Abstract
Coronavirus disease 2019 (COVID-19) is as an emerging infectious disease (EID) that has caused the worst public health catastrophe of the 21st century thus far. In terms of impact, the COVID-19 pandemic is second only to the Spanish Flu pandemic of 1918 in modern world history. As of 7 September 2021, there have been 220 million confirmed cases of COVID-19 and more than 4.5 million deaths. EIDs pose serious public health and socio-economic risks, and 70% of EIDs originate from wildlife. Preventing development of EIDs such as COVID-19 is a pressing concern. Here, taking the COVID-19 pandemic as an example, we illustrate the disastrous effects of EIDs and assess their emergence and evolution from a One Health perspective. We propose a One Health strategy, centered on ‘moving the gates forward’, for EID prevention and control at the human–animal–environment interface. This strategy may be instructive and provide early warnings of EIDs in the future.
Keywords: COVID-19, Emerging infectious diseases, Zoonotic, One Health, Human–animal–environment interface, Early warning
1. Introduction
By 1918, the Spanish influenza (H1N1) pandemic had infected about 1 billion people worldwide and caused more than 25 million deaths. The 1918 Spanish Flu has been described as one of the ‘the deadliest infectious diseases in the world’.1 Coronavirus disease 2019 (COVID-19), caused by a novel coronavirus called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),2 spread worldwide within weeks of an initial outbreak in Wuhan, China in December 2019. As of 7 September 2021, there have been 220 million confirmed cases of COVID-19 and more than 4.5 million deaths.3 Both the 2018 Spanish Flu and COVID-19 represent examples of deadly EIDs, defined as diseases caused by a newly discovered species or a new pathogenic microorganism.
The history of humankind can be viewed as a struggle against infectious diseases. In the past 20 years, EIDs including H5N1 avian influenza, H7N9 avian influenza, severe acute respiratory syndrome (SARS), Ebola fever, and Middle East respiratory syndrome (MERS) have posed serious threats to human health and global economic development. Considering their potential to cause high morbidity and mortality and their rapid spread, EIDs cannot always be controlled using traditional strategies before novel approaches can be developed. Moreover, the development of drugs and vaccines is a complex, time-consuming, and costly process. Therefore, a key question is how to avoid the next EID pandemic.
By the 1990s, researchers had begun to investigate similarities among EIDs by identifying the origins and emergence patterns of pathogens. Overall, 60% of global EIDs are of zoonotic origin, and 70% originate from wildlife.4 To the best of our knowledge, most EID emergence has been related to anthropogenic activities that place people in increased contact with wildlife carrying numerous previously unfamiliar microbes, promoting pathogen spillover.4, 5, 6 With ongoing globalization, the emergence of EIDs has been exacerbated by increased rates of human travel and global trade.7 Various factors at the human–animal–environment interface jointly promote the emergence of EIDs; in turn, the adverse impacts of EID affect humans, animals, and the environment. Preventing EIDs from occurring cannot be achieved by any one discipline alone; intersectoral, interregional, and interdisciplinary cooperation is needed. This viewpoint is known as the One Health strategy, a new concept to address zoonoses at the human-animal-environment interface.8 A key focus of a One Health strategy for EIDs is establishing interdisciplinary, cross-sectoral, and cross-regional collaborations to provide early warnings of EID outbreaks. Metaphorically speaking, we call this goal ‘moving the gates forward’, and it can be accomplished by focusing on the course of animal-to-human transmission to stop pathogen spillover and development of epidemics.9
Here, we illustrate the disastrous effects of EIDs and analyze their emergence and evolution from a One Health perspective. We propose a One Health strategy centered on moving the gates forward for EID prevention and control.
