Abstract
Acanthamoeba keratitis (AK) is a rare but serious infection of the eye and can lead to blindness. The effective and safe medical therapy remains unclear for AK until present. Antimicrobial activity and biological characteristic of chitosan encourage screening of it against Acanthamoeba. Thus, in vitro anti-amoebic activities of commercial chitosan and nano-chitosan were tested on pathogenic Acanthamoeba genotype T4, a causative agent of human AK. The Acanthamoeba spp. was isolated from the keratitis patient. The Acanthamoeba genotype T4 was approved using PCR method followed by sequencing technique. Chitosan nanoparticles was prepared using ionic gelation method and characterized by their physicochemical properties. In the present study, the in vitro activity of serial dilutions (12.5, 25, 50, 100, and 200 µL/mL) of commercial chitosan and nano-chitosan were evaluated against Acanthamoeba trophozoites and cysts. The finding of nano-chitosan particle size by DLS was 118 nm with a PDI of about 0.134. Zeta potential value was found to be 42.7 mV. The obtained results showed that the tested chitosan and nano-chitosan presented anti-amoebic activities dependent to time and concentration. The inhibitory effect of the chitosan and nano-chitosan is enhanced by increasing the concentration and incubation time. The inhibitory effect of nano-chitosan on both trophozoites and cyst was more than chitosan. According to the results, nano-chitosan shows the potent activity against Acanthamoeba T4 and could be used for the development of novel and safe therapeutic approaches in the future.
Keywords: Chitosan, Nano-chitosan, Acanthamoeba, In vitro, Inhibitory effect
Introduction
Acanthamoeba spp. are ubiquitous and pathogenic microorganism widely distributed in various habitats, including water, air, soil, dust, rivers, or medical instruments like, dental treatment units, hemodialysis unit and ophthalmology yard (Saberi et al. 2019a, b). Acanthamoeba spp. present two main stages in its life cycle: vegetative and motile form or trophozoite stage, and the latent and non-motile form or cyst stage that both forms can enter the body through various routs (Siddiqui and Khan 2012). Acanthamoeba spp. is capable of causing granulomatous amoebic encephalitis (GAE), a severe cerebral infection affecting mostly immunocompromised patients (Kilvington and White 1994). They can either cause Acanthamoeba keratitis (AK), primarily in contact lens wearers and may also occur in association with skin lesions (Kilvington and White 1994; Panjwani 2010). Although AK is still considered as a rare disease and is usually diagnosed late, the incidence in soft contact lens wearers has increased to reach 1 in 21,000 in 2015 (Randag et al. 2019).
Diagnosis of AK is difficult and in most cases diagnosed as bacterial, fungal or viral keratitis. The cultivation of Acanthamoeba from the corneal biopsy or from contact lenses can be used to diagnose AK. Also, confocal microscopy, and Immunofluorescence assays and multiplex real-time PCR methods were used for diagnosis of AK (Siddiqui and Khan 2012).
Treatment of AK is difficult due to the lack of standardized diagnostic tests and awareness among clinicians, since diagnosis of the condition is frequently elusive (Dart et al. 2009). Failure to achieve the optimum dose of select drug and phenotypic switching in Acanthamoeba spp. could be the causes of failure to treat Acanthamoeba infections (Khan 2006). Treatment of AK includes a combination of antimicrobial agents such as biguanides and diamidines, while in some cases a topical azole and/or neomycin is added in combination (Maycock and Jayaswal 2016). On the other hand, effective treatment with a combination of topical biguanide and diamidine therapy has been reported mostly when initiated in early stages of the disease (Clarke et al. 2012). Polyhexamethylene biguanide (PHMB) and chlorhexidine are the two biguanides that leading to loss of cellular components and inhibition of enzymes necessary for cell respiration. PHMB and chlorhexidine are effective at low concentrations (0.02%) but PHMB is toxic to human corneal cells (Turner et al. 2004). Propamidine-isethionate (0.1%), hexamidine-diisethionate (0.1%), and dibromopropamidine are diamidines used for the treatment of AK that by altering the structure and permeability of the cell membrane, causing denaturation of cytoplasmic contents (Szentmáry et al. 2019). The effort for safe and effective drugs remains a challenge.
