Abstract
COVID-19 is a major problem with an increasing incidence and mortality. The discovery of Volatile Organic Compounds (VOCs) based on breath analysis offers a reliable, rapid, and affordable screening method. This study examined VOC-based breath analysis diagnostic performance for SARS-COV-2 infection compared to RT-PCR. A systematic review was conducted in 8 scientific databases based on the PRISMA guideline. Original English studies evaluating human breaths for COVID-19 screening and mentioning sensitivity and specificity value compared to RT-PCR were included. Six studies were included with a total of 4093 samples from various settings. VOCs-based breath analysis had the cumulative sensitivity of 98.2% (97.5% CI 93.1%−99.6%) and specificity of 74.3% (97.5% CI 66.4%−80.9%). Subgroup analysis on chemical analysis (GC-MS) and pattern recognition (eNose) revealed higher sensitivity in the eNose group. VOC-based breath analysis shows high sensitivity and promising specificity for COVID-19 public screening.
Keywords: COVID-19, screening, diagnosis, volatile organic compound; breath analysis, breath-testing
Introduction
The COVID-19 pandemic has been a major world health problem with more than 230 million confirmed cases and 4 million deaths worldwide by September 26, 2021[1] (2021). COVID-19, a disease caused by the SARS-CoV-2 virus, is highly infectious, and can be transmitted through various modes (Long et al., 2020, McArthur et al., 2020). Therefore, WHO highly recommends a consistent, and mass-scaled screening approach to contain the pandemic (WHO, 2020).
Currently, COVID-19 screening and diagnosis rely on RT-PCR on nasopharyngeal and oropharyngeal swabs as the gold standard. Despite its high sensitivity and specificity, RT-PCR requires sophisticated facilities, complex, time-consuming procedures, and trained staff. Moreover, being an invasive sampling method, obtaining clinical samples is considered to be uncomfortable for the recipients (Berna et al., 2021). Hence, it is considered unsuitable for a rapid and large-scale diagnosis (Wintjens et al., 2020). Therefore, a more affordable and applicable screening and diagnosis method is currently needed for successful screening strategies (Kurstjens et al., 2020).
The interaction between pathogenic viruses in respiratory tract infection and the body's microenvironment produces distinctive volatile organic compounds (VOCs) (Broza et al., 2015, Chen et al., 2020). VOCs are gaseous molecules released as a degradation product of metabolic processes in the body whose composition changes directly as a result of pathologic processes, such as an infection or a malignancy (Haick et al., 2014).
These VOCs could be obtained through exhalation and further tested using 2 basic methods, namely examination of chemical compounds such as gas-chromatography and mass-spectrometry (GC-MS) which is capable of analyzing individual compounds or using an electronic nose (eNose) with pattern-recognition of chemical compounds using multivariate analysis (Boots et al., 2015, Farraia et al., 2019). Previous studies have shown the typical presence of VOCs in the early stages of infection and differ between patients with certain diseases from lung infections to cancer (Ahmed et al., 2017, Traxler et al., 2018, Broza et al., 2019, Ratiu et al., 2020, van Keulen et al., 2020). These findings support the development of a VOC-based COVID-19 screening. This technique appears as a promising alternative that provides a simple, fast, non−invasive, point-of-care diagnostic tool that can be used for mass screening, and ultimately optimizes COVID-19 control strategies (Lamote et al., 2020). However, the evidence regarding the effectiveness of VOC-based breath analysis for the diagnosis of COVID-19 has not been systematically reviewed. Therefore, this study aimed to analyze the diagnostic performance of VOC-based breath analysis for COVID-19 screening in comparison to RT-PCR.
Materials & methods
This systematic review was presented following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) protocol (Moher et al., 2009).
Search strategy
The literature search was performed in 8 databases: PubMed, ScienceDirect, Cochrane, ProQuest, EBSCOhost, Wiley, SpringerLink for published articles, and MedXRiv for preprints articles up to February 21, 2021. Search terms were related to electronic nose, volatile organic compound, VOC, breath test, breath analysis, SARS-CoV-2, and COVID-19 with other related MeSH terms.
Eligibility criteria
Prospective or retrospective cohorts, cross-sectional, and case-control studies of human breath analysis in suspected or confirmed COVID-19 patients, of which evaluated the sensitivity and specificity of VOCs-based breath analysis (index test) for COVID-19 compared to RT-PCR test (reference test), were viewed as eligible. Studies were excluded if any of the accompanying attributes were found: (1) Review articles, case series, or letters to editors; (2) in-vitro studies without clinical examples; (3) studies not reporting diagnostic evaluation.
Study selection
All literature search results were assessed autonomously by 3 investigators, such as screening of titles and abstracts based on eligibility criteria. In case of uncertainty or unclear studies, it was incorporated for the next selection stage, and was discussed openly. Subsequently, the full text was reviewed to exclude ineligible studies. All included studies were validated by all investigators.
