dundas I, Beardsmore C, Wellman T, & Stocks J (1998). A collaborative study of infant respiratory function testing. European Respiratory Journal, 12(4), 944–53. [DOI] [PubMed] [Google Scholar]
- Eckberg DL (2003). The human respiratory gate. Journal of Physiology, 548, 339–352. 10.1111/j.1469-7793.2003.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egizio VB, Eddy M, Robinson M, & Jennings JR (2011). Efficient and cost-effective estimation of the influence of respiratory variables on respiratory sinus arrhythmia. Psychophysiology, 48(4), 488–494. doi: 10.1111/j.1469-8986.2010.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel GL (1978). Psychologic stress, vasodepressor (vasovagal) syncope, and sudden death. Annals of Internal Medicine, 89(3), 403–412. 10.7326/0003-4819-89-3-403. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, & Kaye D (2003). Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiologica Scandinavia, 177, 275–284. 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Frijda NH (1986). The emotions. Cambridge University Press. [Google Scholar]
- Gartstein MA, Knyazev GG, & Slobodskaya HR (2005). Cross-cultural differences in the structure of infant temperament: United States of America (U.S.) and Russia. Infant Behavior & Development, 28, 54–61. doi: 10.1016/j.infbeh.2004.09.003. [DOI] [Google Scholar]
- Gartstein MA, Peleg Y, Young BN, & Slobodskaya HR (2009). Infant temperament in Russia, United States of America, and Israel: differences and similarities between Russian-speaking families. Child Psychiatry and Human Development, 40, 241–256. doi: 10.1007/s10578-009-0123-3. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development, 26, 64–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp L & Ram N (2018). Developmental dynamics of autonomic function in childhood. Psychophysiology, 55(11):e13218. doi: 10.1111/psyp.13218. [DOI] [PubMed] [Google Scholar]
- Gerin W, Pickering TG, Glynn L, Christenfeld N, Schwartz A, Carroll D, & Davidson K (2000). An historical context for behavioral models of hypertension. Journal of Psychosomatic Research, 48, 369–377. 10.1016/S0022-3999(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, & Jennings JR (2018). Host in the machine: A neurobiological perspective on psychological stress and cardiovascular disease. American Psychologist, 73(8), 1031–1044. 10.1037/amp0000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Kraynak TE, Fisher JP, & Gianaros PJ (2017). Cardiovascular and autonomic reactivity to psychological stress: Neurophysiological substrates and links to cardiovascular disease. Autonomic Neuroscience, 207, 2–9. 10.1016/j.autneu.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SAO, Jones CW, Theall KP, Glackin E, & Drury SS (2017). Thinking across generations: Unique contributions of maternal early life and prenatal stress to infant physiology. Journal of the American Academy of Child and Adolescence Psychiatry, 56(11), 922–929. 10.1016/j.jaac.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P (1992). Respiratory and cardiac rhythms as windows to central and autonomic biobehavioral regulation: selection of window frames, keeping the panes clean and viewing the neural topography. Biological Psychology, 34, 131–61. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, & Wieling W (1991). Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology, 28, 201–216. 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, & Taylor EW (2007). Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285. 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Grossman P, van Beek J, & Wientjes CA (1990). Comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology, 27(6), 702–714. 10.1111/j.1469-8986.1990.tb03198.x [DOI] [PubMed] [Google Scholar]
- Haley DW, & Stansbury K (2003). Infant stress and parent responsiveness: regulation of physiology and behavior during still-face and reunion. Child Development, 74(5), 1534–1546. 10.1111/1467-8624.00621. [DOI] [PubMed] [Google Scholar]
- Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, & Osborn JW (2017). Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. American Journal of Physiology Heart Circulatory Physiology, 312(5), 1031–1051. 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslegrave RJ, & Furedy JJ (1979). Sensitivities of HR and T-wave amplitude for detecting cognitive and anticipatory stress. Physiology and Behavior, 22(1), 17–23. 10.1016/0031-9384(79)90397-4. [DOI] [PubMed] [Google Scholar]
