Abstract
Background
Ventilation has emerged as an important strategy to reduce indoor aerosol transmission of coronavirus disease 2019. Indoor air carbon dioxide (CO2) concentrations are a surrogate measure of respiratory pathogen transmission risk.
Objectives
To determine whether CO2 monitors are necessary and effective to improve ventilation in hospitals.
Methods
A randomized, placebo (sham)-controlled, crossover, open label trial. Between February and May 2021, we placed CO2 monitors in twelve double-bed patient rooms across two geriatric wards. Staff were instructed to open windows, increase the air exchange rate and reduce room crowding to maintain indoor air CO2 concentrations ≤800 parts per million (ppm).
Results
CO2 levels increased during morning care and especially in rooms housing couples (rooming-in). The median (interquartile range, IQR) time/day with CO2 concentration > 800 ppm (primary outcome) was 110 min (IQR 47–207) at baseline, 82 min (IQR 12–226.5) during sham periods, 78 min (IQR 20–154) during intervention periods and 140 min (IQR 19.5–612.5) post-intervention. The intervention period only differed significantly from the post-intervention period (P = 0.02), mainly due to an imbalance in rooming-in. Significant but small differences were observed in secondary outcomes of time/day with CO2 concentrations > 1000 ppm and daily peak CO2 concentrations during the intervention vs. baseline and vs. the post-intervention period, but not vs. sham. Staff reported cold discomfort for patients as the main barrier towards increasing ventilation.
Discussion
Indoor air CO2 concentrations in hospital rooms commonly peaked above recommended levels, especially during morning care and rooming-in. There are many possible barriers towards implementing CO2 monitors to improve ventilation in a real-world hospital setting. A paradigm shift in hospital infection control towards adequate ventilation is warranted.
Trial registration
ClinicalTrials.gov Identifier: NCT04770597
Keywords: Carbon dioxide, Coronavirus disease 2019, Geriatrics, Healthcare-associated infections, Hospitals, Ventilation
Graphical abstract
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused considerably morbidity and mortality worldwide, especially among frail older adults (De Smet et al., 2020). While vaccination protects against severe COVID-19, death and to some extent against transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, the virus which causes COVID-19), other mitigation strategies will likely continue to be required. Among such strategies, ventilation and purification of indoor air feature prominently (Allen and Ibrahim, 2021). Indeed, there is growing consensus and accumulating evidence for respiratory transmission or SARS-CoV-2, not only via larger droplets but also via smaller particles in aerosols, mainly in closed spaces (Greenhalgh et al., 2021; Meyerowitz et al., 2021; Morawska et al., 2021; Noorimotlagh et al., 2021; Tang et al., 2021).
One convenient surrogate parameter for ventilation in this context is the indoor carbon dioxide (CO2) concentration (Rudnick and Milton, 2003). Humans exhale CO2 at concentrations of almost 40,000 parts per million (ppm) (Rudnick and Milton, 2003) compared to outdoor concentrations which have increased in recent years to ~450 ppm (fluctuating between 400 and 500 ppm). Thus, the increase in CO2 concentrations above outdoor levels mainly reflects the indoor concentration of human exhaled air. Under average circumstances, an indoor CO2 concentration > 380 ppm above outside levels implies that inhaled air contains 1% exhaled air (Rudnick and Milton, 2003). In turn, this rebreathed fraction or “shared air” correlates with airborne microbial counts (Hiwar et al., 2021; Tavera Busso et al., 2020) and the basic reproductive number of respiratory infections (Du et al., 2020; Rudnick and Milton, 2003).
There is no universal agreement on optimal cut-offs for indoor CO2 concentrations in general, or in the context of COVID-19 in particular. Regulatory bodies in several countries have proposed guidelines to maintain indoor CO2 concentrations below values ranging from 800 to 1000 ppm (European Centre for Disease Prevention and Control, 2020; Superior Health Council of Belgium, 2020). One COVID-19 outbreak in a Dutch nursing home was associated with the use of an energy-saving CO2-driven ventilation system, which maintained indoor CO2 concentrations around 1000 ppm (de Man et al., 2021).
Modern hospitals, particularly operating and delivery rooms, intensive care units and microbiology laboratories, are equipped with superior heating, ventilation and air conditioning (HVAC) systems, which also remove particles via high-efficiency particulate air (HEPA) filters (Allen and Ibrahim, 2021). For normal patient rooms however, the recommended outdoor and total air change rates per hour are only 2 and 4–6, respectively (Allen and Ibrahim, 2021). While these rates may be sufficient to meet indoor air quality requirements under basal conditions (i.e. patients alone in their rooms), they may be temporarily insufficient when rooms are crowded by healthcare staff and/or visitors (Ramos et al., 2015; Tang et al., 2009). Indeed, physical distancing and limiting occupant density are challenging in hospitals, since patients (especially frail older adults) depend on caregivers. Moreover, opening windows in hospital may be avoided due to thermal and draft discomfort (Shajahan et al., 2019; Zhou et al., 2015) or risk of patient injury from falls out of open windows.
