Introduction
The word androgen is derived from the Greek words andros and genao, which translate to “a man” and “produce or create” respectively. Hyperandrogenism is therefore any state with an excess production of “male” hormones, although these hormones are normally found in women at lower levels. The most clinically relevant hormone in hyperandrogenism is testosterone, which is converted peripherally to dihydrotestosterone (DHT), its biologically active form. The most common symptom of hyperandrogenism in women is hirsutism, and the most prevalent cause is polycystic ovarian syndrome (PCOS).1 The approach to hyperandrogenism in women differs depending on the stage of the woman’s life. This article will serve as a concise review of hyperandrogenism in women at various stages of adult life.
Physiology of Androgens in Women
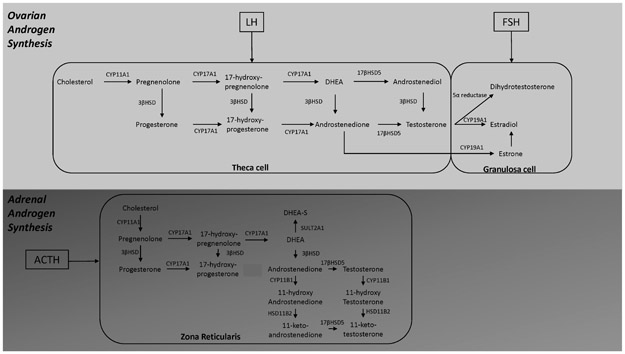
In women during the reproductive years, the two sources of androgens are the adrenal glands and ovaries (Figure 1). It is estimated that 33% of circulating testosterone is produced by the theca cells of the ovaries.2 The remaining testosterone is derived from androstenedione (A4), which is produced by both the ovaries and adrenal glands, and converted to testosterone in peripheral tissues. Testosterone is then converted by 5-α reductase to dihydrotestosterone (DHT) both in the granulosa cell of the ovary and in peripheral tissues such as the skin.
Figure 1.
Androgen synthesis in ovaries and adrenal glands. LH – luteinizing hormone; FSH – follicle stimulating hormone; CYP11A1 - cytochrome P450 cholesterol side-chain cleavage; CYP17A1 - cytochrome P450 17α-hydroxylase/17,20-lyase; 3βHSD - 3β-hydroxysteroid dehydrogenase; CYP19A1 - cytochrome P450 aromatase demethylation/A-ring aromatization; SULT2A1 – sulfotransferase 2A1; CYP11B1 - cytochrome P450 11β-hydroxylase; HSD11B2 - 11β-hydroxysteroid dehydrogenase type 2
A4 and dehydroepiandrosterone (DHEA) are secreted by the ovaries and the adrenal glands. Dehydroepiandrosterone sulfate (DHEA-S) is only produced in the zona reticularis of the adrenal gland.3 DHEA is converted to A4. Adrenal androgen production is under ACTH (adrenocorticotrophic hormone) control whereas ovarian androgen production is under control of luteinizing hormone (LH). The circulating concentration of all androgens, except DHEA-S, is therefore influenced by the phase of the menstrual cycle. Of note, androgens have a circadian rhythm, albeit mild, in women.4
DHEA, DHEA-S and A4 are considered pre-androgens as their action at the androgen receptor is far less potent than testosterone. In women they can play a role in hyperandrogenic symptoms and signs because overall testosterone levels are relatively low.5 The DHEA-S concentration in a female increases from the age of 7-8 years (adrenarche), peaks in her 20’s, and then decreases and plateaus in her 50-60’s. While the control of DHEA-S production is mainly by ACTH, it has a long half-life and is the best biomarker for adrenal hyperandrogenism in most situations. DHEA-S levels are increased by prolactin and insulin-like growth factor 1, which may explain the hyperandrogenism associated with other disorders.5 Interestingly, circulating DHEA-S can be used as a precursor by ovarian follicles to produce DHEA, testosterone and DHT.6
Androgens have direct effects on reproduction via the androgen receptor and indirect effects through conversion to estrogen. The androgen receptor is present on cells throughout the hypothalamic-pituitary-ovarian axis.2 Therefore, high androgen levels can suppress hypothalamic and pituitary secretion of GnRH, LH and FSH directly and via aromatization to estradiol. Androgens also play a role in ovarian function, directly. Androgen receptor knockout mouse populations (complete,7, 8 granulosa cell specific9 and gonadotroph specific10) show decreased fertility, change in cycle length, poor follicle health, and decreased ovulation. Clinically and experimentally, excess androgens either from an endogenous11 or exogenous source12 result in increased follicle recruitment but then arrested follicle development. Androgen pretreatment during in vitro fertilization has resulted in improved ovarian response leading to increased pregnancy and live birth rates in some studies.13, 14 Optimal androgen concentrations are therefore required for ovarian follicle initiation, follicle growth, ovulation and oocyte maturation.
Genetics
PCOS is by far the most common cause of hyperandrogenism in women, thus most of the genetic data on hyperandrogenism in women stem from PCOS studies. Genome-wide association studies of testosterone levels in women provide additional insight into testosterone production.15 PCOS is a heritable, polygenic disorder with multiple risk loci contributing to disease.16 The International PCOS Consortium performed a meta-analysis of more than 10,000 PCOS cases, with a control to case ratio of approximately 10:1, resulting in the identification of 19 loci that confer risk for PCOS in Han Chinese and European women.17 Of those identified, 8 were associated with increased testosterone and/or hyperandrogenism phenotypes. Both THADA and FSHβ loci are associated with testosterone levels or regulation.18, 19 DENND1A is associated with hyperandrogenism (biochemical and clinical). However, the exact underlying mechanism by which it influences androgen concentrations and actions is unclear.15 The IRF1/RAD50 locus is associated with testosterone levels in the European genome-wide association studies (GWAS),17 however its effect is likely indirect due to the lack of association in the GWAS of testosterone levels.18 The other loci associated with hyperandrogenism are SOD2, ERBB3/RAB5, TOX3 and C9orf3.17
History/Physical
The three most important aspects when taking a history in the woman with hyperandrogenism are age, ethnicity and duration of symptoms. The top differential diagnoses change depending on these features.
Age
Premenopausal women are more likely to have PCOS or non-classic congenital adrenal hyperplasia (NCCAH). The top diagnoses would shift to ovarian hyperthecosis and androgen producing tumors in post-menopausal women. If the woman is pregnant, gestational hyperandrogenism would be the likely culprit.
Ethnicity
Ethnicity influences the color, normal distribution and quantity of body hair. Women of Mediterranean, Middle Eastern, South Asian and Hispanic ethnicities have higher cut offs for the modified Ferriman-Gallwey Score (mFG) (Figure 2) compared to East Asians and Caucasians of Northern European ancestry.20 Further, self-perceived hirsutism scores are higher than clinician perceived scores.21 This should not negate the symptom of hirsutism, especially if it is of concern to the woman.1
Figure 2.
