Abstract
Heterotopic ossification (HO) is the formation of extraskeletal bone in non-osseous tissues. It is caused by an injury that stimulates abnormal tissue healing and regeneration, and inflammation is involved in this process. It is worth noting that macrophages are crucial mediators of inflammation. In this regard, abundant macrophages are recruited to the HO site and contribute to HO progression. Macrophages can acquire different functional phenotypes and promote mesenchymal stem cell (MSC) osteogenic differentiation, chondrogenic differentiation, and angiogenesis by expressing cytokines and other factors such as the transforming growth factor-β1 (TGF-β1), bone morphogenetic protein (BMP), activin A (Act A), oncostatin M (OSM), substance P (SP), neurotrophin-3 (NT-3), and vascular endothelial growth factor (VEGF). In addition, macrophages significantly contribute to the hypoxic microenvironment, which primarily drives HO progression. Thus, these have led to an interest in the role of macrophages in HO by exploring whether HO is a “butterfly effect” event. Heterogeneous macrophages are regarded as the “butterflies” that drive a sequence of events and ultimately promote HO. In this review, we discuss how the recruitment of macrophages contributes to HO progression. In particular, we review the molecular mechanisms through which macrophages participate in MSC osteogenic differentiation, angiogenesis, and the hypoxic microenvironment. Understanding the diverse role of macrophages may unveil potential targets for the prevention and treatment of HO.
Subject terms: Immunological disorders, Trauma
Introduction
Heterotopic ossification (HO) is the formation of extraskeletal bone in non-osseous tissues caused by abnormal differentiation of progenitor cells, because of local or systemic pathological imbalances1. In general, HO is classified into two types2: acquired HO (AHO) and genetic HO (GHO). Inflammation contributes to the onset and progression of these HO types3. Studies show that macrophages, as critical mediators of the immune system4, are interesting players that link inflammation and HO pathogenesis5. In fact, increasing research evidence chronicles a close relationship between macrophages and HO, showing that the recruitment and activation of macrophages drive HO6–8. It is the activation of macrophages that regulates the critical processes of HO development, including osteogenic differentiation and chondrogenic differentiation of mesenchymal stem cells (MSCs)8, a suitable hypoxic microenvironment9, and angiogenesis10. In this review, we discuss the important role of macrophages in HO and highlight macrophage-centered therapeutic strategies, which effectively modulate inflammation and macrophages, including immunotherapy and nanomedicine. Gaining insights into these issues will shed light on the critical role of macrophages in HO pathogenesis and lead to novel therapeutic strategies for HO.
Heterotopic ossification
HO is largely thought to be caused by injuries that stimulate abnormal tissue healing and regeneration mediated by inflammation11. AHO, a non-genetic form, is the most common and constitutes a severe complication of musculoskeletal trauma, including severe burns, fractures, joint arthroplasty (traumatic HO, THO)12, and cerebral or spinal insult (neurogenic HO, NHO)13 (Fig. 1). The pathogenesis of AHO is complex and remains unclear. Studies14,15 have shown that local inflammation and physical damage cause AHO. These lead to the recruitment of progenitor cells and release of numerous osteogenesis-inducing factors, which, accompanied by fibrosis and angiogenesis, eventually induce cartilage intermediates to form ectopic bone14,15. Fibrodysplasia ossificans progressiva (FOP) and progressive osseous heteroplasia (POH) are two autosomal dominant genetic forms of HO (GHO)16. Patients with FOP carry a mildly activating mutation of ACVR1, a gene which encodes the cell surface type I bone morphogenetic protein (BMP) receptor also named ALK2 (activin receptor-like kinase 2)17. To date, several mutations have been identified, all of which located in the glycine-serine (GS) region of ACVR1. The most common mutation in FOP patients is a single-point mutation of ACVR1 R206H17. This mutation decreases the stability of the glycine-serine region, which leads to the continuous activation of ACVR1, and ultimately HO and joint fusion in FOP patients18. Other rare missense mutations in the GS or protein kinase (PK) domain of ACVR1 (G365D, c.617 G > A, c.605 G > T, c.983 G > A) have been reported in FOP patients19–22. Genetically POH is caused by loss of function mutations in the Gs-α isoform of the GNAS1 gene23. Gene mutations in FOP and POH induce abnormal homeostasis and cell differentiation processes, triggering ectopic intrachondral or intramembranous ossification24. It should be noted that the pathogenesis and progression of AHO are influenced by injury and accompanied by a robust inflammation25. On the other hand, the two rare FOP and POH autosomal dominant genetic HO forms have different presentations and clinical severities16. The patients with FOP frequently present with redness, swelling, heat, and pain similar to local inflammation before the onset of the disease26, and tissue injury enhances the aggravation of FOP development. However, POH is not usually relevant to trauma or inflammation27. Studies have reported that the connective tissues, including blood vessels and skeletal muscles at AHO and FOP lesion sites have elevated levels of macrophages, mast cells, MSCs, osteocytes, chondrocytes, fibroblasts, and cytokines8,28–30.
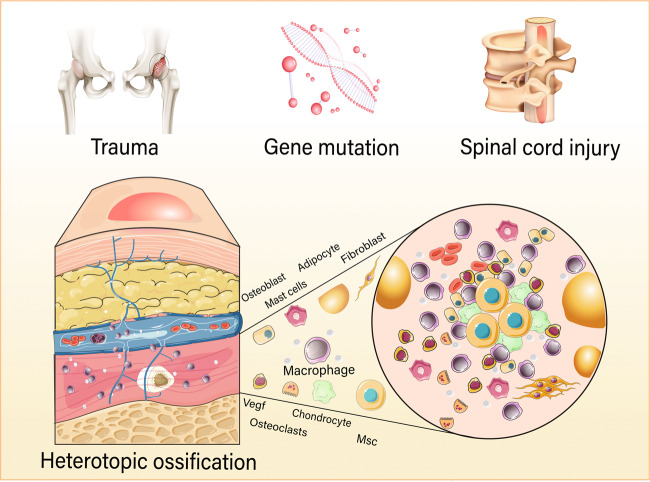
Fig. 1. Schematic illustration of heterotopic ossification (HO) formation.
HO is the formation of extraskeletal bone in soft tissues, which is caused by neurogenic trauma (spinal cord injury, encephalitis, etc.), gene mutation, and severe skeletal muscle trauma. HO is characterized as a multifactorial pathology and inflammatory disease. Inflammation is a common feature of acquired and genetic HO, which manifests as pain, warmth, redness, and swelling. Macrophages, mast cells, MSCs, chondrocytes, bone cells, fibroblasts, osteoclasts, and osteoblasts exist in lesion areas of vessels and muscles. Macrophages in the inflammatory environment influence osteogenic differentiation of MSCs and angiogenesis, the key steps of HO development.
In the HO mouse model, co-depletion of macrophages and mast cells effectively blocks the occurrence and development of FOP8. Several studies have investigated the role of mast cells in FOP and NHO14,31. For example, mast cells participate in the formation of FOP lesions. Factors released by degranulated mast cells stimulate inflammatory edema, fibrosis, and angiogenesis and enhance the progression of FOP and NHO lesions14,31. Moreover, in the AHO mouse models, macrophage depletion alone has been reported to significantly impair HO6. Studies propose that inflammation is a major risk factor in the development of HO1,3,32, and macrophages play a major role in this process6–8,33.
Inflammation and macrophages
Inflammation is a normal physiological response of the body to infections and injuries34, but it is also often associated with the occurrence and deterioration of HO35,36. Inflammation is a process of damage and repair tightly regulated by the immune and vascular systems, which can be classified into two kinds, namely acute and chronic34. In non-onset FOP patients, studies have reported a pro-inflammatory state36. This implies that FOP is caused by prolonged and hyper-activation of the immune system mediated by chronic inflammation36, whereas AHO occurrence and development are caused by acute or chronic inflammation37,38. Inflammation is an important trigger factor for FOP and AHO. Inflammation induces osteogenic gene expression, angiogenesis, and hypoxic microenvironment by stimulating immune cells to secrete numerous inflammatory factors, thereby jointly promoting cartilage differentiation and bone formation in tissues35,39,40. In FOP, only minor local inflammation is sufficient to trigger HO26. In particular, the effect of activin A (Act A) in an inflammatory environment is unique to FOP, as the binding of Act A to ACVR1 mutants described previously drives HO development in FOP8,41. AHO is caused by inflammation with injury, and HO caused by trauma most often occurs as a result of extensive soft tissue injury and inflammation42. While it is difficult to attribute HO pathogenesis and progression to a specific type of inflammation21, it is vital to point out that macrophages, as the most critical sentinels and regulators of the immune system to inflammation43, actually play central roles in this inflammatory disease8,44. Macrophages serve as immune cells, as well as active secretory cells that secrete diverse mediators, which regulate the host defense system, inflammation, and homeostasis45. Tissue-resident macrophages are derived from the embryonic or adult hematopoietic stem cell (HSC) progenitor cells under homeostatic condition46. The study has shown that monocyte-derived macrophages originating from the bone marrow cells are mostly associated with responses to tissue repair and inflammation47. Monocyte-macrophage lineage cells are characterized by considerable diversity and plasticity, which not only play a crucial role in regulating homeostasis and promote normal tissue development, including bone morphology repair48, fiber formation, branch recovery49, and regulation of angiogenesis50. However, when these functions are not coordinated, macrophages can also become the root cause of many bone metabolic diseases, such as HO51 and rheumatoid arthritis52. Although tissue-resident macrophages are evenly distributed in various tissues, mononuclear-macrophages change with time and are highly heterogeneous in vivo53, in response to numerous signals. At the most basic level, the M1 macrophages are characterized by high levels of pro-inflammatory cytokine expression and promote inflammation and the killing of microorganisms54. M2 macrophages are believed to be involved in the promotion of tissue remodeling and tumor progression55. Macrophages in the human body do not exist in the form of a single phenotype. They will dynamically change during injury, regeneration, and healing. M1 macrophages commonly initiate inflammatory response immediately after injury, then during the repair inflammation stage, infiltrating macrophages have an M2 phenotype56,57. A study has highlighted the heterogeneity that presents in the macrophages at the site of extremity injury during HO, including different types of macrophages being recruited to the site of HO5. Depletion of macrophages is shown to greatly suppress the development of AHO and FOP in some studies6,8, but to promotes HO in another study58. Although many depleted macrophages were M2, these studies6,8 have failed to assess which of the two the macrophages, M1 or M2, is depleted first and more severely. Notably, depletion of M1 may greatly weaken the phagocytic function51,59. Therefore, these may indicate that different types of macrophages play roles along with different stages of HO, but not those macrophages must have an inhibitory effect on HO. The appropriate phenotype of macrophages exhausted at a precise time could be key in the prevention and treatment of HO51. Because of such a sophisticated and complex system of macrophage polarization, the mechanism by which macrophages affect HO is elusive.
