Abstract
A recent theory posits that prediction deficits may underlie the core symptoms in autism spectrum disorder (ASD). However, empirical evidence for this hypothesis is minimal. Using a visual extrapolation task, we tested motion prediction abilities in children and adolescents with and without ASD. We examined the factors known to be important for motion prediction: the central-tendency response bias and smooth pursuit eye movements. In ASD, response biases followed an atypical trajectory that was dominated by early responses. This differed from controls who exhibited response biases that reflected a gradual accumulation of knowledge about stimulus statistics. Moreover, while better smooth pursuit eye movements for the moving object were linked to more accurate motion prediction in controls, in ASD, better smooth pursuit was counterintuitively linked to a more pronounced early response bias. Together, these results demonstrate atypical visual prediction abilities in ASD and offer insights into possible mechanisms underlying the observed differences.
Keywords: Autism, prediction, perception, vision, eye movements
Introduction
Prediction is a fundamental brain function that enables more effective interaction with an inherently dynamic environment (Heeger, 2017). It allows us to not only make inferences about the future, but also to interpret the current events in the context of past history. A recent theory proposes that this ability to make predictions is impaired in individuals with autism spectrum disorder (ASD; Gomot & Wicker, 2012; Hellendoorn, Wijnroks, & Leseman, 2015; Pellicano & Burr, 2012; Sinha, Kjelgaard, Gandhi, Tsourides, & Cardinaux, 2014). The theory posits that the seemingly distinct core deficits associated with ASD—social communication challenges and the presence of restricted and repetitive behaviors—may be explained by fundamental deficits in detecting predictive relationships in the environment (Sinha et al., 2014). For example, theory of mind, a well-known challenge for individuals with ASD (e.g., Baron-Cohen, 2000), requires predicting another individual’s emotional or cognitive states based on both immediately available social cues and one’s previous history of observations about this person. Similarly, ASD symptoms related to insistence on sameness have been conceptualized as a way to cope with the unpredictability in our world (Markram & Markram, 2010; Sinha et al., 2014).
The present study examined the integrity of visual motion prediction abilities in ASD—a domain where events unfold rapidly, sometimes within a millisecond timescale. A prediction impairment on such a brief time can have negative consequences on everyday visual tasks where individuals interact with dynamic objects. For instance, children with ASD may have difficulty catching balls (Jasmin et al., 2009; Whyatt & Craig, 2012), which is often attributed to gross motor difficulties. However, deficits in visual prediction may offer an alternative explanation, one that is more consistent with first-hand accounts of such difficulties (Sinha et al., 2014). Also, visual motion processing is a well-characterized sensory function and has been used in a wide range of studies to make inferences about higher-level cognitive functions (Park & Tadin, 2018; Pasternak & Tadin, 2020). Thus, testing visual motion prediction offers a tractable way to study prediction mechanisms in ASD, with possible implications for both perceptual behavior and general prediction abilities in ASD.
Prediction, even in its arguably simpler visual form, is not a unitary process. Motion prediction performance is affected by several key perceptual and oculomotor factors (Bosco et al., 2015). A growing number of studies emphasizes the role of extra-retinal signals (i.e., sources of motion information available outside of retinal visual signals), such as those from eye movements, in facilitating motion prediction. Typically, observers visually track a moving object using smooth pursuit eye movements. As the object disappears behind an occluder, smooth pursuit velocity diminishes and people make predictive saccadic eye movements to the target location where the object would arrive (Bennett & Barnes, 2003, 2004; Diaz, Cooper, Rothkopf, & Hayhoe, 2013; Orban de Xivry, Bennett, Lefèvre, & Barnes, 2006). Critically, studies have shown that better quality of pursuit is associated with better prediction performance (Delle Monache, Lacquaniti, & Bosco, 2014; Spering, Dias, Sanchez, Schütz, & Javitt, 2013; Spering, Schütz, Braun, & Gegenfurtner, 2011). The rationale is that extra-retinal signals from smooth pursuit provide additional information about object motion (Spering et al., 2011), which is especially useful when visual stimulation is absent (e.g., during occlusion).
Motion prediction relies not only on current sensory inputs, but also on the recent history of object motion (Kwon & Knill, 2013). As we gain knowledge on the statistics of a stimulus distribution, we integrate that with the current sensory information to make perceptual judgements (Knill & Pouget, 2004). As a result, our responses are subject to different sources of bias, depending on the statistics of the learned stimulus distributions. One particular example is the central-tendency bias (Hollingworth, 1910), where our perceptual estimates are biased towards (or away from) the mean of the stimulus distribution. This pattern is observed not only in motion prediction (Kwon & Knill, 2013), but also in a wide range of tasks and domains (Hollingworth, 1910; Jazayeri & Shadlen, 2010; Makin, Stewart, & Poliakoff, 2009; Verstynen & Sabes, 2011). While the existence of bias might seem maladaptive on the first glance, it reflects the visual system’s strategy to make the best possible predictions under conditions of uncertainty by using prior observations. In fact, overall performance error decreases when the magnitude of the central-tendency bias is optimal (e.g., Jazayeri & Shadlen, 2010).
Thus, together with the extra-retinal signals, prior knowledge of stimulus distributions is a cue we can rely on for visual motion prediction. This may be of particular importance in ASD given recent proposals on how individuals with ASD integrate current sensory estimates with past knowledge. Specifically, the theory postulates that individuals with ASD are less influenced by prior information when making perceptual judgments (Pellicano & Burr, 2012; although see Brock, 2012 for an alternative hypothesis). Similar patterns of behavior (i.e., increased sensitivity to current sensory stimulation and less emphasis on the past) have been more recently hypothesized and characterized by a broader framework of predictive processing in ASD as a tendency for greater weighting on prediction errors (e.g., Van de Cruys et al., 2014; for a review, see Palmer, Lawson, & Hohwy, 2017).
Studies in ASD show mixed findings on whether motion prediction abilities and the associated ocular responses are impaired in this population. A recent study reported an impairment in young adults with ASD when making predictions during straight self-motion (i.e., forward motion) but not curved trajectories (Sheppard, Van Loon, Underwood, & Ropar, 2016), while another study reported no deficits in children and young adolescents with ASD for horizontal motion (Tewolde, Bishop, & Manning, 2018). Some evidence suggests atypicalities in a variety of basic eye movement characteristics across a wide age range in ASD (Freedman & Foxe, 2017; Sweeney, Takarae, Macmillan, Luna, & Minshew, 2004; Takarae, Minshew, Luna, Krisky, & Sweeney, 2004), which in turn can deteriorate prediction where extra-retinal signals might be essential. In tasks where eye movements reflected predictions, fewer predictive saccades in adolescents with ASD have been reported (Goldberg et al., 2002). However, other studies have shown intact anticipatory pursuit (Aitkin, Santos, & Kowler, 2013; Ego, Bonhomme, et al., 2016), and intact pursuit gain and predictive acceleration during occlusion (Ego, Bonhomme, et al., 2016) in adolescents and young adults with ASD. These studies used highly ‘predictable’ conditions (e.g., saccade targets alternated at regular intervals, or use of a fixed occlusion duration) to specifically assess whether the eye movements reflect or aid predictions. Such regularity, however, makes it difficult to assess possible group differences in more dynamic conditions that are common in the natural environment.
Here, we investigated motion prediction abilities in children and adolescents with ASD and age- and IQ-matched typically developing (TD) controls. We used a simple hitting task (Fig. 1A) where participants extrapolated the motion of a briefly presented stimulus (constant speed) and made a key-press response when they thought the stimulus had arrived at a target location (Kwon & Knill, 2013). The task is similar to natural interception behaviors, which often directly impose prediction demands. Imagine catching a ball in baseball. The available visual information is limited by various sources (e.g., occlusion from other players or changes in one’s own gaze) such that one needs to predict the motion trajectory for appropriate action. To evaluate individuals’ prediction performance and understand how they make use of available information, we manipulated the stimulus duration before occlusion (visible duration) as well as the time-to-arrival (occluded duration).
Figure 1.