2. Current global pandemic and disease burden of COVID-19
In early December 2019, the media reported sporadic and clustered cases of a ‘pneumonia of unknown origin’ in the city of Wuhan in Hubei Province, China.10 The pneumonia spread quickly, affecting other countries and regions globally within a short time. On 11 February 2020 the World Health Organization (WHO) named the syndrome COVID-19 and declared this infectious disease a global pandemic.11 At present, COVID-19 is still spreading at an unrelenting pace worldwide (Fig. 1 ). As of 7 September 2021, there had been more than 220 million infections and 4.5 million related deaths, and 30 countries had exceeded 1 million cumulative confirmed cases. The United States, India, and Brazil have recorded the highest numbers of both COVID-19 confirmed cases and deaths. The rapid development of this pandemic has taxed healthcare systems worldwide and also wreaked havoc on the global economy and society, with far-reaching effects on global market trading, job markets, tourism, and transportation.12, 13 The disability adjusted life year (DALY) is an important indicator that combines disease incidence and mortality to quantify the burden of a disease, and can be used to better understand the impact of COVID-19 on health and the economy.14, 15 As of the end of April 2021, the number of DALYs (in thousands) lost because of COVID-19 was estimated at 31 930,16 very close to the burden (in thousands) of 45 000 DALYs lost because of tuberculosis and malaria combined and 4.61-fold the 6340 (thousand) DALYs lost because of upper respiratory diseases.17
Fig. 1.
Global map of coronavirus disease 2019 cases. Figures represent the cumulative number of confirmed cases as of 7 September 2021. The ten countries with the highest numbers of cumulative confirmed cases are labeled.
3. Origins and emergence of COVID-19 and zoonotic links
3.1. Wildlife origins and intermediate hosts of SARS-CoV-2
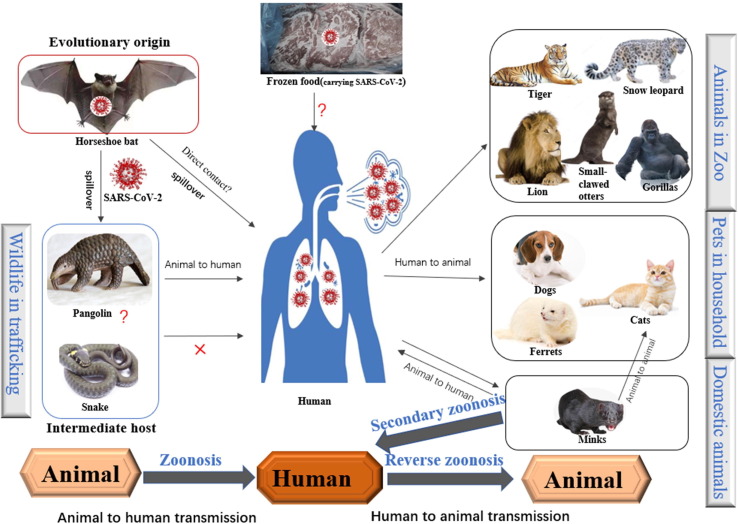
Epidemiological investigations established that the first COVID-19 patient hospitalized in the Central Hospital of Wuhan on 26 December 2019 was a worker at the Wuhan Huanan seafood wholesale market. A wide variety of live wild animals are sold at the market, including hedgehogs, badgers, snakes, and birds (turtledoves), as well as animal carcasses and animal meat.18 In addition, about 49% of early COVID-19 cases had a history of contact with the Huanan seafood market.19 Given these epidemiological findings, the source of the causative agent of COVID-19 was suspected to be related to wild animals at this seafood market. This hypothesis was supported by a report that 33 of 585 environmental samples from the Huanan seafood market tested positive for SARS-CoV-2, and most of these samples were obtained from stalls selling wildlife.20 Moreover, sequence homology of SARS-CoV-2 with SARS-CoV and MERS-CoV (79% and 50%, respectively) suggests the virus probably originated in bats, which acted as the original hosts.21, 22 Whole genome sequencing has shown that SARS-CoV-2 shares 96.2% sequence identity with BatCoV RaTG13 and 88% similarity with two bat-derived SARS-like coronaviruses (bat-SL-COVZC45 and bat-SL-COVZC21), suggesting that bats are the most likely hosts of SARS-CoV-2.23 The Chinese horseshoe bat (genus Rhinolophus, identified in Yunnan province) is believed to be the natural reservoir for SARS-CoV-2 based on genomic and evolutionary analyses.23
It is noteworthy that there was no clear evidence that bats were being sold at the Huanan seafood market and there is a geographic discrepancy between the site where SARS-CoV-2 was proposed to originate (Yunnan) and where the outbreak occurred (Wuhan). Thus, scientists have hypothesized the existence of intermediate hosts promoting spillover to humans from bats. Snakes sold at the market were initially suspected of being intermediate hosts24; however, this hypothesis was disproved on the basis of the composition of snake ACE and its differences from human ACE2.25 Previously, Malayan pangolins were considered likely intermediate hosts because a coronavirus isolated from pangolins (pangolin-CoV) shared 91.02% sequence identity with SARS-CoV-2 and 90.55% identity with BatCoV RaTG13 at the whole genome level.26 Comparative genomics analyses suggested that SARS-CoV-2 might be derived from recombination between a BatCoV RaTG13-like virus and a pangolin CoV-like virus,27 which may have represented the critical step for the virus to infect humans. This hypothesis would also explain why SARS-related coronavirus-specific antibodies are present at higher levels in individuals living in rural locations compared with those living near bat caves.28 Further studies are required to identify the definitive intermediate host(s) and elucidate the exact mechanisms underlying the origin of SARS-CoV-2.
