Abstract
Background:
Anxiety and irritability frequently co-occur in youth and are mediated by aberrant threat responses. However, empirical evidence on neural mechanisms underlying this co-occurrence is limited. To address this, we apply data-driven latent phenotyping to data from a prior report of a well-validated threat extinction recall fMRI paradigm.
Methods:
Participants included 59 youth (28 anxiety disorder, 31 healthy volunteers; Mage=13.15yrs) drawn from a transdiagnostic sample of 331 youth, in which bifactor analysis was conducted to derive latent factors representing shared vs. unique variance of dimensionally-assessed anxiety and irritability. Participants underwent threat conditioning and extinction. Approximately three weeks later, during extinction recall fMRI, participants made threat-safety discriminations under two task conditions: current threat appraisal and explicit recall of threat contingencies. Linear mixed-effects analyses examined associations of a “negative affectivity” factor reflecting shared anxiety and irritability variance with whole-brain activation and task-dependent amygdala connectivity.
Results:
During recall of threat-safety contingencies, higher negative affectivity was associated with greater prefrontal (ventrolateral/ventromedial, dorsolateral, orbitofrontal), motor, temporal, parietal, and occipital activation. During threat appraisal, higher negative affectivity was associated with greater amygdala-inferior parietal lobule connectivity to threat/safety ambiguity.
Limitations:
Sample included only healthy youth and youth with anxiety disorders. Results may not generalize to other diagnoses for which anxiety and irritability are also common, and our negative affectivity factor should be interpreted as anxiety disorders with elevated irritability. Reliability of some subfactors was poor.
Conclusions:
Aberrant amygdala-prefrontal-parietal circuitry during extinction recall of threat-safety stimuli may be a mechanism underlying the co-occurrence of pediatric anxiety and irritability.
Keywords: fMRI, threat, anxiety, irritability, bifactor, negative affectivity
1. Introduction
Comorbidity is pervasive in psychiatry and complicates pathophysiological research by yielding nonspecific correlates, impeding target identification and development of putative mechanistic treatment interventions (Insel, 2014; Kaczkurkin et al., 2020; Kapur, Phillips, & Insel, 2012; Kotov et al., 2017; Milham, Craddock, & Klein, 2017). In youth, anxiety and irritability are common and often co-occur (Merikangas et al., 2010; Stoddard et al., 2014). Recently, in a clinical sample of 230 youth diagnosed with a primary anxiety disorder, approximately 53% presented with elevated irritability (Shimshoni et al., 2020); in other samples with both treatment-seeking and typically developing youth, irritability and anxiety are significantly correlated (r’s=.42–.43; Cornacchio et al., 2016; Crum et al., 2020; Stoddard et al., 2017).
Both anxiety and irritability involve high-arousal, phasic negative affect states (e.g., “negative affectivity”) (Cardinale et al., 2019; Lee et al., 2017; Rothbart, 2007), and show overlapping and distinct neural mechanisms of threat orienting (Kircanski et al., 2018). While a large literature links anxiety to aberrant threat responses (Craske, Hermans, & Vervliet, 2018; LeDoux & Pine, 2016; Milad & Quirk, 2012; Shin & Liberzon, 2010), a smaller literature examines threat responding in irritability (Brotman, Kircanski, Stringaris, Pine, & Leibenluft, 2017; Leibenluft, 2017). Aberrant threat response, both in irritability and anxiety, is associated with perturbations in the amygdala-prefrontal-hypothalamic-periaqueductal gray circuitry (Brotman et al., 2017; Craske et al., 2018; LeDoux & Pine, 2016; Leibenluft, 2017; Milad & Quirk, 2012; Shin & Liberzon, 2010). Given the overlapping neural circuitry and high co-occurrence of irritability with anxiety, examining the neural mechanisms underlying this co-occurrence may improve the development of evidence-based treatment of these commonly co-occurring conditions (Kircanski et al., 2019; Shimshoni et al., 2020). In the present study, we use a computational latent phenotyping approach with a threat learning functional Magnetic Resonance Imaging (fMRI) paradigm to examine the neural mechanisms of threat learning underlying the commonality between anxiety and irritability in youth.
Increasingly, researchers use latent phenotyping approaches to address the challenge of parsing specific and shared pathophysiology underlying clinical comorbidity (Flagel et al., 2016; Friston, Redish, & Gordon, 2017; Kaczkurkin et al., 2020). Bifactor modeling represents one such approach (Bornovalova, Choate, Fatimah, Petersen, & Wiernik, 2020; Caspi et al., 2014; Castellanos-Ryan et al., 2014; Harrewijn et al., 2021; Kaczkurkin et al., 2020; Kircanski et al., 2018; Zald & Lahey, 2017). Bifactor analysis uses observed data as indicators to estimate latent constructs (Caspi et al., 2014; Kaczkurkin et al., 2020; Reise, Moore, & Haviland, 2010). It is well-suited to handle correlated symptoms, such as irritability and anxiety (Stoddard et al., 2014), to reveal a hierarchical structure of symptoms including a general/common factor and unique, orthogonal subfactors (Cardinale et al., 2019; Harrewijn et al., 2021; Kircanski et al., 2018). Thus, it is an appealing approach for research on pathophysiology of correlated domains to disentangle distinct and common variances (Castellanos-Ryan et al., 2014; Kircanski et al., 2018; Reise, 2012; Shanmugan et al., 2016; Zald & Lahey, 2017). While the use of bifactor and other latent models in psychopathology research is increasing, its application to task-based fMRI data is in its infancy (Castellanos-Ryan et al., 2014; Kircanski et al., 2018; Shanmugan et al., 2016).
Prior work has begun to disentangle neural correlates of threat-related processing in anxiety and irritability. Two studies examined the neural correlates underlying the co-occurrence of anxiety and irritability by testing the interactions between irritability and anxiety symptoms (Crum et al., 2020; Stoddard et al., 2017). These studies found that high levels of both symptoms were associated with decreased anterior cingulate cortex (ACC) activation during viewing of negative images (Crum et al., 2020) and decreased amygdala-medial prefrontal cortex (mPFC) connectivity during implicit threat processing (Stoddard et al., 2017). In a recent study, Kircanski et al. (2018) applied bifactor analysis to parse the unique and shared variances of dimensionally-assessed irritability and anxiety symptoms in youth as it related to attentional threat orienting. Using an fMRI dot-probe task to examine attentional threat orienting, a common factor of irritability and anxiety (i.e., negative affectivity) was associated with increased thalamic activation during threat orienting (Kircanski et al., 2018). Together, these studies (Crum et al., 2020; Kircanski et al., 2018; Stoddard et al., 2017) provide preliminary data supporting the investigation of the co-occurrence of irritability and anxiety, which may present with unique neural markers distinct from those of either irritability or anxiety presenting alone.
Findings from Kircanski et al. (2018) illustrate the promise of a bifactor modeling approach to parse the unique and common variance of irritability and anxiety and relate them to distinct neural correlates of threat orienting. The current study expands Kircanski et al. (2018) by focusing on threat-safety discrimination, rather than threat orienting. The ability to differentiate threat and safety through associative learning has been studied extensively in anxiety disorders using threat conditioning, extinction, and extinction recall paradigms (Craske et al., 2018; Shechner, Hong, Britton, Pine, & Fox, 2014). Such processes, mediated by amygdala-prefrontal cortex (PFC) function, are conserved across species (Milad & Quirk, 2012) and offer a useful means of understanding the pathophysiology underlying threat-safety learning and discrimination in anxiety disorders (Graham & Milad, 2011). In particular, threat extinction recall paradigms are sensitive to detecting anxiety-related differences in memory recall of threat/safety signals, eliciting greater fronto-temporal activation (i.e., ventromedial PFC [vmPFC], subgenual and medial ACC, dorsolateral prefrontal cortex [dlPFC], inferior temporal cortex) (Britton et al., 2013; Gold et al., 2020; Michalska et al., 2019) and altered amygdala-vmPFC connectivity during extinction recall across specific conditions/stimuli (Gold et al., 2016, 2020) in youth with anxiety. Currently, the literature on such processes is scarce in irritability (Kircanski et al., 2018; Stoddard et al., 2017). Indeed, to date, no studies have examined threat conditioning, extinction, or extinction recall in irritability alone or in combination with anxiety.
