Abstract
A wide range of xenobiotic compounds are metabolized by cytochrome P450 (CYP) enzymes, and the genes that encode these enzymes are often induced in the presence of such compounds. Here, we show that the nuclear receptor CAR can recognize response elements present in the promoters of xenobiotic-responsive CYP genes, as well as other novel sites. CAR has previously been shown to be an apparently constitutive transactivator, and this constitutive activity is inhibited by androstanes acting as inverse agonists. As expected, the ability of CAR to transactivate the CYP promoter elements is blocked by the inhibitory inverse agonists. However, CAR transactivation is increased in the presence of 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP), the most potent known member of the phenobarbital-like class of CYP-inducing agents. Three independent lines of evidence demonstrate that TCPOBOP is an agonist ligand for CAR. The first is that TCPOBOP acts in a dose-dependent manner as a direct agonist to compete with the inhibitory effect of the inverse agonists. The second is that TCPOBOP acts directly to stimulate coactivator interaction with the CAR ligand binding domain, both in vitro and in vivo. The third is that mutations designed to block ligand binding block not only the inhibitory effect of the androstanes but also the stimulatory effect of TCPOBOP. Importantly, these mutations do not block the apparently constitutive transactivation by CAR, suggesting that this activity is truly ligand independent. Both its ability to target CYP genes and its activation by TCPOBOP demonstrate that CAR is a novel xenobiotic receptor that may contribute to the metabolic response to such compounds.
A very diverse array of naturally occurring and synthetic compounds are metabolized by cytochrome P450 (CYP) monooxygenases in the liver. These CYP substrates range from endogenous compounds such as steroids and cholesterol to drugs and carcinogens such as phenobarbital (PB) and aromatic hydrocarbons. The oxidized products are more polar, and the result is generally detoxification. However, a number of compounds, notably procarcinogens, are metabolized to more active forms.
The regulation of expression of the CYP genes is dependent on a number of different factors, including cell type and hormonal status. Of particular importance for those enzymes involved in metabolism of foreign or xenobiotic compounds is the induction of the expression of the appropriate CYP genes by such xenobiotics (31). It is now relatively well established, for example, that the expression of CYP1 genes is induced by the aryl hydrocarbon receptor, a member of the PAS family of transcription factors, in response to dioxin or other polycyclic or halogenated aromatic hydrocarbon ligands (9). Several members of the nuclear hormone receptor superfamily have also been implicated in responses of CYP gene expression to xenobiotics. The first of these was the peroxisome proliferator-activated receptor (PPAR), which was initially found (15) to be activated by fibrates and other compounds previously known to increase the levels of peroxisomes in rodents and to increase expression of CYP genes (23). Definitive confirmation of the role of PPARα in CYP gene regulation was recently provided by the demonstration that PPARα-deficient mice are unable to activate CYP4A gene expression in response to peroxisome proliferators (18).
More recently, the receptor variously termed PXR (17, 19) SXR (3), and PAR (2) has been proposed to mediate the induction of expression of the CYP3A gene by a number of different steroids, steroid antagonists, and xenobiotic compounds. This is based on both the activation of the receptor by such compounds, apparently as a consequence of direct ligand binding, and the identification of PXR response elements in CYP3A promoters (3, 17, 19, 25). Similarly, the receptor CAR has also been proposed to mediate the PB induction of CYP2B10 gene expression, based on the demonstration that CAR-RXR heterodimers bind a DNA element capable of mediating PB response (14, 28). In contrast to the apparently direct effect of the xenobiotic compounds as PXR ligands, PB reportedly has an indirect effect on CAR subcellular localization, promoting nuclear translocation (16). Since CAR behaves as an apparently constitutive transcriptional activator (1, 5), this translocation should be sufficient to result in activation of CAR target genes.
We have examined the potential interaction of CAR-RXR heterodimers with previously described receptor response elements in CYP and other genes. We find that such heterodimers interact not only with the previously described CYP2B elements (14, 28) but also with two quite different elements from the CYP3A gene and one element from the mouse mammary tumor virus (MMTV). The identification of these binding sites expands the range of potential CAR target genes. More importantly, we have found that the xenobiotic 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) is a CAR ligand. The agonist activity of this compound, which is a highly potent PB-like inducer of CYP gene expression (27), increases CAR transactivation above the constitutive level. This contrasts directly with the effects of previously described inverse agonists, which act to inhibit CAR transactivation (7). Interestingly, mutation of putative ligand contact residues in the CAR ligand binding domain (LBD) blocks both the stimulatory effect of TCPOBOP and the inhibitory effect of inverse agonists but does not affect constitutive transactivation, suggesting that this activity is truly ligand independent. Based on both its ability to target CYP genes and its activation by TCPOBOP, we propose that CAR functions directly as a xenobiotic receptor in vivo.
MATERIALS AND METHODS
Cell culture and transfections.
