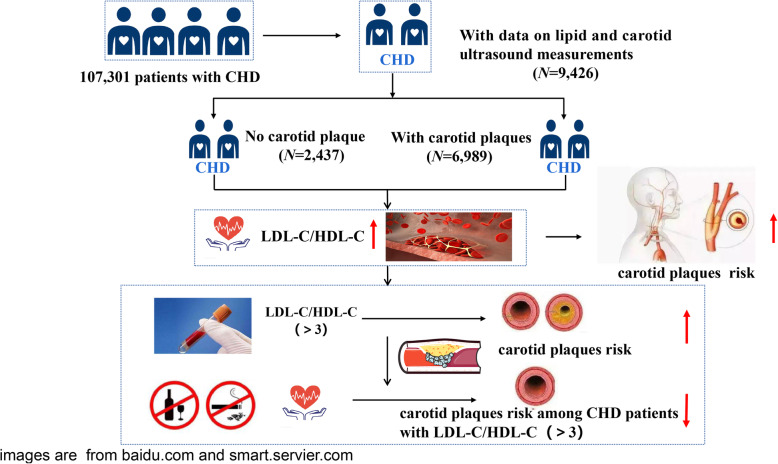
Abstract
Background
Evidence on the relationship between the low−/high-density lipoprotein cholesterol ratio (LDL-C/HDL-C) and carotid plaques remains limited. This study aimed to examine the association between LDL-C/HDL-C and carotid plaques in participants with coronary heart disease (CHD) and to further explore the extent to which a healthy lifestyle reduces the risk of LDL-C/HDL-C-related carotid plaques.
Methods
This large-scale and multi-centre retrospective study included 9426 CHD patients (aged 35–75 years) between January 1, 2014 and September 30, 2020. The LDL-C/HDL-C values were converted to the following tertiles: lowest (< 2.15), middle (2.15–3), and highest (> 3). Healthy lifestyle-related factors referred to whether or not the participant was a non-smoker and non-drinker. Participants were divided into an unfavourable group (those who did not adhere to healthy lifestyle factors), intermediate (only one unhealthy factor), and favourable (neither of the two unhealthy factors). Logistic regression was used for statistical analyses.
Results
Of the 9426 participants, 6989 (74.15%) CHD patients had carotid plaques. After adjustment for confounders, each unit increase in the LDL-C/HDL-C was significantly associated with carotid plaques (OR: 1.61; 95%CI: 1.43–1.84; P < 0.001). Multivariate logistic regression revealed that carotid plaques risk for the highest tertile (> 3) was 1.18 times that of the lowest quartile (< 2.15). Compared with an unfavourable lifestyle, an intermediate or a favourable lifestyle was associated with a significant 30% (OR: 0.70; 95%CI: 0.64–0.78; P < 0.001) or 67% (OR: 0.33; 95%CI: 0.29–0.37; P < 0.001) reduction in carotid plaques risk, respectively, among CHD patients with high LDL-C/HDL-C. There were significantly additive and multiplicative interactions between lifestyle and LDL-C/HDL-C with regards to carotid plaques.
Conclusion
A high LDL-C/HDL-C is associated with a risk of carotid plaques developing in CHD patients. Adhering to a healthy lifestyle has additive beneficial effects on reducing the risk of carotid plaques, especially in relation to the highest LDL-C/HDL-C.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-021-01575-w.
Keywords: Coronary heart disease, Carotid plaques, LDL-C/HDL-C, Healthy lifestyle
Introduction
The statistical report of the American Heart Association, updated in 2021, indicates that cardiovascular disease is associated with a substantial global health and economic burden [1]. The incidence and mortality of coronary heart disease (CHD) is also increasing and has become the key cause of death and disease in China. Coronary atherosclerosis (AS), as the basis of CHD, usually occurs concurrently with carotid AS. Coronary AS is the basis of coronary artery stenosis, in which abnormal lipid metabolism, the coagulation system, inflammatory factor stimulation, and other risk factors damage endothelial cells promoting an inflammatory reaction and lipid deposition, and thus, accelerating plaque formation [2]. Studies have shown that peripheral vascular AS is predictive of cardiovascular diseases (CVD) with the highest mortality rates.
It has been reported that when the conventional lipid parameters of triglycerides (TG), namely high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and total cholesterol (TC) remain apparently normal, other lipid parameters, such as lipid ratios, including TC/HDL-C, LDL-C/HDL-C, TG/HDL-C, and non-HDL-C/HDL-C, are the diagnostic alternatives that predict the risk of a cardiovascular event [3–5]. However, clinical studies have suggested that despite treatment to reduce LDL-C, significant major adverse cardiovascular events may continue to occur, and may be residual risks associated with lipid abnormalities, particularly dyslipidaemia causing AS [6, 7]. Therefore, new goals are required to complement the measures used to prevent CVD. Previous studies have shown that high LDL-C/HDL-C is associated with an increased risk of carotid plaque in obese adults [8]. LDL-C/HDL-C is positively associated with the presence of carotid plaques in male patients with type 2 diabetes [9]. Furthermore, LDL-C/HDL-C was found to be associated with different differences in carotid plaque properties [10]. Nevertheless, there are limited data on the association of LDL-C/HDL-C with carotid plaques and carotid plaque composition in CHD. Therefore, methods for reducing the risk of carotid plaques in CHD patients comprise an important research topic.
It is evident that healthy lifestyle factors can prevent CVDs. Drinking and smoking, two very common and concurrent risk factors, are associated with a significant proportion of mortality due to CVD [11]. Studies have shown that altering many health-related factors are required to effectively reduce the risk of CVD complications [12]. However, the extent to which a healthy lifestyle can reduce the risk of carotid plaques in CHD patients remains unclear.
Therefore, this study aimed to explore the relationship between LDL-C/HDL-C and carotid plaques in CHD patients, and to investigate the extent to which a healthy lifestyle could mitigate the risk of carotid plaques in CHD patients.
