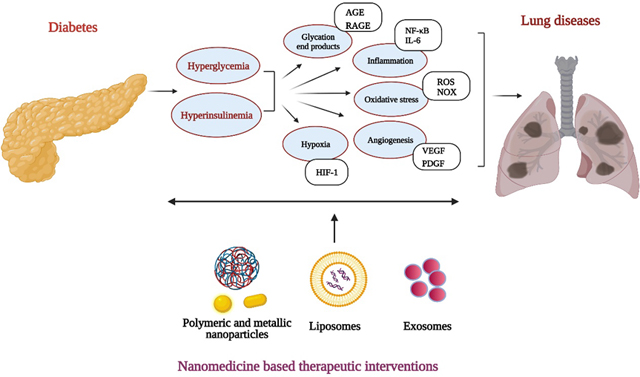
Abstract
Diabetes mellitus (DM), is the most common metabolic disease and is characterized by sustained hyperglycemia. Accumulating evidences supports a strong association between DM and numerous lung diseases including chronic obstructive pulmonary disease (COPD), fibrosis, and lung cancer (LC). The global incidence of DM-associated lung disorders is rising and several ongoing studies, including clinical trials, aim to elucidate the molecular mechanisms linking DM with lung disorders, in particular LC. Several potential mechanisms, including hyperglycemia, hyperinsulinemia, glycation, inflammation, and hypoxia, are cited as plausible links between DM and LC. In addition, studies also propose a connection between the use of anti-diabetic medications and reduction in the incidence of LC. However, the exact cause for DM associated lung diseases especially LC is not clear and is an area under intense investigation. Herein, we review the biological links reported between DM and lung disorders with emphasis on LC. Furthermore, we report common signaling pathways (eg: TGF-β, IL-6, HIF-1, PDGF) and miRNAs that are dysregulated in DM and LC and serve as molecular targets for therapy. Finally, we propose a nanomedicine based approach for delivering therapeutics (eg: IL-24, HuR siRNA) to disrupt signaling pathways common to DM and LC and thus potentially treat DM-associated LC. Finally, we conclude that the effective modulation of commonly regulated signaling pathways would help design novel therapeutic protocols for treating DM patients diagnosed with LC
Keywords: Diabetes, drug delivery, HuR, IL-24, lung cancer, nanomedicine
Graphical abstract.
Signaling pathways shared between diabetes and chronic lung disease including lung cancer offer avenues for therapeutic interventions with various nanoformulations. Image created with BioRender.com
1. Introduction
Diabetes Mellitus (DM) is a pathological condition characterized by increase in blood sugar levels and the loss of functional β-cell mass in the pancreas is identified to be the key mechanism underlying DM. The β-cells of the pancreas produce the hormone insulin which in turn promotes absorption of glucose into cells as an energy source. There are two forms of DM is which differ from each other pathogenically: 1) Type 1 DM, or T1DM, wherein the pancreas cannot produce insulin due to the depletion of pancreatic β-cells mediated by autoimmune reactions (insulin deficiency); and 2) Type 2 DM, or T2DM, which is a metabolic disorder that arises due to loss of insulin secretion coinciding with insulin resistance by the pancreas. Both types of DM ultimately lead to hyperglycemia and predispose individuals to other physiological disorders. Measurement of fasting plasma glucose (FPG) and plasma glycated hemoglobin (HbA1c) levels, the latter of which reflects the mean ambient fasting and postprandial glycemic values over a 2- to 3-month period, are widely used to determine the status of long-term glyco-regulation in patients.
The burden of DM has increased worldwide with over 450 million cases reported as of 2019 and a predicted expected total of 700 million by 2045 [1]. T1DM, arising largely due to an individual's inherent genetic predisposition, is most often diagnosed in children ranging from 0 to 14 years, but can also occur in individuals at any age. T1DM can be triggered by environmental factors (viral infections, vitamin D levels, hygiene, and exposure to ultraviolet rays or pollutants) and diet/lifestyle factors (physical activity, nutrition, stress, socioeconomic conditions) [2, 3]. In most cases of T1DM, onset is sudden and is characterized by symptoms of polydipsia, polyuria, lack of energy/extreme tiredness, polyphagia, sudden weight loss, slow-healing wounds, recurrent infections and dehydration [4]. In summary, T1DM arises due to complex interplay of genetic, immune and environmental factors leading to destruction of pancreatic β-cell function. Infiltrating immune cells, suggestive of increased inflammation, have also been seen in T1DM patients [5]. In contrast, T2DM, occurs predominantly in adults, although numerous studies report increasing numbers in children and adolescents [6]. The numbers of people with T2DM is significantly higher than those with T1DM; the increasing incidence of T2DM is largely attributable to sedentary lifestyles, unhealthy dietary habits, stressful lifestyles, and environmental conditions in addition to any underlying putative hereditary influences. Based on these combined factors, T2DM occurs at varying rates in different racial and/or ethnic groups.
Irrespective of DM type, increased blood glucose levels affect multiple organs, including the kidneys, eyes, and liver, and, thus, DM patients require continuous ongoing evaluation to assess diabetes related complications. Morbidity and mortality from DM results from both macrovascular disease (eg: coronary artery disease, cerebrovascular accident) and microvascular disease (eg: retinopathy, nephropathy, and neuropathy) [7]. Both diabetes itself and diabetic medications are known to induce a wide variety of pulmonary complications, and over the last few years many studies have identified the lung as an impacted organ in diabetes [8]. DM patients reportedly have an increased risk of asthma, chronic obstructive pulmonary disease (COPD), pneumonia, fibrosis and lung cancer [9].
The data suggests potentially overlapping or shared regulatory mechanism(s) between the two pathologically different disease states i.e. DM and lung disease. This premise is further substantiated by the ongoing clinical trials focused on DM and lung diseases (Table 1), for example, one clinical trial (NCT02967406) is studying the role of a sedentary lifestyle as a cause of DM, COPD and cancers. However, the relationships between diabetes and lung disorders are not clearly understood, and elucidating how the two disease states operate and influence each other will provide avenues to develop new treatment strategies. This review will largely focus on the role of diabetes in lung disorders with special emphasis on lung cancer (LC), and lead to suggestive therapeutic interventions for better management of diabetes associated LC.
Table 1.
Current clinical trials on DM and lung diseases registered in clinicaltrials.gov
| Trial No. | Study Title | Condition | Intervention | Status | Phase |
|---|---|---|---|---|---|
| NCT04001725 | Efficacy of metformin in preventing glucocorticoid-induced diabetes in patients with brain metastases | Brain metastases, melanoma, LC, breast cancer | Dexamethasone, Metformin | Recruiting | Phase 2 |
| NCT00782366 | Predictive genetic risk assessment trial | Colon cancer, LC, T2DM, atrial fibrillation, obesity | SNP analysis | Completed | N.A. |
| NCT02967406 | Impact of lifestyle modification the development of dementia, chronic kidney disease, diabetes, COPD, cancers and cardiovascular disease in a Thai general population | Dementia DM, chronic kidney disease, cardiovascular diseases, malignancy COPD | Behavioral | Active, not recruiting | N.A. |
| NCT01236053 | Cancer in patients with Gabapentin (GPRD) | Diabetes and cancers | Gabapentin | Completed | N.A. |
1.1. Diabetes and COPD
COPD, a condition characterized by irreversible airway obstruction, is mostly caused by long-term exposure to harmful substances such as cigarette smoke. COPD is the third leading cause of death, it affects 380 million patients worldwide [10], is classified as a disease of elderly, and its prevalence increases with age. Cardiovascular disease, diabetes, obesity and hypertension are all reported co-morbidities in COPD patients [11]. Approximately 10% of diabetes patients have COPD [12, 13] indicating a potential relationship between the two diseases. Studies indicate that DM can worsen the disease progression in COPD patients and increase their mortality (1.62 times) [14, 15]. Patients with T2DM have a higher incidence of COPD, an association that is stronger in women than in men and in those over 65 years of age [16, 17]. The chronic hyperglycemia of diabetes increases a patient’s risk of developing chronic airflow obstructions that subsequently impair efficient breathing by causing mechanical dysfunction of the lungs and airways. A meta-analysis by Bram van den Borst et al. showed that reduced lung function manifests as a reduction in forced expiratory volume in one second (FEV1%, 2.8% in T1DM and 4.9% in T2DM) and reduction of forced vital capacity (FVC %, 3.8% in T1DM and 6.7% in T2DM) [18]. Additionally, HbA1c levels (<6% and >10%) are associated with a greater risk (HR: 1.19, 95% CI: 1.06–1.34) of COPD in patients with T2DM [19], suggesting that both, hyperglycemia and hypoglycemia can support development of COPD. While hyperglycemia, oxidative stress, inflammation, and use of corticosteroid therapy could all potentially contribute to the link between DM and COPD, the exact mechanisms and effect of DM over COPD is not well elucidated.
Equally, manifestations of COPD can also lead to DM. The increased expression of C-reactive protein (CRP), reflecting an inflammatory process, that is seen in the lungs of COPD patients [20] that could further lead to development of diabetes. COPD is characterized by increased inflammation and oxidative stress, as well as upregulation of mammalian target of rapamycin (mTOR) [21]. A higher risk of developing T2DM in COPD patients (1.8; 95% CI:1.1–2.8) than in asthma patients (1.0; 0.8–1.2) may be attributable to the different inflammatory and immune cell patterns observed in COPD (dominated by neutrophils, macrophages, and Th1 cells) and asthma (eosinophils and Th2 cells) respectively [22]. Interestingly, a retrospective case control study has reported a beneficial role of T2DM on COPD and attributed this to lifestyle changes [23].
The effects of anti-diabetic medications on COPD and anti-COPD treatment modalities on DM have also been widely studied. Metformin, a common drug used in T2DM, reduced the risk of COPD (HR 0.46; 95% CI) when used for more than 2 years [15, 24]. Furthermore, an observational study evaluated the effects of six-month metformin treatment in T2DM patients with moderate-to-severe COPD and identified improved dyspnea and health status [25]. The survival benefit observed with metformin reportedly arose from a reduction in airways infections; metformin use leads to reduced airways glucose permeability and decreases Staphylococcus aureus load induced under hyperglycemia [26]. In addition, gut microbiota dysbiosis plays a role in in the progression and exacerbation of COPD [27], and use of metformin induces compositional changes in the gut microbiota which lead to improved insulin resistance and reduced tissue inflammation [28]. However, use of insulin, or sulphonylurea did not associate with COPD [23]. Inhaled corticosteroids (ICS) are in used to manage COPD and exposure to systemic corticosteroids reportedly causes a dose-dependent increase in serum glucose concentration (hyperglycemia) in DM patients [29]. However, O’Byrne et al., [30] found no significant association between budesonide usage and onset of diabetes or hyperglycemia in COPD patients. It is evident from these reports that the contribution of DM to COPD remains unclear and investigations into the underlying molecular mechanisms to identify potential causal effects are warranted.
1.2. Diabetes and Asthma
Asthma is a chronic respiratory disease that affects approximately 8% of the population in the United States [https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm].Diabetes (T1DM and T2DM) contributes to an increased risk of asthma, with the incidence of asthma being 47% higher in the T1DM cohort than in non-diabetic controls [31]. Wu et al., showed that insulin resistance and metabolic syndrome or metabolic features common in pre-diabetes/diabetes, can further influence asthma morbidity and that such patients have higher (27% - prediabetes and 33% - diabetes) asthma exacerbation rates [32]. Another study reported that asthma incidence was significantly higher (HR 1.30; 95% CI: 1.24–1.38) in a diabetic cohort than a non-diabetic cohort after adjusting for age, sex, and obesity [33]. Increased HbA1c (aOR; 1.38 95% CI: 1.03 – 1.84) and insulin levels (aOR 1.02; 95% CI: 1.01 – 1.04) also lead to increased occurrence of asthma [34]. Yang et al., reported an association between HbA1c, asthma-related hospitalizations, and lung function among adults in the United Kingdom. HbA1c levels in the prediabetes/diabetes range (≥42 mmol/mol) were found to be associated with 1 or more asthma hospitalizations and inversely associated with FEV1 and FVC [35]. Single nucleotide polymorphisms (SNPs) common to T1DM and asthma have also been reported; both T1DM and allergic asthma were significantly associated with the TLR2 rs3804100 T allele and this may be a susceptibility locus common to the two diseases [36]. Analysis of Th1/Th2 cytokine balance in patients with T1DM and asthma revealed a similar cytokine expression pattern but lower Th1/Th2 ratio when compared to patients with T1DM only [37].
Chen et al., [33] investigated whether use of various anti-diabetic agents associate with the risk of asthma; insulin use increased (OR 2.23; 95% CI: 1.52–3.58) the risk of asthma among diabetic patients. Insulin, via activation of the phosphoinositide 3-kinase (PI3K) pathway, promotes mast cell survival and degranulation, which in turn facilitates bronchoconstriction and enhances airway hyper-responsiveness, an observed clinical phenotype of asthma [38–40]. Insulin also modulates the T cell differentiation by promoting a shift toward a Th2 type response, which is key mechanism to the pathogenesis of asthma [41]. Inhaled insulin in diabetic patients is associated with decreased lung function as determined by FEV1 [42]. In contrast, the use of either metformin (HR 0.80; 95% CI 0.69–0.93) or sulphonylurea (HR 0.76; 95% CI: 0.60–0.97) was beneficial and reduced the risk of asthma [43, 44] and related outcomes, including asthma-related hospitalization (OR 0.43; 95% CI : 0.14–1.29), asthma-related emergency room visits (OR 0.81; 95% CI: 0.74–0.89) and asthma exacerbations (OR 0.65; 95% CI: 0.28–1.48) [45]. Metformin also reduces both airway inflammation and remodelling in vitro and in vivo [33, 46, 47]. In another study, the effect of glucagon like peptide-1 receptor (GLP-1R) agonist on T2DM patients with asthma was compared to sodium glucose linked transporter 2 (SGLT-2) and dipeptidylpeptidase 4 (DPP-4) inhibitors, sulphonylureas and basal insulin [48]. The study results showed that GLP-1R agonist administration in adult asthmatics with T2DM was beneficial and reduced the number of asthma exacerbations compared to all other drugs used for treatment intensification. It is evident that there are some therapeutic benefits in using anti-diabetic medicines for asthma, however, the mechanism of action underlying these beneficial effects are yet to be determined.
1.3. Diabetes and Lung Fibrosis
Diabetes reportedly induces pathological changes including inflammation and fibrosis in the lung tissue. Mechanisms believed to contribute to hyperglycemia-associated tissue fibrosis include oxidative stress, deregulation of the nuclear factor-kappa B (NF-κB) and transforming growth factor beta (TGF-β) pathways, apoptosis and endoplasmic reticulum (ER) stress [49–52]. Additionally, deposition of extracellular matrix (ECM) proteins, including collagen and fibronectin, by lung fibroblasts and inflammatory myofibroblasts also contributes to lung fibrosis. Chen et al., using a diabetic rat model showed that sirtuin 3 (SIRT3) is important for mitigating lung fibrosis. In their study, diabetic rats developed lung fibrosis and the fibrotic lung tissue had increased levels of N-cadherin and alpha smooth muscle actin (α-SMA), proteins associated with epithelial to mesenchymal transition (EMT), along with increased collagen I, collagen III, and fibronectin expression. Administration of bone marrow derived mesenchymal stem cells (MSCs) to the diabetic rats resulted in increased SIRT3 and superoxide dismutase (SOD) expression with concomitant reduction in lung fibrosis. Molecular studies revealed that SIRT3 expression by MSCs suppressed EMT, as evidenced by increased E-Cadherin expression, and inhibited the NF-κB/HMGB1/RAGE/NLRP3-mediated inflammatory response, and reduced apoptotic cell death and ER stress by modulating the PI3K/AKT signaling pathway [49]. Thus, the use of MSCs as a therapeutic approach for diabetes associated lung fibrosis was proposed. Talakatta et al., demonstrated that both TGF-β signaling and high glucose (HG) played a role in diabetes induced lung fibrosis [53]. TGF-β signaling promoted EMT leading to inflammation and fibrosis while reducing glucose caused reversion to the mesenchymal to epithelial transition (MET) phenotype. The study showed that sustained exposure to HG conditions as a consequence of diabetes supported cell proliferation and EMT that led to lung fibrosis and pulmonary dysfunction. Additionally, uncontrollable cell proliferation and inflammation can increase the risk of LC development. From a therapeutic stand point, treatment with the GLP-1R agonist exendin-4 partially reduced the hyperglycemia-induced tissue damage by reducing glucose levels and oxidative stress. However, use of this molecule also reduced insulin signaling and collagen accumulation which in turn increased lung injury thereby limiting its usefulness [54]. The studies described show a clear association between diabetes and lung fibrosis, and several signaling pathways contributing to the disease have been identified. However, there are significant gaps in the field that require study, and improved treatments to manage diabetes-induced lung fibrosis are needed.
1.4. Diabetes and Cancer
Cancer primarily occurs due to gene alterations and mutations that confers uncontrolled proliferation and survival on tumor cells. In recent years, both metabolism and aberrant metabolic activity have been linked to cancer. Epidemiologic and clinical studies indicate a complex relationship between diabetes, obesity and the incidence of cancer. Both T1DM and T2DM are associated with an increased risk of cancer progression. For example, diabetes may perturb various cell signaling mechanisms that are crucial for the initiation and or progression of tumorigenesis; it does so via hyperglycemia, which drives malignant cell growth, hyperinsulinemia, which impacts cell survival and mitogenesis, and inflammation [55, 56]. Thus, understanding the molecular changes occurring in DM, especially at the cellular level, will provide insights into their role in cancer and offer therapeutic strategies to mitigate cancer growth. While DM is associated with a broad spectrum of human cancers (including pancreatic, ovarian, breast, and uterine among others) [57], in the following sections we will discuss the putative association of LC with DM with particular emphasis on molecular signaling pathways shared between the two diseases and therapeutic strategies to disrupt such signaling pathways using nanomedicine.
1.4.1. Lung cancer (LC)
LC is the leading cause of cancer related mortality worldwide. Epidemiologic studies suggest that diabetic patients are at a significantly higher risk both for developing LC and for LC-associated mortality (HR 1.27; 95% CI: 1.07–1.50) [58, 59]. Pre-existing diabetes had an adverse effects on cancer patient survival, including those with LC, and was associated with increased mortality (HR 1.44; 95% CI: 1.40–1.49) compared to cancer patients without diabetes [57]. Higher fasting blood glucose (>126 mg/dL) is an independent prognostic factor contributing to higher risk (69% greater) and mortality rates in non-small cell lung carcinoma (NSCLC) patients [60]. Furthermore, the hyperglycemic environment of diabetes was associated with different survival outcomes in patients diagnosed with NSCLC and those with small cell lung cancer (SCLC) [61]. NSCLC patients with DM who underwent surgery as first line treatment had poor survival compared to those patients without DM undergoing surgery; for NSCLC patients receiving chemotherapy DM-status did not impact survival. In contrast, SCLC patients with DM and receiving chemotherapy or chemo-radiation therapy had poor survival compared to SCLC patients without DM. These studies show that DM, in addition to playing an important role in LC development, also influences treatment response and survival outcomes. While the mechanism by which DM influences LC growth and treatment response is unclear, one explanation put forth is that higher expression of insulin receptor (IR) and insulin-like growth factor (IGF), in addition to apart increasing serum insulin levels, may contribute to LC growth and metastases; higher plasma IGF-I levels was associated with increased (OR 2.06; 95% CI:1.19–3.56) risk of LC [62]. Another study reported that greater expression of IGF-1 receptor (IGF-1R) and insulin receptor substrate 2 (IRS-2) in NSCLC patients with T2DM, negatively correlated with lymph node metastases and tumor staging [63].
Additional factors contributing to LC progression include HG. Increased HG levels suppressed miR-194 and concomitantly increased miR-194 regulated nuclear factor of activated T cells 5 (NFAT5) in NSCLC cell lines resulting in increased cell proliferation, invasion and migration [64]. NFAT5 is known to play a role in cell differentiation and inflammation during embryogenesis and has an oncogenic role in human cancers. In NSCLC with DM, miR-194 was significantly reduced with concomitant increase in NFAT5 expression compared to NSCLC without DM. HG was also shown to promote LC cell proliferation, migration and angiogenesis-related proteins (VEGF, HIF-1α) by modulating the receptor for advanced glycation end-products (RAGE)/Nicotinamide adenine dinucleotide phosphate oxidase (NOX)-4 [65]. Both, RAGE and NOX4 are associated with DM and reactive oxygen species (ROS)-mediated oxidative stress, and inhibiting RAGE/NOX4 reduces the proliferative and migratory activity of NSCLC cell lines. Thus, HG-mediated increased ROS and inflammation in DM could support LC progression. Alisson et al., reported that exposure of A549 LC cells to HG (25 mM) increased TGF-β secretion and expression of O-glycosylated oncofetal fibronectin that promoted EMT and tumorigenesis [66]. HG also confers proliferative and cell survival advantages by inhibiting the growth arrest-specific 5 (GAS5) –TRIB 3 axis [67] and potentially promotes resistance to anticancer drugs via JNK-mediated downregulation of the p53 pathway [68]. Finally, a retrospective study showed increased peritumoral inflammation (r=0.377; P<0.05), programmed death-ligand 1 (PD-L1) positivity (<50%), and disease recurrence (HR: 3.08; 95%CI: 1.027–9.23) in diabetic NSCLC compared to non-diabetic NSCLC [69]. The authors concluded that metabolic reprogramming due to DM likely contributed to increased PD-L1 expression and immune suppression leading to disease aggressiveness and recurrence. Thus, investigating how DM modulates the immune cell milieu will likely offer new avenues for incorporating immunotherapy for diabetic LC patients.
