Abstract
Mounting evidence indicates that vitamin C has the potential to be a potent anti-cancer agent when administered intravenously and in high doses (high-dose IVC). Early phase clinical trials have confirmed safety and indicated efficacy of IVC in eradicating tumour cells of various cancer types. In recent years, the multi-targeting effects of vitamin C were unravelled, demonstrating a role as cancer-specific, pro-oxidative cytotoxic agent, anti-cancer epigenetic regulator and immune modulator, reversing epithelial-to-mesenchymal transition, inhibiting hypoxia and oncogenic kinase signalling and boosting immune response. Moreover, high-dose IVC is powerful as an adjuvant treatment for cancer, acting synergistically with many standard (chemo-) therapies, as well as a method for mitigating the toxic side-effects of chemotherapy. Despite the rationale and ample evidence, strong clinical data and phase III studies are lacking. Therefore, there is a need for more extensive awareness of the use of this highly promising, non-toxic cancer treatment in the clinical setting. In this review, we provide an elaborate overview of pre-clinical and clinical studies using high-dose IVC as anti-cancer agent, as well as a detailed evaluation of the main known molecular mechanisms involved. A special focus is put on global molecular profiling studies in this respect. In addition, an outlook on future implications of high-dose vitamin C in cancer treatment is presented and recommendations for further research are discussed.
Keywords: Ascorbic acid, Vitamin C, IVC, Cancer, Clinical trials, Proteomics, Transcriptomics, Metabolomics
Background
Vitamin C (VitC), also known as ascorbic acid or ascorbate, is an essential water-soluble vitamin that plays an important role in human physiology. Most of its physiological functions involve its ability to act as an antioxidant or as a cofactor for a wide variety of enzymatic reactions, thereby contributing to stabilisation of the tertiary structure of collagen, norepinephrine synthesis and iron absorption [1, 2]. Emerging data show that VitC is also a cofactor for newly characterised hydroxylases of the family of Fe-containing 2-oxoglutarate-dependent dioxygenases that regulate gene transcription and cell signalling pathways [3, 4]. In addition, immune cells accumulate high concentrations of VitC, underlining its key function in various processes within the immune system [5]. Importantly, while most vertebrate species can synthesize ascorbic acid, humans cannot, and they are therefore dependent on oral consumption of VitC.
The concept of utilizing VitC as a therapeutic agent for cancer care was first introduced by double Nobel Prize winning chemist Linus Pauling and physician Ewan Cameron almost 50 years ago [6–8]. Specifically, Pauling and Cameron published a number of clinical reports that indicated significantly prolonged survival rates of terminal cancer patients treated with pharmacological doses of VitC (10 g/ day by intravenous infusion for about 10 days and orally thereafter) compared to matched historical controls that did not receive VitC. The same amounts of high-dose VitC administered orally only in randomized double blind placebo control studies could not confirm this favourable response in advanced human cancer [9, 10]. Herein lies the essence of much controversy concerning the implementation of VitC in cancer treatment over the past decades. An important distinction must therefore be made between orally administered VitC (OC), achieving maximum plasma concentrations of no more than 220 μmol/L of blood, and pharmacological or high-dose IVC, generating plasma concentrations up into the millimolar range (≥ 15 mmol/L) [11–13], which is needed to kill cancer cells based on pre-clinical studies.
In light of this, high-dose IVC has re-emerged as a potent anti-cancer agent over the past two decades, with several phase I and a few phase II clinical trials reporting high tolerability and safety with promising signs of efficacy in the treatment of various cancer types, either as monotherapy or as a combination therapy [14–16]. In addition, there is strong clinical evidence for IVC’s ability to reduce chemotherapy-related side effects, such as fatigue, and to improve quality of life also in the palliative care setting [17–19].
The aim of this review is to create an up-to-date overview of the most important research conducted within the field of high-dose VitC and cancer therapy. First, the use of high-dose VitC mono- and combination therapy in the pre-clinical and clinical setting is discussed, followed by a discussion of the molecular mechanisms found to be involved in the anti-cancer activity portrayed by VitC. Specifically, the contribution of emerging global profiling studies based on proteomics, transcriptomics and metabolomics to these insights will be highlighted. In this regard, our findings will provide an outlook on future research, examining current gaps in our knowledge and addressing the limitations of research in the clinical setting and the need for more extensive clinical trials. Also, future implications of high-dose VitC in cancer therapy will be discussed in both treatment and palliative care.
High-dose VitC as a single agent
The pioneering clinical studies that initiated the interest in VitC as anticancer agent [6–8] employed VitC as single agent. Since then, a great number of clinical and pre-clinical studies have explored high-dose VitC. In this section, we briefly summarize the pre-clinical and clinical studies of VitC as monotherapy before elaborating more on the combination therapy studies.
Pre-clinical VitC monotherapy studies
A vast number of studies have shown encouraging anti-cancer activity of VitC at millimolar concentrations (~ 1–20 mM) in pre-clinical models of various cancer types [15]. The most investigated have been leukaemia [20–24], colon cancer [25–32], melanoma [33–37], pancreatic cancer [14, 31, 38] and prostate cancer [39–41]. Similar results have been described for the treatment of non-small-cell lung cancer (NSCLC) [16], breast cancer [31, 42], ovarian cancer [31, 43, 44], hepatocellular carcinoma [45, 46], malignant mesothelioma [47, 48], thyroid cancer [49, 50], oral squamous cell carcinoma [51], neuroblastoma [52] and glioma, including the difficult-to-treat glioblastoma multiform (GBM) [16, 53, 54].
One notable example of the progress in VitC pre-clinical research is the recent work in hard-to-treat Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) driven tumours, such as KRAS mutant colorectal cancer (CRC) [25, 27, 32]. Based on prior studies by Yun et al. [32] and Aguilera et al. [25], Cenigaonandia-Campillo et al. [27] used elevated doses of VitC (5–10 mM) in KRAS mutant CRC tumours, both in vitro and in vivo. They showed that VitC was able to target common metabolic aberrancies by decreasing adenosine triphosphate (ATP) and glucose transporter 1 (GLUT-1) levels, as well as by dissipating the mitochondrial membrane potential, which could sensitize KRAS mutant CRC cells to current treatments such as chemotherapy. Given the importance of developing better treatments for patients with KRAS driven tumours, non-toxic combinations with VitC are also being explored and will be discussed in the following section 2.
In the majority of cancer types, most of the in vivo studies have shown inhibition of tumour growth (40–60%) by using elevated doses of ascorbate (1-4 g/kg) either intravenously (IV) or intraperitoneally (IP) [15, 55–57]. Importantly, in order to maintain VitC levels inside the tumour, daily administration is the most optimal schedule [56]. By using these doses and frequency, VitC also successfully reduced and/or impaired metastasis formation (50–90%) [33, 39, 43, 58–61].
In terms of safety and tolerability, several studies have shown that high-dose VitC does not increase toxicity levels in vivo yet protects from other treatment side-effects when used as an adjuvant agent [15, 62–64].
Overall, the studies performed in vitro and in vivo using high-dose VitC as single agent in a large number of cancer types, have shown that it is a promising anti-cancer agent impairing both tumor growth and metastasis.
Clinical VitC monotherapy studies
Clinical monotherapy studies administering high-dose VitC in patients with various types of advanced malignancies report this therapy to be safe, showing no significant toxicity at doses of up to 3 g/kg [13] (Table 2). These studies additionally demonstrated that at the given doses, ascorbate plasma levels of over 10 mM could be sustained for several hours, and observed maximum achievable blood concentrations of up to 49 mM [13]. Grade 3 or higher adverse events possibly related to IVC treatment were reported in only 1–2 cases per study (with 17–24 patients included per study, see Table 2), the most common being hypokalemia [13, 65], hypernatremia [13], hypertension and anemia [66]. Riordan et al. [65] additionally reported one case of kidney stones in a metastatic CRC patient with a history of renal calculi, suggesting IVC may be contraindicated for patients with renal dysfunction. Nielsen et al. [66] reported one case of pulmonary embolism and pneumonia each, both of which can also be attributed to the underlying disease, since cancer is known to increase the risk of thromboembolic events. Hoffer at al [12]. reported no grade 3 or higher toxicities.
Table 2.
16 published clinical studies using medium-to-high dose IVC as anti-cancer therapy
| Cancer type (s) | Allocation/Phase | Interventions | VitC IV dosea | VitC dosage and injection scheme | No. patients | Results | Conclusions/Comment | Ref. |
|---|---|---|---|---|---|---|---|---|
| IVC monotherapy | ||||||||
| Advanced cancers | Single group, Phase 1 | IVC monotherapy | high |
30–110 g/m2 (0.8–3.0 g/kg), 4x/week, 4 weeks (both consecutive), rate of 1 g/min |
17 | All doses were well tolerated. Doses of 70, 90, and 110 g/m2 maintained levels at or above 10–20 mM for 5–6 h (Cmax 49 mM). No objective antitumor response | Recommended dose for future studies is 70–80 g/m2 (= 1.9–2.2 g/kg) based on Cmax | [13, 125] |
| Single group, Phase 1 | IVC monotherapy | high | 0.4–1.5 g/kg, 3x/week, 4 week treatment cycles; oral dose of 500 mg twice daily on non-infusion days | 24 | Well tolerated, without significant toxicity; dose of 1.5 g/kg sustains plasma ascorbic acid concentrations > 10 mM for > 4 h (Cmax 26 mM); 2 patients with unexpected stable disease | The recommended phase 2 dose is 1.5 g/kg; ascorbate may need to be combined with cytotoxic or other redoxactive molecules to be an efficacious treatment | [12], no ClinicalTrial.gov Identifier | |
| Single group, Phase n.s. | IVC monotherapy | medium | 0.15–0.71 g/kg/day, continuous infusion for up to 8 weeks | 24 | IVC therapy relatively safe, only few and minor adverse events observed; plasma ascorbate concentrations in the order of 1 mM attained | Further clinical studies with high dose IVC are warranted | [65], no ClinicalTrial.gov Identifier | |
| Prostate | Phase 2 | IVC monotherapy | medium | 5 g week 1, 30 g week 2 and 60 g weeks 3–12; daily oral dose of 500 mg starting after first infusion for 26 weeks | 23 | No patient achieved the primary endpoint of 50% PSA reduction; instead, a median increase in PSA of 17 μg/L was recorded at week 12; no signs of disease remission were observed; target dose of 60 g AA IV produced a peak plasma AA concentration of 20.3 mM [126] | This study does not support the use of intravenous AA outside clinical trials | [66, 126, 127] |
| IVC combination therapy - Chemotherapy and radiation therapy | ||||||||
| Advanced cancers | Single group, Phase 1/2 | IVC + standard care cytotoxic chemotherapy | high | 1.5 g/kg, 2 or 3x per week | 14 | IVC-chemotherapy is non-toxic and generally well tolerated; individual highly favourable responses found in biliary tract, cervix and head and neck cancer patients, colorectal cancer patients without benefit | Neither proves nor disproves IVC’s value in cancer therapy; illustrates potential for “discovery in clinical practice” | [83, 128] |
| Glioblastoma | Single group, Phase 1 | IVC + RT + temozolomide (TMZ) | high | Radiation phase: 15–125 g, 3x weekly, 7 weeks; Adjuvant phase: dose-escalation until plasma level of 20 mM was achieved, 2x weekly, 28 weeks | 13 | Safe and well tolerated; targeted ascorbate plasma levels of 20 mmol/L achieved in the 87.5 g cohort; favourable OS and PFS compared to historical controls (RT + TMZ only) | Phase 2 clinical trial initiated (NCT02344355), currently active, not recruiting | [16, 129, 130] |
| NSCLC | Single group, Phase 2 | IVC + carboplatin + paclitaxel | high | 75 g, 2x weekly | 14 | Increased disease control and objective response rates | Still recruiting (NCT02420314), see Table 3 | [16, 133] |
| Ovarian | Phase 1/2a, randomized |
Arm 1: IVC + carboplatin + paclitaxel Arm 2: carboplatin + paclitaxel only |
high | Dose escalation up to 75 or 100 g, with target peak plasma concentration of 350 to 400 mg/dl (20 to 23 mM), 2x/week, for 12 months (of which the first 6 months in conjunction with chemotherapy) | 25 | Longer PFS and substantially decreased toxicities compared to control arm w/o Vit C; trend toward improved median OS |
Study not powered for detection of efficacy, larger clinical trials warranted |
[63, 145] |
| Pancreatic | Single group, Phase 1/2a | IVC + gemcitabine | high | 25–100 g dose escalation in phase I, 75–100 g in phase II, 3x weekly, for 4 weeks | 14 | Well tolerated, no clinically significant influence on gemcitabine pharmacokinetics |
Phase 2/3 trial needed to detect efficacy and benefit of IVC |
[14, 146] |
| Single group, Phase 1 | IVC + RT + gemcitabine | high | 50–100 g daily during RT, 6 weeks | 16 | Safe and well tolerated with suggestions of efficacy; increased OS and PFS compared to institutional average; 100 g determined to be MTD, 75 g selected as a recommended phase II dose | Phase 2 trial is indicated | [110, 147] | |
| Phase 2, randomized |
Arm 1: IVC + G-FLIP/G-FLIP-DM Arm 2: G-FLIP/G-FLIP-DM only |
high | 75–100 g, 1–2x per week, with GFLIP every every 2 weeks until progression | 26 | Safe and well tolerated. May avoid standard 20–40% rates of severe toxicities | Abstract only, no data shown | [148, 149] | |
| Single group, Phase 1 | IVC + gemcitabine | high | 50–125 g, 2x weekly to achieve target plasma level of ≥350 mg/dL (≥20 mM) | 9 | Well-tolerated with suggestion of some efficacy; plasma levels of 20–30 mM were reached with doses ranging from 0.75–1.75 g/kg | Phase 2 trial is indicated | [82, 150] | |
| IVC combination therapy - Targeted therapy | ||||||||
| Colorectal, Gastric | Single group, Phase 1 |
IVC + mFOLFOX6 or FOLFIRI (part 1); IVC + mFOLFOX6 ± bevacizumab (part 2) |
high |
Dose escalation phase (part 1): 0.2–1.5 g/kg, once daily, days 1–3, in a 14-day cycle until MTD was reached; Speed expansion phase (part 2): MTD or at 1.5 g/kg if MTD not reached |
36 (30 colorectal, 6 gastric) |
MTD not reached; no DLT; favourable safety profile and preliminary efficacy | Recommended dose for future studies 1.5 g/kg/day; extended to phase 3 study | [151, 152] |
| Pancreatic | Single group, Phase 1 | IVC + gemcitabine + erlotinib | high | 50–100 g, 3x/week, 8 weeks | 9 | Tumor shrinkage in 8/9 patients; peak ascorbic acid concentrations as high as 30 mmol/L in the highest dose group | Phase 2 trial with longer treatment period 100 g dosage warranted | [153, 154] |
| B-cell non-Hodgkin’s lymphoma | Single group, Phase 1 | IVC + CHASER regimen | high | 75 g or 100 g 5x in 3 weeks | 3 | Whole body dose of 75 g safe and sufficient to achieve an effective serum concentration (> 15 mM (264 mg/dl) | No NCT number; Phase II trial is indicated | [155], no ClinicalTrial.gov Identifier |
| IVC combination therapy - Combinations with emerging non-pharmaceutical therapies | ||||||||
| NSCLC | Phase 1/2, randomized |
Arm 1: IVC + mEHT + BSC Arm 2: BSC alone |
high | 1 g/kg, 1.2 g/kg or 1.5 g/kg, 3x/week for 8 weeks (Phase 1); 1 g/kg, 3x/week, 25 treatments in total (Phase 2) | 97 | IVC treatment concurrent with mEHT is safe and improved the QoL of NSCLC patients (Phase 1, Ou et al., 2017); significantly prolonged PFS, OS and QoL (Phase 2) | IVC + mEHT is a feasible treatment in advanced NSCLC | [156–158] |
Shown are the 16 published trials using medium-to-high dose IVC out of a total 34 published trials. All 34 trials, including those using low-dose or oral VitC, are summarized in Fig. 3. Entries are ordered primarily by kind of combination treatment, and secondarily by cancer type
aHigh dose ≥1 g/kg, low dose ≤10 g whole body dose
n.s., not specified; g/kg × 37 = g/m2 (1.5 g/kg = 56 g/m2); G-FLIP/G-FLIP-DM: low dose Gemcitabine, fluorouracil, leucovorin, irinotecan, and oxaliplatin/ G-FLIP + low dose docetaxel and mitomycin C; CHASER regimen: Rituximab, cyclophosphamide, cytarabine, etoposide and dexamethasone; mFOLFOX6/FOLFIRI, oxaliplatin, leucovorin and 5-fluorouracil/irinotecan, leucovorin and 5-fluorouracil
Beyond being safe and well-tolerated, objective anti-tumor response was not observed in any of these IVC monotherapy studies. While Stephenson et al. [13], Hoffer et al. [12] and Riordan et al. [65] reported 3 (out of 16), 2 (out of 24) and 1 (out of 24), and patients with stable disease, respectively, the study by Nielsen et al. [66] reported no signs of disease remission or stabilization. Latter result is likely related to the fact that both dose and administration frequency (maximum of 60 g whole body dose given 1 time per week for 12 weeks) was considerably lower compared to the other studies (here, up to 3 g/kg were administered at least 3 times per week, for up to 8 weeks, see Table 2). That being said, a number of promising case reports have reported unexpectedly long survival time and in some cases even complete tumour regression of advanced or metastatic disease [67–72]. In future studies, molecular profiling of these exceptional responders would be of high value to explore molecular features that make certain tumors more sensitive to IVC.
Currently, one phase II study is ongoing whereby the effect of high-dose (1.25 g/kg) VitC monotherapy is being studied in resectable or metastatic colorectal, pancreatic and lung tumors (Table 3). The objective of the study is to investigate the effect on pathological tumor response in resectable tumors and to observe objective tumor response in KRAS or BRAF mutant metastatic tumors (NCT03146962) [73]. In addition, one medium-dose effort in bladder cancer (NCT04046094) [74] as well as several oral and/or low-dose monotherapy studies in non-solid tumors (NCT03682029)(NCT03613727)(NCT03964688) [75–77] are currently ongoing in line with the promising pre-clinical data concerning these latter cancer types [21, 78].
Table 3.
16 ongoing clincal studies using medium-to-high dose IVC as anti-cancer therapy
| Cancer type(s) | NCT Number | Allocation/ Phase |
Interventions | Type of combination therapy | VitC IV dose* | VitC dose and administration schedule | Estimated enrollment | Primary outcome(s) |
|---|---|---|---|---|---|---|---|---|
| Colorectal |
[131] |
Randomized, Phase 3 |
Arm 1: Ascorbic acid + chemotherapy Arm 2: Chemotherapy alone (FOLFOXIRI+/− bevacizumab) |
Chemo + Targeted | high | 1.5 g/kg/day, D1–3, every 2 weeks | 400 | Objective Response Rate |
| Colorectal, Pancreatic, Lung |
[73] |
Single group, Phase 2 |
Cohort A: VitC for 2–4 consecutive weeks Cohort 2: VitC up to 6 months Cohort 3: VitC for 1–2 weeks prior to and following Y90 radioembolization of hepatic metastases |
RE | high | 1.25 g/kg for 4 days/week | 50 |
Pathologic response (cohort A) 3-month disease control rate (DCR) (cohort B) Maximal tolerated dose (cohort C) |
| Hepatocellular, Pancreatic, Gastric, Colorectal |
[132] |
Single group, Phase 2 | VitC + metformin | Targeted | high | 1.5 g/kg, D1–3, every 2 weeks | 30 | Progression-free survival |
| Lung |
[133] |
Single group, Phase 2 | Ascorbic acid + paclitaxel + carboplatin | Chemo | high | 75 g, two times/week | 57 | Tumor response |
| Lung |
[134] |
Single group, Phase 2 | Ascorbate + chemoRT (radiation therapy + paclitaxel + carboplatin) | Chemo-RT | high | 75 g, 3 times/week | 46 | Progression rate |
| Lymphoma |
[135] |
Single group, Phase 1 | VitC + melphalan | Chemo | high |
50 g, 75 g and 100 g (3 + 3 cohort method) |
9 | Number of treatment related adverse events |
| Lymphoma |
[136] |
Randomized, Phase 2 |
Arm 1: Ascorbic acid + combination chemotherapy Arm 2: Placebo + combination chemotherapy (rituximab + ifosfamide + carboplatin + etoposide D1–3; rituximab + cisplatin + cytarabine + dexamethasone if MR or SD after 2 courses) Arm 3: Ascorbic acid + combination chemotherapy (ifosfamide + carboplatin + etoposide or cisplatin + cytarabine + dexamethasone or gemcitabine + dexamethasone + cisplatin or gemcitabine + oxaliplatin or oxaliplatin + cytarabine + dexamethasone) |
Chemo + Targeted + Corticosteroid | high |
High dose (n.s.) on D1, 3, 5, 8, 10, 12, 15, 17 and 19, combination chemotherapy on D1–3; treatment repeats every 21 days for up to 4 courses |
151 | Overall response rate |
| Pancreatic |
[137] |
Randomized, Phase 2 |
Arm 1: Ascorbate + chemotherapy Arm 2: Chemotherapy alone (gemcitabine + nab-paclitaxel) |
Chemo | high | 75 g, three times/weekly for 4 weeks | 65 | Overall survival |
| Pancreatic |
[138] |
Single group, Phase 1 | VitC + chemotherapy/stem cell treatment (melphalan + carmustine + vitamin B12B + ethanol) | Chemo + Dietary suppl. | high | Dose-escalation beginning with 3 g/m^2 and escalating to a maximum of 8 g/m^2 | 10 |
Rate of mucositis, rate of engraftment of Neutrophils + adverse events, among others |
| Pancreatic |
[139] |
Single group, Phase 1/2 | Ascorbic acid + nab-paclitaxel + cisplatin + gemcitabine | Chemo | high | ≥ 20 mM plasma concentration | 36 |
Phase IB: recommended phase II dose (to reach ≥20 mM) Phase II: disease control rate |
| Prostate |
[140] |
Randomized, Phase 2 |
Arm 1: Ascorbate + Docetaxel Arm 2: Placebo + Docetaxel |
Chemo | high | 1 g/kg, 3 times/ week | 69 | Occurrence of PSA decline of > = 50% + adverse events |
| Renal Cell |
[141] |
Randomized, Phase 2 |
Arm 1: Ascorbic acid + tyrosine kinase inhibitor Arm 2: Tyrosine kinase inhibitor alone (Pazopanib) |
Targeted | high | 1 g/kg 3 times/week | 91 | Treatment failure-free rate |
| Sarcoma |
[142] |
Single group, Early phase 1 | Ascorbate + gemcitabine | Chemo | high | 75 g dose on D1–2, until target serum concentration between 20 and 30 mM (otherwise maximum dose of 125 g) | 20 | Progression-free survival |
| Sarcoma |
[143] |
Single group, Phase 1/2 | Ascorbate + radiation therapy | RT | high | 75 g, three times/week | 25 | Incidence of dose limiting toxicities (DLTs) + tumor response |
| Bladder |
[74] |
Single group, Phase 1/2 | Ascorbic acid | – | medium | 25 g, 2 times/week for 4 weeks | 21 | Post treatment pathological staging |
| Lung |
[144] |
Randomized, Phase 1/2 |
Arm 1: VitC + tyrosine kinase inhibitor Arm 2: Tyrosine kinase inhibitor alone (osimertinib, erlotinib or gefitinib) |
Targeted | medium | 30 g once/week | 150 | Progression-free survival |
Shown are the 16 trials using medium-to-high dose IVC out of a total 23 studies currently recruiting (status February 2021), as retrieved from the clinicaltrials.govdatabase (see also Fig. 3). Entries are ordered primarily by high-to-medium IVC dose, and secondarily by cancer type
In general, high-dose VitC monotherapy has not been clinically assessed in patients that have not received (heavy) prior systemic treatment and that are not terminally ill. This fact may explain the limited response effects observed. Finding a feasible clinical setting to include less heavily pre-treated patients however is complicated, as it would involve denying patients standard of care. For this reason, future applications of high-dose VitC as cancer therapy may rather be in combination strategies and we will focus more on this application in the sections below. However, important lessons regarding administration frequency can be learned from these monotherapy studies, whereby only those studies that administered IVC at least 3 times per week warranted further clinical trials. The recommended doses ranged from 1.5 g/kg [12] to 1.9–2.2 g/kg [13].
