Abstract
Emerging evidence suggest a possible association between immune thrombocytopenia (ITP) and some formulations of COVID-19 vaccine. We conducted a retrospective case series of ITP following vaccination with Vaxzevria ChadOx1-S (AstraZeneca) and mRNA Comirnaty BNT162b2 COVID-19 (Pfizer-BioNTech) vaccines and compare the incidence to expected background rates for Victoria during the first six months of the Australian COVID-19 vaccination roll-out in 2021. Cases were identified by reports to the Victorian state vaccine safety service, SAEFVIC, of individuals aged 18 years or older presenting with thrombocytopenia following COVID-19 vaccination without evidence of thrombosis. Twenty-one confirmed or probable cases of ITP were identified following receipt of AstraZeneca (n = 17) or Pfizer-BioNTech (n = 4) vaccines. This translates to an observed incidence of 8 per million doses for AstraZeneca vaccine, twice the expected background rate of 4.1 per million. The observed rate for Pfizer-BioNTech was consistent with the expected background rate. The median time to onset for the cases post AstraZeneca vaccination was 10 days (range 1–78) and median platelet nadir 5 × 109/L (range 0–67 × 109/L). Hospital presentations or admissions for management of symptoms such as bleeding occurred in 18 (86%) of the cases. The majority of cases (n = 11) required intervention with at least 2 therapy modalities. In conclusion, we observed a substantially higher than expected rate of ITP following AstraZeneca vaccination. ITP is the second haematological adverse event, distinct from that of thrombosis with thrombocytopenia syndrome (TTS), observed following AstraZeneca vaccination.
Keywords: Immune thrombocytopenia, Vaccination, Vaccine, COVID-19
1. Introduction
With the emergence of SARS-CoV-2 variants, such as the B.1.617.2 Delta variant, high vaccine uptake is an even more crucial component of the global pathway out of the Coronavirus disease (COVID-19) pandemic, including in Australia. The Vaxzevria ChadOx1-S (AstraZeneca) and mRNA Comirnaty BNT162b2 (Pfizer-BioNTech) COVID-19 vaccines are both integral parts of the current Australian vaccine strategy [1], and are generally well tolerated with mild, common, and expected adverse effects such as fever, fatigue, headache and myalgia [2]. However, recent studies identified Thrombosis with Thrombocytopenia Syndrome (TTS) as a rare but serious condition associated with AstraZeneca vaccine [3], [4]. Early research suggests that TTS is likely an auto-immune phenomenon and may have similar pathophysiology to heparin-induced thrombocytopenia (HIT) [3].
While TTS is a new idiosyncratic adverse event of special interest (AESI), with low platelets and associated thrombosis, another haematological condition, immune thrombocytopenia (ITP) has been closely monitored as an AESI [5], given its similar autoimmune basis, and known occurrence following the Measles-Mumps-rubella (MMR) vaccine [6], [7]. This is considered biologically plausible because ITP has also been associated with SARS-CoV-2 infection [8]. Emerging evidence suggests a causal link between ITP and COVID-19 vaccines, particularly AstraZeneca vaccine [9], [10]. Other studies have investigated ITP following mRNA vaccines and to date found no association [11], [12], [13]. In our analysis, we examine a series of reported cases of ITP following vaccination with AstraZeneca and Pfizer-BioNTech vaccines and compare these to expected background rates in Victoria, Australia.
2. Methods
In the Australian jurisdiction of Victoria [population ∼ 6.6 million] [14], adverse events following immunisation (AEFI) are spontaneously reported by patients or health-care providers to SAEFVIC, the state-wide vaccine safety service [15]. SAEFVIC comprises a central reporting enhanced passive and active surveillance system integrated with clinical services. Vaccine providers and recipients are encouraged to report AEFI following COVID-19 vaccination, regardless of vaccine brand. SAEFVIC is responsible for the follow-up of AEFI, including referral to the Victorian Specialist Immunisation Services (VicSIS), a network of COVID-19 specialist immunisation clinics, for further management as required [16]. All AEFI are also forwarded to the national regulator, the Therapeutic Goods Administration (TGA), which is responsible for pharmacovigilance and national collation of spontaneous (passive) adverse event reports [17], [18].