In this study, we used a data-driven latent phenotyping approach, i.e., bifactor model, with a well-validated threat extinction recall paradigm (Britton et al., 2013; Gold et al., 2016, 2020) to identify the neural correlates of extinguished threat recall underlying the commonality between pediatric anxiety and irritability symptoms. During the task, participants are asked to make threat-safety discriminations under two attention conditions: threat appraisal and memory recall (Britton et al., 2013; Gold et al., 2016, 2020). Specifically, participants rate their current levels of fear evoked by, and memory for, facial morph stimuli falling along a continuum with parametrically varying degrees of similarity to the extinguished threat (CS+) and safety (CS−) cues. This paradigm was designed to measure the response gradients, and capture the transitional boundary, between threat and safety. Previous research of extinction recall in clinical anxiety has used passive viewing to mimic non-human animal research; however, passive viewing does not measure psychological processes relevant to clinical anxiety, such as subjective fear levels (e.g., threat appraisal). For each attention condition, participants provide continuous Likert scale ratings in response to one of two questions regarding each facial morph stimulus: 1) How afraid are you? (threat appraisal condition); 2) How likely was she to scream? (memory recall condition).
This is the first study to examine threat conditioning, extinction, and extinction recall in irritability and anxiety. Prior work using variants of our threat extinction recall paradigm (Britton et al., 2013; Glenn, Fox, Pine, Peters, & Michalska, 2020; Gold et al., 2016, 2020; Michalska et al., 2019; Shechner et al., 2018) found that anxiety diagnosis or risk for anxiety (i.e., behavioral inhibition) was associated with perturbed function in frontal (vmPFC, dlPFC, ACC) and temporal (inferior temporal cortex) regions, as well as thalamus during memory recall of threat/safety signals and altered amygdala-vmPFC functional connectivity during extinction recall across conditions/stimuli. Using a different extinction recall paradigm, Marin et al. (2017) also reported reduced vmPFC activation and greater amygdala-vmPFC connectivity during extinction recall in adults with anxiety. Although prior behavioral research reveals similar patterns of discriminative learning during conditioning and extinction among anxious and non-anxious youth, anxious relative to non-anxious youth exhibit heightened behavioral and physiological fear responses to all conditioned stimuli (Abend et al., 2020; Dvir, Horovitz, Aderka, & Shechner, 2019). During extinction recall, anxiety diagnosis or risk for anxiety (i.e., behavioral inhibition) was associated with elevated fear (Britton et al., 2013) and skin conductance response (SCR; Michalska et al., 2019) to extinguished threat/safety cues during threat appraisal and greater memory to extinguished threat cues (CS+; Britton et al., 2013; Shechner et al., 2018). Based on these findings and studies on the co-occurrence of anxiety and irritability (Crum et al., 2020; Kircanski et al., 2018; Stoddard et al., 2017), we hypothesized that the commonality between anxiety and irritability in youth (i.e., “negative affectivity”) would be associated with dysfunction in the vmPFC, ACC, thalamus, and temporal regions during memory recall of threat/safety signals. We also hypothesized that negative affectivity would be associated with altered amygdala-based functional connectivity during extinction recall across conditions. Behaviorally, negative affectivity would be associated with heightened fear responses (in physiological measures and subject ratings) to all conditioned stimuli during conditioning and extinction and greater fear ratings during threat appraisal to, and better memory during recall of, the extinguished threat (CS+) relative to safety (CS−) cues.
2. Method
2.1. Participants
Participants were recruited by the National Institute of Mental Health (NIMH) Intramural Research Program; written consent/assent was obtained from parents/children, respectively. Recruitment occurred in several steps. First, in a large transdiagnostic sample of youth (N=331; Mage=13.57 yrs; 54.08% female) with primary disruptive mood dysregulation disorder (DMDD; n=70), anxiety disorder(s) (n=95), attention-deficit/hyperactivity disorder (ADHD; n=39), and healthy volunteers (n=127), we conducted bifactor analysis to derive latent factors representing the common and unique variance associated with dimensionally-assessed anxiety and irritability symptoms (see below and eResults 1 in the Supplement for details). Second, of the 331 youth included in the bifactor analysis, fMRI data on extinction recall were available in 59 youth who completed a threat conditioning and extinction paradigm, followed by the extinction recall fMRI paradigm. These 59 youth constituted the final sample of this study and included 28 participants with anxiety disorder(s) and 31 healthy volunteers (Mage=13.15 yrs; 66.10% female), reflecting a subsample from a report by Gold et al. (2020). Additional bifactor model was not conducted in the final sample of 59 youth given the small sample size. We used the bifactor-derived factor scores as our clinical phenotypes; all reported analyses relating threat conditioning, extinction, and extinction recall to the bifactor-derived clinical phenotypes are new. See Table 1 for the sample characteristics across the entire sample of N=59 and the Supplement (eMethods 1) for detailed diagnostic/clinical assessments.
Table 1.
Sample Characteristics
| N (%) or Mean (SD) | |
|---|---|
| Age, mean (SD), y | 13.15 (2.68) |
| Female, n (%) | 39 (66.10) |
| IQ, mean (SD)a | 113.73 (12.23) |
| SES, mean (SD)b | 36.16 (19.58) |
| Motion, mean (SD)c | 0.09 (0.06) |
| Days between visits, mean (SD) | 19 (6.75) |
| Irritability Measures, mean (SD) | |
| Child-reported ARI | 1.56 (2.42) |
| Parent-reported ARI | 1.54 (2.57) |
| Anxiety Measures, mean (SD) | |
| Child-reported SCARED | 20.75 (16.03) |
| Parent-reported SCARED | 17.90 (17.21) |
Abbreviations: ARI = Affective Reactivity Index; SCARED = Screen for Child Anxiety Related Emotional Disorders; SES = Socioeconomic status.
Measured by the Wechsler Abbreviated Scale of Intelligence.
Measured by the Hollingshead 2-factor index. Missing data for 2 participants.
Calculated as the mean Euclidean distance of framewise volume shift after censoring.
2.2. Bifactor-derived clinical latent factors of anxiety and irritability
Detailed results of the bifactor analysis of anxiety and irritability symptoms assessed using parent- and child-reports of the Screen for Child Anxiety Related Emotional Disorders (SCARED) (Birmaher et al., 1997, 1999) and parent- and child-reports of the Affective Reactivity Index (ARI) (Stringaris et al., 2012), respectively, have been described in our prior work (Cardinale et al., 2019). In this study, we applied the bifactor-derived factor scores reported in Cardinale et al. (2019) to the behavioral and imaging analyses in the 59 youth who completed the extinction recall fMRI paradigm. The bifactor analysis revealed four factors: a general factor (termed “negative affectivity”), anxiety, parent-reported irritability, and child-reported irritability (see the eResults 1 in the Supplement for details).
Analyses indicated that only the general factor showed satisfactory reliability in terms of the index of construct replicability (H) and omega (ωh, omega hierarchical, i.e., the proportion of variances in the indicators attributable to the common factor) – H=.94 and ωh=.77 (H >.80 and ωh >.75 indicate acceptable reliability) (Hancock & Mueller, 2001; Levin-Aspenson, Watson, Clark, & Zimmerman, 2020; Reise, Bonifay, & Haviland, 2013; Rodriguez, Reise, & Haviland, 2016). See the Supplement (eResults 1 & eFigure 1) for more details. Therefore, in this study, we focused on findings with the general factor (i.e., “negative affectivity,” standardized factor score M±SD = −.10±0.82), reflecting shared variance in anxiety and irritability symptoms. Exploratory findings regarding the other symptom-specific latent subfactors (i.e., anxiety, parent-reported irritability, and child-reported irritability) are presented in the Supplement (eResults 3–4 and eTable 1–3). See Discussion for methodological considerations and limitations regarding the other subfactors.
