Abstract
Using recombinant 15- to 30-kDa fragments and fusion with glutathione S-transferase (GST), we investigated the seroreactivity of three large structural proteins of Epstein-Barr virus (EBV), p150 (BcLF1, capsid), p143 (BNRF1, tegument), and gp125 (BALF4, membrane) in Western blots. None of 13 fragments tested, however, was qualified for diagnostic application. In contrast, the two small viral capsid antigens (VCA), p18 (BFRF3) and p23 (BLRF2), demonstrated sensitive (100%) EBV-specific immunoglobulin G (IgG) reactivities. While p18 additionally showed maximum sensitivity for IgM detection, the IgM sensitivity of p23 was restricted (44%). An autologous fusion protein, p23-p18, which consists N-terminally of full-length p23, followed by the carboxy half of p18, was constructed. This antigen was subjected to indirect VCA enzyme-linked immunosorbent assays (ELISAs), for IgG and IgM, as well as to a μ-capture (μc) IgM ELISA. All assays were found to be 100% specific when EBV-negative sera were tested. Using sera from previously infected individuals, the p23-p18 fusion revealed an improved IgG sensitivity of 99% compared to sensitivities of 97 and 93% for the single antigens p18 and p23, respectively. The sensitivity and specificity of the indirect IgM ELISA with samples of primary and past infections, respectively, were 100%. The μc principle for IgM overcame completely the interference by rheumatoid factors. Compared to the specificity of the indirect IgM version, the specificity with sera collected from rheumatoid arthritis patients increased from 48 to 100%. In summary, the p23-p18 IgG and μc IgM ELISAs showed excellent performances and are promising new diagnostic tests for the detection of EBV-specific antiviral capsid antibodies.
To date, the predominantly performed diagnostic assays for confirmation of suspected Epstein-Barr virus (EBV) primary infection, which can manifest itself as infectious mononucleosis, detect specific antibodies directed against the virus capsid antigen (VCA). Indirect immunofluorescence assays (IFA), when performed according to the original methods (6), still serve as the “gold standard” of EBV serodiagnosis. However, these assays are time-consuming and are not suitable for automatic handling. Furthermore, due to the variability of antigen-producing cells as well as subjective reading of results, they are also difficult to standardize. The VCA complex, as serologically defined by IFA, reacts with immunoglobulin M (IgM) and IgG antibodies during the course of EBV primary infection. While anti-VCA IgM disappears after convalescence and typically does not emerge a second time in life, anti-VCA IgG shows lifelong persistence. The replacement of VCA IFA by more convenient methods like enzyme-linked immunosorbent assays (ELISAs) requires defined polypeptides, for example, provided by recombinant DNA technology. Early antigen (EA)- and nuclear antigen (EBNA)-specific ELISAs based on recombinant antigens are commercially available and have been successfully used in EBV diagnosis for some time (3, 8). VCA IFA serologically defines antigens which are more difficult to replace by recombinant proteins. This is related to the complexity of the VCA family of proteins, lack of its complete definition at the protein level, and the quality of antigens in in vitro assays.
Early studies showed that the proteins p150 (BcLF1) and gp125 (BALF4) are immunogenic. The latter is believed to be a dominant immunogen of the VCA complex (11). The cell culture-derived natural antigens p150 and gp125 have been isolated and used for diagnostic application (11). Likewise, the major component of the tegument, p143 (BNRF1), has been identified as a seroactive antigen by immuno precipitation experiments (23). By using lambda cDNA libraries, two small capsid proteins, p18 (BFRF3) and p40 (BdRF1), have been identified and characterized with respect to their serodiagnostic properties (16). In particular, p18 is highly immunogenic in humans, and the essential B-cell epitopes have been mapped to the carboxy region (17). More recently, a second small capsid protein, p23 (BLRF2), has been expressed in Escherichia coli as a heterologous fusion protein and serological evaluation has demonstrated VCA-like antibody profiles (12).
Using heterologous gene fusion of antigenic fragments encoded by VCA open reading frames, we attempted to define serologically reactive antigens in Western blots. IgM as well as IgG reactivity was considered by using sera either from infectious mononucleosis patients or from previously infected healthy individuals. In this first part of the study, we investigated the major capsid protein, p150 (BcLF1); the tegument protein, p143 (BNRF1); the glycoprotein B (gB) homologue gp125 (BALF4); and the two small capsid proteins, p18 (BFRF3) and p23 (BLRF2). Because full-length expression is difficult in E. coli for large proteins like p150, p143, and gp125, we subdivided the corresponding genomic regions in DNA fragments of similar sizes, encoding 15- to 30-kDa proteins. These fragments have been cloned and expressed in fusion with glutathione S-transferase (GST) in order to obtain stable expression and hence rapid information about their serodiagnostic potential. This approach has been successfully used in the identification of diagnostically useful antigens of human cytomegalovirus (HCMV) (20).
