Abstract
Formins stimulate actin polymerization by promoting both filament nucleation and elongation. Because nucleation and elongation draw upon a common pool of actin monomers, the rate at which each reaction proceeds influences the other. This interdependent mechanism determines the number of filaments assembled over the course of a polymerization reaction, as well as their equilibrium lengths. In this study, we used kinetic modeling and in vitro polymerization reactions to dissect the contributions of filament nucleation and elongation to the process of formin-mediated actin assembly. We found that the rates of nucleation and elongation evolve over the course of a polymerization reaction. The period over which each process occurs is a key determinant of the total number of filaments that are assembled, as well as their average lengths at equilibrium. Inclusion of formin in polymerization reactions speeds filament nucleation, thus increasing the number and shortening the lengths of filaments that are assembled over the course of the reaction. Modulation of the elongation rate produces modest changes in the equilibrium lengths of formin-bound filaments. However, the dependence of filament length on the elongation rate is limited by the number of filament ends generated via formin’s nucleation activity. Sustained elongation of small numbers of formin-bound filaments, therefore, requires inhibition of nucleation via monomer sequestration and a low concentration of activated formin. Our results underscore the mechanistic advantage for keeping formin’s nucleation efficiency relatively low in cells, where unregulated actin assembly would produce deleterious effects on cytoskeletal dynamics. Under these conditions, differences in the elongation rates mediated by formin isoforms are most likely to impact the kinetics of actin assembly.
Significance
Dynamic remodeling of the actin cytoskeleton enables cells to perform essential processes including migration, division, and intracellular transport. Here, we dissected the mechanism of actin polymerization mediated by formin, a protein that stimulates the nucleation and elongation of actin filaments. Nucleation and elongation draw upon a common pool of actin monomers, so the rate at which each reaction proceeds influences the other. We found that robust nucleation by formins limits the availability of actin monomers that are required to generate long filaments. A low rate of formin-mediated nucleation is therefore most likely to amplify the effects of observed differences in the elongation activities of formin isoforms on the intracellular kinetics of actin assembly.
Introduction
Actin polymerization is a fundamental biological reaction that supports a broad range of essential cellular functions, including cell growth, division, and motility. Actin assembly is tightly regulated in cells, both at the initial step of filament nucleation and during subsequent elongation. Spontaneous nucleation requires the assembly of energetically unstable actin trimers that become stable filaments through the association of an additional monomer (1, 2, 3). A diverse cohort of actin-binding proteins control filament nucleation either through monomer sequestration or nucleation-promoting mechanisms, ensuring that filament assembly occurs at the appropriate time and subcellular location (4,5). Once assembled, filament nuclei continue to bind actin monomers at their barbed and pointed ends at rates that are modulated by elongation-promoting proteins (5).
Whereas many proteins that regulate actin assembly do so by influencing either filament nucleation or elongation, the formin family of proteins stimulates both processes (6, 7, 8). Formins promote filament nucleation by encircling and binding actin nuclei via their dimeric formin homology 2 (FH2) domains (9, 10, 11, 12). This interaction stabilizes nascent actin nuclei and enables elongation through subsequent actin-binding events (9,11,13, 14, 15, 16, 17). After nucleation, FH2 dimers remain bound at filament barbed ends and step processively onto incoming actin subunits to incorporate them into the filament (17). Conformational fluctuations of the FH2 dimer “gate” the barbed end by regulating its availability for actin monomer binding, ultimately slowing elongation (17, 18, 19). Formins overcome the effects of gating on elongation through transient interactions with the actin monomer-binding protein profilin (17,18). Profilin-actin complexes bind polyproline tracts located within formin FH1 domains (20,21), enabling their rapid delivery to the barbed end via diffusion of these flexible domains (18,22).
Most eukaryotes express at least two formin isoforms that assemble unbranched actin filaments that are incorporated into cytoskeletal structures including cytokinetic rings, filopodia, and stress fibers (23,24). Each formin isoform mediates a specific rate of filament elongation that depends on both the extent to which its FH2 domain gates the barbed end and the efficiency with which its FH1 domain delivers profilin-actin to the barbed end (17,25). Formin isoforms have also been shown to possess specific nucleation activities, though the mechanism underlying these differences is not well understood (26,27).
Actin polymerization proceeds at a rate that depends on the concentration of available actin monomers (2,28). This rate decreases as monomers are consumed over the course of polymerization. Because filament nucleation and elongation draw upon a common pool of actin monomers, both reactions contribute to the depletion of the monomer concentration, and the rate at which one reaction proceeds influences the rate of the other. Modulation of the efficiency of one process can therefore alter the number of filaments assembled over the course of the reaction, as well as the lengths the filaments ultimately attain. Because formins mediate both nucleation and elongation, this interdependent mechanism might enable formin isoforms with differing polymerization activities to assemble filament networks with specific physical properties.
In this study, we dissected the mechanism of formin-mediated actin polymerization to determine the contributions of filament nucleation and elongation to the process of actin assembly. Using kinetic modeling, we found that the rates of both nucleation and elongation evolve over the course of a polymerization reaction. The period over which each process occurs is a key determinant of the number of filaments that are ultimately assembled, as well as their average equilibrium lengths. Inclusion of formin in polymerization reactions speeds filament nucleation, thus increasing the number and shortening the lengths of the filaments that are assembled. Analysis of in vitro polymerization assays confirmed the effects of varying the reactant concentrations and the filament elongation rate on polymerization. Modulation of the elongation rate produces modest changes in the equilibrium lengths of formin-bound filaments. However, the dependence of filament length on the elongation rate is limited by the number of filament ends generated via formin’s nucleation activity. Sustained elongation of small numbers of formin-bound filaments therefore requires inhibition of nucleation via monomer sequestration and a low concentration of activated formin. Our results underscore the mechanistic advantage for keeping formin’s nucleation efficiency relatively low in physiological conditions (29). This strategy also maximizes the impact of differences in the elongation properties of formin isoforms on the kinetics of actin network assembly.
Materials and methods
Kinetic modeling
Kinetic schemes described by Sept and co-workers (2), Vavylonis and co-workers (18), and Paul and Pollard (10) were combined to generate a single model of actin polymerization that integrates the nucleation and elongation activities of formin in the absence and presence of profilin (Fig. 1). Consistent with the published kinetic schemes (2,10,18), reaction parameters for ATP hydrolysis are not explicitly included in our model but can be accounted for via adjustment of the elongation rate constants (18). Mathematical modeling of actin polymerization time courses was performed using the program COPASI (30) using previously published rate constants (Table S1) (10,18). Modeling was performed in a simulated reaction volume of 1000 μm3 (1 pL). Time courses were calculated in deterministic mode using the LSODA algorithm (31,32) at fixed initial bulk actin, profilin and/or formin concentrations.
Figure 1.
Kinetic model of spontaneous and formin-mediated actin filament nucleation and elongation. Profilin binds actin monomers (purple panel). Assembly of filaments with free barbed ends occurs via a nucleation phase and elongation at barbed and pointed ends (yellow panels). Assembly of filaments with formin-bound barbed ends occurs via a nucleation phase, direct binding of actin and profilin-actin to barbed ends, FH1-mediated delivery of profilin-actin to the barbed end, and binding of actin monomers to pointed ends (blue panels). Rate constants corresponding to each reaction are described in Table S1. To see the figure in color, go online.
Protein purification
Actin was purified from a chicken skeletal muscle acetone powder by one cycle of polymerization and depolymerization (33). Monomeric actin was isolated by gel filtration on Sephacryl S-300 resin (GE Healthcare, Chicago, IL) in G-Buffer (2 mM Tris (pH 8.0), 0.2 mM ATP, 0.5 mM DTT, 0.1 mM CaCl2) and stored at 4°C. The concentration of actin was calculated using an extinction coefficient of 26,600 M−1 cm−1 at 290 nm.
