Abstract
The high mortality of coronavirus disease 2019 (COVID-19) patients is due to their progression to cytokine-associated organ injuries, primarily the acute respiratory distress syndrome (ARDS). The uncertainties in the molecular mechanisms leading to the switch from the early virus infection to the advanced stage ARDS is a major gridlock in therapeutic development to reduce mortality. Previous studies in our laboratory have identified matrix metalloprotease-3 (MMP3) as an important mediator of bacterial lipopolysaccharide (LPS)-induced ARDS, particularly in the exudative phase. Our studies have also reported elevated plasma MMP3 activity levels in the ARDS patients and that inhibition of MMP3 can reduce the severity of LPS-induced ARDS in mice. Given these observations, targeting MMP3 could be a potential option to treat COVID-19 patients with ARDS, and measurement of MMP3 activity in the plasma may serve as a biomarker for the early detection of ARDS in COVID-19 patients.
Keywords: COVID-19, MMP3, stromelysin1, ARDS, biomarker, syndrome
1. INTRODUCTION
Identification of therapeutic targets to inhibit the progress of coronavirus disease 2019 (COVID-19) to acute respiratory distress syndrome (ARDS) is critical. COVID-19 [1] has emerged as a worldwide health concern due to its high infection rate1, prolonged asymptomatic incubation period, and high mortality [2, 3]. Several early trials for COVID-19 on medications such as hydroxychloroquine showed no benefits [4, 5]. COVID-19 is expected to infect several million people, and the advanced stage of COVID-19 is associated with acute respiratory distress syndrome (ARDS) and increased mortality [6]. According to the recent reports cited by the US Centers for Disease Control and Prevention (CDC), among all COVID-19 patients, ARDS develops between 3-17%. In contrast, ARDS develops between 20-42% of hospitalized patients and 67-85% of ICU patients [7-12]. Thus, targeting the mechanism of COVID-19 associated ARDS development is a key therapeutic strategy to decrease the high mortality rate. Further, the timely identification of patients at the highest risk of disease progression supports the use of early lung-protective strategies.
The exudative phase of ARDS is characterized by the outflow of plasma and cells from the circulation to the extravascular tissues setting up alveolar edema, mediated by inflammatory cytokines [13, 14]. Without therapeutic intervention, this process can cause irreversible damage to the lungs. Matrix metalloproteinases (MMPs) play an important role in innate immunity and inflammatory response, and various inflammatory lung diseases, such as ARDS, asthma, and pulmonary fibrosis, are characterized by an increase in the expression of one or more of the MMPs [15, 16]. MMP3, also known as stromelysin1, is a matricellular protease that not only degrades the extracellular matrix in the basement membrane but also promotes the cell-cell barrier disruption due to its enzymatic proteolysis of junctional proteins, particularly the tight junction proteins claudins and occludins [17]. The structure of MMP3 is shown in Fig. (1) [18]. Endothelial cells [19, 20], bronchial and alveolar epithelial cells and inflammatory cells [21] are reported to secrete MMP3 in the lungs, suggesting its potential role in the early stages of ARDS. Epithelial cells that line the bronchus and the alveoli, and endothelial cells that line the innermost later of the bllod vessels are the primary sites of infection and replication of the severe acute respiratory syndrome causing coronavirus-2 (SARS-CoV2) [22]. Notably, MMP3 inhibition reversed MMP3s association with inflammation via increased interleukin-1β and monocyte chemoattractant protein-3 expression [23]. In agreement with the finding that MMP3 is associated with inflammation and lung injury, studies from our laboratory have demonstrated that FoxO-regulated expression of MMP3 in endothelial cells promotes bacterial lipopolysaccharide (LPS)-induced endothelial-barrier disruption in vitro and lung injury and edema in vivo [19].
Fig. (1).
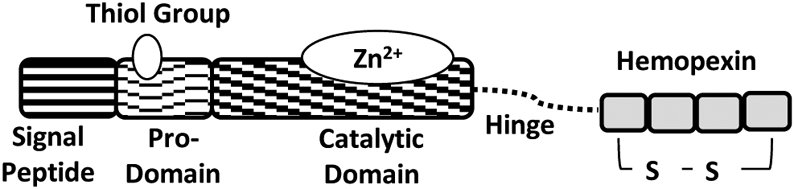
Structure of MMP3 (stromelysin1) showing the signal peptide, pro-domain, catalytic domain, hinge (linker) region, and hemopexin domain.
