Abstract
Background.
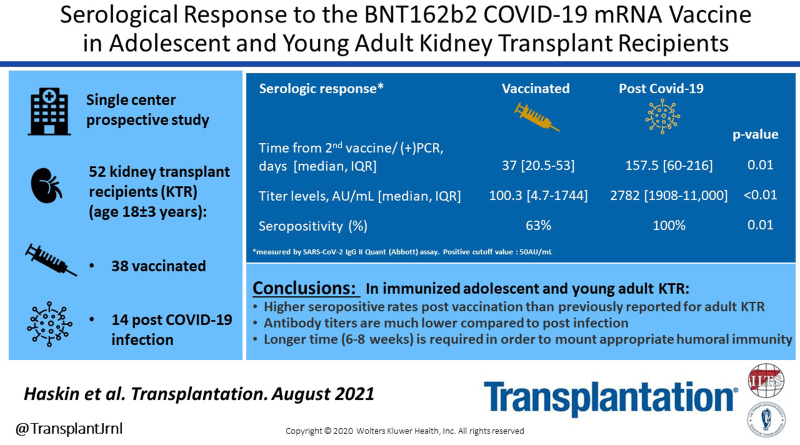
Initial reports in adult kidney transplant recipients (KTR) indicate low immunogenicity after 2 doses of the BNT162b2 COVID-19 mRNA vaccine. We describe the immunogenicity of this vaccine compared to the serologic response in naturally infected COVID-19 positive adolescent and young adult KTR.
Methods.
For this prospective observational study, the study group included 38 KTR who received 2 doses of the tested vaccine, and the control group included 14 KTR who had a previous polymerase chain reaction–confirmed COVID-19 infection.
Results.
The mean age was 18 ± 3 y. Positive serologic responses were observed in 63% and 100% of the study and control groups, respectively (P = 0.01). Antibody titers were almost 30-fold higher in the control than the study group (median [interquartile range (IQR)]: 2782 [1908–11 000] versus 100.3 [4.7–1744] AU/mL, P < 0.001), despite the longer time from the COVID-19 infection to serologic testing compared to time from vaccination (median [IQR]: 157.5 [60–216] versus 37 [20.5–53] d, P = 0.011). Among vaccinated patients, higher proportions of those seronegative than seropositive were previously treated with rituximab (50% versus 8%, P = 0.01). Time from the second vaccine dose to serologic testing was longer in seropositive than seronegative patients (median [IQR]: 24.5 [15–40] versus 46 [27–56] d, P = 0.05). No patient developed symptomatic COVID-19 disease postvaccination.
Conclusions.
The BNT162b2 COVID-19 mRNA vaccine yielded higher positive antibody response in adolescent and young adult KTR than previously reported for adult KTR. Antibody titers after vaccination were significantly lower than following COVID-19 infection. Longer time may be required to mount appropriate humoral immunity to vaccination in KTR.
INTRODUCTION
Currently, the approved available vaccines against severe acute respiratory coronavirus type 2 (SARS-CoV-2) infection include mRNA vaccines and adenovirus-based replication-incompetent vector vaccines. International recommended guidelines for coronavirus disease 2019 (COVID-19) vaccine distribution have given priority to immunocompromised patients including kidney transplant recipients (KTR).1,2 However, this population was not included in the original vaccine clinical trials.3,4 Humoral immunity against previously developed vaccines such as influenza or hepatitis B is decreased in solid organ transplant recipients.5,6 Initial reports indicate low immunogenicity of COVID-19 vaccines in adult KTR. Only 11%–17% of patients had detectable anti-spike antibodies 20–28 d after the first dose of COVID-19 vaccine,7,8 and 36%–59% developed detectable antibodies 28 d after the second dose.9-12 Predictors for an improved immune response among the adult transplant patients included: first allograft receipt versus multiple previous renal transplants, longer duration from transplantation, higher estimated glomerular filtration rate (eGFR), and an overall lower level of immunosuppression.9-12 COVID-19 infection has been reported after SARS-CoV-2 vaccination in solid organ transplant recipients with low or undetectable titers of anti-spike antibodies.13-15 This suggests that COVID-19 vaccinated but seronegative patients may be more vulnerable to future infection or disease.
Adolescents and young adults generally experience a less severe course of COVID-19 disease than do older individuals.16 Contrary to adults, the overall morbidity and mortality from COVID-19 infection in the pediatric kidney transplant population is low.17,18 Among healthy individuals, the antibody quantity and quality of the immunogenic response to COVID-19 infection were shown to be the lowest for the 19–24 y age group.19 Adolescents have lower SARS-CoV-2 immunoglobulin G (IgG) levels postinfection than do younger children or older adults.19 This lower immunogenic response, in addition to immunosuppression among KTR, raises concerns that adolescent KTR may have a lower humoral response to the vaccine.