Constructs encoding the FH1 and FH2 domains of Cdc12p (residues 882–1375) and Bni1p (residues 1228–1766), and the FH2 domain of Bni1p (residues 1329–1766) were expressed from pGEX-4T-3 plasmids (GE Healthcare) in BL21-CodonPlus (DE3)-RP cells cells (Agilent Technologies, Santa Clara, CA) and purified as previously described (34). The Bni1p FH2 construct includes the 19 C-terminal residues of the FH1 domain, which increase the stability of the FH2 domain without altering its activity (34). We used ProtParam (www.web.expasy.org/protparam (35)) to calculate extinction coefficients. Saccharomyces cerevisiae profilin was expressed from a pMW172 vector in BL21(DE3)pLysS cells and purified as described (10,34). We used an extinction coefficient of 19,060 M−1 cm−1 at λ = 280 nm to calculate the concentration of purified profilin.
Both the kinetic model and in vitro experiments are based on a heterologous system consisting of chicken skeletal muscle actin and yeast formins and profilins. This system was selected because its polymerization properties have been extensively characterized (10,17,36). However, rates of assembly mediated by formins and profilin have been demonstrated to be actin isoform-specific (37, 38, 39). It is therefore likely that each formin mediates different rates of nucleation and elongation when paired with its homologous yeast actin.
Microscopy and data analysis
Glass coverslips (22 mm × 50 mm; Thermo Fisher Scientific, Waltham, MA) were sonicated in 2% Hellmanex III (MilliporeSigma, Burlington, MA), rinsed extensively and sonicated in ddH20. The imaging surface was constructed by placing Scotch Tape (3M) around the perimeter of a 4.5 mm × 4.5 mm region of the coverslip. Coverslips were flamed before use. The imaging surface was incubated with 0.5% Tween 20 in HS-TBS (600 mM NaCl, 50 mM Tris, (pH 7.5)) and 100 mg/mL bovine serum albumin in HS-TBS, and equilibrated with KMEI buffer (50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0)) before introduction of the sample.
Ca2+-ATP-actin monomers were incubated with 0.05 mM MgCl2 and 0.2 mM EGTA for 3 min to generate Mg2+-ATP-actin. Polymerization of 2 μM actin monomers was initiated in the absence or presence of formin and/or profilin via incubation in KMEI buffer for 1–2 h. Samples were imaged every 30 min and analyzed via total internal reflection fluorescence (TIRF) microscopy to determine when polymerization had reached equilibrium.
Assembled actin filaments were stabilized and fluorescently labeled via the addition of 4 μM fluorescein-isothiocyanate (FITC) phalloidin (Sigma-Aldrich, St. Louis, MO). After a 10-min incubation, samples were diluted to a final concentration of 2–10 nM actin in 2× microscopy buffer (1× microscopy buffer: 10 mM imidazole (pH 7.0), 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 50 mM DTT, 0.2 mM ATP, 15 mM glucose, 20 μg/mL catalase, 100 μg/mL glucose oxidase, 0.5% (w/v) methylcellulose (4000 cP at 2%)). 10–15 μL of each sample was loaded onto an imaging surface using pipette tips that were cut to reduce filament shearing. Filaments were visualized by through-objective TIRF microscopy on an Olympus Ti83 motorized microscope equipped with a CellTIRF system using a 60×, 1.49 NA objective and a 488-nm laser. Images were acquired using a Hamamatsu C9100-23B ImagEM X2 EMCCD camera and CellSens Dimension software (Olympus).
Filament numbers and lengths were quantified from TIRF micrographs using a MATLAB (The MathWorks, Natick, MA) program developed in-house. Filaments were detected from noise-filtered and background-subtracted images using MATLAB’s image thresholding algorithm. Detected filaments were skeletonized to facilitate length measurements. The number of filaments and their corresponding lengths were quantified for three replicates at each formin concentration using at least five fields of view per replicate. Single exponential fits were applied to filament length distributions (2). For an exponential distribution, the fraction of filaments (fi) with length l was determined by the relation fi = λexp(−λli), where the mean length is 1/λ and the variance (li) = (1/λ)2.
Results
To dissect the contributions of filament nucleation and elongation to the dynamics of formin-mediated actin polymerization, we constructed a kinetic model composed of reaction schemes for both formin activities (Fig. 1). Our model accounts for interactions among actin monomers, actin nuclei, filament barbed ends, filament pointed ends, formin and profilin. Consistent with published studies, we consider spontaneous filament assembly to occur through self-association of actin monomers into trimers (Fig. 1, Reactions 2 and 3) (1,2,10). Binding of a fourth monomer establishes a stable filament (Fig. 1, Reactions 4 and 5) that can elongate via the association of additional, free actin monomers at both ends and profilin-bound monomers only at the barbed end (Fig. 1, Reactions 6–9) (1,28,40, 41, 42). Formin-mediated nucleation occurs via the association of a formin FH2 dimer with two actin monomers (Fig. 1, Reaction 10) (9,10). After this step, the formin remains bound to the barbed end of the filament (17,43). FH2-mediated gating slows barbed end elongation by decreasing the frequency of actin monomer binding (Fig. 1, Reactions 11–13) (18,19). Profilin-actin complexes bind formin FH1 domains and are delivered to the barbed end in a diffusion-limited reaction (Fig. 1, Reactions 14–19) (21,22). This process speeds elongation in a profilin-dependent manner (17,18). Formins do not influence pointed end elongation (Fig. 1, Reaction 20) (17).
We used published values for the rate constants that govern each set of intermolecular associations (Table S1) (10,18). We employed rate parameters that were obtained from Brownian dynamics simulations to define the monomer dimerization, trimerization, and filament formation steps (44). We used experimentally-determined rates to describe the elongation of formin-bound filaments and filaments with free barbed ends (17,28). Our model quantifies the number of filaments that are assembled over time and tracks subunit addition at filament ends. These measurements enable quantification of the nucleation and elongation rates as they evolve over the course of each simulated polymerization reaction.
Actin polymerization in the absence of formin
To establish kinetic baselines for nucleation and elongation, we first simulated time courses of polymerization for reactions containing only actin monomers. Examination of 100,000 s trajectories enabled us to determine appropriate limits for making observations within the simulated framework. Consistent with published experimental observations (1), our simulations generated time courses of spontaneous actin polymerization that progress at rates that depend on the initial monomer concentration (Fig. 2 A). The total number of filaments assembled in each reaction increases over time and is largest at the highest sampled actin concentration at all time points throughout the course of the trajectory (Fig. 2 B). At actin concentrations exceeding 1 μM, polymerization trajectories reach stable values within ∼15,000 s. Reactions containing less than 1 μM actin require more time to arrive at equilibrium.
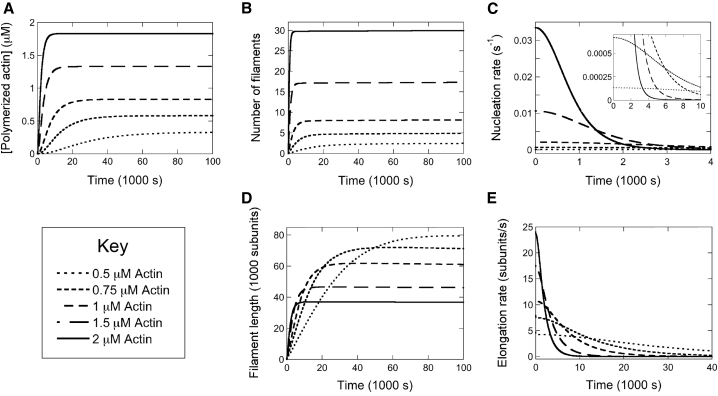
Figure 2.
