Abstract
Introduction:
Acanthamoeba encompasses several species of free-living ameba encountered commonly throughout the environment. Unfortunately, these species of ameba can cause opportunistic infections that result in Acanthamoeba keratitis, granulomatous amebic encephalitis, and occasionally systemic infection.
Areas Covered:
This review discusses relevant literature found through PubMed and Google scholar published as of January 2021. The review summarizes current common Acanthamoeba keratitis treatments, drug discovery methodologies available for screening potential anti-Acanthamoeba compounds, and the anti-Acanthamoeba activity of various azole antifungal agents.
Expert Opinion:
While several biguanide and diamidine antimicrobial agents are available to clinicians to effectively treat Acanthamoeba keratitis, no singular treatment can effectively treat every Acanthamoeba keratitis case. Efforts to identify new anti-Acanthamoeba agents include trophozoite cell viability assays, which are amenable to high-throughput screening. Cysticidal assays remain largely manual and would benefit from further automation development. Additionally, the existing literature on the effectiveness of various azole antifungal agents for treating Acanthamoeba keratitis is incomplete or contradictory, suggesting the need for a systematic review of all azoles against different pathogenic Acanthamoeba strains.
Keywords: Acanthamoeba, amebicidal, antifungal, assay, azole, cell viability, cysticidal, drug screening, drugs, keratitis treatments
1. Introduction
Acanthamoeba are several species of ubiquitous free-living ameba found throughout the world. While Acanthamoeba spp. are most commonly found in wet environments, such as lakes and swimming pools, they can be found in less hospitable environments, such as heating, ventilation, and air conditioning vents, and contact lens solutions.
Depending on environmental conditions, Acanthamoeba can exist as a trophozoite or cyst (Figure 1A, 1B). Trophozoites are the motile, reproductive stage of Acanthamoeba. This stage exists in favorable environments with ample nutrients and appropriate temperature. Trophozoites can range in size from 25-40 μm in length [1]. Trophozoites move via pseudopodia and ingest bacteria, yeast, and cell debris through phagocytosis or food cup formation [2]. Trophozoites utilize pinocytosis to ingest nutrients present in the liquid environment [1].
Figure 1. Life cycle and pathogenesis of A. castellanii.
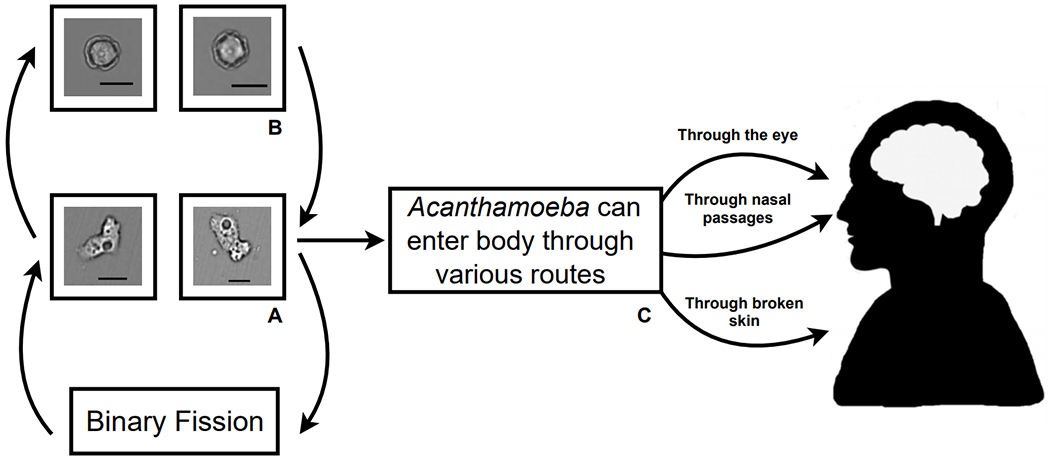
(A) Acanthamoeba spp. proliferate via binary fission as motile trophozoites. (B) Under sub-optimal growth conditions, Acanthamoeba spp. can encyst to form dormant, double-walled cysts. (C) Acanthamoeba spp. trophozoites or cysts can be opportunistic pathogens and infect humans through the eyes, nose, or broken skin. Magnification: 200x; Scale bar: 30 μm.
Cysts are dormant and are composed of a cell within a double cyst wall composed of chitin, protein, and cellulose. Cyst walls are rigid and cysts are spherical in shape with a size ranging from 13-20 μm in diameter. Cysts are resistant to harsh environmental conditions that would be fatal to trophozoites. The outer wall of a cyst contains a high amount of cellulose, which results in a high degree of drug resistance [3]. This drug resistance allows cysts to survive otherwise effective medical therapies for killing the parasite [4].
Acanthamoeba trophozoites reproduce through binary fission. Excystation from a cyst to a trophozoite usually follows after an environment becomes suitable. The trophozoite stage of the parasite is considerably less durable than its cyst counterpart, and is sensitive to antimicrobial agents [5].
While Acanthamoeba spp. are typically free-living, they can be opportunistic pathogens and produce disease in humans. Trophozoites and cysts are both able to infect humans and produce Acanthamoeba keratitis, granulomatous amebic encephalitis, and disseminated infections (Figure 1C) [6].
Acanthamoeba spp. are often associated with Acanthamoeba keratitis, a severe corneal infection. This infection typically occurs in contact-lens wearers, but it can also occur in patients with recent corneal trauma. Most keratitis infections are caused by the T4 genotype. Clinical testing has been performed on strains of this genotype, taken from the affected patients [7]. While effective therapies, such as topical combinations of chlorhexidine gluconate and polyhexamethylene biguanide (PHMB), exist to treat Acanthamoeba keratitis, the parasite can encyst in the ocular tissue to resist current standard-of-care therapies and lead to recurrent keratitis. As such, discovering and identifying therapeutics that are effective against both stages of the parasite would be critical to reducing Acanthamoeba keratitis recurrence and improving existing therapies. This review will discuss current treatments and advances in screening and identifying novel therapeutics for Acanthamoeba keratitis treatment.
2. Clinical symptoms
Acanthamoeba keratitis can trigger a number of symptoms in patients. Common early symptoms or signs of infection include ocular pain and irritation, excessive tear production, light sensitivity, blurry vision, pseudodendritiformic epitheliopathy, corneal opacity, conjunctival hyperemia, multifocal stromal infiltrates, and ring and perineural infiltrates [1,8,9]. While the parasites are largely confined to the cornea and active infections typically do not penetrate past the Descemet’s membrane of the cornea, the presence of Acanthamoeba parasites in the cornea may trigger further inflammation through molecular mimicry or stimulating delayed type hypersensitivity [10–12]. In advanced cases of Acanthamoeba keratitis, patients can suffer from iris atrophy, secondary glaucoma, cataract, uveitis, scleritis, and chorioretinitis [1,9,13,14].
3. Diagnosis
Early on, 75 - 90% of Acanthamoeba keratitis are misdiagnosed and inappropriately treated as bacterial, mycotic, or viral keratitis [9]. Since delays in proper treatment can allow the infection to progress and negatively affect patient outcomes, proper diagnosis is crucial. To this end, clinicians employ a number of diagnostic techniques, including microbial culture, histopathology, confocal microscopy, and PCR analysis to identify Acanthamoeba keratitis in patients [1,9].
Microbial culture is still one of the primary diagnostic techniques for identifying Acanthamoeba keratitis. In microbial culture, samples collected from corneal scrapings, biopsies, or contact lens swabs are cultured on E. coli plated non-nutrient agar plates [15]. The cultures are observed under microscopy to morphologically identify if Acanthamoeba trophozoites or cysts are present [9,15]. While Acanthamoeba trophozoites can become apparent on the culture medium within several days, this diagnostic technique can take up to several weeks to return a definitive diagnosis depending on the initial parasite load [9,15].
Histopathological techniques rely upon staining corneal samples with various dyes and observing for evidence of Acanthamoeba trophozoites and cysts. Periodic acid Schiff, Masson, Gram, Giemsa, Grocott-methenamine-silver, hematoxylin and eosin, or calcofluor white stains can all be used to enhance contrast of trophozoites and cysts for detection [9,15–17]. Since this diagnostic technique is subjective and dependent on the skill of the examiner, diagnostic accuracy can vary significantly.
In vivo confocal microscopy is another diagnostic technique that allows for rapid diagnosis of Acanthamoeba keratitis [18,19]. Care providers will examine the patient’s eye using a confocal microscope to try identifying cysts, which appear as hyper-reflective circular structures; trophozoites can be more difficult to identify using in vivo confocal microscopy as they can resemble leukocytes and keratocytes [9,15,19]. In vivo confocal microscopy can have detection sensitivities as high as 90 - 100%, but diagnostic outcomes are highly dependent upon the training and experience of the examiner [9,19–21].
In PCR analysis, Acanthamoeba-specific 18S rRNA primers, such as Nelson, ACARNA, JDP1/JDP2, and Acant, can be used to detect Acanthamoeba parasites to inform a diagnosis [22–26]. PCR has added advantages of rapid results and the sensitivity to detect low numbers of parasites that may be missed by other techniques [27]. PCR diagnostic results can vary depending on the primers used and assay conditions, but diagnostic sensitivities have been reported to be >70% and can be further improved by using multiple primers to validate diagnostic assay results [22,28]. While PCR can be a useful diagnostic, it is limited by its inability to distinguish between genetic material from viable and nonviable parasites and clinicians should consider this during determining a diagnosis [9,29].
4. Current treatments
Acanthamoeba keratitis is a severe corneal infection that, if left untreated, can result in permanent visual impairment or blindness. Acanthamoeba spp. parasites adhere to the surface of the corneal epithelium through a mannose-binding protein [30,31]. Once adhered, the parasites secrete proteases that kill corneal epithelial cells and allow further invasion into the corneal stroma [31].
Since untreated Acanthamoeba keratitis can lead to severe and life-altering consequences, clinicians have focused on removing infected tissue and killing the parasites. A number of chemotherapeutic and surgical interventions have been utilized.
There are a whole suite of chemotherapeutic agents available to treat Acanthamoeba keratitis, including biguanide, diamidine, antiseptic, antiparasitic, photodynamic, antibiotic, and antifungal agents (Table 1) [1,16,32,33]. The most commonly used biguanides are chlorhexidine gluconate and PHMB while diamidines include propamidine isethionate and hexamidine isethionate [16]. In vitro data suggest antiseptic povidone-iodine can have anti-Acanthamoeba activity, but these results have not been clinically validated [16,34,35]. Antiparasitic drug miltefosine has also been utilized clinically in managing Acanthamoeba keratitis and given orphan drug status by the FDA [16,36–41]. Photodynamic therapy utilizes photosensitive agents to kill parasites [42]. Antibiotics, such as neomycin and polymyxin B, and antifungal agents, including amphotericin B, natamycin, and azoles, have also been clinically utilized to manage and treat Acanthamoeba keratitis [1,9,16,43].
Table 1.
Common drugs and concentrations used for treating Acanthamoeba keratitis.
| Drug (treatment concentration) | Reference |
|---|---|
| Chlorhexidine (0.02 - 0.1% topical eye drops) | [50] |
| PHMB (0.02 - 0.06% topical eye drops) | [50] |
| Hexamidine (0.1% topical eye drops) | [50,55] |
| Propamidine (0.1% topical eye drops) | [50] |
| Natamycin* | [56] |
| Amphotericin B* | [32] |
| Neomycin* | [57–59] |
| Polymyxin B* | [32] |
| Povidone-iodine* | [16] |
| Miltefosine (50 mg oral) | [36,39,40] |
Treatment dosing not well standardized.
While exact treatment regimens can vary among clinical practitioners, Acanthamoeba keratitis therapies frequently employ chlorhexidine or PHMB as monotherapy or in combination with propamidine isethionate and hexamidine [44,45]. Commonly reported combinations include, but are not limited to, PHMB with propamidine, chlorhexidine with propamidine, chlorhexidine with PHMB, and PHMB with propamidine and neomycin [46]. Typically, these therapies are initially administered topically hourly for several days. Afterwards, the frequency of dosing is adjusted per patient needs and can last up to 6 to 12 months for successful keratitis resolution [32,44,47].
Surgical interventions focus on physically removing corneal tissue to remove the nidus of infection or physical methods of killing Acanthamoeba parasites. Debridement involves scraping or irritating the cornea using a blade in an effort to surgically remove infected tissue [48]. Penetrating keratoplasty involves removing infected tissue and replacing it with a clean donor cornea [45,49,50]. It has been shown to resolve cases that other methods were unable to [51]. Other surgical treatments, such as cryotherapy, seek to physically treat the infection by freezing portions of the cornea to directly eradicate the parasites [52–54]. In advanced cases where surgery and chemotherapies are unsuccessful at resolving the infection, clinicians may resort to enucleation of the infected eye [1,49].
