Abstract
Young adults are at high risk for suicide, yet there is limited ability to predict suicidal thoughts and behaviors. Machine learning approaches are better able to examine a large number of variables simultaneously to identify combinations of factors associated with suicidal thoughts and behaviors. The current study used LASSO regression to investigate extent to which a number of demographic, psychiatric, behavioral, and functional neuroimaging variables are associated with suicidal thoughts and behaviors during young adulthood. 78 treatment seeking young adults (ages 18–25) completed demographic, psychiatric, behavioral, and suicidality measures. Participants also completed an implicit emotion regulation functional neuroimaging paradigm. Report of recent suicidal thoughts and behaviors served as the dependent variable. Five variables were identified by the LASSO regression: Two were demographic variables (age and level of education), two were psychiatric variables (depression and general psychiatric distress), and one was a neuroimaging variable (left amygdala activity during sad faces). Amygdala function was significantly associated with suicidal thoughts and behaviors above and beyond the other factors. Findings inform the study of suicidal thoughts and behaviors among treatment seeking young adults, and also highlight the importance of investigating neurobiological markers.
Keywords: machine learning, amygdala, implicit emotion regulation, suicide prediction
1. Introduction
Suicide is a serious global health issue, accounting for more than half of all violent deaths (World Health Organization, 2014). Young adults are at especially high risk; suicide is the second leading cause of death worldwide among those between the ages of 18–25 (World Health Organization, 2014). The majority of studies identifying risk for suicidal thoughts and behaviors to date have focused on single factors in isolation (Franklin et al., 2016). There is a need for more complex models that simultaneously include a range of risk factors across multiple levels of analyses, so that many sources of available information can be considered (Nock, 2016). Least Absolute Shrinkage and Selection Operator (LASSO) regression is a machine learning statistical technique that allows for the testing of a large number of potential variables relative to the number of study participants. LASSO regression has been commonly used in genetic studies (Kohannim et al., 2012; Luo et al., 2015; Zemmour et al., 2015), and has more recently been applied to psychiatric research and fMRI studies (Bertocci et al., 2016; Bertocci et al., 2017; Ramsay et al., 2018). In particular, Bertocci and colleagues used LASSO regression in two novel studies to examine which combinations of neural, demographic, and clinical measures are associated with mental health outcomes in psychiatrically unwell youth. In the current study, we extended the use of this approach to investigate extent to which a number of relevant demographic, psychiatric, behavioral, and functional neuroimaging factors are associated with a measure of recent suicidal thoughts and behaviors during young adulthood.
The current study included several functional neuroimaging variables associated with emotion regulation. Researchers posit that poor emotion regulation abilities contributes to risk for suicidal thoughts and behaviors (Nock et al., 2008c). Moreover, many individuals report uncontrollable emotional distress as an antecedent to suicidal ideation and attempts. Previous studies suggest that suicidal individuals may exhibit increased activation in affective salience regions (e.g., insula, ACC) and limbic regions (e.g., amygdala, thalamus) during a range of emotionally evocative tasks. However, most studies of suicidality have not measured neural function during tasks that are designed specifically to tap into emotion regulation abilities. Phillips et al. (2003a, b) developed a neural model of emotion regulation that distinguishes between emotion regulation that is voluntary and requires effortful regulation of emotions of which the individual is consciously aware, and emotion regulation that is automatic and an individual to successfully perform a task when emotional aspects exert their influence in a more implicit way. Miller and colleagues (2018) found that adolescents with a lifetime history of suicidal ideation exhibited greater activation in the dlPFC while engaging in effortful regulation of negative emotions during an explicit emotion regulation task. In one other recent study, Chase and colleagues (2019) used the emotional dynamic faces implicit emotion regulation task requiring adolescents to identify a color flash while presented with changing emotional faces that are task irrelevant. The authors found that adolescents with a history of suicide attempts exhibited lower activation in the dlPFC compared to healthy controls, but no difference in neural activation compared to depressed adolescents without a suicide attempt history. However, more studies are needed to investigate potential neural markers of emotion dysregulation in young adulthood when risk for suicide attempts peaks. The current study examined neural function during the emotional dynamic faces implicit emotion regulation task to investigate potential neural markers of suicidal thoughts and behaviors. We investigated neural function in a range of a priori defined cortical and subcortical brain regions during the implicit emotion regulation task implicated in emotion processing and associated with suicidal thoughts and behaviors. Specifically, we included key emotion processing related regions, such as the amygdala, thalamus, cingulate cortex, insula, and frontal regions (Phillips et al., 2008), that also have linked to suicidal thoughts and behavior (Schmaal et al., 2020). In addition, recent neuroimaging evidence suggests that altered function within parietal regions to social-emotional stimuli, such as the somatosensory cortex and supramarginal gyrus, may also be associated with suicidality (Harms et al., 2019; Olié et al., 2017; Pan et al., 2013). Thus, we included these regions in our analyses.
We considered other factors along with neural correlates of suicidal thoughts and behaviors within the LASSO regression. Given that suicidal thoughts and behaviors are associated with a number of psychiatric conditions, including depressive, anxiety, bipolar, and impulse-control disorders (Nock et al., 2008b), we simultaneously investigated a range of psychiatric symptoms and behaviors associated with these disorders (e.g., depressive, anxiety, manic, impulsive behaviors, psychological distress), as well as multiple demographic factors, and association with suicidal thoughts and behaviors. We also examined the relative proportion of suicidal thoughts and behaviors accounted for by functional neuroimaging and other variables. We investigated these factors in a sample of non-medicated, young adults seeking mental health care for variety of emotion problems or life stressors irrespective of psychiatric diagnosis.