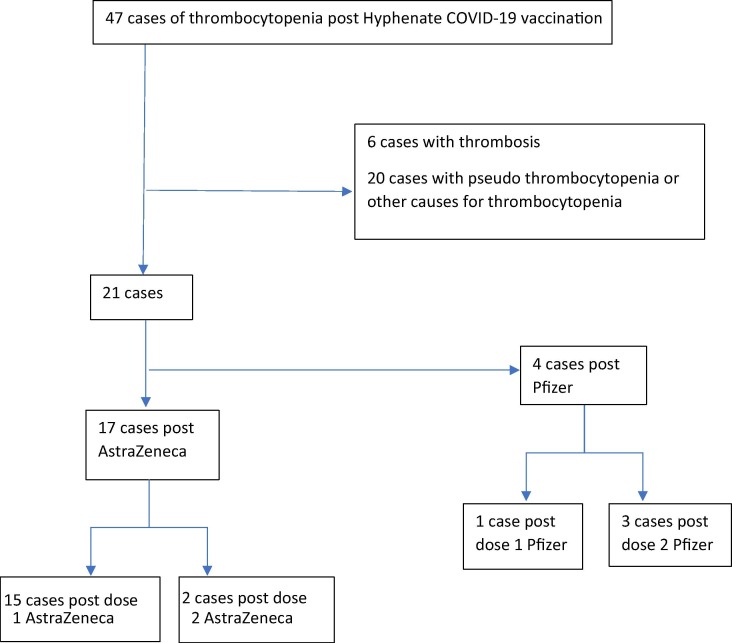
Identified reports of thrombocytopenia submitted to SAEFVIC within the first six months of the Australian COVID-19 vaccination roll-out, between 22 February and 20 August 2021, were assessed. Of the forty-seven cases of thrombocytopenia identified, 6 were excluded due to evidence suggestive of TTS and a further 15 because they either had pseudo-thrombocytopenia (defined as platelet < 150 × 109/L with clumping on specimen) or had a clear alternate diagnosis (e.g. sepsis, malignancy). Patient demographics were described by vaccine brand and dose, with clinical review undertaken of platelet nadir, time to onset, existing history of ITP and possible alternate causes, associated symptoms/signs, severity, and response to treatment for the 26 remaining cases (Fig. 1 ). Additional information was collated from treating clinicians and hospital sites.
Fig. 1.
Consort diagram.
Thrombocytopenia was defined as platelets < 150 × 109/L and Brighton Collaboration criteria utilised to allocate level of diagnostic certainty [19]. Platelet nadir was based on the lowest platelet count documented as serial full blood counts were not performed on all cases. The remaining 26 cases were classified as possible, probable or confirmed ITP by at least one independent haematologist, after considering clinical history, comorbidities, investigations, and response to treatment. The World Health Organization (WHO) bleeding score was used to grade severity [20]. Similarly, for comparison, cases of TTS were identified by reports submitted to SAEFVIC, as well as direct reports from VicSIS clinicians and haematology colleagues. TTS cases were classified locally by at least one independent haematologist as either likely, unlikely or excluded with aid of the Brighton Collaboration criteria for TTS, and then deemed as probable or confirmed by the TGA [21].
Statistical analysis Data were analysed using Microsoft® PowerBI (version 2.91.701.0) with 90% Poisson confidence intervals calculated.