Data extraction
Included studies were extracted, such as study characteristics, the sensitivity, specificity, true positives (TP), false negatives (FN), false positives (FP), and true negatives (TN), either directly provided from sources or calculated from the available data. All results were confirmed by the fourth investigator to ensure objectivity and avoid bias among previous investigators.
Quantitative analysis
Diagnostic values from each index test were compared to RT-PCR. Specifically, sensitivity, specificity, TP, FN, FP, and TN were included for quantitative analysis. Meta-analysis in MetaDTA software (University of Leicester, Leicester, England) used random effect models with 95% confidence interval and extrapolated them into tables and forest plots. Sub-group analysis was performed to discriminate between chemical analysis and pattern analysis of VOC.
Quality assessment
Three review authors independently assessed methodological quality using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) (Whiting et al., 2011). QUADAS-2 examines 4 regions of bias: patient selection, index test, reference standards, and flow/timing. The quality assessment results were then classified into low, unclear, or high risk of bias, and extrapolated into charts from the Review Manager (RevMan 5.4.1). The research model of each study was scored by the TRIPOD statement (Collins et al., 2015).
Results
Study selection
The literature search process was summarized in Fig. 1 . Seventy-eight articles were obtained from the initial electronic search, with a total of 6 articles finally included through the study selection process. Studies were excluded because they only listed elevated VOCs in COVID-19 patients’ breath, without analyzing diagnostic performance compared to RT-PCR. Authors excluded studies from which data to calculate sensitivity or specificity could not be extracted.
Fig. 1.
Literature search flowchart.
Patients’ characteristics
Six articles were included with a total of 4093 participants. Patients’ recruited in the studies are individuals with suggestive symptoms of COVID-19 (Wintjens et al., 2020, Grassin-Delyle et al., 2021, Ruszkiewicz et al., 2020, de et al., 2021); acute respiratory distress syndrome (ARDS) patients requiring mechanical ventilation (Grassin-Delyle et al., 2021); COVID-19 patients confirmed with RT-PCR (Berna et al., 2021, Shan et al., 2020), computed tomography (CT), and laboratory (antibody test) (Shan et al., 2020). The study participants were then grouped into 2 based on their COVID-19 status (positive or negative).
Three studies (Ruszkiewicz et al., 2020, de et al., 2021, Shan et al., 2020) classified their subjects into specific sets to provide thorough diagnostic evaluation. Ruszkiewicz et al (2020) categorized their study participants based on geographic locations (Ruszkiewicz et al., 2020). Vries et al. (2021) divided their studies into 4 sets: training set, validation set, replication set, and asymptomatic set (de et al., 2021). Shan et al. (2020) performed their research on 2 episodes of the training set and testing set, on 3 separate groups of COVID-19 patients, healthy controls, and non−COVID-19 lung infection. The testing set provides a comparison and validates the training set (Shan et al., 2020).
Index and reference tests characteristics
Methods used to detect VOCs vary between studies, as summarized in Table 1 , with some already used patented breath analyzing devices. Three studies analyzed chemical constituents of exhaled breath using GC-MS breath analysis (Berna et al., 2021, Grassin-Delyle et al., 2021, Ruszkiewicz et al., 2020). Three other studies made use of the electronic nose with a pattern recognition method (Wintjens et al., 2020, de et al., 2021, Shan et al., 2020). Regarding the reference test, all studies used RT-PCR as the gold standard for COVID-19 diagnosis.
Table 1.
Included studies characteristics.
