Adult mammals are unable to regenerate the cardiac muscle lost to myocardial injury.1 However, we have previously shown that hearts of neonatal pigs can recover from acute myocardial infarction (MI) occurring on postnatal day (P) 1, with little evidence of scar formation or wall thinning 30 days later,1,2 and cardiac tissue removed during apical resection in P1 mice was shown to have fully regenerated 3 weeks later.1 Because the primary mechanism of regeneration in both early postnatal animal species appeared to involve the proliferation of preexisting cardiomyocytes located near the injury site, we were curious whether the proliferative cardiomyocytes could be induced to undergo further proliferation after a second cardiac injury. We hypothesized that because of the state of activated cellular proliferation machinery in the proliferative cardiomyocytes and potential extension of the developmentally regulated cardiomyocyte proliferative window resulting from activation of injury repair mechanisms, a second injury induced by left anterior descending coronary artery (LAD) ligation distal to the second diagonal 4 weeks after initial apex resection (AR) at P1 (AR+MI) would result in significantly less myocardial structural and functional damage when evaluated 4 weeks later.
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and performed in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (Publication No. 85–23). The study consisted of 44 neonatal piglets. Of the 25 P1 AR+MI piglets, 9 died of lethal arrhythmia within 14 days after AR. The ventricular apex (≈0.2 g) was resected at P1 (n=9), with a recovery period consisting of 27 days until P28, when MI was surgically induced via permanent occlusion of the LAD in a subset of animals (n=6; Figure [A through C]), and the terminal study was performed on P56 (Figure [A through C]). The control group consisted of age-matched intact piglets (n=16). Echography was performed before and after MI at P28 and the terminal study on P56 (Figure [A]). Evaluation of explanted hearts at P56 confirmed that the apex had reformed (Figure [B through E]). At P56, although the LAD occlusion remained visible in coronary angiographic images (Figure [C]), there was no visible left ventricular (LV) infarct in any of the LV anterior wall (Figure [D and E]). Typical cardiac echography before and after LAD ligation on P28 is shown in Figure (Hi). Immediately after LAD ligation, significant myocardial damage was evidenced by LV bulging (Figure [Hi], echo2). Compiled data of pre-MI and post-MI echocardiography on P28 and P56 are shown in Figure (Hii and Hiii), demonstrating that LV anterior wall systolic thickness and fractional shortening did not differ significantly between AR+MI and control animals at P56 (Figure [Hii and Hiii]). Because of critical care requirements immediately after LAD ligation on P28, only 2 of the 3 animals underwent post-MI echography data acquisition. Cardiac magnetic resonance imaging also found no evidence of infarction in the hearts of AR+MI animals (data not shown).
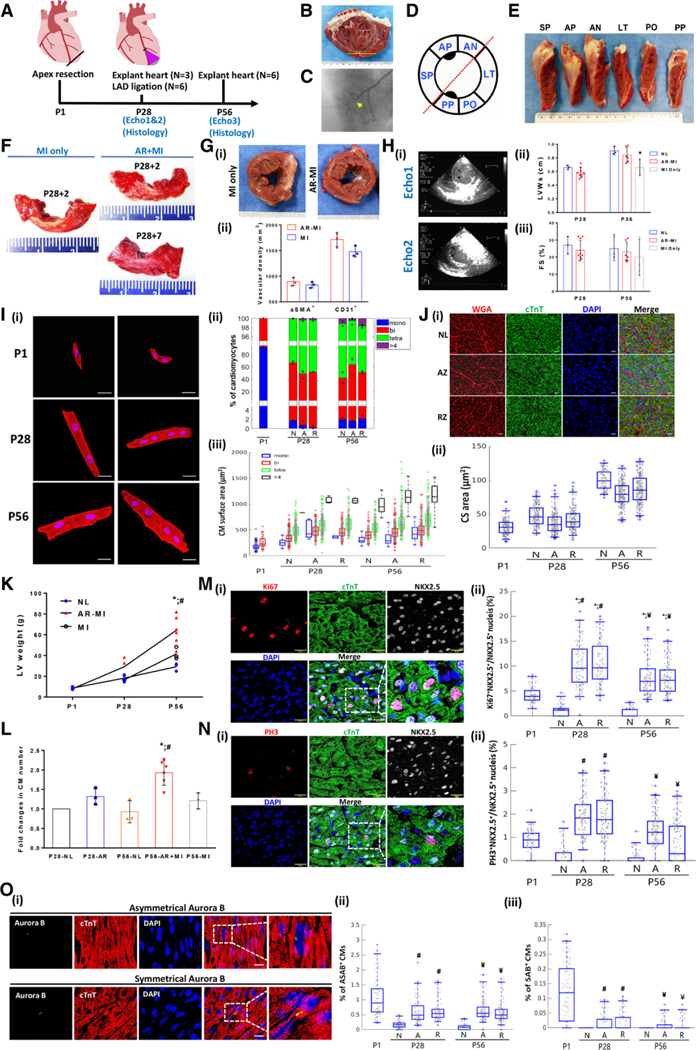
Figure. Myocardial remuscularization occurs after LAD ligation in 28 piglet received apical resection at P1.