Few studies have reported indoor CO2 concentrations in hospitals (Shajahan et al., 2019). Inappropriately high CO2 concentrations were measured in hospitals without modern HVAC systems (Hwang and Park, 2020; Lee et al., 2020; Pereira et al., 2020; Zaman et al., 2021; Zhou et al., 2015). In contrast, excellent CO2 values have been reported in COVID-19 wards without detectable airborne viral genomes (Vosoughi et al., 2021) and an Iranian intensive care unit (where viral genomes were still detected in N = 2/14 air samples) (Kenarkoohi et al., 2020). A French study found maximal CO2 concentrations of 1121 to 1325 ppm in a hospital nursing care room and plaster room, respectively (Baures et al., 2018). In a Taiwanese four-bed intensive care unit room, CO2 levels (range 828–1570 ppm) were above 1000 ppm during visitor hours 92% of the year (Tang et al., 2009). Another study from Taiwan reported that patient wards had the highest average CO2 concentration (1063 ± 483 ppm, N = 3 hospitals) (Jung et al., 2015). Increases in CO2 of almost 400 ppm above outside levels during working hours were observed in two chemotherapy units (Palmisani et al., 2021).
Thus, it is clear that despite guidelines for hospital HVAC systems and CO2 targets in buildings, ventilation in hospital wards may be worse than commonly appreciated. More research is needed not only to provide a more detailed overview of indoor CO2 fluctuations in hospital rooms, but even more so to define optimal strategies to maintain CO2 below recommended maximum levels. One before-after implementation study reported an average CO2 of 1211 ppm in a psychiatric nursing station, which decreased after an intervention to 997 ppm (Chang et al., 2013). Another recent study reported that monitors revealed insufficient ventilation (CO2 up to 963 ppm) in clinical areas and guided implementation of effective mediation strategies (Lu et al., 2021).
CO2 monitors are increasingly deployed and recommended (for example, in countries like Germany, Norway and Belgium (European Centre for Disease Prevention and Control, 2020)) to monitor ventilation and prevent aerosol transmission of SARS-CoV-2 in schools, offices and public buildings including hotels, bars and restaurants (Superior Health Council of Belgium, 2020). Some experts have called for the use of CO2 monitors by nurses in hospitals and nursing homes too (Ahlawat et al., 2020). In schools, one controlled study showed that CO2 warning devices improved the average daily CO2 concentration in classrooms, whereas advice alone did not (Geelen et al., 2008). Other before-after observational studies in school settings support the effectiveness of CO2 monitors (Wargocki and Da Silva, 2015) and other interventions to improve ventilation (Du et al., 2020; Rosbach et al., 2013; Sa et al., 2017). In the hospital environment, Yang et al. reported daily CO2 peak concentrations > 1000 ppm, which could be mitigated using an integrated monitoring system, which alerted medical supervisors and automatically activated ventilation (Yang et al., 2014). However, randomized trials supporting the use of CO2 monitors to improve ventilation, in any setting, remains lacking.
Given this background, we investigated (1) whether indoor air CO2 exceeded threshold levels in double-bed hospital rooms, and (2) whether CO2 monitors are feasible and effective to maintain indoor CO2 concentrations below the commonest thresholds of 800 and 1000 ppm.
2. Methods
This report follows the Consolidated Standards of Reporting Trials (CONSORT) statement's extension to randomized crossover trials (see checklist in the Supplementary Data) (Dwan et al., 2019).
2.1. Setting
This single-center trial ran at the Geriatrics Department of Imelda general hospital in the rural area of Bonheiden, Belgium (Supplementary Fig. 1). A green environment surrounds the hospital and there is no traffic or other nearby CO2 source. All rooms had type D ventilation (mechanical inlet, mechanical outlet) with the possibility to tilt the windows (fully open only by key), additional ventilation grilles, and recently had air-conditioning installed (cooling mode only).
Among general acute wards with standard air exchange rates, we selected the Geriatric Department for several reasons. First, bed occupancy rate in the department is typically >90%. Secondly, almost all patients require assistance from staff for activities of daily living such as washing, dressing, meals etc. Thirdly, geriatrics has higher staffing levels than other acute wards and many nursing students (occasionally up to 10/rotation/ward). All these elements increase room crowding.
On the other hand, many older patients have low levels of physical activity and thus low respiratory minute volumes. Visitors were allowed very restrictively due to the ongoing COVID-19 epidemic, typically only 1 h/week during most of the study period. Thus, older patient age and visitor restrictions may be associated with lower indoor CO2 concentrations.