4.1. Biguanides
Most cases of Acanthamoeba keratitis are treated with a combination of biguanides, which is a class of cationic antimicrobial compounds. This class of antimicrobials disrupts negatively charged cell membranes to kill microbes. The two most effective and widely used biguanides are PHMB and chlorhexidine gluconate [45]. Initial dosing for Acanthamoeba keratitis treatment typically begins at 0.02% for both chlorhexidine and PHMB. In refractory cases, clinicians can increase the dosage of PHMB up to 0.06% [47].
Chlorhexidine gluconate is a broad-spectrum antiseptic effective against bacteria and fungi [60]. It is commonly found in hygiene products, such as hand-cleaning products and oral antiseptics [61]. Chlorhexidine is effective against both trophozoites and cysts [15]. While the mechanism of action for chlorhexidine is to disrupt the cell membrane and cause cell lysis, cyst walls can help physically block uptake of chlorhexidine and act as a resistance mechanism [60,61].
In vitro studies have reported chlorhexidine to be highly potent against both trophozoites and cysts. Chlorhexidine has been found to be trophocidal at concentrations as low as 8 μg/mL (8 x 10−4 %) [62]. Alizadeh et al (2009) identified chlorhexidine to be trophocidal at a concentration of 10 μg/mL (1 x 10−3 %) [63]. Chlorhexidine has been found to be cysticidal at concentrations as low as 1.56 μg/mL (1.56 x 10−4 %) [64]. Lee et al (2007) reported chlorhexidine to have a minimum cysticidal concentration of 7.02 μg/mL (7.02 x 10−4 %) [65].
There has been a limited number of clinical trials evaluating chlorhexidine’s effectiveness in treating Acanthamoeba keratitis. Kosrirukvongs et al (1999) treated six eyes with 0.006% chlorhexidine and reported curing infection in five of the eyes [66]. Lim et al (2008) reported chlorhexidine monotherapy administered as 0.02% eye drops resolved 85.7% of keratitis cases [67]. Additionally, chlorhexidine treatment resulted in greater visual acuity improvement and less corneal scarring than PHMB monotherapy, which suggests chlorhexidine may be more tolerable for patients [67].
PHMB is a wide-spectrum antiseptic utilized in a wide variety of applications, such as wound care, cosmetic preservatives, contact lens cleaners, and pool cleaners [68–70]. In Acanthamoeba, PHMB is taken up into the parasite, where it disrupts the cell membrane and causes cell death [71].
PHMB is commonly used in conjunction with other biguanides and diamidines in the treatment of Acanthamoeba keratitis [45]. In vitro, PHMB has demonstrated excellent potency against trophozoites and cysts with reported minimum trophozoite amebicidal concentrations as low as 2.5 μg/mL (2.5 x 10−4 %) [62]. Heaselgrave et al (2019) reported PHMB to be amebicidal against trophozoites at 3.9 μg/mL [35]. In terms of cysticidal activity, PHMB was found to have a minimum cysticidal concentration of 2.37 μg/mL (2.37 x 10−4 %) while Narasimhan et al (2002) demonstrated a minimum cysticidal concentration of 25 μg/mL (2.5 x 10−3 %) [64,65].
Clinically, PHMB has been evaluated as a monotherapy agent for treating Acanthamoeba keratitis. Lim et al (2008) reported PHMB monotherapy in 0.02% eye drops resolved 78% of keratitis cases and was comparable in efficacy to chlorhexidine-based monotherapy, but they also cautioned that PHMB appeared to cause more corneal scarring, which may limit its utility [67].
Chlorhexidine and PHMB have been clinically evaluated and reported to be efficacious in resolving Acanthamoeba keratitis [66,67]. In vitro, the reported minimum trophozoite amebicidal or cysticidal concentrations vary between reports, but the independently reported values demonstrate chlorhexidine and PHMB are extremely potent against Acanthamoeba and suggest even the starting clinical dose of 0.02% is sufficient to kill Acanthamoeba trophozoites and cysts. Despite this, reports of refractory Acanthamoeba keratitis cases following prolonged biguanide treatment and reports detailing clinical isolates with high resistance reveal biguanides are not a guaranteed panacea [72–74]. Furthermore, these antiseptic agents are nonspecific and have cytotoxicity against corneal epithelial cells and keratocytes at clinically relevant doses. This may limit the maximum tolerable dose [65,75].
4.2. Diamidines
Diamidines are a class of cationic compounds with broad-spectrum antimicrobial activity against bacteria, fungi, ameba, and other protozoa [76]. Diamidines are believed to exert their antimicrobial activity by disrupting cell membranes, denaturing cytosolic proteins, and inhibiting DNA synthesis [47,77]. In the treatment of Acanthamoeba keratitis, commonly used diamidines include 0.1% propamidine isethionate and hexamidine at 0.1% [47].
Propamidine isethionate is a DNA synthesis inhibitor, and it was one of the first agents shown to be effective for treating Acanthamoeba keratitis [32,57,77]. In a retrospective study of 111 cases of Acanthamoeba keratitis, it was used in conjunction with PHMB to significantly improve visual acuity of the majority (>79%) of Acanthamoeba keratitis cases [78]. Propamidine has also been combined with a number of other general purpose antimicrobials, such as neomycin, PHMB, chlorhexidine, polymyxin B, and gramicidin for Acanthamoeba keratitis treatment [58,59,79].
Reports of propamidine’s minimum trophozoite amebicidal concentration range from values as low as 15.6 μg/mL (1.56 x 10−3 %) to estimates as high as 1,000 μg/mL (1 x 10−1 %) [35,80]. Its minimum cysticidal concentration has also been reported to be as low as 250 μg/mL (2.5 x 10−2 %) and as high as 421 μg/mL (4.21 x 10−2 %) [35,72]. Clinically, propamidine and neomycin as a combination therapy has been evaluated and reported to have high efficacy. Hargrave et al (1999) treated Acanthamoeba keratitis patients with 0.1% propamidine solution and neomycin and reported 50 of 60 eyes (83%) resolved successfully [59]. These clinical and in vitro reports suggest propamidine is broadly efficacious against both Acanthamoeba trophozoites and cysts and can be utilized to alleviate Acanthamoeba keratitis.
Hexamidine was originally developed as a trypanocidal agent [81]. More recently, it is now formulated into a number of over-the-counter medications as an antiseptic and antimicrobial agent [82]. For Acanthamoeba keratitis treatment, it was first reported by Brasseur et al (1994) that topical 0.1% hexamidine successfully cleared a case of Acanthamoeba keratitis recalcitrant to propamidine treatment [55]. Since then, hexamidine has been utilized in conjunction with a number of other antimicrobials, such as chlorhexidine, PHMB, and propamidine to successfully treat patients [79].
In vitro, hexamidine isethionate has been reported to be effective against Acanthamoeba cysts and trophozoites [35,77]. Hexamidine’s minimum trophozoite amebicidal concentration has been reported to range from 7.5 μg/mL (7.5 x 10−4 %) to 31.3 μg/mL (3.13 x 10−3 %) [35,80]. It has also been reported to be cysticidal at significantly higher concentrations, with reports of cysticidal activity at concentrations of 222 μg/mL (2.22 x 10−2 %) and 250 μg/mL (2.5 x 10−2 %) [35,72].
Despite various promising reports that chlorhexidine, PHMB, propamidine, and hexamidine are effective against A. castellanii trophozoites and cysts, clinical reports of 5-10% of cases being refractory even to extended courses of these combination therapies suggest the need for compounds with better efficacy and less clinical resistance [32,72].
4.3. Antiseptic agents
Povidone-iodine is an iodophor that penetrates microorganisms and oxidizes cytosolic cell components to cause death [83]. It is generally well tolerated and is used as a broad-spectrum antiseptic agent for wound care [84]. Furthermore, povidone-iodine has also been adapted for ophthalmic use in contact lens cleaning and care [85–87].
In terms of in vitro characterization, povidone-iodine has been found to be cysticidal and trophocidal [34,85–88]. Roongruangchai et al (2009) reported povidone-iodine had a minimum cysticidal concentration of 0.04% and identified structural damage to treated cysts, such as the breakdown of cyst walls and the parasite cell membranes [89]. Sunada et al (2014) demonstrated povidone-iodine was cysticidal against 56 different Acanthamoeba spp. clinical isolates even at concentrations as low as 0.1% [90]. Shi et al (2020) reported 0.25% povidone-iodine completely trophocidal and had significant cysticidal effects (~80% inhibition) [91].
However, several in vitro reports suggest povidone-iodine to be ineffective. Pelletier et al (2011) reported 0.4% povidone-iodine and 0.1% dexamethasone were not cysticidal [92]. Lim et al (2000) reported povidone-iodine had poor activity against trophozoites with a minimum inhibitory concentration exceeding 100 μg/mL (0.01%) and no detectable cysticidal activity against clinical isolates collected in Australia [93]. These conflicting reports may be due to differences in strain susceptibility and testing conditions. While povidone-iodine may be useful for Acanthamoeba keratitis management, its efficacy has yet to be clinically validated and strain susceptibility tests should be considered before widespread usage [16].
4.4. Antiparasitic agents
Miltefosine is an alkylphosphocholine antiparasitic agent commonly used for treating leishmaniasis and other parasitic infections [94]. Miltefosine is believed to induce apoptosis in Acanthamoeba through inhibiting proteinase kinase B [32]. As an anti-Acanthamoeba agent, it is administered orally at 50 mg three times daily, and clinical case reports have reported miltefosine treatment successfully resolved Acanthamoeba keratitis cases [36–41].
In vitro, miltefosine has been verified to have trophocidal and cysticidal properties [95,96]. Chao et al (2020) reported miltefosine to be cysticidal at 2.42 mM [95]. Mrva et al (2011) previously reported miltefosine to be weakly active against Acanthamoeba sp. and Acanthamoeba lugdunensis clinical isolates with minimum trophocidal concentrations of 250 μM and 500 μM, respectively [97]. Miltefosine’s dual activity against trophozoites and cysts makes it potentially useful for treating recalcitrant Acanthamoeba keratitis cases.
4.5. Photodynamic agents
Photodynamic therapy (PDT) is a form of chemotherapy in which photosensitive compounds are stimulated by light to kill cells of interest, and this therapy can be used to treat a number of diseases [98]. A number of in vitro, animal, and clinical case studies highlight the potential for applying photosensitive agents, including tetracationic phthalocyanine RLP068, hypocrellin B, tin porphyrin, methylene blue, riboflavin, titanium dioxide, chorin derivative TONS504, and rose bengal against Acanthamoeba [99–112].
Most photodynamic therapy studies have been limited to in vitro testing on Acanthamoeba isolates. Kassab et al (2003) reported RLP068-PDT caused nuclear damage and cell death in A. palestinensis trophozoites [99]. Chen et al (2008) found hypocrellin B-PDT had a dose-dependent inhibition of trophozoites and cysts, but also noted corneal cytotoxicity [100]. Siddiqui and Khan (2012) reported tin porphyrin-PDT as amebostatic [101]. Mito et al (2012) found methylene blue-PDT to be trophocidal and synergized with PHMB and amphotericin B [102]. Del Buey et al (2012) evaluated riboflavin-PDT on Acanthamoeba isolates and reported a single dose did not completely eradicate all parasites [103]. Lamy et al (2016) used riboflavin-PDT in doses up to ten times higher than recommended for treatment and found it did not enhance the efficacy of PHMB or chlorhexidine [104]. Gomart et al (2018) evaluated titanium dioxide-PDT and found it yielded dual activity against Acanthamoeba trophozoites and cysts [105]. Pertiwi et al (2019) evaluated chorin-derivative TONS504 and found it to cause necrosis in trophozoites and apoptosis in cysts [107]. Collectively, these in vitro studies highlight the trophocidal and cysticidal potential of photodynamic agents.
While several agents have been evaluated in vitro, only a few agents have been evaluated in rabbit animal models. Pertiwi et al (2020) evaluated TONS504-PDT in rabbit models and reported the treatment resolved 58% of infections [106]. Atalay et al (2020) evaluated rose bengal-PDT and reported the therapy decreased parasite loads in the rabbit corneas [108].
