Abstract
Therapy with nucleic acid polymers (NAPs), tenofovir disoproxil fumarate (TDF), and pegylated interferon (pegIFN) achieve high rates of HBsAg loss/seroconversion and functional cure in chronic hepatitis B virus (HBV) infection. The role of hepatitis B surface antigen (HBsAg) seroconversion and inactivation of covalently closed circular DNA (cccDNA) in establishing functional cure were examined. Archived serum from the REP 401 study was analyzed using the Abbott ARCHITECT HBsAg NEXT assay (Chicago, IL), Abbott research use–only assays for HBsAg immune complexes (HBsAg ICs), circulating HBV RNA, and the Fujirebio assay for hepatitis B core‐related antigen (HBcrAg; Malvern, PA). HBsAg became < 0.005 IU/mL in 23 participants during NAP exposure, which persisted in all participants with functional cure. HBsAg IC declined during lead‐in TDF monotherapy and correlated with minor declines in HBsAg. Following the addition of NAPs and pegIFN, minor HBsAg IC increases (n = 13) or flares (n = 2) during therapy were not correlated with HBsAg decline, hepatitis B surface antibody (anti‐HBs) titers, or alanine aminotransferase. HBsAg IC universally declined during follow‐up in participants with virologic control or functional cure. Universal declines in HBV RNA and HBcrAg during TDF monotherapy continued with NAP + pegIFN regardless of therapeutic outcome. At the end of therapy, HBV RNA was undetectable in only 5 of 14 participants with functional cure but became undetectable after removal of therapy in all participants with functional cure. Undetectable HBV RNA at the end of therapy in 5 participants was followed by relapse to virologic control or viral rebound. Conclusion: Anti‐HBs‐independent mechanisms contribute to HBsAg clearance during NAP therapy. Inactivation of cccDNA does not predict functional cure following NAP‐based therapy; however, functional cure is accompanied by persistent inactivation of cccDNA. Persistent HBsAg loss with functional cure may also reflect reduction/clearance of integrated HBV DNA. Clinicaltrials.org number NCT02565719.
Abbreviations
- ALT
alanine aminotransferase
- anti‐Hbs
hepatitis B surface antibody
- cccDNA
covalently closed circular DNA
- HBcrAg
hepatitis B core‐related antigen
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBsAg IC
HBsAg immune complex
- HBV
hepatitis B virus
- HDV
hepatitis D virus
- IgG
immunoglobulin
- LLOD
lower limit of detection
- LLOQ
lower limit of quantification
- NAP
Nucleic acid polymer
- NUC
nucleos(t)ide
- pegIFN
pegylated interferon
- pgRNA
pregenome RNA
- qHBsAg
quantitative HBsAg
- RLU
relative light unit
- RUO
Research Use Only
- SVP
subviral particle
- TDF
tenofovir disoproxil fumarate
- TND
target not detected
- ULN
upper limit of normal
Chronic hepatitis B virus (HBV) infection affects approximately 300 million people worldwide,( 1 ) and the onset of fibrosis, cirrhosis, and hepatocellular carcinoma accompanying chronic HBV infection( 2 ) is responsible for 887,000 deaths annually.( 3 ) During chronic HBV infection, abundant circulating concentrations of the hepatitis B surface antigen (HBsAg) are maintained through the production of noninfectious subviral particles (SVPs) produced separately from infectious virus,( 4 ) and which outnumber virus by a ratio of approximately 10,000:1.( 5 ) The chronicity of HBV‐derived hepatitis is maintained in good part by suppression of innate and adaptive immune function against HBV infection( 6 ) by this persistently circulating pool of SVP‐derived HBsAg.( 6 , 7 )
Restoring immune control of HBV infection so that treatment is no longer required to control liver disease (functional cure, with normal alanine aminotransferase [ALT], HBsAg < 0.05 IU/mL, and undetectable HBV DNA) requires clearance of HBsAg that persists in the absence of therapy. High rates of functional cure of HBV are not achievable with currently approved therapies.( 8 ) While nucleos(t)ide‐based (NUC) antiviral agents effectively inhibit viral replication and prevent progression of liver disease in most cases, NUCs have little effect on the circulating HBsAg due to their inability to target the production of SVP. Additionally, integration of the HBV viral genome into host chromosomes( 9 , 10 ) provides a durable reservoir of SVP production, which is not impacted by the inhibition of viral replication or inhibition of covalently closed circular DNA (cccDNA). The use of pegylated interferon (pegIFN) alone can achieve low rates of functional cure only marginally improved by its use in combination with NUCs.( 11 )
