Abstract
Optic nerve health is essential for proper function of the visual system. However, the pathophysiology of certain neurodegenerative disease processes affecting the optic nerve, such as glaucoma, is not fully understood. Recently, it was hypothesized that a lack of proper clearance of neurotoxins contributes to neurodegenerative diseases. The ability to clear metabolic waste is essential for tissue homeostasis in mammals, including humans. While the brain lacks the traditional lymphatic drainage system identified in other anatomical regions, there is growing evidence of a glymphatic system in the central nervous system, which structurally includes the optic nerve. Named to acknowledge the supportive role of astroglial cells, this perivascular fluid drainage system is essential to remove toxic metabolites from the central nervous system. Herein, we review existing literature describing the physiology and dysfunction of the glymphatic system specifically as it relates to the optic nerve. We summarize key imaging studies demonstrating the existence of a glymphatic system in the optic nerves of wild-type rodents, aquaporin 4-null rodents, and humans; glymphatic imaging studies in diseases where the optic nerve is impaired; and current evidence regarding pharmacological and lifestyle interventions that may help promote glymphatic function to improve optic nerve health. We conclude by highlighting future research directions that could be applied to improve imaging detection and guide therapeutic interventions for diseases affecting the optic nerve.
Key Words: aquaporin-4, cerebrospinal fluid, glaucoma, glymphatic system, hydrocephalus, imaging, metabolic waste clearance, optic nerve, perivascular, pharmacological and lifestyle interventions
Introduction
Waste clearance is essential for the maintenance of tissue homeostasis in mammals, including humans. This physiological function is largely facilitated by the lymphatic system which also controls extracellular fluid volume and plays an essential role in immune response modulation (Margaris and Black, 2012). However, this conventional lymphatic network has not been identified within the central nervous system (CNS) (Jessen et al., 2015), and it was previously hypothesized that individual cells possess their own capacity for the processing of metabolic byproducts. Recently, a waste clearance system called the glymphatic system was discovered in the brain (Iliff et al., 2012). This system comprises a macroscopic extracellular network involving the flow of cerebrospinal fluid (CSF) through the subarachnoid space (SAS) into the brain parenchyma. Entering along para-arterial spaces, CSF drives the clearance of interstitial fluid (ISF) from the brain towards perivenous drainage pathways (Iliff et al., 2012). The entrance of CSF into the ISF space is facilitated by the polarized expression of aquaporin-4 (AQP4) water channels in the endfeet of astrocytes surrounding arterial vessels (Mestre et al., 2018). Regular pulsation of systolic and diastolic blood pressure during physiologic arterial pulsation can drive the CSF-ISF fluid exchange (Iliff et al., 2013). In addition to normal cardiovascular rhythms, other factors that can impact these fluid dynamics and waste clearance include intracranial pressure (ICP), hydrostatic pressure, sleep and wakeful states, and standing versus horizontal posture (Iliff et al., 2013; Xie et al., 2013; Lee et al., 2015; Gakuba et al., 2018; Benveniste et al., 2019).
As the eye is an extension of the diencephalon in the CNS, the optic nerve and the retina bear resemblances to the brain from embryology to physiology in terms of high-rate metabolism, confinement within limited space, fluid homeostasis, fluid pressure ranges and immune privilege. In particular, the eye also lacks the classical lymphatic vessels for metabolic waste clearance. Therefore, researchers have been searching for glymphatic-like components in the eye and optic nerve that may facilitate paravascular clearance similar to the CNS (Hu et al., 2016; Mathieu et al., 2017; Wostyn et al., 2017; Jacobsen et al., 2019; Wang et al., 2020) (Figure 1). Along the optic nerve, the surrounding SAS contains an intricate system of arachnoid trabeculae and septae that are distinct architecturally between the bulbar segment, mid-orbital segment, and canalicular portion, which may play important roles in CSF dynamics (Figure 2) (Killer et al., 2003).
Figure 1.
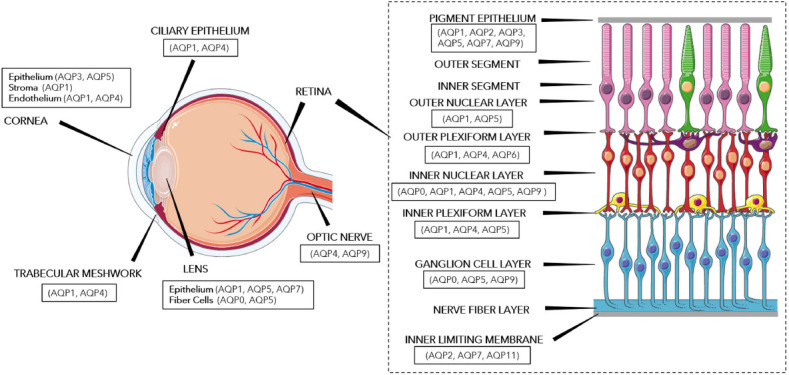
Aquaporin distribution in ocular tissues.
This diagram portrays the aquaporins expressed in various ocular tissues, including the cornea, ciliary epithelium, trabecular meshwork, lens, optic nerve, and the various layers of the retina. Aquaporin distribution data was adapted from Schey et al. (2014) and images of the eye and the retina were modified from SMART (Servier Medical Art), licensed under a Creative Common Attribution 3.0 Generic License. http://smart.servier.com/. AQP: Aquaporin.
Figure 2.
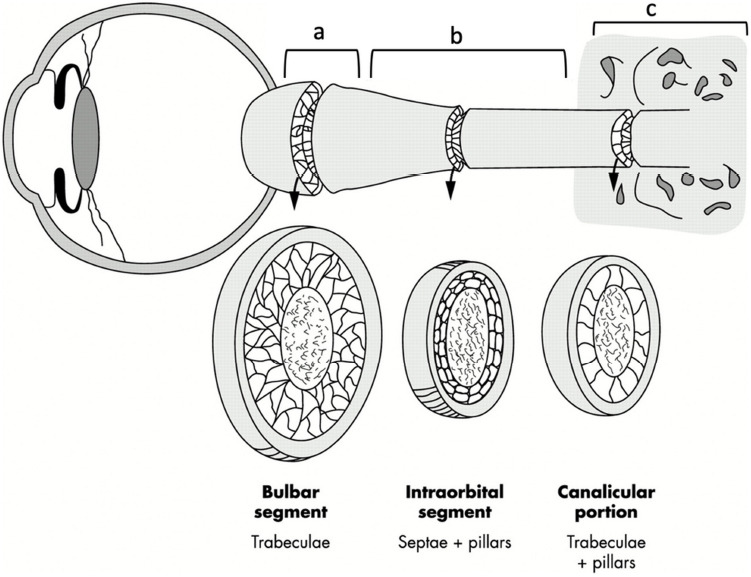
Anatomy of optic nerve subarachnoid space.
Schematic drawing of the optic nerve demonstrating the location of the (a) bulbar segment, (b) mid-orbital segment, and (c) canalicular portion. The bulbar segment (a) and the mid-orbital segment (b) together form the orbital portion. The SAS in the bulbar segment is composed of trabeculae, the SAS in the mid-orbital segment mainly comprises the septae and pillars, and the SAS in the canalicular portion contains both trabeculae and pillars. Reproduced with permission from Killer et al. (2003). SAS: Subarachnoid space.
Within the optic nerve, several studies have indicated the presence of CSF-ISF exchange, yet it has not been fully elucidated whether this reflects a glymphatic pathway in the optic nerve itself or an interconnection between the glymphatic pathway of the brain and SAS of the optic nerve (Wostyn et al., 2015). The retina may also possess an apparent glymphatic system with fluid entering the retina through the internal limiting membrane and plexus, after which it is absorbed via the plexus and Müller cells, thus tightly controlling retinal fluid homeostasis (Petzold, 2016). Aquaporin expressions have also been found in various ocular tissues, including the retina and optic nerve, as pictured in Figure 1. It is important to note that AQP4 expression in the retina is heterogeneous, localized only within the inner plexiform, inner nuclear, and outer plexiform layers (Schey et al., 2014). Given the possible presence of the glymphatic system in the brain, optic nerve, and retina, clearance of toxic solutes from these regions may be relevant to the pathogenesis of age-related, amyloidogenic diseases of the brain and eye, such as Alzheimer's disease (AD), glaucoma, and age-related macular degeneration (Wostyn et al., 2016).
With burgeoning evidence for the existence of a CNS glymphatic system and its involvement in various disease processes, imaging the glymphatic system is of importance to explore the role of the glymphatic system in neurological and ophthalmic pathology, such as glaucoma, hydrocephalus, and neuromyelitis optica spectrum disease (NMOSD). Several animal models are currently employed to investigate the CNS glymphatic system using gadolinium-enhanced magnetic resonance imaging (MRI) as well as fluorescence-based imaging, whereas human glymphatic imaging via gadolinium-enhanced MRI and other non-invasive measures is also emerging (Hu et al., 2016; Wostyn et al., 2017; Jacobsen et al., 2019). Herein, we review pertinent literature regarding the imaging of glymphatic system in normal and impaired optic nerves with an overview of methods for glymphatic modulation that may be applied to the visual system using pharmacological interventions and lifestyle/behavioral interventions.
Search Strategy and Selection Criteria
We used PubMed and Google Scholar to search for articles published from 1998 to 2021 with keywords aquaporin, cerebrospinal fluid, glymphatic, or optic nerve.
Glymphatic Imaging of the Optic Nerve
Recent studies have employed various imaging modalities to suggest the existence of a glymphatic system in the visual pathways of wild-type rodents, AQP4-null rodents, and humans. This glymphatic system via the optic nerve has been explored both ex vivo (Figure 3) and in vivo (Figure 4).
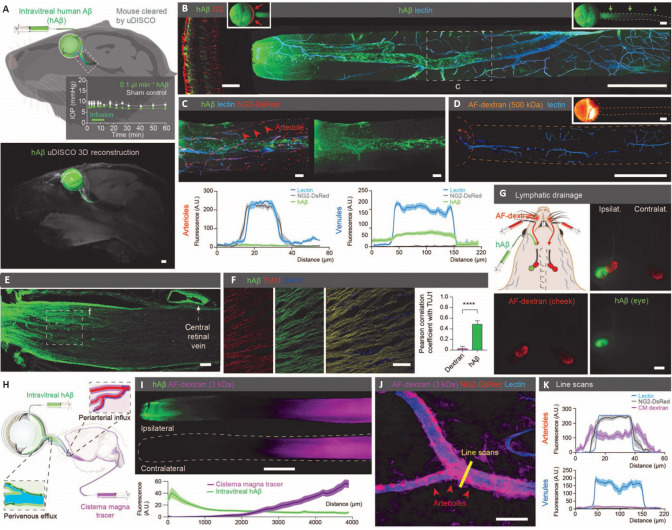
Figure 3.
Ex vivo imaging of the glymphatic system in the optic nerve.
(A) The top image visualizes the intravitreal injection of human amyloid beta (hAß); the graph displays the mouse intraocular pressure (IOP) stabilizing during injection; the bottom image displays the uDISCO-cleared transparent mouse heads 1 hour after hAβ injection, showing that hAβ tracer exited the eye along the optic nerve. (B) These confocal images of ipsilateral retina (left) and optic nerve (right) after intravitreal hAβ injection confirmed that hAβ tracer is transported anterogradely along the nerve. (C) Confocal images from reporter mouse with DsRed-tagged mural cells 30 minutes after intravitreal hAβ injection showed that hAβ tracer preferentially accumulated in the perivascular space along the optic nerve veins. (D) Confocal image of mouse optic nerve 30 minutes after intravitreal Alexa Fluor-dextran injection showed no entrance of this tracer into the nerve. (E, F) Confocal images of optic nerve co-labeling with TUJ1 after tracer administration. Note tracer accumulation in the dural lining of the nerve. (G) The cervical lymph nodes are exhibiting intense hAβ labeling 3 hours after injection. (H) Schematic of double injections of hAβ intravitreally and fluorescent tracer intercisternally. (I) Representative image and quantification of the double injections described in (H), which highlights that tracers transported within the optic nerve in both anterograde and retrograde directions. (J) Confocal images of the optic nerve from reporter mouse with DsRed-tagged mural cells (vascular smooth muscle cells and pericytes) after intracisternal dextran injection with line scan quantified in (K). (J) and (K) show that the tracer injected intercisternally predominantly transported along the periarterial and pericapillary spaces. Reproduced with permission from Wang et al. (2020). AF: Alexa Fluor; DAPI: 4′,6-diamidino-2-phenylindole; DsRed: Discosoma sp. red fluorescent protein; GS: glutamine synthetase; hAß: human amyloid beta; IOP: intraocular pressure; NG2: neuron-glial antigen 2; TUJ1: neuron-specific class III beta-tubulin; uDISCO: ultimate 3D imaging of solvent-cleared organs.
Figure 4.
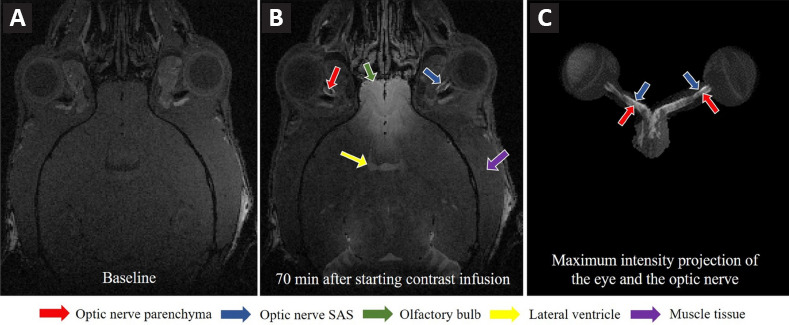
In vivo gadolinium-MRI of the cerebrospinal fluid dynamics in the mouse optic nerve.
3D dynamic contrast-enhanced MRI at the level of the eye and the optic nerve before (A) and at 70 minutes (B) after starting gadolinium contrast infusion into the subarachnoid space of the lumbar spine. Arrows indicate the corresponding brain and muscle regions of interest. Maximum intensity projection after image segmentation of the eyes and the optic nerves in (C). Note the apparent signal enhancement not only in the optic nerve SAS but also the optic nerve parenchyma. Reproduced with permission from Faiq et al. (2020). SAS: Subarachnoid space.