In regards to human clinical data, both riboflavin and rose bengal have been reported as effective. Despite equivocal in vitro data, several reports have noted Acanthamoeba keratitis resolution following riboflavin-PDT [109–111]. Moren et al (2010), Khan et al (2011), and Price et al (2012) all reported successfully treating Acanthamoeba keratitis patients with riboflavin-PDT [109–111]. Naranjo et al (2019) used rose bengal-PDT to treat a number of keratitis cases caused by various bacterial, fungal, and protozoal agents and reported a 72% case resolution rate [112]. Considering several reports on riboflavin-PDT have reported successful treatment suggests this agent warrants further investigation and attention as a potential Acanthamoeba keratitis therapeutic.
Photodynamic therapy could potentially expand Acanthamoeba keratitis management options, but currently existing literature is mostly restricted to in vitro work. As such, photodynamic therapy warrants further investigation. Additionally, a significant effort should be made to expand the number of randomized clinical trials evaluating photodynamic agents.
4.6. Antibiotics
Several antibiotics, particularly neomycin and polymyxin-B, have been commonly used in Acanthamoeba keratitis management. Neomycin is a broad-spectrum aminoglycoside antibiotic that binds the ribosomal subunit to inhibit translation and protein synthesis [16]. Polymyxin-B binds and disrupts microbial cell membranes [32]. Neomycin, polymyxin-B, and bacitracin are also combined to form the triple antibiotic (Neosporin), which is in common ophthalmic use [43,113].
Clinically, several case reports have detailed usage of neomycin and polymyxin-B with propamidine isethionate to resolve Acanthamoeba keratitis [113–117]. Most notably, Hargrave et al (1999) performed a clinical trial evaluating neomycin-polymyxin B-gramicidin in conjunction with 0.1% propamidine isethionate and reported favorable Acanthamoeba keratitis outcomes [59]. As such, while there is only one notable clinical trial documenting the usage of neomycin and polymyxin-B, case studies do support antibiotics being helpful in Acanthamoeba keratitis management. Furthermore, the use of antibiotics in treatment of Acanthamoeba keratitis can be prudent, as infected corneas often have co-infection with bacterial organisms as well.
4.7. Antifungal agents
Natamycin is an antifungal agent that binds sterols in fungal cell membranes, leading to cell permeabilization and death [118]. In vitro, natamycin has demonstrated cysticidal effects against Acanthamoeba [90]. Clinically, natamycin can be used in conjunction with other antimicrobial agents. Kitagawa et al (2003) reported applying 1% natamycin ointment six times daily in combination with 0.02% chlorhexidine steadily improved cases of Acanthamoeba keratitis over the course of a week [56]. The report also suggested the combination therapy of chlorhexidine, natamycin, and debridement as a useful keratitis management strategy [56].
Amphotericin B is a polyene antifungal agent that binds ergosterol and causes membrane instability and cell death of the pathogen [119]. In vitro, Taravaud et al (2017) evaluated amphotericin B against A. castellanii and reported A. castellanii gradually displayed higher resilience to amphotericin B over time [120]. Apart from in vitro data, clinical data and case reports on amphotericin B’s use for treating Acanthamoeba keratitis is limited. However, amphotericin B is commonly used to resolve keratitis cases caused by other microorganisms. Biser et al (2004) reported 0.5% topical amphotericin B rapidly resolved a case of Arthrographis keratitis that mimicked Acanthamoeba keratitis [121]. 0.3 - 0.5% amphotericin B has been considered for fungal keratitis intervention, but its utility is limited due to its toxicity [122]. Behrens-Baumann et al (1990) evaluated amphotericin B in a rabbit Candida keratitis model and recommended a combination of amphotericin B and fluconazole for Candida keratitis [123]. As such, amphotericin B is a commonly used keratitis drug that can be considered as a potential antimicrobial agent for Acanthamoeba keratitis management.
5. Azoles as anti-Acanthamoeba agents
Azoles are a class of antifungal agents originally developed to target sterol 14a demethylase (CYP51) and inhibit ergosterol biosynthesis [124]. Since Acanthamoeba spp. encode for CYP51 with 31-35% sequence identity to fungal CYP51, antifungal azoles have been considered and evaluated for treating Acanthamoeba keratitis [125]. Clinically, several azoles have been evaluated in very limited clinical cases for treating Acanthamoeba keratitis. These include imidazole (clotrimazole, miconazole, and ketoconazole) and triazole (itraconazole, fluconazole, and voriconazole) class antifungal azoles (Table 2). Several azoles have been identified and suggested to have potent amebicidal and cysticidal properties against Acanthamoeba spp., suggesting new treatments for Acanthamoeba keratitis [126]. Azoles are attractive for Acanthamoeba keratitis treatment as they are generally well tolerated [125]. However, considerations into the method of administration, ophthalmic formulation, and adjunctive surgical preparations have to be made as topically applied imidazoles poorly penetrate the corneal epithelium [127–131].
Table 2.
Acanthamoeba keratitis experimental therapies.
| Experimental azole-based drug combinations | Reference |
|---|---|
| 0.02% PHMB + 0.1% propamidine | [78] |
| 0.02% chlorhexidine + 0.1% propamidine | [79] |
| 200 mg ketoconazole + 0.2% chlorhexidine + ciprofloxacin + 1% atropine | [132] |
| Oral itraconazole + 0.1% topical miconazole + surgical debridement | [133] |
| 0.1% miconazole + 150 mg oral itraconazole | [133] |
| 0.02% chlorhexidine + 50 mg ketoconazole | [134] |
| 1% voriconazole + 0.02% PHMB | [135] |
| Cryosurgery + 300 mg oral fluconazole | [52] |
| 4 mg/mL topical posaconazole | [136] |
5.1. Clotrimazole
Clotrimazole is an antifungal therapy that has proven to be cysticidal in vitro against various Acanthamoeba strains [137]. As such, clotrimazole is commonly used as a primary therapy against Acanthamoeba keratitis in combination with propamidine [138].
Four Acanthamoeba keratitis patients were treated with a combination therapy of 1% clotrimazole with topical neomycin sulfate-polymyxin B sulfate-gramicidin and propamidine isethionate. This therapy was coupled with 0.25% fluorometholone, four times a day, and systemic 200 mg of ketoconazole therapy twice daily [137]. 1% clotrimazole was suspended in artificial tears and found to be well tolerated by patients [137].
In terms of in vitro data, Duma and Finley (1976) reported clotrimazole inhibited trophozoite motility at concentrations as low as 50-100 μg/mL at 24 hours and ≥100 μg/mL at 48 hours [139]. Elder et al (1994) reported low cysticidal activity of clotrimazole with a minimum cysticidal concentration >500 μg/mL [4]. Despite seemingly contradictory in vitro data with clotrimazole alone, a combination therapy with clotrimazole has demonstrated clinical efficacy in clearing Acanthamoeba keratitis cases.
There is no reported data on the corneal penetration of clotrimazole following systemic application. However, topical clotrimazole has been demonstrated to readily penetrate the cornea in rabbit models [123]. Furthermore, when paired with penetrating keratoplasty, 1% clotrimazole suspended in artificial tears successfully controlled recurrent Acanthamoeba keratitis, suggesting adjunctive surgical interventions can enhance clotrimazole’s ocular bioavailability [137].
5.2. Miconazole
Miconazole is a relatively new antifungal agent, with limited testing outside of research settings. Several patients affected with AK were treated with 0.1% miconazole and 150 mg of oral itraconazole hourly [133]. Ishibashi et al (1990) reported the patients showed improvement with the resolution of the Acanthamoeba keratitis following treatment with miconazole and itraconazole [133]. In this clinical study, the patients tolerated the treatment with no adverse effects.
Miconazole’s effectiveness was tested in comparison to topical neomycin-polymyxin B-bacitracin (Neosporin). Neosporin, given as four drops hourly was compared to Neosporin with 10 mg/mL miconazole, and Sharma et al (1990) reported miconazole did not significantly affect patient recovery [43]. Ultimately, this study confirmed that miconazole did not show promise as a new antifungal therapy in the clinical setting.
Nagington et al (1976) reported miconazole had a minimum inhibitory concentration towards trophozoite ranging from 10-100 μg/mL and a minimum cysticidal concentration of 1000 μg/mL in vitro [140]. In contrast, Saunders et al (1992) reported that 1% miconazole was not cysticidal against Acanthamoeba cysts [141]. The mixed clinical and in vitro efficacy data on miconazole may be due to varying susceptibility of different Acanthamoeba strains.
Miconazole is not commonly administered orally, so there is little data about its circulation following oral administration. In rabbit models, de-epithelializing corneas prior to topical miconazole administration significantly improved miconazole’s bioavailability in the aqueous humor [142]. Additionally, topical miconazole paired with oral itraconazole and debridement has been utilized successfully to resolve Acanthamoeba keratitis [133]. These reports suggest adjunctive surgical preparations can enhance the bioavailability of topically applied miconazole.
5.3. Ketoconazole
Ketoconazole has been commonly prescribed as a combination therapy for the treatment of Acanthamoeba keratitis. Demirci et al (2006) reported ketoconazole administration to a patient affected with Acanthamoeba keratitis [134]. 0.02% chlorhexidine was given hourly along with 50 mg ketoconazole administered orally daily [134]. This study demonstrated the effectiveness of ketoconazole in eliminating the corneal inflammation [134]. Wynter-Allison et al (2005) reported that the treatment with 200 mg oral ketoconazole daily, topical 0.02% chlorhexidine hourly, ciprofloxacin every four hours, and 1% atropine twice daily resulted in rapid clearance of the keratitis within a week [132]. However, recurrent Acanthamoeba keratitis occurred, which suggests ketoconazole to be ineffective [132].
Ketoconazole’s bioavailability has been evaluated in rabbit models. Hemady et al (1992) reported detecting high concentrations of ketoconazole in the cornea following topical or oral administration and confirmed this could be further enhanced by debriding the eyes prior to topical treatment [143]. Clinically, systemic administration of ketoconazole in combination with topical miconazole has also been used successfully to prevent recurrent Acanthamoeba keratitis following penetrating keratoplasty [144].
Ketoconazole’s contrasting results suggest further characterization of it as monotherapy and combination therapy is required to identify if ketoconazole is effective in treating Acanthamoeba keratitis. Furthermore, additional characterization of ketoconazole’s ocular bioavailability would help inform clinicians of its treatment potential.
5.4. Itraconazole
Itraconazole is a relatively new azole that has demonstrated extensive, broad-spectrum antimicrobial activity [145]. However, Hernèndez-Martínez et al (2019) reported that A. castellanii was minimally sensitive to itraconazole in vitro at a concentration of 20.14 ± 4.93 μM [146]. After comparing itraconazole to voriconazole, they concluded that voriconazole should be used in place of itraconazole since voriconazole had more potent cysticidal effects [146].
Clinically, itraconazole has been used to treat several Acanthamoeba keratitis patients. Three patients were administered 150 mg oral itraconazole along with 0.1% topical miconazole following debridement, and the patients showed improvement and no sign of recurrent infection [133].
Oral itraconazole was used in combination with topical miconazole and debridement and successfully resolved Acanthamoeba keratitis [133]. Even though oral itraconazole is commonly used for ocular infections, it appears to have poor corneal availability. 1% topical itraconazole had poor corneal penetration, suggesting that a suitable vehicle to prepare itraconazole may improve corneal penetration [147].
5.5. Fluconazole
Fluconazole is a commonly prescribed, readily available oral antifungal with broad-spectrum antimicrobial activity [148]. Amoils et al (1999) reported administering 300 mg of fluconazole orally daily for 8 weeks following cryosurgery and found the Acanthamoeba keratitis resolved in that time [52].
In vitro, Lamb et al (2015) reported fluconazole as a weak inhibitor (IC50: 30 μM) that did not inhibit cell proliferation since its minimum inhibitory concentration toward trophozoites exceeded 64 μg/mL [125]. Anwar et al (2018) reported that conjugating fluconazole to silver nanoparticles did not improve its anti-Acanthamoeba activity [149]. Interestingly, Anwar et al (2019) reported that conjugating fluconazole to gold nanoparticles improved the drug’s inhibition of Acanthamoeba by 11% at 5 μM [150]. In summary, in vitro data suggests fluconazole has minimal activity against Acanthamoeba, but chemical modifications may improve its overall activity.