Participants and methods
Participants
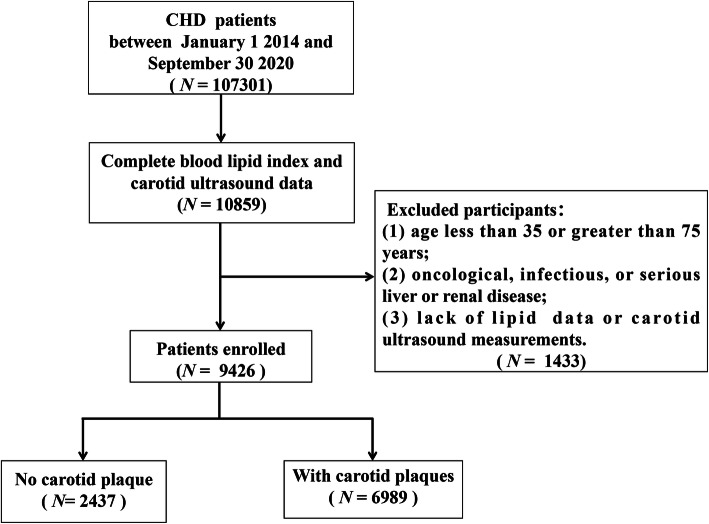
This large-scale and multi-centre retrospective study included 107,301 patients with CHD who were hospitalized in the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, the Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Nankai Hospital: Tianjin Hospital of Integrated Traditional Chinese and Western Medicine, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Tianjin Chest Hospital, and Tianjin Medical University General Hospital between January 1, 2014, and September 30, 2020. Participants were excluded if they: (1) were aged less than 35 or greater than 75 years; (2) had oncological, infectious, or serious liver or renal disease; or (3) lacked lipid data or carotid ultrasound measurements. Finally, a total of 9426 participants were enrolled in the study. A flow chart of the patient selection process is shown in Fig. 1. This study was approved by the ethics committee of the Tianjin University of Traditional Chinese Medicine (approval number TJUTCM-EC20190008) and registered with the Chinese Clinical Trial Registry on July 14, 2019 (registration number ChiCTR-1900024535) and in ClinicalTrials.gov on July 18, 2019 (registration number NCT04026724).
Fig. 1.
Flow chart of patient recruitment
Data collection
Recordings of personal history were collected by trained medical staff. This included information on age, sex, smoking, drinking, medical history, and prior medication history, all of which were investigated by means of a standard structured questionnaire. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by trained physicians using an electronic device. Hypertension was defined as an elevated SBP ≥ 130 mmHg or a DBP ≥ 80 mmHg [13]. Type 2 diabetes was defined as elevated glycated haemoglobin (HbA1c) ≥ 6.5% [14].
Fasting venous blood samples were obtained from all participants on the second day of hospitalization. The levels of TC, TG, HDL-C, LDL-C and other indicators were measured directly by an automatic haematology analyser. Quality control was conducted by the laboratory according to standard procedures. The lipid related variables were calculated as follows: Non-HDL-C = TC – HDL-C; Non-HDL-C/HDL-C = (TC – HDL-C)/HDL-C.
Carotid artery ultrasound was conducted by trained and certified technicians using a diagnostic ultrasound system. Participants were imaged at the common carotid artery, internal carotid artery, and the carotid artery bifurcation while positioned supine. They were carefully scanned from multiple directions under B-mode imaging. The carotid ultrasound results were evaluated by trained and certified technicians. The carotid intima-media thickness (CIMT) was defined as the mean of the IMT of the right and left common carotid arteries [10]. The number and echo properties of carotid plaques were recorded by a professional physician, based on analyses of carotid colours on the Doppler ultrasound results. The number of carotid plaques were classified as single (n = 1) and multiple (n ≥ 2). Echo properties of carotid plaques varied from hypoechoic, isoechoic, hyperechoic, and mixed. In this study, strict quality control procedures were implemented to monitor and test the consistency of image acquisition and analysis. The quality evaluations between laboratories were all conducted by licenced personnel.
Data on smoking and drinking in the current study was collected by means of a question regarding smoking/drinking habits with two response options: 1) “no;” 2) “yes.” “Never smoked/non-smoker” and “no/mild drinking” were defined as “no,” “current smoker/former smoker” and “heavy drinking” were defined as “yes” [15, 16]. In the study, no smoking and no alcohol were considered to be healthy lifestyle factors. Participants were divided into one of three lifestyles categories based on their smoking and drinking habits. The unfavourable group comprised participants who both smoked and drank; the intermediate group was constituted of participants who either only smoked or only drank; and the favourable group consisted of participants who neither smoked nor drank alcohol.
Statistical analyses
The characteristics of participants in the different groups were compared using χ2 tests, t-test and the Mann–Whitney test. The odds ratios (ORs) and 95% confidence intervals (CIs) of carotid plaques were estimated for LDL-C/HDL-C indicators using logistic regressions. Age, sex, SBP, DBP, HbA1c, smoking, drinking, TC, TG, HDL-C, LDL-C, TC/HDL-C, TG/HDL-C, Non-HDL-C, Non-HDL-C/HDL-C, hypertension, type 2 diabetes, and current use of antilipidemic medication were considered potential confounders in this association. The collinearity between independent variables was evaluated to ensure that it was appropriate to include them in the same model. Missing values for smoking (n = 19), drinking (n = 19), SBP (n = 30), DBP (n = 30), HbA1c (n = 765), and carotid plaque echogenicity (n = 104) were credited by chained equations to create five completed datasets.
The combined impact of LDL-C/HDL-C and lifestyle on the risk of carotid plaques was evaluated by assessing dummy variables, based on a coupled exposure to both factors. The existence of an additional interaction was examined by estimating the relative excess risk due to interaction (RERI), the attributable proportion (AP), and the synergy index (SI). Furthermore, the multiplicative interaction incorporating the two variables and their cross-product term in the same model were estimated. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and SPSS 24.0 (IBM Corp, New York, NY, USA).
Results
Baseline characteristics
Of the 9426 participants, the average age was 59.00 ± 5.00 years, males constituted 48.96%, and CHD patients with carotid plaques accounted for 74.15%. The baseline characteristics of the participants were divided into three groups: LDL-C/HDL-C < 2.15 (T1), 2.15 ≤ LDL-C/HDL-C ≤ 3 (T2), and LDL-C/HDL-C > 3 (T3), according to the tertiles of LDL-C/HDL-C. Those in the T3 group were more likely to be male. They had higher SBP, DBP, HbA1c, TG, TC, LDL-C, TC/HDL-C, TG/HDL-C, LDL-C/HDL-C, and non-HDL-C/HDL-C. This group also had a higher number of CHD patients who smoked and drank and had hypertension, type 2 diabetes, and carotid plaques, than participants in the T1 group. The odds of carotid plaques, the number of carotid plaques, and carotid plaque echogenicity also differed among the different LDL-C/HDL-C groups (P < 0.001) (Table 1).
