Abstract
Chemically modified mRNA represents a unique, efficient, and straightforward approach to produce a class of biopharmaceutical agents. It has been already approved as a vaccination-based method for targeting SARS-CoV-2 virus. The COVID-19 pandemic has highlighted the prospect of synthetic modified mRNA to efficiently and safely combat various diseases. Recently, various optimization advances have been adopted to overcome the limitations associated with conventional gene therapeutics leading to wide-ranging applications in different disease conditions. This review sheds light on emerging directions of chemically modified mRNAs to prevent and treat widespread chronic diseases, including metabolic disorders, cancer vaccination and immunotherapy, musculoskeletal disorders, respiratory conditions, cardiovascular diseases, and liver diseases.
Keywords: Chemically Modified mRNA, COVID-19, Vaccination, Chronic diseases, Cancer, Diabetes
Graphical Abstract
1. Introduction
Although the COVID-19 pandemic shifted the attention when it started late in 2019 towards utilizing chemically modified mRNA technology to prevent the spread of the disease, the applications of this technology in the prevention and treatment of other conditions should not be overlooked. Indeed, the story of this new-generation method began over twenty years ago with a chief focus on cancer treatment [1], [2], [3]. The first-ever FDA approval of mRNA based vaccines happened swiftly in less than a year from the start of the COVID-19 pandemic, with an emergency use authorization (EUA) granted to Pfizer-BioNTech and Moderna COVID-19 vaccines [4], [5]. Most recently, on August 23, 2021, the FDA has granted the first full approval to the Pfizer-BioNTech COVID-19 vaccine, which is now marketed under the name of COMIRNATY® [6], [7]. These novel vaccines have provided a proof of concept for the readiness of this method in handling difficult disease conditions, and many research groups have already started working on the development of agents from this class of biologics to manage various diseases [2], [8], [9], [10]. Actually, different types of chemically modified mRNA can play dominant roles when employed for different therapeutic purposes like genome engineering, immunotherapy, genetic reprogramming, and protein replacement therapies in several disease conditions [10], [11].
Utilizing naked mRNA to treat diseases is challenging because of its high susceptibility to degradation by the extracellular RNases, and the great difficulty in transporting this large molecule through cellular membranes, resulting in compromised stability and poor delivery of mRNA molecules [12]. In order to promote better delivery of synthetic mRNAs, various pharmaceutical delivery systems have been developed [12]. The gold standard method involves using lipid nanoparticles (LNPs) formulated through electrostatic binding of polymers or cationic lipids with the negatively charged modified mRNA [2], [12]. The modified mRNA-coated lipid nanoparticles were proven to be biodegradable, biocompatible, and able to prolong the therapeutic effect [2], [12]. In addition, mRNA-enclosed lipid nanoparticles proficiently interact with cell membranes, leading to facilitated endocytosis and cellular uptake, which can be employed as a targeted delivery system to various sites of interest [2], [10], [12], [13]. Administration methods can involve systemic blood injections, local injections in organs or muscles, and inhalation [2], [10], [13].
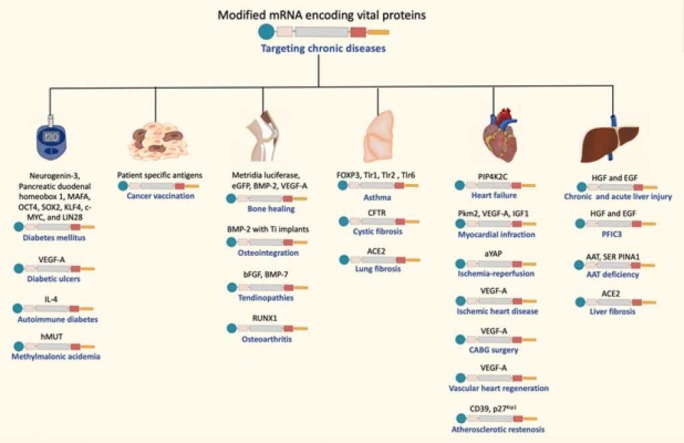
When compared to the traditional DNA-based delivery, modified mRNA offers numerous advantages. One of the main advantages is that mRNA does not enter the nucleus or integrate into the host’s genome. Moreover, mRNA does not possess a risk of mutagenicity, which is the main drawback of the DNA-based delivery systems [14]. Furthermore, modified mRNA can efficiently utilize the cell’s translation machinery to produce the therapeutic proteins of interest in a dose dependent-manner [13]. Additionally, the safety profile of the chemically modified mRNA is better as the risk for immunogenicity remains low due to the structural and nucleotide modifications [2], [10], [13]. Moreover, synthetic mRNA-based therapy is considered cheaper, faster, and easily manufactured on a large-scale [10]. Due to the outstanding characteristics and features of modified mRNA, it has been investigated for many preventive and therapeutic pursuits in different disease states, including chronic diseases. These devastating diseases include diabetes, methylmalonic acidemia, cancer vaccination and immunotherapy, osteoporosis, osteoarthritis, osteointegration, tendinopathies, asthma, cystic fibrosis, ischemic heart disease (IHD), heart failure, myocardial infraction, atherosclerotic restenosis, chronic liver injury, progressive familial intrahepatic cholestasis type 3 (PFIC3), alpha 1-antitrypsin (AAT) deficiency, and fibrosis of the liver and lung.
1.1. An insight into the mRNA technology and the COVID-19 case
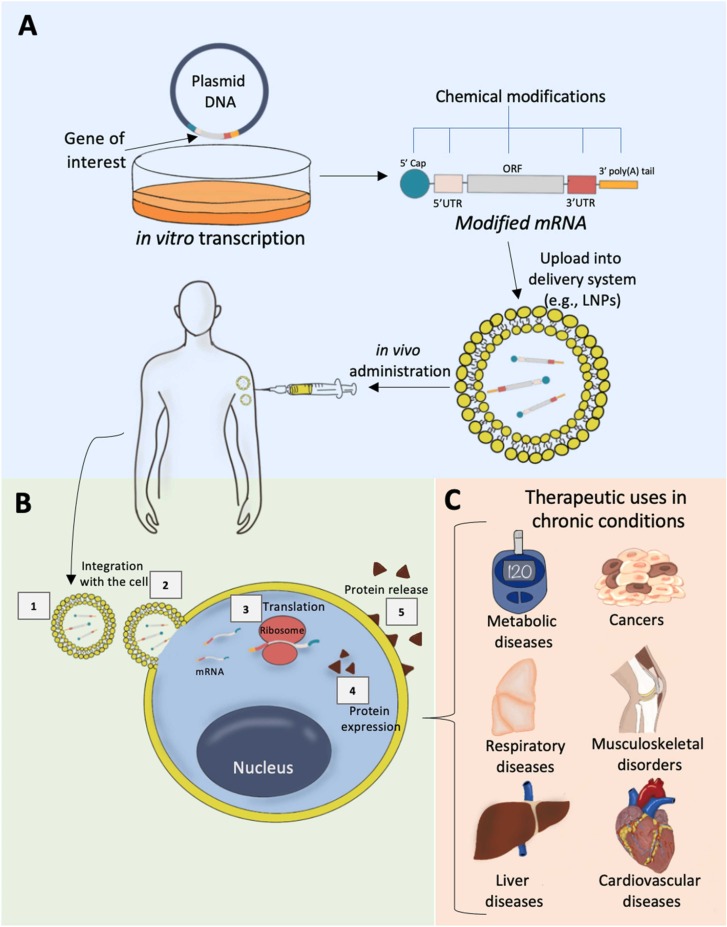
The basic structure of modified mRNA resembles the natural mRNA found in eukaryotic cells [12], [13]. It consists of five major building blocks that start with the 5′ cap structure and end with the 3′ poly(A) tail ( Fig. 1A). In between, there are the 5′ untranslated region (5′UTR), open reading frame (ORF), and the 3′ untranslated region (3′UTR) [12], [13]. Particularly, the ORF region would encode the protein of interest which typically integrates between the start and the stop codons [12], [13] (Fig. 1A).
Fig. 1.
Chemically modified mRNA development stages, biological actions, and applications. A) modified mRNA structure, in vitro transcription, modifications, formulation, and delivery; B) Mechanism of action of synthetic modified mRNA; C) Therapeutic applications for the prevention and treatment of different chronic diseases.
To enhance the translation and/or half-life of the mRNA, chemical modifications can be deployed within different regions of the mRNA structure to optimize its integrity, efficiency, stability, and safety. A functional 5′ cap structure is required for efficient translation of mRNA, which happens through 5′ cap binding to eukaryotic translation initiation factor 4E (EIF4E) [15]. Binding of 5′ cap to the mRNA decapping enzymes (DCP1, DCP2 or DCPS), on the other hand, controls mRNA decay [15]. In a study, new cap analogs carrying a 1,2-dithiodiphosphate moiety along a tri- or tetraphosphate bridge were designed as reagents for mRNA modification [15]. These cap analogs were shown to have a high affinity towards EIF4E and resistance against the SpDcp1/2 decapping complex [15]. Besides, the poly(A) tail is located at the end of mRNA and functions by regulating the efficiency of the translation and the stability [16]. One method to modify the poly(A) tail is through using recombinant poly(A) polymerase which helps extends the synthesized mRNA enzymatically through incorporating modified nucleotides into the poly(A) tail [16]. Karikó et al., reported that in vitro transcription of mRNAs alongside poly(A) tail modifications and incorporation of pseudouridines led to a superior biological stability, increased translational capacities, and no immunogenicity [16]. Furthermore, optimizing the 5′- and 3′-UTRs regions can be achieved through addition of regulatory sequence components known to regulate the stability and translation of endogenous mRNA, such as β-globin 3′-UTR sequences [17]. Finally, the ORF (the coding region) optimization can occur through replacement of rare codons with common ones to accelerate the translation efficiency [18]. Different optimizations may also be incorporated to improve the safety of mRNA based therapeutics. For example, Toll-like receptors (TLRs) can easily recognize unmodified mRNA nucleotides in the synthetic mRNA, making it highly immunogenic [19], [20], [21]. To optimize the mRNA structure and reduce its TLRs medicated immunogenicity, merging modified nucleotides like 5-methylcytidine, pseudouridine, N1-methylpseudouridine, 5-methyluridine, or N6-methyladenosine was demonstrated as a successful modality for escaping TLRs activation and preventing immunogenicity [16], [22].