The aim of this study was to identify and combine the most reactive antigens or antigenic fragments for the development of recombinant VCA ELISAs. If possible, a single polyantigen, constructed by autologous gene fusion, would be generated. Recently, this strategy has been followed for the development of recombinant HCMV antigens (21). Thus, in the second part of the study, p23 and p18 were selected for further investigation. We constructed an autologous fusion protein consisting of full-length p23 at the N terminus followed by the carboxy half of p18. This antigen has been expressed in E. coli, highly purified, and compared with the individual antigens p18 and p23 in ELISA-based assays. IgM and IgG were detected separately by using sera from infectious mononucleosis patients, rheumatoid arthritis (RA) patients, and healthy blood donors. Together, the results show that the combination of p18 and p23 is superior to each single antigen. Moreover, the p23-p18 ELISAs, IgG and μ-capture (μc) IgM, are promising novel diagnostic tests for detecting VCA-specific antibodies and provide the means for improved specific, sensitive, and rapid serodiagnosis of infectious mononucleosis.
MATERIALS AND METHODS
Reference tests, serum panels, and definition of serostatus.
Reference serology was provided by the EBV ELISA systems from Biotest, Dreieich, Germany (EA IgM, EA IgG, and EBNA-1 IgG); Gull, Bad Homburg, Germany (VCA IgM, VCA IgG, and EBNA-1 IgG); and DiaSorin, Saluggia, Italy (VCA IgM, VCA IgG, and EBNA-1 IgG). Two serum panels were used: panels 1 and 2 for the experiments referred to in Tables 2 and 4, respectively. Those sera used for the experiments presented in Tables 1 and 3 were selected from panel 1. Sera from seronegative individuals (panel 1, n = 7; panel 2, n = 10) and previously infected individuals (panel 1, n = 102; panel 2, n = 185) were collected from healthy blood donors living in the region of Frankfurt am Main, Germany. The patients with primary infections (panel 1, n = 22; panel 2, n = 28) were clinically and serologically diagnosed as having infectious mononucleosis, and sera were collected from different laboratories in Germany. Half of the patients whose sera were included in panel 2 (n = 14) were followed up serologically for up to 12 months. Most primary infections were also confirmed with VCA IgM and IgG IFA. The criteria for the confirmation of a primary infection were EA IgM positive, VCA IgM positive, EBNA-1 IgG negative, and typical symptoms, i.e., lymphadenopathy, pharyngitis, and fever. For the definition of previous infections, the conditions were VCA IgG and EBNA-1 IgG positive and no symptoms. The sera from RA patients (panel 2, n = 23) were kindly provided by Agostino Bazicchi, University of Pisa, Pisa, Italy. These patients had all been previously infected with EBV.
TABLE 2.
Comparison of diagnostic performances of the VCA IgG and VCA IgM (indirect) ELISAs based on GST-p18, p23, and p23-p18
| ELISA (indirect) | Cutoff [OD] | Sera from patients with:
|
Seronegatives (n = 7)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Primary infections (n = 22)
|
Previous infections (n = 102)
|
|||||||
| No. pos.a | Sensitivity (%) | No. pos. | Sensitivity (%) | Specificity (%) | No. pos. | Specificity (%) | ||
| GST-p18 IgG | 0.20 | 12 | 54.5 | 99 | 97.1 | 0 | 100.0 | |
| p23 IgG | 0.15 | 17 | 77.3 | 95 | 93.1 | 0 | 100.0 | |
| p23-p18 IgG | 0.20 | 18 | 81.8 | 101 | 99.0 | 0 | 100.0 | |
| GST-p18 IgM | 0.40 | 22 | 100.0 | 1 | 99.9 | 0 | 100.0 | |
| p23 IgM | 0.20 | 8 | 36.4 | 0 | 100.0 | 0 | 100.0 | |
| p23-p18 IgM | 0.40 | 22 | 100.0 | 0 | 100.0 | 0 | 100.0 | |
pos., positive.
TABLE 4.
Diagnostic performance of the p23-p18 (μc)-IgM ELISA
| Serum panel source | Serum samples
|
ELISA
|
||
|---|---|---|---|---|
| No. | No. positive | Sensitivity (%) | Specificity (%) | |
| Seronegative subjects | 10 | 0 | 100.0 | |
| Patients with primary infections | ||||
| Early, 0 to 3 mo | 28 | 28 | 100.0 | |
| Recent, 6 to 12 mo | 14 | 3 | 78.6 | |
| Donors with previous infections | 185 | 1 | 99.5 | |
TABLE 1.