Actin polymerization in the absence of formin. Quantification of simulated polymerization reactions containing 0.5 μM (dotted line), 0.75 μM (short dashes), 1 μM (medium dashes), 1.5 μM (long dashes), and 2 μM (solid line) actin monomers. Each reaction was simulated over 100,000 s in a reaction volume of 1000 μm3. (A) Concentration of polymerized actin over time. (B) Number of filaments assembled over time. (C) Actin filament nucleation rate over time. For clarity, data are plotted over a range of 4000 s. (Inset) Actin nucleation data plotted with different axis scales to highlight the shapes of the curves for the reactions containing 0.5 and 0.75 μM actin. (D) Average length of polymerized filaments over time. Lengths were calculated by dividing the concentration of polymerized actin by the number of filaments at each time point. (E) Rate of filament elongation over time. For clarity, data are plotted over a range of 40,000 s.
As expected, filament nucleation rates are fastest at early time points and decrease over time as monomers are consumed by polymerization (Fig. 2 C). The initial nucleation rate is faster at high actin concentrations than at low concentrations. However, the shape of the nucleation trajectory broadens in reactions containing lower actin concentrations, leading to faster nucleation rates at later points in the trajectory. At the lowest actin concentrations we simulated, the nucleation rate trajectory is nearly flat, but its value overtakes the nucleation rates of the higher concentrations as the reaction progresses (Fig. 2 C, inset).
In each polymerization reaction, filament elongation takes place over a longer period than nucleation (Fig. 2 E). The initial filament elongation rate is fastest at the highest actin concentration. However, reactions containing lower actin concentrations exhibit broadened trajectories with faster elongation rates at later times. Thus, a decrease in the rate at which monomers are consumed prolongs the time over which individual filaments elongate through monomer binding. As a result, filament lengths measured at the ends of the simulated trajectories are inversely proportional to the initial concentration of monomeric actin (Fig. 2 D).
To assess the relevance of our kinetic modeling to experimental observations, we compared our simulated polymerization reactions to in vitro measurements of assembled filaments. We incubated purified actin monomers in conditions mimicking those in our simulated reactions. Once the reactions reached equilibrium, we added fluorescent phalloidin, imaged the filaments using TIRF microscopy and quantified filament numbers and lengths.
In addition to nucleation and elongation, actin filaments assembled in vitro undergo length-dependent fragmentation and annealing (2,45). These reactions can alter both the number and lengths of the actin filaments. The probability of severing and annealing both increase with the concentration of filaments (2). To minimize the likelihood of these events occurring, we used actin monomer concentrations in the low micromolar range.
Each in vitro reaction robustly assembled into filaments of varying lengths (Fig. 3 A). As previously reported (2), the distributions of filament lengths are well characterized by single exponential fits (Fig. 3 B), which yield both an average filament length and a variance (see Materials and methods). The average lengths of filaments assembled in our reactions containing 2 μM actin agree with published measurements performed on similar reactions, confirming that our sample preparation and visualization methods minimize filament breakage (2). As the concentration of actin monomers included in each reaction increases, the number of assembled filaments increases and the average filament length decreases (Fig. 3, C and D; data points). The magnitudes of the concentration-dependent changes in filament number and length are similar to the trends predicted by our simulations (Fig. 3, C and D; lines), confirming that our model produces physiologically relevant insights at these actin concentrations. Despite this general agreement between our simulated and experimental data sets, the average filament length measured at 2 μM actin slightly exceeds the value predicted by our simulations. This discrepancy likely arises from our use of fluorescence measurements to quantify filament lengths. This technique is limited in its ability to detect and accurately measure very short filaments.
Figure 3.
In vitro actin polymerization in the absence of formin. The experimental conditions were as follows: A range of concentrations of actin monomers in polymerization buffer. The filaments were labeled with FITC-phalloidin and visualized by TIRF microscopy. (A) Representative TIRF micrographs of filaments assembled in reactions containing a range of actin concentrations. (B) Histogram of filament lengths measured at equilibrium for a representative polymerization reaction containing 2 μM actin. The line is an exponential fit to the data. The fitted value for λ is 0.156, and the mean filament length is 1/λ, or 6.4 μm. (C) Dependence of the number of actin filaments visualized per 10,000 μm2 on the actin concentration. Error bars are standard errors of the mean of at least 15 micrographs collected via three independent assays. Simulated data (solid line) were normalized and plotted on the same y axis scale as the experimental data. (D) Dependence of the average filament length on the actin concentration. Error bars are standard errors of the mean of at least 15 micrographs collected via three independent assays. Simulated data (solid line) were normalized and plotted on the same y axis scale as the experimental data.
Formins shorten the time frame for nucleation
Our simulated trajectories indicate that varying the concentration of the components in a polymerization reaction can alter the number of filaments that are assembled, as well as their lengths at equilibrium. To investigate how the nucleation and elongation activities of formins influence the distribution of actin monomers into populations of filaments, we simulated actin polymerization in the presence of formin. We initially sampled a range of formin concentrations spanning six orders of magnitude. In the absence of profilin, FH2 domain gating limits the rate at which actin monomers bind barbed ends, thus slowing the elongation of formin-bound filaments relative to filaments with free barbed ends (17). To create distinct rates of elongation for our simulated formin-bound and free filaments, we used an FH2 gating factor of 0.5. To ensure that our reaction time courses arrive at equilibrium within a reasonable time frame, we performed our simulations with 2 μM actin monomers. Under these conditions, most of our simulated trajectories approach equilibrium within 10,000 s (∼2.8 h).
In each simulated reaction, the number of assembled filaments increases over time, and reactions that include larger concentrations of formin contain more filaments at all time points than reactions that include lower concentrations of formin (Fig. 4 A). The initial formin-mediated nucleation rate is linearly proportional to the concentration of formin, consistent with a nucleation reaction that involves the association of one preassembled, stable formin dimer with actin monomers (Fig. 4 B, y-intercept values). In contrast, nucleation of filaments with free barbed ends (i.e., not formin-bound) occurs at the same initial rate independent of the formin concentration (Fig. S1 A). The formin-mediated and spontaneous nucleation rates both decrease over time, and the time period over which both types of nucleation events occur depends on the formin concentration (Fig. 4 B; Fig. S1 A). At each formin concentration we sampled, formin-mediated nucleation occurs over a period that is approximately twice as long as the time period for spontaneous nucleation.
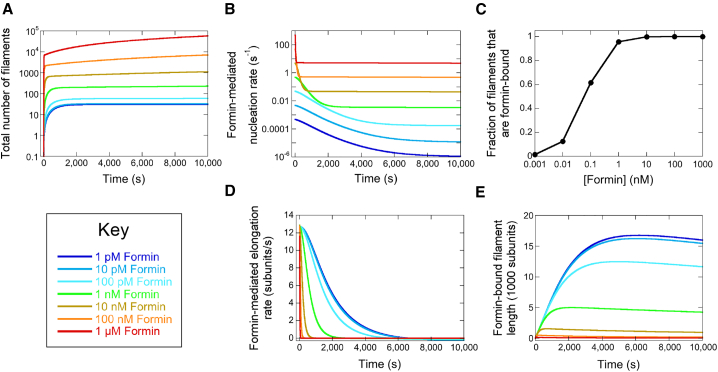
Figure 4.
Formins assemble short filaments. Quantification of simulated polymerization reactions containing 2 μM actin and 1 pM (dark blue lines), 10 pM (light blue lines), 100 pM (cyan lines), 1 nM (green lines), 10 nM (tan lines), 100 nM (orange lines), or 1 μM (red lines) formin. The formin gating factor was set at 0.5. Each simulation was carried out over 10,000 s. (A) Total number of filaments assembled over the course of each trajectory. The total number of filaments was determined by summing the numbers of formin-bound and free filaments at each time point. For clarity, the y axis is represented on a log scale. (B) Formin-mediated nucleation rates over time. For clarity, the y axis is represented on a log scale. (C) The dependence of the fraction of filaments that are formin-bound on the concentration of formin. For clarity, the x axis is represented on a log scale. (D) Formin-mediated elongation rate over time. (E) Average lengths of formin-bound filaments over time. To see the figure in color, go online.