Chitosan (poly-b-(1e4)-D-glucosamine), is a non-toxic polymer, obtained by N-deacetylation of chitin, which is mainly made from crustacean shells and is very much similar to cellulose (Younes and Rinaudo 2015). Chitin and chitosan are widely used as health foods in several countries. The application of chitosan includes a variety of areas such as pharmaceutical and medical applications, paper production, textile wastewater treatment, biotechnology, cosmetics, food processing and agriculture (Elieh-Ali-Komi and Hamblin 2016).
Chitosan has numerous pharmacological actions including immunopotentiating, antihypertensive, hypocholesterolemic and antibacterial activities. It has antioxidant activity and as an antibacterial agent can be used to help deliver drugs through the skin (Tripathi and Singh 2018). Previous studies on antimicrobial activity of chitosan have been proved against many microorganisms (Goy et al. 2009).
Nano-chitosan was obtained from chitosan by crosslinking with sodium tripolyphosphate (TPP). The effectiveness of nano-chitosan films in the treatment of cutaneous leishmaniasis was confirmed in animal models (Goy et al. 2009). Due to its antimicrobial activity, biocompatibility and biodegradability, chitosan has attained increasing commercial interest as suitable resource (Goy et al. 2009).
Antimicrobial activity and biological characteristic of chitosan encourage screening of it against Acanthamoeba. Hence, based on above literature on chitosan, current study was designed to test the anti-acanthamoebic effects of the chitosan and nano-chitosan against trophozoites and cysts of pathogenic Acanthamoeba genotype T4 that was isolated from a patient with keratitis.
Materials and methods
Preparation of the chitosan and nano-chitosan
Chitosan nanoparticles were prepared using ionic gelation method (Chabra et al. 2019). Briefly, 0.1 g chitosan (Mw 165 kD) were melted in acetic acid (0.1%) and stirred overnight at 25 °C. Tripolyphosphate (TPP) and double-distilled water were mixed together to reach a concentration of 0.05% w/v and the solution was added to chitosan (1:5 ratio) using an insulin syringe and was stirred at 25 °C. Ultrasonication was applied for 5 min to diminish the size of nanoparticles. Photon correlation spectroscopy of the chitosan nanoparticles was carried out by a Dynamic Light Scattering (DLS) instrument (Nano-ZS; Malvern, UK) to determine the size of the chitosan nanoparticles and zeta potential and polydispersity index (PDI).
Acanthamoeba isolate
Our study was performed with the Acanthamoeba genotype T4 that was isolated from a keratitis patient with Accession Number (KU877552). Before the experiments, the identification of the Acanthamoeba genotype was checked using PCR and sequencing techniques, which results displayed an Acanthamoeba genotype T4 (Saberi et al. 2019). The main reasons for choosing this genotype are abundant cases and its global distribution and high transmission.
Acanthamoeba cultivation
Amoeba were cultivated in 1% non-nutrient agar (Difco, USA) enriched with TYM (Trypticase, Yeast and Maltose) and incubated at room temperature. Plates were examined daily for 7–14 days.
Trophozoites and cysts preparation
The non-nutrient agar surface was carefully scraped using a sterile cell scraper, trophozoites and cysts were collected by pouring 4–5 mL of sterile phosphate-buffered saline (PBS) into the culture plates; the suspension was centrifuged at 2000 rpm for 5 min, the supernatant was discharged, and the pellet was washed twice with PBS. Trophozoites and cysts in a suspension were counted in a hemocytometer slice and the suspension was adjusted to 2 × 105 amoeba/mL and was subjected to drug testing (Dodangeh et al. 2017).