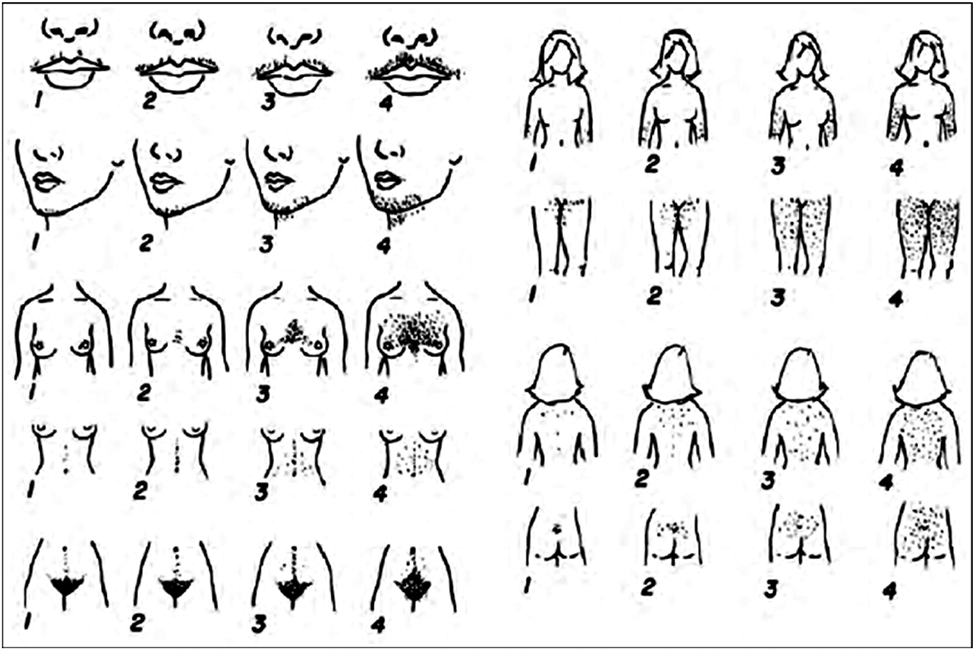
Modified Ferriman Gallwey Score.
Duration of symptoms
Rapid onset (over months) of increased hair growth is concerning for an androgen producing tumor compared with PCOS (12 months vs 42 months in one study).22
The most common symptoms of hyperandrogenism in women are:
Hirsutism
Hirsutism is defined as male pattern terminal hair growth in a woman.23 Both the location of hair growth that is bothersome to the woman and the type of hair growth influence treatment plan. Distribution of terminal hair growth should be clearly documented. The modified Ferriman Gallwey score (mFG) grades nine androgen sensitive areas on the quantity of hair growth and is useful for documentation (Figure 2). Scores range from 0 (absence of terminal hair) to 4 (fully covered). Abnormal total scores are as follows: ≥9 in Middle Eastern, Mediterranean, South Asian and Hispanic women; ≥8 in Blacks and European Caucasians; ≥7 in Southern Chinese women; ≥6 in South American women; and ≥2 in Han Chinese women.20, 24 The mFG has significant inter-user variability and is subjective to the scorer.25, 26 As noted earlier, self-reported scores are often higher than clinician scores and are limited by a patient’s previous attempts at hair removal. In addition, the score limits the importance of local areas. For example, a woman with new onset increased terminal hair growth on her face due to an androgen producing tumor will receive a score of only 4.27 It also fails to include the side burns and lower buttocks.1 Nevertheless, it remains the most common metric to score hirsutism.
Alopecia
The typical pattern of hair loss in women with hyperandrogenism follows a male pattern with vertex thinning/balding (male pattern hair loss or MPHL). MPHL or androgenic alopecia is commonly associated with elevated levels of circulating androgens. Female pattern hair loss (FPHL) usually occurs in the central scalp with preservation of the frontal hairline.28 Elevated circulating androgens may or may not be associated, although the level of 5α reductase activity at the hair follicle cannot be easily assessed and may play a role in FPHL.29, 30 Interestingly, biochemical hyperandrogenism was found in 26-84% of women presenting with FPHL.31-33
Acne
Sebaceous glands are able to convert testosterone to DHT as they exhibit 5α reductase activity.34 In addition, they express 3β-hydroxysteroid dehydrogenase, 17β hydroxysteroid dehydrogenase and P450 side chain cleavage activity,35 all contributing to an increased androgenic environment which promotes sebum formation in the presence of a pro-androgenic environment.36
Oligomenorrhea/Amenorrhea
Documentation of age at menarche, menstrual cycle history and use of any hormonal contraception are important aspects in further elucidating an underlying etiology for hyperandrogenism. In the postmenopausal woman, hyperandrogenism can often lead to postmenopausal bleeding due to the increased conversion of testosterone to estrogen.
Of note, a thorough drug history should be performed to ensure there was no exposure to oral minoxidil, danazol, antiepileptics, anabolic steroids, and exogenous androgens. These exogenous androgens can include transfer from a family member’s prescription,37 DHEA supplements, bioidentical hormone creams/pills/pellets, hormone boosters or anti-aging cocktails supplied by wellness/anti-aging clinics.38
Symptoms and signs of virilization are more likely to indicate an ovarian or adrenal tumor. These signs include deepening of the voice, clitoromegaly and increased muscle mass. Clitoromegaly is defined as a clitoris length >10mm or a clitoral index (length x width) >35 mm2.39
Important clues to other underlying endocrine disorders include signs of Cushing syndrome (purple striae, supraclavicular fullness, facial plethora, easy bruising) and acromegaly (enlarged jaw, macroglossia, and swollen hands/feet with an increase in shoe/ring size).
Diagnostic Evaluation (Figure 3)
Figure 3.
