Abstract
The use of cold preservation solutions to rapidly flush and cool the kidney followed by static cold storage in ice has been the standard kidney preservation technique for the last 50 y. Nonetheless, changing donor demographics that include organs from extended criteria donors and donation after circulatory death donors have led to the adoption of more diverse techniques of preservation. Comparison of hypothermic machine perfusion and static cold storage techniques for deceased donor kidneys has long been debated and is still contested by some. The recent modification of hypothermic machine perfusion techniques with the addition of oxygen or perfusion at subnormothermic or near-normothermic temperatures are promising strategies that are emerging in clinical practice. In addition, the use of normothermic regional perfusion to resuscitate abdominal organs of donation after circulatory death donors in situ before cold flushing is also increasingly being utilized. This review provides a synopsis of the different types of preservation techniques including their mechanistic effects and the outcome of their application in clinical practice for different types of donor kidney.
INTRODUCTION
Techniques of kidney preservation traditionally rely on the principle of hypothermia. Hypothermic temperatures inhibit enzymatic processes and reduce metabolism by 2- to 3-fold for every 10°C reduction in temperature.1 This slows the depletion of ATP and also inhibits the degrading processes. Nonetheless, over time, the gradual depletion of energy substrates leads to a loss of cell viability and the development of necrosis.1,2 The cold ischemic time (CIT) alone is an independent risk factor for graft failure and is directly associated with delayed graft function (DGF).3 The risk of DGF increases by 23% for every 6 h of cold ischemia (CI) and if the CIT is limited to <12 h, the risk of DGF is reduced by 15%.4 The CIT also significantly increases allograft immunogenicity, provoking acute and chronic rejection.5,6
In the last decade, driven by rising transplant waiting lists, more extended criteria donor (ECD) and donation after circulatory death (DCD) donor kidneys are considered for transplantation. Older donor organs have higher immunogenicity and increased risk of graft dysfunction and failure.5,6 The use of uncontrolled DCD (uDCD) donors is also increasing, with firmly established programs in several European countries.7,8 ECD, controlled DCD (cDCD), and uDCD kidneys are all particularly susceptible to CI.9-11 Efforts are made to limit the preservation interval where possible. Nonetheless, geographical and logistical constraints often result in more prolonged durations of CI, particularly in the United States. Different modes of mechanical preservation using modified hypothermic and normothermic techniques are being investigated to minimize or reverse the effects of CI and some of these have found their way into clinical practice.
This review summarizes the outcomes of kidney transplantation from different types of organ donor, then details the different kidney preservation techniques, exploring their mechanistic actions and application in clinical practice stratified by organ donor type.
KIDNEY DONORS
Live Donor Kidney Transplantation
Living related or unrelated kidney donor transplantation has excellent outcomes. Rates of DGF, graft, and patient survival are detailed in Table 1.12-15 Kidneys have a short period of warm ischemia (WI; 4–10 min) under normal circumstances followed by a brief period of CI (mean, 2–6 h). Nonetheless, kidney exchange programs and donation have increased over the last few years and without the capacity to transfer patients in some centers, CIT can be much longer. Evidence from the United States, Australia, the United Kingdom, and Europe has demonstrated that shipping kidneys is safe with CITs of up to 16 h. However, this increases the risk of DGF, which may reduce graft and patient survival and increase incidences of acute rejection.12,34-37
TABLE 1.
Outcomes of kidney transplantation from different donor types (% range)
| Donor type | DGF rate (%) | Graft survival at 1 y (%) | Graft survival at 5 y (%) | Patient survival at 1 y (%) | Patient survival at 5 y (%) | References |
|---|---|---|---|---|---|---|
| Living | 2–19 | 96–98 | 85–92 | 98–99 | 93–95 | 12-15 |
| DBD | 10–28 | 89–97 | 80–85 | 96–99 | 82–89 | 16-19 |
| ECD | 28–38 | 82–92 | 49–80 | 90–96 | 70–93 | 4,9-11,20 |
| DCD | 23–58 | 85–92 | 77–87 | 95–99 | 82–89 | 7,16-19,21-26 |
| uDCD | 42–93 | 85–100 | 60–87 | 83–98 | 78–94 | 8,23,27-33,38 |
DBD, donation after brain death; DCD, donation after circulatory death; DGF, delayed graft function; ECD, extended criteria donor; uDCD, uncontrolled DCD
Deceased Donor Kidney Transplantation
Rates of DGF in deceased donor kidney transplantation range from 10% in standard donation after brain death (DBD) kidneys to 93% in uDCD4,7,8,10,16-23,27,38 (Table 1). The outcomes of graft and patient survival at 1 y are good but slightly lower in comparison to living donor kidneys.8-11,18,20,21,24-33,38 At 5 y, there is a marked reduction in graft and patient survival, in particular in ECD kidney transplants (Table 1).8-11,18,20,21,24-33,38
KIDNEY PRESERVATION TECHNIQUES
Static Cold Storage
Static cold storage (SCS) is the simplest form of organ preservation. Organs are flushed with cold preservation solution at approximately 4 °C, then stored in solution on ice until transplanted. Preservation solutions are designed to allow adequate flushing of the microcirculation and rapidly cool the kidney. They contain a number of critical components to maintain cellular viability during storage at 4 °C: an impermeant to counteract swelling and to provide stability of the cellular ultrastructure, a buffer to prevent the buildup of intracellular acidosis and a balanced electrolyte composition with either a high or low Na+/K+ ratio, again to counteract cellular swelling. Solutions with a high K+ concentration are classified as intracellular and those with a high Na+ as extracellular solutions (Table 2). There are a number of different SCS preservation solutions available for kidney preservation.
TABLE 2.
Components commonly used in organ preservation solutions
| Impermeants | Glucose, lactobionate, mannitol, raffinose, and sucrose |
| Colloid | HES and PEG |
| Buffers | Citrate, histidine and phosphate |
| Electrolytes | Calcium, chloride, magnesium, magnesium sulfate, potassium, and sodium |
| Antioxidants | Allopurinol, glutathione, mannitol, and tryptophan |
| Additives | Adenosine, glutamic acid, and ketoglutarate |
HES, hydroxyethyl starch; PEG, polyethylene glycol.
Collins solution was one of the first acellular preservation solutions used in clinical transplantation in 1969.39 It was later modified and renamed Euro-Collins solution.40
Hyperosmolar citrate (HOC), more commonly known as Soltran or Marshall’s solution, was first developed in the 1970s.41 HOC uses citrate as a buffer to prevent accumulation of calcium within the cell (Table 2). Its hypertonicity is also designed to prevent fluid entry into cells. It is a relatively inexpensive, nonviscose intracellular solution.
University of Wisconsin (UW) solution developed by Belzer and Southard in the 1980s contains a vast array of ingredients including antioxidants (glutathione) to scavenge oxygen-free radicals, allopurinol to block the activity of xanthine oxidase, and adenosine, an ATP precursor.42,43 It is an intracellular solution and also contains the colloid hydroxyethyl starch to prevent cellular swelling (Table 2). It is considered the “gold standard” preservation solution.
Histidine-tryptophan-ketoglutarate (HTK) solution was originally developed as a cardioplegic solution but because of its low viscosity was quickly adopted for abdominal organs.44 It is an extracellular solution and uses the impermeant mannitol and histidine as a buffer. It also contains 2 amino acids: tryptophan, to stabilize cellular membranes and prevent oxidant damage; and ketoglutarate, a substrate to support anaerobic metabolism (Table 2).
Celsior is an extracellular solution and was initially designed for heart transplantation. It contains histidine as a buffer, lactobionate and mannitol to prevent edema, and glutathione as an antioxidant.45
Other less well-known solutions include Institut George Lopex-1 solution (IGL-1) and Solution de Conservation des Organes et des Tissus (SCOT, organ and tissue preservation solution).46,47 They are extracellular solutions containing polyethylene glycol as a colloid. Hypertonic citrate adenine solution containing citrate and adenine,48 phosphate-buffered sucrose 140, developed in the United Kingdom49, and Perfudex50 and Polysol51 are less common solutions. The composition of the more common preservation solutions are listed in several other reviews.52-54
Live Donor Kidneys
SCS is the main method of preservation for live donor (LD) kidney transplants. There is little evidence for the use of one preservation solution over another in LD kidney transplantation (Table 3). Lynch et al55 reported in a series of 475 LD kidneys the rate of DGF was significantly reduced in kidneys preserved in HTK versus UW (3.2% versus 8.2%, P = 0.001). UW, HOC, HTK, or Celsior solutions are the most commonly used. In some centers where the CITs are very short, kidneys are simply flushed with a cold crystalloid solution.77
TABLE 3.