Seroreactivities of recombinant VCA fragments in Western blots developed with sera from infectious mononucleosis patients (IgM) or previously infected donors (IgG)
| Antigen | Reading frame | Cloned sequence (aa) | Fusion | No. of positive sera
|
Intensity of reactiona
|
||
|---|---|---|---|---|---|---|---|
| IgM (n = 9) | IgG (n = 9) | IgM | IgG | ||||
| gp125/1 | BALF4 | 433–580 | GST | 0 | 0 | ||
| gp125/2 | BALF4 | 580–729 | GST | 0 | 0 | ||
| gp125/3 | BALF4 | 752–857 | GST | 0 | 0 | ||
| p143/1 | BNRF1 | 1–253 | GST | 8 | 9 | (+) | + |
| p143/2 | BNRF1 | 254–488 | GST | 0 | 0 | ||
| p143/3 | BNRF1 | 489–739 | GST | 5 | 3 | (+) | (+) |
| p143/4 | BNRF1 | 811–1088 | GST | 6 | 5 | (+) | (+) |
| p143/5 | BNRF1 | 1089–1322 | GST | 1 | 5 | (+) | (+) |
| p150/1 | BcLF1 | 1–199 | GST | 3 | 1 | (+) | + |
| p150/2 | BcLF1 | 303–455 | GST | 0 | 0 | ||
| p150/3 | BcLF1 | 450–556 | GST | 0 | 0 | ||
| p150/4 | BcLF1 | 450–659 | GST | 3 | 3 | (+) | (+) |
| p150/5 | BcLF1 | 659–857 | GST | 3 | 2 | (+) | + |
| p18 | BFRF3 | 105–176 | GST | 9 | 9 | ++ | ++ |
| p23 | BLRF2 | 1–162b | None | 4 | 9 | ++ | ++ |
Symbols indicating intensity of stained bands: (+), weak bands only; +, moderate bands; ++, strong reaction.
Full length.
TABLE 3.
Comparison of the p23-p18 μc ELISA with the standard indirect IgM ELISA
| IgM ELISA principle | Components
|
Sera from patients with:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Solid phase | Conjugate | Primary infections (n = 12)
|
Previous infections (n = 20)
|
RA (n = 23)
|
||||
| No. pos.a | Sensitivity (%) | No. pos. | Specificity (%) | No. pos. | Specificity (%) | |||
| Indirect | p13-p18 | Anti-human IgM-POD | 12 | 100.0 | 0 | 100.0 | 12 | 47.8 |
| μc | Anti-human IgM | p23-p18-POD | 12 | 100.0 | 0 | 100.0 | 0 | 100.0 |
pos., positive.
Recombinant GST fusion proteins.
Recombinant 15- to 30-kDa fragments of p150 (BcLF1), p143 (BNRF1), and gp125 (BALF4), as well as the carboxy half of p18 (BFRF3), have been cloned and expressed in E. coli in fusion with GST. The expressed amino acids are given in Table 1. The general cloning strategy and the methods have been described in detail previously (19). Briefly, amplification was performed with pairs of PCR primers containing recognition sites for the endonucleases BamHI and EcoRI (AvaI), 5′ and 3′ of the original priming sequence, respectively, to facilitate the subsequent cloning steps. Cosmids and plasmids harboring defined genomic fragments or cDNAs of EBV genome B95-8 served as templates for PCR amplification. After initial cloning in vector pUC8 and identification of recombinant clones, DNA fragments were subcloned without modification into vector pGEX-3X, which enables the expression of polypeptides in fusion with GST (14). The GST proteins have been purified to at least 90% purity. Most of the fusion proteins were insoluble and after lysis of bacteria has been isolated from the sediment. Purification was then performed by washing steps and ion-exchange or gel chromatography in 8 M urea. Soluble GST proteins were purified by affinity chromatography using glutathione-Sepharose (Pharmacia, Uppsala, Sweden). Detailed protocols for upstream procedures and purification of GST proteins consisting of viral antigen fragments have been published recently (7).
Directly expressed proteins.
The p23 sequence and the p23-p18 gene fusion have been cloned and directly expressed in E. coli by using the T7 vector pET5c, which permits expression with an N-terminal amino acid leader sequence of 14 amino acids (15). Both antigens had similar biochemical properties and could be purified according to an identical purification scheme from 6 liters of E. coli culture. The primarily insoluble antigens were solubilized by a pH shift to 9.5 from the sediment fraction of the lysate. After an ammonium sulfate fractionation, the antigens were purified by cation-exchange chromatography (SP-Sepharose; Pharmacia), followed by a gel chromatography step (Superdex 200, HiLoad; Pharmacia). The final purity was >99% as demonstrated by sodium dodecyl sulfate-polyacrylamide disc electrophoresis, anti-E. coli Western blotting, and capillary electrophoresis.
Western blot study.