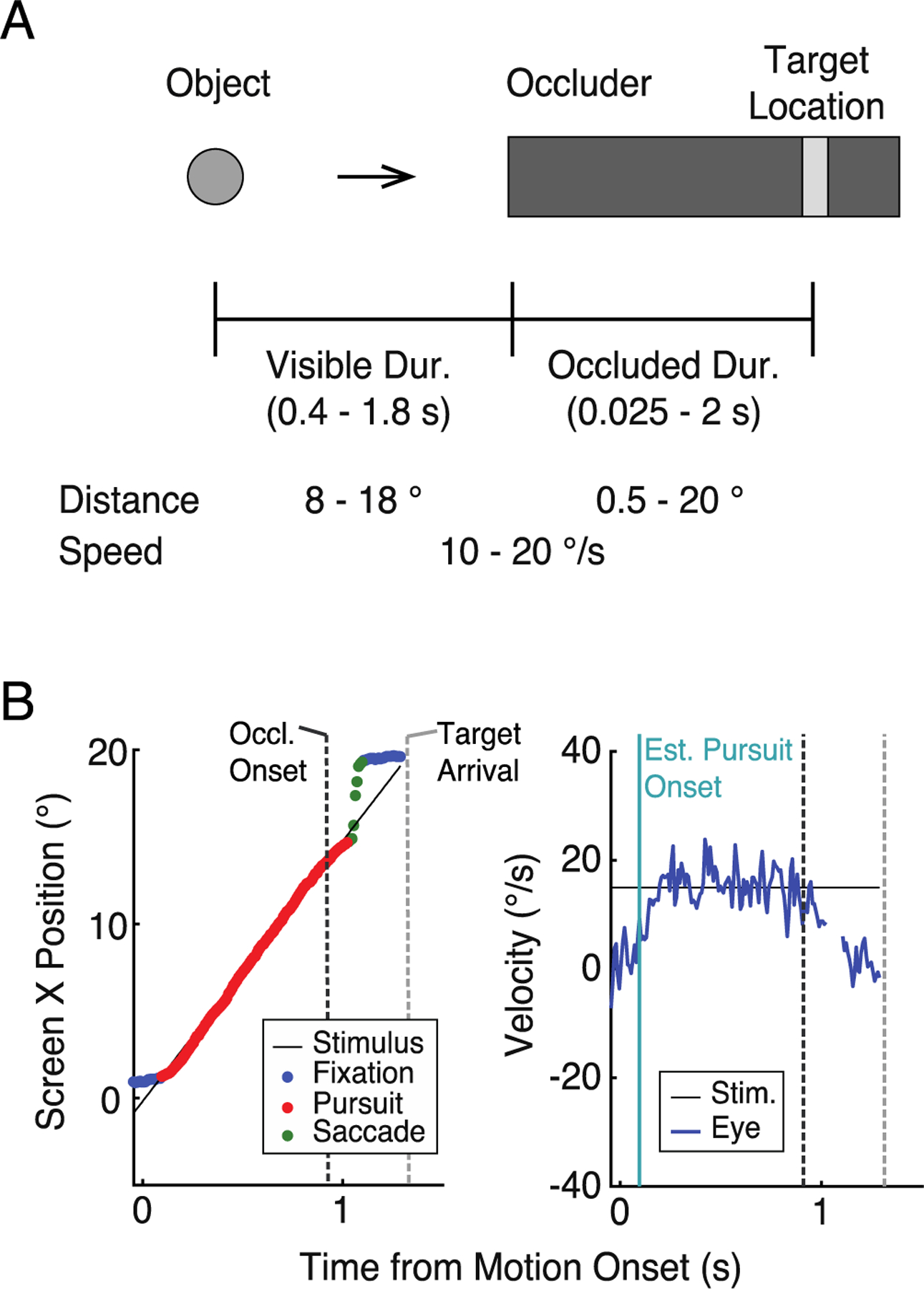
A: Schematic illustrations of experimental setting. The actual moving object was a bird from a popular smartphone game (Angry Birds; Rovio Entertainment, 2009), here depicted as a grey circle. The object moved for varying durations (visible duration) before it disappeared behind the occluder (darker grey bar). Participants responded when they thought that the object had arrived at the target location (lighter grey bar). The actual target arrival time (occluded duration) also varied from trial to trial. The visible and occluded durations were determined by sampling the distance and speed from uniform distributions (see Methods). B: An example eye movement trace from one participant. Darker and lighter vertical dashed lines indicate occluder onset and the actual target arrival time, respectively. Left: Horizontal eye position. Each dot is an eye movement sample with the color reflecting the classification based on our analysis (see Methods). Right: Eye velocity trace, with saccade removed.
The goal of the study was twofold. First, we tested whether individuals with ASD are impaired in motion prediction. For this, we measured the different types of prediction errors, that is, bias (non-random error) and variability (random error) within each participant’s behavioral key-press responses. Based on proposals of prediction in ASD, we hypothesized worse prediction performance (i.e., larger bias and/or greater intra-individual variability in behavioral responses) in this population. Such impairment should be more prominent at longer occluded durations where there is greater prediction demand.
Second, we investigated whether the prediction performance in ASD is related to atypicalities in the use of relevant information that are known to affect motion prediction: a) central-tendency bias and b) smooth pursuit eye movements. Specifically, we examined whether individuals with ASD learn and integrate the statistics of past hitting time (i.e., occluded duration) into their predictions. This effect would manifest itself as a progressive development of central-tendency bias, where their behavioral responses are biased towards the mean of the distribution throughout the experiment (Kwon & Knill, 2013). For smooth pursuit eye movements, we tested whether individuals with ASD take advantage of smooth pursuit in making predictions. Past studies have shown that, in typical populations, prediction performance is better when participants visually pursuit a moving object (vs. when they fixate; Spering et al., 2013, 2011). Moreover, these studies have also observed that, as stimulus presentation (visible duration) becomes longer, both the smooth pursuit quality and prediction performance increases, suggesting that participants benefit from smooth pursuit in making motion predictions. Based on this finding, we examined the integrity of such relationship between smooth pursuit and prediction performance in ASD across visible durations. Together, these analyses allowed us to better parse out the prediction errors driven by different sources and gain further insights on possible differential use of relevant cues for motion prediction in ASD.
Methods
Participants
Twenty-six children and adolescents (ages 9–17 years) with ASD (25 male) and 20 TD controls (18 male) participated in the study. Participants were recruited to be within this age range with IQ > 80. Exclusion criteria for both groups included uncorrected vision (screened over the phone and confirmed at the first lab visit), diagnosis of a neurological disorder or injury, or injuries affecting eye movements. TD participants were further excluded if they had received other mental health (e.g. ADHD, depression, anxiety) or learning/behavioral diagnoses or if they had a 1st degree relative with an ASD. Participants recruited for the ASD group were required to have a previous clinical diagnosis of ASD.
We confirmed or ruled out an ASD diagnosis at the research visit with a combination of the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999), and either the Autism Diagnostic Interview-Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003) with parents of participants with ASD, or the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003) with parents of TD participants. A research-reliable examiner administered the ADOS and ADI-R, and a licensed clinical psychologist made final diagnostic decisions. In a subset of ASD participants (n = 19), we also collected a 20-item parent-report measure of symptoms of inattention and impulsivity, the SNAP-IV (Bussing et al., 2008). IQ was measured by abbreviated versions of the Wechsler Intelligence Scale for Children, 4th Edition (Wechsler, 2003) or the Wechsler Adult Intelligence Scale, 4th Edition (Wechsler, 2008), selected based on participant age.
The groups were matched both on age (ASD: mean = 13.3, standard deviation (SD) = 2.0; TD: mean = 13.6, SD = 2.3; t(44) = .46, p = .65) and full scale IQ (ASD: mean = 106.1, SD = 16.7; TD: mean = 113.5, SD = 14.6; t(44) = 1.6, p = .12). The mean ADOS severity score for ASD was 6.85 (SD = 1.41) and for TD was 1.45 (SD = .89). Parents reported on their child’s race/ethnicity and annual household income. 87% identified as White/Caucasian (18 TD and 22 ASD) and 13% as more than one race (2 TD and 4 ASD). Annual household income was distributed as follows: 13% under $50,000 (5 ASD), 13% $50–75,000 (4 TD and 2 ASD), 26% $75–100,000 (6 TD and 6 ASD), 32.6% $100–200,000 (6 TD and 9 ASD), 4.3% over $200,000 (1 TD and 1 ASD). Income information for five participants (2 ASD and 3 TD) was not reported. ASD participants were generally high-functioning (Full Scale IQ > 80 for all but one participant) and all had fluent and complex speech. Several participants were excluded from eye tracking analyses due to calibration difficulties with individuals wearing glasses (5 ASD and 1 TD) or fewer than 15% of trials being deemed as usable (3 ASD and 1 TD; see Eye movement analysis for details). Thus, the sample for all eye movement analyses consisted of 18 participants with ASD and 18 TD controls. This subsample was also matched on age (t(34) = −.25, p = .81) and full scale IQ (t(34) = −1.07, p = .29). The mean ADOS severity score for this subset of ASD participants was 6.61.
All participants had normal or corrected-to-normal visual acuity (20/40) as assessed with the Snellen eye chart. Parents gave written informed consent, and participants gave assent. All participants were paid for participation. Procedures were approved by the Research Subjects Review Board at the University of Rochester in accordance with the Declaration of Helsinki.
Apparatus
Stimuli were created in MATLAB and Psychophysics Toolbox (Brainard, 1997; Pelli, 1997), and were shown on a customized linear DLP projector (DepthQ WXGA 360 at 1280 × 720 resolution). The projector presents greyscale images at a frame rate of 120 Hz. Viewing distance was 135 cm, with each pixel subtending 2 arcmin of visual angle. Eye position was recorded using a desk-mounted Eyelink 1000 video-based eye tracker (SR Research) at a sampling rate of 120 Hz, matching the frame rate of our display. Recording was binocular, but only the data from one eye (selected based on which eye had the smaller overall SD in eye velocity on a given trial) was analyzed. We recorded eye position from 250 ms before the onset of stimulus motion until participants’ response. Calibration was performed at the beginning of each block. A chin rest was used to support a still seated position, and an experimenter was in the experiment room with participants to encourage on-task behavior. An interactive visual schedule, where participants marked the completion of each block, was used to facilitate progress and maintain motivation. Subjectively, we observed a high degree of participant motivation throughout the experiment. We speculate that this is, in part, due to the game-like task design of our experiment.