HepG2 cells were maintained in Dulbecco's modified Eagle medium, and LLC-PK1 cells were maintained in Medium 199 (Life Technologies), supplemented with 10% fetal bovine serum (Hyclone). For the transfections, 105 HepG2 cells or 0.5 × 105 LLC-PK1 cells were plated in 24-well dishes supplemented with charcoal-stripped serum and transfected using the calcium phosphate precipitation method as described previously (29). The next morning, cells were washed with phosphate-buffered saline and ligands were added. Typically, transfections included 100 ng of luciferase or CAT reporter plasmids, 50 ng of human growth hormone or β-galactosidase (β-Gal) internal control plasmids (pTKGH or cytomegalovirus β-Gal, respectively), and 100 ng of cdm8 expression vectors for receptors or VP16 or Gal fusion proteins. A total of 500 ng of plasmids was used per well. Cells were assayed for luciferase (Promega) or CAT (Boehringer Mannheim) activity 24 h after the addition of the ligands, and reporter expression was normalized to the medium human growth hormone (Quest Institute Diagnostics) concentration or β-Gal (Tropix) activity, according to the respective manufacturer's directions. Similar results were obtained from at least three independent experiments. Finally, for the generation of the CAR permanent cell line (HepG2-CAR), HepG2 cells were transfected with the pCDNA3.1-mCAR expression vector and CAR-positive clones were selected by neomycin resistance.
Plasmids.
The βRARE-TK-Luc reporter plasmid with three copies of the direct repeat 5 (DR-5) motif from the promoter of the mouse RARβ2 gene has been described previously (1). The ER6-TK-CAT reporter contains three copies of the everted repeat 6 (ER-6) element from the human CYP3A4 promoter, and the DR3-TK-CAT reporter contains two copies of the DR-3 motif from the rat CYP3A1 promoter (generous gifts of Steve Kliewer). The LXRE-TK-Luc reporter contains three copies of the DR-4/5 element from the MMTV promoter (so designated because it can be considered either a DR-4 or a DR-5 element) (generous gift of D. J. Mangelsdorf). Finally, the CYP2B10-TK-Luc construct contains one copy of the 51-bp PB-responsive enhancer module (PBREM) from the mouse CYP2B10 promoter (14), with two DR-4-type elements. Expression vectors for mCAR (5), the Gal4-mCAR-LBD fusion (7), the Gal4-RXR-LBD fusion and the GE1bLuc reporter (8) have been described previously. The Gal4-mCARΔ8 fusion construct contains a deletion of the last 8 amino acids. The ligand binding domain of mCAR was subcloned into pCMX-VP16 as a SalI/NotI fragment, in order to generate a VP16-mCAR fusion protein. The receptor interaction domain (RID) of SRC-1 was subcloned into both pGex2-TK (Pharmacia) and pCMX-Gal4 as a SalI/NotI fragment. Substitution mutations in helix 3 of mCAR were introduced by PCR, using the wild-type cdm8-mCAR plasmid as a template and primers containing the appropriate nucleotide changes. The plasmid with the F171L mutation was generated first and was then used as a template for the introduction of the I174A mutation. The presence of the expected mutations and no others in the mutant expression vector was confirmed by extensive sequencing prior to use.
Electrophoretic mobility shift assays.
pT7-Lac-His vectors containing full-length cDNAs of mCAR and hRXRα were used with the TNT-coupled transcription translation system (Promega) to prepare [35S]methionine-labeled proteins. The double-stranded oligonucleotides listed in Table 1 were end labeled with [γ-32P]ATP (NEN) and incubated with 1 to 2 μl of [35S]methionine-labeled CAR and RXR. The complexes were resolved on 4% nondenaturing polyacrylamide gel as described previously (29) and visualized by autoradiography. An approximately 100-fold excess of specific or nonspecific competitors was included, as indicated below.
TABLE 1.
CAR response elementsa
| Promoter | Type of repeat | Sequence |
|---|---|---|
| mRARβ2 | DR5 | −530GATCCGGGTAGGGTTCACCGAAAGTTCACTCGA−502 |
| MMTV | DR4/5 | −123GGTTCCCAGGGTTAAAATAAGTTCATCTAGA−94 |
| rCYP3A1 | DR3 | −1262GTAGATGAACTTCATGAACTGTCTA−1238 |
| hCYP3A4 | ER6 | −930AGAATATGAACTCAAAGGAGGTCAGTGAG−902 |
| mCYP2B10-1 | DR4 | −2336CCGAACTGTACTTTCCTGACCTTGGCAC−2316 |
| mCYP2B10-2 | DR4 | −2304CCACCATCAACTTGCCTGACACAGGAA−2278 |
Promoter sequences are from other references, as follows (reference numbers are in parentheses): mouse RARβ2 (mRARβ2) (37), MMTV (35), rat CYP3A1 (rCYP3A1) (22), human CYP3A4 (hCYP3A4) (10), and mouse CYP2B10 (mCYP2B10) (14). Boldface type indicates the hexamer consensus sequence, and underlining indicates the response element. The superscript numbers are nucleotide positions.
Western blot.
For the Western blot experiment, confluent HepG2 parental cells or HepG2-CAR cells were maintained in either dimethyl sulfoxide (DMSO) or 250 nM TCPOBOP for 48 h. Portions (10 μg) of cell extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were blotted, and CYP2B6 was identified with the monoclonal antibody 49-10-20 (6) generously provided by H. Gelboin, National Institutes of Health, Bethesda, Md.
In vitro interactions.
Glutathione S-transfesrase (GST) protein purification and coactivator association assays were carried out as previously described (7). Briefly, a GST–SRC-1 RID fusion protein was bound to glutathione-Sepharose beads and incubated with [35S]methionine-labeled CAR or CAR double mutant (CARdm) in the presence of 1 μM TCPOBOP, 50 μM androstanol, both TCPOBOP and androstanol, or solvent alone. The beads were extensively washed, and bound proteins were eluted with 15 mM glutathione. The eluted proteins were resolved by SDS-PAGE and visualized by autoradiography. The amount of CAR protein bound to the GST–SRC-1 RID fusion protein in the presence of TCPOBOP corresponded to approximately 12.5% of the total input.