The association between T2DM and LC is also attributable to a sedentary lifestyle that results in the formation and accumulation of advanced glycation end products (AGEs). AGE derivatives (carboxymethyllysine (CML), argpyrimidine, and pentosidine) are formed by the glycosylation of sugars with macromolecules including proteins and lipids. The interaction of AGE with their receptor (RAGE) results in aberrant cell signaling and ROS generation leading to inflammation which culminates in cell damage [70, 71]. RAGE, which is highly expressed in normal lung tissues, was markedly reduced in NSCLC and that was associated with higher tumor stage (TNM) [72]. Similarly, higher levels of CML were observed in a LC animal model suggesting its involvement in the development and progression of LC [73]. All of the above reports indicate that DM plays an important role in the growth and progression of LC.
1.5. Molecular signaling pathways common to diabetes and LC
Diabetes and cancer, albeit differing in their patho-physiology, have significant overlap in the important molecular signaling pathways and associated proteins. For example, inflammation, angiogenesis, cell proliferation, cell death, and DNA damage, along with the various proteins (eg: TGF-β, IL6, PDGF, HIF-1, VEGF, c-myc, p53, NF-κB, ROS, AKT, mTOR) and nucleic acids (miRNA, mRNA, lncRNA) that regulate these processes, are both shared and are reported to be deregulated in diabetes and LC (Figure 1). Therefore, a better understanding of these commonly altered pathways will help in identifying molecules as therapeutic targets for use in diabetic LC patients. The following sections provide examples of few dysregulated molecular signaling mechanisms in DM and LC; the information provided herein is not exhaustive and readers are advised to refer additional literature.
Figure 1.
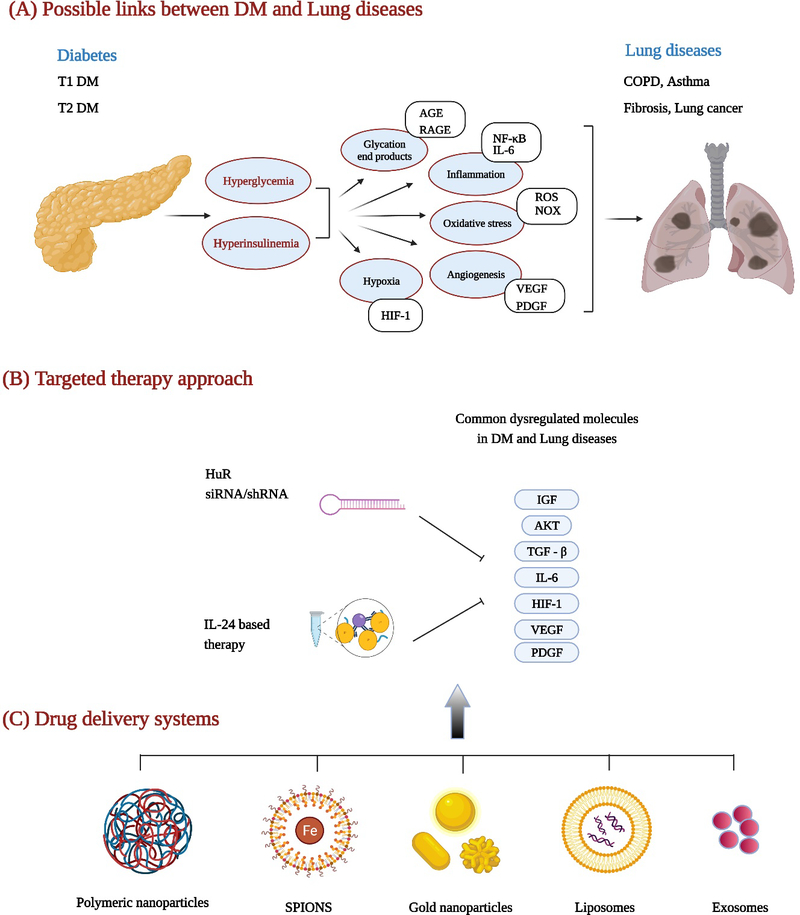
Schematic showing mechanistic links between, and therapeutic approaches for treating, diabetes and lung diseases. (A) signaling pathways and molecules triggered in diabetes that are shared in cancer and contribute to lung diseases. (B) molecules that are dysregulated in diabetes and cancer and regulated by both HuR and IL-24. Delivery of siRNA targeted to HuR or IL-24 plasmid DNA for inhibiting molecules expressed in both diabetes and LC. (C) nanoformulations that can be used for delivering HuR siRNA or IL-24 plasmid DNA. Images were created with Biorender.com.
1.5.1. Transforming growth factor (TGF) - β signaling
TGF-β is involved in various biological processes that includes regulating cell growth and differentiation, apoptosis, angiogenesis, and remodeling of the ECM [74]. TGF-β/Smad3 signaling regulates glucose tolerance and energy homeostasis as well as insulin gene transcription in the pancreatic islet β-cells. Dhawan et al., showed that TGF-β/Smad3 signaling activates and maintains p16Ink4a expression to prevent β-cell replication [75], and that inhibiting TGF-β signaling with small molecule inhibitors reduces p16Ink4a and promotes β-cell replication in human islets transplanted into non obese diabetic (NOD) - SCID IL-2Rg, null mice [75]. Finally, diabetes induced fibrotic changes in the lung occurs via the TGF-β1 signaling-mediated EMT pathway [53]. Thus, TGF-β signaling blockade is likely to be beneficial in both diabetes and diabetes-induced lung fibrosis [76].
TGF-β signaling is also important in cancer, including LC; TGF-β primarily promotes tumorigenesis and metastases by suppressing the immune system. In LC, high TGF-β expression is associated with advanced tumor stage, lymph node metastases, and worse survival, and thus, higher TGF-β expression is an indicator of poor prognosis [77]. In a 3D co-culture model of cancer-associated fibroblasts and LC cells, knockdown of TGF-β (1 and 2) caused anti-proliferative effects and attenuated fibroblast-mediated collagen gel contraction, resulting in reduced LC cell invasion [78]. Abrogation of TGF-β (1 & 2) using neutralizing antibodies, antisense oligonucleotides, and small molecule inhibitors has been tested as a potential cancer therapeutic in several preclinical studies [79, 80]. Assessment of anti-TGF therapies for LC has also proceeded to the clinical trials stage (clinicaltrials.gov). An ongoing phase I/II clinical study is currently testing stereotactic ablative radiotherapy in combination with the anti-TGF antibody (fresolimumab) in stage I/II LC (NCT02581787). In another study, a bispecific fusion protein targeting PD-L1 and TGF-β receptor II (M7824) is being assessed in combination with radiation for treatment of SCLC (NCT03554473). For additional clinical trials focused on TGF-targeted therapy in LC, readers are advised to visit www.clinicaltrials.gov.
It is evident that TGF-β plays an important role in DM, DM induced lung fibrosis, and LC. Thus, modulation of TGF-β will prove beneficial in improving DM status by restoring pancreatic β-cell function and in the process reducing LC tumor progression and metastases.
1.5.2. Interleukin (IL)-6 signaling
The pleiotropic cytokine IL-6 is primarily involved in inflammatory and immunological processes and dysregulation of IL-6 signaling is implicated in the pathogenesis of T1DM and T2DM [81, 82]. Systemic IL-6 is elevated in T1DM and T2DM patients [83, 84], and high circulating levels of IL-6 is considered to be an independent predictor of T2DM [85]. In addition, the IL-6 gene polymorphisms −174G>C (rs1800795) and −573G>C (rs1800796) also have a significant association with the incidence of T2DM [86]. In addition to the role of the pro-inflammatory cytokine IL-6 in insulin resistance, studies also report the involvement of IL-6 inhibition in improving insulin sensitivity, glucose homeostasis and inflammation in T2DM [87–89]. The effects of IL-6 on insulin sensitivity and glucose metabolism is context specific and appears to vary among different tissues. Akbari et al., [90] have suggested exploiting the therapeutic potential of selective IL-6 trans-signaling inhibition for management of T2DM; targeting IL-6-mediated chronic inflammation and signaling may be beneficial in T2DM treatment.
IL-6 is also well studied for its role in EMT, tumor progression and metastasis of NSCLC [91]; higher systemic IL-6 levels are prognostic factor for NSCLC patients [92]. High expression of IL-6 and the IL-6 receptor components gp80 and gp130 in NSCLC tissues indicate a possible role for IL-6-mediated signaling of downstream molecules (i.e. JAK/STAT, AKT, ERK) in promoting NSCLC [93]. The IL-6 promoter polymorphism (rs1800796), associated with T2DM, is also associated with increased risk of LC as well as prostate and colorectal cancers [94]. IL-6 reportedly enhances interactions between NSCLC cells and lung fibroblasts leading to increased EMT, chemoresistance and tumor progression by enhancing TGF-β signaling [95]. IL-6 also modulates the NF-κB signaling pathway which increases T-cell immunoglobulin and mucin domain containing 4 (TIM-4) expression and promotes migration, invasion and EMT of NSCLC cells [96]. Blockade of IL-6, on the other hand, apparently reprograms the lung tumor microenvironment and reduces tumor progression in K-Ras mutant lung tumors [97]. Nanomedicine-based treatment strategies for reducing IL-6 signaling in cancer has also been investigated. Polymeric and liposome based delivery of IL-6 siRNA, and IL-6R antibodies reduced cell proliferation in breast and colorectal cancer cells, and melanoma [98] and inhibited metastasis in mouse models of breast cancer [99]. Although the role of IL-6 in LC is well established, a significant knowledge gap exists regarding the complex molecular mechanisms of IL-6 signaling in the development of T2DM. Since a humanized anti-IL-6 antibody (ALD518) was tested in a Phase I clinical trials for treatment of NSCLC with fatigue and cachexia (NCT00866970) [100], it would be worthwhile to study the therapeutic effect of ALD518 in T2DM. In summary, therapeutic interventions capable of inhibiting the pro-inflammatory and deleterious effects of IL-6, without compromising its beneficial effects, including metabolic functions, would undoubtedly help treat T2DM and T2DM-associated LC.
1.5.3. Hypoxia inducible factor (HIF)-1 signaling
Cells must adapt to various physiological and pathological conditions to survive; under hypoxic conditions, rapidly proliferating cells (eg: embryogenesis, tumorigenesis) adapt to survive by activating the DNA binding transcription factor, HIF. In hypoxia, the HIF family of proteins induce the expression of many genes (eg: VEGF, PDGF, erythropoietin, and angiopoietin, among others) that facilitate metabolic reprogramming of the cell and enhance angiogenesis thereby promoting cell survival. Among the HIF family members, HIF-1α is the most studied and is known to play an important role in several human diseases including atherosclerosis, pulmonary hypertension, diabetes and cancer. Under normoxia, HIF-1α undergoes proteasomal degradation in the cytoplasm. However, under hypoxia, HIF-1α translocates to the nucleus where it dimerizes with HIF-1β thus becoming functional and transcriptionally activating its downstream targets that are essential for survival, angiogenesis, metabolism, and EMT. Similar functions can be assigned to HIF-2α due based on homology with HIF-1α, however, this review will focus on HIF-1α in the context of DM and cancer. For in-depth information on HIFs and their role in hypoxia, readers should refer to the literature.
HIF-1 signaling is required to maintain the normal homeostasis and function of the pancreas and pancreatic β-cells. However, HIF-1 signaling is deregulated in diabetes and diabetes-associated disorders [101]. In diabetes, the pancreatic islets are hypoxic and HG exacerbates hypoxia [102]. In T1DM, activation of HIF-1α signaling in the pancreatic β-cells protects against β-cell depletion [103]. Furthermore, deletion of HIF-1α in a NOD mouse model led to increased incidence of virus- and toxin-mediated T1DM, thus highlighting the importance of HIF-1α in pancreatic β-cells in preventing T1DM [104]. HIF-1α signaling and HIF-1α also reduce diabetic nephropathy and increase wound healing by promoting angiogenesis; nephropathy and poor wound healing are important complications of DM [105–107]. Thus, HIF-1 activation in diabetic tissues may be important in treating DM and diabetes-associated sequalae.
In contrast, HIF-1 activation in cancer is deleterious. HIF-1α activation in cancer cells especially under hypoxia, is associated with cell survival, angiogenesis, EMT and metastasis. In addition, in the hypoxic center of a tumor, HIF-1α activation promotes drug resistance. HIF-1α expression has been reported in many human cancers and regulates numerous growth factors and oncogenes [108]. In NSCLC, HIF-1α mRNA expression positively correlates with VEGF and hexokinase type II expression [109]; additionally, HIF-1α expression associates with disease progression and poor prognosis in node-positive patients. Gao et al., reported that HIF-2α, but not HIF-1 α, contributed to poor prognosis in NSCLC [110]. HIF-1α was recently shown to modulate the tumor microenvironment and create an immunosuppressive environment in LC cells by upregulating PD-L1 through the involvement of NF-κB and EZH2 [111, 112]. Finally, resistance to chemotherapy in LC has been attributed to HIF-1 and HIF-1-induced glycolysis [113]. Thus, HIF (−1α, −2α) are important in cancer development and progression and represent a potential therapeutic target.
Since HIF (−1α, −2α) plays an important role in cancer, nanomedicine-based drug delivery systems have been tested to knockdown HIF-1 in many cancers including LC. Delivery of echinomycin, an inhibitor of HIF-1α in a liposomal formulation showed suppression of TNBC metastases in tumor xenograft models with no treatment-related toxicity [114]. Chen et al., showed treatment of nasopharyngeal carcinoma with HIF-1α siRNA encapsulated in TPGS-b-poly(ε-caprolactone-ran-glycolide) NPs reduced HIF-1α levels and inhibited tumor growth both, in vitro and in vivo [115]. These studies suggest similar HIF-1α targeted nanomedicine approaches can be applied for LC treatment.
While HIF-1 expression is associated with deleterious effects in cancer, it does have a beneficial role in diabetes. Hence, the management and treatment of diabetes-associated LC using HIF-1 targeted therapeutics would need to be carefully designed and tested.
1.5.4. Platelet derived growth factor (PDGF) signaling
PGDF, via multiple mechanisms, promotes cell proliferation, growth, and migration. Deregulation of PDGF signaling occurs in several human diseases including diabetes, fibrosis, cancer, and atherosclerosis, among others [116]. Activation of PDGF occurs by binding of four PDGF polypeptides (A, B, C, and D) to form five functional growth factors (PDGF-AA, -BB, -AB, -CC and –DD). Binding of functional PDGF (AA, -BB, -AB, -CC and –DD) to its receptors (PDGFR-α and PDGFR-β) results in activation of downstream signaling and elicits appropriate functions. For more information on PDGF, readers are directed to an available comprehensive review [117]. Herein, we briefly discuss PDGF role in diabetes and cancer.
PDGF signaling in diabetes, especially in gestational diabetes, causes dedifferentiation of β cells which leads to β-cell dysfunction [118]. Overexpression of PDGF-AA, as well as hypomethylation at a CpG site in PDGF-AA, both reportedly increase T2DM risk, hyperinsulinemia, insulin resistance, and steatohepatitis risk [119]. Similarly, overexpression of PDGF-A and PDGF-B contribute to fibrosis in diabetic nephropathy [120], and PDGF-BB-mediated P38 MAPK activation leads to chronic inflammation in the vascular smooth muscle cells of diabetic rats [121].
Altered PDGF expression and signaling is also reported in lung diseases, including LC. High tumor-cell expression of PDGF-β and PDGFR-α correlated with poor survival and is a negative prognostic indicator for survival [122]. In contrast, high PDGFR- α expression in stromal cells was an indicator of increased survival while high PDGFR-β expression was associated with poor survival [123, 124]. It is important to note that PDGFR expression varies in NSCLC subtypes and in squamous cell carcinoma [123], suggesting a careful analysis of PDGF/PDGFR is warranted for diagnostic and prognostic assessments. In the context of PDGF/PDGFR-targeted therapeutics, small molecule kinase inhibitors, antibodies, and aptamers have been tested and show efficacy in preclinical studies [125–127]. Olaratumab, a human anti-PDGFR-α monoclonal antibody (IMC-3G3) is undergoing clinical testing in human cancers (NCT02783599, NCT02677116, NCT03086369). In a phase II clinical study, treatment of NSCLC patients with olaratumab in combination with chemotherapy showed no improvements in progression free survival (PFS) or overall survival (OS) [128]. In summary, aberrant PDGF/PDGFR signaling is a common feature in both diabetes and lung diseases, including LC, and targeted therapy to modulate PDGF levels in both, LC and T2DM has promise.
1.6. Therapeutic interventions for DM associated LC
1.6.1. Anti-diabetic drugs for LC
Metformin and thiazolidinediones (TZDs) are two anti-diabetic drugs that have been tested for their anticancer properties and have shown efficacy against LC in preclinical studies [129, 130]. In a mouse model of LC, metformin treatment reduced the tumor burden by 53–72% [131]. Metformin chemotherapy significantly improved survival (PFS: 8.4 months and OS: 20.0 months) in diabetic NSCLC patients [132]. Similarly, exposure to either metformin and/or TZD in T2DM patients reduced the likelihood of developing LC [130]. However, an aggressive disease phenotype was likely in T2DM patients who did develop LC while receiving metformin. Metformin/insulin treatment was also shown to lower the risk (metformin HR 0.82; 95% CI: 0.72–92 and insulin HR 0.65; 95% CI: 0.44–95) of LC-specific mortality [133]. Metformin in combination with epidermal growth factor-tyrosine kinase inhibitors (EGFR-TKI) show an enhanced therapeutic effect and increased PFS (9.2 months; 95% CI: 8.6–11.7) and OS (33.4 months; 95% CI: 29.4–40.2) in T2DM patients with LC. Additionally, metformin plus EGFR-TKI combinatorial therapy was superior to that of EGFR-TKI plus other hypoglycemic agents in NSCLC patients with T2DM [134, 135]. TZD, like metformin, reportedly reduces the risk of LC by 33% in diabetic patients [136]; TZD treatment reduced the expression of the anti-apoptotic, immunosuppressive and inflammatory prostaglandin E2 in NSCLC cell lines [137] and reduces the risk of LC by 33% [138]. Additional anti-diabetic agents such as SGLT2 and DPP4 inhibitors have also been reported to have beneficial effects on LC. While each of these anti-diabetic agents have shown antitumor activity, the molecular mechanism by which they operate varies and extensive and discussion of such is beyond the scope of this review. A single center, randomized Phase 2 trial of metformin in treating glucocorticoid-induced diabetes in patients with melanoma, LC and breast cancer with brain metastases is ongoing (NCT04001725). Results from the clinical study may elucidate the mechanism by which anti-diabetic drugs regulate cancer growth or prevention. In summary, use of anti-diabetic treatment modalities is anticipated to have beneficial effects in the management and treatment of LC, the efficacy of such treatments could be improved by using novel delivery systems described in section 1.7
1.6.2. Drug delivery approach targeting molecules/pathways to mitigate DM associated LC
1.6.2.1. Molecular targeting of human antigen R (HuR)
HuR is an RNA-binding protein which regulates the stability and transcription of numerous mRNAs whose protein products are known to promote cell growth (c-myc, c-Fos), cell cycle (p21, cyclin D, E), survival (Bcl-2, XIAP, Mcl1), angiogenesis (VEGF, HIF1), cytokines and growth factors (TNF, PDGF, IL-6, TGF-β), migration and invasion (MMP-9, uPA and uPAR), EMT (Snail, TGF-β), inflammation (COX-2, iNOS, IL-8) and immune evasion (TGF-β, MKP-1, PD-L1), etc. [139]. Thus, HuR likely plays an important roles in various normal and pathological conditions in humans.
In the context of lung diseases, Wang et al., reported that induction of PDGF elevated HuR expression in cultured airway smooth muscle cells [140]. The increase in HuR expression further led to enhanced expression of TGF-β1 and inflammatory cells indicating a HuR/TGF-β1 feedback loop that regulated airway remodelling in vivo and in vitro in asthmatic conditions. Thus, targeting HuR and interrupting the HuR/TGF-β1 circuit may make an impact in the management of asthma. HuR is also reported to mediate TGF-β1-induced pro-fibrogenic actions in hepatic stellate cells [141]. Finally, HuR knockout in skeletal muscle of mice results in a metabolically inflexible phenotype with mild obesity and impaired glucose tolerance [142], while decreased expression of oxidative phosphorylation, fatty acid transport, and mitochondrial fatty acid metabolism genes along with decreases in protein levels of molecules in the mTOR signaling pathway was reported [142]. All of these findings support a role for HuR in lung diseases, fibrosis and metabolism.
