Abstract
BACKGROUND:
Ovarian cancer patients often present with late-stage disease and non-specific symptoms, but little is known about factors affecting time to diagnosis in the United States.
METHODS:
A retrospective population-based study of the Surveillance, Epidemiology, and End Results-Medicare database was conducted. We included women 66 years and older with stage II–IV epithelial ovarian cancer with at least one code for abdominal/pelvic pain, bloating, difficulty eating, or urinary symptoms within 1 year of cancer diagnosis. Time to diagnosis was defined from the first claim with a pre-specified symptom to ovarian cancer diagnosis. Kruskal-Wallis tests were used to assess for differences in TTD by group medians. Univariate and generalized linear models with log-link function evaluated TTD by covariables.
RESULTS:
In 13872 women analyzed, mean and median times to diagnosis were 2.9 and 1.1 months. Median TTD differed significantly by first symptom (p<0.001), number of symptoms (p<0.001), and first physician specialty seen (p<0.001). On multivariable analysis, TTD differed significantly according to race/ethnicity (p<0.001), geographic region (p=.001), urban-rural location (p=.031), emergency room presentation (p<0.001), and number of specialties seen (p<0.001). Relatively shorter time to diagnosis was associated with diagnosis in 2006–2010 (RR 0.92, 95% CI 0.87–0.98) and 2011–2015 (RR 0.87, 95% CI 0.81–0.93) compared to 1992–1999.
CONCLUSIONS:
The time from symptomatic presentation to care to ovarian cancer diagnosis is influenced by clinical and demographic variables. Our findings reinforce the importance of educating all physicians on ovarian cancer symptoms to aid diagnosis.
Keywords: Epithelial ovarian cancer, late diagnosis, symptom assessment, SEER program, gynecologic neoplasm
LAY SUMMARY
Ovarian cancer is often diagnosed once disease has spread because the classic symptoms of ovarian cancer – abdominal or pelvic pain, bloating, difficulty eating, and urinary issues – can be mistaken for other problems. Our study examined the time between when women with classic ovarian cancer symptoms went to a doctor and when they received a cancer diagnosis in a large database population. We found that the time to diagnosis differed based on the type and number of symptoms and what type of doctor a woman went to, as well as factors like race, geographic location, and year of diagnosis.
PRECIS
In this retrospective population-based cancer registry database study of 13872 women with ovarian cancer, the time between symptomatic presentation to medical care and cancer diagnosis differed significantly by presenting symptom, number of symptoms, physician specialty, and clinical and demographic variables, including significant racial disparities. Our findings reinforce the importance of educating all physicians on ovarian cancer symptoms to facilitate timely workup and referrals.
INTRODUCTION
Ovarian cancer remains the most lethal gynecologic cancer in the United States (US), with the majority of cases diagnosed at late stages after distant spread of disease.1 Multiple studies have identified that the symptoms most associated with ovarian cancer include abdominal or pelvic pain, bloating, loss of appetite, and urinary symptoms,2–10 which led to the creation of the modified Goff symptom index as a screening tool for ovarian cancer. The modified Goff index has a reported sensitivity of 65.5% and specificity of 84.7% overall,6, 11 which makes it an imperfect tool. However, because there are no acceptable screening methods for ovarian cancer for asymptomatic women, it is currently the best tool we have to guide workup of concerning symptoms. Furthermore, most women who are eventually diagnosed with ovarian cancer first present to non-gynecologic physicians,2, 3 which makes it imperative that all providers are aware of the association of these symptoms with ovarian cancer. Because ovarian cancer symptoms are frequently non-specific at presentation, women often have delayed diagnoses of ovarian cancer,2, 3, 12 which not only worsens survival but can also create frustration and increase symptom burden for patients.13
Despite knowing that ovarian cancer diagnosis is often delayed due to non-specific symptoms, no recent studies have examined the time between symptom presentation and ovarian cancer diagnosis in the US or what factors may impact the time to diagnosis (TTD). Studies suggest that the mean time from ovarian cancer symptom onset to diagnosis ranges from 1.4 to 10.0 months and depends on the type and number of symptoms, route to diagnosis, and diagnostic workup.2, 14–17 However, these reports are largely small observational studies or have been conducted internationally and thus represent dissimilar healthcare systems compared to the US. While controversial,18–22 recent studies have suggested that faster time to diagnosis may positively affect cancer survival.14, 19, 22, 23 Thus, understanding the factors affecting time to ovarian cancer diagnosis in the US could not only elucidate gaps in the diagnostic workup of ovarian cancer but also provide actionable targets to improve ovarian cancer detection and care.
The purpose of the present study was to describe the time from presentation to medical care with “classic” ovarian cancer symptoms as defined in a validated symptom screening tool to the diagnosis of ovarian cancer and identify any factors associated with decreased TTD in the US using a retrospective population-based review of diagnosis codes and claims data in the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.
METHODS
The SEER-Medicare database, which collects population-based cancer registry information from nearly 35% of the US population,24 was searched for patients with a new diagnosis of stage II-IV primary epithelial ovarian cancer from 1992–2015 who were 66 years and older. International Classification of Diseases (ICD) for Oncology 3rd edition histology codes were used to identify adenocarcinomas of the ovary; borderline, stromal, and germ cell Cancer Stage Group, sixth edition.25 Because the Goff index has higher sensitivity and specificity for locally advanced disease,6, 11 Stage I disease was excluded; in the final study population, 8.8% of women had stage II disease, 47.% had stage III disease, 36.9% had stage IV disease, and 6.6% were unknown stage. Patients were included only if they had Medicare full A and B coverage and no health maintenance organization enrollment from 12 months before diagnosis to 12 months after diagnosis. Patients were excluded if they had no pathologic confirmation of ovarian adenocarcinoma, if ovarian cancer was not their first cancer diagnosis, or if the diagnosis was made at autopsy or by death certification alone (Table 1).