Increased endothelial MMP3 levels in our studies correlated with decreased expression of predominant endothelial tight junction protein claudin-5, whose decrease is associated with ARDS, suggesting the potential role of MMP3 in the promotion of vascular permeability [24]. Further, LPS-induced lung injury also correlated with increased expression of MMP3 in the bronchoalveolar lavage fluid (BALF) associated with increased neutrophil myeloperoxidase activity in the plasma, lung tissue, and BALF [19]. Another study from our group confirmed this further in human ARDS patients where significantly elevated MMP3 activity was observed in the plasma/serum samples of ARDS patients compared to the samples collected from the healthy control subjects [20]. The mechanisms by which MMP3 expression is regulated in inflammatory cells are currently unknown. Nevertheless, cotreatment with MMP3 inhibitor, UK356618, effectively reversed LPS-induced endothelial-barrier disruption in vitro and lung injury and edema in vivo [19], indicating the role of MMP3 in mediating pathological inflammation and tissue injury (Fig. 2). Although several compounds have been tested for their potency and selectivity for MMP3 inhibition (Table 1), UK356618 has demonstrated the highest efficacy and specificity for MMP3 [25].
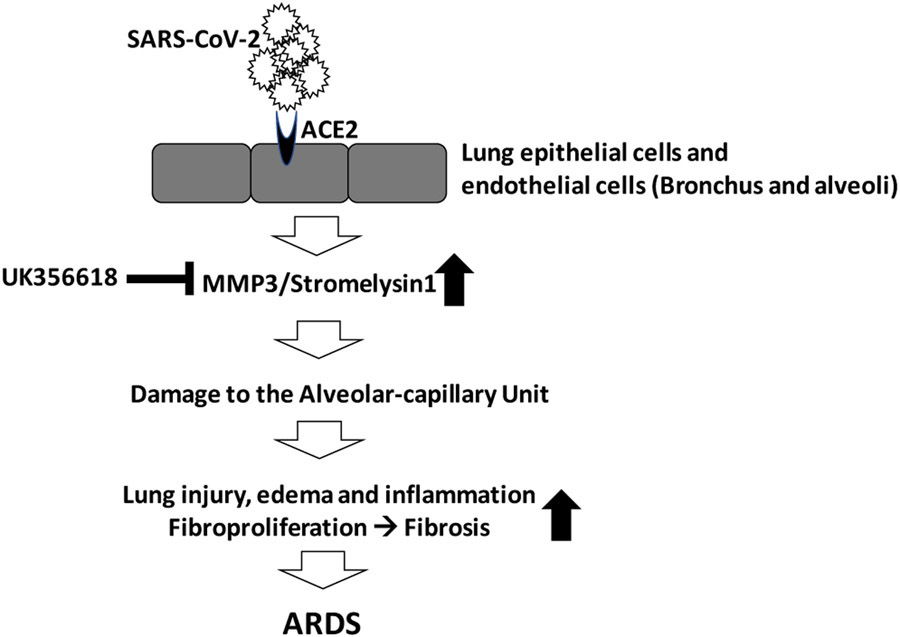
Fig. (2).
MMP3 inhibition during the exudative phase of ARDS will reduce vascular injury and alveolar edema and prevent the disease progression to the irreversible stages of the disease.
Table 1.