2. Method
Participants
84 young adults ages 18–25 years old (70% female) seeking help from mental health counseling or psychiatric services for psychological distress, such as behavioral and emotional problems, coping with stressors, or interpersonal relationships, were recruited for this study. Participants were recruited via community advertisement, student counseling services, and a participant registry. Exclusion criteria for the study were: history of head injury, neurological, pervasive developmental disorder or systemic medical disease, cognitive impairment (Mini-Mental State Examination score < 24 (Crum et al., 1993), and premorbid North American Adult Reading Test (Johnstone et al., 1996), IQ estimate < 85, visual disturbance that is < 20/40 Snellen visual acuity (Holladay, 1997), left or mixed handedness using Annett’s criteria (Dragovic and Hammond, 2007), alcohol/substance use disorder (SUD, including nicotine) and/or illicit substance use (except cannabis) over the last 3 months determined by the Structured Clinical Interview for DSM-5 (SCID; First et al., 2015) and current use tested with urine and saliva tests. We did not exclude for lifetime/present cannabis use (non-SUD levels), given its common usage in 18–25 year-olds. Additional MRI exclusion criteria included positive pregnancy test/report for female participants, ferromagnetic objects in the body, and any current psychotropic medication use for > 2 weeks in distressed individuals. We did not exclude for previous medication use, but individuals needed to be free from such medication for at least 3 months. 3 participants did not complete the neuroimaging scan, 2 participants were removed for excessive movement during the scan, and 1 participant was removed due to incomplete questionnaire data. Thus, a total of 78 participants were included in analyses for this study. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human participants were approved by the University of Pittsburgh Institutional Review Board, approval number 19040176. Written informed consent was obtained from all participants.
Clinical and Behavioral Assessments
Participants were asked about basic demographic information (gender, age, and education). Education scores ranged from 1 (less than 7th grade education) to 8 (graduate or professional degree). Participants were also assessed on the following psychiatric and behavioral measures: Clinician-administered Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959), Hamilton Rating Scale for Depression (HAM-D; Hamilton, 1960), Mood and Anxiety Symptom Questionnaire (MASQ; Watson and Clark, 1991) Barratt Impulsiveness Scale (BIS-11; Attentional Impulsiveness, Motor Impulsiveness, and Nonplanning Impulsiveness scales; Patton et al., 1995), UPPS-P Impulsive Behavior Scale (Negative Urgency, Lack of Premeditation, Lack of Perseverance, Sensation Seeking, and Positive Urgency scales; Lynam et al., 2006), Young Mania Rating Scale (Young et al., 2000), and Kessler Psychological Distress Scale (K10; Kessler et al., 2002). In addition, the 6 item suicidality subscale of the short form of the Mood Spectrum Self-Report questionnaire (MOODS-SR; Fagiolini et al., 1999) was administered to participants and measured suicidal thoughts and behaviors. The MOODS-SR inquires whether participants experienced in the last month a period when he/she a) felt like life was not worth living, b) hoped to die, c) wanted to die, d) made suicide plans, e) committed a suicide attempt, and f) whether medical attention was required after an attempt. Participants answer in a yes/no dichotomous format and possible scores range from 0–6.
Neuroimaging Paradigm
Participants completed the dynamic faces task (12 minutes 45 secs), which has been previously described in detail (Perlman et al., 2012). Briefly, stimuli were grayscale emotional faces (happy, angry, fearful, and sad) taken from the NimStim face database, and grayscale ovals (matched in luminance with the face stimuli) that served as control stimuli. The task included three, 12-trial blocks for each emotional face type and six 6-trial blocks of shapes presented pseudorandomly. During the face trials, a face changed in emotional expression from neutral to emotional over 1 second in 5% increments. During shape trials, an oval shape changed in size to parallel the changes in the face trials. In the middle of each trial (200 to 6500 ms), a semitransparent foreground color flash (blue, orange or yellow) overlaid the image. Participants identified the color of the foreground color flash using a response pad.
Neuroimaging Data Acquisition and Processing
Functional neuroimaging data for the majority of participants (N=53) were collected using a 3.0 Tesla Siemens Trio 2 MRI scanner in the Magnetic Resonance Research Center (MRRC) at the University of Pittsburgh Medical Center. The remaining 25 participants were scanned on the 3T Siemens Magnetom Prisma system. For each participant, a total of 504 blood-oxygenation-level-dependent (BOLD) images were acquired with a simultaneous multi-slice (SMS) gradient echo EPI sequence (18 slices, SMS factor= 3, TR= 1500, TE= 30 ms, Field of View (FOV)= 220 × 220 mm, matrix = 64 × 64, Flip Angle= 55°, Bandwidth= 1860 Hz/Px). In addition, we acquired structural 3D axial MPRAGE images on the Trio/Prisma scanners (TR= 1500/1520 ms, TE= 3.19/3.17 ms, Flip Angle 8°, FOV= 256 × 256 mm, 1 mm isotropic voxels, 176 continuous slices) and fieldmaps for the Trio/Prisma (TR= 500/554 ms, TE1= 4.92/4.08 ms, TE2= 7.38/6.54 ms, FOV= 220 × 220 mm, matrix = 64 × 64, Flip Angle= 45/60°, Bandwidth= 1302/1532 Hz/Px).