Meta-analyses, systematic reviews, randomized controlled trials, case series, and registry studies comparing different preservation techniques in each type of kidney donor
| Method of preservation | Donor type | Outcome | References |
|---|---|---|---|
| SCS vs SCS | LD | Case series: HTK reduced DGF vs UW | 55 |
| DBD | Systematic review: Euro-Collins increased the risk of DGF. UW equivalent risk of DGF vs HTK and Celsior | 56 | |
| Registry data: IGL-1 reduced the risk of DGF | 57 | ||
| Randomized study: Celsior improved graft survival vs UW | 58 | ||
| ECD | Randomized study: UW equivalent rates of DGF vs Celsior | 59 | |
| Registry data: Lower DGF with SCOT solution vs IGL-1 | 35 | ||
| Registry data: HTK increased the risk of graft loss vs UW | 60 | ||
| DCD | Meta-analysis: Solutions equivalent | 56 | |
| HMP vs SCS | LD | Case series: Reduced vascular resistance | 61 |
| DBD | Systematic review and RCT: Reduced incidences and risk of DGF | 62,63 | |
| Systematic review: Improved graft survival at 1 + 3 y | 64 | ||
| ECD | Meta-analysis: Reduced the risk of DGF and improved 1-y graft survival | 65 | |
| RCT: Reduced the risk of DGF, PNF, and improved 1 + 3-y graft survival | 66 | ||
| Registry data: Reduced DGF and improved 1-y graft survival | 67 | ||
| Retrospective study: Equivalent rates of DGF | 68 | ||
| DCD | Meta-analysis: Reduced DGF and improved graft survival at 1 + 3 y | 69 | |
| Meta-analysis: Reduced DGF | 62 | ||
| RCT: Reduced DGF | 70 | ||
| RCT: Equivalent rates of DGF | 71,72 | ||
| Registry data: Reduced DGF | 73 | ||
| Oxygenated HMP vs HMP | DCD | RCT: No significant difference in eGFR at 12 mo. Reduced the incidences of severe complications and acute rejection | 74 |
| NMP vs SCS | |||
| ECD | Case series: Reduced DGF | 75 | |
| NRP vs standard in situ cooling | uDCD | Registry study: Reduced PNF and graft loss | 76 |
DBD, donation after brain death; DCD, donation after circulatory death; DGF, delayed graft function; ECD, extended criteria donor; eGFR, estimated glomerular filtration rate; HMP, hypothermic machine perfusion; HTK, histidine-tryptophan-ketoglutarate; IGL-1, Institut George Lopex-1 solution; LD, living donor; NMP, normothermic machine perfusion; NRP, normothermic regional perfusion; PNF, primary nonfunction; RCT, randomized controlled trial; SCOT, Solution de Conservation des Organes et Tissus; SCS, static cold storage; uDCD, uncontrolled DCD; UW, University of Wisconsin solution.
DBD Kidneys
The main method of kidney preservation is SCS for standard criteria DBD donor kidneys. The most commonly used solutions for SCS include UW, HTK, HOC, and Celsior solutions. Several countries have their own particular preference of solution, for example, IGL-1 is used widely in France but not in other countries.57
Evaluation of the best solution for deceased donor kidneys was the subject of much clinical research from the 1970s to early 2000s (Table 3). O’Callaghan et al56 conducted a systematic review of 15 trials (3584 kidney transplants) comparing different preservation solutions in all types of deceased donor kidneys. These included 10 randomized controlled trials (RCTs) and 5 non-RCT trials. The authors concluded that the type of preservation solution used influenced the risk of DGF. However, it was not possible to calculate the relative risk (RR) of DGF with any individual solution. Euro-Collins solution was associated with a higher risk of DGF than UW and HTK solutions. UW solution was found to have an equivalent risk of DGF to HTK in 2 RCTs and Celsior in 3 RCTs.56 One study found no increased risk of DGF with UW or Euro-Collins solutions with extended CITs.78 In agreement, several studies have found similar outcomes with extended CITs between HTK and UW solutions.55,79 In the past, HTK solution has been associated with an increased risk of graft loss particularly if the CIT exceeded 24 h.40,80
Most recently, Legeai et al57 reviewed the outcome of 5 different SCS solutions used for DBD kidneys in France between 2010 and 2014 (n = 7649). The majority (45.5%) were preserved using IGL-1 solution, 9.6% SCOT solution, 29% Celsior solution, 9.6% UW solution, and 5.9% HTK solution. The risk of DGF was significantly lower with the use of IGL-1 solution.
The impact of different preservation solutions on graft survival appears to be minimal, with only 1 study demonstrating improved graft survival with Celsior solution compared to UW solution.58
ECD Kidneys
There are few well-conducted studies examining the effects of different SCS preservation solutions in ECD kidneys (Table 3). In a single multicenter randomized study including a total of 50 kidneys, Montalti et al59 found no difference in rates of DGF when comparing UW (52%) and Celsior solutions (48%). The majority of evidence comes from registry data. Legeai et al57 found that the association with DGF and SCOT solutions versus IGL-1 was lower in ECD kidneys (odds ratio [OR] 2.72, 95% confidence interval [CI] 1.98-3.75) compared to standard DBD.57 HTK and UW performed similarly irrespective of the CIT. Stewart et al60 found using the UNOS registry database that the risk of death-censored graft loss increased when using HTK solution compared with UW solution for ECD kidneys.
cDCD Kidneys
SCS is becoming less common for DCD kidneys. However, in the United Kingdom, the majority of cDCD kidneys undergo SCS with UW solution. There are very few studies examining the effects of different preservation solutions for SCS in DCD kidneys (Table 3). In an early series from Japan (2004), Euro-Collins solution was used for SCS of 108 cDCD kidneys. Ninety percent of the kidneys were transplanted but the DGF rate was 86%. The death-censored graft survival was 80% at 5 y and 62.9% at 10 y.81
Historically, concerns with the use of HTK solution have been raised regarding its use for DCD kidneys. Some clinical studies have associated its use with the increased risk of primary nonfunction (PNF) and early graft loss. However, meta-analysis including all donor types found no evidence of this in DCD kidneys.56
uDCD Kidneys
In a UK series of 112 DCD kidneys, the majority uDCD (Maastricht category II donors), Barlow et al81 compared the results to 164 matched DBD kidney transplants during the same era (1992–2003). In the DCD series, kidneys were rapidly cooled in situ followed by SCS with HOC solution at 4 °C. The rate of DGF was 83.9% versus 22% (P < 0.001) and the PNF rate was 5.4%, similar to the DBD kidneys (1.8%, P = 0.164). There was no significant difference in graft survival between the groups up to 15 y posttransplant.
Hypothermic Machine Perfusion
Hypothermic machine perfusion (HMP) involves the continuous recirculation of cold preservation solution through the kidney at a low pressure. The temperature is maintained between 2 and 8 °C. HMP allows a continual flush of the microcirculation, preventing the accumulation of toxic metabolites. UW solution modified for machine perfusion (MP), UW-MP, is used in most cases for HMP. It was developed by Belzer in the 1960s and provides a source of nutrients and metabolites to support a low level of cellular metabolism to reduce the formation of lactic acid within the cell.82 The addition of a colloid (hydroxyethyl starch) is a crucial ingredient to prevent cellular edema during perfusion. The mechanical passage of fluid through the microcirculation also protects against depolarization of the endothelial cell membrane and reduces free radical formation.83 Studies have also shown that HMP can increase the release of nitric oxide and reduce endothelin-1, a potent vasoconstrictor produced by vascular endothelial cells.61,84 HMP can also reduce levels of inflammatory cytokines62,85 and increase phosphorylation of the Akt-Erk signaling pathway to increase the expression of anti-apoptotic and decrease the expression of proapoptotic genes.86
Assessment
HMP also allows an assessment of viability, which includes the measurement of flow and resistance parameters, analyses of a range of biomarkers in the circulating perfusate and imaging technologies to examine structure. These viability assessments are listed in Table 4 and have been reviewed extensively in several recent publications.87,88 The most commonly reported are glutathione S-transferase, lactate dehydrogenase, fatty acid-binding protein, interleukin 18, and measures of lipid peroxidation. Although useful in determining a level of injury, their ability to determine outcome following transplantation is limited.87,88
TABLE 4.
Perfusion characteristics, biomarkers, and imaging techniques used for kidney assessment during HMP and NMP
| HMP | NMP | |
|---|---|---|
| Perfusion characteristics | ||
| Flow | Flow | |
| Intrarenal resistance | Intrarenal resistance | |
| Macroscopic appearance | ||
| Biomarkers | ||
| GST (tGST, αGST, pi-GST) | Urine production | |
| LDH | Creatinine clearance | |
| Lactate | Lactate | |
| AST | Potassium concentration | |
| Matrix metalloproteinase 9 + 2 | Acid-based homeostasis | |
| Alanine-aminopeptidase | ET-1 | |
| FABP (H-FABP, L-FABP) | IL-6, IL-8, IL-18 | |
| NGAL | FMN | |
| Redox-active iron | Lactate | |
| Lipid peroxidation | AST | |
| Ionized calcium | Nanoparticle release | |
| KIM-1 | FMN | |
| FMN | Proteomics | |
| IL-18 | Glucose oxidation | |
| MicroRNA 21 | Transcriptional analysis | |
| H-NMR | ||
| Histone H3 | ||
| Imaging | ||
| MRI | MRI | |
| Contrast-enhanced ultrasound | ||
αGST, alpha GST; AST, aspartate transaminase; ET-1, endothelin-1; FABP, fatty acid–binding protein; FMN, flavin mononucleotide; GST, glutathione S-transferase; HMP, hypothermic machine perfusion; H-NMR, proton nuclear magnetic resonance; IL, interleukin; KIM-1, kidney injury molecule-1; LDH, lactate dehydrogenase; NGAL, neutrophil gelatinase–associated lipocalin; NMP, normothermic machine perfusion; tGST: total GST.
Technologies
In the last 2 decades, there has been growing support for HMP in kidney transplantation. There are a number of commercially available portable HMP systems such as the LifePort Kidney Transporter (Organ Recovery System), Kidney Assist (Organ Assist, Groningen, the Netherlands), and WAVES machine (Institut Georges Lopez, Lissieu, France). The RM3 system (Waters Medical Systems, Birmingham, AL) is not portable but is still widely used in the United States.
Live Donor Kidneys
There is only 1 report of using HMP in LD kidney transplantation (Table 3). Moser et al61 analyzed a series of 16 LD kidneys that underwent HMP and compared them to 16 kidneys that had SCS. The study was powered to detect a difference in vascular resistance measured by Doppler ultrasound. After laparoscopic retrieval, HMP kidneys were flushed with HTK solution and placed on the LifePort Kidney Transporter and perfused with kidney perfusion solution 1. High levels of injury markers were detected in the perfusate (neutrophil gelatinase lipocalin and lactate dehydrogenase) and compared similarly to levels measured in deceased donor kidneys. There were no incidences of DGF or acute rejection in the SCS or HMP kidneys. Resistive indices measured by Doppler ultrasound were significantly less for HMP kidneys postoperatively. The authors concluded that there was a sufficient amount of injury in LD kidneys to warrant the wider use of HMP, particularly in cases where kidneys are shipped and have more prolonged CITs.
