Abstract
Acute pelvic pain (APP) requires urgent medical evaluation and treatment. Differential diagnosis of APP is broad, including a variety of gynecologic and non-gynecologic/ urinary, gastrointestinal, vascular and other entities. Close anatomical and physiological relations of pelvic structures, together with similar clinical presentation of different disorders and overlapping of symptoms, especially in the emergency background, make the proper diagnosis of APP challenging. Imaging plays a crucial role in the fast and precise diagnosis of APP. Ultrasonography is the first-line imaging modality, often accompanied by CT, while MRI is utilized in specific cases, using short, tailored protocols. Recognizing the cause of APP in females is a challenging task, due to the wide spectrum of possible origin and overlap of their imaging features. Therefore, the radiologist has to be familiar with the possible causes of APP, and, relying on clinical presentation, together with laboratory findings, choose the best imaging strategy in order to establish a fast and accurate diagnosis.
Introduction
Pelvic pain is a common condition affecting patients of all age groups and is defined as acute when it lasts for less than 3 months. Acute pelvic pain (APP) requires urgent medical evaluation and treatment. Differential diagnosis of APP is broad, including a variety of gynecologic and non-gynecologic entities. Close anatomical and physiological relations of pelvic structures, together with a similar clinical presentation of different disorders and overlapping of symptoms, especially in the emergency background, make the proper diagnosis of APP challenging.
The diagnosis of APP is based on anamnesis, clinical and laboratory findings, together with diagnostic imaging, which significantly increases the speed and accuracy, as well as confidence in the patient management.1,2 In an emergency setting, both transvaginal and transabdominal ultrasound is the first-line imaging modality for initial evaluation of the patients presenting with APP, with rather high sensitivity and specificity for detection of pelvic pathology. It is a low-cost diagnostic modality, widely available, and lacks ionizing radiation.3,4 Nevertheless, many urgent conditions require further diagnostic imaging.
CT is a powerful diagnostic tool, widely utilized in patients with APP, especially in cases when ultrasound findings are inconclusive or urinary and gastrointestinal pathology is suspected. According to the American College of Radiology appropriateness criteria, a contrast-enhanced CT scan is the preferred imaging given the high diagnostic performances, widespread availability, and fast acquisition.5 It has been proven that CT findings changed the referring diagnosis in more than half of all patients administered at ED and significantly influenced the treatment planning.6 However, due to the potential harmful effects of ionizing radiation, MRI is the method of choice for young and pregnant patients, whenever available, using shorter, tailored protocols depending on the suspected diagnosis.5,7
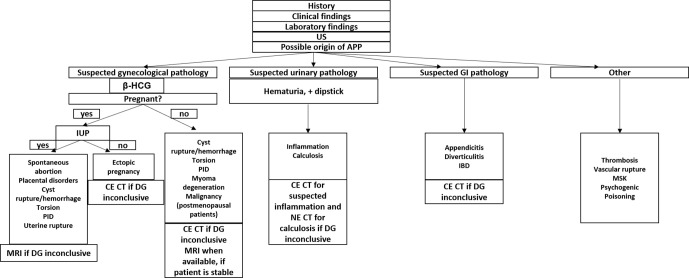
The list of the most common causes of APP is summarized in Figure 1, whereas the most common radiological findings and suggested differential diagnosis were listed in Table 1.
Figure 1.
Diagnostic approach to the most common causes of APP -ultrasonography. APP, acute pelvic pain; CECT, contrast-enhanced computed tomography; DG, diagnosis; GI, Gastrointestinal; IUP, Intrauterine pregnancy; NECT, non-enhanced computed tomography.
Table 1.
Common radiological findings in APP and suggested differential diagnosis
| Radiological finding | Differential diagnosis | |
|---|---|---|
| Peritoneal fluid | Ovarian cyst rupture, ovarian torsion, ovarian hyperstimulating syndrome, ruptured ectopic pregnancy, mittelschmerz, PID, appendicitis, colonic diverticulitis, ovarian carcinoma | |
| Fat stranding | Ileocoecal | Appendicitis, Crohn`s disease |
| Pericolic | Colonic diverticulitis, epiplioc appendagitis, omental infarction | |
| Periileal | Crohn`s disease, regional enteritis, omental infarction | |
| Pelvic | PID, endometriosis, iliac aneurysm rupture | |
| Perirenal | Pyelonephritis | |
| Bowel distension | Volvulus, incarcerated hernia, intususception | |
| Pneumoperitoneum | Intestinal perforation, colonic diverticulitis, appendicitis | |
| Retropneumoperitoneum | Emphysematous pyelonephritis, perinephric abscess | |
| Pelviceal mass | Gynecological malignancy, degenerated myoma, endometrioma, extrauterine pregnancy, periappendicular abscess, peridiverticular abscess, iliac aneurysm | |
| Kidney enlargement | Perinephritic abscess, pyelonephritis | |
| Retroperitoneal heamathoma | Aortic/iliac aneurysm rupture, perirenal haemathoma | |
APP, acute pelvic pain; PID, pelvic inflammatory disease.
Gynecological causes
Gynecological emergencies are among the most common conditions causing APP.–8,9 The underlying pathological conditions leading to the onset of pain are rather diverse—10and can be didactically divided according to the age group, pregnancy status, and organ of origin.11
The most common causes in non-pregnant females of reproductive age are rupture or hemorrhage of ovarian cysts, inflammation, ovarian torsion, and myoma degeneration or torsion.
Ovarian cysts are a common finding in pre-menopausal females and usually are not related to intensive pain unless they undergo hemorrhage or rupture.12 They are easily identified with ultrasonography and CT as a thin-walled serous follicular cyst, with a diameter of 3 cm or larger, or corpus luteum cysts, which have a thick irregular wall of increased peripheral vascularity, presented as a “ring of fire” on Color Doppler ultrasound or peripheral enhancement on CT.13
Physiological changes during the menstrual cycle lead to increased ovarian vascularity during the luteal phase, which may lead to hemorrhage or rupture.14 Ultrasonographicaly, hemorrhagic cyst can have a diverse presentation, depending on the evolutional stage of blood products, however, lace-like internal echoes with peripheral vascularization on CD and no internal signal, with possible fluid–fluid levels are typical. On CT, high-density content and thick, enhancing walls are observed.
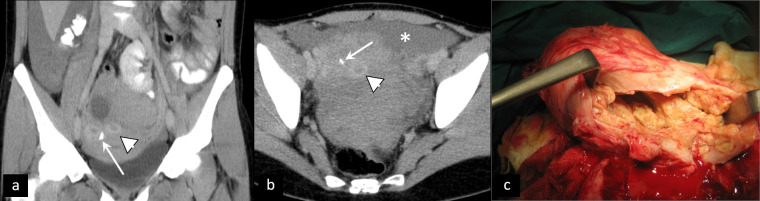
In cases of rupture, hematoperitoneum (high density peritoneal free fluid) is observed, sometimes accompanied by active extravasation of contrast agent in the proximity of the cyst (Figure 2).
Figure 2.
Rupture of corpus luteum cyst. Contrast-enhanced CT (a, b) shows multiple cysts with thick, enhancing walls (arrowhead), corresponding to the “ring of fire“ sign on ultrasound, and active extravasation of contrast agent (arrow), accompanied by hematoperitoneum (asterisk). (c) Intraoperative specimen.
MRI is not a method of choice for the evaluation of ovarian cysts in the acute background due to the fact that it is not widely available and fast. However, hemorrhagic cysts are easily identified by MRI as T1W hyperintense lesions without a drop of the signal on T1W fat saturation sequences, shading sign (which is characteristic for endometrioma), and contrast enhancement.15,16
Some females may experience APP of different intensity at the moment of ovulation, caused by the rupture of the follicle, called “Mittelschmerz”.17 Such pain is physiological and does not require significant medical attention. It is presented as unilateral discomfort or pain of the lower abdomen, usually spontaneously resolved within several hours, sometimes accompanied by a small amount of free pelvic fluid.