2.3. Procedures
2.3.1. Visit 1: Threat conditioning and extinction task
Participants completed an established threat conditioning and extinction paradigm as reported in Gold et al. (2020) (eFigure 2A). Briefly, participants were presented with visual stimuli showing two women displaying neutral facial expressions as the conditioned threat and safety stimuli (CS: CS+ and CS−, respectively). The task consisted of three phases: pre-conditioning, conditioning, and extinction. SCR and startle response using electromyography (EMG; collected continuously), and self-rated fear (collected between phases) were recorded. For more details about this visit and psychophysiological measures, see the Supplement (eMethods 2) and Gold et al. (2020).
2.3.2. Visit 2: Threat extinction recall during fMRI
Approximately 3 weeks following Visit 1 (M±SD = 19±6.75 days; range = 10–35 days), participants returned for the fMRI scan during the threat extinction recall task (Gold et al., 2020) (eFigure 2B). Participants made threat-safety discriminations under two task conditions: threat appraisal and explicit memory. Specifically, participants rated, along a 7-point Likert scale from 0 (not at all) to 6 (extremely), their current levels of fear evoked by, and memory for, facial morph stimuli falling along a continuum with varying degrees of similarity to the extinguished threat (CS+) and safety (CS−) cues (with 9 morphed images in between; eFigure 2B). See Supplement for details regarding the fMRI task procedures (eMethods 2).
2.4. Imaging Data Acquisition and Preprocessing
Neuroimaging data were acquired on a 3-T General Electric scanner using a 32-channel head coil. Data were preprocessed using the Analysis of Functional NeuroImages (AFNI) (Cox, 1996). The first four volumes of each functional run were discarded to allow for the magnetization to reach a steady state, resulting in 268 TRs per run. Standard preprocessing procedures were applied to the fMRI data (e.g., despiking, slice-timing correction, co-registration, normalization, and smoothing). TR pairs with a Euclidean norm motion derivative >1mm or an outlier fraction >0.1 were censored during linear regression (Gold et al., 2020; Kircanski et al., 2018). See eMethods 3 in the Supplement for acquisition and preprocessing parameters.
Processed, scaled fMRI data were entered into three General linear models (GLMs) to estimate voxelwise blood oxygen level-dependent (BOLD) signal change and task-related functional connectivity of the amygdala (bilateral amygdala seeds) in the first-level analysis. One GLM estimated BOLD signal change and included the following parameters: a quadratic detrending polynomial, a regressor for each of 6 translational and rotational motion parameters, and 22 gamma-convolved regressors of interest for modeling task conditions. These 22 task regressors corresponded to each morphed image (11 images: 0% [=CS−], 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100% [=CS+]) for each task condition (2 conditions: explicit memory vs. threat appraisal). The other two GLMs applied generalized psychophysiological interaction (gPPI) (McLaren, Ries, Xu, & Johnson, 2012) methods to identify brain regions that differed in their functional connectivity with each amygdala seed (left, right) as a function of task conditions. For details, see eMethods 3 in the Supplement and Gold et al. (2020).
2.5. Statistical analysis
Analyses used participants’ continuous latent factor scores on four factors derived from the bifactor analysis: negative affectivity (common factor), anxiety, parent-reported irritability, and child-reported irritability. These scores were used as between-subjects variables in the group-level analyses of behavioral (i.e., ratings and RT) and fMRI data.
2.5.1. Behavioral data
Behavioral data were analyzed using the lme package in R (Rstudio, 2020). Two linear mixed-effects models were tested for behavioral data during fMRI, one for ratings and the other for RT. Models tested task condition (threat appraisal, explicit memory) by morph (1–11) by each bifactor-derived latent factor (negative affectivity, anxiety, parent-reported irritability, child-reported irritability) to examine associations between the phenotypes and ratings/RT. The number of days between visits was modeled as a nuisance variable.
2.5.2. Imaging data
Group-level fMRI data were analyzed with linear mixed-effects modeling using 3dLME (Chen, Saad, Britton, Pine, & Cox, 2013), which relied on the lme function in R. We tested three models (one for activation and two for bilateral amygdala functional connectivity) of task condition (threat appraisal, explicit memory) by morph (1–11) by each latent factor (negative affectivity, anxiety, parent-reported irritability, child-reported irritability) to examine associations between the bifactor-derived phenotypes and whole-brain neural activation and amygdala functional connectivity. Linear and quadratic trends of the morph stimuli (based on weights generated from orthogonal polynomials) served as within-subject variables. We tested linear trends to identify regions that showed activation/connectivity as a linear function of threat and quadratic trends to identify regions that responded more to ambiguous threat relative to CS− and CS+. The number of days between visits was modeled as a nuisance variable.
A whole-brain gray matter mask was used in the analyses, as was a mask that included voxels for which data existed for at least 90% of participants (Abend et al., 2020; Gold et al., 2020). The initial voxel-wise threshold was set at p<.001, two-sided. Multiple-testing cluster-extent correction was set at family-wise error rate of α<.05/60=.00083 to correct for 60 F-tests (involving latent factors) via Monte Carlo cluster-size simulation by using methods designed recently to address concerns regarding inflated false positive rates (Cox, Chen, Glen, Reynolds, & Taylor, 2017). These 60 tests represents the three above-described models (one for activation and two for bilateral amygdala functional connectivity) for eight three-way interactions of task condition by morph (linear/quadratic) by each latent factor, eight two-way interactions of morph (linear/quadratic) by each latent factor, and four two-way interactions of task condition by each latent factor, i.e., 3*(8+8+4)=60. The resulting cluster-size threshold was k≥76 (1187.5 mm3). At this threshold, we did not find significant three-way interactions, i.e., task condition by morph (linear/quadratic) by any latent factors in the activation analysis; however, we observed large clusters of 907 to 4757 voxels (14,172 to 74,328 mm3) for the two-way interactions of task condition by latent factors. To facilitate interpretation, we extracted clusters from the activation analysis by using a more stringent voxelwise threshold of p<.0001 (cluster size = 28 voxels, i.e., 437.5 mm3). To characterize significant interactions, we extracted mean percent signal change and connectivity values averaged from each cluster using AFNI’s 3dROIstat program and conducted post-hoc analyses in RStudio using the lme function.
3. Results
3.1. Behavioral data
Psychophysiological measures during threat conditioning and extinction demonstrated successful threat conditioning and extinction. There was a significant negative affectivity by phase by stimulus interaction on the EMG data, F2,92=4.76, p=.01. That is, higher negative affectivity was related to lower EMG to CS+ vs. CS− during pre-conditioning (r=−.43, p=.002), but not during conditioning (r=.11, p=.479) or extinction (r=−.05, p=.738). For detailed out-of-scanner behavioral results, see eResults 2 in the Supplement.
3.1.1. Task Ratings and RT during fMRI
Analysis of task ratings indicated a three-way interaction of negative affectivity by task condition by morph (linear trend) (F1,1214=7.72, p=.006) on ratings during the task. Follow-up analyses within each task condition indicated a significant negative affectivity by morph (linear trend) interaction in the threat appraisal condition (F1,580=5.24, p=.02), but not in the explicit memory condition (F1,580=0.07, p=.79). Specifically, during threat appraisal, fear ratings increased as the proportion of threat (% CS+ in the morphs) linearly increased, and this association was stronger in those with high vs. low negative affectivity (median split for visualization purposes; B=1.33, t(289)=4.30, p<.001 and B=1.16, t(298)=3.44, p<.001, respectively; see eFigure 3 in the Supplement). No association between negative affectivity and RT was noted (ps>.11).
