Abstract
The spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has triggered a global effort to rapidly develop and deploy effective and safe coronavirus disease 2019 (COVID-19) vaccinations. Vaccination has been one of the most effective medical interventions in human history, although potential safety risks of novel vaccines must be monitored, identified, and quantified. Adverse events must be carefully assessed to define whether they are causally associated with vaccination or coincidence. Neurologic adverse events following immunizations are overall rare but with significant morbidity and mortality when they occur. Here, we review neurologic conditions seen in the context of prior vaccinations and the current data to date on select COVID-19 vaccines including mRNA vaccines and the adenovirus-vector COVID-19 vaccines, ChAdOx1 nCOV-19 (AstraZeneca) and Ad26.COV2.S Johnson & Johnson (Janssen/J&J).
Introduction
The spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has triggered a massive global effort in vaccine development. Over 200 vaccines have entered preclinical development since the beginning of the pandemic, of which at least 40 have entered clinical trials.1 Two mRNA vaccines and the Ad26.COV2.S Johnson & Johnson (Janssen/J&J) vaccine are now approved by the US Food and Drug Administration (FDA) in the United States, with several others in the pipeline. The need to rapidly develop vaccines for SARS-CoV-2 comes at a time when scientific innovation and technology is enabling a new era of rapid vaccine development, shortening a process that previously stretched over 10–15 years to several months.
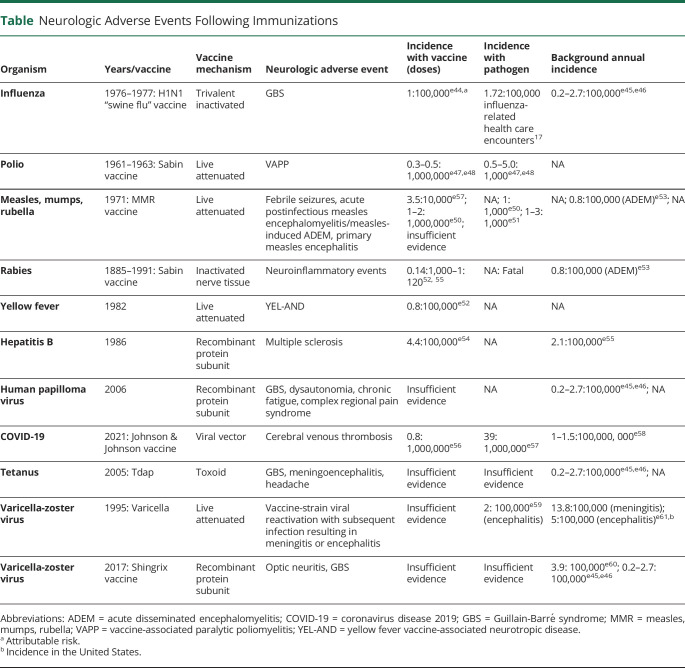
Vaccination has been one of the most effective public health and medical interventions in human history. An essential part of developing any vaccine is to ensure safety risks are monitored, identified rapidly, and quantified. Causality between suspected adverse events (AEs) and vaccines are first evaluated during phase 2 and 3 randomized controlled trials to determine whether the vaccine group has a higher incidence of AEs than the placebo group. After the vaccine is released, phase 4 observational studies are continually performed to evaluate whether rare AEs are due to the vaccine or would have occurred in the absence of the vaccine. Surveillance systems are utilized in postmarketing safety monitoring as well. When any suspected AEs are reported, a causal relationship must be assessed through temporal association, biological plausibility, and experimental evidence.2 Neurologic AEs following immunizations are overall rare, though with significant morbidity and mortality when they occur3 (Table).
Table.
Neurologic Adverse Events Following Immunizations
Here, we review past neurologic events in the context of prior vaccine programs. Current trial safety data with regards to neurologic events with approved coronavirus disease 2019 (COVID-19) vaccines in the United States are also discussed.
Historical Aspects of Vaccine Safety: Neurologic Conditions
Vaccinations have been the most successful tool against infectious diseases, although overall vaccine hesitancy and perception of risk have limited the success of some disease eradication measures. While the advent of national vaccine campaigns coincided with a fall in the percentage of childhood deaths from infection, from 61.6% in 1900 to 2% in 1998,4 there was much public skepticism with traditional whole or partial organism-based vaccines. For example, vaccine-associated paralytic poliomyelitis (VAPP) is a known side effect of the oral polio vaccine (OPV), occurring in about 1 in 2.4 million doses.5 Newer platforms of subunit, recombinant polysaccharide, and conjugate vaccines are believed to be much safer. Most recently, novel nucleic acid–based vaccines, including mRNA vaccines used against COVID-19,6 have emerged using targeted mechanisms that often do not even require use of adjuvants.7,8 Vaccine safety remains a primary concern to the general public during the COVID-19 vaccinations campaigns now occurring worldwide. Evaluating whether there may be a causal association between vaccinations and neurologic conditions, rather than isolated temporal events, is critical to addressing public perception of vaccine risk.