Data were preprocessed using a combination of software packages (SPM, FSL, AFNI) implemented in Nipype (Gorgolewski et al., 2011). Preprocessing included realignment, coregistration, distortion correction, normalization, despiking, and smoothing. A first-level fixed-effect general linear model (GLM) was constructed for each participant using Statistical Parametric Mapping software, Version-8 (SPM8), with the four emotions (anger, fear, sad, happy) and shape conditions. Motion parameters were included as covariates of no interest to control for participant movement. Participants were removed from analyses when movement was excessive (greater than 4mm translation or 5° rotation). A regressor to correct for physiological fluctuations was also included, derived from the mean signal within white matter, CSF and high temporal standard deviation voxels (Fournier et al., 2014). A high pass filter (256 seconds), and autoregressive AR(1) modeling were also implemented at the first level.
Extraction of Neural Measures
A first-level single subject statistical parametric map was generated for each emotion>shape (fear> shape; happy>shape; sad>shape; anger>shape) contrast and used in second-level (group) analysis to identify neural activity across participants during face emotion processing. We extracted the first eigenvariate from the entire individual contrast images for all significant clusters in a priori regions implicated in both emotion processing and suicide risk (anatomically defined using Automated Anatomical Labeling atlas; (Tzourio-Mazoyer et al., 2002). We included bilateral amygdala, thalamus, anterior and midcingulate regions, insula, front regions (including superior, middle, and inferior areas) and relevant parietal regions, including the secondary somatosensory cortex/(rolandic operculum; Hu et al., 2015) and supramarginal gyrus (see Table 1).
Table 1.
Means and Standard Deviation for Variables included in LASSO Regression
| Demographic and Clinical/Behavioral Variables | M (SD) | fMRI Variables | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| - | Region | Angry | Fear | Sad | Happy | |
| Gender | M (SD) | M (SD) | M (SD) | M (SD) | ||
| Education | 5.13 (1.04) | R. Insula | −.07 (.29) | −.06 (.24) | −.02 (.22) | −.04 (.24) |
| Age | 21.66 (2.18) | L. Insula | −.06 (.22) | −.01 (.21) | −.03 (.21) | −.03 (.21) |
| BIS-11 Attentional Impulsiveness | 17.96 (3.88) | R. Thalamus | −.01 (.23) | .00 (.20) | −.01 (.21) | .00 (.22) |
| BIS-11 Motor Impulsiveness | 21.72 (4.62) | L. Thalamus | −.01 (.23) | .02 (.22) | .00 (.21) | −.01 (.21) |
| BIS-11 Nonplanning Impulsiveness | 23.36 (5.06) | R. Rol. Oper. | −.07 (.27) | −.01 (.23) | −.02 (.24) | −.03 (.25) |
| HAM-A Anxiety | 12.49 (6.52) | L. Rol. Oper. | −.06 (.21) | .00 (.20) | −.04 (.22) | −.03 (.22) |
| HAM-D Depression | 15.49 (6.64) | R. SMG | −.12 (.33) | −.11 (.29) | −.03(.35) | −.03 (.29) |
| Kessler Psychological Distress (K10) | 27.26 (7.27) | L. SMG | −.08 (.28) | −.05 (.27) | −.03 (.31) | −.06 (.29) |
| MASQ Anhedonic Depression | 75.73 (14.37 | R. Med. CC | −.06 (.22) | .00 (.22) | −.01 (.20) | −.04 (.24) |
| MASQ Anxious Arousal | 29.69 (11.83) | L. Med. CC | −.05 (.23) | .03 (23) | .00 (.22) | −.04 (.23) |
| MASQ General Distress | 42.77 (11.49) | R. ACC | −.02 (.29) | .03 (.28) | −.01 (.31) | −.03 (.30) |
| UPPS-P Negative Urgency | 2.63 (.62) | L. ACC | .00 (.33) | .07 (.24) | .01 (.36) | −.01 (.38) |
| UPPS-P Lack of Premeditation | 1.92 (.52) | R. Amygdala | .12 (.25) | .08 (.23) | .09 (.26) | .09 (.24) |
| UPPS-P Lack of Perseverance | 2.28 (.52) | L. Amygdala | .15 (.25) | .12 (.26) | .09 (2.6) | .08 (.27) |
| UPPS-P Sensation Seeking | 2.68 (.70) | R. Mid. Front. | −.10 (.33) | −.10 (.32) | −.04 (.29) | −.04 (.32) |
| UPPS-P Positive Urgency | 1.85 (.70) | L. Mid. Front. | −.01(.31) | −.01 (.30) | −.03 (.28) | .01 (.32) |
| Young Mania Rating Scale | 3.03 (2.63) | R. Inf. Front (oper.) | −.02 (.31) | −.03 (.29) | −.01 (.33) | .05 (.36) |
| - | - | L. Inf. Front (oper.) | .01 (.27) | .00 (.32) | −.05 (.30) | .01 (.31) |
| - | - | R. Infer. Front (tri.) | .03 (.34) | .01 (.29) | .00 (.33) | .05 (.36) |
| - | - | L. Infer. Front (tri.) | .02 (.27) | .00 (.28) | −.03 (.30) | .01 (.34) |
| - | - | R Sup. Front. (med.) | .08 (.39) | .09 (.42) | .04 (.40) | .01 (.31) |
| - | - | L. Sup. Front. (med.) | .14 (.47) | .11 (50) | .04 (.49) | .07 (.41) |
| - | - | R Sup. Front. (med. orb.) | .03 (.29) | .07 (.28) | .04 (.32) | .01 (.41) |
| - | - | L. Sup. Front. (med. orb.) | .06 (.33) | .16 (.39) | .10 (.42) | .03 (.54) |
Note. BIS-11= Barratt Impulsiveness Scale; HAM-A = Hamilton Anxiety Rating Scale; HAM-D = Hamilton Rating Scale for Depression; MASQ = Mood and Anxiety Symptom Questionnaire; UPPS-P = UPPS-P Impulsive Behavior Scale; R = Right; L = Left; Rol. Oper. = Rolandic Operculum; SMG = Supramarginal Gyrus; Med. CC = Median Cingulate Cortex (midcingulate cortex); ACC = Anterior Cingulate Cortex; Mid. Front. = Middle Frontal Gyrus; Inf. Front. (oper.) = Inferior Frontal Gyrus, opercular part; Inf. Front. (tri.) = Inferior Frontal Gyrus, triangular part; Sup. Front. (med) = Superior Frontal Gyurs, medial part; Sup. Front. (med. orb.) = Superior Frontal Gyurs, medial orbital part.