In terms of corneal bioavailability, O’day et al (1990) found 20 mg/kg oral fluconazole easily reached all ocular tissues in rabbit models [151]. Abbasoglu et al also reported topical 0.2% fluconazole easily penetrated into the aqueous humor [152]. Additionally, in case reports, fluconazole was effective when paired with cryosurgery, which broke the walls of the cornea, and the infection resolved in eight weeks following the treatment [52]. Collectively, these reports suggest fluconazole can reach sufficiently high concentrations in the cornea.
5.6. Isavuconazole
Isavuconazole is a relatively new second-generation broad spectrum triazole [153]. Isavuconazole is a promising antifungal therapy that has demonstrated cysticidal activity against A. castellanii, and has proven fast-acting against mycotic keratitis in clinic [154,155]. Furthermore, isavuconazole has a favorable safety profile and is well tolerated in patients [153,156].
Isavuconazole is well tolerated and has shown success clinically. Mada et al (2018) reported a patient with mycotic keratitis was administered 372 mg of isavuconazole daily for six weeks [155]. After three months, the patient’s eyes were reported to be free of microorganisms [155].
In vitro, Shing et al (2020) reported isavuconazole as amebicidal and cysticidal with an IC50 of 4.65 nM and a minimum cysticidal concentration of 70 μM [154]. Shing et al (2020) also reported isavuconazole prevented excystation, suggesting isavuconazole could potentially be used to resolve recurrent Acanthamoeba keratitis [154]. Isavuconazole’s amebicidal activity was also confirmed by Rice et al (2020), who reported the prodrug isavuconazonium had an IC50 of 0.09 ± 0.02 μM [157]. Interestingly, Brunet et al (2020) reported isavuconazole to only be mildly amebostatic with no cysticidal activity [158]. While isavuconazole has been utilized to treat fungal keratitis and in vitro data exists to suggest isavuconazole’s effectiveness against Acanthamoeba, there does not appear to be clinical or in vitro data evaluating its corneal penetration or clinical efficacy following topical or systemic administration, suggesting the need for further evaluation.
5.7. Voriconazole
Voriconazole is an antifungal used for treating fungal infections that do not respond to other antifungal therapies [159]. It has been utilized in a limited number of cases of Acanthamoeba keratitis.
Hou et al (2017) showed that while a topical treatment of 0.02% chlorhexidine and 1% voriconazole was unsuccessful, an oral administration of 200 mg of voriconazole twice daily was successful in resolving a case of Acanthamoeba keratitis [160]. Tu et al (2010) also demonstrated successful treatment of three Acanthamoeba keratitis patients with oral voriconazole [161]. Masselam et al (2008) reported that five patients treated with 1% voriconazole and 0.02% PHMB showed improvement in Acanthamoeba keratitis [135].
Voriconazole has been reported to have a minimum inhibitory concentration versus trophozoites of 1-2 μg/mL [125]. Voriconazole’s cysticidal activity has been debated in conflicting reports. Iovenio et al (2014) reported voriconazole’s minimum cysticidal concentrations to be 33.13 ± 22.83 μg/mL for clinical isolates and 46.25 ± 23.26 μg/mL for cell culture strains [162]. However, Talbott et al (2019) reported voriconazole was not cysticidal and even antagonized chlorhexidine and propamidine activity [163]. Gueudry et al (2018) could not recapitulate voriconazole’s cysticidal activity even at concentrations of 200 μg/mL [164]. Since studies of voriconazole convey equivocal findings, further characterization is necessary to evaluate voriconazole’s potential clinical efficacy for Acanthamoeba keratitis.
Voriconazole has demonstrated corneal penetration in rabbit models and patients. 10 μg/mL topical voriconazole penetrated the cornea in rabbit models, which suggests locally applied voriconazole may penetrate the human eye as well [165]. This has been validated by Lau et al (2008) who found 1% voriconazole eye drops readily penetrated the corneal epithelium in humans [166].
5.8. Posaconazole
Posaconazole is another broad-spectrum antifungal agent commonly used for treating recalcitrant fungal infections [167]. Posaconazole has shown promise as a potential Acanthamoeba keratitis treatment due to its significant Acanthamoeba cysticidal activity [162]. After identifying two cases of keratitis resistant to other antifungal treatments, Altun et al (2014) prioritized treating these patients with posaconazole [136]. 4 mg/mL of topical posaconazole was applied hourly and the patients demonstrated significant improvement within 5 days [136].
In vitro, reports of posaconazole’s IC50 against trophozoites range from estimates as low as 3 nM to as high as 7.50 ± 0.39 μM depending upon strain [154,168]. Iovenio et al (2014) reported posaconazole as cysticidal with a minimum cysticidal concentration of 43.75 ± 25.04 μg/mL for clinical and 52.5 ± 23.75 μg/mL for cell culture strains [162]. Sifaoui et al (2019) showed posaconazole’s cysticidal effects against three strains at lower concentrations [168]. Interestingly, Shing et al (2020) reported not being able to recapitulate posaconazole’s cysticidal activity even at 200 μM [154]. It is possible that the activity of posaconazole against cysts may depend on the use of different Acanthamoeba strains.
Posaconazole has excellent tissue penetration and was successful in resolving three cases of keratitis infections [169]. Oral posaconazole is preferred for keratitis treatment as it has demonstrated excellent ocular availability as compared to relatively insoluble topical formulations [170]. While in vitro data suggest posaconazole to be highly potent against trophozoites and potentially even cysts, there are still no clinical case studies into posaconazole’s effectiveness or corneal bioavailability in treating Acanthamoeba keratitis.
6. Drug screening methodologies
While existing treatments and therapies effectively treat most cases of Acanthamoeba keratitis, a small fraction of patients suffer recurrence. Furthermore, current treatments are nonspecific and can result in cytotoxicity to the cornea. Identifying new more-targeted agents against Acanthamoeba spp. would yield better treatments.
6.1. Amebicidal screening
Acanthamoeba trophozoites are proliferative and metabolically active, which are useful properties to utilize for high-throughput screening. Several cell viability assays have commonly been utilized to screen compound libraries and identify amebicidal compounds (Figure 2). In particular, trypan blue exclusion staining, Alamar Blue, and CellTiter-Glo cell viability assays have been utilized to assay trophozoite viability and identify amebicidal compounds.
Figure 2. Current amebicidal screening techniques.
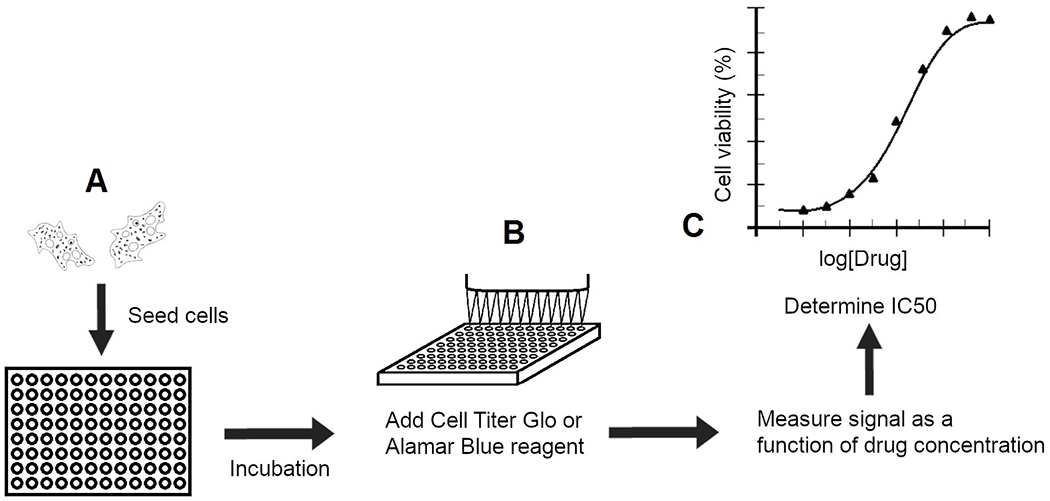
(A) Current high-throughput amebicidal drug screening techniques incubate trophozoites in multiwell screening plates with compounds of interest. (B) The screening plates are then assayed through Alamar Blue or CellTiter-Glo cell viability assays to determine cell viability as a function of drug concentration. (C) Compounds with potent activity at low drug concentrations are considered hits and assessed further to determine the IC50 through secondary screens.
In trypan blue exclusion staining, trophozoites treated with compounds of interest are stained with trypan blue. Live trophozoites will exclude the dye while dead trophozoites will take up the dye. Investigators can then manually determine the cell viability percentage as a function of drug concentration and identify if a compound is amebicidal. This technique has been utilized by several groups to evaluate the amebicidal effects of antimicrobials. For instance, Alizadeh et al (2009) assessed the efficacy of Alexidine on trophozoites by counting the number of viable trophozoites after 24 hours of Alexidine exposure [63]. Padzik et al (2018) treated patient-isolated Acanthamoeba parasites with povidone iodine, chlorhexidine, and toyocamycin and counted the viable trophozoites over the course of six days [88].
While manual counting of cells in trypan blue exclusion staining is relatively inexpensive and can be easily performed with minimal equipment, it is a labor-intensive technique that is not amenable for high-throughput screening. As such, this technique is best reserved for secondary screening on lead compounds that have already been identified through other higher-throughput screening techniques.
In addition to trypan blue exclusion staining, several cell viability assays, such as Alamar Blue and CellTiter-Glo cell viability assays have also been utilized to screen amebicidal compounds against Acanthamoeba spp. These assays have the benefit of being amenable to high-throughput screening.
The Alamar Blue cell viability assay is a colorimetric and fluorescent method that relies upon the reduction of resazurin [171]. In this assay, metabolically active Acanthamoeba trophozoites reduce resazurin, which is blue and weakly fluorescent, to resorufin, which is pink and strongly fluorescent [171]. This reduction can be read using an automated colorimetric or fluorescence plate reader by measuring absorbance (ƛAbs 570 nm and 600 nm or 540 nm and 630 nm) or fluorescence (ƛex 530 - 560 nm; ƛEm 590 nm) [171].
McBride et al (2005) developed a high-throughput assay by adapting the Alamar Blue cell viability assay [172]. Since its development, the Alamar Blue assay has been utilized routinely to assess the viability of Acanthamoeba trophozoites in primary and secondary screens to evaluate potential amebicidal compounds [146,173–177]. While the Alamar Blue cell viability assay provides higher-throughput screening than manual counting techniques, the time required for cells to reduce resazurin and provide a measurable signal can span from several hours to a day [178]. This lengthy incubation period can decrease the overall throughput of a screen performed using Alamar Blue.
As an alternative to the Alamar Blue assay, the CellTiter-Glo assay is a luminescence-based cell viability assay [179]. In this assay, the ATP from lysed live cells is utilized by luciferase to catalyze the conversion of luciferin to oxyluciferin and provide a luminescent signal that can be measured by a luminescence plate reader [179].
Since Acanthamoeba trophozoites are highly metabolically active, measuring ATP levels can determine the relative inhibition of proliferation as a function of drug concentration. Rice et al (2020) screened the MMV pandemic response box with the CellTiter-Glo cell viability assay (Promega) and identified several amebicidal compounds against A. castellanii [157]. CellTiter-Glo has also been utilized to screen azole and statin activity against A. castellanii [154,180]. Since CellTiter-Glo has a significantly shorter incubation time than Alamar Blue, utilizing this viability assay in primary screening of a compound library can help increase throughput [178,179].
6.2. Cysticidal screening
Considering that 5 to 10% of Acanthamoeba keratitis patients suffer recurrence due to cyst resistance and persistence through therapy, identifying drugs that are both highly amebicidal and cysticidal is of the utmost importance for improving Acanthamoeba keratitis outcomes [32,72].
Currently, two techniques are commonly used to determine if a drug is cysticidal. The first technique revolves around trypan blue exclusion staining of drug-treated cysts [95,174,181]. The second technique involves an excystation assay, where treated cysts are manually observed over a time course for evidence of excystation or release of trophozoites from cysts and multiplication of metabolically active trophozoites (Figure 3) [7,63,162].
Figure 3. Traditional cysticidal screening workflow.