Normal optic nerve
Wild type rodents
Early studies identified the distinct polarization of AQP4 expression and the translated effects on water redistribution in both the Müller cells of the retina and the fibrous astrocytes of the optic nerve (Nagelhus et al., 1998). More recent studies further supported the significance of AQP4 in both the retina and optic nerve, as increases in intraocular pressure (IOP) were found to affect AQP4 activity in both regions (Gan et al., 2012). However, the first study to provide evidence of a glymphatic pathway in the optic nerves of rodents was not until 2017. Fluorescent dextran tracers of differing sizes were injected into the CSF of adult mice, and one hour later, all tracer sizes were detected in the SAS surrounding the orbital optic nerve (Mathieu et al., 2017). All of the smaller tracers, 10 kDA and 40 kDA in size, were found in the optic nerve, while the largest tracer of size 500 kDa was not found within the optic nerve. This suggests that CSF entry into paravascular spaces of the optic nerve is molecular size-dependent. Recent in vivo MRI studies have further elucidated the existence of this glymphatic pathway in rodent optic nerves by using gadolinium-diethylenetriamine pentaacetic acid contrast to characterize the spatiotemporal CSF dynamics in and around the optic nerve (Faiq et al., 2020). This study demonstrated signal enhancement not only in the optic nerve SAS but also the optic nerve parenchyma, further supporting the evidence of the presence of a glymphatic system in the optic nerve. Using a different administrative route via intravitreal amyloid beta (Aβ) injection, confocal imaging of the healthy mouse optic nerves showed that Aβ exited the eye along the optic nerve (Wang et al., 2020). Interestingly, this Aβ clearance from the intraocular space was found to be driven by light-induced pupil constriction, which may reflect constriction of the ciliary body enhancing fluid dispersion along the optic nerve.
AQP4-null rodents
If a glymphatic system of the optic nerve facilitated by AQP4 water channels does exist, then it could be assumed that AQP4 deletion would largely impact clearance of waste products from the retina, optic nerve, and the brain. A study in 2020 found that AQP4-null mice experienced significantly reduced retinal penetration and clearance of Aß along the optic nerve (Wang et al., 2020). AQP4 deletion also enhanced the expression of pro-inflammatory cytokines and exacerbated retinopathic phenotype in diabetic rats (Cui et al., 2012). Such inflammatory effects of AQP4 deletion may be equally as prevalent in healthy rodents, though this premise awaits experimental evidence.
Researchers have also explored the relationship between AQP4 deletion and IOP. A study comparing wild-type mice with mice lacking AQP1 and/or AQP4 found that AQP1/4-null mice had significantly lower IOP and aqueous fluid production (Zhang et al., 2002). This effect may presumably be due to the AQP4 expression in the ciliary bodies that produce aqueous humor, and loss of AQP4 might lead to less aqueous production with consequent fall in IOP. However, in this study, AQP deletion did not significantly impact outflow, volume, or compliance. In contrast, when both wild-type and AQP4-null mice were exposed to 3 days of IOP elevation via microbead injection into the anterior chamber to obstruct the aqueous outflow through the trabecular meshwork, it was found that baseline IOP and degree of IOP elevation in AQP4-null mice were not significantly different from those of wild-type mice (Kimball et al., 2020). This raises the question of how glymphatic clearance and IOP are related, especially in the pathogenesis of glaucoma. Future studies are needed to elucidate how inhibition or facilitation of glymphatic clearance may affect IOP and subsequent diseases of the optic nerve.
Humans
While studying human, non-human primate, rat, and mouse retinas, researchers found that an AQP4-positive glial network forms a sheath around the retinal vascular system, presumably constituting the glymphatic system of the optic nerve (Hu et al., 2016). Similarly, a post-mortem study found paravascular spaces in the human optic nerve, further suggesting the presence of a glymphatic system in the visual pathway (Wostyn et al., 2017). The authors hypothesized that glaucoma develops from restriction of normal glymphatic clearance at the lamina cribrosa, in combination with a low ICP. Further studies, however, must be done to test this hypothesis. Likewise, when subjects underwent intrathecal gadolinium-enhanced MRI, researchers found CSF contrast signal enhancement within the optic nerve, optic chiasm, optic tract, and primary visual cortex (Jacobsen et al., 2019). Given the extravascular entry of the CSF tracers, this further supports the evidence of a paravascular waste clearance system of the human optic nerve.
Impaired optic nerve
There is much about the relationship between AQP4 and the impaired optic nerve that remains to be elucidated. These questions range from debris clearance, toxin removal, nutrient supply, IOP/ICP maintenance, to disease etiology and pathogenesis. For example, in a rat model of optic nerve crush injury, AQP4 was downregulated, and it is unclear whether this AQP4 inhibition was protective for or detrimental to the survival of retinal ganglion cells (RGC) (Suzuki et al., 2014). Investigations aimed at understanding this phenomenon have important implications in diseases where the optic nerve is impaired, like glaucoma, hydrocephalus, NMOSD, retinopathies, or other optic neuropathies.
Glaucoma
Imaging the optic nerve in glaucomatous rodents has begun to identify and elucidate vision-related maladies involving glymphatic clearance. For example, IOP elevation in rat eyes has been reported to be associated with decreased AQP4 expression in the retina, but increased expression in the optic nerve head (ONH) (Dibas et al., 2008). The authors hypothesized that increased AQP4 levels in the ONH promoted glial activation and upregulation of water channels, thus possibly leading to astrocyte hypertrophy in glaucoma. Likewise, in response to IOP elevation, the ONH astrocyte process orientation was significantly altered, suggesting that ONH astrocyte process orientation may be a good indicator of early axonal injury in glaucoma (Tehrani et al., 2014). Interestingly, AQP4 labelling was not found to be a good indicator of astrocyte processes in early glaucoma, likely due to the specific localization of AQP4 in astrocyte endfeet. However, in severe glaucoma, actin bundles were located parallel to areas of high AQP4 expression, suggesting greater involvement of AQP4 in increased severity of glaucoma progression.
There is also initial evidence that CSF entry into the optic nerve is impaired in glaucomatous mice (Mathieu et al., 2018). When fluorescent dextran tracer was injected into the CSF space of mice, the tracer signal in the optic nerve was significantly lower in the glaucomatous mice than in age-matched controls. There are several possible explanations, such as increased resistance to ocular CSF flow resulting from elevated IOP or a global disruption of CSF flow in glaucomatous mice. Since AQP4 is expressed in the fourth and lateral ventricle choroid plexus, it can be hypothesized that reduced production of CSF can also give rise to decreased tracer signal though direct evidence of this phenomenon is lacking (Speake et al., 2003). If this is true, then lowering of ICP and therefore alteration of translaminar pressure difference may imbibe further support as one of the hypotheses for glaucoma. Additionally, approximately 80% of aged glaucomatous mice, lacking CSF tracer at the glial lamina, had decreased RGC axonal phosphorylated neurofilament staining of the laminar optic nerves (Mathieu et al., 2018). This suggests a potential relationship between CSF flow and axon pathology in glaucoma, though aging may have also contributed to these findings.
A recent study further supported the disruption of ocular glymphatic flow in glaucomatous mice. Aβ tracer was injected into the vitreous body of mice and while the tracer was found to have an intra-axonal distribution in controls, the tracer was located mostly outside RGC axons in glaucomatous mice (Wang et al., 2020). This suggests that glaucomatous defects in the glial lamina barrier diverted flow of ocular fluid from the axonal to the extracellular area. However, since rodents and primates have different morphology at the optic nerve head including presence of laminar cribrosa in primate but only glial lamina in rodents, further research is needed to confirm the generalizability of the above findings across species.
Recent studies have also imaged CSF exchange in human glaucoma. In a study, patients with normal-tension glaucoma (NTG) (Innes et al., 2018) underwent computed tomography cisternography of the brain and orbits (Killer et al., 2012). In comparison to the control patients who did not have NTG or any other form of glaucoma, NTG patients presented significant differences between the density of contrast-loaded CSF in intracranial spaces and in the SAS of the optic nerves. This suggests that NTG is associated with disturbed CSF dynamics due to optic nerve compartmentation. Another study found a statistically significant reduction in CSF flow towards the retrobulbar segment in NTG patients, as compared to no reduction in CSF flow in the orbital segments of healthy controls (Pircher et al., 2018). Taken together, these studies indicated that impaired CSF dynamics in the optic nerve may contribute to NTG.
More recent studies have proposed potential distinctions between NTG and high-tension glaucoma (Wostyn et al., 2015; Wostyn, 2020). Given that NTG patients tend to have lower ICPs than patients with high-tension glaucoma, this may suggest that changes in CSF dynamics are more pronounced in NTG than in high-tension glaucoma. Furthermore, given that the glymphatic system of the optic nerve is likely continuous with the glymphatic system of the brain, NTG may result from the glymphatic dysfunction in the brain, while high-tension glaucoma may result from ocular glymphatic dysfunction. This distinction of glymphatic dysfunction between the different types of glaucoma calls for further exploration.
Hydrocephalus
Idiopathic normal pressure hydrocephalus (iNPH), a common form of hydrocephalus, is characterized by increased resistance to CSF outflow and unstable ICP in the brain, with the most effective treatment being a CSF shunt (Williams and Malm, 2016). Given the abnormal state of CSF dynamics in this disease, imaging the glymphatic system of iNPH patients can reveal how CSF flow is disrupted, alluding to the development and progression of this condition. MRI studies of iNPH patients have demonstrated significantly decreased clearance of gadobutrol and delayed enhancement at the Sylvian fissure (Ringstad et al., 2017). A follow-up study indicated that delayed clearance of CSF tracer, specifically at the entorhinal cortex, may lead to neurodegeneration and eventually dementia in iNPH patients (Eide and Ringstad, 2019). Interestingly, patients with iNPH also experienced delayed CSF tracer clearance in the optic chiasm, optic tract, prechiasmatic optic nerve, and primary visual cortex (Jacobsen et al., 2020). Reduced tracer clearance in these regions suggests decreased removal of metabolic waste molecules from the visual pathway, which might in turn implicate the increased incidence of glaucoma in iNPH patients (Jacobsen et al., 2020). However, further exploration is needed to clarify the relationship between iNPH and the visual system.
Although these imaging studies have demonstrated that glymphatic clearance is affected in iNPH, current literature remains inconclusive as to whether AQP4 expression changes in hydrocephalus. The relationship between AQP4 expression and hydrocephalus may not be a simple, direct association, but rather a complex and severity-specific phenomenon. For instance, AQP4 was not found to be upregulated in rats with mild hydrocephalus (Aghayev et al., 2012), yet there was indeed significant upregulation of AQP4 in rats with severe hydrocephalus (Mao et al., 2006). Moreover, in rats with kaolin-induced hydrocephalus, AQP4 expression in the periventricular region was significantly dependent on the severity and duration of hydrocephalus (Skjolding et al., 2010). Thus, it may be worthwhile for future studies to investigate the nature of AQP4 function, its polarization, and its change in expression in iNPH patients, and how these characteristics correlate to biomarkers of neurodegeneration.
Interestingly, patients with iNPH have a much higher risk of developing NTG, which likely relates back to the finding of impaired glymphatic function in the visual pathway of iNPH patients (Jacobsen et al., 2020). In fact, patients whose ICP have been lowered through shunt treatment for iNPH were found to be almost 40 times more likely to suffer from NTG than patients without hydrocephalus (Gallina et al., 2018). This observation supports the trans-lamina cribrosa gradient hypothesis, in which the pressure gradient increases due to high IOP or low ICP or both, thus leading to optic nerve damage and optic nerve head cupping. The intricate relationship between ICP and IOP affirms that further imaging studies of the glymphatic systems of the brain and optic nerve are necessary to clarify this mechanism.
Neuromyelitis optica spectrum disease
NMOSD occurs when anti-AQP4 antibodies (NMO-immunoglobulin G; NMO-IgG) enter the brain through blood-brain barrier (BBB)-deficient sites, thus gaining access to the brain parenchyma, inducing deposition of IgG, and precipitating lesions with loss of AQP4 and upregulation of glial fibrillary acidic protein (GFAP) (Mørch et al., 2018). There is currently no treatment for NMOSD, and it can only be ameliorated by immunosuppressants. Moreover, it is unclear whether and how NMO-IgG flows in the CSF to the brain parenchyma. AQP4 channels may play a role in the transcellular transport of this antibody given its approximate molecular weight of 150 kDa, however there is currently insufficient evidence supporting this hypothesis and more experiments are necessary for confirmation. Changes in cerebral arterial pulsatility may impact paravascular CSF flow into the brain parenchyma thus leading to the deposition of toxic solutes (Iliff et al., 2013). Given that NMOSD is associated with primary damage to both the optic nerves and retinal cells (Zeka et al., 2016), future studies should aim to clarify the existence of the glymphatic system and its relevance to NMO-IgG deposition and lesions.
Glymphatic Modulation of the Optic Nerve
The role of the glymphatic system in the pathogenesis of neurodegenerative diseases continues to be elucidated as recent studies have explored potential ways to alter AQP4 activity, through pharmacological interventions, such as AQP4 inhibitory and facilitatory agents (Additional Table 1) and gamma aminobutyric acid (GABA) agonists (Additional Table 2); dietary supplements, such as omega-3 polyunsaturated fatty acids (PUFAs) and vitamin B3 (Additional Table 3) and flavonoids (Additional Table 4); and complementary integrative approaches, such as physical exercise (Additional Table 5), intermittent fasting, and sleep (Additional Table 6). As the optic nerve is an extension of the diencephalon of the CNS, these approaches that act on the CNS may be translated to explore glymphatic modulation of the optic nerve in future studies.
Additional Table 1.