Identical amounts of the 15 different purified antigens (Table 1) were put into separate lanes of sodium dodecyl sulfate-polyacrylamide gels. After electrophoresis and subsequent transfer onto polyvinylidene difluoride membranes under semidry conditions, the blot membranes were developed by using defined sera from primary infected patients (n = 9) for IgM detection or sera from previously infected donors (n = 9) for IgG detection. Only sera which were devoid of anti-GST antibodies, proven with purified GST control protein in a previous experiment, were considered. Details of the methods have been described elsewhere (20). Positivity was defined visually by the appearance of a stained band at the position of the GST antigen. As a positive control, we used an anti-GST rabbit serum.
ELISA experiments.
Three antigens, GST-p18, p23, and p23-p18, have been considered for ELISA studies. Microtest plates (96 wells, Maxisorb; Nunc, Roskilde, Denmark) were coated with 10 μg of antigen per plate. Serum incubation was for 60 min at 37°C at a dilution of 1:21. Peroxidase (POD)-labelled monoclonal antibodies, anti-IgG or -IgM (Biotest), were used as conjugates and incubated for 30 min at 37°C. The enzyme reaction was performed with tetramethylbenzidine-H2O2 (Sigma, Munich, Germany) for 30 min at room temperature. Cutoffs have been fixed individually to obtain maximum performance by using the statistical program MedCalc version 4.2 (MedCalc Software). Precise protocols for the ELISA methods used have been published recently (7).
The method described above is referred to as indirect ELISA. For the p23-p18 IgM detection, a μc test was chosen additionally as an alternative assay principle. As capture antibody, polyclonal anti-IgM (Cappel, Turnhout, Belgium) immobilized on the solid phase (20 μg/plate) was used. Captured serum IgM antibodies specific for p23-p18 were detected by using an antigen-POD conjugate, which was prepared by directly and covalently linking the enzyme to lysine residues of p23-p18 by using the periodate chemistry (10). All other conditions were the same as for the indirect ELISA.
RESULTS
Expression and purification of GST-VCA proteins.
The large VCA proteins, gp125, p143, and p150, were subdivided in fragments of 106 to 278 amino acids. The fragment analysis covered the entire sequence of p143 and p150, which are represented by five clones each. In contrast, for gp125, only the carboxy half was considered for the cloning of three nonoverlapping fragments, thereby sparing a putative transmembrane region between amino acids 730 and 750 (Table 1). The carboxy region has less homology to other herpesviruses than the amino half. For p18, the 72 carboxy-terminal amino acids were used according to the previously described location of immunodominant regions (17). All recombinant fusion proteins showed strong and stable expression. With the exception of gp125/2, p143/1, and p18, which were soluble, all other fusion proteins were strongly insoluble and were solubilized and chromatographed in urea. Propagation of the E. coli cultures and the upstream procedures were performed at production scale. The yields of purified antigen varied between 2 and 70 mg/liter. The final purities were at least 90% for insoluble proteins and 99% for soluble proteins.
Evaluation of VCA fragments in Western blots.
In order to get rapid information about the serodiagnostic potential of the antigen fragments, we performed Western blot experiments utilizing a limited panel of well-defined sera. The sera were carefully selected according to an unequivocal serostatus. Those used for IgM blots had to be positive for VCA IgM, VCA IgG, EA IgM, and EA IgG and negative for EBNA IgG, whereas sera used for IgG blots were positive for VCA IgG and EBNA IgG and negative for VCA IgM and EA IgM. In addition, all sera were devoid of anti-GST antibodies, as proven with purified control antigen in Western blots. The results obtained with the 14 different VCA fragments fused to GST and the one directly expressed full-length protein, p23, are shown in Table 1. Considering both frequency and reactivity, p18 and p23 (full length) were superior to all other fragments. The carboxy region of p18 yielded full sensitivity and reactivity for IgG and IgM detection. Although p23 had an IgG sensitivity and intensity of reaction similar to those of p18, its IgM reactivity was restricted. The p18 was clearly the better IgM antigen. The results with all the other antigens were disappointing. They lack either sensitivity or reactivity, i.e., intensity of bands. The best antigen out of these was the N-terminal part of the tegument protein, p143/1. Surprisingly, none of the gp125 fragments showed any reactivity in IgM or IgG blots, respectively. In summary, the small VCA proteins, p18 and p23, were highly reactive and superior to any other fragment. These two antigens have been selected for ELISA evaluations.
Direct expression and purification of p23 and p23-p18.
p23 was expressed in its full length and purified to apparent homogeneity in production scale. The recombinant antigen showed remarkable expression levels and good stability. The final yield of pure protein was 28 mg/liter of E. coli culture. The purification was supported by the unusual biochemical properties of p23. The protein is extremely basic, with a pI of 10.92, thus allowing a highly selective cation-exchange chromatography. Moreover, p23 lacks the amino acids phenylalanine, tyrosine, tryptophan, and histidine. The antigen appeared as a dimer in electrophoresis and gel chromatography under nonreducing conditions. The dimerization is best explained by the single cysteine at position 46. The molecular mass of the monomer was determined to be 22.4 kDa; that of the dimer was determined to be 45.0 kDa. p23 fullfills many requirements of an ideal fusion moiety, such as stability, expression level, and easy purification.