In cancer, HuR is overexpressed in multiple human cancers and is associated with poor prognosis. HuR overexpression correlated with tumor progression, metastasis and drug resistance [143], and suppressing HuR expression specifically in cancer cells would result in global knockdown of oncoproteins and pronounced antitumor activity. Thus, HuR is a promising molecular target for cancer therapy [144]. Wang et al., used small interfering RNA (siRNA) based approaches to target HuR in LC cells [145]. Although there was reduced HuR expression along with changes in cellular localization, the siRNA based interventions have certain limitations that restrict their use. First, the efficiency/bioavailability of siRNA largely depends on the route of administration, while their net negative charge makes it difficult to penetrate biological membranes and their relatively small size leads to rapid renal clearance by the kidneys. Their relatively short half-life along with threat of unmodified or naked siRNAs undergoing nucleases-mediated extracellular degradation poses another challenge. The many barriers to siRNA delivery are detailed in a recent review by Sajid et al. [146]. Incorporating various chemical modifications into siRNAs or the use of nanoformulations can ameliorate, at least partially, the above drawbacks. Studies from our own laboratory, as well as others, have developed and tested various drug delivery strategies targeting HuR for cancer treatment. Muralidharan et al., using a tumor-targeted cationic lipid-based nanoformulation demonstrated that systemic delivery of HuR-specific siRNA (Figure 2A) significantly suppressed tumor cell growth and migration in vitro and inhibited growth of local and experimental lung metastases in vivo [147, 148]. Interestingly, they showed that silencing of HuR not only reduced expression of several oncoproteins in the tumors but also produced complete tumor regression in few of the mice (Figure 2B) [148]. In another study, co-delivery of cisplatin and HuR siRNA encapsulated in a folate receptor targeted dendrimer nanoparticle showed additive to synergistic antitumor activity against human LC cells [149]. Combining HuR siRNA-nanotherapy with the CXCR4 inhibitor AMD3100 disrupted the CXCR4/SDF signaling and resulted in inhibition of LC cell migration and invasion [150]. Incorporating radiation therapy with HuR siRNA nanotherapy induced oxidative stress and DNA damage resulting in enhanced antitumor activity [151]. Molecular studies showed that silencing HuR prevented efficient repair of radiation induced DNA damage making the tumor cell undergo apoptotic cell death [152]. In ovarian cancer, delivery of HuR siRNA using a folic acid derivative DNA dendrimer (3D nanocarrier) resulted in anticancer activity both in vitro and in vivo [153].
Figure 2.
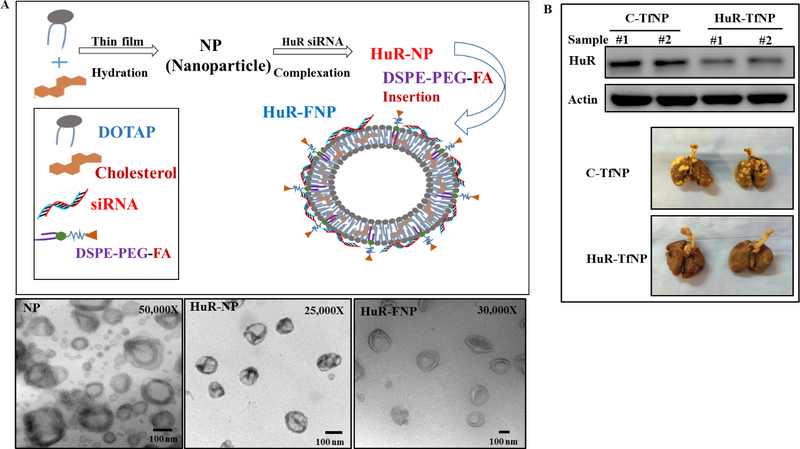
Nanomedicine based approaches for delivering HuR siRNA in LC cells. (A) Schematic representation of synthesis and TEM characterization of HuR siRNA – folate nanoparticle (FNP) prepared with DOTAP: Cholesterol, and (B) reduced expression of HuR in mouse A549 LC models treated with HuR – transferrin liposomal nanoparticle (TfNP) when compared to control. Image reproduced from Muralidharan et al., 2016 [148] and Muralidharan et al., 2017 [149] that is under an open access Creative Commons CC BY 4.0 licence.
Targeting HuR using small molecule inhibitors (CMLD2, MS-444) also results in antitumor activity in glioma, thyroid cancer and colorectal cancer [154–157]. Romeo, et al., showed MS-444-mediated inhibition of HuR restored TRAIL sensitivity in pancreatic tumor cells [158]. More recently, the HuR inhibitor KH-3 was demonstrated to interrupt the interaction between HuR and FOXQ1 and suppress breast cancer invasion and metastasis [157]. Recent studies also indicate a role for HuR in modulating the immune response. Depletion of HuR from macrophages and glial cells resulted in significant reduction in glioblastoma growth and was associated with reduction in tumor cell PD-L1 expression with concomitant increases in macrophages of the M1 phenotype and CD4+ T cells infiltration [159]. Liu et al., recently reported a direct association between HuR and PD-L1, and treatment with MS-444 resulted in reduction in both HuR and PD-L1 and suggested that incorporating PD-L1/PD-1 immunotherapy will likely restore the host immune response and improve the efficacy of MS-444 and other HuR-targeted therapeutics[160]. We have demonstrated that silencing of HuR while having inherent antitumor activity, also increased cell surface PD-L1 expression in LC cells (unpublished data) supporting the incorporation of anti-PD-L1 immunotherapy with HuR-targeted therapy. However, further detailed studies focused on the molecular mechanism(s) by which HuR regulates PD-L1, and possibly other immune checkpoint proteins, are essential before incorporating immunotherapy with HuR-targeted therapy.
As well as playing a role in cancer progression, HuR is also associated with muscle wasting and atrophy. Muscle wasting, or cachexia is a debilitating and common condition among cancer patients that is aggravated by cancer treatments. Sanchez et al., using muscle-specific HuR knockout mice showed that loss of HuR prevented muscle loss due to cancer cachexia [161]. In another study, HuR was shown to regulate inflammation induced muscle wasting by competing with micro (mi) RNA-330 for binding to the 3’ untranslated region (UTR) of STAT-3 [162]. miR-330 promotes muscle wasting, and its displacement by HuR suppresses STAT-3 mediated muscle wasting. All of these studies convincingly demonstrate HuR is a valid molecular target for cancer therapy. A careful design of drug delivery approaches to selectively disrupt HuR functions in cancer cells, immune cells and skeletal muscles could significantly impact in cancer management and treatment outcomes.
Another important aspect to consider is that many of the HuR-regulated mRNAs and their protein products identified and reported as overexpressed in cancer are also known to be modulated in DM and DM-associated lung diseases. For example, Amadio et al., showed that lipoplexes-mediated HuR siRNA delivery to the eye of streptozotocin (STZ)-induced diabetic rats significantly reduced vascular endothelial growth factor (VEGF), a molecular target of HuR and prevented diabetes induced retinopathy [163]. The study established the proof-of-concept of targeting HuR for managing diabetes-associated retinal diseases. Thus, availability of HuR-targeted therapeutics that specifically suppress HuR in the desired target cells (i.e. cancer and skeletal muscle among others) will be beneficial in treating both DM independent LC and DM-associated LC. Additionally, HuR-targeted therapeutics will potentially be beneficial in treating DM-associated lung diseases and other complications (eg: retinopathy).
1.6.2.2. Interleukin (IL)-24
IL-24 also referred to as melanoma differentiation associated gene-7 (mda-7) is a member of the IL-10 cytokine superfamily and is located on chromosome 1 at 1q32. [164, 165]. Members of the IL-10 family, in addition to IL-10 and IL-24, include IL-19, IL-20, IL-22, and IL-26. IL-24 was originally identified in human melanoma cells that were allowed to differentiate with IFN-γ [166]. The IL-24 cDNA encodes an evolutionarily conserved protein of 206 amino acids with a predicted size of 23.8 kDa. Presence of three glycosylation and five phosphorylation sites in the IL-24 protein sequence have been predicted, and to a certain extent validated by functional studies [164, 167]. Additionally, demonstration that the IL-24 protein undergoes ubiquitination and proteasomal degradation suggests that the protein is susceptible to a variety of post-translational modifications, a feature that is typically observed in a classical tumor-suppressor gene [168]. Finally, a secretory signal sequence present in the IL-24 cDNA enables the protein to be secreted and available in soluble form to exert its autocrine and paracrine functions by binding to one of the two heterodimeric receptors complexes, IL-20R1/IL-20R2 and IL-22R1/IL-20R2 [164, 165]. These unique features of IL-24 distinguishes it from other family members, leading to its testing as a cancer therapeutic and as a pro-inflammatory Th1 cytokine.
The first report of IL-24 to functioning as a tumor suppressor gene involved overexpressing exogenous IL-24 in human breast cancer cells and showing tumor growth inhibition [169]. Follow-up studies showed IL-24 mRNA, but not the protein, was detected in human cancer cell lines and tissues. Furthermore, loss of IL-24 protein expression in melanoma and LC was associated with disease stage and poor prognosis [170, 171]. In addition, intravenous delivery of IL-24 plasmid DNA containing DOTAP: Cholesterol NPs suppressed tumor growth and metastasis in A549 LC xenograft model (Figure 3) [172]. These results indicated that IL-24 is required to suppress tumor growth and its loss likely contributes to tumor progression. No IL-24 gene mutations or polymorphisms have been reported to-date. Based on these initial reports, our laboratory as well as several others, have tested IL-24 as a cancer therapeutic against numerous human cancer models both in vitro and in vivo [167, 173–177]. These studies conclusively demonstrate exogenous expression of IL-24 results in antitumor activity thus confirming IL-24 is a bona fide tumor suppressor. Additionally, combinatorial therapies with radiation, chemotherapy and small molecule inhibitors can enhance the antitumor activity of IL-24 [177–186]. The molecular mechanism by which IL-24 exerts its anticancer activities varies among the cancer cell types tested and is cell type dependent. Modulation of several molecules and pathways (PKR [187], PERK, Fas/FasL [188], MAPK [JNK, ERK], AKT, mTOR, beta-catenin/Wnt signaling, NF-κB, STAT-3, and Gli-Hedgehog signaling [189], and iNos [190] among others) by IL-24 have been reported [168, 173, 176–179, 182, 184]. However, studies of the role of IL-24 mediating tumor cell death converge on the mitochondria, where it activates pro-apoptotic proteins and caspases resulting in apoptotic cell death.
Figure 3.
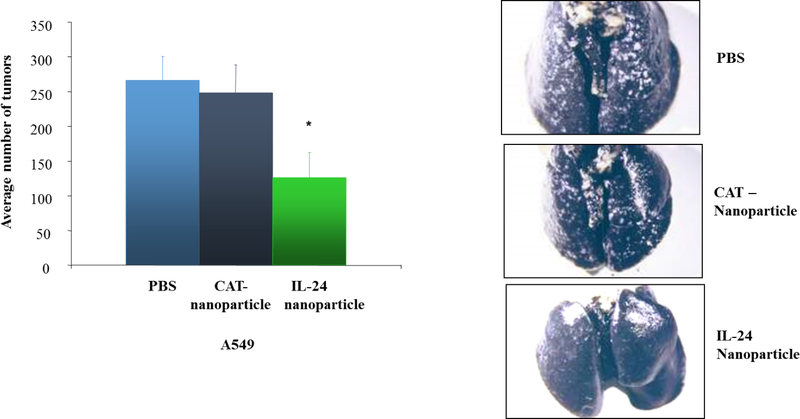
Delivery of DOTAP: Cholesterol based IL-24 nanoparticle reduced tumor burden and metastases in A549 mouse lung tumor models in comparison to control PBS treated and empty vector treated control (CAT – nanoparticle). Image modified and reproduced from Ramesh et al., 2004 [172].
As well as from inducing tumor cell death, IL-24 inhibits cell migration and invasion by downregulating MMPs (MMP-2 and -9), PI3K, FAK, CXCR4 chemokine, and modulating EMT (cadherin, TWIST, TGF-β) [191, 192]. IL-24 also inhibits tumor angiogenesis by both intracellular and extracellular mechanisms of action [182, 193–195]. The extracellular mode of action involves binding of IL-24 to its receptors and the resulting inhibitory activity on VEGF-mediated angiogenesis. The intracellular mode of action involves inhibiting the Src-STAT3 pathway leading to reduction of VEGF. In addition to VEGF, IL-24 also modulates IL-8 and HIF-1α expression in LC cells (unpublished data). Finally, IL-24 inhibits inducible nitric oxide synthase (iNOS) and an inverse correlation between IL-24 and iNOS was reported in melanoma [190].Since iNOS can also regulate angiogenesis it is possible that IL-24 mediated antiangiogenic activity is in part, due to iNOS inhibition, a possibility that has not been tested to date. Finally, IL-24 has pro-immune properties and stimulates the host immune response to effectively suppress tumor growth [196–198] The anti-tumor response involved activation and infiltration of CD8+ T cells along with release of pro-inflammatory cytokines (TNF, IL-1, IFN-γ, IL-12). The studies clearly demonstrate IL-24-mediated antitumor activity involves multiple mechanisms and pathways, that underly it’s testing in the clinic for cancer treatment [199, 200].
Of note, many of the signaling molecules and pathways and cytokines/chemokines regulated by IL-24 are also reported in lung diseases [201–204] and diabetes, suggesting a potential overlap of the signaling mechanisms in LC lung diseases and diabetes. For example, deregulation of the angiogenic process is common in both, cancer and diabetes leading to severity of the diseases. Since, IL-24 can inhibit angiogenesis, its application in the treatment of diabetes-associated retinopathy and poor wound healing is a possibility [205]. Thus, with careful experimental design for each disease setting, it is worthwhile to test the therapeutic benefits of IL-24-based therapy not only in LC but also in COPD, lung fibrosis, and diabetes. The caveats associated with these therapeutic approaches would be ensuring a suitable route of administration (i.e, intraperitoneal, intravenous, intraocular, subcutaneous, intradermal, etc.) to achieve maximum bioavailability. In addition, the drug delivery vehicle (i.e. liposomes, dendrimers, exosomes, metals, polymers, etc.) will need to be optimized for each disease condition to achieve maximum benefit and clinical relevance. Furthermore, adequate precautions must be incorporated in order to limit toxicities associated with use of these therapeutic approaches.
1.7. Nanomedicine based approaches for DM and Cancer
Owing to their small size, biocompatibility, and ease of multi-functionalization nanotechnology-based applications are increasingly used to deliver therapeutics and monitor treatment response. The potential of nanotechnology-based therapy in medicine has supported the development of multiple novel drug and nanoparticle formulations incorporating metallic nanoparticles, polymeric nanoparticles, dendrimers, liposomes, extracellular vesicles or exosomes containing drugs and other target moieties [206, 207].
Diabetes research is one of the few areas where nanotechnology has been effectively transformed from basic research findings to the clinic. The hand-held glucometer is one of the most sophisticated product of nanotechnology-based engineering products available to date. Following this success, research is ongoing to develop tools for sustainable delivery of insulin [208], and for reactivation of damaged β-cells in the pancreas. One approach aims to re-initiate insulin production for T1DM management by delivering biologics such DNA (for gene therapy) and siRNA [209]. Similar approaches are being assessed for T2DM to sensitize cells to insulin and induce glycolytic metabolism. Nanomedicine approaches have been actively explored for diabetes [210] and cancer research [207] in context of therapy as well as for diagnostics. Doxil (Doxorubicin hydrochloride liposome) is a widely used nanomedicine application for the treatment of ovarian cancer and multiple myeloma. Delivery of biologics, including small molecule inhibitors or ligands for certain receptors that are overexpressed in cancer or diabetes and described in the earlier sections of this review, can be actively pursued via nanotechnology-based approaches. Our laboratory has developed several nanomaterial-based therapeutic delivery strategies for targeting one key oncogenic molecule, namely HuR, in LC [147] and melanoma [211]. Using nanotechnology, the role of several regulatory molecules like miRNAs could also be deciphered to explore their potential role in cancer and diabetes. Table 2 describes a list of miRNAs that are involved in regulation of LC and DM physiology. Effective modulation of these miRNAs would alter the expression of key tumor suppressor genes and oncogenes and lead to desired therapeutic effect. In the following subsections, we will discuss many technologies in different categories of nanotechnology involved in therapeutic delivery and diagnostics applied for DM and LC.
Table 2:
Common miRNAs dysregulated in diabetes and LC
| miRNA | Role in diabetes | References | Role in LC | References |
|---|---|---|---|---|
| miR-20b-5p | Elevated expression of miR-20b-5p in T2DM modulates insulin-stimulated glucose metabolism via AKT signaling. | [271] | Biomarker for NSCLC, overexpression of miR-20b promote NSCLC cell proliferation and migration | [272] |
| Knock out of miR-20b-5p enhances diabetes associated wound healing and promotes wound angiogenesis | [259] | [273] | ||
| miR-126 | Loss of endothelial miR-126 impairs peripheral angiogenic signaling in patients with DM | [274] | Downregulation of miR-126 in LC. Ectopic expression of miR-126 suppresses EMT and metastasis of LC cells, biomarker for NSCLC | [275] |
| Downregulation of miR-126 regulates angiogenesis and vascular integrity, therapeutic agent for DM | [276] | [277] | ||
| miR-150 | Higher expression of miR-150, miR-30a-5p seen in T2DM, predictors of T2DM and prediabetes | [278] | miR-150 overexpressed in LC. Knockdown of miR-150 inhibit LC cell viability | [279] |
| miR-30a-5p | miR-30a-5p downregulated in LC and p53/miR-30a-5p/SOX4 feedback loop mediates cellular proliferation, migration of NSCLC cells | [280] [281] |
||
| miR-194 | Modulator of glucose metabolism, decreased expression in T2DM | [282] | Downregulated in HG condition, introduction of miR-194 suppressed the proliferation, migration, and invasion of LC cells induced by HG and suppresses metastasis of LC cells | [64] [283] |
| miR-27b | Negative regulator of hepatic gluconeogenesis, decreased in T2DM conditions | [284] | miR-27b expression downregulated in LC. Overexpression of miR-27b further suppresses EMT in LC | [285] |
| miR-375 | Increased expression in STZ treated mice, marker of β-cell death and potential predictor of diabetes. | [286] | Upregulation of miR-375 predicts chemoresistance induction in SCLC | [287] |
| miR-214-3p | Decreased expression in T2DM, correlates with serum insulin and HbA1c level | [288] | Downregulation of miR-214-3p in LC negatively correlates with FGFR1 in LC patients. Overexpression of miR-214-3p inhibits EMT and Wnt/MAPK/AKT pathway | [289] |
| miR-let-7f-5p | Decreased expression of miR-let-7f-5p in plasma exosomes of NSCLC patients | [290] | ||
| miR-103 | Decreased expression reported in prediabetes and diabetes rats | [291] | Reduced miR-103 expression negatively correlating with tumor grade seen in NSCLC tissues and cells | [292] |
1.7.1. Polymer-based nanomaterials
Owing to their high biocompatibility, ease of fabrication in a variety of structures, biomimetic nature polymeric materials are a preferred choices for developing drug delivery vehicles. Biodegradable polymers such as poly (lactic acid) (PLA), poly (lactic-co-glycolic) acid (PLGA), gelatin, albumin, and chitosan have consistently demonstrated sustainable release of drugs and are widely used as delivery vehicles for therapeutics as approved by Food and Drug Administration (FDA) [212–214]. Nanoparticles have been widely used both for insulin delivery and management of diabetes associated complications [208, 210]. Attaining efficient bioavailability of insulin is an essential requirement for effectively treating insulin-dependent T1DM. To address this, Damge et al., developed a polymer based vehicle using poly (ε-caprolactone) and a polyacrylic polymer, Eudragit RS for oral delivery of insulin. They achieved high encapsulation efficiency (96%) and anti-diabetic effects characterized by decreased fasting glycemic values and increased serum insulin levels which were attributed to the mucoadhesive properties of Eudragit RS [215]. Malathi et al., also developed a D-α-tocopherol poly (ethylene glycol) 1000 succinate (TPGS)-emulsified poly (ethylene glycol) (PEG) - PLGA nanoparticles (NPs) for oral administration of insulin and demonstrated a two-fold decrease in serum glycemic levels in diabetic rats [216]. Diabetic retinopathy (DR), a diabetes-associated complication causes vision impairment, and VEGF-A and matrix metalloproteinase-9 (MMP-9) are two crucial proteins involved in its initiation and progression. Zeng et al., utilized a PLGA based nanoparticle, prepared by double emulsion method, to deliver IL-12. The IL-12 NPs reduced MMP-9 and VEGF-A levels and decreased retinal neovascularization in DR mice [217]. Furthermore, fenofibrate - an agonist of peroxisome proliferator-activated receptor-α (PPAR-α) reportedly protects against DR. The drawbacks of conventional systemic administration of fenofibrate were avoided by encapsulating fenofibrate within a PLGA nanoparticle. This system, by reducing retinal dysfunctions in terms of vascular leakage and VEGF levels, has showed promising results using STZ-induced diabetic rats [218]. Diabetic ischemic ulcers are another complication in diabetic patients, often leading to neuropathy and resulting in amputations of the feet. The angiogenic process is pivotal in the process of wound healing and delivery of VEGF-A and PDGF-B through PLGA nanospheres resulted in improved repair of diabetic foot ulcers in STZ induced rat models [219]. Interestingly, angiogenesis is also a hallmark of cancer progression, and in many tumors VEGFR is overexpressed. Thus, targeting of the tumor with VEGF/VEGF-conjugated polymeric NPs can be beneficial for developing anti-cancer based therapeutics. Several polymer-based vehicles have been developed and assessed for delivery of anti-angiogenic therapeutics in different cancer models. In addition to VEGF, PLGA based NPs have been developed for carrying various chemotherapeutic drugs like paclitaxel (PTX), cisplatin (CDDP), and therapeutic biomolecules such as siRNA, miRNA, peptides and plasmid DNAs (Figure 4) [220]. Recently, Varani et al., developed a VEGF-PLGA-based theranostic and tested its diagnostic ability in BALB/c mice bearing a syngeneic tumor. VEGF-PLGA-NPs exhibited a 1.75 times higher accumulation at the tumor site when compared to PLGA-NPs [221]. Polymeric nanoparticles have great potential for application in cancer theranostics and is an area of extensive active investigation. A detailed review of PLGA based therapeutic interventions in cancer was presented by Rezvantalab et al. [222].