Chemically modified mRNA works principally by delivering defined genetic information into patients’ cells to mitigate or prevent certain disease conditions (Fig. 1). First, an in vitro transcription of mRNA that carries the genetic material of interest begins [23] (Fig. 1A). These synthesized mRNAs can then get encapsulated within optimized delivery systems, such as lipid nanoparticles (LNPs) before injection, which could be administered directly by injection into the patient’s cells in vivo or integrated with the patient’s cells ex-vivo and transferred back into the patient [13] (Fig. 1A). Following administration, LNPs will cross the cytoplasmic membrane, and the LNP-loaded mRNA will be delivered to the cytoplasm of the cell without entering the nucleus or integrating with the cell’s genome [2], [10], [13] (Fig. 1B). After the delivery of the mRNA to the cytoplasm of the cell, ribosomal translation of the mRNA occurs, expression of the desired protein subsequently takes place, and the desired proteins get released through the cell's membrane [2], [10], [13] (Fig. 1B). As discussed in subsequent sections and represented in Fig. 1C, this recent method can play a role in preventive and therapeutic applications in various chronic diseases.
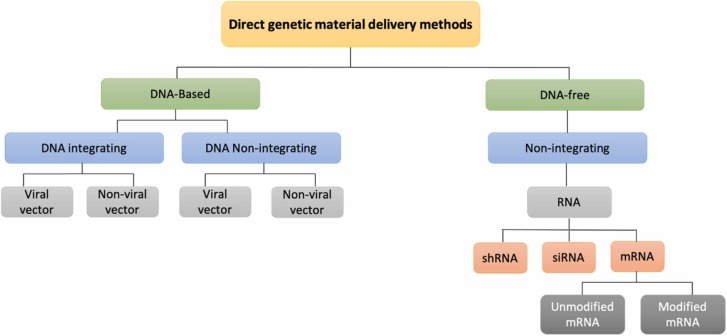
Different gene delivery approaches can introduce genetic material into somatic cells, comprising direct and indirect transfer methods ( Fig. 2). The direct techniques can be divided into DNA-based or DNA-free methods, including mRNA as one of the DNA-free approaches [24], [25], [26], [27] (Fig. 2). DNA-based delivery methods are classified into DNA integrating and DNA non-integrating [24] (Fig. 2). The integrative approach embraces the insertion of foreign genetic material into the host genome. In contrast, the non-integrative techniques are achieved without integrating exogenous genetic material into the genome of the host cells [24], [25], [26], [27]. The first generation of DNA-based gene therapy utilized viral vectors to penetrate into the nucleus and insert the carried genetic material into the differentiating cells nucleic acid [28]. These integrated pieces of the genetic material can permanently stay in the host’s cells genome and may cause cell damage, tumorigenesis, and cell death [28]. Besides, the left behind viral footprints may induce or enhance the probability of different infectious diseases [28]. The second generation of DNA-based gene therapy does not integrate with the cell genome. Instead, they interact with the DNA molecules located outside the genome [29]. However, they can still integrate with the cells’ DNA and may lead to significant cell damage or cell death [29]. The most recent method of direct gene editing and transfer include RNA-based therapies; particularly, short hairpin RNA (shRNA), small interfering (also known as short interfering or silencing) RNA (siRNA), and messenger RNA (mRNA) [30], [31], [32] (Fig. 2). Both shRNA and siRNA are considered interfering RNAs that act through binding to the mature mRNA and interfering with its translation [30].
Fig. 2.
Representation of available gene therapy delivery approaches.
Since the beginning of the COVID-19 pandemic in late 2019 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the vaccine race has started with hundreds of vaccines being investigated at different stages of clinical trials [33]. These developed vaccines employed diverse approaches, including inactivated viruses [34], nonreplicating viral vectors [35], protein subunits [36], DNA plasmids [37], and RNA-based methods [38]. Amidst them, modified messenger RNA vaccines gained the greatest attention and demonstrated outstanding potential [38], [39], [40]. Even though this technology is relatively new and no similar vaccines were ever approved in the past, Pfizer-BioNTech and Moderna were able to gain the first FDA approval for modified mRNA-based vaccines under Emergency Use Authorization (EUA) [4], [5]. Very recently, Pfizer-BioNTech received the full FDA authorization for their COVID-19 vaccine, COMIRNATY® [6], [7]. Besides, Moderna has submitted their rolling applications to the FDA to gain a full biologics application license for their COVID-19 vaccines after more clinical efficacy and safety data were collected from phase III clinical trials [41]. These two vaccines were made through an in vitro transcription of modified mRNA encoding an essential SARS-CoV-2 protein, the spike protein, loaded into lipid nanoparticles (LNPs) to optimize its cellular delivery [38], [42]. In addition to COVID-19, mRNA vaccines can effectively be utilized to protect from several other viral infections such as Ebola, influenza, hepatitis C, papilloma, zika, cytomegalovirus, and human immunodeficiency virus (HIV), and numerous of them are now at different stages of development stages [43].
2. Synthetic modified mRNA for preventing and treating chronic diseases
Based on the aforementioned information on mRNA, this new innovative technology does not only target viral infections, but can also represent a milestone for preventing and treating a range of chronic diseases, as discussed in the subsequent sections ( Table 1).
Table 1.
Summary of clinical and preclinical studies on modified mRNA uses in chronic diseases.
| Target indication | Encoded gene/protein | Study design | Main findings | Ref. |
|---|---|---|---|---|
| Metabolic disorders | ||||
| Diabetes mellitus | Neurogenin-3 and small chemical molecules | Preclinical, in vitro | The reprogrammed pancreatic organoid cells retained the normal physiologic function of pancreatic β-cells | [44] |
| Diabetes mellitus | Pancreatic duodenal homeobox 1, neurogenin3, and MAFA | Preclinical, in vitro, in vivo | HDDCs were turned into insulin-producing cells in response to different stimuli | [45] |
| Diabetes mellitus | OCT4, SOX2, KLF4, c-MYC, and LIN28 | Preclinical, in vitro | modified mRNA engineered iPSCs led to a significant upregulation of pancreatic specific microRNAs | [46] |
| Diabetic ulcers; wound healing | VEGF-A | Preclinical, in vivo | A dose-depended improvement in vasodilation, vascularization, and wound bed oxygenation | [48] |
| Diabetic ulcers; wound healing | VEGF-A | Clinical, RCT, phase 1 | In DM type 2 patients, it promoted VEGF-A expression and blood flow; with no remarkable side effects | [49] |
| Autoimmune diabetes | IL-4 | Preclinical, in vivo | Glucose hemostasis was maintained following hyperglycemia; disease onset was prevented in 50% of prediabetic mice | [50] |
| Methylmalonic acidemia/aciduria | hMUT | Preclinical, in vivo | hMUT expression in the liver was leveled up; plasma methylmalonic acid level declined by 75–85% | [52] |
| Oncology | ||||
| Vaccination and immunotherapy* | Reprograming natural killer cells and T cells to target specific cancer antigens | Clinical, RCTs, phase II & III | Have shown promise in numerous cancer types and stages, including melanoma, renal cell carcinoma, prostate cancer, acute myeloid leukemia, non-small-cell lung cancer, glioblastoma, and colorectal cancer | [53], [54], [55], [56], [57], [58], [59] |
| Musculoskeletal disorders | ||||
| Bone healing | Metridia luciferase, eGFP, and BMP-2 | Preclinical, in vitro | Osteogenic differentiation in preosteoblast cells; expression of osteogenic markers | [61] |
| Bone healing | BMP-2 | Preclinical, in vitro, in vivo | Osteogenic differentiation; bone regeneration in defective femoral bones | [62] |
| Bone healing | BMP-2 | Preclinical, in vitro, in vivo | Upregulation of angiogenic and osteogenic genes; bone formation and endochondral osteogenesis in defective femoral bones | [63] |
| Bone healing | BMP-2 plus VEGF-A | Preclinical, in vivo | Synergistically fostered osteogenesis and healing in defective skull | [64] |
| Osteointegration | BMP-2 with Ti implants | Preclinical, in vitro | Osteogenesis in a dose-dependent manner and increased mineralization and ALP activity | [69] |
| Achilles tendon defects | bFGF | Preclinical, in vivo | Significant construct stiffness; no side effects; no side effects | [72] |
| Tendon injuries | BMP-7 | Preclinical, in vivo | Greater expression of BMP-7 protein and less collagen type III formation in severely inflamed tendons | [73] |
| Osteoarthritis | RUNX1 | Preclinical, in vivo | Suppression of OA progression and amplified expression of RUNX1 and other associated cartilage-anabolic markers | [76] |
| Respiratory conditions | ||||
| Asthma | FOXP3 | Preclinical, in vivo | Preventive and therapeutic responses; protected against goblet cell metaplasia, airway hyperresponsiveness, and allergen-induced tissue inflammation | [80] |
| Asthma | Tlr1, Tlr2 and Tlr6 | Preclinical, in vivo | Protected against airway inflammation through preventing TLR activation | [81] |
| Cystic fibrosis | CFTR | Preclinical, in vitro | Reestablished cAMP-induced CFTR currents by a twofold increase | [85] |
| Cystic fibrosis | CFTR | Preclinical, in vitro, in vivo | CFTR expression was significantly increased, and its function as a chloride channel was restored | [86] |
| Cardiovascular Diseases | ||||
| Heart failure | PIP4K2C | Preclinical, in vitro, in vivo | Improved heart function, reverse cardiac hypertrophy, cardiac fibrosis; increased survival | [89] |
| Myocardial infraction | Pkm2 | Preclinical, in vivo | Promoted cardiomyocyte cell division, cardiac function, and survival | [90] |
| Myocardial infraction | VEGF-A, IGF1 | Preclinical, in vivo | Intramyocardial injection caused differentiation of endogenous heart progenitors; amended heart function; prolonged survival | [93] |