On December 20, 2020, Israel launched a vaccination campaign with the BNT162b2 COVID-19 mRNA vaccine. The aim of our study was to describe the immunogenicity and safety profile of the Pfizer-BioNTech COVID-19 mRNA vaccine in adolescent and young adult KTR. Their immunologic response was compared to the serologic response observed in KTR who were naturally infected with COVID-19.
MATERIALS AND METHODS
Patients and Study Design
The study was approved by the institutional review board of Schneider Children’s Medical Center protocol number 0017-21-RMC. All patients or their legal guardians signed informed consent and assent.
For this prospective observational study, KTR were recruited between February 20, 2021, and June 6, 2021, at Schneider Children’s Medical Center, a tertiary-care pediatric hospital that houses a nephrology institute. The latter serves as a national, full-service referral center for children with kidney disease, including transplantation. The inclusion criterion for the study group was completion of the 2-dose regimen of the Pfizer-BioNTech COVID-19 vaccine according to the manufacturer’s recommended dose and time schedule (2 vaccine doses at a 3-wk interval). The inclusion criterion for the control group was a previous COVID-19 infection that was confirmed by nasopharyngeal swab real-time quantitative reverse transcription–polymerase chain reaction (PCR) authorized by the Israeli Ministry of Health. Swabs were obtained for COVID-19 infection that was suspected either from patient symptoms or contract history with a person who was infected with COVID-19. Patients were categorized according to the time from transplantation, the time from COVID-19 diagnosis or the second vaccination, and the absence or presence of serologic response to vaccination.
Immunogenicity was evaluated in the study and control groups by measuring COVID-19 antibody titer levels. The SARS-CoV-2 IgG II Quant assay (Abbott, Abbott Park, IL) was used for quantitative measurement of IgG antibodies against the spike protein of SARS-CoV-2. A test was considered positive if IgG was >50 antibody unit (AU)/mL.20 Patients who were vaccinated had their antibody levels checked at least 1 wk post second vaccine dose. COVID-19 serology before vaccination was not routinely obtained. Some patients were tested for COVID-19 IgG antibodies before study entry using a different indirect in-house enzyme-linked immunoassay (The Central Virology Laboratory of the Ministry of Health at Sheba Medical Center, Tel Hashomer, Israel). Results of these tests were recorded as well.
Safety was evaluated using a structured questionnaire that addressed side effects in study patients at their first clinic visit following the second vaccine. Additional tests included total IgG level, blood count, full chemistry, and tacrolimus trough levels.
Clinical Data
Demographic and clinical data were abstracted from electronic medical records, including age, gender, baseline kidney disease, time and type of transplant, and additional medical diagnoses. The dates of the first and second COVID-19 vaccines were recorded for the study group, and the date of the first positive PCR was recorded for those who were infected with COVID-19 disease. For each patient, anthropometric and clinical parameters including height, weight, body mass index, type and dose of current immunosuppression medications, previous immunosuppressive medications, additional medications, white blood cell count, serum creatinine, and trough tacrolimus drug levels were recorded from the clinic visit before the COVID-19 first vaccine or disease and at the visit when COVID-19 antibody titer levels were drawn. The eGFR was calculated using the 2009 modified Schwartz formula.21
According to our center’s clinical practice, the immunosuppression protocol in transplant patients consists of induction treatment with basiliximab, or anti-thymocyte globulin, depending on the patient’s immunologic risk. Maintenance immunosuppression consists of a triple-drug regimen with calcineurin inhibitor (mainly tacrolimus), mycophenolate mofetil, and steroids. Two patients in the study cohort received azathioprine instead of mycophenolate mofetil.
Statistical Analysis
Continuous variables are presented by means ± SD and by medians and interquartile ranges (IQRs) as appropriate, based on distribution. Categorical variables are presented using percentages. Continuous parameters were compared between groups using the 2-tailed independent t test and the Kruskal-Wallis test for parametric and nonparametric variables, respectively. Fisher’s exact test was used to compare categorical variables between groups. For comparison of patients at different time points, the 2-tailed t test for 2 dependent means was used. Multiple logistic regression analysis was used to assess the potential parameters associated with poor serologic response postvaccination. Data were analyzed using SAS statistical software (SAS, Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
Cohort Characteristics
Our center follows 100 KTR. Forty-one KTR who met the study’s inclusion criteria and received 2 doses of the Pfizer-BioNTech COVID-19 vaccine were eligible to participate in the study group. Of these, 38 were available for blood tests and comprised the study group. The control group included all KTR who were previously diagnosed with COVID-19 infection at our center (14 patients). One patient in the control group received 1 dose of vaccine 5.5 mo after a positive PCR test for COVID-19. The characteristics of the study and control groups are presented in Table 1. Mean age of the entire cohort was 18 ± 3 y (range 10–26.8). Vaccinated KTR were older than the control group (mean age 18.6 ± 2.8 versus 16.5 ± 3.1 y, P = 0.02). Otherwise, baseline characteristics did not differ between the groups.