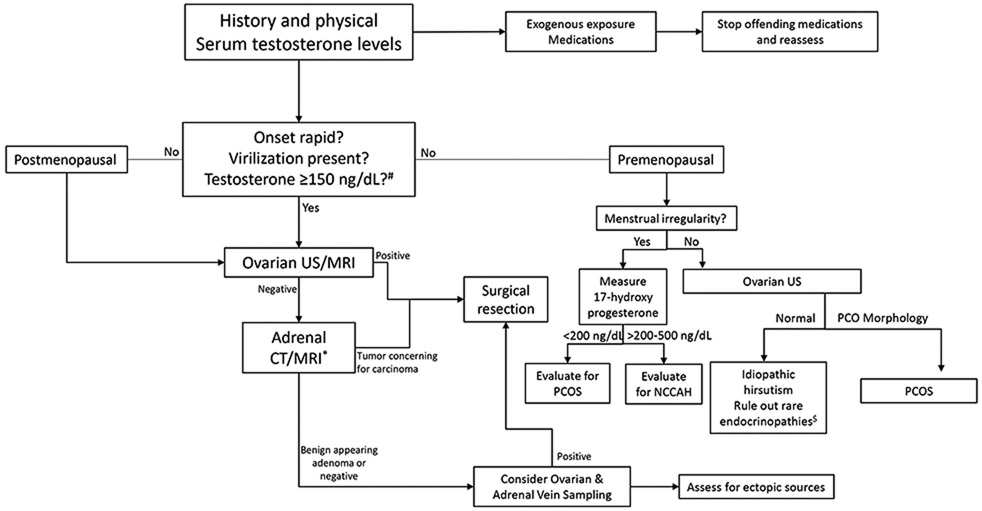
Diagnostic approach to Hyperandrogenism in Women
US – ultrasound; MRI – magnetic resonance imaging; CT – computed tomography; PCOS – polycystic ovarian syndrome; NCCAH – non-classic congenital adrenal hyperplasia
*CT contraindicated in pregnancy
$See text for details #Measured by LC-MS/MS
Laboratory Investigation
The most useful laboratory test is a total testosterone concentration. The method of assay, however, influences the accuracy of this measurement. Liquid chromatography/mass spectrometry (LC-MS/MS) is the most reliable method in quantifying androgen excess in women.40 Measurement of total testosterone by direct radio-immunoassay (RIA) is the most widely available method. RIA was designed to measure total testosterone concentrations in men. The interassay variation at low testosterone levels is high.41 Therefore, while RIAs and other immunoassays are sufficient to identify moderate to severe androgen excess (as is seen in tumors), it often fails to detect mild elevations in PCOS.41, 42 Free testosterone correlates strongly with hyperandrogenism. However, its measurement is fraught with inaccuracies.1 Free testosterone is most accurately measured by equilibrium dialysis, which is not readily available in most clinical laboratories.41 Low levels of sex hormone binding globulin (SHBG) can be used as an indirect marker of higher free testosterone levels.43 The clinician should bear in mind that obesity, hypothyroidism, inflammation, SHBG polymorphisms and mutations can all cause low levels of SHBG and influence free testosterone.44-47 Of note, laboratory testing should not be performed until at least 3 months after stopping hormonal contraception of any type and in the absence of progestin coated IUDs. Hormones will suppress endogenous androgens and make measurements inaccurate for clinical diagnostic purposes.
In a premenopausal woman, a pregnancy test should be performed. If negative, measurement of prolactin, follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH) and an early morning 17-hydroxyprogesterone (7-9 AM) should be evaluated. Cushing’s syndrome and acromegaly should be ruled out based on the presenting clinical symptoms and signs.
Measuring DHEA-S can be helpful to assess adrenal hyperandrogenism. DHEA-S can be elevated in both PCOS and adrenal tumors.48 If the DHEA-S is >700 μg/dL, an adrenal tumor should be ruled out.1 Concentrations of A4 and DHEA are not part of the routine evaluation for hyperandrogenism. The biochemical pattern of A4, DHEA and DHT are variable and measurements add little value except in specific clinical cases.49-51
Imaging
If the physical examination reveals virilization or the laboratory measurements show severe biochemical androgen excess (total testosterone by LC/MS ≥150 ng/dL in premenopausal woman or ≥ 64 ng/dL in a postmenopausal woman),22 pelvic imaging should be the next step in evaluation. An ovarian source is the etiology in approximately 80% of cases.22, 52 Unless there is concern for an ovarian tumor, imaging should also be delayed until at least 3 months after stopping hormonal contraception of any type and in the absence of progestin coated IUDs. Hormones will cause the ovarian volume to decrease and make measurements inaccurate for PCOS clinical diagnostic purposes.
Due to lower cost, transvaginal ultrasonography with color Doppler should be the first line of imaging.53 However, ovarian tumors are generally small in size (Leydig cell tumors <3cm) and isoechoic. They are easily missed by transabdominal ultrasonography. Magnetic resonance imaging (MRI) would be the next best step if pelvic ultrasonography is negative. 18 fluorodeoxyglucose-positron emission tomography (18FDG-PET) imaging is usually reserved for select cases.54, 55 Of note, ovarian hyperthecosis can be described on ultrasonography as bilateral increase in ovarian size, a single ovarian nodule, or it can also appear normal.56 The data comparing the differing imaging techniques in the evaluation of severe hyperandrogenism are scant.53, 57 The absence of an ovarian tumor on imaging does not rule out the presence of an ovarian androgen producing tumor. The presence of 12 or more antral follicles and/or ovarian volume >10 cm3 would meet the Rotterdam criteria for PCOS.58
When pelvic imaging is negative or if androgen levels suggest an adrenal etiology (DHEA-S >700 μg/dL), adrenal computed tomography (CT) would be the next step. Adrenal CT should be performed with and without contrast so that the Hounsfield units, absolute and relative wash out can be calculated. If CT is contraindicated, MRI with the measurement of chemical shift can be performed.59 18FDG-PET/CT can also be considered second line in select cases.60 Adrenal incidentalomas are common, especially in the postmenopausal age group.61 Adrenal imaging should therefore only be pursued if clinically indicated. Adrenal imaging may reveal a solitary benign nodule, adrenal carcinoma or bilateral hyperplasia. In the presence of an adrenal tumor, the woman should also be assessed for excess endogenous cortisol secretion. If cortisol excess is present, surgery for Cushing syndrome will decrease morbidity and mortality.62 Bear in mind that the excess adrenocorticotrophic hormone (ACTH) production in NCCAH would lead to bilateral adrenal cortical hyperplasia but there would not be excess cortisol present. 63
Lastly, in the setting of severe hyperandrogenism, ovarian and adrenal vein sampling can be used when both pelvic and adrenal imaging are negative.52 Right and left ovarian and adrenal veins are accessed and testosterone is measured to determine a left to right difference. This requires the skillset of a highly experienced interventional radiologist. Ovarian and adrenal vein sampling is useful in two scenarios: (1) in the premenopausal woman where preservation of fertility is desired and localization of one ovary for resection is required, and (2) in the postmenopausal woman with a small adrenal nodule but a suspected ovarian source.
Differential Diagnoses (Table 1)
Table 1.
Differential Diagnoses of Hyperandrogenism
| Stage of Life | Adrenal | Ovarian | Other |
|---|---|---|---|
| Premenopausal | Congenital Adrenal Hyperplasia (classic and non-classic) Glucocorticoid Resistance Cortisone Reductase deficiency Adrenal Adenoma Adrenal Carcinoma Bilateral Macronodular Adrenal Hyperplasia |
PCOS Ovarian tumors: Sertoli-Leydig cell tumors Granulosa-theca cell tumors Hilus cell tumors |
Idiopathic Hirsutism Exogenous exposure Hyperprolactinemia Cushing’s disease Acromegaly Insulin resistance syndromes Medications (danazol, valproic acid, oxcarbazepine) |
| Gestational | Adrenal Adenoma Adrenal carcinoma |
Luteoma Theca lutein cysts Sertoli-Leydig cell tumors |
Exogenous exposure Placental aromatase deficiency |
| Postmenopausal | Adrenal Adenoma Adrenal carcinoma Bilateral Macronodular Adrenal Hyperplasia |
Ovarian Hyperthecosis Ovarian tumors: Sertoli-Leydig cell tumors Granulosa-theca cell tumors Teratomas Krukenberg tumors |
Exogenous exposure Hyperprolactinemia Cushing’s disease Acromegaly Medications (danazol, valproic acid, oxcarbazepine) |
The top differential diagnoses differ based on the stage of the woman’s life. We discuss the 3 most common diagnoses in premenopausal and postmenopausal women. In addition, gestational hyperandrogenism is briefly explored.
