Abstract
Meat is a vital source of nutrients for human wellbeing and health; however, recent studies suggest a decline in meat preference as a protein source due to some health problems associated with meat intake. This study evaluates the levels of essential elements (Mn, Mg, P, Ca, K, Cu, Na, Zn and Fe) and proximate composition (protein, fat, moisture, carbohydrate, fibre and ash) of three snail species namely; Achatina achatina, Achatina fulica and Archachatina marginata species from the Kumasi Central Market. The mineral content and proximate composition of the three land snail species' shell and meat were analysed using Atomic Absorption Spectrometry and other Standard Methods of Analysis. The study revealed that the three snail species differed in the composition of significant nutrients and trace elements in meat. It is evident from this study that the consumption of snail meat can promote good human health, with Archachatina marginata having the best nutritional value. In the meat from the three snail species, a strong significant positive correlation between Ca and P levels was observed, and the Cu and Fe (p < 0.05). In both the proximate and mineral analysis, each meat parameter was correspondingly higher than that of the shell for all the snail species, except for ash and Ca contents. The estimated daily intakes of Zn, Fe, Mn and Cu were lower compared with the tolerable daily intake, suggesting that the essential elements are at acceptable levels. Therefore, consumers of these land snails will gain the benefits of the proximate and mineral constituents. The shells can also be utilised as food supplements for livestock and eliminate the burden of managing snail shell waste.
Keywords: Proximate composition, Estimated daily intake, Mineral content, Land snail
Proximate composition, Estimated daily intake, Mineral content, Land snail.
1. Introduction
Meat is an excellent source of protein and several vital elements of high biologic value, as well as lipids rich in vitamin B12 and linoleic acid (Higgs 2000; Bošković et al., 2015). Non-pristine protein foods, such as eggs, meat, and milk obtained from the poultry and livestock sectors, are very important for human diets in many parts of the world because they provide essential trace elements, vitamins, minerals, amino acids, and a rich amount of dietary proteins for safe human health (Alturiqi and Albedair 2012). Generally, meat is a suitable carrier of several essential micronutrients, such as Fe, Se, folic acid and vitamins in an easily absorbable form. The intake of meat can be an excellent approach to addressing the optimum necessities of these micronutrients (Williamson et al., 2005). The optimal intake of elements such as Se, Zn, Cu, Fe, and Mn is critical since they are necessary for the effective performance of nearly all enzymatic and biochemical activities in the human body (Higgs 2000). Meat intake ensures adequate distribution of essential amino acids and micronutrients involved in the regulatory mechanisms of energy metabolism (Cabrera and Saadoun 2014; Bauchart et al., 2007; Biesalski 2005).
Most animal protein is obtained from farm animals in pork, mutton, beef and poultry (Fleck 1976). Globally, pork is the most consumed meat, followed by poultry, beef and mutton with a consumption rate of 15.8, 13.6, 9.6 and 1.9 kg/capita/year (FAOSTAT 2014). However, these sources have been reduced because of drought, the high cost of feed, diseases, mean output of local animal breeds and primitive animal management techniques. This has resulted in the use of a non-typical source of meat protein, including land snail meat. At the moment, there is a lot of interest in the production and sale of land snail meat. Snails are invertebrate animals, which belong to the class Gastropods and phylum Molluscs (Brunt et al., 1999). A snail is composed of a soft body and a shell. The shell is the protective casing, which consists of calcium carbonate, the body consists of protein (60–70 % on dry basis) and water (70 %) (Adeyeye and Afolabi 2004). The shell in most species consists of one-third of the bodyweight of snail (Cobbinah 1994).
Snails are predominantly found in Africa and the most popular snail species are the Achatina achatina (A. achatina), Archachatina marginata (A. marginata) and Achatina fulica (A. fulica) species (Apata et al., 2015). The A. marginata does not have a solid shell colour but has a dark brown foot (flesh), while A. fulica, on the other hand, is small and could have a whitish or dark brown flesh. A. fulica is low in economic value than the A. marginata and A. achatina. The most consumed snail in Ghana is the A. achatina, locally known as “nwapa”. Globally, A. achatina is the largest land snail and are more challenging to breed than other snail species (Cobbinah 1994). The A. fulica has been regarded as the most widely invasive and significant land snail pest (Otten et al., 2006; Raut and Barker 2002). Because of its impact on horticulture and agricultural crops, A. achatina is regarded as one of the most disturbing species (Civeyrel and Simberloff 1996; Mead 1961).
According to popular understanding, sea snail meat has a high carbohydrate content, a low fat and high protein content, and is an important source of vitamin E, phosphorus, potassium, calcium, and sodium (Felici et al., 2020; Ghosh et al., 2017). Additionally, like shell fishes, such as mussels (Özyurt et al., 2013) and oyster (Özyurt et al., 2013), snail meat is high in polyunsaturated fatty acids (Özyurt et al., 2013), which are important nutrients. As a result, snail meat is a great high-protein, low-fat diet. A kilogram of gigantic snail intended for human consumption might be recovered for roughly half a kilogram of high-value animal feed (Piba et al., 2014). Indeed, snail shell powder is utilized as a calcium source in animal diets for broilers and layers, small animals, and cattle (Tchakounte et al., 2019). The shell can also be utilized to decrease soil acidity in crop cultivation (Kouakou et al., 2014). Snails have a high mineral and protein content while being low in cholesterol and fat (Murphy 2001), which is why they are consumed in large quantities in numerous European nations, particularly France (Yildirim et al., 2004). Cernuella virgata, also known as Helicella virgata is an exclusive dish in top European restaurants, where fresh snail meat is used in food preparation (Scheifler et al., 2002). Several snail meat studies and its products have described the toxicological and microbiological human health risks (Tremlova 2000). Proximate analysis of Helix pomatia (H. pomatia) revealed low lipids, high protein and major minerals contents (Özogul et al., 2005). Moreover, snail meat has medicinal value and is relatively low in cholesterol level and high in mineral content (Akinnusi 1998). The proximate and mineral analysis of snails have been broadly reported (Adeyeye and Afolabi 2004; Ademolu et al., 2004; MC Milinsk et al., 2003a, Milinsk et al., 2003b; Menta and Parisi 2001; Ghosh et al., 2017; Fiordelmondo et al., 2020). Snails are fried in Indonesia, and the meal is known as sate kakul. Snails are baked with rice or fried in a skillet with red paprika powder and vegetable oil. However, data on the nutritional value of land snails related to proximate and mineral content in Ghana is limited. As a result, the objectives were to (1) evaluate the mineral and proximate composition of the flesh or meat and shell of A. achatina, A. marginata and A. fulica species from Kumasi in Ghana and (2) examine the non-carcinogenic adverse health risk associated with the consumption of these snail species. Based on the objectives, the study estimated the following null hypothesis: (1) the percentage moisture attained was relatively low for all meat samples; (2) the target population could face no serious adverse effects from consuming snail species from the study area; and (3) there are few significant correlations between proximate and nutrient components of the snail meat.