Table 1:
Database population selection
| Step | Selection Criteria | Excluding | Remaining n |
|---|---|---|---|
| 1 | Ovarian cancer | 0 | 79486 |
| 2 | 1st cancer-ovarian cancer | 8571 | 70915 |
| 3 | Year of diagnosis 1992–2015 | 1423 | 69492 |
| 4 | Age 66 and older | 25685 | 43807 |
| 5 | AJCC Stage II-IV and Stage Unknown | 5323 | 38484 |
| 6 | Selected patients whose ICD-O-3 histology codes were consistent with the adenocarcinomas of the ovary but excluding borderline tumors, stromal and germ cell tumors | 9978 | 28506 |
| 7 | Patients with pathologic confirmation | 318 | 28188 |
| 8 | Exclude patients diagnosed at autopsy or by death certificate only | 31 | 28157 |
| 9 | Full Medicare A and B coverage and No HMO enrollment 12 months before diagnosis to 12 months after diagnosis | 9464 | 18693 |
| 10 | ICD code for abdominal/pelvic pain (625.0,625.5,625.9,789.01–789.09), bloating (789.30–789.39), urinary symptoms (788.1,788.3,788.4), or difficulty eating (536.0,536.9,564.0,738.0,787.02,787.3,787.9,787.91) | 4821 | 13872 |
AJCC, American Joint Committee on Cancer; ICD-O, International Classification of Diseases for Oncology; HMO, Health Maintenance Organization; ICD, International Classification of Diseases
Based on previous studies and the modified Goff index,2–10 only women who had an outpatient diagnosis code for at least 1 of the 4 most common classic ovarian cancer symptoms in the year prior to diagnosis of ovarian cancer were included. The 4 pre-specified symptoms were classified according to International Classification of Diseases 9th and 10th Revision Clinical Modification (ICD-9-CM, ICD-10-CM) codes into abdominal/pelvic pain (625.0,625.5,625.9,789.01–789.09), bloating (789.30–789.39), urinary symptoms (788.1,788.3,788.4), and difficulty eating, which included nausea and early satiety (536.0,536.9,564.0,738.0,787.02,787.3,787.9,787.91).
Medicare claims data for visits that contained any of the 4 pre-specified symptoms were extracted from the SEER-Medicare database for analysis. Provider specialty types were identified based on Health Care Financing Administration codes from the Physician/Supplier Data File. We included all physician specialty visits that met our inclusion criteria, and also specifically examined visits to obstetrics and gynecology, gastroenterology, urology, emergency medicine, and internal medicine/family practice/general practice due to the relevance of these specialties to our symptom codes.2, 3 We accumulated the number of physician types if a patient had more than one type of physician visit. The specialty codes for internal medicine, family practice, and general practice specialty codes were combined into 1 category for analysis. All provider visits were based on outpatient presentation.
The primary outcome of interest was TTD, defined from the date of the first coded pre-specified ovarian cancer symptom in a claim (index date) to the date of ovarian cancer diagnosis. The primary reason for an index date visit did not have to be one of the pre-specified symptoms. Covariables were selected for clinical significance and included age group at diagnosis (66–70, 71–75, 76–80, and ≥81 years), race/ethnicity, Charlson Comorbidity Index,26 marital status, US geographic region (Northeast, Midwest, South, West), percent of the subjects’ county living below the federal poverty level, year of diagnosis (1992–1999, 2000–2005, 2006–2010, and 2011–2015), urban-rural continuum (all urban, mostly urban, mostly rural, all rural), emergency room visit for any reason between index date and diagnosis, and number of physician specialty types seen for any reason between index date and diagnosis (0, 1–4, and >4). The percentage of women with incomes below the poverty level were based on Census ACS variables, with recorded groupings based on quartiles. Rural-urban continuum codes were based on US Department of Agriculture classifications.27 Patients coded as seeing zero physician specialty types either had the same index date and date of cancer diagnosis or were diagnosed in the inpatient setting.
Clinical and demographic characteristics of our cohort were summarized. Missing data were categorized as unknown. The frequency of codes for abdominal/pelvic pain, bloating, difficulty eating, and urinary symptoms was summarized. The frequency of provider specialty type visits was reported for the one year prior to diagnosis and from index date to cancer diagnosis. Descriptive statistics were reported for TTD according to first coded symptom type, number of symptoms in the year prior to diagnosis, and first provider specialty visit (defined as first specialty visit among the 5 pre-selected specialties which included a diagnosis code for at least one of the 4 pre-specified symptoms), and covariables.
To assess for differences between group medians, nonparametric Kruskal-Wallis tests were performed for variables with ≥3 groups and Wilcoxon rank-sum tests were performed for variables with 2 groups. Univariable and multivariable analyses were used to assess factors affecting TTD from index date to cancer diagnosis using a saturated generalized linear model with gamma distribution and log-link function. Logistic regression with a generalized logit model was performed to evaluate factors affecting the number of physician specialty type visits from index date to cancer diagnosis by covariables.
Comparisons were considered statistically significant using a baseline 2-sided alpha level of 0.05 for single testing with a Bonferroni correction for multiple comparisons testing. Data analysis was done using SAS enterprise guide 7.1. (SAS Institute, Cary, NC). This study was exempted by the Institutional Review Board at MD Anderson Cancer Center. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used in the preparation of this manuscript.