| Studies, y | Location | Settings | Design | Sample size | COVID-19 +ve | COVID-19 -ve | Sample population | Index Test | Reference Test | Sample Collection |
|---|---|---|---|---|---|---|---|---|---|---|
| Wintjens et al., 2020 | Maastricht, Netherlands | Hospital | prospective, proof-of-principle cohort | 219 | 57 | 162 | Outpatient, clinic employees with COVID-19 symptom | Aeonose, (The Aeonose Company, Zutphen, the Netherlands | RT-PCR | Participants breathed for 5 consecutive min through a disposable mouthpiece containing both a carbon filter and a high-efficiency particulate air (HEPA) filter to prevent contamination of the internal tubing |
| Berna, et al 2020 | Pennsylvania, USA | Hospital | cross sectional | 25 | 10 | 15 | Children confirmed positive/negative by NP RT-PCR | Three-bed Universal sorbent tubes containing Tenax, Carbograph, and Carboxen | NP RT-PCR | SARS-CoV-2-infected and -uninfected subjects exhaled through a disposable cardboard mouthpiece connected to a chamber. |
| Grassin-Delyle 2020 | Garches, France | Hospital | prospective observational study | 40 | 28 | 12 | ARDS patients, requiring invasive mechanical ventilation | proton-transfer-reaction quadrupole time-of-flight mass spectrometer | RT-PCR | Heated transfer line connected directly to the end of endotracheal tube |
| Vries et al 2020 | Amsterdam | Public | Prospective case control | 1948 | 1718 | 230 | Early symptoms suggestive of COVID-19 and/or who had been in contact with someone diagnosed with COVID-19 | cloud-connected eNose (SpiroNose®) | RT-PCR | Exhaled breathing during nasopharyngeal swab |
| Ruszkiewicz et al., 2020 | Edinburgh, UK, and Dortmund, Germany | Hospital | observational prospective case control | 65 | 55 | 10 | Emergency patient or outpatient clinic; respiratory symptoms; possible COVID-19. | GC-IMS (BreathSpec, G.A.S. Dortmund) | RT- qPCR | single breath-sample (forced exhale) with a single use sampling device |
| Shan et al., 2020 | Wuhan, China | Hospital | case-control | 69 | 30 | 39 | COVID-19 patients; confirmed by CT, nasal and pharyngeal swab specimens RT-PCR, and antibody tests | Breathalyzer (Nanovation, Israel) | RT-PCR | Breath samples were collected by the study subjects breathing directly into the aperture of the instrument for at least 4 seconds, keeping the instrument approximately 1–2 cm from the mouth. |
Sample collection
Wintjens (2020) utilized Aeonose (The Aeonose Company, Netherland) which required the participants to breathe for 5 consecutive minutes through a disposable mouthpiece containing a carbon filter and a high-efficiency particulate air (HEPA) (Wintjens et al., 2020). Using Breathalyzer (Nanovation, Israel), Shan (2020) collected the breath samples by requiring the subjects to breathe directly into an aperture of the instrument for at least 4 seconds (Shan et al., 2020). SpiroNose was used by Vries et al (de et al., 2021). Another device, MCC-IMS from STEP Sensortechnik, used by Ruszkiewicz et al requires patients to exhale slowly for 10 seconds into a foam cuffed oxygen catheter via filter (Ruszkiewicz et al., 2020). Berna et al used a 3-bed Universal sorbent tube and the patients were required to exhale through a disposable cardboard mouthpiece connected to a chamber (Berna et al., 2021). Using a more complex, proton-transfer-reaction quadrupole time-of-flight mass spectrometer, Grassin-Delyle et al collected the samples through a heated transfer line connected directly to the end of an endotracheal tube (Grassin-Delyle et al., 2021).
Breathe analysis
Six candidate breath biomarkers were significantly elevated in the breath of children with SARS-CoV-2 infection: 3 aldehydes (octanal, nonanal, and heptanal), as well as decane, tridecane, and 2-pentyl furan (Berna et al., 2021). Grassin-Delyle et al. (2021) identified elevated substances in COVID-19 suspected patients, such as methylpent-2-enal, 2,4-octadiene 1-chloroheptane, and nonanal (Grassin-Delyle et al., 2021). In the study by Ruszkiewicz et al. (2020), breath analysis identified aldehydes (ethanal, octanal), ketones (acetone, butanone), and methanol that discriminated COVID-19 from other conditions (Ruszkiewicz et al., 2020).
Risk of bias assessment
Methodological quality assessment was summarized in Fig. 2 . All studies returned unclear risk of bias for inpatient selection, as there was an unclear issue whether each study had avoided case-control design. No studies reported inappropriate exclusion. In the scope of the index test, 4 studies reported a high risk of bias, and 2 others of unclear risk. All studies did not mention if the breath analysis was interpreted independently to RT-PCR result. Also, all studies did not determine a pre-specified threshold, as the use of VOCs-based breath analysis for COVID-19 screening was still under primary development, and the breath biomarkers for COVID-19 had not been established. Two studies (de et al., 2021, Shan et al., 2020) later mentioned proposed cut off values based on their trials. Regarding the reference standard, only one study (Wintjens et al., 2020) returned a high risk of bias as the authors stated low sensitivity of RT-PCR procedure, leading to false-negative results which solved by antibody tests. The reference standards interpretation should be blinded to breathe analysis. In the aspect of flow and timing, a study (de et al., 2021) with several sets was at risk of dropout since the participants were contacted after a while for additional tests.
Fig. 2.
QUADAS-2 assessment on the risk of bias and concerns of applicability.
Based on the TRIPOD model development (Collins et al., 2015), most studies reported on validation of published model within the population, consisting of type III using 1 data set (Berna et al., 2021, Wintjens et al., 2020, Grassin-Delyle et al., 2021, Shan et al., 2020). One study used several datasets for external validation (Vries et al., 2021). One study reported model development and validation (type IIb and III) in 2 different non−randomized datasets (Ruszkiewicz et al., 2020).