RESULTS
CAR binding to response elements from CYP promoters.
The numerous nuclear hormone receptors that bind DNA as heterodimers with RXR can recognize a wide range of hormone response elements (20). These elements consist of two copies of a hexamer related to the 5′ RGGTCA 3′ consensus, arranged as either DRs inverted repeats (head-to-head), or ERs (tail-to-tail) and separated by from 0 to 8 bp. In general, DNA binding specificity is imposed by the spacing and relative arrangement of the hexamers. Initial studies (1, 5) demonstrated that CAR-RXR heterodimers recognize DRs of the binding consensus hexamer with a 5-bp spacer (DR-5), particularly the well-studied DR-5 from the RARβ2 promoter (βRARE). More recent studies have expanded the list of potential CAR-RXR targets with the identification of two DR-4 sites in the promoter of the CYP2B10 gene (28). To further explore the range of potential targets, a series of additional hormone response elements, particularly those identified in other CYP genes, was examined for binding to CAR-RXR heterodimers. These included the DR-3 and ER-6 elements from the promoters of the CYP3A1 and CYP3A4 genes (17), respectively, a complex element from the promoter of the MMTV, previously described as a binding site for LXR-RXR heterodimers (35) and the two DR-4 elements from the promoter of the CYP2B10 gene (28) (Table 1).
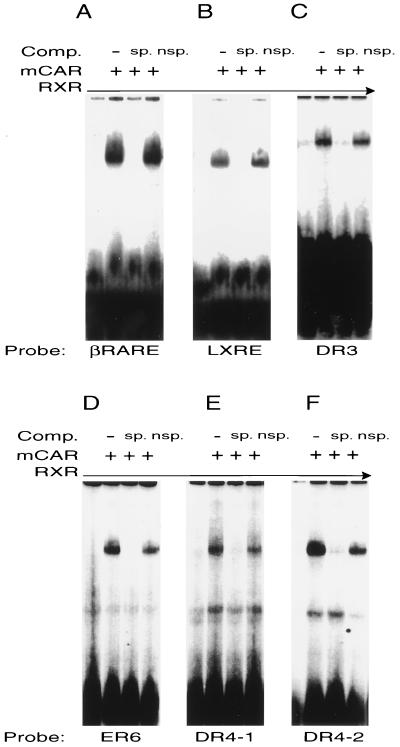
As demonstrated in Fig. 1, heterodimers of RXR and murine CAR specifically bound to the DR-5 βRARE element (Fig. 1A) and the LXRE element on the MMTV promoter (Fig. 1B), which could be considered either a DR-5 or a DR-4 element. CAR-RXR heterodimers also bound to both of the CYP3A elements (Fig. 1C and D), previously identified as binding sites for PXR-RXR heterodimers as well as both of the CYP2B10 elements (Fig. 1E and F). Based on a series of competition experiments, the apparent binding affinity of the CAR-RXR heterodimers was highest for the βRARE and LXRE elements, followed by the DR-3-, the two DR-4-, and the ER-6-type elements (data not shown). Addition of RXR was essential for binding to all elements, as expected from previous results demonstrating that CAR functions as a heterodimer (1, 5).
FIG. 1.
CAR-RXR heterodimers bind a variety of response elements. CAR and RXR proteins were produced by coupled in vitro transcription and translation and incubated with labeled oligonucleotides. (A) βRARE (DR-5) element from the promoter of the mouse RARβ2 gene; (B) LXRE (DR-4/5) element from the promoter of MMTV; (C) DR-3 element from the rat CYP3A1 promoter; (D) ER-6 element from the promoter of the human CYP3A4 gene; (E and F) the two DR-4 motifs (DR-4-1 and DR-4-2, respectively) from the PBREM of the mouse CYP2B10 promoter. Incubations were carried out in the presence or absence of either specific (sp.) (i.e., self) competitor or nonspecific (nsp.) (i.e., SP1) oligonucleotides, as indicated. Binding of the CAR-RXR heterodimer to the βRARE, the LXRE, and the DR-3 elements was stronger than binding to the ER-6 element and the two DR-4 elements. The autoradiograms in panels D, E, and F were exposed for approximately 12 h and those in panels A, B, and C were exposed for approximately 6 h.
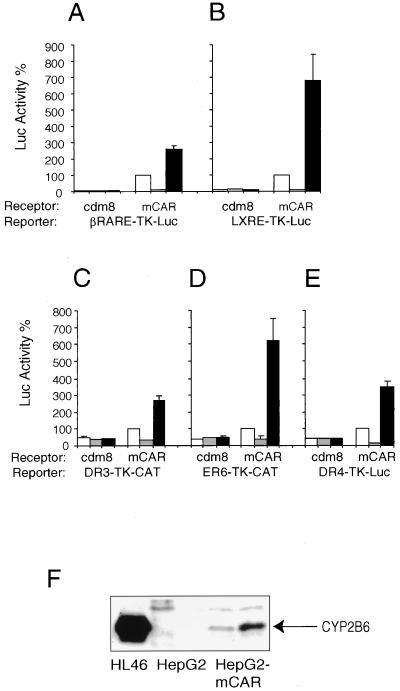
To determine whether these in vitro DNA binding sites could also function as active response elements, a series of appropriate luciferase or CAT reporter plasmids was constructed by inserting the various sites upstream of the TK promoter. As expected, all of the CAR binding sites were transactivated in an apparently constitutive manner by CAR (Fig. 2). For βRARE-TK-Luc (Fig. 2A) and the LXRE element (Fig. 2B), transactivation activities were observed to be 20- and 15-fold above background, respectively, whereas a more modest twofold stimulation was observed for the DR-3 (Fig. 2C), ER-6 (Fig. 2D), and DR-4 (Fig. 2E)-type elements. Overall, these results demonstrate that CAR, like other RXR partners, can recognize a range of hormone response elements and raise the possibility that CAR may have a broad role in regulation of CYP genes in the liver.