DBD Kidneys
HMP is becoming more common practice for DBD kidneys in some countries. There have been a number of studies comparing HMP to SCS for DBD kidneys (Table 3). Peng et al62 recently performed a systematic review and meta-analysis of RCTs comparing HMP with SCS. A total of 13 RCTs were included in the study. In a subgroup analysis of DBD kidneys, HMP reduced the incidence of DGF compared to SCS (RR 0.78, 95% CI 0.67-0.92, P = 0.003) but there was no difference in graft survival at 1 y (RR 1.04, 95% CI 0.99-1.09, P = 0.10). Tingle et al64 conducted a recent meta-analysis including 971 patients from 4 studies. HMP was found to reduce the risk of DGF in DBD kidneys (RR = 0.77, 95% CI 0.67-0.90, P = 0.006). HMP also had a graft survival benefit at 1 and 3 y posttransplant.
Moers et al63 reported the largest RCT comparing HMP and SCS from Europe including 672 kidneys from 336 deceased donors. The majority of the kidneys included in the study were from DBD standard criteria donors (SCDs; 78%). They found that HMP reduced the risk of DGF compared to SCS (OR 0.57, P = 0.01).
In a further analysis of the European RCT, Kox et al89 found that kidneys with short CITs also benefited from HMP, with low rates of DGF. They recommended that HMP should be used for all deceased donor kidneys regardless of the duration of CI. Some centers advocate the use of HMP for more prolonged CI periods to prevent the increased risk of DGF.73,90
ECD Kidneys
There is growing support for HMP for ECD kidneys and it has become standard practice in some centers. Again, a number of studies have compared HMP to SCS for ECD kidneys (Table 3). Jiao et al performed a meta-analysis of 7 studies involving 2374 HMP and 8716 ECD kidneys comparing HMP with SCS. There was a significant reduction in the OR of 0.59 (95% CI 0.54-0.66, P < 0.001) of DGF following HMP and improved graft survival at 1 y (OR 1.12, 95% CI 1.03-1.21, P = 0.005).65
In a subanalysis of the European RCT of 91 ECDs (182 kidneys), Gallinat et al66 demonstrated that HMP reduced the risk of DGF (OR 0.460, P = 0.047), lowered PNF rates by 9%, and improved graft survival at 1 y (92.3% versus 80.2%, P = 0.02) compared to SCS. This benefit in graft survival persisted at 3 y posttransplant.
From French registry data, Savoye et al67 found that the rate of DGF was significantly reduced in HMP kidneys (24%; n = 801) compared with SCS kidneys (38%; n = 3515) (OR 0.49 [0.40–0.60]). This result was also confirmed in a subanalysis of 66 pairs of ECD kidneys. HMP also reduced the risk of 1-y graft loss (OR 0.77 [0.60–0.99]).
In contrast in a US study, Basu et al68 found HMP had no significant effect on DGF (HMP 20.8% versus cold storage 25.8%). Kidney graft survival at 1 y was similar but at 3 y it was improved in the SCS kidneys. Six-year graft survival was 64.3% in HMP kidneys and 51.5% in SCS kidneys (P = 0.22). A possible explanation for the lack of effect was that the study included a high percentage of imported kidneys which were only placed on HMP after a significant period of CI. The CIT was also significantly longer in the HMP kidneys (28.9 h versus 24 h, P = 0.003).68
cDCD Kidneys
HMP with UW-MP solution is used for the majority of cDCD kidneys in Europe and in the United States. There have been a number of studies assessing the effects of HMP compared to SCS in cDCD kidneys (Table 3). In a recent meta-analysis, Tingle et al concluded from analysis of 7 studies including 772 DCD kidneys that HMP reduced the risk of DGF compared to SCS (RR 0.75 [0.64–0.87], P = 0.0002). There was strong evidence that HMP also improved graft survival at 1 and 3 y posttransplant.69 The systematic review and meta-analysis reported by Peng et al62 found that the risk of DGF in HMP DCD kidneys was significantly reduced (RR 0.73, 95% CI 0.61-0.88, P = 0.0010).
Using data from the European RCT comparing HMP and SCS in 82 pairs of DCD kidneys, Jochmans et al70 reported that the rate of DGF was reduced from 69.5% in the SCS group to 53.7% in the HMP group (OR 0.43, 95% CI 0.20-0.89, P = 0.025). However, 1-y graft survival was similar (93.9% versus 95.1%).
The PPART study reported by Watson et al71 from the United Kingdom included 45 pairs of kidneys from DCD donors. There was no significant difference in DGF (cold storage 56% versus HMP 58%). The main criticism of the trial was that kidneys were only placed on the HMP system (LifePort Kidney Transporter) on arrival at the recipient center after a period of SCS. Summers et al conducted a following RCT of pairs of kidneys from 51 DCD donors: kidneys underwent HMP (n = 51) for the entire preservation period and were compared to SCS kidneys (n = 51).71 DGF rates in the SCS group were 62.8% versus HMP 58.8% (P = 0.69). PNF in the SCS group was 5.9% and HMP 3.9% (P = 0.65). It is difficult to draw a conclusion from this study as the trial was underpowered and ended prematurely due to difficulty in recruiting. Of note, 53% of the DCD kidneys were from older donors.72 Other smaller studies have found significant reduction in the rate of DGF.91 Patel et al73 published the results of a cohort of kidneys undergoing HMP in the United Kingdom between 2007 and 2015. Rates of DGF were significantly lower than for SCS kidneys (34.2% versus 42.0%; P < 0.001). A marginal functional benefit was found at 1 y but there was no difference in graft survival.
uDCD Kidneys
Pieter Hoogland et al8 reported the outcome of the earliest series of uDCD kidney transplants (n = 135) from 1981 to 2009 in the Netherlands: the DGF rate was 61% and the PNF rate 22%. All kidneys were flushed in situ with HTK solution and the majority preserved by HMP with Belzer UW solution from 1985. Before that Euro-Collins solution was used for HMP.
In a series of uDCD kidneys in France from 2007 to 2010, Abboud et al33 reported a DGF rate of 95% and PNF rate of 5%. All kidneys were preserved by HMP. Patient and graft survival at 1 y were 98% and 91.4%, respectively. Sanni et al92 reported on a series of 100 DCD kidneys, of which 46% were uDCD. Kidneys were retrieved after rapid cooling in situ using HTK solution with 1.5 million units of streptokinase added in a preflush. All kidneys were then machine perfused with Belzer solution at 4 °C. The incidence of DGF was 62%, significantly higher than in a control group of 100 DBD kidneys (P = 0.0002).
Hypothermic Oxygenated Machine Perfusion
Experimental studies suggest that even at hypothermic temperatures the addition of oxygen during perfusion can support ATP synthesis and prevent the deterioration of mitochondrial redox homeostasis to protect against preservation injury.93 This has been studied extensively in the liver.94 In the kidney, the addition of oxygen appears to be particularly beneficial when organs have been subjected to a period of WI injury.95 Buchs et al95 found that levels of ATP were restored in porcine kidneys after 30 min of WI injury. However, oxygenation had no added benefit in kidneys without injury.
In a recent experimental study, Kaminski et al96 monitored cortical tissue levels of ATP and oxygen during HMP and SCS. They found that in kidneys with WI injury, levels of ATP were higher during HMP and oxygen consumption increased compared to SCS.
It also appears that supplementing HMP with oxygen, recently termed hypothermic oxygenated machine perfusion (HOPE), for short durations can be used to resuscitate and condition organs.97 ATP can be replenished to reduce levels of oxidative stress and improve organ viability. Koetting et al97 demonstrated the advantage of adding oxygen during a 90-min period of hypothermic reconditioning to recover organs after ischemic injury with a 3-fold improvement in renal clearance of creatinine. In a rodent model, Kron et al98 found that 1 h of HOPE reduced cytokine release and resulted in less T-cell and macrophage activation. They also demonstrated improved function and less early fibrosis compared to untreated controls.
Darius et al99 advocated the use of a high concentration of oxygen during HMP and found that conditioning with oxygen at the beginning or for the entire 22 h of HMP was preferable to 2 h of HOPE at the end. The mitochondria were better preserved and levels of succinate, lactate, and flavin mononucleotide were significantly reduced compared to kidneys undergoing HMP without oxygen. The optimal concentration of oxygen administered during HMP is still debated. Although studies have shown benefit with the administration of high concentrations (100% and 95%), a concentration of 21% was also shown to reduce oxidative stress and improve the energy status.100
These preliminary research studies have led to the adaption and development of commercially available HMP systems that support oxygenation. LifePort, Kidney Assist, RM3, and WAVES either have membrane oxygenators incorporated into the circuit or they can be easily adapted with an external oxygenator added. There is also some evidence that oxygen can be simply bubbled into the perfusate during HMP to maintain an adequate partial pressure of oxygen, although lower than is achieved with an added oxygenator.99 A new system VitaSmart (Bridge to Life) is a multiorgan pump (liver and kidney) designed for use in the operating theater. The system relies on the simplicity of placing the kidney in a basin of cooled preservation solution on the back table in preparation for benching. The kidney is connected to the system via the renal artery and oxygenated preservation solution continually recirculated using a roller pump until ready for transplantation.