Premenopausal Hyperandrogenism
PCOS
PCOS is the most common endocrine disorder in reproductive age women, affecting approximately 10% of the population.64 Two out of 3 Rotterdam criteria are required in order to achieve the diagnosis: 1) oligomenorrhea/amenorrhea, 2) clinical or biochemical hyperandrogenism and/or 3) polycystic ovaries on ultrasound, which is defined as 20 or more antral follicles and/or ovarian volume >10 cm3. 58 In women with hyperandrogenism, 57-82% meet the criteria for PCOS.65, 66 In women with PCOS, 65-75% have hyperandrogenism, with hirsutism being the most common symptom.67 In one prospective study, only 2.3% of women with PCOS had another identifiable endocrine disorder to explain symptoms.68
Idiopathic Hirsutism
When there are no abnormalities found on investigation, i.e. elevated testosterone or DHEA-S and normal ovaries on ultrasound in a woman under the age of 40 years and not on hormonal contraception, and there are no menstrual abnormalities, the diagnosis of idiopathic hirsutism is made.69 Treatment is directed toward controlling hirsutism with shared decision making to ensure the woman’s perceived areas of concern are adequately addressed.
NCCAH
Women with NCCAH present with menstrual irregularities and hyperandrogenism, often leading to a misdiagnosis of PCOS. NCCAH is due to 21-hydroxyase deficiency (P450c21) leading to increased 17-hydroxyprogesterone precursor available for the androgen pathway with increased production of androstenedione and testosterone.70 The diagnosis can be made if the 7-9 am 17-hydroxyprogesterone is > 500 ng/dL. If the am level is between 200-500 ng/dL, a stimulated 17-hydroxyprogesterone level 60 min after a 250 mcg injection of cosyntropin is needed, with a diagnostic 17-hydroxyprogesterone level ≥1500 ng/mL. Unlike the classic form, NCCAH rarely manifests with cortisol deficiency and thus glucocorticoid replacement and ACTH suppression is not needed unless fertility is desired. General hyperandrogenism treatments can be used (see section on Treatment: Glucocorticoids below).
Postmenopausal Hyperandrogenism
Ovarian Hyperthecosis
Ovarian hyperthecosis is a histologic diagnosis noted when there is the presence of nests of luteinized theca cells throughout the ovarian stroma. Postmenopausal women present with slow onset and progressive symptoms of hyperandrogenism. In severe cases, virilization can occur. Typical signs of insulin resistance are often present (acanthosis nigricans, skin tags, central obesity). The abundance of luteinized cells is thought to be due to increased gonadotrophin levels which result in increased androgen production.71 Postmenopausal bleeding, due to endometrial hyperplasia from testosterone aromatization to estrogen can also be a presenting symptom. Women often have elevated testosterone and estradiol concentrations with inappropriately low LH and FSH for a menopausal woman.72 Ultrasonography often reveals bilaterally enlarged, solid ovaries for the woman’s stated age.73 Therefore, it is important to evaluate ovarian size compared to age-based references as ovaries that are normal in size compared to a reproductive aged woman are likely enlarged compared to normative data in postmenopausal women. In premenopausal women with hyperthecosis, ovaries may demonstrate an absence of small follicles on ultrasound in addition to enlarged ovaries.
Ovarian and Adrenal Neoplasms
Androgen producing tumors are more common in postmenopausal women. Most ovarian sources are benign whereas adrenal tumors can be either benign or malignant. Adrenocortical carcinoma often co-secretes other hormones (cortisol, aldosterone) in addition to excess androgens thereby warranting further assessment. Women present with rapidly, progressive symptoms of hyperandrogenism, often with virilization.56, 74 Surgical resection results in rapid resolution of symptoms.
Iatrogenic Hyperandrogenism
Androgens, including DHEA, are often prescribed to treat postmenopausal symptoms.75 The currently available testosterone replacements were developed for male hypogonadism, but can be a cause of hyperandrogenism in women exposed to a partner’s topical testosterone. If these products are used in women, they can lead to frank hyperandrogenism.72 Antiepileptics (valproic acid and oxcarbazepine) have been associated with biochemical hyperandrogenism.76, 77
Gestational Hyperandrogenism
Hyperandrogenism in pregnancy is extremely rare.78 During normal pregnancy, testosterone and A4 concentrations rise progressively in each trimester, returning to baseline concentrations postpartum.79, 80 The increase in androgens promotes cervical ripening81 and contributes to the relaxation of the myometrium during pregnancy.82 The increase in androgen concentrations is offset by an increase in SHBG, thereby limiting the biologically active fraction.83 Rarely a pregnancy luteoma, the physiologic remnant of the corpus luteum from the menstrual cycle of conception, produces testosterone at high levels and results in hyperandrogenism. Placental aromatase cytochrome P450 converts androgens to estradiol which is then metabolized to estriol by the fetal liver, protecting the fetus from maternal hyperandrogenism.83 In rare cases of recessive placental aromatase deficiency, the placenta is unable to aromatize androgens and both the mother and fetus experience virilization.
In general, only hyperandrogenism internal to the fetus or placental aromatase deficiency cause fetal virilization.84 Female fetal exposure to excess androgens between the 7th and 12th weeks of gestation can result in labial fusion and clitoromegaly. The hormonal milieu may also play a role in long term fetal development with potential influence on fetal growth85, metabolism86, cardiovascular function87, reproductive function88 and behavior.78, 89 The most common fetal cause is fetal 21-hydroxylase CAH.90 Treatment options are limited and dependent upon fetal sex and fetal virilization risk.83, 91
Treatment
The goals of management are two fold: (1) Identify and surgically treat severe virilization and (2) decrease any perceived symptoms/signs of hyperandrogenism.1
Ovarian and adrenal tumors should undergo complete surgical resection if possible. Bilateral oophorectomy is the treatment of choice for ovarian hyperthecosis. If no tumor is identified in the postmenopausal woman, bilateral oophorectomy should be performed given the high likelihood of an ovarian source.22, 56 In the premenopausal woman, fertility sparing cytoreductive surgery should be considered. 92 For adrenal tumors, non-surgical techniques can be employed if surgery is contraindicated or not desired. Radiofrequency ablation and CT-guided cyroablation have been utilized in functional adrenal adenomas with similar outcomes compared to surgical resection.93, 94
Medical Management
Lifestyle
In obese women with PCOS, weight loss results in a small decrease in testosterone as measured by the free androgen index (mean difference −1.11, 95% confidence interval (CI) −1.96 to −0.26) and a concomitant mild improvement in hirsutism (Mean difference −1.12 [95% confidence interval (CI) (−2.16 to −0.08)].95 While lifestyle intervention is necessary for overall health, it has a limited impact in improving hyperandrogenism. It should not be the sole management strategy.96
Oral Contraceptives
Oral contraceptives containing ethinyl estradiol (EE) suppress LH production and increase SHBG concentrations resulting in decreased ovarian androgen production and decreased free testosterone concentrations, respectively.97 The dose of ethinyl estradiol, whether low dose (20 mcg) or average dose (30-35 mcg), both work to suppress androgens and all progestins seem to work equally well regardless of the underlying androgenicity.1 Hormonal contraception can also cause a small reduction in adrenal androgen secretion, inhibition of androgen binding to its receptor and inhibition of 5α-reductase.1 There is an increased risk of venous thromboembolism with oral contraceptives, with greater risk in obese women over the age of 39 years who smoke.98 Obesity itself is not a contraindication to using oral contraceptives.99 In women with hirsutism or androgenic acne, oral contraceptives should be first line medical management if fertility is not desired.1, 96 The effect on hyperandrogenic symptoms/signs should be reassessed in six months.1
Antiandrogens
After 6 months of oral contraceptive use, an antiandrogen can be added to improve symptoms of hyperandrogenism. The vailable anti-androgens in the United States are spironolactone (100-200 mg/day) and finasteride (2.5-5mg/day). Use of any anti-androgen in women is considered off-label use.