RESULTS
We identified 18,693 women with stage II-IV primary epithelial ovarian cancer in the SEER-Medicare database who met our initial inclusion criteria, of which 13,872 (74.2%) had codes for at least 1 pre-specified classic ovarian symptom and were included in subsequent analyses (Table 1). In the year prior to diagnosis, 9438 (68.0%) of women had codes for abdominal/pelvic pain, 8466 (61.0%) for bloating, 5199 (37.5%) for difficulty eating, and 1060 (7.6%) for urinary symptoms. Additionally, 5968 (43.0%) had codes for just 1 symptom between the index date and cancer diagnosis, 5679 (40.9%) for 2 symptoms, 2063 (14.9%) for 3 symptoms, and 162 (1.2%) for all 4 symptoms.
Of all visit types in the one year prior to diagnosis among our pre-selected specialty types, internal medicine comprised the most visits (47.3%), followed by obstetrics and gynecology (5.5%), gastroenterology (3.8%), urology (1.7%), and emergency medicine (0.6%). Similarly, the most common visits between index date and cancer diagnosis were to internal medicine/family practice (46.1%), obstetrics/gynecology (9.1%), gastroenterology (6.6%), urology (2.2%), and emergency medicine (0.7%). The distribution of visit frequency to all physician specialties are provided in Supplementary Table 1. Among all women analyzed, 73.6% saw one of our pre-specified specialty types at their index date visit.
The median overall TTD was 1.1 months and mean overall TTD was 2.9 months (Figure 1, Table 2). When examined according to first coded symptom type, median TTD was 0.5 months for bloating, 1.3 months for abdominal/pelvic pain, 1.3 months for difficulty eating, and 5.3 months for urinary symptoms. The mean and median TTD increased as the number of symptoms increased, from a median of 0.7 months with 1 symptom to 7.0 months with 4 symptoms. Median TTD based on first physician specialty visit was 1.1 months for obstetrics and gynecology, 0.8 months for emergency medicine, 1.6 months for internal medicine, 2.4 months for gastroenterology, and 3.3 months for urology. The median TTD differed significantly according to first coded symptom type (p< 0.001), number of symptoms (p< 0.001), and first physician specialty seen (p< 0.001) (Table 2). Additional analyses for all examined covariables are provided in Supplementary Table 2.
Figure 1. Mean and median TTD by first symptom, number of symptoms, and first physician specialty.
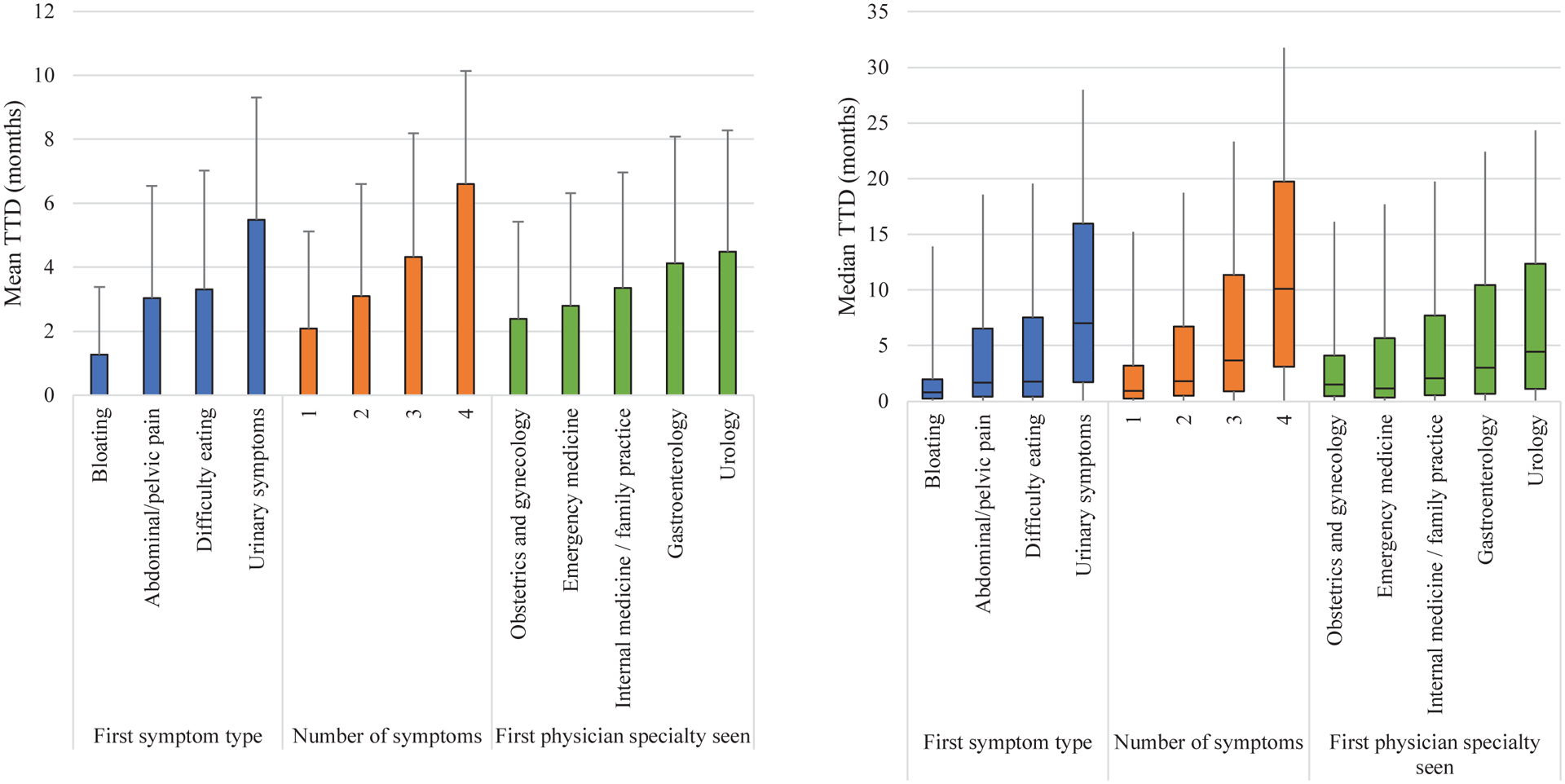
Left: Mean TTD is indicated by the top of each colored bar. One-sided capped lines represent positive SD from mean.