Study outcome
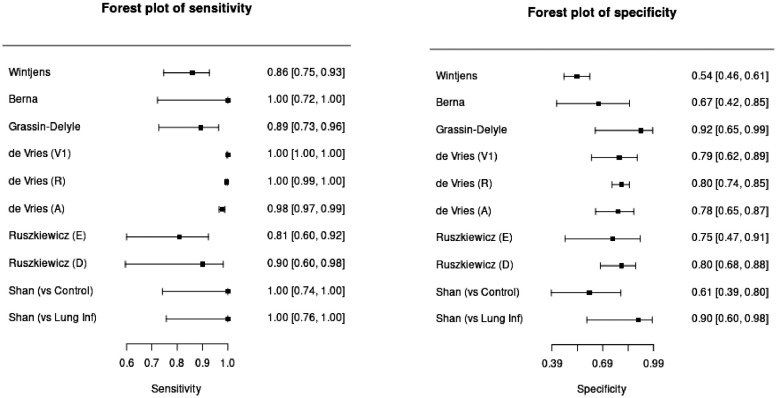
Study outcomes are extracted to calculate sensitivity and specificity ( Table 2 ). Validation, replication, and asymptomatic sets results were included in the pooled analysis. Regarding the sensitivity value, almost all studies showed a high degree of sensitivity exceeding 80% for all studies, ranging from 81% to 100%, with 2 studies having a 100% sensitivity value (Berna et al., 2021; Shan et al., 2020). Specificity values were ranging from 53.7% to 94%. Out of all 6 studies, 3 studies had a specificity value of over 80% (Grassin-Delyle et al., 2021, Ruszkiewicz et al., 2020, de et al., 2021, Shan et al., 2020).
Table 2.
Study outcomes.
| Studies, y | Annotation | True Positive (TP) | False Negative (FN) | False Positive (FP) | True Negative (TN) | Total Sample | Sensitivity (Sn; %) | Specificity (Sp; %) |
|---|---|---|---|---|---|---|---|---|
| Wintjens et al. 2020 | N/A | 49 | 8 | 75 | 87 | 219 | 86 | 53.7 |
| Berna et al 2020 | N/A | 10 | 0 | 5 | 10 | 25 | 100 | 66.6 |
| Grassin-Delyle et al 2020 | N/A | 25 | 3 | 1 | 11 | 40 | 90 | 94 |
| Vries et al 2020 | Validation Set | 871 | 0 | 7 | 26 | 904 | 100 | 78.8 |
| Replication Set | 1711 | 7 | 46 | 184 | 1948 | 99.6 | 80 | |
| Asymptomatic Set | 689 | 15 | 11 | 39 | 754 | 97.9 | 78 | |
| Ruszkiewicz et al 2020 | Edinburgh Set | 17 | 4 | 3 | 9 | 33 | 81 | 75 |
| Dortmund Set | 9 | 1 | 11 | 44 | 65 | 90 | 80 | |
| Shan et al 2020 | COVID-19 vs Control Testing Set | 11 | 0 | 7 | 11 | 29 | 100 | 61.1 |
| COVID-19 vs Lung Infection Testing Set | 12 | 0 | 1 | 9 | 22 | 100 | 90 |
In general, all studies yielded similar results in sensitivity value with a slight difference in the specificity among the sets. In Vries et al (2020), the high sensitivity value of the validation test was confirmed by both the replication, and asymptomatic set. However, the specificity was considered to be lower in the asymptomatic set (de et al., 2021). Shan et al. (2020) reported that VOCs based breath analysis yielded a perfect performance of sensitivity in both population control of healthy and other lung infection participants. It successfully discriminated SARS-CoV-2 infection among the other lung infections (Shan et al., 2020).
Meta-analysis of sensitivity and specificity
Pooled analysis of 6 included studies was extrapolated into forest plots (Fig. 3 ) to visualize the sensitivity and specificity of VOC-based breath analysis on each study and its significance. Cumulative outcomes were summarized in Table 3 with sensitivity and specificity values of 98.2% (97.5% CI 93.1%−99.6%) and 74.3% (97.5% CI 66.4%−80.9%) respectively. Subgroup analysis on chemical analysis (GC-MS) and pattern recognition (eNose) revealed cumulative sensitivity of 88.4% (97.5% CI 78.5%−94.1%) and 99.1% (97.5% CI 95.3%−99.8%) respectively, with specificity value of 78.7% (97.5% CI 69.3%−85.8%), and 74.6% (97.5% CI 63.6%−83.2%). The result has shown higher sensitivity in the pattern recognition (eNose) tool while GC-MS offers higher specificity in diagnosing SARS-COV-2 infection. The positive and predictive value of each test was also calculated by assuming the prevalence value of 2.91% for COVID-19 based on the WHO COVID-19 Dashboard (2021).