Data Analytic Plan
Given that our outcome variable was a count variable and therefore did not show the properties of a normal distribution, a Poisson distribution was used to model the number of suicidality items endorsed using the MOODS questionnaire. In addition, given that we have a large number of variables (p= 114) relative to participants (n=78), we used a LASSO regression analysis for data selection and reduction using the GLMNET package in R (Friedman et al., 2014). LASSO is a modified form of least squares regression that regularizes complex models with a parameter that shrinks the coefficients toward zero, and eliminates unimportant terms. This method minimizes prediction error and enforces sparsity in the solution (Tibshirani, 1996). We used a k=10 fold cross validation to identify the optimal model that minimized the mean cross validated error.
A test statistic or p value for LASSO that has a simple and exact asymptotic null distribution has been proposed by Lockhart et al (2014). However it is not yet available for use with Poisson distributions. We thus calculated several other measures that are meaningful for data inference: 1) rate ratio (exponentiated coefficients) of the nonzero coefficients identified in the LASSO model; 2) R-square for variance explained by the model; 3) Wald Chi-Square from a Poisson Regression with five independent variables from the LASSO regression.
For our analysis, MOODS-SR suicide risk scores served as the dependent variable (DV), and independent variables were clinical and demographic variables acquired on scan-day and emotion processing neural activity described above. We included scanner effects (Trio or Prisma) in the model, given that not all participants used the same scanner.
3. Results
Approximately 70% of participants endorsed at least one suicidal thought or behavior related event on the MOODS-SR (M =2.19, SD=1.84). Descriptive information for independent variables included in LASSO regression are presented in Table 1.
Results from the LASSO regression showed that five factors together optimized the fit of the model using the minimum λ after cross validation. This minimum λ corresponds to the penalty at which the minimal mean squared error (MSE) is achieved (Friedman et al., 2014). Of these, two were demographic variables (age and level of education), two were psychiatric variables (Hamilton Rating Scale for Depression; HAM-D, and the Kessler Psychological Distress Scale; K10), and one was a neuroimaging variable (left amygdala activity during sad faces, see Table 2). Specifically, younger age and level of education, and higher levels of depression, psychiatric distress, and left amygdala activation, was associated with suicidal thoughts and behaviors.
Table 2.
Non-zero predictors of suicide risk events identified by LASSO regression.
| Predictor | LASSO coefficient | Rate Ratio | Wald Chi-Square | 95% Confidence Interval |
|---|---|---|---|---|
|
| ||||
| Age | −.004 | 1.00 | 1.68 | −.15 to .03 |
| Level of education | −.01 | .99 | 1.37 | −.33 to .08 |
| HAM-D Depression | .01 | 1.01 | 1.5 | −.01 to .05 |
| Kessler Psychological Distress (K10) | .002 | 1.00 | 1.3 | −.01 to .05 |
| L. amygdala to sad | .08 | 1.08 | 8.61* | .08 to .42 |
Note.
< .01
HAM-D = Hamilton Depression Rating Scale; Kessler distress scale (K10); L = Left.
The five factors identified by the LASSO regression were next entered into a Poisson regression for further inference testing in accordance with our Data Analytic Plan (Table 2). The five factors in total explained 21.2% of the variance in suicidal thoughts and behaviors. Demographic variables alone (age and education) explained 7% of the variance, and psychiatric variables (depression and Kessler psychiatric distress) explained 9.9% of the variance. Left amygdala function explained 9.3% of the variance, and was significantly associated with suicidal thoughts and behaviors in this regression above and beyond the other factors (p <.01).
4. Discussion
The current study used LASSO regression, a machine learning statistical technique, to help identify the combination of variables that is most strongly associated with recent suicidal thoughts and behaviors, among a large number of demographic, psychiatric, behavioral, and neuroimaging variables in a sample of unmedicated treatment-seeking young adults with a range of behavioral problems and psychiatric symptoms. Five factors were identified using this technique: two demographic variables (age and education level), two psychiatric variables (HAM-D17 depression and Kessler psychological distress scores), and one neuroimaging variable (neural function in the amygdala during emotion processing of sad faces). Follow up Poisson regression analyses showed that these five factors together explained 21.2% of the variance in suicidal thoughts and behaviors. Moreover, the neuroimaging predictor alone was associated with suicidal thoughts and behaviors above and beyond all other variables identified in the LASSO regression, and explained 9.3% of the variance (i.e. a little less than half the explained variance). Study findings pinpoint a combination of five key factors that may be strongly associated with suicidal thoughts and behaviors in young adults. Future longitudinal studies are needed to investigate the extent to which this combination of factors may improve the ability to detect risk for suicidal thoughts and behaviors. Findings also suggest that the consideration of neural factors, in combination with other factors, may be especially important to improving risk detection.