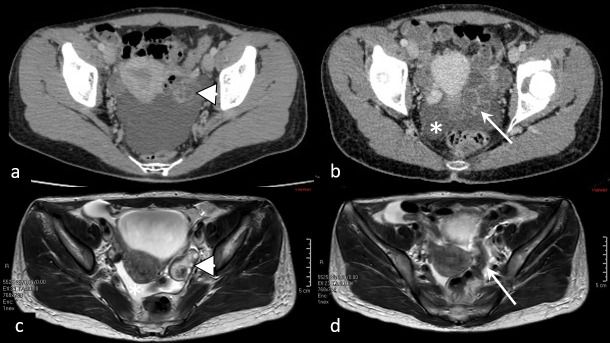
Pelvic inflammatory disease (PID) is defined as an acute clinical syndrome associated with ascending spread of microorganisms unrelated to pregnancy or surgery, typically arising in sexually active pre-menopausal females.18 It has a broad spectrum of presentation, depending on the extent of the inflammation and affected genital structures. Ultrasound findings may be inconclusive, comprising thickening and hypervascularity of genital organs, especially Fallopian tubes, accompanied by ascites and presence of cogwheel sign and beads on a string sign,19 and should therefore always be interpreted with clinical and laboratory correlation. CT findings indicating PID include thickening and enhancement of Fallopian tube wall exceeding 5 mm, thickening of the uterosacral ligaments, obliteration of fascial planes, free fluid in the cul-de-sac, loss of definition of the uterine border, pelvic fat infiltration and pelvic edema, reactive lymphadenopathy, and signs of peritonitis–20 (Figure 3).
Figure 3.
Pelvic inflammatory disease. Contrast-enhanced CT (a, b) shows slightly enlarged, inhomogenous left ovary (arrowhead), together with left parauterine tubular structure with enhancing walls (arrow), resembling inflamed fallopian tube, accompanied by ascites (asterisk). MRI (c, d) performed 3 days later confirmed oophoritis (arrowhead), however, parauterine mass was confirmed to be sigmoid colon loop (arrow).
Ovarian torsion is defined as torsion of the ovary and part of the tube around the vascular pedicle, leading to partial or complete vascular compromisation. It is most commonly caused by ovarian lesions but can occur in females with no underlying ovarian pathology, probably due to ovarian hypermobility.21 Ultrasonography is the first-line imaging modality, showing enlarged ovary with engorged central ovarian parenchyma and peripherally displaced follicles, sometimes accompanied by “whirlpool sign” presenting twisted vascular pedicle and free fluid. A complete absence of Doppler signal is not mandatory due to the dual arterial supply of ovaries. Ovarian torsion has a similar presentation on CT and MRI, with MRI being preferable due to the absence of ionizing radiation and higher specificity whenever available (Figure 4). Since it most commonly affects young females, a fast and precise diagnosis is essential for the preservation of ovaries and the prevention of complications, which may include abscess formation and peritonitis.22
Figure 4.
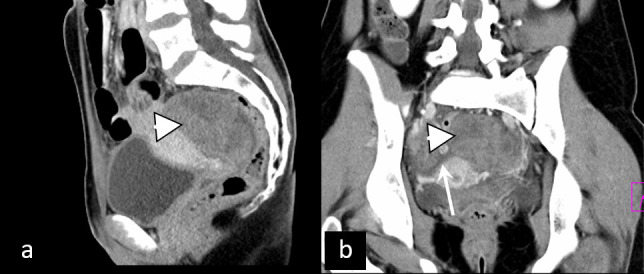
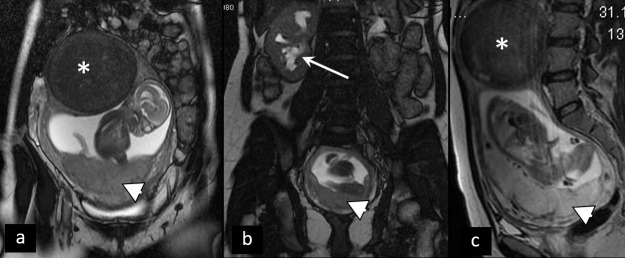
Ovarian torsion. Inhomogenous retrouterine pelvic mass (arrowhead) presenting edematous, displaced ovary due to ovarian torsion, accompanied by ascites (arrow).
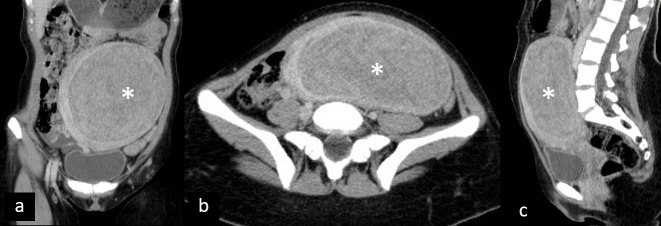
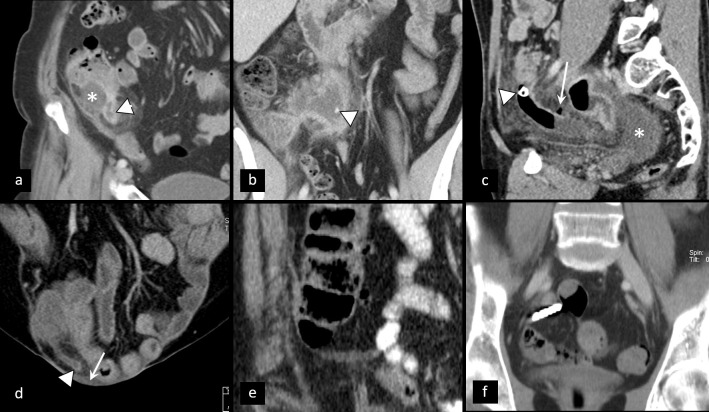
Most myomas are asymptomatic or with mild symptoms. However, in about one-third of patients, myomas can cause pain either due to degeneration or torsion. Degeneration occurs when myoma outgrows its vascular supply and can be hyaline, myxoid, red, cystic, and hemorrhagic.23 Although hyaline is the most common, the hemorrhagic one is most likely to cause APP and is frequently related to pregnancy or contraceptive intake. Depending on the type of degeneration, ultrasonography can demonstrate inhomogeneous uterine mass, with possible cystic areas and partial absence of Doppler signal in infarction areas.24 CT and MRI show a similar presentation of degenerated myomas, which are inhomogeneous, with cystic appearance and areas of low post-contrast attenuation due to necrosis and infarction25 (Figure 5). Areas of high attenuation on CT and high signal intensity on MRI may suggest hemorrhage. Rapid growth and irregular pattern may pose a diagnostic challenge towards myosarcoma.26
Figure 5.
Degenerated myoma. Coronal (a), axial (b), and sagittal (c) contrast-enhanced CT shows large myoma (asterisk) with signs of extensive degeneration.
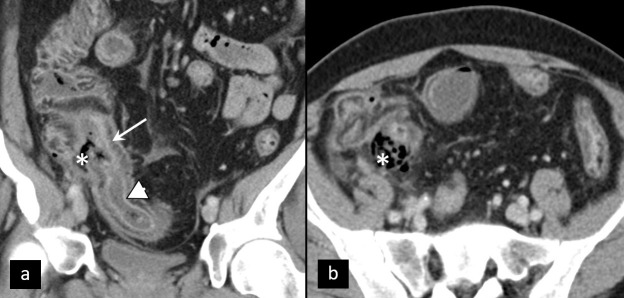
Torsion of pedunculated myoma, which is a rare cause of APP, is ultrasonographically presented as heterogeneous pedunculated parauterine mass, with twisted pedicle and no or diminished blood flow within the mass on CD. CT findings include enlarged, heterogenous parauterine mass, while twisted pedicle is best depicted using MRI27 (Figure 6).
Figure 6.