Fig. 3.
Forest plot of sensitivity and specificity of VOC-based breath analysis on included studies.
Table 3.
Pooled analysis outcome of meta-analysis on 6 studies, summarized in this table with sensitivity, specificity, false positive rate, logit of sensitivity and specificity, positive predictive value, and negative predictive value.
| Parameter | VOC vs RT-PCR (97.5% CI) | GC-MS Breath Analysis (97.5% CI) | Pattern Recognition Breath Analysis (97.5% CI) |
|---|---|---|---|
| Sensitivity | 98.2% (93.1%−99.6%) | 88.4% (78.5%−94.1%) | 99.1% (95.3%−99.8%) |
| Specificity | 74.3% (66.4%−80.9%) | 78.7% (69.3%−85.8%) | 74.6% (63.6%−83.2%) |
| logit(sensitivity) | 4.008 (2.602−5.413) | 2.031 (1.294−2.768) | 4.701 (3.002−6.401) |
| logit(specificity) | 1.062 (0.68−1.445) | 1.308 (0.814−1.802) | 1.08 (0.556−1.603) |
| False Positive Rate | 25.7% (19.1%−33.6%) | 21.3% (14.2%−30.7%) | 25.4% (16.8%−36.4%) |
| Positive Predictive Value | 10.28% | 11.06% | 10.47% |
| Negative Predictive Value | 99.93% | 99.56% | 99.96% |
Predictive value is calculated with assumption of COVID-19 prevalence of 2.91% based on the recent COVID-19 report (WHO COVID-19 Dashboard) as per September 2021.
Discussion
This is the first systematic review and meta-analysis examining the diagnostic performance of VOCs-based breath analysis for diagnosing SARS-CoV-2 infection, according to the authors’ latest search. Currently available studies of VOC-based breath analysis provided a vast array of variables ranging from different populations, analytical methods, and settings. The included studies utilized various products of breath analyzer as the index test and RT-PCR as the reference standard. Cumulative results were calculated from pooled outcomes of 6 included studies. Sensitivity value is reported to be at 98.2% (97.5% CI 93.1%−99.6%). This high sensitivity value supports the potential of this screening method to identify more people with COVID-19 and fewer missing ones. In studies with several sets, both 2 studies consistently reported high sensitivity among all sets, and varied participant characteristics (de et al., 2021, Shan et al., 2020). Although all studies reported variety in specificity value, VOCs based breath analysis showed a moderately satisfying performance to distinguish non−SARS-COV-2 infections, with the cumulative specificity value of 74.3% (97.5% CI 70.3%−83.9%). Concerning the current conventional diagnostic tool of the COVID-19 rapid antigen test, the minimum threshold is at 90% sensitivity, and 97% specificity (European Commission Directorate-General for Health and Food, 2021). Although the specificity value did not meet the requirement, it is considered to be acceptable as a screening method since false-positive results will later be confirmed by the gold standard. Quoting Shan et al (Shan et al., 2020), the decision to delay a plane passenger for a confirmatory test is better than permitting him to get on the aircraft with the risk of infecting 300 passengers.
Subgroup analysis for the use of analytical platforms and pattern recognition (eNose) groups aimed to examine the diagnostic value of each method. The analysis had shown a higher sensitivity in the pattern recognition group, while the GC-MS group showed higher specificity. However, the value might not differ on a major scale. The analysis however had shown high potential of pattern recognition breath analysis for screening diagnostic tests.
The Positive (PPV) and negative predictive value (NPV) of each respective tests have shown essential clinical aspects for applicable interpretation. Both tests yielded more than 99% NPV, which can be interpreted to have a high predictive value in predicting non−infected patients out of the negative test result. This value is applicable to reassure both population and healthcare facilities in ruling out SARS-CoV-2 infection in patients. However, PPV of around 10% in VOC analysis has underlined the need for a confirmatory test to the positive result. VOCs-based breath analysis can sensitively detect SARS-CoV-2 infection due to its high sensitivity. It is an important trait for early detection and screening, especially in community settings with a high transmission rate. However, COVID-19 should not be diagnosed solely by the breath analysis result. Conventional confirmatory tests are still needed to rule out infections. Hence, in general, it can be safely assumed that VOCs-based breath analysis shows a good performance with potentially high diagnostic value for public health COVID-19 screening.
Besides testing patients with suggestive symptoms of SARS-CoV-2 infection, detecting infection in an asymptomatic population is also crucial to manage and contain the disease transmission (Ruszkiewicz et al., 2020). Screening for SARS-CoV-2 takes place in various settings, such as the first presentation to health care services, and in the public facilities which are prone to community transmission. The urgency of mass screening relies on the fact that not only infected patients have an increased risk of developing adverse outcomes, but the asymptomatic ones might also form a risk for hospital workers particularly during procedures generating aerosols, and for other nearby people in public (Berna et al., 2021, Wintjens et al., 2020, Ruszkiewicz et al., 2020).