| Myocardial infraction | IGF1 | Preclinical, in vivo | Cardiomyocyte survival under hypoxia-induced apoptosis states; downstream increase in Akt and Erk phosphorylation; downregulation of IGF1 specific miRNA-1 and -133 expression | [95] |
| Ischemia-reperfusion | aYAP | Preclinical, in vitro, in vivo | Decreased cardiomyocyte necrosis, inflammation, and hypertrophic remodeling following Ischemia-reperfusion stress. | [91] |
| Ischemic heart disease | VEGF-A | Preclinical, in vivo | Intradermal injection induced a local and rapid production of VEGF-A protein | [92] |
| CABG surgery | VEGF-A | Clinical, RCTs, phase II | The safety and tolerability of epicardial injection of a modified mRNA encoding VEGF-A is currently being evaluated in patients undergoing CABG surgery | [97] |
| Vascular heart regeneration | VEGF-A | Preclinical, in vivo | Promoted endothelial specification; engraftment, survival and proliferation of the human Isl1 + progenitors | [94] |
| Atherosclerotic restenosis | CD39 | Preclinical, in vitro | Bioactive protective stent coating was developed and led to CD39 overexpression in endothelial cells; ADP hydrolysis; prevention of platelet activation | [88] |
| Atherosclerotic restenosis | p27Kip1 | Preclinical, in vivo | p27Kip1, containing sequence of endothelial cell-specific miR-126, prevented restenosis and led to vessel reendothelialization. | [96] |
| Liver diseases | ||||
| Chronic and acute liver injury | HGF and EGF | Preclinical, in vivo | Proliferation of hepatocytes; reversal of steatosis of non-alcoholic fatty liver disease; hepatocytes regeneration following acute paracetamol-induced liver injury; normalization of ALT | [99] |
| PFIC3 | hABCB4 | Preclinical, in vivo | Returned the phospholipid transport in PFIC3 livers; liver regeneration; normalization clinical parameters, including liver fibrosis, inflammation ductular reaction | [101] |
| AAT deficiency | AAT | Preclinical, in vitro, in vivo | Efficient uptake into hepatocytes and de novo expression of functioning AAT | [103] |
| AAT deficiency | SER PINA1 | Preclinical, in vitro, in vivo | Augmented SERPINA1 expression and biodistribution in lung and liver | [104] |
| Liver and lung fibrosis | ACE2 | Preclinical, in vitro , in vivo | Targeted liver and lung translation of substantial quantities of ACE2 protein; efficient selective uptake of in different carrier systems | [107] |
2.1. Metabolic disorders: diabetes and methylmalonic acidemia
Several studies have demonstrated that modified mRNA can direct non-endocrine pancreatic cells to produce insulin endogenously to treat diabetes. In a study conducted by Koblas et al., it has been reported that pancreatic organoid cells could be stimulated to act as insulin-producing cells by administering modified mRNA that encodes the transcription factor neurogenin-3 and small chemical molecules to reprogram the epigenetic state [44]. Their results showed that the reprogrammed cells retained the normal physiologic function of pancreatic β-cells, which was evident with the expression of insulin, glucokinase, and vital transcription factors [44]. However, the insulin-secretory efficiency was partially enhanced when the extracellular glucose concentrations were elevated [44]. These findings indicate that the investigated modified mRNA can partially modulate pancreatic cells to reproduce insulin; thus, it may be only effective as adjuvant therapy for the treatment of diabetes mellitus (DM).
Further studies may be warranted to promote the use of chemically modified mRNA as a monotherapy function in DM, particularly type 1, by introducing different or dual genetic material encoding other proteins to increase the secretory capability of insulin-secreting cells and maintain glucose homeostasis. In another study with a similar pursuit, human pancreatic duct-derived cells (HDDCs) were turned into insulin-producing cells by transfection with synthetic modified mRNA carrying vital pancreatic transcription factors (neurogenin3, pancreatic duodenal homeobox 1, and V-Maf musculoaponeurotic fibrosarcoma oncogene homolog A) [45]. This transfection was shown to induce β-cell differentiation of HDDCs in vitro [45]. As reported, 37% insulin positive cells were detected within one week [45]. The HDDC-derived β-cells were capable of producing C-peptide and insulin in response to different stimuli, such as glucose [45]. In the in vivo study, when HDDC-derived β-cells were administered into a diabetic mouse model, hyperglycemia was alleviated, and insulin was prompted to be produced in response to the elevated blood sugar level [45]. Likewise, another study determined that induced pluripotent stem cells (iPSCs) were successfully generated through a modified mRNA reprogramming method [46]. Compared with parental fibroblasts, the modified mRNA engineered iPSCs led to a significant upregulation of pancreatic specific microRNAs, which are known to play a central role in promoting the proliferation of pancreatic β-cells and insulin secretion [46]. These findings support the critical role that chemically modified mRNA may represent a promising therapeutic strategy to reverse and treat type 1 DM.
Among the overwhelming complications of diabetes are diabetic ulcers with compromised wound healing [47]. Targeting angiogenesis and vascularization had long represented an attractive strategy to promote and accelerate wound healing. Remarkably, vascular endothelial growth factor A (VEGF-A) has been extensively studied for its beneficial attributes in gene transfer; whereby, modified mRNA represented a possible methodology to overcome many limitations associated with different genetic and reprogramming routines. A group of researchers has tested the effects of administering a modified mRNA carrying genetic material encoding the vascular endothelial growth factor A (VEGF-A) protein using a diabetic wound healing mouse model [48]. Interestingly, a dose-depended improvement in vasodilation, vascularization, and wound bed oxygenation was observed in this study [48]. This has, in turn, resulted in an augmented re-epithelialization throughout the early stage of diabetic wound healing [48]. When studied in humans for the first time, transdermal injection of modified mRNA encoding vascular endothelial growth factor A (VEGF-A) was administered to patients with type 2 DM in a double-blind randomized, placebo-controlled, phase 1 study [49]. It was shown to effectively promote VEGF-A expression after 4–24 h following mRNA administration and was well tolerated with no remarkable side effects [49]. Furthermore, a twofold increase in blood flow was noted at 4 h post administration compared to the control group and was maintained for 7 days [49]. These findings may indicate that modified mRNA holds a promise in promoting wound healing in diabetic patients and may potentially mitigate other ischemic disease conditions. However, the study did not directly assess whether the established results occurred merely due to the administered modified VEGF-A mRNA translation. Further investigations are demanded to precisely determine the exact direct and indirect effects of the administered mRNA.
When the role of synthetically modified mRNA was investigated in autoimmune diabetes, it has been shown to alleviate the symptoms and delay or prevent the disease onset [50]. Creusot et al., studied the role of modified mRNA in modulating the immune system through interfering with the expression of immunoregulatory products derived from dendritic cells in the bone marrow [50]. In this study, a single injection of modified mRNA carrying interleukin 4 (IL-4) into a nonobese diabetic mouse model was done. Glucose hemostasis was maintained immediately following the onset of hyperglycemia with a continuous expression of IL-4 for around 24 h [50]. Nonetheless, these effects were reported to have been maintained for several months after the transfection in most of the mice that were injected with the modified mRNA in the study [50]. The same research team also reported that these modified mRNA effects delayed and prevented the disease onset in a prediabetic nonobese mouse model by approximately 50% [50]. This, again, provides some an evidence that modified mRNA can target many forms of diabetes from diverse angles and could be an efficient therapeutic approach to treat diabetes and diabetes-related disorders.
Another metabolic disorder that can be tackled with modified mRNA is methylmalonic acidemia/aciduria (MMA). MMA is a metabolic disorder characterized primarily by enzymatic deficiency of methylmalonyl-CoA mutase (hMUT), which consequently leads to the buildup of methylmalonic acid [51]. To overcome the current limitations of management and treatment approaches of MMA, a novel modified mRNA was developed to encode for hMUT and was encapsulated in LNPs [52]. When administered intravenously into two MMA mouse models, hMUT expression in the liver was leveled up and the plasma methylmalonic acid levels were declined by 75–85% [52]. Repetitive doses of the mRNA decreased the number of circulating metabolites and considerably promoted weight gain and improved survival rate [52]. Concerning safety, no evident side effects were noted, and the modified mRNA was reported to be well tolerated [52]. Synthetic mRNA has been shown to be a potentially successful therapeutic strategy in targeting metabolic diseases and may be tested to prevent and treat other metabolic disorders.