MMP3 inhibitors and their specificity.
| S.No. | MMP3 Inhibitor | Other MMPs Inhibited | References |
|---|---|---|---|
| 1. | Actinonin | MMP-1/2/3/7/8/9/10/12/13 | Mitchell et al., 2007 [33] |
| 2. | PD166793 | MMP-2/3/13 | Paolocci et al., 2006 [34] |
| 3. | MMP Inhibitor V | MMP-2/3/8/9/12/13 | Okamoto et al., 2007 [35] |
| 4. | MMP-3 Inhibitor VIII | MMP-3/12 | Sindermann et al., 2008 [36] |
| 5. | MMP-3 Inhibitor V | MMP-3 (Untested for other MMPs) | Johnson et al., 1999 [37] |
| 6. | MMP-2/3 Inhibitor II | MMP-2/3 | Jacobsen et al., 1999 [38] |
| 7. | UK 370106 | MMP-3 (Untested for other MMPs) | Matthews et al., 2008 [39] |
| 8. | UK 356618 | MMP-3 (Potent and highly specific) | Fray and Dickinson 2001 [25] |
COVID-19 has been associated with upregulated proinflammatory cytokines in addition to hemodynamic instability, multi-system organ failure, and notably the development of ARDS [26]. In this process of excessive production of proinflammatory cytokines, macrophages are activated (macrophage activation syndrome) and cause secondary hemophagocytic lymphohistiocytosis, further disrupting immune homeostasis [26]. In this stage, COVID-19 patients are at a higher risk of fatality at the hands of their immune system, as an indirect effect of the virus, than the actual virus. This maladaptive immune response poses a key target to prevent ARDS if damage to the alveolar-capillary unit and infiltration of inflammatory cells to the extravascular tissues can be prevented. Based on our experimental models of LPS-induced ARDS, inhibiting the MMP3 activity appears to have the potential to restrict the damage to the alveolar epithelial and endothelial-barriers, thus limiting lung edema, injury, and inflammation [19].
Beyond the potential therapeutic benefits of targeting MMP3 in COVID-19, the measurement of MMP3 activity in COVID-19 patients may also have utility as a prognostic marker, which has been demonstrated in other disease states. Previous studies have determined that the cutoff value used for Kaplan Meier survival risk shows an association between MMP3 expression and severity of cardiovascular disease [27]. A recent study conducted in a cohort of male patients with and without acute coronary syndrome used MMP3 expression level to predict myocardial infarction (MI) [28]. In pulmonary fibrosis, a correlation between the expression of MMP3, endostatin, inflammatory cytokines in BALF, and lung functionality of idiopathic pulmonary fibrosis patients has been established [29]. Although a similar study has yet to be undertaken in ARDS or COVID-19 patients, our recent report on the elevated MMP3 activity in the serum/plasma samples of ARDS patients is an indication that MMP3 activity measurement may serve as an important prognosis marker for the disease severity of COVID-19 patients.
A correlation between MMP3 and the activity of plasminogen, an important precursor in breaking down clots, has also been reported suggesting its potential involvement in coagulopathy [30]. As such, investigating the effect of targeting MMP3 in hypercoagulopathic COVID-19 patients would be necessary; however, the administration of an MMP3 inhibitor via inhalation could avoid this potential pitfall [31, 32]. Given this compelling early evidence, the use of an MMP3 activity assay as a marker for COVID-19 associated ARDS prognosis deserves further investigation.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support received from NHLBI, NCATS, Georgia Clinical and Translational Science Alliance.