This study reveals the potential of breath testing to screen SARS-CoV-2 infection. Respiratory viral infections induce metabolic changes and lead to VOC profile changes. Hence, infection-associated volatile organic compounds (VOCs) are potentially applied to develop noninvasive diagnostic tools by sensor arrays or electronic noses (Berna et al., 2021). Previously, VOCs have been proven to successfully detect, not only malignancies, but also bacterial (E. coli, M. tuberculosis), fungal (Aspergillus spp.), and viral infections (influenza-A, rhinovirus) (Wintjens et al., 2020, Grassin-Delyle et al., 2021, Steppert et al., 2021). Sethi et al. compiled the use of VOC-based breath analysis to detect several respiratory infectious diseases. Even though the samples were taken from deep alveolar breaths and discarding the air volume from the upper respiratory tract, which might seem quite broad; and were analyzed by varying equipment; the results still aligned with each other and were able to differentiate healthy controls to M. tuberculosis, P. aeruginosa, A. fumigatus infections (Sethi et al., 2013).
Studies on COVID-19 breath analysis were able to indicate COVID-19 status. Several gaseous components have been markedly elevated in the exhaled breath COVID-19 patients compared to healthy controls, which are potentially used as biomarkers. COVID-19 diagnosed patients breath shows a greater amount of aldehydes: methylpent-2-enal (Grassin-Delyle et al., 2021), octanal and nonanal, in comparison to patients with other acute respiratory illnesses, such as COPD, and pneumonia (Berna et al., 2021, Ruszkiewicz et al., 2020). Chen et al (Chen et al., 2020) revealed higher levels of ethyl butanoate from COVID-19 patients compared to controls and lung cancer patients. Moreover, the isopropanol component is distinctively higher, up to 100-fold difference in COVID-19 patients’ breath. Acetone levels were lower in COVID-19 patients than in other subjects. On the other hand, non−COVID-19 infections (other upper respiratory tract infections) yielded markedly higher volatiles amount of butyraldehyde and isopropanol (Chen et al., 2020). The concentration of VOCs did not represent illness severity and was not correlated with viral load. Hence, the exhaled breath roled as a SARS-CoV-2 infection per se (Grassin-Delyle et al., 2021).
These distinct breath borne VOCs characteristics, which can be recognized by both analytical method and electronic nose, can be considered to support screening and alert the presence of COVID-19 patients. Specific unique receptors for cell entry of virus (ACE-2 receptor for SARS-CoV-2) leads to distinct downstream pathways inside infected cells, which are followed by the release of virus-specific VOCs even at the disease's early stage. Despite unspecific initial symptoms of SARS-CoV-2 infection, which look alike to other viral respiratory infections, COVID-19 is characterized by its ability to develop systemic complications, and to induce elevated cytokine release. Therefore, these interactions lead to distinct VOC patterns to discriminate SARS-CoV-2 from other viral respiratory infections (de et al., 2021). Some organic compounds appeared as a sub-product of cell membrane destruction due to oxidative stress reactive oxygen species (ROS) which were generated by various types of inflammatory, immune, and structural cells in the airway in SARS-CoV-2 pathogenesis (Berna et al., 2021).
VOC-based breath analysis showed high sensitivity and specificity which is vital for a screening test in the attempt to control transmission (de et al., 2021). This method eliminated the need for deep sampling (Wintjens et al., 2020) and replaced it with a non−invasive and painless sampling method, being more feasible to be performed in critically ill, pediatric, and geriatric patients (Sethi et al., 2013). Strategies based on mass spectrometry analysis can identify all compounds in breath samples, being valuable for pathophysiologic research. However, these strategies are time-consuming, costly, and depend on a properly skilled operator which makes them unconventional to be implemented in clinical settings (Farraia et al., 2019).
Electronic nose (eNose) has the potential to overcome these impediments as they are moderately affordable, support rapid and real-time analysis, and eliminate the need for specific personnel (Wintjens et al., 2020, de et al., 2021). It uses pattern recognition and identifies different subjected odours by comparing them with previously learnt patterns (Farraia et al., 2019). When a chemical input of exhaled breath is subjected to eNose, it will induce physical change in the sensors which are subsequently detected by transducers, and converted into an electrical signal-producing a unique signature or breath print (Farraia et al., 2019). eNose is expected to detect even minor VOCs composition changes (Wintjens et al., 2020, Shan et al., 2020). To attain this goal, it is fundamental to equip a prediction model with a training set of samples and external validation for further research (Farraia et al., 2019). Prospectively, a cloud-connected breath analysis integrated with artificial intelligence, and a large database could diagnose COVID-19 within seconds (de et al., 2021). In short, VOC-based breath analysis could potentially be used as the point-of-care screening tool for COVID-19 in public setting before secondary confirmatory molecular tests.