TABLE 1.
Baseline characteristics and serologic response of the cohort
| Study group: vaccinated patients (n = 38) | Control group: COVID-19 infected (n = 14) | P | |
|---|---|---|---|
| Age, mean ± SD (y) | 18.6 ± 2.8 (range, 13.5–26.8) | 16.5 ± 3.1 (range, 10–20.6) | 0.02 |
| Males, n (%) | 25 (66) | 10 (71) | 1 |
| Background renal disease, n (%) | |||
| CAKUT/PUV | 10 (26) | 9 (69) | |
| Glomerulonephritis | 5 (13) | 1 (7) | |
| Nephrotic syndrome | 10 (26) | 3 (21) | |
| Nephronophthisis | 9 (24) | 0 | |
| Other | 4 (10.5) | 1 (7) | |
| Additional medical conditions, n (%) | |||
| Hypertension | 12 (32) | 1 (7) | |
| Lung disease | 4 (10.5) | 3 (21) | |
| Diabetes | 0 | 1 (7) | |
| Immunodeficiency | 2 (5) | 2 (14) | |
| Additional organ transplant | 2 (5) | 0 | |
| Obesity (BMI >95%) | 5 (13) | 2 (14) | |
| Second transplantation, n (%) | 4 (10.5) | 3 (21) | 0.37 |
| Type of transplantation, n (%) | |||
| Living related | 16 (42) | 5 (36) | |
| Living unrelated | 7 (18) | 4 (29) | |
| Deceased donor | 15 (40) | 5 (36) | |
| Baseline therapy/renal function | |||
| Tacrolimus through levels, mean ± SD (ng/mL) | 6.8 ± 2 | 6 ± 1.2a | 0.14 |
| Mycophenolate dose, mean ± SD (mg/m2/dose) | 359 ± 60b | 364 ± 61 | 0.79 |
| Previous rituximab treatment, n (%) | 9 (23.7) | 3 (21.4) | 1.00 |
| Indication for rituximab: | |||
| Treatment for rejection | 3 | 1 | |
| Treatment for PTLD | 1 | 0 | |
| Transplant preparation | 2 | 1 | |
| Glomerulonephritis | 3 | 1 | |
| eGFR, mean ± SD (mL/min/1.73m2) | 57.4 ± 22.1 | 62.7 ± 15.7 | 0.42 |
| Vaccination/infection data | |||
| Time from transplantation to vaccination/infection, mean ± SD (y) | 7.3 ± 5.6 | 7 ± 5.41 | 0.95 |
| Time from second vaccine dose/infection to serologic testing, median (IQR) (d) | 37 (20.5–53) | 157.5 (60–216) | <0.001 |
| COVID-19 IgG titers, median (IQR) (AU/mL) | 100.3 (4.7–1744) | 2782 (1908–11 000)c | 0.00081 |
aOne patient was treated with cyclosporine.
bTwo patients were treated with azathioprine.
cExcluding the patient who was vaccinated post–COVID-19 infection.
AU, antibody unit; BMI, body mass index; CAKUT, congenital anomalies of kidney and urinary tract; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; IgG, immunoglobulin G; IQR, interquartile range; PUV, posterior urethral valve; PTLD, posttransplant lymphoproliferative disorder.
Serological Response Among Vaccinated Patients
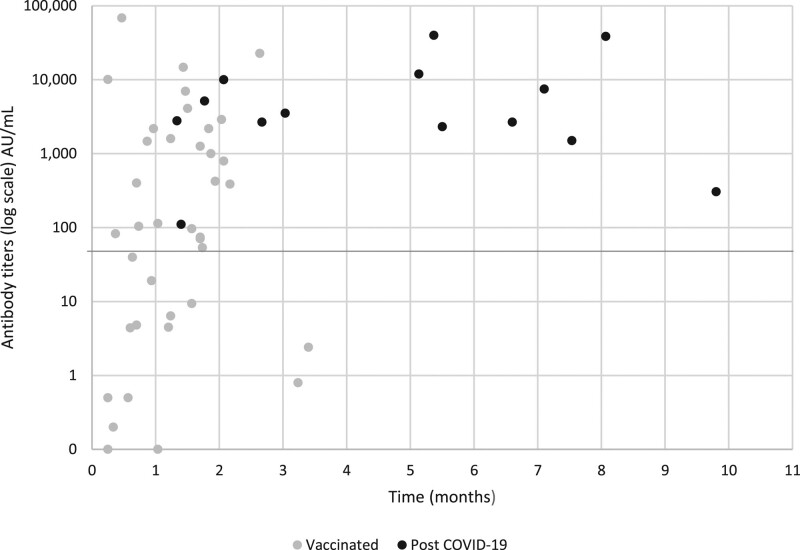
The majority of vaccinated KTR showed a positive serologic response (24 [63%]) at a median of 37 (IQR: 20.5–53) d after the second vaccine dose. Postvaccine antibody levels peaked at 1–2 mo after the second vaccine dose (Figure 1).