2.2. Trial design and randomization
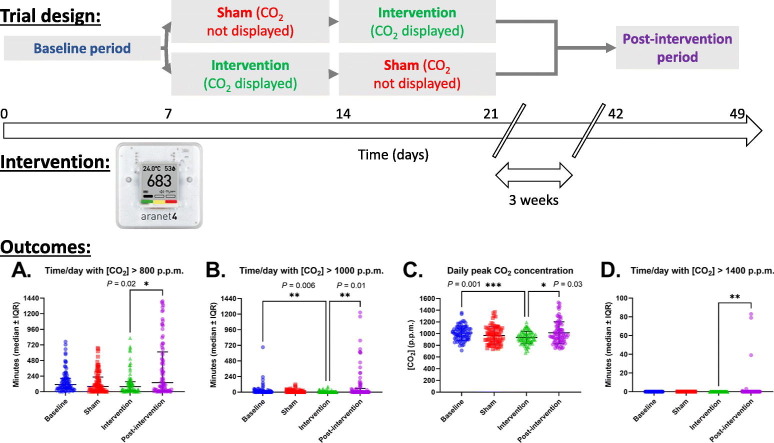
The study design and protocol have been reported (Laurent and Frans, 2021). We performed a randomized, placebo (sham)-controlled, open label, crossover trial aimed to demonstrate superiority of the intervention. Between February 21st and May 2nd, 2021, we placed CO2 monitors in twelve double-bed patient rooms across two geriatric wards. Following a baseline period, monitors were randomized 1:1 to an intervention or sham (monitor display visible or not) AB/BA crossover period. Three weeks later, post-intervention measurements were obtained. Each period lasted seven days (Fig. 1 ).
Fig. 1.
Study design overview.
Thus, the study consists of four periods:
-
1.
Baseline (pre-intervention) period (duration 1 week): all monitors facing downwards, staff blinded to the CO2 values.
-
2.
Sham period (duration 1 week): monitors facing downwards, staff blinded to the CO2 values.
-
3.
Intervention period (duration 1 week): monitors facing forward, measurements visible to the clinical staff for one week.
-
4.
Post-intervention period (duration 1 week): after a three-week interval, the sensors are again installed in six double-bed rooms, with their display facing downwards and staff blinded to the measurements.
Given the open-label design, we chose the sham-control group to take potential observation bias (Hawthorne effect) and carryover effects (influence of the intervention on the sham periods due to lack of blinding) into account.
We randomized the six sensors to the sham/intervention or intervention/sham arm respectively (AB/BA crossover design), just before the end of the baseline period, using an online random sequence generator (1:1 ratio, N = 1 block size; https://www.random.org/sequences/?min=1&max=6&col=3&format=html&rnd=). Due to the nature of the intervention, allocation concealment is not possible. Investigators performing the analysis were also not blinded to allocation. However, the a priori statistical analysis plan (Laurent and Frans, 2021) and the availability of data for independent review by other scientists mitigates the risk of bias.
2.3. Intervention
This study used six Aranet4 Home® and one Airthings Wave Plus® monitor (measurement ranges 0–9999 ppm and 400–5000 ppm, respectively). Both monitors are commercially available in Europe and use non-dispersive infrared CO2 sensors with ±3% accuracy reported by the manufacturer, and raw data logging with time stamps. The Aranet® sensors are factory-calibrated, recommended for use in schools by the Federation of European HVAC Associations, and have been shown to be reliable compared to research-grade instruments and to reflect ventilation rates in a clinical environment (Huang et al., 2021). The Airthings® instrument was used after the seven-day self-calibration period. When we cross-calibrated all devices, they displayed near-identical values. We also compared the CO2 values from Aranet4 Home® to a professional indoor climate multimeter (Testo® 435-1) which again showed identical CO2 values. The sensors were placed at a height between 1 and 2 m and not near the window or door.
Pilot data suggested that the highest CO2 concentrations were observed in patient rooms, in line with previous literature (Baures et al., 2018). Compared to the Airthings Wave Plus®, Aranet4 Home® monitors were more responsive (a slight delay can be seen as an offset with the Airthings Wave Plus® sensor, Supplementary Fig. 2). We used the Airthings Wave Plus® to monitor outdoor CO2 levels continuously during the trial.
At the start of the randomized sham/intervention period, clinical staff on each ward (nurses, nurse assistants, physiotherapists, occupational therapists, social workers etc.) were educated by the principal investigator on the purpose and methods of the trial and strategies to improve ventilation. Staff were instructed to maintain CO2 levels ≤ 800 ppm. During the two-week sham/intervention period, staff were interviewed at least every three days regarding difficulties in achieving CO2 targets, and additional solutions were sought to improve implementation. For example, in the intervention group, signs in the patient rooms alerted clinical staff to the ongoing experiment, CO2 targets and strategies on how to improve ventilation (open window, increase HVAC ventilation rate, reduce room crowding). We also discussed with staff how they could improve ventilation to remove CO2, or alter their care routines to prevent CO2 accumulation.