3.2. Frozen foods as a potential source of SARS-CoV-2
Imported frozen foods, especially meat products and animal carcasses contaminated or infected with zoonotic pathogens, are other controversial potential sources of SARS-CoV-2. This hypothesis originated from a report by the WHO stating that wildlife carcasses were left behind in freezers at the Huanan market as frozen food and sold in late December 201929 and that during the pandemic, SARS-CoV-2 has been identified from frozen foods, packaging, and storage surfaces.30 One notable event in China (the shortage of pork caused by the African swine fever virus pandemic)31 facilitated the use of cold-chain transport for a variety of other meats to meet the massive demand for meat products. Frozen meat, especially lower-priced or illegally produced meat, is more likely to carry pathogens.32 Higher rates of pathogen contamination combined with low transport temperatures enables long-term survival of such pathogens, masking considerable infection risks to susceptible persons. Some cases of COVID-19 in China were reported to be linked to imported frozen foods.30 Frozen foods are worth our attention as potential trans-regional sources of SARS-CoV-2 and other pathogens.
3.3. Reverse and secondary zoonosis of SARS-CoV-2
The COVID-19 pandemic among humans has shown reverse zoonotic properties (human-to-animal transmission; Fig. 2 ). Naturally occurring reverse SARS-CoV-2 zoonosis events have been documented in several animal species and in different countries.33 An increasing number of pets, including cats, ferrets, and dogs, live in close contact with humans and have been reported to be infected with COVID-19; clinical manifestations ranging from asymptomatic infection to severe respiratory illness.34, 35, 36, 37 Studies have reported that cats and ferrets appear to be more susceptible to COVID-19 than dogs.38 Fortunately, no strong evidence exists for pet-to-human or sustained pet-to-pet transmission of SARS-CoV-2. In addition to pets, multiple zoo animal species have been observed to suffer from COVID-19, including tigers, lions, gorillas, Asian small-clawed otters, and cougars.39, 40 These animals appear to have been infected by zoo staff members with COVID-19. Reverse zoonotic transmission of SARS-CoV-2 in farmed minks is alarming. Minks are highly susceptible to SARS-CoV-2 infection and have transmitted the virus back to humans as a secondary zoonosis. Although additional investigations are necessary, evidence contained in one report indicated that a SARS-CoV-2 variant in Danish farmed mink could be spread to mink-farm workers.41 Cats near these farms were infected with COVID-19 by minks, providing proof of animal-to-animal transmission. Transmission of SARS-CoV-2 from humans to numerous animals, occasionally from animals to animals, and even from animals to humans, makes it clear that the virus can infect and be transmitted between a wide range of distantly related mammal species. This increases the risk of cross-species transmission on a large scale.
Fig. 2.
Zoonotic links of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The horseshoe bat has been reported as the source of SARS-CoV-2. The intermediate host is unclear, although snakes or pangolins have been suggested to be most likely. Animals that have been associated with reverse zoonoses are shown above. Secondary zoonotic events have been reported at mink farms.