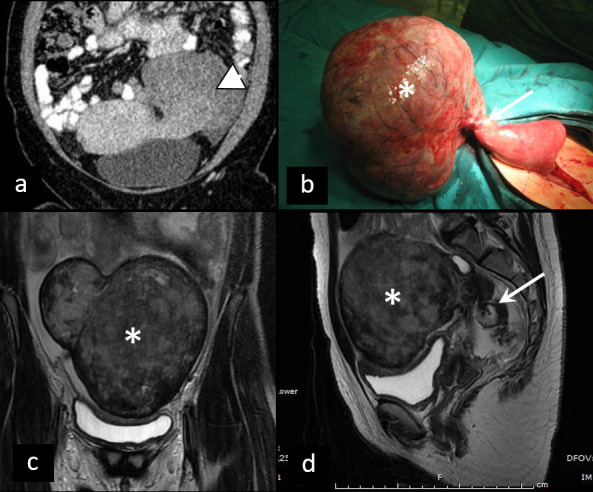
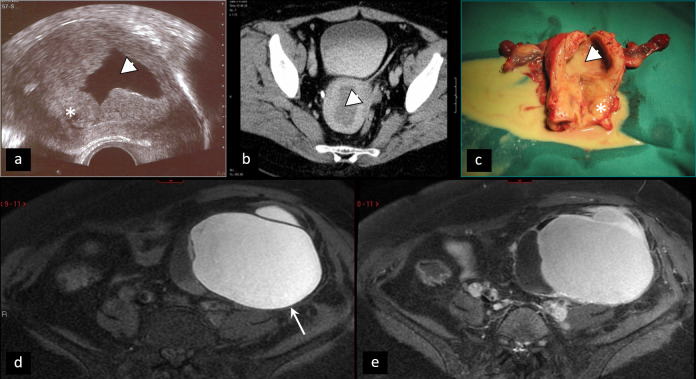
Torsion of pedunculated myoma. (a) Coronal contrast-enhanced CT of torsion of pedunculated subserous myoma (arrowhead). Differential diagnosis towards torsion of an ovarian lesion can be challenging. Intraoperative specimen (b) and T2W MRI (c, d) of partly necrotic solid ovarian tumor (asterisk), due to torsion, with typical ”whirlpool“ sign (arrow).
Endometriosis, defined as as the presence of functional endometrial glands and stroma outside the uterus, is relatively common cause of acute and chronic pelvic pain, which is usually cyclic, related to secretory phase of menstrual cycle.28 Both ultrasound and MRI are excellent diagnostic tools for depicting ovarian endometriomas—unilocular “ground glass” cystic lesions without vascularization on ultrasound and with typical “shading sign” on T2W images, whereas MRI has shown better sensitivity for deep pelvic form.29
APP during pregnancy can have obstetric etiology which comprises ectopic pregnancy, spontaneous abortion, preterm or normal labour, placental disorders, predominantly placental abruption or inflammation, either pre- or post-partum. However, other disorders, such as ovarian cyst rupture or torsion, myoma degeneration, or torsion, occurring in young females can concur with pregnancy and cause acute onset of pain30 (Figure 7). Due to the harmful effect of ionizing radiation on the fetus, especially in early pregnancy, ultrasonography, both pelvic and vaginal, is the method of choice, accompanied by MRI whenever strongly needed and available.31
Figure 7.
Patient in the second trimester of pregnancy presenting with acute abdominopelvic pain. Coronal (a, b) and sagittal (c) T2W MRI show partly degenerated intramural myoma (asterisk), placenta previa (arrowhead), and right hydronephrosis (arrow), caused by compression of the right ureter by the enlarged uterus. Acute pain in this patient is caused by hydronephrosis.
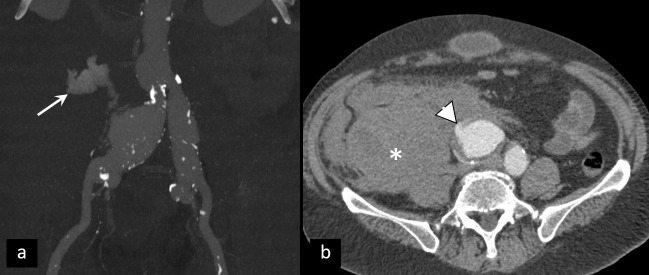
The important diagnostic step of patients in early pregnancy with pelvic pain or vaginal bleeding is the exclusion of ectopic pregnancy (EP).32 Transabdominal and transvaginal ultrasound in most cases show empty cavum and extrauterine mass, usually separate from an ovary, since the vast majority of EPs have tubal localization.33 CT is not a preferable imaging modality due to radiation. However, it is occasionally utilized due to the inaccessibility of MRI and inconclusiveness of ultrasound.34,35 In cases of rupture, hematoperitoneum is observed, clinically accompanied by signs of hemorrhagic shock (Figure 8).
Figure 8.
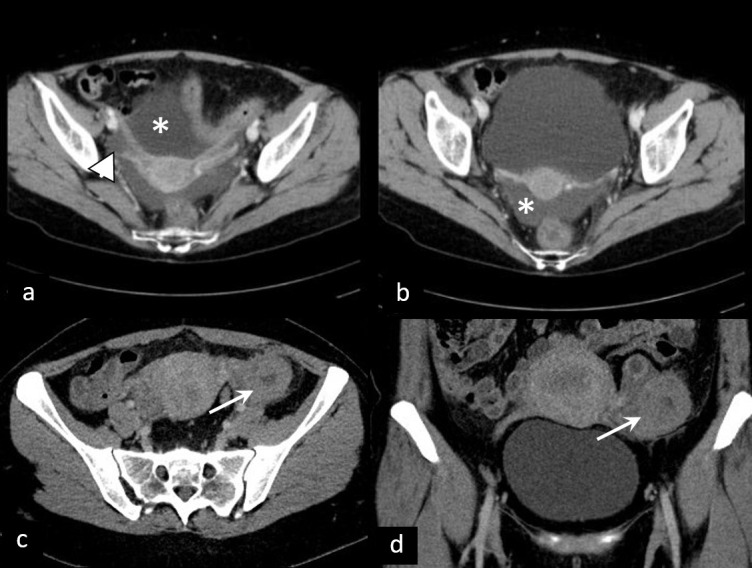
Ectopic pregnancy. (a, b) Ruptured right tubal ectopic pregnancy (arrowhead) with massive hematoperitoneum (asterisk). (c, d) Unperforated left tubal ectopic pregnancy (arrow)—CT shows gestational sac with no signs of rupture.
Placental disorders comprise placental abruption and placental adhesive disorders. The sensitivity of ultrasonography for placental abruption is low, while MRI is superior to ultrasound in the evaluation of placental hemorrhage due to better resolution and larger field of view. Three types of placental adhesive disorders (PAD), placenta accreta, placenta increta and placenta percreta show different degrees of chorionic invasion into the myometrium. The initial diagnostic tool is ultrasonography, with the typical presence of lacunae in the placenta, while MRI shows dark T2W placental bands.30
In females undergoing fertility treatment, ovarian hyperstimulation syndrome, a possible life-threathening iatrogenic condition, should be considered. It is characterized by enlarged ovaries with multiple follicular cysts arranged in a spoke-wheel pattern and acute fluid shift out of the intravascular space, leading to ascites, pleural od pericardial effusions36 (Figure 9).
Figure 9.
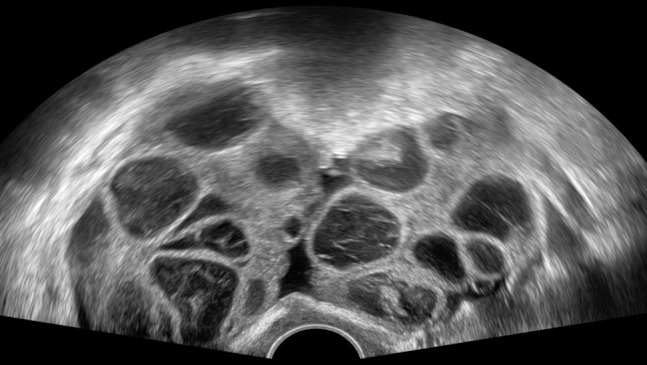
Ovarian hypertimulation syndrome. Ultrasonography depicts enlarged ovaries with multiple follicular cysts arranged in a spoke-wheel pattern.