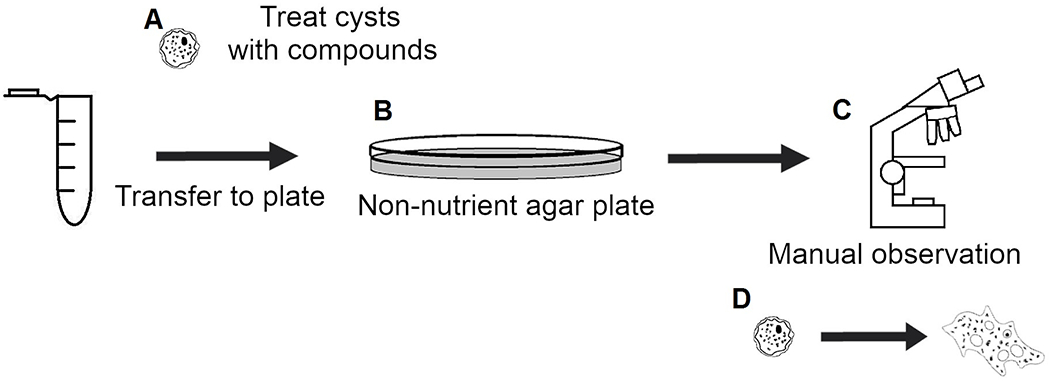
(A) Cysts are treated and incubated with compounds of interest. (B) Treated cysts are transferred to non-nutrient agar plates with E. coli. (C) Plates are manually imaged and observed daily for evidence of excystation. (D) Observe excystation and proliferation of trophozoites or trails left behind in agar media to manually determine if compound of interest was cysticidal.
Trypan blue exclusion staining is a cell viability assay where intact, live cells exclude the trypan blue dye. In this assay, drug-treated cysts are stained and manually assessed for viability by counting to determine the percentage of viable cysts. This technique has been utilized to assay the cysticidal activity of a number of compounds. For instance, Mafra et al (2013) utilized trypan blue exclusion staining with an automated cell counter to determine the viability of cysts following chlorhexidine and PHMB treatment [75]. Jha et al (2014) utilized this technique to assay Acanthamoeba cyst viability following chloroquine treatment [182].
In an excystation assay, Acanthamoeba trophozoites are cultured on non-nutrient agar plates containing heat-killed Escherichia coli to generate cysts [64]. The cysts are collected, treated with compounds of interest for 48 hours, plated onto new non-nutrient agar plates, and observed daily under a microscope for a week [64]. Compounds are considered cysticidal if there is no evidence of excystation, such as proliferating trophozoites, even after 7 days on the new agar plates [64]. This technique has been utilized to evaluate numerous potentially cysticidal antimicrobials. Alizadeh et al (2009) evaluated Alexidine’s cysticidal activity [63]. Iovieno et al (2014) assayed several azole and antifungal agents to identify two potentially cysticidal compounds using this technique [162].
Evaluating the cysticidal effects of antimicrobial compounds can be inherently challenging, as manual counting and observation of cysts under a regular microscope is labor-intensive, subject to human error, and low-throughput. These limitations prevent trypan blue viability and excystation assays from being effectively utilized to screening large compound libraries. As such, development of a high-throughput cysticidal assay amenable to automation would significantly increase the rate at which anti-Acanthamoeba cysticidal compounds can be identified.
7. Conclusions
Acanthamoeba keratitis is a relatively rare infection and while biguanide and diamidine-based treatments are quite effective at resolving most cases, there are some clinical reports of recalcitrance and resistance [32,72]. As such, discovery of new chemotherapeutic agents could significantly expand Acanthamoeba keratitis treatment options.
Current efforts to identify new anti-Acanthamoeba compounds rely primarily upon trophocidal assays that utilize commercially available cell viability assays while cysticidal assays require manual observation of drug-treated cysts for evidence of excystation or counting viable cysts through trypan blue cell viability staining [64,95,154,157,172,174]. Since no Acanthamoeba keratitis panacea currently exists and cysts likely contribute to treatment failure, assays that can identify cysticidal compounds will be crucial. Excystation-based assays that measure if cysts can excyst following drug treatment provide direct evidence of a drug’s cysticidal potential. As such, any drug leads that are identified to be trophocidal should also be screened through cysticidal assays, such as excystation assays or trypan blue viability assays, to identify drugs with dual activity against trophozoites and cysts.
Clinical studies and in vitro drug screens on the efficacy of azole antifungal agents against Acanthamoeba spp. have yielded varying results with reports suggesting a number of azoles are effective against Acanthamoeba spp. Compounds, such as posaconazole, isavuconazole, clotrimazole, voriconazole, itraconazole, and miconazole have been reported to be effective against trophozoites [125,139,140,146,154,168]. Posaconazole and isavuconazole have been reported to be cysticidal as well [154,162]. Fluconazole and ketoconazole have conflicting reports regarding their activities against trophozoites and cysts [125,132,150].
Considering the availability and general tolerability of azole antifungal agents, they could be worthwhile additions to the clinical armamentarium against Acanthamoeba keratitis if evaluated further. While antifungal azoles are generally believed to not penetrate the corneal epithelium well when topically applied, alterations in the method of administration, formulation changes, and adjunctive surgical preparations could enhance corneal penetration and improve the efficacy of azole antifungal agents.
8. Expert opinion
Within the next five to ten years, research into screening and identifying new compounds effective against Acanthamoeba will likely continue to be driven by academic institutions rather than the pharmaceutical or contact lens industries. Since trophozoites are less labor-intensive to work with and typically more susceptible to drugs than cysts are, the rate of discovery for trophocidal compounds will likely far exceed that of cysticidal compounds. Despite this, identifying cysticidal compounds will still be paramount as cysts are a likely contributor to recalcitrance and treatment failure in Acanthamoeba keratitis.
Considering the difficulty and laborious nature of cysticidal assays, this could be a key area for research and development. Current cysticidal assays either rely upon trypan blue viability staining or manual observation of cysts for evidence of trophozoite excystation. Since both of these approaches are manual and low-throughput, any developments in automation and miniaturization could significantly increase the throughput of cysticidal drug screens to yield new cysticidal compounds. Developing a commercially available biochemical viability assay that is unaffected by the cysts’ metabolic dormancy and resilient cyst walls and highly amenable to high-throughput screening would also further speed up cysticidal drug discovery.
Since combination therapies have proven more successful than single-drug monotherapies, researchers should strongly consider employing drug combination experiments in both trophocidal and cysticidal assays. While combination experiments in in vitro trophocidal assays are straightforward and the dose-effect relationships between two drugs can be assessed by classical isobolograms, development of a combination experiment in the cysticidal assay in vitro may prove challenging due to trypan blue staining being low-throughput and excystation-based cysticidal assays revolving on a “cysticidal-or-not” basis rather than percentage inhibition. Additionally, testing the efficacy of combination therapies in vivo introduces an additional layer of complexity. Despite these challenges, it is worthwhile to test compounds in vitro in monotherapy and combination therapy during the early stages of drug discovery and development as this will provide researchers direction on the selection of compounds that may bring a greater chance of success in in vivo efficacy experiments.
Several hurdles remain in discovering and implementing new anti-Acanthamoeba agents clinically. Acanthamoeba keratitis is relatively rare with estimates as low as one to two per million contact lens wearers annually developing Acanthamoeba keratitis [183]. Since there are relatively few Acanthamoeba keratitis patients, there is minimal need and financial incentive for the pharmaceutical and contact lens industries to invest in the discovery of new antimicrobial agents. However, considering the rarity of the disease, identification of a lead that is efficacious in the animal model of Acanthamoeba keratitis also brings an advantage of receiving orphan drug status from the FDA, which may encourage more research efforts into identifying anti-Acanthamoeba therapeutics.
Medical precedence also poses an additional hurdle to implementing any newly discovered antimicrobial agents as biguanide and diamidine therapies work well for the majority of Acanthamoeba keratitis patients. In spite of these challenges, it is possible that the infection is underdiagnosed as a retrospective study from a single center in Iowa showed that the average number of new Acanthamoeba keratitis cases per year among Iowa residents doubled during 2010-2017 [183]. The rapid increase in Acanthamoeba keratitis case numbers has led the National Institutes of Health to label Acanthamoeba keratitis as an Emerging Infectious Disease.
Since Acanthamoeba keratitis is an Emerging Infectious Disease, this re-emphasizes the importance for the discovery of better therapies for its treatment. Identifying novel antimicrobial agents with dual activity against Acanthamoeba trophozoites and cysts would yield more potential therapy options for clinicians to treat Acanthamoeba keratitis and reduce the prevalence of patient relapse and recalcitrant infections to improve patient outcomes.
Article Highlights.
Acanthamoeba spp. are causative agents of Acanthamoeba keratitis, granulomatous amebic encephalitis, and systemic infections.
Acanthamoeba keratitis treatments heavily rely on a combination of biguanide and diamidine antimicrobial agents, but other antiseptic, antiparasitic, photodynamic, antibiotic, and antifungal drugs have been evaluated as potential treatment options.
Azole antifungal agents are well tolerated in patients and have varying levels of trophocidal and cysticidal activity.
Several azole antifungal agents have been tested in limited cases of Acanthamoeba keratitis. Further evaluation is needed to determine the ocular bioavailability and effectiveness of azole therapy for Acanthamoeba keratitis.
Several high-throughput cell viability assays exist for identifying trophocidal compounds while cysticidal assays rely on manual observation or trypan blue staining.
Funding
A Debnath was supported by the grant R21AI146460 from NIH.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
References of interest (“*”) and of considerable interest (“**”) have been highlighted for readers.
- 1.Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003. April;16:273–307. [DOI] [PMC free article] [PubMed] [Google Scholar]; *General, extensive review that discusses all aspects of Acanthamoeba spp. as disease-causing agents.
- 2.Acanthamoeba castellanii - an overview | ScienceDirect Topics [Internet]. [cited 2020 Sep 9]. Available from: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/acanthamoeba-castellanii.
- 3.Coulon C, Collignon A, McDonnell G, Thomas V. Resistance of Acanthamoeba cysts to disinfection treatments used in health care settings. J. Clin. Microbiol. 2010. August;48:2689–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder MJ, Kilvington S, Dart JK. A clinicopathologic study of in vitro sensitivity testing and Acanthamoeba keratitis. Investigative ophthalmology & … 1994; . [PubMed] [Google Scholar]
- 5.Sheng W-H, Hung C-C, Huang H-H, et al. First case of granulomatous amebic encephalitis caused by Acanthamoeba castellanii in Taiwan. Am. J. Trop. Med. Hyg. 2009. August;81:277–279. [PubMed] [Google Scholar]
- 6.CDC. Pathogen & Environment | Acanthamoeba | Parasites | CDC [Internet]. 2019. [cited 2020 Sep 8]. Available from: https://www.cdc.gov/parasites/acanthamoeba/pathogen.html.
- 7.Baig AM, Iqbal J, Khan NA. In vitro efficacies of clinically available drugs against growth and viability of an Acanthamoeba castellanii keratitis isolate belonging to the T4 genotype. Antimicrob. Agents Chemother. 2013. August;57:3561–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. Illness & Symptoms | Acanthamoeba | Parasites | CDC [Internet]. 2010. [cited 2021 Feb 17]. Available from: https://www.cdc.gov/parasites/acanthamoeba/illness.html.
- 9.Szentmáry N, Daas L, Shi L, et al. Acanthamoeba keratitis - Clinical signs, differential diagnosis and treatment. Journal of Current Ophthalmology. 2019. March;31:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke DW, Alizadeh H, Mayhew E, et al. Why Does Acanthamoeba castellanii Not Progress Beyond the Cornea to Produce Intraocular Infections? Invest. Ophthalmol. Vis. Sci. 2005. May; . [DOI] [PubMed] [Google Scholar]
- 11.Neelam S, Niederkorn JY. Pathobiology and Immunobiology of Acanthamoeba Keratitis: Insights from Animal Models. Yale J Biol Med. 2017. June;90:261–268. [PMC free article] [PubMed] [Google Scholar]
- 12.McClellan K, Howard K, Mayhew E, et al. Adaptive immune responses to Acanthamoeba cysts. Exp. Eye Res. 2002. September;75:285–293. [PubMed] [Google Scholar]
- 13.Kelley PS, Dossey AP, Patel D, et al. Secondary glaucoma associated with advanced acanthamoeba keratitis. Eye Contact Lens. 2006. Jul;32:178–182. [DOI] [PubMed] [Google Scholar]
- 14.Herz NL, Matoba AY, Wilhelmus KR. Rapidly progressive cataract and iris atrophy during treatment of Acanthamoeba keratitis. Ophthalmology. 2008. May;115:866–869. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015. Feb;22:10. [DOI] [PMC free article] [PubMed] [Google Scholar]; *General review that specifically addresses Acanthamoeba keratitis.
- 16.Szentmáry N, Shi L, Daas L, Seitz B. Diagnostics and management approaches for Acanthamoeba keratitis. Expert Opin. Orphan Drugs. 2020. July;8:227–236. [Google Scholar]; *Recent review that addresses clinical management aspects of Acanthamoeba keratitis.