Summary of inhibitory and facilitating agents on AQP4 activity and neurological effects
| Inhibitor/Facilitator | Compounds of interest | Exp. Type | Model/Species | Dosage and Admin. Route | Exp. Protocols | Main findings | Citation |
|---|---|---|---|---|---|---|---|
| Inhibitor | AER-270 | in vivo | C57BL/6J mice | 0.8 mg/kg in 6 mL water IP | - | ↓ Cerebral edema by 2.5× ↑ Survival by 3.3× in water intoxication models |
Farr et al. (2019) |
| AER-271 | in vivo | C57BL/6J mice | 10 mg/kg in 0.2 mL Tris IP | - | ↓ Cerebral edema by 2.6× ↑ Neurological outcomes in ischemic stroke models |
||
| in vivo | Sprague- Dawley rats | various dosages, IV and EJV | 48 h infusion 30 min post- injection | ° Minimum effective dosage to reduce cerebral edema is 4 mg/kg loading with 0.03 mg/kg/h infusion | |||
| in vivo | Sprague- Dawley rats | 5 mg/kg IP with 0.08 mg/kg/h SQ | 24 h infusion | ↓ Cerebral edema, neuronal death, and neuroinflammation 3 h post-cardiac arrest | Wallisch et al. (2019) | ||
| AAZ | in vivo | Wistar/ST rats | 10 mg/kg IP | Injection 3 or 12 h post- TBI/cerebral edema | ↑ Survival rate from 53% to 75% in 3 h post-TBI group but no significant decrease in cerebral edema ↓ Cerebral edema with 100% survival in 12 h post- TBI group |
Katada et al. (2012) | |
| in vivo | C57BL/6J mice | 15 mg/kg in DMSO IP | Injection 30 min post-TBI | ° Limited AQP4 reorganization ° No significant effect on astrocyte activation ↓ Post-TBI cerebral edema |
Glober et al. (2019) | ||
| in vitro | Sprague- Dawley rat astrocytes, Human astrocytes | 0.2 mg/mL in DMSO | - | ° AAZ prevented AQP4 aggregation and redistribution post-OGD | |||
| in vitro | C8D1A astrocyte mTBI model | 20 μM | Added 15 min before injury | ↑ Viability of astrocytic cells post-TBI with AAZ pre-treatment | Sturdivant et al. (2016) | ||
| AAZ, MZA | in vitro rat AQP4, Human AQP1 | Recombinant | - | - |
° AAZ: ↓ water permeability through AQP4 but not AQP1 channels by 46.7%, recovered to 88.6% water permeability ° MZA: no significant results of water conduction through AQP1 or AQP4 |
Tanimura et al. (2009) | |
| Carbonic anhydrase inhibitors TGN-020 | in vitro | Xenopus oocytes | - | Oocyte functional assay used | ° AAZ showed 80%, EZA 68%, 4- acetamidobenzsulfonamide 23% AQP4 inhibition | Huber et al. (2007) | |
| TGN-020 | in vivo | C57BL/6J mice | 200 mg/kg in 0.1 mL saline IP | Injection 15 min pre-ischemia | ↓ Cerebral edema volume in pretreated TGN-020 brain ischemia mice models | Igarashi et al. (2011) | |
| in vivo | Wistar rats | 5.0 mg/kg in DMSO IP | Injection immediately post-ON crush | ↑ RGC loss by changes in glutamate synthetase levels ↑ Extracellular glutamate levels post-crush |
Nishikawa et al. (2016) | ||
| in vivo | Wistar rats | 2 μL of 10 ng/μL in PBS IV | Injection 2 wk post-diabetes induction | ↓ Swelling of Müller cells in diabetes-induced rats that showed increase in VEGF | Kida et al. (2017) | ||
| in vivo | Wistar rats | 2 μL of 10 ng/μL in PBS IV | Injection 8 wk post-diabetes induction | ↓ Swelling of Müller cells ↓ Thickening of retina diabetes-induced rats |
Oosuka et al. (2020) | ||
| Compounds structurally similar to AQP4 inhibitors | in vitro in silico | X. laevis oocytes, rat AQP4b | 5 μL of 200 μM | Compounds used had unrelated biological properties | ° Thiadiazole, sumatriptan, and rizatriptan showed highest AQP4 inhibitory activity ° Suggests heteroaromatic groups may be favored over aliphatic groups, hydrogen bonds essential to inhibit AQP4 |
Huber et al. (2009) | |
| Facilitator | TGN-073 | in vivo | C57BL/6J mice | 20 or 200 mg/kg in 0.2 mL saline IP | 30 min before study | ↑ Water flux turnover of interstitial fluid managed by AQP4 system | Huber et al. (2018) |
AQP1: Aquaporin-1; AQP4: aquaporin-4; AAZ: acetazolamide; EJV: external jugular vein; EZA: 6-ethoxybenzothiazole-2-sulfonamide; IP: intraperitoneal; IV: intravitreal; MZA: methazolamide; OGD: oxygen glucose deprivation; ON: optic nerve; RGC: retinal ganglion cells; SQ: subcutaneous; TBI: traumatic brain injury; VEGF: vascular endothelial growth factor.
Additional Table 2.
Summary of various agents on GABA activity and associated neurological changes related to the visual system
| Compounds/Agents of interest | Type of study | Exp. Method(s) | Model/Species/Culture | Exp. Protocols | Relevant findings | Citation |
|---|---|---|---|---|---|---|
| DAMGO, DPDPE | Animal | in vivo | New Zealand white rabbits | ° 2 μmol/μL or 4 μmol/μL into ARC or SC ° IOP before and every 5 min for 1 h after injection |
↓ IOP post-ARC injection of μ/δ agonists ↓ IOP post-ARC and SC injection of naloxone but not in conjunction with μ/δ agonist injections |
Jin et al. (2014) |
| GABA | Animal | in vivo | C57BL/6J, AQP4−/− mice | ° Dissolved in CSF tracer ° Via cisterna magna |
° GABA promotes glymphatic drainage in wild-type but not AQP4-/-mice ° No effects on paravascular movement |
Wu et al. (2020) |
| in vivo | Wistar rats | ° 1 g/kg IP ° 32.5 min or 62.5 min before sacrifice | ↓ GABA steady state concentrations and accumulation in post-3 wk IOP increase group | Moreno et al. (2008) | ||
| in vivo, histochemistry | DBA/2J and C57BL/6J mice, Sprague Dawley rats | ° IHC or IF staining to visualize GABA receptor expression in ARC | ↑ Expression of GABA receptor in ARC in high IOP rat and DBA/2J mice models ↓ IOP and GABA receptor expression in high IOP models with GABA antagonists |
Gong et al. (2018) | ||
| GABA, butanoate | Genomic | PARIS | - | ° 3108 POAG cases and 3430 controls total | ° Specific genes of the butanoate metabolism pathway found to contribute to GABA metabolism ° Suggests association with glaucoma pathogenesis |
Bailey et al. (2014) |
| PNU-282987 ((α7- nAChR) agonist) | Animal | in vivo | Wistar rats | ° 5 μL of 100 μM IV ° IOP elevation by EVC |
↑ GAD65/67 levels ↑ Presynaptic release of GABA onto RGCs |
Zhou et al. (2017) |
| - | Case studies | Humans | ° 10-d stress reducing and residual vision activating treatments | ↑ Humphrey VF test scores post-10 day treatment | Sabel et al. (2018) | |
| - | Animal | in vitro, histochemistry | Sprague-Dawley rats | ° Rats sacrificed 60 d post- RYGB ° LCM, RNA sequencing, IHC staining |
° Aqp4 gene among most down-regulated genes after RYGB ° Suggests gliosis reversal and decrease in glia cell function |
Barkholt et al. (2019) |
ARC: Arcuate nucleus; α7-nAChR: alpha7 nicotinic acetylcholine; CSF: cerebrospinal fluid; DAMGO: [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin; DPDPE: [D-Pen2,D-Pen5]- enkephalin; EVC: external jugular vein; GABA: gamma-aminobutyric acid; IF: immunofluorescence; IHC: immunohistochemical; IOP: intraocular pressure; IP: intraperitoneal; IV: intravitreal; LCM: laser capture microdissection; PARIS: pathway analysis by randomization incorporating structure; POAG: primary open-angle glaucoma; RGC: retinal ganglion cells; RYGB: Roux-en-Y gastric bypass; SC: subconjunctival.
Additional Table 3.
Summary of the effects of omega-3 and vitamin B3 on glymphatic activity and the visual system
| Type of supplement | Specific supplement(s) | Exp. Type | Model/Species | Dosage and Admin. Route | Exp. Protocols | Main findings | Citation |
|---|---|---|---|---|---|---|---|
| Omega-3 | Safflower, flaxseed, and tuna oils | in vivo | Sprague- Dawley rats | ° ω-3−: 7% saff. oil ° ω-3+: 5.5% saff., 1.00% flax., 0.50% tuna oils ° Oral |
° IOP measured at 5, 10, 20, 40 wk of age |
° 20+ wk old mice: ↓ IOP more in ω-3+ group than ω-3− group ° May be correlated with decreased aqueous outflow facility in the ω-3− group |
Nguyen et al. (2007) |
| DHA | in vivo | fat-1, C57BL/6J, Aqp4-/-mice | ° 30 mg/kg ° Fish oil with 52.4% DHA ° Oral |
° Daily oral gavage for 3 wk °Intracisternal infusion of FITC-d3 tracer to track CSF movement |
° fat-1: ↑ endogenous n-3 PUFA levels ∝ faster and more efficient Aβ clearance, ↓ impairment of hippocampus-dependent memory and AQP4 polarization post-Aβ ICV injection ° C57BL/6J: ↑ CSF influx and clearance, hippocampus CA3 region neuron survival, spatial learning and memory ° Aqp4-/-: confirmed involvement of AQP4 channels in PUFA promoting CSF clearance |
Ren et al. (2017) | |
| in vivo | Humans | ° 1050 mg/d ° Oral |
° 6 mon treatment ° Evaluated at baseline, 3 and 6 mon |
↓ IOP by 2-3 mmHg in both eyes in treatment group ↑ DHA content in erythrocyte membrane, TAC, MDA, plasma IL-6 levels |
Romeo Villadóniga et al. (2018) | ||
| DHA, EPA | in vivo | APP/PS1, C57BL/6J mice | ° 50 μL of fish oil (13 μM EPA, 99 μM DHA) ° Intragastrical |
° daily for 4 wk | TAC, MDA, plasma IL-6 levels ↑ Aβ1-40 levels in plasma ∝↑ Aβ transport from brain by transport proteins ↓ Overactivation of astrocytes and microglia by inhibiting NF-κB phosphorylation |
Yan et al. (2020) | |
| DHA, EPA, ALA | in vivo | Humans | ° 500-600 mg DHA, 1000 mg EPA, +/-900 mg ALA daily ° Oral |
° 90 d treatment ° IOP measured at baseline and day 90 |
↓ IOP by at least 8% in non-glaucomatous adults consuming typical Western diet with ω-3 supplementation | Downie and Vingrys (2018) | |
| DHA, EPA, | in vivo | Sprague- | ° 10% EPA + 7% | ° 6 mon treatment | ↑ IOP increase post-laser treatment | Schnebelen et al. (2009) | |
| GLA | Dawley rats | DHA ° 10% GLA or ° 10% EPA + 7% DHA + 10% GLA ° Oral |
° IOP elevation at 3 mon via laser photocoagulation of episcleral veins, limbus, and TM ° IOP measured weekly |
° PUFA supplementation did not affect rate of IOP change post-op, did not prevent loss of RGCs or retinal function ° EPA+DHA+GLA: ↓ glial cell activation and ↑ retinal cells preservation ∝↓ retinal stress post-photocoagulation |
|||
| DHA, EPA, timolol | in vivo | DBA/2J, C57BL/6J mice | ° 270 mg/g/d (400 mg EPA + 200 mg DHA) ° 0.5% timolol ° Oral and/or topical |
° Daily treatment for 3 mon total ° Disease progression monitored via tonometry and slit lamp biomicroscopy |
° PUFA+timolol: preserved RGC density but no effect on retinal microglial activation ° PUFA or timolol: ↓ expression of retinal pro-inflammatory cytokines and M1/M2 macrophages ° timolol only: ↓ IOP |
Kalogerou et al. (2018) | |
| Vitamin B3 | NAM | in vivo, in vitro | DBA/2J mice | ° 550 or 2000 mg/kg ° Oral |
° Received NAM treatment at 6 mon old (pre-IOP elevation) or at 9 mon old (post-IOP elevation) | ↓ ON degeneration, RGC soma loss, RNFL thinning ↑ Protection of visual and metabolic functions ↓ Age-related gene expression changes in RGCs ° Higher dosage led to no ON damage in 93% of treated eyes and less IOP elevation |
Williams et al. (2017) |
| in vivo, in vitro | DBA/2J mice | ° 550 mg/kg/d ° Oral |
° NAM added to drinking water | ° Resulted in protection from synapse and RGC loss, RGC activity declines, anterograde axon transport and decreased incidences of remodeling and atrophy of ONH | Williams et al. (2018) | ||
| in vivo | Humans diagnosed with glaucoma | ° 1.5 g/d, then 3.0 g/d ° Oral |
° 6 wk of each dosage, then crossover without washout | ° Significant improvement in inner retinal function in high dosage most likely via metabolic rescue of RGCs ° No significant changes in IOP or RNFL thickness |
Hui et al. (2020) | ||
| in vivo | DBA/2J mice | ° 200 mg/kg/d Oral |
° Chow/water enriched with NAM ° Mice monitored from 3 to 12 mon old ° PERG with flickering field to assess RGC autoregulation |
↑ Preservation of RGC function ↓ IOP ° At least 2x more preservation of RGCs and mitochondria than in control mice |
Chou et al. (2020) |
Aβ: Amyloid-beta; ALA: α-linolenic acid; AQP4: aquaporin-4; CSF: cerebrospinal fluid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; FITC: fluorescein isothiocyanate; GLA: γ-linolenic acid; ICV: intracerebroventricular; IL: interleukin; IOP: intraocular pressure; MDA: (plasma) malondialdehyde; NAM: nicotinamide; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; ω-3: omega-3; ON: optic nerve; ONH: optic nerve head; PERG: pattern electroretinogram; PUFA: polyunsaturated fatty acids; RGC: retinal ganglion cells; RNFL: retinal nerve fiber layer; TAC: total antioxidant capacity; TM: trabecular meshwork.
Additional Table 4.