Anti-Acanthamoeba assay of chitosan and nano-chitosan
In vitro evaluation was performed in sterile 96-well plates (Sigma-Aldrich). In this way 250 µL of the adjusted 2 × 105 amoeba/mL was added with the same volume of serial dilutions of the chitosan and nano-chitosan (12.5, 25, 50, 100, and 200 µL/mL) in microcentrifuge tubes were vortexed and incubated at 37 °C for 24, 48, and 72 h. After the incubation periods, 20 µL of the amoebic suspensions were mixed with the same volume of 1% Methylene blue (MB) to detect and count the viable amoeba. Unstained (viable) and stained (non-viable) cells were then counted and all tests were performed in duplicate and were repeated at least three times. Negative and positive controls always monitored the experiment: 0.02% chlorhexidine digluconate and acetic acid were used as positive and negative controls, respectively.
Cytotoxicity test
Mouse Macrophages cell line J774A.1 (ATCC® TIB-67™) were seeded into 96-well plates (Nunc, Roskilde, Denmark) in 100 µl RPMI-1640 medium (Gibco, Life Technologies GmbH, Germany) supplemented with 10% fetal bovine serum (Gibco, Germany) and 1% Gentamycine (Gibco, Germany). Cells then were incubated at 37°Cwith 5% CO2 for 24 h. Different concentrations (12.5, 25, 50, 100, and 200 µL/mL) of nano-chitosan were added to each well in triplicate. After 24, 48 and 72 h of incubation time, 10 µL of MTT solution (Sigma, UK) (5 mg/mL in PBS) was added to each well, including controls. After 3 h of incubation at 37 °C, the supernatant was removed and 100 µL of DMSO (Darmstadt, HE, Germany) was added. Lastly, the absorbance at 570 nm was measured by a microtiter plate reader (BioTek ELX800, Winooski, VT, USA) and the viability was calculated by the following formula: Viability (%) = OD test /OD control × 100.
Statistical analysis
In this study to analyze the data, we first examine the assumption of normality of values using the Kolmogorov–Smirnov test with Z approximation. Parametric statistical methods were used for data analysis. Repeated measurement test, analysis of variance, independent t-test and LSD test were used. Significance level was set at < 0.05.
Results
Particle size and zeta potentials
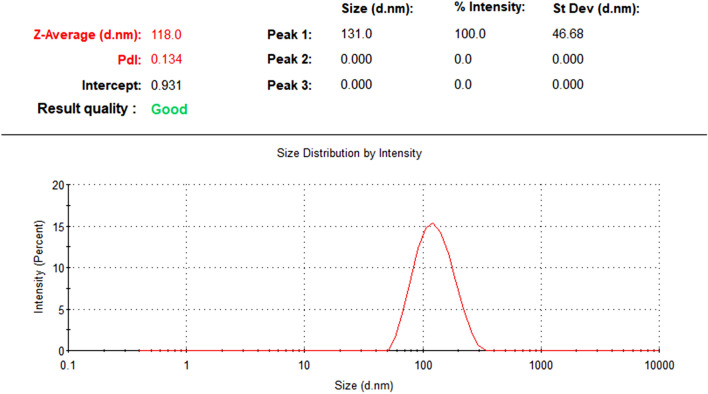
The particle size result of nano-chitosan by DLS was 118 nm with a PDI of about 0.134 and these data were shown in Fig. 1. Surface charge and thereby the stability of the prepared nanoparticle systems was determined by zeta potential measurements. Zeta potential value was found to be 42.7 mV (Fig. 2). This value lies in the stable range, indicating that this nanoparticle is stable during experiment period.
Fig. 1.
DLS showing the size distribution of nano-chitosan with narrow size distribution
Fig. 2.
Zeta potential of chitosan nanoparticles showed positive charge
Effects of chitosan on trophozoites and cysts
The concentrations of 12.5, 25, 50, 100 and 200 µl/mL of chitosan were used against trophozoites and cysts at three different times (24, 48 and 72 h). Trypan blue dye exclusion test revealed that the viable Acanthamoeba cyst and trophozoites contained clear cytoplasm whereas a nonviable amoeba will have a blue cytoplasm. After adding the lowest concentration of the chitosan (12.5 µl/mL), 72.31% of trophozoites and 91.88% cysts were viable after 24 h, respectively. At another concentration and time, 25 µl/mL of chitosan after 72 h, 44.65% trophozoites and 54.29% cysts survived. Anti-Acanthamoeba activity at 100 µl/mL of the chitosan reveled survival of 28.84% of trophozoites and 34.18% of cysts after 72 h. The mean of trophozoites and cysts survival rate when incubated (for 72 h) at concentrations of 200 µl/mL of the chitosan was 17.03 and 16.02, respectively. The results of the effect of chitosan in different concentrations and times and survival percentage of trophozoites and cysts are tabulated in Fig. 3 and Table 1.