Figure 4.
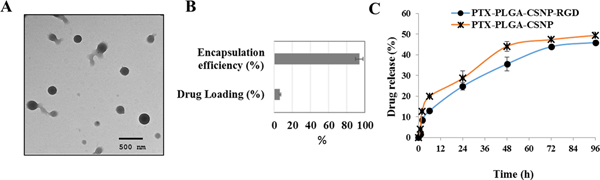
Nanomedicine based approaches for delivering therapeutics to LC cells. (A) TEM images of PLGA - Chitosan nanoparticle (CSNP) for co-delivering RGD (arginine-glycine-aspartic acid) peptide and PTX to integrin αvβ3 receptors, (B) % drug loading and encapsulation efficiency of the nanocarrier and (C) in vitro drug release data from unmodified and modified nanocarrier. Image reproduced from Babu et al., 2017 [220] that is under an open access Creative Commons CC BY 4.0 licence.
1.7.2. Metallic Nanoparticles
Over the past few years, nanoparticles synthesized from metals, including gold, silver and iron, have been used to carry therapeutics and/or serve as contrast agents for imaging and theranostics. Metallic nanoparticles offer various advantages including: a) their inert nature offers high degree biocompatibility and low immunogenicity; b) the enhanced permeability and retention (EPR) effect contributes to sustained release of drugs leading to enhanced therapeutic effect; c) their ease of multi-functionalization for delivering multiple therapeutics/target moieties simultaneously; and d) ease of size /shape tuning for altering their loading capacity [223].
Zhang et al., created gold nanoclusters (AuNCs) and loaded them with 3-aminophenylboronic acid (PBA) (glucose responsive factor) for sustainable and targeted release of the loaded insulin to create an AuNC-PBA-insulin complex. This complex aided in rapid insulin release based on changes in glucose concentration levels in a T1DM mouse model for up to 3 days [224]. Islet transplantation is a current treatment modality for management of T1DM, and use of conventional delivery methods to deliver therapeutic cargo to islet cells has been challenging due to poor penetration capability. To circumvent this problem, Cy5-gold NPs (GNPs) oligonucleotide conjugates were utilized and this model demonstrated enhanced uptake of molecular cargo in pancreatic islets without perturbing the normal cellular function [225]. GNPs have also been used to deliver anti-VEGF drug Sorafenib tosylate for management of DR [226]. Insulin loaded chitosan iron oxide nanoparticles (IONPs) [227] and superparamagnetic iron oxide nanoparticles (SPIONs) [228] have also yielded favourable results in the management of diabetes. Studies demonstrate SPIONS function by activating hepatic PPAR-gamma coactivator 1-α, adipocytokines, or by regulating the lipid metabolism directly, and deliver profound anti-diabetic effects in T2DM mouse models [229]. SPIONs are also being exploited as contrast-imaging agent in Magnetic Resonance Imaging (MRI) to determine successful islets transplantation [230]. MRI was also used to study the bio-distribution of nanoparticle and drug 2‐(1′H‐indole‐3′‐carbonyl)‐thiazole‐4‐carboxylic acid methyl ester based delivery system in T1DM mouse models[231] SPIONs conjugated with quercetin have also been successfully tested to treat DM associated cognitive decline [232]. In addition to use as an imaging agent, IONPs have also been incorporated in development of nanosensors for monitoring blood glucose levels [233].
Metallic nanoparticles have also been used in the field of cancer therapeutics. In LC research, both GNPs [234] and IONPs [235, 236] have been extensively studied for its use in drug delivery, imaging, and theranostics. Ramalingam et al., utilized doxorubicin conjugated GNPs stabilized with polyvinylpyrrolidone to inhibit growth of LC cells. This system upregulated the expression of various tumor suppressor genes and induced apoptosis in LC cells [237]. Folic acid (FA) - conjugated PEG modified SPIONS labelled with Cy5.5 have been used to develop optical imaging system that was used for the diagnosis of LC in mice models [236].
1.7.3. Lipid based nanoparticles / liposomes
Liposomes have emerged as a promising means of drug and gene delivery. Liposomes are small nano-sized vesicles made of single or multiple layers of lipids, and they can efficiently carry hydrophilic or hydrophobic drugs encapsulated within them. Liposomes are synthetic NPs that very closely resembles biological vesicles and are considered an efficient drug carrier. Current methods of drug administration in DM, i.e. oral or subcutaneous injections, have limitations in terms of enzymatic degradation and poor absorption, which can result in instability and skin allergies. Liposomes that can encapsulate and protect drugs and deliver them to the intended site, avoiding such limitations [238]. Liposomes have been used for oral delivery of insulin to hepatocytes in order to enhance insulin absorption, however, there are limitations that restrict their optimal use [239]. Kapoor et al., showed that PEGylation of liposomes provided greater stability in systemic circulation, leading to prolonged insulin release in diabetic rats and a hypoglycaemic effect; these findings reflect the true potential of liposomes as drug delivery vehicles [240]. Genetic manipulation by genome engineering is another treatment option that is being actively explored to treat DM (both T1DM and T2DM). The challenge of this treatment method is ensuring precise delivery of modified therapeutic cargo to the target site. Genome alteration using the CRISPR/Cas9 complex for DM treatment has been attempted by Cho et al.; they used lecithin liposomes to deliver Cas9 with a single-stranded guide RNA (sgRNA) ribonucleoprotein (Cas9-RNP) directed towards DPP-4 with an aim of regulating GLP-1 function in T2DM db/db mice [241].
In the context of LC, Lipoplatin (liposomally-encapsulated form of cisplatin) is currently under fast-track approval by the U.S. FDA for clinical use. Devarajan et al., showed use of lipoplatin therapy had significantly lower nephrotoxicity compared to cisplatin alone in rat models [242]. PTX is another chemotherapeutic drug widely used for treating LC. Wang et al., conducted a phase 1 clinical trial of PTX delivery by liposome in 12 NSCLC patients and identified better pharmacokinetics, efficacy, and lower toxicity associated with intrapleural PTX liposome injections [243]. A liposome based targeted PTX delivery system to strategically direct the drug to tumor sites, especially those with multidrug resistance (MDR), enhanced efficacy and specificity; Jiang et al., developed a dual liposome delivery system that releases drug based on extracellular pH and has a modified lipid peptide - D[KLAKLAK]2 (KLA) attached to it. This system showed an efficacious therapeutic effect in LC A549 cells and A549/Taxol drug resistant cells [244]. In our own laboratory, we have developed and successfully tested tumor-targeted folate receptor-α (FRA)-targeted DOTAP: Cholesterol lipid nanoparticles containing HuR siRNA (HuR-FNP) in LC cells and preclinical LC models [147, 148]. In addition to the above-mentioned examples, liposomes-based treatment strategy have been adopted for other treatment modalities like cancer vaccines, adjuvant and neo-adjuvant interventions, and theranostics. For more details on nanoparticle- and liposome-based delivery systems in LC, readers are directed to a review by Babu et al. [207].
1.7.4. Exosomes for drug delivery
Exosomes are tiny nano-sized vesicles produced by all cells and they have the unique ability to carry biomolecules between different cells in the body. As an analogy, exosomes are often considered as cargo carriers in the body and, due to this property, they are also being exploited as drug carrier vehicles [245]. Several studies have loaded therapeutics on exosomes and evaluated their ability to deliver drugs to the desired site [246, 247]. Exosomes reportedly have significant structural similarities with liposomes. However, exosomes have an edge over conventional synthetic liposomes, particularly as regards an absence of immunogenic response, ease in loading hydrophilic and hydrophobic drugs, and multi-functionalization of surface among others.
Exosomes normally have a profound effect on the surrounding cells, culminating in perturbation of cellular physiology [248]. Such effects can be seen in both normal and pathological conditions such as diabetes and cancer. In many instances, exosomes serve as an integral component of various cellular events in DM including glucose homeostasis. In an interesting study, when miRNAs were packaged into exosomes derived from the adipose tissue macrophages of obese mice and the exosomes administered to lean mice, the recipient animals developed glucose intolerance and insulin resistance. In contrast, when exosomes isolated from lean mice were administered to obese mice, their glucose tolerance and insulin sensitivity improved [249]. Exosomes are also known to impart immunoregulatory functions, leading to autoimmune diabetes. In T1DM intracellular membrane proteins usually act as target autoantigens. Cianciaruso et al., showed that exosomes derived from rat and human pancreatic islets contained membrane proteins, including glutamic acid decarboxylase 65, islet-associated protein 2 and proinsulin, that act as autoantigens and in turn activate dendritic cells which may lead to initiation of autoimmune responses in T1DM [250]. The insulin resistance in T2DM develops by dysregulation of obesity and accumulation of fat in the adipose tissue. Recently, exosomes isolated from adipocytes have been shown to carry sonic hedgehog which modulates the immune system and induces pro-inflammatory signaling leading to insulin resistance. In addition, several exosomal miRNAs and lncRNAs have been identified and shown to have a putative role in pathogenesis of T1DM and T2DM [251]. Many of such miRNAs and proteins are also being explored as biomarkers for diagnosis of DM and to evaluate the therapeutic responses.
Exosomes, similarly to liposomes, can be engineered or tailored for their therapeutic use as drug delivery carriers. Loading of therapeutics into exosomes can be broadly divided into two basic approaches: (a) exogenous loading which involves loading of therapeutic cargo (like small RNAs, proteins, DNA and drugs) into the lumen of exosomes by various physical methods including incubation, electroporation, and sonication among others; or (b) endogenous loading, which involves using de novo synthesis machinery to package the chosen therapeutic cargo into the exosomes. Alvarez-Erviti et al., performed the pioneering study of adding siRNA exogenously into exosomes. They used dendritic cell derived exosomes and electroporation to load exogenous siRNA into exosomes which resulted in loading efficiencies of up to 25%. The loaded exosomes showed a successful therapeutic effect on the targeted brain cells after crossing the blood–brain barrier [252]. In our laboratory we have successfully employed incubation technique to exogenously load GNPs-Doxorubicin conjugates into exosomes (nanosomes) for selectively delivering drug moiety to LC cells [253]. The second method of endogenous loading encompasses transfecting shRNA/miRNA/siRNAs in donor cells for expressing the target moiety and further harvesting exosomes from the transfected donor cells for therapeutic purposes. Using the exogenous loading strategy, Ohno et al., stably transfected human embryonic kidney (HEK) cells with pDisplay GE11 peptide to generate GE11 peptide expressing exosomes. Subsequently, the GE11 transfected HEK cells were subjected to second round of transfection with let-7a miRNA and the exosomes isolated from the cells expressed GE11 and contained let-7a miRNA. Administration of the GE11 positive exosomes containing let-7 miRNA to mice bearing breast cancer resulted in tumor suppression [254]. Izco et al., combined exogenous and endogenous loading approach for delivering shRNA minicircles (shRNA-MCs) into the brain for preventing Parkinson disease. In their study primary dendritic cells were transfected with the Rabies Virus Glycoprotein (RVG)-Lamp2b peptide to produce RVG expressing exosomes. The exosomes were subsequently exogenously loaded with alpha-synuclein shRNA-MCs by electroporation. Systemic administration of the alpha-synuclein shRNA-MCs loaded exosomes decreased the aggregation of alpha-synuclein normally seen in Parkinson conditions and offered therapeutic benefits in disease management [255].
In context of diabetes, human urine-derived stem cell exosomes also offered a protective role against diabetes induced kidney injury [256] and hind limb ischemia [257]. Urine-derived stem cell exosomes in T1DM rats reportedly carried TGF-β1, angiogenin and bone morphogenetic protein (BMP)-7 in their lumen and gave favourable response for protection from diabetes-induced kidney injury [256]. The regenerative property of exosomes derived from stem cells has been extensively explored for preventing damage and repairing organs by DM. The regenerative and immunomodulatory properties of stem cell exosomes have been proposed to have a protective role against autoimmune destruction of pancreatic islets in T1DM [258]. Exosomes have also been implicated in diabetic wound healing [259]. Geiger et al., used human fibrocyte-derived exosomes to demonstrate an increased rate of wound healing in diabetic mice. The study identified exosome cargo to contain HSP-90α, activated STAT3, proangiogenic miRNAs (namely miR-126, miR-130a, miR-132), anti-inflammatory miRNAs (miR124a, miR-125b), and a microRNA regulating collagen deposition (miR-21). This cargo content mainly responsible for promoting angiogenesis, migration, and proliferation of keratinocytes resulting in enhanced wound healing. This study gives strong evidence of using fibrocyte cell-derived exosomes to treat diabetic ulcers or wounds [260].
Exosomes have also been widely studied in almost all cancer types including LC. Over the last 20 years, exosomes and more specifically the molecules contained in their lumen, have been implicated in various tumor-related pathways including initial inflammation, EMT, angiogenesis, metastasis, and resistance to therapy [261, 262]. Multiple studies have also explored the role of exosomes in pathogenesis of lung morbidities, such as COPD, and further substantiated their use as biomarkers and diagnostics [263]. Tan et al., isolated and quantified exosomes from stable COPD patients and patients with acute COPD exacerbations. The found elevated levels of plasma exosomes in both types of COPD patients when compared to healthy, non-smoking controls; the levels of plasma exosomes were further reported to correlate with markers of inflammation [264]. Bazzan et al., took a more specific approach, and isolated exosomes from bronchoalveolar lavage fluids of non-smokers, smoker with or without COPD, and idiopathic pulmonary fibrosis. They found higher levels exosomes in smokers irrespective of COPD status compared to the non-smokers [265]. In addition to having significant intrinsic health-associated roles, exosomes have also shown their potential for therapy.
Exosomes have recently garnered much attention in the management of lung and other cancers, largely based on their ability to deliver therapeutic cargoes in a targeted fashion. In one of the earliest study, Kim et al., studied exosome-based PTX drug delivery, for treating MDR cancer; they reported a 50 fold increase in activity against drug resistant MDCKMDR1 (Pgp+) cells when PTX was delivered in exosomes. The study also showed that exosomes delivered to the airways co-localized with cancer cells in a mouse model of Lewis lung carcinoma pulmonary metastases, thus substantiating a potent therapeutic effect [266]. In a follow up study, the same group delivered PTX exosomes conjugated with aminoethylanisamide – PEG and demonstrated enhanced therapeutic efficacy [267]. Our laboratory also works at the forefront of developing exosome based delivery system for anti-cancer drugs. We have established a unique and novel approach of combining drug-conjugated metallic nanoparticles, such as GNPs, SPIONS with exosomes (Figure 5). Using this approach, we have sustainably delivered therapeutics selectively to H1299 and A549 LC cells with minimal toxicity to non-cancerous cells [253]. Other investigators have examined the de novo effect of exosomes in cancer progression. Mesenchymal NSCLC cells derived exosomes exhibit the potential to transfer their acquired chemoresistance phenotype to surrounding epithelial NSCLC cells. The study further reported that levels of ZEB1 mRNA, a transcription factor involved in the process of EMT, were increased in the parental human bronchial epithelial cells (HBECs) when exosomes from oncogenic mesenchymal HBECs were added [268].
Figure 5.
Exosomes as carrier of therapeutics. Schematic representation of exosomes functionalized with SPIONS carrying anti-cancer drugs such as doxorubicin/cisplatin for targeting transferrin receptors in LC cells.
Although exosomes have recently engendered much interest as delivery vehicles, there remain several pitfalls that must be addressed before they can transition to the clinic. Issues including scaling up of exosome production, isolation of pure homogenous populations of exosomes, and methods to develop controlled loading of therapeutics remain unresolved. Nevertheless, the progress made in the last decade clearly indicates the bright future of this technology. With the rapid addition of investigators and scientific groups to the field of exosome research, we anticipate these hurdles can readily and rapidly be overcome.
In summary, we have demonstrated that there are many common targets between DM and LC (Figure 1) and the above-described nanoparticles can be effectively used for developing efficient and precise therapeutic modalities.
1.8. Conclusions and future perspectives
Diabetes mellitus (DM) incidence continues to increase around the world and poses major challenges, in particular when associated with secondary diseases. Effective management of DM in order to maintain normal blood glucose levels includes dietary management and daily exercise supplemented with anti-diabetic drugs (metformin sulfonylureas, TZD, GLP-1 agonists, DPP-4 inhibitors) or insulin. The evidence increasingly supports the role of diabetes in contributing to additional pathological conditions such as fibrosis and many cancers including LC. However, the precise mechanisms that link DM (T1DM, T2DM) with lung diseases and LC is not clear and is an area under intense investigation. Molecular studies reveal significant overlap in the signaling pathways in DM and cancer suggesting that early onset of diabetes with sustained inflammation may provide an environmental niche for cells to become susceptible to fibrosis and transformation and/or promote tumor cell survival, growth, and metastasis. Thus, inhibiting or disrupting signaling pathways shared by diabetes and cancer could be effective in managing diabetes-associated cancers. This contention is supported by the observation that diabetic patients treated with metformin exhibit significant reduction in the incidence of LC. In addition to metformin, other anti-diabetic drugs have also been reported to exhibit anticancer activities in preclinical studies. In the context of biologics, many have been tested specifically geared towards either DM or LC, but very few to none have been investigated in the context of DM and cancer. In this review we have given examples of two biologics (IL-24 and HuR) that modulate molecules shared by DM and LC and that are potentially be useful in treating DM-associated LC. However additional studies testing IL-24 and HuR-targeted therapies in relevant experimental models with diabetes and LC are necessary. Additionally, applying multi-omics approaches such as genomics, transcriptomics, proteomics, and metabolomics will lead to identification of new therapeutic targets; bridge existing knowledge gaps and provide novel information relevant to disease progression. For instance, a recent investigation by Chen et al., utilized a combination of metabolomics, proteomics and peptidomic to decipher the biological characteristics and signatures involved in metabolic disorders like DM and obesity [269]. A review by Gan et al., reported various miRNAs and protein biomarkers identified using transcriptomics- and proteomics-based approaches for diagnosis of diabetes [270]. Table 2 lists certain miRNAs obtained from high throughput genomic or transcriptomic technologies that are commonly dysregulated in LC and DM and can thus serve as potential targets. These commonly regulated miRNAs could be further analysed to provide valuable insights into the pathogenesis of diabetes associated LC. Finally, the availability and testing of additional therapeutics (biologics, synthetic small molecule inhibitors) targeting the identified new targets shared between DM and LC and using innovative and novel nanodrug delivery platforms (Table 3) would potentially have a significant impact in managing DM-associated LC in the clinic.
Table 3:
Nanomedicine approaches targeted towards signaling pathways in DM and LC
| Nanomedicine for DM | References | Nanomedicine approaches for LC | References | |
|---|---|---|---|---|
| IL6 | Information not available | Information not available | ||
| TGF-β | Use of AUNPs in diabetic nephropathy reduced TGF-β1, VEGF and decreased renal damage | [293] | Nanoemulsion formulation of neobavaisoflavone repressed TGF-β/SMAD pathway and triggered immune microenvironment in LC | [294] |
| HIF-1 | Wound dressings made from polycaprolactone based nanofibers continuously released oxygen, increased HIF-1α and accelerated angiogenesis and wound healing in diabetic rats. | [295] | PLGA-NP with curcumin decreased HIF-1α in A549 LC cells Microporous organosilica shell coated cisplatin for inhibiting HIF-1 signalling in LC cells led to improved efficacy of cisplatin and decreased LC metastasis. |
[296, 297] |
| PDGF | PLGA scaffolds loaded with PDGF and antibiotics (gentamycin, vancomycin) aided diabetic wound healing and tissue regeneration | [298] | Information not available |
Acknowledgments
This study was supported in part by grants received from the National Cancer Institute (NCI) of the National Institutes of Health (NIH; R01 CA167516 and R01CA233201); from the National Institute of General Medical Sciences (P20 GM103639) of the National Institutes of Health; a Merit Grant (101BX003420A1) from the Department of Veterans Affairs; a Pilot Grant and Seed Grant funded by the National Cancer Institute Cancer Center Support Grant P30CA225520 awarded to the University of Oklahoma Stephenson Cancer Center; from the Department of Defense (DOD) through the Lung Cancer Research Program (LCRP) under award no.W81XWH-18-1-0637 & W81XWH-19-1-0647; a grant (HR18-088) from the Oklahoma Center for Advanced Science and Technology (OCAST); funds received from the Presbyterian Health Foundation Seed Grant, Presbyterian Health Foundation Bridge Grant, Oklahoma Tobacco Settlement Endowment Trust (TSET) awarded to the University of Oklahoma Stephenson Cancer Center, and the Jim and Christy Everest Endowed Chair in Cancer Developmental Therapeutics.