Stimuli, task, and experimental design
The stimulus was a moving bird (2 × 2°) from a popular mobile game, Angry Birds (Rovio Entertainment, 2009) presented on a grey background (Fig. 1A). The initial position of the stimulus was always at the left most side of the screen, and the moving direction was always left to right. The occluder was a long rectangle (dark grey) with 5° height which extended to the right most side of the screen. Within the occluder, a bar (light grey; 1° width and same height as the occluder) was placed to indicate the target location.
We manipulated the main variables of our study—visible and occluded durations—by independently and randomly sampling the stimulus speed, visible distance, and occluded distance (i.e., target location). Across trials, speed and visible distance were randomly sampled from a uniform distribution (speed: 10–20°/s; visible distance: 8–18°). For each trial, the stimulus moved at a constant speed and fixed distance before it disappeared behind an occluder. Together, this resulted in a distribution of visible durations that ranged between 0.4 and 1.8 s. The occluded distance was randomly sampled from a uniform distribution between 0.5 and 20°, yielding a range of occluded durations between 0.025 and 2 s.
Each trial began with a dynamic circle (Foss-Feig, Tadin, Schauder, & Cascio, 2013) that shrank in size from 0.63 to 0.1° in radius over 250 ms at the initial stimulus position and disappeared. After 250 ms, the stimulus appeared on the screen, moved horizontally rightward and disappeared behind the occluder. Participants were instructed to press the space bar when they thought the stimulus had arrived at the target location. A trial was counted as ‘correct’ if the distance between participants’ response (i.e., as indicated by the location of the stimulus center when the space bar was pressed) and the designated target location was less than 1.5°. On these correct trials, the bird visually bounced by increasing and decreasing its size repetitively over 1 s, paired with a sound. When participants were ‘incorrect’, the position of the bird at the time of participants’ button press was shown for 1 s as visual feedback. Note that ‘correct’ and ‘incorrect’ trials were used only for feedback; participants’ keyboard responses were recorded and analyzed as a continuous measure (i.e., when they pressed the space bar in relation to the actual arrival time at the target location).
To evaluate the central-tendency bias and ensure that the statistical properties of the stimulus distributions are appropriately learned (Berniker, Voss, & Kording, 2010; Kwon & Knill, 2013), participants completed 400 trials total within a single day, distributed across 4 experimental blocks (100 trials each). Each block took approximately 7–8 minutes, and breaks were given in between.
Prediction performance analysis
We report two types of error in prediction performance: bias and variability. Both absolute (responded-actual time) and relative (responded/actual time) biases were calculated, and variability was estimated by taking the SD of absolute and relative biases accordingly. The use of relative bias allowed us to average the data across trials and conditions when needed. Given that our stimulus (a bird) was inherently asymmetric in visual features, the centroid of the bird was used as its position in obtaining these bias and variability measures (see Supplemental Online Material S1 for the centroid extraction method).
We discarded the trials where the target location was too close to the starting point of the occluder such that participants could make a response while the stimulus was still visible (i.e., no prediction required; occluded distance < 1°; < 2.4% of trials). We also excluded outlier trials that were greater than 3 SD in relative bias (< 3% of trials). Note that trials excluded for these reasons were also excluded in all eye movement analyses. When analyzing prediction performance alone, we used an average of 393 trials per participant (out of 400).
To examine the changes in overall prediction performance with increasing prediction demand, we binned the trials into 10 or 5 bins (depending on the analysis) based on occluded duration such that each bin contained an approximately equal number of trials. Absolute bias and variability in participants’ key-press responses were calculated for each bin.
Analyses of the development of central-tendency bias
To evaluate the possible differences in the central-tendency bias between ASD and TD, we first compared the pattern of prediction bias in blocks 1 and 4 across occluded durations. This was based on the assumption that we would observe the largest difference in response bias in these two blocks if participants had learned the stimulus distribution throughout the experiment. We also examined the development of prediction bias in the shortest and the longest occluded duration bins (i.e., where the biases typically develop in the opposite directions—an indication that the responses are biased towards or away from the mean). For this, we averaged the prediction bias every 20 trials in the two occluded duration bins for each participant and performed a linear regression analysis to determine the slope of the trajectory.
Eye movement analysis
Eye movement data were analyzed offline. All trials were visually inspected, and the trials with eye blinks were removed from the analysis. On average, this eliminated 25.07% of the trials in ASD and 23.19% in TD (t(34) = 0.29, p = .77).
The primary goal of the eye movement analysis was to obtain pursuit gain that characterizes smooth pursuit quality for each participant and condition. To do this, we classified the eye movement samples into different eye movement types (saccades, smooth pursuits, and fixations; Fig. 1B, left). Detailed steps are explained in Supplemental Online Material (S2). Briefly, saccades were first detected from the eye velocity traces and removed (Fig. 1B, right). The removed velocity samples were linearly interpolated. From these saccade-removed velocity traces, smooth pursuit was classified (vs. fixations) by a) detecting the pursuit onset using a piecewise linear regression and b) applying a position dispersion criteria (Komogortsev & Karpov, 2013). A trial was counted as a “pursuit trial” if the pursuit onset was detected and pursuit lasted more than five samples as determined by dispersion.
For the eye movement analyses, we only used the “pursuit trials” where the pursuit was detected. Despite the fact that we did not provide specific instructions for eye movements in the task, on average, we were able to detect smooth pursuit behavior in a large number of the trials in both groups (ASD: 3.85 times greater number of pursuit than no pursuit trials; TD: 5.01 times greater; t(34) = −.89, p = .38). This suggests that smooth pursuit is a natural strategy to use in this task. On average, smooth pursuit was present in 223 trials per participant in ASD and 217 trials in TD. The number of removed eye movement trials was not different between groups (F(1, 34) = .07, p = .8) and across blocks (F(2.5, 85.08) = 2.49, p = .08), and there was no significant interaction between block and group (F(2.5, 85.08) = 1.62, p = .20).
To characterize the quality of smooth pursuit, we calculated the pursuit gain by dividing the eye velocity by the object velocity—a measure that accounts for differences in object speed. We separately calculated the gain across three different periods of smooth pursuit, open-loop, closed-loop, and occluded. Open-loop is the first 100–150 ms after pursuit onset when the eyevelocity begins to increase, driven by the feedforward signals from the retina (Lisberger, 2015; Lisberger & Medina, 2015). During closed-loop, the eye-velocity reaches a steady state closely following the object motion. We defined the open-loop period to be the first 100 ms after the pursuit onset, and closed-loop was from 200 ms after the pursuit onset until the occluder onset. We also ran the analyses using a more constrained definition of closed-loop (200–400 ms after the pursuit onset), and the results did not change. Occluded period (time from occluder onset to participants’ response) was included to probe potential differences in the strategies that individuals with ASD may use for prediction in the absence of visual stimulation. The peak pursuit gain, time-to-peak, and the gain at the end of the closed-loop period were also obtained to better understand possible group differences in the temporal dynamics of the smooth pursuit behavior. Additional analyses indicated that our paradigm and measurements yielded data that are of sufficient quality (see Supplemental Online Material S3).
Testing the relationship between prediction errors and smooth pursuit
To examine the relationship between smooth pursuit and prediction performance, we tested whether the quality of pursuit, when present, was related to prediction performance. To test this, we exploited a well-established finding in typical populations that both the smooth pursuit quality during the closed-loop and prediction performance become better with increasing visible duration (Spering et al., 2013, 2011). Thus, we divided our data into shorter vs. longer visible duration trials using a median split (median = 0.86 s), and separately estimated the closed-loop pursuit gain in these two visible duration conditions (shorter and longer). Note that this analysis is analogous to experimentally inducing pursuit gain changes within a participant by manipulating stimulus duration (Spering et al., 2011).
We then examined the relationship between pursuit quality and prediction performance in three ways. We first assessed if smooth pursuit quality, on average, becomes better with increasing visible duration in both groups. To do this, we tested the changes in closed-loop pursuit gain in the shorter and longer visible duration conditions. Next, we examined whether pursuit gain is a significant predictor of prediction performance across visible durations. For this, we fit linear mixed effects models to prediction performance from both the shorter and longer visible duration conditions, with the pursuit gain as a fixed effect and participant as a random effect (intercept). We fit the models separately for each group and prediction error (bias and variability), yielding 4 models in total. All these models had lower BIC values (see Supplemental Online Material, Table S1) compared to the ones which also included visible duration as a predictor. Finally, we investigated if there is a relationship between pursuit quality and prediction performance within an individual, by correlating closed-loop pursuit gain and prediction bias on a trial-by-trial basis in each participant.
For statistical analyses, we tested the effects of group and conditions at the significance level of 0.05. Hyunh-Feldt and Bonferroni corrections were used as necessary. Welch’s t-test was used for tests involving unequal variances. For linear mixed effects models, p-values were estimated via t-tests using the Satterthwaite approximations to degrees of freedom.