2. Materials and methods
2.1. Materials
The reagents used were as follows: 1.25 % sodium hydroxide (NaOH), 1.25 % sulphuric acid (H2SO4), nitric acid (HNO3) and 70% perchloric acid (HClO4), lanthanum chloride, ammonium metavanadate and ammonium heptamolybdate. These chemicals were all procured from Sigma-Aldrich. All the reagents were used as received with no purification.
2.2. Sampling and sample preparation
Three different snail species commonly found in Ghana were purchased in November 2018 and March 2019 from the Kumasi Central Market and 10 snails per each, A. achatina, A. marginata and A. fulica species (Figure 1), were obtained for analysis and processed following an earlier method (Felici et al., 2020). The snail meats were removed from the shell, washed separately with deionised water and dilutes acid prepared from 1 M solution to remove any adhering contamination. The shell was washed clean of blood and slime, let to dry, and then weighed. To get the dry weight, the snail samples weighing between 20 and 30 g were dried in an oven at 75–80 °C for 24 h. Dried samples were milled separately into powder and sieved with a 0.5 μm mesh size to achieve homogeneity in particle size.
Figure 1.
Species of Achatina achatina, Archachatina marginata and Achatina fulica.
2.3. Proximate and energy composition analysis
-
-
The protein, moisture, total ash, fat, fibre and carbohydrate content of the powdered snail meat and shell were determined in duplicate for each species. A thermostatically controlled forced-air oven was used to analyse the moisture content of the samples by drying.
-
-
The Kjeldahl method was employed to evaluate the protein composition of 2.0 g of the dried snail powders with a catalyst (Kjeldahl 1883). The protein content was calculated by multiplying the nitrogen content by 6.25.
-
-
The soxhlet extraction apparatus was used in the determination of crude fat. The fat was thoroughly extracted from about 2 g of each dried snail powder (W0), using petroleum ether as a solvent for the extraction. Crude fibre content was determined from the de-fatted samples by acid hydrolysis (1.25 % H2SO4) followed by base hydrolysis (1.25 % NaOH). The residue was then dried at 100 °C and weighed (W1). For 30 min, the dried sample was ignited in a muffle furnace at 600 °C. The resulting ash was weighed, cooled in a desiccator, and labelled W2. The loss in weight of the crucible following ignition was expressed as percentage crude fibre (CF). The percentage crude fibre was calculated as indicated in Eq. (1) (Jatto et al., 2010):
| (1) |
where W0 is the weight of each dried snail powder, W1 is the weight of dish + sample and W2 is the weight of dish + ash.
-
-
The ash composition was determined by incineration at 600 °C for 2 h in a muffle furnace and the weight of the sample remained after ashing was calculated as percentage ash content. The percentage total ash was calculated as % in Eq. (2) (Jatto et al., 2010):
| Total ash = (W1 – W2) x 100 | (2) |
-
-
The carbohydrate content was calculated by subtracting 100 from the total of all the other proximate measurements (moisture, protein, fat, fibre and ash).
-
-
The energy value of the snail samples was obtained by multiplying the percentage composition of protein, fat and carbohydrate by their corresponding values of 17, 37 and 17, respectively (James 1995).
2.4. Mineral analysis
Each milled snail sample was weighed into three separate digestion flasks, and 10 mL of nitric acid (HNO3) was added to each flask before the samples were placed in a fume chamber overnight. The flasks were then heated in a fume room until no red nitrogen dioxide (NO2) fumes were produced. After cooling the flasks, 4 mL of 70% perchloric acid (HClO4) was added to each flask. The mixture was heated once more to dry off the contents. Each digested sample was then diluted to 50 mL. The absorbance was recorded using atomic absorption spectrophotometer system Model Nov AA 400p (Analytik Jena GmbH, Jena, Germany) against a blank. During the Ca determination, 1 mL of lanthanum chloride was added to the original solution to unmask Ca from Mg. The concentration of each mineral (ppm) was recorded and the total mineral concentration in mg/100 g was then calculated according to Eq. (3) (Akinnusi et al., 2018):
| (3) |
2.5. Determination of phosphorus (P)
In a 100 mL volumetric flask, a 2 g aliquot of sample was dry-ashed and 5 mL of ammonium metavanadate and 5 mL of ammonium heptamolybdate were added. The addition of 4 mL of HNO3 was then made. Phosphorus concentration was measured from the calibration curve of its standard according to Beer-Lambert's Law.
2.6. Human health risk analysis
The health risks due to chronic exposure to trace elements were evaluated in the present study.
2.6.1. Estimated daily intakes
The estimated daily intakes (EDIs) of trace elements from snail consumption are determined by the consumers' body weight, the daily consumption rate of the snails and the concentration of elements. Estimation was done using Eq. (4) (Guo et al., 2016):
| (4) |
where Cmetal, Cfactor and Dfood intake are the mean essential metal concentration in the various snail species (mg/100 g), the conversion factor (0.085), the daily consumption rate of snail (kg day−1), respectively. The body weight was considered as 70 kg in the present study (Omar et al., 2013).
2.6.2. Non-carcinogenic risk
The non-carcinogenic risks associated with snail consumption were evaluated using the target hazard quotients (THQs), where the calculation was done following Eq. (5) (Guo et al., 2016):
| (5) |
Oral reference dose (RfD) values of 0.014, 0.7, 0.3 and 0.04 mg kg−1 day−1 for Mn, Fe, Zn and Cu, respectively, were used in this study (USEPA 2010). THQ <1 suggest that the exposed population is within the safe limit (Guo et al., 2016; Wang et al., 2005).
It has been observed that exposure to two or more contaminants causes interactive and/or additive effects (Hallenbeck 1993). The total THQ (TTHQ) of trace element for each snail species was calculated by adding the THQ value of the individual heavy elements as in Eq. (6) (Guo et al., 2016):
| (6) |
A hazard index (HI) method established by USEPA (1986) was employed to assess the total adverse effects for non-carcinogenic risk posed by each element. The HI was evaluated following Eq. (7) (Guo et al., 2016):
| (7) |
When HI > 1, there can be a worry for potential adverse health risk.