2.4. Outcomes
Our primary hypothesis was that CO2 monitors would record less time/day (in minutes) with elevated CO2 levels (>800 ppm, primary endpoint) during the intervention period, compared to the sham period. Secondary outcomes include the time with CO2 > 1000 ppm or >1400 ppm (which are the built-in cut-off levels for orange and red warning lights on the Aranet® devices).
Following the two-week sham/intervention phase, an anonymous online survey was sent via e-mail to ward staff to collect quantitative feedback regarding feasibility and preference to use CO2 monitors (using a 10-point Likert scale; predefined additional endpoints), as well as regarding their knowledge and behavior regarding ventilation and possible barriers towards implementation.
2.5. Statistical analysis
The four periods (baseline, sham, intervention and post-intervention) were analyzed by non-parametric Kruskal-Wallis test (because the residuals were not normally distributed) comparing the intervention period against the baseline, sham and post-intervention periods (3 comparisons). A priori, we assumed that if the intervention vs. baseline together with the intervention vs. post-intervention comparison would be significant (but not intervention vs. sham, which could be masked by carry-over effects), a pre-post effect of the intervention could be assumed.
The only exclusion criterion was incomplete occupancy: when the double-bed rooms were not fully occupied before 12 a.m., measurements from that day were excluded. Missing data were not planned to be imputated. Data were analyzed using the intention-to-treat principle, even though monitors in the sham group were occasionally noticed to be unblinded by staff during the study.
Statistical analyses were performed using GraphPad Prism v.9.1.1. Gaussian distribution of the data was assessed by the D'Agostino-Pearson (omnibus K2) normality test. Two-tailed, multiplicity-adjusted α below 0.05 was considered significant.
Due to lack of sufficient pilot data for robust power calculation, we assumed a conventional moderate effect size (f = 0.25). With an α error probability of 0.05, power of 0.95 and four groups, we calculated a total sample size of N = 280 measurements. With four groups, six sensors, two wards, seven days of measurements and expecting 15% excluded values due to unoccupied rooms (N = 4 × 6 × 2 × 7 × 0.85 = 285.6), our trial should be powered to detect a moderate effect size. Power calculation was performed using G*Power software version 3.1.9.7 (Kiel University, Germany).
2.6. Ethics and trial registration
On February 9th, 2021, our Institutional Ethical Committee decided that the study did not require informed consent since the design did not qualify as a human clinical trial according to applicable national and European regulations. Only basic demographic data about the room occupants (i.e. age and sex) as well as bed occupancy were collected anonymously via the electronic health records by the principal investigator. The head nurses of each ward provided verbal consent to participate voluntarily in the study, and all staff members were free to apply mitigation strategies to avoid high indoor CO2 levels or proceed with usual care. There was no funding involved in this trial.
The trial protocol was registered with ClinicalTrials.gov on February 21st 2021 (and published on February 25th 2021), and later amendments were registered at: https://clinicaltrials.gov/ct2/show/NCT04770597.
3. Results
3.1. Study sample characteristics
During the study, rooms were occupied 95.2% of the time, yielding N = 320/336 days or 7680 h of data. Room occupants were 97 women and 30 men, mean age (±standard deviation) 86.6 ± 5.6 years. The median outdoor CO2 concentration was 447 ppm (interquartile range [IQR] 424–470 ppm, see red dotted line in Supplementary Figs. 3–6).
3.2. Primary and secondary outcomes
CO2 levels increased mainly during morning care or when couples were hospitalized together. In both circumstances, we noticed that not only windows but also room doors -the main ventilation inlet- were usually closed (see Supplementary Figs. 3–6). During the baseline period, we noticed that our staff never operated the air-conditioning system in the rooms, since they received no prior instructions how to do so.
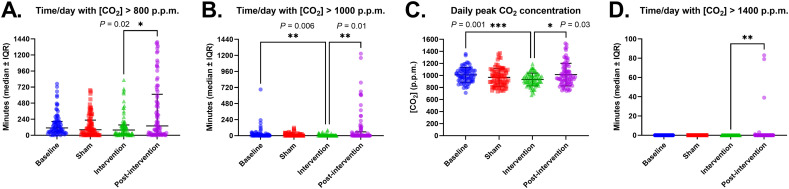
The median (IQR) time/day with CO2 concentration > 800 ppm (primary outcome) was 110 min (7.6% of the day; IQR 47–207 min, P = 01511 vs. intervention) at baseline, 82 min (5.7%, IQR 12–226.5 min, P > 0.99 vs. intervention) in the sham period, 78 min (5.4%, IQR 20–154 min) in the intervention period and 140 min (9.7%, IQR 19.5–612.5 min, P = 0.0167 by Dunn's multiple comparisons test) post-intervention (Fig. 2A).
Fig. 2.