4. Similarities between EIDs occurring in the past and present
4.1. EIDs are a persistent human catastrophe
The history of humankind can be viewed as the history of our struggle against infectious diseases. In the mid to late 14th century, a plague known as the ‘Black Death’ swept across Europe and killed 25 million people, corresponding to approximately one-third of the population at the time.42 In the early 20th century, a process was invented to replace mink skins with otter skins to increase profits. This led to a culling frenzy and transmission of the plague to humans through otters, resulting in the death of nearly 60 000 people in northeastern China.43 In 1918/1919, the Spanish Flu infected about 1 billion people and killed at least 25 million worldwide, far exceeding the death toll of the First World War.1 With gradual improvements in the public health system, significant progress has been made in the prevention and control of infectious diseases. However, the threat to humanity from EIDs is still present: multiple EIDs have emerged worldwide in the last 20 years alone, placing a heavy burden on public health systems and the economy. For example, in 2002/2003, the SARS epidemic caused at least 8096 infections and 774 deaths; the mortality rate was 11% and the global economic cost was over US$30 billion.44 In March 2009, an outbreak of influenza A (H1N1) in Mexico and the United States spread to 214 countries and territories worldwide, infecting 1.3 million people and killing more than 18 000.45 In 2012, an outbreak of MERS occurred in Saudi Arabia with a 39% mortality rate; the virus caused 1401 infections and 543 deaths globally, contributing to anxiety in more than half of the general population of Saudi Arabia at the end of the epidemic.46, 47 In 2014, outbreaks of Ebola occurred several countries across West Africa, killing 11 310 people with mortality rates of 50% to 90%.48 These EIDs demonstrated rapid spread and high mortality, and represented major public health problems endangering human health.
Immunization is one of the most economical and effective means for the prevention and control of infectious diseases. However, owing to the time required and high costs of vaccine development before market, vaccines are inadequate for the prevention of EIDs. For these reasons, we need to focus on how to avoid the occurrence of EIDs and enable early warning of EID outbreaks to improve prevention and control.
4.2. Wildlife is a major source of EIDs
Many uncharacterized viruses are zoonotic and understanding these viruses, particularly those of wild animal origin, is vital in the management of EIDs. Of the 180 RNA viruses recognized as potentially harmful to humans, 89% are zoonotic,49 suggesting that animal-derived viruses play an important role in the occurrence of EIDs. Zoonotic EIDs such as human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), Ebola, Marburg hemorrhagic fever, West Nile fever, Nipah fever, Hendra, human monkeypox, various subtypes of avian influenza, SARS, MERS, and COVID-19 are caused by viral spillover from wildlife to humans. Anthropogenic activities play an important role in this process. Wild apes and rodents are common animal sources of EIDs. A precursor of HIV was identified in monkeys in central Africa and was originally contracted by African people preying on monkeys.50 Cases of monkeypox were reported in the United States in 2003, with the majority of patients having had close contact with imported pet prairie dogs infected by African rodents.51 Bats are the primary natural hosts of SARS-CoV-2 as well as SARS-CoV and MERS-CoV. According to the Global Bat Virus Database, scientists have identified more than 4100 viruses in nearly 200 species of bat, including more than 500 coronaviruses.52 More and more people are eating wild animals to satisfy a demand for high-protein foods. The fruit civet, which is often eaten by humans, and the dromedary, which is the most traded animal in Saudi Arabia, are important intermediate hosts of SARS-CoV and MERS-CoV, respectively.53 Wild waterfowl are natural reservoirs for all types of avian influenza viruses.54 In summary, rodents, primates, and wild birds are important sources of pathogens causing EIDs. These animals carry viruses that have adapted to coexist within their hosts and have low or no pathogenicity, but can spill over into humans or domesticated animals and become highly pathogenic to humans. In recent years, a complex mixture of human activities, ecological damage, and socio-economic factors including illegal hunting, wildlife farming, and illegal trade, have contributed to promotion of direct human–and domestic animal–wildlife interactions, resulting in contact with wildlife-derived EIDs.6
SARS-CoV-2 and other past EIDs have reminded us of the need to pay careful attention to the close links between humans, animals, and the environment and their role in the occurrence and development of EIDs. Wildlife origins are important features of EIDs. Anthropogenic activities and their impact on the environment, including hunting and the wildlife trade, urbanization, ecological tourism, climate change, and habitat destruction, play critical roles in facilitating spillover of emerging pathogens.55 In turn, the emergence of EIDs can have severe impacts on humans, animals, and the environment. The time has arrived for us to combine multisectoral and multidisciplinary efforts to tackle EIDs.