FUNDING
The paper has been funded by the NHLBI (R01HL-103952) and NCATS (UL1TR-002378) to Payaningal R. Somanath. Funds from the NCATS supported Georgia Clinical and Translational Science Alliance (KL2-TR000455) to Andrea Sikora Newsome is acknowledged.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Biswas A; Bhattacharjee U; Chakrabarti AK; Tewari DN; Banu H; Dutta S Emergence of novel coronavirus and COVID-19: Whether to stay or die out? Crit. Rev. Microbiol, 2020, 46(2), 182–193. 10.1080/1040841X.2020.1739001 PMID: 32282268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mohamed AA; Mohamed N; Mohamoud S; Zahran FE; Khattab RA; El-Damasy DA; Alsayed E; Abd-Elsalam S SARS-CoV-2: The path of prevention and control. Infect. Disord Drug Targets, 2021, 21(3), 358–362. 10.2174/1871526520666200520112848 PMID: 32433010 [DOI] [PubMed] [Google Scholar]
- [3].Singhal T A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatr, 2020, 87, 281–286. 10.1007/s12098-020-03263-6 PMID: 32166607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abd-Elsalam S; Elkadeem M; Glal KA Chloroquine as chemoprophylaxis for COVID-19: Will this work? Infect. Disord. Drug Targets, 2020. (Online ahead of print). 10.2174/1871526520666200726224802 PMID: 32713336 [DOI] [PubMed] [Google Scholar]
- [5].Abd-Elsalam S; Esmail ES; Khalaf M; Abdo EF; Medhat MA; Abd El Ghafar MS; Ahmed OA; Soliman S; Serangawy GN; Alboraie M Hydroxychloroquine in the treatment of COVID-19: A multicenter randomized controlled study. Am. J. Trop. Med. Hyg, 2020. 10.4269/ajtmh.20-0873 PMID: 32828135 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [6].Li X; Ma X Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit. Care, 2020, 24(1), 198. 10.1186/s13054-020-02911-9 PMID: 32375845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guan WJ; Ni ZY; Hu Y; Liang WH; Ou CQ; He JX; Liu L; Shan H; Lei CL; Hui DSC; Du B; Li LJ; Zeng G; Yuen KY; Chen RC; Tang CL; Wang T; Chen PY; Xiang J; Li SY; Wang JL; Liang ZJ; Peng YX; Wei L; Liu Y; Hu YH; Peng P; Wang JM; Liu JY; Chen Z; Li G; Zheng ZJ; Qiu SQ; Luo J; Ye CJ; Zhu SY; Zhong NS China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med, 2020, 382(18), 1708–1720. 10.1056/NEJMoa2002032 PMID: 32109013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen N; Zhou M; Dong X; Qu J; Gong F; Han Y; Qiu Y; Wang J; Liu Y; Wei Y; Xia J; Yu T; Zhang X; Zhang L Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 2020, 395(10223), 507–513. 10.1016/S0140-6736(20)30211-7 PMID: 32007143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huang C; Wang Y; Li X; Ren L; Zhao J; Hu Y; Zhang L; Fan G; Xu J; Gu X; Cheng Z; Yu T; Xia J; Wei Y; Wu W; Xie X; Yin W; Li H; Liu M; Xiao Y; Gao H; Guo L; Xie J; Wang G; Jiang R; Gao Z; Jin Q; Wang J; Cao B Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 2020, 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 PMID: 31986264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang D; Hu B; Hu C; Zhu F; Liu X; Zhang J; Wang B; Xiang H; Cheng Z; Xiong Y; Zhao Y; Li Y; Wang X; Peng Z Clinical characteristics of 138 hospitalized patients with. Novel coronavirus-infected pneumonia in Wuhan, China. JAMA, 2020, 323(11), 1061–1069. 10.1001/jama.2020.1585 PMID: 32031570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu C; Chen X; Cai Y; Xia J; Zhou X; Xu S; Huang H; Zhang L; Zhou X; Du C; Zhang Y; Song J; Wang S; Chao Y; Yang Z; Xu J; Zhou X; Chen D; Xiong W; Xu L; Zhou F; Jiang J; Bai C; Zheng J; Song Y Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease. Pneumonia in Wuhan, China. JAMA Intern. Med, 2020, 180(7), 934–943. 10.1001/jamainternmed.2020.0994 PMID: 32167524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang X; Yu Y; Xu J; Shu H; Xia J; Liu H; Wu Y; Zhang L; Yu Z; Fang M; Yu T; Wang Y; Pan S; Zou X; Yuan S; Shang Y Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med, 2020, 8(5), 475–481. 10.1016/S2213-2600(20)30079-5 PMID: 32105632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sweeney RM; McAuley DF Acute respiratory distress syndrome. Lancet, 2016, 388(10058), 2416–2430. 10.1016/S0140-6736(16)00578-X PMID: 27133972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thompson BT; Chambers RC; Liu KD Acute respiratory distress syndrome. N. Engl. J. Med, 2017, 377(6), 562–572. 10.1056/NEJMra1608077 PMID: 28792873 [DOI] [PubMed] [Google Scholar]