Influenza Vaccinations
Influenza vaccination is crucial for controlling seasonal influenza spread and preventing possible pandemics. The first inactivated influenza vaccine was developed in 1940 by Dr. Thomas Francis, Jr. and Dr. Jonas Salk as a monovalent vaccine, containing inactivated influenza A virus.9 The vaccine has continued to evolve with the discovery of additional influenza viruses, resulting in the current inactivated vaccine, a quadrivalent formulation containing 4 inactivated strains of influenza.9
Guillain-Barré syndrome (GBS) was first reported as an adverse effect of the influenza vaccine in 1976 during a rapid mass vaccination effort in the United States in response to the novel A/NJ/76 (Hsw1N1) influenza virus emergence at a military base in New Jersey.10 Approximately 43 million people were vaccinated in a 3-month period through the National Influenza Immunization Program until the program's suspension in December 1976. Several cases of GBS were identified through Centers for Disease Control and Prevention (CDC) vaccine safety surveillance.10,11 Nationwide surveillance identified GBS in approximately 1 in 100,000 vaccine recipients and found that the risk of GBS among vaccinated individuals was 7.3 times the risk of that in nonvaccinated individuals.12,13 The baseline incidence of GBS is estimated to be approximately 1.7/100,0000 individuals. The period of 5 weeks after vaccination was identified as a period of increased risk, though cases of GBS were reported rarely up to 9–10 weeks after vaccination.12 Since this time, multiple studies internationally have sought to identify an association between the seasonal influenza vaccine and GBS, with conflicting evidence. Some studies have shown higher GBS incidence with pandemic rather than seasonal vaccines, while others have found no association, citing multiple confounding variables.13,14 A prospective study in Quebec with the influenza A (H1N1) vaccine found a relative risk of 3.02 of all confirmed cases and of 2.33 for cases, with the number of cases attributed to vaccination being approximately 2 per 1,000,000 doses.15 A 2015 systematic review and meta-analysis of 39 studies between 1981 and 2014 reported the overall relative risk of GBS from any influenza vaccine was 1.41, with pandemic vaccines having a higher relative risk of 1.84 as compared to seasonal vaccines with risk of 1.22, ultimately concluding there was an association between GBS and influenza vaccine.16 The pathogenesis for the development of GBS after influenza vaccination is thought to be due to antigenic cross-activity that triggers antibody production against human neuronal cells.14 Most importantly perhaps is the evidence from multiple studies that influenza infection has a stronger association with GBS than vaccines.17,18
Narcolepsy was reported in association with the European H1N1 influenza vaccination campaign in 2009–2010.19 Pandemrix was the most widely distributed of the 8 influenza vaccines, with over 30.5 million doses administered.20 Surveillance of vaccine-related AEs was notable for an increase in narcolepsy predominantly reported in children and adolescents a few months after initiation of Pandemrix vaccination.19,20 The Swedish vaccine safety agency reported 87 verified cases of narcolepsy with cataplexy in patients age 19 and younger and found the relative risk of narcolepsy after vaccination with Pandemrix was 6.6 compared to that of unvaccinated individuals.21 In 2012, The Finnish vaccine safety agency investigated 71 new diagnoses of narcolepsy in patients ages 4–19 and found a 12.7-fold increased risk of narcolepsy within 8 months after receiving the Pandemrix vaccine as compared to unvaccinated individuals of the same age.20 A 2017 systematic review and meta-analysis further examined the association between Pandemrix and narcolepsy in all age groups and determined that the relative risk of narcolepsy was 5- to 14-fold higher in children and adolescents, with an absolute risk of about 3 cases of narcolepsy in 100,000 vaccinated adolescents/children.22 The proposed mechanism of the effect was a higher prevalence of haplotype HLA-DQB1*0602 in Northern European countries, which is associated with narcolepsy. In patients with this haplotype and thus predisposition for narcolepsy, antibodies from the vaccine cross-reacted with both influenza nucleoprotein and hypocretin receptor 2, associated with wakefulness.23,24 Multicountry population-based cohort studies have since explored the risk of narcolepsy associated with other H1N1 pandemic influenza vaccines as well as seasonal influenza vaccines and have found no evidence of association.25,26
Poliomyelitis Vaccinations
Poliomyelitis cases have been successfully reduced >99.9% worldwide, with only 1 serotype remaining in 2 countries.27 This has been accomplished through the introduction of the inactivated vaccine by Salk in 1955, the live, attenuated oral vaccine by Sabin in 1961, and the formation of the Global Polio Eradication Initiative in 1988.28 Not including a technical failure to completely inactivate the Salk vaccine in 1955 at Cutter Laboratories, no neurologic adverse reactions have been conclusively and solely linked to the inactivated polio vaccine.28 There have been individual case reports of acute disseminated encephalomyelitis (ADEM), encephalopathy, anti-NMDA receptor encephalitis, and optic neuritis following the inactivated polio vaccine, but these cases have involved combination vaccines and while temporally associated with their administration, case reports are considered the lowest level of evidence-based medicine.28-33
A well-documented risk of the OPV is the development of VAPP.28 VAPP results from the oral vaccine strain returning to a neurovirulent strain as it replicates in the intestinal tract.34 This risk is highest in adults and immunocompromised individuals, particularly those with B-cell disorders.35 Those in close contact with individuals who receive the OPV can also be at risk for contracting VAPP as the virus is excreted through stool for up to 6 weeks, when it can revert to its infective form.36 It then has the potential to cause community outbreaks as a neurovirulent circulating vaccine-derived poliovirus (cVDPV).28,34 cVDPVs are similar to wild-type poliovirus, most common in regions with low immunity, and have the potential to cause paralytic polio.37 The United States has exclusively used the inactive polio vaccine since 2000 and the only known cases of cVDPVs have been nonparalytic and associated with an undervaccinated community exposed to an immunodeficient individual who had received the OPV outside the United States.38,39 Some individuals with B-cell disorders can become chronically infected and develop immunodeficiency-associated vaccine-derived polioviruses, which can be paralytic or lead to meningoencephalitis.40 In 1994, a causal relationship between the OPV and GBS was hypothesized, but not established, by the Institute of Medicine of the National Academy of Sciences in Washington, DC, due to biologic plausibility.2
Measles, Mumps, Rubella Vaccine
The live attenuated measles, mumps, rubella (MMR) vaccine has been previously and incorrectly linked to autism in a now widely debunked 1998 report.39,41 Case-control, cohort, and meta-analyses of epidemiologic studies have consistently shown that there is no association between the MMR vaccine and autism, leading the Institute of Medicine to conclude in 2004, and again in 2011, that no link exists.41-43 Despite this evidence, speculation and fear about an association between MMR vaccination and autism persists.