FIG. 2.
CAR transactivation. HepG2 cells were cotransfected with a CAR expression vector and reporters containing various CAR DNA binding sites, as indicated, in the presence or absence of androstanol or TCPOBOP. Luciferase activity is relative to that directed by CAR alone (100%). CAR is a strong constitutive activator of the βRARE-TK-Luc (A) and the MMTV-LXRE-TK-Luc (B) reporters and a weak activator of the rat CYP3A1 DR3-TK-CAT (C), the human CYP3A4 ER6-TK-CAT (D), and the mouse CYP2B10 DR4-TK-Luc (E) reporter constructs in the absence of ligands (white bars). Constitutive activity is inhibited in the presence of a 5 μM concentration of the inverse agonist androstanol (gray bars). In all cases, an increase in the CAR-mediated activation was observed upon addition of 250 nM TCPOBOP (black bars). (F) Lanes: the leftmost lane, HL46, a positive control containing high amounts of CYP2B6 protein; the next two lanes, confluent HepG2 cells treated for 48 h with 250 nM TCPOBOP and solvent, respectively; the two rightmost lanes, HepG2-CAR cells treated for 48 h with 250 nM TCPOBOP and solvent, respectively. A 10-μg quantity of cell lysate was resolved by SDS-PAGE, and immunoblots were developed with a monoclonal antibody specific for CYP2B6.
Stimulation of CAR transactivation by TCPOBOP.
Transactivation mediated by murine CAR is blocked by binding of inverse agonist ligands, such as androstanol (7) (Fig. 3A). However, this inhibitory effect is lost in the presence of several compounds known to activate expression of CYP genes, particularly TCPOBOP (14, 28) (Fig. 3B). This compound is the most potent of a class of xenobiotics that is exemplified by PB. Observations that PB responses are dependent on signaling processes involving phosphorylation (13) suggest a secondary mechanism for such effects. However, the functional antagonism between androstanol and TCPOBOP is also consistent with the possibility that the latter compound acts as a direct agonist ligand for CAR. To further examine this possibility, CAR-mediated transactivation of various response elements was tested in the presence of TCPOBOP or androstanol. CAR transactivation of the LXRE (Fig. 3B)- and the ER-6 (Fig. 3D)-containing constructs was increased by more than fivefold above the apparently constitutive level in the presence of TCPOBOP (Fig. 2). Similar, though less-marked, effects were observed with the βRARE (Fig. 3A), the DR-3 (Fig. 3C), and the DR-4 (Fig. 3E) elements. As expected from previous results, CAR-dependent transactivation of these elements was strongly decreased in the presence of androstanol (Fig. 2). TCPOBOP and androstanol had competing effects when added together (data not shown).
FIG. 3.
Structures of androstanol (A) and TCPOBOP (B).
In order to investigate the effect of CAR and TCPOBOP on endogenous CYP gene expression, we examined responses of CYP2B6 protein levels in control HepG2 cells or a stable derivative of these cells expressing CAR (HepG2-CAR). The promoter of the human CYP2B6 gene contains sequences highly homologous to the PBREM of the murine CYP2B10, shown in Fig. 1 to bind CAR-RXR and in Fig. 2E to be activated by CAR and TCPOBOP. The parental HepG2 cells do not express detectable levels of CYP2B6 protein. However, CAR expression significantly increased basal levels of expression, and this expression was further induced upon treatment with TCPOBOP (compare the two rightmost lanes of the gel shown in Fig. 2F).
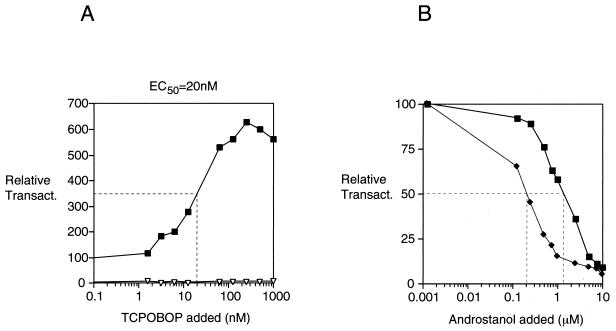
TCPOBOP is a relatively potent activator of CAR, with a 50% effective concentration (EC50) of approximately 20 nM (Fig. 4A). This is quite consistent with the very low concentrations required for TCPOBOP effects on CYP gene expression in primary hepatocytes (13) and is below the approximately 250 to 400 nM EC50 for the inhibitory effect of the inverse agonist androstanol (7) (Fig. 4B). To determine whether TCPOBOP behaves as a direct competitor with androstanol, increasing amounts of the inverse agonist were added in either the presence or absence of 100 nM TCPOBOP. As indicated in Fig. 4B, TCPOBOP shifted the androstanol dose-response curve to the right. The approximately fivefold magnitude of this effect, as indicated by the EC50 for androstanol inhibition, agrees well with that expected based on the EC50 for TCPOBOP stimulation. Thus, these results suggest that these two compounds compete directly for occupancy of the CAR LBD.