The equilibrium composition of each polymerization reaction depends on the formin concentration. At 1 μM formin, the rate of formin-mediated nucleation is ∼105 times faster than nucleation of filaments with free barbed ends (Fig. 4 B; Fig. S1 A). Under these conditions, the polymerized reaction contains mostly formin-bound filaments (Fig. 4 C). In contrast, spontaneous actin nucleation is faster than formin-mediated nucleation at 1 pM formin. Thus, the majority of filaments assembled in this reaction have free barbed ends. At intermediate concentrations of formin, the rate of formin-mediated filament nucleation approaches the spontaneous nucleation rate. As a result, polymerized reactions contain mixtures of both formin-bound and free filaments.
Formins assemble short filaments
Nucleated filaments elongate at rates that depend on whether their barbed ends are formin-bound or free (17). In our simulated reactions, elongation of both formin-bound and free filaments slows over time as monomers are consumed (Fig. 4 D; Fig. S1 B). The elongation rate trajectory narrows as the formin concentration increases and reactions containing lower concentrations of formin exhibit faster elongation rates at later times. As a result, the average filament length quantified once each reaction has attained equilibrium is inversely dependent on the formin concentration (Fig. 4 E; Fig. S1 C).
Consistent with our kinetic modeling, inclusion of an FH1FH2 construct of the S. cerevisiae formin Bni1p (which has a gating factor of ∼0.5 (10,17)) increases the number of filaments assembled in polymerization reactions performed in vitro (Fig. 5 A, top row; and Fig. 5 B, solid circles). The observed increase in filament nucleation is matched by a decrease in the average filament length (Fig. 5 C, solid circles). Varying the concentration of Bni1p produces hyperbolic effects on both filament number and length that reach a plateau at concentrations above 500 nM Bni1p.
Figure 5.
Bni1p and Cdc12p stimulate nucleation at the expense of filament length. The experimental conditions were as follows: 2 μM actin monomers and a range of concentrations of FH1FH2 constructs of Bni1p or Cdc12p in polymerization buffer. The filaments were labeled with FITC-phalloidin and visualized by TIRF microscopy. (A) Representative TIRF micrographs of filaments assembled in the absence and presence of Bni1p (top row) or Cdc12p (bottom row). (B) Dependence of the number of actin filaments visualized per 10,000 μm2 on the concentration of Bni1p (solid circles) or Cdc12p (open circles). Error bars are standard errors of the mean of at least 15 micrographs collected via three independent assays. (C) Dependence of the average filament length on the concentration of Bni1p (solid circles) or Cdc12p (open circles). Error bars are standard errors of the mean of at least 15 micrographs collected via three independent assays. (D) Simulated dependence of the number of filaments on the concentration of formin with a gating factor of 0.4 (solid line) or 0.05 (dashed line). (E) Simulated dependence of the average filament length on the concentration of formin with a gating factor of 0.4 (solid line) or 0.05 (dashed line).
The concentration-dependent effects of Bni1p on the number and lengths of the actin filaments it assembles are more subtle than predicted by our modeling when performed using published parameters (Fig. S2). Although this formin’s elongation properties have been extensively characterized, its affinity for actin nuclei has not been experimentally determined. Thus, to obtain simulated data that reproduce our experimental measurements, we tested the effects of slowing the association rate constant for formin-mediated nucleation (Fig. 1, reaction 10). We found that slowing this rate by a factor of ∼6 generates a simulated trendline that more closely reproduces the effects of Bni1p on actin assembly. To further increase the similarity between our simulated and experimental data, we also decreased the gating factor to 0.4, which is slightly below the published value (10,17) (Fig. 5, D and E; gating = 0.4).
Filament lengths and numbers depend nonlinearly on FH2 domain gating
Our simulations demonstrate that inclusion of a formin that both stimulates nucleation and slows elongation increases the number and shortens the lengths of actin filaments assembled over the course of polymerization. To assay the dependence of filament lengths on the rate of elongation, we varied the gating factor of our simulated formin. The gating factor “p” is a measure of the probability that a formin FH2 dimer adopts a polymerization-competent conformation (18). Formins with gating factors near 0 inhibit subunit addition at barbed ends nearly completely. In contrast, barbed ends bound by formins with a gating factor of 1 elongate at the same rate as do filaments with free barbed ends. To simplify our analysis, we performed our simulations at a formin concentration of 1 nM, which ensures that over 95% of filaments assembled over the course of each trajectory are formin-bound.
At each gating factor we sampled, formin-mediated actin assembly reaches equilibrium within 5000 s. Filaments nucleated by formin elongate over time and attain lengths that depend nonlinearly on the gating factor (Fig. 6 A). Filament length is most sensitive to changes in gating when the gating factor is small. For example, decreasing the gating factor from 0.5 to 0.1 decreases the average filament length by ∼55%. In contrast, increasing the gating factor from 0.5 to 0.9 increases the average length by only ∼33%.
Figure 6.
Filament lengths and numbers depend nonlinearly on FH2 domain gating. Quantification of simulated polymerization reactions containing 2 μM actin and 1 nM formin at a range of gating factors. The gating factor “p” values were 0.1 (dark blue lines), 0.3 (light blue lines), 0.5 (cyan lines), 0.7 (green lines), 0.9 (orange lines), and 1.0 (red lines). Each simulation was carried out over 10,000 s. (A) Average filament length over time. (B) Number of filaments assembled in each reaction over time. To see the figure in color, go online.
The number of filaments assembled in each polymerization reaction also depends nonlinearly on the gating factor (Fig. 6 B). A formin with a gating factor of 0.1 nucleates 50% more filaments than a formin with a gating factor of 0.5, which in turn nucleates ∼33% more filaments than a formin with a gating factor of 0.9.
To assess the consequences of varying the gating factor, we repeated our in vitro actin polymerization reactions in the presence of a range of concentrations of the Schizosaccharomyces pombe formin Cdc12p (Fig. 5 A, bottom row). This formin has a gating factor of ∼0.05 and therefore, filaments nucleated by this formin elongate mainly through pointed end elongation (17). Inclusion of Cdc12p stimulates the assembly of shorter and more numerous filaments than observed in reactions containing identical concentrations of Bni1p (Fig. 5, B and C). The differences in the numbers and lengths of the filaments assembled by these formins exceed the differences predicted by our model (Figs. S2 and S3), suggesting that Cdc12p nucleates filaments more efficiently than does Bni1p. Indeed, doubling the association rate constant for formin-mediated nucleation relative to the rate used for Bni1p generates a simulated curve that reproduces the effects of Cdc12p on actin assembly (Fig. 5, D and E; Fig. S3).
Inhibition of nucleation by profilin promotes the assembly of long filaments
Formins overcome the limitations on elongation imposed by FH2 gating by binding and delivering profilin-actin complexes to barbed ends via their FH1 domains (18). In addition to speeding formin-mediated elongation, profilin also sequesters monomers and inhibits both nucleation and pointed end elongation (42,46,47). It is therefore likely that profilin influences the distribution of actin monomers into polymerized filaments in both the absence and presence of formin.
To obtain baseline measurements for the effects of profilin on polymerization, we simulated the effects of a range of profilin concentrations on actin assembly in the absence of formin. We set the affinity at which profilin binds to actin monomers to 3 μM, which matches the measured binding constant for S. cerevisiae profilin (48). Consistent with its role in monomer sequestration, we found that profilin decreases the initial nucleation rate relative to the rate observed for actin alone (Fig. 7 A). The magnitude of this decrease is proportional to the fraction of actin monomers that are profilin-bound in each reaction. Reactions containing at least 10 μM profilin undergo minimal nucleation owing to the near-complete binding of actin monomers to profilin. Concomitantly, the time period over which nucleation occurs increases as the concentration of profilin increases.
Figure 7.