Results
Coarse measures of prediction performance
To understand the differences in prediction performance between ASD and TD, we first analyzed the data from all experimental blocks together, thus, for now, ignoring temporal dynamics associated with the development of the central-tendency bias. Here, we calculated the absolute bias (responded - actual time) and variability (SD in prediction bias) in their behavioral responses across occluded durations. Across measures of prediction bias and variability, we observed the expected relationship between occluded duration and performance in both groups (Fig. 2A). Specifically, as occluded duration became longer, there was an increasing tendency to respond almost 100 ms earlier than the actual hitting time (F(2.74, 120.7) = 37.83, p < .001; Fig. 2A, left) and increased response variability (F(3.98, 175.22) = 235.87, p < .001; Fig. 2A, right). There were no group differences in either of the measures (all p’s > .11). Although we did observe a tendency for individuals with ASD to respond earlier than TD at the longest occluded duration bin, this interaction, however, did not reach statistical significance (F(2.74, 120.7) = 2.3, p = .086). Overall, these results demonstrate that longer occluded durations are associated with increased prediction demand, similarly in both groups.
Figure 2.
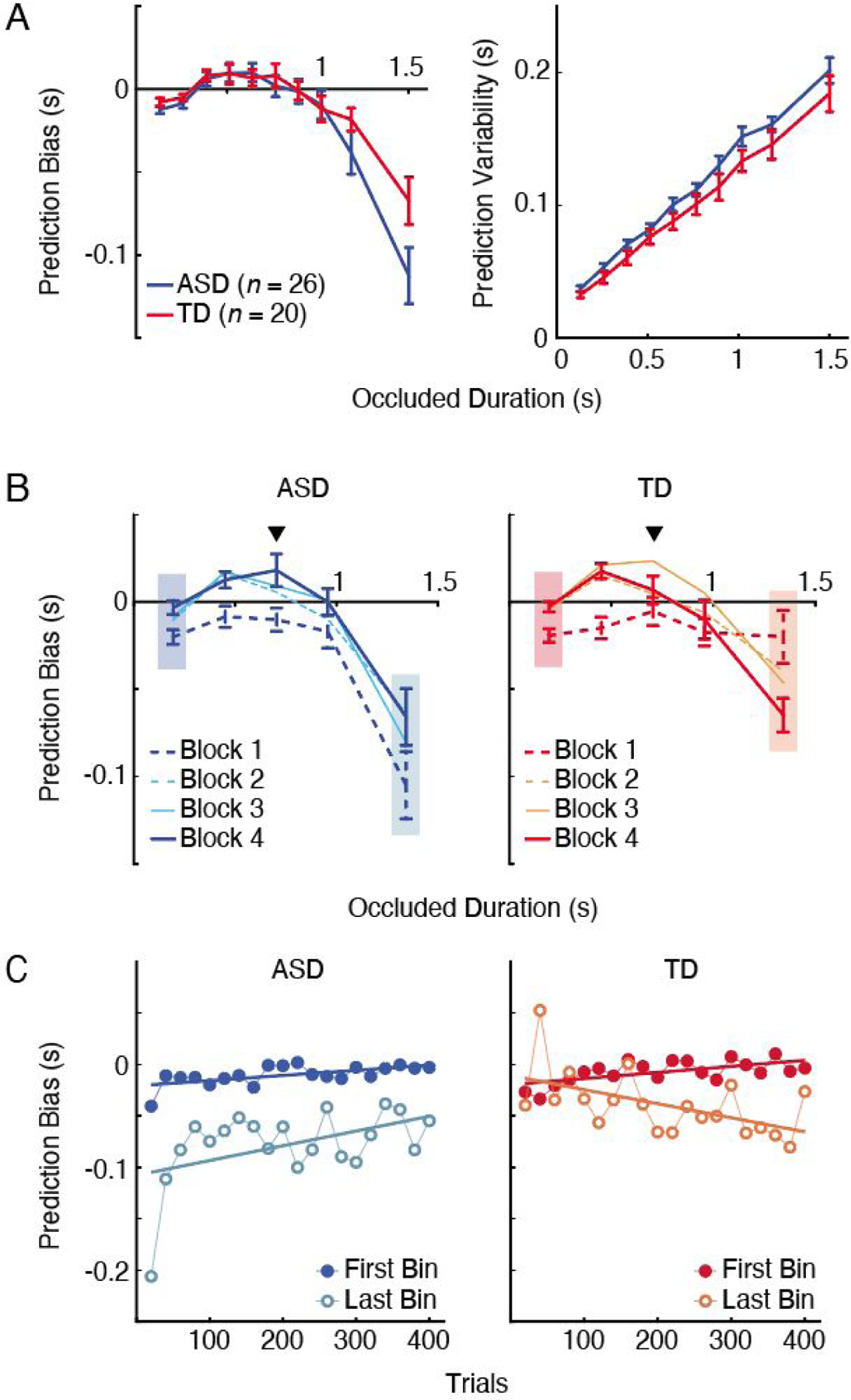
Prediction performance across varying occluded durations for ASD (blue) and TD (red). A: Group average for prediction bias (left) and variability (right) across all trials (0 indicates accurate prediction). Error bars are the standard error of the mean. B: Average prediction bias in each block across occluded durations for ASD (left) and TD (right). Inverted triangles show the mean occluded duration for the stimuli used in the study. Error bars are the standard error of the mean. The shaded rectangles indicate the bins used for the analyses in Panel C. C: The development of prediction bias (averaged every 20 trials) over time in ASD (left) and TD (right) at the first (darker colors) and last (lighter colors) bins of the occluded durations. Data for each bin is fit with a standard linear regression.
Development of central-tendency bias across blocks
While the above results may suggest that individuals with ASD are not impaired at the level of motion prediction performance, an investigation of how individuals make predictions over time revealed notable differences. Specifically, we conducted a planned analysis on the changes in prediction bias between blocks 1 and 4, which revealed atypicalities in ASD in regard to the development of central-tendency bias. There was a three-way interaction among group, occluded duration and block (F(2.95, 129.99) = 6.60, p < .001). To better understand this, we separately analyzed the data in each group. In TD (Fig. 2B, right), we found a significant interaction between block and occluded duration (F(3.2, 60.76) = 9.06, p < .001). In block 1, there was an overall small early bias (i.e., tendency to respond earlier than the actual target arrival time) that was similar in magnitude across occluded durations. In other words, initially, TD individuals treated different occluded durations similarly—an expected result given that there is little past experience to affect predictive behavior at this early stage. However, by block 4 the pattern changed. At longer occluded durations, the early bias strengthened, while at shorter occluded durations, the early bias weakened and turned into a late bias (responding later than the target arrival time). This pattern in TD is consistent with the central-tendency bias reported in previous studies (Jazayeri & Shadlen, 2010; Kwon & Knill, 2013), where the responses are biased towards the mean of the learned stimulus distribution. In fact, the pattern of the prediction bias observed in block 4 closely resembles that generated by an ideal observer model (Supplemental Online Material S4), suggesting an optimal behavior in TD.
On the other hand, in ASD, we did not find a typical progression toward a central tendency bias. Here, while ASD individuals showed a pattern similar to TD in terms of central-tendency bias in the end, the strong early bias at longer occluded durations was already present in block 1 (Fig. 2B, left). There was a main effect of occluded duration in ASD (F(1.77, 44.29) = 25.92, p < .001) with the largest early bias appearing at the longest occluded duration bin. Moreover, we found a main effect of block (F(1, 25) = 17.63, p < .001), where the early bias overall became weaker in block 4. Contrary to the pattern found in TD, the interaction between block and occluded duration was not significant in ASD (F(2.77, 69.66) = .84, p = .47). These results suggest that, unlike in TD, the early bias in the longer occluded duration bin was present from the first block of the experiment in ASD.
One possible explanation for the presence of such early bias in ASD could be that the ASD individuals learned the stimulus statistics substantially faster than TD. However, the analysis of how the prediction bias developed across trials (Fig. 2C) revealed a pattern that cannot be fully explained by this account. In TD (Fig. 2C, right), there was a clear central-tendency bias, with a progressive development of the biases in the opposite directions for the shorter and longer occluded durations (the first and last bins in Fig. 2B, respectively; first bin: slope = .000059 (5.9 ms increase per 100 trials), t(18) = 3.35, p = .004; last bin: slope = −.00014 (−14 ms decrease per 100 trials), t(18) = −2.68, p = .02). Consistent results were found when using the second occluded duration bin, where a late bias eventually appears in block 4. These indicate that TD participants, over time, learned the stimulus statistics and integrated this knowledge into their predictions, resulting in stronger biases over time. Such strategy is thought to be optimal because it can help reducing the uncertainty in sensory estimates (Jazayeri & Shadlen, 2010).
In contrast, in ASD, the early bias was present at the very beginning and was numerically the strongest in as early as the first 20 trials in both the first and the last occluded duration bins (Fig 2C, left). This is an insufficient number of samples to learn statistical properties of a distribution that they had never encountered before (Berniker et al., 2010; Kwon & Knill, 2013). Moreover, the magnitude of the bias did not remain the same; it changed in the same direction in the two occluded duration bins, weakening over the course of the experiment (first bin: slope = .00005 (5 ms increase per 100 trials), t(18) = 3.26, p = .004); last bin: slope = .00014 (14 ms increase per 100 trials), t(18) = 2.24, p = .04). These differences in the pattern of how the biases developed over time suggest that the prediction bias in ASD, at least initially in the experiment, likely have been affected by a source that is different from the one used by TD individuals (see Discussions). Additional analyses ruled out the possibility that such atypical patterns of prediction bias in ASD were a result of impulsivity or subjective difficulty (or motivation) across blocks (Supplemental Online Material S5).