Table 1.
General characteristics of study participants
| Characteristic | Total (N = 9426) |
LDL-C/HDL-C tertile | P- value | ||
|---|---|---|---|---|---|
| T1 (n = 3168) | T2 (n = 3128) | T3 (n = 3168) | |||
| Sex, n (%) | < 0.001 | ||||
| Male | 4615(49.0) | 1438(45.9) | 1466(46.3) | 1711(54.7) | |
| Female | 4811(51.0) | 1692(54.1) | 1702(53.7) | 1417(45.3) | |
| Age, years, median (IQR) | 64.0(59.0–69.0) | 65.0(60.0–70.0) | 64.0(58.0–69.0) | 64.0(58.0–69.0) | < 0.001 |
| SBP, mmHg, median (IQR) | 140.0(128.0–156.0) | 139.0(125.0–154.0) | 140.0(129.0–157.0) | 141.0(130.0–160.0) | < 0.001 |
| DBP, mmHg, median (IQR) | 82.0(77.0–90.0) | 80.0(75.0–90.0) | 83.0(77.0–90.0) | 85.0(80.0–91.0) | < 0.001 |
| HbA1c, %, median (IQR) | 6.1(5.6–7.0) | 5.9(5.6–6.7) | 6.0(5.6–7.0) | 6.2(5.7–7.4) | < 0.001 |
| Smoking, n (%) | 4161(44.1) | 1251(40.0) | 1317(41.6) | 1593(50.9) | < 0.001 |
| Drinking, n (%) | 5316(56.4) | 1591(50.8) | 1665(52.6) | 2060(65.9) | < 0.001 |
| TG, mmol/L, median (IQR) | 1.4(1.0–2.0) | 1.1(0.9–1.6) | 1.5(1.0–2.0) | 1.8(1.3–2.4) | < 0.001 |
| TC, mmol/L, median (IQR) | 4.6(3.9–5.3) | 3.9(3.2–4.5) | 4.6(4.0–5.1) | 5.2(4.6–6.0) | < 0.001 |
| HDL-C, mmol/L, median (IQR) | 1.0(0.9–1.2) | 1.2(1.0–1.4) | 1.01(0.96–1.21) | 1.0(0.8–1.1) | < 0.001 |
| LDL-C, mmol/L, median (IQR) | 2.8(2.0–3.4) | 2.0(1.6–2.4) | 2.8(2.3–3.2) | 3.5(3.0–4.0) | < 0.001 |
| Non-HDL-C, mmol/L, median (IQR) | 3.5(2.8–4.2) | 2.6(2.1–3.1) | 3.5(3.0–4.0) | 4.3(3.7–4.9) | < 0.001 |
| TC/HDL-C, median (IQR) | 4.2(3.5–5.2) | 3.2(2.8–3.6) | 4.2(3.9–4.6) | 5.5(5.0–6.2) | < 0.001 |
| LDL-C/HDL-C, median (IQR) | 2.6(2.0–3.3) | 1.7(1.4–2.0) | 2.6(2.4–2.8) | 3.6(3.3–4.2) | < 0.001 |
| TG/HDL-C, median (IQR) | 1.4(0.9–2.1) | 0.9(0.6–1.4) | 1.3(1.0–2.1) | 1.9(1.4–2.7) | < 0.001 |
| Non-HDL-C/HDL-C, median (IQR) | 3.2(2.5–4.2) | 2.2(1.8–2.6) | 3.2(2.9–3.6) | 4.5(4.0–5.2) | < 0.001 |
| CIMT, mm, median (IQR) | 1.0(0.9–1.2) | 1.0(0.9–1.2) | 1.0(0.9–1.2) | 1.0(0.9–1.2) | < 0.001 |
| Hypertension, n (%) | 7443(79.0) | 2300(73.5) | 2518(79.5) | 2625(83.9) | < 0.001 |
| Type 2 diabetes, n (%) | 4320(45.8) | 1281(73.5) | 1452(45.8) | 1587(50.7) | < 0.001 |
| Current antilipidemic medication, n (%) | 6218(65.9) | 2086(66.6) | 2130(67.2) | 2002(64.0) | < 0.001 |
| Current antihypertensive medication, n (%) | 6869(72.8) | 2314(73.9) | 2331(73.5) | 2224(71.0) | < 0.001 |
| Carotid plaque, n (%) | 6989(74.1) | 2228(71.2) | 2244(70.8) | 2517(80.5) | |
| No. of carotid plaque, n (%) | < 0.001 | ||||
| 1 | 365(5.2) | 98(4.4) | 110(4.9) | 157(6.2) | |
| ≥ 2 | 6624(94.8) | 2130(95.6) | 2134(95.1) | 2360(93.8) | |
| Carotid plaque echo property, n (%) | < 0.001 | ||||
| Hypoechoic plaque | 454(6.5) | 136(6.1) | 164(7.3) | 154(6.1) | |
| Isoechoic plaque | 510(7.3) | 140(6.3) | 150(6.7) | 220(8.7) | |
| Hyperechoic plaque | 3950(56.5) | 1293(58.0) | 1276(56.7) | 1381(54.9) | |
| Mixture plaque | 2075(29.7) | 659(29.6) | 654(29.1) | 762(30.3) | |
T1: LDL-C/HDL-C < 2.15; T2: 2.15 ≤ LDL-C/HDL-C ≤ 3; T3: LDL-C/HDL-C > 3. Data are presented as median (interquartile) or number (proportion, %); P-value was calculated by Kruskal-Wallis test
SBP Systolic blood pressure, DBP Diastolic blood pressure, TC Total cholesterol, TG Triglycerides, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, IQR Interquartile range
Associations between univariate and the risk of carotid plaques
Compared to CHD patients without carotid plaques, male sex, age, SBP, HbA1c LDL-C, non-HDL-C-HDL-C, smoking, drinking, hypertension, type 2 diabetes, and current antilipidemic medication were associated with carotid plaques. All lipid variables were risk factors for carotid plaque formations. HDL-C might have been negatively associated with the risk of carotid plaques in CHD, although LDL-C/HDL-C remained the highest risk factor associated with carotid plaque formation in CHD patients (Table 2).