Right: The median TTD is indicated by the horizontal bar across each colored box. The interquartile ranges are indicated by the colored boxes. The lower whiskers extend to the minimum values and the upper whiskers extend to the maximum values.
Table 2:
Distribution of TTD by symptom and physician specialty variables
| Category | Variable | N (%) | Mean TTD (mos) | SD (mos) | Median TTD (mos) | Lower quartile (mos) | Upper quartile (mos) | P-valuea |
|---|---|---|---|---|---|---|---|---|
| Total cohort | 13872 (100.0) | 2.88 | 3.49 | 1.12 | 0.39 | 4.44 | ||
| First symptom typeb | Bloating | 4081 (26.1) | 1.26 | 2.12 | 0.53 | 0.26 | 1.18 | |
| Abdominal / pelvic pain | 7272 (46.6) | 3.03 | 3.51 | 1.25 | 0.43 | 4.88 | ||
| Difficulty eating | 3496 (22.4) | 3.31 | 3.72 | 1.32 | 0.43 | 5.79 | ||
| Urinary symptoms | 762 (4.9) | 5.49 | 3.81 | 5.28 | 1.74 | 8.95 | ||
| Group | 15611 | <0.001 | ||||||
| Number of symptoms | 1 | 5968 (43.0) | 2.08 | 3.04 | 0.66 | 0.26 | 2.27 | |
| 2 | 5679 (40.9) | 3.09 | 3.51 | 1.32 | 0.49 | 4.90 | ||
| 3 | 2063 (14.9) | 4.32 | 3.87 | 2.8 | 0.89 | 7.66 | ||
| 4 | 162 (1.2) | 6.6 | 3.54 | 6.99 | 3.13 | 9.61 | ||
| Group | 13872 | <0.001 | ||||||
| First physician specialty seenc | Obstetrics / gynecology | 1764 (17.3) | 2.39 | 3.04 | 1.05 | 0.46 | 2.60 | |
| Emergency medicine | 1235 (12.1) | 2.79 | 3.53 | 0.82 | 0.33 | 4.54 | ||
| Internal medicine / family practice | 5642 (55.3) | 3.35 | 3.61 | 1.55 | 0.53 | 5.63 | ||
| Gastroenterology | 1373 (13.4) | 4.13 | 3.96 | 2.37 | 0.66 | 7.40 | ||
| Urology | 195 (1.9) | 4.48 | 3.80 | 3.32 | 1.12 | 7.93 | ||
| Group | 10209 | <0.001 | ||||||
TTD, time to diagnosis. SD, standard deviation. Significant values signified with bold text.
P-value is from Kruskal-Wallis test comparing variable medians. P-values were considered significant if <0.0167 using Bonferroni correction for multiple comparison testing using a baseline alpha of 0.05 and a k of 3.
Some patients had codes for multiple symptoms occurring as first symptoms.
Excludes patients who did not see 1 of these 5 physician specialties at their index date
On multivariable analysis (Table 3), TTD differed significantly according to race/ethnicity (p<0.001), geographic region (p=.001), year of diagnosis (p<0.001), emergency room presentation (p<0.001), and number of physician specialties seen between index date and diagnosis (p<0.001). There was a higher relative risk of longer TTD in Hispanic women (RR 1.21, 95% CI 1.12–1.32) compared to non-Hispanic Whites; living in the West (RR 1.10, 95% CI 1.05–1.16) compared to the Northeast; presentation to the emergency room (RR 1.12, 95% CI 1.03–1.21); and seeing more than 4 physician specialty types compared to 1–4 physician specialty types between index date and diagnosis (RR 2.99, 95% CI 2.87–3.12). Lower relative risk of longer TTD was associated with diagnosis in 2011–2015 (RR 0.87, 95% CI 0.81–0.93) compared to 1992–1999.