Inhibition of nucleation by profilin promotes the assembly of long filaments. Quantification of simulated polymerization reactions containing 2 μM actin and 0 μM (dark blue lines), 0.5 μM (light blue lines), 1 μM (cyan lines), 2.5 μM (green lines), 5 μM (tan lines), 10 μM (orange lines), or 25 μM (red lines) profilin. Profilin’s affinity for actin monomers was set at 3 μM. Each simulation was carried out over 10,000 s. (A) Filament nucleation rate over time. (B) Filament elongation rate over time. (C) Number of filaments assembled in each reaction over time. (D) Average filament length over time. To see the figure in color, go online.
The initial rate of filament elongation also slows in the presence of profilin, but this effect is less sensitive to the profilin concentration compared to the nucleation rate (Fig. 7 B). Unlike nucleation, which depends on the concentration of free (i.e., not profilin-bound) monomers, filament elongation is limited by the dissociation of profilin from the barbed end (49,50). Profilin’s affinity for barbed ends is 100-fold weaker than its affinity for monomers (41). The weaker dependence of the initial elongation rate on the profilin concentration is consistent with this difference in affinity. In contrast to the modest effect on the initial rate of filament elongation, profilin significantly increases the time over which elongation proceeds. As a result, filaments elongate at near-constant rates for at least 5000 s in reactions containing at least 10 μM profilin. These reactions also require longer than 10,000 s to reach equilibrium. In combination, profilin’s effects on nucleation and elongation decrease the number of assembled actin filaments and increase filament length (Fig. 7, C and D).
Profilin modulates the lengths of filaments assembled by formin
Inclusion of 1 nM formin in polymerization reactions containing profilin significantly increases the initial rate of filament nucleation compared to reactions conducted in the absence of formin (Fig. 8 A). As the profilin concentration increases, the magnitude of formin’s stimulatory effect on the initial nucleation rate increases (Fig. 8 B). Formin also shortens the period over which nucleation occurs at all concentrations of profilin, and all reactions approach equilibrium within 1500 s (Fig. 8 A).
Figure 8.
Profilin modulates the lengths of filaments assembled by formins. Quantification of simulated polymerization reactions containing 2 μM actin, 1 nM formin, and 0 μM (dark blue lines), 0.5 μM (light blue lines), 1 μM (cyan lines), 2.5 μM (green lines), 5 μM (tan lines), 10 μM (orange lines), or 25 μM (red lines) profilin. Profilin’s affinity for actin monomers was set at 3 μM. Each simulation was performed over 10,000 s. (A) Filament nucleation rate over time. (B) Dependence of the ratio of the initial nucleation rate measured in the presence of formin to the rate measured in the absence of formin on the concentration of profilin. (C) Filament elongation rates over time. (D) Dependence of the initial filament elongation rate (corresponding to the y-intercept in C) on the concentration of profilin. (E) Number of filaments assembled in each reaction over time. (F) Dependence of the ratio of the number of filaments assembled in the presence of formin to the number measured in the absence of formin on the concentration of profilin. Filament numbers were quantified once each reaction reached equilibrium. (G) Average filament length over time. (H) Dependence of the average equilibrium filament length on the concentration of profilin. To see the figure in color, go online.
Profilin produces a well-characterized biphasic change in the rate of formin-mediated elongation (10,17,34). Subsaturating concentrations of profilin speed elongation by producing profilin-actin complexes that bind formin FH1 domains, enabling their delivery to the barbed end. At concentrations of profilin that exceed the concentration of actin monomers, competition among profilin-actin complexes and free profilin for binding to FH1 domains slows filament elongation (18). Consistent with these established effects on elongation, the initial elongation rate in simulations that include formin depends nonlinearly on the profilin concentration and is fastest at 5 μM profilin (Fig. 8, C and D). Elongation slows over time, and the period over which elongation proceeds increases as a function of profilin (Fig. 8 C). At all concentrations of profilin, elongation takes place over a longer period of time than does nucleation.
Together, the effects of profilin on filament nucleation and elongation decrease the total number of filaments assembled over the course of a polymerization reaction (Fig. 8 E). Despite this effect, all reactions containing formin nucleate at least six times as many filaments as the same reaction conducted in the absence of formin (Figs. 7 C and 8 F). Formin’s relative effect on the number of assembled filaments increases with increasing profilin concentration. Filaments assembled in reactions containing formin also attain their maximal lengths much faster than filaments assembled in the absence of formin (Figs. 7 D and 8 G). These filament lengths are ∼8 times shorter than filaments polymerized in the absence of formin, consistent with the significantly larger number of filament ends generated by nucleation across which to distribute actin monomers. As the concentration of profilin increases, the equilibrium filament lengths increase until a plateau is reached at 10 μM profilin (Fig. 8 H). This plateau likely results from the slow rate of formin-mediated filament elongation at high profilin concentrations, which compensates for a slow rate of nucleation.
In vitro polymerization reactions capture the effects of formin and profilin on filament assembly
We compared our simulated results to measurements of actin assembly reactions performed in vitro in the presence of 5 μM profilin. We introduced a range of concentrations of our FH1FH2 construct of Bni1p and quantified the number of filaments and their average lengths once each reaction reached equilibrium (Fig. 9 A). Bni1p FH1FH2 produces a concentration-dependent increase in the number of filaments assembled in our reactions (Fig. 9 B; solid circles). This increase in filament number is matched by a shortening of the average filament length (Fig. 9 C). Both effects phenomenologically reproduce the trends predicted by our simulations (Fig. 9, D and E). In reactions containing at least 250 nM formin, assembled filaments are approximately twice as long as filaments polymerized in reactions containing the identical concentration of Bni1p but lacking profilin (Figs. 5 C and 9 C).
Figure 9.
In vitro polymerization reactions capture the effects of formin and profilin on filament assembly. The experimental conditions were as follows: 2 μM actin monomers, 5 μM S. cerevisiae profilin and a range of concentrations of Bni1p FH1FH2 or FH2 in polymerization buffer. The filaments were labeled with FITC-phalloidin and visualized by TIRF microscopy. (A) Representative TIRF micrographs of filaments assembled in the absence and presence of Bni1p FH1FH2. (B) Dependence of the number of actin filaments visualized per 10,000 μm2 on the concentration of Bni1p FH1FH2 (solid circles) or Bni1p FH2 (open circles). Error bars are standard errors of the mean of at least 15 micrographs collected via three independent assays. (C) Dependence of the average filament length on the concentration of Bni1p FH1FH2 (solid circles) or FH2 (open circles). Error bars are standard errors of the mean of at least 15 micrographs collected via three independent assays. (D) Dependence of the number of filaments on the concentration of FH1FH2 (solid line) or FH2 (dashed line) in simulated reactions. (E) Dependence of the average filament length on the concentration of FH1FH2 (solid line) or FH2 (dashed line) in simulated reactions. Simulations were performed using the association rate constant for formin-mediated nucleation that was found to reproduce Bni1p’s effects on actin assembly in the absence of profilin (Fig. 5, D and E; Table S1).
To test the relationship between the FH1-mediated filament elongation rate and filament lengths measured at equilibrium, we compared the results of our experiments conducted with Bni1p FH1FH2 to measurements performed in the presence of Bni1p FH2. These constructs contain identical FH2 domains and differ only in their ability to bind and deliver profilin-actin to barbed ends. Because the FH2 construct lacks profilin-binding sites, it mediates slower filament elongation than does the FH1FH2 construct in the presence of profilin (10,17,22,34). Use of this construct therefore enabled us to modulate the formin-mediated elongation rate while keeping the profilin concentration constant at 5 μM.