FIGURE 1.
COVID-19 antibody titer levels in kidney transplant recipients. Levels are shown for patients who were vaccinated (gray dots) and who were infected with COVID-19 (black dots), according to the time from the second vaccine dose or from a positive polymerase chain reaction result. The solid line represents the positive cutoff value of 50 AU/mL. AU, antibody unit; COVID-19, coronavirus disease 2019.
For 7 patients, serum antibody titers were checked after vaccination, before study entry. Three of these patients who were negative at a first test (at 15–25 d following their second vaccine) became positive on a second test (at 47–56 d following their second vaccine). Three patients were positive at the first test, 13–32 d after the second dose. One patient was negative before study entry (33 d following the second vaccine) and remained negative at 97 d after the second vaccine.
Table 2 compares patients who developed antibody levels above the designated cutoff point of 50 AU/mL postvaccination with patients who did not. More time elapsed from the second vaccine dose to serologic testing in patients who developed antibodies than in those who did not develop antibodies (median time [IQR]: 46 d [27–56] versus 24.5 d [15–40], P = 0.05).
TABLE 2.
Comparison between patients with negative vs positive serologic response to coronavirus disease 2019 vaccination, according to the cutoff point of 50 AU/mL
| Serologic response | Negative (n = 14) | Positive (n = 24) | P |
|---|---|---|---|
| Age, mean ± SD (y) | 17.9 ± 2.6 | 19.1 ± 2.9 | 0.21 |
| Males, n (%) | 7 (50) | 18 (75) | 0.16 |
| Type of transplantation | |||
| Second transplantation, n (%) | 3 (21) | 1 (4) | 0.13 |
| Multiple organ transplantations, n (%) | 0 | 2 (8)a | |
| Living related, n (%) | 6 (43) | 10 (42) | |
| Living unrelated, n (%) | 3 (21) | 4 (17) | |
| Deceased donor, n (%) | 5 (36) | 10 (42) | |
| Time from transplantation to serologic testing, mean ± SD (y) | 5.9 ± 5.2 | 8.2 ± 5.8 | 0.23 |
| Time from second vaccine to serologic testing, median (IQR) (d) | 24.5 (15–40) | 46 (27–56) | 0.05 |
| Prevaccine medications and labs | |||
| Tacrolimus trough levels, mean ± SD (ng/mL) | 7.4 ± 2 | 6.5 ± 1.9 | 0.17 |
| Mycophenolate dose, mean ± SD (mg/m2/dose) | 365 ± 69 | 324 ± 113b | 0.24 |
| Rituximab treatment, n (%) | 7 (50) | 2 (8) | 0.01 |
| ATG treatment, n (%) | 3 (21) | 2 (8) | 0.34 |
| eGFR, mean ± SD (mL/min/1.73m2) | 53.3 ± 28.5 | 59.8 ± 17.7 | 0.39 |
| eGFR <30 mL/min/1.73m2, n (%) | 4 (29) | 0 (0) | 0.014 |
| IgG levels, mean ± SD (mg/dL) | 877.5 ± 377 | 1078.9 ± 367.5c | 0.12 |
| WBC count, mean ± SD (K/mL) | 6.33 ± 1.31 | 7.09 ± 2.34 | 0.27 |
aOne patient with kidney and liver transplant and another with kidney and lung transplant.
bTwo patients were on azathioprine.
cIgG levels were obtained for 21 of 24 patients.
ATG, anti-thymocyte globulin; AU, antibody unit; eGFR, estimated glomerular filtration rate; IgG, immunoglobulin G; IQR, interquartile range; K, thousands; WBC, white blood cell.
As shown in Table 2, higher proportions of patients who did not develop antibodies had an eGFR <30 mL/min/1.73m2 (29% versus 0%, P = 0.014) and were previously treated with rituximab (50% versus 8%, P = 0.01). On multivariate logistic regression analysis, only the association with prior rituximab treatment remained statistically significant (odds ratio 0.08; 95% confidence interval, 0.013-0.48; P = 0.006). In KTR who received rituximab, median time from last rituximab infusion to first vaccination was 40 mo (IQR: 21–62) in the 7 seronegative patients and 12 and 62 mo in the 2 seropositive patients. None of the vaccinated patients developed symptomatic COVID-19 infection after a mean follow-up period of 142 ± 31 d from the first vaccine dose.