Fig. 3.
Percentage viability of Acanthamoeba trophozoites and cysts after treating with the chitosan
Table 1.
Effects of the chitosan on the proliferation of Acanthamoeba trophozoites and cysts
| Time | Concentrations | Trophozoites Mean ± SD | Cyst Mean ± SD | P-value |
|---|---|---|---|---|
| 24 h | 12.5 µL/mL | 72.31 ± 2.906 | 91.88 ± 0.370 | 0.000 |
| 25 µL/mL | 62.23 ± 1.211 | 84.18 ± 0.740 | 0.000 | |
| 50 µL/mL | 55.57 ± 2.414 | 76.28 ± 1.110 | 0.000 | |
| 100 µL/mL | 47.61 ± 5.639 | 70.39 ± 2.018 | 0.003 | |
| 200 µL/mL | 32.41 ± 2.855 | 63.24 ± 2.906 | 0.000 | |
| Control − | 99.00 ± 1.732 | 99.33 ± .667 | 0.795 | |
| Control + | 10.47 ± 4.268 | 40.99 ± 2.273 | 0.000 | |
| 48 h | 12.5 µL/mL | 62.08 ± 3.265 | 80.69 ± 3.350 | 0.002 |
| 25 µL/mL | 51.28 ± 7.480 | 72.22 ± 4.811 | 0.015 | |
| 50 µL/mL | 45.70 ± 4.172 | 62.23 ± 1.211 | 0.003 | |
| 100 µL/mL | 38.88 ± 9.632 | 54.07 ± 0.403 | 0.112 | |
| 200 µL/mL | 19.94 ± 4.438 | 45.94 ± 4.170 | 0.002 | |
| Control − | 97.33 ± 2.516 | 99.00 ± 1.00 | 0.398 | |
| Control + | 0.000 ± 0.000 | 15.44 ± 1.191 | 0.000 | |
| 72 h | 12.5 µL/mL | 50.00 ± 4.545 | 67.67 ± 4.628 | 0.009 |
| 25 µL/mL | 44.65 ± 2.590 | 54.29 ± 4.172 | 0.027 | |
| 50 µL/mL | 39.92 ± 2.537 | 47.22 ± 6.159 | 0.131 | |
| 100 µL/mL | 28.84 ± 3.330 | 34.18 ± 3.916 | 0.146 | |
| 200 µL/mL | 17.03 ± 3.846 | 16.02 ± 13.911 | 0.910 | |
| Control − | 97.00 ± 2.645 | 97.67 ± 1.202 | 0.749 | |
| Control + | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 |
Effects of nano-chitosan on trophozoites and cysts
The results of study showed that survival percentage of trophozoites and cysts in treated with nano-chitosan was decreased in compared with chitosan (Table 2). The viability rate of the trophozoites and cysts treated with nonochitosan, after 24 h of exposure at concentration of 12.5 μl/mL was 57.07% and 80.55%, at 25 μl/mL was 48.48% and 68.37%, at 50 μl/mL was 38.88%, 57.50% and at 100 μl/mL was 27.68% and 55.57%, respectively. This was followed by adding 200 µl/mL of nano-chitosan after 24 h, survival rate of trophozoites and cysts was 19.96% and 39.92%. Trophozoites survival rate decreased with increasing concentration of the nano-chitosan and it was statistically significant (see Table 2). In addition, the nano-chitosan is more effective than the chitosan. In total, in the presence of 100 µL/mL of the nonochitosan, eliminate the whole population of trophozoites after 72 h, whereas only 2.56% of cysts were viable when incubated at 200 µL/mL concentration after 72 h (see Fig. 4 and Table 2).