Nucleic acid polymers (NAPs) selectively block the assembly of SVP,( 12 ) effectively inhibiting HBsAg release from hepatocytes harboring either cccDNA or integrated HBV DNA.( 13 ) This effect is accompanied by reduction in intracellular HBsAg due its increased exposure to degradation as dimers.( 12 , 14 ) The effect of NAPs is limited to the inhibition of SVP assembly: no inhibition of HBV RNA or HBV DNA synthesis or production/secretion of HBV core antigen, HBV e antigen, or infectious Dane particles occurs.( 14 ) In vivo, NAP therapy leads to reduction of both viral replication in the liver and clearance of HBsAg from the blood and liver and multilog reductions in liver HBV DNA and cccDNA, effects that persist after removal of NAP therapy.( 15 , 16 , 17 ) Several clinical trials have demonstrated the reproducibility and genotype‐independent nature of this effect in patients with hepatitis B e antigen (HBeAg)–positive or HBeAg‐negative HBV mono‐infection and HBeAg‐negative HBV/hepatitis D virus (HDV) co‐infection.( 13 , 18 , 19 ) In these trials, the speed of HBsAg clearance and the proportion of patients achieving HBsAg loss was markedly improved by the addition of thymosin α1 or pegIFN, which was correlated by the appearance of restored immune function including rapid increases in hepatitis B surface antibodies (anti‐HBs) and the appearance of asymptomatic host–derived transaminase flares. The addition of pegIFN also increased the proportion of participants achieving functional cure of HBV. When used in combination with TDF and pegIFN, NAPs achieved functional cure in 39% of participants completing therapy in the REP 401 study.( 19 ) The maintenance of functional cure of HBV and HDV after removal of NAP‐based therapy has been shown to persist for at least 5 years.( 18 , 19 , 20 ) These clinical benefits were recently shown to be linked to the high prevalence of therapeutic transaminase flares in the REP 401 study.( 21 )
The persistence of cccDNA in the liver during chronic HBV infection( 22 ) and its simultaneous rapid turnover( 23 ) promote both the continued production of virions and the ability of chronic HBV infection to establish viral mutations capable of evading the immune response( 24 , 25 ) and/or the action of many direct‐acting antiviral therapies, either approved( 26 ) or in development.( 27 ) Both circulating HBV RNA( 28 , 29 ) and HBcrAg( 30 , 31 ) have been shown to be indirect markers of transcriptional activity of cccDNA within the liver. In patients co‐infected with HBV/HDV receiving a suboptimal NAP‐based combination therapy regimen, inactivation of cccDNA achieved during therapy was durably maintained after removal of therapy in 4 of 11 participants completing therapy.( 20 ) In these participants, HBsAg was maintained < 0.005 IU/mL (as determined by Abbott ARCHITECT HBsAg NEXT with lower limit of detection (LLOD) of 0.005 IU/mL) in these participants in the absence of measurable HBsAg immunocomplexes (HBsAg IC).( 20 ) To better understand the virologic responses occurring during and persisting after NAP‐based combination therapy in the REP 401 study, HBsAg reduction was evaluated using the Abbott ARCHITECT HBsAg NEXT assay, and the role of anti‐HBs in this clearance was evaluated by examining HBsAg immunocomplexes. Transcriptional inactivation of cccDNA was examined through circulating HBV RNA and HBcrAg.
Patients and Methods
The REP 401 study was a randomized, controlled, phase 2 study examining the safety and efficacy of NAP‐based combination therapy with either REP 2139‐Mg or REP 2165‐Mg, TDF, and pegIFN( 19 ) in treatment‐naïve HBeAg‐negative chronic HBV infection. The trial design is summarized in Fig. 2A and consisted of randomization of 40 participants without cirrhosis after completion of 24 weeks of TDF monotherapy into control (receiving TDF + pegIFN) or experimental (receiving TDF + pegIFN + NAPs) groups. NAP‐based combination therapy proceeded for 48 weeks in the experimental group and in the control group following crossover after HBsAg response during 24 weeks of TDF + pegIFN indicated futility of achieving functional cure in the absence of NAPs. Following removal of all therapy, all participants were followed for 48 weeks.( 19 ) The REP 401 protocol and participant recruitment and management during the study have been previously published.( 19 ) Briefly, 1 participant withdrew from therapy after the introduction of pegIFN due to depression, and 2 participants withdrew after 24 weeks of pegIFN for personal reasons not related to safety. All 3 completed follow‐up. Follow‐up of 48 weeks was completed in 33 participants; follow‐up of 24 weeks was completed in 6 participants; and follow‐up of 12 weeks was completed in 1 participant.( 19 ) The primary safety and efficacy endpoints in this study have been previously published.( 19 )
FIG. 2.
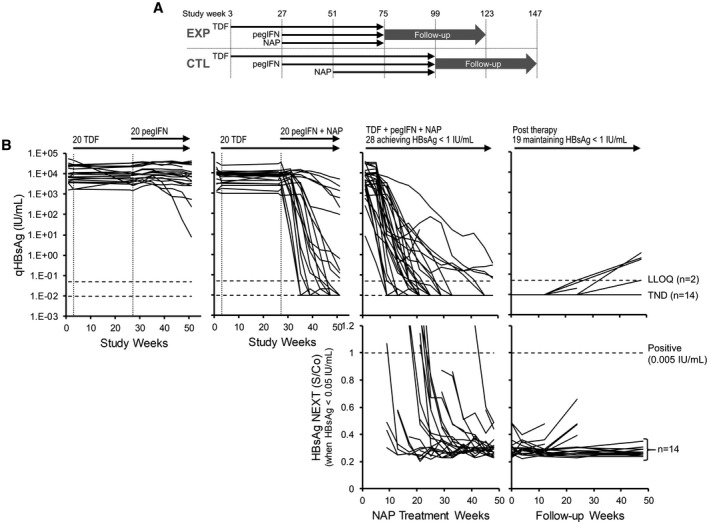
Analysis of HBsAg decline in the REP 401 study through high‐sensitivity HBsAg NEXT assay. (A) Design of the REP 401 trial. (B) qHBsAg response (top row) for all control (left) and experimental (middle left) participants during the first 48 weeks of treatment, for all participants achieving HBsAg < 1 IU/mL during 48 weeks of NAP therapy (middle right) and maintaining HBsAg < 1 IU/mL during follow‐up (right). All qHBsAg < 0.1 IU/mL were evaluated by HBsAg NEXT (bottom row). All participants achieving TND through qHBsAg also achieved HBsAg < 0.005 IU/mL by HBsAg NEXT. The number of participants maintaining HBsAg < LLOQ or TND and no increase in HBsAg S/Co during follow‐up are indicated on the right. HBsAg responses in participants experiencing viral rebound during follow‐up are presented in Supporting Fig. S1. Treatment paradigms are indicated at the top. qHBsAg responses were previously published.( 19 ) Abbreviations: CTL, control; EXP, experimental.