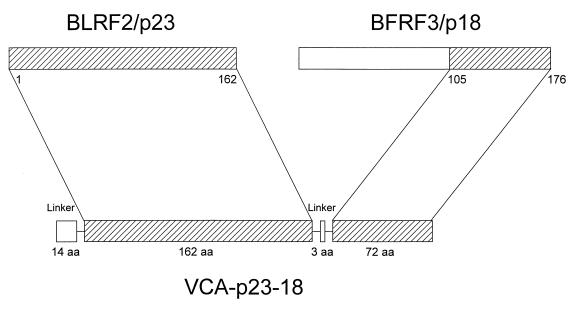
The construction of the autologous fusion of p18 and p23 is shown in Fig. 1. In total, the recombinant protein consists of 251 amino acids. Interestingly, the fused p18 carboxy moiety has an amino acid composition similar to that of p23, i.e., it also lacks phenylalanine, tyrosine, and tryptophan. Likewise, the basic nature is the same (pI = 10.95). p23-p18 was propagated by using identical methods, which were successful for p23 production. Final yield of pure protein was 17 mg/liter of E. coli culture. The molecular mass of the monomer was determined to be 32.4 kDa. Dimerization occurred under nonreducing conditions to a low extent only. The molecular mass of the dimer was 77.4 kDa.
FIG. 1.
Construction of the VCA p23-p18 by autologous gene fusion. aa, amino acid.
Evaluation of selected VCA proteins in ELISA.
The autologous fusion protein p23-p18 has been subjected to VCA IgG and IgM prototype ELISAs in direct comparison with the individual antigen assays based on the heterologous fusion protein GST-p18 and the directly expressed p23, respectively. The antigens were coated onto the solid phase and used for indirect ELISA experiments. In total, six different tests were evaluated with different panels of sera (Table 2). Sera from patients with primary infections were used for demonstrating the sensitivity of the antigens for IgG and IgM. According to the reference serology, a panel of sera from healthy donors were separated into seronegative and seropositive samples, i.e., those from previously infected individuals. The negative samples were used for calculation of specificities and the level of the cutoffs. Sera from previously infected individuals indicated sensitivity of IgG as well as specificity of IgM. The results of Table 2 show that p23-p18 revealed an improved IgG sensitivity compared to that of each single antigen. Interestingly, the optical densities of the IgG ELISAs were relatively low among early stages of primary infection, sometimes even negative, especially for p18. This is due to a certain delay in IgG seroconversion (data not shown). Among the IgM ELISA results, p23-p18 had the same sensitivity but a better specificity than GST-p18. In agreement with the finding of the Western blot analysis, the sensitivity of anti-p23 IgM was again limited.
Development and evaluation of the p23-18 μc ELISA.
p23 introduces a high number of free amino groups, provided by 11 lysine residues. This facilitates covalent coupling via Schiff’s base reaction (10). With the highly reactive and stable p23-p18-POD conjugate, a μc IgM assay was developed. Interference by rheumatoid factors (RF) is a well-known problem of indirect VCA IgM assays (5). Indeed, the p23-p18 indirect IgM ELISA yielded 52% false positives within a serum panel from rheumatoid arthritis patients (Table 3). In contrast, the μc IgM test overcame completely the RF interference. Specificity increased to 100% by keeping the full sensitivity for primary infections. Hence, we chose the p23-p18 μc IgM ELISA for all further investigations. Table 4 summarizes the results obtained so far for this type of VCA IgM ELISA. These experiments are a supplementation of those presented in Table 2. The serum panels used for evaluation of the μc ELISA, however, are more extensive. The panel of sera from patients with primary infections included 14 samples from follow-ups, for which we selected one early sample each, i.e., at most up to 3 months after onset of symptoms. In addition, a subsequent sample from the postacute stage, i.e., between 6 to 12 months after the onset of symptoms, was collected. This panel of recent acute infections proved the rapid seroreversion of anti-p23-p18 IgM during convalescence. Except for three patients, all others (79%) reverted to undetectable levels between 3 and 12 months. The specificity with sera from previously infected healthy donors was 99.5%. This reflects a very low prevalence of anti-p23-p18 IgM in the healthy population.
DISCUSSION
The VCA IFA, up to now, still serve as a “gold standard” of EBV serodiagnosis (6). VCA serology, with respect to its time course during primary infection, is composed of three typical characteristics: first, an immediate and transient IgM response, which rarely emerges a second time in life; second, an early IgG response in coincidence with IgM, which typically increases during the acute stage of primary infection; and third, late IgG antibodies persisting for life with respectable titers in every EBV-infected individual. One may speculate whether these different aspects are related to different antigens or not. Besides true capsid antigens, many other immunogenic structural proteins, for instance, of the envelope and of the tegument, are presented by the EBV-infected P3HR1 cells used for VCA IFA and likely contribute to the seroreactivity. In this study, we reevaluated the most important structural proteins described in the literature by using recombinant DNA technology in order to define a set of antigens or part of antigens for ELISA development, thereby matching the VCA IFA-defined serology as closely as possible.