Spironolactone
Spironolactone is an aldosterone receptor antagonist with a dose-dependent competitive inhibition of the androgen receptor. It is effective in reducing hirsutism scores.100 It is contraindicated in pregnancy owing to blockade of androgen action critical for formation of the external male genitalia. Therefore, it should be used with a reliable contraceptive method unless the woman is abstinent. It is a potassium sparing diuretic and therefore can cause hypotension, dizziness and hyperkalemia. Potassium levels should be monitored and caution should be taken in the setting of renal impairment.
Finasteride
Finasteride is a 5α reductase inhibitor, with specific activity against type 2 5α reductase. It decreases local DHT levels in hair follicles with comparable effects to other antiandrogens. The effect of finasteride is dose dependent, with 2.5 mg and 5 mg having a similar reduction in hirsutism scores and 7.5 mg slightly more effective.101 The most common side effects are low libido, depression/asthenia and orthostatic hypotension.102 Dutasteride is a 5α reductase inhibitor with activity against both type 1 and 2 5α reductase enzymes. There is limited data on the use of dutasteride in women.103 While there is no direct comparison of spironolactone to finasteride, pooled data suggest spironolactone to be more effective, although finasteride data are limited.100
Cyproterone Acetate
Cyproterone acetate is a competitive inhibitor at the androgen receptor and commonly used outside of the United States.69 It is available in combination with ethinyl estradiol in the form of combined oral contraceptive pills. At a dose of 2 mg, its effect is equivalent to 50 mg of spironolactone.104 However, the risk of venous thromboembolism is 50-100% higher than for oral contraceptives with levonorgestrel.105 The use of an oral contraceptive containing cyproterone acetate results in a mildly better mFG on follow up compared to the use of other oral contraceptives.106 The Endocrine Society notes its effect on hirsutism is not clinically significant. Cyproterone acetate containing hormonal contraceptives are not recommended over other oral contraceptives.1
Local/Topical Treatment
If medical management is contraindicated or symptoms are not worrisome enough to the woman to warrant systemic therapy, local treatment options should be discussed. In addition, local treatment is an important adjunct to hormonal treatment as the improvement in hirsutism with hormonal treatment takes over 6 months, reflecting the cycle of the hair follicle. Direct hair removal via shaving, waxing, bleaching, chemical creams, photoepilation or electrolysis can be utilized.1 Topical antiandrogens have limited efficacy in hirsutism. Acne should be treated by a dermatologist. First line therapy for alopecia is topical minoxidil, noting a synergistic effect when combined with systemic antiandrogens.28 Other treatment options for alopecia are low level laser light therapy,107 hair transplantation108 and platelet rich plasma.109
Glucocorticoids
In classic 21-hydroxylase congenital adrenal hyperplasia, glucocorticoids are effective in suppressing ACTH stimulated adrenal androgen production.70 In NCCAH, glucocorticoids should not be the first line therapy to treat symptoms/signs of hyperandrogenism other than for ovulation induction. In fact, oral contraceptives are more effective in improving hirsutism compared to dexamethasone.110
GnRH Agonists
GnRH agonists are equally effective as oral contraceptives in decreasing hirsutism.111 These long-acting, modified GnRH peptides initially stimulate, but then suppress LH and FSH after approximately 1 week owing to desensitization. GnRH agonists therefore induce hypoestrogenism, resulting in bone loss and hot flashes.112 Its use should be reserved for the rare cases of virilization that are not amenable to other therapies.
Medications that Reduce Insulin Levels or Improve Insulin Action
In multiple meta-analyses, medications that reduce insulin levels or improve insulin action (metformin, troglitazone and rosiglitazone) were no more effective than placebo in treating hyperandrogenism in PCOS.1, 96, 100, 113 Therefore, metformin should be reserved for the treatment of prediabetes or type 2 diabetes in women with PCOS, as it is not as effective as other medications for cosmetic concerns or for uterine protection in the case of irregular menses.
Conclusion
The approach to hyperandrogenism in women varies depending on the woman’s age and severity of symptoms. Once tumorous hyperandrogenism is excluded, the most common cause is PCOS. Hirsutism is the most common presenting symptom. The woman’s concern about her symptoms plays an important role in the management of disease. While measurement of testosterone is useful in identifying an underlying cause, care must be taken when interpreting the less accurate assays that are available commercially. Surgical resection is curative in tumorous etiologies while medical management is the mainstay for non-tumorous causes.