Oxygen can also be applied during SCS or HMP with the use of an extracellular hemoglobin called M101 isolated from the marine lugworm Arenicola marina (HEMO2life; Hemarina, Morlaix, France). M101 can release oxygen across a gradient over a range of temperatures. It also has antioxidant properties to protect against ischemia-reperfusion injury.101
ECD Kidneys
To reduce rates of DGF and improve outcome, there have been several recent studies examining the effect of HOPE on ECD kidneys (Table 3). In 2019, the Consortium for Organ Preservation in Europe (COPE) completed a RCT investigating HOPE after SCS versus SCS (COPE-POMP ISRCTN 63853508). The primary end point was 1-y graft survival and the results are awaited. In Germany, Meister et al102 demonstrated the feasibility of HOPE in a preliminary report of 2 pairs of ECD kidneys, one of each pair treated with 2–3 h of HOPE following SCS using the Kidney Assist Transporter and the other SCS. The same group is currently extending this series to include 15 ECD kidneys with a primary end point of dialysis within the first week of transplantation.103
In a recent safety study, Le Meur et al104 added the M101 oxygen carrier during HMP of ECD kidneys. A series of DBD kidneys undergoing SCS with added M101 was also included in the study. No adverse effects were noted and there was a small decrease in the rate of DGF.
cDCD Kidneys
The COPE consortium recently reported the results of an international double-blind multicenter trial comparing HMP with and without oxygenation using the Kidney Assist Transporter device in pairs of kidneys from >50 y DCD donors (COPE-COMPARE ISRCTN32967929; Table 3). The primary outcome measure was renal function at 12 mo posttransplant. There was no significant difference in graft function when comparing the 83 pairs that reached the primary end point (mean difference in estimated glomerular filtration rate [eGFR] 3.7 mL/min/1.73 m2, 95% CI 1.0-8.4; P = 0.12).74 The incidences of severe complications and episodes of acute rejection were lower in the oxygenated HMP group suggesting a reduction in the immunogenicity of the grafts.105,106 In a recent Italian series, 10 DCD kidneys were preserved by HOPE after normothermic regional perfusion (NRP). The rate of DGF was 30% with no reports of PNF.107
Normothermic Machine Perfusion
Perfusing organs at sub- or near-normal temperatures has become the subject of much research over the last decade. Rather than suppressing metabolism, the conditions are designed to support aerobic metabolism and restore cellular function. This has a number of potential advantages over SCS and standard hypothermic techniques. Injury caused by CI during SCS can be avoided or minimized, it may allow the upregulation of repair mechanisms and also provides the opportunity to make a functional assessment of the kidney. The disadvantage of normothermic machine perfusion (NMP) is that the restoration of cellular function also insights the upregulation of inflammatory mediators that can potentially cause harm.108 Experimental studies have explored techniques using red blood cell–based solutions to support oxygen delivery109 or artificial oxygen carriers such as Hemopure (hemoglobin glutamer-250 [bovine]; HBOC-201, Hemoglobin Oxygen Therapeutics LLC)110 and pyridoxylated bovine hemoglobin reported by Brasile et al.111 Brasile et al111 conducted a significant amount of research from the 1990s using an exsanguinous metabolic support medium (Breonics) made up of a highly enriched tissue culture-like medium containing essential and nonessential amino acids, lipids, and carbohydrates supplemented with bovine hemoglobin to perfuse canine, porcine, and nontransplanted human kidneys.112 They demonstrated that perfusing kidneys at 32 °C for short periods could protect against ischemic injury and that more prolonged periods of perfusion (24 h) could promote recovery and repair.111,112 The addition of growth factor or mesenchymal stromal cells to the kidneys during perfusion could further promote recovery.113,114
Nicholson and Hosgood used more physiological conditions by perfusing porcine and human kidneys with a red blood cell–based solution at 35–36 °C.109,115 NMP was performed for a short 1–2 h period after hypothermic preservation. Experimental evidence showed that ATP could be replenished and protective mechanisms such as heat shock protein 70 were upregulated.115 More recently using a similar protocol Hameed et al116 examined the transcriptional changes in gene expression in pairs of nontransplanted human kidneys. A 1-h period of NMP-activated protective stress responses and promoted cell survival and proliferation.
Kaths et al117 found that more prolonged periods of NMP (8–16 h) were necessary to recover function after WI and CI injury. They used a red blood cell–based solution mixed with STEEN solution. STEEN solution was originally formulated for the lung and contains a high concentration of albumin and dextran to create a high osmotic pressure. For NMP of the kidney, it is necessary to dilute it with a crystalloid solution to prevent cellular damage and diffuse vacuolation of the tubular cells. Weissenbacher et al118,119 also advocated the use of prolonged periods of NMP and demonstrated that by recirculating the urine the kidney could maintain a more stable environment.
Gallinat et al120 also used a preservation medium based on STEEN solution to gradually rewarm kidneys after WI and CI injury. The protocol involved a 90-min controlled phase of rewarming before NMP at 35 °C using STEEN solution. The experimental evidence demonstrated the adequate delivery of oxygen without the addition of an oxygen carrier.121 Gradual rewarming protected against mitochondrial and cellular injury by reducing levels of damage-associated molecular patterns (toll-like receptor 4 and high mobility group box 1 protein) during reperfusion.122 Furthermore, it could more efficiently optimize ATP and oxygen consumption levels during reperfusion compared to the immediate transition from 4 °C.122
Assessment
The increasing interest in NMP for kidney preservation is also focused on its use as a device to assess the viability or quality of the kidney. The higher level of cellular function compared to HMP allows a functional assessment in addition to the cellular biomarkers used during HMP (Table 4).123 More recently, the application of metabolomic and transcriptional analysis has been investigated and may help to reveal future biomarkers (Table 4).
Technologies
At present, the Kidney Assist device made by Organ Assist based in the Netherlands is the only commercially available CE-marked NMP system for the kidney. Other reported systems are in-house adaptations of current cardiopulmonary bypass technology or custom-made perfusion devices. The UK-based company OrganOx has designed a prototype portable system for maintaining a kidney under NMP conditions for prolonged periods but it is not yet available on the market.
DBD Kidneys
NMP has not been reported for the preservation of standard DBD kidneys. However, the Toronto group is conducting a pilot study (n = 25) investigating the effects of 1–10 h of normothermic ex vivo kidney perfusion with a blood-based solution in DBD and DCD kidneys.
ECD Kidneys
NMP using a red blood cell–based perfusate and adapted cardiopulmonary bypass system was first introduced into clinical practice in December 2010 in the United Kingdom.109 A kidney from an ECD rejected by 5 other centers in the United Kingdom was transplanted successfully after 1-h NMP. The recipient had slow graft function for 1 mo but remained dialysis-free.
In 2013, the feasibility and safety of NMP was established in a series of 18 ECD kidneys.75 The DGF rate was 11% compared to 36.2% in a matched series of historical control kidneys that had SCS only; 1-y graft survival and patient survival were similar (Table 3). Hosgood and Nicholson124 also reported that it was feasible and safe to perform NMP then place an ECD kidney back on ice for a further 5 h until transplantation.
Most recently Minor et al used the Kidney Assist device and the controlled rewarming approach with STEEN solution to perfuse an ECD kidney. After 90 min of controlled rewarming, the kidney was successfully transplanted with immediate graft function.125
In the Netherlands, a pilot study has recently been completed that assessed the effects of 2–6 h of NMP using the Kidney Assist device in ECD kidneys.126
cDCD Kidneys
Nicholson and Hosgood have reported several cases of DCD kidneys undergoing 1-h NMP. In each of the cases, NMP was used to salvage the kidneys after they were inadequately flushed during retrieval.127,128 In 1 case, the kidney was retrieved after NRP. In both cases, the kidneys performed well during NMP and were transplanted successfully.
With the promising results from the series of ECD kidneys, the NMP technology is being assessed in a RCT of DCD kidneys.129 Kidneys are randomized to receive either SCS or NMP in a 1:1 ratio. The trial is due to report in 2021 (ISRCTN15821205).
There is 1 report of using NMP technology to avoid any exposure to CI. He et al130 adapted the Kidney Assist device to perfuse a DCD kidney by cannulating the infrarenal abdominal aorta and suprarenal inferior renal cava and transferring to the Kidney Assist device while maintaining circulation. The kidney was perfused for 110 min with a red blood cell–based solution before implantation into the recipient. Circulation was maintained throughout and the recipient had immediate graft function.
Normothermic Regional Perfusion
Traditionally, organs from deceased and DCD donors are rapidly flushed in situ with cold preservation solution to reduce the temperature and lower the metabolism as quickly as possible to prevent cellular injury. NRP involves the restoration of circulation with the donor’s own blood using extracorporeal membrane oxygenation technology after confirmation of circulatory arrest. NRP can be carried out over a range of temperatures, subnormothermic (4–22 °C) or normothermic (35–37 °C). In the United Kingdom, the donor is heparinized after cardiac death to prevent clotting, but heparinization is permitted before cardiac death in some European protocols. Circulation is restored for a variable period of time (1–6 h). NRP is thought to condition the organs by upregulating adenosine receptors which may protect against preservation injury. In a recent porcine study, NRP was found to enhance the expression of protective mechanisms (erythropoietin, heme-oxygenase-1, glucose transport 1, and vascular endothelial growth factor) via the hypoxia-inducible factor 1α signaling pathway.131 They found levels of aspartate aminotransferase decreased over 4 h of NRP, which suggested a reduction in injury and a possible reconditioning effect. NRP was difficult to maintain after 4 h and an increase in inflammatory markers with high levels of monocyte chemoattractant protein-1, interleukin 1β and significant macrophage infiltration, together with increased platelet activation and expression of thrombomodulin, suggest that it may have some detrimental effects.131
Donor Assist (Organ Assist) is the one commercially available dedicated NRP system. Other systems are adapted extracorporeal membrane oxygenation or cardiac bypass systems (Maquet, Medtronic).