In particular, this study focused on neural function that may underlie emotion regulation dysfunction. We examined neural correlates during an implicit emotion regulation paradigm during which young adults were presented with task irrelevant emotional stimuli. Findings showed that heightened activation to sad faces in the left amygdala, a key region involved in the processing of emotional stimuli, was most strongly associated with suicidal thoughts and behaviors in this sample. We speculate that implicit emotion regulation tasks may be relevant for understanding neural correlates of automatic emotion regulation processes required for young adults to manage emotions while completing everyday tasks successfully, such as academic schoolwork or job-related activities. Young adults who experience suicidal thoughts and behaviors exhibit poor emotion regulation abilities and more intense negative emotions from day-to-day (Kovacs and George, 2020; Scott et al., 2015). Thus, amygdala activation during implicit emotion regulation may be a marker for poor emotional functioning while attending to everyday tasks and risk for suicidal thoughts and behaviors among young adults with emotional distress.
Our neuroimaging findings contribute to a burgeoning area of research suggesting that structural and functional alteration of the amygdala is linked with suicidal thoughts and behaviors (Johnston et al., 2017; Miller et al., 2018). Several prior studies highlight structural and functional abnormalities in the amygdala and suicide risk (Alarcón et al., 2019; Kang et al., 2017). Specifically, larger amygdala volumes and decreased amygdala-prefrontal connectivity, as well as heightened amygdala activation to negative emotional faces, has been shown to be associated with greater severity of suicide risk or suicidal behavior (Balcioglu and Kose, 2018; Johnston et al., 2017; Quevedo et al., 2016). The finding that amygdala function in the context of sad faces was most relevant to suicidal thoughts and behaviors is consistent with theory and research suggesting that the desire for suicide frequently emerges in response to sad emotions (Nock et al., 2009). Moreover, neuroimaging studies have previously identified abnormally elevated left amygdala activation to sad faces relative to other emotional faces, as an important marker of mood problems (de Almeida and Phillips, 2013).
Our findings showing that younger age and lower levels of education in this sample explained 7% of the variance also builds on prior studies examining demographic predictors of suicide risk (Nock et al., 2008a). Early adulthood generally has been identified as a high risk developmental period for suicide. Furthermore, our age finding (within our restricted young adulthood age range) is in line with recent studies suggesting that risk for suicide attempts may peak during the transition from late adolescence into early adulthood, and then decline over time as youth progress through adulthood (Cha et al., 2018). In addition, our finding that less education was an important factor associated with suicidal thoughts and behaviors is consistent with the World Health Organization World Mental Health Survey finding that lower educational attainment compared to same aged-peers among young adults is linked to suicidal thoughts and behaviors (Mortier et al., 2018). Together, findings highlight that the early period of young adulthood is a particularly high risk developmental phase, particularly for those who may have less college education compared to peers.
Finally, results identifying that non-specific psychological distress as measured by the Kessler 10 (K10) is associated with suicidal thoughts and behaviors is consistent with both past and recent theories of suicide that emphasize the role of intense emotional distress in the desire for suicide (Joiner Jr et al., 2005; Klonsky and May, 2015). Findings contribute to other evidence for the clinical utility of the Kessler K10 scale in screening for suicidal thoughts and behaviors (O’Connor et al., 2012), especially when considered in conjunction with additional factors identified in this study. Finally, in addition to general psychological distress, greater severity of depressive symptoms was a second clinical variable identified with the LASSO regression data analytic approach. Major depressive disorder (MDD) has been shown to be among the most prevalent lifetime disorders among suicidal individuals, and is consistent with evidence that mood disorders are one of the strongest predictors of suicidal thoughts and behaviors in youth and adults (Nock et al., 2008a; Nock et al., 2013). Overall, results are in line with commonly studied risk factors for suicide related outcomes (Nock et al., 2008a). However prior studies have typically investigated these factors in isolation, and thus our study advances this work by using a data driven approach to identify the combination of factors that are strongly associated with suicidal thoughts and behaviors in a sample of young adults.
Although this study is important in that it demonstrates the application of novel machine learning statistical techniques to the study of suicidal thoughts and behaviors, findings must be considered within the context of several limitations. First, although we were able to include a large number of risk factors in our LASSO regression, there are other potentially relevant factors associated with suicidal thoughts and behaviors in the literature that were not measured in this study, such as gender identity, same-sex attraction or behavior, or environmental factors (e.g., life stressors). Findings from machine learning approaches may vary depending on whatever factors were measured and included in the analyses. Thus, we have identified factors that were most strongly associated with suicidal thoughts and behaviors in the context of the set of factors and sample characteristics associated with this specific study. Of note, the study sample included more women than men. It is possible that the high proportion of women is the reason why sex was not identified as a major demographic factor related to suicidality in this study, despite well-established sex differences in suicidal ideation, behavior, and death (Nock et al., 2008b). Alternatively, sex may not be a meaningful correlate when considered along with a high number of other relevant factors across levels of analyses. Moreover, the sex difference in this sample is consistent with higher rates of females in populations seeking treatment (Oliver et al., 2005) and exhibiting suicidal ideation and behavior (Glenn et al., 2017), and is thus more representative of at risk populations within clinical outpatient settings.
