Abstract
Immunotherapy for cancer has moved from pre-clinical hypothesis to successful clinical application in the past 15 years. However, not all cancers have shown response rates in clinical trials for these new agents, high-grade gliomas in particular have proved exceedingly refractory to immunotherapy. In adult patients, there has been much investigation into these failures, and researchers have concluded that an immunosuppressive microenvironment combined with low mutational burden render adult glioblastomas “immune cold”.
Pediatric cancer patients develop gliomas at a higher rate per malignancy than adults, and their brain tumors bear even fewer mutations. These tumors can also develop in more diverse locations in the brain, beyond the cerebral hemispheres seen in adults, including in the brainstem where critical motor functions are controlled. While adult brain tumor immune infiltration has been extensively profiled from surgical resections, this is not possible for brainstem tumors which can only be sampled at autopsy.
Given these limitations, there is a dearth of information on immune cells and their therapeutic and prognostic impact in pediatric high-grade gliomas (pHGGs), including hemispheric tumors in addition to brainstem. In this report we use computational methods to examine immune infiltrate in pHGGs and discover distinct immune patterns between hemispheric and brainstem tumors. In hemispheric tumors, we find positive prognostic associations for regulatory T-cells, memory B-cells, eosinophils, and dendritic cells, but not in brainstem tumors. These differences suggest that immunotherapeutic approaches must be cognizant of pHGG tumor location and tailored for optimum efficacy.
Keywords: pediatric high-grade glioma, DIPG, CIBERSORT, immune infiltration, prognostic biomarker, tumor location, immunosuppression
Introduction
Pediatric high-grade gliomas (pHGGs) are aggressive brain tumors in children with poor prognoses and limited therapeutic options. A frequent mutation in pHGG subtypes are amino acid substitutions in histone tails, specifically on histone H3.1 and H3.3. Lysine-to-methionine (H3.1/3.3-K27M) mutations occur in brainstem and midline tumors almost exclusively, and indicate the worst prognosis among pHGGs. Hemispheric tumors arise in the cerebral cortex and are often H3-WT but sometimes feature H3.3-G34R/V mutations, which have worse prognosis than H3-WT but significantly better than H3.1/3.3-K27M [1]. Radiotherapy is the standard of care for brainstem tumors, while hemispheric tumors may add chemotherapy or targeted therapy in combination with radiotherapy depending on detected mutations [2]. There have been no significant advances in pHGG therapy and these cancers are in desperate need of inventive and efficacious modalities.
Clinical trials have recently begun investigating immuno-modulating therapies for pHGG, including vaccines (NCT01130077, NCT03334305, NCT03615404), immune checkpoint blockade (NCT03690869), and cytokine therapy (NCT03330197). For these interventions to work properly, there must be cytotoxic immune cells present either in the tumor, or in the peripheral blood that can traffic to the tumor site, to be stimulated and become more active. These trials are not designed to account for mutational and anatomical differences among pHGG patients, which may play a role in efficacy of immunotherapies if immune infiltration differs by these factors. At present, there have been limited investigations on the immune status of pHGGs that include hemispheric and brainstem tumors, and how different immune cell subtypes may contribute to patient prognosis.
Within this report we use the computational methods CIBERSORT, MCP Counter and CIBERSORTx to investigate a pHGG patient dataset, which includes both H3-WT hemispheric and H3-K27M brainstem gliomas. We find that distributions of immune cells differ between these tumor locations and that positive patient prognoses can be predicted by immune cell types. Presence of regulatory T-cells, memory B-cells, eosinophils, and dendritic cells are positively prognostic for hemispheric tumors, but not brainstem tumors. We further investigate immunosuppressive factors in the RNA-Seq data and find brainstem tumors possess a gene signature consistent with a more immunosuppressive microenvironment. We also determine genetic correlates of patient survival that suggest cytokines and growth factors are influential in the progression of pHGGs and may represent targets for developmental therapeutics.