2.7. Statistical analysis
The data was statistically analysed using the IBM Statistical Package for the Social Sciences (Version 20.0, Armonk, NY, USA). The relations between the obtained proximate and mineral contents in the analysed snail samples were evaluated using Pearson's correlation analysis. To characterize and distinguish the observed sample, Principal Component Analysis (PCA) was applied to the experimental data.
3. Results
3.1. Data from proximate and energy composition analysis of snails
The proximate analysis and energy calculation results of both meat and shell of A. achatina, A. marginata and A. fulica species are presented in Table 1.
Table 1.
The proximate and energy content of the dried snail meat and shell samples (% ± SD).
| Parameter | Meat (mean ± SD) |
Shell (mean ± SD) |
||||
|---|---|---|---|---|---|---|
| A. marginata | A. achatina | A. fulica | A. marginata | A. achatina | A. fulica | |
| Moisture (%) | 5.2 ± 0.05 | 6.1 ± 0.01 | 4.88 ± 0.01 | 0.27 ± 0.04 | 0.43 ± 0.13 | 0.21 ± 0.01 |
| Fat (%) | 4.37 ± 0.06 | 5.06 ± 0.14 | 2.27 ± 0.16 | 0.68 ± 0.05 | 0.59 ± 0.03 | 0.62 ± 0.13 |
| Protein (%) | 85.12 ± 2.14 | 71.66 ± 1.24 | 62.56 ± 1.23 | 2.1 ± 0.41 | 3.18 ± 0.58 | 2.06 ± 0.25 |
| Fibre (%) | 1.32 ± 0.15 | 1.21 ± 0.03 | 0.03 ± 0.01 | 0.5 ± 0.05 | 0.63 ± 0.02 | 0.36 ± 0.04 |
| Ash (%) | 3.06 ± 0.02 | 3.49 ± 0.01 | 3 ± 0.01 | 96.31 ± 0.01 | 94.85 ± 0.11 | 95.85 ± 0.08 |
| Carbohydrate (%) | 2.25 ± 0.11 | 13.69 ± 0.15 | 27.29 ± 1.21 | 0.64 ± 0.03 | 0.95 ± 0.01 | 1.26 ± 0.02 |
| Energy (KJ/100g) | 1646.98 ± 8.68 | 1638.17 ± 6.53 | 1611.44 ± 8.6 | 71.74 ± 1.49 | 92.04 ± 1.57 | 79.38 ± 1.4 |
SD - Standard deviation.
3.2. Data on mineral content of snails
Although snail meat is high in protein, it is also a vital source of essential minerals. The mineral contents of A. achatina, A. marginata and A. fulica purchased from the Kumasi Central Market, Ghana, are given in Table 2.
Table 2.
Mineral composition in the meat and shell of A. achatina, A. marginata and A. fulica.
| Mineral | Meat (mean ± SD) |
Shell (mean ± SD) |
||||
|---|---|---|---|---|---|---|
| A. marginata | A. achatina | A. fulica | A. marginata | A. achatina | A. fulica | |
| Na | 67.64 ± 0.19 | 58.09 ± 0.12 | 73.38 ± 0.27 | 5.92 ± 0.04 | 6.82 ± 7.29 | 21.83 ± 0.15 |
| K | 111.43 ± 0.46 | 114.65 ± 0.44 | 111.02 ± 0.3 | 24.95 ± 0.31 | 22.32 ± 0.15 | 26.41 ± 0.26 |
| Ca | 701.79 ± 4.32 | 656.9 ± 5.46 | 402.29 ± 5.18 | 13716.09 ± 99.56 | 14188.53 ± 607.3 | 14375 ± 288.89 |
| Mg | 308.7 ± 0.42 | 304.62 ± 0.19 | 301.2 ± 0.33 | 50.09 ± 0.53 | 65.06 ± 0.99 | 119.71 ± 0.81 |
| P | 268.53 ± 5.34 | 241.9 ± 11.3 | 61.29 ± 11.34 | BDL | BDL | BDL |
| Zn | 8.41 ± 0.12 | 6.28 ± 0.1 | 5.81 ± 0.1 | BDL | 0.3 ± 0.01 | BDL |
| Fe | 6.33 ± 0.05 | 5.75 ± 0.05 | 26.64 ± 0.26 | 1.37 ± 0.04 | 2.34 ± 0.09 | 3.8 ± 0.08 |
| Mn | 0.73 ± 0.04 | 0.17 ± 0.03 | 1.29 | 0.33 ± 0.01 | 0.14 ± 0.04 | BDL |
| Cu | 0.98 ± 0.03 | 0.73 ± 0.02 | 3.83 ± 0.08 | BDL | BDL | BDL |
Concentrations are in mg/100 g ± standard deviation; BDL - below detection limit.
3.3. Correlation analysis
Tables 3 and 4 showed a few significant correlations between the snail meat's proximate and nutrient components were observed.
Table 3.
Pearson's correlation matrix of proximate and nutrient components in the meat and shell of A. achatina, A. marginata and A. fulica species.
| Moisture | Fat | Protein | Fibre | Ash | Carbohydrate | Energy | Na | K | Ca | Mg | P | Zn | Fe | Mn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fat | 0.852 | 1 | |||||||||||||
| Protein | 0.144 | 0.641 | 1 | ||||||||||||
| Fibre | 0.646 | 0.95 | 0.848 | 1 | |||||||||||
| Ash | 0.99 | 0.768 | 0.001 | 0.53 | 1 | ||||||||||
| Carbohydrate | -0.301 | -0.756 | -0.987 | -0.922 | -0.162 | 1 | |||||||||
| Energy | 0.513 | 0.887 | 0.923 | 0.987 | 0.386 | -0.973 | 1 | ||||||||
| Na | -0.992 | -0.91 | -0.266 | -0.736 | -0.964 | 0.417 | -0.616 | 1 | |||||||
| K | 0.988 | 0.762 | -0.008 | 0.523 | 1∗∗ | -0.152 | 0.377 | -0.962 | 1 | ||||||
| Ca | 0.597 | 0.929 | 0.88 | 0.998∗ | 0.477 | -0.945 | 0.995 | -0.692 | 0.468 | 1 | |||||
| Mg | 0.204 | 0.687 | 0.998∗ | 0.879 | 0.062 | -0.995 | 0.945 | -0.324 | 0.053 | 0.907 | 1 | ||||
| P | 0.614 | 0.937 | 0.87 | 0.999∗ | 0.495 | -0.937 | 0.993 | -0.707 | 0.487 | 1∗ | 0.898 | 1 | |||
| Zn | -0.097 | 0.439 | 0.971 | 0.697 | -0.238 | -0.92 | 0.804 | -0.028 | -0.247 | 0.74 | 0.955 | 0.726 | 1 | ||
| Fe | -0.72 | -0.977 | -0.791 | -0.995 | -0.613 | 0.878 | -0.965 | 0.801 | -0.606 | -0.987 | -0.826 | -0.99 | -0.621 | 1 | |
| Mn | -0.964 | -0.96 | -0.401 | -0.825 | -0.917 | 0.542 | -0.722 | 0.99 | -0.913 | -0.788 | -0.455 | -0.801 | -0.17 | 0.878 | 1 |
| Cu | -0.753 | -0.986 | -0.76 | -0.989 | -0.651 | 0.854 | -0.951 | 0.829 | -0.644 | -0.978 | -0.798 | -0.982 | -0.582 | 0.999∗ | 0.9 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Table 4.