Aseptic meningitis had historically been reported following MMR vaccination.44 Retrospective studies and systematic reviews have supported an association between Leningrad 3 and Urabe strain–containing MMR and aseptic meningitis, but not with the Jeryl Lynn mumps strain, which is now commonly used and the only available strain in the United States.44,45
Systematic reviews have supported a likely association between the MMR vaccine and febrile seizures, often self-limiting events, in children the first few weeks after vaccination.45 This risk appears limited to children younger than 2 years following their first MMR vaccination.46 Notably, there is no association between the MMR vaccine and the development of epilepsy.47
There was a case report of an immunocompromised child who developed measles inclusion body encephalitis following MMR vaccination.48 RT-PCR and sequencing revealed the measles strain isolated in the brain was identical to the vaccine strain. There have been concerns regarding an association between the MMR vaccine and encephalitis or encephalopathy, but further evaluation and studies show no evidence of such a link.44 This has led the Institute of Medicine to conclude in 2011 that there is inadequate evidence of a causal relationship between the MMR vaccine and encephalitis or encephalopathy.43 Neither MMR nor other vaccines have been linked to late development of AEs, akin to the encephalitis that may develop following a natural measles infection.
Rabies Vaccination
Rabies is a preventable disease with a fatality rate of almost 100%.49 The development of an inactivated vaccine for preexposure prophylaxis in high-risk individuals including veterinarians and postexposure prophylaxis in individuals with possible exposure has significantly reduced the incidence of rabies and associated mortality in humans.50 Multiple preparations of the vaccine exist with varying safety profiles: (1) nerve tissue vaccines; (2) embryonated egg-based vaccines; and (3) cell culture vaccines.
The first rabies vaccine contained crude preparations of animal nerve tissue, developed in 1885 by Dr. Louis Pasteur and later modified by Dr. David Semple in 1911.51 In 1889, a case of postvaccine nerve paralysis was reported, followed by a high volume of case reports of postvaccination meningoencephalomyelitis, mononeuritis multiplex affecting multiple cranial nerves, dorsolumbar transverse myelitis, and GBS.52-54 The reported incidence of neurologic complications following these nerve tissue vaccines reported in the literature varies significantly, from 0.14 per 1,000 cases to 1 per 120 cases per treatment.52,54,55 A center in Thailand published multiple retrospective cohort studies of patients admitted with neurologic AEs after receiving the Semple vaccine to better characterize these events and mechanisms.52,56 One study published in 1987 reviewed 61 patients who experienced complications after receiving the Semple vaccine from 1984 to 1985: 25 only had minor AEs such as headache, while the other 36 had major AEs within 6–14 days of the first injection, including 6 patients with meningoencephalomyelitis, 2 patients with meningoencephalitis, 7 patients with myelitis, 13 patients with meningitis, and 4 patients with GBS.52 All 36 with major AEs were found to have elevated levels of both serum and CSF antibody to myelin basic protein compared to the 25 patients with minor AEs and 39 other patients with no complications after immunization, though the study lacked appropriate controls.52,56 This study and others clarified that the mechanism of neurologic injury observed after the Semple vaccine is most likely mediated by antibodies such as anti–myelin basic protein that form in response to exogenous nerve tissue.