Materials and Methods
RNA-Seq dataset and CIBERSORT/CIBERSORTx/MCP Counter analysis
Raw RNA-Seq from dataset published by Mackay et al [1] was analyzed by the CIBERSORT algorithm with the standard LM22 matrix [3; 4]. Patient demographic characteristics of the population analyzed are summarized in Table 1. The dataset was used for MCP Counter analysis [5] and CIBERSORTx analysis. Individual patients were segmented into H3-WT hemispheric tumors and H3-K27M brainstem tumors for further analysis and matched to survival data. Midline and G34R/V hemispheric tumors were not used for survival analyses due to lack of statistical power. CIBERSORT values per patient and immune cell type were classified as significant (p < 0.05), non-significant (p > 0.05), and undetectable (p-value could not be computed). The p-value from CIBERSORT output is a p-value for the global deconvolution of each sample. MCP Counter scores are presented with a cut-off value of 10, excluding <2% of all data points. CIBERSORTx in B-mode (batch correction) was applied to the normalized bulk RNA-seq gene expression dataset with the LM22 gene signature to estimate the relative fractions of 22 immune cell types.
Table 1.
Description of patient population examined by CIBERSORT. Age, gender and survival of patients studied are shown for the entire group and by histone mutation status and tumor location. These data are compiled from Mackay et al.
| Characteristics | All | Brainstem | Midline | Hemispheric | |||
|---|---|---|---|---|---|---|---|
| WT | K27M | WT | K27M | WT | G24RV | ||
|
| |||||||
| Median age, yr (range) | 9.7 (0.1–30) | 8 (1.8–15.6) | 7.7 (2–17.2) | 8.3 (0.5–20) | 9.9 (1.2–17.9) | 10.1 (0.1–22.7) | 15 (7–30) |
| Number of patients | 220 | 9 | 54 | 21 | 23 | 85 | 17 |
| Female no. (%) | 95 (49) | 3 (33) | 31 (65) | 8 (38) | 13 (57) | 30 (43) | 7 (47) |
| Male no. (%) | 97 (51) | 6 (67) | 17 (35) | 13 (62) | 10 (43) | 39 (57) | 8 (53) |
| Survival, months (range) | 21.1 (0.1–160.8) | 12.4 (0.1–33.3) | 12 (0.3–36.2) | 36.3 (3.4–160.8) | 17.9 (0.1–30.1) | 30.1 (1.7–137.6) | 20.2 (2.3–65.9) |
Statistical analysis and graphing software
Statistical tests such as Log-Rank survival, Wilcoxon survival, Spearman correlation, ANOVA, and unpaired t-tests were performed with GraphPad Prism 8. Alpha was set at 0.05 and data shown in Figures 2A and 6A were corrected for multiple hypothesis testing whereas all other figures are depicting independent comparisons and do not require multiple hypothesis testing. All graphs were made in GraphPad Prism 8. For CIBERSORTx data, graphs were made in R (v.3.6.0) with R packages ggplot2 and ggpubr. P values were calculated by a two-sided Wilcoxon rank-sum test.
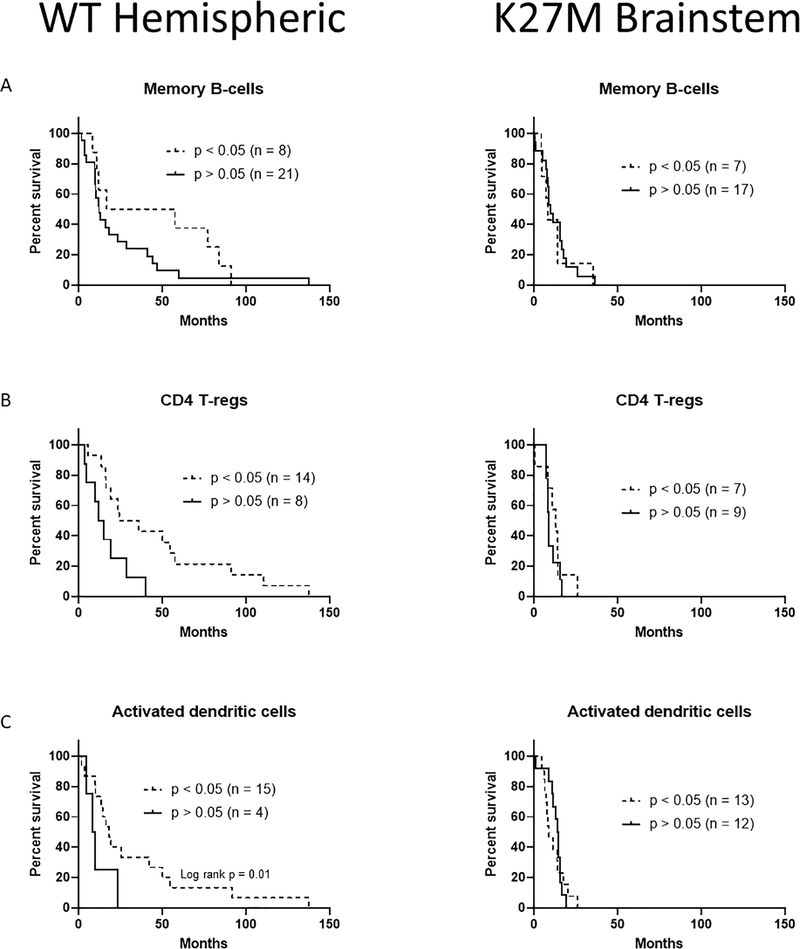
Figure 2.