Pearson's correlation matrix of proximate and nutrient components in the shell of A. achatina, A. marginata and A. fulica species.
| Moisture | Fat | Protein | Fibre | Ash | Carbohydrate | Energy | Na | K | Ca | Mg | Zn | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fat | -0.556 | 1 | |||||||||||
| Protein | 0.972 | -0.735 | 1 | ||||||||||
| Fibre | 0.962 | -0.307 | 0.871 | 1 | |||||||||
| Ash | -0.836 | 0.921 | -0.941 | -0.654 | 1 | ||||||||
| Carbohydrate | -0.264 | -0.655 | -0.031 | -0.518 | -0.308 | 1 | |||||||
| Energy | 0.797 | -0.945 | 0.916 | 0.6 | -0.998∗ | 0.373 | 1 | ||||||
| Na | -0.674 | -0.238 | -0.484 | -0.851 | 0.159 | 0.89 | -0.091 | 1 | |||||
| K | -0.996 | 0.477 | -0.947 | -0.983 | 0.782 | 0.352 | -0.737 | 0.74 | 1 | ||||
| Ca | -0.021 | -0.819 | 0.212 | -0.295 | -0.53 | 0.97 | 0.587 | 0.753 | 0.114 | 1 | |||
| Mg | -0.552 | -0.386 | -0.342 | -0.76 | 0.005 | 0.95 | 0.064 | 0.988 | 0.627 | 0.845 | 1 | ||
| Zn | 0.965 | -0.756 | 1∗ | 0.855 | -0.951 | 0 | 0.928 | -0.456 | -0.936 | 0.243 | -0.313 | 1 | |
| Fe | -0.374 | -0.563 | -0.147 | -0.614 | -0.196 | 0.993 | 0.263 | 0.937 | 0.458 | 0.935 | 0.98 | -0.116 | 1 |
| Mn | 0.179 | 0.718 | -0.056 | 0.442 | 0.39 | -0.996 | -0.452 | -0.847 | -0.269 | -0.988 | -0.919 | -0.087 | -0.979 |
Correlation is significant at the 0.05 level (2-tailed).
3.4. The principal component analysis
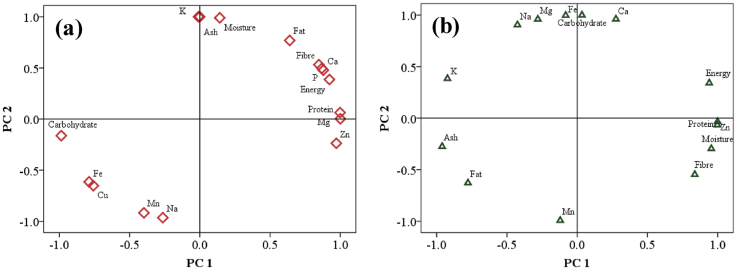
The proximate and nutritional components of the shell and meat of A. achatina, A. marginata, and A. fulica species were classified using PCA. PCA decomposes the original matrix into several products of score matrices and component loading (Otto 1999; Kaiser and Rice 1974). Also, PCA allows a substantial decrease in the number of variables between the different snail parts and measured parameters (Otto 1999; Kaiser and Rice 1974). The number of components retained in the original matrix loading and score matrices was evaluated by applying Kaiser-Meyer Olkin and Bartlett's test, which retains only principal components (PC) with Eigenvalues greater than 1. Component loadings of <0.5, 0.5 and >0.5 signify poor, moderate and high loadings. The PCA results in the shells and meat of A. achatina, A. marginata and A. fulica species are given in Figure 2.
Figure 2.
The distribution pattern of proximate and nutrient components in the (a) meat and (b) shell of A. achatina, A. marginata and A. fulica species by using PCA.
3.5. Estimated daily intake
The EDI of Mn, Cu, Fe and Zn was estimated based on the mean concentration of each trace element in A. achatina, A. marginata and A. fulica species and the results are presented in Table 5.
Table 5.
Estimated daily intakes and hazard analysis of trace elements from the intake of edible land snails.