Conclusion
COVID-19 patients showed a distinct pattern of VOCs. Through many studies, VOC-based breath analysis is having high sensitivity and negative predictive value, supported with the rapid and more feasible procedure, yielding a high potential for COVID-19 screening in public settings. In the future, VOC-based breath analysis can be integrated with cloud databases, and artificial intelligence as a promising point-of-care COVID-19 mass-scaled screening.
Study limitations
Due to the novelty of the topic discussed, there are limited resources of studies, and evidence regarding the evaluation of VOC-based breath analysis for COVID-19 screening. The currently available studies were considered to be preliminary researches to examine breath analysis for COVID-19 screening. As a result, the included studies showed a wide heterogeneity in some aspects, such as varied population, and patients characteristics. Patients recruited in studies were diverse, from asymptomatic people, patients with suggestive symptoms of COVID-19, people having close contact history with COVID-19 confirmed patients, to critically ill ARDS patients needing ventilators. An extreme variation could also be seen in sample age, ranging from pediatric patients to elderly subjects, which may correlate with different clinical manifestations. Also, the use of analytical methods and various electronic nose products may give different performances of breath analysis, which may introduce bias. The breath sampling technique was varied between each study and a study instructed the participants with specific preparation before obtaining the sample. Variations between the use of real-time and stored breath samples may cause bias. Furthermore, the usage of different artificial intelligence methods for pattern recognition features in electronic noses may lead to different performances between the eNose. Subsequently, the threshold, and specific biomarkers of SARS-CoV-2 infection had not been established. Therefore, each study may report its own cut off value and propose some volatile compounds which potentially be used as markers to detect COVID-19 patients.
Declaration of competing interests
The authors declare there is no conflict of interest.
Funding
This paper was not supported by any funding agency nor grant.
Declaration of data availability statement
The data that support the findings of this study are available from the corresponding author, ADS, upon reasonable request.
Author's contribution
Anita Dominique Subali: Conceptualization; Methodology; Validation; Writing- Original Draft; Writing- Review & Editing; Visualization Supervision; Project Administration; Lowilius Wiyono: Methodology; Formal analysis; Investigation; Writing - Review & Editing; Visualization; Supervision; Muhammad Yusuf: Methodology; Investigation; Writing - Original Draft; Muhammad Fathi Athallah Zaky: Methodology; Investigation; Writing - Original Draft.
Footnotes
Address: Veteran Street, Malang City, East Java, Indonesia. Postal Code: 65145.
References
- Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126 doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur L, Sakthivel D, Ataide R, Chan F, Richards JS, Narh CA. Review of burden, clinical definitions, and management of Covid-19 Cases. Am J Trop Med Hyg. 2020;103(2):625–638. doi: 10.4269/ajtmh.20-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Advice for the public. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed June 16, 2021.
- WHO. WHO coronavirus (COVID-19) dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data. 2021. Available at: https://covid19.who.int/. Accessed September 26, 2021.
- Berna AZ, Akaho EH, Harris RM, Congdon M, Korn E. Reproducible breath metabolite changes in children with SARS-CoV-2 infection. ACS Infect Dis. 2021;7(9):2596–2603. doi: 10.1021/acsinfecdis.1c00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Rutjes AW, Westwood ME, Mallet S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- Wintjens AGWE, Hintzen KFH, Engelen SME, Lubbers T, Savelkoul PHM, Wesseling G, et al. Applying the electronic nose for pre-operative SARS-CoV-2 screening. Surg Endosc. 2020;35(12):6671–6678. doi: 10.1007/s00464-020-08169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurstjens S, van der Horst A, Herpers R, Geerits MWL, Kluiters-de Hingh YCM, Göttgens EL, et al. Rapid identification of SARS-CoV-2-infected patients at the emergency department using routine testing. Clin Chem Lab Med. 2020;58(9):1587–1593. doi: 10.1515/cclm-2020-0593. Accessed at: June 16, 2021. [DOI] [PubMed] [Google Scholar]
- Broza YY, Mochalski P, Ruzsanyi V, Amann A, Haick H. Hybrid Volatolomics and Disease Detection. Angew Chemie - Int Ed. 2015;54(38):11036–11048. doi: 10.1002/anie.201500153. [DOI] [PubMed] [Google Scholar]