Molecular mechanism of macrophages in HO
Enrichment of macrophages has been reported in damaged tissue in AHO and FOP, their clearance effectively inhibits HO development, and both M1 and M2 macrophages promote ectopic bone formation in different ways5. The three factors necessary for the occurrence and development of HO include an inciting inflammatory incident, osteogenic and chondrogenic differentiation of MSCs, and an appropriate microenvironment60,61. Various studies have reported that macrophages contribute critically in modulating these multiple aspects of HO progression from initiation to MSC chondrogenic and osteogenic differentiation, hypoxia microenvironment, and angiogenesis.
Macrophages regulate osteogenic differentiation of MSCs
Ectopic bones are formed in soft tissues after severe injury, inflammation, or genetic diseases. Except for POH which is a type of intramembranous ossification, AHO and FOP are associated with endochondral ossification62. This usually occurs through initial cartilage formation followed by endochondral ossification. Ectopic bone formation includes four stages: inflammation, cartilage formation, osteogenesis, and ectopic bone maturation. In the inflammatory phase of AHO and FOP, immune cells infiltrate the injury site63. In the chondrogenesis stage, MSCs differentiate into chondrocytes64. During the osteogenesis stage, chondrocytes undergo hypertrophy and calcification65. In the final stage of maturation, the bone marrow matures to form a cancellous bone. Therefore, inflammation is an important factor that initiates ectopic bone formation. Inflammation has different roles during osteogenic differentiation and chondrogenic differentiation. For example, several inflammatory factors such as TGF-β promote cartilage and bone formation39. A study on patients with osteoarthritis reports that M1 macrophages induce cartilage apoptosis and then M2 macrophages promote cartilage hypertrophy and ectopic bone formation66,67. However, the effect of different types of macrophages on chondrogenic differentiation in HO patients remains unclear. MSCs are multipotent stem cells with self-renewal ability and can differentiate into chondrocytes or osteoblasts68. Osteogenic and chondrogenic differentiation of MSCs is one of the key processes responsible for the occurrence and development of HO60. It has been chronicled those macrophages of different phenotypes promote the osteogenic differentiation of MSCs69, and the exhaustion of macrophages evidently impairs the osteogenic differentiation potential of MSCs70. In return, the MSCs release hormone such as prostanoid prostaglandin E-2 (PGE-2) to regulate macrophage polarization71. MSCs also regulate macrophage chemotaxis by secreting chemokines such as MIP-1 and 2 and MCP-5, which are chemoattractants for monocytes/macrophages and play a key role in macrophage infiltration during wound healing72. Different types of macrophages modulate inflammation and accelerate bone repair growth and HO progression7,73. Therefore, the interaction between MSCs and macrophages promotes the development of HO. Currently, it is believed that macrophages secrete cytokines and factors, including TGF-β1, BMP, Act A, OSM, SP, and NT-3. They play a vital role in mediating MSCs osteogenesis differentiation and are associated with both acquired and inherited HO5,7,8,74–76 (Fig. 2).
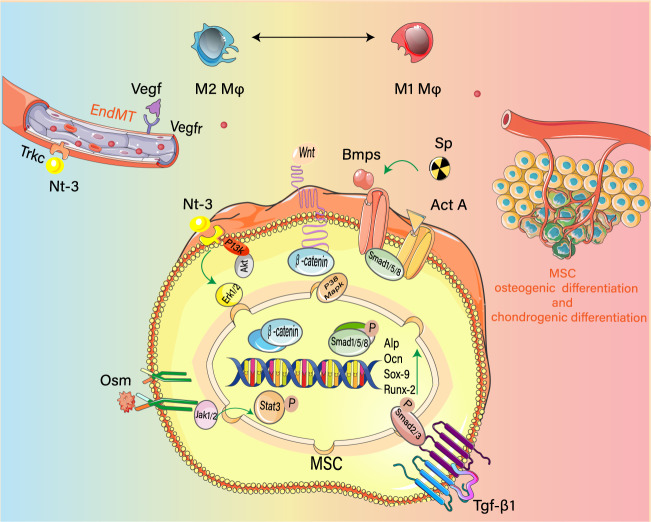
Fig. 2. Molecular mechanisms of HO induced by different phenotypes macrophages.
Mononuclear macrophages are polarized to the M1-M2 macrophages in injured tissues in responding to injury. Macrophages secrete OSM and TGF-β1, BMPs, Act A, SP, and VEGF, and promote the expression of NT-3 and other factors. These signal molecules through different signal pathways, including TGF-β1-Smad2/3 and BMP-Smad1/5/8, jointly induce osteogenesis of MSCs, angiogenesis, and proper microenvironment formation, promoting the occurrence and development of HO through multiple pathways.
TGF-β1
TGF-βs constitute three major isoforms (TGF-β1, TGF-β2, and TGF-β3), all of which participate in the pathogenesis and progression of HO formation5. However, macrophages only secrete TGF-β15, a key regulator of chondrogenic differentiation and monocyte/macrophage function77,78. In the early stages of inflammation, TGF-β1 has been found to be co-localized with F4/80+ and CD68 + macrophages in THO sites of mice and human THO samples, and TGF-β1 is upregulated in many macrophage clusters at HO sites5. The high levels of TGF-β1 induce osteogenic differentiation of MSCs through the TGF-β1-Smad2/3 pathway, promoting HO occurrence and progression5. Matrine inhibits TGF-β/smad2/3 pathway and induces MSC migration and osteogenic differentiation, and significantly suppresses HO progression in an ATP mouse model79 (Fig. 2). The study has also found that the CD47-activating peptide that blocks TGF-β1 for systemic therapy inhibits the formation of HO by altering the monocyte/macrophage phenotype and reducing the levels of chondrogenic and osteogenic markers5. However, further studies are needed to understand how phenotypes of macrophages are altered and to determine whether the body produces other heterogeneous macrophages other than M1/M2. The formation of AHO is prevented in macrophage-deficient colony-stimulating factor 1 (CSF-1) deficient (Csf1−/−) mice, which are depleted in macrophages39. In addition, HO is not formed in TGF-β1−/− mice whose macrophages/monocyte lineages and neutrophils do not produce TGF-β1, while signs of HO formation in control mice is noted39. Therefore, TGF-β1 secreted by immune cells in the inflammatory phase is an important inducer of HO. However, the problem of the study39 is that the effect of neutrophils on TGF-β1 is not ruled out, and it is unclear whether macrophages also secrete other cytokines to regulate the process of HO. Hence, at this time, cannot fully confirm that TGF-β1 secreted by macrophages participates in the occurrence and development of AHO. Similar findings are made in hereditary HO36. A previous study has reported thatM2 macrophages secretes TGF-β1 during the inflammatory phase in FOP36, indicating that TGF-β1 also contributes to the fibrosis and ossification process of FOP.
BMPs
BMPs are multifunctional growth factors that are involved in bone development. Among them, BMP-2, BMP-4, and BMP-7 are primarily involved in the occurrence of HO39,80,81. It is documented that BMPs participate in HO development by promoting the proliferation and differentiation of MSCs into cartilage and bone tissue through the BMP/Smad and p38 MAPK signaling pathways36,80. BMPs facilitate the assembly of BMP type I and type II receptors to activate downstream Smad effector proteins 1, 5, and 8. After being phosphorylated by type II receptors, such as BMPRII and ACTRIIA, activated BMP type I receptors, including ALK2, ALK3, and ALK6, can in turn phosphorylate SMAD proteins to induce FOP82 and AHO83. Another study has found that the interaction between BMPs and the Wnt/β-catenin signaling pathway related to epithelial-mesenchymal transition (EMT) that mediate HO84. In addition, modulation of Smad1/5/8 phosphorylation, via small molecule inhibitors targeting BMP type I receptor kinase activity, could mitigate traumatic HO formation resulting from Achilles tenotomy and severe body burn83. Knocking out BMP type I receptors (ALK2 and ALK3), as well as using BMP ligand trap A3Fc, have significantly reduced HO formation following Achilles tenotomy85. Taken together, these losses of function studies indicate that BMP signaling is critical in traumatic HO and that BMPs represent the most likely candidate injury‐induced osteogenic factor(s) in THO85. Trauma is not enough to induce HO, and other factors, such as gene mutation86 and neurogenic injury87, are needed. For example, the induction of NHO requires either a TBI or a spinal cord injury with muscle damage88,89. Blast injury alone cannot cause THO90, but when combined with concomitant fracture91 or wound infection, such as bacterial infection, it can induce severe HO92. On the other hand, hereditary HO, such as FOP, usually only requires gene mutation and mild inflammation, including injury-mediated inflammation and noninjury-mediated inflammation, such as viral infection26. BMPs are strongly correlated with the participation of macrophages in inflammation (Fig. 2). Studies have found that the microenvironment recruited by macrophages might well promote the expression of BMP in MSCs and even express BMP, thereby inducing HO15,44,80. Histological analysis80 confirms the expression of BMP-7 in BMP-2–induced AHO mice muscle tissue at 48 h after injury, but not in control uninjured muscle tissue. It also reveals severe inflammatory cell infiltration into the muscle tissue, indicating the possibility that BMP-7 is a result of the interplay between inflammation and activation of BMP-2. Double immunofluorescence staining indeed confirm the macrophage marker, CD68, to be localized in close proximity to BMP-7 signals80. In the AHO mouse model, following the decrease in macrophages, BMP-7 level in injury muscle tissue are also greatly reduced at the early stage80. Although these studies have not profiled the macrophage phenotypes that secrets BMPs, both M1 and M2 macrophages could secrete BMP93,94 and indirectly enhance the expression of BMPs. In animal studies, TGF-β1 secreted by macrophages has been reported to promote the expression of BMPs in MSCs, inducing ectopic bone formation95. Furthermore, the effect of TGF-β1 on BMP is macrophage-dependent95. Importantly, the BMP signaling pathway coordinates the polarization of macrophages and subsequently affects inflammation8. The secretion of BMP-2 at the remodeling site, which can potentially stimulate M2 commitment, may induce an anti-inflammatory response by polarized macrophages96. Furthermore, BMP-4 has been implicated in promoting the secretion of pro-inflammatory factors, e.g., TNF-α, by mast cells to activate inflammation in an FOP mouse model8. Therefore, the etiology of FOP is considered as a frequent spontaneous inflammation and an abnormal repair program8. The interaction between macrophages and BMPs promotes the recruitment and polarization of macrophages that express high levels of BMPs, which mediate the dysregulation of osteogenic differentiation of MSCs and prompting HO occurrence and progression15.