In post-menopausal females presenting with APP, malignancy has to be ruled out. Pelvic malignancies, most commonly ovarian or uterine cancers, can induce pain of different intensity and duration, which tends to have sudden onset in cases of complications such as perforation, venous thrombosis or inflammation (Figure 10).
Figure 10.
Gynecological malignancies presenting as APP. (a, b and c) Cervical cancer. (a) Ultrasound of cervical cancer (asterisk) causing cervical stenosis and consequent pyometra (arrowhead). (b) CT shows a distended uterine cavum filled with hypodense thick fluid (arrowhead). (c) Intraoperative specimen confirms the diagnosis of pyometra caused by tumor inducing cervical stenosis. (d) and (e) hemorrhagic ovarian metastasis presenting as T1FS hyperintense ovarian mass (arrow). Pre- (d) and post-contrast (e) T1FS tomograms.
Urinary causes
The origin of APP can be related to urinary system pathology, among other sites. Pain resulting from renal and ureteral stones is a common cause for patients presenting in the acute setting.37 According to current guidelines, low dose CT is the preferred first-line imaging modality for nephrolithiasis in adults, while ultrasound is reserved for children and pregnant females30,38 (Figure 11).
Figure 11.
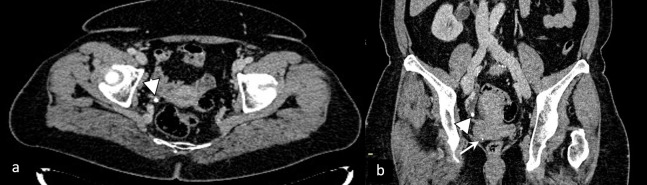
Calculus (arrowhead) in the distant segment of the right ureter, causing obstruction and proximal dilatation of ureter and hydronephrosis. Pelvic ureterolithiasis can easily be misdiagnosed as phleboliths (arrow).
Acute pyelonephritis may well be demonstrated on non-contrast CT as unilateral perinephric stranding or renal enlargement—mild to severe grade.39 It is mainly a clinical diagnosis with symptoms such as flank or suprapubic pain, high temperature, dysuria, or vomiting.40 If there are no calculi, one should think of more severe complications, and intravenous iodinated contrast media must be applied. Typically, acute pyelonephritis on contrast-enhanced CT may be demonstrated as focal or wedge-shaped areas of hypoattenuation of the renal parenchyma, or it may show striated nephrogram pattern41 (Figure 12). More serious complications of acute pyelonephritis such as a renal or perinephric abscess (Figure 13) or even vascular complications may also be seen.42
Figure 12.
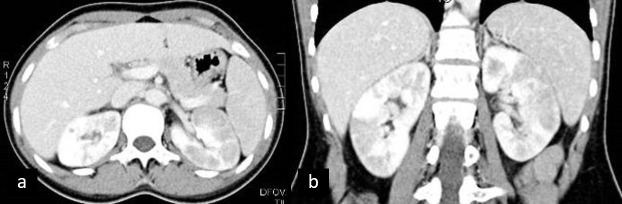
Pyelonephritis. Contrast-enhanced CT appreciation of bilateral hypodense wedge-shaped areas within thickened renal parenchyma.
Figure 13.
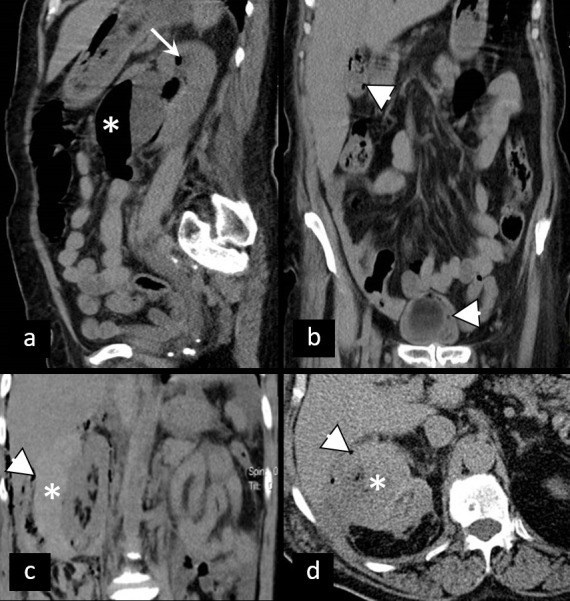
Non-contrast CT in evaluation of urinary emergencies. (a) Patient with emphysematous pyelonephritis presenting with gas collections in pyelon (asterisk) and upper calyx (arrow). (b) The same patient with gas collections in the wall of the gallbladder and urinary bladder (arrowheads)—simultaneous emphysematous cholecystitis and cystitis. (c, d) Perinephric abscess—thick perirenal collection (asterisk) with gas particles (arrowhead).
MRI appearance of acute pyelonephritis is similar to CT signs of the affected kidney: renal enlargement, striated nephrogram, and perinephric fluid reaction. Areas of focal pyelonephritis have lower signal intensity on T2 weighted and show restricted proton diffusion. Calculi or air bubbles in the urinary tract may be depicted as MRI signal void.40,43
The renal ultrasound is also the first-line imaging modality in symptomatic pregnant females. The transabdominal ultrasound can visualize calculi in the renal pelvis and proximal and distal parts of the ureter (the ureteropelvic and the ureterovesical junction).44 The transvaginal ultrasound may be useful for visualizing stones in the distal part of the ureter and at the ureterovesical junction.45 If the ultrasound is negative for urolithiasis, a non-contrast MRI may be performed. MRI resolution tends to be less than optimal, and small stones can be missed. In unresolved cases, ultra-low-dose CT is suggested for depicting obstructing urinary tract calculi in pregnant females. Comparing to standard non-contrast CT of the abdomen and pelvis, which delivers an estimated effective radiation dose of 8–10 mSv, ultra-low-dose CT of the same region reduces radiation dose below teratogenic threshold levels significantly.43,44
Unsuspected urinary tract infections (UTI) may be detected on CT performed for other clinical reasons. Complicated UTI occurs in patients with structural or functional risk factors. The key aims of imaging are confirmation of urological cause, detection of obstruction and abscesses requiring interventional or surgical treatment, and detection of urolithiasis and retained foreign bodies such as catheters.46
Diagnosis of acute infective cystitis (AIC) is suggested when inflammatory changes are present in the bladder. Diffuse mural bladder thickening is significant if the circumferential wall is over 1 cm in thickness. On CT, the muscular layer shows poor enhancement from intramural edema. There is also "hazy "increased attenuation of the extraperitoneal perivesical fat planes.47,48
Emphysematous cystitis as well as emphysematous pyelonephritis, particularly seen in diabetic patients, are a form of complicated life-threatening UTI, in which gas-forming microorganisms lead to the formation of characteristic air-attenuation linear changes within the bladder wall, air bubbles in the renal parenchyma, collecting system, bladder lumen and sometimes in the perirenal and perivesical tissue46 (Figure 13).
At MRI, AIC can be depicted as focal or diffuse mural oedema and as inflammatory T2 hyper signal of the perivesical fat, which is most appreciated with fat suppression techniques.49 When a soft-tissue irregularity is visualized at the interface between mural thickening and perivesical fat, the differential diagnosis of AIC must include bladder carcinoma and certain non-neoplastic disorders. Because of better tissue characterization and diffusion-weighted sequences, MRI is superior to CT in detecting tumor tissue.46
Post-treatment bladder aspect due to chemotherapy (particularly with cyclophosphamide) and irradiation, as well as rare inflammatory diseases of the bladder such as cystitis cystica, cystitis glandularis, eosinophilic cystitis, can be confused with AIC or bladder neoplasm.50–52.