Table 3:
Association between patient characteristics and time to diagnosis on multivariable generalized linear model
| Variable | N (%) | RR | 95% CI | P-valuea | |
|---|---|---|---|---|---|
| Age group | 66–70 | 3397 (24.5) | REF | ||
| 71–75 | 3444 (24.8) | 1.00 | 0.95–1.05 | 0.85 | |
| 76–80 | 3269 (23.6) | 0.98 | 0.93–1.03 | 0.44 | |
| 81+ | 3762 (27.1) | 1.03 | 0.98–1.08 | 0.26 | |
| Group | 0.25 | ||||
| Race/Ethnicity | White, non-Hispanic | 11767 (84.8) | REF | ||
| Black, non-Hispanic | 852 (6.1) | 1.08 | 1.00–1.17 | 0.043 | |
| Hispanic | 735 (5.3) | 1.21 | 1.12–1.32 | <0.001 | |
| Other/Unknown | 518 (3.7) | 1.18 | 1.07–1.29 | 0.001 | |
| Group | <0.001 | ||||
| Charlson Comorbidity Index score | 0 | 8704 (62.7) | REF | ||
| 1 | 3078 (22.2) | 1.05 | 1.00–1.10 | 0.037 | |
| ≥ 2 | 2090 (15.1) | 1.00 | 0.94–1.05 | 0.89 | |
| Group | 0.09 | ||||
| Marital status | Married | 5732 (41.3) | REF | ||
| Unmarried | 7646 (55.1) | 0.99 | 0.96–1.03 | 0.77 | |
| Unknown | 494 (3.6) | 0.89 | 0.81–0.99 | 0.027 | |
| Group | 0.09 | ||||
| Geographic region | Northeast | 2642 (19.0) | REF | ||
| Midwest | 2150 (15.5) | 1.03 | 0.97–1.10 | 0.29 | |
| South | 3050 (22.0) | 1.08 | 1.02–1.15 | 0.011 | |
| West | 6030 (43.5) | 1.10 | 1.05–1.16 | <0.001 | |
| Group | 0.001 | ||||
| Census Tract Population % Below Poverty | 1st quartile: 0–<5.43 | 3446 (24.8) | REF | ||
| 2nd quartile: 5.43–<11.07 | 3421 (24.7) | 0.99 | 0.94–1.04 | 0.59 | |
| 3rd quartile: 11.07–<19.74 | 3491 (25.2) | 1.04 | 0.98–1.09 | 0.18 | |
| 4th quartile: 19.74–<100 | 3456 (24.9) | 1.00 | 0.95–1.06 | 0.94 | |
| Unknown | 58 (4.2) | 1.14 | 0.86–1.50 | 0.37 | |
| Group | 0.32 | ||||
| Year of diagnosis | 1992–1999 | 3073 (22.2) | REF | ||
| 2000–2005 | 4653 (33.5) | 0.97 | 0.91–1.03 | 0.31 | |
| 2006–2010 | 3327 (24.0) | 0.92 | 0.87–0.98 | 0.014 | |
| 2011–2015 | 2819 (20.3) | 0.87 | 0.81–0.93 | <0.001 | |
| Group | <0.001 | ||||
| Urban-rural continuum | All urban | 8134 (58.6) | REF | ||
| Mostly urban | 2555 (18.4) | 1.01 | 0.96–1.06 | 0.66 | |
| Mostly rural | 849 (6.1) | 1.00 | 0.92–1.08 | 0.97 | |
| All rural | 990 (7.1) | 0.95 | 0.88–1.02 | 0.18 | |
| Unknown | 1344 (9.7) | 1.12 | 1.03–1.21 | 0.005 | |
| Group | 0.031 | ||||
| Emergency room presentation | No | 8630 (62.2) | REF | ||
| Yes | 5242 (37.8) | 1.12 | 1.03–1.21 | <0.001 | |
| Group | <0.001 | ||||
| Number of physician specialty type visits from index date to cancer diagnosis | 1–4 | 2100 (15.1) | REF | ||
| 0 | 7063 (50.9) | 0.31 | 0.30–0.33 | <0.001 | |
| >4 | 4709 (33.9) | 2.99 | 2.89–3.12 | <0.001 | |
| Group | <0.001 | ||||
Significant values signified with bold text.
P-values were considered significant if <0.005 using Bonferroni correction for multiple comparison testing using a baseline alpha of 0.05 and a k of 10.
In our logistic regression model comparing the number of physician specialty type visits from index date to cancer diagnosis (Supplementary Table 3), the number of specialties seen differed significantly by patient age (p<0.001), CCI score (p<0.001), geographic region (p<0.001), year of diagnosis (p<0.001), urban-rural location (p<0.001), and emergency room presentation (p<0.001). The odds of seeing more than 4 physician specialties increased significantly over time when compared to 1992–1999 (2000–2005 OR 1.17, 95% CI 1.02–1.33; 2006–2010 OR 1.5, 95% CI 1.31–1.72; 2011–2015 OR 1.6, 95% CI 1.39–1.85).
DISCUSSION
This study used a population-based cancer registry data to retrospectively analyze the time from classic ovarian symptom presentation to ovarian cancer diagnosis in the US. We found that TTD differed significantly by presenting symptom, physician visit type, and clinical and demographic variables.
Ours is the first study we know of use a large database to examine the time to diagnosis of ovarian cancer in the US, which allowed us to review patterns within the larger healthcare system as opposed to at an individual patient level. Our study confirms at a population level that abdominal/pelvic pain, bloating, and difficulty eating are common symptoms leading up to a diagnosis of ovarian cancer.2, 7, 8, 16 Urinary symptoms were less common and associated with a longer TTD in our study, and may thus have less clinical utility as a screening symptom, which has also been suggested by prior studies.10 Our study demonstrated that many women present with multiple ovarian cancer symptoms, with 57% of our cohort having 2–4 symptoms coded in the year prior to their diagnosis. Notably, the mean and median TTD increased as the number of symptoms increased. We did not require that a classic ovarian cancer symptom was the primary reason for a physician visit; however, because we examined billing claims with diagnosis codes for these symptoms, they were deemed important enough for a physician to create an assessment and plan for that claim. Our finding that the majority of women with an eventual diagnosis of ovarian cancer had one of the classic symptoms included on a validated symptom screening index speaks to the importance of having a high index of suspicion for ovarian cancer, even when these symptoms may not be the primary reason for a visit. Even though the absolute differences in TTD in our study were often small, even small differences in TTD may affect cancer survival14, 19, 22 and efforts should be taken to minimize any delays in diagnosis.