Table 2.
Effects of the nano-chitosan on the proliferation of Acanthamoeba trophozoites and cysts
| Time | Concentrations | Trophozoite Mean ± SD | Cyst Mean ± SD | P-value |
|---|---|---|---|---|
| 24 h | 12.5 µL/mL | 57.07 ± 2.186 | 80.55 ± 4.811 | 0.002 |
| 25 µL/mL | 48.48 ± 4.656 | 68.37 ± 1.480 | 0.002 | |
| 50 µL/mL | 38.88 ± 4.811 | 57.50 ± 3.859 | 0.006 | |
| 100 µL/mL | 27.68 ± 2.906 | 55.57 ± 2.414 | 0.000 | |
| 200 µL/mL | 19.96 ± 4.050 | 39.92 ± 2.537 | 0.002 | |
| Control − | 99.00 ± 1.732 | 99.33 ± .667 | 0.795 | |
| Control + | 10.47 ± 4.268 | 40.99 ± 2.273 | 0.000 | |
| 48 h | 12.5 µL/mL | 41.16 ± 4.566 | 61.16 ± 2.670 | 0.003 |
| 25 µL/mL | 32.47 ± 1.480 | 52.56 ± 2.220 | 0.000 | |
| 50 µL/mL | 26.19 ± 4.123 | 40.99 ± 2.273 | 0.006 | |
| 100 µL/mL | 17.94 ± 4.441 | 32.32 ± 4.628 | 0.018 | |
| 200 µL/mL | 7.72 ± 0.595 | 19.30 ± 3.350 | 0.004 | |
| Control − | 97.33 ± 2.516 | 99.00 ± 1.00 | 0.398 | |
| Control + | 0.000 ± 0.000 | 15.44 ± 1.191 | 0.000 | |
| 72 h | 12.5 µL/mL | 23.71 ± 1.110 | 38.38 ± 6.308 | 0.017 |
| 25 µL/mL | 15.44 ± 1.191 | 31.36 ± 1.745 | 0.000 | |
| 50 µL/mL | 5.34 ± 4.637 | 25.75 ± 1.312 | 0.002 | |
| 100 µL/mL | 00.00 ± 0.000 | 18.80 ± 3.700 | 0.013 | |
| 200 µL/mL | 00.00 ± 0.000 | 2.56 ± 4.441 | 0.423 | |
| Control − | 97.00 ± 2.645 | 97.67 ± 1.202 | 0.749 | |
| Control + | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 |
Fig. 4.
Percentage viability of Acanthamoeba trophozoites and cysts after treating with the nano-chitosan
Cytotoxicity activity
The MTT assay indicated that chitosan and nano-chitosan in different concentrations had no toxicity effects on the J774A.1 viability after 24, 48 and 72 h.
Discussion
According to the results, the tested chitosan and nano-chitosan have been shown to possess anti-Acanthamoeba effects. Despite progress in anti-Acanthamoeba therapeutic agents, such as combination therapies and keratoplasty, cysts are still resistant to therapeutic agents. This is a challenge that has not yet been addressed. For example, the morbidity and mortality associated with AK and GAE have remained high (Lorenzo-Morales et al. 2015; Visvesvara et al. 2007). Many of the available drugs lead to ineffective and unsafe treatment, due to their unwanted side effects. Therefore, the need for drug compounds that are effective against Acanthamoeba and also do not cause side effects in host cells seems necessary (Roshni et al. 2020; Trabelsi et al. 2012).