Footnotes
Disclosure statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin KA, Anderson RR, Chang RJ, et al. Evaluation and Treatment of Hirsutism in Premenopausal Women: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2018;103(4):1233–1257. [DOI] [PubMed] [Google Scholar]
- 2.Walters KA, Handelsman DJ. Role of androgens in the ovary. Molecular and Cellular Endocrinology 2018;465:36–47. [DOI] [PubMed] [Google Scholar]
- 3.Schiffer L, Arlt W, Storbeck K-H. Intracrine androgen biosynthesis, metabolism and action revisited. Molecular and Cellular Endocrinology 2018;465:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davison SL, Bell R. Androgen Physiology. Semin Reprod Med 2006;24(02):071–077. [DOI] [PubMed] [Google Scholar]
- 5.Burger HG. Androgen production in women. Fertil Steril 2002)77 Suppl 4:S3–5. [DOI] [PubMed] [Google Scholar]
- 6.Haning RV Jr., Flood CA, Hackett RJ, et al. Metabolic Clearance Rate of Dehydroepiandrosterone Sulfate, Its Metabolism to Testosterone, and Its Intrafollicular Metabolism to Dehydroepiandrosterone, Androstenedione, Testosterone, and Dihydrotestosterone in Vivo*. The Journal of Clinical Endocrinology & Metabolism 1991;72(5):1088–1095. [DOI] [PubMed] [Google Scholar]
- 7.Shiina H, Matsumoto T, Sato T, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A 2006;103(1):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng XB, Jimenez M, Desai R, et al. Characterizing the neuroendocrine and ovarian defects of androgen receptor-knockout female mice. Am J Physiol Endocrinol Metab 2013;305(6):E717–726. [DOI] [PubMed] [Google Scholar]
- 9.Walters KA, Middleton LJ, Joseph SR, et al. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod 2012;87(6):151. [DOI] [PubMed] [Google Scholar]
- 10.Wu S, Chen Y, Fajobi T, et al. Conditional knockout of the androgen receptor in gonadotropes reveals crucial roles for androgen in gonadotropin synthesis and surge in female mice. Mol Endocrinol 2014;28(10):1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hague WM, Adams J, Rodda C, et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clinical Endocrinology 1990;33(4):501–510. [DOI] [PubMed] [Google Scholar]
- 12.Becerra-Fernández A, Pérez-López G, Menacho Román M, et al. Prevalence of hyperandrogenism and polycystic ovary syndrome in female to male transsexuals. Endocrinología y Nutrición (English Edition) 2014;61(7):351–358. [DOI] [PubMed] [Google Scholar]
- 13.Wiser A, Gonen O, Ghetler Y, et al. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: A randomized prospective study. Human Reproduction 2010;25(10):2496–2500. [DOI] [PubMed] [Google Scholar]
- 14.Kim C-H, Howles CM, Lee H-A. The effect of transdermal testosterone pretreatment on controlled ovarian stimulation and IVF outcome in low responders. Fertility and Sterility 2011;95(2):679–683. [DOI] [PubMed] [Google Scholar]
- 15.Welt CK. Genetics of polycystic ovary syndrome: What is new? Endocrinol Metab Clin North Am. 2021;50(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vink JM, Sadrzadeh S, Lambalk CB, et al. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab 2006;91(6):2100–2104. [DOI] [PubMed] [Google Scholar]
- 17.Day F, Karaderi T, Jones MR, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet 2018;14(12):e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruth KS, Day FR, Tyrrell J, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med 2020;26(2):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZJ, Zhao H, He L, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet 2011;43(1):55–59. [DOI] [PubMed] [Google Scholar]
- 20.Escobar-Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Human Reproduction Update 2011;18(2):146–170. [DOI] [PubMed] [Google Scholar]
- 21.Pasch L, He SY, Huddleston H, et al. Clinician vs Self-ratings of Hirsutism in Patients With Polycystic Ovarian Syndrome: Associations With Quality of Life and Depression. JAMA Dermatol 2016;152(7):783–788. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Kapoor E, Singh RJ, et al. Diagnostic Thresholds for Androgen-Producing Tumors or Pathologic Hyperandrogenism in Women by Use of Total Testosterone Concentrations Measured by Liquid Chromatography-Tandem Mass Spectrometry. Clinical Chemistry 2018;64(11):1636–1645. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med 2005;353(24):2578–2588. [DOI] [PubMed] [Google Scholar]
- 24.Afifi L, Saeed L, Pasch LA, et al. Association of ethnicity, Fitzpatrick skin type, and hirsutism: A retrospective cross-sectional study of women with polycystic ovarian syndrome. Int J Womens Dermatol 2017;3(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wild RA, Vesely S, Beebe L, et al. Ferriman Gallwey self-scoring I: performance assessment in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005;90(7):4112–4114. [DOI] [PubMed] [Google Scholar]
- 26.Yildiz BO, Bolour S, Woods K, et al. Visually scoring hirsutism. Hum Reprod Update 2010;16(1):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adefris M, Fekadu E. Postmenopausal mild hirsutism and hyperandrogenemia due to granulosa cell tumor of the ovary: a case report. J Med Case Rep 2017;11(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmina E, Azziz R, Bergfeld W, et al. Female Pattern Hair Loss and Androgen Excess: A Report From the Multidisciplinary Androgen Excess and PCOS Committee. The Journal of Clinical Endocrinology & Metabolism 2019;104(7):2875–2891. [DOI] [PubMed] [Google Scholar]
- 29.Montalto J, Whorwood CB, Funder JW, et al. Plasma C19 steroid sulphate levels and indices of androgen bioavailability in female pattern androgenic alopecia. Clin Endocrinol (Oxf) 1990;32(1):1–12. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez P, Serrano-Falcón C, Torres JM, et al. 5α-Reductase isozymes and aromatase mRNA levels in plucked hair from young women with female pattern hair loss. Arch Dermatol Res 2018;310(1):77–83. [DOI] [PubMed] [Google Scholar]
- 31.Vexiau P, Chaspoux C, Boudou P, et al. Role of androgens in female-pattern androgenetic alopecia, either alone or associated with other symptoms of hyperandrogenism. Arch Dermatol Res 2000;292(12):598–604. [DOI] [PubMed] [Google Scholar]
- 32.Karrer-Voegeli S, Rey F, Reymond MJ, et al. Androgen dependence of hirsutism, acne, and alopecia in women: retrospective analysis of 228 patients investigated for hyperandrogenism. Medicine (Baltimore) 2009;88(1):32–45. [DOI] [PubMed] [Google Scholar]
- 33.Futterweit W, Dunaif A, Yeh HC, et al. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J Am Acad Dermatol 1988;19(5 Pt 1):831–836. [DOI] [PubMed] [Google Scholar]
- 34.Makrantonaki E, Zouboulis CC. Testosterone metabolism to 5alpha-dihydrotestosterone and synthesis of sebaceous lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. Br J Dermatol 2007;156(3):428–432. [DOI] [PubMed] [Google Scholar]
- 35.Thiboutot D, Jabara S, McAllister JM, et al. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1). J Invest Dermatol 2003;120(6):905–914. [DOI] [PubMed] [Google Scholar]