Physician specialty also mattered in obtaining a timely diagnosis of ovarian cancer in our study. Most patients in our study saw an internal medicine or family practice doctor prior to diagnosis, in line with previous studies.3, 4, 17 Despite the fact that these specialties may not have exposure to the care of ovarian cancer, they had a similar TTD as obstetrician-gynecologists, which may be due to their gatekeeper role in the US medical system. Nearly 26% of our study population did not present to one of our prespecified physician specialties at their index visit, but by billing for a classic ovarian cancer symptom, the symptom warranted enough physician attention to require a plan and could have been an opportunity for workup or referral. Perhaps reflecting the increasing subspecialization of medical care in the US,28–30 women in our study saw an increasing number of physician specialties over the time period of our study, and seeing more physicians was associated with longer TTD.
As medical care in the US becomes more specialized, all health care providers must recognize warning signs of ovarian cancer. The National Comprehensive Cancer Network provides guidelines for the workup of the classic ovarian cancer symptoms we examined31, and previous studies have demonstrated that patients who receive guideline-adherent care after initial presentation have shorter referral times to a gynecologic oncologist32 and improved survival.33 While this has not specifically been examined in ovarian cancer, improved adherence to guideline recommendations may also minimize cost inefficiencies both to patients and the healthcare system by reducing unnecessary testing, multiple physician visits, and time costs due to delayed care.32, 34 Targeted education on the modified Goff index and other signs and symptoms of ovarian cancer for providers outside of obstetrics and gynecology, reimbursement incentives for adherence to evidence-based guidelines, and expedited referral pathways to gynecologic oncology providers15 could be ways to improve guideline recommendation adherence and decrease the TTD for ovarian cancer.
While patients who presented to an emergency room physician at their index date had a relatively shorter TTD compared to other specialties, our regression model suggests that presentation to an emergency department between index date and diagnosis was associated with longer TTD compared to no emergency department presentation. We speculate that the relatively short TTD for patients with first presentation to an emergency room physician may be a function of worse symptom severity, greater access to imaging in the emergency department than in office-based practices, and/or sociodemographic variables that we cannot account for. However, because the workup of ovarian cancer is generally an outpatient endeavor, repeated emergency room visits does not hasten the diagnosis even if the initial presentation was to an emergency room physician.
Our regression models provided several other interesting results. TTD differed significantly by race and ethnicity once other covariables were accounted for, which has been demonstrated in other cancers35, 36 and reflects the broader racial and ethnic disparities in ovarian cancer care in the US.1, 37–41 Increased TTD for Hispanic patients is likely the result of complex social determinants of health and elements of structural racism within the US medical system leading up to diagnosis.42 These structural problems within the medical system are especially important to address in a disease like ovarian cancer, where adherence to guideline recommendations can mitigate survival disparities between races.43 We hypothesize that differences in TTD by geographic region may reflect regional differences in practice or access to care including travel time, which has been associated with ovarian cancer treatment and outcomes.44, 45 Patients diagnosed more recently had a relatively shorter TTD, which may reflect improved tumor antigen immunoassays and imaging, improved physician awareness, or better referral pathways developed over time. Notably, we observed no differences in TTD based on county poverty level, which may be a function of universal coverage in this database and speaks to the importance of improved insurance for all patients.
A major strength of our study is the large cohort due to our use of the SEER-Medicare linked database,24 which would not be possible with an experimental or observational study design. Because we looked at claims prior to ovarian cancer diagnosis, our study is a good “real world” view of how women present prior to diagnosis, which is difficult to ascertain in studies in which recall bias can have a large influence on results such as survey and case-control studies.46 SEER-Medicare is a longitudinal database, allowing for evaluation of claims prior to a diagnosis of cancer as well as cancer-related care, which we used to examine patterns of care in the time leading up to a diagnosis of ovarian cancer. Furthermore, the database allowed us to compare claims over a 23-year time period, a span which covers many changes in ovarian care diagnosis and care, which allowed us to examine temporal changes in TTD of ovarian cancer.9
One limitation of our study is our use of a retrospective deductive study design, which used established diagnoses to look backwards at pre-existing symptoms. This is opposed to what providers do when patients present with symptoms before a diagnosis is established and is reflected in our finding that 26% of our original study population did not present with classic symptoms at their index date visit. However, while our study is inherently limited by this retrospective study design, it was not our goal to test the sensitivity or specificity of classic ovarian cancer symptoms, which has been done previously.5, 6, 11, 47 Similarly, it was outside the scope of our study design to determine how frequently women who presented with a classic ovarian cancer symptom had a non-ovarian cancer diagnosis.
Other limitations include those inherent to large database studies such as SEER-Medicare.48 Specific to the SEER-Medicare database, we were only able to include women over age 66, which includes only 46.6% of new ovarian cancer cases49 and thus may not be representative of all women with ovarian cancer, although it is worth noting that the Goff index has a higher specificity for older populations.6 Because we only examined data through 2015, our conclusions may not be generalizable to the present, although our analysis does cover important changes in ovarian cancer workup and care including the use of tumor antigen immunoassay testing and the Affordable Care Act. Our data is based on coding during physician visits; thus, it does not tell us the frequency, severity, or duration of symptoms nor factors that led the individual to seek medical care. The lack of this information in the SEER-Medicare database limits the interpretation of our results, especially at an individual patient level, but has previously been described.3, 6, 8, 11 Our results are likely underestimates of how long women experience symptoms before they receive a diagnosis because we based on analysis on symptomatic presentation to care instead of patient-reported onset of symptoms. Although we tried to be inclusive in the codes that made up our symptom groupings, we may have inadvertently omitted important codes. Because all women in our study had insurance under non-HMO Medicare plans, our results may have limited generalizability in uninsured populations and for patients with HMO insurance plans, although ovarian cancer stage at diagnosis has not been previously shown to depend on HMO inclusion based on limited data.50 It is possible that our significant statistical findings were due to our large cohort size. Finally, the small absolute differences in TTD in our analyses may ultimately have limited clinical significance, although the impact of TTD on ovarian cancer survival remain largely unknown. In response to this need, our group is planning a follow-up study to examine the association between TTD and survival in ovarian cancer.