Serum samples obtained every 4 weeks during therapy and at each follow‐up visit were analyzed for a variety of experimental endpoints. Where quantitative HBsAg (qHBsAg; Abbott ARCHITECT) was < 0.05 IU/mL, samples were additionally tested using the qualitative Abbott ARCHITECT HBsAg NEXT assay( 32 ) with LLOD of 0.005 IU/mL. All available time points were evaluated with the Abbott Research Use Only (RUO) assay for HBsAg immunocomplexes (HBsAg IC), Abbott RUO HBV RNA (lower limit of quantification [LLOQ] = 1.65 log10 copies/mL) through quantitative real‐time polymerase chain reaction and HBcrAg (upper limit of quantification = 6.8 log10 U/mL; LLOQ = 3 log10 U/mL) using the Fujirebio Lumipulse assay.
Circulating HBV RNA was detected using the Abbott RealTime HBV‐RNA RUO assay (Abbott Diagnostics, Abbott Park, IL) as previously described.( 33 ) This dual‐target assay is designed to quantify HBV RNA from highly conserved targets in the core and X genes located at the 5′ and 3′ ends of the full‐length pregenome RNA (pgRNA), respectively. Primers are capable of amplifying RNA from both pgRNA and HBV messenger RNA. Assay values are reported as log units per milliliter, determined by calibration of the assay with a DNA secondary standard (Abbott Molecular, Des Plaines, IL) that is traceable to the World Health Organization HBV‐DNA standard, where 1 U of HBV RNA is equal to 1 IU of HBV DNA. LLOQs were calculated to be 1.81 and 1.65 log U/mL for core and X targets, respectively.
The HBsAg/anti‐HBs IC assay is an RUO assay developed for the automated Abbott ARCHITECT platform. The assay is a two‐step chemiluminescent microparticle immunoassay used for the qualitative detection of HBsAg/anti‐HBs immune complexes. In the first step, a mouse anti‐HBs monoclonal antibody is used to capture uncomplexed HBsAg or HBsAg complexed with patient‐derived anti‐HBs antibodies. In the second step, an acridinium‐labeled monoclonal antibody directed against human immunoglobulin (IgG) is added to the reaction. After a wash step to remove unbound material, pretrigger and trigger are added to the reaction to induce a fluorescent emission of the acridinium, which is measured by the instrument and reported as relative light units (RLUs). Changes in RLU signal over time in individual patients can be used to show increasing or decreasing amounts of HBsAg/anti‐HBs immune complexes.
These experimental virologic markers were evaluated in comparison with previously published qHBsAg (Abbott ARCHITECT; LLOQ = 0.05 IU/mL), anti‐HBs (Abbott ARCHITECT), HBV DNA (Abbott RealTi me HBV; LLOQ = 10 IU/mL), and ALT (upper limit of normal [ULN] = 50 U/L)( 19 ) data, which are presented in a different arrangement to facilitate interpretation.
For predictive testing of outcomes from on‐therapy milestones, published outcomes from the REP 401 study were used except that the single participant (02‐005), which was not assigned an outcome, was assigned to the virologic control group with qHBsAg = 3.04 IU/mL, anti‐HBs = 8.41 mIU/mL, HBV DNA < LLOQ, and ALT = 60 U/L (ULN = 50 U/L) at 48 weeks of follow‐up.( 19 )
For the purposes of plotting and statistical analysis, HBV RNA values of target not detected were right‐censored to 1 log10 copies/mL, and when < LLOQ were left censored to 1.5 log10 copies/mL. HBcrAg > 6.8 log10 U/mL was right‐censored to 7 log10 U/L, and < 3 log10 U/mL was left‐censored to 2.5 log10 U/mL. Statistical differences between discrete variables were assessed by Fisher’s exact test, and between means of continuous variables were assessed by Student t test or analysis of variance where appropriate. Statistical significance was considered met with P < 0.05. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Correlates Between Baseline Virology and Therapeutic Outcome
As previously published,( 19 ) 40 participants with treatment‐naïve HBeAg‐negative chronic HBV infection received 24 weeks of TDF in the REP 401 study, followed by randomization to receive 48 weeks of TDF + pegIFN + NAPs or TDF + pegIFN. After receiving 24 weeks of TDF + pegIFN, these participants were crossed over to 48 weeks of TDF + pegIFN + NAPs. At the end of 48 weeks of treatment‐free follow‐up in the REP 401 study, 14 participants had achieved functional cure, 15 had achieved virologic control, and 11 had experienced rebound.( 19 ) There was no correlation between baseline qHBsAg, HBsAg IC, ALT, HBV DNA, HBV RNA, or HBcrAg and therapeutic outcome (Fig. 1). Additionally, the levels of HBsAg IC present at baseline were not correlated with baseline HBsAg or ALT or HBsAg response during therapy (Fig. 1).
FIG. 1.