Primary and secondary trial outcomes. A. The primary outcome of time/day (in minutes) with CO2 concentration > 800 ppm. Secondary outcomes: B. Time/day (in minutes) with CO2 concentration > 1000 ppm, C. daily peak CO2 concentration, and D. Time/day (in minutes) with CO2 concentration > 1400 ppm. Each point represents measurements from one 24 h period (N = 77, 82, 79 and 82 days in the baseline, sham, intervention and post-intervention period, respectively). Bars represent median and interquartile range. The intervention period was compared to the baseline, sham, and post-intervention period by Kruskal-Wallis test followed by Dunn's multiple comparisons test with multiplicity-adjusted P values as indicated.
The median time/day with CO2 levels > 1000 ppm was 2 min (IQR 0–19, P = 0.0064 vs. intervention) at baseline, 0 min (IQR 0–20, P = 0.2366 vs. intervention) in the sham period, 0 min (IQR 0–2) during intervention and 0 min (IQR 0–57, P = 0.0100 vs. intervention) post-intervention (Fig. 2B). The median daily peak CO2 concentration was 1010 ppm (IQR 926.5–1086, P = 0.0010 vs. intervention) at baseline, 964 ppm (IQR 846–1075, P = 0.5143) during the sham period, 932 ppm (IQR 861–1002) during intervention, and 977.5 ppm (IQR 873.5–1127, P = 0.0298) post-intervention (Fig. 2C). By Mann-Whitney U test, the differences in these three outcomes between the sham and intervention groups were not significant (P = 0.77, P = 0.052 and P = 0.22, respectively), confirming the results by the Kruskal-Wallis tests.
CO2 concentrations exceeding 1400 ppm were only observed during the post-intervention period, for 3 min on one day in one room and for a total of 202 min over four days in another room (out of 12 total rooms, see Supplementary Fig. 6). By Kruskal-Wallis test, the medians differed significantly (P = 0.0021), and by Dunn's multiple comparisons test, the intervention period differed significantly from the post-intervention period (P = 0.0055) but not from the baseline and sham periods (P > 0.9999, Fig. 2D).
3.3. Other outcomes
Staff members (N = 32 anonymous survey respondents) gave high ratings (median 8/10) for feasibility and preference to use CO2 monitors (Supplementary Fig. 7).
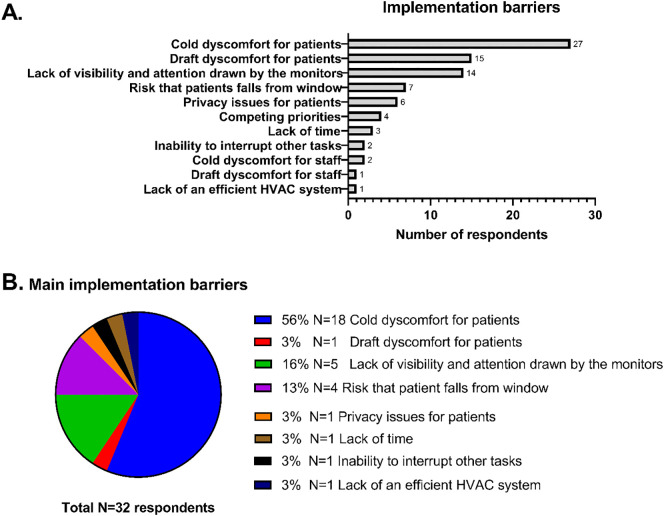
The main barriers for implementation were cold discomfort for patients (N = 19, 59%), lack of visibility and attention drawn by the monitors (N = 5) and risk that the patient would fall out of an open window (N = 4) (Fig. 3 ).
Fig. 3.
Staff-reported barriers towards increasing ventilation using CO2 monitors. A. Any implementation barriers identified by staff (N = 32) responding to post-study survey. B. The most important barrier indicated by each of the respondents. HVAC = heating, ventilation and air conditioning.
4. Discussion
Our findings show that indoor air CO2 concentrations in our hospital commonly peaked above recommended levels, providing a rationale for widespread use of CO2 monitors in patient rooms and clinical areas. These results are in line with previous reports in the literature (Baures et al., 2018; Chang et al., 2013; Jung et al., 2015; Pereira et al., 2020; Tang et al., 2009; Zhou et al., 2015) and are thus likely widely generalizable to many acute hospital wards worldwide. Of course, there are also many modern hospital wards with superior HVAC systems and excellent overall ventilation (as seen in intensive care units for example) (Kenarkoohi et al., 2020; Vosoughi et al., 2021).
Nosocomial transmission contributes to the incidence and mortality of COVID-19 (De Smet et al., 2020; Klompas et al., 2021). According to one estimate, ~11% of patients hospitalized with COVID-19, had hospital-acquired infection (Read et al., 2021). It is surprising how little attention has been paid to indoor CO2 monitoring in hospitals during the pandemic, and prevention of nosocomial transmission of SARS-CoV-2 via the airborne route. While most attention concerning COVID-19 in hospital infection control has focused on traditional hand hygiene, surface disinfection and droplet precautions, a paradigm shift in hospital infection control towards a focus on improving ventilation (“air hygiene”) is warranted (Greenhalgh et al., 2021; Morawska et al., 2021).