Summary of various flavonoids on glymphatic/neurological activity and the visual system
| Type(s) of flavonoid | Exp. Type | Model/Species | Dosage and Admin. Route | Exp. Protocols | Main findings | Citation |
|---|---|---|---|---|---|---|
| Baicalin | in vivo | C57BL/6J mice | ° 0.5 mL of 10, 30, or 100 mg/kg ° Oral |
° Administered once daily for 2 consecutive days post-brain injury | ° 30 and 100 kg/mg groups: ↓ Brain inflammation, infarction, and overexpression of AQP4 protein levels post-stroke | Lee et al. (2019) |
| in vivo | Sprague-Dawley rats | ° 4 mL/kg of 2.5 mg/mL or 12.5 mg/mL ° IP |
° SAH induced via endovascular perforation ° Baicalin administered 2 and 12 h post-SAH |
↓ EBI, MMP-9, AQP4 levels in both dosages ↑ Nrf2/NQO1/HO-1 antioxidative signaling pathway ↑ BBB integrity improved post-SAH |
Zhang et al. (2020) | |
| Pinocembrin | in vivo | Sprague-Dawley rats | ° 3, 10, or 30 mg/kg ° IV |
° Cerebral ischemia induced by MCAO ° Injection at 0, 8, 16 h post-MCAO |
↓ Cerebral edema ↑ Preservation of ultrastructure of the neurovascular unit ↓ Elevated levels of cytokines, AQP4, MMP-9, and inflammatory mediators |
Gao et al. (2010) |
| Qctn, taxifolin, kaempferol | in vitro | RGC-5 cells | ° 25 μM Qctn ° 100 μM taxifolin or 15 μM kaempferol |
° 30 min post-flavonoid treatment, oxidative stress via GSH depletion (10 mM glutamate + 500 μM BSO), 0.5 mM t-BOOH, or 650 μM H2O2 | ° ~85% apoptosis in all 3 oxidative stress methods ° Qctn prevented cell death in all three methods ° Taxifolin prevented cell death in all but H2O2 treatment ° Kaempferol only effective against cell death by GSH depletion |
Maher and Hanneken (2005) |
| Qctn | in vivo | Wistar rats | ° 25 and 50 mg/kg ° Oral |
° 6 mon treatment ° Diabetes induced via IP injection of streptozotocin (45 mg/kg) |
↓ TNF-α and IL-1β levels post-Qctn, greater in 50 kg/mg dosage ↓ AQP4 overexpression in Müller cell endfeet and caspase-3 |
Kumar et al. (2014) |
| in vivo | Wistar rats | ° 2 μL of 10 μM ° Intravitreal |
° Glaucoma model via cauterization of scleral veins OD ° IOP measured before and weekly post-surgery for 4 wk ° Qctn injection once weekly for 4 wk |
° RGC function restored to 72% of normal eye ↑ Frequency and amplitude of mIPSCs in Off-type glaucomatous RGCs ↑ Frequency only of GABAergic mIPSCs in On-type RGCs ° Inhibited frequency only of glutamatergic mEPSCs in On-and Off-type RGCs |
Zhou et al. (2019) | |
| in vivo | Wistar rats | ° 2 μL of 10 μM ° Intravitreal |
° AC injection of 5 μL paramagnetic polystyrene microbeads to induce COHTN OD ° IOP measured pre-COHTN, 1 and 3 d, end of 1, 2, 3, 4 wk post-COHTN |
↓ Loss of RGC function as early as 3 days post-COHTN ↓ RGC apoptosis |
Gao et al. (2017) | |
| in vitro | Sprague-Dawley rat RGCs | ° 1, 10, 20, 50 or 100 μM | ° Qctn added for 24, 48, or 72 h ° 200 μM COCl2 to induce hypoxia |
↑ RGC survival at 10, 20, 50 μM ° High toxicity at 100 μM ° Optimum level of RGC viability: 20 μM at 48 h |
||
| Fisetin | in vivo, in vitro | DBA/2J, C57BL/6J mice | ° 5, 10, 20, or 30 mg/kg |
° Visual function assessed via PERG, VEPs, and tonometry ° Immunocytochemistry, ELISA, and Western blot used to determine expression levels of TNF-α, IL-1β, IL-6, NK-κB |
↓ IOP ↑ Restoration of RGC function ↑ RGC viability ↓ Retinal microglial activation ↓ Inflammatory cytokine activations by inhibiting the NF-κB signaling pathway |
Li et al. (2019) |
AC: Anterior chamber, AQP4: aquaporin-4, BBB: blood brain barrier, BSO: buthionine sulfoximine, COHTN: chronic ocular hypertension, EBI: early brain injury, ELISA: enzyme-linked immunosorbent assay, GSH: glutathione, IL: interleukin, IOP: intraocular pressure, IP: intraperitoneal, IV: intravenous, MCAO: middle cerebral artery occlusion, mEPSC: miniature excitatory postsynaptic currents, mIPSC: miniature inhibitory postsynaptic currents, MMP-9: matrix metalloproteinase-9, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, OD: oculus dexter, PERG: pattern electroretinogram, Qctn: quercetin, RGC: retinal ganglion cells, SAH: subarachnoid hemorrhage, t-BOOH: t-butyl peroxide, TNF-α: tumor necrosis factor alpha, VEP: visual evoked potentials.
Additional Table 5.
Effects of exercise on glymphatic/neurological activity and the visual system
| Exp. Type | Model/Species | Exp. Protocols | Main findings | Citation |
|---|---|---|---|---|
| in vivo | C57BL/6J mice | ° 5 wk voluntary wheel running ° 6 km daily on avg |
↑ CSF influx and efflux during wakefulness and when under ketamine-xylazine anesthesia ↓ Glymphatic activity when actively running |
von Holstein-Rathlou et al.(2018) |
| in vivo, in vitro | C57BL/6J, Thy1–GFP transgenic mice | ° 6 wk voluntary wheel running ° Assessed via Morris water maze, in vivo two-photon imaging, histology |
↓ Time to reach platform in running group ↑ Time spent in target quadrant than control mice ↑ Paravascular CSF-ISF exchange in aging brain ↑ AQP4 polarity in cortex, hippoc., and perivascular regions ↓ Amt of astrocytes and microglia in the cortex, CA1, DG, CA3 regions of hippoc. ↓ Aβ deposition in hippoc. and cortex ↑ Number of dendritic spines in cortex and hippoc. |
He et al. (2017) |
| in vivo, in vitro | APP/PS1 mice | ° 10 wk treadmill training at 10-12 m/min for 20-60 min/d ° Assessed via Pavlovian fear conditioning and testing, immunohistochemistry |
↓ Body weight gain ↑ Citrate synthase activity in soleus muscles ↑ And restored amyg.-and hippoc.-associated long-and short-term memories ↑ Dendritic complexity and field of the hippoc. and BLA ↑ BDNF signaling pathways and levels of LRP1 in hippoc. and amyg. ↓ Conc of Aβ in hippoc. and amyg. |
Lin et al. (2015) |
| in vivo, in vitro | Sprague-Dawley rats | ° 2.4 mg/kg STZ bilateral ICV injection for AD rat model ° 4 wk treadmill exercise of 30 min/d, 5 d/wk at 1 wk post-STZ ° Assessed via Barnes maze task, passive avoidance test, novel object recognition test, immunohistochemistry, histology |
↑ And restored memory function and novel object recognition ability ↓ Latency in completing tasks ↓ STZ-induced hippoc. neuronal degeneration ↓ Levels of Aβ, AβPP amyloidogenic processing, hyperphosphorylation of tau protein in hippoc. regions ↑ Anti-inflammatory cytokine (IL-4, IL-10) levels ↓ Pro-inflammatory cytokine (IL-1β, TNF-α) levels ↓ Neuronal damage by oxidative stress and caspase activity ↑ CCO activity and ATP production |
Lu et al. (2017) |
| in vivo | Humans | ° 5 yr study ° Exercise frequency and period varied ° IOP measured at beginning and end of study |
° More frequent exercise per week and longer exercise time significantly associated with greater decrease in IOP | Fujiwara et al. (2019) |
| in vivo | Humans | ° Yoga positions (Adho Mukha Svanasana, Uttanasana, Halasana, Viparita Karani) held for 2 min each ° IOP measured before, during, and after the exercises |
↑ IOP during yoga poses w/in 2 min, greatest increase in Adho Mukha Svanasana position ↓ IOP to baseline after poses in seated position ° Glaucoma pts had IOPs 1-2 mmHg higher on avg compared to controls but not statistically significant |
Jasien et al. (2015) |
| in vivo | Glaucoma patients | ° Pts of ages 60-80 yr wore accelerometers for 1 wk ° Assessed via Humphrey VF at baseline and 1 wk |
↓ VF sensitivity ∝↑ daily steps and non-sedentary activity ° Covariates associated with faster sensitivity loss include age, non-Caucasian race, h/o glaucoma or cataract surgery, worse VF loss at baseline |
Lee et al. (2019b) |
Aβ: Amyloid-beta, AβPP: amyloid-beta precursor protein, AD: Alzheimer’s disease, AQP4: aquaporin-4, ATP: adenosine triphosphate, BDNF: brain-derived neurotrophic factor, BLA: basolateral amygdala, CCO: cytochrome c oxidase, CSF: cerebrospinal fluid, DG: dentate gyrus, ICV: intracerebroventricular, IL: interleukin, IOP: intraocular pressure, ISF: interstitial fluid, LRP1: low density lipoprotein receptor-related protein 1, STZ: streptozotocin, TNF-α: tumor necrosis factor alpha, VF: visual field.
Additional Table 6.
Effects of intermittent fasting and sleep on glymphatic/neurological activity and the visual system
| Lifestyle component | Exp. Type | Model/Species | Exp. Protocols | Main findings | Citation |
|---|---|---|---|---|---|
| Intermittent Fasting | in vivo | APP/PS1 mice | ° IF group fed every other day, fasted the following day for 5 mon ° Assessed via Morris water maze test, immunohistochemistry, histology |
↓ Cognitive dysfunction, prevented brain from increase of Aβ deposition, and restored the AQP4 polarity to localize in astrocytic endfeet ↓ Expressions of AQP4-M1 and HDAC3, and ratio of AQP4-M1/M23 ratio in cerebral cortex |
Zhang et al. (2017) |
| in vitro | Human U251 glioma cells | ° Cells cultured for 3 h with βOHB, βOHB + Aβ, Aβ only, or medium only ° After 3 h, cells with Aβ further treated with Aβ25−35 for 12 h ° Various DNA and protein expression |
↓ Expressions of AQP4-M1 and HDAC3, AQP4-M1/M23 ratio by βOHB ↑ Expressions of AQP4-M23 and miR-130a via HDAC3 expression inhibition |
||
| in vivo | Humans | ° 27 healthy adults fasting during month of Ramadan ° Full ophthalmic exam and OCTA done at AM and PM of wk 2 and 3 of Ramadan, and 2 mon later |
↓ Lower IOP, CCT, and average RNFL thickness during evening of fasting ↑ Whole and peripapillary RPC density and ONH vCDR during fasting |
Nilforushan et al. (2020) | |
| Sleep | in vivo | C57BL/6J mice | ° 3 groups: naturally asleep to awake, awake to ketamine/xylazine anesthesia, awake to norepinephrine receptor antagonists ° CSF tracer infusion via cisterna magna ° Visualized CSF tracer influx via in vivo two-photon imaging |
↑ 60% of interstitial space in naturally asleep and anesthetic mice ↓ ~95% of influx in periarterial and parenchymal spaces of awake mice ° Sleeping and anesthetized mice showed influx of CSF tracers in periarterial spaces, subpial regions, brain parenchyma ° Aβ cleared 2× faster in sleeping/anesthetized mice than in awake mice ↑ Cortical interstitial volume when adrenergic receptors are inhibited |
Xie et al. (2013) |
| in vivo | Humans | ° Glaucoma and healthy participants ° Assessed via sleep survey, retinal photos, 2 VF tests per eye |
° Suggest that worse sleep parameters may be risk factor/consequence of glaucoma ° Lowest disc defined glaucoma and/or VFD prevalence associated with 7 h/night ° Highest disc defined glaucoma associated with ≥ 10 h/night ° Highest VFDs associated with ≤ 3 h and ≥ 10 h/night ° Association between disc defined glaucoma and ≤ 9 min and ≥ 30 min sleep latency ↑ VFD ∝ likely to not perform optimally in daytime ° No association found between glaucoma and any sleep disorders |
Qiu et al. (2019) |
Aβ: Amyloid-beta, AQP4: aquaporin-4, BDNF: brain-derived neurotrophic factor, βOHB: β-hydroxybutyrate, CCT: central corneal thickness, CSF: cerebrospinal fluid, HDAC3: histone deacetylase 3, IF: intermittent fasting, IOP: intraocular pressure, OCTA: optical coherence tomography angiography, ONH: optic nerve head, POAG: primary open angle glaucoma, RNFL: retinal nerve fiber layer, RPC: radial peripapillary capillary, vCDR: vertical cup/disc ratio, VF: visual field, VFD: visual field defects.
Pharmacological interventions
AQP4 inhibitors
One compound found to inhibit AQP4 function and consequently reduce cerebral edema is AER-271. This prodrug was developed in order to achieve higher (> 5000-fold improved) aqueous solubility in aqueous solution at pH 7, in comparison to its predecessor, AER-270. In a rat stroke model administered with 10 mg/kg AER-271 through intravenous route, the animals showed reduced cerebral edema and improved neurological outcomes (Farr et al., 2019). Similarly, in a rat model of asphyxial cardiac arrest, treatment with AER-271 showed protection against early cerebral edema, neurological deficits, and neuroinflammation (Wallisch et al., 2019).
Another potential inhibitor of AQP4 function is acetazolamide (AAZ), a carbonic anhydrase inhibitor, typically used to treat epilepsy and idiopathic intracranial hypertension. AAZ inhibits AQP4-mediated water transport by around 80% and significantly reduces the permeability of AQP4 water channels (Huber et al., 2007; Tanimura et al., 2009). AAZ has proven effective in several rodent models of traumatic brain injury, mediating its effects through prevention of AQP4 reorganization after injury and consequent reduction in cytotoxic edema (Katada et al., 2012; Sturdivant et al., 2016; Glober et al., 2019). Future studies should explore the mechanism in which AAZ inhibits AQP4 function, to determine its potential uses beyond epilepsy and cerebral edema.
Another potent inhibitor of AQP4-mediated water transport is TGN-020 (Huber et al., 2009), a compound with IUPAC nomenclature as 2-(Nicotinamide)-1,3,4-thiadiazole, N-(1,3,4-Thiadiazol-2-yl)-3-pyridinecarboxamide, N-(1,3,4-Thiadiazol-2-yl)pyridine-3-carboxamide, N-(1,3,4-Thiadiazolyl)-m-nicotinamide. In a classical study, pretreatment with TGN-020 blocked water movement through AQP4 channels, thus reducing water influx and delaying the development of cytotoxic edema in murine models of focal cerebral ischemia (Igarashi et al., 2011). In studies of diabetic rat models, TGN-020 reduced retinal edema and associated swelling of Müller cells (Kida et al., 2017; Oosuka et al., 2020). In addition to Müller cells’ involvement in fluid redistribution, these cells also play a significant role in retinal cell reprogramming and regeneration, possibly indicating their importance for proper RGC function (Goldman, 2014).