Better smooth pursuit quality during occluded period in ASD
To characterize the quality of smooth pursuit, we first separately analyzed the pursuit gain in each group. The eye velocity during pursuit is typically slower than the object such that the resulting pursuit gain yields a number less than 1. In other words, a pursuit gain closer to 1 reflects faster and better pursuit, more similar to the object velocity.
The mean pursuit gain (eye velocity / object velocity; with saccades removed) over time for each group is shown in Fig. 3A. In both groups, the mean pursuit gain increased rapidly after the onset of the object motion, reached a steady-state and decreased as the object became occluded. While the overall shape of the pursuit gain pattern over time was similar between the two groups, we observed a difference in the timing of pursuit onset, with individuals with ASD being slower in initiating the smooth pursuit (t(34) = 2.8, p = .008; ASD: mean = .174 s, SD = .016; TD: mean = .156 s, SD = .022). Also, in ASD, the time when the pursuit gain reached the peak was slower than that in TD (t(34) = 3.23, p = .003; ASD: mean = .645 s, SD = .041; TD: mean = .594 s, SD = .054), although the two groups were not different in terms of the magnitude of pursuit gain at the peak (calculated by averaging five samples around a time point; t(34) = 1.11, p = .28; ASD: mean = 1.24, SD = .18; TD: mean = 1.17, SD = .21), nor at the time before the stimulus occlusion (average of five samples before the occluder onset; t(34) = 1.6, p = .12; ASD: mean = .56, SD = .25; TD: mean = .44, SD = .19).
Figure 3.
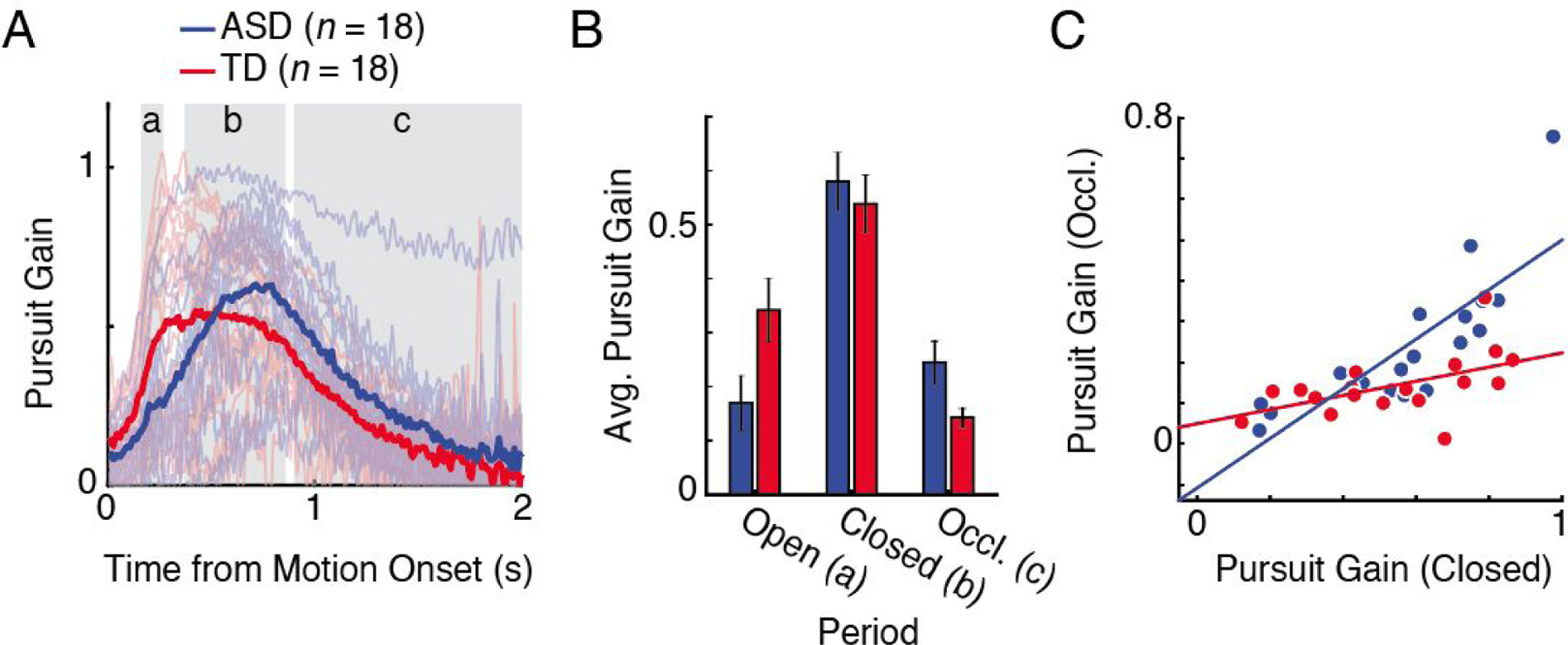
Results from the smooth pursuit analysis for ASD (blue) and TD (red). A: Mean pursuit gain traces from the stimulus motion onset, with saccades excluded. Light curves show the mean traces for each participant, and dark curves are the group mean. Different pursuit periods in B are indicated by shaded gray areas. Note that these pursuit periods were calculated using the average pursuit onset across all individuals, and thus are only an approximation for visualization. B: Average pursuit gain at different pursuit periods for each group. Error bars are standard error of the mean. C: Correlation between pursuit gain during closed-loop and occluded periods. Each data point represents the mean from an individual participant. Colored lines are regression lines for each group.
To better characterize the differences in smooth pursuit over time, we compared the mean pursuit gain in three different time periods (open-loop, closed-loop, and occluded period; see Eye Movement Analysis) between ASD and TD (Fig. 3B). As expected, we observed a significant main effect of pursuit period (F(2, 68) = 90.37, p < .001), where the pursuit gain was the largest during the closed-loop period. Notably, there was a significant interaction between group and pursuit period (F(2, 68) = 12.22, p < .001). Post hoc t-tests revealed that, compared to TD, pursuit gain was smaller in ASD during the open-loop (t(34) = −2.22, p = .03), but larger during the occluded period (t(23.26) = 2.29, p = .03). The two groups showed similar pursuit gain during closed-loop (t(34) = .55, p = .59). The smaller open-loop gain in ASD appears to be related to their slower pursuit onset; there was a strong negative correlation between open-loop gain and pursuit onset in both of the groups (ASD: r = −.87, p < .001; TD: r = −.94, p < .001), implying that those who were slower at initiating the pursuit also had overall worse smooth pursuit quality during the open-loop period.
An unexpected aspect of the results was significantly better smooth pursuit in ASD during stimulus occlusion. There was one ASD participant who maintained unusually high pursuit velocity during both closed-loop and occluded periods (Fig. 3A, topmost pale blue line). Even when this participant was excluded, the results remained statistically identical (see Supplemental Online Material, S6). One possible account for increased occluded gain in ASD is that, similar to open-loop period, their occluded pursuit gain may have been affected by slower pursuit onset. That is, in order to catch up with th stimulus, slower initiation of smooth pursuit in ASD may have resulted in faster smooth pursuit, leading to larger pursuit gain during the occluded period. However, pursuit onset, in fact, was negatively related to occluded pursuit gain in both ASD (r = −.574, p = .01) and TD (r = −.575, p = .01). In other words, slower pursuit onset was linked to worse smooth pursuit during the occluded period. This indicates that better smooth pursuit during the occluded period in ASD was not a compensation for their slower pursuit onset.
Instead, better pursuit during the occluded period in ASD seem to be closely related to that in the closed-loop period (Fig. 3C). In both groups, we found a positive correlation in pursuit gain between the two periods (ASD: r = .81, p < .001; TD: r = .52, p = .03), suggesting that those who followed the object better with their eyes during closed-loop also had higher pursuit gain during stimulus occlusion. In fact, the group difference in occluded-period gain was not significant when controlling for the gain at the end of the closed-loop period (F(1, 33) = 2.56, p = .12), although it was still significant when controlling for the peak closed-loop gain (F(1, 33) = 4.31, p = .046). These results indicate that the quality of pursuit just before the stimulus disappeared was likely carried through to when the stimulus was occluded. The two groups were not different in the number and cumulative amplitude of saccades in all periods (all p’s > .10). Together with the differences in the development of prediction bias in ASD, these results point to a possibility that there may have been differences in how individuals with ASD predicted object motion compared to TD, with a differential use of eye-movement signals being a possible candidate. In the next section, we explore this possibility.
Smooth pursuit quality is differently related to prediction performance in ASD
Motion prediction performance typically benefits from smooth pursuit eye movements (Spering et al., 2013, 2011): As pursuit quality improves with longer visible duration, prediction performance also improves. Deviations from this link would suggest an atypical relationship between smooth pursuit and prediction performance. Here, we specifically examine the hypothesis that prediction performance is differently related to smooth pursuit quality in ASD. If smooth pursuit in ASD is related to prediction, then we should observe an atypical link between prediction performance and pursuit gain in closed-loop or occluded periods (i.e., when the eye-velocity is likely to be influenced by both the feedforward and feedback signals). To test this, we investigated the changes in closed-loop pursuit gain and prediction performance across visible durations (as determined based on the median of 0.86 s; see Testing the relationship between prediction errors and smooth pursuit).