The use of CO2 monitors did not affect our primary trial outcome, highlighting the many implementation barriers in a real-world hospital setting. Carryover effects might have influenced the results during the sham period. Still, key secondary outcomes showed significant (albeit small) improvements during intervention compared to baseline and post-intervention. These differences were driven mainly by situations of rooming-in (Supplementary Figs. 3 and 6). In these circumstances, couples in hospitals were noticed to spend prolonged time with their room door closed (especially at night), whereas the main inlet of fresh outside air in our (type D) balance ventilation system was located in the corridor (direction of air flow from the corridor into patient rooms). Thus, we identified hospital rooming-in as a situation of particular concern for aerosol transmission of COVID-19.
There is little doubt that monitors can measure CO2 levels relatively cheaply and accurately in a hospital environment. However, like any technology, implementation requires nurses and other clinicians to look at the monitors and increase ventilation, e.g. by operating available HVAC systems, opening windows and/or altering their work routines (e.g., not washing two patients in the same room consecutively). Simply recommending monitors with the expectation that they by themselves maintain appropriate CO2 levels in a clinical environment appears unrealistic. We identified significant barriers towards implementation, mainly patients complaining from cold and draft discomfort from increased ventilation as well as lack of attention drawn by the monitors. Indeed, nurses and other clinical staff have many other responsibilities and may resist alterations in their work routines e.g. due to privacy concerns from leaving the door open while giving a bed bath to a patient behind a curtain, or concern that windows must remain closed to prevent patients from falling out. Further coaching and training staff in using CO2 monitors and applying ventilation strategies may be required to obtain sustained behavioral changes (perhaps using a “CO2 steward”), ideally in combination with engineered solutions like CO2-driven ventilation (Yang et al., 2014). In any case, the risks for healthcare workers and patients of insufficient indoor air quality in hospitals should not continue to be ignored.
Our study has several limitations, mainly due to its single-center, open-label design. A major limitation is that our trial is not designed nor powered to determine whether CO2 monitors reduce the risk of hospital-acquired infections including COVID-19. Moreover, CO2 is an excellent though imperfect proxy of respiratory infection risk. Mask wearing, HEPA filters, loud vocalization or physical distancing for example, affect pathogen transmission risk (Bazant and Bush, 2021; Miller et al., 2021) without influencing CO2 levels, while metabolism (i.e., respiratory quotient) influences CO2 production independent of exhaled air volume.
5. Conclusions
To the best of our knowledge, this is the first randomized trial investigating the use of CO2 monitors in the hospital environment. At least during this short-term intervention, the use of CO2 monitors did not affect the primary outcome, although significant but small reductions in secondary outcomes were observed. Staff generally reported positive attitudes towards using CO2 monitors although several possible barriers towards manually improving ventilation in the real-world hospital setting were identified. Further research is required to determine the clinical significance of indoor CO2 concentrations, and to define optimal strategies to achieve target CO2 levels in hospitals.
Data availability statement
Following publication, all data supporting this manuscript will be made available to established investigators upon simple request.
Ethics approval statement
The study protocol was reviewed and approved by the Institutional Review Board of Imelda Hospital, Bonheiden, Belgium.
Patient consent statement
Not applicable.
Clinical trial registration
ClinicalTrials.gov Unique Identifier NCT04770597
CRediT authorship contribution statement
Conceptualization: Laurent, Frans. Resources: Laurent, Frans. Data curation: Laurent. Formal analysis: Laurent. Investigation: Laurent, Frans. Project administration and supervision: Laurent. Writing - original draft: Laurent. Writing - review & editing: Laurent, Frans. Approval of the final manuscript: Laurent, Frans.
Declaration of competing interest
Dr. Laurent has received consultancy and lecture fees from Alexion, Amgen, Kyowa Kirin, Menarini, Orifarm, Sandoz, Takeda, UCB and Will Pharma, all unrelated to this work. Dr. Frans reports no conflicts of interest.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Editor: Anastasia Paschalidou
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.151349.
Appendix A. Supplementary data
Supplementary figures
CONSORT checklist of information to include when reporting randomised crossover trials.