5. A One Health strategy for EIDs
5.1. Animal-based reverse etiology studies and surveillance
Wild animals carry large numbers of microorganisms, which are not only important sources of EIDs but also key targets for interrupting cross-species transmission. Unfortunately, research on specific species of wild animals is lacking, and thus prevention and control efforts for EIDs are currently passive. China is one of the richest countries in the world in terms of wildlife species: the estimated number of undescribed virus species in the country is over 1.2 million.56 Xu Jianguo, an academic at the Chinese Academy of Engineering, proposed the concept of ‘reverse microbial etiology’ research. The aim of this field is to investigate the relationships between animal-derived pathogens and human pathogenesis through wildlife microbial research, to assess the probability that animal-derived pathogens will cause major outbreaks of infectious diseases, and to provide forewarning of potential EID outbreaks.57 Wildlife microbiology research is essential to understand wildlife microbial communities and for the prediction, timely detection, and early warning of potential emerging pathogens. Furthermore, findings on potential emerging pathogens should be shared with experts across different disciplines and sectors to fully assess the risk of cross-species transmission and to assist in providing early warnings. For example, a researcher isolated a potentially pathogenic novel sandy virus, known as the Wenzhou mammarenavirus virus (WENV), from five species of rodents and one species of shrew in Wenzhou, Zhejiang Province, China; a number of patients with respiratory symptoms in Southeast Asian countries subsequently tested positive for the virus.58 Subsequently, it was discovered that the prevalence of WENV in wild rodents was about 1.0%,59 providing a meaningful baseline for this newly zoonotic pathogen. Investigations into potential pathogenic agents carried by domestic animals should also be taken seriously. Pseudorabies virus (PRV) is extremely infectious in pigs, causing catastrophic economic losses in the swine industry; the role of PRV as a potential zoonotic pathogen has been controversial for some time.60 Recently, Chinese scientists isolated porcine PRV from human cerebrospinal fluid for the first time, providing direct and strong evidence of cross-species transmission of PRV to humans.61 More importantly, we need to establish a systematic surveillance system for zoonotic viruses in a wide range of animal species based on microbial etiology research, and establish stable animal surveillance sentinel sites to detect wildlife and domesticated animal epidemics in a timely manner. This will help shift from a reactive response to EIDs to a proactive one to enable ‘moving the gates forward’ in the prevention and control of EIDs.
5.2. A One Health strategy based on animal practitioner populations
Animal workers are those who are involved in animal breeding, slaughtering, processing, and marketing; these individuals have the most frequent contacts with animals. These populations are at high risks of EIDs and are often sentinels for the spillover of emerging pathogens to humans. This elevated risk has been confirmed by multiple studies: pig farm workers have 3.4 times higher risk of H3N2 influenza virus infection than the general population,62 and mink farmers have elevated risks of infection with mink-associated coronavirus variants.63 The composition of the animal workforce in China is large and complex. The National Bureau of Statistics has estimated that the number of individuals working with animals in China is between 50 and 70 million. Therefore, regular monitoring of animal practitioner populations is of significant strategic importance. Fever is a common early symptom of EID and may be the primary indicator for surveillance of animal workers. With the help of intelligent positioning systems, anomalies can be detected and sources of infection can be quickly traced, thus interrupting the spread of EIDs in a timely manner. This strategy involves keeping a close eye on the health status of animal workers and facilitates rapid traceability of pathogens to enable early warning and response in the early stages of an outbreak. These factors are critical stopping EIDs from spreading to larger-scale populations and causing epidemics. We need to establish a stable cohort of animal workers, moving away from the current reactive model based on hospital surveillance to a proactive surveillance model with animal workers as the target population, to improve early warning capabilities and early detection of outbreaks.
5.3. Establishing a One Health early warning information platform
Under the One Health strategy, an early warning information platform should be established to accommodate research and monitoring information arising from the human–animal–environment interface. This platform should involve different sectors and regions and span multiple disciplines. The platform would include information on the distribution and disease surveillance in animals (including wildlife and domestic animals); microorganism baselines and monitoring of microorganism frequencies in animals; population ecology data (including population migration and ecological damage); environment and climate data; disease monitoring in animal workers; and other factors related to the human–animal–environment interface. Above all, this information ought to be shared and integrated by relevant authorities to evaluate disease risk and assess economic benefit using a mathematical modelling approach. This will enable these bodies to provide early warnings regarding EIDs and guide decisions and responses. Formulating appropriate laws and regulations is critical to enable the government to protect this shared information platform and coordinate the interests of various sectors.