Background rates were estimated from the Victorian Admitted Episodes Dataset (VAED) of hospital admissions ICD-10-AM coded as idiopathic thrombocytopenia (D693) or other primary thrombocytopenia (D694) during 2016–2019 inclusive, excluding admissions similarly coded within the preceding 12 months to exclude readmissions [22]. This dataset has previously been found to be an important tool for using health outcome administration data to inform background rates and safety surveillance of key AESI [23]. Vaccine doses administered were obtained from the Australian Immunisation Register (AIR) as all doses reported until 14 days prior to the end date of the review period (as at day of data extraction on 1 September 2021). 1,101 AstraZeneca and 650 Pfizer-BioNTech doses had unknown dose number and were excluded from the analysis. Rates were calculated based on expected admissions within 28 days following vaccination and considered by vaccine-relevant age-group stratification of 20–49 years (Pfizer-BioNTech), ≥50 years (AstraZeneca) and ≥ 20 overall. The calculation of rates using the VAED was thought to be appropriate given 80% (n = 15) of our cases were hospitalised (88% following AstraZeneca and 50% following Pfizer-BioNTech vaccination). Relevant population denominator was obtained from Australian Bureau of Statistics (ABS) estimated residential population, by year and age [24].
2.1. Ethics approval
No ethics approval was required for this study as cases are de-identified; and follow-up of cases was undertaken as part of public health AEFI management.
3. Results
A total of 21 patients were classified as confirmed (15 cases) or probable (6 cases) ITP (Table 1 ). Seventeen of these were following AstraZeneca vaccine (15 following dose 1 and 2 following dose 2). There were also 5 possible cases of ITP in which other concurrent medical issues may have also contributed to thrombocytopenia and so these were excluded from the analysis.
Table 1.
Confirmed and Probable cases of ITP following vaccination with AstraZeneca and Pfizer-BioNTech vaccines from 22 February to 20 August 2021.
| Astrazeneca | Pfizer | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases and rates | |||||||||
| Cases | Doses | Rate per 100,000 (90 %CI) | Cases | Doses | Rate per 100,000 (90 %CI) | Cases | Doses | Rate per 100,000 (90 %CI) | |
| Observed Total | 17 | 2,009,966 | 0.8 (0.54–1.27) | 4 | 1,608,323 | 0.2 (0.08–0.57) | 21 | 3,618,289 | 0.6 (0.39–0.84) |
| Dose 1 | 15 | 1,434,925 | 1.0 (0.64–1.61) | 2 | 978,255 | 0.2 (0.04–0.64) | 17 | 2,413,180 | 0.7 (0.45–1.06) |
| Dose 2 | 2 | 575,041 | 0.3 (0.06–1.1) | 2 | 630,038 | 0.3 (0.06–1.00) | 4 | 1,205,109 | 0.3 (0.11–0.76) |
| Expected background cases (hospital admissions, within 28 days) | Dose 1 = 6 Dose 2 = 2 Total = 8 | Adults 50 + years 28 days = 0.41 | Dose 1 = 2 Dose 2 = 1 Total = 3 | Adults 20–49 years 28 days = 0.19 | Dose 1 = 7 Dose 2 = 3 Total = 10 | Adults 20 + years 28 days = 0.28 | |||
| Demographics | |||||||||
| Age range (median) | 56–97 (72) | 20–40 (38) | 20–97 (68) | ||||||
| Male:female | 8:9 | 0:4 | 8:13 | ||||||
| Clinical results | |||||||||
| plat nadir (median) | 0–67 (5) | 1–87 (47) | 0–87 (5) | ||||||
| Days to plt nadir (median) | 2–67 (14) | 2–56 (10) | 2–67 (14) | ||||||
| Days to symptom onset (median) | 1–78 (10) | 2–21 (9) | 1–24 (10) | ||||||
| Brighton Collaboration Level | |||||||||
| Brighton Collaboration level 1 | 14 | 3 | 17 | ||||||
| Brighton Collaboration level 2 | 3 | 1 | 4 | ||||||
90% Confidence intervals [poisson].
Expected background rate (calculated from adult hospital admissions with primary ICD-10 code thrombocytopenia) cases within 28 days of vaccination.