- 36.Ju Q, Tao T, Hu T, et al. Sex hormones and acne. Clin Dermatol 2017;35(2):130–137. [DOI] [PubMed] [Google Scholar]
- 37.Merhi ZO, Santoro N. Postmenopausal virilization after spousal use of topical androgens. Fertil Steril 2007;87(4):976.e913–975. [DOI] [PubMed] [Google Scholar]
- 38.Stuenkel CA, Manson JE. Compounded Bioidentical Hormone Therapy: Does the Regulatory Double Standard Harm Women? JAMA Internal Medicine 2017;177(12):1719–1720. [DOI] [PubMed] [Google Scholar]
- 39.Tagatz GE, Kopher RA, Nagel TC, et al. The clitoral index: a bioassay of androgenic stimulation. Obstet Gynecol 1979;54(5):562–564. [PubMed] [Google Scholar]
- 40.Rosner W, Auchus RJ, Azziz R, et al. Utility, Limitations, and Pitfalls in Measuring Testosterone: An Endocrine Society Position Statement. The Journal of Clinical Endocrinology & Metabolism 2007;92(2):405–413. [DOI] [PubMed] [Google Scholar]
- 41.Legro RS, Schlaff WD, Diamond MP, et al. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab 2010;95(12):5305–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang WY, Knochenhauer ES, Bartolucci AA, et al. Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertility and Sterility 2005;83(6):1717–1723. [DOI] [PubMed] [Google Scholar]
- 43.Rosenfield RL. Plasma testosterone binding globulin and indexes of the concentration of unbound plasma androgens in normal and hirsute subjects. J Clin Endocrinol Metab 1971;32(6):717–728. [DOI] [PubMed] [Google Scholar]
- 44.Goldman AL, Bhasin S, Wu FCW, et al. A Reappraisal of Testosterone's Binding in Circulation: Physiological and Clinical Implications. Endocr Rev 2017;38(4):302–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simó R, Barbosa-Desongles A, Lecube A, et al. Potential role of tumor necrosis factor-α in downregulating sex hormone-binding globulin. Diabetes 2012;61(2):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 1991;72(1):83–89. [DOI] [PubMed] [Google Scholar]
- 47.Hogeveen KN, Cousin P, Pugeat M, et al. Human sex hormone-binding globulin variants associated with hyperandrogenism and ovarian dysfunction. J Clin Invest 2002;109(7):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elhassan YS, Idkowiak J, Smith K, et al. Causes, Patterns, and Severity of Androgen Excess in 1205 Consecutively Recruited Women. J Clin Endocrinol Metab 2018;103(3):1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welt CK, Arason G, Gudmundsson JA, et al. Defining constant versus variable phenotypic features of women with polycystic ovary syndrome using different ethnic groups and populations. J Clin Endocrinol Metab 2006;91(11):4361–4368. [DOI] [PubMed] [Google Scholar]
- 50.O'Reilly MW, Taylor AE, Crabtree NJ, et al. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione. J Clin Endocrinol Metab 2014;99(3):1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livadas S, Pappas C, Karachalios A, et al. Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine 2014;47(2):631–638. [DOI] [PubMed] [Google Scholar]
- 52.Kaltsas GA, Mukherjee JJ, Kola B, et al. Is ovarian and adrenal venous catheterization and sampling helpful in the investigation of hyperandrogenic women? Clin Endocrinol (Oxf) 2003;59(1):34–43. [DOI] [PubMed] [Google Scholar]
- 53.Outwater EK, Wagner BJ, Mannion C, et al. Sex cord-stromal and steroid cell tumors of the ovary. Radiographics 1998;18(6):1523–1546. [DOI] [PubMed] [Google Scholar]
- 54.McCarthy-Keith DM, Hill M, Norian JM, et al. Use of F 18-fluoro-D-glucose-positron emission tomography-computed tomography to localize a hilar cell tumor of the ovary. Fertil Steril 2010;94(2):753.e711–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prassopoulos V, Laspas F, Vlachou F, et al. Leydig cell tumour of the ovary localised with positron emission tomography/computed tomography. Gynecol Endocrinol 2011;27(10):837–839. [DOI] [PubMed] [Google Scholar]
- 56.Yance VRV, Marcondes JAM, Rocha MP, et al. Discriminating between virilizing ovary tumors and ovary hyperthecosis in postmenopausal women: clinical data, hormonal profiles and image studies. European Journal of Endocrinology 2017;177(1):93. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka YO, Tsunoda H, Kitagawa Y, et al. Functioning ovarian tumors: direct and indirect findings at MR imaging. Radiographics 2004;24 Suppl 1:S147–166. [DOI] [PubMed] [Google Scholar]
- 58.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018;110(3):364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nandra G, Duxbury O, Patel P, et al. Technical and Interpretive Pitfalls in Adrenal Imaging. Radiographics 2020;40(4):1041–1060. [DOI] [PubMed] [Google Scholar]
- 60.Delivanis DA, Bancos I, Atwell TD, et al. Diagnostic performance of unenhanced computed tomography and (18) F-fluorodeoxyglucose positron emission tomography in indeterminate adrenal tumours. Clin Endocrinol (Oxf) 2018;88(1):30–36. [DOI] [PubMed] [Google Scholar]
- 61.Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. European Journal of Endocrinology 2016;175(2):G1. [DOI] [PubMed] [Google Scholar]
- 62.Javanmard P, Duan D, Geer EB. Mortality in Patients with Endogenous Cushing's Syndrome. Endocrinol Metab Clin North Am 2018;47(2):313–333. [DOI] [PubMed] [Google Scholar]
- 63.Kok HK, Sherlock M, Healy NA, et al. Imaging features of poorly controlled congenital adrenal hyperplasia in adults. Br J Radiol 2015;88(1053):20150352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016;31(12):2841–2855. [DOI] [PubMed] [Google Scholar]
- 65.Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 2004;89(2):453–462. [DOI] [PubMed] [Google Scholar]
- 66.Carmina E, Rosato F, Jannì A, et al. Extensive clinical experience: relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab 2006;91(1):2–6. [DOI] [PubMed] [Google Scholar]
- 67.Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89(6):2745–2749. [DOI] [PubMed] [Google Scholar]
- 68.O'Driscoll JB, Mamtora H, Higginson J, et al. A prospective study of the prevalence of clear-cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol (Oxf) 1994;41(2):231–236. [DOI] [PubMed] [Google Scholar]
- 69.Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocr Rev 2000;21(4):347–362. [DOI] [PubMed] [Google Scholar]
- 70.Merke DP, Auchus RJ. Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. N Engl J Med 2020;383(13):1248–1261. [DOI] [PubMed] [Google Scholar]
- 71.Krug E, Berga SL. Postmenopausal hyperthecosis: functional dysregulation of androgenesis in climacteric ovary. Obstet Gynecol 2002;99(5 Pt 2):893–897. [DOI] [PubMed] [Google Scholar]
- 72.Markopoulos MC, Kassi E, Alexandraki KI, et al. MANAGEMENT OF ENDOCRINE DISEASE: Hyperandrogenism after menopause. European Journal of Endocrinology 2015;172(2):R79. [DOI] [PubMed] [Google Scholar]
- 73.Rousset P, Gompel A, Christin-Maitre S, et al. Ovarian hyperthecosis on grayscale and color Doppler ultrasound. Ultrasound Obstet Gynecol 2008;32(5):694–699. [DOI] [PubMed] [Google Scholar]
- 74.Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med 1994;331(15):968–973. [DOI] [PubMed] [Google Scholar]