Table 2.
Associations between univariate and the risk of carotid plaques
| Variables | Carotid plaques | ||
|---|---|---|---|
| OR(95% CI) | β | P-value | |
| Sex | |||
| Female | Reference | ||
| Male | 2.30(2.22–2.40) | 0.835 | < 0.001 |
| Age, years | 1.082(1.079–1.085) | 0.079 | < 0.001 |
| SBP, mmHg | 1.013(1.012–1.014) | 0.013 | < 0.001 |
| DBP, mmHg | 0.996(0.995–0.998) | −0.004 | < 0.001 |
| HbA1c, % | 1.30(1.28–1.32) | 0.265 | < 0.001 |
| Smoking | |||
| No | Reference | ||
| Yes | 2.33(2.24–2.43) | 0.847 | < 0.001 |
| Hypertension | |||
| No | Reference | ||
| Yes | 2.03(1.94–2.12) | 0.707 | < 0.001 |
| Type 2 diabetes | |||
| No | Reference | ||
| Yes | 1.61(1.55–1.67) | 0.474 | < 0.001 |
| Current antilipidemic medication | |||
| No | Reference | ||
| Yes | 0.87(0.84–0.91) | −0.138 | < 0.001 |
| Current antihypertensive medication | |||
| No | Reference | ||
| Yes | 0.99(0.95–1.03) | −0.009 | 0.689 |
| TC, mmol/L | 0.96(0.94–0.97) | −0.043 | < 0.001 |
| TG, mmol/L | 1.00(0.99–1.01) | 0.000 | 0.974 |
| HDL-C, mmol/L | 0.58(0.55–0.62) | −0.544 | < 0.001 |
| LDL-C, mmol/L | 1.08(1.06–1.10) | 0.075 | < 0.001 |
| Non-HDL-C, mmol/L | 1.00(0.98–1.02) | 0.000 | 0.957 |
| TC/HDL-C | 1.12(1.10–1.14) | 0.113 | < 0.001 |
| TG/HDL-C | 1.03(1.02–1.04) | 0.027 | < 0.001 |
| Non-HDL-C/HDL-C | 1.12(1.10–1.14) | 0.113 | < 0.001 |
| LDL-C/HDL-C | 1.26(1.24–1.29) | 0.233 | < 0.001 |
OR Odds ratio, CI Confidence interval, β Regression coefficient
Association between LDL-C/HDL-C and carotid plaques
Three logistic regression models were constructed to assess the impact of LDL-C/HDL-C on carotid plaques (Table 3). In the unadjusted model, LDL-C/HDL-C was shown as a continuous variable, significantly associated with the presence of carotid plaques (OR: 1.27; 95% CI: 1.24–1.29). After further adjustment, the chance of developing carotid plaques increased by 23% (OR: 1.23; 95% CI: 1.20–1.26) and 61% (OR: 1.61; 95% CI: 1.41–1.84). In the unadjusted and further adjustment model, logistic regression suggested that the carotid plaques risk of the T3 group were 1.67 and 1.75, respectively and 1.18-fold higher than that of the T1 group. On further analyses, in which continuous LDL-C/HDL-C were converted to classified variables (tertiles), the P for the trend of the LDL-C/HDL-C with carotid plaques in the unadjusted or adjusted model was consistent with the results obtained when the LDL-C/HDL-C served as a continuous variable (P < 0.001 or P < 0.05).
Table 3.
Association of LDL-C/HDL-C and carotid plaques
| Variables | Carotid plaques | |||||
|---|---|---|---|---|---|---|
| OR (95% CI)a | P-value | OR (95% CI)b | P-value | OR (95% CI)c | P-value | |
| LDL-C/HDL-C | 1.27(1.24–1.29) | < 0.001 | 1.23(1.20–1.26) | < 0.001 | 1.61(1.41–1.84) | < 0.001 |
| < 2.15 | Reference | Reference | Reference | |||
| [2.15–3] | 0.98(0.94–1.03) | 0.455 | 1.04(1.00–1.07) | 0.081 | 0.92(0.86–0.98) | 0.012 |
| > 3 | 1.67(1.59–1.75) | < 0.001 | 1.75(1.66–1.84) | < 0.001 | 1.18(1.06–1.31) | 0.003 |
| P-trend | < 0.001 | < 0.001 | 0.020 | |||
aModel 1: unadjusted;
bModel 2: adjusted for age, sex, SBP, DBP, HbA1c;
cModel 3: adjusted for age, sex, SBP, DBP, HbA1c, smoking, drinking, TC, TG, HDL-C, LDL-C, TC/HDL-C, TG/HDL-C, Non-HDL-C, Non-HDL-C/HDL-C, hypertension, type 2 diabetes, current antilipidemic medication
Association between lifestyle-related factors and carotid plaques
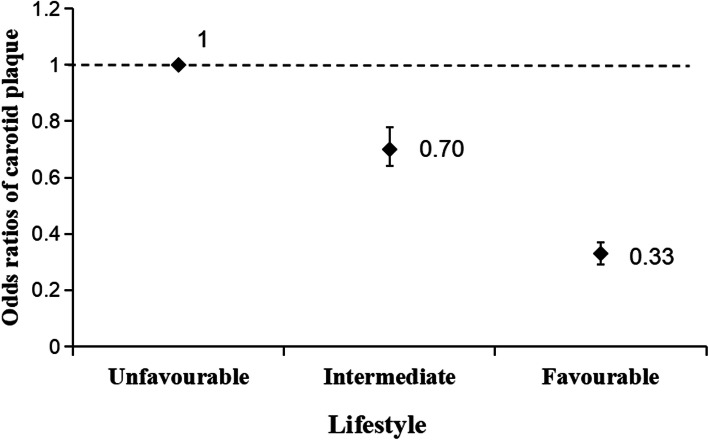
In the unadjusted and adjusted models for age, sex, SBP, DBP, and HbA1c, no smoking and no drinking were correlated with a reduced risk of carotid plaques. An intermediate lifestyle and a favourable lifestyle were significantly relevant with regards to a reduced risk of carotid plaques, after sex, age, SBP, DBP, and HbA1c were adjusted for (Table 4). Compared with an unfavourable lifestyle, an intermediate or a favourable lifestyle was associated with a significant 30% (OR: 0.70; 95%CI: 0.64–0.78; P < 0.001) or 67% (OR: 0.33; 95%CI: 0.29–0.37; P < 0.001) reduction in carotid plaques risk, respectively, among CHD patients with high LDL-C/HDL-C (Fig. 2).