The observed rate of confirmed or probable ITP following Pfizer-BioNTech was 2 per million, similar to the expected background rate of 1.9 per million (20–49 years of age) within 28 days of vaccination. The observed rate following AstraZeneca was 8 per million, and as high as 10 per million following dose 1, which is more than twice the expected background rate of 4.1 per million among persons aged ≥ 50 years of age within 28 days of vaccination.
Of the seventeen cases following AstraZeneca vaccination, following expert haematology review, sixteen were de novo ITP, while one is thought to have chronic ITP. Two had some bruising noted prior to vaccination. Eleven of the cases (65%) had bleeding, with a WHO bleeding score of 2 or 3. As expected, the most common signs reported were mucocutaneous bleeding (53%). One case had ocular bleeding and subdural haematoma, and there was one fatality in, secondary to myocardial infarction 7 weeks after the vaccine and was deemed as unrelated to the diagnosis of ITP.
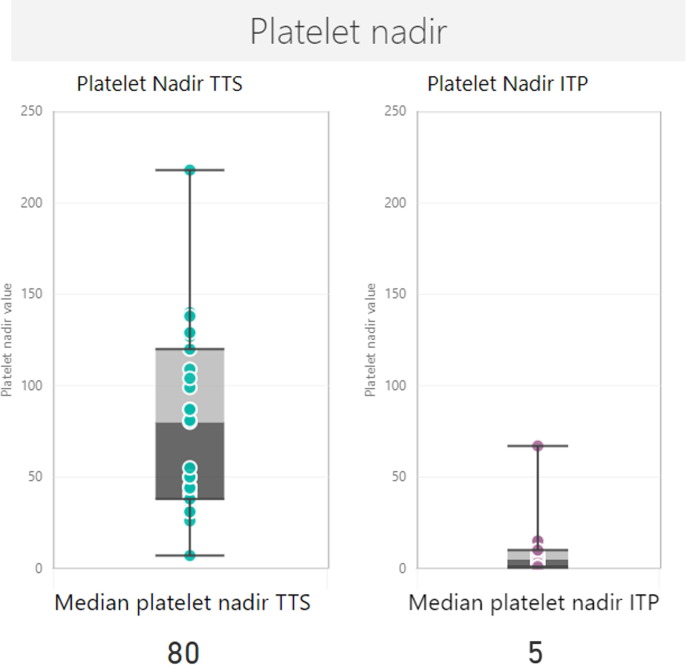
Cases reported following AstraZeneca (n = 17) had onset within 28 days of vaccination, except for one case that presented late on day 78. Platelet nadir was extremely low in these confirmed and probable cases, with a median nadir of 5 × 109/L (range 0–67) (Table 1). The median platelet nadir for ITP cases was markedly lower when compared to the median platelet nadir of 80 × 109/L for 36 confirmed and probable cases of TTS reported to date in Victoria (Fig. 2 ).
Fig. 2.
Box and whisker plot for the platelet nadir of confirmed and probable TTS and ITP cases.
Sixteen (94%) received treatment with corticosteroids. Eight patients also received between 1 and 2 g/kg intravenous immunoglobulin (IVIG) as part of first-line therapy. Three patients were significantly refractory to treatment requiring the addition of a thrombopoietin receptor agonist. Four cases (24%) recovered (defined as platelets > 100 × 109/L) without ongoing anti-platelet treatment (e.g. corticosteroids and/or IVIG), and 5 (29%) had platelet recovery with ongoing treatment with corticosteroids and/or thrombopoietin receptor agonists. Seven (41%) remained thrombocytopenic (platelets < 100 × 109) at the time of the review, four of which were despite ongoing anti-platelet treatment, two which did not require ongoing treatment, and one which was mild and never received treatment. The final case was the fatality in our series, and the individual remained thrombocytopenic at time of death (platelets 76 × 109/L).