Comparison of the Serologic Response Between Vaccinated and COVID-19–positive Patients
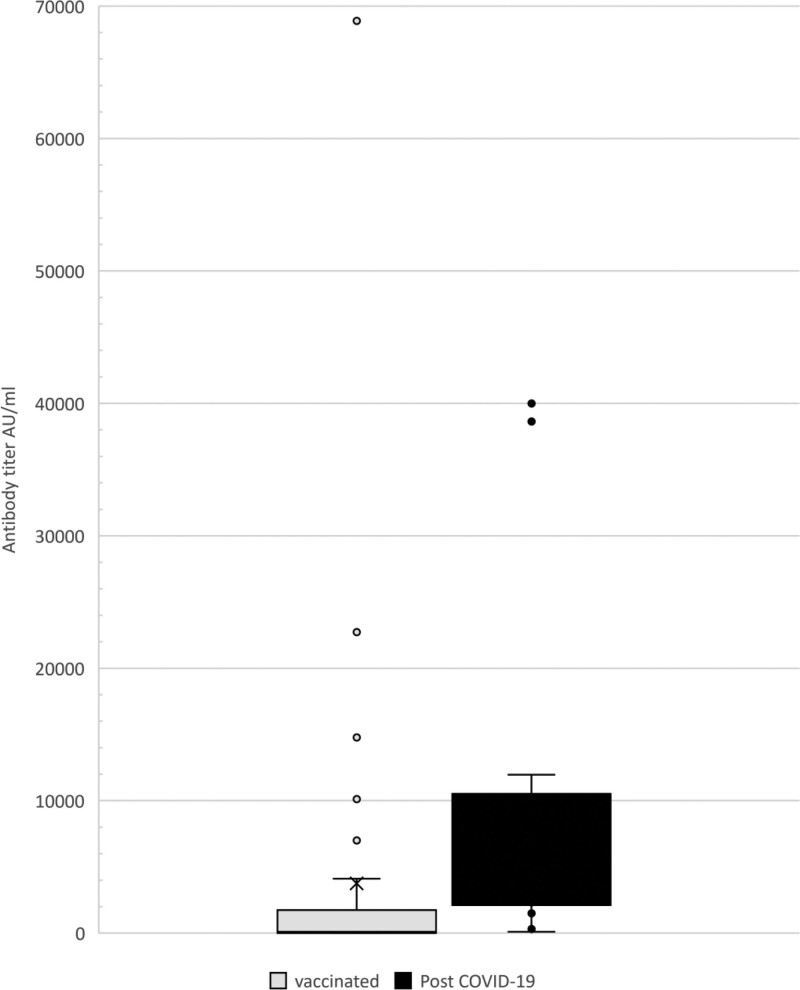
A higher proportion of patients developed a positive serologic response after infection than after COVID-19 vaccination [14/14 (100%) versus 24/38 (63%), P = 0.01]. The median viral antibody titer level was almost 30-fold higher among COVID-19 positive patients compared to vaccinated patients: 2782 AU/mL (IQR: 1908–11 000) versus 100.3 AU/mL (IQR: 4.7–1744), P = 0.0008 (Figure 2). This difference was witnessed despite the longer time that had elapsed from the COVID-19 infection to serologic testing than from vaccination to testing (median [IQR]: 157.5 [60–216] versus 37 [20.5–53] d, P < 0.001) (Figure 2). Five vaccinated patients had very high antibody titers of 7000 AU/mL and above, similar to patients who were infected with COVID-19 disease. The patient who contracted COVID-19 disease and subsequently received 1 dose of vaccine had a qualitative positive SARS-CoV-2 Ab before vaccination and a titer of 5149 AU/mL postvaccination. Antibody levels among COVID-19 positive patients continued to remain high, even 6–8 mo following positive PCR results (Figure 1).
FIGURE 2.
Comparison of severe acute respiratory syndrome coronavirus 2 antibody titers in kidney transplant recipients, between those who were vaccinated and those who were post–COVID-19 infection. Antibody titer is shown in gray for vaccinated patients and black for post–COVID-19 patients. The solid line represents median titer level; X represents mean titer level; the boxes represent the interquartile range; whiskers represent the lowest and highest values within the range. Additional dots represent outliers that are 1.5 times above the third quartile. AU, antibody unit; COVID-19, coronavirus disease 2019.