6. Conclusion
Avoiding future EIDs is a pressing concern. An important shared property of historical EIDs is their animal origins. The outbreak of COVID-19 has re-emphasized the human–animal interface as an important source of EIDs. This suggests that prevention and control of EIDs requires a shift from traditional thinking to action at the human–animal–environment interface. The novel One Health strategy is described here as a response to the characteristics EIDs; the goals of this strategy are to avoid the emergence of EIDs or to stop their spread early. Using the One Health strategy, we need to establish a cross-sectoral, interdisciplinary, and cross-regional collaborative mechanism, focusing on populations at risk of EIDs and infectious wildlife sources, to carry out active surveillance of animal workers, strengthen ‘reverse microbial etiology’ research in animals, and establish stable and systematic multi-species animal surveillance. From this foundation, a well-established, integrated One Health early warning information platform shared across sectors and countries can take shape. Only in this way can we avoid the recurrence of EIDs and promote the common health of human, animals, and the environment.
Conflict of interest
We claim that there is no conflict of interest associated with the paper entitled “A One Health strategy for emerging infectious disease based on the COVID-19 outbreak”.
Acknowledgment
This work was funded by the the National Key Research and Development Project (2018YFE0208000), funder is Jiahai Lu.
References
- 1.Van Bergen L. Een nieuwe Spaanse vijand [A new Spanish enemy: the Spanish flu in the Netherlands in the period 1918-1920]. Ned Tijdschr Geneeskd. 202013;164:D5224. [Article in Dutch]. [PubMed]
- 2.World Health Organization. Novel Coronavirus 2019. Geneva: WHO; 2019. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Accessed on: September 7, 2021.
- 3.One Health Research Center Emerging Infectious Disease Surveillance. Global new coronary pneumonia. http://covid.eidonehealth.com/templates/world.html Accessed on: September 7, 2021.
- 4.Krause R.M. Dynamics of emergence. J Infect Dis. 1994;170(2):265–271. doi: 10.1093/infdis/170.2.265. [DOI] [PubMed] [Google Scholar]
- 5.Morse S.S. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1(1):7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones K.E., Patel N.G., Levy M.A., et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden N.H., AbdelMalik P., Pulliam J. Emerging infectious diseases: prediction and detection. Can Commun Dis Rep. 2017;43(10):206–211. doi: 10.14745/ccdr.v43i10a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J., Liu L., Wang G., Lu J. One Health in China. Infect Ecol Epidemiol. 2016;6(1):33843. doi: 10.3402/iee.v6.33843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amuasi J.H., Lucas T., Horton R., Winkler A.S. Reconnecting for our future: The Lancet One Health Commission. Lancet. 2020;395(10235):1469–1471. doi: 10.1016/S0140-6736(20)31027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To K.W., Sridhar S., Chiu K.Y., et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg. Microbes Infect. 2021;10(1):507–535. doi: 10.1080/22221751.2021.1898291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., et al. novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyman M., Mark C., et al. Data analytics to evaluate the impact of infectious disease on economy: case study of COVID-19 pandemic. Patterns (N Y) 2021;2(8) doi: 10.1016/j.patter.2021.100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayittey F.K., Ayittey M.K., Chiwero N.B., et al. Economic impacts of Wuhan 2019-nCoV on China and the world. J Med Virol. 2020;92(5):473–475. doi: 10.1002/jmv.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salinas-Escudero G., Toledano-Toledano F., García-Peña C., et al. Disability-adjusted life years for the COVID-19 pandemic in the Mexican Population. Front Public Health. 2021;9:686700. doi: 10.3389/fpubh.2021.686700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John D., Narassima M.S., Menon J., et al. Estimation of the economic burden of COVID-19 using disability-adjusted life years (DALYs) and productivity losses in Kerala, India: a model-based analysis. BMJ Open. 2021;11(8):e049619. doi: 10.1136/bmjopen-2021-049619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan CY, Fann JCY, et al., Estimating global burden of COVID-19 with disability-adjusted life years and value of statistical life metrics. J Formos Med Assoc 2021;120:S106e17. [DOI] [PMC free article] [PubMed]
- 17.GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Nov 10;392(10159):1859-1922. [DOI] [PMC free article] [PubMed]
- 18.Wu F., Zhao S., Yu B., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekpenyong M.E., Edoho M.E., et al. A hybrid computational framework for intelligent inter-continent SARS-CoV-2 sub-strains characterization and prediction. Sci Rep. 2021;11(1):14558. doi: 10.1038/s41598-021-93757-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y., Peng F., et al. The deadly coronaviruses: The, SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2003;2020(109) doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciotti M., Angeletti S., Minieri M., et al. COVID-19 outbreak: an overview. Chemotherapy. 2019;64:215–223. doi: 10.1159/000507423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji W., Wang W., Zhao X., et al. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92(4):433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luan J., Jin X., Lu Y., Zhang L. SARS-CoV-2 spike protein favors ACE2 from Bovidae and Cricetidae. J Med Virol. 2020;92(9):1649–1656. doi: 10.1002/jmv.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020;30(7):1346–1351.e2. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong MC, Javornik Cregeen SJ, et al. Evidence of recombination in coronaviruses implicating pangolin origins of nCoV-2019. bioRxiv [Preprint]. 2020 Feb 13:2020.02.07.939207.