| Snail species | EDI |
THQ |
TTHQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Zn | Fe | Mn | Cu | Zn | Fe | Mn | Cu | ||
| A. marginata | 2.06E-06 | 1.54E-06 | 1.78E-07 | 2.4E-07 | 6.85E-06 | 2.21E-06 | 1.27E-05 | 5.99E-06 | 2.78E-05 |
| A. achatina | 1.54E-06 | 1.41E-06 | 4.16E-08 | 1.78E-07 | 5.12E-06 | 2.01E-06 | 2.97E-06 | 4.46E-06 | 1.46E-05 |
| A. fulica | 1.42E-06 | 6.51E-06 | 3.15E-07 | 9.36E-07 | 4.73E-06 | 9.3E-06 | 2.25E-05 | 2.34E-06 | 3.89E-05 |
| Hazard index | 8.12E-05 | ||||||||
4. Discussion
Snails' growth performance and nutritional value enhanced by feeding them a compounded balanced diet rich in vitamins, energy, protein and calcium. This study compared the mineral content and proximate composition of the shell and meat of the three land snail species with those of other studies. To the best of our knowledge, this is one of only a few papers that deal with the flesh quality characteristics and shell characterisation of snail species obtained from the Kumasi Central Market in Ghana. The decision to collect snail samples at two distinct times of the year (November and March) was made to analyse the quality and biometric characteristics of the snails during the collecting season. The amount of protein, moisture, crude fat, crude fibre, ash and carbohydrate varied according to sample species. The proximate compositions were significantly higher in meat compared to shells except for the ash contents. The protein, fat and ash contents in the meat of A. achatina, A. marginata and A. fulica were lower compared with the report by Özogul et al. (2005) (16.35 ± 0.67, 0.41 ± 0.02 and 1.89 ± 0.7%, respectively), but the moisture content in this study was lower. Ademolu et al. (2004), reported a fat content in the range of 1.18–1.7 % for A. marginata fed on different nitrogen-based foods. Milinsk et al., 2003a, Milinsk et al., 2003b also reported a 0.91–1.29 % fat content for Helix aspersa. Engmann et al. (2013) also obtained a protein composition of 82.96% for dried meat samples of A. achatina in Ghana. Protein has many other functions that include repairing worn-out tissues (Krafts 2010; Ademolu et al., 2004) and may be responsible for snail meat being used to enhance amputated limbs by traditional healers (Engmann et al., 2013). Many factors, including drought, diseases, and the mean output of local animal breeds, have decreased typical animal protein sources such as livestock in the form of pork, mutton, beef, and poultry (Fleck 1976). Thus, meat from a land snail can serve as a good source of protein to reduce high demand on regular sources. Wosu (2003) reported protein content of beef (17.5 %), pork (11.9 %), chicken (20.2 %), turkey (20.2 %), and dried fish (60 %) which are all lower than the least amount recorded in the meat of A. fulica (62.56 %) in this study. The crude fat contents from the results are comparatively low (2.27–5.06 %). Fagbuaro et al. (2006) and Adeyeye (1996) obtained lower fat content of snail meat (1.23–1.43% and 1.2–4.3%, respectively) which confirm findings in this work that snail meat has low-fat content when compared with that found in egg (9.6 %), mutton (21.4 %), duck (23%) and beef (22 %) (FAO 1995). A. fulica meat will be a good source from this work if one wants to reduce fat intake since it had the least amount of fat of 2.27 % among the three snail species analysed. From the results, ash was the main component of the snail shell ranging from 94.8 to 96.31 % for A. marginata. The ash content of a snail shell represents the quantity and amount of inorganic components and carbon compounds in the form of oxides and salts (Ameh 2006). Because of its absorbent nature, carbon plays an important role in molecular absorption. Therefore, the snail shell ash can play an important role in removing heavy metals from aqueous media. Moisture in a sample is an indication of how long that sample can be stored. The percentage of moisture attained was quite low for all meat samples and was 4.88–6.1 %. This means that dried snail meat, when well packaged, can be kept for a significant period. The energy values obtained were high for all the meat samples and they did not differ much from each other. The higher energy value suggests that snail meat can offer substantial calories in human nutrition. Engmann et al. (2013) reported an energy value of 1613 KJ/100 g for A. achatina meat.
The studies showed that all the minerals analysed were found in the meat of the three snail species in different amounts, whereas few were not detectable in the shell samples. Generally, meats are low in Ca content (from 9 to 11 mg/100g) even though the body quickly absorbs it (Muller 1988). This is not the case with snail flesh, since the results revealed that calcium was the most plentiful mineral in all of the snail species studied. Ca content, the major element, was in the range of 402.29 ± 5.18 to 701.79 ± 4.32 mg/100 g. The P, K, Mg and Na contents were in the range of 61.29 ± 11.34 to 268.53 ± 5.34, 111.02 ± 0.3 to 114.65 ± 0.44, 301.2 ± 0.33 to 308.7 ± 0.42 and 67.64 ± 0.19 to 58.09 ± 0.12 mg/100 g, respectively, in the snail meat. Mn content of A. achatina, A. marginata and A. fulica meat was less than 2 mg/100 g. The Zn and Fe contents in this study were higher compared with H. pomatia (1.35 and 1.71 mg/100 g, respectively).
The higher content of Ca compared with the minerals was comparable with other studies. Ademolu et al. (2004) found that A. fulica had a higher content of Ca (780 mg/100 g) and Özogul et al. (2005) also observed a higher content of Ca (750 mg/100 g) in H. pomatia from Cukurova region, Turkey. Moreover, Engmann et al. (2013) obtained 585.8 mg/100g of Ca in dried A. achatina (meat), whiles Watson (1971) also reported values of 650–700 mg/100g which were all consistent with the values obtained in this study.
The elevated Ca level in the snail flesh might be attributed to the salt treatment of the snails, which is often used to remove the shells of the snails (Özogul et al., 2005). The mineral contents of snail meat can be influenced by several factors, including biological cycle, season, species, environment and nutrient availability (Özogul et al., 2005). Compared with other animal products, such as milk, eggs, liver and beef, whose calcium content is 120, 54, 6 and 7 mg/100 g, respectively, confirmed the richness of Ca in snail meat (Fox and Cameron 1977). Thus, it is highly recommended that infants be fed on diets blended with powdered snail meat since the development of bones and teeth during infancy and childhood demands a high amount of calcium (Engmann et al., 2013).
Magnesium was the next abundant mineral obtained in both the meat and shell samples after calcium. The results obtained in this study were higher than the 45.59 and 46.15 mg/100 g recorded in A. achatina and A. marginata species, respectively (Fagbuaro et al., 2006). According to Cruz and Tsang (1992), P and Ca are essential for sustaining optimal bone formation during childhood and developmental phases of humans, while Mn, Zn and Fe are considered essential minerals for diseases prevention, growth and fundamental cellular activities (Sherman 1992; Lukaski 2004).
The Fe content of both meat and shell was relatively low, but the meat of A. fulica recorded a considerable high amount of iron (26.64 mg/100 g), which was virtually quadruple that of the meat of A. marginata. Even though the iron levels were relatively low (Table 2), it compared well with conventional meat products, such as kidney (6 mg/100 g) and liver (11.4 mg/100 g) reported by Fox and Cameron (1977). Engmann et al. (2013) found a Fe concentration of 9.8 mg/100g in dried A. achatina flesh, which was quite close to the 6.33 mg/100 g found in this study for the same species. Fe plays a crucial role in processes, such as oxygen transport and cellular respiration. Fe content in a meat product is usually absorbed more quickly than that from vegetables and cereals, as these sources produce Fe in the form of phytate and oxalate complexes (Ramakrishnan and Semba 2008). This makes snail meat a good source of Fe in combating anaemia, which is widespread in developing countries, including Ghana. Zn has many roles in the human system, such as dark adaptation and night vision (Burton and Foster 1988; Christian and West Jr 1998). The present study demonstrated that Zn was present in significant amounts in all of the meat samples, with A. marginata having the highest concentration (8.41 mg/100 g). However, only the shell of A. achatina contained a detectable amount of Zn (0.3 mg/100 g).