- Chen H, Qi X, Ma J, Zhang C, Feng H, Yao M. Breath-borne VOC Biomarkers for COVID-19. MedRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.06.21.20136523v1. Accessed 21 June 2021. [Google Scholar]
- Haick H, Broza YY, Mochalski P, Ruzsanyi V, Amann A. Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev. 2014;43(5):1423–1449. doi: 10.1039/c3cs60329f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots AW, Bos LD, van der Schee MP, van Schooten FJ, Sterk PJ. Exhaled molecular fingerprinting in diagnosis and monitoring: validating volatile promises. Trends Mol Med. 2015;21(10):633–644. doi: 10.1016/j.molmed.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Farraia MV, Cavaleiro Rufo J, Paciência I, Mendes F, Delgado L, Moreira A. The electronic nose technology in clinical diagnosis: a systematic review. Porto Biomed J. 2019;4(4):e42. doi: 10.1097/j.pbj.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed WM, Lawal O, Nijsen TM, Goodacre R, Fowler SJ. Vol. 3. American Chemical Society; 2017. Exhaled volatile organic compounds of infection: a systematic review; pp. 695–710. (ACS Infectious Diseases). Available at: https://pubs.acs.org/doi/full/10.1021/acsinfecdis.7b00088. Accessed 21 April 2021. [DOI] [PubMed] [Google Scholar]
- Traxler S, Bischoff AC, Saß R, Trefz P, Gierschner P, Brock B, et al. VOC breath profile in spontaneously breathing awake swine during Influenza A infection. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-33061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broza YY, Zhou X, Yuan M, Danyao Q, Zheng Y, Vishinkin R, et al. 22nd. Vol. 119. American Chemical Society; 2019. Disease detection with molecular biomarkers: from chemistry of body fluids to nature-inspired chemical sensors.https://pubs.acs.org/doi/abs/10.1021/acs.chemrev.9b00437 (Chemical Reviews). p. 11761-817. Accessed from: Accessed 30 November 2021. [DOI] [PubMed] [Google Scholar]
- Ratiu IA, Ligor T, Bocos-Bintintan V, Mayhew CA, Buszewski B. Volatile organic compounds in exhaled breath as fingerprints of lung cancer, asthma and COPD. J Clin Med. 2020;10(1):32. doi: 10.3390/jcm10010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Keulen KE, Jansen ME, Schrauwen RWM, Kolkman JJ, Siersema PD. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment Pharmacol Ther. 2020;51(3):334–346. doi: 10.1111/apt.15622. Available at: https://pubmed.ncbi.nlm.nih.gov/31858615. Accessed 20 December 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamote K, Janssens E, Schillebeeckx E, Lapperre TS, De Winter BY, van Meerbeeck JP. Vol. 14. Institute of Physics Publishing; 2020. The scent of COVID-19: Viral (semi-)volatiles as fast diagnostic biomarkers? [Internet] (Journal of Breath Research). Available at: https://pubmed.ncbi.nlm.nih.gov/32599571/. Accessed 16 June 2021. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J J, Altman DG, Prisma Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- Grassin-Delyle S, Roquencourt C, Moine P, Saffroy G, Carn S, Heming N, et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: a pilot study. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszkiewicz DM, Sanders D, O’Brien R, Reed MJ, Riepe AC, Bailie K, et al. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry - a feasibility study. EClinicalMedicine [Internet] 2020;29–30 doi: 10.1016/j.eclinm.2020.100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries R, Vigeveno R, Mulder S, Farzan N, Vintges DR, Goeman JJ, et al. Ruling out SARS-CoV-2 infection using exhaled breath analysis by electronic nose in a public health setting. medRxiv. 2021 doi: 10.1101/2021.02.14.21251712. [DOI] [Google Scholar]
- Shan B, Broza YY, Li W, Wang Y, Wu S, Liu Z, et al. Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano. 2020;14(9):12125–12132. doi: 10.1021/acsnano.0c05657. [DOI] [PubMed] [Google Scholar]
- Steppert C, Steppert I, Sterlacci W, Bollinger T. Rapid detection of SARS-CoV-2 infection by multicapillary column coupled ion mobility spectrometry (MCC-IMS) of breath. A proof of concept study. J Breath Res. 2021;15(2) doi: 10.1088/1752-7163/abe5ca. Available at: https://iopscience.iop.org/article/10.1088/1752-7163/abe5ca. Accessed 16 June 2021. [DOI] [PubMed] [Google Scholar]
- Sethi S, Nanda R, Chakraborty T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin Microbiol Rev. 2013;26(3):462–475. doi: 10.1128/CMR.00020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission Directorate-General for Health and Food Safety. EU health preparedness: a common list of COVID-19 rapid antigen tests and a common standardised set of data to be included in COVID-19 test result certificates. Public Heal Ctry knowledge, Cris Manag Heal Secur. 2021. Available at: https://ec.europa.eu/health/sites/default/files/preparedness_response/docs/covid-19_rat_common-list_en.pdf. Accessed November 30, 2021.