In the 1950s, new vaccine strains were introduced containing purified duck and chick embryos, and a further paradigm shift occurred in the 1970s with the development of cell culture rabies vaccines derived from human diploid cell cultures.49,57 These newer vaccines had favorable safety and efficacy, with incidence of reported neurologic complications following these vaccines respectively of 0.03 per 1,000 for embryonic egg-based vaccines and 0.01 per 1,000 for cell culture vaccines.52,58,59 In 1984, the WHO recommended replacing all nerve tissue vaccines with embryonated egg-based and cell culture vaccines.51 High-income countries quickly discontinued production of nerve tissue vaccines, but several low-income countries throughout Africa and Asia continued to use nerve tissue vaccines due to availability and relative ease of production. In 2004, the WHO Expert Consultation issued a statement that all nerve tissue vaccine production must be discontinued, and by 2015 all countries phased out the nerve tissue vaccine.60
Yellow Fever
The live attenuated yellow fever vaccine is highly effective and systematic reviews suggest the vaccine provides lifelong immunity.e1 However, some cases may call for booster doses of the vaccine to be administered. This includes those who were pregnant when first immunized, those who have received a stem cell transplant in the interim, and individuals traveling to high-risk settings who received the vaccine more than 10 years ago. Historically, the French neurotropic yellow fever vaccine strain was reportedly associated with encephalitis after a temporal association was seen with an increase in cases in children following a mass vaccination campaign in Nigeria in the 1950s.e2,e3 A more recently reported neurologic AE is the development of yellow fever vaccine-associated neurotropic disease (YEL-AND), for which older age is a risk factor.e4 YEL-AND includes GBS, ADEM, or meningoencephalitis that occurs within a month of vaccination with yellow fever virus or antibodies found in the CSF.e5,e7-e10 Incidence is reported at 0.4–0.8 per 100,000 vaccinations and it is more common in men, individuals younger than 6 months or older than 60 years, and people with an underlying immunocompromised state.e1,e6
Other Vaccines
The hepatitis B vaccine has been under scrutiny for possible association with multiple sclerosis (MS) since 1998, but most reported cases occurred in a population genetically predisposed to MS and the number of reported cases (1,100/25,000,000) does not exceed the prevalence of MS in the unvaccinated population (about 5/100,000).e11 Thus, current epidemiologic data do not support this proposed association. Though the human papillomavirus (HPV) vaccine has been linked to GBS, dysautonomia, chronic fatigue, and complex regional pain syndrome cases, in 2018 the American Autonomic Society conducted a detailed literature review including safety surveillance data and released a statement reporting no causal relationship between HPV vaccination and these conditions.e12 The Shingrix herpes zoster vaccine has had a few case reports of postvaccination optic neuritis and GBS, but lack of robust data precludes the ability to determine a causal relationship.e13
Overview of Current COVID-19 Vaccine Candidates
The main SARS-CoV-2 vaccine delivery systems include mRNA-based vaccines, DNA-based vaccines, viral vector vaccines, and protein subunit vaccines. There are currently 9 vaccines in phase III or IV trials. Most COVID-19 vaccines being developed contain copies of the spike surface protein, a unique feature of the SARS-CoV-2 virus.e14
mRNA-Based Vaccines
Moderna/National Institute for Allergy and Infectious Diseases developed a candidate vaccine mRNA-1273 that encodes the SARS-CoV-2 spike glycoprotein.e15 The phase 3, randomized placebo-controlled trial published in December 2020 enrolled 30,420 volunteers. Exclusion categories relevant to patients with underlying neurologic conditions included those in an “immunosuppressive or immunodeficient state, those who had received systemic immunosuppressants or immune-modifying drugs for >14 days in total within 6 months prior to screening (for corticosteroids ≥20 milligram [mg]/day of prednisone equivalent), or those who had received systemic immunoglobulins or blood products within 3 months prior to the day of screening.” Exclusion of these participants likely relates to concern with efficacy of the vaccine and monitoring, rather than a safety concern with this and other nonlive vaccine candidates. The study population consisted of 24.8% who were 65 years of age or older. There are no published data on underlying neurologic conditions in the cohort. The most common treatment-related AEs (those reported in at least 1% of participants) in the placebo group and the mRNA-1273 group included headache (0.9% and 1.4%). Bell palsy was reported in the vaccine group (3 participants [<0.1%]) and the placebo group (1 participant [<0.1%]) during the observation period of the trial (through day 759).e15 Of note, the baseline incidence of Bell palsy is ∼2.3/10,000, so these events do not exceed what we would expect in the population.
BioNTech together with Pfizer developed an mRNA-based vaccine candidate, BNT162b2, which includes a modified mRNA and a t2 fibritin-derived trimerization domain to enhance immune response.e16 In the phase 2/3 multinational trial, individuals over 16 years of age were randomly assigned to receive 2 doses, 21 days apart, of either placebo or the BNT162b2 vaccine candidate (30 μg per dose). Key exclusion criteria included under 16 years of age, a medical history of COVID-19, treatment with immunosuppressive therapy (prior to trial or planned receipt during the trial, “including cytotoxic agents or systemic corticosteroids, e.g., for cancer or an autoimmune disease”), receipt of immunoglobulin 60 days prior to or planned receipt during the study, or diagnosis with an immunocompromising condition (including HIV or any “known or suspected immunodeficiency as determined by history, labs/physical exam”).e17 Between July 27, 2020, and November 14, 2020, 43,448 participants were randomized, with 21,720 receiving BNT162b2 and 21,728 receiving placebo. The median age was 52 years, 42% of participants were older than 55 years, and overall, 21% of the cohort had at least 1 coexisting condition. Underlying neurologic conditions noted included dementia (7 in experimental arm, 11 in placebo arm), cerebrovascular disease (195 participants in experimental arm, 166 in placebo arm), and “hemiplegia or paraplegia” (13 in experimental arm, 21 in placebo arm). In terms of reactogenicity, the most commonly reported systemic events included headache (52% among younger vaccine recipients, 39% among older recipients after the second dose). Bell palsy was reported by 4 participants in the vaccine group and none in the placebo group, occurring at days 3, 9, 37, and 48 after vaccination. Rates were consistent with the expected background rate in the general population. Of the 4 related serious AEs reported among BNT162b2 recipients, 1 had reported neurologic symptoms of “right leg paresthesias,” but no further information on evaluation and etiology of paresthesias is given in the study.