Table 4.
Association between lifestyle-related factors and carotid plaques
| Lifestyle factor | No. of participants | Carotid plaques | |
|---|---|---|---|
| OR (95% CI)a | OR (95% CI)b | ||
| Smoking | |||
| Yes | 4158 | Reference | Reference |
| No | 5268 | 0.43(0.41–0.45)** | 0.51(0.48–0.54)** |
| Dringking | |||
| Yes | 5297 | Reference | Reference |
| No | 4129 | 0.46(0.44–0.48)** | 0.53(0.51–0.56)** |
| Lifestyle | |||
| Unfavourable | 2869 | Reference | Reference |
| Intermediate | 3736 | 0.71(0.67–0.75)** | 0.70(0.66–0.75)** |
| Favourable | 2821 | 0.28(0.27–0.29)** | 0.31(0.29–0.33)** |
aModel 1: unadjusted;
bModel 2: adjusted for age, sex, SBP, DBP, HbA1c;
Compared with no carotid plaque, * P < 0.05, ** P < 0.01
Fig. 2.
Multi-adjusted ORs (95% CIs) of carotid plaque in relation to lifestyle among patients with the highest LDL-C/HDL-C(> 3) in CHD
Joint effect of LDL-C/HDL-C and lifestyle-related factors on the risk of carotid plaques
There was a significant additive interaction between LDL-C/HDL-C and lifestyle-related factors on the risk of carotid plaques in the joint effect analysis (RERI: 2.777; 95% CI: 1.681–3.872; AP: 0.209; 95% CI: 0.142–0.276; SI: 1.291; 95% CI: 1.178–1.416). The multi-adjusted OR for LDL-C/HDL-C, multiplied by unfavourable lifestyle, was 1.17 (95% CI: 1.05–1.30; P = 0.004) for carotid plaques in CHD (Table 5).
Table 5.
Additive interaction between lifestyle-related factors and LDL-C/HDL-C on the risk of carotid plaques
| Joint exposure | No. of participants | Carotid plaques | |||
|---|---|---|---|---|---|
| Lifestyle | LDL/HDL(> 3) | n | OR (95% CI)a | OR (95% CI)b | |
| Intermediate/Favourable | NO | 4522 | 3015 | reference | reference |
| Unfavourable | NO | 1776 | 1457 | 2.29(2.17–2.42)** | 1.86(1.74–2.00)** |
| Intermediate/Favourable | Yes | 2035 | 1574 | 1.71(1.62–1.79)** | 1.63(1.54–1.72)** |
| Unfavourable | Yes | 1093 | 943 | 3.15(2.92–3.39)** | 2.59(2.37–2.82)** |
aModel 1: unadjusted;
bModel 2: adjusted for age, sex, SBP, DBP, HbA1c;
Compared with no carotid plaque, * P < 0.05, ** P < 0.01
Measures of additive interaction for carotid plaques:
Relative excess risk due to interaction (RERI): 2.777; 95% CI: 1.681–3.872;
Attributable proportion due to interaction (AP): 0.209; 95% CI: 0.142–0.276;
Synergy index (SI): 1.291; 95% CI: 1.178–1.416
Supplementary analysis
Further investigations were completed using electronic supplementary material (ESM). Considering possible sex differences in CHD, this study performed stratified analyses, revealing that the associations between LDL-C/HDL-C and the risk of carotid plaques in CHD was not affected by sex (Additional file 1: Table S1). The association of all lipid variables with the number and echo properties of carotid plaques was evaluated, and LDL-C/HDL-C exhibited the highest correlation (Additional file 1: Table S2 and Additional file 1: Table S3). The association of lifestyle and the number and echo properties of carotid plaques was evaluated. The correlation of drinking with the number of carotid plaques was far greater than that of smoking (Additional file 1: Table S4). Smoking had the highest correlation with mixture echoes of carotid plaques, and drinking had the highest correlation with isoechoic carotid plaques (Additional file 1: Table S5). Excluding missing values for covariates, the results did not change substantially compared with those obtained from the initial analysis.
Discussion
Studies indicate that coronary AS and carotid stenosis are closely related [17]. Most acute cardiovascular events are attributed to vulnerable carotid atherosclerotic plaque ruptures and secondary thromboses [18, 19]. Dyslipidaemia is the main risk factor for AS [20, 21]. LDL-C is considered a major cardiovascular risk factor, with a denser reduction in LDL-C more greatly associated with reduced total and cardiovascular mortality in patients at baseline [22]. Studies have shown that HDL can help cholesterol flow out of macrophages, prevent them from becoming foam cells, prevent the oxidation of LDL, and prevent platelets from migrating and gathering at plaque sites. Therefore, once LDL penetrates a dysfunctional endothelium, high HDL can have an anti-atherosclerotic role [23]. Other studies have shown that HDL from healthy individuals can stimulate the production of endothelial nitric oxide, reduce the production of endothelial reactive oxygen species, prevent the inflammatory reactions of endothelium, and improve endothelial function [24]. However, there is evidence that the protective effect of HDL on atherosclerosis will lead to atherosclerosis when the inflammatory process increases. Studies have suggested a reverse relationship between the plasma HDL-C concentration and CHD [25]. Therefore HDL-C-mediated protection remains controversial. Studies have proved that LDL-C/HDL-C is closely related to the onset of CHD and the progression of atherosclerosis [4, 26, 27]. A prospective study showed that the LDL-C/HDL-C predicted the progress of CIMT better than HDL-C or LDL-C alone [28]. These findings are consistent with the results of this research, which further investigated the relationship between LDL-C/HDL-C and the risk of carotid plaque formation, the number of carotid plaques, and carotid plaque echogenicity, and found that LDL-C/HDL-C > 3 was a risk factor for carotid plaque formation in CHD.