3.2. Brain imaging data
3.2.1. Whole-brain Activation
Analysis indicated two-way interactions of negative affectivity by task condition in multiple brain regions (Table 2; Figures 1 & 2). These included prefrontal cortex (i.e., ventrolateral prefrontal cortex [vlPFC] extending to vmPFC, inferior frontal gyrus, OFC, dlPFC), premotor cortex, insula, temporal regions (e.g., superior temporal gyrus, fusiform gyrus), parietal regions (e.g., inferior and superior parietal lobule), occipital regions, cerebellum, and brain stem. Follow-up analyses indicated that, in general, higher negative affectivity was associated with greater activation in these regions during the explicit memory but not threat appraisal condition (Table 2). Additional findings with the other factors are reported in the Supplement (eResults 4).
Table 2.
Negative affectivity x task condition interaction from the whole-brain activation analysis
| Regionsa | BA | Size (k) | Peak (x, y, z)b | Analysisc | r d | ||
|---|---|---|---|---|---|---|---|
| F 1,1214 | p value | TA | EM | ||||
| Prefrontal | |||||||
| L vlPFC/vmPFCe | 47 | 179 | (−36, 39, −6) | 26.85 | <.0001 | .00 | .28 |
| L inferior frontal gyrus | 44 | 50 | (−44, 11, 4) | 30.35 | <.0001 | −.08 | .24 |
| R orbitofrontal cortex | 11/47 | 45 | (26, 41, −6) | 39.07 | <.0001 | −.08 | .26 |
| R dlPFC | 9/46 | 30 | (46, 24, 26) | 26.37 | <.0001 | .09 | .30 |
| Motor | |||||||
| L premotor cortex | 6 | 103 | (−19, −1, 56) | 31.74 | <.0001 | −.01 | .21 |
| R premotor cortex | 6 | 76 | (39, 1, 56) | 34.28 | <.0001 | −.10 | .20 |
| Insula | |||||||
| R insula | 13 | 28 | (36, 19, 14) | 29.70 | <.0001 | −.10 | .21 |
| Temporal | |||||||
| L anterior STG | 22 | 93 | (−49, −1, −4) | 40.70 | <.0001 | −.13 | .24 |
| L fusiform face area | 37 | 52 | (−44, −59, −9) | 27.17 | <.0001 | −.09 | .09 |
| L fusiform face area | 30 | 36 | (−36, −41, −14) | 29.37 | <.0001 | −.07 | .16 |
| Parietal | |||||||
| L inferior parietal lobule | 40/7 | 356 | (−29, −46, 44) | 39.85 | <.0001 | −.06 | .28 |
| R superior parietal lobule | 7 | 70 | (34, −61, 56) | 29.10 | <.0001 | .09 | .27 |
| L S2 | 43 | 46 | (−51, −16, 19) | 40.04 | <.0001 | −.09 | .21 |
| Occipital | |||||||
| R V2 | 18 | 34 | (16, −86, −11) | 30.53 | <.0001 | −.02 | .22 |
| R cuneus | 19 | 31 | (31, −74, 39) | 26.66 | <.0001 | .06 | .30 |
| Cerebellum | |||||||
| L cerebellum | - | 689 | (−16, −81, −26) | 62.33 | <.0001 | −.18 | .27 |
| Brainstem | |||||||
| Brainstem | - | 41 | (−6, −24, −9) | 33.02 | <.0001 | −.18 | .19 |
Abbreviations: BA = Brodmann area; dlPFC = dorsolateral prefrontal cortex; L = left; EM = explicit memory; R = right; S2 = secondary somatosensory cortex; STG = superior temporal gyrus; TA = threat appraisal; V2 = secondary visual cortex; vlPFC = ventrolateral prefrontal cortex; vmPFC = ventromedial prefrontal cortex.
At voxelwise p=.001, the largest significant cluster was of 1598 voxels. To facilitate interpretation, we extracted clusters using the more stringent voxelwise p=.0001. At this threshold, clusters ≥ 28 voxels survive whole-brain correction at α=.05/60=.00083. Region comprising the greatest portion of the cluster extent.
Coordinates are in Talairach space.
Post-hoc linear mixed models on mean BOLD (blood oxygenation level-dependent) signal for extracted cluster.
Post-hoc correlations between negative affectivity factor and brain activation for each task condition (explicit memory [EM] and threat appraisal [TA]) after adjusting for days between visits and anxiety, parent-rated irritability, and child-rated irritability factors.
Two subjects were excluded from post-hoc analyses because of outliers (i.e., 3 SDs below the mean).
Figure 1. Negative affectivity by task condition interaction on vlPFC/vmPFC activation.
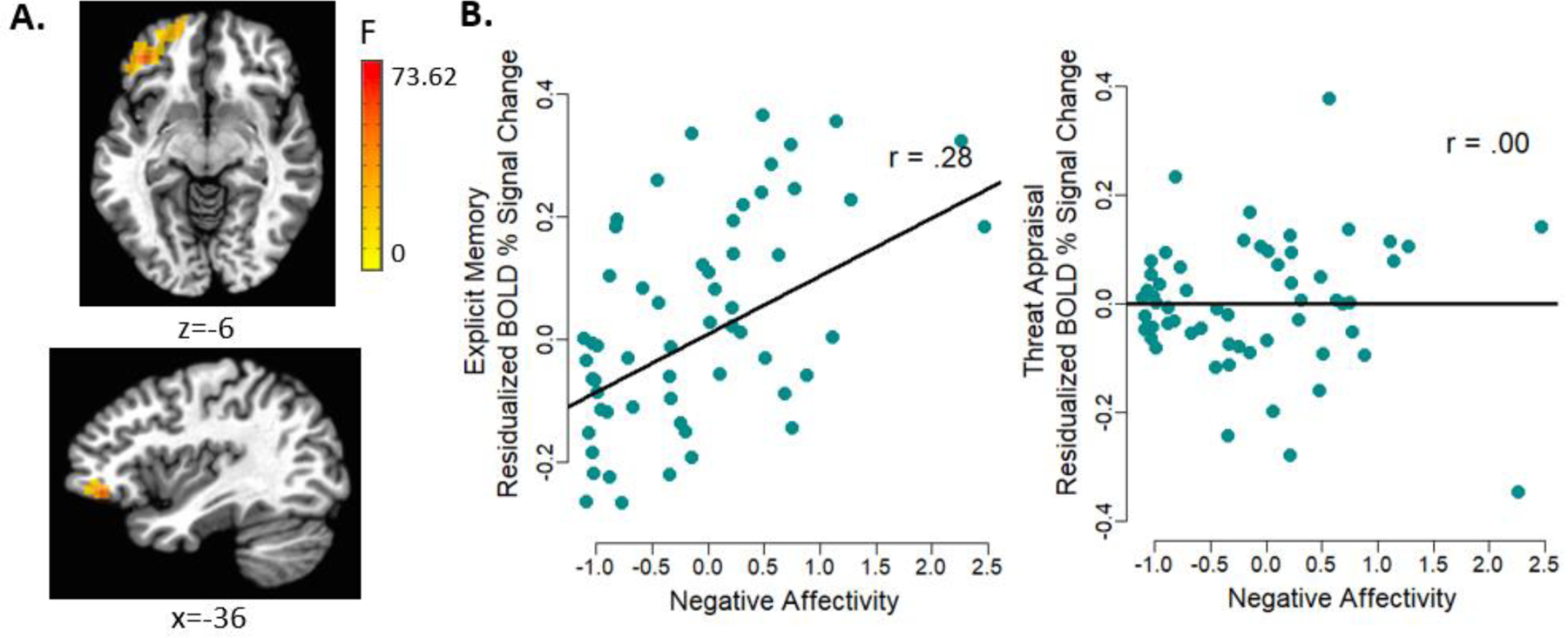
(A) Whole-brain analyses revealed negative affectivity by task condition interaction on activation in the left vlPFC extending to vmPFC (Talairach: x, y, z = −36, 39, −6; 179 voxels). (B) Residualized scatterplot (adjusting for other latent factors and days between visits) depicting individual data points and the association between negative affectivity and the BOLD (blood oxygenation level-dependent) % signal change during the explicit memory and threat appraisal conditions. Higher negative affectivity was related to greater vlPFC/vmPFC activation during explicit memory but not threat appraisal.