Rajagopal Ramesh is an Oklahoma TSET Research Scholar and holds the Jim and Christy Everest Endowed Chair in Cancer Developmental Therapeutics.
We thank Dr. Danny Morton for editorial assistance.
The content is solely the responsibility of the authors. The opinions, interpretations, conclusions and recommendations are those of the authors and not necessarily endorsed by or representative of the official views by the NIH, DOD, Department of Veterans Affairs, or the Oklahoma Tobacco Settlement Endowment Trust.
List of Abbreviations
- AGE
Advanced glycation end products
- aOR
Average odds ratio
- AUNCs
Gold nanoclusters
- CI
Confidence interval
- CML
Carboxymethyllysine
- COPD
Chronic obstructive pulmonary disease
- CRP
C-Reactive protein
- DM
Diabetes mellitus
- DPP4
Dipeptidylpeptidase-4
- DR
Diabetic retinopathy
- ECM
Extracellular matrix
- EGFR-TKI
Epidermal growth factor – tyrosine kinase inhibitors
- EMT
Epithelial to mesenchymal transition
- EPR
Enhanced permeability and retention
- ER
Endoplasmic reticulum
- FDA
Food and Drug Administration
- FEV1%
Forced expiratory volume in one second
- FPG
Fasting plasma glucose
- FVC %
Forced vital capacity
- GAS5
Growth arrest-specific 5
- GLP-1
Glucagon lowering peptide -1
- GLP-1R
Glucagon lowering peptide -1 receptor
- GNPs
Gold nanoparticles
- HbA1c
Plasma glycated hemoglobin
- HG
High glucose
- HIF1
Hypoxia-inducible factor 1
- HR
Hazard ratio
- HuR
Human antigen R
- ICS
Inhaled corticosteroids
- IGF
Insulin growth factor
- IGF-1R
IGF 1 receptor
- IL-24
Interleukin 24
- IL-6
Interleukin 6
- iNOS
Inducible nitric oxide synthase
- IONPs
Iron oxide nanoparticles
- IR
Insulin receptor
- IRS-2
Insulin receptor substrate 2
- LC
Lung cancer
- MDR
Multidrug resistance
- MET
Mesenchymal to epithelial transition
- miRNA
Micro RNA
- MMP
Matrix metalloproteinase
- MMP-9
Matrix metalloproteinase-9
- MRI
Magnetic Resonance Imaging (MRI)
- mTOR
Mammalian target of rapamycin
- NFAT5
Nuclear factor of activated T cells - 5
- NF-kB
Nuclear factor kappa B
- NOD
Non obese diabetic
- NOX4
NADPH Oxidase 4
- NPs
Nanoparticles
- NSCLC
Non-small cell lung carcinoma
- OR
Odds ratio
- OS
Overall survival
- PDGF
Platelet derived growth factor
- PD-L1
Programmed death ligand 1
- PEG
poly (ethylene glycol)
- PFS
Progression free survival
- PI3K
Phosphoinositide 3-kinases
- PLA
Poly (lactic acid)
- PLGA
Poly (lactic-co-glycolic) acid
- PTX
Paclitaxel
- RAGE
Receptor advanced glycation end products
- ROS
Reactive oxygen species
- SCLC
Small cell lung carcinoma
- SGLT2
Sodium glucose linked transporter - 2
- siRNA
Small interfering RNA
- SIRT3
Sirtuin 3
- SNP
Single nucleotide polymorphism
- SOD
Superoxide dismutase
- SPIONS
Superparamagnetic iron oxide nanoparticles
- STZ
Streptozotocin
- T1 DM
Type 1 diabetes
- T2 DM
Type 2 diabetes
- TGF- β
Transforming growth factor beta
- TIM-4
T-cell immunoglobulin and mucin domain containing 4
- TZDs
Thiazolidinediones
- UTR
Untranslated region
- VEGF
Vascular endothelial growth factor
- αSMA
Alpha smooth muscle actin
Footnotes
Conflict of interest
The authors report no conflicts of interest in this work
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R, Committee IDFDA, Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition, Diabetes Res Clin Pract, 157 (2019) 107843. [DOI] [PubMed] [Google Scholar]
- [2].Knip M, Simell O, Environmental triggers of type 1 diabetes, Cold Spring Harb Perspect Med, 2 (2012) a007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pilacinski S, Zozulinska-Ziolkiewicz DA, Influence of lifestyle on the course of type 1 diabetes mellitus, Arch Med Sci, 10 (2014) 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kahanovitz L, Sluss PM, Russell SJ, Type 1 Diabetes - A Clinical Perspective, Point Care, 16 (2017) 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li M, Song LJ, Qin XY, Advances in the cellular immunological pathogenesis of type 1 diabetes, J Cell Mol Med, 18 (2014) 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reinehr T, Type 2 diabetes mellitus in children and adolescents, World J Diabetes, 4 (2013) 270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J, Emerging Risk Factors C, Diabetes mellitus, fasting glucose, and risk of cause-specific death, N Engl J Med, 364 (2011) 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sandler M, Is the lung a ťarget organ' in diabetes mellitus?, Arch Intern Med, 150 (1990) 1385–1388. [PubMed] [Google Scholar]
- [9].Khateeb J, Fuchs E, Khamaisi M, Diabetes and Lung Disease: A Neglected Relationship, Rev Diabet Stud, 15 (2019) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Schmid V, Buist S, Chronic obstructive pulmonary disease: current burden and future projections, Eur Respir J, 27 (2006) 397–412. [DOI] [PubMed] [Google Scholar]
- [11].Gupta KK, Singh J, Gupta P, Patel ML, Kumar V, Chaudhary SC, Uncovering Metabolic Syndrome among Chronic Obstructive Pulmonary Disease Patients in a Tertiary Care Hospital, India, J Clin Diagn Res, 11 (2017) OC08–OC11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kerr EA, Heisler M, Krein SL, Kabeto M, Langa KM, Weir D, Piette JD, Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients' treatment priorities and self-management?, J Gen Intern Med, 22 (2007) 1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Caughey GE, Roughead EE, Vitry AI, McDermott RA, Shakib S, Gilbert AL, Comorbidity in the elderly with diabetes: Identification of areas of potential treatment conflicts, Diabetes Res Clin Pract, 87 (2010) 385–393. [DOI] [PubMed] [Google Scholar]
- [14].Goldman MD, Lung dysfunction in diabetes, Diabetes Care, 26 (2003) 1915–1918. [DOI] [PubMed] [Google Scholar]
- [15].Ho TW, Huang CT, Tsai YJ, Lien AS, Lai F, Yu CJ, Metformin use mitigates the adverse prognostic effect of diabetes mellitus in chronic obstructive pulmonary disease, Respir Res, 20 (2019) 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ehrlich SF, Quesenberry CP Jr., Van Den Eeden SK, Shan J, Ferrara A, Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer, Diabetes Care, 33 (2010) 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hsu IL, Lu CL, Li CC, Tsai SH, Chen CZ, Hu SC, Li CY, Population-based cohort study suggesting a significantly increased risk of developing chronic obstructive pulmonary disease in people with type 2 diabetes mellitus, Diabetes Res Clin Pract, 138 (2018) 66–74. [DOI] [PubMed] [Google Scholar]
- [18].van den Borst B, Gosker HR, Zeegers MP, Schols AM, Pulmonary function in diabetes: a metaanalysis, Chest, 138 (2010) 393–406. [DOI] [PubMed] [Google Scholar]
- [19].Li CI, Li TC, Liu CS, Lin WY, Chen CC, Yang SY, Lin CC, Extreme values of hemoglobin a1c are associated with increased risks of chronic obstructive pulmonary disease in patients with type 2 diabetes: a competing risk analysis in national cohort of Taiwan diabetes study, Medicine (Baltimore), 94 (2015) e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aksu F, Capan N, Aksu K, Ofluoglu R, Canbakan S, Yavuz B, Akin KO, C-reactive protein levels are raised in stable Chronic obstructive pulmonary disease patients independent of smoking behavior and biomass exposure, J Thorac Dis, 5 (2013) 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pasini E, Flati V, Comini L, Olivares A, Bertella E, Corsetti G, Vitacca M, Mammalian Target of Rapamycin: Is It Relevant to COPD Pathogenesis or Treatment?, COPD, 16 (2019) 89–92. [DOI] [PubMed] [Google Scholar]
- [22].Rana JS, Mittleman MA, Sheikh J, Hu FB, Manson JE, Colditz GA, Speizer FE, Barr RG, Camargo CA Jr., Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women, Diabetes Care, 27 (2004) 2478–2484. [DOI] [PubMed] [Google Scholar]
- [23].Rayner LH, McGovern AP, Sherlock J, Gatenby P, Correa A, Creagh-Brown B, de Lusignan S, Type 2 diabetes: A protective factor for COPD?, Prim Care Diabetes, 12 (2018) 438–444. [DOI] [PubMed] [Google Scholar]
- [24].Tseng CH, Metformin and risk of chronic obstructive pulmonary disease in diabetes patients, Diabetes Metab, 45 (2019) 184–190. [DOI] [PubMed] [Google Scholar]
- [25].Sexton P, Metcalf P, Kolbe J, Respiratory effects of insulin sensitisation with metformin: a prospective observational study, COPD, 11 (2014) 133–142. [DOI] [PubMed] [Google Scholar]
- [26].Garnett JP, Baker EH, Naik S, Lindsay JA, Knight GM, Gill S, Tregoning JS, Baines DL, Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose, Thorax, 68 (2013) 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mammen MJ, Sethi S, COPD and the microbiome, Respirology, 21 (2016) 590–599. [DOI] [PubMed] [Google Scholar]
- [28].Hur KY, Lee MS, New mechanisms of metformin action: Focusing on mitochondria and the gut, J Diabetes Investig, 6 (2015) 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Slatore CG, Bryson CL, Au DH, The association of inhaled corticosteroid use with serum glucose concentration in a large cohort, Am J Med, 122 (2009) 472–478. [DOI] [PubMed] [Google Scholar]
- [30].O'Byrne PM, Rennard S, Gerstein H, Radner F, Peterson S, Lindberg B, Carlsson LG, Sin DD, Risk of new onset diabetes mellitus in patients with asthma or COPD taking inhaled corticosteroids, Respir Med, 106 (2012) 1487–1493. [DOI] [PubMed] [Google Scholar]
- [31].Hsiao YT, Cheng WC, Liao WC, Lin CL, Shen TC, Chen WC, Chen CH, Kao CH, Type 1 Diabetes and Increased Risk of Subsequent Asthma: A Nationwide Population-Based Cohort Study, Medicine (Baltimore), 94 (2015) e1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wu TD, Brigham EP, Keet CA, Brown TT, Hansel NN, McCormack MC, Association Between Prediabetes/Diabetes and Asthma Exacerbations in a Claims-Based Obese Asthma Cohort, J Allergy Clin Immunol Pract, 7 (2019) 1868–1873 e1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen CZ, Hsu CH, Li CY, Hsiue TR, Insulin use increases risk of asthma but metformin use reduces the risk in patients with diabetes in a Taiwanese population cohort, J Asthma, 54 (2017) 1019–1025. [DOI] [PubMed] [Google Scholar]
- [34].Lee KH, Lee HS, Hypertension and diabetes mellitus as risk factors for asthma in Korean adults: the Sixth Korea National Health and Nutrition Examination Survey, Int Health, 12 (2020) 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang G, Han YY, Forno E, Yan Q, Rosser F, Chen W, Celedon JC, Glycated Hemoglobin A1c, Lung Function, and Hospitalizations Among Adults with Asthma, J Allergy Clin Immunol Pract, 8 (2020) 3409–3415 e3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bjornvold M, Munthe-Kaas MC, Egeland T, Joner G, Dahl-Jorgensen K, Njolstad PR, Akselsen HE, Gervin K, Carlsen KC, Carlsen KH, Undlien DE, A TLR2 polymorphism is associated with type 1 diabetes and allergic asthma, Genes Immun, 10 (2009) 181–187. [DOI] [PubMed] [Google Scholar]
- [37].Rachmiel M, Bloch O, Bistritzer T, Weintrob N, Ofan R, Koren-Morag N, Rapoport MJ, TH1/TH2 cytokine balance in patients with both type 1 diabetes mellitus and asthma, Cytokine, 34 (2006) 170–176. [DOI] [PubMed] [Google Scholar]
- [38].Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H, Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype, Am J Respir Cell Mol Biol, 41 (2009) 494–504. [DOI] [PubMed] [Google Scholar]
- [39].Vianna EO, Garcia-Leme J, Allergen-induced airway inflammation in rats. Role of insulin, Am J Respir Crit Care Med, 151 (1995) 809–814. [DOI] [PubMed] [Google Scholar]
- [40].Singh S, Prakash YS, Linneberg A, Agrawal A, Insulin and the lung: connecting asthma and metabolic syndrome, J Allergy (Cairo), 2013 (2013) 627384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Viardot A, Grey ST, Mackay F, Chisholm D, Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype, Endocrinology, 148 (2007) 346–353. [DOI] [PubMed] [Google Scholar]
- [42].McMahon GT, Arky RA, Inhaled insulin for diabetes mellitus, N Engl J Med, 356 (2007) 497–502. [DOI] [PubMed] [Google Scholar]
- [43].Wen L, Zhong W, Chai Y, Zhong Q, Gao J, Guan L, Zhang M, Huaiquan L, Haiyang Y, Qingxue W, Changfu Y, Yunzhi C, Association of Metformin Use with Asthma Exacerbation in Patients with Concurrent Asthma and Diabetes: A Systematic Review and Meta-Analysis of Observational Studies, Can Respir J, 2020 (2020) 9705604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rayner LH, McGovern A, Sherlock J, Gatenby P, Correa A, Creagh-Brown B, deLusignan S, The impact of therapy on the risk of asthma in type 2 diabetes, Clin Respir J, 13 (2019) 299–305. [DOI] [PubMed] [Google Scholar]
- [45].Li CY, Erickson SR, Wu CH, Metformin use and asthma outcomes among patients with concurrent asthma and diabetes, Respirology, 21 (2016) 1210–1218. [DOI] [PubMed] [Google Scholar]
- [46].Park CS, Bang BR, Kwon HS, Moon KA, Kim TB, Lee KY, Moon HB, Cho YS, Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase, Biochem Pharmacol, 84 (2012) 1660–1670. [DOI] [PubMed] [Google Scholar]
- [47].Pan Y, Liu L, Li S, Wang K, Ke R, Shi W, Wang J, Yan X, Zhang Q, Wang Q, Chai L, Xie X, Li M, Activation of AMPK inhibits TGF-beta1-induced airway smooth muscle cells proliferation and its potential mechanisms, Sci Rep, 8 (2018) 3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN, Asthma Exacerbations in Type 2 Diabetics with Asthma on Glucagon-like Peptide-1 Receptor Agonists, Am J Respir Crit Care Med, 203 (2020) 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen Y, Zhang F, Wang D, Li L, Si H, Wang C, Liu J, Chen Y, Cheng J, Lu Y, Mesenchymal Stem Cells Attenuate Diabetic Lung Fibrosis via Adjusting Sirt3-Mediated Stress Responses in Rats, Oxid Med Cell Longev, 2020 (2020) 8076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hosseinzadeh A, Javad-Moosavi SA, Reiter RJ, Yarahmadi R, Ghaznavi H, Mehrzadi S, Oxidative/nitrosative stress, autophagy and apoptosis as therapeutic targets of melatonin in idiopathic pulmonary fibrosis, Expert Opin Ther Targets, 22 (2018) 1049–1061. [DOI] [PubMed] [Google Scholar]
- [51].Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D, Apoptosis in lung injury and fibrosis, Eur Respir J, 32 (2008) 1631–1638. [DOI] [PubMed] [Google Scholar]
- [52].Yue Y, Meng K, Pu Y, Zhang X, Transforming growth factor beta (TGF-beta) mediates cardiac fibrosis and induces diabetic cardiomyopathy, Diabetes Res Clin Pract, 133 (2017) 124–130. [DOI] [PubMed] [Google Scholar]
- [53].Talakatta G, Sarikhani M, Muhamed J, Dhanya K, Somashekar BS, Mahesh PA, Sundaresan N, Ravindra PV, Diabetes induces fibrotic changes in the lung through the activation of TGF-beta signaling pathways, Sci Rep, 8 (2018) 11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Oztay F, Sancar-Bas S, Gezginci-Oktayoglu S, Ercin M, Bolkent S, Exendin-4 partly ameliorates - hyperglycemia-mediated tissue damage in lungs of streptozotocin-induced diabetic mice, Peptides, 99 (2018) 99–107. [DOI] [PubMed] [Google Scholar]
- [55].Sciacca L, Vigneri R, Tumminia A, Frasca F, Squatrito S, Frittitta L, Vigneri P, Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients, Nutr Metab Cardiovasc Dis, 23 (2013) 808–815. [DOI] [PubMed] [Google Scholar]
- [56].Wojciechowska J, Krajewski W, Bolanowski M, Krecicki T, Zatonski T, Diabetes and Cancer: a Review of Current Knowledge, Exp Clin Endocrinol Diabetes, 124 (2016) 263–275. [DOI] [PubMed] [Google Scholar]
- [57].van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR, Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis, Int J Cancer, 120 (2007) 1986–1992. [DOI] [PubMed] [Google Scholar]
- [58].Luo J, Hendryx M, Qi L, Ho GY, Margolis KL, Pre-existing diabetes and lung cancer prognosis, Br J Cancer, 115 (2016) 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tseng CH, Diabetes but not insulin increases the risk of lung cancer: a Taiwanese population-based study, PLoS One, 9 (2014) e101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Luo J, Chen YJ, Chang LJ, Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients, Lung Cancer, 76 (2012) 242–247. [DOI] [PubMed] [Google Scholar]
- [61].Nakazawa K, Kurishima K, Tamura T, Ishikawa H, Satoh H, Hizawa N, Survival difference in NSCLC and SCLC patients with diabetes mellitus according to the first-line therapy, Med Oncol, 30 (2013) 367. [DOI] [PubMed] [Google Scholar]
- [62].Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X, Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis, J Natl Cancer Inst, 91 (1999) 151–156. [DOI] [PubMed] [Google Scholar]
- [63].Ding J, Tang J, Chen X, Men HT, Luo WX, Du Y, Ge J, Li C, Chen Y, Cheng K, Qiu M, Liu JY, Expression characteristics of proteins of the insulin-like growth factor axis in non-small cell lung cancer patients with preexisting type 2 diabetes mellitus, Asian Pac J Cancer Prev, 14 (2013) 5675–5680. [DOI] [PubMed] [Google Scholar]
- [64].Meng X, Li Z, Zhou S, Xiao S, Yu P, miR-194 suppresses high glucose-induced non-small cell lung cancer cell progression by targeting NFAT5, Thorac Cancer, 10 (2019) 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liao YF, Yin S, Chen ZQ, Li F, Zhao B, High glucose promotes tumor cell proliferation and migration in lung adenocarcinoma via the RAGENOXs pathway, Mol Med Rep, 17 (2018) 8536–8541. [DOI] [PubMed] [Google Scholar]
- [66].Alisson-Silva F, Freire-de-Lima L, Donadio JL, Lucena MC, Penha L, Sa-Diniz JN, Dias WB, Todeschini AR, Increase of O-glycosylated oncofetal fibronectin in high glucose-induced epithelial-mesenchymal transition of cultured human epithelial cells, PLoS One, 8 (2013) e60471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ding CZ, Guo XF, Wang GL, Wang HT, Xu GH, Liu YY, Wu ZJ, Chen YH, Wang J, Wang WG, High glucose contributes to the proliferation and migration of non-small cell lung cancer cells via GAS5-TRIB3 axis, Biosci Rep, 38 (2018) BSR20171014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang L, Zhong N, Liu S, Zhu X, Liu Y, High glucose stimulates proliferation and inhibits apoptosis of non-small-cell lung cancer cells by JNK-mediated downregulation of p53 pathway, Acta Biochim Biophys Sin (Shanghai), 49 (2017) 286–288. [DOI] [PubMed] [Google Scholar]
- [69].Febres-Aldana CA, Poppiti R, Varlotto JM, Voland R, Zaleski M, Sharzehi S, Rassaei N, Diabetes mellitus type 2 is associated with increased tumor expression of programmed death-ligand 1 (PD-L1) in surgically resected non-small cell lung cancer-A matched case-control study, Cancer Treat Res Commun, 23 (2020) 100170. [DOI] [PubMed] [Google Scholar]