Atherosclerotic plaques are rich in lipids, and lipid composition is considered to affect the stability of atherosclerotic plaques. Furthermore, lipid content strongly determines plaque impenetrability and the rate of stenosis [29]. Recent studies have found that neovascularization in plaques is an important marker for evaluating plaque stability [30, 31]. A relationship between neovascularization in plaques and different types of plaque echogenicity, revealed by conventional ultrasound, has been reported. Moreover, neovascularization in isoechoic plaques and hypoechoic fibrous lipid plaques is more obvious than that in calcified plaques [32]. Homogeneous hypoechoic plaques are mostly unstable plaques, rich in lipids, and prone to inflammatory reactions, which may then promote the production of neovascularization. Neovascularization in hyperechoic plaques is rare [33]. In this study, an elevated LDL-C/HDL-C ratio was significantly correlated with the occurrence of hypoechoic and isoechoic plaques. A possible explanation may be that the characteristics of unstable plaques are associated with a denser LDL subgroup typing. The greater the LDL density, the easier the spread to the lower endothelium, where oxidization is more likely. Furthermore, high-density LDL particles have a higher affinity for proteinosan, which extends its stay on the wall and induces the development of AS. Moreover, the HDL-C particle size generally decreases with the increase in LDL-C/HDL-C, suggesting a blockade of HDL maturity, which may be responsible for the progression of AS [34, 35].
However, studies have shown that moderate drinking is associated with a reduction in the risk of CHD. A case-controlled study has revealed that changes in alcohol consumption during life could distort the relationship between real alcohol and CHD [14, 36]. A case-cohort study showed that drinking was inversely associated with non-fatal CHD risk, but positively associated with the risk of different stroke subtypes. A multivariable mendelian randomization study found that smoking was a risk factor for CVD even after adjusting for drinking [37]. Studies have shown that smoking is closely associated with the occurrence of carotid plaques, especially in current smokers [38, 39]. However, this study acknowledged that information regarding the amount of drinking and smoking was not available. It may introduce self-report bias. In accordance with these results, lifestyle interventions aimed at stopping smoking and alcohol may greatly reduce the risk of carotid plaques in CHD.
In summary, in this large-scale and multi-centre retrospective database of CHD, the study findings provided evidence of an independent positive correlation between LDL-C/HDL-C and carotid plaques, which is consistent with the findings of recent studies [8]. A high LDL-C/HDL-C > 3 (T3) was independently associated with an increased risk of carotid plaques in CHD patients, with a higher risk value than the other lipid variables. Moreover, the study indicated that CHD patients with LDL-C/HDL-C > 3 (T3), adhering to a healthy lifestyle has additive beneficial effects on reducing the risk of carotid plaques. This study provides more reliable evidence for the prevention and clinical treatment of carotid plaques in patients with CHD. This study provides more reliable evidence for the prevention and clinical treatment of carotid plaques in patients with CHD.
Strengths and limitations
This study has several limitations. First, ultrasound may not be as accurate as high-resolution magnetic resonance imaging or computed tomography in assessing the presence of a plaque. However, the safety and non-invasive nature of ultrasound should be acknowledged. Furthermore, body mass index (BMI) is an important confounding factor in CHD and carotid plaques. Because much BMI data were missing from this study, it was not included in the model. Additionally, this study only included hospitalized patients. The purpose of this study was to provide standardized and stable research results, but there might have been a selection bias. Finally, in multi-centre studies, methods may differ across the locations. However, as the external quality assessments among the clinical laboratories at each centre were conducted by licenced personnel, the measurement results of each centre’s laboratory were comparable in this study. Finally, the results were recorded and described by professional doctors, which may increase their reliability.
Conclusion
A high LDL-C/HDL-C was independently associated with an increased risk of carotid plaques in CHD patients, with a higher risk value than the other lipid variables. In clinical treatment, the impact of a high LDL-C/HDL-C should be considered. In addition to basic hypolipidemic treatment, attention should be paid to strengthen self-management, promote a healthy lifestyle, and reduce the risk of carotid plaques in CHD patients.
Supplementary Information
Additional file 1: Table S1. Association of LDL-C/HDL-C and carotid plaquesin male and female. Table S2. Association of lipid and carotid plaques number. Table S3. Association between lipid and carotid plaques echogenicity. Table S4. Association of lifestyle and carotid plaque number. Table S5. Association between lifestyle and carotid plaques echogenicity.
Acknowledgments
We thank all the participants in the study and the members of the survey teams, as well as the financial support.
Abbreviations
- AP
Attributable proportion
- AS
Atherosclerosis
- BMI
Body mass index
- CHD
Coronary heart disease
- CI
Confidence interval
- CIMT
Carotid intima-media thickness
- CVD
Cardiovascular diseases
- DBP
Diastolic blood pressure
- ESM
Electronic supplementary material
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- LDL-C/HDL-C
Low−/high-density lipoprotein cholesterol ratio
- OR
Odds ratio
- RERI
Relative excess risk due to interaction
- SI
Synergy index
- SBP
Systolic blood pressure
- TC
Total cholesterol
- TG
Triglycerides
Authors’ contributions
CY, SG and RY were responsible for the study protocol and statistical analysis; ZL and QC analysed the data together and drafted the article; YL, XC, SW, YH, MH, YL, XX conducted data collection; YX, LL and YZ revised the article critically. All authors revised the article for important intellectual content and approved the article. The authors read and approved the final manuscript.
Funding
This work was supported by the National Basic Research Program of China (973 project, grant numbers: 2014CB542902).
Availability of data and materials
The datasets used in the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Tianjin University of Traditional Chinese Medicine (approval number: TJUTCM-EC20190008) and registered with the Chinese Clinical Trial Registry on July 14, 2019 (registration number ChiCTR-1900024535) and in Clinical Trials.gov on July 18, 2019 (registration number: NCT04026724).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhu Li, Qi Cheng and Yijia Liu are co-first authors.