- 17.Hahn TW, O’Brien TP, Sah WJ, Kim JH. Acridine orange staining for rapid diagnosis of Acanthamoeba keratitis. Jpn J Ophthalmol. 1998. April;42:108–114. [DOI] [PubMed] [Google Scholar]
- 18.Chew SJ, Beuerman RW, Assouline M, et al. Early diagnosis of infectious keratitis with in vivo real time confocal microscopy. CLAO J. 1992. July;18:197–201. [PubMed] [Google Scholar]
- 19.Villani E, Baudouin C, Efron N, et al. In vivo confocal microscopy of the ocular surface: from bench to bedside. Curr. Eye Res. 2014. March;39:213–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanavi MR, Javadi M, Yazdani S, Mirdehghanm S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea. 2007. August;26:782–786. [DOI] [PubMed] [Google Scholar]
- 21.Tu EY, Joslin CE, Sugar J, Booton GC, Shoff ME, Fuerst PA. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis. Cornea. 2008. August;27:764–772. [DOI] [PubMed] [Google Scholar]
- 22.Yera H, Ok V, Lee Koy Kuet F, et al. PCR and culture for diagnosis of Acanthamoeba keratitis. Br. J. Ophthalmol. 2020. Oct; . [DOI] [PubMed] [Google Scholar]
- 23.Mathers WD, Nelson SE, Lane JL, et al. Confirmation of confocal microscopy diagnosis of Acanthamoeba keratitis using polymerase chain reaction analysis. Arch. Ophthalmol. 2000. February;118:178–183. [DOI] [PubMed] [Google Scholar]
- 24.Maubon D, Dubosson M, Chiquet C, et al. A one-step multiplex PCR for acanthamoeba keratitis diagnosis and quality samples control. Invest. Ophthalmol. Vis. Sci. 2012. May;53:2866–2872. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder JM, Booton GC, Hay J, et al. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 2001. May;39:1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasricha G, Sharma S, Garg P, Aggarwal RK. Use of 18S rRNA gene-based PCR assay for diagnosis of acanthamoeba keratitis in non-contact lens wearers in India. J. Clin. Microbiol. 2003. July;41:3206–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivière D, Szczebara FM, Berjeaud J-M, et al. Development of a real-time PCR assay for quantification of Acanthamoeba trophozoites and cysts. J. Microbiol. Methods. 2006. January;64:78–83. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann OJ, Green SM, Morlet N, et al. Polymerase chain reaction analysis of corneal epithelial and tear samples in the diagnosis of Acanthamoeba keratitis. Invest. Ophthalmol. Vis. Sci. 1998. June;39:1261–1265. [PubMed] [Google Scholar]
- 29.Fittipaldi M, Pino Rodriguez NJ, Adrados B, et al. Discrimination of viable Acanthamoeba castellani trophozoites and cysts by propidium monoazide real-time polymerase chain reaction. J. Eukaryot. Microbiol. 2011. August;58:359–364. [DOI] [PubMed] [Google Scholar]
- 30.Panjwani N Pathogenesis of acanthamoeba keratitis. Ocul. Surf. 2010. Apr;8:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke DW, Niederkorn JY. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006. April;22:175–180. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui R, Aqeel Y, Khan NA. The Development of Drugs against Acanthamoeba Infections. Antimicrob. Agents Chemother. 2016. October;60:6441–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Review that addresses drug development and different druggable targets for Acanthamoeba spp. organisms.
- 33.Ting DSJ, Henein C, Said DG, Dua HS. Photoactivated chromophore for infectious keratitis - Corneal cross-linking (PACK-CXL): A systematic review and meta-analysis. Ocul. Surf. 2019. Aug;17:624–634. [DOI] [PubMed] [Google Scholar]
- 34.Gatti S, Cevini C, Bruno A, et al. In vitro effectiveness of povidone-iodine on Acanthamoeba isolates from human cornea. Antimicrob. Agents Chemother. 1998. September;42:2232–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heaselgrave W, Hamad A, Coles S, Hau S. In vitro evaluation of the inhibitory effect of topical ophthalmic agents on acanthamoeba viability. Transl. Vis. Sci. Technol. 2019. Sep;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirabayashi KE, Lin CC, Ta CN. Oral miltefosine for refractory Acanthamoeba keratitis. Am. J. Ophthalmol. Case Rep. 2019. December;16:100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thulasi P, Saeed HN, Rapuano CJ, et al. Oral miltefosine as salvage therapy for refractory acanthamoeba keratitis. Am. J. Ophthalmol. 2020. October;223:75–82. [DOI] [PubMed] [Google Scholar]
- 38.Avdagic E, Chew HF, Veldman P, et al. Resolution of Acanthamoeba Keratitis with Adjunctive Use of Oral Miltefosine. Ocul. Immunol. Inflamm. 2019. December;1–4. [DOI] [PubMed] [Google Scholar]
- 39.Dewan N, Ming W, Holland SP, et al. Oral miltefosine as adjunctive treatment for recalcitrant acanthamoeba keratitis. Cornea. 2019. July;38:914–917. [DOI] [PubMed] [Google Scholar]
- 40.Tavassoli S, Buckle M, Tole D, et al. The use of miltefosine in the management of refractory Acanthamoeba keratitis. Cont. Lens Anterior Eye. 2018. Aug;41:400–402. [DOI] [PubMed] [Google Scholar]
- 41.Naranjo A, Martinez JD, Miller D, et al. Systemic miltefosine as an adjunct treatment of progressive acanthamoeba keratitis. Ocul. Immunol. Inflamm. 2020. May;1–9. [DOI] [PubMed] [Google Scholar]
- 42.Jori G, Fabris C, Soncin M, et al. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg. Med. 2006. Jun;38:468–481. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S, Srinivasan M, George C. Acanthamoeba keratitis in non-contact lens wearers. Arch. Ophthalmol. 1990. May;108:676–678. [DOI] [PubMed] [Google Scholar]
- 44.Haburchak DR. Acanthamoeba Infection Treatment & Management: Medical Care, Surgical Care, Consultations [Internet]. Medscape. 2017. [cited 2020 Sep 9]. Available from: https://emedicine.medscape.com/article/211214-treatment.
- 45.CDC. Acanthamoeba Keratitis Fact Sheet for Healthcare Professionals | Acanthamoeba | Parasites | CDC [Internet]. 2017. [cited 2020 Sep 9]. Available from: https://www.cdc.gov/parasites/acanthamoeba/health_professionals/acanthamoeba_keratitis_hcp.html.
- 46.Oldenburg CE, Acharya NR, Tu EY, et al. Practice patterns and opinions in the treatment of acanthamoeba keratitis. Cornea. 2011. December;30:1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maycock NJR, Jayaswal R. Update on acanthamoeba keratitis: diagnosis, treatment, and outcomes. Cornea. 2016. May;35:713–720. [DOI] [PubMed] [Google Scholar]
- 48.Stepp MA, Zieske JD, Trinkaus-Randall V, et al. Wounding the cornea to learn how it heals. Exp. Eye Res. 2014. April;121:178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dart JKG, Saw VPJ, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am. J. Ophthalmol. 2009. October;148:487–499.e2. [DOI] [PubMed] [Google Scholar]
- 50.Saidel MA. Acanthamoeba Keratitis Treatment [Internet]. American Academy of Opthalmology. 2006. [cited 2020 Oct 20]. Available from: https://www.aao.org/current-insight/acanthamoeba-keratitis-treatment.
- 51.Kitzmann AS, Goins KM, Sutphin JE, Wagoner MD. Keratoplasty for treatment of Acanthamoeba keratitis. Ophthalmology. 2009. May;116:864–869. [DOI] [PubMed] [Google Scholar]
- 52.Amoils SP, Heney C. Acanthamoeba keratitis with live isolates treated with cryosurgery and fluconazole. Am. J. Ophthalmol. 1999. June;127:718–720. [DOI] [PubMed] [Google Scholar]
- 53.Meisler DM, Ludwig IH, Rutherford I, et al. Susceptibility of Acanthamoeba to cryotherapeutic method. Arch. Ophthalmol. 1986. January;104:130–131. [DOI] [PubMed] [Google Scholar]
- 54.Binder PS. Cryotherapy for medically unresponsive acanthamoeba keratitis. Cornea. 1989;8:106–114. [PubMed] [Google Scholar]
- 55.Brasseur G, Favennec L, Perrine D, et al. Successful treatment of Acanthamoeba keratitis by hexamidine. Cornea. 1994. September;13:459–462. [DOI] [PubMed] [Google Scholar]
- 56.Kitagawa K, Nakamura T, Takahashi N, et al. A novel combination treatment of chlorhexidine gluconate, natamycin (pimaricin) and debridement for a Acanthamoeba keratitis. Jpn J Ophthalmol. 2003. December;47:616–617. [DOI] [PubMed] [Google Scholar]
- 57.Wright P, Warhurst D, Jones BR. Acanthamoeba keratitis successfully treated medically. Br. J. Ophthalmol. 1985. October;69:778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varga JH, Wolf TC, Jensen HG, et al. Combined treatment of Acanthamoeba keratitis with propamidine, neomycin, and polyhexamethylene biguanide. Am. J. Ophthalmol. 1993. April;115:466–470. [DOI] [PubMed] [Google Scholar]
- 59.Hargrave SL, McCulley JP, Husseini Z. Results of a trial of combined propamidine isethionate and neomycin therapy for Acanthamoeba keratitis. Brolene Study Group. Ophthalmology. 1999. May;106:952–957. [DOI] [PubMed] [Google Scholar]; **Notable clinical trial evaluating propamidine isethionate in combination with neomycin for Acanthamoeba keratitis treatment.
- 60.Kuyyakanond T, Quesnel LB. The mechanism of action of chlorhexidine. FEMS Microbiol. Lett. 1992. December;100:211–215. [DOI] [PubMed] [Google Scholar]
- 61.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999. January;12:147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turner NA, Russell AD, Furr JR, Lloyd D. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J. Antimicrob. Chemother. 2000. July;46:27–34. [DOI] [PubMed] [Google Scholar]
- 63.Alizadeh H, Neelam S, Cavanagh HD. Amoebicidal activities of alexidine against 3 pathogenic strains of acanthamoeba. Eye Contact Lens. 2009. Jan;35:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narasimhan S, Madhavan HN, K LT. Development and application of an in vitro susceptibility test for Acanthamoeba species isolated from keratitis to polyhexamethylene biguanide and chlorhexidine. Cornea. 2002. March;21:203–205. [DOI] [PubMed] [Google Scholar]
- 65.Lee J-E, Oum BS, Choi HY, et al. Cysticidal effect on acanthamoeba and toxicity on human keratocytes by polyhexamethylene biguanide and chlorhexidine. Cornea. 2007. July;26:736–741. [DOI] [PubMed] [Google Scholar]
- 66.Kosrirukvongs P, Wanachiwanawin D, Visvesvara GS. Treatment of acanthamoeba keratitis with chlorhexidine. Ophthalmology. 1999. April;106:798–802. [DOI] [PubMed] [Google Scholar]; **Clinical trial evaluating chlorhexidine treatment of Acanthamoeba keratitis.
- 67.Lim N, Goh D, Bunce C, et al. Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am. J. Ophthalmol. 2008. January;145:130–135. [DOI] [PubMed] [Google Scholar]; **Comparative clinical trial evaluating polyhexamethylene biguanide versus chlorhexidine for Acanthamoeba keratitis treatment.