Lymphoid immune cell infiltrates can predict survival benefit in hemispheric pHGG. (A) Survival curves of hemispheric and brainstem pHGG patients with detectable amounts of memory B-cells. (B) Survival curves of hemispheric and brainstem pHGG patients with detectable amounts of regulatory T-cells. (C) Survival curves of hemispheric and brainstem pHGG patients with detectable amounts of activated dendritic cells. Significant Wilcoxon or Log-Rank results are indicated.
Figure 6.
MCP Counter analysis complements CIBERSORT immune infiltration survival benefits. (A) Distribution of MCP Counter scores for cell types and segmented by tumor location. (B) Survival curves of hemispheric and brainstem pHGG patients segmented by top and bottom 20% of MCP Counter scores for each cell type. * = p < 0.05 by one-way ANOVA or Log-Rank, no multiple comparison correction.
Results
We first examined distributions of immune cells that could be detected by the CIBERSORT platform and their differences between pHGG tumor location. Significant differences were found between WT hemispheric pHGGs and K27M brainstem pHGGs for regulatory T-cells, activated dendritic cells, and eosinophils (Fig 1A, * = p < 0.05). Additionally, G34R/V hemispheric pHGGs displayed fewer regulatory T-cells and NK cells than WT hemispheric pHGGs (Fig 1A, ** = p < 0.01). As sample sizes were highest for WT hemispheric and K27M brainstem patients, we further analyzed cell type distribution by normalizing significance to total cell number. We found more detectable amounts of CD8, NK, M1 macrophages, and activated mast cells in hemispheric tumors, but more detectable amounts of activated dendritic cells (DCs) and neutrophils in brainstem tumors (Fig 1B). Of the detectable total for each cell type, hemispheric tumors had more significant amounts of regulatory T-cells and eosinophils.
Figure 1.
Immune cell type prevalence varies by pHGG tumor location. (A) Distribution of CIBERSORT p-values (assessed by were t-tests with multiple comparison correction) for immune cell types and segmented by tumor location. (B) Distribution of CIBERSORT p-values for immune cell types, normalized to total sample number, and segmented by WT hemispheric or K27M brainstem location. * = p < 0.05 by one-way ANOVA, no multiple comparison correction.
We next compared if the significant immune infiltrate of each cell type held prognostic value for patient survival outcomes. Our previously published work examined NK and CD8+ T cells[6], therefore, here we examined additional cell types. In the lymphoid compartment, we found that memory B-cells (Fig 2A), CD4+ regulatory T-cells (Fig 2B, p = 0.01), and activated DCs (Fig 2C) were positively prognostic in hemispheric pHGGs when patients had presence of each cell type. Notably, this did not hold true for brainstem DIPG patients, who showed no survival benefit for these cell types.