Although AQP4 inhibition by TGN-020 has been shown to reduce edema, it has also been implicated in impaired clearance of Aß via the peri-/para-vascular routes of the brain (Rosu et al., 2020). This suggests that AQP4 inhibition may favor the deposition of Aβ characterized by AD. TGN-020 has also been shown to be associated with neurodegenerative effects in a rat model of optic nerve crush injury by downregulating the uptake of glutamate by optic nerve astrocytes and AQP4, thus increasing loss of RGC after optic nerve crush injury (Nishikawa et al., 2016). Future studies must be conducted to confirm these neurodegenerative effects of TGN-020 on the optic nerve.
The AQP4 inhibitors discussed above and listed in Additional Table 1 are restricted to investigations in conditions of edema, thereby prompting future experiments to be conducted in order to evaluate their potential as therapeutic agents in diseases like glaucoma, hydrocephalus, and NMOSD.
AQP4 facilitators
While several potential AQP4 inhibitors have been identified, there is only one currently documented AQP4 facilitator in the literature, TGN-073, which was reported recently and is listed in Additional Table 1. TGN-073 significantly increases turnover of interstitial fluid through AQP4 water channels (Huber et al., 2018). The hypothesized molecular mechanism is that the ligand binds to AQP4, leading to a conformational shift, which in turn, facilitates water flux. Future studies may explore the role of TGN-073 in potential use against diseases of the optic nerve where glymphatic clearance is impaired.
GABA agonists
Recently, GABA was found to modulate glymphatic clearance of Aß from the brain (Wu et al., 2020). GABA enhanced glymphatic clearance in wild-type mice but not in AQP4-null mice, thus indicating that GABA-mediated clearance is dependent upon AQP4 channels. This is further supported by the fact that the GABA-A receptor is co-localized with AQP4 in brain tissue. Although GABA and AQP4 appear to be linked, the effects of GABA upregulation in the optic nerve have not yet been comprehensively elucidated. Furthermore, one study hypothesized that GABA-A receptor agonist would induce AQP4 upregulation, thus promoting autoimmune brain inflammation, as seen in NMOSD (Jarius et al., 2015). Therefore, careful modulation of GABA levels is necessary. Experimental protocols and results included in this section on GABA agonists are included in further detail in Additional Table 2.
GABA has often been implicated in the pathogenesis of glaucoma (Bailey et al., 2014). For example, Bailey et al. found that the butanoate metabolism pathway (specifically the components that contribute to GABA and acetyl-CoA metabolism) was significantly associated with primary open-angle glaucoma and NTG. Furthermore, in a model of ocular hypertension, GABA uptake in the retina was significantly increased, while glutamate- and high K+ -induced GABA release displayed a significant decrease (Moreno et al., 2008). This disruption of glutamate-GABA balance in hypertensive eyes could indicate a key role of GABA in glaucoma.
With regards to investigations in CNS, the expression of GABA-A/B receptors in the arcuate nucleus of glaucomatous mice was found to be significantly upregulated compared to the control group (Gong et al., 2018). The arcuate nucleus of the hypothalamus plays an imperative role in stress management, as well as in IOP regulation through cortisol secretion (Jin et al., 2014; Leon-Mercado et al., 2017). Both of these factors are crucial in glaucoma etiopathogenesis (Sabel et al., 2018). This may link to AQP4 expression, as transcriptomic analysis of rat arcuate nucleus previously demonstrated region-specific alterations in AQP4 expression (Barkholt et al., 2019).
In support of the above discussed findings, PNU-282987, an alpha7 nicotinic acetylcholine receptor (α7-nAChR) agonist, was found to modulate GABAergic synaptic transmission, thus protecting retinal ganglion cell function (Zhou et al., 2017). Elevated IOP was found to downregulate retinal α7-nAChR expression, suggesting that α7-nAChR expression relates to GABA, IOP, and glaucoma. Future studies should aim to explore how GABA is involved in glymphatic clearance and whether the aforementioned GABA agonists could be used to promote waste clearance in the optic nerve.
Dietary supplements
Omega-3
There is growing evidence supporting omega-3 PUFAs acting by a glymphatic mechanism in the alleviation of neurodegenerative conditions (Ren et al., 2017). For example, Ren et al. (2017) found that in a mouse model of AD, oral administration of fish oil promoted clearance of Aß from the brains of transgenic mice but not AQP4-null mice. Likewise, imaging of brain tissues indicated that during injection of Aβ, omega-3 PUFAs protected AQP4 polarization and inhibited astrocytes, thus supporting the hypothesis that omega-3 PUFAs acted through an AQP4-dependent glymphatic mechanism. It is important to note that fish oil contains a variety of other beneficial moieties (like Vitamin A, Vitamin D, calcium, etc.) that have nutritional as well as disease-ameliorating effects. Whether the observed effects are due to PUFAs or due to the combination of various nutrients in fish oil needs to be resolved. Furthermore, omega-3 PUFAs were found to improve BBB transport and promote Aβ clearance from the brain to systemic circulation (Yan et al., 2020). BBB transport and glymphatic clearance appear to work hand-in-hand to prevent protein accumulation and the corresponding initiation of neurodegenerative disorders (Verheggen et al., 2018).
Omega-3 PUFAs have been suggested as a potential protective agent against optic nerve damage in glaucoma. In a mouse model of glaucoma, omega-3 diet supplementation through oral gavage prevented RGC degeneration, especially when combined with treatment of timolol eyedrops (Kalogerou et al., 2018). These neuroprotective effects of omega-3 treatment are accompanied with decrease in IOP. For instance, Sprague-Dawley rats that were raised on omega-3-sufficient diets had a 13% decrease in IOP at 40 weeks of age, compared to rats raised on omega-3-deficient diets (Nguyen et al., 2007). In a study on patients with pseudoexfoliative glaucoma, docosahexaenoic acid, an omega-3 PUFA, led to significant decreases in IOP (Romeo Villadóniga et al., 2018). Similarly, in young normotensive adults, three months of systemic omega-3 supplementation significantly reduced IOP by 8% (Downie and Vingrys, 2018). Interestingly, when supplementation of omega-3 was compounded with omega-6, rat eyes were more efficiently protected from retinal damage induced by IOP increase (Schnebelen et al., 2009), thereby making a case for neuroprotective effects. The mechanism by which omega-3 PUFAs act on the optic nerve is yet to be elucidated but prevention of gliosis (inflammatory activation of astroglia) may be considered as a candidate process for investigative studies. This is because inflammation and consequent activation of astroglia precipitate dysregulation and mislocalization of AQP4 which may, in all possibilities, affect glymphatic clearance. Given the growing information about the glymphatic hypothesis, future studies aiming to explore the potential effect and mechanism of PUFAs on AQP4 function and glymphatic clearance are needed. Experimental protocols and results included in this section on omega-3 PUFAs are included in further detail in Additional Table 3.
Flavonoids
There is some evidence that flavonoids, such as baicalin, pinocembrin, quercetin, and fisetin, are involved in AQP4 expression in the brain and eye (Tesse et al., 2018). In cerebral ischemic mice, baicalin downregulated AQP4 expression, thus reducing edema and inflammation (Lee et al., 2019a). Baicalin showed similar effects in rats with experimental subarachnoid hemorrhage, thereby reducing the expression of AQP4 and inflammatory cytokines (Zhang et al., 2020). Another flavonoid, pinocembrin, was found to have similar results. Pinocembrin inhibited production of inflammatory signals and expression of AQP4 in cerebral ischemic rats, protecting the rat brain as a therapeutic effect (Gao et al., 2010). Similarly, quercetin treatment inhibited diabetes-induced upregulation of GFAP and AQP4 expression in diabetic retinas (Kumar et al., 2014). The inhibition of AQP4 expression in Müller cell endfeet and perivascular space apparently prevented diabetic retinal edema and RGC death.
Just as flavonoids demonstrated neuroprotective effects in models of cerebral ischemia and diabetic retinopathy, flavonoids have also been found to protect RGCs from oxidative stress-induced death in diseases like glaucoma (Maher and Hanneken, 2005). However, to the best of our knowledge, no studies have suggested a glymphatic mechanism of flavonoids for treatment of glaucoma. Instead, one study suggested that quercetin reduces glutamate from nerve terminals, thus protecting against excitatory neurotoxicity (Zhou et al., 2019). Another study suggested that quercetin improves mitochondrial function thereby preventing RGC apoptosis (Gao et al., 2017). Others suggested that fisetin regulates cytokine production and inhibits pro-inflammatory pathways like nuclear factor kappa B (Li et al., 2019). Under this backdrop, it appears that these moieties may mediate their effects through both glymphatic-dependent and glymphatic-independent mechanisms. There may also be an indirect relationship between these flavonoids and glymphatic modulation which may or may not depend on AQP4. Future studies are needed to have better answers to the questions regarding protective mechanism of flavonoids. In particular, it would be worthwhile to determine whether the effects involve AQP4 inhibition and glymphatic clearance, anti-inflammatory and anti-apoptotic properties, a combination thereof, or an entirely disparate mechanism. Experimental protocols and results included in this section on flavonoids are included in further detail in Additional Table 4.
Vitamin B3
Vitamin B3 has been proposed as a potential therapeutic agent for degeneration of the optic nerve in glaucoma (Williams et al., 2017). Retinal levels of nicotinamide adenine dinucleotide (NAD+), a key molecule for mitochondrial metabolism, decline as one ages, thus rendering neurons susceptible to degenerative insults. Williams et al. (2017) found that supplementation of vitamin B3, also known as NAD+ precursor nicotinamide, can protect against mitochondrial dysfunction, thus making RGCs relatively resilient to IOP-induced stress. Likewise, at the highest dosage tested, vitamin B3 decreased the likelihood of developing glaucoma by 10-fold (Williams et al., 2018). In addition to protecting RGCs in preclinical models, vitamin B3 has also been found to improve inner retinal function in glaucomatous patients (Hui et al., 2020). In glaucomatous mice, vitamin B3 was found to help RGCs autoregulate their function in response to a flicker-induced metabolic challenge (Chou et al., 2020). This demonstrates that vitamin B3 helps preserve mitochondrial function in glaucoma.
Mitochondrial function may also be linked to the glymphatic system. A significant association between an increase in pathological mitochondria in astrocytic endfeet and loss of perivascular AQP4 was found in patients with iNPH (Hasan-Olive et al., 2019). Pathological mitochondria may indicate defective energy metabolism in endfeet processes of iNPH, which may impair glymphatic clearance of brain metabolites. Vitamin B3 has been found to protect against mitochondrial toxicity and reduce oxidative stress through its antioxidant properties (Depeint et al., 2006). Therefore, future studies should further explore whether and how vitamin B3 affects glymphatic clearance along the visual pathways in glaucoma, hydrocephalus, and NMOSD. Experimental protocols and results included in this section on vitamin B3 are included in further detail in Additional Table 3.
Complementary and integrative approaches
Exercise
While neuroprotective effects of exercise are well-documented in literature, the effects of physical exercise on glymphatic clearance in the brain are a novel area of study. Experimental protocols and results included in this section on physical exercise are included in further detail in Additional Table 5.
Many recent studies involving voluntary exercise in AD rodent models have found promising results of enhanced glymphatic clearance. Voluntary exercise was found to increase glymphatic influx of CSF tracers in the hypothalamus, ventral, and lateral cortices of young awake mice (von Holstein-Rathlou et al., 2018). Similarly, voluntary running effectively increased AQP4 expression and polarization in the brains of mice, consequently reduced accumulation of Aβ deposits, and ameliorated inflammatory activation of microglia and astrocytes (He et al., 2017). Furthermore, exercise was found to preserve hippocampal-dependent cognitive functioning and memory via increased clearance of Aβ, attenuation of mitochondrial dysfunction, and inhibition of neuronal apoptosis in the hippocampus (Lin et al., 2015; Lu et al., 2017). These studies suggest several possible hypotheses for exercise-induced neuroprotection in the aging brain, including glymphatic clearance.
Physical exercise has been suggested as a modifiable risk factor and potential therapeutic intervention in glaucoma. For example, increased exercise frequency and time were strongly associated with reduced IOP levels (Fujiwara et al., 2019), though the effects depended on the type of exercise, such as running or yoga (Jasien et al., 2015). It is pertinent to note that for every 5000 daily steps or 2.6 hours of non-sedentary physical activity, the average rate of visual field loss decreased in glaucoma patients by 10% (Lee et al., 2019b). However, the mechanism by which physical exercise acts on the optic nerve has yet to be elucidated, though relaxation response, reduction in cortisol, improvement in vagal tone, parasympathetic activation, and endorphin release can all be postulated. Given the growing information about the glymphatic hypothesis, future studies should explore the effects of voluntary exercise on glymphatic clearance in diseases like glaucoma where the optic nerve is affected, while being mindful of the type of exercise.
Intermittent fasting
Recent literature has suggested that intermittent fasting has the potential to influence glymphatic clearance (Additional Table 6). In a mouse model of AD, alternate-day fasting was found to have restored AQP4 polarity, which improved cognitive function and reduced Aβ deposition (Zhang et al., 2017). Given the involvement of AQP4, this may indicate that fasting affects glymphatic clearance. Furthermore, in healthy human subjects, Ramadhan fasting was found to be associated with decrease in IOP and central corneal thickness; interestingly, retinal nerve fiber layer thickness and ONH vertical cup-to-disc ratio were significantly different during fasting visits versus non-fasting visits (Nilforushan et al., 2020). Together, these studies indicate that fasting may have a protective effect on the optic nerve, perhaps through a mechanism of glymphatic clearance. Exploration of this association between fasting, glymphatic function, and optic nerve health may be an important area of research for the near future.