References
- Ahlawat A., Mishra S.K., Birks J.W., Costabile F., Wiedensohler A. Preventing airborne transmission of SARS-CoV-2 in hospitals and nursing homes. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17228553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.G., Ibrahim A.M. Indoor air changes and potential implications for SARS-CoV-2 transmission. JAMA. 2021;325:2112–2113. doi: 10.1001/jama.2021.5053. [DOI] [PubMed] [Google Scholar]
- Baures E., Blanchard O., Mercier F., Surget E., le Cann P., Rivier A., et al. Indoor air quality in two french hospitals: measurement of chemical and microbiological contaminants. Sci. Total Environ. 2018;642:168–179. doi: 10.1016/j.scitotenv.2018.06.047. [DOI] [PubMed] [Google Scholar]
- Bazant M.Z., Bush J.W.M. A guideline to limit indoor airborne transmission of COVID-19. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2018995118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.L., Lee L.L., Weng C.H., Tsai C.Y. A project to improve psychiatric nursing station air quality. Hu Li Za Zhi. 2013;60:73–81. doi: 10.6224/JN.60.5.73. [DOI] [PubMed] [Google Scholar]
- De Smet R., Mellaerts B., Vandewinckele H., Lybeert P., Frans E., Ombelet S., et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J. Am. Med. Dir. Assoc. 2020;21(928–932) doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C.R., Wang S.C., Yu M.C., Chiu T.F., Wang J.Y., Chuang P.C., et al. Effect of ventilation improvement during a tuberculosis outbreak in underventilated university buildings. Indoor Air. 2020;30:422–432. doi: 10.1111/ina.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwan K., Li T., Altman D.G., Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366 doi: 10.1136/bmj.l4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control . 2020. Heating, Ventilation and Air-conditioning Systems in the Context of COVID-19: First Update. Stockholm, Sweden. [Google Scholar]
- Geelen L.M., Huijbregts M.A., Ragas A.M., Bretveld R.W., Jans H.W., van Doorn W.J., et al. Comparing the effectiveness of interventions to improve ventilation behavior in primary schools. Indoor Air. 2008;18:416–424. doi: 10.1111/j.1600-0668.2008.00542.x. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T., Jimenez J.L., Prather K.A., Tufekci Z., Fisman D., Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397:1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwar W., King M.F., Shuweihdi F., Fletcher L.A., Dancer S.J., Noakes C.J. What is the relationship between indoor air quality parameters and airborne microorganisms in hospital environments? A systematic review and meta-analysis. Indoor Air. 2021;31(5):1308–1322. doi: 10.1111/ina.12846. [DOI] [PubMed] [Google Scholar]
- Huang Q., Marzouk T., Cirligeanu R., Malmstrom H., Eliav E., Ren Y.-F. Ventilation Rate Assessment by Carbon Dioxide Levels in Dental Treatment Rooms. medRxiv. 2021. pp. 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.H., Park W.M. Indoor air concentrations of carbon dioxide (CO2), nitrogen dioxide (NO2), and ozone (O3) in multiple healthcare facilities. Environ. Geochem. Health. 2020;42:1487–1496. doi: 10.1007/s10653-019-00441-0. [DOI] [PubMed] [Google Scholar]
- Jung C.-C., Wu P.-C., Tseng C.-H., Su H.-J. Indoor air quality varies with ventilation types and working areas in hospitals. Build. Environ. 2015;85:190–195. [Google Scholar]
- Kenarkoohi A., Noorimotlagh Z., Falahi S., Amarloei A., Mirzaee S.A., Pakzad I., et al. Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci. Total Environ. 2020;748 doi: 10.1016/j.scitotenv.2020.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M., Baker M.A., Rhee C., Tucker R., Fiumara K., Griesbach D., et al. A SARS-CoV-2 cluster in an acute care hospital. Ann. Intern. Med. 2021;174:794–802. doi: 10.7326/M20-7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent M.R., Frans J. 2021. Monitors to Improve Indoor Carbon Dioxide Concentrations in the Hospital: Background, Rationale and Protocol for a Randomized, Sham-controlled, Cross-over, Open Label Trial. medRxiv. [Google Scholar]
- Lee H.J., Lee K.H., Kim D.K. Evaluation and comparison of the indoor air quality in different areas of the hospital. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000023942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Li Y., Zhou H., Lin J., Zheng Z., Xu H., et al. Affordable measures to monitor and alarm nosocomial SARS-CoV-2 infection due to poor ventilation. Indoor Air. 2021;31(6):1833–1842. doi: 10.1111/ina.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man P., Paltansing S., Ong D.S.Y., Vaessen N., van Nielen G., Koeleman J.G.M. Outbreak of coronavirus disease 2019 (COVID-19) in a nursing home associated with aerosol transmission as a result of inadequate ventilation. Clin. Infect. Dis. 2021;73:170–171. doi: 10.1093/cid/ciaa1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E.A., Richterman A., Gandhi R.T., Sax P.E. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann. Intern. Med. 2021;174:69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.L., Nazaroff W.W., Jimenez J.L., Boerstra A., Buonanno G., Dancer S.J., et al. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2021;31:314–323. doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Allen J., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., et al. A paradigm shift to combat indoor respiratory infection. Science. 2021;372:689–691. doi: 10.1126/science.abg2025. [DOI] [PubMed] [Google Scholar]