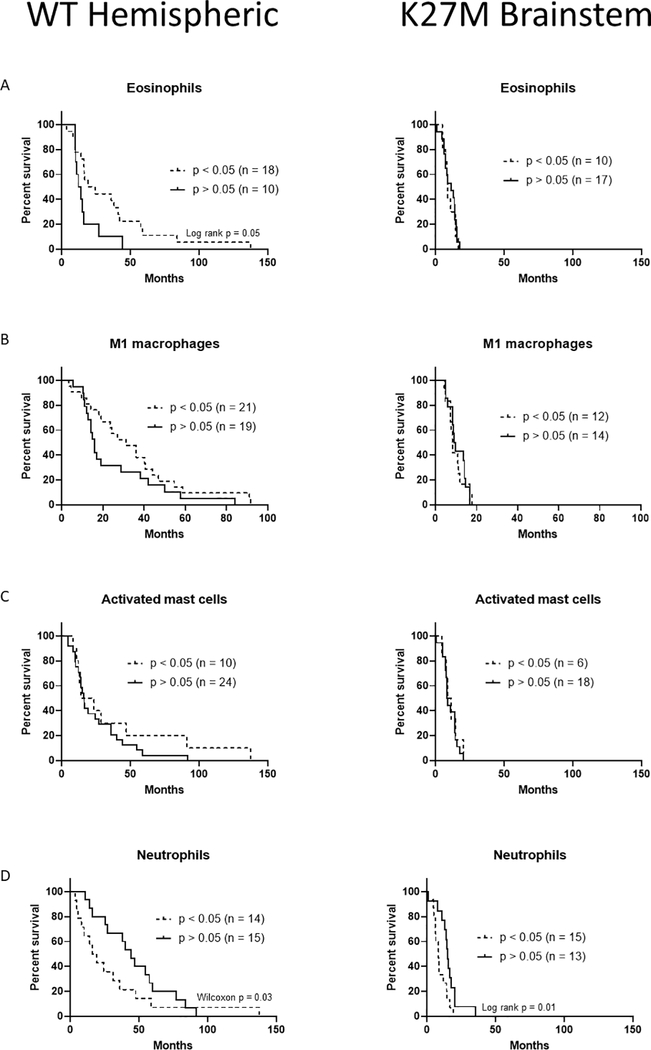
We next examined if cell types in the myeloid compartment were prognostic and varied by pHGG tumor location. We again found that brainstem tumors never benefit from immune infiltrate, but positive associations were found in hemispheric tumors for eosinophils (Fig 3A, p = 0.05), M1 macrophages (Fig 3B), and activated mast cells (Fig 3C). Interestingly, neutrophils were negatively prognostic for both hemispheric (Fig 3D, p = 0.03) and brainstem (Fig 3D, p = 0.01) locations. We compared hemispheric to brainstem tumors using the average patient survival for each type, aiming to profile which immune cells types could predict long-term survivors, or the “long-tail” seen in immunotherapy regimens [7]. Here we found significant differences by tumor location for NK cells, regulatory T-cells, dendritic cells, memory B-cells, eosinophils, monocytes, and M1 macrophages (Figs 4A and 4B, * = p < 0.05, ns = not significant). Cytotoxic CD8 T-cells and M2 macrophages could predict small numbers of long-term survivors, but the differences were non-significant, and detectable presence of these cells pushed survival below the average for hemispheric pHGGs. However it is important to note that macrophage and microglia gene expression profiles in brain as assessed by CIBERSORT may overlap and M1 values may be inclusive of other cell types present in the brain.
Figure 3.
Myeloid immune cell infiltrates can predict survival benefit in hemispheric pHGG. (A) Survival curves of hemispheric and brainstem pHGG patients with detectable amounts of eosinophils. (B) Survival curves of hemispheric and brainstem pHGG patients with detectable amounts of M1 macrophages. (C) Survival curves of hemispheric and brainstem pHGG patients with detectable amounts of activated mast cells. (D) Survival curves of hemispheric and brainstem pHGG patients with detectable amounts of neutrophils. Significant Wilcoxon or Log-Rank results are indicated.
Figure 4.
Immune infiltration only correlates with long-term survivors in hemispheric pHGG. (A) All detectable lymphoid cell types plotted as hemispheric v. brainstem by patient survival. (B) All detectable myeloid cell types plotted as hemispheric v. brainstem by patient survival. Average survival calculated using all patients regardless of detectable CIBERSORT output. * = p < 0.05 by unpaired t-test, no multiple comparison correction; ns = no significance.
Given the complete lack of survival benefit in brainstem tumors across several immune cell types, we hypothesized the local tumor microenvironment may be immunosuppressive and lacking in inflammatory signals. We investigated the RNA-Seq data used for CIBERSORT and plotted immunosuppressive genes segregated by tumor location. Brainstem tumors uniformly expressed more immunosuppressive genes, with significant differences (Fig 5A, * = p < 0.05) found for IDO2, IL10, FASLG, IL6, VEGFA, and VEGFC. These genes were chosen based on reports linking them to immune suppression in brain tumors[8; 9; 10; 11; 12]. We next sought to examine if we could detect secretory cytokines from immune cells within the bulk RNA-Seq data and if they differed by tumor location. Using patients with significant relative NK cell infiltrate as a model, we found hemispheric tumors expressed significantly more TGFβ1, but less IFNG and GZMB, than brainstem tumors (Fig 5B, * = p < 0.05). TGFβ family members are well known to suppress NK function [13], but NK cells are able to confer survival benefit in hemispheric tumors [14], suggesting NK activating signals are expressed highly enough in hemispheric pHGG to compensate. For tumors with NK infiltrate, the immunomodulatory genes GZMB and SLAMF6 correlate significantly with survival in hemispheric tumors (Fig 5C, p < 0.05), and no immunosuppressive genes correlated in brainstem tumors. When examining all patients together, we found that IL10, FGL2, VEGFB, and VEGFC were significantly correlated with hemispheric pHGG survival (Fig 5D, p < 0.05). In brainstem tumors, IL10 and IDO2 were significantly correlated (Fig 5E, p < 0.05), suggesting that IL10 may be a common immunomodulator across pHGG subtypes.