Act A, a member of the Activin family, is secreted by immune cells, such as Th2 cells97, neutrophils98, and natural killer (NK) cells99. Although no studies have detected the release of Act A from macrophages53, Act A is involved in regulating the polarization of macrophages by inducing the expression of arginase-1 required for the M2 phenotype and inhibiting the expression of the IFN-γ-inducing NO synthase required for the M1 phenotype97. As a crucial mediator of inflammation, immunity, and fibrosis100, Act A is potentially associated with the occurrence and development of FOP41. Act A cannot induce ectopic bone formation in wild-type mice41. In contrast, Act A can activate ACVR1 (R206H) to contribute to HO41. Hatsellet et al.41 has shown that a monoclonal antibody against Act A antibody prevents the occurrence of FOP. Activation of the ACT A alone is not enough to induce the formation of ectopic bones in skeletal muscle, as injury or other types of tissue damage is also required63. In an inflammatory environment, by amplifying mutated ACVR1 signaling, Act A induces differentiation of MSCs into osteoblasts and chondrocytes by activating the Smad1/5/8 and mTOR signaling pathways101 (Fig. 2), while inhibiting osteoclast formation102 causing the FOP form of HO. Moreover, Act A regulates immune cells (including monocytes, macrophages, microglia, mast cells, NK cells, and dendritic cells) and the functions of B cells and T cells. It exhibits pro-inflammatory and anti-inflammatory multifunctional effectors103. Experimental evidence8 has shown that after introducing the ACVR1 R206H mutation to enhance Act A signaling in the FOP model, the number of mononuclear—macrophages and mast cells increases at the early and middle stages. However, only the mast cells secrete more cytokines, while no difference in cytokine secretion in macrophages8 is found. Although the effect of Act A on macrophages and HO is not fully understood, we speculate that the complex interaction among Act A, macrophages, and ACVR1 mutations is responsible for the development of FOP. As Act A only activates the signaling from mutant ACVR1, but not wild-type ACVR1104, Act A does not induce the formation of post THO104.
OSM
OSM105, a member of the IL-6 cytokine family, is secreted by monocytes, macrophages, and bone cells, and promotes the development of HO76 (Fig. 2). Some studies have shown that macrophage-derived OSM plays a key role in NHO76 and that elevated levels of macrophage-derived OSMs mediate NHO by activating osteogenic differentiation of MSCs76.This is due to the high expression of OSM mRNA in the damaged muscles of NHO mice and patients, and the drastic increase in OSM expression after stimulating monocyte/macrophage with LPS76. Treatment with anti-OSM antibody can greatly reduce the mineralization of NHO-MSC induced by osteogenic differentiation medium supplemented with LPS-activated monocytes/macrophages. In vivo and in vitro studies have shown that the binding of the activated macrophage-derived OSM to OSMR indeed promotes the osteogenic and chondrogenic differentiation of MSCs in muscle tissue, thereby enhancing NHO76. Deleting the OSMR gene dramatically reduces the chance of NHO development compared with wild-type controls following spinal cord injury and muscle injury in vivo76, confirming that signaling through the OSMR promotes HO formation. A subsequent study74 has proposed that macrophage-derived OSMs mediate NHO via the binding of OSM to the GP130/OSMR complex on MSCs and then tyrosine-phosphorylation of STAT3 by activating Janus kinase1/2 (JAK1/2)74. The signaling pathways synergistically promote osteogenic differentiation of MSCs74. STAT3 is significantly higher and remains persistently activated in injured muscles of mice with central nervous system lesion and developing NHO, but not in injured muscles without spinal cord injury74. The result shows that this persistent STAT3 phosphorylation and high activation in the injured muscle is an important driver of NHO74. The long-lasting activation of STAT3 may require the stimulation of spinal cord injury. The use of a specific inhibitor of JAK1/2 kinases greatly inhibits the progress of NHO in the mice, which supports that the OSM—JAK/STAT3 pathway indeed induces the occurrence and development of HO74. However, limitations of this study are that phosphorylation of STAT3 cannot be confirmed in muscle satellite cells or mesenchymal cells in vivo, and that the phenotype of macrophages was not investigated. Furthermore, activation of STAT3 in MSCs upregulates the expression of OSMR and LIFR, which enhances the effect of OSM on MSCs, and improves the osteogenic efficiency of MSCs106. Of note, deletion of the OSMR gene has not completely ablated NHO76, indicating that in addition to OSM, other cytokines or mechanisms involving in macrophages are also at play.
SP
SP, a neuropeptide produced by macrophages, is involved in transmitting information during noxious stimulus response, such as pain107. In recent years, studies have shown that SP facilitates the proliferation and mineralization of MSCs and inflammation, which are closely linked to AHO and FOP30. They also emphasize that damage induces the upregulation of SP, which promotes HO via BMP14,30 (Fig. 2). This has been illustrated by the accumulation of high levels of M1 macrophages and SP at the HO site in THO rat models with Achilles tendon injury75. Macrophages appear to be involved in the infiltration of substance P108, which may stimulate M1 macrophages to secrete high levels of inflammatory factors, resulting in overexpression of BMP-275. CGRP counteracted the effect of SP has been shown to suppress ectopic bone formation effectively75. Therefore, SP secreted by M1 macrophages after injury amplifies inflammation and may have an effect on upregulating BMP-2 to mediate THO75. BMP-2, in turn, induces the expression of the neuroinflammatory mediators, SP, and CGRP14. The role of SP in FOP and NHO has received significant attention. High levels of SP are secreted in the early lesions of NHO, and non-neurogenic SPs are mainly produced by macrophages and mast cells6, which significantly promote the osteogenic differentiation of MSCs by upregulating BMP-430.
NT-3
NT-3 plays an essential role in the growth and development of the nervous system and bones109. In the THO mouse models110, NT-3 activated by TGF-β cytokines acts on TrkC to induce endothelial to mesenchymal transition (EndMT), leading to MSCs generation. NT-3 accelerates the differentiation of MSCs into bone and cartilage by upregulating Sox9, RUNX2, and other110. NT-3 is also able to stimulate neovascularization to promote HO110. While inhibition of NT‐3 suppressed the induction of EndMT and bone formation in HO110. A recent study indicates that activated macrophages mediate the secretion of NT-3 from chondrocytes that play a vital role in HO formation7. Interestingly, NT-3 is always co-localized with M1 and M2 macrophages throughout the formation of HO7 (Fig. 2). An in vitro study verified that macrophages mediate the secretion of NT-37. In addition, macrophage-derived NT-3 accelerates osteogenic differentiation of tendon stem cells (TDSC) of mesenchymal lineages by activating the ERK1/2 and PI3K/Akt signaling pathways7. Moreover, NT-3 enhances the expression of BMP (especially BMP-2) and VEGF in mineralized cells to mediate bone and blood vessel formation111.
TGF-β1, BMPs, Act A, OSM, SP, and NT-3 upregulated by different macrophage phenotypes are involved in the occurrence of HO by regulation of the osteogenic differentiation of MSCs. The soft tissues of HO patients have been shown to harbor elevated levels of inflammatory factors, including MCP-1, IGF-1, and IL-23112,113, that can induce MSC osteogenic differentiation via different pathways114,115. Further studies are needed to understand the roles of these factors in macrophages and HO.