In the first part of this study, 15 recombinant antigen fragments encoded by five different late reading frames of EBV were analyzed for their usefulness in serodiagnosis. Previously, we successfully applied the same strategy for the identification and selection of HCMV antigens (20). In general, heterologous fusion with GST allows stable expression and rapid purification of viral antigen fragments. This approach is hampered, however, if insoluble fusion proteins are produced. They need special purification protocols and refolding conditions (7). Unfortunately, the majority of VCA fragments in this study are expressed as very insoluble GST fusion proteins, likely due to numerous cysteines and high overall hydrophobicities. Altered or improper folding may be one explanation for the insufficient seroreactivities of fragments from gp125, p143, and p150, antigens which are known to be immunogenic in humans (11, 23). Moreover, gp125 (BALF4), otherwise known as gp110 or gB homologue, is believed to be the major immunogen of the VCA complex (11). The lack of glycosylation in E. coli and fragmentation may equally account for a loss of important confirmational epitopes within the recombinant gp125 fragments. N-link glycosylated, full-length recombinant gp125, derived from baculovirus expression in insect cells, maintained the same seroreactivity as its natural counterpart (13). It must be emphasized, however, that we have considered only polypeptides from the carboxy half, and it cannot be excluded that essential epitopes are located in the residual part of gp125. Although EBV-specific reactions have been obtained in Western blots with some fragments of p143 and p150, for example with the N terminus of p143, this was not preserved in ELISAs (data not shown). For p150, the weak reactions are in concordance with results obtained previously, using a recombinant truncated p150 for ELISA (4). This antigen was constructed by autologous fusion of genomic regions covering roughly p150/1 and p150/4 from this study.
Nevertheless, besides the negative findings with 13 fragments from the large VCA proteins, we obtained powerful reactions for the small VCA proteins, p18 and p23. These results confirm previous reports (16, 17, 12). The p18 was represented by the 72 carboxy-terminal amino acids, which span the essential epitopes (17). Unfortunately, for p18, direct expression in E. coli is difficult, and other groups used either heterologous fusion with β-galactosidase (18), GST (2), or combinations of synthetic peptides from the carboxy region (17). In contrast, p23 has been expressed directly in its full length. A dihydrofolate reductase (DHFR) fusion protein has been characterized recently (12) and proved to be a useful diagnostic ELISA antigen, yielding high IgG and IgM sensitivities. In our hands, the p23 nonfusion protein had a more restricted IgM sensitivity with sera from mononucleosis patients with early infection. The IgG sensitivity, however, was as high as that reported for the DHFR protein. The discrepancy for IgM is best explained by the different ELISA conditions and serum panels used. We used conditions for which the specificity in all control panels was 100%. With respect to detection of IgG antibodies, p23 and GST-p18 had similar sensitivities. While GST-p18 was more sensitive in detecting previous infections, p23 detects more primary infections. Typically, sera from early acute infections are weakly positive or even negative, especially for anti-p18 IgG. Although the majority of sera reacted simultaneously in the p23- and GST-p18 IgG ELISAs, some sera that react exclusively with either p23 or p18 exist. This strongly suggests the use of a combination of both antigens.
Besides the apparent diagnostic improvement by combination, the gene fusion with p23 is also effective for production and POD labelling of recombinant p18. The p23 moiety introduces several biochemical advantages, like high expression levels, easy purification, good stability, and solubility. Moreover, p23 provides a high number of lysines, which enables effective enzyme coupling, yielding a highly reactive and stable p23-p18-POD conjugate. As already mentioned, the unusual amino acid composition and biochemical properties of p23 and p23-p18 could be used to advantage for efficient purification. The biochemical similarity of p23 and p18 is evident. Both polypeptides contained a cysteine at a similar position, and a dimeric structure may likely occur for both proteins in vivo. Moreover, protein sequence alignments suggest a weak homology in the carboxy regions (data not shown). For IgM assays, p18 is sufficient; however, its production is difficult. In principle, heterologous fusion with GST, DHFR, or β-galactosidase bears the risk of false-positive reactions. Gene fusion to p23, however, guarantees more sensitive IgG detection and more specific IgM detection, whereas production of the recombinant protein is facilitated. Very recently, GST-p18 has been suggested for application in a VCA IgM ELISA (2). Although anti-GST antibodies do not occur very frequently in human sera, exceptions exist, especially for IgM, which disqualifies GST proteins for commercial application. Our donor panel contained one positive anti-GST IgM sample. This reflects a frequency of 1%. In general, heterologous fusion proteins should not be considered for specific diagnosis without further controls.