Figure 5.
Brainstem pHGG exhibit greater immunosuppression which can be correlated with survival. (A) Gene expression of immunosuppressive factors from all patients regardless of detectable CIBERSORT output. (B) Gene expression of secreted cytokines from patients with significant NK infiltration by CIBERSORT. (C) Expression v. survival plot of immunosuppression genes with significant Spearman correlations (p < 0.05) in hemispheric pHGG patients with significant NK infiltration by CIBERSORT. (D) Expression v. survival plot of immunosuppression genes with significant Spearman correlations (p < 0.05) in all hemispheric pHGG patients. (E) Expression v. survival plot of immunosuppression genes with significant Spearman correlations (p < 0.05) in all brainstem pHGG patients. * = p < 0.05 by unpaired t-test, no multiple comparison correction. Inset table shows values for Spearman correlations and for 5C-E.
We concluded our study by using alternate computational analyses of the same pHGG RNA-Seq data and performed MCP Counter analysis as well as CIBERSORTx. The MCP Counter method claims to offer superior inter-sample analytical capability versus CIBERSORT’s intra-sample leukocyte resolution [5]. We first plotted MCP Counter scores for pHGG tumor types, which directly correlate to proportion of the indicated cell type in the tumor sample. We found K27M brainstem tumors to have significantly more monocytes, while WT hemispheric tumors had significantly more fibroblasts and significantly less neutrophils compared G34R/V hemispheric and WT midline tumors, respectively (Fig 6A, * = p < 0.05). We next probed survival data by taking the top 20% and bottom 20% of MCP Counter scores for each immune cell type and plotted WT hemispheric and K27M brainstem tumor survival. Like our CIBERSORT findings, we found NK cells and CD8 T-cells to benefit WT hemispheric survival but have no or detrimental effects for K27M brainstem survival (Fig 6B, * = p < 0.05). Monocytes displayed a similar survival prediction pattern, while endothelial cells displayed no difference in either tumor location. Fibroblasts and dendritic cells show clear positive benefits for WT hemispheric tumors and possible positivity for K27M brainstem. Intriguingly, neutrophils were positively prognostic for WT hemispheric tumors but a negative factor for K27M brainstem tumors. CIBERSORTx is a relatively newly designed informatics platform and was built based on single-cell RNA-seq data and is expected to be more accurate for RNA-seq data deconvolution relative to CIBERSORT which uses bulk expression array data. Table 2 shows the 22 cell types assessed by CIBERSORTx and p values as calculated by a two-sided Wilcoxon rank-sum test in WT versus K27M tumors. Both absolute abundance and fractional abundance were examined and reveals that K27M tumors have significantly more CD4 memory activated T cells, resting NK cells, activated DCs, eosinophils and neutrophils than WT tumors.
Table 2.
CIBERSORTx analyses of immune cells. P values for absolute and fractional abundance are shown for 22 cell types using B-mode (batch correction) as applied to the normalized bulk RNA-seq gene expression dataset. Bolded p values (p < 0.05) are significant in the indicated subtypes. P values were calculated by a two-sided Wilcoxon rank-sum test.