Nonreplicating Viral Vector Vaccines
Oxford-AstraZeneca's vaccine ChAdOx1 nCoV-19 is an adenovirus vector vaccine containing the gene for the spike protein.e18 Efficacy and safety were compared to a control of either meningococcal vaccine or saline.e18,e19 Phase III interim results were initially published in early December 2020 and included participants 18 years and older.e20 An interim analysis of the ChAdOx1 nCoV-19 vaccine included data from 4 ongoing randomized trials in the United Kingdom, South Africa, and Brazil. A total of 23,848 participants were enrolled at the time of the interim report. Primary efficacy was assessed in participants who received 2 doses of the vaccine. All 4 studies included participants who received 2 doses. Key exclusion criteria relevant to individuals with neurologic conditions included “administration of immunoglobulins and/or any blood products within the 3 months preceding the planned administration of the vaccine candidate, confirmed or suspected immunosuppressive or immunodeficient state and use of immunosuppressant medication within the past 6 months, except topical steroids or short-term oral steroids (course lasting <14 days), any autoimmune conditions not requiring immunosuppressive or immunomodulatory therapy, and chronic neurologic illness excluding migraine.”e18 Safety data were based on 74,341 person-months. Serious neurologic side effects were noted in 2 participants: (1) transverse myelitis occurring 14 days after booster dose and (2) transverse myelitis occurring 10 days after initial dose in a participant who was later assessed and diagnosed with MS. A third case of transverse myelitis occurred in a participant in the control group who had received meningococcal vaccine 68 days previously. There were 6 cases of facial paralysis noted: 3 in the vaccine arm and 3 in the control arm. Several other nervous system AEs were reported in both the experimental and control arm (n = 64, 0.5% in the experimental arm; n = 79, 0.7% in the control arm); those only reported in the experimental arm or reported at a higher rate than in the control arm (all <0.1%). There was no evidence of causality from the vaccine and timing of the AEs was not available. The neurologic events reported only in the control arm are not included here. Public health surveillance has identified cases of thromboses, including cerebral venous thrombosis (CVT), after the ChAdOx1 nCoV-19 vaccine.e21 Thrombotic events have been associated with low platelet counts with events resembling autoimmune heparin-induced thrombocytopenia (aHIT). Many individuals have been positive for anti–platelet factor 4 (PF4) antibodies, as in aHIT.e22 Recent updates have reported 309 cases of thrombosis in Europe, with approximately 40% of cases having evidence of CVT.e23-e25 The majority of cases reported were in women younger than 60 years, which led some countries to restrict the age range for administration of the ChAdOx1 nCoV-19 vaccine.e24 Overall risk is thought to be very rare, especially in the context of the significant risk of thrombotic events in the context of acute COVID-19 itself.
Ad26.COV2.S Johnson & Johnson (Janssen/J&J)
The Ad26.COV2.S vaccine is a human adenovirus vector vaccine encoding the SARS-CoV-2 spike protein.e26-e28 In the interim analysis of the phase 3 data, 19,630 participants received Ad26.COV2.S and 19,691 received placebo. Ad26.COV2.S protected against moderate to severe critical COVID-19 “with onset at least 14 days after administration (116 cases in the vaccine group vs 348 in the placebo group; efficacy, 66.9%; adjusted 95% CI, 59.0–73.4) and at least 28 days after administration (66 vs 193 cases; efficacy, 66.1%; adjusted 95% CI, 55.0–74.8).”e26 In the vaccine group, the most common systemic reactions were headache (in 38.9%), fatigue (in 38.2%), and myalgia (in 33.2%). Venous thromboembolic events (11 in the vaccine group vs 3 in the placebo group), seizure (which occurred in 4 participants in the vaccine group vs 1 in the placebo group), and tinnitus (in 6 vs 0) were among the serious AEs thought to be related to vaccination. There was 1 participant who developed GBS in the active vaccine arm and 1 participant developing GBS in the placebo arm.e29,e30 In postmarketing surveillance, additional GBS cases were identified by FDA medical officers’ daily review of incoming serious AE reports and automated query of the Vaccine Adverse Event Reporting System (VAERS) for AE terms for GBS. As of June 30, 2021, preliminary data from the US CDC identified 100 GBS cases, with 95 patients hospitalized, 10 patients intubated, and 1 death.e31 Sixty-one (61%) were male, mean age 53.6 years (12.46), with median time of onset 13 days (range 0–75 days) after vaccination, with 98 cases in a 42-day window after vaccination. With 98 cases of GBS in a 42-day risk window from 12,235,978 vaccine doses given, the risk ratio of GBS based on these preliminary reports is 5.010 (95% confidence interval 4.07, 6.11). Twenty-four reports described bilateral facial palsy, 12 unilateral Bell palsy, and 6 mentioned a recent illness including generalized rash, upper respiratory infection, or flu-like symptoms 1–2 weeks before GBS. On July 12, 2021, authorized emergency use authorization fact sheets by the FDA were updated to include new information about GBS.e32 Ongoing analysis of cases is being conducted as well as further surveillance to define possible causal association with vaccination. The annual incidence of GBS prior to the COVID-19 pandemic is estimated to be 1.7/100,000.e33