Regulation of hypoxic microenvironment by macrophages
Hypoxic microenvironment refers to a condition where the damage of the vasculature and/or the infiltration of immune cells decreases oxygen supply and/or increases oxygen consumption in cells and tissues116. Increasing evidence indicates that hypoxia is a critical determining factor for the appropriate microenvironment for acquired and hereditary HO35,117. Studies show that the primary cause of hypoxia in FOP patients is inflammation35. Both hypoxia and inflammation stabilize HIF-1α, and HIF-1α and HIF-1β complex upregulates the expression of BMP and VEGF, inducing MSC chondrogenic and osteogenic differentiation and angiogenesis, ultimately leading to FOP118 (Fig. 2) and AHO119. MSCs regulate hypoxia during bone development. One study120 has shown that the aggregation of MSCs induces local hypoxia, thereby stabilizing the transcriptional activity of HIF-1α and inducing transcription factors such as SOX9 to regulate the expression of cartilage matrix protein. The lack of HIF-1α during embryonic development may lead to joint deformities120. In vitro experiments have found that hypoxia can enhance the proliferation and ossification ability of MSCs117. Therefore, it is reasonable that inflammation-induced hypoxic microenvironment and MSCs may have a certain cascading effect in HO patients. As macrophages have been shown to be enriched in inflammation sites with severe hypoxia121, we propose that the immune cells that mediate the effects of hypoxia in HO patients are mainly macrophages. There is causal crosstalk between the macrophages and hypoxic microenvironment122. In the early phase of host response, high monocyte-macrophage extravasation, infiltration and accumulation in the inflammation site, and increased metabolic activity of infiltrating cells, together with vascular damage and edema, all contribute to reduced oxygen tension in the inflamed tissue123. Hypoxia, in turn, activates the inflammatory pathways to aggravate inflammation124. Therefore, macrophage-mediated inflammation and hypoxia signals jointly activate HIF-1α.M1 macrophages stabilize the transcription of HIF-1α in various inflammatory diseases125,126. For instance, the enhanced transcriptional activity of HIF-1α by macrophages has been reported in human peritoneal scar tissue127, suggesting that HIF-1α is expressed by different phenotypes of macrophages. In summary, it is likely that macrophages provide a suitable hypoxic microenvironment for HO and utilize multiple pathways to enhance the stability of HIF-1α to induce HO development3.
Macrophages promote angiogenesis
Hypoxia and angiogenesis are inseparable processes in the body. Hypoxia response initiates the process of restoring oxygen supply found in many diseases128,129. At the cellular level, offsetting the lack of oxygen is achieved through actions such as angiogenesis, proliferation, and self-renewal. Hypoxia and blood vessel formation contribute to bone healing or normal bone formation130. For example, increased VEGF expression has been shown to promote angiogenesis and fracture healing131. Mice with mutant HIF-α have narrow bones and a thin cortex, which is considered to be a secondary factor of damage to the vascular system132. However, hypoxia and blood vessel formation are harmful processes that lead to the pathogenesis of HO. The development of HO is highly dependent on neovascularization10,133. Macrophages are vital factors that promote angiogenesis. Importantly, physiological angiogenesis mainly depends on tissue macrophages134, and numerous types of macrophages are involved in pathologic angiogenesis135. Under hypoxia, macrophages achieve a pro-angiogenic response directly by upregulating angiogenesis molecules (VEGF, FGF2, IL-8, VEGF type I receptors, angiogenin) or indirectly by upregulating angiogenesis regulators (SSP1, F3, MMP1)136. VEGF constitutes the primary vascular growth factor regulating normal and pathological angiogenesis in the body137. In THO and FOP mice models, macrophages have been shown to coordinate the expression of VEGF A10 (Fig. 2). In contrast, vascular defects have been reported in macrophage-deficient mice134. Mononuclear macrophages could be involved in angiogenesis and promote vascular anastomosis in HO. However, the mechanism by which macrophages of different phenotypes promote angiogenesis and induce HO remains unknown. A previous study has shown that M1 macrophages promote blood vessel generation after secreting inflammatory factors such as VEGF and TNF-α138, while M2 macrophages stabilize angiogenesis138. Therefore, VEGF A is most likely derived from M1 macrophages, and M2 macrophages assist angiogenesis in HO development. Meanwhile, the M1-M2 induced blood vessel formation is found to play an important role in the normal tissue repair process139. For example, after muscle injury, M1 macrophages first infiltrate the damaged site, and then M2 macrophages undergo an anti-inflammatory response and secrete cytokines (such as MMP9) to maintain fiber remodeling and vascular remodeling function138. In particular, IL-10 is mainly produced by macrophages, and its secretion induces blood vessel and muscle fiber formation to repair damages140. Depletion of M2 leads to poor injury healing139,141. In addition, M2 modulates EndMT in damaged and malnourished muscles. Inhibition of the M2 formation may induce EndMT in mice by activating TGF-β and promote blood vessels remodeling in the damaged muscle tissue, leading to the accumulation of collagen and the replacement of muscle by fibrotic tissue142. Therefore, proper expansion/maintenance of M2 in the early stage of inflammation may be needed to prevent EndMT over activity143 and HO. The effect of macrophages on angiogenesis could depend on when and the duration of action of different phenotypes. In conclusion, M1 and M2 macrophages are potential targets for developing an antiangiogenic therapy design for HO.
Taken together, the mechanisms that macrophages mediate HO are complex, diverse, and apparently involving the entire process of HO formation. We aim to highlight that macrophages occupy a central role by illustrating how different phenotypes macrophages mediate HO and how this will provide strategies for the prevention and treatment of HO.
Macrophage targeting strategies in HO therapies
Macrophages are quickly recruited and activated in the inflammatory microenvironment after injury144. Studies have shown that different macrophage subtypes affect the inflammation outcome by secreting pro-inflammatory and/or anti-inflammatory cytokines and jointly promote the initiation and development of HO5. Due to the important role of macrophages in HO, the regulation of macrophage function and inflammation could effectively prevent the first episode, as well as the relapse of HO8.
Immunosuppressive agents
Immunosuppressive agents repress immune cells, e.g., macrophages preventing an overactive immune system145. Currently, many clinical and experimental studies have shown that non-steroidal anti-inflammatory drugs146 and imatinib147 reduce inflammation and tissue edema, and are hence utilized as prophylaxis drugs for both acquired and inherited HO. These anti-inflammatory drugs act by inhibiting the inflammatory responses mediated by macrophages, mast cells, and other immune cells148, effectively fighting inflammation and suppressing the progression of HO149. Although these drugs are curative, they cause some adverse side effects due to their broad-spectrum immunosuppression150. In recent years, medications such as pexidartinib, PLX7486, and clodronate, have been used to treat cancer by suppressing the tumor-associated macrophages151. Interestingly, a previous study has shown that a key underlying mechanism of HO constitutes injury-induced increase of immune checkpoint proteins (ICs) expressed by macrophages, which include stimulatory ICs (CD40 and CD134) and inhibitory ICs (PD1, PD-L1, etc.)51 (Fig. 3). The use of neutralizing antibodies (Abs) against inhibitory ICs not only increases the expression of inflammatory cytokines (IFN-γ and TNF-α) in BMP4-induced macrophages, but also effectively decreases the population of CD206+/F4/80+(M2) macrophages51. The expression of anti-inflammatory cytokines nearly prevents or drastically limits the extent of AHO51, while the loss function of stimulatory ICs (CD40 and CD134) promotes HO formation51. It is important to note that accurate immunosuppression management suppresses the polarization of macrophages and retains mononuclear macrophages in the human body, which maintains immunity and minimizes complications152. Altogether, these novel immunosuppressants constitute promising drugs for the prevention and treatment of HO.
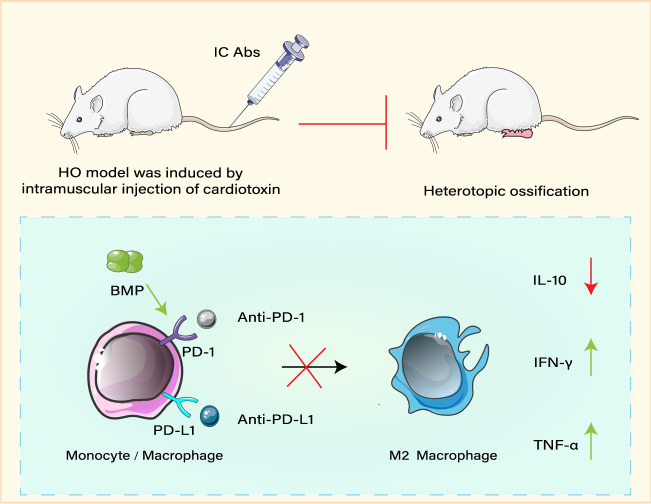
Fig. 3. Regulation of macrophages by immune checkpoint inhibitors to block HO.
Neutralizing antibodies (Abs) such as anti-PD1 and anti-PDL1 can be used to inhibit the proliferation and polarization of macrophages by targeting inhibitory ICs. These upregulate IFN-γ and TNF-α in BMP4-induced macrophages and downregulate IL-10 and decrease the population of M2, ultimately leading to the effective inhibition of HO by Abas.
Macrophages targeted nanomedicine
At present, nanomedicine mainly treats diseases with inflammation backgrounds, such as HO153, multiple sclerosis154, and others, through the development of well-designed therapies. It opens up new prospects for the treatment of inflammatory-related bone metabolism diseases. Specially designed nanoparticles (NPs) such as liposomes, solid lipid NPs, and inorganic NPs, can be used as drug carriers, targeting specific cells by identifying molecules expressed on the surface of activated macrophages155 or endothelial cells153 (Fig. 4). They thereby accurately mediate drugs to the desired cell population and regulating cell activity and functions156, avoiding the conventional problems of traditional medicine, such as the systemic toxicity of the drug156. NPs are also well-known for their enhanced permeability and retention effect. One recent study has shown that in the HO model, SPIO nanoparticles containing Smad 7 can inhibit EMT of EPCs, which is involved in PI3K/Akt signaling, depress the expression of TGF-β1 and BMP in EPC, and prevent the development of HO153. HO is significantly inhibited by using chlorophosphate-containing liposomes to deplete macrophages8,157. Nanoparticle targeting macrophages can go through two pathways-receptor-mediated endocytosis and non-specific phagocytosis158. M1 macrophages can be targeted by hybrid lipid–latex (LiLa) nanoparticles-bearing phagocytic signals159, while M2 macrophages can be targeted by mannose-decorated F127-TA hybrid nanoparticles160. Therefore, it may be possible to target activated macrophages through nanoparticles and administer drugs to inhibit the activation of different types of macrophages or reprogram the macrophages155, thereby inhibiting the development of HO. Nanomedicine not only can consume macrophages, but also can adjust the polarization bias of M1-M2 by reprogramming macrophages161. It is worth noting that siRNA-carrying nanoparticles also bring new possibilities for gene therapy targeting macrophages162. Macrophages at the injury site are recruited and differentiated from monocytes163. Importantly, chemokines, including CCL2 and CCL5, are vital drivers of the infiltration of monocytes/macrophages164, as well as of acquired and hereditary HO29,165. Therefore, targeting chemokines and their receptors (e.g., CCL2-CCR2 and CCL5-CCR5)164, which effectively block the recruitment of monocytes and activation of macrophages164, constitutes an excellent strategy of gene therapy curing inflammatory diseases, such as HIV and multiple sclerosis29,164. It may be an effective way to potentially prevent and treat HO. The promising RNA-based strategies include the application of RNA silencing and antisense RNAs delivered by NPs to edit chemokine genes genetically166. For example, PNP/PRSsi significantly inhibits the CCR2 gene and other inflammatory genes (such as those encoding Chi3l3 and Il1rap, which are usually produced by single nuclear cell/macrophage expression), thereby reducing the recruitment of macrophages and tissue fibrosis167.