In this study, chitosan and nano-chitosan were tested as therapeutic agents at concentrations of 12.5, 25, 50, 100 and 200 µl/mL, as well as positive (chlorhexidine 20 µl/mL) and negative (acetic acid) controls always used in this study. Comparative assessment of the viability of trophozoites and cysts of Acanthamoeba T4 after exposure to the chitosan and nano-chitosan showed changes in the number of trophozoites and cysts, that with increased concentrations and time, the trend of viable trophozoites and cysts was decreasing. Anti-Acanthamoeba effects of chitosan and nano-chitosan on trophozoites showed that there was a significant difference in all concentrations and three times (see Tables 1 and 2); it means that the effect of of nano-chitosan on trophozoites was more efficient than chitosan. The results of this study showed that chitosan and nano-chitosan on trophozoites are more effective than cysts. Rigid double-layered wall of cyst causes a difference in the sensitivity to drug in the trophozoites and cysts (Dodangeh and Fakhar 2016). Acanthamoeba cysts are highly resistant and according to switch phenotypes, amoeba into a dormant cyst form is a major barrier in the development of effective treatment. Results of previous studies showed that the addition of cellulose enzyme to disrupt cyst wall structure present amoeba cysts susceptible to the effects of anti- Acanthamoeba drugs (Abjani et al. 2016). In other hand, mammalian cells lack chitosan and cellulose synthesis, so there is a need to investigate on the role of cellulose-degrading molecules in contact lens disinfectants as well as in drug formulations (Abjani et al. 2016).
Severe AK often treated with a combination of drugs such as PHMB or chlorhexidine (Alkharashi et al. 2015). One of the limitations of this treatment with the mentioned drugs was the toxic effects biguanides have on human keratocytes. Moreover, the treatment has a very lengthy course of the disease and it has a low effect on the cyst (Eldin et al. 2019). Finding a natural compound with anti Acanthamoeba activity and non-toxic to human cells is essential to treatment of AK. There were viability of Acanthamoeba isolated from keratitis patient cultivated in vitro examined after exposure to chitosan and nonochitosan. Natural products such as chitosan and nano-chitosan can be evaluated as a new generation of antimicrobial agents against Acanthamoeba infections, especially, if investigated with cyst targeting (2007). Many studies accomplished to date on chitin and derivatives as a source of bioactive material (Benhabiles et al. 2012; Salah et al. 2013). Surprisingly, there are no previous reports in the literature about bacterial resistance to chitosan. In addition, chitosan and its derivatives have demonstrated their promising application for the treatment of cancer (Kim 2010). Chitosan is applauded for its nontoxic nature, antimicrobial activity, polycationic, antitumor activity, antioxidative activity, anticholesterolemic, and analgesic effect (Kim and Rajapakse 2005). Using the incorporation of polyanion like TPPchitosan nanoparticle (ChNP) can be prepared easily in chitosan solution (Divya and Jisha 2018). Studies have reported antibacterial activity of ChNP against different pathogen bacteria (Qi et al. 2004). The antibacterial activity of chitosan is dependent on various factors included disruption and altering the membrane permeability, inhibition of DNAreplication and subsequently cell death and chelating agent that selectively binds to trace metal elements causing toxin production. In addition, other factors such as molecular weight and degree of deacetylation of parent chitosan, size and concentration of nanoparticle and pH, temperature and time are important to mechanism of chitosan (Kong et al. 2010).
The results of chitosan and nano-chitosan effects on cysts showed that in 25, 50, 100 and 200 µl/mL concentrations of both drugs at time 24 h was significant difference (P < 0.05), but in 12.5 µl/mL in time was no significant difference (P > 0.05). All concentrations at time 48 h showed that there was a significant difference (P < 0.05) between the efficacies of the chitosan and nano-chitosan on cysts. In 12.5, 25, 50 and 100 µl/mL concentrations of both drugs at time 72 h was significant difference (P < 0.05) while in 200 µl/mL in time was no significant difference (P > 0.05) between the efficacies of the both drugs on cysts. However, the effectiveness of nano-chitosan on cysts was more prominent than chitosan. In Siddiqui et al., study, liposomal complexation of pentamidine isethionate and ergosterol is shown to enhance its antiacanthamoebic activity (Siddiqui et al. 2009). In another study, Willcox et al., reported that silver nanoparticles have been successfully used to inhibit microbial growth and colonization on contact lenses, which shows the potential of nanoparticles in the development of contact lens solutions (Willcox et al. 2010).