Contributor Information
Rongrong Yang, Email: rongrong0423@hotmail.com.
Shan Gao, Email: bianjibugs@163.com.
Chunquan Yu, Email: ycqtjutcm@foxmail.com.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW, On behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee American Heart Association Council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke Statistics-2021 update: A report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Hirata T, Arai Y, Takayama M, Abe Y, Ohkuma K, Takebayashi T. Carotid plaque score and risk of cardiovascular mortality in the oldest old: results from the TOOTH study. J Atheroscler Thromb. 2018;25(1):55–64. doi: 10.5551/jat.37911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappelletti A, Astore D, Godino C, Bellini B, Magni V, Mazzavillani M, Pagnesi M, Agricola E, Chiesa R, Colombo A, Margonato A. Relationship between syntax score and prognostic localization of coronary artery lesions with conventional risk factors, plasma profile markers, and carotid atherosclerosis (CAPP study 2) Int J Cardiol. 2018;257:306–311. doi: 10.1016/j.ijcard.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XX, Wei M, Shang LX, Lu YM, Zhang L, Li YD, Zhang JH, Xing Q, Tu-Erhong ZK, Tang BP, Zhou XH. LDL-C/HDL-C is associated with ischaemic stroke in patients with non-valvular atrial fibrillation: a case-control study. Lipids Health Dis. 2020;19(1):217. doi: 10.1186/s12944-020-01392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturlaugsdottir R, Aspelund T, Bjornsdottir G, Sigurdsson S, Thorsson B, Eiriksdottir G, Gudnason V. Prevalence and determinants of carotid plaque in the cross-sectional REFINE-Reykjavik study. BMJ Open. 2016;6(11):e012457. doi: 10.1136/bmjopen-2016-012457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi Z, Chen H, Wen Z, Yuan F, Ni H, Gao W, Shen J, Li J, Lin Y, Shan Y, Jin B, Yan P, Shi H, Luo X. Relation of low-density lipoprotein cholesterol to ischemic stroke in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2017;119(8):1224–1228. doi: 10.1016/j.amjcard.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Tziomalos K, Giampatzis V, Bouziana SD, Spanou M, Kostaki S, Papadopoulou M, Angelopoulou SM, Tsopozidi M, Savopoulos C, Hatzitolios AI. Prognostic significance of major lipids in patients with acute ischemic stroke. Metab Brain Dis. 2017;32(2):395–400. doi: 10.1007/s11011-016-9924-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Q, Liu F, Wang YH, Lai HM, Zhao Q, Luo JY, Ma YT, Li XM, Yang YN. LDL-C:HDL-C ratio and common carotid plaque in Xinjiang Uygur obese adults: a cross-sectional study. BMJ Open. 2018;8(10):e022757. doi: 10.1136/bmjopen-2018-022757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rong D, Li M, Wang X, et al. LDL-C/HDL-C ratio associated with carotid intima-media thickness and carotid plaques in male but not female patients with type 2 diabetes. Clin Chim Acta. 2020;511:215–220. doi: 10.1016/j.cca.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Zhu Y, Jia W, Sun D, Zhao L, Zhang C, Wang C, Chen G, Fu S, Bo Y, Xing Y. Association between lipid profiles and presence of carotid plaque. Sci Rep. 2019;9(1):18011. doi: 10.1038/s41598-019-54285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katcher HI, Hill AM, Lanford JL, et al. Lifestyle approaches and dietary strategies to lower LDL-cholesterol and triglycerides and raise HDL-cholesterol. Endocrinol Metab Clin N Am. 2009;38(1):45–78. doi: 10.1016/j.ecl.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Rosoff DB, Davey Smith G, Mehta N, Clarke TK, Lohoff FW. Evaluating the relationship between drinking, tobacco use, and cardiovascular disease: A multivariable Mendelian randomization study. PLoS Med. 2020;17(12):e1003410. doi: 10.1371/journal.pmed.1003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160–164. doi: 10.1016/j.tcm.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 15.Barua RS, Nancy A. Rigotti, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: A report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol. 2018;72(25):3332–3365. doi: 10.1016/j.jacc.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Fan AZ, Ruan WJ, Chou SP. Re-examining the relationship between alcohol consumption and coronary heart disease with a new lens. Prev Med. 2019;118:336–343. doi: 10.1016/j.ypmed.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cademartiri F, Balestrieri A, Cau R, et al. Insight from imaging on plaque vulnerability: similarities and differences between coronary and carotid arteries-implications for systemic therapies. Cardiovasc Diagn Ther. 2020;10:1150–1162. doi: 10.21037/cdt-20-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu GM, Jason H, Li Y, et al. Carotid plaque imaging and the risk of atherosclerotic cardiovascular disease. Cardiovasc Diagn Ther. 2020;10:1048–1067. doi: 10.21037/cdt.2020.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Zhu GM, Ding V, Huang Y, Jiang B, Ball RL, Rodriguez F, Fleischmann D, Desai M, Saloner D, Saba L, Hom J, Wintermark M. Assessing the relationship between atherosclerotic cardiovascular disease risk score and carotid artery imaging findings. J Neuroimaging. 2019;29(1):119–125. doi: 10.1111/jon.12573. [DOI] [PubMed] [Google Scholar]