| Cell type | Absolute abundance | Fractional abundance |
|---|---|---|
| Naïve B-cells | 0.059 | 0.017 (WT) |
| Memory B-cells | 0.22 | 0.43 |
| Plasma cells | 0.8 | 0.68 |
| CD8 T-cells | 0.91 | 0.87 |
| CD4 naïve T-cells | 0.65 | 0.92 |
| CD4 memory resting T-cells | 0.85 | 0.84 |
| CD4 memory activated T-cells | 0.023 (K27M) | 0.096 |
| Follicular helper T-cells | 0.08 | 0.012 (WT) |
| Regulatory T-cells | 0.4 | 0.21 |
| Gamma-delta T-cells | 0.81 | 0.77 |
| Resting NK cells | 0.042 (K27M) | 0.3 |
| Activated NK cells | 0.13 | 0.13 |
| Monocytes | 0.77 | 0.88 |
| M0 macrophages | 0.099 | 0.21 |
| M1 macrophages | 0.21 | 0.084 |
| M2 macrophages | 0.21 | 0.032 (WT) |
| Resting DCs | 0.3 | 0.15 |
| Activated DCs | 0.0041 (K27M) | 0.0081 (K27M) |
| Resting mast cells | 0.54 | 0.82 |
| Activated mast cells | 0.04 (WT) | 0.018 (WT) |
| Eosinophils | 0.018 (K27M) | 0.063 |
| Neutrophils | 0.013 (K27M) | 0.03 (K27M) |
Discussion
A handful of studies have investigated neuro-oncology immune infiltrates using a combination of computational and live tissue methods, as well as cohorts of adult glioblastoma, pediatric gliomas, or a mixture of the two. Tang et al recently published an immune risk score (IRS) based upon CIBERSORT data in cohorts of adult glioblastoma [15]. In their analysis, they found low numbers of activated NK cells correlated with poor patient prognosis, matching our observations in hemispheric pHGG [14]. However, they also found that significant infiltration of memory B-cells, activated dendritic cells, and M1 macrophages were negatively prognostic, the opposite of our observations. They also did not report on eosinophils, neutrophils, or regulatory T-cells, possibly because these datasets did not report significant infiltration of these cell types. For single gene correlations, they found IDO and GZMB to be negatively prognostic with regards to their IRS score. This was again opposite of our observations, however we correlated expression directly with patient survival, not risk score. Other reports have shown distinct phenotypes of immune cells present in adult gliomas compared to pediatric [16] which may explain these observational differences.
Bockmayr et al developed their own immune signature algorithm to analyze a dataset of over 1,000 samples that included both adult glioma and pHGG [17]. Their analysis found that H3-WT gliomas had a significant enrichment in endothelial gene signatures compared to H3-mutated pHGGs, suggesting increased vascularization of H3-WT tumors. This hypothesis is being investigated using mouse models of DIPG and pHGG [18]. They also found that tumors rich in antigen-presenting cells (APCs), such as dendritic cells and helper T-cells, had a favorable prognosis if the tumor also contained cytolytic cells (CD8 T-cells and NK). H3-WT tumors in this cohort contain 6–7X more adult gliomas than pHGGs, and the authors did not separate these cases in their analyses. However, by examining H3-G34R/V pHGGs compared to H3-K27M pHGGs, we can make partial conclusions based upon tumor location in pediatric patients from this data. H3-G34R/V tumors presented much higher proinflammatory signaling compared to H3-K27M, and tumors with a DIPG diagnosis followed the same trend when compared to anaplastic astrocytoma and glioblastoma. However, we cannot rule out the contribution of the H3-G34R/V mutation in these observed phenotypes compared to H3-WT hemispheric pHGG, as we also observed significant differences in immune cell infiltration comparing G34R/V vs. WT hemispheric pHGGs. It is also important to note the differences in algorithms across platforms and the consequences on data generated. This was apparent in our current study since using CIBERSORT, the presence of neutrophils (p<0.05) related with poor survival, but in MCP counter having high (top 20%) neutrophils correlated with improved survival.
Lieberman et al used both gene expression as well as IHC and functional assays to assess immune infiltrate in pediatric tumors exclusively, allowing direct comparisons of DIPG and hemispheric pHGG [19]. This type of hybrid approach of examining cell histological features as well as genomic data will be important in confirming our macrophage data from CIBERSORT analyses given overlaps in gene expression signatures for microglia and macrophage populations. Regardless, our data was concurrent with that of Lieberman et al in finding that DIPG express lower amounts of TBFβ1 but higher amounts of VEGFα compared to pHGG. Their overarching hypothesis was that lack of immunosurveillance in DIPG was responsible for the low number of immune infiltrates, particularly T-cells. However, we did not find that increased presence of dendritic cells correlated with improved survival in DIPG patients. Furthermore, it has been shown that DIPG patients produce tumor-specific T-cells for the K27M antigen [20], suggesting that tumor microenvironment and trafficking of cytotoxic leukocytes plays a larger role. Recent investigations suggest roles for resident macrophages [21] and chemoattractant gradients [22] in immune reactivity of gliomas, and further studies are needed to probe cytokine secretion capabilities of various pHGG tumor types and their associated resident macrophages.