If a patient is middle-aged or elderly, with symptoms of flank pain and hematuria, one should always consider renal neoplasms. On unenhanced images, one could easily overlook urinary tract cancers as they could have discrete non-specific presentations such as subtle, focal contour abnormalities of the kidney or focal ureteral or bladder wall thickening. Close attention should be paid to an isolated subcapsular or perinephric hemorrhage because there can be underlying neoplasm.39,53
As for acute urethritis, imaging is preferred to exclude complications such as a periurethral abscess. A periurethral abscess can be visualized by ultrasound, but because of inflammatory swelling and tenderness of the penile and perineal structures, MRI is more often used. MRI appearance of acute urethritis is demonstrated as diffuse thickening of the urethra and periurethral tissues, with intermediate to high signal intensity on T2 weighted images and intense contrast enhancement. A urethral diverticulum can mimic the urethral abscess. It is most commonly located in the distal urethra. Other complications of acute urethritis can be visualized on CT and MRI, such as urethroperineal fistula, Fournier gangrene, and fasciitis.54
Gastrointestinal causes
Many intestinal pathological conditions are causes of APP. The localization of the coecum, sigmoid colon, and rectum inside the pelvis and proximity of the intestine with the utero-ovarian complex in females can lead to challenging differential diagnosis in the acute setting.
Acute appendicitis is a common cause of predominantly lower-right quadrant pain. After initial ultrasound evaluation as second-line imaging test ultrasound, CT, and MRI evaluation have comparable and high sensitivity and specificity in children and adults, including pregnant females.55 The concept of appendectomy as the only therapeutical measure for therapy of acute appendicitis has been challenged, thus making us regard uncomplicated and complicated appendicitis as two discrete entities where conservative therapy can be sufficient for the former.56 Hallmarks of complicated appendicitis include contrast enhancement defect in the appendiceal wall, abscess, extraluminal air, intraluminal air, extraluminal appendicolith, intraluminal appendicolith, periappendiceal fat stranding, periappendiceal fluid, ileus, and ascites57 (Figure 14). Although all imaging modalities have difficulties in discriminating complicated from uncomplicated appendicitis CT was reported to have a high negative predictive value for complicated appendicitis.58 With the increased number of patients primarily treated conservatively, the role of interventional radiology in drainage of periapendicular abscesses is emerging with the reported clinical and technical success of 90%.59 The contemporary state of knowledge on acute appendicitis places radiology in a central position in its diagnostics with proper classification of complications. Also, interventional radiology has an important role in minimally invasive treatment in selected cases.
Figure 14.
Appendicitis—a spectrum of appearances. (a) Enhancement of appendiceal wall (arrowhead) with periappendicular fat stranding and abscess collection (asterisk) below the insertion of appendix to the coecum. (b) Extraluminal appendicolith (arrowhead) inside periappendicular abscess. (c) Intraluminal appendicolith (arrowhead) at the insertion of the appendix—note the intraluminal air in the proximal appendix, extraluminal air bubble (arrow), contrast enhancement defect of the appendiceal wall just posterior to the air bubble, and intraperitoneal liquid (asterisk). (d) Appendicitis in a large ventral hernia—contrast enhancement of appendiceal wall (arrowhead), periappendicular fat stranding and intraperitoneal free fluid (arrow). (e) The same patient as in (d) 7 years before with horizontally positioned appendix with no signs of inflammation. (f) Imaging pitfalls—dense appendicoliths or contrast from previous imaging causing artifacts which make evaluation of the appendiceal wall and adjacent fat difficult with no evident signs of appendicitis (operative findings indicated acute gangrenous appendicitis).
Acute colonic diverticulitis is another cause of pelvic pain which can be classified into complicated and uncomplicated type. Uncomplicated diverticulitis is characterized by the thickening of the colonic wall and the edematous reaction of surrounding fat. CT is a leading imaging modality in imaging of acute colonic diverticulitis and evaluation of its complications which include perforation, abscess, bleeding, fistula, peritonitis, and stenosis,60 with different grading systems based on CT findings. Signs of complicated disease with an increasing gravity have been proposed: pericolic air bubbles or little pericolic fluid, presence of peridiverticular abscess collection smaller and than larger than 4 cm, gas further than 5 cm from an inflamed diverticulum, and at the end of spectrum diffuse fluid collection with distant free air, as a sign of a persistent hole in the colon61 (Figure 15). Ultrasound is usually performed as a first-line imaging modality, but its limitation in the evaluation of free gas along with difficulties in evaluation of deep abscesses and potential differential diagnosis concerning vascular and ovarian pathology stay its main limitations in characterization and grading of acute diverticulitis.62
Figure 15.
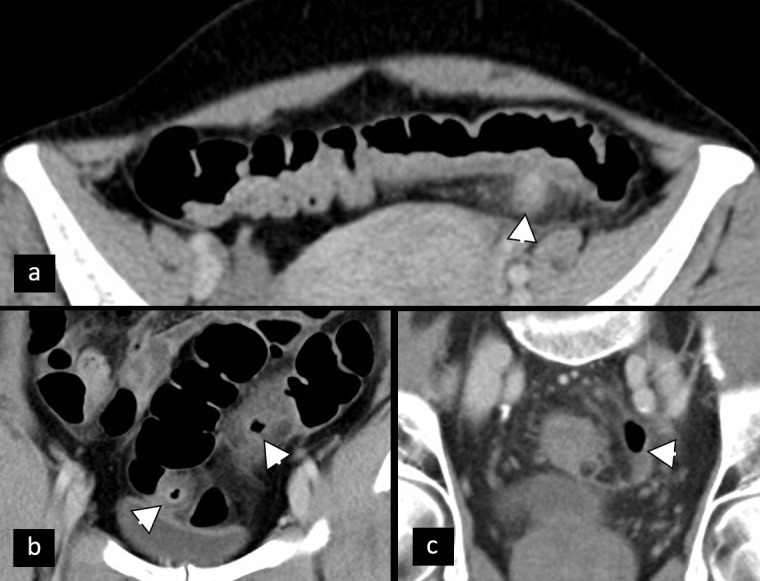
Acute diverticulitis of sigmoid colon—a spectrum of appearances. (a) Non-complicated inflamed diverticulum of the sigmoid colon (arrowhead) with stranding of adjacent fat and edema of the sigmoid wall. (b) Two simultaneous diverticula with signs of non-complicated inflammation (arrowheads). (c) Liquid–fluid collection contained within mesosigmoid fat not bigger than 4 cm (arrowhead).
Acute epiploic appendagitis is often a forgotten differential diagnosis concerning APP of intestinal origin. Torsion of cecal appendages with consequent edema and potential ischemic necrosis and aseptic inflammation leads to characteristic radiological signs on all modalities. The most specific sign on CT imaging is a fatty ovoid pericolic mass with a hyperdense ring which depicts the inflammation of the peritoneal covering of the appendage63 (Figure 16). Secondary acute epiploic appendagitis represents inflammation of the appendage located in the proximity of another inflammatory process such as colonic diverticulitis, appendicitis, or cholecystitis.64 If not complicated epiploic appendagitis is a self-limiting condition that resolves within 3–14 days without the need for antibiotics and surgery.65
Figure 16.
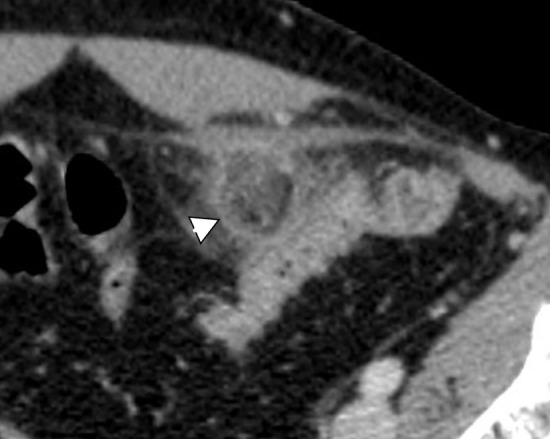
Epiploic appendagitis. Ovoid fatty formation with fat stranding as a sign of edema next to the sigmoid wall. Demarcation of a hyperattenuating ring of visceral peritoneum (arrowhead). Note the lack of edema of the sigmoid wall contrary to the findings of acute diverticulitis.