As long as we did not look at RF-positive sera, the standard indirect IgM assay, i.e., with p23-p18 immobilized onto the solid phase and anti-human IgM-POD as a tracer, proved to be very specific. It was not surprising, however, that RF-containing sera from RA patients disclosed a well-known interference and strongly reacted in this type of p23-p18 IgM ELISA in more than 50% of cases. RFs are autoantibodies that bind to the constant region of IgG and have been demonstrated frequently in sera from RA patients and other clinical entities (for a review, see reference 1). RF as a cause of false-positive reactions in VCA IgM IFA has been known for a long time (5). Different strategies to solve this problem have been reported. Most of them are related to absorption or precipitation of IgG-RF complexes, for instance, by preincubation with anti-human IgG (9) or with IgG-coated latex beads (5). However, these additional steps are expensive and often time-consuming and are not suitable for ELISA processors. The most convenient method to circumvent the RF problem is the use of IgM capture ELISAs. VCA μc IgM ELISAs based on natural antigens have been established previously (22). The p23-p18 μc ELISA presented in this study utilizes a directly labelled antigen conjugate. This is an improvement over assays using indirectly labelled antigen, constructed by adding labelled antigen-specific antibodies (22). Such assays still keep the risk of residual susceptibility for false positivity since some of the captured RFs can bind the antigen-antibody complex. The μc ELISA of this study completely prevented the RF reactivity while keeping its full sensitivity. Moreover, the analysis of sera from patients with recent infections showed that the undesired long-standing IgM positive serology, which is often amplified by capture-type IgM ELISAs, is not a problem in the p23-p18 ELISA. Approximately 80% of patients seroreverted between months 3 and 12 after the emergence of symptoms, and detectable antibody levels persist in only 0.5% of latent carriers.
It must be emphasized that for this first evaluation, only unequivocally classified sera, selected by stringent conditions, were used. Therefore, the real challenge for the diagnostic usefulness of these novel tests will be the direct comparison with VCA IFA in routine diagnosis. This kind of study will be performed soon. So far, both ELISAs showed excellent performance and are promising alternative diagnostic methods for the diagnosis of infectious mononucleosis and EBV seropositivity by sensitive and specific detection of anti-viral capsid IgM and IgG antibodies.
ACKNOWLEDGMENTS
We thank Bernd Deissler, Andrea Heim, Marianne Nashir-Heyer, Christiane Rhode, Petra Volland, and Astrid Wiegleb-Führer for skillful technical assistance.
REFERENCES
- 1.Carson D A, Chen P P, Fox R I, Kipps T J, Jirik F, Goldfine R D, Silverman G, Radoux V, Fong S. Rheumatoid factors and immune networks. Annu Rev Immunol. 1987;5:109–126. doi: 10.1146/annurev.iy.05.040187.000545. [DOI] [PubMed] [Google Scholar]
- 2.Chan K H, Luo R X, Chen H L, Ng M H, Seto W H, Peiris S M. Development and evaluation of an Epstein-Barr virus (EBV) immunoglobulin M enzyme-linked immunosorbent assay based on the 18-kilodalton matrix protein for diagnosis of primary EBV infection. J Clin Microbiol. 1998;36:3359–3361. doi: 10.1128/jcm.36.11.3359-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Färber I, Wutzler P, Wohlrabe P, Wolf H, Hinderer W, Sonneborn H-H. Serological diagnosis of infectious mononucleosis using three anti-Epstein-Barr virus recombinant ELISAs. J Virol Methods. 1993;42:301–308. doi: 10.1016/0166-0934(93)90041-o. [DOI] [PubMed] [Google Scholar]
- 4.Gorgievski-Hrisoho M, Hinderer W, Nebel-Schickel H, Horn J, Vornhagen R, Sonneborn H-H, Wolf H, Siegl G. Serodiagnosis of infectious mononucleosis by using recombinant Epstein-Barr virus antigens and enzyme-linked immunosorbent assay technology. J Clin Microbiol. 1990;28:2305–2311. doi: 10.1128/jcm.28.10.2305-2311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henle G, Lenette E T, Alspaugh M A, Henle W. Rheumatoid factor as a cause of positive reactions in tests for Epstein-Barr virus-specific IgM. Clin Exp Immunol. 1979;36:415–422. [PMC free article] [PubMed] [Google Scholar]
- 6.Henle W, Henle G, Horwitz C A. Epstein-Barr virus-specific diagnostic tests in infectious mononucleosis. Hum Pathol. 1974;5:551–565. doi: 10.1016/s0046-8177(74)80006-7. [DOI] [PubMed] [Google Scholar]