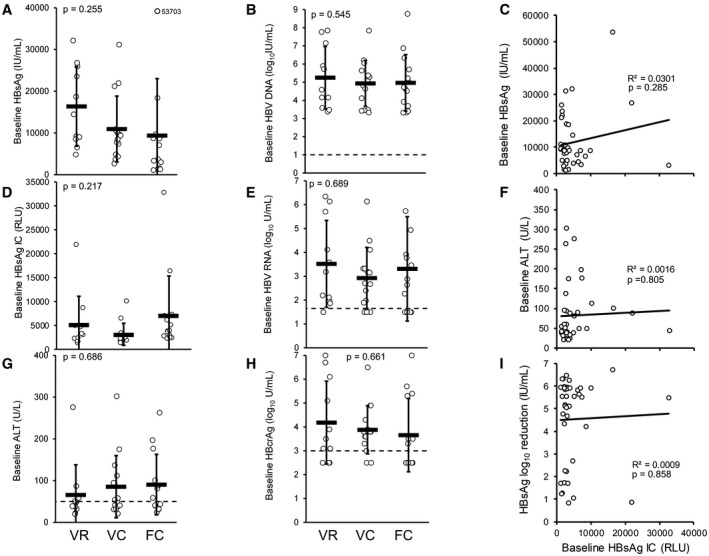
Baseline virologic parameters are not correlated with HBsAg response or therapeutic outcome in the REP 401 study. Differences between baseline HBsAg (A), HBsAg IC (B), ALT (C), HBV DNA (D), HBV pgRNA (E), and HBcrAg (F) in participants experiencing viral rebound (n = 11), virologic control (n = 15), or functional cure (FC, n = 14) after removal of therapy are presented. No significant difference in any baseline parameter between therapeutic outcomes was observed as determined by analysis of variance. Baseline levels of HBsAg IC were not correlated with baseline HBsAg (G), baseline ALT (H), or HBsAg reduction from baseline (I) during therapy. Abbreviations: FC, functional cure; VC, virologic control; VR, viral rebound.
Extent of HBsAg Reduction
During therapy in the REP 401 study, qHBsAg was observed to decline and persistently remain at levels reported as 0.00 IU/mL in 60% of participants. qHBsAg remained at this level throughout follow‐up in 35% of participants.( 19 ) For those qHBsAg results reported as < 0.05 IU/mL, HBsAg was additionally determined by HBsAg NEXT (Fig. 2B). In nine cases, the initial result of < 0.05 IU/mL during the HBsAg decline on therapy was > 0.005 IU/mL; however, the subsequent HBsAg decline during therapy in all participants achieving HBsAg < 0.05 IU/mL was shown to be < 0.005 IU/mL within 4 weeks. Additionally, HBsAg declines in all participants maintaining HBsAg < 0.05 IU/mL during follow‐up were also confirmed to be < 0.005 IU/mL (Fig. 2B). All 14 participants achieving functional cure had HBsAg < 0.005 IU/mL at the end of follow‐up (Table 1).
TABLE 1.
Virologic Status at End of Treatment and Follow‐up With Different Therapeutic Outcomes in the REP 401 Study
| Virologic Milestone | Rebound n = 11 (%) | Virologic Control n = 15* (%) | Functional Cure n = 14 (%) | P Value † | P Value † (Virologic Control vs. Functional Cure) | |
|---|---|---|---|---|---|---|
| Status at end of treatment | ||||||
| HBsAg | <10 IU/mL | 3 (27) | 11 (73) | 14 (100) | <0.001 | 0.099 |
| <1 IU/mL | 3 (27) | 11 (73) | 14 (100) | <0.001 | 0.099 | |
| <0.05 IU/mL | 1 ‡ (9) | 9 (60) | 14 (100) | <0.001 | 0.016 | |
| <0.005 IU/mL | 1 ‡ (9) | 8 (53) | 14 (100) | <0.001 | 0.006 | |
| Anti‐HBs | >10 IU/mL | 3 (27) | 8 (53) | 13 (93) | 0.002 | 0.035 |
| >100 IU/mL | 0 | 8 (53) | 10 (71) | <0.001 | 0.450 | |
| >1,000 IU/mL | 0 | 4 (27) | 8 (71) | 0.006 | 0.139 | |
| >10,000 IU/mL | 0 | 2 (13) | 8 (57) | 0.001 | 0.021 | |
| HBsAg IC < 3,000 RLU | 4 (36) | 6 (40) | 5 (29) | 1 | 1 | |
| HBV DNA | <LLOQ | 9 (82) | 11 (73) | 12 (86) | 0.792 | 0.651 |
| TND | 5 (45) | 8 (53) | 10 (71) | 0.155 | 0.217 | |
| HBV RNA | <LLOQ | 3 (27) | 12 (80) | 13 (93) | 0.001 | 0.597 |
| TND | 2 (18) | 3 (20) | 5 (36) | 0.591 | 0.427 | |
| HBcrAg < LLOQ | 6 (55) | 10 (66) | 13 (93) | 0.093 | 0.169 | |
| Status at end of follow‐up | ||||||
| HBsAg | <10 IU/mL | 0 | 8 (53) | 14 (100) | <0.001 | 0.006 |
| <1 IU/mL | 0 | 5 (33) | 14 (100) | <0.001 | 0.002 | |
| <0.05 IU/mL | 0 | 3 (20) | 14 (100) | <0.001 | <0.001 | |
| <0.005 IU/mL | 0 | 2 (13) | 14 (100) | <0.001 | <0.001 | |
| Anti‐HBs | >10 IU/mL | 1 (9) | 5 (33) | 14 (100) | <0.001 | <0.001 |
| >100 IU/mL | 0 | 2 (13) | 14 (100) | <0.001 | <0.001 | |
| >1,000 IU/mL | 0 | 0 | 14 (100) | <0.001 | <0.001 | |
| >10,000 IU/mL | 0 | 0 | 7 (50) | <0.001 | <0.001 | |
| HBsAg IC < 3,000 RLU | 3 (27) | 12 (80) | 11 § (79) | 0.014 | 1 | |
| HBV DNA | <LLOQ | 0 | 8 (53) | 14 (100) | <0.001 | <0.001 |
| TND | 0 | 4 (27) | 14 (100) | <0.001 | <0.001 | |
| HBV RNA | <LLOQ | 3 (27) | 13 (87) | 14 (100) | <0.001 | <0.001 |
| TND | 2 (18) | 5 (33) | 13 (93) | <0.001 | 0.002 | |
| HBcrAg < LLOQ | 6 (55) | 13 (87) | 14 (100) | 0.010 | 0.483 | |
Participant 02‐005 included HBsAg = 3.04 IU/mL, anti‐HBs = 8.41 mIU/mL, DNA < LLOQ, and ALT 60 U/L (ULN = 50 U/L).