2.2. Oncology: cancer vaccination and immunotherapy
Chemically modified mRNA has been extensively explored as a vaccination modality in cancer immunotherapy, in which both the genetic material is delivered alongside immune stimulation of the innate immune system [9], [10]. Unlike prophylactic viral vaccines, cancer vaccines are mainly utilized as a therapeutic approach which act through targeting distinct tumor antigens or growth-associated factors that are typically expressed by malignant cells [9], [10]. To boost their efficacy and potency against cancer cells, these vaccines are often combined with immune modulators such as OX40, CD83, and 4-1BBL [10]. Immunostimulant action was found to be important as it can prevent immune tolerance while targeting cancer self-antigens [9], [10]. Modified mRNA was deployed to reprogram natural killer cells and T cells to target specific cancer antigens after identifying unique cancer mutations [9], [10]. To effectively deliver the mRNA, various direct and indirect delivery methods could be utilized. The indirect delivery can be done through ex vivo mRNA injection into dendritic cells. Direct injection of mRNA could also be done using different routes of administration, like intramuscular, intradermal, intranodal, and intratumoral [9], [10]. However, systemic administration is avoided mainly to protect against protein aggregation and cellular degradation. Optimized formulations should also be employed to prevent RNase enzyme from degrading the chemically modified mRNA [2]. This technology was developed several years ago, and currently many mRNA cancer vaccines for various malignancies have moved into phase II and III clinical trials using direct or indirect delivery methods. These trials involved testing in numerous cancer types and stages, including melanoma [53], renal cell carcinoma [54], prostate cancer [55], acute myeloid leukemia [56], non-small-cell lung cancer [57], glioblastoma [58], and colorectal cancer [59]. However, the most significant challenge with cancer vaccination is the presence of various genetic variants in different types of cancer and among patients. Thus, this tactic may need to be tailored through a personalized therapeutic strategy on a case-by-case basis, which would add extra cost and effort.
2.3. Musculoskeletal disorders: bone, tendon, and cartilage healing
Disease modeling with modified mRNA can be beneficial in several different musculoskeletal disorders. One of the crucial biomarkers is bone morphogenetic protein 2 (BMP-2), which plays an integral role in bone and cartilage growth and development [60]. Based on this, mRNA coding for BMP-2 can potentially have significant applications in treating different bone-related disorders. Two studies have reported that when loaded into transcript activated matrices (TAMs), synthetic mRNA encoding BMP-2 produced a sustained steady-state protein expression for around seven days with a continuous residual production for about ten days following transfection [61], [62]. Consequently, BMP-2-mRNA expression led to osteogenic differentiation in a preosteoblast cell line in vitro and expression of osteogenic markers [61], [62]. Furthermore, bone regeneration has been noticed when tested in a femoral bone defect rat model [62]. A third study confirmed these findings with a more optimized mRNA encoding BMP-2 [63]. This new chemically modified mRNA induced a robust expression of BMP-2 protein in vitro and in primary muscle-derived mesenchymal stem cells [63]. Moreover, angiogenic and osteogenic genes were reported to have been upregulated in the stem cells following transfection with BMP-2 encoded mRNA [63]. When investigated in vivo in a femoral defect rat model, treated animals with BMP-2 mRNA expressed superior bone formation and endochondral osteogenesis compared to the control group [63]. Significant new tissue formation and vascularization were also particularly noted with higher doses [63]. Moreover, improved vascularization was noted in the healing area of BMP-2 mRNA treated animals [63]. Furthermore, engineering bone marrow stem cells with a modified mRNAs encoding BMP-2 plus VEGF-A was shown to synergistically foster osteogenesis and healing in an in vivo skull defect model [64]. Together, these findings illustrate the significant role that might be played via modified mRNA and the potential therapeutics application in impaired bone healing illnesses such as osteoporosis.
Another application of chemically modified mRNA is in the area of osteointegration. Osteointegration is a fundamental process in the attainment of implant integration with the adjacent bone tissue [65], [66]. Biocompatible titanium (Ti) implants have successfully been developed to restore bone defects in orthopedics [67], [68]. Since synthetic mRNA holds a great promise in stimulating bone healing, they have been investigated for the delivery of osteoinductive proteins to promote implant osteointegration. A research team was able to develop Ti implants coated with different biomaterials to deliver mRNA in a controlled release pattern [69]. The best transfection and expression capabilities were noted with fibrinogen and fibrin coatings, with the former being the superior [69]. Remarkably, they dramatically induced the expression of BMP-2 by 24-fold, which had lasted for seven days post-transfection [69]. It has been reported that the expression of BMP-2 promoted osteogenesis in a dose-dependent manner and increased mineralization and alkaline phosphatase (ALP) activity in vitro [69]. With such findings, synthetically modified mRNA could find its way in various applications related to osteointegration including joint replacement surgeries and dental implants.
Chronic tendon disorders are also common musculoskeletal ailments, especially with athletes, and their treatment embraces some challenges for reconstructive surgeons [70], [71]. Among the most affected tendons is the Achilles tendon, which links the calf muscle with the heel bone and is considered the strongest tendon present in the body [70], [71]. Notably, modified mRNA is a safe, encouraging alternative for promoting soft tissue healing. In a study conducted by Herbst et al., treatment with modified mRNA encoding for the basic fibroblast growth factor (bFGF) was explored in the early healing of Achilles tendon defects in rats [72]. The protein expression was evident for three days post-transfection [72]. Treated animals displayed a significant construct stiffness very similar to the healthy contralateral side compared to the control group [72]. Additionally, no side effects such as necrosis or inflammation were seen [72].
A modified mRNA encoding a different protein, bone morphogenetic protein 7 (BMP-7), was also investigated for its promise in treating tendon injuries [73]. Compared to the control group, BMP-7 encoded mRNA treated animals had a considerably greater expression of BMP-7 protein and less collagen type III formation in severely inflamed tendons [73]. Higher concentrations of type III collagen are known to be associated with a decline in the tensile strength and may consequently lead to total tendon rupture [74]. The reduction of type III collagen noted with BMP-7-mRNA supports its efficacy in targeting chronic tendinopathies.
Other chronic musculoskeletal disorders involve those with cartilage wear and tear. Particularly, Osteoarthritis (OA) represents one of the most prevalent chronic degenerative arthritis illnesses. OA is characterized by a breakdown of the cartilage within the joints where the underlying bones may then start to change and deform [75]. The major concern with OA is the unavailability of effective disease modifying agents [75]. Hence, modified mRNA can be a great tool to assist with the treatment of OA. The role of modified mRNA has been demonstrated through the effective delivery of a cartilage-anabolic, runt-related transcription factor 1 (RUNX1) in mice diseased with OA knee joints [76]. The transfection with the synthetic RUNX1-mRNA resulted in suppression of OA progression and amplified expression of RUNX1 and other associated cartilage-anabolic markers in the articular chondrocytes of the knees of the treated animals [76]. These findings represent an evidence that optimized mRNAs can be employed to deliver vital disease-modifying proteins in order to manage various degenerative cartilage diseases.
2.4. Respiratory conditions: asthma and cystic fibrosis
Chronic asthma has a high morbidity rate and can affect both children and adults. In addition, asthmatic patients were found to be highly susceptible to infectious diseases and chronic co-morbidities [77]. Furthermore, most of the medications regularly used to treat asthma are associated with several adverse effects [78], [79]. Utilizing chemically modified mRNA as a gene therapy for asthma could be a more efficient approach than the current ordinary therapies. In a study conducted by Mays et al., a modified FOXP3 mRNA was tested for its potential in enhancing the expression of a regulatory T cell transcription factor, FOXP3, in vivo in murine allergic asthma model [80]. It was possible to prove that this synthetic mRNA can potentiate both preventive and therapeutic responses [80]. Particularly, protective effects against goblet cell metaplasia, airway hyperresponsiveness, and allergen-induced tissue inflammation were demonstrated [80]. These results conferred both before and after the administration of the mRNA, proving its potential use for treating and preventing asthmatic exacerbations [80]. It has also been demonstrated that these effects occurred partly in an IL-10-dependent pathway, and could be reversed through the diminution of IL-10 or by the administration of recombinant IL-17A or IL-23 [80]. Correspondingly, another study supported that synthetic mRNA can protect against airway inflammation by demonstrating its mechanism against Toll-like receptor (TLR) activation in an asthmatic mouse model [81].
Another respiratory disorder that was tackled with chemically modified mRNA is cystic fibrosis. Cystic fibrosis is an inherited disorder characterized principally by damage of the pulmonary epithelium and digestive system [82]. The disease affects vital cells responsible for the production of mucus, digestive juices and sweat [82]. The main physiologic characteristic involves a defective mutation in the gene responsible for cystic fibrosis transmembrane and conductance regulator (CFTR) [82]. A group of gene therapy techniques have been investigated in clinical trials for the treatment of Cystic fibrosis. However, the efficacy of the tested gene therapies remained low [83], [84]. On the other hand, chemically modified mRNA has displayed a significant potential for enhancing CFTR protein expression in the lungs. Bangel-Ruland et al., aimed to investigate the role of a modified CFTR-mRNA in restoring the functional attributes of lung epithelia of cystic fibrosis cells in vitro [85]. The optimized mRNA was able to upregulate CFTR expression, which in turn had reestablished cAMP-induced CFTR currents by a twofold increase [85]. In another study, synthetically modified CFTR mRNA was packaged into LNPs and tested for its potential in treating cystic fibrosis in patient-derived bronchial epithelial cells in vitro [86]. Interestingly, CFTR expression was significantly increased, and its function as a chloride channel was restored [86]. Additionally, when the CFTR-mRNA was investigated in CFTR knockout mice, the same effects were reported and maintained for an average of 14 days with a peak at 55% on the third day post-administration [86]. Together, these findings indicate that mRNA represents a unique approach to correct CFTR impaired function and may potentially serve as a promising treatment in other monogenic disorders.