cDCD Kidneys
In situ cooling has been the standard method of cooling before organ retrieval for all deceased donors. However, there is growing interest in NRP for DCD donors. Early series of NRP in cDCDs demonstrated that its application could increase the number of available organs with good outcomes. DGF rates were reported at 8.3% and 11%.132,133 In comparison to in situ perfusion or total body cooling the incidence of DGF and PNF was lower in the NRP group (Table 3).134
More recently, in the first series from the United Kingdom, the outcomes of 14 kidneys from 8 cDCD donors were reported: 1 pair of kidney was not used due to a high Remuzzi score; 1 pair had PNF and were removed 5 d posttransplant; and 2 recipients had DGF (18%).135 In the second study from the United Kingdom, Oniscu et al136 reported on 21 NRP retrievals from 3 centers in the United Kingdom. Thirty-two kidneys were transplanted with a DGF rate of 40%. Miñambres et al23 reported the first Spanish series of 27 NRP donors from which 37 kidneys were transplanted and reported a DGF rate of 27%. They compared the results to a series of 51 DBD donors and found no statistically significant difference in graft survival 18 mo posttransplant (91.8% versus 97.2%).23 In an early series from the United States, between 2000 and 2013, the rate of DGF in 48 kidneys retrieved from 37 cDCD donors was 31%.38 Several other studies have found comparable outcomes with DBD kidneys.23,27,28,76,137 The largest study from France included 92 kidneys from NRP donors and 5176 DBD donors and reported significantly lower levels of DGF (9% versus 19%, P < 0.05).76 Kidneys in the NRP cohort all underwent HMP following NRP.
uDCD Kidneys
Some European countries advocate the application of NRP in their uDCD programs to reduce the risk of PNF and increase utility of organ donors.27
Reznik et al138 reported the application of subnormothermic NRP (27–32 °C) in uDCD donors using leukocyte-depleted blood. Forty-four kidneys were transplanted from 22 donors. The DGF rate was 52.3% and there were no incidences of PNF. Graft survival at 1 y was 95.5%.
Results from large studies in Spain and France are encouraging. In Madrid, 237 kidneys were transplanted after NRP and compared to 237 DBD kidneys. The rate of DGF was 73.4% versus 46.4% (P < 0.01) and graft and patient survival similar at 10 y (82.1% versus 80.5%, P = 0.623; 86.2% versus 87.6%, P = 0.454). PNF rate was 6.8% versus 4.2% in the DBD kidneys.28 In another study including 517 kidneys from 445 uDCD donors across different centers in Spain, the incidence of DGF was 76%, PNF rate was 10%, and graft survival at 1 y was 87%.137 A proportion of the donors in this study were >60 years of age and therefore the risk of PNF enhanced. Overall, NRP strategies were preferable to in situ cooling.
In a study from France including 499 kidneys from 414 uDCD donors across 15 different transplant centers from 2007 to 2014, Antoine et al76 found that the risk of PNF and graft loss was significantly higher if NRP was not used (OR 2.6, CI 1.5-4.6). In a smaller study from Portugal of 44 kidneys from 40 uDCD donors, the incidence of DGF was 68% and PNF 9%.139
CONCLUSIONS
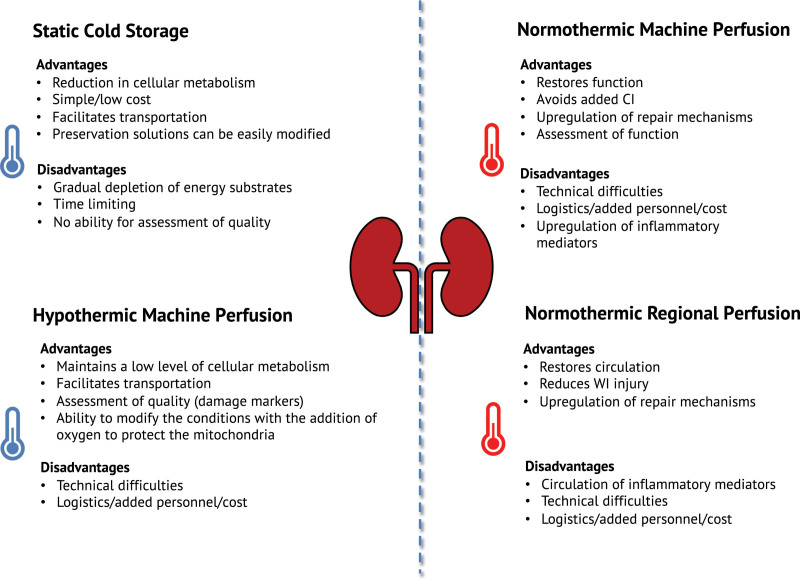
This review provides an overview of the different techniques of kidney preservation and evidence for their application in clinical transplantation for each type of organ donor. Each technique has advantages and disadvantages listed in Figure 1. SCS is a robust method of preservation and results suggest that for living donor and SCD donor kidneys it is a satisfactory method of preservation with no clear advantage of 1 type of preservation solution over another. Nonetheless, UW solution remains the gold standard and most widely used in deceased donor transplantation. There has been little emphasis on the development of new SCS preservation solutions since the 1990s and in recent times few studies have assessed the effects of different solutions, particularly in DCD kidneys. Much of the research has focused on comparing HMP with SCS techniques, with many studies showing reduced risk of DGF.
FIGURE 1.
Advantages and disadvantages of different types of kidney preservation. CI, cold ischemia; WI, warm ischemia.
Since 2016, HMP has become standard practice in the Netherlands for all deceased donor kidneys including SCDs. HMP has received significant support from industry, with a number of commercially available systems on the market. Nonetheless, HMP is not supported by all studies. Furthermore, there is limited evidence for the benefits of HMP on graft survival compared to SCS techniques.
Active oxygenated HMP is an encouraging technique of preservation and has shown significant advantages in liver transplantation. In the kidney, the results of the COPE consortium RCT in DCD donors >50 y demonstrated the safety and feasibility of added oxygen which also may transfer some health-economic benefit of the technique. The results from the COPE led in ECD kidneys are eagerly awaited. The novel application of the oxygen carrier M101 to the preservation solution during SCS or HMP is also a promising new strategy to reduce the effects of CI and warrants further assessment.
NMP is also a promising new technique of preservation that offers more than a simple means of preservation. The ability to assess function, reverse the effects of ischemic injury and its potential use as platform for the delivery of therapeutic agents have significant advantages (Figure 1). However, at present, there is a limited amount of evidence for clinical benefits compared to hypothermic techniques. The utilization of NMP is increasing, with results from small case series of ECD kidneys in the Netherlands and DBD/DCD kidneys in Canada expected this year. NMP can be carried out over a range of temperatures, for different durations and using different compositions of perfusate.140 Further experimental work is required to optimize NMP conditions and to investigate the mechanistic actions before more widespread use in clinical practice.
The results from the application of NRP are also encouraging particularly for increasing the utility of uDCD donors. Although the rates of DGF and PNF are high, graft survival is similar to other deceased donor kidneys. Nonetheless, the rate of organ utilization could be improved and there is no firm evidence of the benefits compared to standard cold in situ organ retrieval.
At present, the number of different preservation techniques is growing and, in the future, there are likely to be a range of different modes of preservation available in kidney transplantation tailored for specific types of donor kidney. Logistics and costs are factors that remain challenging and the application of these technologies may not be available to all (Figure 1). There is a lack of well-designed RCTs comparing different techniques of preservation as highlighted in Table 4. More RCTs are needed to determine the best mode of preservation for specific types of donor kidney, with particular focus on improving long-term graft function and survival. The development of reliable biomarkers to assessment viability and predict outcome is underway, with interest in exosomes and nanoparticles. The application of transcriptional and metabolomic approaches, particularly with the use of NMP, will allow the refinement of preservation techniques and improve outcomes.
Footnotes
The authors declare no conflicts of interest.
The research was funded by the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The views expressed are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care, or NHSBT.
S.A.H. designed the research and wrote the article. R.J.B. designed the research, co-wrote, and reviewed the article. M.L.N. co-wrote and reviewed the article.
REFERENCES
- 1.van’t Hoff MJH. Etudes de dynamique chimique. Recueil des Travaux Chimiques des Pays-Bas. 2010;3:333–336. [Google Scholar]
- 2.Kalogeris T, Baines CP, Krenz M, et al. Ischemia/reperfusion. Compr Physiol. 2016;7:113–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summers DM, Johnson RJ, Hudson A, et al. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet. 2013;381:727–734. [DOI] [PubMed] [Google Scholar]
- 4.Locke JE, Segev DL, Warren DS, et al. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant. 2007;7:1797–1807. [DOI] [PubMed] [Google Scholar]
- 5.Rincon Cintra da Cruz P, Cabral Dias Filho A, Santana VBBM, et al. Donor age amplifies the detrimental effects of cold ischemia time on long-term kidney allograft survival independently of the occurrence of delayed graft function or early acute rejection. Exp Clin Transplant. 2020;18:436–443. [DOI] [PubMed] [Google Scholar]
- 6.Kvietkauskas M, Leber B, Strupas K, et al. Machine perfusion of extended criteria donor organs: immunological aspects. Front Immunol. 2020;11:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lomero M, Gardiner D, Coll E, et al. ; European Committee on Organ Transplantation of the Council of Europe (CD-P-TO). Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020;33:76–88. [DOI] [PubMed] [Google Scholar]
- 8.Pieter Hoogland ER, van Smaalen TC, Christiaans MH, et al. Kidneys from uncontrolled donors after cardiac death: which kidneys do worse? Transpl Int. 2013;26:477–484. [DOI] [PubMed] [Google Scholar]
- 9.Collins MG, Chang SH, Russ GR, et al. Outcomes of transplantation using kidneys from donors meeting expanded criteria in Australia and New Zealand, 1991 to 2005. Transplantation. 2009;87:1201–1209. [DOI] [PubMed] [Google Scholar]
- 10.Fraser SM, Rajasundaram R, Aldouri A, et al. Acceptable outcome after kidney transplantation using “expanded criteria donor” grafts. Transplantation. 2010;89:88–96. [DOI] [PubMed] [Google Scholar]
- 11.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–125. [DOI] [PubMed] [Google Scholar]
- 12.Mogulla MR, Bhattacharjya S, Clayton PA. Risk factors for and outcomes of delayed graft function in live donor kidney transplantation - a retrospective study. Transpl Int. 2019;32:1151–1160. [DOI] [PubMed] [Google Scholar]
- 13.Nafar M, Ahmadpoor P, Al Otaibi T, et al. The frequency and risk factors of delayed graft function in living donor kidney transplantation and its clinical impact on graft and patient survival in part of Middle East. Urol J. 2020;17:55–60. [DOI] [PubMed] [Google Scholar]
- 14.NHSBT kidney transplantation annual report 2018/19. 2019. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/16778/nhsbt-kidney-transplantation-annual-report-2018-19.pdf. Accessed August 2020.