VitC monotherapy in palliative care and quality of life
In palliative care, high-dose VitC is currently gaining ground due to its highly safe and tolerable profile. Not only is high-dose VitC known to relieve pain in cancer patients [79], vast clinical evidence suggests that it has a significant positive impact on patients’ well-being [14, 17–19, 63, 80–83]. This might be due to the frequent hypovitaminosis and VitC deficiency in cancer patients [79, 84, 85], which are commonly enhanced by anti-neoplastic treatments [18].
For instance, a retrospective, multicentre, epidemiological cohort study [18] showed amelioration of appetite, fatigue, depression and sleep disorders in breast cancer and terminal cancer patients suffering from a wide variety of cancer types that received complementary 7.5 g IVC while being treated by respective standard regimens. More recently, a single-center, parallel-group, single-blind interventional study also in breast cancer patients [86] showed a similar and significant reduction of symptoms such as nausea, fatigue, tumor pain and loss of appetite by administering 25 g of IVC per week in addition to their current standard treatment. Favourably, no new side effects were reported after initiation of IVC treatment.
Moreover, another retrospective study showed that patients with radiotherapy-resistant bone metastasis did not only have less pain and better performance measures when given high-dose VitC, they had a median survival time of 10 months as compared to the 2 months median survival time within the control group [80].
Overall, high dose VitC administered as a single agent has not only been shown to be safe and well-tolerated in cancer patients, but also to ameliorate pain and to improve quality of life in the palliative care setting.
High-dose VitC in combination treatments
Many studies in the past years have investigated high dose VitC as an adjuvant pro-oxidative agent mainly in chemo- and radiotherapy. In addition, other combination treatments have been investigated as well. In this section, we review the pre-clinical and clinical literature of high dose VitC in combination treatments.
For pre-clinical studies, we provide detailed information per study and per combination (i.e. cancer type, VitC doses, route of administration, sample size, etc), and describe the observed effects such as synergism, enhanced efficacy and/or reduced toxicity (Table 1, Figs. 1, 2). Particularly for clinical studies, completed and on-going trials using IVC as monotherapy and combination treatment are described in detail (Tables 2, 3, Fig. 3). We examine relevant information on phase of study, type of interventions, IVC dose, injection scheme and number of patients enrolled. In addition, results of completed studies and primary outcomes of ongoing trials are thoroughly discussed.
Table 1.
Combinations of anti-cancer agents and high-dose VitC in pre-clinical in vitro and in vivo studies
| Combination Treatment(s) | Type Drug | Cancer type(s) | Type of Study | Sample Size | Dose In vitro | Tx duration | Dose, Administration In vivo | Schedule In vivo | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2Gy | Radiotherapy | Pancreatic | In vitro | n = 1 cell line | 4 mM | 24 h | – | – | Radio-sensitizing | [87] |
| 5-FU | Chemotherapy | Colorectal | In vitro, In vivo | n = 3 cell lines, n = 48 Balb/c nu/nu mice | 0.15–13.3 mM | 24, 48, 72, 96 h | 150 mg/kg IP | Daily | In vitro synergy, in vivo no benefit | [88] |
| Gastric | In vitro, In vivo | n = 2 cell lines, n = 60 athymic-nu/nu mice | 1 mM | 1 h | 4 g/kg IP | Daily (20–30 days) | Enhanced efficacy | [89] | ||
| Anti-PD-1 | Immunotherapy | B cell lymphoma | In vivo | n = 40 immunocompetent syngenic BALB/c mice | – | N/S | 1500 mM IP | Daily (dose-escalated, 10-19 days) | Synergy | [90] |
| Anti-PD-1/Anti-CTL-4 | Immunotherapy | Breast, Colorectal, Pancreatic | In vivo | n = 13 immunocompetent syngeneic mice | – | N/S | 4 g/kg IP | Daily 5x/week | Synergy and effective antitumor immune memory | [91] |
| ATO | Chemotherapy | Colorectal | In vitro | n = 2 cell lines | 2 mM | 24 h | – | – | Synergy | [92] |
| Colorectal, Pancreatic (mKRAS) | In vitro, In vivo | n = 7 cell lines, n = 30 mice | 1 mM | 48, 72 h | 1.5 g/kg IV | Daily | Enhanced efficacy | [93] | ||
| AML and APL | In vitro | n = 5 cell lines, n = 48 primary cells | 3 mM | 72 h | – | – | Enhanced efficacy | [94] | ||
| CLL | In vitro | Primary cells of n = 18 patients | 1 mM | 24, 72 h | – | – | Enhanced efficacy | [95] | ||
| ATO + vitE | Chemotherapy | APL | In vitro | n = 1 cell line | 0.1 mM | 48 h | – | – | Synergy | [96] |
| Auranofin | Anti-inflammatory | Triple-Negative Breast | In vitro, In vivo | n = 5 cell lines, n = 25 swiss Nude Mice | 2.5 mM | 24 h | 4 g/kg IP | Daily (15 days) | Synergy | [97] |
| Azacytidine | Chemotherapy | Colorectal | In vitro | n = 1 cell line | 0.01, 0.05 mM | 72 h | – | – | Synergy | [98] |
| Carboplatin | Chemotherapy | Gastric | In vitro, In vivo | n = 2 cell line, n = 60 athymic-nu/nu mice | 1 mM | 1 h | 4 g/kg IP | Daily (20–30 days) | Enhanced efficacy | [89] |
| Cetuximab | Targeted therapy | Colorectal (mKRAS) | In vitro, In vivo | n = 5 cell lines, n = N/S athymic nude mice | 0.3, 0.5, 0.7 mM | 6 h | 0.5 g/kg IP | Daily (14 days) | Synergy and abrogates resistance via SVCT-2 | [99] |
| Cisplatin | Chemotherapy | Gastric | In vitro | n = 1 cell line | 0.000284, 0.000568 mM | 48 h | – | – | Synergy | [100] |
| Cervical | In vitro | n = 2 cell lines | 0.000568 mM | 24, 48, 72 h | – | – | Synergy | [101] | ||
| Oral squamous | In vitro, In vivo | n = 8 cell lines, n = 24 C57BL/6 mice | 0.125, 0.25, 0.5, 1 mM | 72 h | 4 g/kg IP | Daily (21 days) | Synergy | [51] | ||
| Ovarian | In vitro | n = 1 cell line | 2 mM | 2 h | – | – | Enhanced efficacy | [102] | ||
| Cervical | In vitro | n = 2 cell lines | 1, 2.5, 3.3, 16 mM | 24, 48, 72 h | – | – | Synergy | [103] | ||
| Gastric | In vitro, In vivo | n = 2 cell lines, n = 60 athymic-nu/nu mice | 1 mM | 1 h | 4 g/kg IP | Daily (20–30 days) | Enhanced efficacy | [89] | ||
| CPI-613 | Targeted therapy | CLL | In vitro | n = 2 cell lines | 0.1–2 mM | 24 h | – | – | Synergy | [104] |
| Decitabine | Chemotherapy | AML | In vitro | n = 2 cell lines | 0.3 mM | 24, 48, 72 h | – | – | Synergy | [105] |
| Colorectal | In vitro | n = 1 cells line | 0.01, 0.05 mM | 72 h | – | – | Synergy | [98] | ||
| Doxorubicin | Chemotherapy | Cervical | In vitro | n = 2 cell lines | 1, 2.5, 3.3, 16 mM | 24, 48, 72 h | – | – | Synergy | [103] |
| Doxycycline | Targeted therapy | Cancer Stem Cells | In vitro | n = 1 cells line | 0.25–0.5 mM | 5 days | – | – | Synergy | [106] |
| Doxycycline + Azithromycin | Targeted therapy | Cancer Stem Cells | In vitro | n = 1 cell line | 0.25 mM | 5 days | – | – | Synergy | [107] |
| Eribulin mesylate | Chemotherapy | Breast | In vitro | n = 6 cell lines | 5, 10, 20 mM | 2 h (×1 or ×2) | – | – | Enhanced efficacy | [108] |
| Etoposide | Chemotherapy | Glioblastoma | In vitro | n = 1 cell line | 1 mM | 48, 96, 144 h | – | – | Enhanced efficacy | [54] |
| Fulvestrant | Hormonal therapy | Breast | In vitro | n = 6 cell lines | 5, 10, 20 mM | 2 h (×1 or ×2) | – | – | Enhanced efficacy | [108] |
| Gefitinib | Targeted therapy | Non-small cell Lung | In vitro | n = 3 cell lines | 0.5, 1, 2.5, 5, 10 mM | 1 h | – | – | Synergy | [109] |
| Gemcitabine | Chemotherapy | Pancreatic | In vitro, In vivo | n = 6 cell lines, n = N/S athymic nude mice | 0.001 mM | 1 h | 4 g/kg IP | Twice daily (6 days) | Radioprotection and radiosensitization | [110] |
| Pancreatic | In vivo | n = 32 mice | – | – | 4 g/kg IP | Daily (45 days) | Enhanced efficacy and VitC equal to combination | [14] | ||
| Gemcitabine + Ionizing radiation (IR) | Chemoradiotherapy | Sarcoma | In vitro, In vivo | n = 2 cell lines, n ≥ 7 per treatment group, athymic-nu/nu mice | 2, 5 mM | 1 h | 4 g/kg IP | Daily (40-60 days) | Radio-chemo sensitizer | [111] |
| Ibrutinib | Targeted therapy | CLL | In vitro | n = 2 cell lines, n = 6 primary cells | 0.1–2 mM | 24 h | – | – | Synergy | [104] |
| Idelalisib | Targeted therapy | CLL | In vitro | n = 2 cell lines, primary cells of n = 6 patients | 0.1–2 mM | 24 h | – | – | Synergy | [104] |
| Irinotecan | Chemotherapy | Colorectal | In vitro, In vivo | n = 3 cell lines, n = 48 Balb/c nu/nu mice | 0.15–13.3 mM | 24, 48, 72, 96 h | 150 mg/kg IP | Daily | Synergy in vitro, enhanced efficacy in vivo | [88] |
| Gastric | In vitro, In vivo | n = 2 cell lines, n = 60 athymic-nu/nu mice | 1 mM | 1 h | 4 g/kg IP | Daily (20–30 days) | Enhanced efficacy | [89] | ||
| Gastric | In vitro, In vivo | n = 5 cell lines, n = 24 ALB/c nude mice | 2, 4 mM | 2 h | 4 g/kg IP | Twice daily | Synergy | [112] | ||
| Melphalan | Chemotherapy | Multiple Myeloma | In vitro, In vivo | Primary cells of n = 13 patients, n = 45 NOD.Cγ-Rag1 mice | 8, 20 mM | 1 h | 4 mg/kg IP | Daily | Synergy | [113] |
| Metformin | Multitargeted Therapy | CLL | In vitro | n = 2 cell lines | 0.1–2 mM | 24 h | – | – | Synergy | [104] |
| Olaparib (PARP inhibitor) | Targeted therapy | AML (TET2-deficient) | In vitro | n = 6 cell lines | 0.125, 0.25, 0.5, 1 mM | 72 h | – | – | Enhanced sensitivity | [22] |
| Oligomycin A | Targeted therapy | CLL | In vitro | n = 2 cell lines | 0.1–2 mM | 24 h | – | – | Synergy | [104] |
| Oxaliplatin | Chemotherapy | Colorectal | In vitro, In vivo | n = 3 cell lines, n = 48 (6 × 8) Balb/c nu/nu mice | 0.15–13.3 mM | 24, 48, 72, 96 h | 150 mg/kg IP | Daily | Synergy in vitro, enhanced efficacy in vivo | [88] |
| Gastric | In vitro, In vivo | n = 5 cell lines, n = 24 ALB/c nude mice | 2, 4 mM | 2 h | 4 g/kg IP | Twice daily | Synergy in vitro, enhanced efficacy in vivo | [112] | ||
| Oxaliplatin + Fasting mimicking diet (FMD) | Chemotherapy + Fasting | Colorectal, Pancreatic, Lung (mKRAS); Prostate, Ovarian | In vitro, In vivo | n = 11 cell lines, n = 38 NSG and BALB/c mice | ≥0.3 mM | 24 h | 4 g/kg IP | Twice daily (36 days) | Synergy | [114] |
| Paclitaxel | Chemotherapy | Oral squamous | In vivo | n = 96 Swiss albino mice | – | N/S | 10 mg oral | – | Enhanced efficacy | [115] |
| Gastric | In vitro, In vivo | n = 2 cell lines, n = 60 athymic-nu/nu mice | 1 mM | 1 h | 4 g/kg IP | Daily (20–30 days) | Enhanced efficacy | [89] | ||
| PLX4032 | Targeted therapy | Thyroid | In vitro, In vivo | n = 3 cell lines; n = 20 nude mice | 0.1–2 mM | 72 h | 3 g/kg IP | Daily (15 days) | Synergy | [64] |
| Sorafenib | Targeted therapy | Liver | In vitro | n = 5 cell lines | 2.5, 5, 7.5, 10, 20 mM | 2 h | – | – | Synergy | [116] |
| Sulfasalazine | Anti-inflammatory | Prostate | In vitro, In vivo | n = 2 cell lines, n = ~ 24 BALB/c nude mice | 1, 2 mM | 2-48 h | 4 g/kg IP | Twice daily (16 days) | Synergy | [117] |
| Sulindac | Anti-inflammatory | Colorectal | In vitro | n = 2 cell lines | 0.5 mM | 48 h | – | – | Synergy | [118] |
| Tamoxifen | Hormonal therapy | Breast | In vitro | n = 6 cell lines | 5, 10, 20 mM | 2 h (×1 or ×2) | – | – | Enhanced efficacy | [108] |
| Temozolomide | Chemotherapy | Glioblastoma | In vitro | n = 1 cell line | 1 mM | 48, 96, 144 h | – | – | Enhanced efficacy | [54] |
| Thieno-triazolo-1,4-diazepine (JQ1) | Targeted therapy | Melanoma | In vitro, In vivo | n = 5 cell lines; n = 10 Gulo−/− and 10 Gulo+/+ mice | 0.00005–0.0001 mM | 72 h | 3.3 g/L and 0.33 g/L, oral | Daily (14 days) | Enhanced efficacy | [119] |
| TMZ/carboplatin + IR | Chemoradiotherapy | Glioblastoma, Non-small cell Lung | In vitro, In vivo | n = 12 cell lines, n = ~ 42 athymic nude mice | 1, 2 mM | 1 h | 4 g/kg IP | Daily | Radio-chemo sensitizer | [16] |
| Topotecan | Chemotherapy | Breast | In vitro | n = 1 cell line | 1 mM | 48 h | – | – | Synergy | [120] |
| TPP derivative dodecyl-TPP (d-TPP) | Targeted therapy | Cancer Stem Cells | In vitro | n = 2 cell lines | 0.25–0.5 mM | 5 days | – | – | Synergy | [121] |
| Trastuzumab | Targeted therapy | Breast | In vitro | n = 6 cell lines | 5, 10, 20 mM | 2 h (×1 or ×2) | – | – | Enhanced efficacy | [108] |
| Triethylenetetramine (TETA) | Targeted therapy | Breast | In vitro, In vivo | n = 9 cell lines, n = 40 BALB/c-nu | 1 mM | 12, 24 h | 3 g/kg IP | Daily (25 days) | Synergy | [122] |
| Vemurafenib | Targeted therapy | BRAF mutant Melanoma | In vitro, In vivo | n = 2 cell lines, n = 18 C57BL/6 mice | 1, 5 mM | 48 h | 0.03 mg/kg oral | Daily | Synergy and abrogates resistance | [123] |
| Venetoclax | Targeted therapy | CLL | In vitro | n = 2 cell lines, primary cells of n = 6 patients | 0.1–2 mM | 24 h | – | – | Synergy | [104] |
| Vit K3 (Menadione) + Everolimus or Barasertib | Vitamin + Targeted therapy | ALL | In vitro | n = 1 cell line | 0.3 mM | 24, 72 h | – | – | Synergy | [124] |
A total of 47 combinations in 44 pre-clinical studies from 2016 to 2021 were retrieved from PubMed using search terms (vitamin c OR ascorbate OR ascorbic acid) AND (combination OR synergy OR combined) AND (cancer)
Tx treatment, mM millimolar, IP intraperitoneal, IV intravenous, JQ1 Thieno-triazolo-1,4-diazepine, 5-FU 5-fluorouracil, Vit vitamin, IR irradiation, TMZ temozolomide, Gem gemcitabine, Dox Doxycycline, Oxa oxaplatin, TETA Triethylenetetramine, BRAF v-raf murine sarcoma viral oncogene homolog B1, PARP poly (ADP-ribose) polymerase, d-TPP TPP derivative dodecyl-TPP, ATO arsenic trioxide, 3-PO 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one, CLL chronic lymphocytic leukemia, AML acute myeloid leukemia, APL acute promyelocytic leukemia, ALL acute lymphoblastic leukemia, TET ten eleven translocation
Fig. 1.
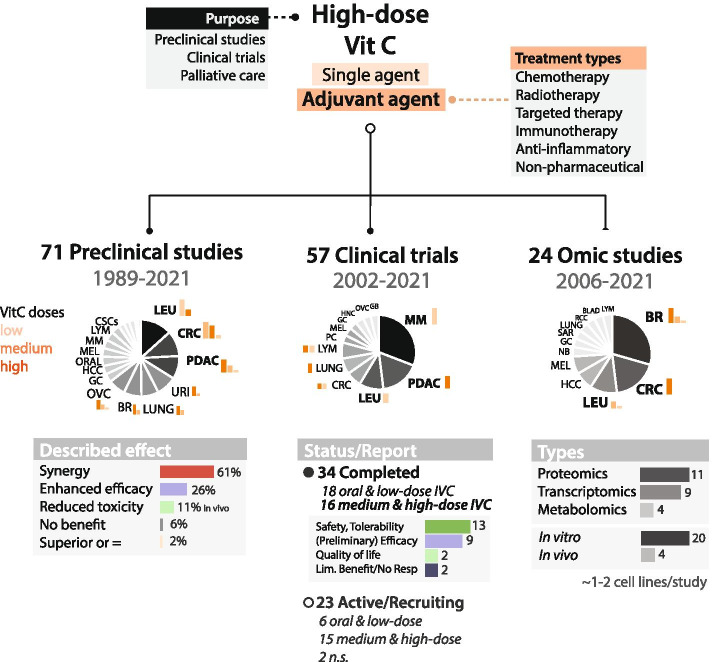
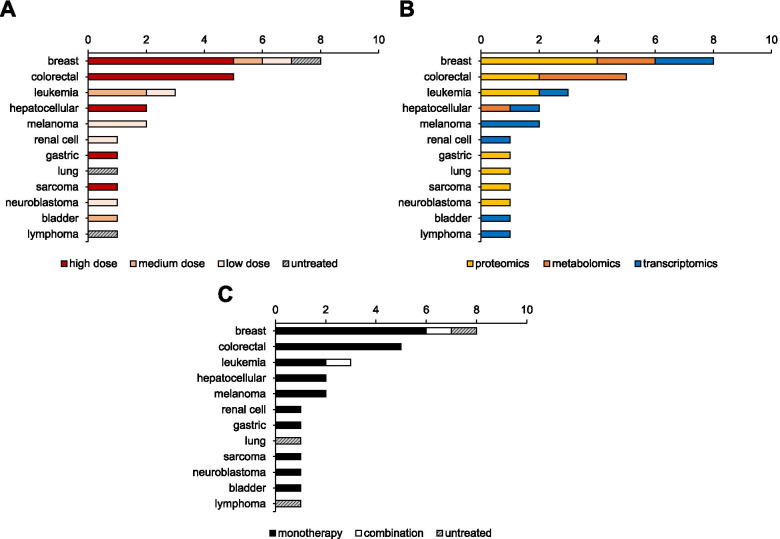
Study overview of pre-clinical, clinical and omics studies using high-dose VitC as anti-cancer agent. Estimated bar graphs of most represented cancer types VitC doses are shown in orange and include high dose (≥ 1 mM in vitro or 1 g/kg in vivo and clinical), medium dose (≤ 0.5 mM in vitro), and low dose (≤ 0.1 mM in vitro,< 1 g/kg in vivo, ≤ 10 g whole body dose clinical). Less represented tumour types are further described in Tables 1, 2, 3 and 4, where oral doses are also included if applicable. Described effect in pre-clinical studies is expressed by percentage of the total number of studies. Reported results in completed clinical trials are expressed by number of studies. Number of studies per global molecular profiling type are also indicated. Omic results include n = 20 in vitro and n = 4 in vivo studies
Fig. 2.