Determined by Fisher’s exact test.
Participant 03‐020 self‐withdrew from therapy early for personal reasons.( 19 )
For the 3 participants with functional cure with HBsAg IC > 3,000 RLU at end of follow‐up, 2 experienced no change from baseline and 1 experienced > 1 log10 decline in HBsAg IC during follow‐up.
Abbreviations: FC, functional control; VC, virologic control.
HBsAg IC During Therapy and Follow‐Up
Given the high rate of HBsAg loss and seroconversion observed during therapy that persisted during treatment‐free follow‐up, the role of anti‐HBs in clearing HBsAg during therapy and maintaining clearance during follow‐up was assessed by analyzing changes in HBsAg IC. HBsAg IC declined significantly during TDF monotherapy in 14 of 40 participants (Figs. 3A and 4). This decline was significantly correlated with declines in HBsAg (Supporting Fig. S1). With the introduction of pegIFN in the control group or pegIFN + NAPs in the experimental group, no additional changes in HBsAg IC were observed in 39 of 40 participants (Fig. 4). During NAP therapy in the 28 participants achieving HBsAg < 1 IU/mL, HBsAg IC “flares” were observed in 2 participants (01‐007 and 02‐050) and minor but gradual increases in HBsAg IC (≥ 1.5× baseline) were observed during the last half of NAP exposure in 13 participants, but no increases in HBsAg IC were observed throughout NAP therapy in the remaining 13 participants (Fig. 4). HBsAg IC dynamics were not correlated with ALT flares or increases in anti‐HBs observed during NAP therapy (Fig. 4) nor with HBsAg declines during NAP therapy (Fig. 2B). During follow‐up, HBsAg IC continually declined in all participants maintaining HBsAg < 1 IU/mL during follow‐up (Fig. 4). No changes in HBsAg IC from baseline were observed in all 11 participants experiencing viral rebound during therapy and follow‐up (Supporting Fig. S2).
FIG. 3.
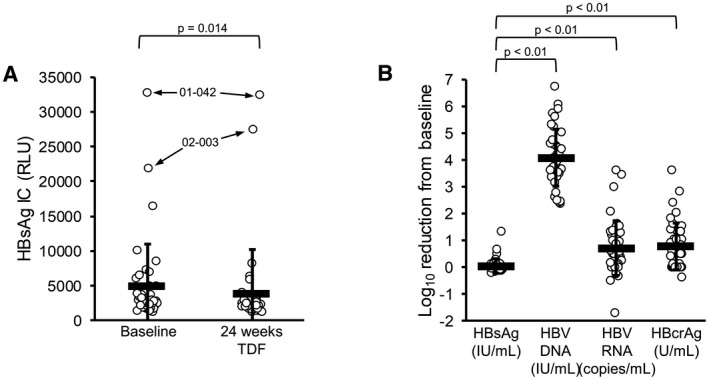
Antiviral responses during TDF monotherapy in the REP 401 study. HBsAg IC decline during TDF monotherapy (A) was statistically significant and universal except for participants 01‐042 and 02‐003. Increases in declines in HBV DNA, HBV RNA, and HBcrAg were significantly greater than for HBsAg (B).
FIG. 4.
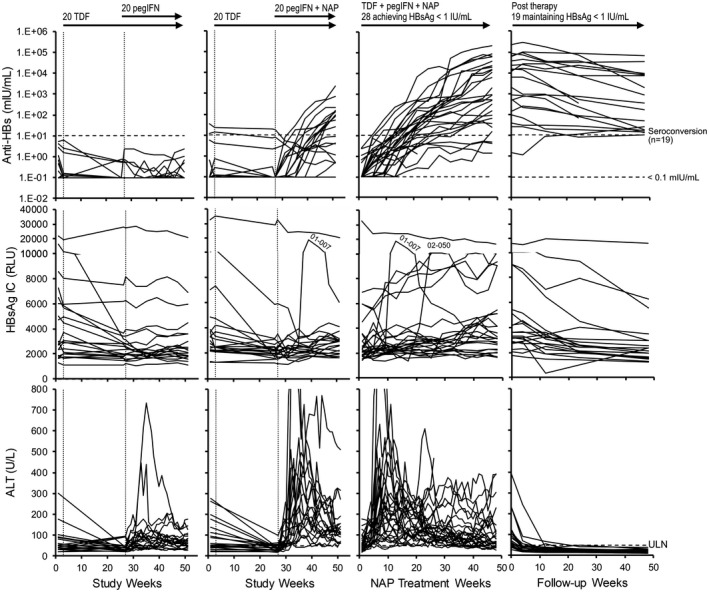
Analysis of HBsAg IC dynamics in the REP 401 study. Anti‐HBs (top row) and ALT dynamics (bottom row) are provided for comparison with HBsAg IC dynamics (middle row). Individual responses for all control (left) and experimental (middle left) participants during the first 48 weeks of treatment, for all participants achieving HBsAg < 1 IU/mL during 48 weeks of NAP therapy (middle right) and maintaining HBsAg < 1 IU/mL after removal of all therapy (right) are provided. Participants with HBsAg IC “flares” (01‐007 and 02‐050) are identified (see also insets). Participants with continuous increases in HBsAg IC during therapy and follow‐up (n = 4) are identified in bold (top, middle) and in inset (*, bottom). Treatment paradigms are indicated at the top (see also Fig. 2A). Anti‐HBs and ALT data were previously published.( 19 ) Anti‐HBs and HBsAg IC responses in all participants experiencing viral rebound during follow‐up are presented in Supporting Fig. S2.