Safety of the Pfizer-BioNTech COVID-19 Vaccine
Thirty-seven participants in the study group filled a questionnaire at a mean of 36.6 ± 25.6 d following their second vaccine. The results are presented in Table 3. The most common adverse reactions reported were pain (65%) and redness (16%) at the injection site. Systemic adverse reactions were fatigue (41%), headache (35%), nausea (16%), and muscle pain (32%). Most adverse reactions were mild to moderate. None of the patients developed myocarditis or other cardiac side effects postvaccination.
TABLE 3.
Adverse reactions to the Pfizer-BioNTech coronavirus disease 2019 vaccine
| Adverse reaction | Degree of the adverse reaction (N = 37), n (%) | |||
|---|---|---|---|---|
| None | Mild | Moderate | Severe | |
| Pain at injection | 13 (35) | 12 (32.5) | 12 (32.5) | |
| Redness | 31 (84) | 4 (11) | 2 (5) | |
| Swelling | 33 (89) | 4 (11) | 0 | |
| Fever | 37 (100) | 0 | 0 | |
| Fatigue | 22 (59) | 9 (24) | 5 (14) | 1 (3) |
| Headache | 24 (65) | 11 (30) | 2 (5) | |
| Chills | 34 (92) | 2 (5) | 1 (3) | |
| Nausea | 31 (84) | 5 (14) | 1 (2) | |
| Vomiting | 37 (100) | 0 | 0 | |
| Diarrhea | 35 (95) | 2 (5) | 0 | |
| Muscle pain | 25 (68) | 6 (16) | 6 (16) | |
| Joint pain | 35 (95) | 0 | 2 (5) | |
| Rash | 37 (100) | |||
| Need for medications | 34 (92) | 3 (8) paracetamol | ||
| Need for ER visit | 37 (100) | |||
| Cardiac side effects | 37 (100) | |||
| Neurologic | 37 (100) | |||
| Autoimmune | 37 (100) | |||
ER, emergency room.
Renal Outcome
One patient developed borderline cellular rejection 2 wk post–COVID-19 vaccine. Mean creatinine levels were higher post- than prevaccination at a mean 79 ± 28 d between the time points (1.48 ± 0.94 versus 1.43 ± 0.93 mg/dL, P < 0.001), though this difference was not clinically significant. The mean eGFR was slightly lower post- than prevaccine, though the difference was not clinically significant (56.6 ± 21.5 versus 57.4 ± 22.1 mL/min/1.73m2; P < 0.001). Similarly for the control group: the mean creatinine level was higher post–COVID-19 versus pre–COVID-19 infection (1.13 ± 0.33 mg/dL versus 1.04 ± 0.26 mg/dL, P = 0.04), and the mean eGFR was lower (59.7 ± 18.8 versus 62.7 ± 15.7 mL/min/1.73 m2, P = 0.068) at a mean 185.2 ± 88.7 d between the time points.
DISCUSSION
This prospective preliminary study evaluated for the first time the antibody response and the safety of the Pfizer-BioNTech COVID-19 vaccine among adolescent and young adult KTR. Their serologic responses were compared to those observed in KTR post–COVID-19 infection. We report a relatively high proportion of detectible anti–SARS-CoV-2 antibodies in 63% of the vaccinees. This is higher than was reported among adult KTR, of whom only 36%–59% became seropositive at a median of 28 d post second vaccine dose.9-12 Also, contrary to adult solid organ transplant recipients, in whom severe COVID-19 disease was described postvaccination,13-15,22 none of the vaccinees in our younger cohort developed symptomatic COVID-19 infection. Diminished antibody levels postvaccine have been suggested as an explanation for the higher postvaccination infection rate observed in adult solid organ transplant recipients.13,15
The younger age of our cohort may explain the higher rate of seroconversion compared to studies of older populations. Older age was shown to be associated with lower seropositivity and lower antibody levels post–COVID-19 vaccine.10,12,23 Muller et al reported decreased development of neutralizing antibodies to SARS-CoV-2 vaccine with increasing age, even in healthy adult volunteers.24 The phenomenon of immunosenescence in older adults has generally been associated with an increased susceptibility to infectious pathogens and poor vaccine response.25 Functional changes that occur in the adaptive immune system with age may explain the reduced antibody production in response to vaccines in the elderly.25 Another explanation for the higher rate of seropositivity observed in our cohort is that we tested our patients after a greater lapse from the second vaccine (a median of 37 compared to 28 d in the abovementioned adult studies).9-12 Attias et al showed that in vaccinated hemodialysis patients who were infection-naive, anti-S1 IgG levels progressively increased over time, even at 3 wk following the second vaccine.26 This is in contrast to the original vaccine efficacy study that reported high neutralization antibody titers already on day 7 post second vaccine dose in healthy individuals.23 Increasing antibody levels over time have also been reported in hemodialysis patients and in KTR infected with COVID-19.26,27 Immunosuppressive treatment was shown to not influence the seroconversion rate over time, though it did lead to a slower rise in antibody levels and longer median time to peak antibody levels.26,27