Consistent with its strong nucleation activity, Bni1p FH2 produces a concentration-dependent increase in the number of filaments assembled in each polymerization reaction, as well as a decrease in the average filament length (Fig. 9, B and C, open circles). At most formin concentrations, Bni1p FH2 assembles shorter filaments than does Bni1p FH1FH2. The difference in the average lengths of filaments polymerized by these two Bni1p constructs is smaller than the difference in their elongation rates but is consistent with the trend predicted by our simulations (Fig. 9 E; (34)). Bni1p FH2 also nucleates more filaments than does Bni1p FH1FH2 at most formin concentrations (Fig. 9, B and D). This suggests that a slower rate of filament elongation boosts the efficiency of filament nucleation even for formin constructs with identical FH2 domains. This increase in nucleation stimulates de novo filament assembly at the expense of filament length.
Discussion
Formins regulate actin filament nucleation and elongation in a profilin-dependent manner. Both processes require and consume actin monomers, suggesting that the nucleation and elongation activities of formins might be interdependent. To dissect the contributions of the nucleation and elongation reactions to formin-mediated actin assembly, we constructed a kinetic model that enables independent examination of each process throughout polymerization. We found that the rates of nucleation and elongation decrease over the course of polymerization and that changes in these rates alter the number and lengths of the resulting actin filaments.
Filament elongation occurs over a longer time period than nucleation
Formins mediate actin polymerization at rates that depend on the concentrations of actin, formin and profilin. To assess the role of each component in actin assembly, we considered each protein individually and in combination. In all of our simulated reactions, the rates of nucleation and elongation are fastest at initial time points and decrease over the course of the reaction. In most reactions, elongation occurs over a longer period than does nucleation, indicating that monomer addition at pre-existing filament ends occurs at low actin concentrations that do not freely support the formation of nuclei.
In reactions containing actin alone, variation of the actin concentration produces changes in the equilibrium lengths of the assembled filaments. Filament lengths become shorter as the concentration of actin increases, consistent with a nonlinear increase in the number of filament ends. This nonlinear relationship arises from the dependence of the nucleation rate on the cube of the monomer concentration (Fig. 1; (2)). An increase in the amount of available actin therefore produces a large increase in the number of assembled filaments. These new filaments in turn increase the number of binding sites across which the monomer pool is distributed via elongation. As a result, each filament incorporates fewer actin monomers and ultimately attains a shorter length at equilibrium.
Inhibition of nucleation via profilin-mediated monomer sequestration decreases the number of filaments that are produced over the course of each reaction. The magnitude of this decrease depends on the fraction of monomers that are profilin-bound, which is dictated by profilin’s affinity for monomers. Once nucleated, filaments can bind profilin-actin complexes at their barbed end. Dissociation of profilin following each profilin-actin binding event regenerates the barbed end binding site and enables sustained filament elongation. Although profilin’s affinity for barbed ends is ∼100-fold weaker than its affinity for monomers (41), profilin’s occupancy at the barbed end increases as its concentration increases (49,50). In combination, these effects on nucleation and elongation dramatically slow polymerization and produce populations of filaments that are smaller in number and attain longer average lengths as the concentration of profilin increases.
In contrast to the effects of profilin, inclusion of formin promotes filament nucleation at the expense of elongation. However, variation of the elongation rate can also modulate the observed filament nucleation rate. Varying the formin gating factor alters the period of time over which both nucleation and elongation occur. At small gating factors, both processes take place over similar time periods. Within this gating regime, small changes in the gating factor produce measurable changes in the number and lengths of filaments assembled over the course of the polymerization reaction. At large gating factors, the time periods for nucleation and elongation diverge. The number and lengths of the polymerized actin filaments also become much less sensitive to changes in the gating factor. These findings suggest that variation of the filament elongation rate produces the most significant changes to the distribution of actin monomers under conditions in which filament nucleation and elongation take place over similar periods of time.
Formin significantly increases the rate of filament nucleation in reactions containing profilin (Fig. 8). The magnitude of this change in nucleation increases as the concentration of profilin increases. As a result, both the rate and the period over which nucleation occurs become less sensitive to profilin in the presence of formin. These effects on nucleation result in an increase in the number of filaments assembled over the course of each polymerization reaction, with the most dramatic changes occurring at profilin concentrations exceeding 5 μM. Formin-bound filaments elongate at rates that exhibit a biphasic dependence on the concentration of profilin (17,18). Despite this dependence, filament lengths increase with increasing profilin concentration until a plateau is reached at 10 μM profilin.
A regime for the assembly of long formin-bound filaments
Our simulations and in vitro experiments demonstrate that reaction conditions dictate not only the rate at which polymerization proceeds, but also the final products of the reaction. By introducing formin into our polymerization reactions, we found that an increase in the nucleation rate produces more filaments at the expense of filament length. We also found that monomer sequestration is an effective way to increase filament length by favoring binding to a pre-existing barbed end over nucleation. As such, we propose that suppression of filament nucleation is a more efficient mechanism for the assembly of long filaments than an increase in the elongation rate. This strategy is consistent with our in vitro observation that Bni1p FH1FH2 assembles filaments that are only up to 2-fold longer than filaments polymerized by Bni1p FH2, despite mediating 4-times faster elongation in the presence of 5 μM profilin (Fig. 9 C; (17,34)).
This mechanism also explains the assembly of very long formin-bound filaments (>20 μm) in elongation experiments monitored by TIRF microscopy (10,17,25). These reactions utilize a low concentration of formin (typically <5 nM) and a concentration of actin monomers that is optimized to ensure a low rate of spontaneous nucleation. Elongation along a surface also protects filaments from shearing. In contrast to these traditional elongation studies, we did not see a measurable increase in the number of long filaments in our bulk in vitro reactions containing low concentrations of formin. However, it is likely that the relatively fast spontaneous nucleation rate at 2 μM actin, coupled with an increased probability of breakage for long filaments, shortened the average filament lengths observed in our assays.
In cells, actin filament lengths are regulated both by the availability of polymerization-competent monomers and by the specific elongation factors that are associated with each filament (5). Cellular concentrations of unpolymerized actin monomers typically range from 10 to 300 μM and thus significantly exceed the critical concentration for polymerization (28,51,52). However, expression of similar concentrations of profilin (50–200 μM in mammalian cells (49)) inhibits spontaneous actin assembly via monomer sequestration and slows filament elongation by increasing the frequency of profilin’s association with barbed ends (49,51). Whereas cellular formin concentrations are relatively low (40 nM for Cdc12p in fission yeast (29)), colocalization within actin structures significantly increases the local concentration of some formins (3 μM for Cdc12p in cytokinetic rings in fission yeast (29)). Regulation of formin activity through autoinhibition (6) is thus an essential mechanism for keeping basal filament nucleation rates low. This strategy enables the assembly of a small number of formin-bound filaments that can elongate rapidly and efficiently. Under these conditions, variations in the elongation rates mediated by different formin isoforms are most likely to impact the lengths of the filaments they assemble (Fig. 10).
Figure 10.
A low nucleation rate amplifies the influence of the elongation rate on filament length. Contour plots depicting the dependence of (A) the number and (B) the equilibrium lengths of filaments assembled in simulated polymerization reactions on the rate at which formins nucleate filaments via FH2-mediated actin monomer binding (kF-Aon) and the elongation rate mediated by the formin. The nucleation rate corresponds to the forward rate constant for reaction 10 in Fig. 1 and Table S1. The elongation rate was varied by altering the gating factor and normalized to the maximal observed elongation rate. Simulations were carried out in the presence of 2 μM actin monomers, 1 nM formin, and 5 μM profilin. Contour lines are shown at intervals of 60 filaments in (A) and 8 μm in (B). To see the figure in color, go online.