- 15.Wang JH, Skeans MA, Israni AK. Current status of kidney transplant outcomes: dying to survive. Adv Chronic Kidney Dis. 2016;23:281–286. [DOI] [PubMed] [Google Scholar]
- 16.Pine JK, Goldsmith PJ, Ridgway DM, et al. Comparable outcomes in donation after cardiac death and donation after brainstem death: a matched analysis of renal transplants. Transplant Proc. 2010;42:3947–3948. [DOI] [PubMed] [Google Scholar]
- 17.Wadei HM, Heckman MG, Rawal B, et al. Comparison of kidney function between donation after cardiac death and donation after brain death kidney transplantation. Transplantation. 2013;96:274–281. [DOI] [PubMed] [Google Scholar]
- 18.Summers DM, Watson CJ, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int. 2015;88:241–249. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Wang C, Ko DS, et al. Comparison of outcomes of kidney transplantation from donation after brain death, donation after circulatory death, and donation after brain death followed by circulatory death donors. Clin Transplant. 2017;31. doi:10.1111/ctr.13110 [DOI] [PubMed] [Google Scholar]
- 20.Ferreira E, Costa J, Romãozinho C, et al. Long-term outcomes of kidney transplantation from expanded-criteria deceased donors: a single-center experience. Transplant Proc. 2017;49:770–776. [DOI] [PubMed] [Google Scholar]
- 21.de Kok MJC, Schaapherder AFM, Alwayn IPJ, et al. Improving outcomes for donation after circulatory death kidney transplantation: science of the times. PLoS One. 2020;15:e0236662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Dinh H, Weekers L, Bonvoisin C, et al. Delayed graft function does not harm the future of donation-after-cardiac death in kidney transplantation. Transplant Proc. 2012;44:2795–2802. [DOI] [PubMed] [Google Scholar]
- 23.Miñambres E, Suberviola B, Dominguez-Gil B, et al. Improving the outcomes of organs obtained from controlled donation after circulatory death donors using abdominal normothermic regional perfusion. Am J Transplant. 2017;17:2165–2172. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RJ, Bradbury LL, Martin K, et al. ; UK Transplant Registry. Organ donation and transplantation in the UK-the last decade: a report from the UK national transplant registry. Transplantation. 2014;97(Suppl 1):S1–S27. [DOI] [PubMed] [Google Scholar]
- 25.Gentil MA, Castro P, Ballesteros L, et al. Survival of kidney allograft of donors after circulatory death is similar to donors after brain death: experience in a regional program. Transplant Proc. 2015;47:2572–2574. [DOI] [PubMed] [Google Scholar]
- 26.Zhu D, McCague K, Lin W, et al. Outcome of kidney transplantation from donor after cardiac death: reanalysis of the US mycophenolic renal transplant registry. Transplant Proc. 2018;50:1258–1263. [DOI] [PubMed] [Google Scholar]
- 27.Coll E, Miñambres E, Sánchez-Fructuoso A, et al. Uncontrolled donation after circulatory death: a unique opportunity. Transplantation. 2020;104:1542–1552. [DOI] [PubMed] [Google Scholar]
- 28.Molina M, Guerrero-Ramos F, Fernández-Ruiz M, et al. Kidney transplant from uncontrolled donation after circulatory death donors maintained by nECMO has long-term outcomes comparable to standard criteria donation after brain death. Am J Transplant. 2019;19:434–447. [DOI] [PubMed] [Google Scholar]
- 29.Viglietti D, Abboud I, Hill G, et al. Kidney allograft fibrosis after transplantation from uncontrolled circulatory death donors. Transplantation. 2015;99:409–415. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Fructuoso AI, Marques M, Prats D, et al. Victims of cardiac arrest occurring outside the hospital: a source of transplantable kidneys. Ann Intern Med. 2006;145:157–164. [DOI] [PubMed] [Google Scholar]
- 31.Metcalfe MS, Butterworth PC, White SA, et al. A case-control comparison of the results of renal transplantation from heart-beating and non-heart-beating donors. Transplantation. 2001;71:1556–1559. [DOI] [PubMed] [Google Scholar]
- 32.Barlow AD, Metcalfe MS, Johari Y, et al. Case-matched comparison of long-term results of non-heart beating and heart-beating donor renal transplants. Br J Surg. 2009;96:685–691. [DOI] [PubMed] [Google Scholar]
- 33.Abboud I, Viglietti D, Antoine C, et al. Preliminary results of transplantation with kidneys donated after cardiocirculatory determination of death: a French single-centre experience. Nephrol Dial Transplant. 2012;27:2583–2587. [DOI] [PubMed] [Google Scholar]
- 34.Allen RDM, Pleass HCC, Woodroffe C, et al. Challenges of kidney paired donation transplants involving multiple donor and recipient surgeons across Australia. ANZ J Surg. 2018;88:167–171. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan AR, Wong G, Chapman JR, et al. Prolonged ischemic time, delayed graft function, and graft and patient outcomes in live donor kidney transplant recipients. Am J Transplant. 2016;16:2714–2723. [DOI] [PubMed] [Google Scholar]
- 36.Simpkins CE, Montgomery RA, Hawxby AM, et al. Cold ischemia time and allograft outcomes in live donor renal transplantation: is live donor organ transport feasible? Am J Transplant. 2007;7:99–107. [DOI] [PubMed] [Google Scholar]
- 37.West MS, Sutherland DE, Matas AJ. Considering death with function as a graft loss in kidney transplant recipients. Transplant Proc. 1997;29:239. [DOI] [PubMed] [Google Scholar]
- 38.Rojas-Peña A, Sall LE, Gravel MT, et al. Donation after circulatory determination of death: The University of Michigan experience with extracorporeal support. Transplantation. 2014;98:328–334. [DOI] [PubMed] [Google Scholar]
- 39.Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours’ ice storage. Lancet. 1969;2:1219–1222. [DOI] [PubMed] [Google Scholar]
- 40.Opelz G, Terasaki PI. Kidney preservation: perfusion versus cold storage-1975. Transplant Proc. 1976;8:121–125. [PubMed] [Google Scholar]
- 41.Jablonski P, Howden B, Marshall V, et al. Evaluation of citrate flushing solution using the isolated perfused rat kidney. Transplantation. 1980;30:239–243. [DOI] [PubMed] [Google Scholar]
- 42.Southard JH, Senzig KA, Hoffman RM, et al. Energy metabolism in kidneys stored by simple hypothermia. Transplant Proc. 1977;9:1535–1539. [PubMed] [Google Scholar]
- 43.Belzer FO, Glass NR, Sollinger HW, et al. A new perfusate for kidney preservation. Transplantation. 1982;33:322–323. [PubMed] [Google Scholar]
- 44.Fridell JA, Mangus RS, Tector AJ. Clinical experience with histidine-tryptophan-ketoglutarate solution in abdominal organ preservation: a review of recent literature. Clin Transplant. 2009;23:305–312. [DOI] [PubMed] [Google Scholar]
- 45.Boku N, Tanoue Y, Kajihara N, et al. A comparative study of cardiac preservation with Celsior or University of Wisconsin solution with or without prior administration of cardioplegia. J Heart Lung Transplant. 2006;25:219–225. [DOI] [PubMed] [Google Scholar]
- 46.Maathuis MH, Ottens PJ, van Goor H, et al. Static cold storage preservation of ischemically damaged kidneys. a comparison between IGL-1 and UW solution. Transpl Int. 2008;21:473–482. [DOI] [PubMed] [Google Scholar]
- 47.Billault C, Vaessen C, Van Glabeke E, et al. Use of the SCOT solution in kidney transplantation: preliminary report. Transplant Proc. 2006;38:2281–2282. [DOI] [PubMed] [Google Scholar]
- 48.Sui M, Zhang L, Yang J, et al. A new HC-A II solution for kidney preservation: a multi-center randomized controlled trial in China. Ann Transplant. 2014;19:614–620. [DOI] [PubMed] [Google Scholar]
- 49.Jamieson RW, Friend PJ. PBS140: new competition in the organ preservation market? Kidney Int. 2006;69:784–785. [DOI] [PubMed] [Google Scholar]
- 50.House AK, Brooks AJ, Woodman K, et al. A comparison of flushing fluids for initial perfusion of kidneys for transplantation. Aust N Z J Surg. 1979;49:705–708. [DOI] [PubMed] [Google Scholar]
- 51.Schreinemachers MC, Bemelman FJ, Idu MM, et al. First clinical experience with polysol solution: pilot study in living kidney transplantation. Transplant Proc. 2013;45:38–45. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Shi J, Xia TC, et al. Preservation solutions for kidney transplantation: history, advances and mechanisms. Cell Transplant. 2019;28:1472–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuller B, Froghi F, Davidson B. Organ preservation solutions: linking pharmacology to survival for the donor organ pathway. Curr Opin Organ Transplant. 2018;23:361–368. [DOI] [PubMed] [Google Scholar]
- 54.Parsons RF, Guarrera JV. Preservation solutions for static cold storage of abdominal allografts: which is best? Curr Opin Organ Transplant. 2014;19:100–107. [DOI] [PubMed] [Google Scholar]
- 55.Lynch RJ, Kubus J, Chenault RH, et al. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin preservation in renal transplantation. Am J Transplant. 2008;8:567–573. [DOI] [PubMed] [Google Scholar]
- 56.O’Callaghan JM, Knight SR, Morgan RD, et al. Preservation solutions for static cold storage of kidney allografts: a systematic review and meta-analysis. Am J Transplant. 2012;12:896–906. [DOI] [PubMed] [Google Scholar]
- 57.Legeai C, Durand L, Savoye E, et al. Effect of preservation solutions for static cold storage on kidney transplantation outcomes: a National Registry Study. Am J Transplant. 2020;20:3426–3442. [DOI] [PubMed] [Google Scholar]