An important finding of this study is the almost 30-fold higher anti–SARS-CoV-2 antibody levels after COVID-19 infection compared to postvaccination. This finding contrasts with studies in healthy adults that showed higher anti-S1 IgG levels in those vaccinated than those who experienced naturally infected COVID-19 disease.23,28 Five vaccinated patients in our study showed exceptionally high levels of SARS-CoV-2 IgG antibodies (≥7000 AU/mL), which were similar to the levels of patients who were infected with COVID-19 (Figure 2). These 5 patients may have had previous subclinical COVID-19 infection, and the vaccine may thus have caused a booster response with significantly increased antibody levels.29
The relatively low postvaccination antibody titers observed in our cohort are similar to those reported by Rozen-Zvi et al,12 who measured postvaccination antibody titers in adult transplant recipients using the same assay as our study. Marinaki et al11 also reported significantly lower antibody titers among solid organ transplant recipients than among age-matched healthcare workers. Treatment with immunosuppressive medications in transplanted patients reduces the immune system’s ability to produce significant antibody levels. Nonetheless, humoral immune response alone is not sufficient to identify vaccine responders. Additional evaluation of cellular immunity in response to vaccination is required among persons who are seronegative.30 Notably, none of our study group developed symptomatic COVID-19 infection postvaccination. This may be because of vaccine-conferred immunity, even in antibody-negative patients, or the massive vaccination campaign in Israel, which dramatically reduced infection rates.31
On multivariate analysis, only prior use of rituximab was found to be a risk factor for failure to develop antibodies post–COVID-19 vaccination. This risk factor was also reported in patients with rheumatic and musculoskeletal diseases after receiving the first COVID-19 vaccine dose.32 Patients treated with rituximab have been reported to demonstrate a poor response to influenza vaccination as well.33 Though time from last rituximab infusion to vaccination was relatively long in our patients (median of 40 mo), there are reports of rituximab causing long-term hypogammaglobulinemia in patients with autoimmune diseases, especially when given with additional immunosuppressive therapy.34 Seronegative KTR in our study had overall lower IgG levels compared to seropositive KTR; however, that was not statistically significant. Other studies described associations of time from transplantation, low eGFR, and high tacrolimus trough levels with failure of developing detectable SARS-CoV-2 antibody levels.9-12 These associations were not found in our study, perhaps because of our small sample size.
Regarding the safety of the Pfizer-BioNTech COVID-19 vaccine in our study population, its administration was not associated with significant side effects or a negative effect on kidney function. Only one patient developed borderline cellular rejection 2 wk after the second vaccine dose. Larger studies in adult transplant recipients also did not show an increased rate of transplant rejection episodes postvaccination.35,36
It is important to note that transplanted patients infected with COVID-19 continued to exhibit strong antibody response even at 6–10 mo postinfection. This corroborates reports from Wuhan, China, that described the maintenance of neutralizing antibodies over a 9-mo period in infected immunocompetent individuals.37 Whether vaccination confers similar immunity is yet to be determined.
Our study has several limitations including the relatively small sample size and lack of an appropriate control group of healthy vaccinated individuals. Comparison of antibody titer results between study and control group may have been different if the number of previously infected COVID-19 KTR was higher. We also did not test for anti–COVID-19 antibodies before vaccine administration to identify patients with previous yet unknown COVID-19 infection. Despite these limitations, we were able to show that the administration of the Pfizer-BioNTech COVID-19 vaccine to adolescent and young adult KTR was safe, and that 63% of the patients achieved an antibody response against SARS-CoV-2. Our findings also showed that in this population, a longer time was required to mount appropriate humoral immunity and that antibody titers after the 2-dose vaccine regimen were significantly lower than those observed following COVID-19 infection. Longer clinical and immunologic follow-up studies are required to determine the duration of vaccine immunity in transplant recipients and whether cellular immunity plays a role in vaccine-induced protection from infection.
ACKNOWLEDGMENTS
We want to thank Sapir Lerman, Cindy Cohen, and Prof Gabriel Hodik for their assistance. They were compensated for their time.
Footnotes
The authors declare no funding or conflicts of interest.