- 75.Davis SR, Baber R, Panay N, et al. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. The Journal of Clinical Endocrinology & Metabolism 2019;104(10):4660–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu X, Wang J, Dong W, et al. A meta-analysis of polycystic ovary syndrome in women taking valproate for epilepsy. Epilepsy Res 2011;97(1-2):73–82. [DOI] [PubMed] [Google Scholar]
- 77.Löfgren E, Tapanainen JS, Koivunen R, et al. Effects of carbamazepine and oxcarbazepine on the reproductive endocrine function in women with epilepsy. Epilepsia 2006;47(9):1441–1446. [DOI] [PubMed] [Google Scholar]
- 78.Hakim C, Padmanabhan V, Vyas AKAK. Gestational Hyperandrogenism in Developmental Programming. Endocrinology 2017;158(2):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuijper EA, Ket JC, Caanen MR, et al. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online 2013;27(1):33–63. [DOI] [PubMed] [Google Scholar]
- 80.Sir-Petermann T, Maliqueo M, Angel B, et al. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 2002;17(10):2573–2579. [DOI] [PubMed] [Google Scholar]
- 81.Ji H, Dailey TL, Long V, et al. Androgen-regulated cervical ripening: a structural, biomechanical, and molecular analysis. Am J Obstet Gynecol 2008;198(5):543.e541–549. [DOI] [PubMed] [Google Scholar]
- 82.Perusquía M, Navarrete E, Jasso-Kamel J, et al. Androgens induce relaxation of contractile activity in pregnant human myometrium at term: a nongenomic action on L-type calcium channels. Biol Reprod 2005;73(2):214–221. [DOI] [PubMed] [Google Scholar]
- 83.Kaňová N, Bičíková M. Hyperandrogenic states in pregnancy. Physiol Res 2011;60(2):243–252. [DOI] [PubMed] [Google Scholar]
- 84.Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod 1998;3(2):130–140. [DOI] [PubMed] [Google Scholar]
- 85.Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol 2006;155(2):365–370. [DOI] [PubMed] [Google Scholar]
- 86.Eisner JR, Dumesic DA, Kemnitz JW, et al. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab 2000;85(3):1206–1210. [DOI] [PubMed] [Google Scholar]
- 87.Vyas AK, Hoang V, Padmanabhan V, et al. Prenatal programming: adverse cardiac programming by gestational testosterone excess. Sci Rep 2016;6:28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abbott DH, Barnett DK, Bruns CM, et al. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 2005; 11(4):357–374. [DOI] [PubMed] [Google Scholar]
- 89.Knickmeyer R, Baron-Cohen S, Fane BA, et al. Androgens and autistic traits: A study of individuals with congenital adrenal hyperplasia. Horm Behav 2006;50(1):148–153. [DOI] [PubMed] [Google Scholar]
- 90.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet 2005;365(9477):2125–2136. [DOI] [PubMed] [Google Scholar]
- 91.Masarie K, Katz V, Balderston K. Pregnancy luteomas: clinical presentations and management strategies. Obstet Gynecol Surv 2010;65(9):575–582. [DOI] [PubMed] [Google Scholar]
- 92.Ertas IE, Taskin S, Goklu R, et al. Long-term oncological and reproductive outcomes of fertility-sparing cytoreductive surgery in females aged 25 years and younger with malignant ovarian germ cell tumors. J Obstet Gynaecol Res 2014;40(3):797–805. [DOI] [PubMed] [Google Scholar]
- 93.Sarwar A, Brook OR, Vaidya A, et al. Clinical Outcomes following Percutaneous Radiofrequency Ablation of Unilateral Aldosterone-Producing Adenoma: Comparison with Adrenalectomy. J Vasc Interv Radiol 2016;27(7):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu YF, Cao C, Shi YB, et al. Computed tomography-guided cryoablation for functional adrenal aldosteronoma. Minim Invasive Ther Allied Technol 2019:1–5. [DOI] [PubMed] [Google Scholar]
- 95.Lim SS, Hutchison SK, Van Ryswyk E, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2019;3(3):Cd007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fitzgerald C, Elstein M, Spona J. Effect of age on the response of the hypothalamo-pituitary-ovarian axis to a combined oral contraceptive. Fertil Steril 1999;71(6):1079–1084. [DOI] [PubMed] [Google Scholar]
- 98.Nightingale AL, Lawrenson RA, Simpson EL, et al. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Reprod Health Care 2000;5(4):265–274. [DOI] [PubMed] [Google Scholar]
- 99.Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep 2016;65(3):1–103. [DOI] [PubMed] [Google Scholar]
- 100.Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: Antiandrogens for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008;93(4):1153–1160. [DOI] [PubMed] [Google Scholar]
- 101.Al-Khawajah MM. Finasteride for hirsutism: a dose finding study. Saudi Med J 1998;19(1):19–21. [PubMed] [Google Scholar]
- 102.Fertig RM, Gamret AC, Darwin E, et al. Sexual side effects of 5-α-reductase inhibitors finasteride and dutasteride: A comprehensive review. Dermatol Online J 2017;23(11). [PubMed] [Google Scholar]
- 103.Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. Journal of the European Academy of Dermatology and Venereology 2018;32(12):2112–2125. [DOI] [PubMed] [Google Scholar]
- 104.Muhn P, Fuhrmann U, Fritzemeier KH, et al. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci 1995;761:311–335. [DOI] [PubMed] [Google Scholar]
- 105.Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. Bmj 2013;347:f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van der Spuy ZM, le Roux PA. Cyproterone acetate for hirsutism. Cochrane Database Syst Rev 2003(4):Cd001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Afifi L, Maranda EL, Zarei M, et al. Low-level laser therapy as a treatment for androgenetic alopecia. Lasers Surg Med 2017;49(1):27–39. [DOI] [PubMed] [Google Scholar]
- 108.Unger WP, Unger RH. Hair transplanting: an important but often forgotten treatment for female pattern hair loss. J Am Acad Dermatol 2003;49(5):853–860. [DOI] [PubMed] [Google Scholar]
- 109.Giordano S, Romeo M, Lankinen P. Platelet-rich plasma for androgenetic alopecia: Does it work? Evidence from meta analysis. J Cosmet Dermatol 2017;16(3):374–381. [DOI] [PubMed] [Google Scholar]
- 110.Frank-Raue K, Junga G, Raue F, et al. [Therapy of hirsutism in females with adrenal enzyme defects of steroid hormone biosynthesis: comparison of dexamethasone with cyproterone acetate]. Klin Wochenschr 1990;68(12):597–601. [DOI] [PubMed] [Google Scholar]
- 111.Heiner JS, Greendale GA, Kawakami AK, et al. Comparison of a gonadotropin-releasing hormone agonist and a low dose oral contraceptive given alone or together in the treatment of hirsutism. J Clin Endocrinol Metab 1995;80(12):3412–3418. [DOI] [PubMed] [Google Scholar]
- 112.Dawood MY, Ramos J, Khan-Dawood FS. Depot leuprolide acetate versus danazol for treatment of pelvic endometriosis: changes in vertebral bone mass and serum estradiol and calcitonin. Fertil Steril 1995;63(6):1177–1183. [DOI] [PubMed] [Google Scholar]
- 113.van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for hirsutism (excluding laser and photoepilation therapy alone). Cochrane Database Syst Rev 2015;2015(4):Cd010334. [DOI] [PMC free article] [PubMed] [Google Scholar]