- 58.Faenza A, Catena F, Nardo B, et al. Kidney preservation with University of Wisconsin and Celsior solution: a prospective multicenter randomized study. Transplantation. 2001;72:1274–1277. [DOI] [PubMed] [Google Scholar]
- 59.Montalti R, Nardo B, Capocasale E, et al. Kidney transplantation from elderly donors: a prospective randomized study comparing Celsior and UW solutions. Transplant Proc. 2005;37:2454–2455. [DOI] [PubMed] [Google Scholar]
- 60.Stewart ZA, Lonze BE, Warren DS, et al. Histidine-tryptophan-ketoglutarate (HTK) is associated with reduced graft survival of deceased donor kidney transplants. Am J Transplant. 2009;9:1048–1054. [DOI] [PubMed] [Google Scholar]
- 61.Moser MAJ, Ginther N, Luo Y, et al. Early experience with hypothermic machine perfusion of living donor kidneys - a retrospective study. Transpl Int. 2017;30:706–712. [DOI] [PubMed] [Google Scholar]
- 62.Peng P, Ding Z, He Y, et al. Hypothermic machine perfusion versus static cold storage in deceased donor kidney transplantation: a systematic review and meta-analysis of randomized controlled trials. Artif Organs. 2019;43:478–489. [DOI] [PubMed] [Google Scholar]
- 63.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19. [DOI] [PubMed] [Google Scholar]
- 64.Tingle SJ, Figueiredo RS, Moir JA, et al. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. 2019;3:CD011671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiao B, Liu S, Liu H, et al. Hypothermic machine perfusion reduces delayed graft function and improves one-year graft survival of kidneys from expanded criteria donors: a meta-analysis. PLoS One. 2013;8:e81826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallinat A, Moers C, Smits JM, et al. Machine perfusion versus static cold storage in expanded criteria donor kidney transplantation: 3-year follow-up data. Transpl Int. 2013;26:E52–E53. [DOI] [PubMed] [Google Scholar]
- 67.Savoye E, Macher MA, Videcoq M, et al. Evaluation of outcomes in renal transplantation with hypothermic machine perfusion for the preservation of kidneys from expanded criteria donors. Clin Transplant. 2019;33:e13536. [DOI] [PubMed] [Google Scholar]
- 68.Basu A, Rosen LM, Tan HP, et al. Outcomes of deceased donor kidney transplantation using expanded criteria donor kidneys following pulsatile preservation. Cureus. 2019;11:e5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tingle SJ, Figueiredo RS, Moir JA, et al. Hypothermic machine perfusion is superior to static cold storage in deceased donor kidney transplantation: a meta-analysis. Clin Transplant. 2020;34:e13814. [DOI] [PubMed] [Google Scholar]
- 70.Jochmans I, Moers C, Smits JM, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. 2010;252:756–764. [DOI] [PubMed] [Google Scholar]
- 71.Watson CJ, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 2010;10:1991–1999. [DOI] [PubMed] [Google Scholar]
- 72.Summers DM, Ahmad N, Randle LV, et al. cold pulsatile machine perfusion versus static cold storage for kidneys donated after circulatory death: a multicenter randomized controlled trial. Transplantation. 2020;104:1019–1025. [DOI] [PubMed] [Google Scholar]
- 73.Patel K, Nath J, Hodson J, et al. Outcomes of donation after circulatory death kidneys undergoing hypothermic machine perfusion following static cold storage: a UK population-based cohort study. Am J Transplant. 2018;18:1408–1414. [DOI] [PubMed] [Google Scholar]
- 74.Jochmans I, Brat A, Davies L, et al. ; COMPARE Trial Collaboration and Consortium for Organ Preservation in Europe (COPE). Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): a randomised, double-blind, paired, phase 3 trial. Lancet. 2020;396:1653–1662. [DOI] [PubMed] [Google Scholar]
- 75.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. 2013;13:1246–1252. [DOI] [PubMed] [Google Scholar]
- 76.Antoine C, Savoye E, Gaudez F, et al. ; National Steering Committee of Donation After Circulatory Death. Kidney transplant from uncontrolled donation after circulatory death: contribution of normothermic regional perfusion. Transplantation. 2020;104:130–136. [DOI] [PubMed] [Google Scholar]
- 77.Tan HP, Vyas D, Basu A, et al. Cold heparinized lactated ringers with procaine (HeLP) preservation fluid in 266 living donor kidney transplantations. Transplantation. 2007;83:1134–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ploeg RJ, van Bockel JH, Langendijk PT, et al. Effect of preservation solution on results of cadaveric kidney transplantation. The European Multicentre Study Group. Lancet. 1992;340:129–137. [DOI] [PubMed] [Google Scholar]
- 79.Agarwal A, Murdock P, Fridell JA. Comparison of histidine-tryptophan ketoglutarate solution and University of Wisconsin solution in prolonged cold preservation of kidney allografts. Transplantation. 2006;81:480–482. [DOI] [PubMed] [Google Scholar]
- 80.Stevens RB, Skorupa JY, Rigley TH, et al. Increased primary non-function in transplanted deceased-donor kidneys flushed with histidine-tryptophan-ketoglutarate solution. Am J Transplant. 2009;9:1055–1062. [DOI] [PubMed] [Google Scholar]
- 81.Nishikido M, Noguchi M, Koga S, et al. Kidney transplantation from non-heart-beating donors: analysis of organ procurement and outcome. Transplant Proc. 2004;36:1888–1890. [DOI] [PubMed] [Google Scholar]
- 82.Lindell SL, Compagnon P, Mangino MJ, et al. UW solution for hypothermic machine perfusion of warm ischemic kidneys. Transplantation. 2005;79:1358–1361. [DOI] [PubMed] [Google Scholar]
- 83.Giraud S, Steichen C, Couturier P, et al. Influence of hypoxic preservation temperature on endothelial cells and kidney integrity. Biomed Res Int. 2019;2019:8572138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nordling S, Brännström J, Carlsson F, et al. Enhanced protection of the renal vascular endothelium improves early outcome in kidney transplantation: preclinical investigations in pig and mouse. Sci Rep. 2018;8:5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tozzi M, Franchin M, Soldini G, et al. Impact of static cold storage vs hypothermic machine preservation on ischemic kidney graft: inflammatory cytokines and adhesion molecules as markers of ischemia/reperfusion tissue damage. Our preliminary results. Int J Surg. 2013;11(Suppl 1):S110–S114. [DOI] [PubMed] [Google Scholar]
- 86.He N, Li JH, Jia JJ, et al. Hypothermic machine perfusion’s protection on porcine kidney graft uncovers greater Akt-Erk phosphorylation [published correction appears in Transplant Proc. 2018; 50(2): 690]. Transplant Proc. 2017;49:1923–1929. [DOI] [PubMed] [Google Scholar]
- 87.Chatauret N, Coudroy R, Delpech PO, et al. Mechanistic analysis of nonoxygenated hypothermic machine perfusion’s protection on warm ischemic kidney uncovers greater eNOS phosphorylation and vasodilation. Am J Transplant. 2014;14:2500–2514. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z, Zhong Z, Lan J, et al. Mechanisms of hypothermic machine perfusion to decrease donation after cardiac death graft inflammation: through the pathway of upregulating expression of KLF2 and inhibiting TGF-β signaling. Artif Organs. 2017;41:82–88. [DOI] [PubMed] [Google Scholar]
- 89.Kox J, Moers C, Monbaliu D, et al. The benefits of hypothermic machine preservation and short cold ischemia times in deceased donor kidneys. Transplantation. 2018;102:1344–1350. [DOI] [PubMed] [Google Scholar]
- 90.Guy A, McGrogan D, Inston N, et al. Hypothermic machine perfusion permits extended cold ischemia times with improved early graft function. Exp Clin Transplant. 2015;13:130–137. [PubMed] [Google Scholar]
- 91.Bellini MI, Charalampidis S, Herbert PE, et al. Cold pulsatile machine perfusion versus static cold storage in kidney transplantation: a single centre experience. Biomed Res Int. 2019;2019:7435248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanni AO, Wilson CH, Wyrley-Birch H, et al. Non-heart-beating kidney transplantation: 6-year outcomes. Transplant Proc. 2006;38:3396–3397. [DOI] [PubMed] [Google Scholar]
- 93.Minor T, Paul A. Hypothermic reconditioning in organ transplantation. Curr Opin Organ Transplant. 2013;18:161–167. [DOI] [PubMed] [Google Scholar]
- 94.Schlegel A, Muller X, Kalisvaart M, et al. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol. 2019;70:50–57. [DOI] [PubMed] [Google Scholar]
- 95.Buchs JB, Lazeyras F, Ruttimann R, et al. Oxygenated hypothermic pulsatile perfusion versus cold static storage for kidneys from non heart-beating donors tested by in-line ATP resynthesis to establish a strategy of preservation. Perfusion. 2011;26:159–165. [DOI] [PubMed] [Google Scholar]
- 96.Kaminski J, Delpech PO, Kaaki-Hosni S, et al. Oxygen consumption by warm ischemia-injured porcine kidneys in hypothermic static and machine preservation. J Surg Res. 2019;242:78–86. [DOI] [PubMed] [Google Scholar]
- 97.Koetting M, Frotscher C, Minor T. Hypothermic reconditioning after cold storage improves postischemic graft function in isolated porcine kidneys. Transpl Int. 2010;23:538–542. [DOI] [PubMed] [Google Scholar]
- 98.Kron P, Schlegel A, Muller X, et al. Hypothermic oxygenated perfusion: a simple and effective method to modulate the immune response in kidney transplantation. Transplantation. 2019;103:e128–e136. [DOI] [PubMed] [Google Scholar]