O.H. was involved in the conception, design, acquisition, and interpretation of the data and in drafting the article. L.A.-H. was involved in the conception, design, and interpretation of the data and in revising the article. N.Z. was involved in the acquisition of the data and revising the article. Y.B. was involved in the acquisition of the data and revising the article. A.D. was involved in the design, acquisition of the data, and revising the article. S.L. was involved in the acquisition of the data and revising the article. G.K. participated in the performance of the research and revising the article. G.H. was involved in the acquisition and interpretation of the data and in revising the article. D.L.-E. was involved in the acquisition of the data and drafting and revising the article. D.L. was involved in the conception and design of the study and in revising the article. H.A. was involved in the conception, design, acquisition, and interpretation of the data and in drafting the article.
REFERENCES
- 1. Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United states, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857–1859. doi: 10.15585/mmwr.mm6949e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control. COVID-19 vaccination and prioritisation strategies in EU/EEA. 2020. Available at https://www.ecdc.europa.eu/en/publications-data/covid-19-vaccination-and-prioritisation-strategies-eueea. Accessed December 22, 2020.
- 3. Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink B, et al. COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birdwell KA, Ikizler MR, Sannella EC, et al. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am J Kidney Dis. 2009;54:112–121. doi: 10.1053/j.ajkd.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manuel O, Pascual M, Hoschler K, et al. Humoral response to the influenza A H1N1/09 monovalent AS03-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52:248–256. doi: 10.1093/cid/ciq104. [DOI] [PubMed] [Google Scholar]
- 7. Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benotmane I, Gautier-Vargas G, Cognard N, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99:1487–1489. doi: 10.1016/j.kint.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21:2913–2915. doi: 10.1111/ajt.16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27:1173.e1–1173.e4. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wadei HM, Gonwa TA, Leoni JC, et al. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021;00:1–4. doi: 10.1111/ajt.16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsapepas D, Paget K, Mohan S, et al. Clinically significant COVID-19 following SARS-CoV-2 vaccination in kidney transplant recipients. Am J Kidney Dis. 2021;78:314–317. doi: 10.1053/j.ajkd.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali N, Alnazari N, Mehta S, et al. Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplantation. 2021;105:e104–e106. doi: 10.1097/TP.0000000000003836. [DOI] [PubMed] [Google Scholar]
- 16. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. International COVID-19 PICU Collaborative. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Varnell C, Jr, Harshman LA, Smith L, et al. COVID-19 in pediatric kidney transplantation: the Improving Renal Outcomes Collaborative. Am J Transplant. 2021;21:2740–2748. doi: 10.1111/ajt.16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marlais M, Wlodkowski T, Al-Akash S, et al. COVID-19 in children treated with immunosuppressive medication for kidney disease. Arch Dis Child. 2021;106:798–801. doi: 10.1136/archdischild-2020-320616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang HS, Costa V, Racine-Brzostek SE, et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw Open. 2021;4:e214302. doi: 10.1001/jamanetworkopen.2021.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbott Core Laboratory. SARS-CoV-2 Immunoassays. 2021. Available at https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2. Accessed June 23, 2021.
- 21. Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tau N, Yahav D, Schneider S, et al. Severe consequences of COVID-19 infection among vaccinated kidney transplant recipients. Am J Transplant. 2021;21:2910–2912. doi: 10.1111/ajt.16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Müller L, Andrée M, Moskorz W, et al. Age-dependent response to the BioNTech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021:ciab381. doi: 10.1093/cid/ciab381. doi:10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crooke SN, Ovsyannikova IG, Poland GA, et al. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Attias P, Sakhi H, Rieu P, et al. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021;99:1490–1492. doi: 10.1016/j.kint.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forbes S, Davari M, Gnanasampanthan S, et al. Persistence of antibody response to SARS-CoV-2 in a cohort of haemodialysis patients with COVID-19. Nephrol Dial Transplant. 2021;36:1292–1297. doi: 10.1093/ndt/gfab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and Th1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 29. Mazzoni A, Di Lauria N, Maggi L, et al. COVID-19 Research Group. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J Clin Invest. 2021;131:e149150. doi: 10.1172/JCI149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dolff S, Zhou B, Korth J, et al. Evidence of cell-mediated immune response in kidney transplants with a negative mRNA vaccine antibody response. Kidney Int. 2021;100:479–480. doi: 10.1016/j.kint.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1098–1099. doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eisenberg RA, Jawad AF, Boyer J, et al. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol. 2013;33:388–396. doi: 10.1007/s10875-012-9813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tieu J, Smith RM, Gopaluni S, et al. Rituximab associated hypogammaglobulinemia in autoimmune disease. Front Immunol. 2021;12:671503. doi: 10.3389/fimmu.2021.671503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105:e56–e57. doi: 10.1097/TP.0000000000003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ou MT, Boyarsky BJ, Motter JD, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105:2170–2174. doi: 10.1097/TP.0000000000003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He Z, Ren L, Yang J, et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. 2021;397:1075–1084. doi: 10.1016/S0140-6736(21)00238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]