Note. vlPFC = ventrolateral prefrontal cortex; vmPFC = ventromedial prefrontal cortex.
Figure 2. Negative affectivity by task condition interaction on activation in regions other than vlPFC/vmPFC.
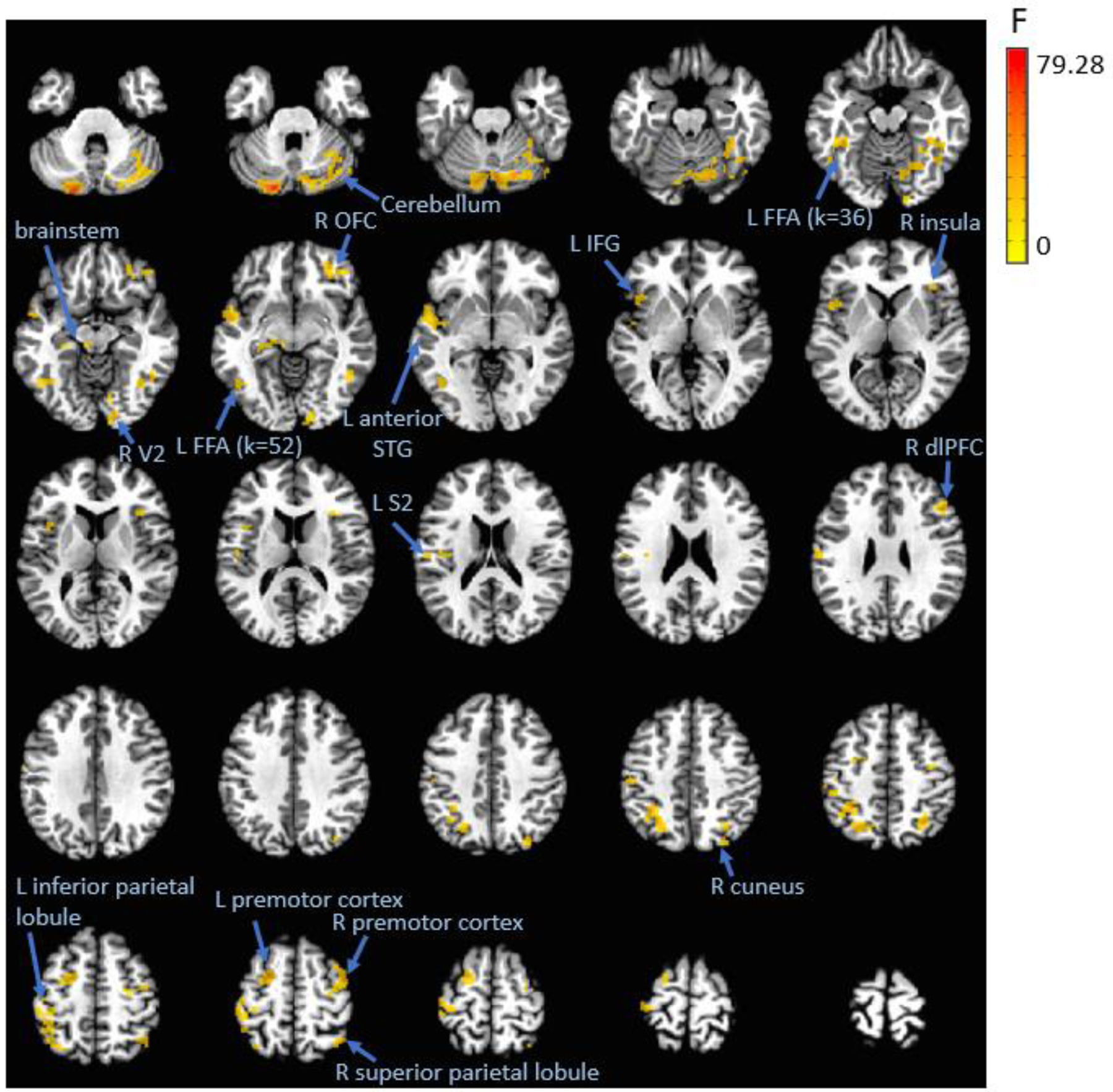
In general, higher negative affectivity was associated with greater activation across these regions during the explicit memory but not threat appraisal condition.
Note. dlPFC = dorsolateral prefrontal cortex; FFA = fusiform face area; IFG = inferior frontal gyrus; L = left; OFC = orbitofrontal cortex; R = right; S2 = secondary somatosensory cortex; STG = superior temporal gyrus; V2 = secondary visual cortex.
3.2.2. Amygdala Functional Connectivity
Analysis revealed a three-way interaction of negative affectivity by task condition by morph (quadratic trend) on the connectivity between the left amygdala and left inferior parietal lobule (Talairach: x, y, z = −51, −61, 26; 79 voxels [1234mm3]; F1,1214=22.54, p<.001). Follow-up analyses by each task condition showed differential effects of negative affectivity by morph (quadratic trend) for the threat appraisal (B=−0.39, F1,580=12.39, p<.001) and explicit memory (B=0.32, F1,580=9.06, p=.003) conditions. Specifically, during threat appraisal but not explicit memory, t-tests of the PPI estimate between the high vs. low negative affectivity group (median spit for visualization purposes) for each morph indicated that the high negative affectivity group, relative to the low group, showed greater connectivity between the left amygdala and left inferior parietal lobule in response to the most ambiguous morph between CS− and CS+ (50%CS+) (Figure 3). No significant associations with negative affectivity were noted for the right amygdala connectivity, and no significant associations with other subfactors were noted for either the left or right amygdala connectivity.
Figure 3. Negative affectivity by task condition by morph (quadratic trend) interaction on the functional connectivity between left amygdala and left inferior parietal lobule.
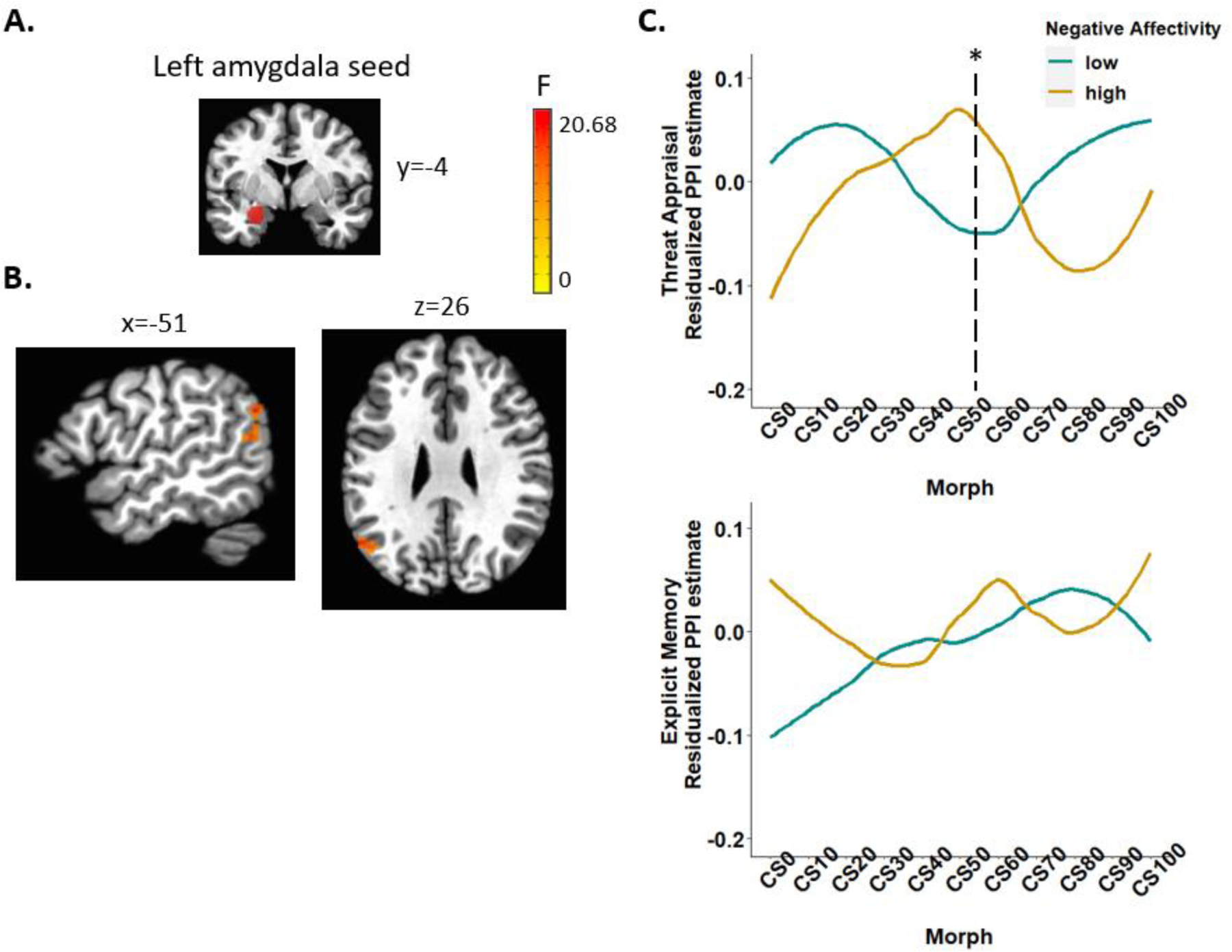
(A) The left amygdala seed. (B) The generalized PPI analysis revealed negative affectivity by task condition by morph (quadratic trend) interaction on the functional connectivity between the left amygdala and left inferior parietal lobule (Talairach: x, y, z = −51, −61, 26; 79 voxels). (C) Residualized PPI estimates (adjusting for other latent factors and days between visits) from CS− to CS+ by each task condition (threat appraisal, explicit memory) and by high vs. low negative affectivity (median split for visualization purposes).