FIG. 4.
TCPOBOP is a high-affinity CAR activator that competes with androstanol for regulation of CAR. (A) A CAR expression vector (cdm8-mCAR) (black squares) or the cdm8 vector alone (open triangles) was cotransfected into HepG2 cells with the LXRE-TK-Luc reporter in the presence of various concentrations of TCPOBOP. Transactivation indicated is relative to that in the absence of TCPOBOP (100%). Half-maximal activation was observed at a concentration of approximately 20 nM TCPOBOP. (B) A transfection similar to that described for panel A was carried out in the presence or absence of 100 nM TCPOBOP and a range of concentrations of androstanol. Transactivation indicated is relative to that in the absence of androstanol in both cases. The amount of androstanol required for half-maximal repression was increased approximately fivefold, from 250 nM in the absence of TCPOBOP (black diamonds) to 1.5 μM in the presence of 100 nM TCPOBOP (black squares).
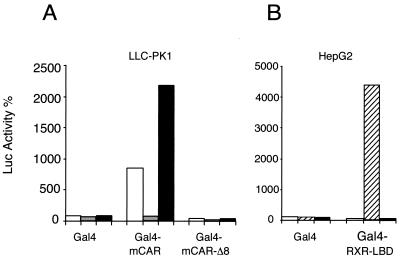
To test the resultant prediction that the effect of TCPOBOP is directed towards the ligand binding domain of CAR, a Gal4-CAR-LBD fusion construct was tested in transient transfection assays. As expected, the constitutively active CAR-LBD was further activated by TCPOBOP and repressed by androstanol (Fig. 5A). Previous results have demonstrated that constitutive transactivation by CAR requires the presence of the conserved C-terminal AF-2 activation helix (5). To test the effect of a deleted CAR AF-2 in the TCPOBOP response, a GAL-CAR protein missing the last 8 amino acids (Gal4-mCAR-Δ8) was used in the same experiment. As expected, deletion of this helix blocked constitutive activity and response to both androstanol and TCPOBOP (Fig. 5A). TCPOBOP had no effect on the ligand binding domain of the heterodimeric partner of CAR, RXR, which was strongly activated by 9-cis-retinoic acid (Fig. 5B).
FIG. 5.
TCPOBOP targets the ligand binding domain of CAR. (A) In LLC-PK1 cells, a Gal4-CAR wild-type fusion protein is constitutively active when bound to a Gal4-luc reporter (GE1b-Luc) in the absence of ligand (white bars). In the presence of 5 μM androstanol (gray bars), activation is blocked. Addition of 250 nM TCPOBOP (black bars) enhances transactivation 2.5-fold. However, a Gal4-CARΔ8 fusion protein lost both constitutive activity and response to ligands. (B) In HepG2 cells, the presence of 0.1 μM 9-cis retinoic acid (hatched bars) strongly activates a Gal4-RXR-LBD fusion protein, while the presence of 250 nM TCPOBOP (black bars) has no effect. White bars, DMSO (absence of ligand).
TCPOBOP is a CAR agonist.
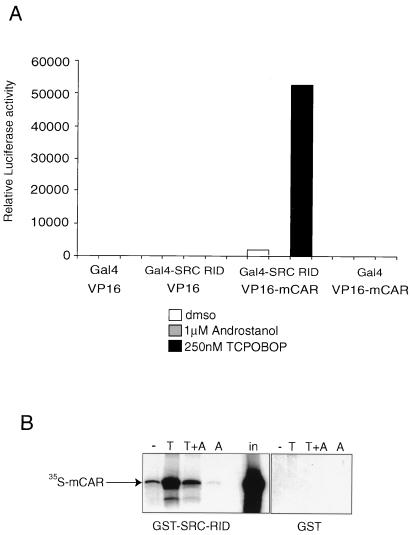
These results suggest that TCPOBOP acts directly as a CAR agonist. Two related approaches based on coactivator association were taken to test this more directly. As previously described, CAR interacts with the coactivator SRC-1 in an apparently constitutive manner, both in vivo and in vitro, and this interaction is lost in the presence of the inverse agonist androstanol (7). As shown in Fig. 6A, the interaction between CAR and SRC-1 in the mammalian two-hybrid system was strongly stimulated in the presence of TCPOBOP. Androstanol alone inhibited the interaction, as previously described, and antagonized the stimulatory effect of TCPOBOP (data not shown). No interaction was observed in the presence of either the Gal fusion proteins alone or the VP16 fusion proteins alone.
FIG. 6.
TCPOBOP enhances CAR interaction with the coactivator SRC-1 in vivo and in vitro. (A) TCPOBOP stimulation of CAR interaction with SRC-1 in vivo. A vector expressing the Gal4–SRC-1 RID fusion protein was cotransfected into HepG2 cells with a vector expressing the VP16-CAR-LBD fusion protein and GE1b-Luc as a reporter, in the absence of ligand or the presence of 5 μM androstanol or 250 nM TCPOBOP, as indicated. Luciferase expression relative to Gal4 alone in the presence of VP16 alone (=1) is indicated. No interaction was observed in the absence of either one or the other fusion protein. (B) TCPOBOP stimulation of CAR interaction with SRC-1 in vitro. 35S-labeled CAR was incubated with beads carrying a GST–SRC-1 RID fusion protein or GST alone in the presence of solvent (DMSO) (−), TCPOBOP (T), or androstanol (A), as indicated. Specifically bound proteins were eluted and resolved by SDS-PAGE. The amount of 35S-labeled CAR corresponding to 20% of the total input (in) is indicated.