- 20.Hou QT, Li SY, Gao Y, Tian H. Relations of lipid parameters, other variables with carotid intima-media thickness and plaque in the general Chinese adults: an observational study. Lipids Health Dis. 2018;17(1):107. doi: 10.1186/s12944-018-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Li Z, Song Y, Liu Y, Zhao H, Liu Y, Zhang T, Yuan Y, Cai X, Wang S, Wang P, Gao S, Li L, Li Y, Yu C. Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clin Chim Acta. 2019;495:365–373. doi: 10.1016/j.cca.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, Tantry U, Kubica J, Raggi P, Gurbel PA. Association between baseline LDL-C level and Total and cardiovascular mortality after LDL-C lowering: A systematic review and Meta-analysis. JAMA. 2018;319(15):1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chainika K, Saini Neeraj K, Sasanka C, et al. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid Med Cell Longev. 2020;15(9):5245308. doi: 10.1155/2020/5245308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konstantinos T, Konstantina K, Marianthi P, et al. Impaired antioxidative activity of high-density lipoprotein is associated with more severe acute ischemic stroke. Metabolism. 2019;98:49–52. doi: 10.1016/j.metabol.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Barbalho SM, Tofano RJ, de Oliveira MB, et al. HDL-C and Non-HDL-C levels are associated with anthropometric and biochemical parameters. J Vasc Bras. 2019;18:e20180109. doi: 10.1590/1677-5449.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong Z, Hou J, Zhang Q, Zhong W, Li B, Li C, Liu Z, Yang M, Zhao P. Assessment of the LDL-C/HDL-C ratio as a predictor of one year clinical outcomes in patients with acute coronary syndromes after percutaneous coronary intervention and drug-eluting stent implantation. Lipids Health Dis. 2019;18(1):40. doi: 10.1186/s12944-019-0979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aizawa M, Inagaki S, Moriyama M, Asano K, Kakehashi M. Correction: modeling the natural history of fatty liver using lifestyle-related risk factors: effects of body mass index (BMI) on the life-course of fatty liver. PLoS One. 2019;14(12):e0226059. doi: 10.1371/journal.pone.0226059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enomoto M, Adachi H, Hirai Y, Fukami A, Satoh A, Otsuka M, Kumagae SI, Nanjo Y, Yoshikawa K, Esaki E, Kumagai E, Ogata K, Kasahara A, Tsukagawa E, Yokoi K, Ohbu-Murayama K, Imaizumi T. LDL-C/HDL-C ratio predicts carotid intima-media thickness progression better than HDL-C or LDL-C alone. J Lipids. 2011;2011:549137–549136. doi: 10.1155/2011/549137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puig N, Jiménez-Xarrié E, Camps-Renom P, Benitez S. Search for reliable circulating biomarkers to predict carotid plaque vulnerability. Int J Mol Sci. 2020;21(21). 10.3390/ijms21218236. [DOI] [PMC free article] [PubMed]
- 30.Mao Y, Liu XQ, Song Y, Zhai CG, Xu XL, Zhang L, Zhang Y. Fibroblast growth factor-2/platelet-derived growth factor enhances atherosclerotic plaque stability. J Cell Mol Med. 2020;24(1):1128–1140. doi: 10.1111/jcmm.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A, Kesavabhotla K, Baradaran H, Kamel H, Pandya A, Giambrone AE, Wright D, Pain KJ, Mtui EE, Suri JS, Sanelli PC, Mushlin AI. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke. 2015;46(1):91–97. doi: 10.1161/STROKEAHA.114.006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mushenkova NV, Summerhill VI, Zhang D, Romanenko EB, Grechko AV, Orekhov AN. Current advances in the diagnostic imaging of atherosclerosis: insights into the pathophysiology of vulnerable plaque. Int J Mol Sci. 2020;21(8):2992. doi: 10.3390/ijms21082992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsu T, Iguchi Y, Arai A, Sakuta K, Sakai K, Terasawa Y, Mitsumura H, Matsushima M. Large but nonstenotic carotid artery plaque in patients with a history of embolic stroke of undetermined source. Stroke. 2018;49(12):3054–3056. doi: 10.1161/STROKEAHA.118.022986. [DOI] [PubMed] [Google Scholar]
- 34.Liu F, Wang Z, Cao X, Pan Y, Zhang E, Zhou J, Zheng L. Relationship between small dense low-density lipoprotein cholesterol with carotid plaque in Chinese individuals with abnormal carotid artery intima-media thickness. BMC Cardiovasc Disord. 2021;21(1):216. doi: 10.1186/s12872-021-02023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.QiaoZhen X, AiGuo M, Tong W, JingJing L, HaiYing L. Correlation between of small dense low-density lipoprotein cholesterol with acute cerebral infarction and carotid atherosclerotic plaque stability. J Clin Lab Anal. 2019;33(6):e22891. doi: 10.1002/jcla.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci C, Wood A, Muller D, Gunter MJ, Agudo A, Boeing H, van der Schouw YT, Warnakula S, Saieva C, Spijkerman A, Sluijs I, Tjønneland A, Kyrø C, Weiderpass E, Kühn T, Kaaks R, Sánchez MJ, Panico S, Agnoli C, Palli D, Tumino R, Engström G, Melander O, Bonnet F, Boer JMA, Key TJ, Travis RC, Overvad K, Verschuren WMM, Quirós JR, Trichopoulou A, Papatesta EM, Peppa E, Iribas CM, Gavrila D, Forslund AS, Jansson JH, Matullo G, Arriola L, Freisling H, Lassale C, Tzoulaki I, Sharp SJ, Forouhi NG, Langenberg C, Saracci R, Sweeting M, Brennan P, Butterworth AS, Riboli E, Wareham NJ, Danesh J, Ferrari P. Alcohol intake in relation to non-fatal and fatal coronary heart disease and stroke: EPIC-CVD case-cohort study. BMJ. 2018;361:k934. doi: 10.1136/bmj.k934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Si J, Li J, Yu C, Guo Y, Bian Z, Millwood I, Yang L, Walters R, Chen Y, du H, Yin L, Chen J, Chen J, Chen Z, Li L, Liang L, Lv J. Improved lipidomic profile mediates the effects of adherence to healthy lifestyles on coronary heart disease. Elife. 2021;10:e60999. doi: 10.7554/eLife.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji X, Leng XY, Dong Y, et al. Modifiable risk factors for carotid atherosclerosis: a meta-analysis and systematic review. Ann Transl Med. 2019;7(22):632. doi: 10.21037/atm.2019.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kianoush S, Yakoob MY, Al-Rifai M, et al. Associations of cigarette smoking with subclinical inflammation and atherosclerosis: ELSA-Brasil (the Brazilian longitudinal study of adult health) J Am Heart Assoc. 2017;6(6):e005088. doi: 10.1161/JAHA.116.005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Association of LDL-C/HDL-C and carotid plaquesin male and female. Table S2. Association of lipid and carotid plaques number. Table S3. Association between lipid and carotid plaques echogenicity. Table S4. Association of lifestyle and carotid plaque number. Table S5. Association between lifestyle and carotid plaques echogenicity.
Data Availability Statement
The datasets used in the present study are available from the corresponding author on reasonable request.