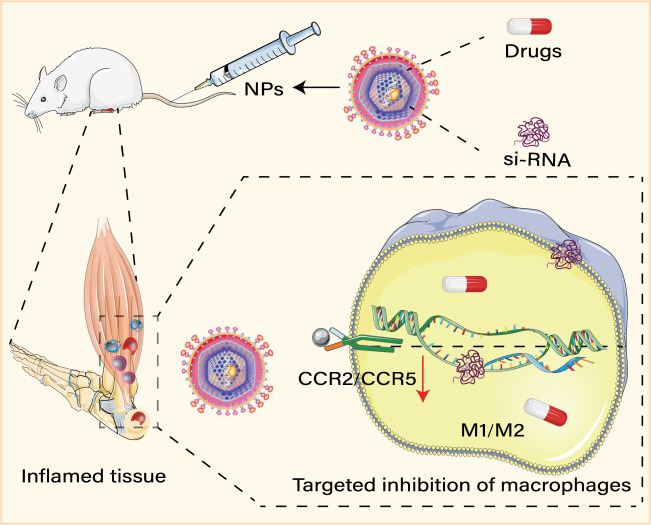
Fig. 4. Macrophages targeted nanomedicine.
Macrophages of different phenotypes in inflamed tissues can be targeted by injecting specific nanoparticles. Drug-loaded NPs, such as chlorophosphate-containing liposomes, blocks HO formation by depleting macrophages. In addition, NPs loaded with siRNA can inhibit the recruitment of macrophages and the secretion of inflammatory mediators by downregulating the expression of CCR2 or CCR5, which may be a new approach for the prevention of HO.
Other targeted therapies may also be effective in inhibiting both acquired HO and hereditary HO. Studies have shown that there is a commonality in the mechanism of AHO development and FOP-ALK2 signaling pathway. The BMP type I ALK2 receptor-specific inhibitors, such as LDN-193819 and LDN-212854, effectively inhibits more than half of AHO82 and FOP63 symptoms in rat models. At the genetic level, the use of RNA interference (RNAi) to target the expression of mutant ALK2 alleles effectively curbs the development of FOP168. The difference is that ACT A-ALK2 only plays a role in FOP. Treatment with anti-activin A antibody can prevent wild-type mice from forming new ectopic bones41. A phase II trial designed to test the efficacy of anti-activin A antibody (REGN2477) in the treatment of abnormal bone formation has recently started169. RARγ agonists have great potential in the prevention of AHO and FOP. RARγ agonists are likely to cause anti-chondrogenesis and prevent intramuscular and subcutaneous regions of HO by maintaining retinoid signal activity and ligand RAR activity while suppressing BMP signal transduction and phosphorylation of Smad170–172. In short, although different types of HO are driven by different mechanisms, the commonality in the development of acquired HO and hereditary HO in terms of inflammation, macrophages, and ALK-2 signaling pathway can be exploited to develop treatments for both types of HO.
Conclusion
The complex pathological mechanisms underlying the occurrence and progress of HO have remained elusive for decades. Interestingly, HO does not present with typical symptoms before its occurrence, and it is irreversible once the ectopic bone is formed. Studies have focused on the critical steps of HO, including the hypoxic microenvironment, MSC osteogenic differentiation, and angiogenesis. Moreover, macrophages are recruited and activated to coordinate these three processes. More importantly, numerous studies have chronicled that the common early manifestation of HO constitutes inflammation. Herein, we elucidate the molecular mechanisms of the different phenotypes of macrophages involved in HO occurrence and progression, and highlight the potential strategies for early prevention and treatment of HO by targeting macrophages.
After an injury, elevated numbers of macrophages are recruited to the injury site, and together with MSCs, mast cells, chondrocytes, osteoblasts, osteoclasts, and fibroblasts form an initial inflammatory environment to mediate HO formation. The recruitment and activation of macrophages during inflammation could be the driver of HO formation and progression.
Macrophages constitute the major inflammation mediators in the occurrence and development of HO. Inflammation is divided into two broad categories, namely, acute and chronic. Although the type of inflammation that mediates HO has remained elusive, it is inevitable that macrophages play important roles in this inflammatory disease. This is because enriched macrophages are recruited at the early stage of inflammation, and different cytokines and factors drive the macrophage M1/M2 polarization. Disturbances in the macrophage function cause an imbalance in tissue damage and repair, leading to aberrant repair. Pro-inflammatory and anti-inflammatory cytokines are involved in HO occurrence and development, which are primarily attributed to different macrophage phenotypes.
Different types of macrophages drive HO by regulating MSC osteogenic differentiation and chondrogenic differentiation, hypoxic microenvironment, and angiogenesis. This is achieved through regulation of TGF-β1, BMPs, Act A, OSM, SP, and NT-3. The hypoxic microenvironment created by macrophages and stabilization of HIF-1α expression induce the formation of bone and vessels.
Inhibition of inflammation and macrophages with immunosuppressants and macrophage-targeting nanomedicine have the potential for HO prevention and treatment.
Many studies have noticed the prominent role of macrophages in the occurrence of HO, but mechanisms of macrophages-mediated HO remain elusive. Given the high heterogeneity and plasticity of macrophages, the inhibitory effects of different phenotypes of macrophages on bone formation and osteoinhibition vary across studies. In this review, we provide insight into the molecular mechanisms by which macrophages induce HO formation. It is likely that the sum of the osteogenic effects of macrophages in HO patients exceed the osteoinhibitory effects under pathological conditions. Proliferation and activation of osteoclasts are the only processes leading to bone resorption, but the mechanisms of ectopic bone formation promoted by macrophages are diverse and complex. Therefore it is important to explore the full landscape of molecular and cellular mechanisms by which macrophages induce HO. This will yield diagnostic and new therapeutic targets for HO.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China [grant numbers: 31900852]; Natural Science Foundation of Jiangxi Province [grant numbers: 2018BAB215012 and 20192ACB21026]; and Development Fund Project from Jiangxi medical college of Nanchang University [grant number: PY201801]. H.L. was supported by a scholarship from the China Scholar Council (201906825051).