Crohn’s disease follow-up is a domain of MR enterography in comparison to CT imaging largely due to ionizing radiation issues. However, in an urgent setting, no significant difference between the two modalities was noted concerning acute findings in Crohn’s disease.66 APP can be caused by terminal ileitis and colitis and its complications such as fistula, abscess formation, or necrosis (Figure 17). Due to the ability of MRI to give functional information on diffusion-weighted imaging, it can be used in patients where ionization or contrast administration should be avoided.67 New fast protocols for MR enterography evaluation have been developed with balanced steady-state free precession imaging offering excellent overall visualization of the small bowel wall, vascular structures, mesentery, and lymph nodes without contrast administration,68 but patient preparation for MR enterography evaluation still stays as a time-consuming obstacle in the urgent setting.
Figure 17.
Necrosis of terminal ileum. (a, b) Submucous fat deposition and edema (arrow) of terminal ileum and colon with mucosal hyperemia (arrowhead); air collection in terminal ileum wall as a sign of necrosis (asterisk) and free air bubbles next to the ileal and cecal wall.
Some other differentials in lower abdominal acute pain of intestinal origin are intestinal obstruction, including volvulus, intestinal perforation, regional enteritis, incarcerated hernia perirectal abscess, intussusception, Meckel diverticulitis, mesenteric arterial or venous thrombosis, omental infarction, and various rare entities such as ileal tumor torsion etc.69–71
Vascular causes
Acute pain in the pelvis in patients with known or newly diagnosed arterial aneurysms prompts urgent evaluation of arterial status and search of signs of potential rupture. CT is a modality of choice for evaluation of aortic and iliac artery rupture based on its speed, availability, ability to depict signs of contained or impending ruptures along with its excellent depiction of arterial anatomy (Figure 18), while bedside ultrasound examination may be helpful for patients whose condition is too unstable to allow their transfer to the CT scanner.72 When periarterial hematoma is depicted on non-contrast CT examination in non-traumatic patients with the arterial aneurysm, an aneurysmatic rupture is highly suspicious, and after that, planning of interventional or surgical treatment is mandatory. Performing CT angiography gives further details concerning the anatomy of arterial tree and depicts possible active extravasation of contrast which is a direct sign of rupture.
Figure 18.
Rupture of the right common iliac artery fusiform aneurysm. (a) VRT MIP - active extravasation of contrast material (arrow). (b) Place of the rupture of the arterial wall (arrowhead) and massive retroperitoneal hematoma (asterisk).
Isolated iliac artery dissection is a relatively rare phenomenon, with only 3 out of 11 patients reported pain as a symptom.73 In patients with such finding, the “satisfaction of search error” could be an issue with the real cause of pain being unidentified. On the side of the venous system, thrombosis of the iliac, mesenteric, or ovarian veins could cause APP,74 and care must be taken to include these structures in the evaluation checklist.
Other miscellaneous conditions that can lead to APP include lead poisoning, porphyria, sickle cell crisis, somatization disorder, malingering, and narcotic seeking.69
Conclusion
Recognizing the cause of APP in females is a challenging task due to the wide spectrum of possible origin and overlap of their imaging features. Therefore, the radiologist has to be familiar with the possible causes of APP and, relying on clinical presentation, together with laboratory findings, choose the best imaging strategy in order to establish a prompt and accurate diagnosis.
Contributor Information
Marijana Basta Nikolic, Email: marijana.basta-nikolic@mf.uns.ac.rs.
Aleksandar Spasic, Email: aleksandar.spasic@mf.uns.ac.rs.
Darka Hadnadjev Simonji, Email: darkahadnadjev@gmail.com.
Sanja Stojanović, Email: sanja.stojanovic@mf.uns.ac.rs.
Olivera Nikolic, Email: olivera.nikolic@mf.uns.ac.rs.
Dragan Nikolic, Email: dragan.nikolic@mf.uns.ac.rs.
REFERENCES
- 1.Stoker J, van Randen A, Laméris W, Boermeester MA, et al. Imaging patients with acute abdominal pain. Radiology 2009; 253: 31–46. doi: 10.1148/radiol.2531090302 [DOI] [PubMed] [Google Scholar]
- 2.Otoni JC, Noschang J, Okamoto TY, Vieira DR, Petry MSM, de Araujo Ramos L, et al. Role of computed tomography at a cancer center emergency department. Emerg Radiol 2017; 24: 113–7. doi: 10.1007/s10140-016-1449-3 [DOI] [PubMed] [Google Scholar]
- 3.Zafar N, Kupesic Plavsic S. Role of ultrasound in the evaluation of acute pelvic pain in nonpregnant reproductive age patients. Reprod Age Patients Donald Sch J Ultrasound Obs Gynecol 2012; 6: 207–17. doi: 10.5005/jp-journals-10009-1244 [DOI] [Google Scholar]
- 4.Alt C, Bharwani N, Brunesch L, Stanza FM, Ma D, El SRF. ESUR quick guide to female pelvis imaging. Lisboa, Portugal: ESUR; 2019. [Google Scholar]
- 5.Scheirey CD, Fowler KJ, Therrien JA, Kim DH, Al-Refaie WB, Camacho MA, et al. ACR Appropriateness Criteria® Acute Nonlocalized Abdominal Pain. J Am Coll Radiol 2018; 15: S217–31. doi: 10.1016/j.jacr.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 6.Pandharipande PV, Reisner AT, Binder WD, Zaheer A, Gunn ML, Linnau KF, et al. CT in the emergency department: a real-time study of changes in physician decision making. Radiology 2016; 278: 812–21. doi: 10.1148/radiol.2015150473 [DOI] [PubMed] [Google Scholar]
- 7.Mervak BM, Wilson SB, Handly BD, Altun E, Burke LM. Mri of acute appendicitis. J Magn Reson Imaging 2019; 50: 1367–76. doi: 10.1002/jmri.26709 [DOI] [PubMed] [Google Scholar]
- 8.Niska R, Bhuiya F, Xu J. National Hospital ambulatory medical care survey: 2007 emergency department summary. Natl Health Stat Report 2010; 26: 1–31. [PubMed] [Google Scholar]
- 9.Fauconnier A, Provot J, Le Creff I, Boulkedid R, Vendittelli F, Doret-Dion M, et al. A framework proposal for quality and safety measurement in gynecologic emergency care. Obstet Gynecol 2020; 136: 912–21. doi: 10.1097/AOG.0000000000004132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hecht S, Meissnitzer M, Forstner R. Akutes Abdomen der Frau: gynäkologische Ursachen [Acute Pelvic pain in women-gynecological causes. Radiologe 2019; 59: 126–32. [DOI] [PubMed] [Google Scholar]
- 11.Bhavsar AK, Gelner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician 2016; 93: 41–8. [PubMed] [Google Scholar]
- 12.Potter AW, Chandrasekhar CA. Us and CT evaluation of acute pelvic pain of gynecologic origin in nonpregnant premenopausal patients. Radiographics 2008; 28: 1645–59. doi: 10.1148/rg.286085504 [DOI] [PubMed] [Google Scholar]
- 13.Sayasneh A, Ekechi C, Ferrara L, Kaijser J, Stalder C, Sur S, et al. The characteristic ultrasound features of specific types of ovarian pathology (review. Int J Oncol 2015; 46: 445–58. doi: 10.3892/ijo.2014.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schallert EK, Abbas PI, Mehollin-Ray AR, Price MC, Dietrich JE, Orth RC. Physiologic ovarian cysts versus other ovarian and adnexal pathologic changes in the preadolescent and adolescent population: US and surgical follow-up. Radiology 2019; 292: 172–8. doi: 10.1148/radiol.2019182563 [DOI] [PubMed] [Google Scholar]