Of the four cases following Pfizer-BioNTech vaccine, two were de novo ITP, while 2 are thought to have chronic ITP. Of the de novo cases, both presented with only skin petechiae and/or bruising. Of the 2 cases of exacerbation of chronic ITP, one presented with severe menorrhagia and the second with haematuria (although investigations as to the cause of the haematuria are ongoing). Two cases required no treatment and two required treatment with corticosteroids, one of whom was also treated with IVIG as part of first line therapy. None of the Pfizer-BioNTech cases were refractory to treatment.
4. Discussion
We describe twenty-one cases of ITP following vaccination with either AstraZeneca or Pfizer-BioNTech vaccines, with the majority (n = 17) associated with AstraZeneca vaccine. For Pfizer-BioNTech vaccine, based on the number of vaccines administered in the study period we would have expected 3 cases, comparable with the 4 cases observed, indicating the robustness of case ascertainment in this series.
The observed rate of ITP following the adenoviral vector vaccine AstraZeneca was 8 per million and as high as 10 per million following dose 1, which was substantially higher than the expected rate of 4.1 per million. This finding is important, because the Therapeutic Goods Administration (TGA) convened an external Vaccine Safety Investigation Group (VSIG) of clinical experts which concluded that a fatal case ITP case in a 61 year old woman from Western Australia was likely linked to the AstraZeneca vaccine [18]. A formal signal investigation into ITP as an AESI in Australia is ongoing.
Our analysis is in keeping with a nested incident-matched case control study in Scotland, which found an association between AstraZeneca vaccine and ITP, with an estimated incidence of 1.13 cases per 100,000 doses [9]. In this study, increased risk was first found at 7–13 days after vaccination. The finding of increased risk of ITP following AstraZeneca vaccine and the window of increased risk was supported by a case control study in the United Kingdom (UK)[10]. The Scottish and UK self-controlled case series both used an adjusted risk ratio to demonstrate increased risk of ITP first occurring within 7–13 and 8–14 days, respectively. Although the methodology within these case series is different, the adjusted risk ratios are consistent with the median time to onset seen in our series [9], [10]. Only two of our confirmed or probable ITP cases underwent testing with Human PF-4 solid-phase sandwich ELISA (enzyme-linked immunosorbent assay) to investigate TTS, both of which were negative, and suggesting that presentation of these cases is different to TTS. These two cases also underwent independent haematology review for other factors, including investigation for thrombosis and d-dimer levels, and did not meet Brighton Collaboration criteria for TTS. Interestingly, median platelet nadir in our ITP cases (median nadir of 5 × 109/L) was significantly lower than the platelet nadir observed in our TTS cases over the same period (median nadir of 81 × 109/L). This suggests a possible different mechanism of action behind these two AESI, in which TTS involves platelet activation rather than platelet destruction [3]. One limitation in these data, however, is that reporting systems are more likely to capture the more severe cases, which present with bleeding or more significant symptoms. There may be milder cases in the community who are asymptomatic and thus never present for care. This contrasts with cases of TTS which are more likely to present to hospital due to thrombotic complications. Both TTS and isolated thrombocytopenia have been added to the product information for AstraZeneca [2]. Another limitation of this study is that background rates were calculated from VAED ICD-10-AM codes for idiopathic thrombocytopenia or other primary thrombocytopenia based on patient diagnosis upon discharge. It is possible that this may over-estimate the background rate of ITP as some individuals may have had alternate causes for their thrombocytopenia.
The United States (US) Vaccine Adverse Event Reporting System (VAERS) reported a series of 28 cases of thrombocytopenia (not all of which were ITP) following vaccination with mRNA (Pfizer-BioNTech and Moderna) COVID-19 vaccines [12]. The observed rate of 0.8 per million doses for both vaccines in this cohort was not higher than expected background rate. This is in keeping with our analysis, which also did not demonstrate an increased rate of ITP following Pfizer-BioNTech vaccination, as well as other studies which have reported similar findings relating to mRNA COVID-19 vaccines [9], [10], [11], [13].