In addition, LASSO regression is one of several machine learning approaches that could be used to identify combinations of factors associated with suicidal thoughts and behaviors, all of which have various strengths and limitations (Wolpert and Macready, 1997). The LASSO regression avoids overfitting and results in simpler, interpretable models. However, the tradeoff of this approach is that some relevant factors among a group of highly correlated variables may not be selected. It will be important for future studies to consider the advantages and disadvantages when applying various machine learning approaches to the understanding of suicide risk. Finally, this was a cross-sectional study that examined potential correlates of recent suicidal thoughts and behaviors at a single timepoint. Data driven, machine learning approaches, such as the LASSO regression, have the ability to examine a large number of variables and identify factors that may be strongly associated with suicidal thoughts and behaviors. However, an important next step in validating these findings is to more carefully and rigorously test identified factors in future studies. Thus, it will be imperative to examine whether these identified variables longitudinally predict suicidal thoughts and behaviors in independent samples.
In conclusion, findings from this study have identified several factors associated with suicidal thoughts and behaviors that have potential to inform future research on detection of suicide risk among treatment seeking young adults. Findings also highlight the importance of investigating neural markers of suicidal thoughts and behaviors. Specifically, this study identifies the amygdala as a potential region that should be further investigated as a target for future non-invasive brain-based interventions, such as transcranial direct current stimulation that can be used to alter amygdala function (indirectly via cortical areas; (Ironside et al., 2019) or real-time fMRI neurofeedback (Young et al., 2014).
Figure 1.
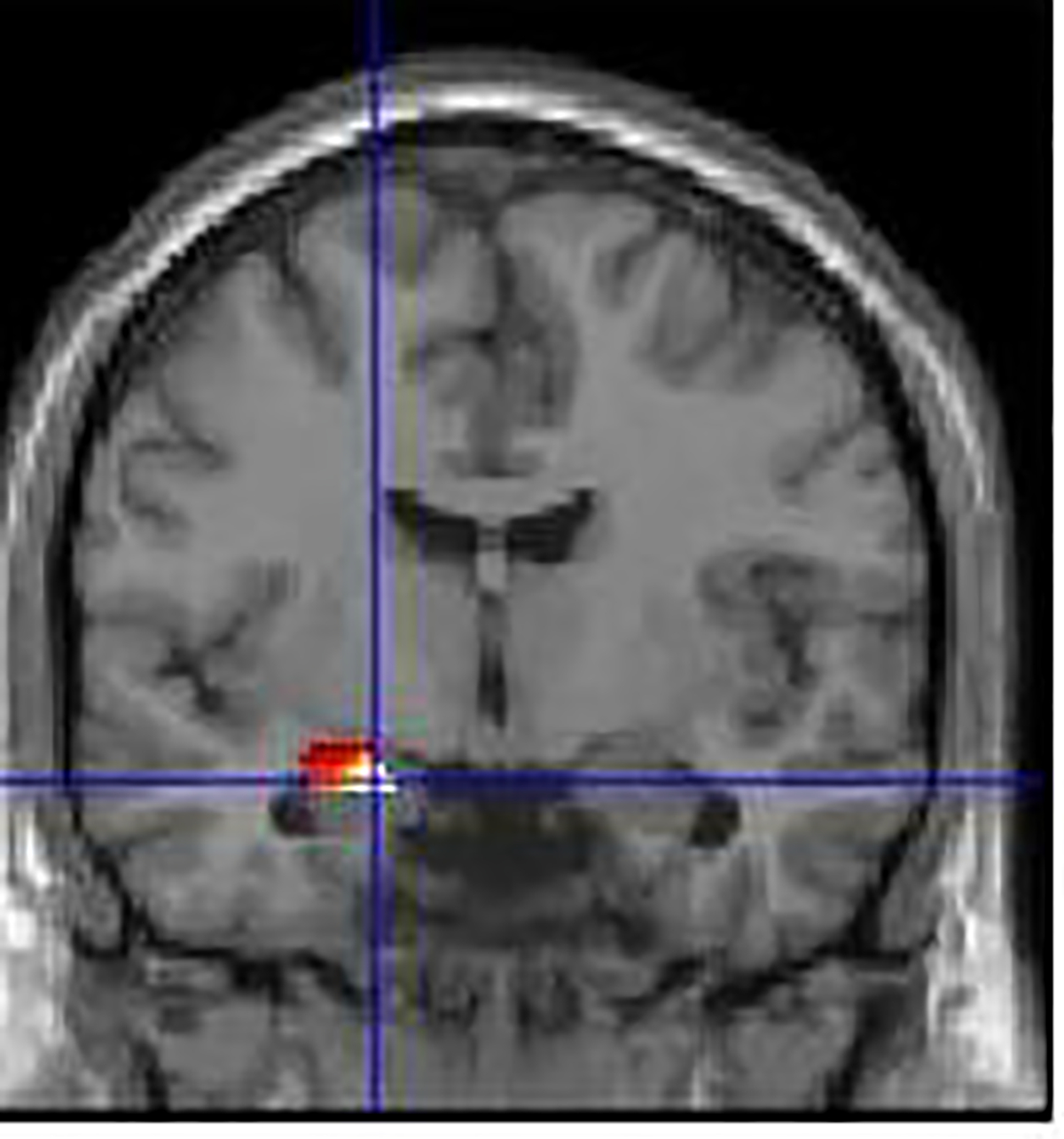
Blood-oxygen-level-dependent (BOLD) response to sad emotional faces in left amygdala identifed by LASSO regression predicting suicide risk.
HIGHLIGHTS.
A combination of 5 variables together across demographic, psychiatric, behavioral, and neural factors was associated with suicidal thoughts and behaviors among treatment-seeking young adults using a machine learning approach
Amygdala function during implicit emotion regulation was associated with suicidal thoughts and behaviors above and beyond the other 4 factors selected by the model
Acknowledgements
This project was supported by the National Insitute of Mental Health (MLP, R01MH100041; CWO, 5K01MH109850), and the National Instutes of Health (Clinical Translational Science Institute at the University of Pittsburgh, UL1 TR001857).