Chitosan could be introduced as a promising compound, as in Shon et al., study, finding showed that growth of Acanthamoeba castellanii was inhibited by chitosan oligosaccharide (up to 20 mg ml−1). In this study, chitosan oligosaccharide inhibited polyamine biosynthesis and ornithine decarboxylase activity (Shon and Nam 2003).
Conclusion
The inhibitory effect of nano-chitosan on both trophozoites and cyst was more than chitosan. According to the results, nano-chitosan show the potent activity against Acanthamoeba T4 and could be used for the development of novel and safe therapeutic approaches in the future. As a whole, further studies are urgently needed to determine and/or confirm its mechanism of action against Acanthamoeba.
Acknowledgements
This project was funded by Toxoplasmosis Research Center, Department of Parasitology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (Grant No: 2892).
Authors’ contributions
HZ, and MF conceptualization and study design. ET, YM, RS samples processing and down experiments. RS manuscript writing. MF and JA manuscript revision and editions.
Data availability
All data generated or analyzed during this study are included in this manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The current study was approved by the Ethical Committee of the Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (IR.MAZUMS.REC.1398.880).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahdi Fakhar, Email: mahdif53@yahoo.com.
Javad Akhtari, Email: javad.akhtari@gmail.com.
References
- Abjani F, Khan NA, Yousuf FA, Siddiqui R. Targeting cyst wall is an effective strategy in improving the efficacy of marketed contact lens disinfecting solutions against Acanthamoeba castellanii cysts. Cont Lens Anterior Eye. 2016;39(3):239–243. doi: 10.1016/j.clae.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Alkharashi M, Lindsley K, Law HA, Sikder S. Medical interventions for AK. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD010792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhabiles MS, Salah R, Lounici H. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012;29(1):48–56. doi: 10.1016/j.foodhyd.2012.02.013. [DOI] [Google Scholar]
- Buzea C, Pachecho II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007 doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Chabra A, Rahimi-Esboei B, Habibi E, et al. Effects of some natural products from fungal and herbal sources on Giardia lamblia in vivo. Parasitology. 2019;146(9):1188–1198. doi: 10.1017/S0031182019000325. [DOI] [PubMed] [Google Scholar]
- Clarke B, Sinha A, Parmar DN, Sykakis E. Advances in the diagnosis and treatment of AK. J Ophthalmol. 2012;2012:484892. doi: 10.1155/2012/484892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148(4):487–499.e2. doi: 10.1016/j.ajo.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Divya K, Jisha MS. Chitosan nanoparticles preparation and applications. Environ Chem Lett. 2018;16(1):101–112. doi: 10.1007/s10311-017-0670-y. [DOI] [Google Scholar]
- Dodangeh S, Fakhar M. Cellulose in acanthamoeba cyst wall: a drug target for Acanthamoeba infection treatment. J Mazandaran Univ Med Sci. 2016;26(141):182–191. [Google Scholar]
- Dodangeh S, Niyyati M, Kamalinejad M, et al. The amoebicidal activity of Ziziphus vulgaris extract and its fractions on pathogenic Acanthamoeba trophozoites and cysts. Trop Biomed. 2017;34(1):127–136. [PubMed] [Google Scholar]
- Eldin HM, Sarhan RM, Khayyal AE. The impact of vinegar on pathogenic Acanthamoeba astronyxis isolate. J Parasit Dis. 2019;43(3):351–359. doi: 10.1007/s12639-019-01098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elieh-Ali-Komi D, Hamblin MR. Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res. 2016;4(3):411–427. [PMC free article] [PubMed] [Google Scholar]
- Goy RC, Britto DD, Assis OB. A review of the antimicrobial activity of chitosan. Polímeros. 2009;19(3):241–247. doi: 10.1590/S0104-14282009000300013. [DOI] [Google Scholar]
- Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30(4):564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- Kilvington S, White DG. Acanthamoeba: biology, ecology and human disease. Rev Med Microbiol. 1994;5(1):12–26. doi: 10.1097/00013542-199401000-00003. [DOI] [Google Scholar]