From the results in Table 2, A. fulica had the highest amount of Na in both the meat and shell samples analysed (73.38 and 21.83 mg/100 g, respectively). Fagbuaro et al. (2006) also obtained similar values for Na in meat samples of A. marginata and A. achatina in Nigeria (52.93 and 60.94 mg/100 g, respectively). Like Ca, phosphorus is a component of teeth and bones, where about 85% of P is found in bones (Otten et al., 2006). From the analysis results, P was detected in the analysed meat of snail species, but P and Cu were below the detection limit in all the shells. Fox and Cameron (1977) reported P content in milk, beef, liver and eggs as 95, 156, 313 and 218 mg/100 g, respectively. Comparing these with the 61.29–268.53 mg/100 g obtained in this study, it can be suggested that snails are an excellent source of P.
Protein and Mg content showed significant positive correlation. Ca and P were positively correlated with fibre (Table 3). Ash content was positively correlated with K. Moreover, Cu and Fe levels were strongly positively correlated (Table 4). Nonetheless, no significant positive correlations were observed among the proximate components. Levels of Zn snail shells were positively correlated with protein, see Table 4. Also, ash and energy were strongly positively correlated.
The PCA results showed that the first two PC accounted for 100% variance for both the meat and shell of A. achatina, A. marginata and A. fulica species. The initial Eigenvalue was 8.862 (accounting for 55.39% of the total variance), while the second Eigenvalue was 7.138 (accounting for 44.61% of the total variance). The levels of Ca, Mg, P, Zn, fat, protein and fibre were the most influential factors for PC1 of the meat samples, while moisture, protein, fibre, energy and Zn level constitute PC1 of the shell samples. The most positively significant parameters for PC2 were Na, moisture, fat, fibre and ash contents in the meat samples, while the carbohydrate, Na, Ca, Mg and Fe showed the high positive influence on PC2 of the shell samples.
Risk assessment is the process that assesses the potential adverse risk via trace element exposure. The non-carcinogenic risks from the intake of snails were evaluated according to the THQs. The THQs assessment is a technique of assessing population risk and establishing exposure limits to offer a possible worst-case scenario of potential adverse health risk (Wang et al., 2005). The nutritional exposure method of non-piscine foodstuff (eggs, milk and meat) intake is a suitable tool for evaluating human health risk based on the consumption levels of contaminants, bioactive compounds and nutrients, as well as offering vital data on the exposure to food contaminants or potential nutritional deficiencies (WHO 1985). For A. fulica, A. achatina and A. marginata species, the EDI decrease in the order of Fe > Zn > Cu > Mn, Zn > Fe > Cu > Mn and Zn > Mn > Fe > Cu, respectively (Table 5). Cu, Mn, Fe, and Zn estimated EDIs were low when compared to their acceptable daily intake levels (FAO/WHO 2010; EC 2006).
The THQs values of the studied trace elements less than 1 suggests that consumers will experience no significant adverse health effects when these snails are used as food (see Table 5). This agreed with those obtained for other meat samples (Darwish et al. 2010, 2018; Bortey-Sam et al., 2015). Furthermore, possible human health hazards from trace element exposure from the consumption of edible land snails were under the acceptable range (THRI <1). The HI value in Table 5 also suggests that the target population could experience no serious adverse risk by only consuming A. achatina, A. marginata and A. fulica species from the study area. Although the EDI, THQ and HI values did not show high adverse health risks, chronic exposure to these toxic trace metals through the intake of edible land snails may reveal possible hazards.
5. Conclusion
For the first time, analyses were performed on the proximate and nutrient components of achatina, A. marginata and A. fulica species purchased from Kumasi Central Market. Nutritional elements present in the studied snail species include protein, fat, P, Ca, Mg, P, Cu and Zn. In both the proximate and mineral analysis, each meat parameter was higher than the corresponding parameter in the shell, except for ash and Ca contents. It is evident from this study that the consumption of snail meat can promote good human health, with Archachatina marginata having the best mineral and proximate components. Considering the components of the meat from the three snail species, a strong significant positive correlation between Ca and P, as well as Cu and Fe levels was observed at p < 0.05. The EDIs and THQ of trace elements (Fe, Zn, Mn and Cu) were higher than tolerable daily intake, suggesting that consumers will not suffer from any health issues associated with consuming excess levels of essential elements. The study has also confirmed the potential of snail shells as a source of feed for livestock, hence a green approach to utilising waste.
Declarations
Author contribution statement
Marian Asantewah Nkansah: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Eric Amakye Agyei: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Francis Opoku: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the Department of Chemistry of the Kwame Nkrumah University of Science and Technology for the use of its facilities for this study.