- 99.Darius T, Gianello P, Vergauwen M, et al. The effect on early renal function of various dynamic preservation strategies in a preclinical pig ischemia-reperfusion autotransplant model. Am J Transplant. 2019;19:752–762. [DOI] [PubMed] [Google Scholar]
- 100.Venema LH, Brat A, Moers C, et al. ; COPE consortium. Effects of oxygen during long-term hypothermic machine perfusion in a porcine model of kidney donation after circulatory death. Transplantation. 2019;103:2057–2064. [DOI] [PubMed] [Google Scholar]
- 101.Kaminski J, Hannaert P, Kasil A, et al. Efficacy of the natural oxygen transporter HEMO2 life® in cold preservation in a preclinical porcine model of donation after cardiac death. Transpl Int. 2019;32:985–996. [DOI] [PubMed] [Google Scholar]
- 102.Meister FA, Czigany Z, Bednarsch J, et al. Hypothermic oxygenated machine perfusion-preliminary experience with end-ischemic reconditioning of marginal kidney allografts. Clin Transplant. 2019;33:e13673. [DOI] [PubMed] [Google Scholar]
- 103.Meister FA, Czigany Z, Bednarsch J, et al. Hypothermic oxygenated machine perfusion of extended criteria kidney allografts from brain dead donors: protocol for a prospective pilot study. JMIR Res Protoc. 2019;8:e14622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Le Meur Y, Badet L, Essig M, et al. First-in-human use of a marine oxygen carrier (M101) for organ preservation: a safety and proof-of-principle study. Am J Transplant. 2020;20:1729–1738. [DOI] [PubMed] [Google Scholar]
- 105.Jochmans I, O’Callaghan JM, Pirenne J, et al. Hypothermic machine perfusion of kidneys retrieved from standard and high-risk donors. Transpl Int. 2015;28:665–676. [DOI] [PubMed] [Google Scholar]
- 106.O’Neill S, Srinivasa S, Callaghan CJ, et al. Novel organ perfusion and preservation strategies in transplantation - where are we going in the United Kingdom? Transplantation. 2020;104:1813–1824. [DOI] [PubMed] [Google Scholar]
- 107.Ravaioli M, De Pace V, Angeletti A, et al. Hypothermic oxygenated new machine perfusion system in liver and kidney transplantation of extended criteria donors: first Italian clinical trial. Sci Rep. 2020;10:6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferdinand JR, Hosgood SA, Moore T, et al. Cytokine absorption during human kidney perfusion reduces delayed graft function-associated inflammatory gene signature. Am J Transplant. 2020. doi:10.1111/ajt.16371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosgood SA, Nicholson ML. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation. 2011;92:735–738. [DOI] [PubMed] [Google Scholar]
- 110.Bhattacharjee RN, Patel SVB, Sun Q, et al. Renal protection against ischemia reperfusion injury: hemoglobin-based oxygen carrier-201 versus blood as an oxygen carrier in ex vivo subnormothermic machine perfusion. Transplantation. 2020;104:482–489. [DOI] [PubMed] [Google Scholar]
- 111.Brasile L, Stubenitsky BM, Booster MH, et al. Hypothermia – a limiting factor in using warm ischemically damaged kidneys [published correction appears in Am J Transplant. 2010; 10(12): 2729]. Am J Transplant. 2001;1:316–320. [DOI] [PubMed] [Google Scholar]
- 112.Brasile L, Glowacki P, Castracane J, et al. Pretransplant kidney-specific treatment to eliminate the need for systemic immunosuppression. Transplantation. 2010;90:1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brasile L, Stubenitsky BM, Haisch CE, et al. Repair of damaged organs in vitro. Am J Transplant. 2005;5:300–306. [DOI] [PubMed] [Google Scholar]
- 114.Brasile L, Henry N, Orlando G, et al. Potentiating renal regeneration using mesenchymal stem cells. Transplantation. 2019;103:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hosgood SA, Patel M, Nicholson ML. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res. 2013;182:153–160. [DOI] [PubMed] [Google Scholar]
- 116.Hameed AM, Lu DB, Patrick E, et al. Brief normothermic machine perfusion rejuvenates discarded human kidneys. Transplant Direct. 2019;5:e502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaths JM, Echeverri J, Linares I, et al. Normothermic ex vivo kidney perfusion following static cold storage-brief, intermediate, or prolonged perfusion for optimal renal graft reconditioning? Am J Transplant. 2017;17:2580–2590. [DOI] [PubMed] [Google Scholar]
- 118.Weissenbacher A, Lo Faro L, Boubriak O, et al. Twenty-four-hour normothermic perfusion of discarded human kidneys with urine recirculation. Am J Transplant. 2019;19:178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weissenbacher A, Huang H, Surik T, et al. Urine recirculation prolongs normothermic kidney perfusion via more optimal metabolic homeostasis – a proteomics study. Am J Transplant. 2020. doi:10.1111/ajt.16334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gallinat A, Lu J, von Horn C, et al. Transplantation of cold stored porcine kidneys after controlled oxygenated rewarming. Artif Organs. 2018;42:647–654. [DOI] [PubMed] [Google Scholar]
- 121.Minor T, von Horn C, Paul A. Role of erythrocytes in short-term rewarming kidney perfusion after cold storage. Artif Organs. 2019;43:584–592. [DOI] [PubMed] [Google Scholar]
- 122.Minor T, von Horn C. Rewarming injury after cold preservation. Int J Mol Sci. 2019;20:2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kabagambe SK, Palma IP, Smolin Y, et al. Combined ex vivo hypothermic and normothermic perfusion for assessment of high-risk deceased donor human kidneys for transplantation. Transplantation. 2019;103:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hosgood SA, Nicholson ML. The first clinical case of intermediate ex vivo normothermic perfusion in renal transplantation. Am J Transplant. 2014;14:1690–1692. [DOI] [PubMed] [Google Scholar]
- 125.Minor T, von Horn C, Gallinat A, et al. First-in-man controlled rewarming and normothermic perfusion with cell-free solution of a kidney prior to transplantation. Am J Transplant. 2020;20:1192–1195. [DOI] [PubMed] [Google Scholar]
- 126.Rijkse E, IJzermans JN, Minnee RC. Machine perfusion in abdominal organ transplantation: current use in the Netherlands. World J Transplant. 2020;10:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Georgiades F, Hosgood SA, Butler AJ, et al. Use of ex vivo normothermic machine perfusion after normothermic regional perfusion to salvage a poorly perfused DCD kidney. Am J Transplant. 2019;19:3415–3419. [DOI] [PubMed] [Google Scholar]
- 128.Hosgood SA, Saeb-Parsy K, Hamed MO, et al. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. 2016;16:3282–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hosgood SA, Saeb-Parsy K, Wilson C, et al. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. 2017;7:e012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He X, Chen G, Zhu Z, et al. The first case of ischemia-free kidney transplantation in humans. Front Med (Lausanne). 2019;6:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kerforne T, Allain G, Giraud S, et al. Defining the optimal duration for normothermic regional perfusion in the kidney donor: a porcine preclinical study. Am J Transplant. 2019;19:737–751. [DOI] [PubMed] [Google Scholar]
- 132.Magliocca JF, Magee JC, Rowe SA, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma. 2005;58:1095–1101. [DOI] [PubMed] [Google Scholar]
- 133.Gravel MT, Arenas JD, Chenault R, II, et al. Kidney transplantation from organ donors following cardiopulmonary death using extracorporeal membrane oxygenation support. Ann Transplant. 2004;9:57–58. [PubMed] [Google Scholar]
- 134.Valero R, Cabrer C, Oppenheimer F, et al. Normothermic recirculation reduces primary graft dysfunction of kidneys obtained from non-heart-beating donors. Transpl Int. 2000;13:303–310. [DOI] [PubMed] [Google Scholar]
- 135.Butler AJ, Randle LV, Watson CJ. Normothermic regional perfusion for donation after circulatory death without prior heparinization. Transplantation. 2014;97:1272–1278. [DOI] [PubMed] [Google Scholar]
- 136.Oniscu GC, Randle LV, Muiesan P, et al. In situ normothermic regional perfusion for controlled donation after circulatory death–the United Kingdom experience. Am J Transplant. 2014;14:2846–2854. [DOI] [PubMed] [Google Scholar]
- 137.Del Río F, Andrés A, Padilla M, et al. ; Spanish Group for the Study of Donation after Circulatory Death. Kidney transplantation from donors after uncontrolled circulatory death: the Spanish experience. Kidney Int. 2019;95:420–428. [DOI] [PubMed] [Google Scholar]
- 138.Reznik ON, Skvortsov AE, Reznik AO, et al. Uncontrolled donors with controlled reperfusion after sixty minutes of asystole: a novel reliable resource for kidney transplantation. PLoS One. 2013;8:e64209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roncon-Albuquerque R, Jr, Gaião S, Figueiredo P, et al. An integrated program of extracorporeal membrane oxygenation (ECMO) assisted cardiopulmonary resuscitation and uncontrolled donation after circulatory determination of death in refractory cardiac arrest. Resuscitation. 2018;133:88–94. [DOI] [PubMed] [Google Scholar]
- 140.Elliott TR, Nicholson ML, Hosgood SA. Normothermic kidney perfusion: an overview of protocols and strategies. Am J Transplant. 2020. doi:10.1111/ajt.16307 [DOI] [PubMed] [Google Scholar]