Collectively, these data combined with our findings suggests brainstem pHGGs possess a harshly immunosuppressive microenvironment lacking in inflammatory signals [16; 17; 19], potentially explaining why immune infiltrate in these tumors is never positively prognostic compared to hemispheric pHGGs. It should be noted that local neuroinflammation caused by infused CAR-T therapy was shown to be fatal in mouse models [23], indicating that caution must be used when attempting to stimulate cytokines in brainstem pHGGs. Another cogent hypothesis is that vascular differences exist between these tumor locations [17; 18; 24], preventing the influx of immune cells to the tumor site. Our data showing mast cell and eosinophil associations with tumors are surprising. Little is known regarding the presence of these immune cells in healthy brain, however reports indicate they are mobilized to the brain during injury, and have been noted in adult glioblastoma patients[25; 26]. Going forward, immunotherapeutic modalities for pHGG will need to consider tumor location when designing new interventions. We suggest that hemispheric pHGGs may respond well to vaccines, checkpoint blockade, and macrophage depletion, while brainstem DIPG will likely benefit more from carefully titrated adoptive cell therapies, epigenetic modulation, and new surgical delivery techniques.
Acknowledgments
The authors thank Dr. Michael Curran for suggestion of CIBERSORT/MCP Counter and Dr. Chris Jones for providing raw RNA-Seq data for analysis.
Funding
This work was supported by the Schissler Foundation Fellowship (to CB) and from the (i) National Institutes of Health: R21 NS093387 (to JC); R61/R33 NS111058 (to JC) and the (ii) Brain Tumor SPORE: P50 CA127001 (Developmental Research Project to JC).
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Statement
This study did not does not contain any human subjects research or animal research performed by any of the authors. Data sets that were probed are publicly available.
References
- [1].Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, Bjerke L, Clarke M, Vinci M, Nandhabalan M, Temelso S, Popov S, Molinari V, Raman P, Waanders AJ, Han HJ, Gupta S, Marshall L, Zacharoulis S, Vaidya S, Mandeville HC, Bridges LR, Martin AJ, Al-Sarraj S, Chandler C, Ng HK, Li X, Mu K, Trabelsi S, Brahim DH, Kisljakov AN, Konovalov DM, Moore AS, Carcaboso AM, Sunol M, de Torres C, Cruz O, Mora J, Shats LI, Stavale JN, Bidinotto LT, Reis RM, Entz-Werle N, Farrell M, Cryan J, Crimmins D, Caird J, Pears J, Monje M, Debily MA, Castel D, Grill J, Hawkins C, Nikbakht H, Jabado N, Baker SJ, Pfister SM, Jones DTW, Fouladi M, von Bueren AO, Baudis M, Resnick A, and Jones C, Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 32 (2017) 520–537 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bailey CP, Figueroa M, Mohiuddin S, Zaky W, and Chandra J, Cutting Edge Therapeutic Insights Derived from Molecular Biology of Pediatric High-Grade Glioma and Diffuse Intrinsic Pontine Glioma (DIPG). Bioengineering (Basel) 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen B, Khodadoust MS, Liu CL, Newman AM, and Alizadeh AA, Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol Biol 1711 (2018) 243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, and Alizadeh AA, Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 12 (2015) 453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman C, Fridman WH, and de Reyniès A, Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol 17 (2016) 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bailey CP, Figueroa M, Gangadharan A, Yang Y, Romero MM, Kennis BA, Yadavilli S, Henry V, Collier T, Monje M, Lee DA, Wang L, Nazarian J, Gopalakrishnan V, Zaky W, Becher OJ, and Chandra J, Pharmacologic inhibition of lysine specific demethylase-1 (LSD1) as a therapeutic and immune-sensitization strategy in pediatric high grade glioma (pHGG). Neuro Oncol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Harris SJ, Brown J, Lopez J, and Yap TA, Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med 13 (2016) 171–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhai L, Ladomersky E, Dostal CR, Lauing KL, Swoap K, Billingham LK, Gritsina G, Wu M, McCusker RH, Binder DC, and Wainwright DA, Non-tumor cell IDO1 predominantly contributes to enzyme activity and response to CTLA-4/PD-L1 inhibition in mouse glioblastoma. Brain Behav Immun 62 (2017) 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhai L, Ladomersky E, Lauing KL, Wu M, Genet M, Gritsina G, Gyorffy B, Brastianos PK, Binder DC, Sosman JA, Giles FJ, James CD, Horbinski C, Stupp R, and Wainwright DA, Infiltrating T Cells Increase IDO1 Expression in Glioblastoma and Contribute to Decreased Patient Survival. Clin Cancer Res 23 (2017) 6650–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rolle CE, Sengupta S, and Lesniak MS, Mechanisms of immune evasion by gliomas. Adv Exp Med Biol 746 (2012) 53–76. [DOI] [PubMed] [Google Scholar]