References
- Ademolu K., Idowu A., Mafiana C., Osinowo O. Performance, proximate and mineral analyses of African giant land snail (Archachatina marginata) fed different nitrogen sources. Afr. J. Biotechnol. 2004;3(8):412–417. [Google Scholar]
- Adeyeye E. Waste yield, proximate and mineral composition of three different types of land snails found in Nigeria. Int. J. Food Sci. Nutr. 1996;47(2):111–116. doi: 10.3109/09637489609012572. [DOI] [PubMed] [Google Scholar]
- Adeyeye E., Afolabi E. Amino acid composition of three different types of land snails consumed in Nigeria. Food Chem. 2004;85(4):535–539. [Google Scholar]
- Akinnusi O. A practical approach to backyard snail-farming. Niger. J. Anim. Prod. 1998;25(1):193–197. [Google Scholar]
- Akinnusi F., Oni O., Ademolu K. Mineral composition of giant African land snail’s (Archachatina marginata) shells from six south West States, Nigeria. Trop. J. Anim. Sci. 2018;20:485–489. [Google Scholar]
- Alturiqi A.S., Albedair L.A. Evaluation of some heavy metals in certain fish, meat and meat products in Saudi Arabian markets. Egypt J. Aquat. Res. 2012;38(1):45–49. [Google Scholar]
- Ameh U. Heinemann Medical Books Ltd; New York: 2006. Standard Operating Procedure National Agency for Food and Drug Administration and Control (NAFDAC) Boriki, Port Harcourt, Nigeria. [Google Scholar]
- Apata E.S., Falola A.R., Sanwo S.K., Adeyemi K.O., Okeowo T.A. Physicochemical and organoleptic evaluation of African giant land snails (Achatina spp.) meat. Int. J. Agric. Sci. Nat. Res. 2015;2(2):24–27. [Google Scholar]
- Bauchart C., Morzel M., Chambon C., Mirand P.P., Reynès C., Buffière C., Rémond D. Peptides reproducibly released by in vivo digestion of beef meat and trout flesh in pigs. Br. J. Nutr. 2007;98(6):1187–1195. doi: 10.1017/S0007114507761810. [DOI] [PubMed] [Google Scholar]
- Biesalski H.-K. Meat as a component of a healthy diet–are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005;70(3):509–524. doi: 10.1016/j.meatsci.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Bortey-Sam N., Nakayama S.M., Ikenaka Y., Akoto O., Baidoo E., Yohannes Y.B., Mizukawa H., Ishizuka M. Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs) Ecotoxicol. Environ. Saf. 2015;111:160–167. doi: 10.1016/j.ecoenv.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Bošković M., Baltić M.Ž., Ivanović J., Đurić J., Dokmanović M., Marković R., Šarčević D., Baltić T. The impact of pork meat and lard on human health. Meat Technol. 2015;56(1):8–15. [Google Scholar]
- Brunt J., Engel Berger K., Rapp G. Secretariat of the Pacific Community; Fiji: 1999. Giant African Snail Plant protection Service. [Google Scholar]
- Burton B.T., Foster W.R. fourth ed. McGraw-Hill Book Company; New York: 1988. Human Nutrition. [Google Scholar]
- Cabrera M., Saadoun A. An overview of the nutritional value of beef and lamb meat from South America. Meat Sci. 2014;98(3):435–444. doi: 10.1016/j.meatsci.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Christian P., West K.P., Jr. Interactions between zinc and vitamin A: an update. Am. J. Clin. Nutr. 1998;68(2):435S–441S. doi: 10.1093/ajcn/68.2.435S. [DOI] [PubMed] [Google Scholar]
- Civeyrel L., Simberloff D. A tale of two snails: is the cure worse than the disease? Biodivers. Conserv. 1996;5(10):1231–1252. [Google Scholar]
- Cobbinah J.R. Technical Centre for Agricultural and Rural Cooperation; Netherlands: 1994. Snail Farming in West Africa. A Practical Guide. [Google Scholar]
- Cruz M., Tsang R. In: Calsium Nutriture for Mothers and Children. Tsang R., Mimorini F., editors. Raven Press; New York: 1992. Introduction to infant mineral metabolism; pp. 1–11. [Google Scholar]
- Darwish W.S., Atia A.S., Khedr M.H., Eldin W.F.S. Metal contamination in quail meat: residues, sources, molecular biomarkers, and human health risk assessment. Environ. Sci. Pollut. Res. 2018;25(20):20106–20115. doi: 10.1007/s11356-018-2182-0. [DOI] [PubMed] [Google Scholar]
- Darwish W.S., Ikenaka Y., El-Ghareeb W., Ishizuka M. High expression of the mRNA of cytochrome P450 and phase II enzymes in the lung and kidney tissues of cattle. Animal. 2010;4(12):2023–2029. doi: 10.1017/S1751731110001394. [DOI] [PubMed] [Google Scholar]
- EC . Off J Eur Union; 2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuff. [Google Scholar]
- Engmann F.N., Afoakwah N.A., Darko P.O., Sefah W. Proximate and mineral composition of snail (Achatina achatina) meat; any nutritional justification for acclaimed health benefits? J. Basic Appl. Sci. Res. 2013;3(4):8–15. [Google Scholar]
- Fagbuaro O., Oso J., Edward J., Ogunleye R. Nutritional status of four species of giant land snails in Nigeria. J. Zhejiang Univ. - Sci. B. 2006;7(9):686–689. doi: 10.1631/jzus.2006.B0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . Food and Agriculture Organization; Rome: 1995. Symposium of the Special Programme of Food Production in Support of Food Security in Low Food Deficit Countries. [Google Scholar]
- FAO/WHO . Food and Agriculture Organization of the United Nations; Geneva, World Health Organization (JECFA/73/SC); Rome: 2010. Summary and Conclusions of the Seventy-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, 8–17 June 2010. [Google Scholar]
- Felici A., Bilandžić N., Magi G.E., Iaffaldano N., Fiordelmondo E., Doti G., Roncarati A. Evaluation of long sea snail hinia reticulata (gastropod) from the middle adriatic sea as a possible alternative for human consumption. Foods. 2020;9(7):905. doi: 10.3390/foods9070905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiordelmondo E., Roncarati A., Vincenzetti S., Pinzaru S.C., Felici A. Sterol and mineral profiles of the common sea snail Hinia reticulata and the long sea snail Nassarius mutabilis (Gastropods) collected from the middle adriatic sea. Curr. Res. Nutr. Food Sci. 2020;8(3):757–764. [Google Scholar]
- Fleck H. third ed. Macmillan; New York: 1976. Introduction to Nutrition. [Google Scholar]
- Food and Agriculture Organization of the United Nations. 2014. http://www.faostat.fao.org/site/610/default.aspx#ancor/ [Google Scholar]
- Fox B.A., Cameron A.G. Hodder & Stoughton Ltd.; London, UK: 1977. Food Science - A Chemical Approach. [Google Scholar]
- Ghosh S., Jung C., Meyer-Rochow V.B. Snail as mini-livestock: nutritional potential of farmed Pomacea canaliculata (Ampullariidae) Agric. Nat. Resour. 2017;51(6):504–511. [Google Scholar]