2.5. Cardiovascular diseases: ischemic heart disease, heart failure, myocardial infarction, and atherosclerotic restenosis
Cardiovascular diseases (CVDs) are considered the leading cause of mortality worldwide, and a main mortality causative [87]. CVDs medications are also known to have persistent drawbacks and endless clinical concerns [87]. Recent studies have suggested the versatile role that synthetically optimized mRNA can play in handling the disease from different angles and promoting prolonged heart repair [88], [89], [90], [91], [92], [93], [94], [95]. Remarkably, recent studies have demonstrated successful delivery, efficient protein expression, and retainment of physiological activities driven through a number of novel chemically modified mRNAs encoding different regulatory proteins that are involved in myocardial regeneration, vascularization, platelet activation, regulation of oxidative stress, hypertrophy, inflammation, and survival [88], [89], [90], [91], [92], [93], [94], [95]. These studies have demonstrated a tremendous preventive and therapeutic potential for managing the regeneration of the heart tissues in different disease conditions including ischemic heart disease [91], [92], myocardial infraction [90], [93], [95], atherosclerotic restenosis [88], [96], heart regeneration [94], and heart failure [89]. The reported activities have been determined through successful protein expression and biological activities of various vital regulatory proteins, which comprised PIP4K2C [89], Pkm2 [90], aYAP [91], VEGF-A [92], [93], [94], IGF1 [93], [95], CD39 [88], and p27Kip1 [96].
Currently, Moderna and AstraZeneca are evaluating the safety and tolerability of epicardial injection of a modified mRNA encoding VEGF-A in patients with impaired systolic function undergoing coronary artery bypass grafting (CABG) surgery (ClinicalTrials.gov: NCT03370887) [97]. The outcomes of this trial are expected to be published by 2023 [97].
Together, these findings highlight the potential therapeutic benefits that can be obtained through the utilization of modified mRNA for different cardiovascular applications. However, since this area remains new, further studies need to be carried out to demonstrate the potential of mRNA in this field by conducting more in vivo studies using experimental animals and then possibly by conducting clinical trials to further evaluate the applicability in treating different CVDs using modified mRNA. One of the challenges involve optimizing the delivery of these molecules through careful consideration of the required doses and pharmacokinetics of various delivery systems, as well as maintaining the heart homeostasis. Ideal delivery routes also have to be explored in order to achieve optimum cardiovascular therapeutic outcomes.
2.6. Liver diseases: chronic liver injury, PFIC3, AAT deficiency, and liver fibrosis
Hepatocyte-growth-factor (HGF) and epidermal-growth-factor (EGF) are known to promote liver tissue regeneration and represent an excellent target for modified mRNA therapy [98]. The function of a modified HGF-mRNA and EGF-mRNA encapsulated in LNPs carrier for hepatocytes regeneration in vivo has been investigated [99]. The administration of both mRNAs promoted transient HGF and EGF functional expression in the liver which was maintained for three days without altering safety [99]. Remarkably, it induced the proliferation of hepatocytes, reversed steatosis of non-alcoholic fatty liver disease, and restored liver function [99]. Furthermore, hepatocytes regeneration was reported to be restored following acute paracetamol-induced liver injury, and Alanine Aminotransferase (ALT) levels were rapidly restored to baseline [99]. Collectively, modified mRNA could be deployed in the management and treatment of chronic and acute liver injuries.
Progressive familial intrahepatic cholestasis type 3 (PFIC3) is an autosomal recessive liver disorder with no existing treatment options other than liver transplantation [100]. The condition is triggered by a loss-of-function mutation of the ABCB4 gene, which encodes phosphatidylcholine transporter (ABCB4/MDR3) [100]. In a recent study, a modified mRNA encoding hABCB4 carried in LNPs was examined for its potential in treating PFIC3 [101]. It has been reported that the targeted delivery of hABCB4-mRNA to the liver resulted in the expression of hABCB4 protein and returned the phospholipid transport in PFIC3 livers [101]. Continuous administration of hABCB4-mRNA doses rescued the phenotype of the disease with dramatic normalization of all relevant clinical parameters, including liver fibrosis, inflammation ductular reaction, along with promoting liver regeneration [101]. These findings support the vital role of modified mRNA in promoting de novo expression of defective proteins and restoring normal physiological function.
Another genetic mutational liver disease arises from alpha 1-antitrypsin (AAT) deficiency in the SERPINA1 gene, leading to liver damage and impaired function [102]. The lack of AAT may also lead to pulmonary emphysema due to the uninhibition of elastolytic activity in the lungs [102]. In a recent 2020 study, AAT-mRNA was developed and tested in cultured human hepatocytes obtained from AAT deficient patients [103]. Remarkably, the expression of AAT protein showed a threefold increase [103]. When tested in AAT deficiency mouse model, systemic administration of the AAT-mRNA-LNPs resulted in an efficient uptake into hepatocytes and de novo expression of functioning AAT [103]. These notions were also supported in another study investigating the role of a modified mRNA encoding SERPINA1 for AAT deficiency treatment, thereby confirming the significant role of modified mRNA in genetic reprogramming and treatment of AAT deficiency [104].
Furthermore, continuous evidence supports the vital role of Angiotensin-converting enzyme-2 (ACE2) in regulating liver and lung fibrosis through inhibiting epithelial cell apoptosis by acting as an antifibrotic epithelial survival factor [105], [106]. The effect of synthetically optimized mRNA was explored for its potential use to treat liver and lung fibrosis. In a study by Schrom et al., ACE2-mRNA promoted the translation of ACE2 protein in lung and liver tissues in vitro and in vivo [107]. There was also an efficient selective uptake of the administered mRNA when administered in different carrier systems [107]. Additionally, targeted liver and lung translation of substantial quantities of ACE2 protein were reported. However, to determine the exact function, further studies need to be carried out to assess the retainment of the functional attributes of ACE2 protein following transcription.
3. Conclusion and future perspectives
Chemically modified mRNAs represent novel and attractive approaches because they are versatile and have broad potential applicability in modern medicine. Through altering the structure, various designs can be adapted to improve the safety, efficacy, delivery, and duration of action. Currently, the technology is being harnessed in the testing of various therapeutic applications in a number of chronic ailments and disorders. Many of them have already successfully moved into testing in clinical trials. FDA approvals of mRNAs for indications like cancer and HIV-1 are nearing, and this technology may soon serve as a great pillar in the field of drug discovery and development.
Despite the availability of enormous safety and efficacy data from clinical and preclinical studies, hurdles with mRNA therapeutics still exist, and efforts must be built to overcome those challenges. For instance, there is a relatively short transient expression of proteins after mRNA transfection and administration; therefore, modified mRNAs with prolonged-expression patterns are warranted especially in certain chronic diseases requiring extended and sustained expression of vital proteins. Targeting the treatment of some pathological conditions that are heterogenous like HIV-1 must be spotlighted without merely focusing on vaccination. Expression kinetics in different disease states should also be explored and optimized for individual diseases. For example, determining whether the protein needs to be constitutively or intermittently expressed. Studies should also determine the dosage or amount of protein required for efficacy without comprimizing the safety. Additionally, further research is necessitated to overcome the instability and storage drawback of synthetically modified mRNA. Moreover, additional studies are also needed to develop advanced drug delivery systems that are capable of optimizing cell targeting. The best delivery route for each condition must also be determined. Since this innovative technology has been recently employed in the clinical setting, a long-term safety profile has to be established, and thorough pharmacovigilance has to be considered.
Funding
This review is part of a project supported by Qatar University (Grant # QUCG-CPH-20/21-4 and QUCG-CPH-21/22-1).
CRediT authorship contribution statement
Dana Elkhalifa: initial draft and figures design & writing and editing, Menatallah Rayan: reviewing the revised the manuscript, Ahmed Negmeldin and Abdelbary Elhissi: reviewing and editing, Ashraf Khalil: reviewing, editing, submission and coordination among the team.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Data availability
No data was used for the research described in the article.
References
- 1.Boczkowski D., Nair S.K., Snyder D., Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 1996;184(2):465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Zhang Z., Luo J., Han X., Wei Y., Wei X. mRNA vaccine: a potential therapeutic strategy. Mol. Cancer. 2021;20(1):1–23. doi: 10.1186/s12943-021-01311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conry R.M., LoBuglio A.F., Wright M., Sumerel L., Pike M.J., Johanning F., et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 1995;55(7) 1397 LP – 1400. [PubMed] [Google Scholar]
- 4.Moderna COVID-19 Vaccine | FDA [Internet]. [cited 2021 Jul 6]. Available from: 〈https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine〉.
- 5.Pfizer-BioNTech COVID-19 Vaccine | FDA [Internet]. [cited 2021 Jul 6]. Available from: 〈https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine〉.
- 6.FDA Approves First COVID-19 Vaccine | FDA [Internet]. [cited 2021 Aug 28]. Available from: 〈https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine〉.