Author contributions
Review conception and design: H.L., Y.F.H., X.Y.W., D.X.Z., W.W.Z., and F.Y.D. Drafting of the article: Y.F.H. and H.L. All authors read and approved the final manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cholok D, et al. Heterotopic ossification and the elucidation of pathologic differentiation. Bone. 2018;109:12–21. doi: 10.1016/j.bone.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mujtaba B, et al. Heterotopic ossification: radiological and pathological review. Radiol. Oncol. 2019;53:275–284. doi: 10.2478/raon-2019-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuo K, Chavez RD, Barruet E, Hsiao EC. Inflammation in fibrodysplasia ossificans progressiva and other forms of heterotopic ossification. Curr. Osteoporos. Rep. 2019;17:387–394. doi: 10.1007/s11914-019-00541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franken L, Schiwon M, Kurts C. Macrophages: sentinels and regulators of the immune system. Cell. Microbiol. 2016;18:475–487. doi: 10.1111/cmi.12580. [DOI] [PubMed] [Google Scholar]
- 5.Sorkin M, et al. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat. Commun. 2020;11:722. doi: 10.1038/s41467-019-14172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genêt F, et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J. Pathol. 2015;236:229–240. doi: 10.1002/path.4519. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, et al. Macrophage-derived neurotrophin-3 promotes heterotopic ossification in rats. Lab. Investig. 2020;100:762–776. doi: 10.1038/s41374-019-0367-x. [DOI] [PubMed] [Google Scholar]
- 8.Convente MR, et al. Depletion of mast cells and macrophages impairs heterotopic ossification in an Acvr1(R206H) mouse model of fibrodysplasia ossificans progressiva. J. Bone Miner. Res. 2018;33:269–282. doi: 10.1002/jbmr.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu D, et al. Expression and significance of hypoxia inducible factor 1α in rat model of heterotopic ossification after Achilles tenotomy. Chin. J. Reparative Reconstr. Surg. 2016;30:1098–1103. doi: 10.7507/1002-1892.20160224. [DOI] [PubMed] [Google Scholar]
- 10.Hwang C, et al. Mesenchymal VEGFA induces aberrant differentiation in heterotopic ossification. Bone Res. 2019;7:36. doi: 10.1038/s41413-019-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Łęgosz P, Drela K, Pulik Ł, Sarzyńska S, Małdyk P. Challenges of heterotopic ossification-Molecular background and current treatment strategies. Clin. Exp. Pharmacol. Physiol. 2018;45:1229–1235. doi: 10.1111/1440-1681.13025. [DOI] [PubMed] [Google Scholar]
- 12.Dey D, et al. The traumatic bone: trauma-induced heterotopic ossification. Transl. Res. 2017;186:95–111. doi: 10.1016/j.trsl.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury. Spinal cord. 2002;40:313–326. doi: 10.1038/sj.sc.3101309. [DOI] [PubMed] [Google Scholar]
- 14.Salisbury E, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J. Cell. Biochem. 2011;112:2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kan L, et al. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27:150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat. Rev. Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shore EM, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 18.Shi X, Zhou L, Shang J, Wang K, Chu CQ. Fibrodysplasia ossificans progressiva-a rare disease with distinctive features yet still a diagnostic challenge: a case report. Medicine. 2020;99:e19933. doi: 10.1097/MD.0000000000019933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuya H, et al. A unique case of fibrodysplasia ossificans progressiva with an ACVR1 mutation, G356D, other than the common mutation (R206H) Am. J. Med. Genet. Part A. 2008;146a:459–463. doi: 10.1002/ajmg.a.32151. [DOI] [PubMed] [Google Scholar]
- 20.Petrie KA, et al. Novel mutations in ACVR1 result in atypical features in two fibrodysplasia ossificans progressiva patients. PloS One. 2009;4:e5005. doi: 10.1371/journal.pone.0005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaliya-Perumal, A. K., Carney, T. J. & Ingham, P. W. Fibrodysplasia ossificans progressiva: current concepts from bench to bedside. Dis. Model Mech. 10.1242/dmm (2020). [DOI] [PMC free article] [PubMed]
- 22.Lin GT, et al. De novo 617G-A nucleotide mutation in the ACVR1 gene in a Taiwanese patient with fibrodysplasia ossificans progressiva. J. Hum. Genet. 2006;51:1083–1086. doi: 10.1007/s10038-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 23.Shore EM, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N. Engl. J. Med. 2002;346:99–106. doi: 10.1056/NEJMoa011262. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan FS, Hahn GV, Zasloff MA. Heterotopic ossification: two rare forms and what they can teach us. J. Am. Acad. Orthop. Surg. 1994;2:288–296. doi: 10.5435/00124635-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Orchard GR, Paratz JD, Blot S, Roberts JA. Risk factors in hospitalized patients with burn injuries for developing heterotopic ossification-A retrospective analysis. J. Burn Care Res. 2015;36:465–470. doi: 10.1097/BCR.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 26.Pignolo RJ, et al. The natural history of flare-Ups in fibrodysplasia ossificans progressiva (FOP): a comprehensive global assessment. J. Bone Miner. Res. 2016;31:650–656. doi: 10.1002/jbmr.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan FS, Shore EM. Progressive osseous heteroplasia. J. Bone Miner. Res. 2000;15:2084–2094. doi: 10.1359/jbmr.2000.15.11.2084. [DOI] [PubMed] [Google Scholar]
- 28.Loder SJ, et al. Characterizing the circulating cell populations in traumatic heterotopic ossification. Am. J. Pathol. 2018;188:2464–2473. doi: 10.1016/j.ajpath.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grgurević L, et al. Elevated plasma RANTES in fibrodysplasia ossificans progressiva - a novel therapeutic target? Med. hypotheses. 2019;131:109313. doi: 10.1016/j.mehy.2019.109313. [DOI] [PubMed] [Google Scholar]
- 30.Kan L, et al. Substance P signaling mediates BMP-dependent heterotopic ossification. J. Cell. Biochem. 2011;112:2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gannon FH, et al. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum. Pathol. 2001;32:842–848. doi: 10.1053/hupa.2001.26464. [DOI] [PubMed] [Google Scholar]
- 32.Mao D, Mi J, Pan X, Li F, Rui Y. Tamoxifen Inhibits the progression of trauma-induced heterotopic ossification in mice. Med. Sci. Monit. 2019;25:7872–7881. doi: 10.12659/MSM.916733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu X, et al. Aberrant RhoA activation in macrophages increases senescence-associated secretory phenotypes and ectopic calcification in muscular dystrophic mice. Aging. 2020;12:24853–24871. doi: 10.18632/aging.202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riegsecker S, Wiczynski D, Kaplan MJ, Ahmed S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013;93:307–312. doi: 10.1016/j.lfs.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, et al. Cellular hypoxia promotes heterotopic ossification by amplifying BMP signaling. J. Bone Miner. Res. 2016;31:1652–1665. doi: 10.1002/jbmr.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barruet E, et al. NF-κB/MAPK activation underlies ACVR1-mediated inflammation in human heterotopic ossification. JCI Insight. 2018;3:e122958. doi: 10.1172/jci.insight.122958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estrores IM, Harrington A, Banovac K. C-reactive protein and erythrocyte sedimentation rate in patients with heterotopic ossification after spinal cord injury. J. Spinal Cord. Med. 2004;27:434–437. doi: 10.1080/10790268.2004.11752233. [DOI] [PubMed] [Google Scholar]
- 38.Roessler PP, et al. Heterotopic Ossification - Complication or Chance? Z. Orthop. Unfall. 2019;157:301–307. doi: 10.1055/a-0732-5946. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, et al. Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice. Nat. Commun. 2018;9:551. doi: 10.1038/s41467-018-02988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo Sicco C, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem cells Transl. Med. 2017;6:1018–1028. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatsell SJ, et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci. Transl. Med. 2015;7:303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alfieri KA, Forsberg JA, Potter BK. Blast injuries and heterotopic ossification. Bone Jt. Res. 2012;1:192–197. doi: 10.1302/2046-3758.18.2000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kan C, et al. BMP-dependent, injury-induced stem cell niche as a mechanism of heterotopic ossification. Stem cell Res. Ther. 2019;10:14. doi: 10.1186/s13287-018-1107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J. Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price JV, Vance RE. The macrophage paradox. Immunity. 2014;41:685–693. doi: 10.1016/j.immuni.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Lu R, Sampathkumar NK, Benayoun BA. Measuring phagocytosis in bone marrow-derived macrophages and peritoneal macrophages with aging. Methods Mol. Biol. 2020;2144:161–170. doi: 10.1007/978-1-0716-0592-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiktor-Jedrzejczak W, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl Acad. Sci. USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Developmental Dyn.: Off. Publ. Am. Assoc. Anatomists. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 50.Lobov IB, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kan C, et al. Inhibition of immune checkpoints prevents injury-induced heterotopic ossification. Bone Res. 2019;7:33. doi: 10.1038/s41413-019-0074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, et al. The inflammation and reactive oxygen species regulated by Nrf2 and NF-κB signaling pathways in 630-nm light-emitting diode irradiation treated THP-1 monocytes/macrophages. Lasers Med Sci. 2020;36:1411–1419. doi: 10.1007/s10103-020-03172-2. [DOI] [PubMed] [Google Scholar]