- [15].Greenlee KJ; Werb Z; Kheradmand F Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol. Rev, 2007, 87(1), 69–98. 10.1152/physrev.00022.2006 PMID: 17237343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Löffek S; Schilling O; Franzke CW Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: a critical balance. Eur. Respir. J, 2011, 38(1), 191–208. 10.1183/09031936.00146510 PMID: 21177845 [DOI] [PubMed] [Google Scholar]
- [17].Sternlicht MD; Werb Z How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol, 2001, 17, 463–516. 10.1146/annurev.cellbio.17.1.463 PMID: 11687497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Madzharova E; Kastl P; Sabino F; Auf dem Keller U Post--translational modification-dependent activity of matrix metalloproteinases. Int. J. Mol. Sci, 2019, 20(12), E3077. 10.3390/ijms20123077 PMID: 31238509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Artham S; Gao F; Verma A; Alwhaibi A; Sabbineni H; Hafez S; Ergul A; Somanath PR Endothelial stromelysin1 regulation by the forkhead box-O transcription factors is crucial in the exudative phase of acute lung injury. Pharmacol. Res, 2019, 141, 249–263. 10.1016/j.phrs.2019.01.006 PMID: 30611853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Artham S; Verma A; Newsome AS; Somanath PR Patients with acute respiratory distress syndrome exhibit increased stromelysin1 activity in the blood samples. Cytokine, 2020, 131, 155086. 10.1016/j.cyto.2020.155086 PMID: 32272349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yamashita CM; Dolgonos L; Zemans RL; Young SK; Robertson J; Briones N; Suzuki T; Campbell MN; Gauldie J; Radisky DC; Riches DW; Yu G; Kaminski N; McCulloch CA; Downey GP Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. Am. J. Pathol, 2011, 179(4), 1733–1745. 10.1016/j.ajpath.2011.06.041 PMID: 21871427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lukassen S; Chua RL; Trefzer T; Kahn NC; Schneider MA; Muley T; Winter H; Meister M; Veith C; Boots AW; Hennig BP; Kreuter M; Conrad C; Eils R SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J, 2020, 39(10), e105114. 10.15252/embj.2020105114 PMID: 32246845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nagase H; Visse R; Murphy G Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res, 2006, 69(3), 562–573. 10.1016/j.cardiores.2005.12.002 PMID: 16405877 [DOI] [PubMed] [Google Scholar]
- [24].Schlingmann B; Molina SA; Koval M Claudins: Gatekeepers of lung epithelial function. Semin. Cell Dev. Biol, 2015, 42, 47–57. 10.1016/j.semcdb.2015.04.009 PMID: 25951797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fray MJ; Dickinson RP Discovery of potent and selective succinyl hydroxamate inhibitors of matrix metalloprotease-3 (stromelysin-1). Bioorg. Med. Chem. Lett, 2001, 11(4), 571–574. 10.1016/S0960-894X(00)00720-4 PMID: 11229774 [DOI] [PubMed] [Google Scholar]
- [26].Mehta P; McAuley DF; Brown M; Sanchez E; Tattersall RS; Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet, 2020, 395(10229), 1033–1034. 10.1016/S0140-6736(20)30628-0 PMID: 32192578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guizani I; Zidi W; Zayani Y; Boudiche S; Hadj-Taieb S; Sanhaji H; Zaroui A; Mechmeche R; Mourali MS; Feki M; Allal-Elasmi M Matrix metalloproteinase-3 predicts clinical cardiovascular outcomes in patients with coronary artery disease: a 5 years cohort study. Mol. Biol. Rep, 2019, 46(5), 4699–4707. 10.1007/s11033-019-04914-4 PMID: 31218540 [DOI] [PubMed] [Google Scholar]