- Hill-Soderlund AL, Mills-Koonce WR, Propper C, Calkins SD, Granger DA, Moore GA, Gariepy JL, & Cox MJ (2008). Parasympathetic and sympathetic responses to the strange situation in infants and mothers from avoidant and securely attached dyads. Developmental Psychobiology, 50(4), 361–376. 10.1002/dev.20302. [DOI] [PubMed] [Google Scholar]
- Hinnant JB, Elmore-Staton L, & El-Sheikh M (2011). Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Developmental Psychobiology, 53(1), 59–68. 10.1002/dev.20487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, & Bishop B (1981). Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. American Journal of Physiology, 241(4), H620–H629. 10.1152/ajpheart.1981.241.4.H620. [DOI] [PubMed] [Google Scholar]
- Homma I, & Masaoka Y (2008). Breathing rhythms and emotions. Experimental Physiology, 93(9), 1011–1021. 10.1113/expphysiol.2008.042424. [DOI] [PubMed] [Google Scholar]
- Hox J (2010). Multilevel analysis: Techniques and applications (2nd ed.). Utrecht: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Johnston DW, & Anastasiades P (1990). The relationship between heart rate and mood in real life. Journal of Psychosomatic Research, 34(1), 21–27. 10.1016/0022-3999(90)90004-N. [DOI] [PubMed] [Google Scholar]
- Klein DF (1993). False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry, 50(4), 306–317. 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Kline KP, Ginsburg GP, & Johnston JR (1998). T-wave amplitude: relationships to phasic RSA and heart period changes. International Journal of Psychophysiology, 29(3), 291–301. 10.1016/S0167-8760(98)00021-X. [DOI] [PubMed] [Google Scholar]
- Koizumi K, & Kollai M (1981). Control of reciprocal and non-reciprocal action of vagal and sympathetic efferents: study of centrally induced reactions. Journal Autonomic Nervous System, 3(2–4), 483–501. 10.1016/0165-1838(81)90082-5. [DOI] [PubMed] [Google Scholar]
- Kreibig SD (2010). Autonomic nervous system activity in emotion: a review. Biological Psychology, 84(3), 394–421. 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Kircher JC, & Webb AK (2007). Multilevel models for repeated measures research designs in psychophysiology: an introduction to growth curve modeling. Psychophysiology, 44(5), 728–736. 10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Lacey JI (1967) Somatic response patterning and stress: Some revisions of activation theory. Psychological Stress: Issues in Research. Appleton-Century-Crofts, 14–44. [Google Scholar]
- Laguna P, Jané R, & Caminal P (1994). Automatic detection of wave boundaries in multilead ECG signals: validation with the CSE database. Computers and Biomedical Research, 27(1), 45–60. 10.1006/cbmr.1994.1006 [DOI] [PubMed] [Google Scholar]
- Lang PJ (1979). Presidential address, 1978. A bio-informational theory of emotional imagery. Psychophysiology, 16(6), 495–512. 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, & Ohman A (2000). Fear and anxiety: animal models and human cognitive psychophysiology. Journal Affective Disorders, 61(3), 137–159. 10.1016/S0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, & Measelle J (2012). Taking stress response out of the box: stability, discontinuity, and temperament effects on HPA and SNS across social stressors in mother-infant dyads. Developmental Psychology, 48(1), 35–45. 10.1037/a0025518. [DOI] [PubMed] [Google Scholar]
- Lovallo WR (2005). Cardiovascular reactivity: mechanisms and pathways to cardiovascular disease. International Journal of Psychophysiology, 58(2–3), 119–132. 10.1016/j.ijpsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Rosenfied D, Wilhelm FH, Zhou E, Conrad A, Ritz T, & Roth WT (2011). Do unexpected panic attacks occur spontaneously? Biological Psychiatry, 70(10), 985–991. 10.1016/j.biopsych.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya P, Brody S, Beck K, Veit R, & Rau H (1997). Differential beta- and alpha-adrenergic activation during psychological stress. European Journal of Applied Physiology and Occupational Physiology, 75, 256–262. 10.1007/s004210050157. [DOI] [PubMed] [Google Scholar]