An important confirmation of the ability of TCPOBOP to act as a CAR ligand was provided by the demonstration that the direct in vitro interaction between CAR and SRC-1 was strongly stimulated by TCPOBOP (Fig. 6B). This biochemical result rules out the possibility of secondary effects of signaling pathways that might be active in cells and demonstrates directly that TCPOBOP behaves as a CAR agonist.
LBD mutations.
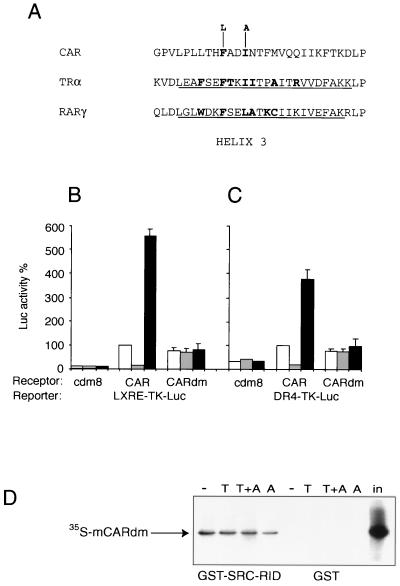
To further confirm that TCPOBOP is a direct ligand, a pair of mutations designed to block ligand binding was introduced into CAR. As indicated in Fig. 7A, these mutations targeted residues in the predicted helix 3 of the CAR ligand binding domain that are conserved in ligand binding domains of TR and RAR. For TR (30), RAR (24) (Fig. 7A), and other receptors (4, 21, 26, 33, 36), these residues are known to make specific contacts with ligands. If mutations of these conserved positions block ligand binding to CAR, they should prevent not only the inhibitory effect of the androstane inverse agonists but also the stimulatory effect of TCPOBOP. They would also block the apparently constitutive activity of CAR, if it were dependent on an as-yet-unidentified, ubiquitous, endogenous ligand. On the other hand, the mutations would not affect the apparently constitutive activity if it were not dependent on ligand binding. As expected, the CAR double mutant was quite resistant to the inhibitory effect of the inverse agonist when assayed on either the MMTV (Fig. 7B) or the CYP2B10 luciferase (Fig. 7C) reporters. The mutant CAR protein was also resistant to the stimulatory effect of TCPOBOP (Fig. 7B and C), providing additional direct evidence that this xenobiotic is a CAR ligand. Importantly, the mutations did not prevent the constitutive activity of CAR, indicating that it may truly be ligand independent (Fig. 7B and C).
FIG. 7.
Mutations in the ligand binding pocket of CAR block effects of CAR ligands. (A) Mutations in potential ligand contact residues of CAR. The sequence of the putative helix 3 of CAR is aligned to those reported for TRα (30) and RARγ (24). Helical residues in the TR and RAR crystal structures are underlined, and residues that make direct contacts with ligands are in boldface. Hydrophobic residues that project into the binding pocket and make contact with ligands are also present in analogous positions in ERα (4, 26), PR (33), PPARγ (21), and PPARδ (36). (B and C) An expression plasmid carrying the CAR double mutant (CARdm) was transfected into HepG2 cells with the LXRE-TK-Luc (B) or the DR4-TK-Luc (C) as the reporter. In both experiments, the double mutant retained approximately 75% of wild-type activity (white bars) and was resistant to both androstanol (gray bars) and TCPOBOP (black bars). (D) CARdm interaction with SRC-1 in vitro. 35S-labeled CARdm was incubated with beads carrying a GST–SRC-1 RID fusion protein or GST alone in the presence of solvent (DMSO) (−), TCPOBOP (T), or androstanol (A), as indicated. Specifically bound proteins were eluted and resolved by SDS-PAGE. The amount of 35S-labeled CARdm corresponding to 20% of the total input (in) is indicated.
To confirm that the mutations block ligand binding, their effect on in vitro binding of coactivator was examined. As expected, the mutations also blocked both the inhibitory effect of androstanol and the stimulatory effect of TCPOBOP on the in vitro binding of SRC-1, but did not alter SRC-1 interaction with the mutant protein in the absence of ligands (Fig. 7D).
DISCUSSION
CAR-RXR heterodimers have previously been found to bind to retinoic acid response elements (RAREs), particularly those of the DR-5 type (1, 5). The studies described here expand the list of potential CAR targets to include a series of response elements from CYP genes and an element from the MMTV promoter, none of which were known to function as RAREs. Both the transient transfection results with isolated elements and the studies with the stable CAR-expressing cell line suggest that the range of CAR targets may include several CYP genes. While these results are consistent with several other recent studies (14, 16, 28), further studies with native promoters will be required to establish the range of CAR targets among these drug-metabolizing enzymes.
Since the DR-3 and ER-6 CYP elements were previously described as binding sites for PXR-RXR heterodimers (3, 17, 19), and the MMTV DR-4/5 element was previously described as a binding site for LXR-RXR heterodimers (34), these results also indicate that the functions of CAR may overlap significantly not only with RAR, but also with these other new receptors. The ability of CAR-RXR heterodimers to bind the DR-4 elements from the CYP2B10 promoter and the ER-6 element from CYP3A4 also suggests a potential overlap with TR-RXR heterodimers, since sites of both types can act as thyroid hormone response elements (20). For each specific element or target gene, the extent of this overlap would be dependent on a number of factors, such as relative affinity for the various receptors and their levels of expression. Overall, however, we conclude that CAR joins the other new receptors, the two more conventional receptors, and, presumably, other nuclear receptors expressed in the liver in an increasingly complex regulatory network. Based on the known functions of these receptors, it appears that many of the genes in this network are involved in the normal metabolism of nutrients (e.g., targets for TR and LXR) and also the metabolic transformation of exogenous compounds (CAR and PXR). As more is learned about these receptors, we anticipate that additional categories of target genes will be added and that their overlapping and specific functional roles will be more clearly defined.