- [70].Ferroni P, Riondino S, Buonomo O, Palmirotta R, Guadagni F, Roselli M, Type 2 Diabetes and Breast Cancer: The Interplay between Impaired Glucose Metabolism and Oxidant Stress, Oxid Med Cell Longev, 2015 (2015) 183928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Walter KR, Ford ME, Gregoski MJ, Kramer RM, Knight KD, Spruill L, Nogueira LM, Krisanits BA, Phan V, La Rue AC, Lilly MB, Ambs S, Chan K, Turner TF, Varner H, Singh S, Uribarri J, Garrett-Mayer E, Armeson KE, Hilton EJ, Clair MJ, Taylor MH, Abbott AM, Findlay VJ, Peterson LL, Magwood G, Turner DP, Advanced glycation end products are elevated in estrogen receptor-positive breast cancer patients, alter response to therapy, and can be targeted by lifestyle intervention, Breast Cancer Res Treat, 173 (2019) 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bartling B, Hofmann HS, Weigle B, Silber RE, Simm A, Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma, Carcinogenesis, 26 (2005) 293–301. [DOI] [PubMed] [Google Scholar]
- [73].Khan H, Khan MS, Ahmad S, The in vivo and in vitro approaches for establishing a link between advanced glycation end products and lung cancer, J Cell Biochem, 119 (2018) 9099–9109. [DOI] [PubMed] [Google Scholar]
- [74].Massague J, TGF-beta signal transduction, Annu Rev Biochem, 67 (1998) 753–791. [DOI] [PubMed] [Google Scholar]
- [75].Dhawan S, Dirice E, Kulkarni RN, Bhushan A, Inhibition of TGF-beta Signaling Promotes Human Pancreatic beta-Cell Replication, Diabetes, 65 (2016) 1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner AE, Finkel T, Rane SG, Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling, Cell Metab, 14 (2011) 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Li J, Shen C, Wang X, Lai Y, Zhou K, Li P, Liu L, Che G, Prognostic value of TGF-beta in lung cancer: systematic review and meta-analysis, BMC Cancer, 19 (2019) 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Horie M, Saito A, Noguchi S, Yamaguchi Y, Ohshima M, Morishita Y, Suzuki HI, Kohyama T, Nagase T, Differential knockdown of TGF-beta ligands in a three-dimensional co-culture tumor- stromal interaction model of lung cancer, BMC Cancer, 14 (2014) 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kage H, Sugimoto K, Sano A, Kitagawa H, Nagase T, Ohishi N, Takai D, Suppression of transforming growth factor beta1 in lung alveolar epithelium-derived cells using adeno-associated virus type 2/5 vectors to carry short hairpin RNA, Exp Lung Res, 37 (2011) 175–185. [DOI] [PubMed] [Google Scholar]
- [80].Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL, Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression, J Clin Invest, 117 (2007) 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Siewko K, Maciulewski R, Zielinska-Maciulewska A, Poplawska-Kita A, Szumowski P, Wawrusiewicz-Kurylonek N, Lipinska D, Milewski R, Gorska M, Kretowski A, Szelachowska M, Interleukin-6 and Interleukin-15 as Possible Biomarkers of the Risk of Autoimmune Diabetes Development, Biomed Res Int, 2019 (2019) 4734063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kristiansen OP, Mandrup-Poulsen T, Interleukin-6 and diabetes: the good, the bad, or the indifferent?, Diabetes, 54 Suppl 2 (2005) S114–124. [DOI] [PubMed] [Google Scholar]
- [83].Chen YL, Qiao YC, Pan YH, Xu Y, Huang YC, Wang YH, Geng LJ, Zhao HL, Zhang XX, Correlation between serum interleukin-6 level and type 1 diabetes mellitus: A systematic review and meta-analysis, Cytokine, 94 (2017) 14–20. [DOI] [PubMed] [Google Scholar]
- [84].Bowker N, Shah RL, Sharp SJ, Luan J, Stewart ID, Wheeler E, Ferreira MAR, Baras A, Wareham NJ, Langenberg C, Lotta LA, Meta-analysis investigating the role of interleukin-6 mediated inflammation in type 2 diabetes, EBioMedicine, 61 (2020) 103062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF, Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study, Diabetes, 52 (2003) 812–817. [DOI] [PubMed] [Google Scholar]
- [86].Huth C, Heid IM, Vollmert C, Gieger C, Grallert H, Wolford JK, Langer B, Thorand B, Klopp N, Hamid YH, Pedersen O, Hansen T, Lyssenko V, Groop L, Meisinger C, Doring A, Lowel H, Lieb W, Hengstenberg C, Rathmann W, Martin S, Stephens JW, Ireland H, Mather H, Miller GJ, Stringham HM, Boehnke M, Tuomilehto J, Boeing H, Mohlig M, Spranger J, Pfeiffer A, Wernstedt I, Niklason A, Lopez-Bermejo A, Fernandez-Real JM, Hanson RL, Gallart L, Vendrell J, Tsiavou A, Hatziagelaki E, Humphries SE, Wichmann HE, Herder C, Illig T, IL6 gene promoter polymorphisms and type 2 diabetes: joint analysis of individual participants' data from 21 studies, Diabetes, 55 (2006) 2915–2921. [DOI] [PubMed] [Google Scholar]
- [87].Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, Rasul A, Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus, Crit Rev Eukaryot Gene Expr, 27 (2017) 229–236. [DOI] [PubMed] [Google Scholar]
- [88].Schultz O, Oberhauser F, Saech J, Rubbert-Roth A, Hahn M, Krone W, Laudes M, Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases, PLoS One, 5 (2010) e14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK, Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction, Diabetes, 53 (2004) 1643–1648. [DOI] [PubMed] [Google Scholar]
- [90].Akbari M, Hassan-Zadeh V, IL-6 signalling pathways and the development of type 2 diabetes, Inflammopharmacology, 26 (2018) 685–698. [DOI] [PubMed] [Google Scholar]
- [91].Shang GS, Liu L, Qin YW, IL-6 and TNF-alpha promote metastasis of lung cancer by inducing epithelial-mesenchymal transition, Oncol Lett, 13 (2017) 4657–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rice SJ, Liu X, Zhang J, Jia B, Zheng H, Belani CP, Advanced NSCLC patients with high IL-6 levels have altered peripheral T cell population and signaling, Lung Cancer, 131 (2019) 58–61. [DOI] [PubMed] [Google Scholar]
- [93].Haura EB, Livingston S, Coppola D, Autocrine interleukin-6/interleukin-6 receptor stimulation in non-small-cell lung cancer, Clin Lung Cancer, 7 (2006) 273–275. [DOI] [PubMed] [Google Scholar]
- [94].Peng X, Shi J, Sun W, Ruan X, Guo Y, Zhao L, Wang J, Li B, Genetic polymorphisms of IL-6 promoter in cancer susceptibility and prognosis: a meta-analysis, Oncotarget, 9 (2018) 12351–12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M, IL-6 Secreted from Cancer-Associated Fibroblasts Mediates Chemoresistance in NSCLC by Increasing Epithelial-Mesenchymal Transition Signaling, J Thorac Oncol, 11 (2016) 1482–1492. [DOI] [PubMed] [Google Scholar]
- [96].Liu W, Wang H, Bai F, Ding L, Huang Y, Lu C, Chen S, Li C, Yue X, Liang X, Ma C, Xu L, Gao L, IL-6 promotes metastasis of non-small-cell lung cancer by up-regulating TIM-4 via NF-kappaB, Cell Prolif, 53 (2020) e12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Caetano MS, Zhang H, Cumpian AM, Gong L, Unver N, Ostrin EJ, Daliri S, Chang SH, Ochoa CE, Hanash S, Behrens C, Wistuba II, Sternberg C, Kadara H, Ferreira CG, Watowich SS, Moghaddam SJ, IL6 Blockade Reprograms the Lung Tumor Microenvironment to Limit the Development and Progression of K-ras-Mutant Lung Cancer, Cancer Res, 76 (2016) 3189–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Masjedi A, Ahmadi A, Atyabi F, Farhadi S, Irandoust M, Khazaei-Poul Y, Ghasemi Chaleshtari M, Edalati Fathabad M, Baghaei M, Haghnavaz N, Baradaran B, Hojjat-Farsangi M, Ghalamfarsa G, Sabz G, Hasanzadeh S, Jadidi-Niaragh F, Silencing of IL-6 and STAT3 by siRNA loaded hyaluronate-N,N,N-trimethyl chitosan nanoparticles potently reduces cancer cell progression, Int J Biol Macromol, 149 (2020) 487–500. [DOI] [PubMed] [Google Scholar]
- [99].Guo C, Chen Y, Gao W, Chang A, Ye Y, Shen W, Luo Y, Yang S, Sun P, Xiang R, Li N, Liposomal Nanoparticles Carrying anti-IL6R Antibody to the Tumour Microenvironment Inhibit Metastasis in Two Molecular Subtypes of Breast Cancer Mouse Models, Theranostics, 7 (2017) 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR, A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer, Expert Opin Biol Ther, 11 (2011) 1663–1668. [DOI] [PubMed] [Google Scholar]
- [101].Catrina SB, Zheng X, Hypoxia and hypoxia-inducible factors in diabetes and its complications, Diabetologia, 64 (2021) 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Sato Y, Endo H, Okuyama H, Takeda T, Iwahashi H, Imagawa A, Yamagata K, Shimomura I, Inoue M, Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro, J Biol Chem, 286 (2011) 12524–12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Nomoto H, Pei L, Montemurro C, Rosenberger M, Furterer A, Coppola G, Nadel B, Pellegrini M, Gurlo T, Butler PC, Tudzarova S, Activation of the HIF1alpha/PFKFB3 stress response pathway in beta cells in type 1 diabetes, Diabetologia, 63 (2020) 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lalwani A, Warren J, Liuwantara D, Hawthorne WJ, O'Connell PJ, Gonzalez FJ, Stokes RA, Chen J, Laybutt DR, Craig ME, Swarbrick MM, King C, Gunton JE, beta Cell Hypoxia-Inducible Factor-1alpha Is Required for the Prevention of Type 1 Diabetes, Cell Rep, 27 (2019) 2370–2384 e2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, Hansell P, Palm F, Activation of hypoxia-inducible factors prevents diabetic nephropathy, J Am Soc Nephrol, 26 (2015) 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Xu J, Liu X, Zhao F, Zhang Y, Wang Z, HIF1alpha overexpression enhances diabetic wound closure in high glucose and low oxygen conditions by promoting adipose-derived stem cell paracrine function and survival, Stem Cell Res Ther, 11 (2020) 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, Pereira T, Yla-Herttuala S, Poellinger L, Brismar K, Catrina SB, Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice, Proc Natl Acad Sci U S A, 105 (2008) 19426–19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Semenza GL, Targeting HIF-1 for cancer therapy, Nat Rev Cancer, 3 (2003) 721–732. [DOI] [PubMed] [Google Scholar]
- [109].Yohena T, Yoshino I, Takenaka T, Kameyama T, Ohba T, Kuniyoshi Y, Maehara Y, Upregulation of hypoxia-inducible factor-1alpha mRNA and its clinical significance in non-small cell lung cancer, J Thorac Oncol, 4 (2009) 284–290. [DOI] [PubMed] [Google Scholar]
- [110].Gao ZJ, Wang Y, Yuan WD, Yuan JQ, Yuan K, HIF-2alpha not HIF-1alpha overexpression confers poor prognosis in non-small cell lung cancer, Tumour Biol, 39 (2017) 1010428317709637. [DOI] [PubMed] [Google Scholar]
- [111].Guo R, Li Y, Wang Z, Bai H, Duan J, Wang S, Wang L, Wang J, Hypoxia-inducible factor-1alpha and nuclear factor-kappaB play important roles in regulating programmed cell death ligand 1 expression by epidermal growth factor receptor mutants in non-small-cell lung cancer cells, Cancer Sci, 110 (2019) 1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhao Y, Wang XX, Wu W, Long H, Huang J, Wang Z, Li T, Tang S, Zhu B, Chen D, EZH2 regulates PD-L1 expression via HIF-1alpha in non-small cell lung cancer cells, Biochem Biophys Res Commun, 517 (2019) 201–209. [DOI] [PubMed] [Google Scholar]
- [113].Sowa T, Menju T, Chen-Yoshikawa TF, Takahashi K, Nishikawa S, Nakanishi T, Shikuma K, Motoyama H, Hijiya K, Aoyama A, Sato T, Sonobe M, Harada H, Date H, Hypoxia-inducible factor 1 promotes chemoresistance of lung cancer by inducing carbonic anhydrase IX expression, Cancer Med, 6 (2017) 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bailey CM, Liu Y, Peng G, Zhang H, He M, Sun D, Zheng P, Liu Y, Wang Y, Liposomal formulation of HIF-1alpha inhibitor echinomycin eliminates established metastases of triple-negative breast cancer, Nanomedicine, 29 (2020) 102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chen Y, Xu G, Zheng Y, Yan M, Li Z, Zhou Y, Mei L, Li X, Nanoformulation of D-alpha-tocopheryl polyethylene glycol 1000 succinate-b-poly(epsilon-caprolactone-ran-glycolide) diblock copolymer for siRNA targeting HIF-1alpha for nasopharyngeal carcinoma therapy, Int J Nanomedicine, 10 (2015) 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Papadopoulos N, Lennartsson J, The PDGF/PDGFR pathway as a drug target, Mol Aspects Med, 62 (2018) 75–88. [DOI] [PubMed] [Google Scholar]
- [117].Andrae J, Gallini R, Betsholtz C, Role of platelet-derived growth factors in physiology and medicine, Genes Dev, 22 (2008) 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Shan Z, Xu C, Wang W, Li W, Enhanced PDGF signaling in gestational diabetes mellitus is involved in pancreatic beta-cell dysfunction, Biochem Biophys Res Commun, 516 (2019) 402–407. [DOI] [PubMed] [Google Scholar]
- [119].Abderrahmani A, Yengo L, Caiazzo R, Canouil M, Cauchi S, Raverdy V, Plaisance V, Pawlowski V, Lobbens S, Maillet J, Rolland L, Boutry R, Queniat G, Kwapich M, Tenenbaum M, Bricambert J, Saussenthaler S, Anthony E, Jha P, Derop J, Sand O, Rabearivelo I, Leloire A, Pigeyre M, Daujat-Chavanieu M, Gerbal-Chaloin S, Dayeh T, Lassailly G, Mathurin P, Staels B, Auwerx J, Schurmann A, Postic C, Schafmayer C, Hampe J, Bonnefond A, Pattou F, Froguel P, Increased Hepatic PDGF-AA Signaling Mediates Liver Insulin Resistance in Obesity-Associated Type 2 Diabetes, Diabetes, 67 (2018) 1310–1321. [DOI] [PubMed] [Google Scholar]
- [120].Langham RG, Kelly DJ, Maguire J, Dowling JP, Gilbert RE, Thomson NM, Overexpression of platelet-derived growth factor in human diabetic nephropathy, Nephrol Dial Transplant, 18 (2003) 1392–1396. [DOI] [PubMed] [Google Scholar]
- [121].Yamaguchi H, Igarashi M, Hirata A, Sugae N, Tsuchiya H, Jimbu Y, Tominaga M, Kato T, Altered PDGF-BB-induced p38 MAP kinase activation in diabetic vascular smooth muscle cells: roles of protein kinase C-delta, Arterioscler Thromb Vasc Biol, 24 (2004) 2095–2101. [DOI] [PubMed] [Google Scholar]
- [122].Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Busund LT, Bremnes RM, Prognostic impact of platelet-derived growth factors in non-small cell lung cancer tumor and stromal cells, J Thorac Oncol, 3 (2008) 963–970. [DOI] [PubMed] [Google Scholar]
- [123].Kilvaer TK, Rakaee M, Hellevik T, Vik J, Petris L, Donnem T, Strell C, Ostman A, Busund LR, Martinez-Zubiaurre I, Differential prognostic impact of platelet-derived growth factor receptor expression in NSCLC, Sci Rep, 9 (2019) 10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Shinohara ET, Gonzalez A, Massion PP, Olson SJ, Albert JM, Shyr Y, Carbone DP, Johnson DH, Hallahan DE, Lu B, PDGFR-beta expression in small cell lung cancer patients, Int J Radiat Oncol Biol Phys, 67 (2007) 431–437. [DOI] [PubMed] [Google Scholar]
- [125].Kanaan R, Strange C, Use of multitarget tyrosine kinase inhibitors to attenuate platelet-derived growth factor signalling in lung disease, Eur Respir Rev, 26 (2017) 170061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang P, Song L, Ge H, Jin P, Jiang Y, Hu W, Geng N, Crenolanib, a PDGFR inhibitor, suppresses lung cancer cell proliferation and inhibits tumor growth in vivo, Onco Targets Ther, 7 (2014) 1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Noskovicova N, Petrek M, Eickelberg O, Heinzelmann K, Platelet-derived growth factor signaling in the lung. From lung development and disease to clinical studies, Am J Respir Cell Mol Biol, 52 (2015) 263–284. [DOI] [PubMed] [Google Scholar]
- [128].Gerber DE, Swanson P, Lopez-Chavez A, Wong L, Dowlati A, Pennell NA, Cronier DM, Qin A, Ilaria R Jr., Cosaert J, Shahir A, Baggstrom MQ, Phase II study of olaratumab with paclitaxel/carboplatin (P/C) or P/C alone in previously untreated advanced NSCLC, Lung Cancer, 111 (2017) 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Blanquicett C, Roman J, Hart CM, Thiazolidinediones as anti-cancer agents, Cancer Ther, 6 (2008) 25–34. [PMC free article] [PubMed] [Google Scholar]
- [130].Mazzone PJ, Rai H, Beukemann M, Xu M, Jain A, Sasidhar M, The effect of metformin and thiazolidinedione use on lung cancer in diabetics, BMC Cancer, 12 (2012) 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA, Metformin prevents tobacco carcinogen--induced lung tumorigenesis, Cancer Prev Res (Phila), 3 (2010) 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Tan BX, Yao WX, Ge J, Peng XC, Du XB, Zhang R, Yao B, Xie K, Li LH, Dong H, Gao F, Zhao F, Hou JM, Su JM, Liu JY, Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes, Cancer, 117 (2011) 5103–5111. [DOI] [PubMed] [Google Scholar]
- [133].Danila E, Linkeviciute-Ulinskiene D, Zablockis R, Gruslys V, Cicenas S, Smailyte G, A Cohort Study of Exposure to Antihyperglycemic Therapy and Survival in Patients with Lung Cancer, Int J Environ Res Public Health, 17 (2020) 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hung MS, Chuang MC, Chen YC, Lee CP, Yang TM, Chen PC, Tsai YH, Yang YH, Metformin Prolongs Survival in Type 2 Diabetes Lung Cancer Patients With EGFR-TKIs, Integr Cancer Ther, 18 (2019) 1534735419869491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Chen H, Yao W, Chu Q, Han R, Wang Y, Sun J, Wang D, Wang Y, Cao M, He Y, Synergistic effects of metformin in combination with EGFR-TKI in the treatment of patients with advanced non-small cell lung cancer and type 2 diabetes, Cancer Lett, 369 (2015) 97–102. [DOI] [PubMed] [Google Scholar]
- [136].Thiazolidinediones reduce the risk of lung cancer in patients with diabetes, Nature Clinical Practice Endocrinology & Metabolism, 3 (2007) 620–621. [Google Scholar]
- [137].Hazra S, Batra RK, Tai HH, Sharma S, Cui X, Dubinett SM, Pioglitazone and rosiglitazone decrease prostaglandin E2 in non-small-cell lung cancer cells by up-regulating 15-hydroxyprostaglandin dehydrogenase, Mol Pharmacol, 71 (2007) 1715–1720. [DOI] [PubMed] [Google Scholar]
- [138].Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP, Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes, J Clin Oncol, 25 (2007) 1476–1481. [DOI] [PubMed] [Google Scholar]
- [139].Srikantan S, Gorospe M, HuR function in disease, Front Biosci (Landmark Ed), 17 (2012) 189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wang N, Yan D, Liu Y, Liu Y, Gu X, Sun J, Long F, Jiang S, A HuR/TGF-beta1 feedback circuit regulates airway remodeling in airway smooth muscle cells, Respir Res, 17 (2016) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ge J, Chang N, Zhao Z, Tian L, Duan X, Yang L, Li L, Essential Roles of RNA-binding Protein HuR in Activation of Hepatic Stellate Cells Induced by Transforming Growth Factor-beta1, Sci Rep, 6 (2016) 22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Warfel JD, HuR Influences Metabolic Flexibility in Skeletal Muscle, 67 (2018) 269–LB. [Google Scholar]
- [143].Wang J, Guo Y, Chu H, Guan Y, Bi J, Wang B, Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis, Int J Mol Sci, 14 (2013) 10015–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wu M, Tong CWS, Yan W, To KKW, Cho WCS, The RNA Binding Protein HuR: A Promising Drug Target for Anticancer Therapy, Curr Cancer Drug Targets, 19 (2019) 382–399. [DOI] [PubMed] [Google Scholar]
- [145].Wang J, Zhao W, Guo Y, Zhang B, Xie Q, Xiang D, Gao J, Wang B, Chen Z, The expression of RNA-binding protein HuR in non-small cell lung cancer correlates with vascular endothelial growth factor-C expression and lymph node metastasis, Oncology, 76 (2009) 420–429. [DOI] [PubMed] [Google Scholar]