- [28].Cavusoglu E; Marmur JD; Kassotis JT; Yanamadala S; Chopra V; Eng C Usefulness of plasma matrix metalloproteinase-3 levels to predict myocardial infarction in men with and without acute coronary syndrome. Am. J. Cardiol, 2016, 117(6), 881–886. 10.1016/j.amjcard.2015.12.022 PMID: 26805660 [DOI] [PubMed] [Google Scholar]
- [29].Craig VJ; Zhang L; Hagood JS; Owen CA Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol, 2015, 53(5), 585–600. 10.1165/rcmb.2015-0020TR PMID: 26121236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Johnson JL; George SJ; Newby AC; Jackson CL Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc. Natl. Acad. Sci. USA, 2005, 102(43), 15575–15580. 10.1073/pnas.0506201102 PMID: 16221765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Levi M; Thachil J; Iba T; Levy JH Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol, 2020, 7(6), e438–e440. 10.1016/S2352-3026(20)30145-9 PMID: 32407672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Becker RC COVID-19 update: Covid-19-associated coagulopathy. J. Thromb. Thrombolysis, 2020, 50(1), 54–67. 10.1007/s11239-020-02134-3 PMID: 32415579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mitchell JK; Pitcher D; McArdle BM; Alnefelt T; Duffy S; Avery V; Quinn RJ Identifying common metalloprotease inhibitors by protein fold types using Fourier transform mass spectrometry. Bioorg. Med. Chem. Lett, 2007, 17(23), 6521–6524. 10.1016/j.bmcl.2007.09.084 PMID: 17933532 [DOI] [PubMed] [Google Scholar]
- [34].Paolocci N; Tavazzi B; Biondi R; Gluzband YA; Amorini AM; Tocchetti CG; Hejazi M; Caturegli PM; Kajstura J; Lazzarino G; Kass DA Metalloproteinase inhibitor counters high-energy phosphate depletion and AMP deaminase activity enhancing ventricular diastolic compliance in subacute heart failure. J. Pharmacol. Exp. Ther, 2006, 317(2), 506–513. 10.1124/jpet.105.099168 PMID: 16436497 [DOI] [PubMed] [Google Scholar]
- [35].Okamoto Y; Satomura K; Nakayama K; Tanaka N; Ohsuzu F; Imaki J; Yoshioka M; Nakamura H A matrix metalloproteinase inhibitor, ONO-4817, suppresses the development of aortic intimal hyperplasia in experimental hyperlipidemic rabbit. Int. Heart J, 2007, 48(3), 369–378. 10.1536/ihj.48.369 PMID: 17592201 [DOI] [PubMed] [Google Scholar]
- [36].Sindermann JR; Kobbert C; Voss R; Ebbing J; March KL; Breithardt G; Weissen-Plenz G Transgenic model of smooth muscle cell cycle reentry: expression pattern of the collageneous matrix. Cardiovasc. Pathol, 2008, 17(2), 72–80. 10.1016/j.carpath.2007.07.003 PMID: 18329551 [DOI] [PubMed] [Google Scholar]
- [37].Johnson LL; Bornemeier DA; Janowicz JA; Chen J; Pavlovsky AG; Ortwine DF Effect of species differences on stromelysin-1 (MMP-3) inhibitor potency. An explanation of inhibitor selectivity using homology modeling and chimeric proteins. J. Biol. Chem, 1999, 274(35), 24881–24887. 10.1074/jbc.274.35.24881 PMID: 10455161 [DOI] [PubMed] [Google Scholar]
- [38].Jacobsen EJ; Mitchell MA; Hendges SK; Belonga KL; Skaletzky LL; Stelzer LS; Lindberg TJ; Fritzen EL; Schostarez HJ; O’Sullivan TJ; Maggiora LL; Stuchly CW; Laborde AL; Kubicek MF; Poorman RA; Beck JM; Miller HR; Petzold GL; Scott PS; Truesdell SE; Wallace TL; Wilks JW; Fisher C; Goodman LV; Kaytes PS Synthesis of a series of stromelysin-selective thiadiazole urea matrix metalloproteinase inhibitors. J. Med. Chem, 1999, 42(9), 1525–1536. 10.1021/jm9803222 PMID: 10229623 [DOI] [PubMed] [Google Scholar]
- [39].Matthews KH; Stevens HN; Auffret AD; Humphrey MJ; Eccleston GM Formulation, stability and thermal analysis of lyophilised wound healing wafers containing an insoluble MMP-3 inhibitor and a non-ionic surfactant. Int. J. Pharm, 2008, 356(1–2), 110–120. 10.1016/j.ijpharm.2007.12.043 PMID: 18280068 [DOI] [PubMed] [Google Scholar]