In general, the substantial direct overlap in potential DNA binding sites for these receptors contrasts with their distinct specificities of ligand binding. However, the results described here also suggest an important functional parallel in ligand binding. Thus, PXR binds an array of xenobiotic activators of CYP gene expression (3, 17) and the results described here demonstrate that the xenobiotic TCPOBOP is a ligand for murine CAR. TCPOBOP has been considered an unusually potent member of the class of PB-like inducers of CYP expression. This group of structurally unrelated compounds is linked functionally by potent inductive effects on expression of CYP2B genes (see reference 32 for a review). The ability of CAR to activate a PB-responsive enhancer element on the promoter of the mouse CYP2B10 gene (14, 28) suggested a direct role for CAR in the PB response. This observation is supported by a recent report suggesting that PB acts via an as-yet-unidentified signaling pathway that induces translocation of CAR from the cytoplasm to the nucleus (16). Such translocation would allow the constitutive activity of CAR (1, 5) to activate expression of CYP2B10 and, presumably, other targets. While this signaling dependent translocation mechanism is consistent with the observation that phosphatase inhibitors block the PB response (13), it is quite different from the direct agonist effects of TCPOBOP postulated here.
Importantly, the translocation mechanism cannot account for some aspects of CAR function, and also for some effects of TCPOBOP and other PB-like inducers. Thus, we have found that either transiently or stably expressed CAR is exclusively nuclear in cultured cell lines, regardless of the presence or absence of various agents such as TCPOBOP or androstanol (I. Tzameli and D. D. Moore, unpublished data). This is consistent with the observation that CAR transactivation is not dependent on treatment with PB or any other agent in a number of transiently transfected cell lines, and also the stably transfected HepG2 derivative line (1, 5, 14). In addition, the proposed translocation mechanism cannot account for the ability of TCPOBOP to reverse the inhibitory effect of androstanol as described here and previously (14). Finally, a detailed characterization of the responses of a series of CYP genes to PB and TCPOBOP showed distinct dose dependencies and time courses (12). These studies, which were consistent with earlier indications of differences in PB and TCPOBOP responses (see, e.g., reference 11), led to the suggestion that different mechanisms were necessary to account for the effects of the two compounds (12).
We are left with the rather surprising possibility that xenobiotics may regulate CAR activity through at least two distinct mechanisms. In hepatocytes, TCPOBOP, PB, and other compounds cause a cytoplasmic-to-nuclear translocation that is sufficient to activate expression of CYP2B and other target genes (16). However, the results described here demonstrate that TCPOBOP and perhaps other compounds can act directly as conventional agonists to increase CAR transactivation above the constitutive level. This additional activity should lead to increased induction of some target genes and could result in response of additional targets. The distinction between these two mechanisms is highlighted by our inability to obtain evidence for direct agonist activity for PB itself, even at a concentration (1 mM [approximately 5 × 104 times higher than that of the EC50 for TCPOBOP]) that is sufficient to induce both nuclear localization and CYP2B10 expression in primary hepatocytes (16). It will be necessary to individually survey the numerous and diverse members of the PB-like class for agonist and translocation effects.
Finally, the results of the mutational analysis of ligand binding of CAR have important implications for both the specific mechanisms of CAR transactivation and the actions of orphan receptors in general. It has been difficult to distinguish between quite different models in which the constitutive activities of CAR and other nuclear receptor superfamily members either are based on an unidentified ubiquitous agonist or are truly ligand independent. The observation that the helix 3 mutations block both TCPOBOP and androstane effects on CAR but leave its constitutive activity essentially unaffected argues strongly for a ligand-independent basis for the constitutive activity in this case. If the hypothetical ubiquitous agonist exists, it must interact with the CAR LBD in a manner distinct from that of either the known agonist or the inverse agonist. Several orphan receptors, such as COUP-TF, NGFI-B, and their relatives, have hydrophobic residues at positions that align well with those mutated in CAR and could also contact their putative ligands. It will be interesting to determine whether similar mutagenesis studies lead to similar conclusions regarding the apparently constitutive actions of these orphans.
ACKNOWLEDGMENTS
We thank S. Kliewer for the ER6-TK-CAT and DR3-TK-CAT constructs and H. Gelboin for the CYP2B6 monoclonal antibody 49-10-20.
This work was supported by NIH grant R01 DK46546 to D.D.M. E.G.S. was supported by R01 ES08658 and R01 GM60346.