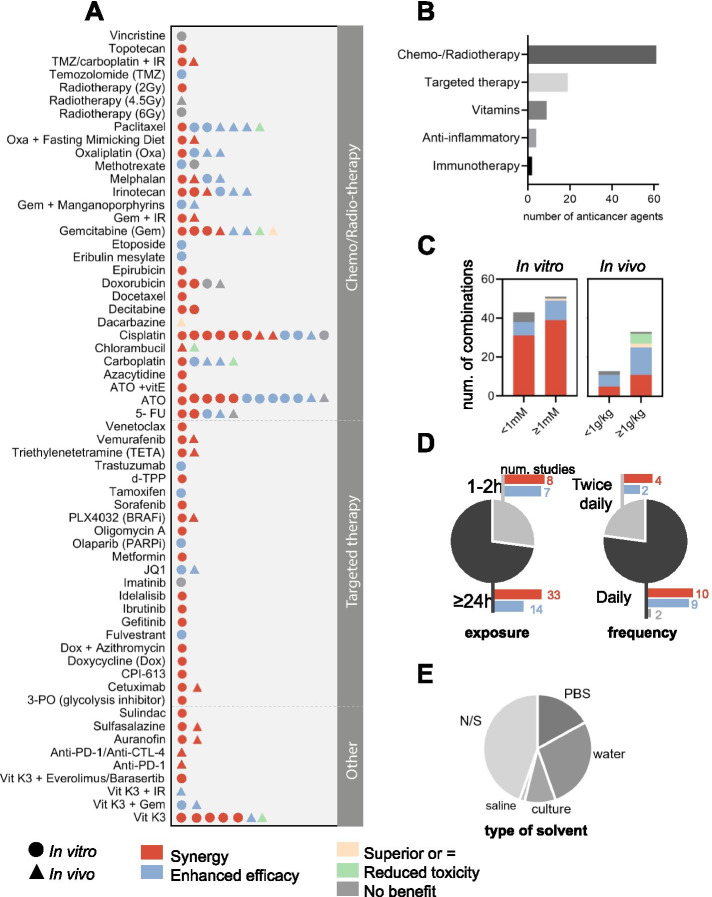
Use of high-dose VitC as adjuvant agent in combination with anti-cancer agents. A Described effect of 59 anti-cancer agents combined with high dose vitC investigated in a total of 71 pre-clinical in vitro and in vivo studies (updated may 2021) describing synergy, enhanced efficacy, superior or equivalent effect, reduced toxicity and/or no benefit. B Number of combinations per treatment type. C Described effect per dose group in vitro and in vivo. D Treatment exposure in vitro in hours and frequency dosage in vivo. E Described solvent used for VitC preparation. Use of water stands for MiliQ water, demi water and sterile water; N/S, not specified
Fig. 3.
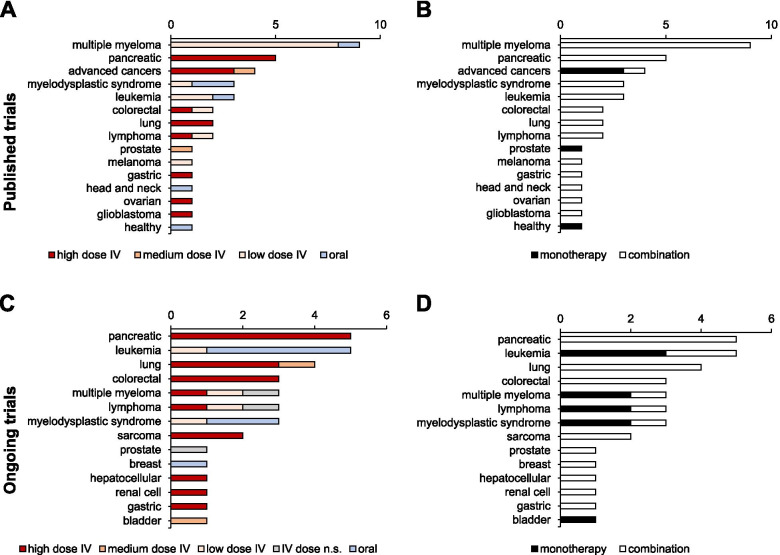
Cancer types investigated in 34 published and 23 ongoing (status February 2021) VitC clinical trials. Annotated are VitC dose group (A and C; high dose ≥1 g/kg, low dose ≤10 g whole body dose) and treatment type (B and D). See Table 2 (medium-to-high-dose published trials; 16/34 of total published trials) and Table 3 (medium-to-high dose ongoing trials; 16/23 of total ongoing trials) for details
Pre-clinical combination studies
A comprehensive overview of all 71 retrieved studies from 1989 to 2021 (Fig. 1), investigating 59 combinations, is shown in Fig. 2, while the 44 studies of the last 5 years are summarized in more detail in Table 1. A division can be made between the highly studied combination with chemotherapy and radiotherapy, the lesser studied with targeted therapies, combinations with immune therapy, which has only more recently gained awareness, and with non-conventional anti-cancer agents (Fig. 2B).
Pre-clinical studies using VitC in combination with chemotherapy and radiation therapy
In pre-clinical models, high-dose VitC is reported to enhance the effectivity of a wide variety of chemotherapeutics such as carboplatin [63, 89], cisplatin [51, 89, 100–103, 179, 180], chlorambucil [181], 5-FU [88, 89, 182], gemcitabine [14, 110, 183, 184] and temozolomide [16, 54] in various cancer cell types, often in a synergistic manner or by enhancing treatment efficacy (Table 1 and Fig. 2).
For example, a recent in vivo study in oral squamous carcinoma described an enhanced therapeutic effect of cisplatin in combination with high-dose VitC (4 g/kg IP twice daily) [51]. A study in pancreatic cancer showed that gemcitabine given in combination with high-dose VitC (4 g/kg IP twice daily) achieved significant tumor growth inhibition in mice bearing pancreatic xenografts compared to control and gemcitabine-only groups [14].
Similarly promising, high-dose VitC has also been found to act as a radio-sensitizer during radiation or chemo-radiation of pre-clinical cancer models, with high specificity for cancer cells over healthy cells [16, 87, 89, 110, 111, 185–190].
A notable example is the study of Schoenfeld et al. [16], which investigated combinations of standard cisplatin chemotherapy with VitC in NSCLC and standard temozolomide and radiation in GBM. To this end, they studied cell line models, performed in vivo studies and a phase I/II clinical trial. Mice injected with high-dose VitC (4 g/kg IP daily) in combination with radio-chemotherapy (5 mg/kg carboplatin weekly, 12 Gy IR/2 fractions (fx)) significantly increased overall survival (~ 50% increase), sensitizing these hard-to-treat NSCLC and GMB tumours to current treatment regimens. Similar results in gastric cancer were described by O’Leary et al., whereby high-dose VitC (4 g/kg IP daily) was injected in combination with carboplatin (15 mg/kg weekly), paclitaxel (10 mg/kg) and 2Gy IR/8fx [89]. An important consideration for pre-clinical combination studies is the clinical standard of care onto which VitC is added, as exemplified by a study in GBM [191] that demonstrated faster tumor progression in tumor-bearing mice treated with a single dose of radiation and daily high-dose ascorbate than in those treated with radiation alone. Here, the authors use a single 4.5 Gy irradiation dose, which does not relate to standard treatment of care in GBM patients who receive daily fractions up to a total of 60 Gy. In addition, the relatively seen lower ascorbate dose of 1 or 2 g/kg compared to the 4 g/kg applied in the GBM study by Schoenfeld et al. [16], possibly promoted VitCs radio-protective rather than radio-sensitizing properties.
Finally, in addition to its enhancing effects in conventional cytotoxic therapies, numerous animal studies have shown decreased off-target toxicity of (chemo-) therapeutic agents following administration of OC and IVC [192]. In this review, Carr and Cook reported that VitC administration typically decreases white blood cell loss, weight loss, ascites accumulation, hepatotoxicity, reticulocytosis, lipid oxidation and cardiomyopathy induced by the chemotherapeutic agents.
Pre-clinical studies using VitC in combination with targeted therapy
A great number of pre-clinical studies have examined the use of high-dose VitC combined with targeted therapies such as kinase inhibitors (i.e. sorafenib, gefitinib, vemurafenib) [109, 116, 123], mitochondrial inhibitors (i.e. doxycycline, venetoclax, oligomycin A, metformin) [104, 106, 107], poly ADP ribose polymerase (PARP) inhibitors [193] and glycolysis inhibitors [194].
Overall, most of the retrieved pre-clinical studies reported synergistic effects in vitro and/or in vivo (Fig. 2A), warranting clinical studies. For instance, an in vitro study showed synergistic anti-cancer action of high-dose VitC in combination with sorafenib, a multi-kinase (eg. Raf-1, B-Raf, VEGFR-1-3 and FLT3) inhibitor, in hepatocellular carcinoma (HCC) cells, and additionally reported a case of prolonged regression of a HCC patient upon combination treatment with IV high-dose VitC and sorafenib [116]. Other studies have reported similar synergistic effects for high-dose VitC combined with EGFR inhibitors cetuximab and gefitinib in KRAS mutated colon cancer and NSCLC cells respectively [99, 109]. Interestingly, Jung et al. [99] showed that medium-dose VitC (0.5 g kg− 1) could abrogate cetuximab resistance in vivo and suggested sodium-dependent vitamin C transporter SVCT2 as a potental marker for enhancing efficacy of the combination treatment of VitC and cetuximab in KRAS-mutant CRC patients. Similarly, resistance to BRAFV600 inhibitor vemurafenib was also abrogated by VitC in melanoma in vivo [123]. Recent findings reinforce the promising synergistic effects of VitC with kinase inhibitors such as BRAFV600 inhibitor PLX4032 in thyroid cancer in vivo [64] and with BTK inhibitor ibrutinib and PI3K inhibitor idelalisib in chronic lymphocytic leukemia (CLL) patient-derived cells [104].
Likewise, emerging anti-cancer compounds targeting telomerases, mitochondrial activity or glycolysis also synergize with high-dose VitC. For instance, telomerase inhibitor triethylenetetramine (TETA) in the treatment of breast cancer [122], glycolysis inhibitor 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3-PO) in NSCLC cells [194], respiratory chain complex I inhibitor metformin, ATP synthase inhibitor oligomycin A and Bcl-2 inhibitor venetoclax in CLL patient-derived cells [104].
Furthermore, enhanced treatment efficacy was confirmed for high-dose VitC in combination with several hormonal treatments such as oestrogen receptor ER and human epidermal growth factor receptor 2 (HER2) inhibitors in breast cancer cells [108], as well as for PARP inhibition in the treatment of AML-TET2 deficient cells [22] and JQ1 (thieno-triazolo-1,4-diazepine), a Bromodomain and extraterminal inhibitor, in the treatment of melanoma [36].
Finally, three recent in vitro studies indicate that high-dose VitC might be of use in eradicating cancer stem cells (CSC) by synergistically targeting mitochondria and causing cell death combined with several targeted agents [106, 107, 121].
All data combined strongly emphasizes the potential of high-dose VitC as adjuvant therapy for targeted therapies.
Pre-clinical studies using VitC in combination with immunotherapy and anti-inflammatory compounds
Little research has been conducted on high-dose VitC in combination with immunotherapy. Two very recent studies show that high-dose VitC synergizes with immune checkpoint inhibitors (ICI) anti-PD-1 and anti-CTL-4 in mouse models, as well as increases the immunogenicity of effector T cells [90, 91]. For instance, Luchtel et al. [90] pre-treated lymphoma cells co-cultured with CD8+ T cells derived from healthy donors with 1 mM VitC. Interestingly, they described a significant 15–21% increase in immunogenicity compared to non-VitC treated cells.
In combination with ICI, high-dose VitC affected tumour growth in a T cell–dependent manner, by attracting effector T-cells and not T regulatory cells. Importantly, in a few mice, complete regressions were observed and mice also acquired immunity after re-injection of tumour cells [91]. Of note, mismatch repair deficient tumours, usually resistant to ICI, showed a very effective response when combined with high-dose VitC. In addition, upon high-dose VitC administration, not only CD8+ T cells, but also macrophages showed increased tumour infiltration, and both enhanced Granzyme B production by cytotoxic T cells and enhanced interleukin 12 production by antigen-presenting cells were observed. These studies are particularly encouraging given the great potential of immunotherapy in anti-cancer treatment, and suggest that high-dose VitC may be a promising combination strategy to convert “cold” tumours into “hot” tumours, further widening the therapeutic scope of immunotherapy.
Furthermore, high-dose VitC strongly enhanced anti-cancer effects of immunosuppressor auranofin in the treatment of triple-negative breast cancer in vitro and in vivo [97]. Similarly, anti-inflammatory compounds such as sulindac [118], sulfasalazine [117] and methotrexate [195] showed strong synergy and enhanced efficacy in the treatment of colon, prostate and liver cancer, respectively.
Pre-clinical studies using VitC in combination with emerging non-pharmaceutical therapies
High-dose VitC has also been combined with other less conventional regimens. One study reports the synergistic effect of fasting-mimicking diet and oxaliplatin in combination with high-dose VitC against KRAS mutated cancers both in vitro and in vivo [114].
In addition, several studies reported synergism of anti-cancer effects of vitamin K3, also known as menadione, combined with VitC in vitro [21, 196–199]. Moreover, one in vivo study found that the combination of these vitamins reduced tumor growth and tumor metastasis in Lewis lung carcinoma [59]. In addition, this vitamin combination was also reported to be synergistic with mTOR inhibitor everolimus and aurora B kinase inhibitor barasertib [124] and sensitized human urothelial tumors to gemcitabine [200] and various solid tumors to radiotherapy in vivo [201], mainly causing cell death upon oxidative stress [202].
Technical considerations and need for standardization
To deduce best practices, we further evaluated dosing schedules, duration of treatment and solvents used in the pre-clinical studies (Table 1, Fig. 2B-E).
First, the type of solvent used for preparing VitC solutions significantly varies, water being the preferred one, followed by phosphate-buffered saline (PBS), culture media -for in vitro studies- and saline -for in vivo studies- (Fig. 2E). Notably, almost 45% of studies did not report the type of solvent used in their methods section. Likewise, most of the studies did not indicate the use of seal to prevent oxygen and light interaction, nor pH range used. In light of VitC chemistry and stability, these are important considerations that should be standardized to get reproducible and robust results [16, 203, 204].
Since VitC effect is dose-dependent, we examined the effect among different dose groups, ≥1 mM vs. < 1 mM in vitro and ≥ 1 g/kg vs. < 1 g/kg in vivo (Fig. 2C). For in vitro studies, a synergistic effect was reported in 80% of all cases and 20% showed enhanced efficacy. Given that 2D and 3D cell culture cannot fully reproduce physiological conditions, in vivo studies provide added value for clinical studies. For in vivo IP injections, synergism was reported two times more often in the studies that used a higher dose ≥1 g/kg, as compared to lower dose < 1 g/kg. Importantly, for the dose group ≥1 g/kg VitC, superior VitC effect [37] as well as reduced toxicity were described [57, 63, 110, 181]. For the dose group < 1 g/kg, several examples that show no added benefit on top of chemotherapeutic agents or even an antagonistic effect were reported [88, 123, 205], highlighting the importance of choosing proper VitC pharmacological doses in vivo, preferably ≥1 g/kg IP, thus reaching sufficient plasma levels to display its anticancer properties [55].
Treatment duration in vitro and frequency in vivo was examined in a similar manner (Fig. 2D). In in vitro studies, cell lines were exposed for long (24-96 h) or short (1–2 h) periods in 74 and 26% of the cases, respectively, generally depending on the type of assay and combination treatment. Although synergism was mostly reported in both cases, short exposures (1–2 h) with a media refresh step are usually preferred to better mimic the physiological conditions in patients [16, 38, 203]. For instance, VitC’s capacity of pH-dependent auto-oxidation and the presence of catalytic metals, such as iron and copper, usually common in cell culture media, can simultaneously increase H2O2 production and impair reproducibility in vitro [206–208]. In order to further improve reproducibility, a dosing per cell scheme has been shown to correct for H2O2 toxicity and accumulation in the media [16, 209] (own observations, unpublished data). In conclusion, and in line with its 2 h half-life in patients, in vitro studies should be carried out thoroughly considering ascorbic acid chemistry with recommended experimental conditions such as avoiding catalytic metals in culture media, using a dosing per cell metric scheme and a 2 h treatment with a media refresh step [13, 126, 156].
In vivo, frequency of high dose VitC was reported as daily in the majority of studies (n = 21), as well as twice daily (n = 6) and twice per week (n = 1). All frequency schedules induced enhanced co-treatment efficacy and synergism in a similar manner. Furthermore, in many studies it was unclear whether combination treatments were co-administered or added in a particular sequence. Altogether, what was clear is that successful in vivo studies used ≥1 g/kg IP VitC mostly on a daily basis with a treatment duration ranging from 2 to 8.5 weeks and a median of 3.5 weeks.
It is noteworthy that most of the in vivo studies use ascorbate-synthesizing models, whose human-mimicking features may be questioned. Contrary to humans, mice can synthesize their own VitC, possibly making them suboptimal models for the evaluation of VitC’s anti-cancer effect [55, 210]. As an alternative model, VitC-deficient mice (i.e. Gulo−/− mice) have recently been used to study VitC in cancer as reviewed by Campbell and Dachs [55]. Nevertheless, the different routes of administration and dose ranges from different studies make these two models difficult to compare. Some data suggests that the μM-range VitC basal concentrations in plasma of ascorbate-synthesizing mice (< 100 μM), similar to plasma VitC levels in (healthy) humans with normal dietary VitC uptake, may have only minimal effects on high-dose (mM-range) VitC tumour killing [211–213]. However, considering the low to scurvy-like levels (often < 10 μM) of plasma VitC in many cancer patients 213–215], the use of VitC-deficient mice may be preferred to allow researchers to better fine-tune physiological cancer conditions [56, 213, 216]. An additional remark is that tumour ascorbate levels, instead of plasma levels, might be more relevant to monitor treatment outcome. Direct evidence addressing these issues may help to better evaluate VitC anti-cancer properties and pave the way for promising and robust clinical trials.
Clinical studies on IVC in combination treatments
Encouraged by the promising results of the pioneering clinical & pre-clinical studies, several phase I and some phase II clinical trials have analysed the use of pharmacologically dosed VitC in combination therapy with conventional cancer treatment agents. A Pubmed database search was performed using search terms “ascorbate OR vitamin C AND cancer AND clinical trial”. In total, 34 completed studies were identified (Fig. 3), 16 of which studied medium-to-high dose IVC (Table 2), and 4 focused on IVC monotherapy specifically, as was discussed in earlier sections of this review. In general, these clinical combination studies have focused on a limited number of cancer types, those including high-dose VitC mainly concerning pancreatic cancer, and lower pharmacological doses mainly concerning non-solid tumors (Fig. 3A). An additional search of the clinicaltrials.gov database using search terms vitamin C or ascorbic acid, and cancer, did not reveal any additional trials that were completed with reported results. Many studies were terminated due to a change in standard of care or, more often, because of poor accrual. The large majority of published studies were carried out with only a limited number of patients, and to date, no large-scale, double blind randomized trials that are imperative in determining the clinical efficacy of IVC have been completed. Having said that, 23 clinical trials, including one phase III study, are currently underway, recruiting patients of several cancer types to investigate the effects of adding IVC in a variety of cancer treatment settings. Sixteen of these ongoing studies use medium-to-high dose IVC, and are reported in Table 3.
Most of the clinical studies presented in this section dose-escalated VitC to achieve ≥20 mM plasma ascorbate concentrations. In general, this was achieved when administering 75 g infusions at least 3 times weekly, and was not significantly further increased at 100 g or more [14, 16, 110]. For those studies administering per kg of body weight, amounts ≥1.0 g VitC/kg [151] were needed to achieve plasma levels of at least 20 mM. We focus in detail only on those studies administering ≥1.0 g/kg or ≥ 75 g (high dose) and ≥ 10 g whole body dose (medium dose).
Clinical studies combining chemotherapy and radiation therapy
The most studied combination treatment using high-dose IVC is together with chemo- and/or radiotherapy (RT) regimens. Eight such studies were identified, of which half were in conducted in the pancreatic cancer setting (Table 2). As with VitC monotherapy, all studies reported favourable toxicity profiles, with 2 randomized trials specifically observing substantially decreased toxicities compared to control arms without IVC [63, 148], although results of latter study are reported as abstract only without showing data. Both studies administered 75–100 g IVC, Ma et al. [63] 2 times a week for 12 months (of which the first 6 months in conjunction with chemotherapy) and Bruckner et al. [148] 1–2 times per week (with GFLIP every 2 weeks until progression). Compared with RT + temozolomide (TMZ) therapy in a single group study in glioblastoma, the addition of IVC possibly provided a protective effect on hematologic toxicities as judged eg. by incidences of thrombocytopenia reported for similar treatment regimens without IVC in other studies [129]. Importantly, Polireddy et al. [14] found no clinically significant influence on gemcitabine pharmacokinetics, suggesting combination treatment is not detrimental to the mechanism of action of standard of care chemotherapies.
Consistent with positive data obtained from animal and other pre-clinical studies, several of these phase I/II studies reported trends towards increased disease control and objective response rates, although all were underpowered for detection of efficacy. In the randomized trial of Ma et al. [63] in ovarian cancer [63], the median time for disease progression was 8.75 months longer with ascorbate addition to standard chemotherapy (carboplatin and paclitaxel) than in chemotherapy alone. Single group studies showed favourable OS and PFS compared to historical controls [82, 111, 129] and institutional averages [110].
Encouragingly, 2 randomized phase 2 trials are currently ongoing in pancreatic (NCT02905578) [137] and prostate (NCT02516670) [140] cancer patients, directly comparing the added benefit of high-dose IVC to standard chemotherapy. Additionally, 7 single group phase 1 and/or 2 trials studying the combination of high-dose IVC with chemo- and/or chemoradiotherapy are currently underway, among others in lung (NCT02420314 and NCT02905591) [133, 134] and pancreatic (NCT03410030) [139] cancer patients.
Clinical studies using VitC in combination with targeted therapy
Three non-randomized clinical studies administered targeted agents on top of chemotherapy and high-dose IVC [151, 153, 155]. Indications of some efficacy were observed in metastatic stage IV pancreatic cancer patients receiving gemcitabine and erlotinib together with IVC [153], with 8/9 patients showing tumour shrinkage after only 8 weeks of treatment. A similar study by Welsh et al. [82], whereby IVC was combined with gemcitabine only, reported similar positive effects, with 6/9 evaluable patients maintaining or improving their performance status. Median overall survival in both studies was 182 days and 13 months, respectively.
Wang et al. [151] combined IVC at 1.5 g/kg once daily for three consecutive days with mFOLFOX6 or FOLFIRI with or without bevacizumab in a 14 day cycle in advanced colorectal and gastric cancer patients (treatment was continued for 12 cycles, disease progression, unmanageable toxic effects, or withdrawal of consent). Besides a favourable safety profile, potential clinical efficacy was observed. Specifically, 14/24 evaluated patients showed PR (objective response rate, ORR, 58.3%) and 9/24 SD (ORR 37.5%), giving a disease control rate of 95.8%. A promising observation was the comparable efficacy in patients with wild-type and with mutant RAS/BRAF tumors. Encouraged by these positive results, this study has since been extended to a randomized phase 3 trial, with an estimated enrolment of 400 mCRC patients (NCT04516681, see Table 3) [131]. To date, this is the only phase 3 trial studying high-dose IVC in anti-cancer treatment.
Ten grade 3 or higher adverse events were reported in the 14 pancreatic cancer patients enrolled in the Monti et al. [153] study, all of which are frequently observed in pancreatic cancer disease progression and/or gemcitabine and erlotinib treatment and thus not likely to be linked to concomitant IVC application. Among the 36 patients enrolled in the Wang et al. study [151], 8 grade 3 or higher adverse events were registered, among which the most common was neutropenia (5 cases), again most likely attributable to the chemotherapy scheme. Likewise, none of the adverse reactions registered in the Kawada et al. [155] study (neutropenia, anemia, and thrombocytopenia) were likely to be directly attributable to IVC treatment.