We found that even when women present with classic ovarian cancer symptoms, the TTD depends on a variety of factors. In exploring differences in TTD based on specialty, we identified an important opportunity for physician education on ovarian cancer symptoms to expedite diagnostic workup and appropriate referrals. In identifying population-wide factors that affect time to diagnosis, we found differences based on race and ethnicity that may point to larger elements of structural racism in our medical system as well as differences based on geographic location that suggest where healthcare resources could be used to improve care pathways. We believe that recognizing the multilevel factors that impact TTD is an important first step toward accomplishing the goal of decreasing the time to ovarian cancer diagnosis. Future research should seek to determine whether TTD is associated with ovarian cancer treatment and survival in the United States.
Supplementary Material
Acknowledgements:
Don Norwood contributed editing assistance through the University of Texas MD Anderson Medical Research Library. No compensation was provided.
Funding Support:
This work was supported in part by the MD Anderson Cancer Center Support Grant from the National Cancer Institute of the National Institutes of Health (NIH/NCI P30 CA016672, CA217685) and the T32 training grant CA101642 (SH). LAM is supported by a NIH-NCIK07-CA201013 grant.
Conflict of Interest:
LAM reports research funding from AstraZeneca, consulting for Glaxo-Smith-Kline, and stocks in Crispr and Bristol-Myers Squibb. The remaining authors report no conflicts of interest related to the subject matter of this manuscript.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2.Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer. 2000;89: 2068–2075. [DOI] [PubMed] [Google Scholar]
- 3.Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. Jama. 2004;291: 2705–2712. [DOI] [PubMed] [Google Scholar]
- 4.Hess LM, Stehman FB, Method MW, Weathers TD, Gupta P, Schilder JM. Identification of the optimal pathway to reach an accurate diagnosis in the absence of an early detection strategy for ovarian cancer. Gynecol Oncol. 2012;127: 564–568. [DOI] [PubMed] [Google Scholar]
- 5.National Collaborating Centre for C. National Institute for Health and Clinical Excellence: Guidance. Ovarian Cancer: The Recognition and Initial Management of Ovarian Cancer. Cardiff (UK): National Collaborating Centre for Cancer (UK) Copyright © 2011, National Collaborating Centre for Cancer., 2011. [Google Scholar]
- 6.Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109: 221–227. [DOI] [PubMed] [Google Scholar]
- 7.Olson SH, Mignone L, Nakraseive C, Caputo TA, Barakat RR, Harlap S. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98: 212–217. [DOI] [PubMed] [Google Scholar]
- 8.Goff B Symptoms associated with ovarian cancer. Clin Obstet Gynecol. 2012;55: 36–42. [DOI] [PubMed] [Google Scholar]
- 9.Orr B, Edwards RP. Diagnosis and Treatment of Ovarian Cancer. Hematol Oncol Clin North Am. 2018;32: 943–964. [DOI] [PubMed] [Google Scholar]
- 10.Lurie G, Thompson PJ, McDuffie KE, Carney ME, Goodman MT. Prediagnostic symptoms of ovarian carcinoma: a case-control study. Gynecol Oncol. 2009;114: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MK, Kim K, Kim SM, et al. A hospital-based case-control study of identifying ovarian cancer using symptom index. J Gynecol Oncol. 2009;20: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baun ML, Jensen H, Falborg AZ, Heje HN, Petersen LK, Vedsted P. Ovarian cancer suspicion, urgent referral and time to diagnosis in Danish general practice: a population-based study. Fam Pract. 2019;36: 751–757. [DOI] [PubMed] [Google Scholar]
- 13.Robinson KM, Christensen KB, Ottesen B, Krasnik A. Diagnostic delay, quality of life and patient satisfaction among women diagnosed with endometrial or ovarian cancer: a nationwide Danish study. Qual Life Res. 2012;21: 1519–1525. [DOI] [PubMed] [Google Scholar]
- 14.Dilley J, Burnell M, Gentry-Maharaj A, et al. Ovarian cancer symptoms, routes to diagnosis and survival - Population cohort study in the ‘no screen’ arm of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Gynecol Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett J, Sharp DJ, Stapley S, Stabb C, Hamilton W. Pathways to the diagnosis of ovarian cancer in the UK: a cohort study in primary care. Bjog. 2010;117: 610–614. [DOI] [PubMed] [Google Scholar]
- 16.Lim A, Mesher D, Gentry-Maharaj A, et al. Time to diagnosis of Type I or II invasive epithelial ovarian cancers: a multicentre observational study using patient questionnaire and primary care records. Bjog. 2016;123: 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan SJ, Francis JE, Nelson AE, Zorbas HM, Luxford KA, Webb PM. Pathways to the diagnosis of epithelial ovarian cancer in Australia. Med J Aust. 2010;193: 326–330. [DOI] [PubMed] [Google Scholar]
- 18.Crawford SC, Davis JA, Siddiqui NA, et al. The waiting time paradox: population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. Bmj. 2002;325: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khorana AA, Tullio K, Elson P, et al. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS One. 2019;14: e0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagle CM, Francis JE, Nelson AE, et al. Reducing time to diagnosis does not improve outcomes for women with symptomatic ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2011;29: 2253–2258. [DOI] [PubMed] [Google Scholar]
- 21.Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112 Suppl 1: S92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman AD, Lambert P, Love AJ, et al. Examining the Effects of Time to Diagnosis, Income, Symptoms, and Incidental Detection on Overall Survival in Epithelial Ovarian Cancer: Manitoba Ovarian Cancer Outcomes (MOCO) Study Group. Int J Gynecol Cancer. 2017;27: 1637–1644. [DOI] [PubMed] [Google Scholar]
- 23.Gomez DR, Liao KP, Swisher SG, et al. Time to treatment as a quality metric in lung cancer: Staging studies, time to treatment, and patient survival. Radiother Oncol. 2015;115: 257–263. [DOI] [PubMed] [Google Scholar]
- 24.Overview of the SEER program. Available from URL: https://seer.cancer.gov/about/overview.html [accessed 11/14/2020.