REFERENCES
- 1.Baes M, Gulick T, Choi H-S, Martinoli M G, Simha D, Moore D D. A new orphan member of the nuclear receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci USA. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter C M, Ong E S, Evans R M. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brzozowski A M, Pike A C, Dauter Z, Hubbard R E, Bonn T, Engstrom O, Ohman L, Greene G L, Gustafsson J A, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 5.Choi H S, Chung M, Tzameli I, Simha D, Lee Y K, Seol W, Moore D D. Differential transactivation by two isoforms of the orphan nuclear hormone receptor CAR. J Biol Chem. 1997;272:23565–23571. doi: 10.1074/jbc.272.38.23565. [DOI] [PubMed] [Google Scholar]
- 6.Ekins S, Vandenbranden M, Ring B J, Gillespie J S, Yang T J, Gelboin H V, Wrighton S A. Further characterization of the expression in liver and catalytic activity of CYP2B6. J Pharmacol Exp Ther. 1998;286:1253–1259. [PubMed] [Google Scholar]
- 7.Forman B M, Tzameli I, Choi H S, Chen J, Simha D, Seol W, Evans R M, Moore D D. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 8.Forman B M, Umesono K, Chen J, Evans R M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 9.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto H, Toide K, Kitamura R, Fujita M, Tagawa S, Itoh S, Kamataki T. Gene structure of CYP3A4, an adult-specific form of cytochrome P450 in human livers, and its transcriptional control. Eur J Biochem. 1993;218:585–595. doi: 10.1111/j.1432-1033.1993.tb18412.x. [DOI] [PubMed] [Google Scholar]
- 11.Heubel F, Reuter T, Gerstner E. Differences between induction effects of 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene and phenobarbitone. Biochem Pharmacol. 1989;38:1293–1300. doi: 10.1016/0006-2952(89)90336-5. [DOI] [PubMed] [Google Scholar]
- 12.Honkakoski P, Auriola S, Lang M A. Distinct induction profiles of three phenobarbital-responsive mouse liver cytochrome P450 isozymes. Biochem Pharmacol. 1992;43:2121–2128. doi: 10.1016/0006-2952(92)90170-n. [DOI] [PubMed] [Google Scholar]
- 13.Honkakoski P, Negishi M. Protein serine/threonine phosphatase inhibitors suppress phenobarbital-induced Cyp2b10 gene transcription in mouse primary hepatocytes. Biochem J. 1998;330:889–895. doi: 10.1042/bj3300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kliewer S A, Moore J T, Wade L, Staudinger J L, Watson M A, Jones S A, McKee D D, Oliver B B, Willson T M, Zetterstrom R H, Perlmann T, Lehmann J M. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee S S, Pineau T, Drago J, Lee E J, Owens J W, Kroetz D L, Fernandez-Salguero P M, Westphal H, Gonzalez F J. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann J M, McKee D D, Watson M A, Willson T M, Moore J T, Kliewer S A. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Investig. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangelsdorf D J, Evans R M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 21.Nolte R T, Wisely G B, Westin S, Cobb J E, Lambert M H, Kurokawa R, Rosenfeld M G, Willson T M, Glass C K, Milburn M V. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 22.Quattrochi L C, Mills A S, Barwick J L, Yockey C B, Guzelian P S. A novel cis-acting element in a liver cytochrome P450 3A gene confers synergistic induction by glucocorticoids plus antiglucocorticoids. J Biol Chem. 1995;270:28917–28923. doi: 10.1074/jbc.270.48.28917. [DOI] [PubMed] [Google Scholar]
- 23.Rao M S, Reddy J K. Peroxisome proliferation and hepatocarcinogenesis. Carcinogenesis. 1987;8:631–636. doi: 10.1093/carcin/8.5.631. [DOI] [PubMed] [Google Scholar]
- 24.Renaud J P, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378:681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- 25.Schuetz E G, Brimer C, Schuetz J D. Environmental xenobiotics and the antihormones cyproterone acetate and spironolactone use the nuclear hormone pregnenolone X receptor to activate the CYP3A23 hormone response element. Mol Pharmacol. 1998;54:1113–1117. doi: 10.1124/mol.54.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiau A K, Barstad D, Loria P M, Cheng L, Kushner P J, Agard D A, Greene G L. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 27.Smith G, Henderson C J, Parker M G, White R, Bars R G, Wolf C R. 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene, an extremely potent modulator of mouse hepatic cytochrome P-450 gene expression. Biochem J. 1993;289:807–813. doi: 10.1042/bj2890807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 29.Tzameli I, Zannis V I. Binding specificity and modulation of the ApoA-I promoter activity by homo- and heterodimers of nuclear receptors. J Biol Chem. 1996;271:8402–8415. doi: 10.1074/jbc.271.14.8402. [DOI] [PubMed] [Google Scholar]
- 30.Wagner R L, Apriletti J W, McGrath M E, West B L, Baxter J D, Fletterick R J. A structural role for hormone in the thyroid hormone receptor. Nature. 1995;378:690–697. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- 31.Waxman D J. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- 32.Waxman D J, Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem J. 1992;281:577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams S P, Sigler P B. Atomic structure of progesterone complexed with its receptor. Nature. 1998;393:392–396. doi: 10.1038/30775. [DOI] [PubMed] [Google Scholar]
- 34.Willy P J, Mangelsdorf D J. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR. Genes Dev. 1997;11:289–298. doi: 10.1101/gad.11.3.289. [DOI] [PubMed] [Google Scholar]
- 35.Willy P J, Umesono K, Ong E S, Evans R M, Heyman R A, Mangelsdorf D J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 36.Xu H E, Lambert M H, Montana V G, Parks D J, Blanchard S G, Brown P J, Sternbach D D, Lehmann J M, Wisely G B, Willson T M, Kliewer S A, Milburn M V. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 37.Zelent A, Mendelsohn C, Kastner P, Krust A, Garnier J-M, Ruffenach F, Leroy P, Chambon P. Differentially expressed isoforms of the mouse retinoic acid receptor beta are generated by usage of two promoters and alternative splicing. EMBO J. 1991;10:71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]