- 28.Wang N., Li S.-Y., et al. Serological evidence of bat SARS-related coronavirus infection in humans, China. Virol Sin. 2018;33:104–107. doi: 10.1007/s12250-018-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO-convened global study of origins of SARS-CoV-2: China Part; www.who.int/publications/i/item/who-convened-global-study-of-origins-ofsars-cov-2-china-part (2021).
- 30.Han J., Zhang X., He S., et al. Can the coronavirus disease be transmittedfrom food? A review of evidence, risks, policies and knowledge gaps. Environ Chem Lett. 2021;19:5–16. doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia W., Hughes J., et al. How one pandemic led to another: Asfv, the disruption contributing to sars-Cov-2 emergence in Wuhan. Preprints. 2021;2021020590 [Google Scholar]
- 32.Lytras S., Xia W., et al. The animal origin of SARS-CoV-2. Science. 2021;373(6558):968–970. doi: 10.1126/science.abh0117. [DOI] [PubMed] [Google Scholar]
- 33.Jo W.K., Oliveira‐Filho E.F., et al. Potential zoonotic sources of SARS-CoV-2 infections. Transbound Emerg Dis. 2021;68(4):1824–1834. doi: 10.1111/tbed.13872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrs V.R., Peiris M., Tam K.W.S., et al. SARS-CoV-2 in quarantined domestic cats from COVID-19 households or close contacts, Hong Kong. China Emerg Infect Dis. 2020;26(12):3071–3074. doi: 10.3201/eid2612.202786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garigliany M., Van-Laere A.S., et al. SARS-CoV-2 natural transmission from human to cat, Belgium, March 2020. Emerg Infect Dis. 2020;26(12):3069–3071. doi: 10.3201/eid2612.202223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gortázar C., Barroso-Arévalo S., Ferreras-Colino E., et al. Natural SARS-CoV-2 infection in Kept Ferrets, Spain. Emerg Infect Dis. 2021;27(7):1994–1996. doi: 10.3201/eid2707.210096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sit T.H.C., Brackman C.J., Ip S.M., et al. Infection of dogs with SARS-CoV-2. Nature. 2020;586(7831):776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dróżdż M., Krzyżek P., Dudek B., et al. Current state of knowledge about role of pets in zoonotic transmission of SARS-CoV-2. Viruses. 2021;13(6):1149. doi: 10.3390/v13061149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goraichuk I.V., Arefiev V., et al. Zoonotic and reverse zoonotic transmissibility of SARS-CoV-2. Virus Res. 2021;302 doi: 10.1016/j.virusres.2021.198473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAloose D., Laverack M., et al. From people to Panthera: natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo. mBio. 2020;11(5):e02220–20. doi: 10.1128/mBio.02220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hossain M.G., Javed A., Akter S., et al. SARS-CoV-2 host diversity: an update of natural infections and experimental evidence. J Microbiol Immunol Infect. 2021;54(2):175–181. doi: 10.1016/j.jmii.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Audoin-Rouzeau F. The black rat (Rattus rattus) and the plague in ancient and medieval western Europe. Bull Soc Pathol Exot. 1999;92(5 Pt 2):422-6. French. [PubMed]
- 43.Zhang Q., Zhang F., et al. Pneumonic plague epidemic in Northeast China in 1910–1911: Dr. Wu Lien-Teh's epidemic preventive system for plague control. Front Med. 2018;12(1):113–115. doi: 10.1007/s11684-018-0613-4. [DOI] [PubMed] [Google Scholar]
- 44.Du L.X. Economic loss, economic burden and economic benefits of health investment in SARS. China Health Economics. 2003;05:2. [Google Scholar]
- 45.World Health Organization. (2009). Situation updates Pandemic (H1N1) [EB/OL], 2009. https://www.who.int/csr/disease/swineflu/updates/en/.