- Kim SK. Chitin, chitosan, oligosaccharides and their derivatives: biological activities and applications. New York: CRC Press; 2010. [Google Scholar]
- Kim SK, Rajapakse N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohydr Polym. 2005;62(4):357–368. doi: 10.1016/j.carbpol.2005.08.012. [DOI] [Google Scholar]
- Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycock NJ, Jayaswal R. Update on Acanthamoeba keratitis: diagnosis, treatment, and outcomes. Cornea. 2016;35(5):713–720. doi: 10.1097/ICO.0000000000000804. [DOI] [PubMed] [Google Scholar]
- Panjwani N. Pathogenesis of AK. Ocul Surf. 2010;8(2):70–79. doi: 10.1016/s1542-0124(12)70071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Xu Z, Jiang X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004;339(16):2693–2700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Randag AC, Van Rooij J, Van Goor AT, et al. The rising incidence of Acanthamoeba keratitis: a 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS ONE. 2019;14(9):222092. doi: 10.1371/journal.pone.0222092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshni Prithiviraj S, Rajapandian SGK, Gnanam H. Clinical presentations, genotypic diversity and phylogenetic analysis of Acanthamoeba species causing keratitis. J Med Microbiol. 2020;69(1):87–95. doi: 10.1099/jmm.0.001121. [DOI] [PubMed] [Google Scholar]
- Saberi R, Fakhar M, Sedighi O, et al. First molecular evidences of Acanthamoeba T3, T4 and T5 genotypes in hemodialysis units in Iran. Acta Parasitol. 2019;64(4):911–915. doi: 10.2478/s11686-019-00122-z. [DOI] [PubMed] [Google Scholar]
- Saberi R, Najafi A, Naserifar R. Detection of Acanthamoeba spp. from dust phenomenon in Ilam Province West Iran. Acta Microbiol Immunol Hung. 2019;66(4):459–468. doi: 10.1556/030.66.2019.011. [DOI] [PubMed] [Google Scholar]
- Salah R, Michaud P, Mati F. Anticancer activity of chemically prepared shrimp low molecular weight chitin evaluation with the human monocyte leukaemia cell line, THP-1. Int J Biol Macromol. 2013;52:333–339. doi: 10.1016/j.ijbiomac.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Shon YH, Nam KS. Inhibiton of polyamine biosynthesis in Acanthamoeba castellanii and 12-O-tetradecanoylphorbol-13-acetate-induced ornithine decarboxylase activity by chitosanoligosaccharide. Biotechnol Lett. 2003;25(9):701–704. doi: 10.1023/a:1023480701270. [DOI] [PubMed] [Google Scholar]
- Siddiqui R, Khan NA. Biology and pathogenesis of Acanthamoeba. Parasit Vectors. 2012;10(5):6. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R, Syed A, Tomas S, et al. Effect of free versus liposomal-complexed pentamidine isethionate on biological characteristics of Acanthamoeba castellanii in vitro. J Med Microbiol. 2009;58(3):327–330. doi: 10.1099/jmm.0.006494-0. [DOI] [PubMed] [Google Scholar]
- Szentmáry N, Daas L, Shi L, et al. AK–Clinical signs, differential diagnosis and treatment. J Curr Ophthalmol. 2019;31(1):16–23. doi: 10.1016/j.joco.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi H, Dendana F, Sellami A. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol. 2012;60(6):399–405. doi: 10.1016/j.patbio.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Tripathi K, Singh A. Chitin, chitosan and their pharmacological activities: a review. Int J Pharm Sci Res. 2018;9:2626–2635. [Google Scholar]
- Turner NA, Russell AD, Furr JR, Lloyd D. Resistance, biguanide sorption and biguanide-induced pentose leakage during encystment of Acanthamoebacastellanii. J Appl Microbiol. 2004;96(6):1287–1295. doi: 10.1111/j.1365-2672.2004.02260.x. [DOI] [PubMed] [Google Scholar]
- Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- Willcox MD, Hume EB, Vijay AK, Petcavich R. Ability of silverimpregnated contact lenses to control microbial growth and colonisation. J Optom. 2010;3(3):143–148. doi: 10.1016/S1888-4296(10)70020-0. [DOI] [Google Scholar]
- Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13(3):1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.