Note. PPI = psychophysiological interaction; CS = conditioned stimuli. *p<.05
3.3. Behavior & brain imaging data
Activations in the 17 significant clusters were not significantly correlated with ratings or RT during explicit memory after Bonferroni correction at alpha=.05/17=.0029 (r’s = −.25 to .29, p’s = .028 to .965). The connectivity between the left amygdala and left inferior parietal lobule in response to the most ambiguous morph (50% CS+) during threat appraisal was also not correlated with either rating or RT to the same stimuli (r = −.13 and .04, respectively).
4. Discussion
This study used a data-driven latent phenotyping approach, i.e., bifactor model, to identify the common variance between pediatric anxiety and irritability symptoms and relate it to neural activation and amygdala connectivity during threat extinction recall. Results indicated that higher negative affectivity (i.e., disordered anxiety symptoms with elevated irritability) was associated with greater activation across multiple brain regions including the prefrontal, motor, insula, temporal, parietal, and occipital regions during memory recall of threat/safety stimuli. Moreover, higher negative affectivity was associated with greater functional connectivity between amygdala and inferior parietal lobule in response to threat/safety ambiguity during threat appraisal. Together, these findings suggest that aberrant amygdala-prefrontal-parietal circuitry during extinction recall of threat-safety discrimination may be a mechanism underlying the co-occurrence of pediatric anxiety and irritability.
Consistent with the literature (see Dvir et al., 2019 for a meta-analysis), participants showed successful discriminative conditioning across all measures (self-report, SCR, and startle response), independent of their negative affectivity (i.e., the bifactor-derived common factor of anxiety and irritability). Specifically, following conditioning, participants reported greater fear and showed greater SCR and startle response to CS+ relative to CS−. The effects for fear rating and startle response, but not SCR, were maintained following extinction; such divergence has been previously reported, potentially indicating that these measures assess related but distinct processes (Lonsdorf et al., 2017).
During extinction recall, fear ratings in the threat appraisal condition (but not ratings in the explicit memory condition) increased as the proportion of threat (% CS+) linearly increased, and this association was stronger in those with high vs. low negative affectivity. This indicates that youth with high negative affectivity, relative to those with low negative affectivity, showed greater discriminative appraisal of extinguished threat vs. safety cues. Of note, behavioral task measures (i.e., ratings and RT) did not correlate with brain activations or amygdala-inferior parietal lobule connectivity measures that were associated with negative affectivity, at the corrected threshold. Studies with larger samples, and thus greater statistical power, may be needed to link behavioral effects, such as ratings and RT, and brain-phenotype associations that emerge during extinction recall, although prior analyses of the larger dataset from which this subsample was derived found no RT modulation on neural activation in youth with anxiety (Gold et al., 2020). While it is possible that neural measures are more sensitive than behavioral measures in detecting phenotype-related associations, future studies should strive to demonstrate convergent effects.
Researchers are beginning to use latent variable models, specifically bifactor modeling, in psychiatric neuroimaging, demonstrating its promise in revealing both common and dissociable neural substrates of psychopathology and symptom dimensions (Cardinale et al., 2019; Kaczkurkin et al., 2020; Kircanski et al., 2018; Shanmugan et al., 2016). Using bifactor modeling with threat extinction recall fMRI data, we found that negative affectivity (i.e., the bifactor-derived common factor of anxiety and irritability) was associated with greater activation across multiple brain regions during memory recall of threat/safety stimuli and greater amygdala-inferior parietal lobule connectivity threat/safety ambiguity during threat appraisal. These findings extend the only previous fMRI study using a bifactor approach with a threat orienting paradigm (i.e., dot-probe task), where negative affectivity was associated with increased thalamic activation (but not with amygdala connectivity) during threat orienting (Kircanski et al., 2018). The current study also extends two other fMRI studies that examined the interaction between irritability and anxiety (Crum et al., 2020; Stoddard et al., 2017) and reported associations between high levels of both anxiety and irritability and less ACC activation during viewing of negative images (Crum et al., 2020) and less amygdala-medial PFC connectivity during social threat processing (Stoddard et al., 2017). Notably, the neural correlates of comorbid irritability and anxiety appeared to be unique compared to those of either irritability or anxiety presenting alone (Crum et al., 2020; Kircanski et al., 2018; Stoddard et al., 2017). Consistent with this, our supplementary results showed that anxiety and irritability subfactors were negatively associated with brain activation during memory recall; the opposite pattern of association manifested in the negative affectivity common factor. However, these results should be interpreted with caution given the low reliability of the subfactors. Nonetheless, the current and past findings (Crum et al., 2020; Kircanski et al., 2018; Stoddard et al., 2017) provide emerging evidence for dissociable neural mechanisms of irritability, anxiety, and their co-occurrence.
Our findings of positive associations between negative affectivity and activation across multiple brain regions, including the prefrontal cortex (e.g., vlPFC extending to vmPFC, a region particularly implicated in extinction recall; Milad & Quirk, 2012), insula, and temporal regions, during memory recall of threat/safety stimuli are largely consistent with our hypotheses and the past literature in pediatric anxiety. Specifically, past research using similar threat extinction recall paradigms found that youth at risk for or with anxiety disorders, relative to healthy volunteers, showed greater fronto-temporal-insular activation (i.e., vmPFC, subgenual and medial ACC, dlPFC, inferior temporal cortex, anterior insula) during memory recall of threat/safety signals (Britton et al., 2013; Gold et al., 2020; Michalska et al., 2019). Young adults with childhood behavioral inhibition (a risk for anxiety disorders) also showed dlPFC dysfunction during memory recall of threat-safety stimuli (Shechner et al., 2018). Memory of threat/safety contingencies is one of the most robust conditioning markers of anxiety (Lissek et al., 2014). Our findings suggest that the neural dysfunction underlying the commonality of youth anxiety and irritability also manifests during explicit recall of extinguished threat/safety contingencies, but not during appraisal of the fear evoked by the threat/safety stimuli. Of note, greater motor, parietal, occipital, and cerebellar activation during memory recall of threat/safety stimuli regions, which have not been found in previous studies in pediatric anxiety, may be distinctly associated with the common variance between anxiety and irritability. The involvement of these regions may reflect the underlying neural dysfunction of irritability in the context of anxiety, as recent work demonstrates the importance of frontoparietal and motor-sensory networks—which support attention control, motor function, and integration of motor-sensory, emotional, and cognitive information—in predicting irritability symptoms (Scheinost et al., 2021).