- 25.American Joint Committee on Cancer (2002) Ovary. In: Greene FL et al. () AJCC Cancer Staging Manual. Springer NY, NY. 10.1007/978-1-4757-3656-4_30. [DOI] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40: 373–383. [DOI] [PubMed] [Google Scholar]
- 27.Rural-urban continuum codes. Available from URL: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/ [accessed December 21, 2020, 2020].
- 28.Cassel CK, Reuben DB. Specialization, subspecialization, and subsubspecialization in internal medicine. N Engl J Med. 2011;364: 1169–1173. [DOI] [PubMed] [Google Scholar]
- 29.Brotherton SE, Rockey PH, Etzel SI. US graduate medical education, 2004–2005: trends in primary care specialties. Jama. 2005;294: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 30.Jeffe DB, Whelan AJ, Andriole DA. Primary care specialty choices of United States medical graduates, 1997–2006. Acad Med. 2010;85: 947–958. [DOI] [PubMed] [Google Scholar]
- 31.Morgan RJ Jr., Armstrong DK, Alvarez RD, et al. Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14: 1134–1163. [DOI] [PubMed] [Google Scholar]
- 32.Boac BM, Xiong Y, Apte SM, et al. Adherence to practice guidelines is associated with reduced referral times for patients with ovarian cancer. Am J Obstet Gynecol. 2018;218: 436.e431–436.e437. [DOI] [PubMed] [Google Scholar]
- 33.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121: 1226–1234. [DOI] [PubMed] [Google Scholar]
- 34.Richards JM, Burgon TB, Tamondong-Lachica D, et al. Reducing Unwarranted Oncology Care Variation Across a Clinically Integrated Network: A Collaborative Physician Engagement Strategy. J Oncol Pract. 2019;15: e1076–e1084. [DOI] [PubMed] [Google Scholar]
- 35.Weiner AB, Matulewicz RS, Tosoian JJ, Feinglass JM, Schaeffer EM. The effect of socioeconomic status, race, and insurance type on newly diagnosed metastatic prostate cancer in the United States (2004–2013). Urol Oncol. 2018;36: 91.e91–91.e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina Y, Silva A, Rauscher GH. Racial/Ethnic Disparities in Time to a Breast Cancer Diagnosis: The Mediating Effects of Health Care Facility Factors. Med Care. 2015;53: 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee S, Gupta D, Caputo TA, Holcomb K. Disparities in Gynecological Malignancies. Front Oncol. 2016;6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karanth S, Fowler ME, Mao X, et al. Race, Socioeconomic Status, and Health-Care Access Disparities in Ovarian Cancer Treatment and Mortality: Systematic Review and Meta-Analysis. JNCI Cancer Spectr. 2019;3: pkz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hildebrand JS, Wallace K, Graybill WS, Kelemen LE. Racial disparities in treatment and survival from ovarian cancer. Cancer Epidemiol. 2019;58: 77–82. [DOI] [PubMed] [Google Scholar]
- 40.Stenzel AE, Buas MF, Moysich KB. Survival disparities among racial/ethnic groups of women with ovarian cancer: An update on data from the Surveillance, Epidemiology and End Results (SEER) registry. Cancer Epidemiol. 2019;62: 101580. [DOI] [PubMed] [Google Scholar]
- 41.Bandera EV, Lee VS, Rodriguez-Rodriguez L, Powell CB, Kushi LH. Racial/Ethnic Disparities in Ovarian Cancer Treatment and Survival. Clin Cancer Res. 2016;22: 5909–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doll KM. Investigating Black-White disparities in gynecologic oncology: Theories, conceptual models, and applications. Gynecol Oncol. 2018;149: 78–83. [DOI] [PubMed] [Google Scholar]
- 43.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Sociodemographic disparities in advanced ovarian cancer survival and adherence to treatment guidelines. Obstet Gynecol. 2015;125: 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villanueva C, Chang J, Bartell SM, Ziogas A, Bristow R, Vieira VM. Contribution of Geographic Location to Disparities in Ovarian Cancer Treatment. J Natl Compr Canc Netw. 2019;17: 1318–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daruvala A, Lucas FL, Sammon J, Darus C, Bradford L. Impact of geography and travel distance on outcomes in epithelial ovarian cancer: a national cancer database analysis. Int J Gynecol Cancer. 2020. [DOI] [PubMed] [Google Scholar]
- 46.Thiese MS. Observational and interventional study design types; an overview. Biochem Med (Zagreb). 2014;24: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst. 2010;102: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40: Iv-3–18. [DOI] [PubMed] [Google Scholar]
- 49.SEER cancer stat facts: ovarian cancer. Available from URL: https://seer.cancer.gov/statfacts/html/ovary.html.
- 50.McGuire V, Herrinton L, Whittemore AS. Race, epithelial ovarian cancer survival, and membership in a large health maintenance organization. Epidemiology. 2002;13: 231–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