- Moore GA (2009). Infants’ and mothers’ vagal reactivity in response to anger. Journal of Child Psychology and Psychiatry, and Applied Disciplines, 50(11), 1392–1400. 10.1111/j.1469-7610.2009.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA, & Calkins SD (2004). Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination or mother-infant interaction. Developmental Psychology, 40(6), 1068–1080. 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Morrison SF (2001). Differential control of sympathetic outflow. American Journal of Physiology, Regulatory Integrative and Comparative Physiology, 281(3), 683–698. 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- Myrtek M, & Brügner G (1996). Perception of emotions in everyday life: studies with patients and normals. Biological Psychology, 42(1–2), 147–164. 10.1016/0301-0511(95)05152-X. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Marceau K, Shirtcliff EA, Hastings PD, & Oldehinkel AJ (2015). Autonomic and Adrenocortical Interactions Predict Mental Health in Late Adolescence: The TRAILS Study. Journal of Abnormal Child Psychology, 43, 847–861. 10.1007/s10802-014-9958-6. [DOI] [PubMed] [Google Scholar]
- Neri E, Agostini F, Baldoni F, Facondini E, Biasini A, & Monti F (2017). Preterm infant development, maternal distress and sensitivity: The influence of severity of birth weight. Early Human Development, 106, 19–24. 10.1016/j.earlhumdev.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Obrist PA (1976). The cardiovascular-behavioral interaction- as it appears today. Psychophysiology, 13(2), 95–107. 10.1111/j.1469-8986.1976.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Oishi K, Kasai T, & Maeshima T (2000) Autonomic response specificity during motor imagery. Journal of Physiological Anthropology and Applied Human Science, 19, 255–261. 10.2114/jpa.19.255. [DOI] [PubMed] [Google Scholar]
- Overbeek TJ, van Boxtel A, & Westerink JH (2012). Respiratory sinus arrhythmia responses to induced emotional states: effects of RSA indices, emotion induction method, age, and sex. Biological Psychology, 91(1), 128–141. 10.1016/j.biopsycho.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Parade SH & Leerkes EM (2008). The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behavior and Development, 31(4), 637–646. doi: 10.1016/j.infbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ (2008). Psychophysiological correlates of aggression and violence: an integrative review. Philosophical Transactions of the Royal Society B Biological Sciences, 63, 2542–2555. 10.1098/rstb.2008.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Hunt K, Der G & Carroll D (2011). Blunted cardiac reactions to acute psychological stress predict symptoms of depression five years later: evidence from a large community study. Psychophysiology, 48(1), 142–148. 10.1111/j.1469-8986.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- Quigley KS, & Stifter CA (2006). A comparative validation of sympathetic reactivity in children and adults. Psychophysiology, 43(4), 357–365. 10.1111/j.1469-8986.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- Rash JA, Campbell TS, Letourneau N, & Giesbrecht GF (2015). Maternal cortisol during pregnancy is related to infant cardiac vagal control. Psychoneuroendocrinology, 54, 78–89. 10.1016/j.psyneuen.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Rau H (1991). Responses of the T-wave amplitude as a function of active and passive tasks and beta-adrenergic blockade. Psychophysiology, 28(2), 231–239. 10.1111/j.1469-8986.1991.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Richards JE, & Casey BJ (1991). Heart rate variability during attention phases in young infants. Psychophysiology, 28(1), 43–53. 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Ritz T (2009). Studying noninvasive indices of vagal control: the need for respiratory control and the problem of target specificity. Biological Psychology, 80(2), 158–168. 10.1016/j.biopsycho.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Ritz T, & Dahme B (2006). Implementation and interpretation of heart rate variability measures in psychosomatic medicine: Practice against better evidence? Psychosomatic Medicine, 68(4), 617–627. 10.1097/01.psy.0000228010.96408.ed. [DOI] [PubMed] [Google Scholar]
- Ritz T, Bosquet Enlow M, Schulz SM, Kitts R, Staudenmayer J, & Wright RJ (2012). Respiratory sinus arrhythmia as an index of vagal activity during stress in infants: Respiratory influences and their control. PLoS One, 7(12), e52729. 10.1371/journal.pone.0052729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Thöns M, & Dahme B (2001). Modulation of respiratory sinus arrhythmia by respiration rate and volume: Stability across postures and volume variations. Psychophysiology, 38(5), 858–862. 10.1111/1469-8986.3850858. [DOI] [PubMed] [Google Scholar]