In contrast, aberrant amygdala-based connectivity mediating the commonality between anxiety and irritability manifested mostly while youth appraised their fear associated with extinguished threat-safety cues. Two previous studies where the current sample was drawn from showed that youth with anxiety disorders, relative to healthy volunteers, exhibited altered amygdala-vmPFC connectivity (Gold et al., 2016, 2020). Using a latent variable approach to examine the commonality between anxiety and irritability, we did not find associations of either the negative affectivity common factor or anxiety subfactor with amygdala-PFC connectivity. Instead, we found that negative affectivity was associated with greater amygdala-inferior parietal lobule connectivity in response to threat/safety ambiguity during threat appraisal. Inferior parietal lobule is part of the dorsal frontoparietal attention network and plays a critical role in sustained attention and attention shifting (Behrmann, Geng, & Shomstein, 2004; Singh-Curry & Husain, 2009). Previous research suggests that inferior parietal hyperactivity may contribute to hypervigilance to threat that is common in anxiety (Balderston et al., 2017). Evidence also shows that reducing inferior parietal cortex activation during threat of shock via transcranial magnetic stimulation is sufficient to reduce physiological arousal related to both fear and anxiety in adults (Balderston et al., 2020). Consistent with these findings in adults, our finding of greater amygdala-inferior parietal lobule connectivity associated negative affectivity may thus reflect greater vigilance and attention to ambiguous threat vs. safety cues when anxious youth with elevated irritability appraise the fear evoked by those cues.
4.1. Limitations
Several limitations are worth noting here. First, we examined irritability in youth with a primary anxiety disorder(s), for whom DMDD (a diagnosis characterized by high, severe levels of irritability) were exclusionary; we did not have extinction recall data from youth with DMDD. As a result, the range of irritability symptoms in this sample was restricted (i.e., low to medium irritability). Therefore, our negative affectivity factor should be interpreted as anxiety disorders with elevated irritability (relative to healthy volunteers with no/little anxiety and irritability). Future studies should include youth with severe, high levels of both irritability and anxiety to evaluate the replicability of our findings. Second, the current sample and their latent factor scores were drawn from a larger transdiagnostic sample (N=331; youth with anxiety disorders, DMDD, ADHD, healthy volunteers) where the bifactor analysis was conducted, and the larger sample did not include all diagnoses that may involve anxiety and/or irritability. Thus, our findings may not generalize to other diagnoses for which anxiety and irritability are also common presenting complaints, e.g., major depressive disorder, bipolar disorder. Third, the reliability of subfactors (i.e., anxiety, parent-reported irritability, child-reported irritability) was poor. Therefore, we only focused on interpreting the results with the general/common negative affectivity factor. It will be essential for future studies to improve the reliability of the subfactors to enable examinations of the neural mechanisms that are specific to anxiety and specific to irritability, independent of those specific to the commonality of anxiety and irritability. Some of the strategies that might improve the reliability of the subfactors include the use of a larger sample size; targeted sampling to recruit youth with high irritability with and without anxiety; and inclusion of clinician ratings in addition to child- and parent-reports to address informant effects. Finally, future work is also needed to determine the extent to which our findings regarding negative affectivity are specific to anxiety and irritability as opposed to a general psychopathology factor (Kotov et al., 2017).
Anxiety and irritability are common in youth and often co-occur (Shimshoni et al., 2020; Stoddard et al., 2014). Children with both elevated irritability and anxiety, compared to children with anxiety alone or healthy volunteers, are more severely impaired in multiple domains of functioning (Shimshoni et al., 2020). However, few data exist to guide treatment for the common and impairing comorbidity of anxiety and irritability (Kircanski et al., 2019; Shimshoni et al., 2020). Exposure therapy, which is based on extinction principles, has been an effective treatment for anxiety disorders (Craske, Treanor, Conway, Zbozinek, & Vervliet, 2014). Our data showed aberrant extinction recall of threat-safety contingencies underlying the co-occurrence of pediatric anxiety and irritability, which may be mediated by alterations in the amygdala-prefrontal-parietal circuitry. Although these findings require replication, they contribute to building an evidence base for treatment development, such as exposure therapy for the comorbidity of anxiety and irritability, by examining the neural mechanisms underlying the commonality between these two phenotypes. This study also adds to the growing literature examining the co-occurrence of irritability and anxiety and its underlying neural correlates, which may be distinct from those presented in either irritability or anxiety alone.
Supplementary Material
Highlights.
Neural mechanisms of the anxiety and irritability co-occurrence is unclear.
Bifactor model identified the commonality between pediatric anxiety and irritability.
Bifactor-derived negative affectivity was related to neural activation and amygdala connectivity during threat extinction recall.
Anxiety and irritability is mediated by aberrant amygdala-prefrontal-parietal circuitry.
Role of the Funding Source:
This study was supported by the Intramural Research Program of the NIMH, and it was conducted under projects ZIA-MH002781 (clinical protocol NCT00018057). Dr. Tseng is supported by a research grant from the NIMH (R00MH110570) and the Fund to Retain Clinical Scientists at the Yale School of Medicine and the Yale Center for Clinical Investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
References
- Abend R, Gold AL, Britton JC, Michalska KJ, Shechner T, Sachs JF, … Pine DS (2020). Anticipatory threat responding: Associations with anxiety, development, and brain structure. Biological Psychiatry, 87, 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend R, Swetlitz C, White LK, Shechner T, Bar-Haim Y, Filippi C, … Pine DS (2020). Levels of early-childhood behavioral inhibition predict distinct neurodevelopmental pathways to pediatric anxiety. Psychological Medicine, 50, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston NL, Beydler EM, Goodwin M, Deng Z. De, Radman T, Luber B, … Grillon C (2020). Low-frequency parietal repetitive transcranial magnetic stimulation reduces fear and anxiety. Translational Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston NL, Hale E, Hsiung A, Torrisi S, Holroyd T, Carver FW, … Grillon C (2017). Threat of shock increases excitability and connectivity of the intraparietal sulcus. ELife, 6, e23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, & Shomstein S (2004). Parietal cortex and attention. Current Opinion in Neurobiology, 14, 212–217. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SMK (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 545–553. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry, 38, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Choate AM, Fatimah H, Petersen KJ, & Wiernik BM (2020, July 1). Appropriate use of bifactor analysis in psychopathology research: Appreciating benefits and limitations. Biological Psychiatry, 88, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, … Pine DS (2013). Response to learned threat: An fMRI study in adolescent and adult anxiety. American Journal of Psychiatry, 170, 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Kircanski K, Stringaris A, Pine DS, & Leibenluft E (2017). Irritability in youth: A translational model. American Journal of Psychiatry, 174, 520–532. [DOI] [PubMed] [Google Scholar]
- Cardinale EM, Kircanski K, Brooks J, Gold AL, Towbin KE, Pine DS, … Brotman MA (2019). Parsing neurodevelopmental features of irritability and anxiety: Replication and validation of a latent variable approach. Development and Psychopathology, 31, 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … Moffitt TE (2014). The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2, 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde ALW, … Conrod PJ (2014). Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. American Journal of Psychiatry, 171, 1310–1319. [DOI] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, & Cox RW (2013). Linear mixed-effects modeling approach to FMRI group analysis. NeuroImage, 73, 176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornacchio D, Crum KI, Coxe S, Pincus DB, & Comer JS (2016). Irritability and severity of anxious symptomatology among youth with anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connectivity, 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Hermans D, & Vervliet B (2018). State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences, 373, 20170025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, & Vervliet B (2014). Maximizing exposure therapy: An inhibitory learning approach. Behaviour Research and Therapy, 58, 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum KI, Hwang S, Blair KS, Aloi JM, Meffert H, … Blair RJR (2020). Interaction of irritability and anxiety on emotional responding and emotion regulation: A functional MRI study. Psychological Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir M, Horovitz O, Aderka IM, & Shechner T (2019). Fear conditioning and extinction in anxious and non-anxious youth: A meta-analysis. Behaviour Research and Therapy, 120, 103431. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Pine DS, Ahmari SE, First MB, Friston KJ, Mathys C, … Thapar A (2016). A novel framework for improving psychiatric diagnostic nosology. In Computational psychiatry: New perspectives on mental illness. (pp. 168–199). Cambridge, MA: MIT Press. [Google Scholar]