First, we replicated the previous finding (Spering et al., 2011) that pursuit gain during the closed-loop is greater for longer than shorter visible durations (F(1, 34) = 25.51, p < .001; Fig. 4A). There was no significant interaction between visible duration and group (F(1, 34) = .37, p = .55), suggesting that the smooth pursuit in both groups became similarly better when the stimulus was visible for a longer period of time.
Figure 4.
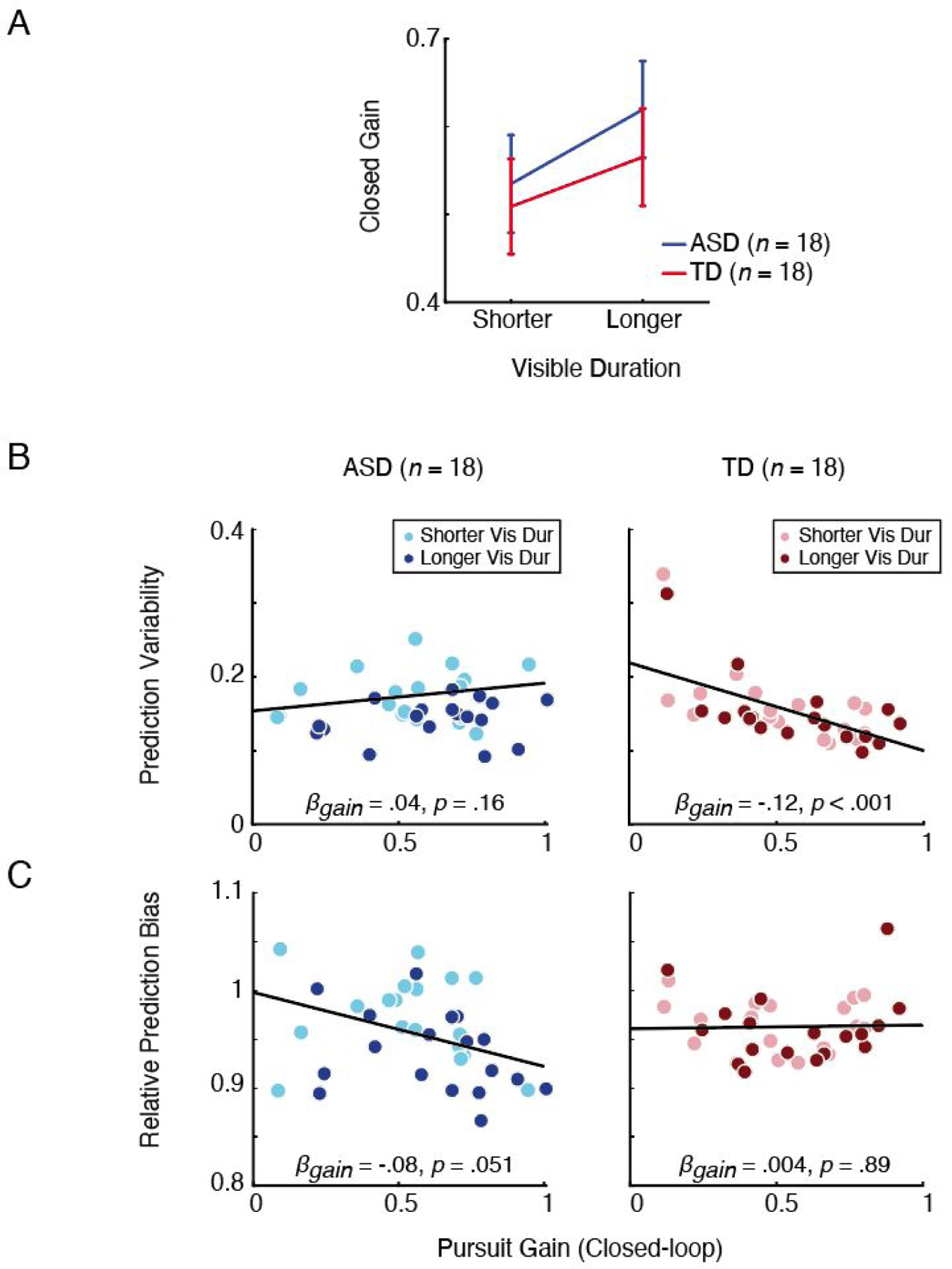
A: The effect of visible duration on closed-loop pursuit gain. Blue and red curves indicate ASD and TD, respectively. Error bars are standard error of the mean. B: Relationship between closed-loop pursuit gain and prediction variability for ASD (left) and TD (right). Each colored dot represents the mean from an individual participant’s data for either shorter (lighter colors) or longer (darker colors) visible durations. Black lines are the fit from linear mixed effects model analyses. C: Relationship between closed-loop pursuit gain and relative prediction bias (< 1 indicates early bias) for ASD (left) and TD (right). Color schemes are identical as in B.
However, linear mixed effects analyses revealed a pattern of relationship between prediction performance and smooth pursuit in ASD that was seemingly different from that observed in TD. Here, we tested whether closed-loop pursuit gain is a significant predictor of prediction performance in each group across visible durations (Figs. 4B & C). In TD, consistent with previous reports in typical populations (Spering et al., 2013, 2011), we found that better pursuit was associated with improved prediction performance. Specifically, pursuit gain was negatively related to prediction variability (Fig. 4B, right; βgain = −.12, SE = .03, t(33.93) = −4.27, p < .001), suggesting that better pursuit was linked to more consistent responses in TD. On the other hand, there was no significant relationship between pursuit gain and prediction bias in TD (Fig. 4C, right; βgain = .004, SE = .03, t(19.89) = .14, p = .89). Interestingly, in ASD, we observed the opposite pattern. Pursuit gain was not significantly related to prediction variability (Fig. 4B, left; βgain = .04, SE = .03, t(24.9) = 1.45, p = .16), but was negatively related to prediction bias, which was marginally significant (Fig. 4C, left; βgain. = −.08, SE = .04, t(18.5) = −2.09, p = .051). This suggests that better smooth pursuit was related to increased tendency to respond early in ASD. Thus, while smooth pursuit became better with longer visible duration in both groups, the enhancement in pursuit quality was associated with different types of prediction errors (variability vs. bias) in different directions (better vs. worse prediction). Similar results were observed when using occluded pursuit gain (Supplemental Online Material, S7).
We also tested if we can find a consistent pattern—negative relationship between pursuit gain and prediction bias in ASD—within an individual by performing a trial-by-trial correlation between prediction bias and closed-loop pursuit gain in each participant. These trial-by-trial correlations tend to be low (as many factors influence task performance), but they can reveal the presence of shared mechanisms (Glasser & Tadin, 2014; Stone & Krauzlis, 2003). Specifically, for each participant, we obtained the correlation coefficient (r) between closed-loop pursuit gain and relative prediction bias and tested whether these correlation coefficients were statistically different from zero in each group. Consistent with our analyses across visible durations (Figs. 4C), in ASD, we indeed found a small but significant negative relationship between closed-loop pursuit gain and relative prediction bias (mean r = −.09, SD = .092; mean r statistically different from zero, t(17) = −4.14, p < .001). In TD, we did not find such a relationship (mean r = −.002, SD = .11; mean r not statistically different from zero (t(17) = −.1, p = .92). We also compared whether the mean correlation coefficients were significantly different between the two groups. The results showed that the mean r in ASD was significantly smaller than that in TD t(34) = −2.59, p = .01). When using occluded-period pursuit gain, we again found a negative trial-by-trial correlation in ASD (mean r = −.15, SD = .12; mean r significantly different from zero: t(17) = −5.21, p < .001). These results together show an atypical pattern in ASD where better smooth pursuit may have been linked to earlier prediction bias. We note that the use of only the “pursuit trials” did not bias our results (Supplemental Online Material S8).
Discussion
The present study reveals atypical motion prediction abilities in ASD. All participants, on average, exhibited a tendency to have higher response variability as the occlusion duration became longer and showed evidence for the central-tendency bias (i.e., late responses for short occlusion durations and early responses for long occlusion durations). However, fine-grained analyses revealed that, in TD, the central-tendency bias developed gradually over the course of the experiment. This is a key feature of central-tendency behavior that reflects accumulating knowledge of the stimulus statistics and is considered to be an adaptive predictive behavior (Jazayeri & Shadlen, 2010; Supplemental Online Material S4). In contrast, the ASD group did not show evidence for a central-tendency bias that grows with experience. The two groups also differed in how eye movements were related in prediction behavior. In ASD, the changes in the prediction bias were related to the quality of smooth pursuit. Specifically, better smooth pursuit during the closed-loop period was counterintuitively related to worse prediction (i.e., larger early bias), whereas in TD, it was linked to better prediction performance (i.e., reduced variability). Overall, these results provide evidence for differences in how individuals with ASD make predictions based on available information. In particular, the findings suggest a possible deficit in ASD in regard to learning or integrating the prior knowledge on the statistics of the environment and an atypical use of extra-retinal signals in predicting visual motion.