Participants With Increased HBsAg IC During Therapy
Gradual increases in HBsAg IC occurred during the last 24 weeks of NAP therapy in 13 of 40 participants with 2 additional participants experiencing HBsAg IC “flares” (Fig. 4). The HBsAg IC flare in participant 01‐007 occurred concomitantly with the initial decline in HBsAg but not in participant 02‐050, in whom the HBsAg IC flare was observed after stable HBsAg reduction to < 0.005 IU/mL had occurred. In participants experiencing gradual minor increases in HBsAg IC near the end of NAP therapy, no changes in HBsAg were correlated with these increases. HBsAg IC declined universally during follow‐up in each participant (Fig. 5).
FIG. 5.
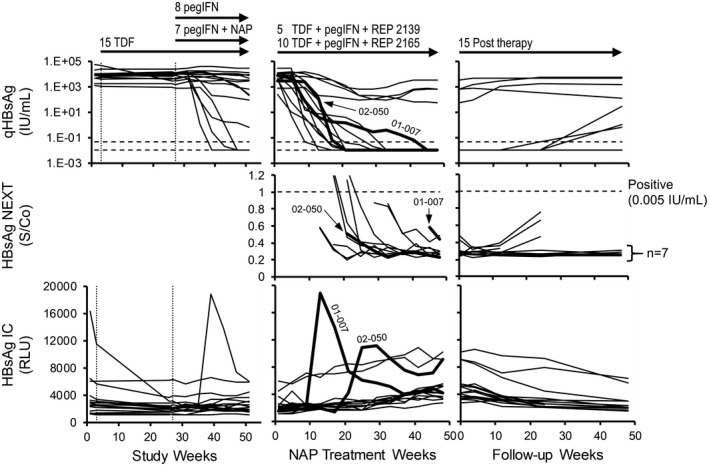
Analysis of HBsAg (top two rows) and HBsAg IC (bottom row) in participants (n = 15) with increase in HBsAg IC (≥ 1.5× baseline) during NAP therapy. Individual responses for all control and experimental participants during the first 48 weeks of therapy (left), for participants during NAP combination therapy (middle) and during follow‐up (left) are provided. Individual responses for the 2 participants experiencing “flares” in HBsAg IC during therapy (01‐007 and 02‐050) are highlighted in bold for all virologic markers examined. Treatment paradigms and proportions of control, experimental, REP 2139‐Mg, and REP 2165‐Mg are indicated at the top (see also Fig. 2A). qHBsAg responses were previously published.( 19 )
HBV RNA and HBcrAg During Therapy and Follow‐Up
Declines in HBV RNA and HBcrAg during TDF monotherapy were significantly greater than the HBsAg declines observed (Fig. 3B). HBV RNA declines ≥ 1 log10 from baseline during TDF monotherapy occurred in 11 of 40 participants with a maximum decline of 3.6 log10 copies/mL observed. HBcrAg declines ≥ 1 log10 from baseline during TDF monotherapy occurred in 15 of 40 participants with a maximum decline of 3.6 log10 U/mL observed (Figs. 3B and 6). Even when declines in HBV RNA and HBcrAg both occurred, HBsAg declines during TDF monotherapy were minimal and exceeded 1 log10 IU/mL from baseline in only 1 of 40 participants (Supporting Fig. S3). Declines in HBV RNA and HBcrAg were correlated with baseline ALT but not baseline HBsAg or HBV DNA (Supporting Fig. S4). Both HBV RNA and HBcrAg continued to decline during therapy following the introduction of pegIFN (Fig. 6), and these additional declines were not significantly different in the presence or absence of NAPs (data not shown). At the end of therapy, HBV RNA was < LLOQ in 30 of 40, target not detected (TND) in 10 of 40, and HBcrAg < LLOQ in 29 of 40 participants (Table 1). In the 29 participants maintaining HBV DNA < 2,000 IU/mL during follow‐up, HBV RNA and HBcrAg were either stable or declined in 23 of 29 and 25 of 29 participants, respectively, and in 4 participants (01‐008, 02‐015, 02‐023, and 03‐023) transient elevations in HBV RNA and HBcrAg followed the transient elevations in HBV DNA, after which virologic control was established with concomitant declines in HBV RNA and HBcrAg (Fig. 6). In the 7 participants with virologic control who experienced mild HBV‐DNA rebound (Table 1; 41‐1212 IU/mL) during follow‐up, 5 of 7 had HBV RNA < LLOQ, 0 of 7 had HBV RNA TND, and 5/7 had HBcrAg < LLOQ at the end of therapy. In the 14 participants establishing functional cure during follow‐up, HBV RNA < LLOQ was present in 13 of 14 participants, HBV RNA was not detected in 5 of 14 participants, and HBcrAg was present in 13 of 14 participants at the end of therapy (Table 1). At the end of follow‐up in the14 participants achieving functional cure, 13 of 14 were TND for HBV RNA and 14 of 14 < LLOQ for HBcrAg at the end of follow‐up (Table 1). In the 11 participants experiencing rebound during follow‐up, declines in HBV RNA and HBcrAg during therapy were indistinguishable from participants establishing virologic control or functional cure, including the observation of HBV RNA and HBcrAg declines during TDF monotherapy (Supporting Fig. S5). Rebound in HBV RNA and HBcrAg generally only occurred when HBV‐DNA rebound exceeded 104 IU/mL (Supporting Fig. S5). In the 11 participants experiencing viral rebound, 4 of 11 had HBV RNA < LLOQ, 2 of 11 had HBV RNA TND, and 6 of 11 had HBcrAg < LLOQ at the end of therapy (Table 1).