Like previous studies, most of our cases were following dose 1 of AstraZeneca vaccine, raising the importance of dose 2 considerations for these patients. The opinion from haematology experts is that an alternate vaccine would be reasonable for these cases. Dose 2 of any COVID-19 vaccine should be deferred until platelet stabilisation (>50 × 109/L and off treatment for > 3 months). Follow-up of these patients at 3 months will be important to assess ongoing thrombocytopenia and need for continuing treatment. The effect of COVID-19 vaccination on pre-existing ITP has not been well characterised. Limited and early data suggests vaccination may worsen thrombocytopaenia in approximately 12% of patients with chronic ITP post-vaccination [25]. However, it is important to note that ITP is most often triggered by a viral infection, and the risk of relapse or worsening of ITP is likely higher following COVID-19 than following vaccination. Chronic ITP patients have been vaccinated safely with careful monitoring. Patients with a history of ITP are therefore recommended to proceed with vaccination and if clinical symptoms worsen post vaccination, then monitoring of platelets and escalation of therapy may be required.
In conclusion, we identified an increased rate of ITP following receipt of AstraZeneca vaccine. This is a second haematological AESI identified following the AstraZeneca vaccine in Australia and is distinct from TTS. This case series highlights the benefit of all cases of thrombocytopenia temporally associated with vaccination being further evaluated by a haematologist. Future studies are required to confirm causality, further evaluate the possible immuno-pathogenic mechanisms, as well as document the outcomes with subsequent COVID19 vaccine doses to provide important information in support of the COVID-19 vaccine programs both in Australia and globally.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Department of Health - Emma Roney, Anita Ona, Anna Power, Thiruverni Ananthanathan, Ngaree Blow, Elise Virah Sawmy, Eleanor Duckworth, Michelle Wolthuizen, Naveen Tenneti.
VicSIS clinic sites.
Michelle Giles (Alfred Health), Sarah Bullen (Alfred Health), Danielle Kennedy (Alfred Health), Kayleigh Malone (Alfred Health), Ciara Burke (Alfred Health), Brian Price (Alfred Health), Joseph de Luca (Austin Health), Jason Trubiano (Austin Health), Kerryn McInnes (Austin Health), Jamie Rotin (Austin Health), Callum Maggs (Barwon Health), Elyse Stevens (Barwon Health), Julie Carlilse (Barwon Health), Loretta Mithen (Barwon Health), Katrina Bellamy (Barwon Health), Caroline Poynder (Barwon Health), Susan Cirillo (Barwon Health), Katherine Gibney (Melbourne Health), Charlotte Slade (Melbourne Health), Elise Wang (Melbourne Health), Tony Korman (Monash Health), Sara Barnes (Monash Health), Karen Bellamy (Monash Health), Jo Hickman (Monash Health), Elizabeth Leahy (Monash Health), Sara Pitts (Monash Health), Craig Aboltins (Northern Health), Jenna Paterson (Northern Health), Hayley Gray (Northern Health), Jade Mertens (Northern Health), Lumnise Gashi (Northern Health), Ben The (Peter MacCallum Cancer Centre), Cindy Yuen (Peter MacCallum Cancer Centre), Marion Kainer (Western Health), Katherine Langan (Western Health), Claire Sanguinetti (Western Health), Kayleen Kraal (Western Health).
All other medical, nursing and administrative staff that support the VicSIS clinics.
SAEFVIC.
Josh Osowicki, Daryl Cheng, Priya Shenton, Mel Addison, Louise Dempsey, Adele Harris, Georgie Lewis, Bianca Penak, Laura Voss, Jaimee Craft, Victoria Scott, Lois Tham.
All other hospital sites and clinicians involved in providing information on cases.