- Guo J., Yue T., Li X., Yuan Y. Heavy metal levels in kiwifruit orchard soils and trees and its potential health risk assessment in Shaanxi, China. Environ. Sci. Pollut. Res. 2016;23(14):14560–14566. doi: 10.1007/s11356-016-6620-6. [DOI] [PubMed] [Google Scholar]
- Hallenbeck W.H. second ed. CRC Press; New York: 1993. Quantitative Risk Assessment for Environmental and Occupational Health. [Google Scholar]
- Higgs J.D. The changing nature of red meat: 20 years of improving nutritional quality. Trends Food Sci. Technol. 2000;11(3):85–95. [Google Scholar]
- James C. Blackie academic and Professional press; Glasgow, UK: 1995. Analytical Chemistry of Foods. [Google Scholar]
- Jatto O., Asia I., Medjor W. Proximate and mineral composition of different species of snail shell. Pac. J. Sci. Technol. 2010;11:416–419. [Google Scholar]
- Kaiser H.F., Rice J. Little jiffy, mark IV. Educ. Psychol. Meas. 1974;34(1):111–117. [Google Scholar]
- Kjeldahl J. Neue Methode zur Bestimmung des Stickstoffs in organischen Korpern. Z. für Anal. Chem. 1883;22:366–382. [Google Scholar]
- Kouakou K., Kouassi D., Kouadio Y. Management of shells of giant African snails (Achatinidae) from the markets of Abidjan (Côte d'Ivoire) J. Appl. Biosci. 2014;83:7625–7634. [Google Scholar]
- Krafts K.P. Tissue repair: the hidden drama. Organogenesis. 2010;6(4):225–233. doi: 10.4161/org.6.4.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaski H.C. Vitamin and mineral status: effects on physical performance. Nutrition. 2004;20(7-8):632–644. doi: 10.1016/j.nut.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Mead A.R. University of Chicago Press; Chicago, IL: 1961. The Giant African Snail: a Problem in Economic Malacology. [Google Scholar]
- Menta C., Parisi V. Metal concentrations in Helix pomatia, Helix aspersa and Arion rufus: a comparative study. Environ. Pollut. 2001;115(2):205–208. doi: 10.1016/s0269-7491(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Milinsk M., Graças Padre R., Hayashi C., Souza NE M.M. Influence of diets enriched with different vegetable oils on the fatty acid profiles of snail Helix aspersa maxima. Food Chem. 2003;82:553–558. [Google Scholar]
- Milinsk M.C., das Graças Padre R., Hayashi C., de Souza N.E., Matsushita M. Influence of diets enriched with different vegetable oils on the fatty acid profiles of snail Helix aspersa maxima. Food Chem. 2003;82(4):553–558. [Google Scholar]
- Muller H.G. Cambridge University Press; Cambridge: 1988. An Introduction to Tropical Food Science. [Google Scholar]
- Murphy B. RIRDC - Rural Industries Research Development Corporation; Kingston: 2001. Breeding and Growing Snails Commercially in Australia. [Google Scholar]
- Omar W.A., Zaghloul K.H., Abdel-Khalek A.A., Abo-Hegab S. Risk assessment and toxic effects of metal pollution in two cultured and wild fish species from highly degraded aquatic habitats. Arch. Environ. Contam. Toxicol. 2013;65(4):753–764. doi: 10.1007/s00244-013-9935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J.J., Hellwig J.P., Meyers L.D. The National Academies Press; Washington, D.C: 2006. Dietary Reference Intakes: the Essential Guide to Nutrient Requirements. [Google Scholar]
- Otto M. Wiley VCH; Weinheim, Germany: 1999. Pattern Recognition and Classification. Chemometrics: Statistics and Computer Application in Analytical Chemistry. [Google Scholar]
- Özogul Y., Özogul F., Olgunoglu A.I. Fatty acid profile and mineral content of the wild snail (Helix pomatia) from the region of the south of the Turkey. Eur. Food Res. Technol. 2005;221(3-4):547–549. [Google Scholar]
- Özyurt G., Kuley E., Etyemez M., Özoğul F. Comparative seasonal sterol profiles in edible parts of Mediterranean fish and shellfish species. Int. J. Food Sci. Nutr. 2013;64(4):476–483. doi: 10.3109/09637486.2012.749836. [DOI] [PubMed] [Google Scholar]
- Piba N.S., Karamoko M., Adou C.F.D., Otchoumou A., Kouassi K.P. Effet du régime et de la teneur en protéines brutes alimentaires sur le rendement en viande de l’escargot Achatina fulica (Bowdich, 1720) Int. J. Biol. Chem. Sci. 2014;8:2296–2305. [Google Scholar]
- Ramakrishnan U., Semba R.D. In: Nutrition and Health in Developing Countries. second ed. Semba R.D., Bloem M.W., editors. Humana Press; Totowa, New Jersey: 2008. Iron deficiency and anaemia; pp. 479–505. [Google Scholar]
- Raut S., Barker G. In: Molluscs as Crop Pests. Barker G., editor. CABI Publishing; Wallingford: 2002. Achatina fulica bowdich and other achatinidae as pests; pp. 55–114. [Google Scholar]
- Scheifler R., Gomot-De Vaufleury A., Badot P.-M. Transfer of cadmium from plant leaves and vegetable flour to the snail Helix aspersa: bioaccumulation and effects. Ecotoxicol. Environ. Saf. 2002;53(1):148–153. doi: 10.1006/eesa.2002.2216. [DOI] [PubMed] [Google Scholar]
- Sherman A.R. Zinc, copper, and iron nutriture and immunity. J. Nutr. 1992;122(suppl_3):604–609. doi: 10.1093/jn/122.suppl_3.604. [DOI] [PubMed] [Google Scholar]
- Tchakounte F.M., Kana J.R., Azine P.C., Meffowoet C.P., Djuidje V.P. Effects of dietary level of calcium on body proportion and nutritional value of African giant snail (Archachatina marginata) J. Anim. Res. 2019;3 [Google Scholar]
- Tremlova B. Histological examination of snail meat specialities. Fleischwirtschaft. 2000;81(12):96–97. [Google Scholar]
- USEPA . U.S. Environmental Protection Agency; Washington, DC: 1986. Guidelines for the Health Risk Assessment of Chemical Mixtures. EPA 630/R-98/002. [Google Scholar]
- USEPA . United State Environmental Protection Agency; Washington, DC: 2010. Risk-based Concentration Table. [Google Scholar]
- Wang X., Sato T., Xing B., Tao S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005;350:28–37. doi: 10.1016/j.scitotenv.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Watson J. Investigations on the nutritive value of some Ghanaian foodstuffs. Ghana J. Agric. Sci. 1971;4(1):95–111. [Google Scholar]
- WHO . World Health Organization; Geneva: 1985. Guidelines for the Study of Dietary Intakes of Chemical Contaminants. WHO Offset Publication No. 87. [PubMed] [Google Scholar]
- Williamson C., Foster R., Stanner S., Buttriss J. Red meat in the diet. Nutr. Bull. 2005;30(4):323–355. [Google Scholar]
- Wosu L. AP Express Publishers Ltd; Nsukka: 2003. Commercial Snail Farming in West Africa: A Guide. [Google Scholar]
- Yildirim M.Z., Ü Kebapçi, Gümüş B.A. Edible snails (terrestrial) of Turkey. Turk. J. Zool. 2004;28(4):329–335. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.