Although most formins mediate both filament nucleation and elongation, the relative efficiency with which each formin isoform performs these activities has been proposed to be tuned for its specific cellular role (53). For example, the cytokinesis-associated formins Cdc12p and mDia2 mediate relatively rapid nucleation and slow elongation (54,55). These assembly properties have been demonstrated to be best suited for the faithful assembly of cytokinetic rings (55). On the other hand, formins with weak nucleation activities such as DAAM1, FMNL2, and FMNL3 have been proposed to bind and elongate the barbed ends of preassembled actin filaments rather than mediating de novo nucleation (53,56,57). Notably, the two formins expressed in budding yeast exhibit dramatically different nucleation activities despite assembling similar actin-based structures and sharing partially overlapping functions (26). To compensate for its relatively weak nucleation activity, the C-terminal tail region of Bni1p binds actin monomers and other actin-binding proteins that stimulate its nucleation activity by localizing monomers near its FH2 domain (15,58). Similar contributions of the C-terminal region to filament nucleation have been observed for other formins (58, 59, 60), suggesting that cells have evolved mechanisms to boost formin-mediated filament nucleation in some cellular contexts. Our study provides insight into the mechanistic origins of these isoform-specific differences in nucleation and elongation activities and establishes a methodology for their quantification.
Use of kinetic modeling to measure affinities of formins for filament nuclei
Visualization of in vitro actin polymerization reactions captured the trends predicted by our simulations on a phenomenological level (Figs. 3, 5, and 9). Introduction of formin into our bulk reactions increased the number of filaments and decreased the average filament lengths (Fig. 5, B and C). Consistent with our simulations, the magnitude of these effects depended on the gating factor. However, both formins produced less dramatic changes in filament distribution than expected (Figs. S2 and S3). Whereas we used experimentally measured values for each formin’s elongation rates (17), the interactions between formins and actin nuclei during filament nucleation are not as well characterized. By varying the association rate constant for formin-mediated nucleation, we generated simulated data sets that more closely reproduced our experimental measurements. In addition to providing more accurate estimates for each formin’s affinity for actin nuclei, these experiments confirmed published reports that Cdc12p nucleates filaments more efficiently than Bni1p (26,27).
Our simulations also serve to highlight portions of the formin mechanism that are not yet well understood. For example, in some reactions containing profilin, Bni1p FH1FH2 assembled shorter filaments than Bni1p FH2 despite mediating faster elongation (Fig. 9, B and C). This is consistent with a role for the FH1 domain in stimulating filament nucleation in the presence of profilin, as has been observed in other experimental studies (10,13,58). These discrepancies reveal a possible application for our model in fitting experimental data sets to extract accurate measurements of the affinities of formin isoforms for filament nuclei and to characterize the roles of FH1 domains and C-terminal-tail regions in mediating nucleation. This could also enable future dissection of the polymerization activities of formin isoforms that possess similar elongation activities but are known to stimulate nucleation at different rates (26,27). These formins would be predicted to generate actin filaments whose lengths and numbers depend both on their gating factor and on their intrinsic affinity for actin nuclei.
Author contributions
M.E.Z. designed research, performed research, analyzed data, and wrote the manuscript. L.A.S. and B.M. performed research and analyzed data. N.C. designed research, analyzed data, wrote the manuscript, and acquired funding.
Acknowledgments
This work was supported by National Institutes of Health research grant R01GM122787.
Editor: Kathleen Trybus.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2021.09.003.
Supporting citations
References (61, 62, 63, 64) appear in the Supporting materials and methods.
Supporting material
References
- 1.Tobacman L.S., Korn E.D. The kinetics of actin nucleation and polymerization. J. Biol. Chem. 1983;258:3207–3214. [PubMed] [Google Scholar]
- 2.Sept D., Xu J., McCammon J.A. Annealing accounts for the length of actin filaments formed by spontaneous polymerization. Biophys. J. 1999;77:2911–2919. doi: 10.1016/s0006-3495(99)77124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frieden C., Goddette D.W. Polymerization of actin and actin-like systems: evaluation of the time course of polymerization in relation to the mechanism. Biochemistry. 1983;22:5836–5843. doi: 10.1021/bi00294a023. [DOI] [PubMed] [Google Scholar]
- 4.Campellone K.G., Welch M.D. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesarone M.A., Goode B.L. Actin nucleation and elongation factors: mechanisms and interplay. Curr. Opin. Cell Biol. 2009;21:28–37. doi: 10.1016/j.ceb.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goode B.L., Eck M.J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 7.Paul A.S., Pollard T.D. Review of the mechanism of processive actin filament elongation by formins. Cell Motil. Cytoskeleton. 2009;66:606–617. doi: 10.1002/cm.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtemanche N. Mechanisms of formin-mediated actin assembly and dynamics. Biophys. Rev. 2018;10:1553–1569. doi: 10.1007/s12551-018-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otomo T., Tomchick D.R., Rosen M.K. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature. 2005;433:488–494. doi: 10.1038/nature03251. [DOI] [PubMed] [Google Scholar]
- 10.Paul A.S., Pollard T.D. The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr. Biol. 2008;18:9–19. doi: 10.1016/j.cub.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pruyne D., Evangelista M., Boone C. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297:612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 12.Zigmond S.H. Formin-induced nucleation of actin filaments. Curr. Opin. Cell Biol. 2004;16:99–105. doi: 10.1016/j.ceb.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Pring M., Evangelista M., Zigmond S.H. Mechanism of formin-induced nucleation of actin filaments. Biochemistry. 2003;42:486–496. doi: 10.1021/bi026520j. [DOI] [PubMed] [Google Scholar]
- 14.Zigmond S.H., Evangelista M., Pring M. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. 2003;13:1820–1823. doi: 10.1016/j.cub.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 15.Moseley J.B., Sagot I., Goode B.L. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol. Biol. Cell. 2004;15:896–907. doi: 10.1091/mbc.E03-08-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y., Moseley J.B., Eck M.J. Crystal structures of a formin homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–723. doi: 10.1016/s0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]
- 17.Kovar D.R., Harris E.S., Pollard T.D. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Vavylonis D., Kovar D.R., Pollard T.D. Model of formin-associated actin filament elongation. Mol. Cell. 2006;21:455–466. doi: 10.1016/j.molcel.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydin F., Courtemanche N., Voth G.A. Gating mechanisms during actin filament elongation by formins. eLife. 2018;7:e37342. doi: 10.7554/eLife.37342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kursula P., Kursula I., Wilmanns M. High-resolution structural analysis of mammalian profilin 2a complex formation with two physiological ligands: the formin homology 1 domain of mDia1 and the proline-rich domain of VASP. J. Mol. Biol. 2008;375:270–290. doi: 10.1016/j.jmb.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Horan B.G., Zerze G.H., Mittal J. Computational modeling highlights the role of the disordered Formin Homology 1 domain in profilin-actin transfer. FEBS Lett. 2018;592:1804–1816. doi: 10.1002/1873-3468.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courtemanche N., Pollard T.D. Determinants of Formin Homology 1 (FH1) domain function in actin filament elongation by formins. J. Biol. Chem. 2012;287:7812–7820. doi: 10.1074/jbc.M111.322958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schönichen A., Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim. Biophys. Acta. 2010;1803:152–163. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Pruyne D. Probing the origins of metazoan formin diversity: evidence for evolutionary relationships between metazoan and non-metazoan formin subtypes. PLoS One. 2017;12:e0186081. doi: 10.1371/journal.pone.0186081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zweifel M.E., Courtemanche N. Competition for delivery of profilin-actin to barbed ends limits the rate of formin-mediated actin filament elongation. J. Biol. Chem. 2020;295:4513–4525. doi: 10.1074/jbc.RA119.012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moseley J.B., Goode B.L. Differential activities and regulation of Saccharomyces cerevisiae formin proteins Bni1 and Bnr1 by Bud6. J. Biol. Chem. 2005;280:28023–28033. doi: 10.1074/jbc.M503094200. [DOI] [PubMed] [Google Scholar]