FIG. 6.
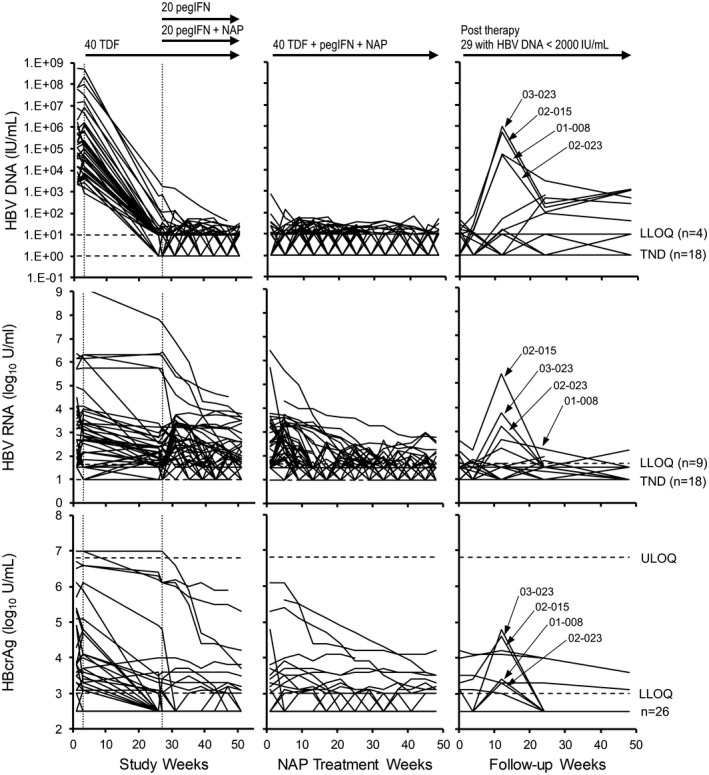
Analysis of HBV RNA and HBcrAg dynamics in the REP 401 study. HBV‐DNA dynamics (top row) are provided for comparison with HBV RNA (middle row) and HBcrAg (bottom row). Individual responses for all control and experimental participants during the first 48 weeks of therapy (left), for all participants during NAP combination therapy (middle) and maintaining HBV DNA < 2,000 IU/mL after removal of all therapy (left) are provided. The number of participants maintaining HBV DNA/HBV RNA < LLOQ or TND and HBcrAg < LLOQ during follow up are indicated on the right. Treatment paradigms are indicated at the top (see also Fig. 2A). HBV DNA, HBV RNA, and HBcrAg responses in participants experiencing viral rebound during follow‐up are presented in Supporting Fig. S2. HBV‐DNA responses were previously published.( 19 )
Relationship Between On‐Therapy Milestones and Outcomes
Differences between on‐therapy virologic milestones and therapeutic outcomes were analyzed in Table 1. The incidence of HBsAg reduction by the end of therapy at the < 10 IU/mL and < 1 IU/mL thresholds was significantly different among outcome groups overall (P < 0.001), but not between virologic control and functional cure groups. However, HBsAg reduction at the < 0.05 IU/mL and < 0.005 IU/mL thresholds was significantly more prevalent in the functional cure versus virologic control groups (P = 0.016 and P = 0.006, respectively). This was also the case for HBsAg seroconversion (>10 mIU/mL), in which anti‐HBs titers were significantly higher in functional cure versus virologic control groups at the end of follow‐up (P = 0.021). Differences in prevalence of HBV DNA < LLOQ or TND, HBV RNA TND, and HBcrAg < LLOQ at the end of therapy were not significantly different overall between outcomes or between virologic control and functional cure. HBV RNA < LLOQ was significantly different between therapeutic outcomes overall (P < 0.001) but not between functional cure and virologic control groups (P = 0.427). By the end of follow‐up, the anti‐HBs titers were greater in functional cure versus virologic control groups (P < 0.001) and the prevalence of HBV RNA < LLOQ (P < 0.001), HBV RNA TND (P < 0.001) was significantly greater in functional cure versus virologic control groups. No significant difference in HBcrAg < LLOQ at the end of follow‐up was observed between functional cure and virologic control groups (P = 0.483).