- 7.Pfizer-BioNTech COVID-19 Vaccine COMIRNATY® Receives Full U.S. FDA Approval for Individuals 16 Years and Older | Pfizer [Internet]. [cited 2021 Aug 28]. Available from: 〈https://www.pfizer.com/news/press-release/press-release-detail/pfizer-biontech-covid-19-vaccine-comirnatyr-receives-full〉.
- 8.Kaur K., Zangi L. Modified mRNA as a therapeutic tool for the heart. Cardiovasc. Drugs Ther. 2020;34(6):871–880. doi: 10.1007/s10557-020-07051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck J.D., Reidenbach D., Salomon N., Sahin U., Türeci Ö., Vormehr M., Kranz L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer. 2021;20(1):69. doi: 10.1186/s12943-021-01348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H., Yang Y., Hong W., Huang M., Wu M., Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct. Target Ther. 2020;5(1):1. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12(2):102. doi: 10.3390/pharmaceutics12020102. (A) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics — developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 14.Fuller D.H., Berglund P. Amplifying RNA vaccine development. N. Engl. J. Med. 2020;382(25):2469–2471. doi: 10.1056/NEJMcibr2009737. [DOI] [PubMed] [Google Scholar]
- 15.Strenkowska M., Grzela R., Majewski M., Wnek K., Kowalska J., Lukaszewicz M., Zuberek J., Darzynkiewicz E., Kuhn A.N., Sahin U., Jemielity J. Cap analogs modified with 1,2-dithiodiphosphate moiety protect mRNA from decapping and enhance its translational potential. Nucleic Acids Res. 2016;44(20):9578–9590. doi: 10.1093/nar/gkw896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16(11):1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y., Xu X.-S., Russell J.E. A nucleolin-binding 3′ untranslated region element stabilizes beta-globin mRNA in vivo. Mol. Cell Biol. 2006;26(6):2419–2429. doi: 10.1128/MCB.26.6.2419-2429.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauro V.P., Chappell S.A. A critical analysis of codon optimization in human therapeutics. Trends Mol. Med. 2014;20(11):604–613. doi: 10.1016/j.molmed.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Dalpke A., Helm M. RNA mediated Toll-like receptor stimulation in health and disease. RNA Biol. 2012;9(6):828–842. doi: 10.4161/rna.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freund I., Eigenbrod T., Helm M., Dalpke A.H. RNA modifications modulate activation of innate toll-like receptors. Genes. 2019;10(2):92. doi: 10.3390/genes10020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaidyanathan S., Azizian K.T., Haque A.K.M.A., Henderson J.M., Hendel A., Shore S., Antony J.S., Hogrefe R.I., Kormann M.S.D., Porteus M.H., McCaffrey A.P. Uridine depletion and chemical modification increase Cas9 mRNA activity and reduce immunogenicity without HPLC purification. Mol. Ther. Nucleic Acids. 2018;12:530–542. doi: 10.1016/j.omtn.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avci-Adali M., Behring A., Steinle H., Keller T., Krajeweski S., Schlensak C., Wendel H.P. In vitro synthesis of modified mRNA for induction of protein expression in human cells. J. Vis. Exp. 2014;(93) doi: 10.3791/51943. e51943–e51943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omole A.E., Fakoya A.O.J. Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ. 2018;6 doi: 10.7717/peerj.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J.S., Choi H.W., Choi S., Do J.T. Reprogrammed pluripotent stem cells from somatic cells. Int. J. Stem Cells. 2011;4(1):1–8. doi: 10.15283/ijsc.2011.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanherkar R.R., Bhatia-Dey N., Makarev E., Csoka A.B. Cellular reprogramming for understanding and treating human disease. Front. Cell Dev. Biol. 2014;2:67. doi: 10.3389/fcell.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang X., Yu Q., Huang Y., Song B., Chen Y., Gao X., He W., Sun X., Fan Y. Effects of integrating and non-integrating reprogramming methods on copy number variation and genomic stability of human induced pluripotent stem Cells. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desfarges S., Ciuffi A. In: Viruses Essent Agents Life. Witzany G., editor. 2012. Viral integration and consequences on host gene expression; pp. 147–175. [Google Scholar]
- 29.Dean D.A., Strong D.D., Zimmer W.E. Nuclear entry of nonviral vectors. Gene Ther. 2005;12(11):881–890. doi: 10.1038/sj.gt.3302534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson B.L., McCray P.B. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 2011;12(5):329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaczmarek J.C., Kowalski P.S., Anderson D.G. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9(1):60. doi: 10.1186/s13073-017-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberman J. Tapping the RNA world for therapeutics. Nat. Struct. Mol. Biol. 2018;25(5):357–364. doi: 10.1038/s41594-018-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur S.P., Gupta V. COVID-19 Vaccine: a comprehensive status report. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., Chen X., Hu Y., Liu X., Jiang C., Li J., Yang M., Song Y., Wang X., Gao Q., Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V.A., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F., Allen E.R., Sharpe H., Schulz J., Holbrook M., Okumura A., Meade-White K., Pérez-Pérez L., Edwards N.J., Wright D., Bissett C., Gilbride C., Williamson B.N., Rosenke R., Long D., Ishwarbhai A., Kailath R., Rose L., Morris S., Powers C., Lovaglio J., Hanley P.W., Scott D., Saturday G., de Wit E., Gilbert S.C., Munster V.J. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586(7830):578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., Chadwick D.R., Clark R., Cosgrove C., Galloway J., Goodman A.L., Heer A., Higham A., Iyengar S., Jamal A., Jeanes C., Kalra P.A., Kyriakidou C., McAuley D.F., Meyrick A., Minassian A.M., Minton J., Moore P., Munsoor I., Nicholls H., Osanlou O., Packham J., Pretswell C.H., San Francisco Ramos A., Saralaya D., Sheridan R.P., Smith R., Soiza R.L., Swift P.A., Thomson E.C., Turner J., Viljoen M.E., Albert G., Cho I., Dubovsky F., Glenn G., Rivers J., Robertson A., Smith K., Toback S. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tebas P., Yang S.P., Boyer J.D., Reuschel E.L., Patel A., Christensen-Quick A., et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31:1–9. doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck RW Jr, Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., Clinical Trial G. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moderna Announces Initiation of Rolling Submission of Biologics License Application (BLA) with U.S. FDA for the Moderna COVID-19 Vaccine | Moderna, Inc. [Internet]. [cited 2021 Jul 24]. Available from: 〈https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-initiation-rolling-submission-biologics/〉.
- 42.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koblas T., Leontovyc I., Loukotova S., Saudek F. Reprogramming of human pancreatic organoid cells into insulin-producing β-like cells by small molecules and in vitro transcribed modified mRNA encoding neurogenin 3 transcription factor. Folia Biol. 2019;65(3):109–123. doi: 10.14712/fb2019065030109. [DOI] [PubMed] [Google Scholar]
- 45.Corritore E., Lee Y.-S., Pasquale V., Liberati D., Hsu M.-J., Lombard C.A., Van Der Smissen P., Vetere A., Bonner-Weir S., Piemonti L., Sokal E., Lysy P.A. V-Maf musculoaponeurotic fibrosarcoma oncogene homolog a synthetic modified mRNA drives reprogramming of human pancreatic duct-derived cells into insulin-secreting cells. Stem Cells Transl. Med. 2016;5(11):1525–1537. doi: 10.5966/sctm.2015-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J., Joglekar M.V., Sumer H., Hardikar A.A., Teede H., Verma P.J. Integration-free human induced pluripotent stem cells from type 1 diabetes patient skin fibroblasts show increased abundance of pancreas-specific microRNAs. Cell Med. 2014;7(1):15–24. doi: 10.3727/215517914X681785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okonkwo U.A., DiPietro L.A. Diabetes and wound angiogenesis. Int. J. Mol. Sci. 2017;18(7):1419. doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun N., Ning B., Hansson K.M., Bruce A.C., Seaman S.A., Zhang C., Rikard M., DeRosa C.A., Fraser C.L., Wågberg M., Fritsche-Danielson R., Wikström J., Chien K.R., Lundahl A., Hölttä M., Carlsson L.G., Peirce S.M., Hu S. Modified VEGF-A mRNA induces sustained multifaceted microvascular response and accelerates diabetic wound healing. Sci. Rep. 2018;8(1):17509. doi: 10.1038/s41598-018-35570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gan L.-M., Lagerström-Fermér M., Carlsson L.G., Arfvidsson C., Egnell A.-C., Rudvik A., Kjaer M., Collén A., Thompson J.D., Joyal J., Chialda L., Koernicke T., Fuhr R., Chien K.R., Fritsche-Danielson R. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019;10(1):871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creusot R.J., Chang P., Healey D.G., Tcherepanova I.Y., Nicolette C.A., Fathman C.G. A short pulse of IL-4 delivered by DCs electroporated with modified mRNA can both prevent and treat autoimmune diabetes in NOD mice. Mol. Ther. 2010;18(12):2112–2120. doi: 10.1038/mt.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou X., Cui Y., Han J. Methylmalonic acidemia: current status and research priorities. Intractable Rare Dis. Res. 2018;7(2):73–78. doi: 10.5582/irdr.2018.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An D., Schneller J.L., Frassetto A., Liang S., Zhu X., Park J.S., et al. Erratum: Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2018:2520. doi: 10.1016/j.celrep.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 53.Weide B., Carralot J.-P., Reese A., Scheel B., Eigentler T.K., Hoerr I., Rammensee H.G., Garbe C., Pascolo S. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J. Immunother. 2008;31(2):180–188. doi: 10.1097/CJI.0b013e31815ce501. [DOI] [PubMed] [Google Scholar]