- 7.Hinderer W, Plachter B, Vornhagen R. Identification of immunoreactive viral antigens. In: Sinclair J, editor. Methods in molecular medicine. 3. Cytomegalovirus protocols, in press. Totowa, N.J: Humana Press; 1999. [DOI] [PubMed] [Google Scholar]
- 8.Hinderer W, Siegl G. Application of recombinant antigens for the diagnosis of acute Epstein-Barr virus infection. In: Kourstak E, Marusyk R G, Murphy F A, Van Regenmortel M H V, editors. Applied virology research. 3. New diagnostic procedures. New York, N.Y: Plenum Press; 1994. pp. 131–143. [Google Scholar]
- 9.Ho D W T, Field P R, Cunningham A L. Rapid detection of acute Epstein-Barr virus infection by an indirect enzyme-linked immunosorbent assay for specific immunoglobulin M (IgM) antibody without rheumatoid factor and specific IgG interference. J Clin Microbiol. 1989;27:952–958. doi: 10.1128/jcm.27.5.952-958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakane P K, Kawoi A. Peroxidase labeled antibody—a new method of conjugation. J Histochem Cytochem. 1974;22:1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- 11.Pearson G R. ELISA tests and monoclonal antibodies for EBV. J Virol Methods. 1988;21:97–104. doi: 10.1016/0166-0934(88)90056-0. [DOI] [PubMed] [Google Scholar]
- 12.Reischl U, Gerdes C, Motz M, Wolf H. Expression and purification of an Epstein-Barr virus encoded 23-kDa protein and characterization of its immunological properties. J Virol Methods. 1996;57:71–85. doi: 10.1016/0166-0934(95)01970-7. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Martinez D, Patton J L, Stewart J A, Pellett P E. Detection of Epstein-Barr virus-specific antibodies by means of baculovirus-expressed EBV gp125. J Virol Methods. 1995;52:145–153. doi: 10.1016/0166-0934(94)00157-c. [DOI] [PubMed] [Google Scholar]
- 14.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 15.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 16.Van Grunsven W M J, Nabbe A, Middeldorp J M. Identification and molecular characterization of two diagnostically relevant marker proteins of the Epstein-Barr virus capsid antigen complex. J Med Virol. 1993;40:161–169. doi: 10.1002/jmv.1890400215. [DOI] [PubMed] [Google Scholar]
- 17.Van Grunsven W M J, Spaan W J M, Middeldorp J M. Localization and diagnostic application of immunodominant domains of the BFRF3-encoded Epstein-Barr virus capsid protein. J Infect Dis. 1994;170:13–19. doi: 10.1093/infdis/170.1.13. [DOI] [PubMed] [Google Scholar]
- 18.Van Grunsven W M J, Van Heerde E C, De Haard H J W, Spaan W J M, Middeldorp J M. Gene mapping of two immunodominant Epstein-Barr virus capsid proteins. J Virol. 1993;67:3908–3916. doi: 10.1128/jvi.67.7.3908-3916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vornhagen R, Plachter B, Hinderer W, Sonneborn H-H, Jahn G. Application of PCR-technology for the generation of DNA-fragments coding for viral antigens. In: Kahl G, Appelhans H, Koempf J, Driesel A J, editors. Bio tech forum. Vol. 10. Heidelberg, Germany: Hüthig; 1992. pp. 279–288. [Google Scholar]
- 20.Vornhagen R, Plachter B, Hinderer W, The T H, Van Zanten J, Matter L, Schmidt C A, Sonneborn H-H, Jahn G. Early serodiagnosis of acute human cytomegalovirus infection by enzyme-linked immunosorbent assay using recombinant antigens. J Clin Microbiol. 1994;32:981–986. doi: 10.1128/jcm.32.4.981-986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vornhagen R, Hinderer W, Sonneborn H-H, Bein G, Matter L, The T H, Enders G, Jahn G, Plachter B. IgM-specific serodiagnosis of acute human cytomegalovirus infection using recombinant autologous fusion proteins. J Virol Methods. 1996;60:73–80. doi: 10.1016/0166-0934(96)02047-2. [DOI] [PubMed] [Google Scholar]
- 22.Wielaard F, Scherders J, Dagelinckx C, Middeldorp J M, Sabbe L J M, Van Belzen C. Development of an antibody-capture IgM-enzyme-linked immunosorbent assay for diagnosis of acute Epstein-Barr virus infections. J Virol Methods. 1988;21:105–115. doi: 10.1016/0166-0934(88)90057-2. [DOI] [PubMed] [Google Scholar]
- 23.Wolf H, Motz M, Kühbeck R, Seibl R, Bayliss G J, Modrow S, Fan J. Selection and production by genetechnological methods of medically relevant EBV antigens. In: Levine P H, Ablashi D V, Pearson G R, Kottaridis S D, editors. Epstein Barr virus and associated diseases. Boston, Mass: Martin Nijhoff Publishing; 1985. pp. 485–494. [Google Scholar]