- [146].Sajid MI, Moazzam M, Kato S, Yeseom Cho K, Tiwari RK, Overcoming Barriers for siRNA Therapeutics: From Bench to Bedside, Pharmaceuticals (Basel), 13 (2020) 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Muralidharan R, Babu A, Amreddy N, Basalingappa K, Mehta M, Chen A, Zhao YD, Kompella UB, Munshi A, Ramesh R, Folate receptor-targeted nanoparticle delivery of HuR-RNAi suppresses lung cancer cell proliferation and migration, J Nanobiotechnology, 14 (2016) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Muralidharan R, Babu A, Amreddy N, Srivastava A, Chen A, Zhao YD, Kompella UB, Munshi A, Ramesh R, Tumor-targeted Nanoparticle Delivery of HuR siRNA Inhibits Lung Tumor Growth In Vitro and In Vivo By Disrupting the Oncogenic Activity of the RNA-binding Protein HuR, Mol Cancer Ther, 16 (2017) 1470–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Amreddy N, Babu A, Panneerselvam J, Srivastava A, Muralidharan R, Chen A, Zhao YD, Munshi A, Ramesh R, Chemo-biologic combinatorial drug delivery using folate receptor-targeted dendrimer nanoparticles for lung cancer treatment, Nanomedicine, 14 (2018) 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Muralidharan R, Panneerselvam J, Chen A, Zhao YD, Munshi A, Ramesh R, HuR-targeted nanotherapy in combination with AMD3100 suppresses CXCR4 expression, cell growth, migration and invasion in lung cancer, Cancer Gene Ther, 22 (2015) 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Mehta M, Basalingappa K, Griffith JN, Andrade D, Babu A, Amreddy N, Muralidharan R, Gorospe M, Herman T, Ding WQ, Ramesh R, Munshi A, HuR silencing elicits oxidative stress and DNA damage and sensitizes human triple-negative breast cancer cells to radiotherapy, Oncotarget, 7 (2016) 64820–64835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Andrade D, Mehta M, Griffith J, Oh S, Corbin J, Babu A, De S, Chen A, Zhao YD, Husain S, Roy S, Xu L, Aube J, Janknecht R, Gorospe M, Herman T, Ramesh R, Munshi A, HuR Reduces Radiation-Induced DNA Damage by Enhancing Expression of ARID1A, Cancers (Basel), 11 (2019) 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Huang YH, Peng W, Furuuchi N, Gerhart J, Rhodes K, Mukherjee N, Jimbo M, Gonye GE, Brody JR, Getts RC, Sawicki JA, Delivery of Therapeutics Targeting the mRNA-Binding Protein HuR Using 3DNA Nanocarriers Suppresses Ovarian Tumor Growth, Cancer Res, 76 (2016) 1549–1559. [DOI] [PubMed] [Google Scholar]
- [154].Wang J, Hjelmeland AB, Nabors LB, King PH, Anti-cancer effects of the HuR inhibitor, MS-444, in malignant glioma cells, Cancer Biol Ther, 20 (2019) 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Allegri L, Baldan F, Roy S, Aube J, Russo D, Filetti S, Damante G, The HuR CMLD-2 inhibitor exhibits antitumor effects via MAD2 downregulation in thyroid cancer cells, Sci Rep, 9 (2019) 7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Blanco FF, Preet R, Aguado A, Vishwakarma V, Stevens LE, Vyas A, Padhye S, Xu L, Weir SJ, Anant S, Meisner-Kober N, Brody JR, Dixon DA, Impact of HuR inhibition by the small molecule MS-444 on colorectal cancer cell tumorigenesis, Oncotarget, 7 (2016) 74043–74058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wu X, Gardashova G, Lan L, Han S, Zhong C, Marquez RT, Wei L, Wood S, Roy S, Gowthaman R, Karanicolas J, Gao FP, Dixon DA, Welch DR, Li L, Ji M, Aube J, Xu L, Targeting the interaction between RNA-binding protein HuR and FOXQ1 suppresses breast cancer invasion and metastasis, Commun Biol, 3 (2020) 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Romeo C, Weber MC, Zarei M, DeCicco D, Chand SN, Lobo AD, Winter JM, Sawicki JA, Sachs JN, Meisner-Kober N, Yeo CJ, Vadigepalli R, Tykocinski ML, Brody JR, HuR Contributes to TRAIL Resistance by Restricting Death Receptor 4 Expression in Pancreatic Cancer Cells, Mol Cancer Res, 14 (2016) 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wang J, Leavenworth JW, Hjelmeland AB, Smith R, Patel N, Borg B, Si Y, King PH, Deletion of the RNA regulator HuR in tumor-associated microglia and macrophages stimulates anti-tumor immunity and attenuates glioma growth, Glia, 67 (2019) 2424–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Liu Y, Li X, Zhang H, Zhang M, Wei Y, HuR up-regulates cell surface PD-L1 via stabilizing CMTM6 transcript in cancer, Oncogene, 40 (2021) 2230–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Janice Sanchez B, Tremblay AK, Leduc-Gaudet JP, Hall DT, Kovacs E, Ma JF, Mubaid S, Hallauer PL, Phillips BL, Vest KE, Corbett AH, Kontoyiannis DL, Hussain SNA, Hastings KEM, Di Marco S, Gallouzi IE, Depletion of HuR in murine skeletal muscle enhances exercise endurance and prevents cancer-induced muscle atrophy, Nat Commun, 10 (2019) 4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Mubaid S, Ma JF, Omer A, Ashour K, Lian XJ, Sanchez BJ, Robinson S, Cammas A, Dormoy-Raclet V, Di Marco S, Chittur SV, Tenenbaum SA, Gallouzi IE, HuR counteracts miR-330 to promote STAT3 translation during inflammation-induced muscle wasting, Proc Natl Acad Sci U S A, 116 (2019) 17261–17270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Amadio M, Pascale A, Cupri S, Pignatello R, Osera C, D.A. V, AG DA, Leggio GM, Ruozi B, Govoni S, Drago F, Bucolo C, Nanosystems based on siRNA silencing HuR expression counteract diabetic retinopathy in rat, Pharmacol Res, 111 (2016) 713–720. [DOI] [PubMed] [Google Scholar]
- [164].Inoue S, Shanker M, Miyahara R, Gopalan B, Patel S, Oida Y, Branch CD, Munshi A, Meyn RE, Andreeff M, Tanaka F, Mhashilkar AM, Chada S, Ramesh R, MDA-7/IL-24-based cancer gene therapy: translation from the laboratory to the clinic, Curr Gene Ther, 6 (2006) 73–91. [DOI] [PubMed] [Google Scholar]
- [165].Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P, mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic, Cancer Biol Ther, 2 (2003) S23–37. [PubMed] [Google Scholar]
- [166].Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB, Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression, Oncogene, 11 (1995) 2477–2486. [PubMed] [Google Scholar]
- [167].Mhashilkar AM, Schrock RD, Hindi M, Liao J, Sieger K, Kourouma F, Zou-Yang XH, Onishi E, Takh O, Vedvick TS, Fanger G, Stewart L, Watson GJ, Snary D, Fisher PB, Saeki T, Roth JA, Ramesh R, Chada S, Melanoma differentiation associated gene-7 (mda-7): a novel anti-tumor gene for cancer gene therapy, Mol Med, 7 (2001) 271–282. [PMC free article] [PubMed] [Google Scholar]
- [168].Panneerselvam J, Munshi A, Ramesh R, Molecular targets and signaling pathways regulated by interleukin (IL)-24 in mediating its antitumor activities, J Mol Signal, 8 (2013) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB, The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice, Proc Natl Acad Sci U S A, 95 (1998) 14400–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, Grimm EA, Loss of MDA-7 expression with progression of melanoma, J Clin Oncol, 20 (2002) 1069–1074. [DOI] [PubMed] [Google Scholar]
- [171].Ishikawa S, Nakagawa T, Miyahara R, Kawano Y, Takenaka K, Yanagihara K, Otake Y, Katakura H, Wada H, Tanaka F, Expression of MDA-7/IL-24 and its clinical significance in resected non-small cell lung cancer, Clin Cancer Res, 11 (2005) 1198–1202. [PubMed] [Google Scholar]
- [172].Ramesh R, Ito I, Saito Y, Wu Z, Mhashikar AM, Wilson DR, Branch CD, Roth JA, Chada S, Local and systemic inhibition of lung tumor growth after nanoparticle-mediated mda-7/IL-24 gene delivery, DNA Cell Biol, 23 (2004) 850–857. [DOI] [PubMed] [Google Scholar]
- [173].Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R, Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro, Gene Ther, 7 (2000) 2051–2057. [DOI] [PubMed] [Google Scholar]
- [174].Liu L, Ma J, Qin L, Shi X, Si H, Wei Y, Interleukin-24 enhancing antitumor activity of chimeric oncolytic adenovirus for treating acute promyelocytic leukemia cell, Medicine (Baltimore), 98 (2019) e15875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Mo Q, Liu L, Bao G, Li T, Effects of melanoma differentiation associated gene-7 (MDA-7/IL-24) on apoptosis of liver cancer cells via regulating the expression of B-cell lymphoma-2, Oncol Lett, 18 (2019) 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM, Zheng M, mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: identification of IL-20 receptor-mediated bystander activity against pancreatic cancer, Mol Ther, 11 (2005) 724–733. [DOI] [PubMed] [Google Scholar]
- [177].Hamed HA, Yacoub A, Park MA, Eulitt P, Sarkar D, Dimitrie IP, Chen CS, Grant S, Curiel DT, Fisher PB, Dent P, OSU-03012 enhances Ad.7-induced GBM cell killing via ER stress and autophagy and by decreasing expression of mitochondrial protective proteins, Cancer Biol Ther, 9 (2010) 526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Chada S, Mhashilkar AM, Liu Y, Nishikawa T, Bocangel D, Zheng M, Vorburger SA, Pataer A, Swisher SG, Ramesh R, Kawase K, Meyn RE, Hunt KK, mda-7 gene transfer sensitizes breast carcinoma cells to chemotherapy, biologic therapies and radiotherapy: correlation with expression of bcl-2 family members, Cancer Gene Ther, 13 (2006) 490–502. [DOI] [PubMed] [Google Scholar]
- [179].Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sarkar D, Settleman J, Fisher PB, Combinatorial treatment of non-small-cell lung cancers with gefitinib and Ad.mda-7 enhances apoptosis-induction and reverses resistance to a single therapy, J Cell Physiol, 210 (2007) 549–559. [DOI] [PubMed] [Google Scholar]
- [180].McKenzie T, Liu Y, Fanale M, Swisher SG, Chada S, Hunt KK, Combination therapy of Ad-mda7 and trastuzumab increases cell death in Her-2/neu-overexpressing breast cancer cells, Surgery, 136 (2004) 437–442. [DOI] [PubMed] [Google Scholar]
- [181].Bocangel D, Zheng M, Mhashilkar A, Liu Y, Ramesh R, Hunt KK, Chada S, Combinatorial synergy induced by adenoviral-mediated mda-7 and Herceptin in Her-2+ breast cancer cells, Cancer Gene Ther, 13 (2006) 958–968. [DOI] [PubMed] [Google Scholar]
- [182].Inoue S, Hartman A, Branch CD, Bucana CD, Bekele BN, Stephens LC, Chada S, Ramesh R, mda-7 In combination with bevacizumab treatment produces a synergistic and complete inhibitory effect on lung tumor xenograft, Mol Ther, 15 (2007) 287–294. [DOI] [PubMed] [Google Scholar]
- [183].Oida Y, Gopalan B, Miyahara R, Inoue S, Branch CD, Mhashilkar AM, Lin E, Bekele BN, Roth JA, Chada S, Ramesh R, Sulindac enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human lung cancer, Mol Cancer Ther, 4 (2005) 291–304. [PubMed] [Google Scholar]
- [184].Shanker M, Gopalan B, Patel S, Bocangel D, Chada S, Ramesh R, Vitamin E succinate in combination with mda-7 results in enhanced human ovarian tumor cell killing through modulation of extrinsic and intrinsic apoptotic pathways, Cancer Lett, 254 (2007) 217–226. [DOI] [PubMed] [Google Scholar]
- [185].Zhang Z, Kawamura K, Jiang Y, Shingyoji M, Ma G, Li Q, Hu J, Qi Y, Liu H, Zhang F, Kang S, Shan B, Wang S, Chada S, Tagawa M, Heat-shock protein 90 inhibitors synergistically enhance melanoma differentiation-associated gene-7-mediated cell killing of human pancreatic carcinoma, Cancer Gene Ther, 20 (2013) 663–670. [DOI] [PubMed] [Google Scholar]
- [186].Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE, Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation, Mol Ther, 9 (2004) 818–828. [DOI] [PubMed] [Google Scholar]
- [187].Pataer A, Vorburger SA, Barber GN, Chada S, Mhashilkar AM, Zou-Yang H, Stewart AL, Balachandran S, Roth JA, Hunt KK, Swisher SG, Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via upregulation of the double-stranded RNA-dependent protein kinase (PKR), Cancer Res, 62 (2002) 2239–2243. [PubMed] [Google Scholar]
- [188].Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S, Ramesh R, Activation of the Fas-FasL signaling pathway by MDA-7/IL-24 kills human ovarian cancer cells, Cancer Res, 65 (2005) 3017–3024. [DOI] [PubMed] [Google Scholar]
- [189].Panneerselvam J, Srivastava A, Mehta M, Chen A, Zhao YD, Munshi A, Ramesh R, IL-24 Inhibits Lung Cancer Growth by Suppressing GLI1 and Inducing DNA Damage, Cancers (Basel), 11 (2019) 1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Ekmekcioglu S, Ellerhorst JA, Mumm JB, Zheng M, Broemeling L, Prieto VG, Stewart AL, Mhashilkar AM, Chada S, Grimm EA, Negative association of melanoma differentiation-associated gene (mda-7) and inducible nitric oxide synthase (iNOS) in human melanoma: MDA-7 regulates iNOS expression in melanoma cells, Mol Cancer Ther, 2 (2003) 9–17. [PubMed] [Google Scholar]
- [191].Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S, Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells, Mol Ther, 9 (2004) 510–518. [DOI] [PubMed] [Google Scholar]
- [192].Huo W, Li ZM, Zhu XM, Bao YM, An LJ, MDA-7/IL-24 suppresses tumor adhesion and invasive potential in hepatocellular carcinoma cell lines, Oncol Rep, 30 (2013) 986–992. [DOI] [PubMed] [Google Scholar]
- [193].Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L, Grimm EA, Renauld JC, Kotenko S, Chada S, Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor, Cancer Res, 63 (2003) 5105–5113. [PubMed] [Google Scholar]
- [194].Inoue S, Branch CD, Gallick GE, Chada S, Ramesh R, Inhibition of Src kinase activity by Ad-mda7 suppresses vascular endothelial growth factor expression in prostate carcinoma cells, Mol Ther, 12 (2005) 707–715. [DOI] [PubMed] [Google Scholar]
- [195].Xie Y, Sheng W, Xiang J, Ye Z, Zhu Y, Chen X, Yang J, Recombinant human IL-24 suppresses lung carcinoma cell growth via induction of cell apoptosis and inhibition of tumor angiogenesis, Cancer Biother Radiopharm, 23 (2008) 310–320. [DOI] [PubMed] [Google Scholar]
- [196].Miyahara R, Banerjee S, Kawano K, Efferson C, Tsuda N, Miyahara Y, Ioannides CG, Chada S, Ramesh R, Melanoma differentiation-associated gene-7 (mda-7)/interleukin (IL)-24 induces anticancer immunity in a syngeneic murine model, Cancer Gene Ther, 13 (2006) 753–761. [DOI] [PubMed] [Google Scholar]
- [197].Ramesh R, Ahmed R, Munshi A, Interleukin (IL)-24: Reconfiguring the Tumor Microenvironment for Eliciting Antitumor Response, Adv Exp Med Biol, 1290 (2021) 99–110. [DOI] [PubMed] [Google Scholar]
- [198].Ma YF, Ren Y, Wu CJ, Zhao XH, Xu H, Wu DZ, Xu J, Zhang XL, Ji Y, Interleukin (IL)-24 transforms the tumor microenvironment and induces anticancer immunity in a murine model of colon cancer, Mol Immunol, 75 (2016) 11–20. [DOI] [PubMed] [Google Scholar]
- [199].Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J, Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study, Mol Ther, 11 (2005) 149–159. [DOI] [PubMed] [Google Scholar]
- [200].Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, van Wart Hood J, Merritt J, Chada S, Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients, Mol Ther, 11 (2005) 160–172. [DOI] [PubMed] [Google Scholar]
- [201].Zdychova J, Komers R, Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications, Physiol Res, 54 (2005) 1–16. [DOI] [PubMed] [Google Scholar]
- [202].Benchoula K, Parhar IS, Wong EH, The crosstalk of hedgehog, PI3K and Wnt pathways in diabetes, Arch Biochem Biophys, 698 (2021) 108743. [DOI] [PubMed] [Google Scholar]
- [203].Reddy S, Ross JM, Fas and Fas ligand immunoexpression in pancreatic islets of NOD mice during spontaneous and cyclophosphamide-accelerated diabetes, Ann N Y Acad Sci, 1005 (2003) 166–169. [DOI] [PubMed] [Google Scholar]
- [204].Domagala-Kulawik J, Hoser G, Dabrowska M, Chazan R, Increased proportion of Fas positive CD8+ cells in peripheral blood of patients with COPD, Respir Med, 101 (2007) 1338–1343. [DOI] [PubMed] [Google Scholar]
- [205].Tahergorabi Z, Khazaei M, Imbalance of angiogenesis in diabetic complications: the mechanisms, Int J Prev Med, 3 (2012) 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Majumder J, Taratula O, Minko T, Nanocarrier-based systems for targeted and site specific therapeutic delivery, Adv Drug Deliv Rev, 144 (2019) 57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Babu A, Templeton AK, Munshi A, Ramesh R, Nanoparticle-Based Drug Delivery for Therapy of Lung Cancer: Progress and Challenges, Journal of Nanomaterials, 2013 (2013) 863951. [Google Scholar]
- [208].Zhang T, Tang JZ, Fei X, Li Y, Song Y, Qian Z, Peng Q, Can nanoparticles and nanoprotein interactions bring a bright future for insulin delivery?, Acta Pharm Sin B, 11 (2021) 651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Chellappan DK, Sivam NS, Teoh KX, Leong WP, Fui TZ, Chooi K, Khoo N, Yi FJ, Chellian J, Cheng LL, Dahiya R, Gupta G, Singhvi G, Nammi S, Hansbro PM, Dua K, Gene therapy and type 1 diabetes mellitus, Biomed Pharmacother, 108 (2018) 1188–1200. [DOI] [PubMed] [Google Scholar]
- [210].Wang Y, Wang C, Li K, Song X, Yan X, Yu L, He Z, Recent advances of nanomedicine-based strategies in diabetes and complications management: Diagnostics, monitoring, and therapeutics, J Control Release, 330 (2021) 618–640. [DOI] [PubMed] [Google Scholar]
- [211].Ahmed R, Muralidharan R, Srivastava A, Johnston SE, Zhao YD, Ekmekcioglu S, Munshi A, Ramesh R, Molecular Targeting of HuR Oncoprotein Suppresses MITF and Induces Apoptosis in Melanoma Cells, Cancers (Basel), 13 (2021) 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Banik BL, Fattahi P, Brown JL, Polymeric nanoparticles: the future of nanomedicine, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 8 (2016) 271–299. [DOI] [PubMed] [Google Scholar]
- [213].Li B, Li Q, Mo J, Dai H, Drug-Loaded Polymeric Nanoparticles for Cancer Stem Cell Targeting, Front Pharmacol, 8 (2017) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Golub JS, Kim YT, Duvall CL, Bellamkonda RV, Gupta D, Lin AS, Weiss D, Robert Taylor W, Guldberg RE, Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth, Am J Physiol Heart Circ Physiol, 298 (2010) H1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Damge C, Maincent P, Ubrich N, Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats, J Control Release, 117 (2007) 163–170. [DOI] [PubMed] [Google Scholar]
- [216].Malathi S, Nandhakumar P, Pandiyan V, Webster TJ, Balasubramanian S, Novel PLGA-based nanoparticles for the oral delivery of insulin, Int J Nanomedicine, 10 (2015) 2207–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zeng L, Ma W, Shi L, Chen X, Wu R, Zhang Y, Chen H, Chen H, Poly(lactic-co-glycolic acid) nanoparticle-mediated interleukin-12 delivery for the treatment of diabetic retinopathy, Int J Nanomedicine, 14 (2019) 6357–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Qiu F, Meng T, Chen Q, Zhou K, Shao Y, Matlock G, Ma X, Wu W, Du Y, Wang X, Deng G, Ma JX, Xu Q, Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration, Mol Pharm, 16 (2019) 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Shi R, Lian W, Han S, Cao C, Jin Y, Yuan Y, Zhao H, Li M, Nanosphere-mediated co-delivery of VEGF-A and PDGF-B genes for accelerating diabetic foot ulcers healing in rats, Gene Ther, 25 (2018) 425–438. [DOI] [PubMed] [Google Scholar]