- Rother M, Witte H, Zwiener U, Eiselt M, & Fischer P (1989). Cardiac aliasing—a possible cause for the misinterpretation of cardiorespirographic data in neonates. Early Human Development, 20(1), 1–12. 10.1016/0378-3782(89)90068-6. [DOI] [PubMed] [Google Scholar]
- Sackner MA, Watson H, Belsito AS, Feinerman D, Suarez M, Gonzalez G, Bizousky F, & Krieger B (1989). Calibration of respiratory inductive plethysmograph during natural breathing. Journal of Applied Physiology, 66(1), 410–420. 10.1152/jappl.1989.66.1.410. [DOI] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Chen MH, & Cohen RJ (1989). Transfer function analysis of autonomic regulation. II. Respiratory sinus arrhythmia. American Journal of Physiology, 256(1), H153–H161. 10.1152/ajpheart.1989.256.1.H153. [DOI] [PubMed] [Google Scholar]
- Scher H, Furedy JJ, & Heslegrave RJ (1984) Phasic T-wave amplitude and heart rate changes as indices of mental effort and task incentive. Psychophysiology, 21(3), 326–333. 10.1111/j.1469-8986.1984.tb02942.x. [DOI] [PubMed] [Google Scholar]
- Schulz S, Ayala ES, Dahme B, & Ritz T (2009). A MATLAB toolbox for correcting within-individual effects of respiration rate and tidal volume on respiratory sinus arrhythmia during variable breathing. Behavior Research Methods, 41, 1121–1126. 10.3758/BRM.41.4.1121. [DOI] [PubMed] [Google Scholar]
- Simon E, Meuret AE, & Ritz T (2017). Sympathetic and parasympathetic cardiac responses to phobia-relevant and disgust-specific emotion provocation in blood-injection-injury phobia with and without fainting history. Psychophysiology, 54(10), 1512–1527. 10.1111/psyp.12900. [DOI] [PubMed] [Google Scholar]
- Sosinsky L, Markovitz S, & Carter A (2004). Parent-child interaction rating scales (PCIRS). Unpublished manual. University of Massachusetts; Boston, 2004. [Google Scholar]
- Søvik S (2000). Quantifying infant respiratory variability: how to capture complexity. Acta Pediatrica, 89(12),1401–1403. 10.1111/j.1651-2227.2000.tb02764.x. [DOI] [PubMed] [Google Scholar]
- Stemmler G, Grossman P, Schmid H, & Foerster F (1991). A model of cardiovascular activation components for studies using autonomic receptor antagonists. Psychophysiology, 28(4), 367–382. 10.1111/j.1469-8986.1991.tb00720.x [DOI] [PubMed] [Google Scholar]
- Suurland J, van der Heijden KB, Huijbregts SCJ, van Goozen SHM, & Swaab H (2017). Interaction between prenatal risk and infant parasympathetic and sympathetic stress reactivity predicts early aggression. Biological Psychology, 128, 98–104. 10.1016/j.biopsycho.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Tibu F, Hill J, Sharp H, Marshall K, Glover V, & Pickles A (2014). Evidence for sex differences in fetal programming of physiological stress reactivity in infancy. Developmental Psychopathology, 26, 879–888. 10.1017/S0954579414000194. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, & Taylor T (2003). Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine, 65, 46–62. 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Turner JR, & Carroll D (2006). Heart rate and oxygen consumption during mental arithmetic, a video game, and graded exercise: further evidence of metabolically-exaggerated cardiac adjustments. Psychophysiology, 22(3), 261–267. 10.1111/j.1469-8986.1985.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Van Diest I, De Peuter S, Devriese S, Wellens E, Van de Woestijne KP, & Van den Bergh O (2005). Imagined risk of suffocation as a trigger for hyperventilation. Psychosomatic Medicine, 67, 813–819. 10.1097/01.psy.0000181275.78903.64. [DOI] [PubMed] [Google Scholar]
- Wang L, & Maxwell SE (2015). On disaggregating between-person and within-person effects in longitudinal data using multilevel models. Psychological Methods, 20(1), 63–83. 10.1037/met0000030. [DOI] [PubMed] [Google Scholar]
- Weinberg MK, & Tronick EZ (1996). Infant affective reactions to the resumption of maternal interaction after the still-face. Child Development, 67(3), 905–914. 10.1111/j.1467-8624.1996.tb01772.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Grossman P, & Coyle MA (2004). Improving estimation of cardiac vagal tone during spontaneous breathing using a paced breathing calibration. Biomedical Sciences Instrumentation, 40, 317–324. [PubMed] [Google Scholar]
- Wong DL, Tai TC, Wong-Faull DC, Claycomb R, Meloni EG, Myers KM, Carlezon WA Jr., & Kyetnansky R (2012). Epinephrine: a short- and long-term regulator of stress and development of illness: a potential new role for epinephrine in stress. Cellular and Molecular Neurobiology, 32, 737–748. 10.1007/s10571-011-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