- 53.Summers KM, Bush SJ, Hume DA. Network analysis of transcriptomic diversity amongst resident tissue macrophages and dendritic cells in the mouse mononuclear phagocyte system. PLoS Biol. 2020;18:e3000859. doi: 10.1371/journal.pbio.3000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shapouri-Moghaddam A, et al. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 55.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucas T, et al. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 57.Wu J, et al. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. EBioMedicine. 2020;58:102920. doi: 10.1016/j.ebiom.2020.102920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tirone M, et al. Severe heterotopic ossification in the skeletal muscle and endothelial cells recruitment to chondrogenesis are enhanced by monocyte/macrophage depletion. Front. Immunol. 2019;10:1640. doi: 10.3389/fimmu.2019.01640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regulatory, Integr. Comp. Physiol. 2010;298:R1173–1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peterson JR, et al. Effects of aging on osteogenic response and heterotopic ossification following burn injury in mice. Stem cells Dev. 2015;24:205–213. doi: 10.1089/scd.2014.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medici D, Olsen BR. The role of endothelial-mesenchymal transition in heterotopic ossification. J. Bone Miner. Res. 2012;27:1619–1622. doi: 10.1002/jbmr.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu R, Hu J, Zhou X, Yang Y. Heterotopic ossification: mechanistic insights and clinical challenges. Bone. 2018;109:134–142. doi: 10.1016/j.bone.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 63.Dey D, et al. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci. Transl. Med. 2016;8:366ra163. doi: 10.1126/scitranslmed.aaf1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dilling CF, et al. Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification. J. Bone Miner. Res. 2010;25:1147–1156. doi: 10.1359/jbmr.091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Brien EJ, Frank CB, Shrive NG, Hallgrímsson B, Hart DA. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int. J. Exp. Pathol. 2012;93:319–331x. doi: 10.1111/j.1365-2613.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandes TL, et al. Macrophage: a potential target on cartilage regeneration. Front. Immunol. 2020;11:111. doi: 10.3389/fimmu.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai M, Sui B, Xue Y, Liu X, Sun J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials. 2018;180:91–103. doi: 10.1016/j.biomaterials.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem cells Transl. Med. 2012;1:142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, et al. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell tissue Res. 2017;369:273–286. doi: 10.1007/s00441-017-2598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vi L, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J. Bone Miner. Res. 2015;30:1090–1102. doi: 10.1002/jbmr.2422. [DOI] [PubMed] [Google Scholar]
- 71.Maggini J, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PloS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS one. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horwood NJ. Macrophage polarization and bone formation: a review. Clin. Rev. Allergy Immunol. 2016;51:79–86. doi: 10.1007/s12016-015-8519-2. [DOI] [PubMed] [Google Scholar]
- 74.Alexander KA, et al. Inhibition of JAK1/2 tyrosine kinases reduces neurogenic heterotopic ossification after spinal cord injury. Front. Immunol. 2019;10:377. doi: 10.3389/fimmu.2019.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tuzmen C, Verdelis K, Weiss L, Campbell P. Crosstalk between substance P and calcitonin gene-related peptide during heterotopic ossification in murine Achilles tendon. J. Orthop. Res. 2018;36:1444–1455. doi: 10.1002/jor.23833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torossian F, et al. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI Insight. 2017;2:e96034. doi: 10.1172/jci.insight.96034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao X, et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc. Natl Acad. Sci. USA. 2014;111:E1211–1220. doi: 10.1073/pnas.1321347111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Kraan PM, Blaney Davidson EN, Blom A, van den Berg WB. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads. Osteoarthr. Cartil. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 79.Mao D, Pan X, Rui Y, Li F. Matrine attenuates heterotopic ossification by suppressing TGF-β induced mesenchymal stromal cell migration and osteogenic differentiation. Biomed. Pharmacother. . 2020;127:110152. doi: 10.1016/j.biopha.2020.110152. [DOI] [PubMed] [Google Scholar]
- 80.Li L, et al. Muscle injury promotes heterotopic ossification by stimulating local bone morphogenetic protein-7 production. J. Orthop. Translat. 2019;18:142–153. doi: 10.1016/j.jot.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barruet E, et al. The ACVR1 R206H mutation found in fibrodysplasia ossificans progressiva increases human induced pluripotent stem cell-derived endothelial cell formation and collagen production through BMP-mediated SMAD1/5/8 signaling. Stem cell Res. Ther. 2016;7:115. doi: 10.1186/s13287-016-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu PB, et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat. Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peterson JR, et al. Treatment of heterotopic ossification through remote ATP hydrolysis. Sci. Transl. Med. 2014;6:255ra132. doi: 10.1126/scitranslmed.3008810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katono N, et al. Involvement of BMP and Wnt signals leading to epithelial-mesenchymal transition in colon adenocarcinoma with heterotopic ossification. Ann. Clin. Lab. Sci. 2021;51:271–276. [PubMed] [Google Scholar]
- 85.Agarwal S, et al. Strategic targeting of multiple BMP receptors prevents trauma-induced heterotopic ossification. Mol. Ther.: J. Am. Soc. Gene Ther. 2017;25:1974–1987. doi: 10.1016/j.ymthe.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chakkalakal SA, Shore EM. Heterotopic ossification in mouse models of fibrodysplasia ossificans progressiva. Methods Mol. Biol. 2019;1891:247–255. doi: 10.1007/978-1-4939-8904-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tőkési N, et al. Pyrophosphate therapy prevents trauma-induced calcification in the mouse model of neurogenic heterotopic ossification. J. Cell. Mol. Med. 2020;24:11791–11799. doi: 10.1111/jcmm.15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Debaud C, et al. Local and systemic factors drive ectopic osteogenesis in regenerating muscles of spinal-cord-injured mice in a lesion-level-dependent manner. J. neurotrauma. 2021;38:2162–2175. doi: 10.1089/neu.2021.0058. [DOI] [PubMed] [Google Scholar]
- 89.Alexander KA, Tseng HW, Salga M, Genêt F, Levesque JP. When the nervous system turns skeletal muscles into bones: how to solve the conundrum of neurogenic heterotopic ossification. Curr. Osteoporos. Rep. 2020;18:666–676. doi: 10.1007/s11914-020-00636-w. [DOI] [PubMed] [Google Scholar]
- 90.Polfer EM, et al. The development of a rat model to investigate the formation of blast-related post-traumatic heterotopic ossification. Bone Jt. J. 2015;97-B:572–576. doi: 10.1302/0301-620X.97B4.34866. [DOI] [PubMed] [Google Scholar]
- 91.Qureshi AT, et al. Early characterization of blast-related heterotopic ossification in a rat model. Clin. Orthop. Relat. Res. 2015;473:2831–2839. doi: 10.1007/s11999-015-4240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pavey GJ, et al. Bioburden increases heterotopic ossification formation in an established rat model. Clin. Orthop. Relat. Res. 2015;473:2840–2847. doi: 10.1007/s11999-015-4272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dube PR, Birnbaumer L, Vazquez G. Evidence for constitutive bone morphogenetic protein-2 secretion by M1 macrophages: constitutive auto/paracrine osteogenic signaling by BMP-2 in M1 macrophages. Biochem. Biophys. Res. Commun. 2017;491:154–158. doi: 10.1016/j.bbrc.2017.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun X, et al. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact. Mater. 2021;6:757–769. doi: 10.1016/j.bioactmat.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Lent PL, et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis rheumatism. 2004;50:103–111. doi: 10.1002/art.11422. [DOI] [PubMed] [Google Scholar]
- 96.Wei F, Zhou Y, Wang J, Liu C, Xiao Y. The immunomodulatory role of BMP-2 on macrophages to accelerate osteogenesis. Tissue Eng. Part A. 2018;24:584–594. doi: 10.1089/ten.tea.2017.0232. [DOI] [PubMed] [Google Scholar]
- 97.Ogawa K, Funaba M, Chen Y, Tsujimoto M. Activin A functions as a Th2 cytokine in the promotion of the alternative activation of macrophages. J. Immunol. (Baltim., Md.: 1950) 2006;177:6787–6794. doi: 10.4049/jimmunol.177.10.6787. [DOI] [PubMed] [Google Scholar]
- 98.Qi Y, et al. Activin A regulates activation of mouse neutrophils by Smad3 signalling. Open Biol. 2017;7:16034. doi: 10.1098/rsob.160342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma C, et al. Activin A regulates activities of peripheral blood natural killer cells of mouse in an autocrine and paracrine manner. Exp. cell Res. 2019;374:114–1213. doi: 10.1016/j.yexcr.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 100.de Kretser DM, O’Hehir RE, Hardy CL, Hedger MP. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol. Cell. Endocrinol. 2012;359:101–106. doi: 10.1016/j.mce.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 101.Hino K, et al. An mTOR signaling modulator suppressed heterotopic ossification of fibrodysplasia ossificans progressiva. Stem cell Rep. 2018;11:1106–1119. doi: 10.1016/j.stemcr.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schoenmaker T, et al. The effect of Activin-A on periodontal ligament fibroblasts-mediated osteoclast formation in healthy donors and in patients with fibrodysplasia ossificans progressiva. J. Cell. Physiol. 2019;234:10238–10247. doi: 10.1002/jcp.27693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aleman-Muench GR, Soldevila G. When versatility matters: activins/inhibins as key regulators of immunity. Immunol. cell Biol. 2012;90:137–148. doi: 10.1038/icb.2011.32. [DOI] [PubMed] [Google Scholar]
- 104.Hwang C, et al. Activin A does not drive post-traumatic heterotopic ossification. Bone. 2020;138:115473. doi: 10.1016/j.bone.2020.115473. [DOI] [PubMed] [Google Scholar]
- 105.Richards CD. The enigmatic cytokine oncostatin m and roles in disease. ISRN Inflamm. 2013;2013:512103. doi: 10.1155/2013/512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nicolaidou V, et al. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PloS One. 2012;7:e39871. doi: 10.1371/journal.pone.0039871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. (Baltim., Md.: 1950) 1997;159:5654–5660. [PubMed] [Google Scholar]
- 108.Debaud C, et al. Peripheral denervation participates in heterotopic ossification in a spinal cord injury model. PloS One. 2017;12:e0182454. doi: 10.1371/journal.pone.0182454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang LJ, Zhang RP, Li JD. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta neurochir (Wien). 2014;156:1409–1418. doi: 10.1007/s00701-014-2089-6. [DOI] [PubMed] [Google Scholar]
- 110.Zhang J, et al. Neurotrophin-3 acts on the endothelial-mesenchymal transition of heterotopic ossification in rats. J. Cell. Mol. Med. 2019;23:2595–2609. doi: 10.1111/jcmm.14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Su YW, et al. Neurotrophin-3 Induces BMP-2 and VEGF Activities and Promotes the bony repair of injured growth plate cartilage and bone in rats. J. Bone Miner. Res. 2016;31:1258–1274. doi: 10.1002/jbmr.2786. [DOI] [PubMed] [Google Scholar]
- 112.Evans KN, et al. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high-energy penetrating war injuries. J. Orthop. trauma. 2012;26:e204–213. doi: 10.1097/BOT.0b013e31825d60a5. [DOI] [PubMed] [Google Scholar]
- 113.Kan C, et al. Microenvironmental factors that regulate mesenchymal stem cells: lessons learned from the study of heterotopic ossification. Histol. Histopathol. 2017;32:977–985. doi: 10.14670/HH-11-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tu B, et al. Macrophages derived from THP-1 promote the osteogenic differentiation of mesenchymal stem cells through the IL-23/IL-23R/β-catenin pathway. Exp. Cell Res. 2015;339:81–89. doi: 10.1016/j.yexcr.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 115.Rico-Llanos GA, Becerra J, Visser R. Insulin-like growth factor-1 (IGF-1) enhances the osteogenic activity of bone morphogenetic protein-6 (BMP-6) in vitro and in vivo, and together have a stronger osteogenic effect than when IGF-1 is combined with BMP-2. J. Biomed. Mater. Res. A. 2017;105:1867–1875. doi: 10.1002/jbm.a.36051. [DOI] [PubMed] [Google Scholar]