- [220].Babu A, Amreddy N, Muralidharan R, Pathuri G, Gali H, Chen A, Zhao YD, Munshi A, Ramesh R, Chemodrug delivery using integrin-targeted PLGA-Chitosan nanoparticle for lung cancer therapy, Sci Rep, 7 (2017) 14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Varani M, Galli F, Capriotti G, Mattei M, Cicconi R, Campagna G, Panzuto F, Signore A, Theranostic Designed Near-Infrared Fluorescent Poly (Lactic-co-Glycolic Acid) Nanoparticles and Preliminary Studies with Functionalized VEGF-Nanoparticles, J Clin Med, 9 (2020) 1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Rezvantalab S, Drude NI, Moraveji MK, Guvener N, Koons EK, Shi Y, Lammers T, Kiessling F, PLGA-Based Nanoparticles in Cancer Treatment, Front Pharmacol, 9 (2018) 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Sharma A, Goyal AK, Rath G, Recent advances in metal nanoparticles in cancer therapy, J Drug Target, 26 (2018) 617–632. [DOI] [PubMed] [Google Scholar]
- [224].Zhang Y, Wu M, Dai W, Chen M, Guo Z, Wang X, Tan D, Shi K, Xue L, Liu S, Lei Y, High drug-loading gold nanoclusters for responsive glucose control in type 1 diabetes, J Nanobiotechnology, 17 (2019) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Vega RA, Wang Y, Harvat T, Wang S, Qi M, Adewola AF, Lee D, Benedetti E, Oberholzer J, Modified gold nanoparticle vectors: a biocompatible intracellular delivery system for pancreatic islet cell transplantation, Surgery, 148 (2010) 858–865; discussion 865–856. [DOI] [PubMed] [Google Scholar]
- [226].Dave V, Sharma R, Gupta C, Sur S, Folic acid modified gold nanoparticle for targeted delivery of Sorafenib tosylate towards the treatment of diabetic retinopathy, Colloids Surf B Biointerfaces, 194 (2020) 111151. [DOI] [PubMed] [Google Scholar]
- [227].Kebede A, Singh AK, Rai PK, Giri NK, Rai AK, Watal G, Gholap AV, Controlled synthesis, characterization, and application of iron oxide nanoparticles for oral delivery of insulin, Lasers Med Sci, 28 (2013) 579–587. [DOI] [PubMed] [Google Scholar]
- [228].Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R, Managing diabetes with nanomedicine: challenges and opportunities, Nat Rev Drug Discov, 14 (2015) 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Ali LMA, Shaker SA, Pinol R, Millan A, Hanafy MY, Helmy MH, Kamel MA, Mahmoud SA, Effect of superparamagnetic iron oxide nanoparticles on glucose homeostasis on type 2 diabetes experimental model, Life Sci, 245 (2020) 117361. [DOI] [PubMed] [Google Scholar]
- [230].Tai JH, Foster P, Rosales A, Feng B, Hasilo C, Martinez V, Ramadan S, Snir J, Melling CWJ, Dhanvantari S, Rutt B, White DJG, Imaging Islets Labeled With Magnetic Nanoparticles at 1.5 Tesla, Diabetes, 55 (2006) 2931–2938. [DOI] [PubMed] [Google Scholar]
- [231].Dubreil C, Sainte Catherine O, Lalatonne Y, Journe C, Ou P, van Endert P, Motte L, Tolerogenic Iron Oxide Nanoparticles in Type 1 Diabetes: Biodistribution and Pharmacokinetics Studies in Nonobese Diabetic Mice, Small, 14 (2018) e1802053. [DOI] [PubMed] [Google Scholar]
- [232].Ebrahimpour S, Esmaeili A, Beheshti S, Effect of quercetin-conjugated superparamagnetic iron oxide nanoparticles on diabetes-induced learning and memory impairment in rats, Int J Nanomedicine, 13 (2018) 6311–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Chen H, Fang A, He L, Zhang Y, Yao S, Sensitive fluorescent detection of H2O2 and glucose in human serum based on inner filter effect of squaric acid-iron(III) on the fluorescence of upconversion nanoparticle, Talanta, 164 (2017) 580–587. [DOI] [PubMed] [Google Scholar]
- [234].Knights OB, McLaughlan JR, Gold Nanorods for Light-Based Lung Cancer Theranostics, Int J Mol Sci, 19 (2018) 3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Huang G, Zhang C, Li S, Khemtong C, Yang SG, Tian R, Minna JD, Brown KC, Gao J, A Novel Strategy for Surface Modification of Superparamagnetic Iron Oxide Nanoparticles for Lung Cancer Imaging, J Mater Chem, 19 (2009) 6367–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Yoo MK, Park IK, Lim HT, Lee SJ, Jiang HL, Kim YK, Choi YJ, Cho MH, Cho CS, Folate-PEG-superparamagnetic iron oxide nanoparticles for lung cancer imaging, Acta Biomater, 8 (2012) 3005–3013. [DOI] [PubMed] [Google Scholar]
- [237].Ramalingam V, Varunkumar K, Ravikumar V, Rajaram R, Target delivery of doxorubicin tethered with PVP stabilized gold nanoparticles for effective treatment of lung cancer, Sci Rep, 8 (2018) 3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Pattni BS, Chupin VV, Torchilin VP, New Developments in Liposomal Drug Delivery, Chem Rev, 115 (2015) 10938–10966. [DOI] [PubMed] [Google Scholar]
- [239].Spangler RS, Insulin administration via liposomes, Diabetes Care, 13 (1990) 911–922. [DOI] [PubMed] [Google Scholar]
- [240].Kapoor N, Bhargava S, Bhargava V, FP432 PEGylated glucose triggered liposomes for insulin delivery, Nephrology Dialysis Transplantation, 33 (2018) i179–i180. [Google Scholar]
- [241].Cho EY, Ryu JY, Lee HAR, Hong SH, Park HS, Hong KS, Park SG, Kim HP, Yoon TJ, Lecithin nano-liposomal particle as a CRISPR/Cas9 complex delivery system for treating type 2 diabetes, J Nanobiotechnology, 17 (2019) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Devarajan P, Tarabishi R, Mishra J, Ma Q, Kourvetaris A, Vougiouka M, Boulikas T, Low renal toxicity of lipoplatin compared to cisplatin in animals, Anticancer Res, 24 (2004) 2193–2200. [PubMed] [Google Scholar]
- [243].Wang X, Zhou J, Wang Y, Zhu Z, Lu Y, Wei Y, Chen L, A phase I clinical and pharmacokinetic study of paclitaxel liposome infused in non-small cell lung cancer patients with malignant pleural effusions, Eur J Cancer, 46 (2010) 1474–1480. [DOI] [PubMed] [Google Scholar]
- [244].Jiang L, Li L, He X, Yi Q, He B, Cao J, Pan W, Gu Z, Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response, Biomaterials, 52 (2015) 126–139. [DOI] [PubMed] [Google Scholar]
- [245].Srivastava A, Amreddy N, Pareek V, Chinnappan M, Ahmed R, Mehta M, Razaq M, Munshi A, Ramesh R, Progress in extracellular vesicle biology and their application in cancer medicine, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 12 (2020) e1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Liang Y, Duan L, Lu J, Xia J, Engineering exosomes for targeted drug delivery, Theranostics, 11 (2021) 3183–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Srivastava A, Amreddy N, Razaq M, Towner R, Zhao YD, Ahmed RA, Munshi A, Ramesh R, Exosomes as Theranostics for Lung Cancer, Adv Cancer Res, 139 (2018) 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Isola AL, Chen S, Exosomes: The Messengers of Health and Disease, Curr Neuropharmacol, 15 (2017) 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM, Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity, Cell, 171 (2017) 372–384 e312. [DOI] [PubMed] [Google Scholar]
- [250].Cianciaruso C, Phelps EA, Pasquier M, Hamelin R, Demurtas D, Alibashe Ahmed M, Piemonti L, Hirosue S, Swartz MA, De Palma M, Hubbell JA, Baekkeskov S, Primary Human and Rat beta-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity, Diabetes, 66 (2017) 460–473. [DOI] [PubMed] [Google Scholar]
- [251].Chang W, Wang J, Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus, Cells, 8 (2019) 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA, Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes, Nature Biotechnology, 29 (2011) 341–345. [DOI] [PubMed] [Google Scholar]
- [253].Srivastava A, Amreddy N, Babu A, Panneerselvam J, Mehta M, Muralidharan R, Chen A, Zhao YD, Razaq M, Riedinger N, Kim H, Liu S, Wu S, Abdel-Mageed AB, Munshi A, Ramesh R, Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells, Sci Rep, 6 (2016) 38541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M, Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells, Mol Ther, 21 (2013) 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Izco M, Blesa J, Schleef M, Schmeer M, Porcari R, Al-Shawi R, Ellmerich S, de Toro M, Gardiner C, Seow Y, Reinares-Sebastian A, Forcen R, Simons JP, Bellotti V, Cooper JM, Alvarez-Erviti L, Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology, Mol Ther, 27 (2019) 2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, Fan Y, Wang Y, Wang NS, Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats, Stem Cell Res Ther, 7 (2016) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Zhu Q, Li Q, Niu X, Zhang G, Ling X, Zhang J, Wang Y, Deng Z, Extracellular Vesicles Secreted by Human Urine-Derived Stem Cells Promote Ischemia Repair in a Mouse Model of Hind-Limb Ischemia, Cell Physiol Biochem, 47 (2018) 1181–1192. [DOI] [PubMed] [Google Scholar]
- [258].Newton WC, Kim JW, Luo JZQ, Luo L, Stem cell-derived exosomes: a novel vector for tissue repair and diabetic therapy, J Mol Endocrinol, 59 (2017) R155–R165. [DOI] [PubMed] [Google Scholar]
- [259].Xiao Y, Zheng L, Zou X, Wang J, Zhong J, Zhong T, Extracellular vesicles in type 2 diabetes mellitus: key roles in pathogenesis, complications, and therapy, J Extracell Vesicles, 8 (2019) 1625677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Geiger A, Walker A, Nissen E, Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice, Biochem Biophys Res Commun, 467 (2015) 303–309. [DOI] [PubMed] [Google Scholar]
- [261].Azmi AS, Bao B, Sarkar FH, Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review, Cancer Metastasis Rev, 32 (2013) 623–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Osaki M, Okada F, Exosomes and Their Role in Cancer Progression, Yonago Acta Med, 62 (2019) 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].O'Farrell HE, Yang IA, Extracellular vesicles in chronic obstructive pulmonary disease (COPD), J Thorac Dis, 11 (2019) S2141–S2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Tan DBA, Armitage J, Teo TH, Ong NE, Shin H, Moodley YP, Elevated levels of circulating exosome in COPD patients are associated with systemic inflammation, Respir Med, 132 (2017) 261–264. [DOI] [PubMed] [Google Scholar]
- [265].Bazzan E, Radu CM, Tinè M, Cocconcelli E, Biondini D, Turato G, Simioni P, Cosio MG, Saetta M, Exosomes are differentially present in bronchoalveolar lavage (BAL) of smokers and patients with COPD and IPF, European Respiratory Journal, 54 (2019) PA1682. [Google Scholar]
- [266].Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV, Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells, Nanomedicine, 12 (2016) 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, Kabanov AV, Batrakova EV, Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations, Nanomedicine, 14 (2018) 195–204. [DOI] [PubMed] [Google Scholar]
- [268].Lobb RJ, van Amerongen R, Wiegmans A, Ham S, Larsen JE, Moller A, Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance, Int J Cancer, 141 (2017) 614–620. [DOI] [PubMed] [Google Scholar]
- [269].Chen D, Zhao X, Sui Z, Niu H, Chen L, Hu C, Xuan Q, Hou X, Zhang R, Zhou L, Li Y, Yuan H, Zhang Y, Wu J, Zhang L, Wu R, Piao HL, Xu G, Jia W, A multi-omics investigation of the molecular characteristics and classification of six metabolic syndrome relevant diseases, Theranostics, 10 (2020) 2029–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Gan WZ, Ramachandran V, Lim CSY, Koh RY, Omics-based biomarkers in the diagnosis of diabetes, J Basic Clin Physiol Pharmacol, 31 (2019). [DOI] [PubMed] [Google Scholar]
- [271].Katayama M, Wiklander OPB, Fritz T, Caidahl K, El-Andaloussi S, Zierath JR, Krook A, Circulating Exosomal miR-20b-5p Is Elevated in Type 2 Diabetes and Could Impair Insulin Action in Human Skeletal Muscle, Diabetes, 68 (2019) 515–526. [DOI] [PubMed] [Google Scholar]
- [272].Peng L, Li S, Li Y, Wan M, Fang X, Zhao Y, Zuo W, Long Y Xuan, Regulation of BTG3 by microRNA-20b-5p in non-small cell lung cancer, Oncol Lett, 18 (2019) 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Zhang ZJ, Song XG, Xie L, Wang KY, Tang YY, Yu M, Feng XD, Song XR, Circulating serum exosomal miR-20b-5p and miR-3187–5p as efficient diagnostic biomarkers for early-stage non-small cell lung cancer, Exp Biol Med (Maywood), 245 (2020) 1428–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M, Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes, Circ Res, 107 (2010) 810–817. [DOI] [PubMed] [Google Scholar]
- [275].Jia Z, Zhang Y, Xu Q, Guo W, Guo A, miR-126 suppresses epithelial-to-mesenchymal transition and metastasis by targeting PI3K/AKT/Snail signaling of lung cancer cells, Oncol Lett, 15 (2018) 7369–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Pishavar E, Behravan J, miR-126 as a Therapeutic Agent for Diabetes Mellitus, Curr Pharm Des, 23 (2017) 3309–3314. [DOI] [PubMed] [Google Scholar]
- [277].Grimolizzi F, Monaco F, Leoni F, Bracci M, Staffolani S, Bersaglieri C, Gaetani S, Valentino M, Amati M, Rubini C, Saccucci F, Neuzil J, Tomasetti M, Santarelli L, Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression, Sci Rep, 7 (2017) 15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Jimenez-Lucena R, Camargo A, Alcala-Diaz JF, Romero-Baldonado C, Luque RM, van Ommen B, Delgado-Lista J, Ordovas JM, Perez-Martinez P, Rangel-Zuniga OA, Lopez-Miranda J, A plasma circulating miRNAs profile predicts type 2 diabetes mellitus and prediabetes: from the CORDIOPREV study, Exp Mol Med, 50 (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Jiang K, Shen M, Chen Y, Xu W, miR150 promotes the proliferation and migration of nonsmall cell lung cancer cells by regulating the SIRT2/JMJD2A signaling pathway, Oncol Rep, 40 (2018) 943–951. [DOI] [PubMed] [Google Scholar]
- [280].Li X, Liu J, Liu M, Xia C, Zhao Q, The Lnc LINC00461/miR-30a-5p facilitates progression and malignancy in non-small cell lung cancer via regulating ZEB2, Cell Cycle, 19 (2020) 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [281].Quan X, Li X, Yin Z, Ren Y, Zhou B, p53/miR-30a-5p/ SOX4 feedback loop mediates cellular proliferation, apoptosis, and migration of non-small-cell lung cancer, J Cell Physiol, 234 (2019) 22884–22895. [DOI] [PubMed] [Google Scholar]
- [282].Latouche C, Natoli A, Reddy-Luthmoodoo M, Heywood SE, Armitage JA, Kingwell BA, MicroRNA-194 Modulates Glucose Metabolism and Its Skeletal Muscle Expression Is Reduced in Diabetes, PLoS One, 11 (2016) e0155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Zhu Y, Das K, Wu J, Lee MH, Tan P, RNH1 regulation of reactive oxygen species contributes to histone deacetylase inhibitor resistance in gastric cancer cells, Oncogene, 33 (2014) 1527–1537. [DOI] [PubMed] [Google Scholar]
- [284].Wang S, Ai H, Liu L, Zhang X, Gao F, Zheng L, Yi J, Sun L, Yu C, Zhao H, Li Y, Micro-RNA-27a/b negatively regulates hepatic gluconeogenesis by targeting FOXO1, Am J Physiol Endocrinol Metab, 317 (2019) E911–E924. [DOI] [PubMed] [Google Scholar]
- [285].Zhang J, Hua X, Qi N, Han G, Yu J, Yu Y, Wei X, Li H, Chen X, Leng C, Liu Q, Lu Y, Li Y, MiR-27b suppresses epithelial-mesenchymal transition and chemoresistance in lung cancer by targeting Snail1, Life Sci, 254 (2020) 117238. [DOI] [PubMed] [Google Scholar]
- [286].Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ, Circulating miR-375 as a biomarker of beta-cell death and diabetes in mice, Endocrinology, 154 (2013) 603–608. [DOI] [PubMed] [Google Scholar]
- [287].Li M, Shan W, Hong B, Zou J, Li H, Han D, Zhang Y, Li L, Li D, Lin W, Circulating miR-92b and miR-375 for monitoring the chemoresistance and prognosis of small cell lung cancer, Sci Rep, 10 (2020) 12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Avgeris M, Kokkinopoulou I, Maratou E, Mitrou P, Boutati E, Scorilas A, Fragoulis EG, Christodoulou MI, Blood-based analysis of 84 microRNAs identifies molecules deregulated in individuals with type-2 diabetes, risk factors for the disease or metabolic syndrome, Diabetes Res Clin Pract, 164 (2020) 108187. [DOI] [PubMed] [Google Scholar]
- [289].Yang Y, Li Z, Yuan H, Ji W, Wang K, Lu T, Yu Y, Zeng Q, Li F, Xia W, Lu S, Reciprocal regulatory mechanism between miR-214–3p and FGFR1 in FGFR1-amplified lung cancer, Oncogenesis, 8 (2019) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Wang N, Guo W, Song X, Liu L, Niu L, Song X, Xie L, Tumor-associated exosomal miRNA biomarkers to differentiate metastatic vs. nonmetastatic non-small cell lung cancer, Clin Chem Lab Med, 58 (2020) 1535–1545. [DOI] [PubMed] [Google Scholar]
- [291].Vatandoost N, Amini M, Iraj B, Momenzadeh S, Salehi R, Dysregulated miR-103 and miR-143 expression in peripheral blood mononuclear cells from induced prediabetes and type 2 diabetes rats, Gene, 572 (2015) 95–100. [DOI] [PubMed] [Google Scholar]
- [292].Yang D, Wang JJ, Li JS, Xu QY, miR-103 Functions as a Tumor Suppressor by Directly Targeting Programmed Cell Death 10 in NSCLC, Oncol Res, 26 (2018) 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Alomari G, Al-Trad B, Hamdan S, Aljabali A, Al-Zoubi M, Bataineh N, Qar J, Tambuwala MM, Gold nanoparticles attenuate albuminuria by inhibiting podocyte injury in a rat model of diabetic nephropathy, Drug Deliv Transl Res, 10 (2020) 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Ye H, He X, Feng X, Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment, Biomed Pharmacother, 129 (2020) 110369. [DOI] [PubMed] [Google Scholar]
- [295].Zehra M, Zubairi W, Hasan A, Butt H, Ramzan A, Azam M, Mehmood A, Falahati M, Chaudhry AA, Rehman IU, Yar M, Oxygen Generating Polymeric Nano Fibers That Stimulate Angiogenesis and Show Efficient Wound Healing in a Diabetic Wound Model, Int J Nanomedicine, 15 (2020) 3511–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Khan MN, Haggag YA, Lane ME, McCarron PA, Tambuwala MM, Polymeric Nano-Encapsulation of Curcumin Enhances its Anti-Cancer Activity in Breast (MDA-MB231) and Lung (A549) Cancer Cells Through Reduction in Expression of HIF-1alpha and Nuclear p65 (Rel A), Curr Drug Deliv, 15 (2018) 286–295. [DOI] [PubMed] [Google Scholar]
- [297].Zhang X, He C, Liu X, Chen Y, Zhao P, Chen C, Yan R, Li M, Fan T, Altine B, Yang T, Lu Y, Lee RJ, Gai Y, Xiang G, One-pot synthesis of a microporous organosilica-coated cisplatin nanoplatform for HIF-1-targeted combination cancer therapy, Theranostics, 10 (2020) 2918–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Lee CH, Liu KS, Cheng CW, Chan EC, Hung KC, Hsieh MJ, Chang SH, Fu X, Juang JH, Hsieh IC, Wen MS, Liu SJ, Codelivery of Sustainable Antimicrobial Agents and Platelet-Derived Growth Factor via Biodegradable Nanofibers for Repair of Diabetic Infectious Wounds, ACS Infect Dis, 6 (2020) 2688–2697. [DOI] [PubMed] [Google Scholar]