While all these completed trials studied combinations of chemo- and targeted therapies only, 3 ongoing trials are now investigating the addition of IVC to targeted agents only (eg. in lung cancer patients in randomized trial NCT03799094) [144].
Clinical studies using VitC in combination with emerging non-pharmaceutical therapies
Finally, one randomized phase II trial compared a combination of high-dose IVC plus modulated electrohyperthermia (mEHT) with best supportive care (BSC) to BSC alone in advanced stage NSCLC patients. Not only quality of life but also PFS and OS were significantly prolonged in the IVC/mEHT arm (PFS: 3 months vs 1.85 months; OS: 9.4 months vs 5.6 months) [157], suggesting this treatment combination may be a non-toxic way of improving the prognosis of patients with advanced NSCLC. Except for one case of grade 3 diarrhea in the active arm (49 patients), the overall adverse effects of IVC and mEHT were marginal.
Anti-cancer mechanisms
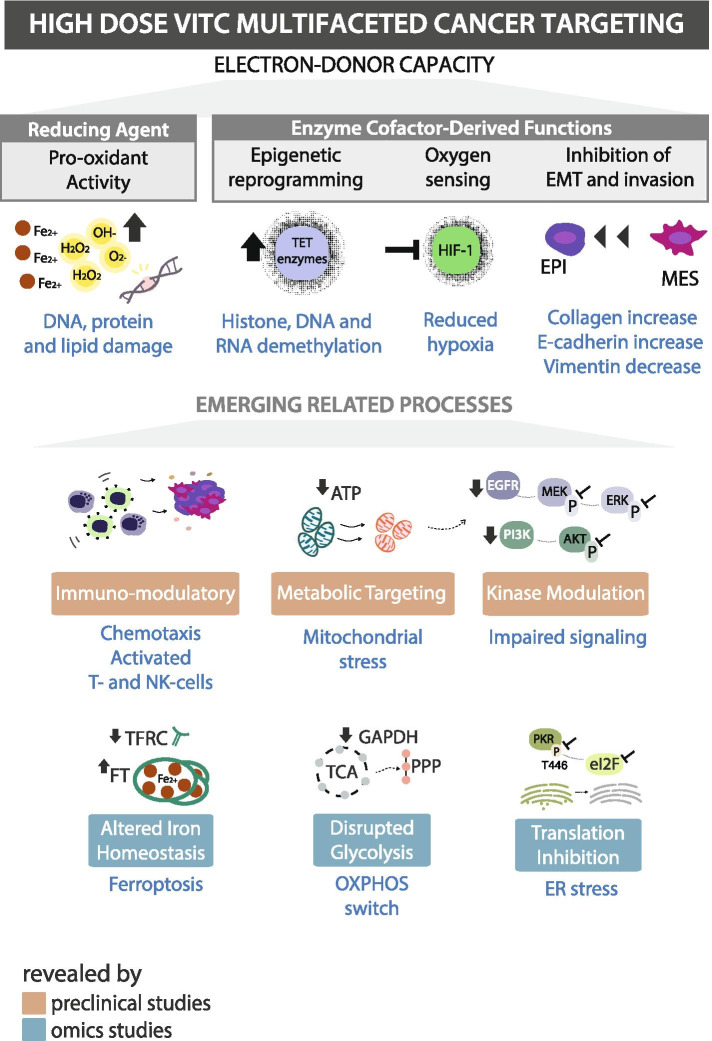
The most widely described mechanism by which VitC is cytotoxic to cancer cells in a selective manner is its pro-oxidant facet, which targets redox imbalance. More recent studies have reported additional mechanisms such as epigenome regulation, oxygen-sensing, immunomodulatory functions, epithelial-to-mesenchymal transition and kinase activity regulation [1, 2, 5, 60, 64, 99, 109, 217, 218] (Figs. 4 and 6). Pre-clinical studies studying VitC in combination with other anti-cancer agents have also contributed significantly to the insight into the potential mechanisms of action (MoA) of VitC. By collecting the described MoA from experimental studies dating from 2016 to 2021, we provide an overview of the various cancer modulatory effects that underline VitC as a multi-targeting agent in relation to the treatment (Fig. 4). In total we identified 14 described effects, of which 7 were recurrent (described more than six times). We also generated an up-to-date comprehensive overview of the multi-faceted targeting effects of VitC in the treatment of cancer (Fig. 6).
Fig. 4.
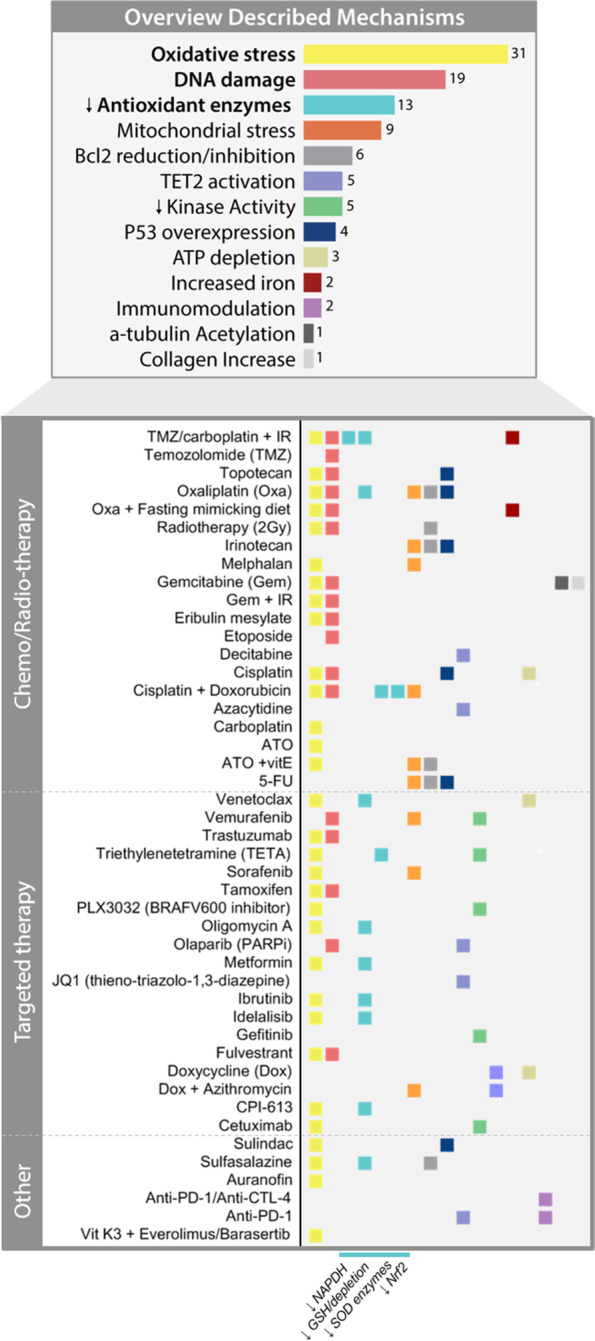
Mechanisms of action described for high-dose VitC in combination with anti-cancer agents in pre-clinical studies. Summary of anti-cancer VitC effects described in vitro and in vivo studies for a total of 45 combinations in the last 5 years (2016–2021). Detailed mechanism of actions per anti-cancer agent are described below. Colour legend corresponds to each mechanism described
Fig. 6.
Overview of high-dose VitC multifaceted cancer effects investigated in pre-clinical and omic studies. Schematic representation of the four most known high-dose VitC modulatory effects in cancer cells and the recently concomitant emerging mechanisms
Pro-oxidant activity
High concentrations of VitC act as a pro-oxidant, eliciting hydrogen peroxide–dependent cytotoxicity in cancer cells without adversely affecting normal cells [15]. This mechanism is based on VitC redox capacity of metals, such as iron or copper, both generally abundant in tumour cells and involved in important enzyme catalytic activities [219–222]. For instance, reduction of iron from Fe3+ to Fe2+, known as Fenton reaction, allows the formation of oxygen radicals such as hydrogen peroxide.
In brief, high dose VitC acts as a pro-oxidant in cancer cells; however, in normal cells its anti-oxidant properties are prevalent [2, 54, 63, 181, 223]. One of the causes of cancer cells being more susceptible to high-dose VitC is their increased level of labile iron (Fe2+ iron amenable to exchange between reactions), which reacts with H2O2 to form the damaging hydroxyl radical (OH•) [224]. Along with increased iron levels, cancer cells generally have a higher metabolic rate than healthy cells and an abundance of defective mitochondria, leading to endogenously higher oxidative stress levels [16, 225–227]. Moreover, cancer cells generally lack catalase activity, making them extra vulnerable to oxidative stress [2, 228–230]. These anti-cancer effects can be abolished by adding the main detoxifying enzyme catalase to the medium, underscoring a role for H2O2 [231].
In addition, cancer cells exhibit increased expression of GLUT1. This transporter can also mediate uptake of oxidized VitC (dehydroascorbic acid, DHA) which is reduced back after uptake by the cell, resulting in depletion of intracellular antioxidants such as glutathione (GSH), nicotinamide adenine dinucleotide phosphate (NAPDH) and SOD enzymes, thereby further increasing reactive oxygen species (ROS) levels in cancer cells [32]. Importantly, these anti-cancer effects have been widely reported as synergistic when combining VitC with targeted therapies (Fig. 2).
Therefore, further increasing oxidative stress is an important anti-cancer strategy which also underlines the effectiveness of cytotoxic therapies such as chemo- and radiation therapy.
Many studies have clearly shown that redox functions of VitC are dose-dependent; acting mainly as an anti-oxidant at normal plasma concentrations that range from 30 to 80 μM, and acting as a pro-oxidant in pharmacological concentrations 0.5-20 mM by increasing ROS (i.e. H2O2 and O2−) [1, 232]. High-dose VitC thus leads to ROS formation and thereby targets redox imbalance, which results in DNA, protein and lipid damage of cancer cells [15, 30, 38]. In combination with chemo/radiation therapies, ROS increase, DNA damage, reduction of antioxidant barriers (eg.. SOD2, Nrf2, NAPDH, GSH) and mitochondrial stress were the most reported MoA (Figs. 4 and 6), which may explain the notorious synergistic effect with VitC (Fig. 2).
In addition, four pre-clinical studies reported overexpression of P53 when VitC was combined with chemotherapeutics such as topotecan, oxaliplatin, irinotecan, cisplatin and 5-FU, as well as with anti-inflammatory compound sulindac [88, 101, 118, 120]. Notably, overexpression of P53 gene is known to play a key role in reducing oxidative stress levels by, for instance, mediating enzyme activity of known ROS scavengers glutathione peroxidase (GPX) and aldehyde dehydrogenase (ALDH) [233]. These findings suggest P53 may be involved in VitC-mediated cytotoxicity.
Interestingly, in a study in thyroid cancer, ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways has been shown to mediate cancer cytotoxicity in vivo [49]. The synergy between kinase inhibitors and high-dose VitC can be partly explained by increased redox imbalance, considering recent data showing that kinase inhibitors induce synergistic toxicity with low-dose H2O2 in colorectal cancer cells [234].
Similarly, a remarkable kinase modulator effect was observed in several studies, mostly by reducing phosphorylation levels of ERK, BRAF and AKT [64, 99, 109, 122, 123] (Figs. 2 and 4). This effect might position VitC as a promising alternative to kinase inhibitors in the treatment of cancer.
In addition, the effect of glycolysis inhibitors may also be enhanced by high-dose VitC in a ROS-dependent manner, since both inhibitors increase oxidative stress levels [235]. The efficacy of combining VitC with immunosuppressor auranofin can also be partly ascribed to redox imbalance targeting, since auranofin was shown to induce intracellular accumulation of H2O2 generated by VitC [236]. Clearly, redox imbalance is a major target involved in the specific anti-cancer activity induced by high-dose VitC.
Co-factor activity
As mentioned previously, VitC acts as a reducing agent of iron, crucial for Fe-containing protein function. These iron sequestering enzymes are involved in numerous metabolic processes such as the mitochondrial respiratory chain (i.e. cytochrome C, NADH-ubiquinone reductase or complex I), synthesis of collagen (prolyl oxygenase) and oxidative stress regulation (i.e. catalase, peroxidases) [237].
Along with its pro-oxidant function, VitC-mediated cytotoxicity toward cancer cells has also been explained by the 1. regulation of collagen synthesis, 2. hypoxia inducible factor (HIF) proteasomal degradation and 3. TET activity regulation.
Collagen synthesis, EMT and invasion
Regulation of collagen synthesis is key for hampering cancer progression. The concept of counteracting decreased collagen synthesis and thereby targeting a potential metastatic vulnerability in cancer by using VitC was first proposed by William McCormick over 60 years ago [238, 239], and subsequently extended by Ewan Cameron [240]. One of the major components of the extracellular matrix are collagen fibrils, which are formed by strong collagen tertiary structures. VitC is known to stabilize these strong cross-links, preventing neoplastic invasion [241, 242]. As mentioned in previous sections, recent pre-clinical studies [14, 33, 39, 43, 58–61] and case report studies [67–72, 243, 244] have shown a significant decrease or depletion of metastasis, and complete tumour regression of advanced or metastatic disease, respectively. Interestingly, Polireddy et al. [14] showed that metastatic reduction in pancreatic cancer was correlated with increased stromal collagen levels in vivo. In their phase I/IIa study, they also found increased collagen levels in a patient who became suitable for tumour resection after 70 doses of IVC (100 g/infusion) and 9 cycles of gemcitabine, compared to untreated, FOLFIRINOX or gemcitabine-treated patients [14].
Another described mechanism by which VitC targets cancer invasion is the reversion of epithelial-to-mesenchymal transition [60, 245]. Zhao et al. [245] reported VitC to inhibit the proliferation, migration and epithelial-mesenchymal-transition of lens epithelial cells through deactivating hypoxia inducible factor. Moreover, Zeng et al. [60] showed a reduction of vimentin and an increase of E-cadherin levels upon high-dose VitC, thereby suppressing EMT and inhibiting cell migration and invasion in breast cancer in vitro and in vivo.
In light of collagen synthesis activation, EMT reversion and invasiveness inhibition, high dose VitC could be an effective solution for the prevention and treatment of advanced disease.
Oxygen-sensing
Many solid tumours become hypoxic when their growth outruns the emergence of new blood vessels around it. To ensure their survival, tumour cells in turn activate the transcription factor HIF-1 [246, 247].
VitC regulates location and function of HIF hydroxylases, which deactivate HIF-1 by ultimately targeting it to proteasomal degradation and thereby suppressing tumour growth [1, 2, 248–250]. In particular, Fischer and Miles [248] showed that VitC was able to decrease the malignant potential of melanoma by hampering HIF-1α activity, and Kawada et al. [155] showed a downregulation of HIF-1 upon high-dose VitC in human leukemic cells in vitro and in vivo. Jóźwiak et al. [249] also found a negative correlation between HIF-1α mRNA expression and VitC levels in human thyroid neoplastic lesions, suggesting that VitC may also interfere with HIF-1 transcriptional activity. Additional pre-clinical [251, 252] and clinical [253, 254] work by Kuiper and colleagues confirmed this inverse relationship between HIF-1 activity and tumor ascorbate levels. For instance, in their human colorectal cancer study [253], higher levels of tumour VitC were inversely correlated with HIF-1 pathway activation and with a significantly improved disease-free survival. Besides this HIF regulatory function, hypoxia is a common phenomenon in tumour cells and not in normal cells, which increases cancer cell susceptibility to VitC [255].
Given the important role of hypoxia in cancer survival and its well-known implications for treatment resistance, VitC-mediated regulation of HIF activity may provide another facet that is key for improving the treatment of solid tumours.
Epigenome regulation
Cancer cells are well known to have aberrant DNA methylation patterns important for survival and tumour progression [256, 257]. Particularly, active DNA demethylation is carried out by the TET enzymes, which are frequently mutated in haematological malignancies. These enzymes are ketoglutarate-, iron- and oxygen-dependent, and belong to the same family as HIF hydroxylases and prolyl hydroxylases crucial for collagen-synthesis as described above.
In the treatment of cancer, high-dose VitC has been shown to induce DNA demethylation by restoring and regulating TET aberrant levels [3]. This anti-cancer VitC role, previously unknown, was widely investigated a couple of years ago in the context of cancer stem cells in leukaemia progression [20, 22]. Sequentially, Vit-C-mediated restoration of TET, also when mutated, enables the re-expression of tumour-suppressor genes in cancer cells [2, 3, 105, 174]. A notable recent study in acute myeloid leukaemia (AML) reported that high-dose VitC activated TET enzymes synergistically with inhibition of mutant isocitrate dehydrogenase 1 (IDH1), resulting in diminished cell growth and increased myeloid differentiation [24].
Vit-C-mediated restoration of TET was also described in four pre-clinical studies combining high dose VitC with chemotherapy [98], targeted therapy [22, 119] and ICI anti-PD-1 [90] (Figs. 4 and 6). Cimmino et al. [22] showed that upon TET2 induced demethylation, high-dose VitC was able to sensitize leukaemia cells to PARP inhibition, mainly due to increased DNA damage.
In addition to TET enzymes, VitC enhances the activity of Jumonji C (JmjC) domain-containing histone demethylases (JHDM) and thereby hinders the aberrant self-renewal of hematopoietic stem cells [3]. Interestingly, these Jumonji histone demethylases are also responsible for epigenetic landscape regulation and for activating cellular responses upon changes in energy metabolism, oxygen and iron levels [219]. In light of the above, VitC can considerably stimulate demethylation in several ways, leading to the re-expression of tumour suppressor genes, and thereby greatly interfering with tumour survival as well as sensitizing to other therapeutic agents.
Immune modulatory effects
VitC is maintained at high levels in most immune cells and can affect many aspects of the immune response [258]. The contribution of ascorbate as an antioxidant in immune cells is well-established while its cofactor activity for Fe- or Cu-containing oxygenases is emerging as a key factor in the functional effects on both the innate and adaptive immune responses [5, 219]. This activity requires mM concentrations of VitC, thereby emphasizing the need for a high intake to enable adequate immune function, especially in conditions of inflammation and cancer when VitC often becomes deficient. VitC-dependent processes in immune cells include myeloid and T cell differentiation and polarisation, T cell maturation and activation, B cell development, chemotaxis, cytokine production and enhanced NK cell mediated cancer killing [5]. Interestingly, and linked to the previous section, VitC seems to also regulate the epigenetic profile of immune cells such as by TET activity restoration in iTreg cells, which leads to Foxp3 re-expression and drives proper immune cell function [259].
Furthermore, two very recent pre-clinical studies showed that high-dose VitC synergizes with immune checkpoint inhibitors anti-PD-1 and anti-CTL-4 [90, 91] (Figs. 4 and 6). Importantly, Magri et al. [91] observed the largest anti-cancer effect only when administering high-dose VitC to immunocompetent mice and not to immunocompromised mice [91]. This indicates that its anti-tumour activity is not solely dependent on its pro-oxidant effects, but also substantially on some of its immunomodulatory functions.
Global molecular profiling studies on high-dose IVC in the cancer context
To gain further insights in VitC’s anti-cancer properties on the molecular level, system-wide approaches that capture the complex interplay of various cellular signalling pathways are warranted. Specifically, transcriptomic and especially proteomic studies have the power to capture phenotypic manifestations of genetic alterations. To date, global RNA and protein expression studies on high-dose VitC action are confined to a few cell line studies in specific cancer types. Here, we summarize these studies and their most important findings, considering both studies specifically looking at the global effects of VitC treatment on its own (i.e. without confounding co-treatments), as well as the effects of combining VitC with other (chemo-) therapies (Fig. 5, Table 4).
Fig. 5.
Cancer types studied using global molecular profiling techniques. Annotated are VitC dose group (A; high dose ≥1 mM or 1 g/kg, low dose ≤0.1 mM), type of profiling method used (B) and treatment type (C)
Table 4.