- Friston KJ, Redish AD, & Gordon JA (2017). Computational nosology and precision psychiatry. Computational Psychiatry, 1, 2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn DE, Fox NA, Pine DS, Peters MAK, & Michalska KJ (2020). Divergence in cortical representations of threat generalization in affective versus perceptual circuitry in childhood: Relations with anxiety. Neuropsychologia, 142, 107416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Abend R, Britton JC, Behrens B, Farber M, Ronkin E, … Pine DS (2020). Age differences in the neural correlates of anxiety disorders: An fMRI study of response to learned threat. American Journal of Psychiatry, 177, 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Shechner T, Farber MJMJ, Spiro CNCN, Leibenluft E, Pine DS, & Britton JC (2016). Amygdala–cortical connectivity: Associations with anxiety, development, and threat. Depression and Anxiety, 33, 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, & Milad MR (2011). The study of fear extinction: Implications for anxiety disorders. American Journal of Psychiatry, 168, 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock GR, & Mueller RO (2001). Rethinking construct reliability within latent variable systems. In Cudeck R, du Toit S, & Sorbom D (Eds.), Structural Equation Modeling: Present and Future: A Festschrift in Honor of Karl Jöreskog (pp. 195–216). Scientific Software International. [Google Scholar]
- Harrewijn A, Abend R, Naim R, Haller SP, Stavish CM, Bajaj MA, … Brotman MA (2021). Attention bias to negative versus non-negative faces is related to negative affectivity in a transdiagnostic youth sample. Journal of Psychiatric Research, 138, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2014). The nimh research domain criteria (rdoc) project: Precision medicine for psychiatry. American Journal of Psychiatry, 171, 395–397. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Moore TM, Sotiras A, Xia CH, Shinohara RT, & Satterthwaite TD (2020). Approaches to defining common and dissociable neurobiological deficits associated with psychopathology in youth. Biological Psychiatry, 88, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Phillips AG, & Insel TR (2012). Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it. Molecular Psychiatry, 17, 1174–1179. [DOI] [PubMed] [Google Scholar]
- Kircanski K, White LK, Tseng WL, Wiggins JL, Frank HR, Sequeira S, … Brotman MA (2018). A latent variable approach to differentiating neural mechanisms of irritability and anxiety in youth. JAMA Psychiatry, 75, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Craske MG, Averbeck BB, Pine DS, Leibenluft E, & Brotman MA (2019). Exposure therapy for pediatric irritability: Theory and potential mechanisms. Behaviour Research and Therapy, 118, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Waszczuk MA, Krueger RF, Forbes MK, Watson D, Clark LA, … Zimmerman M (2017). The hierarchical taxonomy of psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of Abnormal Psychology, 126, 454–477. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, & Pine DS (2016). Using neuroscience to help understand fear and anxiety: A two-system framework. American Journal of Psychiatry, 173, 1083–1093. [DOI] [PubMed] [Google Scholar]
- Lee M, Aggen SH, Carney DM, Hahn S, Moroney E, Machlin L, … Hettema JM (2017). Latent structure of negative valence measures in childhood. Depression and Anxiety, 34, 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E (2017). Pediatric irritability: A systems neuroscience approach. Trends in Cognitive Sciences, 21, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin-Aspenson HF, Watson D, Clark LA, & Zimmerman M (2020). What Is the General Factor of Psychopathology? Consistency of the p Factor Across Samples. Assessment. [DOI] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, & Grillon C (2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75, 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, … Merz CJ (2017). Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience and Biobehavioral Reviews, 77, 247–285. [DOI] [PubMed] [Google Scholar]
- Marin MF, Zsido RG, Song H, Lasko NB, Killgore WDS, Rauch SL, … Milad MR (2017). Skin conductance responses and neural activations during fear conditioning and extinction recall across anxiety disorders. JAMA Psychiatry, 74, 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in U.S. adolescents: Results from the national comorbidity survey replication-adolescent supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry, 49, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska KJ, Feldman JS, Ivie EJ, Shechner T, Sequeira S, Averbeck B, … Pine DS (2019). Early-childhood social reticence predicts SCR-BOLD coupling during fear extinction recall in preadolescent youth. Developmental Cognitive Neuroscience, 36, 100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2012). Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology, 63, 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Craddock RC, & Klein A (2017). Clinically useful brain imaging for neuropsychiatry: How can we get there? Depression and Anxiety, 34, 578–587. [DOI] [PubMed] [Google Scholar]
- Reise SP (2012). The Rediscovery of Bifactor Measurement Models. Multivariate Behavioral Research, 47, 667–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reise SP, Bonifay WE, & Haviland MG (2013). Scoring and modeling psychological measures in the presence of multidimensionality. Journal of Personality Assessment, 95, 129–140. [DOI] [PubMed] [Google Scholar]
- Reise SP, Moore TM, & Haviland MG (2010). Bifactor models and rotations: Exploring the extent to which multidimensional data yield univocal scale scores. Journal of Personality Assessment, 92, 544–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Reise SP, & Haviland MG (2016). Applying Bifactor Statistical Indices in the Evaluation of Psychological Measures. Journal of Personality Assessment, 98(3), 223–237. [DOI] [PubMed] [Google Scholar]
- Rothbart MK (2007). Temperament, development, and personality. Current Directions in Psychological Science, 16, 207–212. [Google Scholar]
- Rstudio, T. (2020). RStudio: Integrated Development for R. Rstudio Team, PBC, Boston, MA: URL Http://Www.Rstudio.Com/. [Google Scholar]
- Scheinost D, Dadashkarimi J, Finn ES, Wambach CG, MacGillivray C, Roule AL, … Tseng W-L (2021). Functional connectivity during frustration: A preliminary study of predictive modeling of irritability in youth. Neuropsychopharmacology, 46, 1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, … Satterthwaite TD (2016). Common and dissociable mechanisms of executive system dysfunction across psychiatricdisorders in youth. American Journal of Psychiatry, 173, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Fox NA, Mash JA, Jarcho JM, Chen G, Leibenluft E, … Britton JC (2018). Differences in neural response to extinction recall in young adults with or without history of behavioral inhibition. Development and Psychopathology, 30, 179–189. [DOI] [PubMed] [Google Scholar]
- Shechner T, Hong M, Britton JC, Pine DS, & Fox NA (2014). Fear conditioning and extinction across development: Evidence from human studies and animal models. Biological Psychology, 100, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshoni Y, Lebowitz ER, Brotman MA, Pine DS, Leibenluft E, & Silverman WK (2020). Anxious-irritable children: A distinct subtype of childhood anxiety? Behavior Therapy, 51, 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, & Liberzon I (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35, 169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Curry V, & Husain M (2009). The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia, 47, 1434–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Stringaris A, Brotman MA, Montville D, Pine DS, & Leibenluft E (2014). Irritability in child and adolescent anxiety disorders. Depression and Anxiety, 31, 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Tseng W-L, Kim P, Chen G, Yi J, Donahue L, … Leibenluft E (2017). Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiatry, 74, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, & Brotman MA (2012). The Affective Reactivity Index: A concise irritability scale for clinical and research settings. Journal of Child Psychology and Psychiatry and Allied Disciplines, 53, 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, & Lahey BB (2017). Implications of the hierarchical structure of psychopathology for psychiatric neuroimaging. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