- 54.Amin A., Dudek A.Z., Logan T.F., Lance R.S., Holzbeierlein J.M., Knox J.J., Master V.A., Pal S.K., Miller W.H., Karsh L.I., Tcherepanova I.Y., DeBenedette M.A., Williams W.L., Plessinger D.C., Nicolette C.A., Figlin R.A. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): phase 2 study results. J. Immunother. Cancer. 2015;3:14. doi: 10.1186/s40425-015-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kübler H., Scheel B., Gnad-Vogt U., Miller K., Schultze-Seemann W., Vom Dorp F., Parmiani G., Hampel C., Wedel S., Trojan L., Jocham D., Maurer T., Rippin G., Fotin-Mleczek M., von der Mülbe F., Probst J., Hoerr I., Kallen K.J., Lander T., Stenzl A. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J. Immunother. Cancer. 2015;3:26. doi: 10.1186/s40425-015-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Tendeloo V.F., Van de Velde A., Van Driessche A., Cools N., Anguille S., Ladell K., Gostick E., Vermeulen K., Pieters K., Nijs G., Stein B., Smits E.L., Schroyens W.A., Gadisseur A.P., Vrelust I., Jorens P.G., Goossens H., de Vries I.J., Price D.A., Oji Y., Oka Y., Sugiyama H., Berneman Z.N. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl. Acad. Sci. USA. 2010;107(31):13824–13829. doi: 10.1073/pnas.1008051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sebastian M., von Boehmer L., Zippelius A., Mayer F., Reck M., Atanackovic D., Thomas M., Schneller F., Stoehlmacher J., Goekkurt E., Bernhard H., Groeschel A., Bals R., Schmidt S., Scheel B., Koch S.D., Lander T., Kallen K., Knuth A. Messenger RNA vaccination in NSCLC: findings from a phase I/IIa clinical trial. J. Clin. Oncol. 2011;29(15_suppl):2584. [Google Scholar]
- 58.Mitchell D.A., Batich K.A., Gunn M.D., Huang M.-N., Sanchez-Perez L., Nair S.K., Congdon K.L., Reap E.A., Archer G.E., Desjardins A., Friedman A.H., Friedman H.S., Herndon II J.E., Coan A., McLendon R.E., Reardon D.A., Vredenburgh J.J., Bigner D.D., Sampson J.H. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lesterhuis W.J., De Vries I.J.M., Schreibelt G., Schuurhuis D.H., Aarntzen E.H., Boer A.D., et al. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010;30(12) 5091 LP – 5097. [PubMed] [Google Scholar]
- 60.Katagiri T., Watabe T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016;8(6) doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balmayor E.R., Geiger J.P., Koch C., Aneja M.K., van Griensven M., Rudolph C., Plank C. Modified mRNA for BMP-2 in combination with biomaterials serves as a transcript-activated matrix for effectively inducing osteogenic pathways in stem cells. Stem Cells Dev. 2016;26(1):25–34. doi: 10.1089/scd.2016.0171. [DOI] [PubMed] [Google Scholar]
- 62.Badieyan Z.S., Berezhanskyy T., Utzinger M., Aneja M.K., Emrich D., Erben R., Schüler C., Altpeter P., Ferizi M., Hasenpusch G., Rudolph C., Plank C. Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration. J. Control Release. 2016;239:137–148. doi: 10.1016/j.jconrel.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W., De La Vega R.E., Coenen M.J., Müller S.A., Peniche Silva C.J., Aneja M.K., et al. An improved, chemically modified RNA encoding BMP-2 enhances osteogenesis in vitro and in vivo. Tissue Eng. Part A. 2018;25(1–2):131–144. doi: 10.1089/ten.TEA.2018.0112. [DOI] [PubMed] [Google Scholar]
- 64.Geng Y., Duan H., Xu L., Witman N., Yan B., Yu Z., Wang H., Tan Y., Lin L., Li D., Bai S., Fritsche-Danielson R., Yuan J., Chien K., Wei M., Fu W. BMP-2 and VEGF-A modRNAs in collagen scaffold synergistically drive bone repair through osteogenic and angiogenic pathways. Commun. Biol. 2021;4(1):82. doi: 10.1038/s42003-020-01606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alghamdi H.S. Methods to improve osseointegration of dental implants in low quality (type-IV) bone: an overview. J. Funct. Biomater. 2018:7. doi: 10.3390/jfb9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parithimarkalaignan S., Padmanabhan T.V. Osseointegration: an update. J. Indian Prosthodont. Soc. 2013;13(1):2–6. doi: 10.1007/s13191-013-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saini M., Singh Y., Arora P., Arora V., Jain K. Implant biomaterials: a comprehensive review. World J. Clin. Cases. 2015;3(1):52–57. doi: 10.12998/wjcc.v3.i1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li K., Wang C., Yan J., Zhang Q., Dang B., Wang Z., Yao Y., Lin K., Guo Z., Bi L., Han Y. Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: an in vivo study. Sci. Rep. 2018;8(1):1843. doi: 10.1038/s41598-018-19742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fayed O., van Griensven M., Tahmasebi Birgani Z., Plank C., Balmayor E.R. Transcript-activated coatings on titanium mediate cellular osteogenesis for enhanced osteointegration. Mol. Pharm. 2021;18(3):1121–1137. doi: 10.1021/acs.molpharmaceut.0c01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ackermann P.W., Renström P. Tendinopathy in sport. Sports Health. 2012;4(3):193–201. doi: 10.1177/1941738112440957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu F., Nerlich M., Docheva D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2017;2(7):332–342. doi: 10.1302/2058-5241.2.160075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herbst E., Imhoff F.B., Foehr P., Milz S., Plank C., Rudolph C., et al. Chemically modified messenger RNA: modified RNA application for treatment of achilles tendon defects. Tissue Eng. Part A. 2018;25(1–2):113–120. doi: 10.1089/ten.TEA.2017.0443. [DOI] [PubMed] [Google Scholar]
- 73.Groth K., Berezhanskyy T., Aneja M.K., Geiger J., Schweizer M., Maucksch L., Pasewald T., Brill T., Tigani B., Weber E., Rudolph C., Hasenpusch G. Tendon healing induced by chemically modified MRNAS. Eur. Cells Mater. 2017;33:294–307. doi: 10.22203/eCM.v033a22. [DOI] [PubMed] [Google Scholar]
- 74.Eriksen H.A., Pajala A., Leppilahti J., Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J. Orthop. Res. 2002;20(6):1352–1357. doi: 10.1016/S0736-0266(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 75.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aini H., Itaka K., Fujisawa A., Uchida H., Uchida S., Fukushima S., Kataoka K., Saito T., Chung U.I., Ohba S. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Sci. Rep. 2016;6(1):18743. doi: 10.1038/srep18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dharmage S.C., Perret J.L., Custovic A. Epidemiology of asthma in children and adults. Front. Pedia. 2019;7:246. doi: 10.3389/fped.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leung J.S., Johnson D.W., Sperou A.J., Crotts J., Saude E., Hartling L., Stang A. A systematic review of adverse drug events associated with administration of common asthma medications in children. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0182738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pinto C.R., Almeida N.R., Marques T.S., Yamamura L.L.L., Costa L.A., Souza-Machado A. Local adverse effects associated with the use of inhaled corticosteroids in patients with moderate or severe asthma. J. Bras. Pneumol. 2013;39(4):409–417. doi: 10.1590/S1806-37132013000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mays L.E., Ammon-Treiber S., Mothes B., Alkhaled M., Rottenberger J., Müller-Hermelink E.S., Grimm M., Mezger M., Beer-Hammer S., von Stebut E., Rieber N., Nürnberg B., Schwab M., Handgretinger R., Idzko M., Hartl D., Kormann M.S. Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism. J. Clin. Investig. 2013;123(3):1216–1228. doi: 10.1172/JCI65351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeyer F., Mothes B., Will C., Carevic M., Rottenberger J., Nürnberg B., Hartl D., Handgretinger R., Beer-Hammer S., Kormann M.S.D. mRNA-mediated gene supplementation of toll-like receptors as treatment strategy for asthma in vivo. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0154001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saint-Criq V., Gray M.A. Role of CFTR in epithelial physiology. Cell Mol. Life Sci. 2017;74(1):93–115. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burney T.J., Davies J.C. Gene therapy for the treatment of cystic fibrosis. Appl. Clin. Genet. 2012;5:29–36. doi: 10.2147/TACG.S8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cutting G.R. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat. Rev. Genet. 2015;16(1):45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bangel-Ruland N., Tomczak K., Fernández Fernández E., Leier G., Leciejewski B., Rudolph C., Rosenecker J., Weber W.M. Cystic fibrosis transmembrane conductance regulator-mRNA delivery: a novel alternative for cystic fibrosis gene therapy. J. Gene Med. 2013;15(11–12):414–426. doi: 10.1002/jgm.2748. [DOI] [PubMed] [Google Scholar]