Sleep
In the past decade, sleep has been increasingly implicated in glymphatic function (Additional Table 6). In the mouse brain, natural sleep was found to increase the interstitial space by 60%, thereby significantly increasing CSF-ISF exchange and thus the rate of Aβ clearance (Xie et al., 2013). The effects of sleep on glymphatic clearance may be linked to circadian rhythms, as the greatest levels of CSF distribution and AQP4 polarization were detected during the mid-rest phase of the mice (Hablitz et al., 2020). This relationship may be supported by clinical symptoms of AD, as sleep disruption can leave the brain vulnerable to amyloidopathy, which may correspondingly alter sleep patterns (Boespflug and Iliff, 2018). Given that both sleep and the glymphatic system degrade with age, this suggests that sleep disturbance may slow glymphatic clearance of toxic solutes in the brain, leading to progression of neurodegenerative diseases resulting in dementia (Nedergaard and Goldman, 2020). Sleep disruption has also been associated with glaucoma, as poor sleep parameters such as abnormal sleep duration, sleep latency, and daytime dysfunction, are more prevalent in glaucoma patients compared to healthy controls (Qiu et al., 2019). Given the increasing evidence for a glymphatic pathogenesis of glaucoma, the role of sleep in glymphatic dysfunction in glaucoma should be explored in future research.
Future Perspectives
Future research into the glymphatic system should explore several different avenues, including noninvasive methods of imaging the glymphatic system, interactions between AQP4 and the cholinergic nervous system, the role that other aquaporins besides AQP4 play in the glymphatic system, and interactions between the glymphatic system and other waste clearance pathways.
For in vivo imaging, the current practice of injecting exogenous gadolinium-based contrast agents to image the glymphatic system has raised safety concerns with renal impairment and gadolinium deposition in brain tissue (Layne et al., 2018). Therefore, exploration of noninvasive methods using intrinsic endogenous contrasts would be useful for imaging the glymphatic system. One potential candidate is multiple echo time arterial spin labeling, where the time it takes for magnetically labeled intravascular water to exchange across the blood-brain interface can act as an indicator of the expression of AQP4 water channels. In AQP4-deficient mice, there was a 31% increase in exchange time compared to their wild-type counterparts, thus demonstrating the imaging technique's sensitivity (Ohene et al., 2019). Another potential candidate is noninvasive diffusion MRI, a technique that was able to monitor changing astrocyte activity in the brain as AQP4 channels were inhibited by TGN-020 (Debacker et al., 2020). These astrocytic dynamics were indicated by a significant decrease in S-index (a marker of diffusion reflecting tissue microstructure) and a concomitant increase in the water diffusion coefficient. Other imaging techniques to consider include blood-oxygenation-level-dependent (BOLD) functional MRI (fMRI) and diffusion fMRI. However, when AQP4 channels were inhibited by TGN-020 and astrocyte function was disrupted as a consequence, only BOLD fMRI responses reflected this and not diffusion fMRI (Komaki et al., 2020). This reflects that BOLD fMRI is sensitive to AQP4 inhibition and disruption of astrocyte function, whereas diffusion fMRI is not, which may have implications for the specificity of diffusion fMRI in detecting changes in the glymphatic system. Another potential candidate is dynamic glucose-enhanced (DGE) MRI via chemical exchange saturation transfer MRI at 3 Tesla (Huang et al., 2020). DGE MRI detected significantly slower CSF clearance in both young and old mice with AD, compared to age-matched wild-type mice. This reflects the restricted glymphatic clearance and accumulation of Aß that is characteristic of AD. Therefore, DGE MRI may have the potential to detect glymphatic clearance in early stages of diseases of glymphatic dysfunction. Future studies are expected to determine and improve the sensitivity and specificity of these noninvasive techniques so that they can be widely used to evaluate glymphatic mechanisms and dysfunction.
Next, the cholinergic nervous system has been suggested to be involved in glaucoma (Faiq et al., 2019; van der Merwe et al., 2021). Not only do choline-containing compounds enhance RGC survival, but they are also precursors for important components of the cell membrane such as phosphatidylcholine, phosphatidylethanolamine, and sphingomyelin. This may be relevant to the glymphatic system because membrane composition directly impacts AQP4 water permeability (Tong et al., 2012), and phosphatidylcholine forms an important component of neuronal and glial cell membranes. In fact, the polarized expression of AQP4 in astrocytic endfeet can possibly be attributed to the different lipid compositions of endfeet membranes and membranes in the rest of the cell including the endomembrane system. Since glymphatic exchange of CSF and ISF is thought to be facilitated by this polarized expression of AQP4, a possible relationship among choline, lipid composition of membranes, and polarization of AQP4 expression would open up new avenues for investigations and therapeutic possibilities.
Beyond AQP4, other aquaporins have demonstrated potential protective involvement during optic nerve damage. For example, in a rat model of optic nerve crush, aquaporin-9 (AQP9) expression in the optic nerve increased post-injury (Suzuki et al., 2014). It has been hypothesized that the amount of AQP9-positive astrocytes augment in counteracting metabolic damage at the injury site. Furthermore, expression of AQP9 in the optic nerve increased and was co-localized with AQP4 and GFAP in a rat model of glaucoma, implying that the damaged RGCs may, in part, be dependent on the surrounding astrocytes (Yang et al., 2013). These findings suggest a potential complementary relationship between AQP4 and AQP9 in the optic nerve that deserves attention. Such approaches would determine the relevance of this AQP4 and AQP9 crosstalk in modulating the glymphatic system. Current limitations in developing AQP4 inhibitors include the many homologous AQP isoforms with broad tissue distribution and functions, predicted and undesired effects of pharmacological agents, and the need for high BBB penetration (Verkman et al., 2017). This, therefore, prompts the application of existing pharmacological interventions and integrative approaches in modulating AQP4 function or even other aquaporins in optic nerve diseases.
Apart from aquaporins, other markers may be implicated for the modulation of the glymphatic system. Some of them include cortisol, α-syntrophin (Snta1), and platelet derived growth factor-B (PDGF-B). Cortisol is a stress hormone whose dysregulation is associated with poor sleep quality. Combined with the recent discovery of sleep mediated activation of the glymphatic system in mice (Hill et al., 2020), it can be inferred that sleep and, by extension, cortisol homeostasis may serve to clear metabolic waste and cellular debris in the CNS and the eye (Xie et al., 2013). Snta1 is a cytoplasmic peripheral membrane scaffold protein that is a component of the dystrophin-associated protein complex at neuromuscular junctions to regulate intracellular calcium ion levels. Snta1 knockout mice lacked perivascular localization of AQP4 without alteration in AQP4 expression levels (Amiry-Moghaddam et al., 2003), while also demonstrating significant reduction in CSF-ISF efflux (Mestre et al., 2018). Lastly, in an experiment mapping the development of the glymphatic system in mice, the PDGF retention motif knockout mouse line Pdgfbret/ret showed a maldeveloped and malfunctioning glymphatic system as compared to control mice, which persisted throughout their adult life. The investigators concluded that PDGF-B plays a critical role in the development of perivascular CSF efflux (Munk et al., 2019).
In addition to the glymphatic system, there are several other potential metabolic waste clearance pathways in the eye and brain, including the ocular lymphatic system, the intramural periarterial drainage system, and the meningeal lymphatic system (Iliff et al., 2012; Louveau et al., 2015; Yucel and Gupta, 2015; Albargothy et al., 2018; Deng et al., 2020). Recent studies have suggested an imperative and coherent connection between meningeal lymphatic vessels and the glymphatic system in the optic nerve (Chan-Ling et al., 2019). Glaucoma has been associated with impairment of both the glymphatic and meningeal lymphatic systems in the retrolaminar optic nerve. Glaucomatous optic nerve meninges showed greater lymphatic filling, which may indicate a link between the meningeal lymphatic and glymphatic systems. Further research should explore these correlates, as they become increasingly relevant in the pathogenesis of optic nerve impairment.
Conclusion
The early findings explicated here indicate the growing evidence for the existence and properties of a glymphatic system in the optic nerve. This is supported by imaging of healthy optic nerves in wild-type rodents, AQP4-null rodents, and humans. There is also an increasing amount of evidence for the potential involvements of glymphatic dysfunction in optic nerve diseases such as glaucoma and hydrocephalus, though more research is necessary to elucidate possible mechanisms. These findings, along with pharmacological interventions and integrative approaches for glymphatic modulation may guide the development of targeted treatment strategies to the optic nerve, and may have implications for ameliorating optic neuropathies to improve vision health outcomes.
Acknowledgments: We thank all collaborators who contributed to our research papers upon which the present commentary is based.
Additional files:
Additional Table 1: Summary of inhibitory and facilitating agents on AQP4 activity and neurological effects.
Additional Table 2: Summary of various agents on GABA activity and associated neurological changes related to the visual system.
Additional Table 3: Summary of the effects of omega-3 and vitamin B3 on glymphatic activity and the visual system.
Additional Table 4: Summary of various flavonoids on glymphatic/neurological activity and the visual system.
Additional Table 5: Effects of exercise on glymphatic/neurological activity and the visual system.
Additional Table 6: Effects of intermittent fasting and sleep on glymphatic/neurological activity and the visual system.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by National Institutes of Health, No. R01-EY028125; BrightFocus Foundation, No. G2019103; Feldstein Medical Foundation; Research to Prevent Blindness/Stavros Niarchos Foundation International Research Collaborators Award; and an unrestricted grant from Research to Prevent Blindness to NYU Langone Health Department of Ophthalmology (to KCC).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Alvaro J Mejia-Vergara, Fundacion Universitaria Sanitas, Colombia.
Funding: This work was supported by National Institutes of Health, No. R01-EY028125; BrightFocus Foundation, No. G2019103; Feldstein Medical Foundation; Research to Prevent Blindness/Stavros Niarchos Foundation International Research Collaborators Award; and an unrestricted grant from Research to Prevent Blindness to NYU Langone Health Department of Ophthalmology (to KCC).
P-Reviewer: Mejia-Vergara AJ; C-Editors: Zhao M, Zhao LJ, Qiu Y; T-Editor: Jia Y
References
- 1.Aghayev K, Bal E, Rahimli T, Mut M, Balci S, Vrionis F, Akalan N. Aquaporin-4 expression is not elevated in mild hydrocephalus. Acta Neurochir (Wien) 2012;154:753–759. doi: 10.1007/s00701-011-1241-9. [DOI] [PubMed] [Google Scholar]
- 2.Albargothy NJ, Johnston DA, MacGregor-Sharp M, Weller RO, Verma A, Hawkes CA, Carare RO. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 2018;136:139–152. doi: 10.1007/s00401-018-1862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 2003;100:2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey JN, Yaspan BL, Pasquale LR, Hauser MA, Kang JH, Loomis SJ, Brilliant M, Budenz DL, Christen WG, Fingert J, Gaasterland D, Gaasterland T, Kraft P, Lee RK, Lichter PR, Liu Y, McCarty CA, Moroi SE, Richards JE, Realini T, et al. Hypothesis-independent pathway analysis implicates GABA and acetyl-CoA metabolism in primary open-angle glaucoma and normal-pressure glaucoma. Hum Genet. 2014;133:1319–1330. doi: 10.1007/s00439-014-1468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkholt P, Rigbolt KTG, Falkenhahn M, Hübschle T, Schwahn U, Fernandez-Cachon ML, Schmidt T, Theis S, Hansen HH, Hay-Schmidt A, Pedersen PJ, Vrang N, Jelsing J. Global transcriptome analysis of rat hypothalamic arcuate nucleus demonstrates reversal of hypothalamic gliosis following surgically and diet induced weight loss. Sci Rep. 2019;9:16161. doi: 10.1038/s41598-019-52257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benveniste H, Heerdt PM, Fontes M, Rothman DL, Volkow ND. Glymphatic system function in relation to anesthesia and sleep states. Anesth Analg. 2019;128:747–758. doi: 10.1213/ANE.0000000000004069. [DOI] [PubMed] [Google Scholar]
- 7.Boespflug EL, Iliff JJ. The emerging relationship between interstitial fluid-cerebrospinal fluid exchange, amyloid-β, and sleep. Biol Psychiatry. 2018;83:328–336. doi: 10.1016/j.biopsych.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan-Ling T, Uddin MN, Koina ME, Behar-Cohen FF, Hu P. Compelling structural and functional evidence for glymphatics and lymphatics in human posterior eye: Enhanced immune response and glymphatic/lymphatic changes in glaucoma pathogenesis. Invest Ophthalmol Vis Sci. 2019;60:4274–4274. [Google Scholar]