A, Schematic overview of the experimental design. Echos1and 2 are echocardiograms taken before and after left anterior descending coronary artery (LAD) ligation distal to the second diagonal branch at postnatal day (P) 28. B, Typical images of the left ventricular (LV) internal view of the LV anterior wall of an apex resection with myocardial infarction (AR-MI) heart at P56. Yellow line indicates the location of the initial P1 apical resection. C, Coronary angiogram obtained at P56 to confirm the successful P28 LAD occlusion. D, Schematic diagram of the LV short-axis view subdivided into 6 circumferential segments according to coronary perfusion/ physiology. E, Typical longitudinal view of the LV at P56 of an AR-MI heart. No evidence of infarct in segments of the septal wall (SP), anterior wall with papillary muscle (AP), and anterior wall (AN) that are perfused by the LAD. F, Typical images of triphenyl tetrazolium chloride–stained LV anterior circumferential segments from the P28 control group of MI only 2 days after LAD ligation (left). AR-MI hearts were explanted 2 (right top) and 7 (right bottom) days after LAD ligation (ie, on P30 or P35); the normal myocardium is red, and the infarct appears white. Gi, Typical images of short-axis LV cross-sections from MI-only hearts (ie, LAD ligation on P28 without previous AR; left) and AR-MI hearts (ie, AR on P1 with LAD ligation on P28; right) that were explanted 28 days after LAD ligation (ie, on P56). Evidence of infarction (ie, white scar) is visible in the section from the MI-only heart but not in the section from the AR-MI heart. Gii, Quantification of myocardial vascular density by α-smooth muscle actin (aSMA) and CD31 staining for MI-only control hearts and AR-MI hearts. Hi, Typical echocardiographic images of an AR-MI heart obtained before (Echo1) and after (Echo2) LAD ligation at P28. Arrows in echo 2 identify anterior-septal systolic bulging secondary to LAD ligation. Hii and Hiii, Compiled echocardiographic data for age-matched normal, AR+MI, and MI-only hearts. *P<0.05 vs normal. Ii, Typical images of disaggregated cardiomyocytes (CMs) stained for cardiac troponin T (red); nuclei were counterstained with DAPI (blue). Bar=20 μm. Iii, Proportion of CMs containing 1 (mono), 2 (bi), 4 (tetra), or >4 nuclei at the indicated time points. Iiii, Quantification of the surface areas of disaggregated CMs (3 hearts per group, 300–500 CMs per heart). At least 1000 CMs were evaluated for each group at each time point. Ji, Typical images of myocyte cross-sectional (CS) area from different areas of the LV at P28. Scale bar=10 μm. Jii, Quantification of CS area over time (P1–P56). A minimal of 200 CMs from each group were counted. K, LV weight in normal, MI only, and AR-MI hearts over time of neonatal hearts (P1–P56). L, Relative changes of total CM counts in each group (normalized to P28 normal heart counts, ie, 4.318×108). *P<0.05 vs P56 normal; #P<0.05 vs P56 MI only. To decipher whether the apical resection was accompanied by an increase in myocyte proliferation, we compared myocardial expression of karyokinesis markers of Ki67, PH3, and the M-phase nuclei division marker Aurora B (AB) in control and AR-MI hearts. M and O, When localized symmetrically between 2 daughter nuclei (symmetrical AB [SAB]), SAB staining is a marker for cytokinesis, whereas asymmetrical localization (asymmetrical AB [ASAB]) identifies a karyokinesis event within a single cell.5 Karyokinesis activity was expressed as percentage of Ki67+NKX2.5+ and PH3+NKX2.5+ nuclei over the total NKX2.5+ nuclei (M and N). The karyokinesis activity increased >10 fold at both P28 and P56 time points compared with postnatal age–matched normal in AR hearts (M and N). Each of the karyokinesis activity markers is similar to or higher than P1 normal hearts (M and N). The karyokinesis/cytokinesis activity was also measured as the percentage of CMs positively expressing ASAB (Oii) and SAB (Oiii) over the total number of cardiomyocytes (O). Again, the karyokinesis/cytokinesis activity as indicated by the percentage of positive ASAB (Oi and Oii) and SAB (Oiii) at P28 and P56 was a few folds higher than the age-matched normal (O) and similar to P1 normal hearts (O). At both P28 and P56 time points, the karyokinesis/cytokinesis activity was not significantly different between the AR cutting zone (A) and remote zone (R), indicating that the activation of myocyte proliferation machinery is expanded beyond the LV AR injury site (M through O). In normal hearts, the proportion of cardiomyocytes displaying Ki67, PH3, and AB expression declined to nearly undetectable levels on P28 and P56 (M through O), which is consistent with the cell cycle arrest (M through O). *P<0.05 vs P1. #P<0.05 vs P28 normal. ¥P<0.05 vs P56 normal. Significance was evaluated with1-way ANOVA when comparing >2 groups and 2-way ANOVA in K. For data displayed in boxplots (I, J, M, and O), significance was evaluated with the Mann-Whitney U test; the 3 lines represent the 25th, 50th (median), and 75th percentiles, and the vertical lines extend from the 5th to the 95th percentile. Values below the 5th percentile or above the 95th percentile were considered outliers. A or AZ indicates apex zone of the AR heart; FS, fractional shortening; LT, lateral wall; LVWs, left ventricular wall thickness during systole; N or NL, normal heart; PO, posterior wall; PP, posterior papillary wall; R or RZ, remote (basal) zone of AR-MI heart; and WGA, wheat germ agglutinin.