References
- 1.Operation COVID Shield. National COVID Vaccine Campaign Plan, https://www.health.gov.au/sites/default/files/documents/2021/08/op-covid-shield-national-covid-vaccine-campaign-plan.pdf.; 2021 [accessed 13 August 2021].
- 2.Therapeutic Goods Administration (TGA). Australian Product Information: COVID-19 Vaccine AstraZeneca (ChAdOx1-S) solution for injection, https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2021-PI-01194-1&d=20210814172310101; 2021 [accessed 13 August 2021].
- 3.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brighton Collaboration. Priority list of Adverse Events of Special Interest: COVID-19, https://brightoncollaboration.us/priority-list-aesi-covid/; 2020 [accessed 13 August 2021].
- 6.Miller E., Waight P., Farrington C.P., Andrews N., Stowe J., Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child. 2001;84(3):227–229. doi: 10.1136/adc.84.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black C., Kaye J.A., Jick H. MMR vaccine and idiopathic thrombocytopaenic purpura. Br J Clin Pharmacol. 2003;55(1):107–111. doi: 10.1046/j.1365-2125.2003.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharjee S., Banerjee M. Immune Thrombocytopenia Secondary to COVID-19: a Systematic Review. SN Compr. Clin Med. 2020;2(11):2048–2058. doi: 10.1007/s42399-020-00521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson C.R., Shi T., Vasileiou E., Katikireddi S.V., Kerr S., Moore E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. Bmj. 2021;374:n1931. [DOI] [PMC free article] [PubMed]
- 11.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh K.J., Baumblatt J., Chege W., Goud R., Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2021;39(25):3329–3332. doi: 10.1016/j.vaccine.2021.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA. 2021;326(14):1390. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austalian Bureau of Statistics. National, state and territory population, https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release#states-and-territories; 2020 [accessed 13 August 2021].
- 15.Clothier H.J., Crawford N.W., Kempe A., Buttery J.P. Surveillance of adverse events following immunisation: the model of SAEFVIC. Victoria Commun Dis Intell Q Rep. 2011;35(4):294–298. [PubMed] [Google Scholar]
- 16.Melbourne Vaccine Education Centre (MVEC), https://mvec.mcri.edu.au/references/the-vicsis-victorian-specialist-immunisation-services-network/; 2021 [accessed 12 October].
- 17.Therapeutic Goods Administration (TGA). Reporting suspected side effects associated with a COVID-19 vaccine. 2021, https://www.tga.gov.au/reporting-suspected-side-effects-associated-covid-19-vaccine; 2021 [accessed 13 August 2021].
- 18.Therapeutic Goods Administration (TGA). COVID-19 vaccine weekly safety report - 08-07-2021, https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-08-07-2021; 2021 [accessed 13 August 2021].
- 19.Brighton Collaboration. Thrombocytopenia: Case Definition Companion Guide, https://brightoncollaboration.us/thrombocytopenia-case-definition-companion-guide/; 2021 [accessed 13 August 2021].
- 20.Miller A.B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Brighton Collaboration. Interim Case Definition of Thrombosis with Thrombocytopenia Syndrome (TTS), https://brightoncollaboration.us/thrombosis-with-thrombocytopenia-syndrome-interim-case-definition/; 2021 [accessed 26 September 2021].
- 22.Victorian Department of Health. VAED manual 2021-22 (all sections), https://www2.health.vic.gov.au/about/publications/policiesandguidelines/VAED-manual-2021-22-all-sections; 2021 [accessed 7 October 2021].
- 23.Clothier H.J., Lee K.J., Sundararajan V., Buttery J.P., Crawford N.W. Human papillomavirus vaccine in boys: background rates of potential adverse events. Med J Aust. 2013;198(10):554–558. doi: 10.5694/mja12.11751. [DOI] [PubMed] [Google Scholar]
- 24.Australian Bureau of Statistics. National, state and territory population, https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population; 2021 [accessed 13 August 2021].
- 25.Kuter D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br J Haematol. 2021 doi: 10.1111/bjh.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]