- 27.Scott B.J., Neidt E.M., Kovar D.R. The functionally distinct fission yeast formins have specific actin-assembly properties. Mol. Biol. Cell. 2011;22:3826–3839. doi: 10.1091/mbc.E11-06-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollard T.D. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 1986;103:2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J.Q., Pollard T.D. Counting cytokinesis proteins globally and locally in fission yeast. Science. 2005;310:310–314. doi: 10.1126/science.1113230. [DOI] [PubMed] [Google Scholar]
- 30.Hoops S., Sahle S., Kummer U. COPASI--a COmplex PAthway SImulator. Bioinformatics. 2006;22:3067–3074. doi: 10.1093/bioinformatics/btl485. [DOI] [PubMed] [Google Scholar]
- 31.Hindmarsh A.C. In: Scientific Computing, IMACS Transactions on Scientific Computation. Stepleman R.S., editor. Vol. 1. North-Holland; 1983. ODEPACK: a systematized collection of ODE solvers; pp. 55–64. [Google Scholar]
- 32.Petzold L. Automatic selection of methods for solving stiff and nonstiff systems of ordinary differential equations. SIAM J. Sci. Statist. Comput. 1983;4:136–148. [Google Scholar]
- 33.Spudich J.A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 34.Sherer L.A., Zweifel M.E., Courtemanche N. Dissection of two parallel pathways for formin-mediated actin filament elongation. J. Biol. Chem. 2018;293:17917–17928. doi: 10.1074/jbc.RA118.004845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasteiger E., Hoogland C., Bairoch A. In: The Proteomics Protocols Handbook. Walker J.M., editor. Humana Press; 2005. Protein identification and analysis tools on the ExPASy server; pp. 571–607. [Google Scholar]
- 36.Kuhn J.R., Pollard T.D. Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys. J. 2005;88:1387–1402. doi: 10.1529/biophysj.104.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel A.A., Oztug Durer Z.A., Quinlan M.E. Drosophila and human FHOD family formin proteins nucleate actin filaments. J. Biol. Chem. 2018;293:532–540. doi: 10.1074/jbc.M117.800888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen K.K., Rubenstein P.A. Differential regulation of actin polymerization and structure by yeast formin isoforms. J. Biol. Chem. 2009;284:16776–16783. doi: 10.1074/jbc.M109.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ti S.C., Pollard T.D. Purification of actin from fission yeast Schizosaccharomyces pombe and characterization of functional differences from muscle actin. J. Biol. Chem. 2011;286:5784–5792. doi: 10.1074/jbc.M110.199794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jégou A., Niedermayer T., Romet-Lemonne G. Individual actin filaments in a microfluidic flow reveal the mechanism of ATP hydrolysis and give insight into the properties of profilin. PLoS Biol. 2011;9:e1001161. doi: 10.1371/journal.pbio.1001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Courtemanche N., Pollard T.D. Interaction of profilin with the barbed end of actin filaments. Biochemistry. 2013;52:6456–6466. doi: 10.1021/bi400682n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollard T.D., Cooper J.A. Quantitative analysis of the effect of Acanthamoeba profilin on actin filament nucleation and elongation. Biochemistry. 1984;23:6631–6641. doi: 10.1021/bi00321a054. [DOI] [PubMed] [Google Scholar]
- 43.Paul A.S., Pollard T.D. Energetic requirements for processive elongation of actin filaments by FH1FH2-formins. J. Biol. Chem. 2009;284:12533–12540. doi: 10.1074/jbc.M808587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sept D., McCammon J.A. Thermodynamics and kinetics of actin filament nucleation. Biophys. J. 2001;81:667–674. doi: 10.1016/S0006-3495(01)75731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrianantoandro E., Blanchoin L., Pollard T.D. Kinetic mechanism of end-to-end annealing of actin filaments. J. Mol. Biol. 2001;312:721–730. doi: 10.1006/jmbi.2001.5005. [DOI] [PubMed] [Google Scholar]
- 46.Markey F., Larsson H., Lindberg U. Nucleation of actin polymerization from profilactin. Opposite effects of different nuclei. Biochim. Biophys. Acta. 1982;704:43–51. doi: 10.1016/0167-4838(82)90130-3. [DOI] [PubMed] [Google Scholar]
- 47.Tobacman L.S., Brenner S.L., Korn E.D. Effect of Acanthamoeba profilin on the pre-steady state kinetics of actin polymerization and on the concentration of F-actin at steady state. J. Biol. Chem. 1983;258:8806–8812. [PubMed] [Google Scholar]
- 48.Eads J.C., Mahoney N.M., Almo S.C. Structure determination and characterization of Saccharomyces cerevisiae profilin. Biochemistry. 1998;37:11171–11181. doi: 10.1021/bi9720033. [DOI] [PubMed] [Google Scholar]
- 49.Funk J., Merino F., Bieling P. Profilin and formin constitute a pacemaker system for robust actin filament growth. eLife. 2019;8:e50963. doi: 10.7554/eLife.50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zweifel M.E., Courtemanche N. Profilin’s affinity for formin regulates the availability of filament ends for actin monomer binding. J. Mol. Biol. 2020;432:166688. doi: 10.1016/j.jmb.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollard T.D., Blanchoin L., Mullins R.D. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- 52.Koestler S.A., Rottner K., Small J.V. F- and G-actin concentrations in lamellipodia of moving cells. PLoS One. 2009;4:e4810. doi: 10.1371/journal.pone.0004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breitsprecher D., Goode B.L. Formins at a glance. J. Cell Sci. 2013;126:1–7. doi: 10.1242/jcs.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neidt E.M., Skau C.T., Kovar D.R. The cytokinesis formins from the nematode worm and fission yeast differentially mediate actin filament assembly. J. Biol. Chem. 2008;283:23872–23883. doi: 10.1074/jbc.M803734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Homa K.E., Zsolnay V., Kovar D.R. Formin Cdc12’s specific actin assembly properties are tailored for cytokinesis in fission yeast. Biophys. J. 2021;120:2984–2997. doi: 10.1016/j.bpj.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaillant D.C., Copeland S.J., Copeland J.W. Interaction of the N- and C-terminal autoregulatory domains of FRL2 does not inhibit FRL2 activity. J. Biol. Chem. 2008;283:33750–33762. doi: 10.1074/jbc.M803156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Block J., Breitsprecher D., Rottner K. FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr. Biol. 2012;22:1005–1012. doi: 10.1016/j.cub.2012.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gould C.J., Maiti S., Goode B.L. The formin DAD domain plays dual roles in autoinhibition and actin nucleation. Curr. Biol. 2011;21:384–390. doi: 10.1016/j.cub.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson M.E., Heimsath E.G., Kull F.J. FMNL3 FH2-actin structure gives insight into formin-mediated actin nucleation and elongation. Nat. Struct. Mol. Biol. 2013;20:111–118. doi: 10.1038/nsmb.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vizcarra C.L., Bor B., Quinlan M.E. The role of formin tails in actin nucleation, processive elongation, and filament bundling. J. Biol. Chem. 2014;289:30602–30613. doi: 10.1074/jbc.M114.588368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vinson V.K., De La Cruz E.M., Pollard T.D. Interactions of Acanthamoeba profilin with actin and nucleotides bound to actin. Biochemistry. 1998;37:10871–10880. doi: 10.1021/bi980093l. [DOI] [PubMed] [Google Scholar]
- 62.Kang F., Purich D.L., Southwick F.S. Profilin promotes barbed-end actin filament assembly without lowering the critical concentration. J. Biol. Chem. 1999;274:36963–36972. doi: 10.1074/jbc.274.52.36963. [DOI] [PubMed] [Google Scholar]
- 63.Perelroizen I., Marchand J.B., Carlier M.F. Interaction of profilin with G-actin and poly(L-proline) Biochemistry. 1994;33:8472–8478. doi: 10.1021/bi00194a011. [DOI] [PubMed] [Google Scholar]
- 64.Lapidus L.J., Eaton W.A., Hofrichter J. Measuring the rate of intramolecular contact formation in polypeptides. Proc. Natl. Acad. Sci. USA. 2000;97:7220–7225. doi: 10.1073/pnas.97.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.