Our findings together show that, despite seemingly similar patterns of performance on the surface, motion prediction appears to be influenced by distinct mechanisms in ASD and TD. The results are seemingly inconsistent with previous studies that tested motion prediction abilities in ASD. The discrepancy may be driven by differences in paradigm and analysis approach, highlighting the importance of isolating individual factors that contribute to motion prediction abilities. Using a driving-simulation, Sheppard et al. (2016) found a deficit in ASD in predicting the time-to-arrival for the other car, only when the observer motion was in a forward and not in a curved trajectory. Here, the results may have been affected by how individuals interpret the optic flow generated by the simulated observer motion (Sheppard et al., 2016) rather than impairments in prediction ability per se. Using a similar visual extrapolation paradigm as ours with three occlusion durations (1, 2, and 4 s), Tewolde et al., (2018) found no group differences in prediction variability in ASD. They also report a similar pattern of prediction bias in both ASD and TD, with a tendency to respond late at shorter occlusion duration and to respond early at longer occlusion duration. It is important to note that this pattern was observed from an aggregate data across all trials, and it is unknown if there was a difference in the development of bias over time as was the case in our data.
We also report a novel finding that smooth pursuit during stimulus occlusion was significantly better in ASD. The occluded pursuit gain was correlated with that in the closed-loop period, suggesting that individuals with ASD may have sustained their pursuit better throughout occlusion. Better smooth pursuit in ASD, however, came at a cost; while it was linked to better performance (i.e., reduced variability) in TD, it was associated with worse performance (i.e. larger bias) in ASD. This counterintuitive result provides insight into potential underlying mechanisms that may be atypical in ASD. We first consider the possibility that this finding reflects a general impairment in the saccadic eye-movement system in ASD. In the absence of visual stimulation, the visual system compensates for the decrease in pursuit velocity with predictive saccades (Orban de Xivry et al., 2006, 2008). This compensation ability becomes better throughout development (Ego, Yüksel, Orban de Xivry, & Lefèvre, 2016). In ASD, previous studies have shown less precise saccades (Johnson et al., 2012; Schmitt, Cook, Sweeney, & Mosconi, 2014), and difficulties adapting to saccadic errors (Freedman & Foxe, 2017). Thus, it is possible that better smooth pursuit in ASD could be a byproduct of a deficit in predictive catch-up saccades, a deficit that may in turn worsen prediction performance. However, we do not find this explanation to be likely. We did not find any group differences in any of our saccade measures. Furthermore, ASD is associated with an intact ability in the alternation between smooth pursuit and saccades in the absence of visual stimulation (Ego, Bonhomme, et al., 2016).
Another possibility considers an atypical influence of extra-retinal signals for motion prediction in ASD. A growing number of studies imply that the perceptual system can rely on both retinal and extra-retinal signals related to eye movements when extrapolating motion trajectories (Bosco et al., 2015; Gauthier, Nommay, & Vercher, 2007; Spering et al., 2011). Such processing is proposed to be mediated by a network of areas (Lisberger, 2009; Ono, 2015), including the medial superior temporal area, supplementary eye field and cerebellum, where neurons respond to visual motion and maintain their response during occlusion. Extra-retinal signals can be particularly useful during occlusion when the retinal motion associated with the object is absent. Here, it is possible that such signals may have been used and interpreted differently by the two groups, resulting in different types of prediction errors. For instance, in TD, greater pursuit gain might contribute to the certainty in sensory signals about object motion, affecting the prediction variability (random error). On the other hand, in ASD, pursuit signals may have been linked with the non-random errors, such as in the estimation of the object speed. Given that time is negatively related to speed (time = distance/speed), faster estimated object motion from pursuit would have resulted in shorter estimated time to target location, and thus earlier bias in ASD which deteriorates the performance (note that smooth pursuit gain is typically smaller than 1 such that better pursuit is achieved by faster eye-velocity). While speculative, such an account may provide an explanation for why an earlier prediction bias is counterintuitively linked with better smooth pursuit in ASD.
Our finding of atypical development of central-tendency bias in ASD may be consistent with a proposal that postulates less influence of Bayesian priors on perceptual experiences in ASD (Palmer et al., 2017; Pellicano & Burr, 2012; Van de Cruys et al., 2014). The central-tendency bias has been suggested to reflect our brain’s optimal strategies for incorporating prior knowledge into perceptual judgments (Jazayeri & Shadlen, 2010). Thus, the lack of this bias in ASD may indicate difficulties integrating prior knowledge with sensory inputs. However, this conclusion should be interpreted with caution. One of the key predictions from this Bayesian hypothesis is that, due to less precise priors, the perceptual estimates in ASD should be more accurate (i.e., less biased) and truer to the current sensory signals. In this context, our finding that the responses in ASD were still erroneous and biased is inconsistent with this hypothesis. Nevertheless, the results suggest that prediction performance in ASD is influenced by a different source of bias that is less dependent on accumulating knowledge of the stimulus statistics. Whether this is due to individuals’ deficits in learning this information or in integrating it into percepts warrants further investigation.
An alternative explanation for atypical motion prediction abilities in ASD may involve motion processing deficits per se in ASD. It is well documented that individuals with ASD have atypicalities in processing motion information (Koh, Milne, & Dobkins, 2010; Schauder, Park, Tadin, & Bennetto, 2017; Spencer et al., 2000; Takarae, Luna, Minshew, & Sweeney, 2008), which may in turn disrupt prediction accuracy in this domain. Our finding that individuals with ASD demonstrated lower pursuit gain during the open-loop period potentially supports this idea. A recent study reported that individuals with ASD require longer stimulus durations to perceive motion for smaller stimuli (Schauder et al., 2017), similar to our stimulus size. This makes it possible that, in our prediction task, more time was needed for them to initiate the smooth pursuit and/or reach a steady-state due to decreased sensitivity to motion. However, it is difficult to conclude that motion processing deficits alone fully explain our results for several reasons. First, we employed longer visible durations (well above threshold for both ASD and TD), which would have minimized the effects of motion sensitivity differences. Second, while we found differences in the open-loop pursuit gain, pursuit quality was similar between groups during the closed-loop period. This indicates that once motion is successfully detected, the internally generated motion information in the ocular motor system for smooth pursuit may be intact. Therefore, although we cannot completely rule out the influence of decreased motion sensitivity in ASD on our findings, it is less likely that general motion processing deficits contributed to our main findings regarding motion prediction.
Conclusions
Our study provides evidence for atypical motion prediction abilities in ASD that may be influenced by differential use of relevant information in motion prediction. Such findings were uncovered using a paradigm that approximates the natural ways individuals interact with dynamic objects. The study provides empirical support for a recent theory that proposes prediction deficits as a global trait in ASD. Future studies should examine whether the atypicalities we observed in the motion domain reflect a broader prediction deficit that can be generalized to other complex areas, and their potential influence on the core behavioral symptoms of ASD.
Supplementary Material
Acknowledgements
This study was supported by R01 EY019295 and T32 EY007125 (to D.T.), R01 DC009439 (to L.B.), and a University of Rochester PumpPrimer II award (to D.T. and L.B.). W.P. was supported by an Autism Science Foundation Pre-doctoral Fellowship 16-006. Computing resources were provided by the Center for Integrated Research Computing at the University of Rochester.