Footnotes
Conflict of Interest: All authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcón G, Sauder M, Teoh JY, Forbes EE, Quevedo K, 2019. Amygdala functional connectivity during self-face processing in depressed adolescents with recent suicide attempt. Journal of the American Academy of Child & Adolescent Psychiatry 58(2), 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcioglu YH, Kose S, 2018. Neural substrates of suicide and suicidal behaviour: from a neuroimaging perspective. Psychiatry and Clinical Psychopharmacology 28(3), 314–328. [Google Scholar]
- Bertocci MA, Bebko G, Versace A, Fournier JC, Iyengar S, Olino T, Bonar L, Almeida JR, Perlman SB, Schirda C, 2016. Predicting clinical outcome from reward circuitry function and white matter structure in behaviorally and emotionally dysregulated youth. Molecular psychiatry 21(9), 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocci MA, Bebko G, Versace A, Iyengar S, Bonar L, Forbes EE, Almeida JR, Perlman SB, Schirda C, Travis M, 2017. Reward-related neural activity and structure predict future substance use in dysregulated youth. Psychological medicine 47(8), 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha CB, Franz PJ, Guzmán M, E., Glenn CR, Kleiman EM, Nock MK, 2018. Annual Research Review: Suicide among youth–epidemiology,(potential) etiology, and treatment. Journal of child psychology and psychiatry 59(4), 460–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF, 1993. Population-based norms for the Mini-Mental State Examination by age and educational level. Jama 269(18), 2386–2391. [PubMed] [Google Scholar]
- de Almeida JRC, Phillips ML, 2013. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biological psychiatry 73(2), 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovic M, Hammond G, 2007. A classification of handedness using the Annett Hand Preference Questionnaire. British Journal of Psychology 98(3), 375–387. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Dell’Osso L, Pini S, Armani A, Bouanani S, Rucci P, Cassano GB, Endicott J, Maser JD, Katherine Shear M, 1999. Validity and reliability of a new instrument for assessing mood symptomatology: the Structured Clinical Interview for Mood Spectrum (SCI-MOODS). International Journal of Methods in Psychiatric Research 8(2), 71–82. [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL, 2015. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association, Arlington, VA. [Google Scholar]
- Fournier JC, Chase HW, Almeida J, Phillips ML, 2014. Model Specification and the Reliability of fMRI Results: Implications for Longitudinal Neuroimaging Studies in Psychiatry. Plos One 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, Musacchio KM, Jaroszewski AC, Chang BP, Nock MK, 2016. Risk Factors for Suicidal Thoughts and Behaviors: A Meta-Analysis of 50 Years of Research. Psychological Bulletin, No Pagination Specified. [DOI] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Simon N, Tibshirani R, 2014. GLMNET, 2.0–2 ed. CRAN. [Google Scholar]
- Glenn CR, Lanzillo EC, Esposito EC, Santee AC, Nock MK, Auerbach RP, 2017. Examining the course of suicidal and nonsuicidal self-injurious thoughts and behaviors in outpatient and inpatient adolescents. Journal of abnormal child psychology 45(5), 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS, 2011. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1959. The assessment of anxiety states by rating. Br J Med Psychol 32(1), 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MB, Casement MD, Teoh JY, Ruiz S, Scott H, Wedan R, Quevedo K, 2019. Adolescent suicide attempts and ideation are linked to brain function during peer interactions. Psychiatry Research: Neuroimaging 289, 1–9. [DOI] [PubMed] [Google Scholar]
- Holladay JT, 1997. Proper method for calculating average visual acuity. Journal of refractive surgery 13(4), 388–391. [DOI] [PubMed] [Google Scholar]
- Hu L, Zhang L, Chen R, Yu H, Li H, Mouraux A, 2015. The primary somatosensory cortex and the insula contribute differently to the processing of transient and sustained nociceptive and non-nociceptive somatosensory inputs. Human brain mapping 36(11), 4346–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside M, Browning M, Ansari TL, Harvey CJ, Sekyi-Djan MN, Bishop SJ, Harmer CJ, O’Shea J, 2019. Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: a randomized clinical trial. JAMA psychiatry 76(1), 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JA, Wang F, Liu J, Blond BN, Wallace A, Liu J, Spencer L, Cox Lippard ET, Purves KL, Landeros-Weisenberger A, 2017. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. American journal of psychiatry 174(7), 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Callahan CD, Kapila CJ, Bouman DE, 1996. The comparability of the WRAT-R reading test and NAART as estimates of premorbid intelligence in neurologically impaired patients. Archives of Clinical Neuropsychology 11(6), 513–519. [PubMed] [Google Scholar]
- Joiner TE Jr, Brown JS, Wingate LR, 2005. The psychology and neurobiology of suicidal behavior. Annu. Rev. Psychol. 56, 287–314. [DOI] [PubMed] [Google Scholar]
- Kang S-G, Na K-S, Choi J-W, Kim J-H, Son Y-D, Lee YJ, 2017. Resting-state functional connectivity of the amygdala in suicide attempters with major depressive disorder. Progress in neuro-psychopharmacology and biological psychiatry 77, 222–227. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand S-L, Walters EE, Zaslavsky AM, 2002. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychological medicine 32(6), 959–976. [DOI] [PubMed] [Google Scholar]
- Klonsky ED, May AM, 2015. The three-step theory (3ST): A new theory of suicide rooted in the “ideation-to-action” framework. International Journal of Cognitive Therapy 8(2), 114–129. [Google Scholar]