- [11].Chahlavi A, Rayman P, Richmond AL, Biswas K, Zhang R, Vogelbaum M, Tannenbaum C, Barnett G, and Finke JH, Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res 65 (2005) 5428–38. [DOI] [PubMed] [Google Scholar]
- [12].Tamura R, Tanaka T, Akasaki Y, Murayama Y, Yoshida K, and Sasaki H, The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: perspectives for therapeutic implications. Med Oncol 37 (2019) 2. [DOI] [PubMed] [Google Scholar]
- [13].Regis S, Dondero A, Caliendo F, Bottino C, and Castriconi R, NK Cell Function Regulation by TGF-β-Induced Epigenetic Mechanisms. Front Immunol 11 (2020) 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bailey CP, Figueroa M, Gangadharan A, Yang Y, Romero MM, Kennis BA, Yadavilli S, Henry V, Collier T, Monje M, Lee DA, Wang L, Nazarian J, Gopalakrishnan V, Zaky W, Becher OJ, and Chandra J, Pharmacologic inhibition of lysine specific demethylase-1 (LSD1) as a therapeutic and immune-sensitization strategy in pediatric high grade glioma (pHGG). Neuro-Oncology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tang G, and Yin W, Development of an Immune Infiltration-Related Prognostic Scoring System Based on the Genomic Landscape Analysis of Glioblastoma Multiforme. Frontiers in Oncology 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lin GL, Nagaraja S, Filbin MG, Suva ML, Vogel H, and Monje M, Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol Commun 6 (2018) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bockmayr M, Klauschen F, Maire CL, Rutkowski S, Westphal M, Lamszus K, Schüller U, and Mohme M, Immunologic Profiling of Mutational and Transcriptional Subgroups in Pediatric and Adult High-Grade Gliomas. Cancer Immunol Res 7 (2019) 1401–1411. [DOI] [PubMed] [Google Scholar]
- [18].Wei X, Hartley R, Bear H, Fuller C, and Phoenix TN, Hgg-13. Determining Regional Differences in High-Grade Glioma Vasculature Phenotype. neuro-oncology 21 (2019) ii89. [Google Scholar]
- [19].Lieberman NAP, DeGolier K, Kovar HM, Davis A, Hoglund V, Stevens J, Winter C, Deutsch G, Furlan SN, Vitanza NA, Leary SES, and Crane CA, Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol 21 (2019) 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chheda ZS, Kohanbash G, Okada K, Jahan N, Sidney J, Pecoraro M, Yang X, Carrera DA, Downey KM, Shrivastav S, Liu S, Lin Y, Lagisetti C, Chuntova P, Watchmaker PB, Mueller S, Pollack IF, Rajalingam R, Carcaboso AM, Mann M, Sette A, Garcia KC, Hou Y, and Okada H, Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med 215 (2018) 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khalsa JK, Cheng N, Keegan J, Chaudry A, Driver J, Bi WL, Lederer J, and Shah K, Immune phenotyping of diverse syngeneic murine brain tumors identifies immunologically distinct types. Nat Commun 11 (2020) 3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Niu B, Zeng X, Phan TA, Szulzewsky F, Holte S, Holland EC, and Tian JP, Mathematical modeling of PDGF-driven glioma reveals the dynamics of immune cells infiltrating into tumors. Neoplasia 22 (2020) 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, Labanieh L, Hulleman E, Woo PJ, Rietberg SP, Vogel H, Monje M, and Mackall CL, Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med 24 (2018) 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Subashi E, Cordero FJ, Halvorson KG, Qi Y, Nouls JC, Becher OJ, and Johnson GA, Tumor location, but not H3.3K27M, significantly influences the blood-brain-barrier permeability in a genetic mouse model of pediatric high-grade glioma. J Neurooncol 126 (2016) 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Curran CS, and Bertics PJ, Eosinophils in glioblastoma biology. J Neuroinflammation 9 (2012) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ribatti D, The crucial role of mast cells in blood-brain barrier alterations. Exp Cell Res 338 (2015) 119–25. [DOI] [PubMed] [Google Scholar]