In the clinical trials, there was also 1 patient with a venous thrombotic event who also had a CVT event, in a 25-year-old man with transverse sinus thrombosis. The clinical trial assessment was that the CVT “most likely resulted from multiple pre-disposing factors including preexisting cerebral sigmoid sinus stenosis and an infection with an unknown organism that started 8 days following vaccination, triggering inflammation and a hypercoagulable state.” Importantly, thrombocytopenia was also observed and subsequent testing identified anti-PF4 antibodies at the time of the event. In postmarketing surveillance, further cases of thromboses with thrombocytopenia have been identified. In mid-April, the CDC recommended a pause in the use of the vaccine, in the context of 6 reports of CVT.e34,e35 The pause was later lifted after thorough safety review. As of May 7, the CDC reported a total of 19 or 28 thrombosis with thrombocytopenia syndrome cases with CVT. Among the 28 cases of thrombosis with thrombocytopenia syndrome, median age was 40 years (range 18–59 years) and 22 (79%) cases were in women.e36 Many individuals have multiple sites of thromboses.e37 The cases closely resembled those described after ChAdOx1 nCOV-19 vaccination with presence of thrombocytopenia and positive anti-PF4 antibodies.
Other viral vector vaccines utilizing adenoviruses include the Ad5-nCoV vaccine (sponsored by CanSino Biologics)e38 and rAd26 and rAd5 vector-based Gam-COVID-Vac (sponsored by Sputnik V).e39,e40 No current reports exist with regards to venous thromboses including CVT with these other adenovirus vector vaccinations though surveillance systems are regionally variable. Vector-based vaccines utilizing inactivated SARS-CoV-2 are also in trial, including Coronovac (sponsored by Sinovac Biotech),e41 which uses an aluminum hydroxide adjuvant, and inactivated COVID-19 (sponsored by Sinopharm).e42 A vector-based trial is underway utilizing TICE Bacille Calmette-Guérin (Merck), which contains live attenuated Mycobacterium bovis.e43
Discussion
In addition to clinical trial data, monitoring of AEs following COVID-19 immunizations in the postmarketing period is an essential strategy for ensuring safety. Historical examples of neurologic conditions in the context of vaccines emphasize the critical need for ongoing and robust pharmacovigilance for investigating possible AEs following immunization. Reports of possible AEs often serve as a major influence on public opinion on vaccine safety, and thus, it is a scientific obligation to perform thorough investigations. The CDC has expanded safety surveillance in the United States on COVID-19 vaccines through a multipronged approach including passive and enhanced-passive surveillance methodologies, new systems including v-safe, and additional information sources, and scaling up existing safety monitoring systems. The importance of monitoring for possible neurologic events in those with and without underlying neurologic conditions is essential, with collection of detailed epidemiologic and clinical data to define whether a causal relationship is plausible.
Glossary
- ADEM
acute disseminated encephalomyelitis
- AE
adverse event
- aHIT
autoimmune heparin-induced thrombocytopenia
- CDC
Centers for Disease Control and Prevention
- COVID-19
coronavirus disease 2019
- cVDPV
circulating vaccine-derived poliovirus
- CVT
cerebral venous thrombosis
- FDA
Food and Drug Administration
- GBS
Guillain-Barré syndrome
- HPV
human papillomavirus
- MMR
measles, mumps, rubella
- MS
multiple sclerosis
- OPV
oral polio vaccine
- PF4
platelet factor 4
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- VAPP
vaccine-associated paralytic poliomyelitis
- YEL-AND
yellow fever vaccine-associated neurotropic disease
Appendix. Authors
Footnotes
Podcast: NPub.org/rc7ke4
COVID-19 Resources: NPub.org/COVID19
Study Funding
Kiran Thakur received grant support from NIH/NINDS 1K23NS105935-01 and the Centers for Disease Control and Prevention.
Disclosure
Dr. Thakur serves as a neurology consultant on the Clinical Immunization Safety Assessment CDC Project on COVID-19 vaccination safety. Go to Neurology.org/N for full disclosures.