- 68.Romanowski EG, Yates KA, O’Connor KE, et al. Evaluation of polyhexamethylene biguanide (PHMB) as a disinfectant for adenovirus. JAMA Ophthalmol. 2013. April;131:495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schnuch A, Geier J, Uter W, et al. The biocide polyhexamethylene biguanide remains an uncommon contact allergen. Contact Derm. 2007. Apr;56:235–239. [DOI] [PubMed] [Google Scholar]
- 70.Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014. Aug;3:511–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khunkitti W, Lloyd D, Furr JR, Russell AD. Aspects of the mechanisms of action of biguanides on trophozoites and cysts of Acanthamoeba castellanii. J. Appl. Microbiol. 1997. January;82:107–114. [DOI] [PubMed] [Google Scholar]
- 72.Pérez-Santonja JJ, Kilvington S, Hughes R, et al. Persistently culture positive acanthamoeba keratitis: in vivo resistance and in vitro sensitivity. Ophthalmology. 2003. August;110:1593–1600. [DOI] [PubMed] [Google Scholar]
- 73.Ferrari G, Matuska S, Rama P. Double-biguanide therapy for resistant acanthamoeba keratitis. Case Rep. Ophthalmol. 2011. Sep;2:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang F-C, Shih M-H, Chang K-F, et al. Characterizing clinical isolates of Acanthamoeba castellanii with high resistance to polyhexamethylene biguanide in Taiwan. J. Microbiol. Immunol. Infect. 2017. October;50:570–577. [DOI] [PubMed] [Google Scholar]
- 75.Mafra CSP, Carrijo-Carvalho LC, Chudzinski-Tavassi AM, et al. Antimicrobial action of biguanides on the viability of Acanthamoeba cysts and assessment of cell toxicity. Invest. Ophthalmol. Vis. Sci. 2013. September;54:6363–6372. [DOI] [PubMed] [Google Scholar]
- 76.Soeiro MNC, De Souza EM, Stephens CE, Boykin DW. Aromatic diamidines as antiparasitic agents. Expert Opin. Investig. Drugs. 2005. Aug;14:957–972. [DOI] [PubMed] [Google Scholar]
- 77.Perrine D, Chenu JP, Georges P, et al. Amoebicidal efficiencies of various diamidines against two strains of Acanthamoeba polyphaga. Antimicrob. Agents Chemother. 1995. February;39:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duguid IG, Dart JK, Morlet N, et al. Outcome of acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology. 1997. October;104:1587–1592. [DOI] [PubMed] [Google Scholar]
- 79.Seal D, Hay J, Kirkness C, et al. Successful medical therapy of Acanthamoeba keratitis with topical chlorhexidine and propamidine. Eye (Lond). 1996;10 ( Pt 4):413–421. [DOI] [PubMed] [Google Scholar]
- 80.Alizadeh H, Silvany RE, Meyer DR, et al. In vitro amoebicidal activity of propamidine and pentamidine isethionate against Acanthamoeba species and toxicity to corneal tissues. Cornea. 1997. January;16:94–100. [PubMed] [Google Scholar]
- 81.James EA, James BH, Nicholson AJ. Process for the preparation of diamidine derivatives. US Patent … 1942; . [Google Scholar]
- 82.Final report on the safety assessment of Hexamidine and Hexamidine Diisethionate. Int. J. Toxicol. 2007;26 Suppl 3:79–88. [DOI] [PubMed] [Google Scholar]
- 83.Lepelletier D, Maillard JY, Pozzetto B, Simon A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob. Agents Chemother. 2020. August;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, et al. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017. Aug;44:260–268. [DOI] [PubMed] [Google Scholar]
- 85.Cho P, Reyes S, Boost MV. Microbiocidal characterization of a novel povidone-iodine based rigid contact lens disinfecting solution. Cont. Lens Anterior Eye. 2018. Aug;41:542–546. [DOI] [PubMed] [Google Scholar]
- 86.Martín-Navarro CM, Lorenzo-Morales J, López-Arencibia A, et al. Acanthamoeba spp.: efficacy of Bioclen FR One Step, a povidone-iodine based system for the disinfection of contact lenses. Exp. Parasitol. 2010. September;126:109–112. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi T, Gibbon L, Mito T, et al. Efficacy of commercial soft contact lens disinfectant solutions against Acanthamoeba. Jpn J Ophthalmol. 2011. September;55:547–557. [DOI] [PubMed] [Google Scholar]
- 88.Padzik M, Baltaza W, Conn DB, et al. Effect of povidone iodine, chlorhexidine digluconate and toyocamycin on amphizoic amoebic strains, infectious agents of Acanthamoeba keratitis - a growing threat to human health worldwide. Ann. Agric. Environ. Med. 2018. December;25:725–731. [DOI] [PubMed] [Google Scholar]
- 89.Roongruangchai J, Sookkua T, Kummalue T, Roongruangchai K. Pouzolzia indica methanolic extract fraction 2 and povidone-iodine induced changes in the cyst of Acanthamoeba spp.: light and electron microscopic studies. J. Med. Assoc. Thai. 2009. Nov;92:1492–1499. [PubMed] [Google Scholar]
- 90.Sunada A, Kimura K, Nishi I, et al. In vitro evaluations of topical agents to treat Acanthamoeba keratitis. Ophthalmology. 2014. October;121:2059–2065. [DOI] [PubMed] [Google Scholar]
- 91.Shi L, Muthukumar V, Stachon T, et al. The Effect of Anti-Amoebic Agents and Ce6-PDT on Acanthamoeba castellanii Trophozoites and Cysts, In Vitro. Transl. Vis. Sci. Technol. 2020. November;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pelletier JS, Miller D, Liang B, Capriotti JA. In vitro efficacy of a povidone-iodine 0.4% and dexamethasone 0.1% suspension against ocular pathogens. J. Cataract Refract. Surg. 2011. Apr;37:763–766. [DOI] [PubMed] [Google Scholar]
- 93.Lim L, Coster DJ, Badenoch PR. Antimicrobial susceptibility of 19 Australian corneal isolates of Acanthamoeba. Clin. Experiment. Ophthalmol. 2000. April;28:119–124. [DOI] [PubMed] [Google Scholar]
- 94.Dorlo TPC, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012. November;67:2576–2597. [DOI] [PubMed] [Google Scholar]
- 95.Chao M, Thongseesuksai T, Boonmars T, Laummaunwai P. Investigation of the in vitro cysticidal activity of miltefosine against Acanthamoeba spp. J. Parasit. Dis. 2020. Jun;44:491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walochnik J, Duchêne M, Seifert K, et al. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 2002. March;46:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mrva M, Garajová M, Lukáč M, Ondriska F. Weak cytotoxic activity of miltefosine against clinical isolates of Acanthamoeba spp. J. Parasitol. 2011. June;97:538–540. [DOI] [PubMed] [Google Scholar]
- 98.Kwiatkowski S, Knap B, Przystupski D, et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018. October;106:1098–1107. [DOI] [PubMed] [Google Scholar]
- 99.Kassab K, Dei D, Roncucci G, et al. Phthalocyanine-photosensitized inactivation of a pathogenic protozoan, Acanthamoeba palestinensis. Photochem. Photobiol. Sci. 2003. June;2:668–672. [DOI] [PubMed] [Google Scholar]
- 100.Chen Z, Xuguang S, Zhiqun W, Ran L. In vitro amoebacidal activity of photodynamic therapy on Acanthamoeba. Br. J. Ophthalmol. 2008. September;92:1283–1286. [DOI] [PubMed] [Google Scholar]
- 101.Siddiqui R, Khan NA. Photochemotherapeutic strategies against Acanthamoeba keratitis. AMB Express. 2012. Sep;2:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mito T, Suzuki T, Kobayashi T, et al. Effect of photodynamic therapy with methylene blue on Acanthamoeba in vitro. Invest. Ophthalmol. Vis. Sci. 2012. September;53:6305–6313. [DOI] [PubMed] [Google Scholar]
- 103.del Buey MA, Cristóbal JA, Casas P, et al. Evaluation of in vitro efficacy of combined riboflavin and ultraviolet a for Acanthamoeba isolates. Am. J. Ophthalmol. 2012. March;153:399–404. [DOI] [PubMed] [Google Scholar]
- 104.Lamy R, Chan E, Good SD, et al. Riboflavin and ultraviolet A as adjuvant treatment against Acanthamoeba cysts. Clin. Experiment. Ophthalmol. 2016. April;44:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gomart G, Denis J, Bourcier T, et al. In Vitro Amoebicidal Activity of Titanium Dioxide/UV-A Combination Against Acanthamoeba. Invest. Ophthalmol. Vis. Sci. 2018. September;59:4567–4571. [DOI] [PubMed] [Google Scholar]
- 106.Dwia Pertiwi Y, Chikama T, Sueoka K, et al. Efficacy of Photodynamic Anti-Microbial Chemotherapy for Acanthamoeba Keratitis In Vivo. Lasers Surg. Med. 2020. December; . [DOI] [PubMed] [Google Scholar]
- 107.Pertiwi YD, Chikama T, Sueoka K, et al. Antimicrobial Photodynamic Therapy with the photosensitizer TONS504 eradicates Acanthamoeba. Photodiagnosis Photodyn. Ther. 2019. December;28:166–171. [DOI] [PubMed] [Google Scholar]
- 108.Atalay HT, Uysal BS, Sarzhanov F, et al. Rose Bengal-Mediated Photodynamic Antimicrobial Treatment of Acanthamoeba Keratitis. Curr. Eye Res. 2020. February;45:1205–1210. [DOI] [PubMed] [Google Scholar]
- 109.Morén H, Malmsjö M, Mortensen J, Ohrström A. Riboflavin and ultraviolet a collagen crosslinking of the cornea for the treatment of keratitis. Cornea. 2010. January;29:102–104. [DOI] [PubMed] [Google Scholar]
- 110.Khan YA, Kashiwabuchi RT, Martins SA, et al. Riboflavin and ultraviolet light a therapy as an adjuvant treatment for medically refractive Acanthamoeba keratitis: report of 3 cases. Ophthalmology. 2011. February;118:324–331. [DOI] [PubMed] [Google Scholar]
- 111.Price MO, Tenkman LR, Schrier A, et al. Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J. Refract. Surg. 2012. October;28:706–713. [DOI] [PubMed] [Google Scholar]
- 112.Naranjo A, Arboleda A, Martinez JD, et al. Rose bengal photodynamic antimicrobial therapy for patients with progressive infectious keratitis: A pilot clinical study. Am. J. Ophthalmol. 2019. September;208:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sharma R, Jhanji V, Satpathy G, et al. Coinfection with Acanthamoeba and Pseudomonas in contact lens-associated keratitis. Optom. Vis. Sci. 2013. February;90:e53–5. [DOI] [PubMed] [Google Scholar]
- 114.Beattie AM, Slomovic AR, Rootman DS, Hunter WS. Acanthamoeba keratitis with two species of Acanthamoeba. Can. J. Ophthalmol. 1990. August;25:260–262. [PubMed] [Google Scholar]
- 115.Skarin A, Florén I, Kiss K, et al. Acanthamoeba keratitis in the south of Sweden. Acta Ophthalmol. Scand. 1996. December;74:593–597. [DOI] [PubMed] [Google Scholar]
- 116.Moore MB, McCulley JP. Acanthamoeba keratitis associated with contact lenses: six consecutive cases of successful management. Br. J. Ophthalmol. 1989. April;73:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berger ST, Mondino BJ, Hoft RH, et al. Successful medical management of Acanthamoeba keratitis. Am. J. Ophthalmol. 1990. October;110:395–403. [DOI] [PubMed] [Google Scholar]
- 118.Natamycin | C33H47O13N - PubChem [Internet]. [cited 2021 Feb 24]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Natamycin.