- 9.Chou TH, Romano GL, Amato R, Porciatti V. Nicotinamide-rich diet in DBA/2J mice preserves retinal ganglion cell metabolic function as assessed by PERG adaptation to flicker. Nutrients. 2020;12:1910. doi: 10.3390/nu12071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui B, Sun JH, Xiang FF, Liu L, Li WJ. Aquaporin 4 knockdown exacerbates streptozotocin-induced diabetic retinopathy through aggravating inflammatory response. Exp Eye Res. 2012;98:37–43. doi: 10.1016/j.exer.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Dada T, Mittal D, Mohanty K, Faiq MA, Bhat MA, Yadav RK, Sihota R, Sidhu T, Velpandian T, Kalaivani M, Pandey RM, Gao Y, Sabel BA, Dada R. Mindfulness meditation reduces intraocular pressure, lowers stress biomarkers and modulates gene expression in glaucoma: a randomized controlled trial. J Glaucoma. 2018;27:1061–1067. doi: 10.1097/IJG.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 12.Debacker C, Djemai B, Ciobanu L, Tsurugizawa T, Le Bihan D. Diffusion MRI reveals in vivo and non-invasively changes in astrocyte function induced by an aquaporin-4 inhibitor. PLoS One. 2020;15:e0229702. doi: 10.1371/journal.pone.0229702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng W, Liu C, Parra C, Sims JR, Faiq MA, Sainulabdeen A, Song H, Chan KC. Quantitative imaging of the clearance systems in the eye and the brain. Quant Imaging Med Surg. 2020;10:1–14. doi: 10.21037/qims.2019.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Depeint F, Bruce WR, Shangari N, Mehta R, O’Brien PJ. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006;163:94–112. doi: 10.1016/j.cbi.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Dibas A, Yang MH, He S, Bobich J, Yorio T. Changes in ocular aquaporin-4 (AQP4) expression following retinal injury. Mol Vis. 2008;14:1770–1783. [PMC free article] [PubMed] [Google Scholar]
- 16.Downie LE, Vingrys AJ. Oral omega-3 supplementation lowers intraocular pressure in normotensive adults. Transl Vis Sci Technol. 2018;7:1. doi: 10.1167/tvst.7.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab. 2019;39:1355–1368. doi: 10.1177/0271678X18760974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faiq MA, Wollstein G, Schuman JS, Chan KC. Cholinergic nervous system and glaucoma: From basic science to clinical applications. Prog Retin Eye Res. 2019;72:100767. doi: 10.1016/j.preteyeres.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faiq MA, Sainulabdeen A, Parra C, Wang X, Lee CH, Zhang J, Liu C, Deng W, Wollstein G, Schuman JS, Chan KC. In vivo contrast-enhanced MRI of cerebrospinal fluid dynamics in mouse optic nerve. Invest Ophthalmol Vis Sci. 2020;61:2767–2767. [Google Scholar]
- 20.Farr GW, Hall CH, Farr SM, Wade R, Detzel JM, Adams AG, Buch JM, Beahm DL, Flask CA, Xu K, LaManna JC, McGuirk PR, Boron WF, Pelletier MF. Functionalized phenylbenzamides inhibit aquaporin-4 reducing cerebral edema and improving outcome in two models of CNS injury. Neuroscience. 2019;404:484–498. doi: 10.1016/j.neuroscience.2019.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiwara K, Yasuda M, Hata J, Yoshida D, Kishimoto H, Hashimoto S, Yoshitomi T, Ninomiya T, Sonoda KH. Long-term regular exercise and intraocular pressure: the Hisayama Study. Graefes Arch Clin Exp Ophthalmol. 2019;257:2461–2469. doi: 10.1007/s00417-019-04441-9. [DOI] [PubMed] [Google Scholar]
- 22.Gakuba C, Gaberel T, Goursaud S, Bourges J, Di Palma C, Quenault A, Martinez de Lizarrondo S, Vivien D, Gauberti M. General anesthesia inhibits the activity of the “glymphatic system”. Theranostics. 2018;8:710–722. doi: 10.7150/thno.19154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallina P, Savastano A, Becattini E, Orlandini S, Scollato A, Rizzo S, Carreras G, Di Lorenzo N, Porfirio B. Glaucoma in patients with shunt-treated normal pressure hydrocephalus. J Neurosurg. 2018;129:1078–1084. doi: 10.3171/2017.5.JNS163062. [DOI] [PubMed] [Google Scholar]
- 24.Gan SW, Ran JH, Chen H, Ren ZQ, Sun SQ, Zhu SJ, Lu WT, Xu J, Zhang B, Huang J, Wang KJ, Chen Z. Lysosomal degradation of retinal glial AQP4 following its internalization induced by acute ocular hypertension. Neurosci Lett. 2012;516:135–140. doi: 10.1016/j.neulet.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 25.Gao FJ, Zhang SH, Xu P, Yang BQ, Zhang R, Cheng Y, Zhou XJ, Huang WJ, Wang M, Chen JY, Sun XH, Wu JH. Quercetin declines apoptosis, ameliorates mitochondrial function and improves retinal ganglion cell survival and function in in vivo model of glaucoma in rat and retinal ganglion cell culture in vitro. Front Mol Neurosci. 2017;10:285. doi: 10.3389/fnmol.2017.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M, Zhu SY, Tan CB, Xu B, Zhang WC, Du GH. Pinocembrin protects the neurovascular unit by reducing inflammation and extracellular proteolysis in MCAO rats. J Asian Nat Prod Res. 2010;12:407–418. doi: 10.1080/10286020.2010.485129. [DOI] [PubMed] [Google Scholar]
- 27.Glober NK, Sprague S, Ahmad S, Mayfield KG, Fletcher LM, Digicaylioglu MH, Sayre NL. Acetazolamide treatment prevents redistribution of astrocyte aquaporin 4 after murine traumatic brain injury. Neurosci J 2019. 2019 doi: 10.1155/2019/2831501. 2831501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong JL, Lou XT, Yuan YX, Chen LW, Ji PT, Li L, Zhao Y, Zhang H. The increased expression of GABA receptors within the arcuate nucleus is associated with high intraocular pressure. Mol Vis. 2018;24:574–586. [PMC free article] [PubMed] [Google Scholar]
- 30.Hablitz LM, Plá V, Giannetto M, Vinitsky HS, Stæger FF, Metcalfe T, Nguyen R, Benrais A, Nedergaard M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11:4411. doi: 10.1038/s41467-020-18115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasan-Olive MM, Enger R, Hansson HA, Nagelhus EA, Eide PK. Pathological mitochondria in neurons and perivascular astrocytic endfeet of idiopathic normal pressure hydrocephalus patients. Fluids Barriers CNS. 2019;16:39. doi: 10.1186/s12987-019-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, Pei Z, Xu GQ, Lan Y. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci. 2017;10:144. doi: 10.3389/fnmol.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill VM, O’Connor RM, Shirasu-Hiza M. Tired and stressed: examining the need for sleep. Eur J Neurosci. 2020;51:494–508. doi: 10.1111/ejn.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu P, Arfuso F, Madigan MC, Adamson S, Shaw LC, Boulton ME, Grant MB, Chan-Ling T. Evidence for a glymphatic system in human, primate, rat and mouse retina. Invest Ophthalmol Vis Sci. 2016;57 [Google Scholar]
- 35.Huang J, van Zijl PCM, Han X, Dong CM, Cheng GWY, Tse KH, Knutsson L, Chen L, Lai JHC, Wu EX, Xu J, Chan KWY. Altered d-glucose in brain parenchyma and cerebrospinal fluid of early Alzheimer's disease detected by dynamic glucose-enhanced MRI. Sci Adv. 2020;6:eaba3884. doi: 10.1126/sciadv.aba3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber VJ, Tsujita M, Nakada T. Identification of aquaporin 4 inhibitors using in vitro and in silico methods. Bioorg Med Chem. 2009;17:411–417. doi: 10.1016/j.bmc.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 37.Huber VJ, Tsujita M, Yamazaki M, Sakimura K, Nakada T. Identification of arylsulfonamides as aquaporin 4 inhibitors. Bioorg Med Chem Lett. 2007;17:1270–1273. doi: 10.1016/j.bmcl.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Huber VJ, Igarashi H, Ueki S, Kwee IL, Nakada T. Aquaporin-4 facilitator TGN-073 promotes interstitial fluid circulation within the blood-brain barrier: [17O]H2O JJVCPE MRI study. Neuroreport. 2018;29:697–703. doi: 10.1097/WNR.0000000000000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hui F, Tang J, Williams PA, McGuinness MB, Hadoux X, Casson RJ, Coote M, Trounce IA, Martin KR, van Wijngaarden P, Crowston JG. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: a crossover randomized clinical trial. Clin Exp Ophthalmol. 2020;48:903–914. doi: 10.1111/ceo.13818. [DOI] [PubMed] [Google Scholar]
- 40.Igarashi H, Huber VJ, Tsujita M, Nakada T. Pretreatment with a novel aquaporin 4 inhibitor, TGN-020, significantly reduces ischemic cerebral edema. Neurol Sci. 2011;32:113–116. doi: 10.1007/s10072-010-0431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Innes KE, Selfe TK, Brundage K, Montgomery C, Wen S, Kandati S, Bowles H, Khalsa DS, Huysmans Z. Effects of meditation and music-listening on blood biomarkers of cellular aging and Alzheimer's disease in adults with subjective cognitive decline: an exploratory randomized clinical trial. J Alzheimers Dis. 2018;66:947–970. doi: 10.3233/JAD-180164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobsen HH, Ringstad G, Jørstad Ø, Moe MC, Sandell T, Eide PK. The human visual pathway communicates directly with the subarachnoid space. Invest Ophthalmol Vis Sci. 2019;60:2773–2780. doi: 10.1167/iovs.19-26997. [DOI] [PubMed] [Google Scholar]
- 45.Jacobsen HH, Sandell T, Jørstad Ø, Moe MC, Ringstad G, Eide PK. In vivo evidence for impaired glymphatic function in the visual pathway of patients with normal pressure hydrocephalus. Invest Ophthalmol Vis Sci. 2020;61:24. doi: 10.1167/iovs.61.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jarius S, Aktas O, Wildemann B. Gamma-aminobutyric acid receptor agonists, aquaporin-4, and neuromyelitis optica: a potential link. Med Hypotheses. 2015;85:628–630. doi: 10.1016/j.mehy.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Jasien JV, Jonas JB, de Moraes CG, Ritch R. Intraocular pressure rise in subjects with and without glaucoma during four common yoga positions. PLoS One. 2015;10:e0144505. doi: 10.1371/journal.pone.0144505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a Beginner's guide. Neurochem Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin J, Xu GX, Yuan ZL. Influence of the hypothalamic arcuate nucleus on intraocular pressure and the role of opioid peptides. PLoS One. 2014;9:e82315. doi: 10.1371/journal.pone.0082315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalogerou M, Kolovos P, Prokopiou E, Papagregoriou G, Deltas C, Malas S, Georgiou T. Omega-3 fatty acids protect retinal neurons in the DBA/2J hereditary glaucoma mouse model. Exp Eye Res. 2018;167:128–139. doi: 10.1016/j.exer.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Katada R, Nishitani Y, Honmou O, Mizuo K, Okazaki S, Tateda K, Watanabe S, Matsumoto H. Expression of aquaporin-4 augments cytotoxic brain edema after traumatic brain injury during acute ethanol exposure. Am J Pathol. 2012;180:17–23. doi: 10.1016/j.ajpath.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Kida T, Oku H, Horie T, Fukumoto M, Okuda Y, Morishita S, Ikeda T. Implication of VEGF and aquaporin 4 mediating Müller cell swelling to diabetic retinal edema. Graefes Arch Clin Exp Ophthalmol. 2017;255:1149–1157. doi: 10.1007/s00417-017-3631-z. [DOI] [PubMed] [Google Scholar]
- 53.Killer HE, Laeng HR, Flammer J, Groscurth P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol. 2003;87:777–781. doi: 10.1136/bjo.87.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Killer HE, Miller NR, Flammer J, Meyer P, Weinreb RN, Remonda L, Jaggi GP. Cerebrospinal fluid exchange in the optic nerve in normal-tension glaucoma. Br J Ophthalmol. 2012;96:544–548. doi: 10.1136/bjophthalmol-2011-300663. [DOI] [PubMed] [Google Scholar]
- 55.Kimball E, Schaub J, Quillen S, Keuthan C, Pease ME, Korneva A, Quigley H. The role of aquaporin-4 in optic nerve head astrocytes in experimental glaucoma. PLoS One. 2021;16:e0244123. doi: 10.1371/journal.pone.0244123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komaki Y, Debacker C, Djemai B, Ciobanu L, Tsurugizawa T, Bihan DL. Differential effects of aquaporin-4 channel inhibition on BOLD fMRI and diffusion fMRI responses in mouse visual cortex. PLoS One. 2020;15:e0228759. doi: 10.1371/journal.pone.0228759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar B, Gupta SK, Nag TC, Srivastava S, Saxena R, Jha KA, Srinivasan BP. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp Eye Res. 2014;125:193–202. doi: 10.1016/j.exer.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Layne KA, Dargan PI, Archer JRH, Wood DM. Gadolinium deposition and the potential for toxicological sequelae - A literature review of issues surrounding gadolinium-based contrast agents. Br J Clin Pharmacol. 2018;84:2522–2534. doi: 10.1111/bcp.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee B, Lim C, Lim S, Cho S. Baicalin administered orally after ischemia/reperfusion alleviated brain injury in mice by inhibiting inflammation and edema. Nat Prod Commun. 2019a;14:1–7. [Google Scholar]
- 60.Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H. The effect of body posture on brain glymphatic transport. J Neurosci. 2015;35:11034–11044. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee MJ, Wang J, Friedman DS, Boland MV, De Moraes CG, Ramulu PY. Greater physical activity is associated with slower visual field loss in glaucoma. Ophthalmology. 2019b;126:958–964. doi: 10.1016/j.ophtha.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leon-Mercado L, Herrera Moro Chao D, Basualdo MD, Kawata M, Escobar C, Buijs RM. The arcuate nucleus: a site of fast negative feedback for corticosterone secretion in male rats. eNeuro. 2017;4 doi: 10.1523/ENEURO.0350-16.2017. doi: 10.1523/ENEURO.0350-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Qin J, Fu T, Shen J. Fisetin rescues retinal functions by suppressing inflammatory response in a DBA/2J mouse model of glaucoma. Doc Ophthalmol. 2019;138:125–135. doi: 10.1007/s10633-019-09676-9. [DOI] [PubMed] [Google Scholar]
- 64.Lin TW, Shih YH, Chen SJ, Lien CH, Chang CY, Huang TY, Chen SH, Jen CJ, Kuo YM. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer's disease (APP/PS1) transgenic mice. Neurobiol Learn Mem. 2015;118:189–197. doi: 10.1016/j.nlm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu Y, Dong Y, Tucker D, Wang R, Ahmed ME, Brann D, Zhang Q. Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer's disease. J Alzheimers Dis. 2017;56:1469–1484. doi: 10.3233/JAD-160869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maher P, Hanneken A. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Invest Ophthalmol Vis Sci. 2005;46:4796–4803. doi: 10.1167/iovs.05-0397. [DOI] [PubMed] [Google Scholar]
- 68.Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci. 2006;23:2929–2936. doi: 10.1111/j.1460-9568.2006.04829.x. [DOI] [PubMed] [Google Scholar]
- 69.Margaris KN, Black RA. Modelling the lymphatic system: challenges and opportunities. J R Soc Interface. 2012;9:601–612. doi: 10.1098/rsif.2011.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathieu E, Gupta N, Ahari A, Zhou X, Hanna J, Yucel YH. Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Invest Ophthalmol Vis Sci. 2017;58:4784–4791. doi: 10.1167/iovs.17-22290. [DOI] [PubMed] [Google Scholar]