References
- 1.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. [DOI] [PubMed] [Google Scholar]
- 2.Stratton KR, Howe CJ, Johnston RB Jr. Adverse events associated with childhood vaccines other than pertussis and rubella: summary of a report from the Institute of Medicine. JAMA. 1994;271(20):1602–1605. [PubMed] [Google Scholar]
- 3.WHO. Adverse events following immunization (AEFI). Accessed February 17, 2020. who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/aefi
- 4.Guyer B. Freedman MA, Strobino DM, Sondik EJ. Annual summary of vital statistics: trends in the health of Americans during the 20th century. Pediatrics. 2000;106(6):1307–1317. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Paralytic poliomyelitis: United States, 1980-1994. MMWR Morb Mortal Wkly Rep. 1997;46:79–83. [PubMed] [Google Scholar]
- 6.Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584–594. [DOI] [PubMed] [Google Scholar]
- 7.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines: a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianchecchi ETC, Piccirella S, Montomoli E. Evaluating influenza vaccines: progress and perspectives. Future Virol. 2016;11(5):379–393. [Google Scholar]
- 10.Babazadeh A, Javanian M, Mohammadnia-Afrouzi M, et al. Influenza vaccination and Guillain-Barré syndrome: reality or fear. J Translational Intern Med. 2019;7(4):137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States. Am J Epidemiol. 1979;110(2):105–123. [DOI] [PubMed] [Google Scholar]
- 13.Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333(1):43–82. [DOI] [PubMed] [Google Scholar]
- 14.Soni RHS, Wiltshire DA, et al. Antigenic variability a potential factor in assessing relationship between Guillain Barré syndrome and influenza vaccine: up to date literature review. Cureus. 2020;12(9):e10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Wals P, Deceuninck G, Toth E, et al. Risk of Guillain-Barre syndrome following H1N1 influenza vaccination in Quebec. JAMA. 2012;308(2):175–181. [DOI] [PubMed] [Google Scholar]
- 16.Martín Arias LH, Sáinz M, Treceño C, Carvajal A. Guillain-Barré syndrome and influenza vaccines: a meta-analysis. Vaccine. 2015;17:37733778. [DOI] [PubMed] [Google Scholar]
- 17.Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis. 2013;13(9):769–776. [DOI] [PubMed] [Google Scholar]
- 18.Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine. 2015;33(29):3288–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkanen TO, Alakuijala APE, Dauvilliers YA, et al. Incidence of narcolepsy after H1N1 influenza and vaccinations: systematic review and meta-analysis. Sleep Med Rev. 2018;38:177–186. [DOI] [PubMed] [Google Scholar]
- 20.Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLOS One. 2012;7(3):e33536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Läkemedelsverket. Medical Products Agency Occurrence of narcolepsy with cataplexy among children and adolescents in relation to the H1N1 pandemic and Pandemrix vaccinations – Results of a case inventory study by the MPA in Sweden during 2009 – 2010. Report no. 1. Sweden: 2011:1–20. Available at http://www.lakemedelsverket.se/upload/nyheter/2011/Fallinventeringsrapport_pandermrix_110630.pdf [Google Scholar]
- 22.Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLOS One. 2012;7(3):e33723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed SS, Volkmuth W, Duca J, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med. 2015;7(294):294ra105. [DOI] [PubMed] [Google Scholar]
- 24.Hallberg P, Smedje H, Eriksson N, et al. Pandemrix-induced narcolepsy is associated with genes related to immunity and neuronal survival. EBioMedicine. 2019;40:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy J, Weintraub E, Vellozzi C, DeStefano F. Narcolepsy and influenza A (H1N1) pandemic 2009 vaccination in the United States. Neurology. 2014;83(20):1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weibel D, Sturkenboom M, Black S, et al. Narcolepsy and adjuvanted pandemic influenza A (H1N1) 2009 vaccines: multi-country assessment. Vaccine. 2018;36(41):6202–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chard AN, Datta SD, Tallis G, et al. Progress toward polio eradication–worldwide, January 2018-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pliaka V, Kyriakopoulou Z, Markoulatos P. Risks associated with the use of live-attenuated vaccine poliovirus strains and the strategies for control and eradication of paralytic poliomyelitis. Expert Rev Vaccine. 2012;11(5):609–628. [DOI] [PubMed] [Google Scholar]
- 29.Mancini J, Chabrol B, Moulene E, Pinsard N. Relapsing acute encephalopathy: a complication of diphtheria-tetanus-poliomyelitis immunization in a young boy. Eur J Pediatr. 1996;155(2):136–138. [DOI] [PubMed] [Google Scholar]
- 30.Ozawa H, Noma S, Yoshida Y, Sekine H, Hashimoto T. Acute disseminated encephalomyelitis associated with poliomyelitis vaccine. Pediatr Neurol. 2000;23:177–179. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien P, Wong RW. Optic neuritis following diphtheria, tetanus, pertussis, and inactivated poliovirus combined vaccination: a case report. J Med Case Rep. 2018;12(1):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann C, Baur MO, Schroten H. Anti-NMDA receptor encephalitis after TdaP-IPV booster vaccination: cause or coincidence?. J Neurol. 2011;258(3):500–501. [DOI] [PubMed] [Google Scholar]