- 119.Noor A, Preuss CV. Amphotericin B. StatPearls Treasure Island (FL): StatPearls Publishing; . [PubMed] [Google Scholar]
- 120.Taravaud A, Loiseau PM, Pomel S. In vitro evaluation of antimicrobial agents on Acanthamoeba sp. and evidence of a natural resilience to amphotericin B. Int J Parasitol Drugs Drug Resist. 2017. December;7:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biser SA, Perry HD, Donnenfeld ED, et al. Arthrographis keratitis mimicking acanthamoeba keratitis. Cornea. 2004. April;23:314–317. [DOI] [PubMed] [Google Scholar]
- 122.Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017. September;124:1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Behrens-Baumann W, Klinge B, Uter W. Clotrimazole and bifonazole in the topical treatment of Candida keratitis in rabbits. Mycoses. 1990. Dec;33:567–573. [DOI] [PubMed] [Google Scholar]
- 124.Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 1999. January;12:40–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lamb DC, Warrilow AGS, Rolley NJ, et al. Azole Antifungal Agents To Treat the Human Pathogens Acanthamoeba castellanii and Acanthamoeba polyphaga through Inhibition of Sterol 14α-Demethylase (CYP51). Antimicrob. Agents Chemother. 2015. August;59:4707–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elsheikha HM, Siddiqui R, Khan NA. Drug Discovery against Acanthamoeba Infections: Present Knowledge and Unmet Needs. Pathogens. 2020. May;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miller D, Alfonso EC. Management of fungal keratitis: Topical or Systemic therapy? Vision Pan-America. 2014; . [Google Scholar]
- 128.Gokhale NS. Medical management approach to infectious keratitis. Indian J. Ophthalmol. 2008. June;56:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moiseev RV, Morrison PWJ, Steele F, Khutoryanskiy VV. Penetration enhancers in ocular drug delivery. Pharmaceutics. 2019. July;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang W, Wiederhold NP, Williams RO. Drug delivery strategies for improved azole antifungal action. Expert Opin. Drug Deliv. 2008. November;5:1199–1216. [DOI] [PubMed] [Google Scholar]
- 131.Lakhani P, Patil A, Majumdar S. Challenges in the Polyene- and Azole-Based Pharmacotherapy of Ocular Fungal Infections. J. Ocul. Pharmacol. Ther. 2019;35:6–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wynter-Allison Z, Lorenzo Morales J, Calder D, et al. Acanthamoeba infection as a cause of severe keratitis in a soft contact lens wearer in Jamaica. Am. J. Trop. Med. Hyg. 2005. July;73:92–94. [PubMed] [Google Scholar]
- 133.Ishibashi Y, Matsumoto Y, Kabata T, et al. Oral itraconazole and topical miconazole with débridement for Acanthamoeba keratitis. Am. J. Ophthalmol. 1990. February;109:121–126. [DOI] [PubMed] [Google Scholar]
- 134.Demirci G, Ay GM, Karabas LV, et al. Acanthamoeba keratitis in a 5-year-old boy without a history of contact lens usage. Cornea. 2006. April;25:356–358. [DOI] [PubMed] [Google Scholar]
- 135.Masselam KL, Pineda R. Topical Voriconazole as an Effective Co-Agent Against Acanthamoeba Keratitis. Investigative Ophthalmology & … 2008; . [Google Scholar]
- 136.Altun A, Kurna SA, Sengor T, et al. Effectiveness of posaconazole in recalcitrant fungal keratitis resistant to conventional antifungal drugs. Case Rep. Ophthalmol. Med. 2014. August;2014:701653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Driebe WT, Stern GA, Epstein RJ, et al. Acanthamoeba keratitis. Potential role for topical clotrimazole in combination chemotherapy. Arch. Ophthalmol. 1988. September;106:1196–1201. [DOI] [PubMed] [Google Scholar]
- 138.Lin H-C, Hsiao C-H, Ma DH-K, et al. Medical treatment for combined Fusarium and Acanthamoeba keratitis. Acta Ophthalmol. 2009. March;87:199–203. [DOI] [PubMed] [Google Scholar]
- 139.Duma RJ, Finley R. In vitro susceptibility of pathogenic naegleria and acanthamoeba species to a variety of therapeutic agents. Antimicrob. Agents Chemother. 1976. August;10:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nagington J, Richards JE. Chemotherapeutic compounds and Acanthamoebae from eye infections. J. Clin. Pathol. 1976. July;29:648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saunders PP, Proctor EM, Rollins DF, Richards JS. Enhanced killing of Acanthamoeba cysts in vitro using dimethylsulfoxide. Ophthalmology. 1992. August;99:1197–1200. [DOI] [PubMed] [Google Scholar]
- 142.Foster CS, Stefanyszyn M. Intraocular penetration of miconazole in rabbits. Arch. Ophthalmol. 1979. September;97:1703–1706. [DOI] [PubMed] [Google Scholar]
- 143.Hemady RK, Chu W, Foster CS. Intraocular penetration of ketoconazole in rabbits. Cornea. 1992. July;11:329–333. [DOI] [PubMed] [Google Scholar]
- 144.Hirst LW, Green WR, Merz W, et al. Management of acanthamoeba keratitis. Ophthalmology. 1984. September;91:1105–1111. [DOI] [PubMed] [Google Scholar]
- 145.Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin. Pharmacokinet. 1998. Dec;35:461–473. [DOI] [PubMed] [Google Scholar]
- 146.Hernèndez-Martínez D, Reyes-Batlle M, Castelan-Ramírez I, et al. Evaluation of the sensitivity to chlorhexidine, voriconazole and itraconazole of T4 genotype Acanthamoeba isolated from Mexico. Exp. Parasitol. 2019. February;197:29–35. [DOI] [PubMed] [Google Scholar]
- 147.Kaur IP, Rana C, Singh H. Development of effective ocular preparations of antifungal agents. J. Ocul. Pharmacol. Ther. 2008. October;24:481–493. [DOI] [PubMed] [Google Scholar]
- 148.Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004. August;351:876–883. [DOI] [PubMed] [Google Scholar]
- 149.Anwar A, Siddiqui R, Hussain MA, et al. Silver nanoparticle conjugation affects antiacanthamoebic activities of amphotericin B, nystatin, and fluconazole. Parasitol. Res. 2018. January;117:265–271. [DOI] [PubMed] [Google Scholar]
- 150.Anwar A, Siddiqui R, Raza Shah M, Ahmed Khan N. Gold nanoparticles conjugation enhances antiacanthamoebic properties of nystatin, fluconazole and amphotericin B. J. Microbiol. Biotechnol. 2019. January;29:171–177. [DOI] [PubMed] [Google Scholar]
- 151.O’Day DM, Foulds G, Williams TE, et al. Ocular uptake of fluconazole following oral administration. Arch. Ophthalmol. 1990. July;108:1006–1008. [DOI] [PubMed] [Google Scholar]
- 152.Abbasoğlu OE, Hoşal BM, Sener B, et al. Penetration of topical fluconazole into human aqueous humor. Exp. Eye Res. 2001. February;72:147–151. [DOI] [PubMed] [Google Scholar]
- 153.Jenks JD, Salzer HJ, Prattes J, et al. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: design, development, and place in therapy. Drug Des. Devel. Ther. 2018. April;12:1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shing B, Singh S, Podust LM, et al. The Antifungal Drug Isavuconazole Is both Amebicidal and Cysticidal against Acanthamoeba castellanii. Antimicrob. Agents Chemother. 2020. April;e02223–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mada PK, Castano G, Gutierrez-Roberts MG. A Case of Rhizopus Keratitis. Clin Med Rev Case …. 2018; . [Google Scholar]
- 156.Falci DR, Pasqualotto AC. Profile of isavuconazole and its potential in the treatment of severe invasive fungal infections. Infect. Drug Resist. 2013. October;6:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rice CA, Troth EV, Russell AC, Kyle DE. Discovery of Anti-Amoebic Inhibitors from Screening the MMV Pandemic Response Box on Balamuthia mandrillaris, Naegleria fowleri, and Acanthamoeba castellanii. Pathogens. 2020. June;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brunet K, Eestermans R, Rodier MH, Cateau E. In vitro activity of isavuconazole against three species of Acanthamoeba. J Fr Ophtalmol. 2020. April;43:330–333. [DOI] [PubMed] [Google Scholar]
- 159.Jeu L, Piacenti FJ, Lyakhovetskiy AG, Fung HB. Voriconazole. Clin. Ther. 2003. May;25:1321–1381. [DOI] [PubMed] [Google Scholar]
- 160.Hou T-Y, Chen Y-C, Hsu C-C. Rapid resolution of stromal keratitis with the assistance of oral voriconazole in resistant acanthamoeba keratitis. Taiwan J. Ophthalmol. 2017. December;7:224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tu EY, Joslin CE, Shoff ME. Successful treatment of chronic stromal acanthamoeba keratitis with oral voriconazole monotherapy. Cornea. 2010. September;29:1066–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Iovieno A, Miller D, Ledee DR, Alfonso EC. Cysticidal activity of antifungals against different genotypes of Acanthamoeba. Antimicrob. Agents Chemother. 2014. September;58:5626–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Talbott M, Cevallos V, Chen MC, et al. Synergy testing of antiamoebic agents for acanthamoeba: antagonistic effect of voriconazole. Cornea. 2019. October;38:1309–1313. [DOI] [PubMed] [Google Scholar]
- 164.Gueudry J, Le Goff L, Compagnon P, et al. Evaluation of voriconazole anti-Acanthamoeba polyphaga in vitro activity, rat cornea penetration and efficacy against experimental rat Acanthamoeba keratitis. J. Antimicrob. Chemother. 2018. July;73:1895–1898. [DOI] [PubMed] [Google Scholar]
- 165.Sponsel W, Chen N, Dang D, et al. Topical voriconazole as a novel treatment for fungal keratitis. Antimicrob. Agents Chemother. 2006. January;50:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lau D, Fedinands M, Leung L, et al. Penetration of voriconazole, 1%, eyedrops into human aqueous humor: a prospective open-label study. Arch. Ophthalmol. 2008. March;126:343–346. [DOI] [PubMed] [Google Scholar]
- 167.Lebeaux D, Lanternier F, Elie C, et al. Therapeutic drug monitoring of posaconazole: a monocentric study with 54 adults. Antimicrob. Agents Chemother. 2009. December;53:5224–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sifaoui I, Martín-Navarro C, López-Arencibia A, et al. Optimized combinations of statins and azoles against Acanthamoeba trophozoites and cysts in vitro. Asian Pac. J. Trop. Med. 2019;12:283. [Google Scholar]
- 169.Tu EY, McCartney DL, Beatty RF, et al. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592). Am. J. Ophthalmol. 2007. February;143:222–227. [DOI] [PubMed] [Google Scholar]
- 170.Tu EY, Park AJ. Recalcitrant Beauveria bassiana keratitis: confocal microscopy findings and treatment with posaconazole (Noxafil). Cornea. 2007. September;26:1008–1010. [DOI] [PubMed] [Google Scholar]
- 171.Rampersad SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012. September;12:12347–12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McBride J, Ingram PR, Henriquez FL, Roberts CW. Development of colorimetric microtiter plate assay for assessment of antimicrobials against Acanthamoeba. J. Clin. Microbiol. 2005. February;43:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martín-Navarro CM, López-Arencibia A, Lorenzo-Morales J, et al. Acanthamoeba castellanii Neff: In vitro activity against the trophozoite stage of a natural sesquiterpene and a synthetic cobalt(II)-lapachol complex. Exp. Parasitol. 2010. September;126:106–108. [DOI] [PubMed] [Google Scholar]
- 174.Fears AC, Metzinger RC, Killeen SZ, et al. Comparative in vitro effectiveness of a novel contact lens multipurpose solution on Acanthamoeba castellanii. J. Ophthalmic Inflamm. Infect. 2018. October;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jha BK, Seo I, Kong H-H, et al. Tigecycline inhibits proliferation of Acanthamoeba castellanii. Parasitol. Res. 2015. March;114:1189–1195. [DOI] [PubMed] [Google Scholar]
- 176.Sifaoui I, Reyes-Batlle M, López-Arencibia A, et al. Evaluation of the anti-Acanthamoeba activity of two commercial eye drops commonly used to lower eye pressure. Exp. Parasitol. 2017. December;183:117–123. [DOI] [PubMed] [Google Scholar]
- 177.Sifaoui I, Reyes-Batlle M, López-Arencibia A, et al. Screening of the pathogen box for the identification of anti-Acanthamoeba agents. Exp. Parasitol. 2019. June;201:90–92. [DOI] [PubMed] [Google Scholar]
- 178.Thermo Fisher Scientific. alamarBlue™ Cell Viability Reagent [Internet]. [cited 2020 Oct 12]. Available from: https://www.thermofisher.com/order/catalog/product/DAL1025#/DAL1025.
- 179.Hannah R, Beck M, Moravec R, Riss T. CellTiter-Glo™ Luminescent cell viability assay: a sensitive and rapid method for determining cell viability. Promega Cell Notes. 2001. January; . [Google Scholar]
- 180.Hahn HJ, Escrig JI, Shing B, Debnath A. In Vitro Effect of Pitavastatin and Its Synergistic Activity with Isavuconazole against Acanthamoeba castellanii. Pathogens. 2020. August;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abjani F, Khan NA, Yousuf FA, Siddiqui R. Targeting cyst wall is an effective strategy in improving the efficacy of marketed contact lens disinfecting solutions against Acanthamoeba castellanii cysts. Cont. Lens Anterior Eye. 2016. June;39:239–243. [DOI] [PubMed] [Google Scholar]
- 182.Jha BK, Jung H-J, Seo I, et al. Chloroquine has a cytotoxic effect on Acanthamoeba encystation through modulation of autophagy. Antimicrob. Agents Chemother. 2014. October;58:6235–6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Scruggs BA, Quist TS, Salinas JL, Greiner MA. Notes from the Field: Acanthamoeba Keratitis Cases - Iowa, 2002-2017. MMWR Morb Mortal Wkly Rep. 2019. May;68:448–449. [DOI] [PMC free article] [PubMed] [Google Scholar]