Global molecular profiling studies investigating VitC in the cancer context
| Cancer type(s) | Model system | Methodology | Treatment(s) | Type of combination therapy | VitC dosea | Aim | Omics results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Proteomics | ||||||||
| Colorectal | DiFi (RS and XM Difi) cell lines | SILAC-based MS (LC–ESI–MS-MS) | 4 h and 24 h treatments with 1 mM VitCC and/or 50 μg/mL cetuximab | Targeted | high | Hypothesis that VitC in combination with cetuximab could restrain the emergence of secondary resistance to EGFR blockade in CRC RAS/BRAF wild-type models |
- Identification of 4147 proteins Switch from glycolysis to oxidative phosphorylation in cetuximab and combo-treated cells at 4 h - downregulation of LDHA/LDHB - upregulation of PDHA1/PDHB and respiratory enzymes Perturbation of iron metabolism in VitC and combo-treated cells at 24 h - downregulation of TFRC - upregulation of FT |
[159] |
| Breast | MDA-MB-231 cell line | Biotin switch approach (enrichment of proteins containing oxidized thiols) followed by LC-MS/MS | 30 min treatment with 10 mM ascorbic acid | – | high | Identify early alterations of the redoxome in cellular response to AA that might be linked to AA-induced cell death |
- Identification of 2910 cysteine-containing proteins Oxidized targets upon AA treatment: - antioxidant enzymes (eg. PRDX1) - glycolysis and gluconeogenesis pathway (eg. PGK1) - tricarboxylic acid cycle (eg. ACOT7) - DNA, RNA and protein metabolism Cell cycle arrest and translation inhibition associated with AA-induced cytotoxicity. PRDX1 expression levels correlated with AA differential cytotoxicity |
[160] |
| Breast | MCF7 cell line | LC-MS/MS | 24 h treatment with 2 mM VitC | – | high | Effect of VitC in itself at different concentration levels on MCF-7 breast cancer cell line |
- Identification of 1694 proteins with differential regulation Processes impacted by VitC treatment included - unfolded protein response and inhibition of the cell translation (eIF2α, PKR/PKR pThr-446) - apoptotic process |
[161] |
| Neuroblastoma | SH-SY5Y cell line | SUMO-1 IP followed by ESI-FT ICR MS | 30 min treatment with 100 μM ascorbate (or 100 μM hydrogen peroxide) | – | low | Identify redox sensitive proteins of the conjugation machinery for SUMOylation. Oxidative stress (hydrogen peroxide), antioxidant (ascorbate) or control conditions were tested |
- Identification of 169 proteins - Great overlap between all treatments - Proteins identified only in the ascorbate sample included DTD2 and MGAT5B - Proteins without predicted SUMOylation site indentified in both ascorabte and hydrogen peroxide treatments included TUBB4A, TUBB1, HNRNPH3, POLG2 and BUB3 |
[162] |
| Gastric | AGS cell line | MALDI-TOF MS | 24 h treatment with 300 μg/mL (~ 1.7 mM) VitC | – | high | Investigate the molecular mechanism of the inhibitory effect of VitC on AGS cell growth, and protein profiles in AGS cells after exposure to VitC treatment |
- 20 differential proteins identified - downregulation eg. of TPM3 and TPM4 - upregulation of PRDX4 and TXND5 - Identified proteins are mainly involved in cell mobility, antioxidant and detoxification, signal transduction and protein metabolism |
[163] |
| Leukemia | NB4 cell line | MALDI–TOF | 30 min treatment with 0.5 mM LAA (ascorbic acid) | – | medium | Identification of early protein targets of LAA in leukemia cells |
- 9 differential proteins identified - changes in pI as a result of phosphorylation of a TPM isoform) - downregulation eg of of SUPT6H and HSPA8 - upregulation eg. of MATN4 and NONO |
[164] |
| Sarcoma | BALB/C mice implanted with S-180 cancer cells | MALDI TOF-MS/MS | Treatment with 1.5 mg/g body weight ascorbate every three days | – | high | Identify proteins involved in the ascorbic acid-mediated inhibition of tumor progression |
- 11 differential proteins identified - upregulastion of RKIP and ANXA5 |
[165] |
| Colorectal | BALB/C mice implanted with CT-26 cancer cells | MALDI TOF-MS/MS | Treatment with 1.5 mg/g body weight ascorbate every three days | – | high | Proteome changes of tumor tissue were investigated after intraperitoneal administration of a high concentration of ascorbic acid |
- 18 differential proteins identified - upregulation eg. of EIF3I, NPM1 and VIM - regulation of cytoskeleton remodeling |
[166] |
| Breast | MCF7 cell line | LC-MS/MS | 18 h treatment with 1 μM DOX (doxorubicin) or DOX + 200 μM of VitC | Chemo | medium | Describe the changes in protein expression and proliferation of the MCF-7 cells induced by the VitC applied with doxorubicin |
- Identification of 229 proteins - Downregulation of cytoskeletal (FLNA), ribosomal (eg. RPL27A), transcriptional (eg. HNRNPH1), immune system and antioxidant (HSP90AA1, SOD1) proteins in DOX + VitC-treated cells - Upregulation of GAPDH, GPI and ACTA1 |
[167] |
| Leukemia | HL-60 cell line | LC-MS/MS | 48 h treatment with 10 μM As2O3 (arsenic trioxide) or As2O3 + 100 μM L-AA (ascorbic acid) + 50 μM α-TOC (α-tocopherol) | Chemo + Dietary suppl. | low | Evaluate the synergistic mechanism of action of vitamins, such as L-ascorbic acid (L-AA) and a-tocopherol (a-TOC) in As2O3 chemotherapy |
- Number of identified proteins n.s. - Downregulation of cell cycle and translation in cells treated with As2O3, L-AA, and a-TOC compared to As2O3-only - Identification of numerous proteins associated with apoptosis and cell stress in combination treatment |
[96] |
| Breast, Lung | A549 and MDA-MB-231 cell lines | SILAC-based MS (LC-MS/MS) | untreated (A549 cell line resistant to 1 mM AUF (auranofin) + 2.5 mM VitC, MDA-MB-231 cell line sensitive) | Anti-inflammatory | untreated | Decipher the underlying mechanisms for differential response of lung and breast cancer cell models to redox-modulating molecule auranofin (AUF) and to combinations of AUF and VitC |
- Identification of f 4131 proteins common to both cell lines - proteins involved in GSH synthesis and reduction, the pentose phosphate pathway and those belonging to other metabolic pathways (eg PGDH and PTGR1) more abundant in A549 (resistant) cells |
[97] |
| Transcriptomics | ||||||||
| Melanoma | A2058 cell line | RNA-seq | 48 h treatment with 0.1 mM VitC | – | low | Examined the possible mechanisms that could reveal how VitC suppresses cell migration and anchorage-independent growth of A2058 cells |
- 66 genes differentially expressed - alterations predominantly in genes involved in extracellular matrix remodeling. - ARGHAP30, TRIM63 and PTPN7 among 10 most differential genes |
[168] |
| Melanoma | A2058 cell line | RNA-seq | 7 days treatment with 100 μM ascorbate | – | low | To elucidate potential mechanism of ascorbate in inducing apoptosis in A2058 cells. Re-analyse data of Gustafson et al., 2015 using updated algorithms |
- 344 genes including 20 non-coding RNAs (ncRNA) differentially expressed - expression of CLU gene one of the most downregulated genes |
[36] |
| Breast | MDA-MB-231 cell line | RNA-seq | 3 days treatment with 100 μM VitC | – | low | Analysis of transcriptomic changes associated with increased 5hmC generation following exposure to VitC |
- 778 differentially expressed genes - TNFSF10, TFRC and PGK1 among 10 most differential genes |
[169] |
| Renal Cell | 786-O cell line | RNA-seq | Treatment for 10 passages with 100 μM AsANa (sodium L-ascorbate; VitC) or 100 μM APM (oxidation-resistant VitC derivative) | – | low | Examine ccRCC phenotype changes at the global transcriptome level after treatment of VitC for 10 passages |
- 81 differentially expressed genes - most notable genes positively enriched in VitC-treated cells belong to multiple metabolic pathways, such as peroxisome and pentose phosphate pathways - most notable gene sets negatively enriched in VitC-treated cells include DNA replication and mismatch repair genes |
[170] |
| Bladder | T24 cell line | RNA-seq | 0.25 mM VitC, time n.s. | – | medium | Explore the role of 5hmC in bladder cancer and the therapeutic efficacy of VitC in increasing the 5hmC pattern |
- 1172 differentially expressed genes were identified - differential genes mainly associated with focal adhesion, DNA replication, cell cycle, and several cancer-related pathways. |
[171] |
| Hepatocellular | Huh-7 cell line xenograft tumour mouse model | Microarray | 3 days treatment of mice with IP injection of 4.0 g/kg or 2.0 g/kg ascorbate | – | high | Assess effects of high-dose ascorbate on hepatoma |
- 192 genes/ncRNAs uniquely differentially expressed in HCC tumour tissue obtained from mice treated specifically with high-dose ascorbate (4.0 g/kg/3 days) - deregulated genes were involved in insulin receptor signalling, metabolism and mitochondrial respiration |
[172] |
| Lymphoma | JLPS and JLPR cell lines (sensitive/resistant to ascorbate) | Microarray | untreated (JLPR cell line resistant to VitC (incubation of JLPS cells with increasing ascorbate concentrations from 100 μM to 1 mM over 6 month), JLPRS cell line sensitive) | – | untreated | Identify possible mechanisms of ascorbate resistance | - Acquired ascorbate resistance associated with downregulation of eg. HMGB1 and MYC and upregulation of eg. ATF5 | [173] |
| Leukemia | HL60 and MOLM13 cell lines | RNA-seq | 12 or 72 h treatment with 250 μM L-AA (ascorbic acid) | – | medium | Analyse expression of genes upregulated by Tet2 restoration in cKit+ cells in HL60 and MOLM13 cells treated with L-AA |
- 14/50 genes upregulated by Tet2 restoration in mouse cKit+ cells also induced in both human leukemia lines after 12 h of VitC treatment, including genes involved in apoptotic and death receptor signaling (eg. BAX) and NOTCH signaling - Of the top genes downregulated by Tet2-restoration, 34/50 were downregulated in both leukemia lines after 12 h of VitC - Hence, VitC treatment can enhance TET2 function in human leukemia cells in a manner similar to the effects of Tet2 restoration in mouse HSPCs |
[174] |
| Breast | MCF-7 cell line | Microarray | 3 days treatment with 100 nM RA (retinoic acid) and/or 1 mM AA (ascorbic acid) | Chemo | high | Elucidate the mechanism by which RA + AA inhibits breast carcinoma proliferation |
- 29 genes were up-regulated and 38 genes were down-regulated after RA + AA treatment - up-regulation of antioxidant enzymes (eg. GPX2) and proteins involved in apoptosis (eg. CDK11B), cell cycle regulation (eg. EDN1) and DNA repair (eg. RAD51C) - RA or AA on their own failed to upregulate antioxidant genes |
[175] |
| Metabolomics | ||||||||
| Breast, Colorectal | MCF-7, MDA-MB231 and HT29 cell lines | LC-MS | 4 h treatment with 3 mM ascorbate | – | high | Gain insight into the cellular effects of high doses of ascorbate |
- Metabolic shift, reversal of Warburg effect, disruption of redox homeostasis - Cell death dependent on ascorbate-induced oxidative stress and accumulation of ROS, DNA damage, and depletion of essential intracellular co-factors including NAD+/NADH - disruption of glycolysis, rapid drop in ATP levels - inhibition the TCA cycle and increased oxygen consumption |
[176] |
| Breast, Colorectal | MCF7 and HT29 cell lines | CE-TOF MS | 1 h treatment with VitC (0.2 mM, 1 mM or 10 mM) | – | high | Understand anticancer mechanisms of VitC |
- Levels of upstream metabolites in the glycolysis pathway and TCA cycle were increased in both cell lines following treatment with VitC - ATP levels decreased concentration-dependently - VitC inhibited energy metabolism through NAD depletion, thereby inducing cancer cell death |
[177] |
| Colorectal | HCT116 and VACO432 cell lines | LC-MS/MS | 2 mM VitC for 30 min to 2 h | – | high | Clarify the mechanism by which VitC kills cancer cells while sparing normal cells. Profile metabolic changes following VitC treatment |
- Glycolytic intermediates upstream of GAPDH accumulated while those downstream were depleted suggesting that GAPDH was inhibited - Oxidative PPP metabolites increased, indicating that the blockage may shift glycolytic flux into the oxidative PPP - Cysteine, the major limiting precursor for GSH biosynthesis, was also dramatically depleted following VitC treatment - As expected, VitC treatment induced a substantial increase in endogenous ROS in KRAS and BRAF mutant cells |
[32] |
| Hepatocellular | SMMC-7721 cell line | NMR spectroscopy | 48 h treatment with 50 μmol/L OXA (oxaliplatin) and/or 1 mmol/L VitC | Chemo | high | Assess the global metabolic changes in HCC cells following VitC treatment |
- VitC treatment led to inhibition of energy metabolism via NAD+ depletion and amino acid deprivation - OXA caused significant perturbation in phospholipid biosynthesis and phosphatidylcholine biosynthesis pathways - Glutathione metabolism and pathways related to succinate and choline may play central roles in conferring the combined effect between OXA and VitC |
[178] |
Twenty-four studies were retrieved from PubMed using search terms (vitamin c OR ascorbate OR ascorbic acid) AND (proteomics OR mass-spectrometry OR metabolomics OR transcriptomics OR RNA-seq OR RNA sequencing OR microarray OR genomics OR DNA sequencing OR WES) AND (cancer). a high dose ≥ 1 mM or 1 g/kg, low dose ≤ 0.1 mM
Proteomic studies
A number of proteomics studies have been performed to study VitC effects in cancer cell lines employing 2D gel-based analysis and more comprehensive mass spectrometry-based proteomics (Table 4). Here we discuss the latter studies based on nano-liquid-chromatography coupled to mass spectrometry. Very recently, a large-scale proteomic analysis (SILAC-based mass spectrometry) was performed in KRAS/BRAF wild-type CRC cells (DiFi) treated with either VitC (1 mM) or anti-EGFR agent cetuximab, or a combination of both [159]. Both short (4 h) and long-term (24 h) exposure was analyzed. Among the most striking observations was a downregulation of glycolysis in cetuximab and combo-treated cells at early time-points, while proteins related to iron metabolism, such as ferritin and transferrin receptor TFRC, were respectively up and downregulated in VitC and combo-treated cells at later time-points. Based on these results as well as additional metabolic profiling experiments, the authors proposed a model whereby the cetuximab-induced switch from glycolysis to oxidative phosphorylation makes cancer cells more susceptible to the oxidative stress induced by VitC. Subsequent mobilization of iron pools and induction of ROS-mediated stress by VitC could ultimately lead to membrane lipid damage and cell death. A breast cancer study in MDA-MB-231 cells used a biotin switch approach to enrich proteins containing oxidized thiols, followed by LC-MS/MS, to identify very early (30 min) alterations of the redoxome in cellular response to 10 mM ascorbic acid [160]. Besides antioxidant enzymes (such as PRDX1) and glycolysis- and TCA cycle-related proteins (eg. PGK1) showing a significant increase in oxidation upon ascorbic acid treatment, analysis of this redoxome dataset additionally suggested that translation inhibition may be one of the possible mechanisms responsible for oxidative stress-based ascorbic acid cytotoxicity. Using a label-free proteomic approach, another breast cancer study analysed the long-term (24 h) effect of 2 mM VitC on the proteome of MCF-7 cells [161]. Besides proteins directly related to apoptosis, proteins involved in protein processing in the ER were upregulated upon VitC treatment. Specifically, eIF2α and PKR/PKR pThr-446 were suggested to be responsible for the unfolded protein response and inhibition of cell translation during endoplasmic reticulum stress, which may be a direct result of increased oxidative stress. A study focusing on the conjugation machinery for SUMOylation in response to low dose (100 μM) ascorbate performed SUMO-1 IP followed by ESI-FT ICR MS in neuroblastoma cell line SH-SY5Y [162]. This study identified, among others, DTD2 and MGAT5B, two proteins without predicted SUMOylation site, related to translation and glycosylation, respectively, with increased abundance following ascorbate (but not hydrogen peroxide) treatment.
Concerning the effect of combining VitC with other (chemo-) therapies, an LC-MS/MS study in breast cancer cell line MCF7 cell line [167] showed that combining topoisomerase II inhibitor doxorubicin with medium dose (200 μM) VitC lead to a down-regulation of ribosomal, transcriptional and translational, as well and anti-oxidant (eg. SOD1) proteins. Decreased expression of proteins regulating cell cycle and translation was also found when treating HL-60 leukemia cell line with a combination of low dose (100 μM) VitC, ATO and tocopherol (vitamin E) [96]. A SILAC-based mass spectrometry study examined proteomic changes in 2 cell lines (A549 and MDA-MB-231) with different sensitivities to anti-inflammatory redox-modulating molecule auranofin (AUF) in combination with pharmacological doses (2.5 mM) of VitC [97]. Most notably, high expression levels of metabolic proteins with oxidoreductase activity such as TXNRD1, ALDH3A2 and PTGR1 were linked to cellular resistance to AUF/VitC combinations, in line with increased antioxidant mechanisms counteracting the anti-cancer activities of high-dose VitC.
Transcriptomic studies
Most studies investigating changes in the transcriptome following VitC treatment used doses of less than 1 mM. Three Studies by the same group analysed the effect of 0.1 mM VitC on breast and melanoma cell lines using RNA-sequencing [36, 168, 169]. These analyses revealed, among others, deregulation of apoptotic gene clusterin as well as genes involved in extracellular matrix remodeling in melanoma cell line A2058, as well as increased TNF-related apoptosis-inducing ligand (TRAIL) transcripts in breast cancer cell line MDA-MB-231. Latter study also identified genes related to iron metabolism (TFRC) and glycolysis (PGK1), in line with VitC-induced changes on the protein level observed in the proteomic studies referred to before [159, 160]. Ge and colleagues [170] investigated the effects of long term (10 passages), low-dose (0.1 mM) VitC exposure on renal cell line 786-O, and found that while metabolic processes such as glutathione and pentose-phosphate metabolism were positively enriched, genes related to DNA replication and mismatch repair showed negative enrichment. A similar strong de-regulation of DNA replication-related genes was seen by the same group when treating bladder cancer cell line T24 with medium doses (0.25 mM) of VitC [171]. One notable study focused on the effects of high-dose ascorbate on the transcriptome of Huh-7 cell line xenograft tumour hepatocellular mouse models, as assayed by microarray analysis [172]. Changes in the transcript levels of genes involved in insulin receptor signalling, metabolism and mitochondrial respiration were identified, among which was the upregulation of advanced glycosylation end product-specific receptor (AGER). Possibly related to this are microarray-derived findings on acquired resistance in Lymphoma cell lines by the same group [173]. Here, ascorbate resistant JLPR cells (that were generated by incubation of sensitive JLPS cells with increasing ascorbate concentrations from 0.1 to 1 mM over 6 months) were characterized not only by increased levels of genes such as ferritin, topoisomerase II and glutathione peroxidase 4, but also by the decreased expression of high-mobility group protein box 1 (HMGB1), one of the ligands of AGER. In general, as expected and as seen in several of the proteomic studies, VitC-induced abundancy changes in apoptotic genes are also reported in many of the transcriptomic studies [36, 169, 173–175].
Taken together, both the proteomic and transcriptomic studies identified many known facets of VitC action in cancer cell killing, including apoptotic, redox and metabolic mechanisms, but also revealed less defined roles of ascorbic acid, such as the regulation of cytoskeleton remodeling and the inhibition of translation (proteomics) as well as DNA replication and repair (transcriptomics). The key processes found to be altered in high-dose VitC studies specifically include alteration of iron homeostasis, disruption of glycolysis and inhibition of translation (Fig. 6). In addition, critical proteins involved in these pathways were identified, which may give leads for future (co-) targeting strategies.
Metabolomic studies
Finally, four studies sought out to globally profile metabolic changes induced by high-dose VitC administration in breast, colorectal and hepatocellular cancer cell line models [32, 176–178]. Although length of treatment and experimental model differed per study, all observed a drop in ATP levels and a depletion of NAD following exposure to high-dose VitC, in line with the inhibition of energy metabolism and multifaceted metabolic rewiring described in numerous pre-clinical studies using alternative approaches. In general, glycolytic metabolites upstream of GAPDH were enriched upon high dose VitC treatment, while those downstream were depleted, in line with an inhibition of GAPDH by VitC, ultimately leading to the disruption of glycolysis and TCA cycle also observed in several proteomics studies (Table 4, Fig. 6).
Conclusions and outlook
In their 1979 review “Ascorbic Acid and Cancer: A Review” [242], Linus Pauling and colleagues expressed their hopes that “properly designed controlled trials” would soon be conducted to “confirm or refute” their clinical findings, and that if confirmed, “ascorbate will soon become an essential part of all practical cancer treatment and cancer prevention regimens”. Although this vision has not become reality yet, the growing number of well-designed, high impact pre-clinical and early stage clinical studies are contributing to moving the field of high-dose VitC in the cancer care context forward. In addition, with the rise of global profiling strategies such as metabolomics, transcriptomics and large-scale proteomics leading to further delineation of the mechanisms of action of vitamin C, future clinical trials may be designed based on more refined rationales.
Based on molecular characterization of tumor cells, it is becoming increasingly evident that patient subgroups harbouring certain genetic mutations or overexpressing certain proteins may be particularly susceptible to benefiting from VitC mono- and combination therapies. This holds true for tumors baring KRAS mutations for instance, which are generally difficult to treat, being resistant to targeted anti-EGFR therapy amongst others. In this respect, a further boost for the implementation of high-dose VitC in cancer care is expected to arise from an initiative of the Stand Up to Cancer (SU2C) - American Association for Cancer Research charity program, which is raising money for translational cancer research via broad media awareness campaigns. One of the collaborative projects set up as a result of SU2C funding is the “SU2C Colorectal Cancer Dream Team: Targeting Genomic, Metabolic and Immunological Vulnerabilities of Colorectal Cancer”, which has since opened a clinical trial testing the safety and efficacy of high-dose IV VitC as a treatment for KRAS mutant cancers [73]. Importantly, genome sequencing and RNA expression profiling of the tumors collected in this phase II study are planned, in an attempt to further translate pre-clinical mechanistic insights on VitC action to the clinical setting. It has been shown that VitC selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH [32], which may also explain why VitC shows to be especially promising in the treatment of pancreatic cancer, where over 90% of the cases harbour KRAS mutations [260] and MM, where RAS family genes also show the most frequent mutations [113]. In addition, tumors baring a TET2 or IDH-1 mutation may be especially sensitive to VitC treatment, and this is also true for cancer types having high concentrations of labile iron, due to low expression of Ferroportin 1 (Fpn1) for instance. Importantly, IDH-1/2 mutations pose an important anti-cancer strategy for hard-to-treat cancer types, these mutations occurring in ~ 70–80% of lower-grade gliomas and the majority of secondary glioblastomas and in up to 20% of patients with AML [261, 262]. Concerning TET2, mutations in this gene are observed in different myeloid malignancies and are related to AML prognosis [263]. In addition, high-dose VitC also has more effect on mismatch repair (MMR)-deficient tumors than on MMR-competent ones, suggesting that the antitumor effect of VitC is enhanced in tumors harbouring increased mutational/neoantigen burdens [91]. Furthermore, sulindac and VitC could be a novel anti-cancer therapeutic strategy for p53 wild-type colon cancers, as this causes apoptosis in a p53-dependent manner [118]. Finally, using high-dose VitC in immune checkpoint therapy may benefit a wide variety of cancer patients, especially those having low PD-1/PDL-1 expression [90].
An absolute necessity in the quest to make high-dose VitC more broadly available to cancer patients is the conduction of randomized phase III clinical trials on large patient groups (typically over 300), with the aim of assessing the effectiveness of VitC (combinations) compared to the current ‘gold standard’ treatment for a given cancer type. Due to their expensive and time-consuming nature, no such trials have been completed for VitC to date. Nevertheless, based on the promising pre-clinical and early-phase clinical trial findings in the colorectal cancer setting [12, 13, 32, 151], a Chinese phase III trial aiming to evaluate the effectiveness of combing high dose IV VitC (1.5 g/kg) with FOLOX +/− bevacizumab versus treatment with FOLFOX +/− bevacizumab alone as first-line therapy in patients with recurrent or advanced colorectal cancer is currently ongoing (ClinicalTrials.gov Identifier: NCT02969681, Table 3, recruiting status unclear). Related to this, another Chinese phase III trial is currently assessing this combination specifically in peritoneal metastatic colorectal cancer patients with high expression of GLUT3 [131] (Table 3).
From a practical point of view, experiences from clinical trials and case reports have made it evident that while adverse events are rare, a few aspects should be considered before administering high doses of IVC. While some side effects, such as a decrease in the levels of potassium (hypokalemia) by VitC may be mitigated by supplementing the formula, certain conditions have to be closely monitored and may be contraindicative for IVC treatment. For example, in patients with renal insufficiency, high dose IVC may lead to kidney stone formation or acute oxalate nephropathy [65, 264], while a red cell glucose-6-phosphate dehydrogenase deficiency (G6PD) has been linked to cases of hemolytic anemia [66, 265] following high dose IVC, sugesting both of these condition should be screened for prior to high dose IVC administration.
Concerning the optimal IVC administration regimen, evidence outlined in this review suggests that 1) anti-cancer effects can only be achieved when VitC is administered intravenously, 2) the dose of IVC has to be sufficiently high in order to generate millimolar concentrations of VitC in the plasma [12, 13]. The recommended effective doses range from 1.5 g/kg [12, 151] to 1.9–2.2 g/kg [13] in the IVC monotherapy studies, while IVC combination therapies indicated 75 g [110, 155] to 87.5 g [16, 129] whole body dose to be sufficient. Furthermore, 3) these doses of IVC should be administered at least twice a week. Almost all clinical trials that present suggestions of efficacy and other favourable clinical outcomes, administered IVC 2–3 times a week, for at least 8 weeks [63, 82, 153, 156, 157].