- 116.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Drouin G, et al. Muscle injury-induced hypoxia alters the proliferation and differentiation potentials of muscle resident stromal cells. Skelet. Muscle. 2019;9:18. doi: 10.1186/s13395-019-0202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu G, Tandang-Silvas MR, Dawson AC, Dawson TJ, Groppe JC. Hypoxia-selective allosteric destabilization of activin receptor-like kinases: a potential therapeutic avenue for prophylaxis of heterotopic ossification. Bone. 2018;112:71–89. doi: 10.1016/j.bone.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin L, et al. Synergistic inhibition of endochondral bone formation by silencing Hif1α and Runx2 in trauma-induced heterotopic ossification. Mol. Ther. 2011;19:1426–1432. doi: 10.1038/mt.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Provot S, et al. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol. 2007;177:451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ke X, et al. Hypoxia modifies the polarization of macrophages and their inflammatory microenvironment, and inhibits malignant behavior in cancer cells. Oncol. Lett. 2019;18:5871–5878. doi: 10.3892/ol.2019.10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Watanabe S, et al. Glucose regulates hypoxia-induced NLRP3 inflammasome activation in macrophages. J. Cell. Physiol. 2020;235:7554–7566. doi: 10.1002/jcp.29659. [DOI] [PubMed] [Google Scholar]
- 123.D’Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016;283:413–424. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maceckova M, et al. Bone marrow-derived macrophages exclusively expressed caveolin-2: the role of inflammatory activators and hypoxia. Immunobiology. 2015;220:1266–1274. doi: 10.1016/j.imbio.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 125.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lian G, et al. Macrophage metabolic reprogramming aggravates aortic dissection through the HIF1α-ADAM17 pathway(✰) EBioMedicine. 2019;49:291–304. doi: 10.1016/j.ebiom.2019.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strowitzki MJ, et al. Pharmacological HIF-inhibition attenuates postoperative adhesion formation. Sci. Rep. 2017;7:13151. doi: 10.1038/s41598-017-13638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu N, et al. HIF-1α regulates angiogenesis via Notch1/STAT3/ETBR pathway in trophoblastic cells. Cell Cycle. 2019;18:3502–3512. doi: 10.1080/15384101.2019.1689481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Madanecki P, et al. Regulation of angiogenesis by hypoxia: the role of microRNA. Cell. Mol. Biol. Lett. 2013;18:47–57. doi: 10.2478/s11658-012-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yellowley CE, Genetos DC. Hypoxia signaling in the skeleton: implications for bone health. Curr. Osteoporos. Rep. 2019;17:26–35. doi: 10.1007/s11914-019-00500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Street, J. et al. Is human fracture hematoma inherently angiogenic? Clin Orthop. Relat. Res. 224–237 (2000).. [DOI] [PubMed]
- 132.Riddle RC, Leslie JM, Gross TS, Clemens TL. Hypoxia-inducible factor-1α protein negatively regulates load-induced bone formation. J. Biol. Chem. 2011;286:44449–44456. doi: 10.1074/jbc.M111.276683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cocks M, et al. Vascular patterning in human heterotopic ossification. Hum. Pathol. 2017;63:165–170. doi: 10.1016/j.humpath.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fantin A, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 136.White JR, et al. Genetic amplification of the transcriptional response to hypoxia as a novel means of identifying regulators of angiogenesis. Genomics. 2004;83:1–8. doi: 10.1016/S0888-7543(03)00215-5. [DOI] [PubMed] [Google Scholar]
- 137.Uccelli A, et al. Vascular endothelial growth factor biology for regenerative angiogenesis. Swiss Med. Wkly. 2019;149:w20011. doi: 10.4414/smw.2019.20011. [DOI] [PubMed] [Google Scholar]
- 138.Spiller KL, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spiller KL, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bosurgi L, et al. Transplanted mesoangioblasts require macrophage IL-10 for survival in a mouse model of muscle injury. J. Immunol. 2012;188:6267–6277. doi: 10.4049/jimmunol.1102680. [DOI] [PubMed] [Google Scholar]
- 141.Ruffell D, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl Acad. Sci. USA. 2009;106:17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zordan P, et al. Macrophages commit postnatal endothelium-derived progenitors to angiogenesis and restrict endothelial to mesenchymal transition during muscle regeneration. Cell death Dis. 2014;5:e1031. doi: 10.1038/cddis.2013.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iavarone F, et al. Cripto shapes macrophage plasticity and restricts EndMT in injured and diseased skeletal muscle. EMBO Rep. 2020;21:e49075. doi: 10.15252/embr.201949075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin F, et al. Canagliflozin alleviates LPS-induced acute lung injury by modulating alveolar macrophage polarization. Int. Immunopharmacol. 2020;88:106969. doi: 10.1016/j.intimp.2020.106969. [DOI] [PubMed] [Google Scholar]
- 145.Hartono C, Muthukumar T, Suthanthiran M. Immunosuppressive drug therapy. Cold Spring Harb. Perspect. Med. 2013;3:a015487. doi: 10.1101/cshperspect.a015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pacifici M. Acquired and congenital forms of heterotopic ossification: new pathogenic insights and therapeutic opportunities. Curr. Opin. Pharmacol. 2018;40:51–58. doi: 10.1016/j.coph.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaplan FS, et al. Early clinical observations on the use of imatinib mesylate in FOP: a report of seven cases. Bone. 2018;109:276–280. doi: 10.1016/j.bone.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 148.Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 2017;122:74–83. doi: 10.1016/j.addr.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Migliorini F, et al. NSAIDs for prophylaxis for heterotopic ossification after total hip arthroplasty: a bayesian network meta-analysis. Calcif. Tissue Int. 2020;108:196–206. doi: 10.1007/s00223-020-00763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kan SL, et al. Nonsteroidal anti-inflammatory drugs as prophylaxis for heterotopic ossification after total hip arthroplasty: a systematic review and meta-analysis. Medicine. 2015;94:e828. doi: 10.1097/MD.0000000000000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He, W., Kapate, N., Shields, C. W. T. & Mitragotri, S. Drug delivery to macrophages: a review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv. Drug Deliv. Rev. 10.1016/j.addr.2019.12.001 (2019). [DOI] [PubMed]
- 152.Kodama T, et al. The effect of a novel immunosuppressive drug, a PAK-2 inhibitor, on macrophage differentiation/polarization in a rat small intestinal transplantation model. Transpl. Immunol. 2019;57:101246. doi: 10.1016/j.trim.2019.101246. [DOI] [PubMed] [Google Scholar]
- 153.Zhang C, et al. Superparamagnetic iron oxide (SPIO) nanoparticles labeled endothelial progenitor cells (EPCs) administration inhibited heterotopic ossification in rats. Nanomed. J. 2019;21:102078. doi: 10.1016/j.nano.2019.102078. [DOI] [PubMed] [Google Scholar]
- 154.Schmidt J, et al. Drug targeting by long-circulating liposomal glucocorticosteroids increases therapeutic efficacy in a model of multiple sclerosis. Brain: a J. Neurol. 2003;126:1895–1904. doi: 10.1093/brain/awg176. [DOI] [PubMed] [Google Scholar]
- 155.Bader JE, et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;314:G22–g31. doi: 10.1152/ajpgi.00229.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brusini, R., Varna, M. & Couvreur, P. Advanced nanomedicines for the treatment of inflammatory diseases. Advanced drug delivery reviews (2020). [DOI] [PMC free article] [PubMed]
- 157.Li J, et al. Quercetin attenuates trauma-induced heterotopic ossification by tuning immune cell infiltration and related inflammatory insult. Front. Immunol. 2021;12:649285. doi: 10.3389/fimmu.2021.649285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Colino CI, Lanao JM, Gutierrez-Millan C. Targeting of hepatic macrophages by therapeutic nanoparticles. Front. Immunol. 2020;11:218. doi: 10.3389/fimmu.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bagalkot V, et al. Hybrid nanoparticles improve targeting to inflammatory macrophages through phagocytic signals. J. Control Release. 2015;217:243–255. doi: 10.1016/j.jconrel.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hatami E, et al. Mannose-decorated hybrid nanoparticles for enhanced macrophage targeting. Biochem. biophysics Rep. 2019;17:197–207. doi: 10.1016/j.bbrep.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Figueiredo, P. et al. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater.10.1016/j.actbio.2020.09.038 (2020). [DOI] [PubMed]
- 162.Mishra S, Vaughn AD, Devore DI, Roth CM. Delivery of siRNA silencing Runx2 using a multifunctional polymer-lipid nanoparticle inhibits osteogenesis in a cell culture model of heterotopic ossification. Integr. Biol.(Camb). 2012;4:1498–1507. doi: 10.1039/c2ib20200j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Frankenberger M, et al. Chemokine expression by small sputum macrophages in COPD. Mol. Med. 2011;17:762–770. doi: 10.2119/molmed.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fantuzzi L, Tagliamonte M, Gauzzi MC, Lopalco L. Dual CCR5/CCR2 targeting: opportunities for the cure of complex disorders. Cell. Mol. life Sci. 2019;76:4869–4886. doi: 10.1007/s00018-019-03255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Y, et al. Identification of biological pathways and genes associated with neurogenic heterotopic ossification by text mining. PeerJ. 2020;8:e8276. doi: 10.7717/peerj.8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leuschner F, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ding W, et al. Evaluation of the antifibrotic potency by knocking down SPARC, CCR2 and SMAD3. EBioMedicine. 2018;38:238–247. doi: 10.1016/j.ebiom.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takahashi M, Katagiri T, Furuya H, Hohjoh H. Disease-causing allele-specific silencing against the ALK2 mutants, R206H and G356D, in fibrodysplasia ossificans progressiva. Gene Ther. 2012;19:781–785. doi: 10.1038/gt.2011.193. [DOI] [PubMed] [Google Scholar]
- 169.Cappato S, Giacopelli F, Ravazzolo R, Bocciardi R. The horizon of a therapy for rare genetic diseases: a “druggable” future for fibrodysplasia ossificans progressiva. Int. J. Mol. Sci. 2018;19:989. doi: 10.3390/ijms19040989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shimono K, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat. Med. 2011;17:454–460. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shimono K, et al. Inhibition of ectopic bone formation by a selective retinoic acid receptor alpha-agonist: a new therapy for heterotopic ossification? J. Orthop. Res. 2010;28:271–277. doi: 10.1002/jor.20985. [DOI] [PubMed] [Google Scholar]
- 172.Singh S, et al. Surgical management of bilateral hip fractures in a patient with fibrodysplasia ossificans progressiva treated with the RAR-γ agonist palovarotene: a case report. BMC Musculoskelet. Disord. 2020;21:204. doi: 10.1186/s12891-020-03240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.