- 46.World Health Organization. (2018). (MERS-CoV) WHO MERS global summary and assessment of risk, August 2018 (WHO/MERS/RA/August18). https://www.who.int/csr/disease/coronavirus_infections/risk-assessment-august-2018.pdf?ua=1.
- 47.AlNajjar NS, Attar LM, et al. (2017). Psychobehavioural responses to the 2014 Middle East respiratory syndrome-novel corona virus (MERS CoV) among adults in two shopping malls in Jeddah, western Saudi Arabia. Eastern Mediterranean Health Journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit, 22(11), 817–823. [DOI] [PubMed]
- 48.World Health Organization. (2016). Ebola virus disease, situation report 10 June 2016. [EB/OL]. http://apps.who.int/ebola/ebola-situation-reports.
- 49.Watsa M. Wildlife disease surveillance focus group. Rigorous wildlife disease surveillance. Science. 2020;369(6500):145–147. doi: 10.1126/science.abc0017. [DOI] [PubMed] [Google Scholar]
- 50.Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1(1):a006841. [DOI] [PMC free article] [PubMed]
- 51.Reed K.D., Melski J.W., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Liu B, et al. DBatVir: the database of bat-associated viruses. Database (Oxford). 2014 Mar 18;2014:bau021. [DOI] [PMC free article] [PubMed]
- 53.Holloway P., Gibson M., et al. Risk factors for Middle East respiratory syndrome coronavirus infection among camel populations, Southern Jordan, 2014–2018. Emerg Infect Dis. 2021;27(9):2301–2311. doi: 10.3201/eid2709.203508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharshov K., Mine J., et al. Characterization and phylodynamics of reassortant H12Nx viruses in Northern Eurasia. Microorganisms. 2019;7(12):643. doi: 10.3390/microorganisms7120643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Institute of Medicine (US) Committee on Emerging Microbial Threats to Health in the 21st Century. Microbial Threats to Health: Emergence, Detection, and Response. Smolinski MS, Hamburg MA, Lederberg J, editors. Washington (DC): National Academies Press (US); 2003. PMID: 25057653 [PubMed]
- 56.Qin T., Xiangdong R., et al. Conducting wildlife microbial research to address emerging infectious diseases in the future. Disease Surveillance. 2021;36(03):209–213. [Google Scholar]
- 57.Xu J. Reverse microbial etiology: A research field for predicting and preventing emerging infectious diseases caused by an unknown microorganism. J Biosaf Biosecur. 2019;1(1):19–21. doi: 10.1016/j.jobb.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li K., Lin X.D., Wang W., et al. Isolation and characterization of a novel arenavirus harbored by Rodents and Shrews in Zhejiang province, China. Virology. 2015;476:37–42. doi: 10.1016/j.virol.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 59.Wu J.Y., Guo C., Xia Y., et al. Genomic characterization of Wenzhou mammarenavirus detected in wild rodents in Guangzhou City. China One Health. 2021;13:100273. doi: 10.1016/j.onehlt.2021.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong G., Lu J., Zhang W., et al. Pseudorabies virus: a neglected zoonotic pathogen in humans? Emerg Microbes Infect. 2019;8(1):150–154. doi: 10.1080/22221751.2018.1563459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Q., Wang X., et al. A novel human acute encephalitis caused by pseudorabies virus variant strain. Clin Infect Dis. 2020:ciaa987. doi: 10.1093/cid/ciaa987. [DOI] [PubMed] [Google Scholar]
- 62.Wu J.Y., Zhu Y.S., Guo C., et al. A comparative study of associated microbiota between pig farm and pig slaughterhouse in Guangdong, China. Curr Microbiol. 2020;77(11):3310–3320. doi: 10.1007/s00284-020-02187-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.European Centre for Disease Prevention and Control . Stockholm, Sweden; ECDC: 2020. Detection of New SARS-CoV-2 Variants Related to Mink. [Google Scholar]