To conclude, a large body of evidence is accumulating suggesting that VitC, when administered intravenously and in high doses, has potent cancer-selective cytotoxic, cancer-therapy sensitizing and toxicity-reducing properties.
High-dose VitC therefore has the potential to expand the therapeutic range of radio-, chemo- and targeted therapies as well as their efficacy. In addition, a wide variety of cancer patients may benefit from the expanded therapeutic scope of immune checkpoint inhibitors by high-dose VitC. Despite this fact, low accrual remains to hamper further clinical examination, most often because the drug combination in question is no longer standard of care while the study is ongoing. Importantly, this is the case even though the assessment of these combinations may still be highly clinically relevant. Fortunately, future clinical studies combining high-dose VitC with immunotherapy may not face this problem, considering the current high interest in this treatment modality and the need to overcome its current limitations.
Considering how the implementation of high-dose VitC may be a breakthrough in the treatment of cancer patients with poor prognosis and few available treatment options, it is fair to conclude that further clinical examination of this promising and non-toxic cancer treatment modality is not only warranted, but is in fact highly needed.
Acknowledgements
This work was supported by a project grant from the Dutch Cancer Society (# 10212) to C.R.J.
Abbreviations
- 3-PO
3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one
- 5-FU
5-fluorouracil
- AGS
Gastric adenocarcinoma cell line
- AML
Acute myeloid leukemia
- APC
Antigen-presenting cell
- ATO
Arsenic trioxide
- AUF
Auranofin
- BLAD
Bladder cancer
- BR
Breast cancer
- BSC
Best supportive care
- CLL
Chronic lymphocytic leukemia
- CRC
Colorectal cancer
- CSCs
Cancer stem cells
- Cmax
Peak serum concentration achieved
- DHA
Dehydroascorbic acid
- DLT
Dose-limiting toxicities
- Dox
Doxycycline
- d-TPP
TPP derivative dodecyl-TPP
- EMT
Epithelial-to-mesenchymal transition
- EPI
Epithelial
- ESI-FT ICR MS
Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry
- GB
Glioblastoma
- GBM
Glioblastoma multiform
- GC
Gastric cancer
- Gem
Gemcitabine
- HCC
Hepatocellular carcinoma
- HNC
Head and neck cancer
- ICI
Immune checkpoint inhibitors
- IR/RT
Irradiation/radiotherapy
- IVC
High-dose intravenous VitC
- JQ1
Thieno-triazolo-1,4-diazepine
- LC-MS/MS
Liquid chromatography–mass spectrometry
- LEU
Leukemia
- LIP
Labile iron pool
- LUNG
Lung cancer
- LYM
Lymphoma
- MALDI-TOF MS
Matrix assisted laser desorption ionization-time of flight mass spectrometry
- mCRC
Metastatic colorectal cancer
- mEHT
Modulated electrohyperthermia
- MEL
Melanoma
- MES
Mesenchymal
- MM
Multiple myeloma
- MMR
Mismatch repair
- MoA
Mechanisms of action
- MTD
Maximum tolerated dose
- NB
Neuroblastoma
- NSCLC
Non-small-cell lung cancer
- OC
Orally administered VitC
- ORAL
Oral cancer
- ORR
Objective response rate
- OS
Overall survival
- OVC
Ovarian cancer
- Oxa
Oxaplatin
- PC
Prostate cancer
- PDAC
Pancreatic ductal adenocarcinoma
- PFS
Progression-free survival
- QoL
Quality of life
- RCC
Renal cell carcinoma
- RT
Radiation therapy
- SAR
Sarcoma
- SILAC
STABLE Isotope Labeling by/with Amino acids in Cell culture
- TET
Ten eleven translocation enzyme
- TETA
Triethylenetetramine
- TMZ
Temozolomide
- URI
Urinary cancers
- VitC
Vitamin C
Authors’ contributions
F.B., A.V.M. and L.C. performed the literature searches. Figures were created by F.B. and A.V.M. All authors wrote, read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Franziska Böttger and Andrea Vallés-Martí contributed equally to this work.
References
- 1.Padayatty S, Levine M. Vitamin C: the known and the unknown and goldilocks. Oral Dis. 2016;22(6):463–493. doi: 10.1111/odi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ngo B, Van Riper JM, Cantley LC, Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer. 2019;19(5):271–282. doi: 10.1038/s41568-019-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Chong T, Ahearn EL, Cimmino L. Reprogramming the Epigenome with vitamin C. Front Cell Dev Biol. 2019;7:128. doi: 10.3389/fcell.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher SC, Coleman ML. Human 2-oxoglutarate-dependent oxygenases: nutrient sensors, stress responders, and disease mediators. Biochem Soc Trans. 2020;48(5):1843–1858. doi: 10.1042/BST20190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang A, Pullar JM, Currie MJ, Vissers MCM. Vitamin C and immune cell function in inflammation and cancer. Biochem Soc Trans. 2018;46(5):1147–1159. doi: 10.1042/BST20180169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron E, Campbell A. The orthomolecular treatment of cancer II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact. 1974;9(4):285–315. doi: 10.1016/0009-2797(74)90019-2. [DOI] [PubMed] [Google Scholar]
- 7.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci. 1976;73(10):3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci. 1978;75(9):4538–4542. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creagan ET, Moertel CG, O’Fallon JR, Schutt AJ, O’Connell MJ, Rubin J, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced Cancer. N Engl J Med. 1979;301(13):687–690. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- 10.Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced Cancer who have had no prior chemotherapy. N Engl J Med. 1985;312(3):137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 11.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for Oral and intravenous use. Ann Intern Med. 2004;140(7):533. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 12.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19(11):1969–1974. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson CM, Levin RD, Spector T, Lis CG. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;72(1):139–146. doi: 10.1007/s00280-013-2179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polireddy K, Dong R, Reed G, Yu J, Chen P, Williamson S, et al. High dose parenteral Ascorbate inhibited pancreatic Cancer growth and metastasis: mechanisms and a phase I/IIa study. Sci Rep. 2017;7(1):17188. doi: 10.1038/s41598-017-17568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci. 2008;105(32):11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. O 2 ·− and H 2 O 2 -Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell. 2017;31(4):487–500.e8. doi: 10.1016/j.ccell.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H, Mizuno H, Yanagisawa A. High-dose intravenous vitamin C improves quality of life in cancer patients. Pers Med Universe. 2012;1(1):49–53. doi: 10.1016/j.pmu.2012.05.008. [DOI] [Google Scholar]
- 18.Vollbracht C, Schneider B, Leendert V, Weiss G, Auerbach L, Beuth J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo−/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo. 2011;25(6):983–990. [PubMed] [Google Scholar]
- 19.Yeom CH, Jung GC, Song KJ. Changes of terminal Cancer patients’ health-related quality of life after high dose vitamin C administration. J Korean Med Sci. 2007;22(1):7. doi: 10.3346/jkms.2007.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. 2017;549(7673):476–481. doi: 10.1038/nature23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonilla-Porras AR, Jimenez-Del-Rio M, Velez-Pardo C. Vitamin K3 and vitamin C alone or in combination induced apoptosis in leukemia cells by a similar oxidative stress signalling mechanism. Cancer Cell Int. 2011;11(1):19. doi: 10.1186/1475-2867-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell. 2017;170(6):1079–1095.e20. doi: 10.1016/j.cell.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iamsawat S, Tian L, Daenthanasanmak A, Wu Y, Nguyen HD, Bastian D, et al. Vitamin C stabilizes CD81 iTregs and enhances their therapeutic potential in controlling murine GVHD and leukemia relapse. Blood Adv. 2019;3(24):4187–4201. doi: 10.1182/bloodadvances.2019000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mingay M, Chaturvedi A, Bilenky M, Cao Q, Jackson L, Hui T, et al. Vitamin C-induced epigenomic remodelling in IDH1 mutant acute myeloid leukaemia. Leukemia. 2018;32(1):11–20. doi: 10.1038/leu.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguilera O, Muñoz-Sagastibelza M, Torrejón B, Borrero-Palacios A, del Puerto-Nevado L, Martínez-Useros J, et al. Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer. Oncotarget. 2016;7(30):47954–47965. doi: 10.18632/oncotarget.10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandt KE, Falls KC, Schoenfeld JD, Rodman SN, Gu Z, Zhan F, et al. Augmentation of intracellular iron using iron sucrose enhances the toxicity of pharmacological ascorbate in colon cancer cells. Redox Biol. 2018;14(July 2017):82–87. doi: 10.1016/j.redox.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cenigaonandia-Campillo A, Serna-Blasco R, Gómez-Ocabo L, Solanes-Casado S, Baños-Herraiz N, Del Puerto-Nevado L, et al. Vitamin C activates pyruvate dehydrogenase (PDH) targeting the mitochondrial tricarboxylic acid (TCA) cycle in hypoxic KRAS mutant colon cancer. Theranostics. 2021;11(8):3595–3606. doi: 10.7150/thno.51265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamede AC, Pires AS, Abrantes AM, Tavares SD, Gonçalves AC, Casalta-Lopes JE, et al. Cytotoxicity of ascorbic acid in a human colorectal adenocarcinoma cell line (WiDr): in vitro and in vivo studies. Nutr Cancer. 2012;64(7):1049–1057. doi: 10.1080/01635581.2012.713539. [DOI] [PubMed] [Google Scholar]
- 29.Nakanishi K, Hiramoto K, Ooi K. High-dose vitamin C exerts its anti-cancer effects in a Xenograft model of Colon Cancer by suppressing angiogenesis. Biol Pharm Bull. 2021;44(6):884–887. doi: 10.1248/bpb.b21-00089. [DOI] [PubMed] [Google Scholar]
- 30.Pires AS, Marques CR, Encarnação JC, Abrantes AM, Mamede AC, Laranjo M, et al. Ascorbic acid and colon cancer: an oxidative stimulus to cell death depending on cell profile. Eur J Cell Biol. 2016;95(6–7):208–218. doi: 10.1016/j.ejcb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Yin T, Wang Y. In vitro and in vivo assessment of high-dose vitamin C against murine tumors. Exp Ther Med. 2016;12(5):3058–3062. doi: 10.3892/etm.2016.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science (80- ) 2015;350(6266):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakanishi K, Hiramoto K, Sato EF, Ooi K. High-dose vitamin C administration inhibits the invasion and proliferation of melanoma cells in mice ovary. Biol Pharm Bull. 2021;44(1):75–81. doi: 10.1248/bpb.b20-00637. [DOI] [PubMed] [Google Scholar]
- 34.Chen XY, Chen Y, Qu CJ, Pan ZH, Qin Y, Zhang X, et al. Vitamin C induces human melanoma A375 cell apoptosis via Bax- and Bcl-2-mediated mitochondrial pathways. Oncol Lett. 2019;18(4):3880–3886. doi: 10.3892/ol.2019.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang JS, Cho D, Kim Y-I, Hahm E, Yang Y, Kim D, et al. L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8?Independent pathway. Cancer Immunol Immunother. 2003;52(11):693–698. doi: 10.1007/s00262-003-0407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mustafi S, Sant DW, Liu Z-J, Wang G. Ascorbate induces apoptosis in melanoma cells by suppressing Clusterin expression. Sci Rep. 2017;7(1):3671. doi: 10.1038/s41598-017-03893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serrano OK, Parrow NL, Violet P-C, Yang J, Zornjak J, Basseville A, et al. Antitumor effect of pharmacologic ascorbate in the B16 murine melanoma model. Free Radic Biol Med. 2015;87:193–203. doi: 10.1016/j.freeradbiomed.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 38.Du J, Martin SM, Levine M, Wagner BA, Buettner GR, Wang S, et al. Mechanisms of Ascorbate-induced cytotoxicity in pancreatic Cancer. Clin Cancer Res. 2010;16(2):509–520. doi: 10.1158/1078-0432.CCR-09-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollard HB, Levine MA, Eidelman O, Pollard M. Pharmacological ascorbic acid suppresses syngeneic tumor growth and metastases in hormone-refractory prostate cancer. In Vivo. 2010;24(3):249–255. [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z, He P, Luo G, Shi X, Yuan G, Zhang B, et al. Increased Tumoral microenvironmental pH improves cytotoxic effect of pharmacologic ascorbic acid in castration-resistant prostate Cancer cells. Front Pharmacol. 2020;11:570939. doi: 10.3389/fphar.2020.570939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen P, Yu J, Chalmers B, Drisko J, Yang J, Li B, et al. Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anti-Cancer Drugs. 2012;23(4):437–444. doi: 10.1097/CAD.0b013e32834fd01f. [DOI] [PubMed] [Google Scholar]
- 42.Ramezankhani B, Taha MF, Javeri A. Vitamin C counteracts miR-302/367-induced reprogramming of human breast cancer cells and restores their invasive and proliferative capacity. J Cell Physiol. 2019;234(3):2672–2682. doi: 10.1002/jcp.27081. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Guo X, Wang G, Zhou C. Vitamin C inhibits metastasis of peritoneal tumors by preventing spheroid formation in ID8 murine epithelial peritoneal Cancer model. Front Pharmacol. 2020;11:645. doi: 10.3389/fphar.2020.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregoraszczuk EL, Zajda K, Tekla J, Respekta N, Zdybał P, Such A. Vitamin C supplementation had no side effect in non-cancer, but had anticancer properties in ovarian cancer cells. Int J Vitam Nutr Res. 2020;3:1–11. doi: 10.1024/0300-9831/a000634. [DOI] [PubMed] [Google Scholar]
- 45.Lv H, Wang C, Fang T, Li T, Lv G, Han Q, et al. Vitamin C preferentially kills cancer stem cells in hepatocellular carcinoma via SVCT-2. npj Precis Oncol. 2018;2(1):1. doi: 10.1038/s41698-017-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alyoussef A, Al-Gayyar MMH. Cytotoxic and partial hepatoprotective activity of sodium ascorbate against hepatocellular carcinoma through inhibition of sulfatase-2 in vivo and in vitro. Biomed Pharmacother. 2018;103:362–372. doi: 10.1016/j.biopha.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 47.Volta V, Ranzato E, Martinotti S, Gallo S, Russo MV, Mutti L, et al. Preclinical Demonstration of Synergistic Active Nutrients/Drug (AND) Combination as a Potential Treatment for Malignant Pleural Mesothelioma. McCormick DL, editor. PLoS One. 2013;8(3):e58051. doi: 10.1371/journal.pone.0058051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranzato E, Biffo S, Burlando B. Selective Ascorbate toxicity in malignant mesothelioma. Am J Respir Cell Mol Biol. 2011;44(1):108–117. doi: 10.1165/rcmb.2009-0340OC. [DOI] [PubMed] [Google Scholar]
- 49.Su X, Shen Z, Yang Q, Sui F, Pu J, Ma J, et al. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics. 2019;9(15):4461–4473. doi: 10.7150/thno.35219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tronci L, Serreli G, Piras C, Frau DV, Dettori T, Deiana M, et al. Vitamin C cytotoxicity and its effects in redox homeostasis and energetic metabolism in papillary thyroid carcinoma cell lines. Antioxidants. 2021;10(5):809. doi: 10.3390/antiox10050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou J, Chen C, Chen X, Fei Y, Jiang L, Wang G. Vitamin C promotes apoptosis and cell cycle arrest in Oral squamous cell carcinoma. Front Oncol. 2020;10:976. doi: 10.3389/fonc.2020.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deubzer B, Mayer F, Kuçi Z, Niewisch M, Merkel G, Handgretinger R, et al. H2O2-mediated cytotoxicity of pharmacologic Ascorbate concentrations to neuroblastoma cells: potential role of lactate and ferritin. Cell Physiol Biochem. 2010;25(6):767–774. doi: 10.1159/000315098. [DOI] [PubMed] [Google Scholar]
- 53.Castro M, Carson G, McConnell M, Herst P. High dose Ascorbate causes both Genotoxic and metabolic stress in Glioma cells. Antioxidants. 2017;6(3):58. doi: 10.3390/antiox6030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gokturk D, Kelebek H, Ceylan S, Yilmaz DM. The effect of ascorbic acid over the Etoposide- and Temozolomide-mediated cytotoxicity in Glioblastoma cell culture: a molecular study. Turk Neurosurg. 2018;28(1):13–18. doi: 10.5137/1019-5149.JTN.19111-16.1. [DOI] [PubMed] [Google Scholar]
- 55.Campbell EJ, Dachs GU. Current limitations of murine models in oncology for Ascorbate research. Front Oncol. 2014;4:282. doi: 10.3389/fonc.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell EJ, Vissers MCM, Wohlrab C, Hicks KO, Strother RM, Bozonet SM, et al. Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radic Biol Med. 2016;99:451–462. doi: 10.1016/j.freeradbiomed.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 57.Chen P, Stone J, Sullivan G, Drisko JA, Chen Q. Anti-cancer effect of pharmacologic ascorbate and its interaction with supplementary parenteral glutathione in preclinical cancer models. Free Radic Biol Med. 2011;51(3):681–687. doi: 10.1016/j.freeradbiomed.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 58.Taper HS, Jamison JM, Gilloteaux J, Summers JL, Calderon PB. Inhibition of the development of metastases by dietary vitamin C:K 3 combination. Life Sci. 2004;75(8):955–967. doi: 10.1016/j.lfs.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Chen MF, Yang CM, Su CM, Liao JW, Hu ML. Inhibitory effect of vitamin C in combination with vitamin K3 on tumor growth and metastasis of Lewis lung carcinoma xenografted in C57BL/6 mice. Nutr Cancer. 2011;63(7):1036–1043. doi: 10.1080/01635581.2011.597537. [DOI] [PubMed] [Google Scholar]
- 60.Zeng L-H, Wang Q-M, Feng L-Y, Ke Y-D, Xu Q-Z, Wei A-Y, et al. High-dose vitamin C suppresses the invasion and metastasis of breast cancer cells via inhibiting epithelial-mesenchymal transition. Onco Targets Ther. 2019;12:7405–7413. doi: 10.2147/OTT.S222702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Leary BR, Alexander MS, Du J, Moose DL, Henry MD, Cullen JJ. Pharmacological ascorbate inhibits pancreatic cancer metastases via a peroxide-mediated mechanism. Sci Rep. 2020;10(1):17649. doi: 10.1038/s41598-020-74806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeom CH, Lee G, Park JH, Yu J, Park S, Yi SY, et al. High dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J Transl Med. 2009;7(1):1–9. doi: 10.1186/1479-5876-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-Dose Parenteral Ascorbate Enhanced Chemosensitivity of Ovarian Cancer and Reduced Toxicity of Chemotherapy. Sci Transl Med. 2014;6(222):222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 64.Su X, Li P, Han B, Jia H, Liang Q, Wang H, et al. Vitamin C sensitizes BRAFV600E thyroid cancer to PLX4032 via inhibiting the feedback activation of MAPK/ERK signal by PLX4032. J Exp Clin Cancer Res. 2021;40(1):34. doi: 10.1186/s13046-021-01831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riordan HD, Casciari JJ, González MJ, Riordan NH, Miranda-Massari JR, Taylor P, et al. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J. 2005;24(4):269–276. [PubMed] [Google Scholar]
- 66.Nielsen TK, Højgaard M, Andersen JT, Jørgensen NR, Zerahn B, Kristensen B, et al. Weekly ascorbic acid infusion in castration-resistant prostate cancer patients: a single-arm phase II trial. Transl Androl Urol. 2017;6(3):517–528. doi: 10.21037/tau.2017.04.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drisko JA, Chapman J, Hunter VJ. The use of antioxidants with first line chemotherapy in two cases of ovarian cancer. J Am Coll Nutr. 2003;22(2):118–123. doi: 10.1080/07315724.2003.10719284. [DOI] [PubMed] [Google Scholar]
- 68.Drisko JA, Serrano OK, Spruce LR, Chen Q, Levine M. Treatment of pancreatic cancer with intravenous vitamin C. Anti-Cancer Drugs. 2018;29(4):373–379. doi: 10.1097/CAD.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.González MJ, Berdiel MJ, Miranda-Massari JR, López D, Duconge J, Rodriguez JL, et al. High dose intravenous vitamin c and metastatic pancreatic cancer: two cases. Integr Cancer Sci Ther. 2016;3(6):1–2. [Google Scholar]
- 70.Padayatty SJ. Intravenously administered vitamin C as cancer therapy: three cases. Can Med Assoc J. 2006;174(7):937–942. doi: 10.1503/cmaj.050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riordan HD, Riordan NH, Jackson JA, Casciari JJ, Hunninghake R, González MJ, et al. Intravenous vitamin C as a chemotherapy agent: a report on clinical cases. P R Health Sci J. 2004;23(2):115–118. [PubMed] [Google Scholar]
- 72.Seo M-S, Kim J-K, Shim J-Y. High-dose vitamin C promotes regression of multiple pulmonary metastases originating from hepatocellular carcinoma. Yonsei Med J. 2015;56(5):1449. doi: 10.3349/ymj.2015.56.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.ClinicalTrials.gov Identifier: NCT03146962. High Dose Vitamin C Intravenous Infusion in Patients With Resectable or Metastatic Solid Tumor Malignancies.
- 74.ClinicalTrials.gov Identifier: NCT04046094. Intravenous (IV) Vitamin C With Chemotherapy for Cisplatin Ineligible Bladder Cancer Patients.
- 75.ClinicalTrials.gov Identifier: NCT03682029. Epigenetics, Vitamin C, and Abnormal Blood Cell Formation - Vitamin C in Patients With Low-Risk Myeloid Malignancies (EVITA).
- 76.ClinicalTrials.gov Identifier: NCT03613727. Therapeutic Use of Intravenous Vitamin C in Allogeneic Stem Cell Transplant Recipients.
- 77.ClinicalTrials.gov Identifier: NCT03964688. Effect of Vitamin C in Autologous Stem Cell Transplantations (VICAST).