- 15.Lupean R-A, Ștefan P-A, Csutak C, Lebovici A, Măluțan AM, Buiga R, et al. Differentiation of endometriomas from ovarian hemorrhagic cysts at magnetic resonance: the role of texture analysis. Medicina 2020; 56: 113. doi: 10.3390/medicina56100487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolic O, Basta Nikolic M, Spasic A, Otero-Garcia MM, Stojanovic S. Systematic radiological approach to utero-ovarian pathologies. Br J Radiol 2019; 92: 20180439. doi: 10.1259/bjr.20180439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Härmä K, Vollmar P. Imaging of ovarian disease-related pain. In: Pain imaging: a clinical-radiological approach to pain diagnosis. New York: Springer International Publishing; 2019. pp. 45169. [Google Scholar]
- 18.Czeyda-Pommersheim F, Kalb B, Costello J, Liau J, Meshksar A, Arif Tiwari H, et al. Mri in pelvic inflammatory disease: a pictorial review. Abdom Radiol 2017; 42: 935–50. doi: 10.1007/s00261-016-1004-4 [DOI] [PubMed] [Google Scholar]
- 19.Amirbekian S, Hooley RJ. Ultrasound evaluation of pelvic pain. Radiol Clin North Am 2014; 52: 1215–35. doi: 10.1016/j.rcl.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 20.Revzin MV, Mathur M, Dave HB, Macer ML, Spektor M. Pelvic inflammatory disease: multimodality imaging approach with clinical-pathologic correlation. Radiographics 2016; 36: 1579–96. doi: 10.1148/rg.2016150202 [DOI] [PubMed] [Google Scholar]
- 21.Gomes MM, Cavalcanti LS, Reis RL, Silva EJdaCe, Dutra JB, Melo-Leite AFde. Twist and shout: magnetic resonance imaging findings in ovarian torsion. Radiol Bras 2019; 52: 397–402. doi: 10.1590/0100-3984.2018.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otjen JP, Stanescu AL, Alessio AM, Parisi MT. Ovarian torsion: developing a machine-learned algorithm for diagnosis. Pediatr Radiol 2020; 50: 706–14. doi: 10.1007/s00247-019-04601-3 [DOI] [PubMed] [Google Scholar]
- 23.Anyanwu M, K G, M K. Diagnostic dilemma of hyaline cystic degeneration of uterine fibroids. OGIJ 2019; 10. doi: 10.15406/ogij.2019.10.00444 [DOI] [Google Scholar]
- 24.Wilde S, Scott-Barrett S. Radiological appearances of uterine fibroids. Indian J Radiol Imaging 2009; 19: 222–31. doi: 10.4103/0971-3026.54887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhuyar S, Sontakke B, Rajbhara PM. Degenerated fibroid - a diagnostic challenge. Int J Reprod Contracept Obstet Gynecol 2016; 6: 292. doi: 10.18203/2320-1770.ijrcog20164677 [DOI] [Google Scholar]
- 26.Arleo EK, Schwartz PE, Hui P, McCarthy S. Review of leiomyoma variants. American Journal of Roentgenology 2015; 205: 912–21. doi: 10.2214/AJR.14.13946 [DOI] [PubMed] [Google Scholar]
- 27.Le D, Dey CB, Byun K. Imaging findings of a torsed pedunculated uterine leiomyoma: a case report. Radiology Case Reports 2020; 15: 144–9. doi: 10.1016/j.radcr.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foti PV, Farina R, Palmucci S, Vizzini IAA, Libertini N, Coronella M, et al. Endometriosis: clinical features, MR imaging findings and pathologic correlation. Insights Imaging 2018; 9: 149–72. doi: 10.1007/s13244-017-0591-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jha P, Sakala M, Chamie LP, Feldman M, Hindman N, Huang C, et al. Endometriosis MRI lexicon: consensus statement from the Society of abdominal radiology endometriosis disease-focused panel. Abdom Radiol 2020; 45: 1552–68. doi: 10.1007/s00261-019-02291-x [DOI] [PubMed] [Google Scholar]
- 30.Masselli G, Brunelli R, Monti R, Guida M, Laghi F, Casciani E, et al. Imaging for acute pelvic pain in pregnancy. Insights Imaging 2014; 5: 165–81. doi: 10.1007/s13244-014-0314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masselli G, Derchi L, McHugo J, Rockall A, Vock P, Weston M, et al. Acute abdominal and pelvic pain in pregnancy: ESUR recommendations. Eur Radiol 2013; 23: 3485–500. doi: 10.1007/s00330-013-2987-7 [DOI] [PubMed] [Google Scholar]
- 32.Lee R, Dupuis C, Chen B, Smith A, Kim YH. Diagnosing ectopic pregnancy in the emergency setting. Ultrasonography 2018; 37: 78–87. doi: 10.14366/usg.17044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chanana C, Gupta N, Bansal I, Hooda K, Sharma P, Gupta M, et al. Different sonographic faces of ectopic pregnancy. J Clin Imaging Sci 2017; 7: 6. doi: 10.4103/jcis.JCIS_105_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kao LY, Scheinfeld MH, Chernyak V, Rozenblit AM, Oh S, Dym RJ, Joshua Dym R. Beyond ultrasound: CT and MRI of ectopic pregnancy. AJR Am J Roentgenol 2014; 202: 904–11. doi: 10.2214/AJR.13.10644 [DOI] [PubMed] [Google Scholar]
- 35.Srisajjakul S, Prapaisilp P, Bangchokdee S. Magnetic resonance imaging in tubal and non-tubal ectopic pregnancy. Eur J Radiol 2017; 93: 76–89. doi: 10.1016/j.ejrad.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 36.Rao A. Potential imaging findings following assisted reproduction: complications and clinical implications. Emerg Radiol 2018; 25: 73–86. doi: 10.1007/s10140-017-1561-z [DOI] [PubMed] [Google Scholar]
- 37.Jha P, Bentley B, Behr S, Yee J, Zagoria R. Imaging of flank pain: readdressing state-of-the-art. Emerg Radiol 2017; 24: 81–6. doi: 10.1007/s10140-016-1443-9 [DOI] [PubMed] [Google Scholar]
- 38. NICE guideline - renal and ureteric stones: assessment and management. BJU International 2019; 123: 220–32. doi: 10.1111/bju.14654 [DOI] [PubMed] [Google Scholar]
- 39.Rucker CM, Menias CO, Bhalla S. Mimics of renal colic: alternative diagnoses at unenhanced helical CT. Radiographics 2004; 24 Suppl 1(SPEC. ISS.): S11–28. doi: 10.1148/rg.24si045505 [DOI] [PubMed] [Google Scholar]
- 40.O`Connor OJ, Tarek E-D, El-Ghar MA, Maher MM. Common uroradiological referrals: haematuria, loin pain, renal failure and infection haematuria, loin pain, renal failure and infection. In: Grainger & allison’s diagnostic radiology: abdominal imaging. 6th Edition. Amsterdam: Elsevier Health Sciences; 2015. pp. 832858. [Google Scholar]
- 41.Rajasekaran S, K V, Cherian M, Mehta P, Radhakrishnan S. Validity of diffusion-weighted magnetic resonance imaging in the evaluation of acute pyelonephritis in comparison with contrast-enhanced computed tomography. Pjr 2020; 85: 137–43. doi: 10.5114/pjr.2020.93669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minotti B, Treglia G, Pascale M, Ceruti S, Cantini L, Anselmi L, et al. Prevalence of microhematuria in renal colic and urolithiasis: a systematic review and meta-analysis. BMC Urol 2020; 20: 119. doi: 10.1186/s12894-020-00690-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicola R, Menias CO, Obstruction U. Stone disease, and infection. In: IDKD springer series. Cham: Springer; 2018. pp. 2238. [PubMed] [Google Scholar]
- 44.Valovska M-TI, Pais VM. Contemporary best practice urolithiasis in pregnancy. Ther Adv Urol 2018; 10: 127–38. doi: 10.1177/1756287218754765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernandez N, Pais VM. Diagnostic and management considerations for nephrolithiasis in the gravid patient. Clin Nephrol 2016; 85: 70–6. doi: 10.5414/CN108770 [DOI] [PubMed] [Google Scholar]