- Kohannim O, Hibar DP, Stein JL, Jahanshad N, Hua X, Rajagopalan P, Toga A, Jack CR Jr, Weiner MW, De Zubicaray GI, 2012. Discovery and replication of gene influences on brain structure using LASSO regression. Frontiers in neuroscience 6, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, George CJ, 2020. Maladaptive mood repair predicts suicidal behaviors among young adults with depression histories. Journal of affective disorders 265, 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, McShan D, Kong F, Schipper M, Haken RT, 2015. TH-AB-304-07: A Two-Stage Signature-Based Data Fusion Mechanism to Predict Radiation Pneumonitis in Patients with Non-Small-Cell Lung Cancer (NSCLC). Medical physics 42(6Part41), 3702–3702. [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, Cyders MA, 2006. The UPPS-P: Assessing five personality pathways to impulsive behavior. West Lafayette, IN: Purdue University. [Google Scholar]
- Miller AB, McLaughlin KA, Busso DS, Brueck S, Peverill M, Sheridan MA, 2018. Neural correlates of emotion regulation and adolescent suicidal ideation. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3(2), 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier P, Auerbach RP, Alonso J, Axinn WG, Cuijpers P, Ebert DD, Green JG, Hwang I, Kessler RC, Liu H, 2018. Suicidal thoughts and behaviors among college students and same-aged peers: results from the World Health Organization World Mental Health Surveys. Social psychiatry and psychiatric epidemiology 53(3), 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, 2016. Recent and needed advances in the understanding, prediction, and prevention of suicidal behavior. Depression and anxiety 33(6), 460–463. [DOI] [PubMed] [Google Scholar]
- Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, Bruffaerts R, Chiu WT, De Girolamo G, Gluzman S, 2008a. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. The British Journal of Psychiatry 192(2), 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S, 2008b. Suicide and suicidal behavior. Epidemiologic reviews 30(1), 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, Kessler RC, 2013. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA psychiatry 70(3), 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Prinstein MJ, Sterba SK, 2009. Revealing the form and function of self-injurious thoughts and behaviors: A real-time ecological assessment study among adolescents and young adults. Journal of abnormal psychology 118(4), 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Wedig MM, Holmberg EB, Hooley JM, 2008c. The emotion reactivity scale: development, evaluation, and relation to self-injurious thoughts and behaviors. Behavior therapy 39(2), 107–116. [DOI] [PubMed] [Google Scholar]
- O’Connor SS, Beebe TJ, Lineberry TW, Jobes DA, Conrad AK, 2012. The association between the Kessler 10 and suicidality: a cross-sectional analysis. Comprehensive psychiatry 53(1), 48–53. [DOI] [PubMed] [Google Scholar]
- Olié E, Jollant F, Deverdun J, de Champfleur NM, Cyprien F, Le Bars E, Mura T, Bonafé A, Courtet P, 2017. The experience of social exclusion in women with a history of suicidal acts: a neuroimaging study. Scientific reports 7(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver MI, Pearson N, Coe N, Gunnell D, 2005. Help-seeking behaviour in men and women with common mental health problems: cross-sectional study. The British Journal of Psychiatry 186(4), 297–301. [DOI] [PubMed] [Google Scholar]
- Organization, W.H., 2014. Preventing suicide: A global imperative. World Health Organization. [Google Scholar]
- Pan L, Hassel S, Segreti A, Nau S, Brent D, Phillips M, 2013. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychological medicine 43(10), 2129–2142. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology 51(6), 768–774. [DOI] [PubMed] [Google Scholar]
- Perlman SB, Almeida JR, Kronhaus DM, Versace A, LaBarbara EJ, Klein CR, Phillips ML, 2012. Amygdala activity and prefrontal cortex–amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar disorders 14(2), 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC, 2008. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry 13(9), 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Ng R, Scott H, Martin J, Smyda G, Keener M, Oppenheimer CW, 2016. The neurobiology of self-face recognition in depressed adolescents with low or high suicidality. Journal of abnormal psychology 125(8), 1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay IS, Ma S, Fisher M, Loewy RL, Ragland JD, Niendam T, Carter CS, Vinogradov S, 2018. Model selection and prediction of outcomes in recent onset schizophrenia patients who undergo cognitive training. Schizophrenia Research: Cognition 11, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, van Harmelen A-L, Chatzi V, Lippard ET, Toenders YJ, Averill LA, Mazure CM, Blumberg HP, 2020. Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Molecular psychiatry 25(2), 408–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LN, Pilkonis PA, Hipwell AE, Keenan K, Stepp SD, 2015. Non-suicidal self-injury and suicidal ideation as predictors of suicide attempts in adolescent girls: a multi-wave prospective study. Comprehensive psychiatry 58, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, 1991. The mood and anxiety symptom questionnaire (MASQ). Unpublished manuscript, University of Iowa, Iowa City. [Google Scholar]
- Wolpert DH, Macready WG, 1997. No free lunch theorems for optimization. IEEE transactions on evolutionary computation 1(1), 67–82. [Google Scholar]
- Young KD, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, Bodurka J, 2014. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PloS one 9(2), e88785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D, 2000. Young mania rating scale. Handbook of psychiatric measures, 540–542. [Google Scholar]
- Zemmour C, Bertucci F, Finetti P, Chetrit B, Birnbaum D, Filleron T, Boher J-M, 2015. Prediction of early breast cancer metastasis from DNA microarray data using high-dimensional cox regression models. Cancer informatics 14, CIN. S17284. [DOI] [PMC free article] [PubMed] [Google Scholar]