- 33.Stewart O, Chang B, Bradbury J. Simultaneous administration of hepatitis B and polio vaccines associated with bilateral optic neuritis. Br J Ophthalmol. 1999;83(10):1200–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minor P. Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine. 2009;27(20):2649–2652. [DOI] [PubMed] [Google Scholar]
- 35.Hamborsky J, Kroger A, Wolfe C. Epidemiology and Prevention of Vaccine-Preventable Diseases, 13th Edition E-Book. Public Health Foundation; 2015. [Google Scholar]
- 36.Alexander JP Jr, Gary HE Jr, Pallansch MA. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: a review of the literature. J Infect Dis. 1997;175(suppl 1):S176–S182. [DOI] [PubMed] [Google Scholar]
- 37.Alleman MM, Jorba J, Greene SA, et al. Update on vaccine-derived poliovirus outbreaks–worldwide, July 2019-February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(16):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prevots DR, Burr RK, Sutter RW, Murphy TV, Advisory Committee on Immunization Practices. Poliomyelitis prevention in the United States: updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-5):1–7. [PubMed] [Google Scholar]
- 39.Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637–641. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Ivanova O, Driss N, et al. Poliovirus excretion among persons with primary immune deficiency disorders: summary of a seven-country study series. J Infect Dis. 2014;210(suppl 1):S368–S372. [DOI] [PubMed] [Google Scholar]
- 41.Di Pietrantonj C, Rivetti A, Marchione P, Debalini MG, Demicheli V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst Rev. 2020;4:CD004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Institute of Medicine (US) Immunization Safety Review Committee. Immunization Safety Review: Vaccines and Autism. National Academies Press; 2004. [PubMed] [Google Scholar]
- 43.Committee to Review Adverse Effects of Vaccines. Adverse effects of vaccines: evidence and causality. In: Stratton K, Ford A, Rusch E, Clayton EW, eds. Adverse Effects of Vaccines: Evidence and Causality. National Academies Press; 2011. [PubMed] [Google Scholar]
- 44.Mäkelä A, Nuorti JP, Peltola H. Neurologic disorders after measles-mumps-rubella vaccination. Pediatrics. 2002;110(5):957–963. [DOI] [PubMed] [Google Scholar]
- 45.Demicheli V, Jefferson T, Rivetti A, Price D. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev. 2005;(4)CD004407. [DOI] [PubMed] [Google Scholar]
- 46.Ma SJ, Xiong YQ, Jiang LN, Chen Q. Risk of febrile seizure after measles-mumps-rubella-varicella vaccine: a systematic review and meta-analysis. Vaccine. 2015;33:3636–3649. [DOI] [PubMed] [Google Scholar]
- 47.Vestergaard M, Hviid A, Madsen KM, et al. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004;292(3):351–357. [DOI] [PubMed] [Google Scholar]
- 48.Bitnun A, Shannon P, Durward A, et al. Measles inclusion-body encephalitis caused by the vaccine strain of measles virus. Clin Infect Dis. 1999;29(4):855–861. [DOI] [PubMed] [Google Scholar]
- 49.Plotkin SKH, Rupprecht CE. Rabies vaccine. In: Vaccines. Saunders Elsevier; 2008:687–714. [Google Scholar]
- 50.Shah I. Acute demyelinating encephalomyelitis due to neural antirabies vaccine. J Trav Med. 2008;15(1):58–59. [DOI] [PubMed] [Google Scholar]
- 51.Hicks DJ, Fooks AR, Johnson N. Developments in rabies vaccines. Clin Exp Immunol. 2012;169(3):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemachudha T, Griffin DE, Giffels JJ, Johnson RT, Moser AB, Phanuphak P. Myelin basic protein as an encephalitogen in encephalomyelitis and polyneuritis following rabies vaccination. N Engl J Med. 1987;316(7):369–374. [DOI] [PubMed] [Google Scholar]
- 53.Redewill FH Jr, Underwood LJ. Neurological complications to treatment with rabies vaccine. Calif Med. 1947;66(6):360–363. [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. Information Sheet Observed Rate of Vaccine Reactions: Rabies Vaccine. World Health Organization; 2012. [Google Scholar]
- 55.Swaddiwuthipong W, Weniger BG, Wattanasri S, Warrell MJ. A high rate of neurological complications following Semple anti-rabies vaccine. Trans R Soc Trop Med Hyg. 1988;82(3):472–475. [DOI] [PubMed] [Google Scholar]
- 56.Hemachudha T, Phanuphak P, Johnson RT, et al. Neurologic complications of Semple‐type rabies vaccine clinical and immunologic studies. Neurology. 1987;37(4):550. [DOI] [PubMed] [Google Scholar]
- 57.Nogueira Y. Adverse effect versus quality control of the Fuenzalida-Palacios antirabies. Rev Inst Med Trop Sao Paulo. 1998;40(5):295–299. [DOI] [PubMed] [Google Scholar]
- 58.Briggs DJ, Banzhoff A, Nicolay U, et al. Antibody response of patients after postexposure rabies vaccination with small intradermal doses of purified chick embryo cell vaccine or purified Vero cell rabies vaccine. Bull World Health Organ. 2000;78(5):693–698. [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization. Grading of scientific evidence: table III: safety of cell-culture-based rabies vaccines. World Health Organization; 2010. [Google Scholar]
- 60.Gongal G, Sampath G. Introduction of intradermal rabies vaccination: a paradigm shift in improving post-exposure prophylaxis in Asia. Vaccine. 2019;37(suppl 1):A94–A98. [DOI] [PubMed] [Google Scholar]
- Additional references e1–e61 available at links.lww.com/WNL/B507