References
- Aitkin CD, Santos EM, & Kowler E (2013). Anticipatory smooth eye movements in autism spectrum disorder. PLoS ONE, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S (2000). Theory of mind and autism: A review. International Review of Research in Mental Retardation, 23, 169–184. [Google Scholar]
- Bennett SJ, & Barnes GR (2003). Human ocular pursuit furing the transient disappearance of a visual target. Journal of Neurophysiology, 90, 2504–2520. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, & Barnes GR (2004). Predictive Smooth Ocular Pursuit During the Transient Disappearance of a Visual Target. Journal of Neurophysiology, 92, 578–590. [DOI] [PubMed] [Google Scholar]
- Berniker M, Voss M, & Kording K (2010). Learning priors for bayesian computations in the nervous system. PLoS ONE, 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Monache SD, Gravano S, Indovina I, La Scaleia B, Maffei V, … Lacquaniti F (2015). Filling gaps in visual motion for target capture. Frontiers in Integrative Neuroscience, 9, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Brock J (2012). Alternative Bayesian accounts of autistic perception: Comment on Pellicano and Burr. Trends in Cognitive Sciences, 16, 573–574. [DOI] [PubMed] [Google Scholar]
- Bussing R, Fernandez M, Harwood M, Wei Hou, Garvan CW, Eyberg SM, & Swanson JM (2008). Parent and teacher SNAP-IV ratings of attention deficit hyperactivity disorder symptoms: Psychometric properties and normative ratings from a school district sample. Assessment, 15, 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle Monache S, Lacquaniti F, & Bosco G (2014). Eye movements and manual interception of ballistic trajectories: effects of law of motion perturbations and occlusions. Experimental Brain Research, 233, 359–374. [DOI] [PubMed] [Google Scholar]
- Diaz G, Cooper J, Rothkopf C, & Hayhoe M (2013). Saccades to future ball location reveal memory-based prediction in a virtual-reality interception task. Journal of Vision, 13, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego C, Bonhomme L, Orban de Xivry J-J, Da Fonseca D, Lefèvre P, Masson GS, & Deruelle C (2016). Behavioral characterization of prediction and internal models in adolescents with autistic spectrum disorders. Neuropsychologia, 91, 335–345. [DOI] [PubMed] [Google Scholar]
- Ego C, Yüksel D, Orban de Xivry J-J, & Lefèvre P (2016). Development of internal models and predictive abilities for visual tracking during childhood. Journal of Neurophysiology, 115, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig J, Tadin D, Schauder KB, & Cascio CJ (2013). A substantial and unexpected enhancement of motion perception in autism. Journal of Neuroscience, 33, 8243–8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, & Foxe JJ (2017). Eye-movements, sensori-motor adaptation and cerebellar-dependent learning in Autism: Towards potential biomarkers and sub-phenotypes. European Journal of Neuroscience, 1–7. [DOI] [PMC free article] [PubMed]
- Gauthier GM, Nommay D, & Vercher J-L (2007). The role of ocular muscle proprioception in visual localization in targets. Science, 249, 58–61. [DOI] [PubMed] [Google Scholar]
- Glasser DM, & Tadin D (2014). Modularity in the motion system: Independent oculomotor and perceptual processing of brief moving stimuli. Journal of Vision, 14, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, & Landa RJ (2002). Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia, 40, 2039–2049. [DOI] [PubMed] [Google Scholar]
- Gomot M, & Wicker B (2012). A challenging, unpredictable world for people with Autism Spectrum Disorder. International Journal of Psychophysiology, 83, 240–247. [DOI] [PubMed] [Google Scholar]
- Heeger DJ (2017). Theory of cortical function. Proceedings of the National Academy of Sciences, 114, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellendoorn A, Wijnroks L, & Leseman PPM (2015). Unraveling the nature of autism: Finding order amid change. Frontiers in Psychology, 6, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth HL (1910). The central tendency of judment. The Journal of Philosophy, Psychology and Scientific Methods, 7, 461–469. [Google Scholar]
- Jasmin E, Couture M, Mckinley P, Reid G, Fombonne E, & Gisel E (2009). Sensori-motor and daily living skills of preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 231–241. [DOI] [PubMed] [Google Scholar]
- Jazayeri M, & Shadlen MN (2010). Temporal context calibrates interval timing. Nature Neuroscience, 13, 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BP, Rinehart NJ, Papadopoulos N, Tonge B, Millist L, White O, & Fielding J (2012). A closer look at visually guided saccades in autism and Asperger’s disorder. Frontiers in Integrative Neuroscience, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill DC, & Pouget A (2004). The Bayesian brain: The role of uncertainty in neural coding and computation. Trends in Neurosciences, 27, 712–719. [DOI] [PubMed] [Google Scholar]
- Koh HC, Milne E, & Dobkins K (2010). Spatial contrast sensitivity in adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders, 40, 978–87. [DOI] [PubMed] [Google Scholar]
- Komogortsev OV, & Karpov A (2013). Automated classification and scoring of smooth pursuit eye movements in the presence of fixations and saccades. Behavior Research Methods, 45, 203–215. [DOI] [PubMed] [Google Scholar]
- Kwon O-S, & Knill DC (2013). The brain uses adaptive internal models of scene statistics for sensorimotor estimation and planning. Proceedings of the National Academy of Sciences, 110, E1064–E1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG (2009). Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience, 162, 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG (2015). Visual Guidance of Smooth Pursuit Eye Movements. Annual Review of Vision Science, 1, 447–468. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, & Medina JF (2015). How and why neural and motor variation are related. Current Opinion in Neurobiology, 33, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, & Risi S (1999). Autism diagnostic observation schedule-WPS edition. Los Angeles: Western Psychological Services. [Google Scholar]
- Makin ADJ, Stewart AJ, & Poliakoff E (2009). Typical object velocity influences motion extrapolation. Experimental Brain Research, 193, 137–142. [DOI] [PubMed] [Google Scholar]
- Markram K, & Markram H (2010). The Intense World Theory – a unifying theory of the neurobiology of autism. Frontiers in Human Nueroscience, 4, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S (2015). The neuronal basis of on-line visual control in smooth pursuit eye movements. Vision Research, 110, 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Bennett SJ, Lefèvre P, & Barnes GR (2006). Evidence for synergy between saccades and smooth pursuit during transient target disappearance. Journal of Neurophysiology, 95, 418–427. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Missal M, Lefevre P, de Xivry J-JOJ, Lefèvre P, & Xivry J de. (2008). A dynamic representation of target motion drives predictive smooth pursuit during target blanking. Journal of Vision, 8, 6.1–13. [DOI] [PubMed] [Google Scholar]
- Palmer CJ, Lawson RP, & Hohwy J (2017). Bayesian approaches to autism: Towards volatility, action, and behavior. Psychological Bulletin, 143, 521–542. [DOI] [PubMed] [Google Scholar]
- Park WJ, & Tadin D (2018). Motion Perception. In Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience, Sensation, Perception, and Attention. 4th Edition. Wiley, 415–488. [Google Scholar]
- Pasternak T, & Tadin D (2020). Linking neuronal direction selectivity to perceptual decisions about visual motion. Annual Review of Vision Science, 6:335–62. [DOI] [PubMed] [Google Scholar]
- Pelli DG (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10, 437–442. [PubMed] [Google Scholar]
- Pellicano E, & Burr DC (2012). When the world becomes “too real”: A Bayesian explanation of autistic perception. Trends in Cognitive Sciences, 16, 504–510. [DOI] [PubMed] [Google Scholar]
- Rovio Entertainment. (2009). Angry Birds. Retrieved from https://www.angrybirds.com/games/
- Rutter M, Bailey A, & Lord C (2003). The social communication questionnaire: Manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Rutter M, Le Couteur A, & Lord C (2003). Autism Diagnostic Interview, Revised. Los Angeles: Western Psychological Services. [Google Scholar]
- Schauder KB, Park WJ, Tadin D, & Bennetto L (2017). Larger receptive field size as a mechanism underlying atypical motion perception in autism spectrum disorder. Clinical Psychological Science, 5, 827–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LM, Cook EH, Sweeney JA, & Mosconi MW (2014). Saccadic eye movement abnormalities in autism spectrum disorder indicate dysfunctions in cerebellum and brainstem. Molecular Autism, 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard E, Van Loon E, Underwood G, & Ropar D (2016). Difficulties predicting time-to-arrival in individuals with autism spectrum disorders. Research in Autism Spectrum Disorders, 28, 17–23. [Google Scholar]
- Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, & Cardinaux AL (2014). Autism as a disorder of prediction. Proceedings of the National Academy of Sciences, 111, 15220–15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J, O’Brien J, Riggs K, Braddick OJ, Atkinson J, & Wattam-Bell J (2000). Motion processing in autism: Evidence for a dorsal stream deficiency. Neuroreport, 11, 2765–2767. [DOI] [PubMed] [Google Scholar]
- Spering M, Dias EC, Sanchez JL, Schütz AC, & Javitt DC (2013). Efference copy failure during smooth pursuit eye movements in schizophrenia. Journal of Neuroscience, 33, 11779–11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering M, Schütz AC, Braun DI, & Gegenfurtner KR (2011). Keep your eyes on the ball: smooth pursuit eye movements enhance prediction of visual motion. Journal of Neurophysiology, 105, 1756–1767. [DOI] [PubMed] [Google Scholar]
- Stone LS, & Krauzlis RJ (2003). Shared motion signals for human perceptual decisions and oculomotor actions. Journal of Vision, 3, 725–736. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Takarae Y, Macmillan C, Luna B, & Minshew NJ (2004). Eye movements in neurodevelopmental disorders. Current Opinion in Neurology, 17, 37–42. [DOI] [PubMed] [Google Scholar]
- Takarae Y, Luna B, Minshew NJ, & Sweeney JA (2008). Patterns of visual sensory and sensorimotor abnormalities in autism vary in relation to history of early language delay. Journal of the International Neuropsychological Society: JINS, 14, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Krisky CM, & Sweeney JA (2004). Pursuit eye movement deficits in autism. Brain, 127, 2584–2594. [DOI] [PubMed] [Google Scholar]
- Tewolde FG, Bishop DVM, & Manning C (2018). Visual motion prediction and verbal false memory performance in autistic children. Autism Research, 11, 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, De-Wit L, & Wagemans J (2014). Precise minds in uncertain worlds: Predictive coding in autism. Psychological Review, 121, 649–75. [DOI] [PubMed] [Google Scholar]
- Verstynen T, & Sabes PN (2011). How each movement changes the next: An experimental and theoretical study of fast adaptive priors in reaching. Journal of Neuroscience, 31, 10050–10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler. (2008). WAIS-IV: Wechsler Adult Intelligence Scale (4th ed.). San Antonia, TX: Pearson. [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children (4th ed.). San Antonio, TX: Pearson. [Google Scholar]
- Whyatt CP, & Craig CM (2012). Motor skills in children aged 7–10 Years, diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders, 42, 1799–1809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