Comparing time course of cardiomyocyte karyokinesis between control and AR-MI hearts showed that >90% of cardiomyocytes were mononucleated in P1 hearts (Figure [Ii and Iii]). As the animals aged, mononucleated cardiomyocytes became less common as the number of binucleated and tetranucleated cells increased (Figure [Ii and Iii]). The proportions of mononucleated, binucleated, and tetranucleated cardiomyocytes did not differ significantly between normal and injured heart (Figure [Iii and Iiii]). Although cardiomyocyte cross-sectional area increased with aging, there was no significant difference in cross-sectional area between the normal and injured hearts (Figure [J]). Using LV weight and myocyte volume,3 we calculated the total number of myocytes in normal and AR-MI hearts at each time point of postnatal age (Figure [K and L]). When myocytes counts of AR-MI hearts (P28 and P56) normalized to control naïve hearts (P28), the total cardiomyocyte counts increased significantly by 40% and 92% (Figure [L]). This progressive increase of total myocyte number in AR-MI hearts is consistent with previous observations in mice exposed to the progressive hypoxia protocol.4 To examine whether the apical resection procedure was accompanied by an increase in myocyte proliferation, we compared myocardial expression of karyokinesis markers Ki67 and PH3 and M-phase nuclei division marker Aurora B (AB)5 in control and AR-MI hearts (Figure [M through O]). In AR-MI hearts at both P28 and P56, karyokinesis activity was increased >10-fold compared with postnatal age–matched controls (Figure [M and N]). It is notable that most of the karyokinesis activity markers at P28 and P56 in AR-MI hearts were found to be at similar or higher levels compared with those seen in P1 control hearts (Figure [M through O]). The karyokinesis/cytokinesis activity was not significantly different between the apical resected zone and remote zone, suggesting that activation of myocyte proliferation machinery had expanded beyond the LV apical resected injury site (Figure [M through O]). In control hearts, the proportion of cardiomyocytes displaying Ki67, PH3, and AB expression declined to nearly undetectable levels by P28 and P56, consistent with cell cycle arrest (Figure [M and O]).
In conclusion, P1 piglets undergoing AR can recover completely from a second injury event consisting of a main LAD ligation occurring 4 weeks after AR, with no evidence of LV structural or functional abnormality. AR appeared to prevent the onset of cardiomyocyte cell cycle arrest, which likely contributed to myocardial structural recovery through an increase in proliferation of preexisting cardiomyocytes.
Acknowledgments
Sources of Funding
This study is supported in part by the US National Institutes of Health National Heart, Lung, and Blood Institute grants RO1 HL131017, HL149137, and UO1 HL134764.
Footnotes
ARTICLE INFORMATION
The data, methods used in the analysis, and materials used to conduct the research are available to any researcher for purposes of reproducing the results or replicating the procedure.
REFERENCES
- 1.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu W, Zhang E, Zhao M, Chong Z, Fan C, Tang Y, Hunter JD, Borovjagin AV, Walcott GP, Chen JY, et al. Regenerative potential of neonatal porcine hearts. Circulation. 2018;138:2809–2816. doi: 10.1161/CIRCULATIONAHA.118.034886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerdes AM, Moore JA, Hines JM, Kirkland PA, Bishop SP. Regional differences in myocyte size in normal rat heart. Anat Rec. 1986;215:420–426. doi: 10.1002/ar.1092150414 [DOI] [PubMed] [Google Scholar]
- 4.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, et al. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–227. doi: 10.1038/nature20173 [DOI] [PubMed] [Google Scholar]
- 5.Hesse M, Doengi M, Becker A, Kimura K, Voeltz N, Stein V, Fleischmann BK. Midbody positioning and distance between daughter nuclei enable unequivocal identification of cardiomyocyte cell division in mice. Circ Res. 2018;123:1039–1052. doi: 10.1161/CIRCRESAHA.118.312792 [DOI] [PubMed] [Google Scholar]