- 71.Mathieu E, Gupta N, Paczka-Giorgi LA, Zhou X, Ahari A, Lani R, Hanna J, Yücel YH. Reduced cerebrospinal fluid inflow to the optic nerve in glaucoma. Invest Ophthalmol Vis Sci. 2018;59:5876–5884. doi: 10.1167/iovs.18-24521. [DOI] [PubMed] [Google Scholar]
- 72.Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, Monai H, Murlidharan G, Castellanos Rivera RM, Simon MJ, Pike MM, Plá V, Du T, Kress BT, Wang X, Plog BA, Thrane AS, Lundgaard I, Abe Y, Yasui M, et al. (2018) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife. 7:e40070. doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mørch MT, Sørensen SF, Khorooshi R, Asgari N, Owens T. Selective localization of IgG from cerebrospinal fluid to brain parenchyma. J Neuroinflammation. 2018;15:110. doi: 10.1186/s12974-018-1159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreno MC, de Zavalía N, Sande P, Jaliffa CO, Fernandez DC, Keller Sarmiento MI, Rosenstein RE. Effect of ocular hypertension on retinal GABAergic activity. Neurochem Int. 2008;52:675–682. doi: 10.1016/j.neuint.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 75.Munk AS, Wang W, Bechet NB, Eltanahy AM, Cheng AX, Sigurdsson B, Benraiss A, Mae MA, Kress BT, Kelley DH, Betsholtz C, Mollgard K, Meissner A, Nedergaard M, Lundgaard I. PDGF-B is required for development of the glymphatic system. Cell Rep. 2019;26:2955–2969. doi: 10.1016/j.celrep.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. J Neurosci. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen CT, Bui BV, Sinclair AJ, Vingrys AJ. Dietary omega 3 fatty acids decrease intraocular pressure with age by increasing aqueous outflow. Invest Ophthalmol Vis Sci. 2007;48:756–762. doi: 10.1167/iovs.06-0585. [DOI] [PubMed] [Google Scholar]
- 79.Nilforushan N, Abolfathzadeh N, Banifatemi M, Miraftabi A, Sardarinia M, Alemzadeh SA, Nilforushan A. Effects of fasting on peripapillary capillary density, peripapillary nerve fiber layer, intraocular pressure and central corneal thickness. Int Ophthalmol. 2020;40:1439–1447. doi: 10.1007/s10792-020-01310-x. [DOI] [PubMed] [Google Scholar]
- 80.Nishikawa Y, Oku H, Morishita S, Horie T, Kida T, Mimura M, Fukumoto M, Kojima S, Ikeda T. Negative impact of AQP-4 channel inhibition on survival of retinal ganglion cells and glutamate metabolism after crushing optic nerve. Exp Eye Res. 2016;146:118–127. doi: 10.1016/j.exer.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Ohene Y, Harrison IF, Nahavandi P, Ismail O, Bird EV, Ottersen OP, Nagelhus EA, Thomas DL, Lythgoe MF, Wells JA. Non-invasive MRI of brain clearance pathways using multiple echo time arterial spin labelling: an aquaporin-4 study. Neuroimage. 2019;188:515–523. doi: 10.1016/j.neuroimage.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oosuka S, Kida T, Oku H, Horie T, Morishita S, Fukumoto M, Sato T, Ikeda T. Effects of an aquaporin 4 inhibitor, TGN-020, on murine diabetic retina. Int J Mol Sci. 2020;21:2324. doi: 10.3390/ijms21072324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peña-Bautista C, Casas-Fernández E, Vento M, Baquero M, Cháfer-Pericás C. Stress and neurodegeneration. Clin Chim Acta. 2020;503:163–168. doi: 10.1016/j.cca.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 84.Petzold A. Retinal glymphatic system: an explanation for transient retinal layer volume changes? Brain. 2016;139:2816–2819. doi: 10.1093/brain/aww239. [DOI] [PubMed] [Google Scholar]
- 85.Pircher A, Montali M, Wostyn P, Pircher J, Berberat J, Remonda L, Killer HE. Impaired cerebrospinal fluid dynamics along the entire optic nerve in normal-tension glaucoma. Acta Ophthalmol. 2018;96:e562–569. doi: 10.1111/aos.13647. [DOI] [PubMed] [Google Scholar]
- 86.Qiu M, Ramulu PY, Boland MV. Association between sleep parameters and glaucoma in the United States population: national health and nutrition examination survey. J Glaucoma. 2019;28:97–104. doi: 10.1097/IJG.0000000000001169. [DOI] [PubMed] [Google Scholar]
- 87.Ren H, Luo C, Feng Y, Yao X, Shi Z, Liang F, Kang JX, Wan JB, Pei Z, Su H. Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. Faseb J. 2017;31:282–293. doi: 10.1096/fj.201600896. [DOI] [PubMed] [Google Scholar]
- 88.Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017;140:2691–2705. doi: 10.1093/brain/awx191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romeo Villadóniga S, Rodríguez García E, Sagastagoia Epelde O, Álvarez Díaz MD, Domingo Pedrol JC. Effects of oral supplementation with docosahexaenoic acid (DHA) plus antioxidants in pseudoexfoliative glaucoma: a 6-month open-label randomized trial. J Ophthalmol 2018. 2018 doi: 10.1155/2018/8259371. 8259371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosu GC, Catalin B, Balseanu TA, Laurentiu M, Claudiu M, Kumar-Singh S, Daniel P. Inhibition of aquaporin 4 decreases amyloid Aβ40 drainage around cerebral vessels. Mol Neurobiol. 2020;57:4720–4734. doi: 10.1007/s12035-020-02044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sabel BA, Wang J, Cárdenas-Morales L, Faiq M, Heim C. Mental stress as consequence and cause of vision loss: the dawn of psychosomatic ophthalmology for preventive and personalized medicine. Epma J. 2018;9:133–160. doi: 10.1007/s13167-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schey KL, Wang Z, L Wenke J, Qi Y. Aquaporins in the eye: expression, function, and roles in ocular disease. Biochim Biophys Acta. 2014;1840:1513–1523. doi: 10.1016/j.bbagen.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schnebelen C, Pasquis B, Salinas-Navarro M, Joffre C, Creuzot-Garcher CP, Vidal-Sanz M, Bron AM, Bretillon L, Acar N. A dietary combination of omega-3 and omega-6 polyunsaturated fatty acids is more efficient than single supplementations in the prevention of retinal damage induced by elevation of intraocular pressure in rats. Graefes Arch Clin Exp Ophthalmol. 2009;247:1191–1203. doi: 10.1007/s00417-009-1094-6. [DOI] [PubMed] [Google Scholar]
- 94.Skjolding AD, Rowland IJ, Søgaard LV, Praetorius J, Penkowa M, Juhler M. Hydrocephalus induces dynamic spatiotemporal regulation of aquaporin-4 expression in the rat brain. Cerebrospinal Fluid Res. 2010;7:20. doi: 10.1186/1743-8454-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Speake T, Freeman LJ, Brown PD. Expression of aquaporin 1 and aquaporin 4 water channels in rat choroid plexus. Biochim Biophys Acta. 2003;1609:80–86. doi: 10.1016/s0005-2736(02)00658-2. [DOI] [PubMed] [Google Scholar]
- 96.Sturdivant NM, Smith SG, Ali SF, Wolchok JC, Balachandran K. Acetazolamide mitigates astrocyte cellular edema following mild traumatic brain injury. Sci Rep. 2016;6:33330. doi: 10.1038/srep33330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suzuki H, Oku H, Horie T, Morishita S, Tonari M, Oku K, Okubo A, Kida T, Mimura M, Fukumoto M, Kojima S, Takai S, Ikeda T. Changes in expression of aquaporin-4 and aquaporin-9 in optic nerve after crushing in rats. PLoS One. 2014;9:e114694. doi: 10.1371/journal.pone.0114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci U S A. 2010;107:15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanimura Y, Hiroaki Y, Fujiyoshi Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J Struct Biol. 2009;166:16–21. doi: 10.1016/j.jsb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 100.Tehrani S, Johnson EC, Cepurna WO, Morrison JC. Astrocyte processes label for filamentous actin and reorient early within the optic nerve head in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2014;55:6945–6952. doi: 10.1167/iovs.14-14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tesse A, Grossini E, Tamma G, Brenner C, Portincasa P, Marinelli RA, Calamita G. Aquaporins as targets of dietary bioactive phytocompounds. Front Mol Biosci. 2018;5:30. doi: 10.3389/fmolb.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tong J, Briggs MM, McIntosh TJ. Water permeability of aquaporin-4 channel depends on bilayer composition, thickness, and elasticity. Biophys J. 2012;103:1899–1908. doi: 10.1016/j.bpj.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van der Merwe Y, Murphy MC, Sims JR, Faiq MA, Yang XL, Ho LC, Conner IP, Yu Y, Leung CK, Wollstein G, Schuman JS, Chan KC. Citicoline modulates glaucomatous neurodegeneration through intraocular pressure-independent control. Neurotherapeutics. 2021 doi: 10.1007/s13311-021-01033-6. doi: 10.1007/s13311-021-01033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verheggen ICM, Van Boxtel MPJ, Verhey FRJ, Jansen JFA, Backes WH. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci Biobehav Rev. 2018;90:26–33. doi: 10.1016/j.neubiorev.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 105.Verkman AS, Smith AJ, Phuan PW, Tradtrantip L, Anderson MO. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin Ther Targets. 2017;21:1161–1170. doi: 10.1080/14728222.2017.1398236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.von Holstein-Rathlou S, Petersen NC, Nedergaard M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci Lett. 2018;662:253–258. doi: 10.1016/j.neulet.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wallisch JS, Janesko-Feldman K, Alexander H, Jha RM, Farr GW, McGuirk PR, Kline AE, Jackson TC, Pelletier MF, Clark RSB, Kochanek PM, Manole MD. The aquaporin-4 inhibitor AER-271 blocks acute cerebral edema and improves early outcome in a pediatric model of asphyxial cardiac arrest. Pediatr Res. 2019;85:511–517. doi: 10.1038/s41390-018-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang X, Lou N, Eberhardt A, Yang Y, Kusk P, Xu Q, Förstera B, Peng S, Shi M, Ladrón-de-Guevara A, Delle C, Sigurdsson B, Xavier ALR, Ertürk A, Libby RT, Chen L, Thrane AS, Nedergaard M. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci Transl Med. 2020;12:eaaw3210. doi: 10.1126/scitranslmed.aaw3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams MA, Malm J. Diagnosis and treatment of idiopathic normal pressure hydrocephalus. Continuum (Minneap Minn) 2016;22:579–599. doi: 10.1212/CON.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Williams PA, Harder JM, Cardozo BH, Foxworth NE, John SWM. Nicotinamide treatment robustly protects from inherited mouse glaucoma. Commun Integr Biol. 2018;11:e1356956. doi: 10.1080/19420889.2017.1356956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, Smithies O, John SW. Vitamin B(3) modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355:756–760. doi: 10.1126/science.aal0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wostyn P. Do normal-tension and high-tension glaucoma result from brain and ocular glymphatic system disturbances, respectively? Eye (Lond) 2020 doi: 10.1038/s41433-020-01219-w. doi: 10.1038/s41433-020-01219-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wostyn P, Killer HE, De Deyn PP. Glymphatic stasis at the site of the lamina cribrosa as a potential mechanism underlying open-angle glaucoma. Clin Exp Ophthalmol. 2017;45:539–547. doi: 10.1111/ceo.12915. [DOI] [PubMed] [Google Scholar]
- 114.Wostyn P, Van Dam D, Audenaert K, Killer HE, De Deyn PP, De Groot V. A new glaucoma hypothesis: a role of glymphatic system dysfunction. Fluids Barriers CNS. 2015;12:16. doi: 10.1186/s12987-015-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wostyn P, De Groot V, Van Dam D, Audenaert K, Killer HE, De Deyn PP. Age-related macular degeneration, glaucoma and Alzheimer's disease: amyloidogenic diseases with the same glymphatic background? Cell Mol Life Sci. 2016;73:4299–4301. doi: 10.1007/s00018-016-2348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu C, Feng Y, Zhang Q, Liang F, Lan Y, Pei Z, Xu G. Glutamate and γ-aminobutyric acid differentially modulate glymphatic clearance of amyloid β through pulsation- and aquaporin-4 dependent mechanisms. bioRxiv. 2020 DOI: 10.1101/2020.01.31.928481. [Google Scholar]
- 117.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yan L, Xie Y, Satyanarayanan SK, Zeng H, Liu Q, Huang M, Ma Y, Wan JB, Yao X, Su KP, Su H. Omega-3 polyunsaturated fatty acids promote brain-to-blood clearance of β-Amyloid in a mouse model with Alzheimer's disease. Brain Behav Immun. 2020;85:35–45. doi: 10.1016/j.bbi.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 119.Yang MH, Dibas A, Tyan YC. Changes in retinal aquaporin-9 (AQP9) expression in glaucoma. Biosci Rep. 2013;33:e00035. doi: 10.1042/BSR20130005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yucel Y, Gupta N. Lymphatic drainage from the eye: a new target for therapy. Prog Brain Res. 2015;220:185–198. doi: 10.1016/bs.pbr.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 121.Zeka B, Hastermann M, Kaufmann N, Schanda K, Pende M, Misu T, Rommer P, Fujihara K, Nakashima I, Dahle C, Leutmezer F, Reindl M, Lassmann H, Bradl M. Aquaporin 4-specific T cells and NMO-IgG cause primary retinal damage in experimental NMO/SD. Acta Neuropathol Commun. 2016;4:82. doi: 10.1186/s40478-016-0355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang D, Vetrivel L, Verkman AS. Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J Gen Physiol. 2002;119:561–569. doi: 10.1085/jgp.20028597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang HB, Tu XK, Song SW, Liang RS, Shi SS. Baicalin reduces early brain injury after subarachnoid hemorrhage in rats. Chin J Integr Med. 2020;26:510–518. doi: 10.1007/s11655-020-3183-7. [DOI] [PubMed] [Google Scholar]
- 124.Zhang J, Zhan Z, Li X, Xing A, Jiang C, Chen Y, Shi W, An L. Intermittent fasting protects against Alzheimer's disease possible through restoring aquaporin-4 polarity. Front Mol Neurosci. 2017;10:395. doi: 10.3389/fnmol.2017.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou X, Li G, Yang B, Wu J. Quercetin enhances inhibitory synaptic inputs and reduces excitatory synaptic inputs to off- and on-type retinal ganglion cells in a chronic glaucoma rat model. Front Neurosci. 2019;13:672. doi: 10.3389/fnins.2019.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou X, Cheng Y, Zhang R, Li G, Yang B, Zhang S, Wu J. Alpha7 nicotinic acetylcholine receptor agonist promotes retinal ganglion cell function via modulating GABAergic presynaptic activity in a chronic glaucomatous model. Sci Rep. 2017;7:1734. doi: 10.1038/s41598-017-02092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]