- 86.Robinson E., MacDonald K.D., Slaughter K., McKinney M., Patel S., Sun C., Sahay G. Lipid nanoparticle-delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol. Ther. 2018;26(8):2034–2046. doi: 10.1016/j.ymthe.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roth G.A., Johnson C., Abajobir A., Abd-Allah F., Abera S.F., Abyu G., Ahmed M., Aksut B., Alam T., Alam K., Alla F., Alvis-Guzman N., Amrock S., Ansari H., Ärnlöv J., Asayesh H., Atey T.M., Avila-Burgos L., Awasthi A., Banerjee A., Barac A., Bärnighausen T., Barregard L., Bedi N., Belay Ketema E., Bennett D., Berhe G., Bhutta Z., Bitew S., Carapetis J., Carrero J.J., Malta D.C., Castañeda-Orjuela C.A., Castillo-Rivas J., Catalá-López F., Choi J.Y., Christensen H., Cirillo M., Cooper L., Criqui M., Cundiff D., Damasceno A., Dandona L., Dandona R., Davletov K., Dharmaratne S., Dorairaj P., Dubey M., Ehrenkranz R., El Sayed Zaki M., Faraon E.J.A., Esteghamati A., Farid T., Farvid M., Feigin V., Ding E.L., Fowkes G., Gebrehiwot T., Gillum R., Gold A., Gona P., Gupta R., Habtewold T.D., Hafezi-Nejad N., Hailu T., Hailu G.B., Hankey G., Hassen H.Y., Abate K.H., Havmoeller R., Hay S.I., Horino M., Hotez P.J., Jacobsen K., James S., Javanbakht M., Jeemon P., John D., Jonas J., Kalkonde Y., Karimkhani C., Kasaeian A., Khader Y., Khan A., Khang Y.H., Khera S., Khoja A.T., Khubchandani J., Kim D., Kolte D., Kosen S., Krohn K.J., Kumar G.A., Kwan G.F., Lal D.K., Larsson A., Linn S., Lopez A., Lotufo P.A., El Razek H.M.A., Malekzadeh R., Mazidi M., Meier T., Meles K.G., Mensah G., Meretoja A., Mezgebe H., Miller T., Mirrakhimov E., Mohammed S., Moran A.E., Musa K.I., Narula J., Neal B., Ngalesoni F., Nguyen G., Obermeyer C.M., Owolabi M., Patton G., Pedro J., Qato D., Qorbani M., Rahimi K., Rai R.K., Rawaf S., Ribeiro A., Safiri S., Salomon J.A., Santos I., Santric Milicevic M., Sartorius B., Schutte A., Sepanlou S., Shaikh M.A., Shin M.J., Shishehbor M., Shore H., Silva D.A.S., Sobngwi E., Stranges S., Swaminathan S., Tabarés-Seisdedos R., Tadele Atnafu N., Tesfay F., Thakur J.S., Thrift A., Topor-Madry R., Truelsen T., Tyrovolas S., Ukwaja K.N. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abraham M.-K., Nolte A., Reus R., Behring A., Zengerle D., Avci-Adali M., Hohmann J.D., Peter K., Schlensak C., Wendel H.P., Krajewski S. In vitro study of a novel stent coating using modified CD39 messenger RNA to potentially reduce stent angioplasty-associated complications. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magadum A., Singh N., Kurian A.A., Sharkar M.T.K., Sultana N., Chepurko E., Kaur K., Żak M.M., Hadas Y., Lebeche D., Sahoo S., Hajjar R., Zangi L. Therapeutic delivery of Pip4k2c-modified mRNA attenuates cardiac hypertrophy and fibrosis in the failing heart. Adv. Sci. 2021;8(10) doi: 10.1002/advs.202004661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Magadum A., Singh N., Kurian A.A., Munir I., Mehmood T., Brown K., Sharkar M.T.K., Chepurko E., Sassi Y., Oh J.G., Lee P., Santos C.X.C., Gaziel-Sovran A., Zhang G., Cai C.L., Kho C., Mayr M., Shah A.M., Hajjar R.J., Zangi L. Pkm2 regulates cardiomyocyte cell cycle and promotes cardiac regeneration. Circulation. 2020;141(15):1249–1265. doi: 10.1161/CIRCULATIONAHA.119.043067. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen J., Ma Q., King J.S., Sun Y., Xu B., Zhang X., Zohrabian S., Guo H., Cai W., Li G., Bruno I., Cooke J.P., Wang C., Kontaridis M., Wang D.Z., Luo H., Pu W.T., Lin Z. aYAP modRNA reduces cardiac inflammation and hypertrophy in a murine ischemia-reperfusion model. Life Sci. Alliance. 2019;3(1) doi: 10.26508/lsa.201900424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pehrsson S., Hölttä M., Linhardt G., Danielson R.F., Carlsson L. Rapid production of human VEGF-A following intradermal injection of modified VEGF-A mRNA demonstrated by cutaneous microdialysis in the rabbit and pig in vivo. Biomed. Res. 2019;2019:3915851–3915857. doi: 10.1155/2019/3915851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zangi L., Lui K.O., von Gise A., Ma Q., Ebina W., Ptaszek L.M., Später D., Xu H., Tabebordbar M., Gorbatov R., Sena B., Nahrendorf M., Briscoe D.M., Li R.A., Wagers A.J., Rossi D.J., Pu W.T., Chien K.R. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31(10):898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lui K.O., Zangi L., Silva E.A., Bu L., Sahara M., Li R.A., Mooney D.J., Chien K.R. Driving vascular endothelial cell fate of human multipotent Isl1+ heart progenitors with VEGF modified mRNA. Cell Res. 2013;23(10):1172–1186. doi: 10.1038/cr.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang C.-L., Leblond A.-L., Turner E.C., Kumar A.H., Martin K., Whelan D., O’Sullivan D.M., Caplice N.M. Synthetic chemically modified mRNA-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Mol. Pharm. 2015;12(3):991–996. doi: 10.1021/mp5006239. [DOI] [PubMed] [Google Scholar]
- 96.Lockhart J.H., VanWye J., Banerjee R., Wickline S.A., Pan H., Totary-Jain H. Self-assembled miRNA-switch nanoparticles target denuded regions and prevent restenosis. Mol. Ther. 2021;29(5):1744–1757. doi: 10.1016/j.ymthe.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anttila V., Saraste A., Knuuti J., Jaakkola P., Hedman M., Svedlund S., Lagerström-Fermér M., Kjaer M., Jeppsson A., Gan L.M. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol. Ther. - Methods Clin. Dev. 2020;18:464–472. doi: 10.1016/j.omtm.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoffmann K., Nagel A.J., Tanabe K., Fuchs J., Dehlke K., Ghamarnejad O., Lemekhova A., Mehrabi A. Markers of liver regeneration—the role of growth factors and cytokines: a systematic review. BMC Surg. 2020;20(1):31. doi: 10.1186/s12893-019-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rizvi F., Everton E., Smith A.R., Liu H., Osota E., Beattie M., Tam Y., Pardi N., Weissman D., Gouon-Evans V. Murine liver repair via transient activation of regenerative pathways in hepatocytes using lipid nanoparticle-complexed nucleoside-modified mRNA. Nat. Commun. 2021;12(1):613. doi: 10.1038/s41467-021-20903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davit-Spraul A., Gonzales E., Baussan C., Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J. Rare Dis. 2009;4:1. doi: 10.1186/1750-1172-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei G., Cao J., Huang P., An P., Badlani D., Vaid K.A., Zhao S., Wang D.Q.H., Zhuo J., Yin L., Frassetto A., Markel A., Presnyak V., Gandham S., Hua S., Lukacs C., Finn P.F., Giangrande P.H., Martini P.G.V., Popov Y.V. Synthetic human ABCB4 mRNA therapy rescues severe liver disease phenotype in a BALB/c.Abcb4(-/-) mouse model of PFIC3. J. Hepatol. 2021;74(6):1416–1428. doi: 10.1016/j.jhep.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seixas S., Marques P.I. Known mutations at the cause of alpha-1 antitrypsin deficiency an updated overview of SERPINA1 variation spectrum. Appl. Clin. Genet. 2021;14:173–194. doi: 10.2147/TACG.S257511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karadagi A., Cavedon A.G., Zemack H., Nowak G., Eybye M.E., Zhu X., Guadagnin E., White R.A., Rice L.M., Frassetto A.L., Strom S., Jorns C., Martini P.G.V., Ellis E. Systemic modified messenger RNA for replacement therapy in alpha 1-antitrypsin deficiency. Sci. Rep. 2020;10(1):7052. doi: 10.1038/s41598-020-64017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Connolly B., Isaacs C., Cheng L., Asrani K.H., Subramanian R.R. SERPINA1 mRNA as a treatment for alpha-1 antitrypsin deficiency. J. Nucleic Acids. 2018;2018:8247935–8247937. doi: 10.1155/2018/8247935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shim K.Y., Eom Y.W., Kim M.Y., Kang S.H., Baik S.K. Role of the renin-angiotensin system in hepatic fibrosis and portal hypertension. Korean J. Intern. Med. 2018;33(3):453–461. doi: 10.3904/kjim.2017.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uhal B.D., Li X., Piasecki C.C., Molina-Molina M. Angiotensin signalling in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2012;44(3):465–468. doi: 10.1016/j.biocel.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schrom E., Huber M., Aneja M., Dohmen C., Emrich D., Geiger J., Hasenpusch G., Herrmann-Janson A., Kretzschmann V., Mykhailyk O., Pasewald T., Oak P., Hilgendorff A., Wohlleber D., Hoymann H.G., Schaudien D., Plank C., Rudolph C., Kubisch-Dohmen R. Translation of angiotensin-converting enzyme 2 upon liver- and lung-targeted delivery of optimized chemically modified mRNA. Mol. Ther. Nucleic Acids. 2017;7:350–365. doi: 10.1016/j.omtn.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.