- 78.Mastrangelo D, Massai L, Lo Coco F, Noguera NI, Borgia L, Fioritoni G, et al. Cytotoxic effects of high concentrations of sodium ascorbate on human myeloid cell lines. Ann Hematol. 2015;94(11):1807–1816. doi: 10.1007/s00277-015-2464-2. [DOI] [PubMed] [Google Scholar]
- 79.Carr AC, McCall C. The role of vitamin C in the treatment of pain: new insights. J Transl Med. 2017;15(1):77. doi: 10.1186/s12967-017-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Günes-Bayir A, Kiziltan HS. Palliative vitamin C application in patients with radiotherapy-resistant bone metastases: a retrospective study. Nutr Cancer. 2015;67(6):921–925. doi: 10.1080/01635581.2015.1055366. [DOI] [PubMed] [Google Scholar]
- 81.Klimant E, Wright H, Rubin D, Seely D, Markman M. Intravenous vitamin C in the supportive care of cancer patients: a review and rational approach. Curr Oncol. 2018;25(2):139–148. doi: 10.3747/co.25.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013;71(3):765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoffer LJ, Robitaille L, Zakarian R, Melnychuk D, Kavan P, Agulnik J, et al. High-Dose Intravenous Vitamin C Combined with Cytotoxic Chemotherapy in Patients with Advanced Cancer: A Phase I-II Clinical Trial. Hills RK, editor. PLoS One. 2015;10(4):e0120228. doi: 10.1371/journal.pone.0120228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carr AC, Spencer E, Das A, Meijer N, Lauren C, Macpherson S, et al. Patients undergoing myeloablative chemotherapy and hematopoietic stem cell transplantation exhibit depleted vitamin C status in association with febrile neutropenia. Nutrients. 2020;12(6):1–9. doi: 10.3390/nu12061879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mayland CR, Bennett MI, Allan K. Vitamin C deficiency in cancer patients. Palliat Med. 2005;19(1):17–20. doi: 10.1191/0269216305pm970oa. [DOI] [PubMed] [Google Scholar]
- 86.Mansoor F, Kumar S, Rai P, Anees F, Kaur N, Devi A, et al. Impact of intravenous vitamin C Administration in Reducing Severity of symptoms in breast Cancer patients during treatment. Cureus. 2021;13(5):e14867. doi: 10.7759/cureus.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dayer D, Tabandeh MR, Kazemi M. The radio-sensitizing effect of pharmacological concentration of ascorbic acid on human pancreatic Cancer cells. Anti Cancer Agents Med Chem. 2020;20(16):1927–1932. doi: 10.2174/1871520620666200612144124. [DOI] [PubMed] [Google Scholar]
- 88.Pires AS, Marques CR, Encarnação JC, Abrantes AM, Marques IA, Laranjo M, et al. Ascorbic acid Chemosensitizes colorectal Cancer cells and synergistically inhibits tumor growth. Front Physiol. 2018;9:911. doi: 10.3389/fphys.2018.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Leary BR, Houwen FK, Johnson CL, Allen BG, Mezhir JJ, Berg DJ, et al. Pharmacological Ascorbate as an adjuvant for enhancing radiation-chemotherapy responses in gastric adenocarcinoma. Radiat Res. 2018;189(5):456. doi: 10.1667/RR14978.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luchtel RA, Bhagat T, Pradhan K, Jacobs WR, Levine M, Verma A, et al. High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc Natl Acad Sci. 2020;117(3):1666–1677. doi: 10.1073/pnas.1908158117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Magrì A, Germano G, Lorenzato A, Lamba S, Chilà R, Montone M, et al. High-dose vitamin C enhances cancer immunotherapy. Sci Transl Med. 2020;12(532):eaay8707. doi: 10.1126/scitranslmed.aay8707. [DOI] [PubMed] [Google Scholar]
- 92.Tian W, Wang Z, Tang N, Li J, Liu Y, Chu W-F, et al. Ascorbic acid sensitizes colorectal carcinoma to the cytotoxicity of arsenic trioxide via promoting reactive oxygen species-dependent apoptosis and Pyroptosis. Front Pharmacol. 2020;21:11. doi: 10.3389/fphar.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu X, Park M, Sarbassova DA, Ying H, Lee MG, Bhattacharya R, et al. A chirality-dependent action of vitamin C in suppressing Kirsten rat sarcoma mutant tumor growth by the oxidative combination: rationale for cancer therapeutics. Int J Cancer. 2020;146(10):2822–2828. doi: 10.1002/ijc.32658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Noguera NI, Pelosi E, Angelini DF, Piredda ML, Guerrera G, Piras E, et al. High-dose ascorbate and arsenic trioxide selectively kill acute myeloid leukemia and acute promyelocytic leukemia blasts in vitro. Oncotarget. 2017;8(20):32550–32565. doi: 10.18632/oncotarget.15925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biswas S, Zhao X, Mone AP, Mo X, Vargo M, Jarjoura D, et al. Arsenic trioxide and ascorbic acid demonstrate promising activity against primary human CLL cells in vitro. Leuk Res. 2010;34(7):925–931. doi: 10.1016/j.leukres.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vineetha RC, Hariharan S, Jaleel A, Chandran M, Nair RH. L-ascorbic acid and α-Tocopherol synergistically triggers apoptosis inducing Antileukemic effects of arsenic trioxide via oxidative stress in human acute Promyelocytic leukemia cells. Front Oncol. 2020;10:65. doi: 10.3389/fonc.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hatem E, Azzi S, El Banna N, He T, Heneman-Masurel A, Vernis L, et al. Auranofin/vitamin C: a novel drug combination targeting triple-negative breast Cancer. JNCI J Natl Cancer Inst. 2019;111(6):597–608. doi: 10.1093/jnci/djy149. [DOI] [PubMed] [Google Scholar]
- 98.Gerecke C, Schumacher F, Edlich A, Wetzel A, Yealland G, Neubert LK, et al. Vitamin C promotes decitabine or azacytidine induced DNA hydroxymethylation and subsequent reactivation of the epigenetically silenced tumour suppressor CDKN1A in colon cancer cells. Oncotarget. 2018;9(67):32822–32840. doi: 10.18632/oncotarget.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jung S-A, Lee D-H, Moon J-H, Hong S-W, Shin J-S, Hwang IY, et al. L-ascorbic acid can abrogate SVCT-2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells. Free Radic Biol Med. 2016;95:200–208. doi: 10.1016/j.freeradbiomed.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 100.Ghavami G, Sardari S. Synergistic effect of vitamin C with Cisplatin for inhibiting proliferation of gastric Cancer cells. Iran Biomed J. 2020;24(2):119–127. doi: 10.29252/ibj.24.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leekha A, Gurjar BS, Tyagi A, Rizvi MA, Verma AK. Vitamin C in synergism with cisplatin induces cell death in cervical cancer cells through altered redox cycling and p53 upregulation. J Cancer Res Clin Oncol. 2016;142(12):2503–2514. doi: 10.1007/s00432-016-2235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kleih M, Böpple K, Dong M, Gaißler A, Heine S, Olayioye MA, et al. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019;10(11):851. doi: 10.1038/s41419-019-2081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu T-M, Liu S-T, Chen S-Y, Chen G-S, Wu C-C, Huang S-M. Mechanisms and Applications of the Anti-cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells. Front Oncol. 2020;10:1483. doi: 10.3389/fonc.2020.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Darwiche W, Gomila C, Ouled-Haddou H, Naudot M, Doualle C, Morel P, et al. Ascorbic acid (vitamin C) synergistically enhances the therapeutic effect of targeted therapy in chronic lymphocytic leukemia. J Exp Clin Cancer Res. 2020;39(1):228. doi: 10.1186/s13046-020-01738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao H, Zhu H, Huang J, Zhu Y, Hong M, Zhu H, et al. The synergy of vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk Res. 2018;66:1–7. doi: 10.1016/j.leukres.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 106.De Francesco EM, Bonuccelli G, Maggiolini M, Sotgia F, Lisanti MP. Vitamin C and doxycycline: a synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs) Oncotarget. 2017;8(40):67269–67286. doi: 10.18632/oncotarget.18428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fiorillo M, Tóth F, Sotgia F, Lisanti MP. Doxycycline, azithromycin and vitamin C (DAV): a potent combination therapy for targeting mitochondria and eradicating cancer stem cells (CSCs) Aging (Albany NY) 2019;11(8):2202–2216. doi: 10.18632/aging.101905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee SJ, Jeong J-H, Lee IH, Lee J, Jung JH, Park HY, et al. Effect of high-dose vitamin C combined with anti-cancer treatment on breast Cancer cells. Anticancer Res. 2019;39(2):751–758. doi: 10.21873/anticanres.13172. [DOI] [PubMed] [Google Scholar]
- 109.Lee KE, Hahm E, Bae S, Kang JS, Lee WJ. The enhanced tumor inhibitory effects of gefitinib and L-ascorbic acid combination therapy in non-small cell lung cancer cells. Oncol Lett. 2017;14(1):276–282. doi: 10.3892/ol.2017.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alexander MS, Wilkes JG, Schroeder SR, Buettner GR, Wagner BA, Du J, et al. Pharmacologic Ascorbate reduces radiation-induced Normal tissue toxicity and enhances tumor Radiosensitization in pancreatic Cancer. Cancer Res. 2018;78(24):6838–6851. doi: 10.1158/0008-5472.CAN-18-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Bradley MD, Wagner BA, Buettner GR, et al. Redox active metals and H2O2 mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol. 2018;14:417–422. doi: 10.1016/j.redox.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu Y-X, Wu Q-N, Chen D, Chen L-Z, Wang Z-X, Ren C, et al. Pharmacological Ascorbate suppresses growth of gastric Cancer cells with GLUT1 overexpression and enhances the efficacy of Oxaliplatin through redox modulation. Theranostics. 2018;8(5):1312–1326. doi: 10.7150/thno.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xia J, Xu H, Zhang X, Allamargot C, Coleman KL, Nessler R, et al. Multiple myeloma tumor cells are selectively killed by pharmacologically-dosed ascorbic acid. EBioMedicine. 2017;18:41–49. doi: 10.1016/j.ebiom.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Di Tano M, Raucci F, Vernieri C, Caffa I, Buono R, Fanti M, et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat Commun. 2020;11(1):2332. doi: 10.1038/s41467-020-16243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bharadwaj R, Sahu BP, Haloi J, Laloo D, Barooah P, Keppen C, et al. Combinatorial therapeutic approach for treatment of oral squamous cell carcinoma. Artif Cells Nanomed Biotechnol. 2019;47(1):571–584. doi: 10.1080/21691401.2019.1573176. [DOI] [PubMed] [Google Scholar]
- 116.Rouleau L, Antony AN, Bisetto S, Newberg A, Doria C, Levine M, et al. Synergistic effects of ascorbate and sorafenib in hepatocellular carcinoma: new insights into ascorbate cytotoxicity. Free Radic Biol Med. 2016;95:308–322. doi: 10.1016/j.freeradbiomed.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng Z, Luo G, Shi X, Long Y, Shen W, Li Z, et al. The xc− inhibitor sulfasalazine improves the anti-cancer effect of pharmacological vitamin C in prostate cancer cells via a glutathione-dependent mechanism. Cell Oncol. 2020;43(1):95–106. doi: 10.1007/s13402-019-00474-8. [DOI] [PubMed] [Google Scholar]
- 118.Gong E-Y, Shin YJ, Hwang I-Y, Kim JH, Kim S-M, Moon J-H, et al. Combined treatment with vitamin C and sulindac synergistically induces p53- and ROS-dependent apoptosis in human colon cancer cells. Toxicol Lett. 2016;258:126–133. doi: 10.1016/j.toxlet.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 119.Mustafi S, Camarena V, Volmar CH, Huff TC, Sant DW, Brothers SP, et al. Vitamin C sensitizes melanoma to BET inhibitors. Cancer Res. 2018;78(2):572–583. doi: 10.1158/0008-5472.CAN-17-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sinha BK, van ‘t Erve TJ, Kumar A, Bortner CD, Motten AG, Mason RP. Synergistic enhancement of topotecan-induced cell death by ascorbic acid in human breast MCF-7 tumor cells. Free Radic Biol Med. 2017;113:406–412. doi: 10.1016/j.freeradbiomed.2017.10.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Francesco EM, Ózsvári B, Sotgia F, Lisanti MP. Dodecyl-TPP targets mitochondria and potently eradicates Cancer stem cells (CSCs): synergy with FDA-approved drugs and natural compounds (vitamin C and Berberine) Front Oncol. 2019;7:9. doi: 10.3389/fonc.2019.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang L, Luo X, Li C, Huang Y, Xu P, Lloyd-Davies LH, et al. Triethylenetetramine synergizes with pharmacologic ascorbic acid in hydrogen peroxide mediated selective toxicity to breast Cancer cell. Oxidative Med Cell Longev. 2017;2017:1–13. doi: 10.1155/2017/3481710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang G, Yan Y, Ma Y, Yang Y. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 2017;56(8):1965–1976. doi: 10.1002/mc.22654. [DOI] [PubMed] [Google Scholar]
- 124.Ivanova D, Zhelev Z, Lazarova D, Getsov P, Bakalova R, Aoki I. Vitamins C and K3: a powerful redox system for sensitizing leukemia lymphocytes to Everolimus and Barasertib. Anticancer Res. 2018;38(3):1407–1414. doi: 10.21873/anticanres.12364. [DOI] [PubMed] [Google Scholar]
- 125.ClinicalTrial.gov Identifier: NCT00441207. Study of High-Dose Intravenous (IV) Vitamin C Treatment in Patients With Solid Tumors.
- 126.Nielsen TK, Højgaard M, Andersen JT, Poulsen HE, Lykkesfeldt J, Mikines KJ. Elimination of ascorbic acid after high-dose infusion in prostate Cancer patients: a pharmacokinetic evaluation. Basic Clin Pharmacol Toxicol. 2015;116(4):343–348. doi: 10.1111/bcpt.12323. [DOI] [PubMed] [Google Scholar]
- 127.ClinicalTrials.gov Identifier: NCT01080352. Vitamin C as an Anti-cancer Drug.
- 128.ClinicalTrials.gov Identifier: NCT01050621. Trial of Chemotherapy Plus Intravenous Vitamin C in Patients With Advanced Cancer for Whom Chemotherapy Alone is Only Marginally Effective.
- 129.Allen BG, Bodeker KL, Smith MC, Monga V, Sandhu S, Hohl R, et al. First-in-human phase I clinical trial of pharmacologic Ascorbate combined with radiation and Temozolomide for newly diagnosed Glioblastoma. Clin Cancer Res. 2019;25(22):6590–6597. doi: 10.1158/1078-0432.CCR-19-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.ClinicalTrials.gov Identifier: NCT01752491. A Phase I Trial of High-Dose Ascorbate in Glioblastoma Multiforme.
- 131.ClinicalTrials.gov Identifier: NCT04516681. IV Ascorbic Acid in Peritoneal Metastatic Colorectal Cancer.
- 132.ClinicalTrials.gov Identifier: NCT04033107. High Dose Vitamin C Combined With Metformin in the Treatment of Malignant Tumors.
- 133.ClinicalTrial.gov Identifier: NCT02420314. Pharmacological Ascorbate for Lung Cancer.
- 134.ClinicalTrials.gov Identifier: NCT02905591. A Phase 2 Study Adding Ascorbate to Chemotherapy and Radiation Therapy for NSCLC (XACT-LUNG).
- 135.ClinicalTrials.gov Identifier: NCT03602235. High Dose Ascorbic Acid for Plasma Cell Disorders.
- 136.ClinicalTrials.gov Identifier: NCT03418038. Ascorbic Acid and Combination Chemotherapy in Treating Patients With Relapsed or Refractory Lymphoma.
- 137.ClinicalTrials.gov Identifier: NCT02905578. A Phase 2 Trial of High-dose Ascorbate for Pancreatic Cancer (PACMAN 2.1).
- 138.ClinicalTrials.gov Identifier: NCT04150042. A Study of Melphalan, BCNU, Vitamin B12b, Vitamin C, and Stem Cell Infusion in People With Advanced Pancreatic Cancer and BRCA Mutations.
- 139.ClinicalTrials.gov Identifier: NCT03410030. Trial of Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) (AA NABPLAGEM).
- 140.ClinicalTrials.gov Identifier: NCT02516670. Docetaxel With or Without Ascorbic Acid in Treating Patients With Metastatic Prostate Cancer.
- 141.ClinicalTrials.gov Identifier: NCT03334409. Pazopanib Hydrochloride With or Without Ascorbic Acid in Treating Patients With Kidney Cancer That Is Metastatic or Cannot Be Removed by Surgery.
- 142.ClinicalTrials.gov Identifier: NCT04634227. Gemcitabine Plus Ascorbate for Sarcoma in Adults (Pilot).
- 143.ClinicalTrials.gov Identifier: NCT03508726. High Dose Ascorbate With Preoperative Radiation in Patients With Locally Advanced Soft Tissue Sarcomas.
- 144.ClinicalTrials.gov Identifier: NCT03799094. Vitamin C and Tyrosine Kinase Inhibitor in Lung Cancer Patients With Epidermal Growth Factor Receptor Mutations.
- 145.ClinicalTrial.gov Identifier: NCT00228319. Treatment of Newly Diagnosed Ovarian Cancer With Antioxidants.
- 146.ClinicalTrial.gov Identifier: NCT01364805. New Treatment Option for Pancreatic Cancer.
- 147.ClinicalTrial.gov Identifier: NCT01852890. Gemcitabine, Ascorbate, Radiation Therapy for Pancreatic Cancer, Phase I.
- 148.Bruckner H, Hirschfeld A, Gurell D, Lee K. Broad safety impact of high-dose ascorbic acid and induction chemotherapy for high-risk pancreatic cancer. J Clin Oncol. 2017;35(15_suppl):e15711. doi: 10.1200/JCO.2017.35.15_suppl.e15711. [DOI] [Google Scholar]
- 149.ClinicalTrial.gov Identifier: NCT01905150. Ph 2 Trial of Vitamin C & G-FLIP (Low Doses Gemcitabine, 5FU, Leucovorin, Irinotecan, Oxaliplatin) for Pancreatic Cancer.
- 150.ClinicalTrial.gov Identifier: NCT01049880. A Research Trial of High Dose Vitamin C and Chemotherapy for Metastatic Pancreatic Cancer.
- 151.Wang F, He M-M, Wang Z-X, Li S, Jin Y, Ren C, et al. Phase I study of high-dose ascorbic acid with mFOLFOX6 or FOLFIRI in patients with metastatic colorectal cancer or gastric cancer. BMC Cancer. 2019;19(1):460. doi: 10.1186/s12885-019-5696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.ClinicalTrials.gov Identifier: NCT02969681. Vitamin C Intravenously With Chemotherapy in Advanced Colorectal Cancer (Vitality).
- 153.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, et al. Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. Perez-Gracia JL, editor. PLoS One. 2012;7(1):e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.ClinicalTrial.gov Identifier: NCT00954525. Intravenous Vitamin C in Combination With Standard Chemotherapy for Pancreatic Cancer.
- 155.Kawada H, Sawanobori M, Tsuma-kaneko M, Wasada I, Miyamoto M, Murayama H, et al. Phase I clinical trial of intravenous L-ascorbic acid following salvage chemotherapy for relapsed B-cell non-Hodgkin’s lymphoma - PubMed. Tokai J Exp Clin Med. 2014;20(39):111–115. [PubMed] [Google Scholar]
- 156.Ou J, Zhu X, Lu Y, Zhao C, Zhang H, Wang X, et al. The safety and pharmacokinetics of high dose intravenous ascorbic acid synergy with modulated electrohyperthermia in Chinese patients with stage III-IV non-small cell lung cancer. Eur J Pharm Sci. 2017;109:412–418. doi: 10.1016/j.ejps.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 157.Ou J, Zhu X, Chen P, Du Y, Lu Y, Peng X, et al. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J Adv Res. 2020;24:175–182. doi: 10.1016/j.jare.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.ClinicalTrial.gov Identifier: NCT02655913. Safety and Efficacy of Vitamin C Infusion in Combination With Local mEHT to Treat Non Small Cell Lung Cancer (VCONSCLC).