- Noorimotlagh Z., Jaafarzadeh N., Martinez S.S., Mirzaee S.A. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisani J., Di Gilio A., Viana M., de Gennaro G., Ferro A. Indoor air quality evaluation in oncology units at two European hospitals: low-cost sensors for TVOCs, PM2.5 and CO2 real-time monitoring. Build. Environ. 2021;205 [Google Scholar]
- Pereira M., Tribess A., Buonanno G., Stabile L., Scungio M., Baffo I. Particle and carbon dioxide concentration levels in a surgical room conditioned with a window/wall air-conditioning system. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos T., Dedesko S., Siegel J.A., Gilbert J.A., Stephens B. Spatial and temporal variations in indoor environmental conditions, human occupancy, and operational characteristics in a new hospital building. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J.M., Green C.A., Harrison E.M., Docherty A.B., Funk S., Harrison J., et al. Hospital-acquired SARS-CoV-2 infection in the UK's first COVID-19 pandemic wave. Lancet. 2021;398(10305):1037–1038. doi: 10.1016/S0140-6736(21)01786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbach J.T., Vonk M., Duijm F., van Ginkel J.T., Gehring U., Brunekreef B. A ventilation intervention study in classrooms to improve indoor air quality: the FRESH study. Environ. Health. 2013;12:110. doi: 10.1186/1476-069X-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick S.N., Milton D.K. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 2003;13:237–245. doi: 10.1034/j.1600-0668.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- Sa J.P., Branco P., Alvim-Ferraz M.C.M., Martins F.G., Sousa S.I.V. Evaluation of low-cost mitigation measures implemented to improve air quality in nursery and primary schools. Int. J. Environ. Res. Public Health. 2017;14 doi: 10.3390/ijerph14060585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajahan A., Culp C.H., Williamson B. Effects of indoor environmental parameters related to building heating, ventilation, and air conditioning systems on patients' medical outcomes: a review of scientific research on hospital buildings. Indoor Air. 2019;29:161–176. doi: 10.1111/ina.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superior Health Council of Belgium . Federal Agency for Public Health, Food Safety and Environment; Brussels, Belgium: 2020. Recommendations on the Use, Outside Hospitals and Care Institutions, of Passive Ventilation Systems, Mechanical Ventilation, Air-conditioning and Filters to Prevent Potential Airborne Transmission of SARS-COV-2. [Google Scholar]
- Tang C.S., Chung F.F., Lin M.C., Wan G.H. Impact of patient visiting activities on indoor climate in a medical intensive care unit: a 1-year longitudinal study. Am. J. Infect. Control. 2009;37:183–188. doi: 10.1016/j.ajic.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Tang J.W., Bahnfleth W.P., Bluyssen P.M., Buonanno G., Jimenez J.L., Kurnitski J., et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Hosp. Infect. 2021;110:89–96. doi: 10.1016/j.jhin.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavera Busso I., Herrera F., Tames M.F., Gonzalez Gasquez I., Camisassa L.N., Carreras H.A. QuEChER method for air microbiological monitoring in hospital environments. J. Infect. Dev. Ctries. 2020;14:66–73. doi: 10.3855/jidc.11563. [DOI] [PubMed] [Google Scholar]
- Vosoughi M., Karami C., Dargahi A., Jeddi F., Jalali K.M., Hadisi A., et al. Investigation of SARS-CoV-2 in hospital indoor air of COVID-19 patients' ward with impinger method. Environ. Sci. Pollut. Res. Int. 2021;28:50480–50488. doi: 10.1007/s11356-021-14260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargocki P., Da Silva N.A. Use of visual CO2 feedback as a retrofit solution for improving classroom air quality. Indoor Air. 2015;25:105–114. doi: 10.1111/ina.12119. [DOI] [PubMed] [Google Scholar]
- Yang C.T., Liao C.J., Liu J.C., Den W., Chou Y.C., Tsai J.J. Construction and application of an intelligent air quality monitoring system for healthcare environment. J. Med. Syst. 2014;38:15. doi: 10.1007/s10916-014-0015-3. [DOI] [PubMed] [Google Scholar]
- Zaman S.U., Yesmin M., MRS Pavel, Jeba F., Salam A. Indoor air quality indicators and toxicity potential at the hospitals' environment in Dhaka, Bangladesh. Environ. Sci. Pollut. Res. Int. 2021;28:37727–37740. doi: 10.1007/s11356-021-13162-8. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Lyu Z., Qian H., Song J., Möbs V.C. Field-measurement of CO2 level in general hospital wards in Nanjing. Procedia Engineering. 2015;121:52–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures
CONSORT checklist of information to include when reporting randomised crossover trials.
Data Availability Statement
Following publication, all data supporting this manuscript will be made available to established investigators upon simple request.