- 46.Tonolini M, Ippolito S. Cross-Sectional imaging of complicated urinary infections affecting the lower tract and male genital organs. Insights Imaging 2016; 7: 689–711. doi: 10.1007/s13244-016-0503-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schull A, Monzani Q, Bour L, Barry-Delongchamps N, Beuvon F, Legmann P. Imaging in lower urinary tract infections epididymo-orchitis General points. Diagn Interv Imaging 2012; 93: 5008. doi: 10.1016/j.diii.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 48.Browne RFJ, Zwirewich C, Torreggiani WC. Imaging of urinary tract infection in the adult. European Radiology Supplements 2004; 14: 1. doi: 10.1007/s00330-003-2050-1 [DOI] [PubMed] [Google Scholar]
- 49.Schull A, Monzani Q, Bour L, Barry-Delongchamps N, Beuvon F, Legmann P. Imaging in lower urinary tract infections epididymo-orchitis General points. Diagn Interv Imaging 2012; 93: 5008. doi: 10.1016/j.diii.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 50.Wong-You-Cheong JJ, Woodward PJ, Manning MA, Davis CJ. From the Archives of the AFIP: inflammatory and nonneoplastic bladder masses: radiologic-pathologic correlation. Radiographics 2006; 26: 1847–68. doi: 10.1148/rg.266065126 [DOI] [PubMed] [Google Scholar]
- 51.Jia JB, Lall C, Tirkes T, Gulati R, Lamba R, Goodwin SC. Chemotherapy-related complications in the kidneys and collecting system: an imaging perspective. Insights Imaging 2015; 6: 479–87. doi: 10.1007/s13244-015-0417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schieda N, Malone SC, Al Dandan O, Ramchandani P, Siegelman ES. Multi-modality organ-based approach to expected imaging findings, complications and recurrent tumour in the genitourinary tract after radiotherapy. Insights Imaging 2014; 5: 25–40. doi: 10.1007/s13244-013-0295-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang ZJ, Westphalen AC, Zagoria RJ. Ct and MRI of small renal masses. Br J Radiol 2018; 91: 20180131. doi: 10.1259/bjr.20180131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Ghar MA, Farg H, Sharaf DE, El-Diasty T. Ct and MRI in urinary tract infections: a spectrum of different imaging findings. Medicina 2021; 57: 32. doi: 10.3390/medicina57010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eng KA, Abadeh A, Ligocki C, Lee YK, Moineddin R, Adams-Webber T, et al. Acute appendicitis: a meta-analysis of the diagnostic accuracy of US, CT, and MRI as second-line imaging tests after an initial us. Radiology 2018; 288: 717–27. doi: 10.1148/radiol.2018180318 [DOI] [PubMed] [Google Scholar]
- 56.Kim HY, Park JH, Lee YJ, Lee SS, Jeon J-J, Lee KH. Systematic review and meta-analysis of CT features for differentiating complicated and uncomplicated appendicitis. Radiology 2018; 287: 104–15. doi: 10.1148/radiol.2017171260 [DOI] [PubMed] [Google Scholar]
- 57.Kim HY, Park JH, Lee SS, Lee WJ, Ko Y, Andersson RE, et al. CT in Differentiating Complicated From Uncomplicated Appendicitis: Presence of Any of 10 CT Features Versus Radiologists’ Gestalt Assessment. American Journal of Roentgenology 2019; 213: W218–27. doi: 10.2214/AJR.19.21331 [DOI] [PubMed] [Google Scholar]
- 58.Bom WJ, Bolmers MD, Gans SL, van Rossem CC, van Geloven AAW, Bossuyt PMM, et al. Discriminating complicated from uncomplicated appendicitis by ultrasound imaging, computed tomography or magnetic resonance imaging: systematic review and meta-analysis of diagnostic accuracy. BJS Open 2021; 5: zraa030. doi: 10.1093/bjsopen/zraa030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marin D, Ho LM, Barnhart H, Neville AM, White RR, Paulson EK. Percutaneous abscess drainage in patients with perforated acute appendicitis: effectiveness, safety, and prediction of outcome. American Journal of Roentgenology 2010; 194: 422–9. doi: 10.2214/AJR.09.3098 [DOI] [PubMed] [Google Scholar]
- 60.You H, Sweeny A, Cooper ML, Von Papen M, Innes J. The management of diverticulitis: a review of the guidelines. Med J Aust 2019; 211: 421–7. doi: 10.5694/mja2.50276 [DOI] [PubMed] [Google Scholar]
- 61.Sartelli M, Catena F, Ansaloni L, Coccolini F, Griffiths EA, Abu-Zidan FM, et al. WSES guidelines for the management of acute left sided colonic diverticulitis in the emergency setting. World J Emerg Surg 2016; 11: 37. doi: 10.1186/s13017-016-0095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sartelli M, Weber DG, Kluger Y, Ansaloni L, Coccolini F, Abu-Zidan F, et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J Emerg Surg 2020; 15: 32. doi: 10.1186/s13017-020-00313-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gourgiotis S, Oikonomou C, Veloudis G, Lardou I, Pittaras G, Villias C. The diagnostic dilemma of primary epiploic appendagitis and how to establish a diagnosis. Oman Med J 2016; 31: 235–7. doi: 10.5001/omj.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giannis D, Matenoglou E, Sidiropoulou MS, Papalampros A, Schmitz R, Felekouras E, et al. Epiploic appendagitis: pathogenesis, clinical findings and imaging clues of a misdiagnosed mimicker. Ann Transl Med 2019; 7: 814. doi: 10.21037/atm.2019.12.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan E, El-Banna A. A case report of epiploic appendagitis as a mimic of acute cholecystitis. Int J Surg Case Rep 2018; 53: 327–9. doi: 10.1016/j.ijscr.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spektor M, Mathur M, Santacana G, Asch D, Huber S, Staib L, et al. Does MR enterography offer added value after a recent CT in the evaluation of abdominal pain in Crohn’s disease patients? Clin Imaging 2019; 54: 78–83. doi: 10.1016/j.clinimag.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 67.Horowitz JM, Hotalen IM, Miller ES, Barber EL, Shahabi S, Miller FH. How can pelvic MRI with diffusion-weighted imaging help my pregnant patient? Am J Perinatol 2020; 37: 577–88. doi: 10.1055/s-0039-1685492 [DOI] [PubMed] [Google Scholar]
- 68.Jhaveri KS, Sagheb S, Guimaraes L, Krishna S, Ahari AF, Espin-Garcia O. Evaluation of Crohn disease activity using a potential abbreviated MRE protocol consisting of balanced steady-state free precession MRI only versus full-protocol MRE. AJR Am J Roentgenol 2021; 216: 384–92. doi: 10.2214/AJR.20.22856 [DOI] [PubMed] [Google Scholar]
- 69.Kruszka PS, Kruszka SJ. Evaluation of acute pelvic pain in women. Am Fam Physician 2010; 82: 141–7. [PubMed] [Google Scholar]
- 70.Nelson MJ, Pesola GR. Left lower quadrant pain of unusual cause. J Emerg Med 2001; 20: 241–5. doi: 10.1016/S0736-4679(00)00316-4 [DOI] [PubMed] [Google Scholar]
- 71.Yang T-W, Tsuei Y-W, Kao C-C, Kuo W-H, Chen Y-L, Lin Y-Y. Torsion of a giant antimesenteric lipoma of the ileum: a rare cause of acute abdominal pain. Am J Case Rep 2017; 18: 589–92. doi: 10.12659/AJCR.903574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rakita D, Newatia A, Hines JJ, Siegel DN, Friedman B. Spectrum of CT findings in rupture and impending rupture of abdominal aortic aneurysms. Radiographics 2007; 27: 497–507. doi: 10.1148/rg.272065026 [DOI] [PubMed] [Google Scholar]
- 73.Liang Z, Guo W, Du C, Xie Y. Effectiveness of the conservative therapy for spontaneous isolated iliac artery dissection: preliminary results. Vascular 2017; 25: 649–56. doi: 10.1177/1708538117710845 [DOI] [PubMed] [Google Scholar]
- 74.Naffaa L, Deshmukh T, Tumu S, Johnson C, Boyd KP, Meyers AB. Imaging of acute pelvic pain in girls: ovarian torsion and beyond☆. Curr Probl Diagn Radiol 2017; 46: 317–29. doi: 10.1067/j.cpradiol.2016.12.010 [DOI] [PubMed] [Google Scholar]