- 159.Lorenzato A, Magrì A, Matafora V, Audrito V, Arcella P, Lazzari L, et al. Vitamin C restricts the emergence of acquired resistance to EGFR-targeted therapies in colorectal Cancer. Cancers (Basel) 2020;12(3):685. doi: 10.3390/cancers12030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.El Banna N, Hatem E, Heneman-Masurel A, Léger T, Baïlle D, Vernis L, et al. Redox modifications of cysteine-containing proteins, cell cycle arrest and translation inhibition: involvement in vitamin C-induced breast cancer cell death. Redox Biol. 2019;26:101290. doi: 10.1016/j.redox.2019.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bober P, Tomková Z, Alexovič M, Ropovik I, Sabo J. The unfolded protein response controls endoplasmic reticulum stress-induced apoptosis of MCF-7 cells via a high dose of vitamin C treatment. Mol Biol Rep. 2019;46(1):1275–1284. doi: 10.1007/s11033-019-04598-w. [DOI] [PubMed] [Google Scholar]
- 162.Grant MM. Identification of SUMOylated proteins in neuroblastoma cells after treatment with hydrogen peroxide or ascorbate. BMB Rep. 2010;43(11):720–725. doi: 10.5483/BMBRep.2010.43.11.720. [DOI] [PubMed] [Google Scholar]
- 163.Nagappan A, Park H, Park K, Kim JA, Hong G, Kang S, et al. Proteomic analysis of differentially expressed proteins in vitamin C-treated AGS cells. BMC Biochem. 2013;14(1):24. doi: 10.1186/1471-2091-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park S, Lee J, Yeom C-H. A proteomic approach to the identification of early molecular targets changed byL-ascorbic acid in NB4 human leukemia cells. J Cell Biochem. 2006;99(6):1628–1641. doi: 10.1002/jcb.20971. [DOI] [PubMed] [Google Scholar]
- 165.Park S, Ahn ES, Lee S, Jung M, Park JH, Yi SY, et al. Proteomic analysis reveals upregulation of RKIP in S-180 implanted BALB/C mouse after treatment with ascorbic acid. J Cell Biochem. 2009;106(6):1136–1145. doi: 10.1002/jcb.22097. [DOI] [PubMed] [Google Scholar]
- 166.Lee J, Lee G, Park JH, Lee S, Yeom C-H, Na B, et al. Proteomic analysis of tumor tissue in CT-26 implanted BALB/C mouse after treatment with ascorbic acid. Cell Mol Biol Lett. 2012;17(1):62–76. doi: 10.2478/s11658-011-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bober P, Alexovic M, Talian I, Tomkova Z, Viscorova Z, Benckova M, et al. Proteomic analysis of the vitamin C effect on the doxorubicin cytotoxicity in the MCF-7 breast cancer cell line. J Cancer Res Clin Oncol. 2017;143(1):35–42. doi: 10.1007/s00432-016-2259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gustafson CB, Yang C, Dickson KM, Shao H, Van Booven D, Harbour JW, et al. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin Epigenetics. 2015;7(1):51. doi: 10.1186/s13148-015-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sant DW, Mustafi S, Gustafson CB, Chen J, Slingerland JM, Wang G. Vitamin C promotes apoptosis in breast cancer cells by increasing TRAIL expression. Sci Rep. 2018;8(1):5306. doi: 10.1038/s41598-018-23714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ge G, Peng D, Xu Z, Guan B, Xin Z, He Q, et al. Restoration of 5-hydroxymethylcytosine by ascorbate blocks kidney tumour growth. EMBO Rep. 2018;19(8):e45401. doi: 10.15252/embr.201745401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peng D, Ge G, Gong Y, Zhan Y, He S, Guan B, et al. Vitamin C increases 5-hydroxymethylcytosine level and inhibits the growth of bladder cancer. Clin Epigenetics. 2018;10(1):94. doi: 10.1186/s13148-018-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang X, Liu T, Li Z, Feng Y, Corpe C, Liu S, et al. Hepatomas are exquisitely sensitive to pharmacologic ascorbate (P-AscH-) Theranostics. 2019;9(26):8109–26. doi: 10.7150/thno.35378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pei Z, Zhang X, Ji C, Liu S-M, Wang J. Transcriptomic and functional pathways analysis of ascorbate-induced cytotoxicity and resistance of Burkitt lymphoma. Oncotarget. 2016;7(39):63950–63959. doi: 10.18632/oncotarget.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cimmino L, Neel BG, Aifantis I. Vitamin C in stem cell reprogramming and Cancer. Trends Cell Biol. 2018;28(9):698–708. doi: 10.1016/j.tcb.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim K, Pie J, Park J, Park Y, Kim H, Kim M. Retinoic acid and ascorbic acid act synergistically in inhibiting human breast cancer cell proliferation. J Nutr Biochem. 2006;17(7):454–462. doi: 10.1016/j.jnutbio.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 176.Ghanem A, Melzer AM, Zaal E, Neises L, Baltissen D, Matar O, et al. Ascorbate kills breast cancer cells by rewiring metabolism via redox imbalance and energy crisis. Free Radic Biol Med. 2021;163:196–209. doi: 10.1016/j.freeradbiomed.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 177.Uetaki M, Tabata S, Nakasuka F, Soga T, Tomita M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci Rep. 2015;5(1):13896. doi: 10.1038/srep13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin C, Dong J, Wei Z, Cheng K-K, Li J, You S, et al. 1 H NMR-based metabolic profiles delineate the anticancer effect of vitamin C and Oxaliplatin on hepatocellular carcinoma cells. J Proteome Res. 2020;19(2):781–793. doi: 10.1021/acs.jproteome.9b00635. [DOI] [PubMed] [Google Scholar]
- 179.Sarna S, Bhola RK. Chemo-immunotherapeutical studies on Dalton’s lymphoma in mice using cisplatin and ascorbic acid: synergistic antitumor effect in vivo and in vitro. Arch Immunol Ther Exp. 1993;41(5–6):327–333. [PubMed] [Google Scholar]
- 180.Kurbacher CM, Wagner U, Kolster B, Andreotti PE, Krebs D, Bruckner HW. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett. 1996;103(2):183–189. doi: 10.1016/0304-3835(96)04212-7. [DOI] [PubMed] [Google Scholar]
- 181.Kalita S, Verma AK, Prasad SB. Chlorambucil and ascorbic acid-mediated anticancer activity and hematological toxicity in Dalton’s ascites lymphoma-bearing mice. Indian J Exp Biol. 2014;52(2):112–124. [PubMed] [Google Scholar]
- 182.Frömberg A, Gutsch D, Schulze D, Vollbracht C, Weiss G, Czubayko F, et al. Ascorbate exerts anti-proliferative effects through cell cycle inhibition and sensitizes tumor cells towards cytostatic drugs. Cancer Chemother Pharmacol. 2011;67(5):1157–1166. doi: 10.1007/s00280-010-1418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, et al. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med. 2011;50(11):1610–1619. doi: 10.1016/j.freeradbiomed.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Martinotti S, Ranzato E, Burlando B. In vitro screening of synergistic ascorbate-drug combinations for the treatment of malignant mesothelioma. Toxicol Vitr. 2011;25(8):1568–1574. doi: 10.1016/j.tiv.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 185.Castro ML, McConnell MJ, Herst PM. Radiosensitisation by pharmacological ascorbate in glioblastoma multiforme cells, human glial cells, and HUVECs depends on their antioxidant and DNA repair capabilities and is not cancer specific. Free Radic Biol Med. 2014;74:200–209. doi: 10.1016/j.freeradbiomed.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 186.Giommarelli C, Corti A, Supino R, Favini E, Paolicchi A, Pompella A, et al. γ-Glutamyltransferase-dependent resistance to arsenic trioxide in melanoma cells and cellular sensitization by ascorbic acid. Free Radic Biol Med. 2009;46(11):1516–1526. doi: 10.1016/j.freeradbiomed.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 187.Du J, Cieslak JA, Welsh JL, Sibenaller ZA, Allen BG, Wagner BA, et al. Pharmacological Ascorbate Radiosensitizes pancreatic Cancer. Cancer Res. 2015;75(16):3314–3326. doi: 10.1158/0008-5472.CAN-14-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cieslak JA, Sibenaller ZA, Walsh SA, Ponto LLB, Du J, Sunderland JJ, et al. Fluorine-18-labeled thymidine positron emission tomography (FLT-PET) as an index of cell proliferation after pharmacological Ascorbate-based therapy. Radiat Res. 2016;185(1):31–38. doi: 10.1667/RR14203.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Alexander MS, O’Leary BR, Wilkes JG, Gibson AR, Wagner BA, Du J, et al. Enhanced pharmacological Ascorbate oxidation Radiosensitizes pancreatic Cancer. Radiat Res. 2018;191(1):43. doi: 10.1667/RR15189.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hosokawa Y, Saga R, Monzen S, Terashima S, Tsuruga E. Ascorbic acid does not reduce the anticancer effect of radiotherapy. Biomed Rep. 2017;6(1):103–107. doi: 10.3892/br.2016.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Grasso C, Fabre M-S, Collis SV, Castro ML, Field CS, Schleich N, et al. Pharmacological doses of daily Ascorbate protect tumors from radiation damage after a single dose of radiation in an intracranial mouse Glioma model. Front Oncol. 2014;15:4. doi: 10.3389/fonc.2014.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carr AC, Cook J. Intravenous vitamin C for Cancer therapy – identifying the current gaps in our knowledge. Front Physiol. 2018;23:9. doi: 10.3389/fphys.2018.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Demiray M. Combinatorial therapy of high dose vitamin C and PARP inhibitors in DNA repair deficiency: a series of 8 patients. Integr Cancer Ther. 2020;19:1–10. doi: 10.1177/1534735420969812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vuyyuri SB, Rinkinen J, Worden E, Shim H, Lee S, Davis KR. Ascorbic Acid and a Cytostatic Inhibitor of Glycolysis Synergistically Induce Apoptosis in Non-Small Cell Lung Cancer Cells. Chellappan SP, editor. PLoS One. 2013;8(6):e67081. doi: 10.1371/journal.pone.0067081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yiang G-T, Chou P-L, Hung Y-T, Chen J-N, Chang W-J, Yu Y-L, et al. Vitamin C enhances anticancer activity in methotrexate-treated Hep3B hepatocellular carcinoma cells. Oncol Rep. 2014;32(3):1057–1063. doi: 10.3892/or.2014.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gilloteaux J, Jamison JM, Arnold D, Taper HS, Summers JL. Ultrastructural aspects of Autoschizis: a new Cancer cell death induced by the synergistic action of Ascorbate/Menadione on human bladder carcinoma cells. Ultrastruct Pathol. 2001;25(3):183–192. doi: 10.1080/019131201300343810. [DOI] [PubMed] [Google Scholar]
- 197.Gilloteaux J, Jamison JM, Neal D, Summers JL. Synergistic antitumor cytotoxic actions of Ascorbate and Menadione on human prostate (DU145) Cancer cells in vitro: nucleus and other injuries preceding cell death by Autoschizis. Ultrastruct Pathol. 2014;38(2):116–140. doi: 10.3109/01913123.2013.852645. [DOI] [PubMed] [Google Scholar]
- 198.Noto V, Taper HS, Yi-Hua J, Janssens J, Bonte J, De Loecker W. Effects of sodium ascorbate (vitamin C) and 2-methyl-1,4-naphthoquinone (vitamin K3) treatment on human tumor cell growth in vitro. I. Synergism of combined vitamin C and K3 action. Cancer. 1989;63(5):901–906. doi: 10.1002/1097-0142(19890301)63:5<901::AID-CNCR2820630518>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 199.Venugopal M. Synergistic antitumour activity of vitamins C and K3against human prostate carcinoma cell lines. Cell Biol Int. 1996;20(12):787–797. doi: 10.1006/cbir.1996.0102. [DOI] [PubMed] [Google Scholar]
- 200.Kassouf W, Highshaw R, Nelkin GM, Dinney CP, Kamat AM. Vitamins C and K3 sensitize human Urothelial tumors to gemcitabine. J Urol. 2006;176(4):1642–1647. doi: 10.1016/j.juro.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 201.Taper HS, Keyeux A, Roberfroid M. Potentiation of radiotherapy by nontoxic pretreatment with combined vitamins C and K3 in mice bearing solid transplantable tumor. Anticancer Res. 1996;16(1):499–503. [PubMed] [Google Scholar]
- 202.Verrax J, Cadrobbi J, Delvaux M, Jamison JM, Gilloteaux J, Summers JL, et al. The association of vitamins C and K3 kills cancer cells mainly by autoschizis, a novel form of cell death. Basis for their potential use as coadjuvants in anticancer therapy. Eur J Med Chem. 2003;38(5):451–457. doi: 10.1016/S0223-5234(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 203.Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta Rev Cancer. 2012;1826(2):443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Michels AJ, Frei B. Myths, artifacts, and fatal flaws: identifying limitations and opportunities in vitamin C research. Nutrients. 2013;5(12):5161–5192. doi: 10.3390/nu5125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Heaney ML, Gardner JR, Karasavvas N, Golde DW, Scheinberg DA, Smith EA, et al. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res. 2008;68(19):8031–8038. doi: 10.1158/0008-5472.CAN-08-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145(5):532–541. doi: 10.2307/3579271. [DOI] [PubMed] [Google Scholar]
- 207.Clément M-V, Ramalingam J, Long LH, Halliwell B. The in vitro cytotoxicity of Ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid Redox Signal. 2001;3(1):157–163. doi: 10.1089/152308601750100687. [DOI] [PubMed] [Google Scholar]
- 208.Mojić M, Pristov JB, Maksimović-Ivanić D, Jones DR, Stanić M, Mijatović S, et al. Extracellular iron diminishes anticancer effects of vitamin C: an in vitro study. Sci Rep. 2015;4(1):5955. doi: 10.1038/srep05955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Doskey CM, van ‘t Erve TJ, Wagner BA, Buettner GR. Moles of a Substance per Cell Is a Highly Informative Dosing Metric in Cell Culture. PLoS One. 2015;10(7):e0132572. doi: 10.1371/journal.pone.0132572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yu R, Schellhorn HE. Recent applications of engineered animal antioxidant deficiency models in human nutrition and chronic disease. J Nutr. 2013;143(1):1–11. doi: 10.3945/jn.112.168690. [DOI] [PubMed] [Google Scholar]
- 211.Verrax J, Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med. 2009;47(1):32–40. doi: 10.1016/j.freeradbiomed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 212.Iwama M, Amano A, Shimokado K, Maruyama N, Ishigami A. Ascorbic acid levels in various tissues, plasma and urine of mice during aging. J Nutr Sci Vitaminol (Tokyo) 2012;58(3):169–174. doi: 10.3177/jnsv.58.169. [DOI] [PubMed] [Google Scholar]
- 213.Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and nutrition examination survey (NHANES) Am J Clin Nutr. 2009;90(5):1252–1263. doi: 10.3945/ajcn.2008.27016. [DOI] [PubMed] [Google Scholar]
- 214.White R, Nonis M, Pearson JF, Burgess E, Morrin HR, Pullar JM, et al. Low vitamin C status in patients with Cancer is associated with patient and tumor characteristics. Nutrients. 2020;12(8):2338. doi: 10.3390/nu12082338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fain O, Mathieu E, Thomas M. Lesson of the week: scurvy in patients with cancer. BMJ. 1998;316(7145):1661–1662. doi: 10.1136/bmj.316.7145.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Campbell EJ, Vissers MC, Dachs G. Ascorbate availability affects tumor implantation-take rate and increases tumor rejection in Gulo−/− mice. Hypoxia. 2016;4:41–52. doi: 10.2147/HP.S103088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vissers MCM, Das AB. Potential mechanisms of action for vitamin C in cancer: Reviewing the evidence. Front Physiol. 2018;9(JUL):809. doi: 10.3389/fphys.2018.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hapke RY, Haake SM. Hypoxia-induced epithelial to mesenchymal transition in cancer. Cancer Lett. 2020;487:10–20. doi: 10.1016/j.canlet.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pfeifhofer-Obermair C, Tymoszuk P, Petzer V, Weiss G, Nairz M. Iron in the Tumor Microenvironment—Connecting the Dots. Front Oncol. 2018;8(NOV):549. doi: 10.3389/fonc.2018.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cao LL, Liu H, Yue Z, Liu L, Pei L, Gu J, et al. Iron chelation inhibits cancer cell growth and modulates global histone methylation status in colorectal cancer. BioMetals. 2018;31(5):797–805. doi: 10.1007/s10534-018-0123-5. [DOI] [PubMed] [Google Scholar]
- 221.Jung M, Mertens C, Tomat E, Brüne B. Iron as a central player and promising target in Cancer progression. Int J Mol Sci. 2019;20(2):273. doi: 10.3390/ijms20020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.De Luca A, Barile A, Arciello M, Rossi L. Copper homeostasis as target of both consolidated and innovative strategies of anti-tumor therapy. J Trace Elem Med Biol. 2019;55:204–213. doi: 10.1016/j.jtemb.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 223.Chen M-F, Yang C-M, Su C-M, Hu M-L. Vitamin C protects against Cisplatin-induced nephrotoxicity and damage without reducing its effectiveness in C57BL/6 mice Xenografted with Lewis lung carcinoma. Nutr Cancer. 2014;66(7):1085–1091. doi: 10.1080/01635581.2014.948211. [DOI] [PubMed] [Google Scholar]
- 224.Du J, Wagner BA, Buettner GR, Cullen JJ. Role of labile iron in the toxicity of pharmacological ascorbate. Free Radic Biol Med. 2015;84:289–295. doi: 10.1016/j.freeradbiomed.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liberti MV, Locasale JW. The Warburg effect: how does it benefit Cancer cells? Trends Biochem Sci. 2016;41(3):211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2(1):17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cockfield JA, Schafer ZT. Antioxidant defenses: A context-specific vulnerability of cancer cells. Cancers (Basel). 2019;11(8):1208. doi: 10.3390/cancers11081208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Oberley TD, Oberley LW. Antioxidant enzyme levels in cancer. Histol Histopathol. 1997;12(2):525–535. [PubMed] [Google Scholar]
- 230.Doskey CM, Buranasudja V, Wagner BA, Wilkes JG, Du J, Cullen JJ, et al. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016;10:274–284. doi: 10.1016/j.redox.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Klingelhoeffer C, Kämmerer U, Koospal M, Mühling B, Schneider M, Kapp M, et al. Natural resistance to ascorbic acid induced oxidative stress is mainly mediated by catalase activity in human cancer cells and catalase-silencing sensitizes to oxidative stress. BMC Complement Altern Med. 2012;12(1):1–10. doi: 10.1186/1472-6882-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pawlowska E, Szczepanska J, Blasiak J. Pro- and antioxidant effects of vitamin C in Cancer in correspondence to its dietary and pharmacological concentrations. Oxidative Med Cell Longev. 2019;2019:1–18. doi: 10.1155/2019/7286737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu D, Xu Y. P53, oxidative stress, and aging. Antioxid Redox Signal. 2011;15(6):1669–1678. doi: 10.1089/ars.2010.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Freund E, Liedtke K-R, Miebach L, Wende K, Heidecke A, Kaushik NK, et al. Identification of two kinase inhibitors with synergistic toxicity with low-dose hydrogen peroxide in colorectal Cancer cells in vitro. Cancers (Basel). 2020;12(1):122. doi: 10.3390/cancers12010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.El Hassouni B, Granchi C, Vallés-Martí A, Supadmanaba IGP, Bononi G, Tuccinardi T, et al. The dichotomous role of the glycolytic metabolism pathway in cancer metastasis: interplay with the complex tumor microenvironment and novel therapeutic strategies. Semin Cancer Biol. 2020;60:238–248. doi: 10.1016/j.semcancer.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 236.Graczyk-Jarzynka A, Goral A, Muchowicz A, Zagozdzon R, Winiarska M, Bajor M, et al. Inhibition of thioredoxin-dependent H2O2 removal sensitizes malignant B-cells to pharmacological ascorbate. Redox Biol. 2019;21:101062. doi: 10.1016/j.redox.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cammack R, Wrigglesworth JM, Baum H, Ponka P, Schulman HM. In Iron Transport and Storage. Boca Raton: CRC Press; 1990. RCW. Iron-dependent enzymes in mammalian systems; pp. 17–39. [Google Scholar]
- 238.McCormick WJ. Cancer: the preconditioning factor in pathogenesis; a new etiologic approach. Arch Pediatr. 1954;71(10):313–322. [PubMed] [Google Scholar]
- 239.McCormick WJ. Cancer: a collagen disease, secondary to a nutritional deficiency. Arch Pediatr. 1959;76(4):166–171. [PubMed] [Google Scholar]
- 240.Cameron E, Rotman D. Ascorbic acid, cell proliferation, and cancer. Lancet. 1972;299(7749):542. doi: 10.1016/S0140-6736(72)90215-2. [DOI] [PubMed] [Google Scholar]
- 241.Boyera N, Galey I, Bernard BA. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int J Cosmet Sci. 1998;20(3):151–158. doi: 10.1046/j.1467-2494.1998.171747.x. [DOI] [PubMed] [Google Scholar]
- 242.Cameron E, Pauling L, Leibovitz B. Ascorbic acid and Cancer: a review. Cancer Res. 1979;39(3):663–681. [PubMed] [Google Scholar]
- 243.Cameron E, Campbell A, Jack T. The orthomolecular treatment of cancer. Chem Biol Interact. 1975;11(5):387–393. doi: 10.1016/0009-2797(75)90007-1. [DOI] [PubMed] [Google Scholar]
- 244.Jackson JA, Riordan HD, Hunninghake RE, Riordan N. High dose intravenous vitamin C and long time survival of a patient with cancer of head of the pancreas. J Orthomol Med. 1995;10(2):87–8. [Google Scholar]
- 245.Zhao L, Quan Y, Wang J, Wang F, Zheng Y, Zhou A. Vitamin C inhibit the proliferation, migration and epithelial-mesenchymal-transition of lens epithelial cells by destabilizing HIF-1α. Int J Clin Exp Med. 2015;8(9):15155–15163. [PMC free article] [PubMed] [Google Scholar]
- 246.Wilkes JG, O’Leary BR, Du J, Klinger AR, Sibenaller ZA, Doskey CM, et al. Pharmacologic ascorbate (P-AscH−) suppresses hypoxia-inducible factor-1α (HIF-1α) in pancreatic adenocarcinoma. Clin Exp Metastasis. 2018;35(1–2):37–51. doi: 10.1007/s10585-018-9876-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fischer AP, Miles SL. Ascorbic acid, but not dehydroascorbic acid increases intracellular vitamin C content to decrease hypoxia inducible factor −1 alpha activity and reduce malignant potential in human melanoma. Biomed Pharmacother. 2017;86:502–513. doi: 10.1016/j.biopha.2016.12.056. [DOI] [PubMed] [Google Scholar]
- 249.Jóźwiak P, Ciesielski P, Zaczek A, Lipińska A, Pomorski L, Wieczorek M, et al. Expression of hypoxia inducible factor 1α and 2α and its association with vitamin C level in thyroid lesions. J Biomed Sci. 2017;24(1):1–10. doi: 10.1186/s12929-017-0388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wohlrab C, MCM V, Phillips E, Morrin H, Robinson BA, Dachs GU. The association between ascorbate and the hypoxia-inducible factors in human renal cell carcinoma requires a functional von Hippel-Lindau protein. Front Oncol. 2018;8(NOV):574. doi: 10.3389/fonc.2018.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wohlrab C, Kuiper C, Vissers MC, Phillips E, Robinson BA, Dachs GU. Ascorbate modulates the hypoxic pathway by increasing intracellular activity of the HIF hydroxylases in renal cell carcinoma cells. Hypoxia. 2019;7:17–31. doi: 10.2147/HP.S201643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kuiper C, Dachs GU, Currie MJ, Vissers MCM. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic Biol Med. 2014;69:308–317. doi: 10.1016/j.freeradbiomed.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 253.Kuiper C, Dachs G, Munn D, Currie M, Robinson B, Pearson JF, et al. Increased tumor Ascorbate is associated with extended disease-free survival and decreased hypoxia-inducible Factor-1 activation in human colorectal Cancer. Front Oncol. 2014;0:10. doi: 10.3389/fonc.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kuiper C, Molenaar IGM, Dachs GU, Currie MJ, Sykes PH, Vissers MCM. Low Ascorbate levels are associated with increased hypoxia-inducible Factor-1 activity and an aggressive tumor phenotype in endometrial Cancer. Cancer Res. 2010;70(14):5749–5758. doi: 10.1158/0008-5472.CAN-10-0263. [DOI] [PubMed] [Google Scholar]
- 255.Tian W, Wang Y, Xu Y, Guo X, Wang B, Sun L, et al. The hypoxia-inducible factor renders cancer cells more sensitive to vitamin C-induced toxicity. J Biol Chem. 2014;289(6):3339–3351. doi: 10.1074/jbc.M113.538157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Klutstein M, Nejman D, Greenfield R, Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016;76:3446–3450. doi: 10.1158/0008-5472.CAN-15-3278. [DOI] [PubMed] [Google Scholar]
- 257.Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019;20:590–607. doi: 10.1038/s41580-019-0159-6. [DOI] [PubMed] [Google Scholar]
- 258.van Gorkom G, Klein Wolterink R, Van Elssen C, Wieten L, Germeraad W, Bos G. Influence of vitamin C on lymphocytes: an overview. Antioxidants. 2018;7(3):41. doi: 10.3390/antiox7030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yue X, Rao A. TET family dioxygenases and the TET activator vitamin C in immune responses and cancer. Blood. 2020;136(12):1394–1401. doi: 10.1182/blood.2019004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci. 2014;39(2):91–100. doi: 10.1016/j.tibs.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kaminska B, Czapski B, Guzik R, Król S, Gielniewski B. Consequences of IDH1/2 mutations in Gliomas and an assessment of inhibitors targeting mutated IDH proteins. Molecules. 2019;24(5):968. doi: 10.3390/molecules24050968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Montalban-Bravo G, DiNardo CD. The role ofID Hmutations in acute myeloid leukemia. Future Oncol. 2018;14(10):979–993. doi: 10.2217/fon-2017-0523. [DOI] [PubMed] [Google Scholar]
- 263.Abdel-Wahab O, Mullally A, Hedvat C, Garcia-Manero G, Patel J, Wadleigh M, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114(1):144–147. doi: 10.1182/blood-2009-03-210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cossey LN, Rahim F, Larsen CP. Oxalate nephropathy and intravenous vitamin C. Am J Kidney Dis. 2013;61(6):1032–1035. doi: 10.1053/j.ajkd.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 265.Quinn J, Gerber B, Fouche R, Kenyon K, Blom Z, Muthukanagaraj P. Effect of high-dose vitamin C infusion in a Glucose-6-phosphate dehydrogenase-deficient patient. Case Rep Med. 2017;2017:1–4. doi: 10.1155/2017/5202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.