The ability of various on‐therapy milestones in predicting functional cure (functional cure vs. virologic control or viral rebound) were analyzed in Table 2. The most specific tests for predicting functional cure from other outcomes were reductions in HBsAg < 1 IU/mL (all 100%) with specificity improving as the HBsAg reduction threshold was lowered to 0.005 IU/mL (from 50% to 60.9%). HBsAg seroconversion was slightly less effective in predicting functional cure (with increased sensitivity but reduced specificity at higher anti‐HBs thresholds) followed by simultaneous HBV RNA and HBcrAg < LLOQ. Clearance of HBV DNA or HBV RNA (< LLOQ or TND) or HBcrAg < LLOQ alone were poorer predictors of functional cure (Table 2). These on‐therapy milestones did not predict other therapeutic outcomes (Supporting Tables S1 and S2).
TABLE 2.
Predictive Ability of On‐Therapy Virologic Milestones for Functional Cure* in the REP 401 Study
| On‐Therapy Milestone | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
| HBsAg | <1 IU/mL | 50.0 | 100.0 | 100.0 | 46.2 |
| <0.05 IU/mL | 58.3 | 100.0 | 100.0 | 61.5 | |
| <0.005 IU/mL | 60.9 | 100.0 | 100.0 | 65.4 | |
| Anti‐HBs | >10 IU/mL | 54.2 | 94.1 | 92.9 | 59.3 |
| >100 IU/mL | 52.6 | 81.0 | 71.4 | 65.4 | |
| >1,000 IU/mL | 66.7 | 78.6 | 57.1 | 84.6 | |
| >10,000 IU/mL | 66.7 | 78.6 | 57.1 | 84.6 | |
| HBV DNA | <LLOQ | 37.5 | 75.0 | 85.7 | 23.1 |
| TND | 43.5 | 76.5 | 71.4 | 50.0 | |
| HBV RNA | <LLOQ | 46.4 | 91.7 | 92.9 | 42.3 |
| TND | 50.0 | 70.0 | 35.7 | 80.8 | |
| HBcrAg < LLOQ | 44.8 | 90.9 | 92.9 | 38.5 | |
| HBV RNA < LLOQ + HBcrAg < LLOQ | 52.2 | 88.9 | 85.7 | 59.3 | |
| HBV RNA TND + HBcrAg < LLOQ | 31.3 | 62.5 | 35.7 | 57.7 | |
Functional cure versus virologic control or viral rebound.
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Discussion
Following NAP‐based combination therapy in the REP 401 study, rebound, virologic control, and functional cure were established in 11 of 40, 15 of 40, and 14 of 40 participants. None of the baseline virologic parameters examined were significantly different in these outcome groups at baseline. Previous evaluation of HBsAg clearance in NAP‐based combination therapy( 13 , 18 , 19 ) was limited to < 0.05 IU/mL due to the LLOQ of available qHBsAg assays.( 34 ) Evaluation of serum samples, in which HBsAg was < 0.05 IU/mL using the Abbott HBsAg NEXT assay( 32 ) with a LLOD of 0.005 IU/mL, established that all HBsAg declines to < 0.05 IU/mL during therapy in the REP 401 study (in 24 of 40 participants) were persistently maintained at < 0.005 IU/mL until the end of therapy. Moreover, the maintenance of HBsAg < 0.05 IU/mL throughout follow‐up in 16 of 40 participants was further shown to be < 0.005 IU/mL. All participants with functional cure had HBsAg < 0.005 IU/mL at the end of therapy. This level of suppression exceeds that detectable by the qualitative HBsAg assay (LLOD = 0.017‐0.022 IU/mL) and indicates that HBsAg clearance achieved during NAP‐based combination therapy and maintained after removal of therapy is profound.
HBsAg IC are commonly present in chronic HBV infection,( 35 , 36 ) and although their impact on circulating HBsAg levels is unclear, they could play a role in maintaining HBsAg suppression, especially given the very high titers of anti‐HBs produced during and maintained after removal of NAP‐based therapy.( 13 , 19 , 20 ) The HBsAg IC assay used in this study provides an aggregate measure of the total population of anti‐HBs IgG bound to HBsAg, regardless of the epitope specificity of the IgG bound. Moreover, it has been well‐established that NAPs do not interact with HBsAg or anti‐HBs,( 13 ) and therefore do not interfere in the HBsAg IC assay. qHBsAg declines occurring during NAP therapy were not correlated with baseline HBsAg IC. However, during TDF monotherapy, HBsAg IC declines in 36 of 40 participants were significantly correlated with very minor reductions in qHBsAg observed during TDF monotherapy. The observation of strong HBsAg IC signals at baseline, which disappear with TDF treatment concomitantly with small reductions in HBsAg, indicates that the mouse monoclonal antibody used is able to capture HBsAg with bound patient‐derived anti‐HBs attached. This also suggests that a small portion of HBsAg present at the start of therapy is recognized by pre‐existing circulating anti‐HBs and that production of this fraction of HBsAg disappeared during TDF monotherapy, leading to the disappearance of HBsAg IC. The rapid multilog declines in qHBsAg, and concomitant multilog increases in anti‐HBs following the first 24 weeks of NAP exposure were not accompanied by increases in HBsAg IC in 34 of 40 participants. Gradual increases in HBsAg IC in 4 of 40 participants during this period and HBsAg IC flares in 2 participants and gradual HBsAg IC increases in 13 participants during the last 24 weeks of NAP therapy were also not correlated with increases in anti‐HBs, transaminase flares, or with declines in qHBsAg except in participant 01‐007. Additionally, HBsAg IC either remained stable at low levels or steadily declined during follow‐up in all participants, indicating that remaining reservoirs for HBsAg production are being eliminated. The overall lack of correlation among HBsAg IC, anti‐HBs, and HBsAg levels in the REP 401 study suggests that the HBsAg IC present at baseline or appearing during therapy may not play a significant role in the initial clearance of HBsAg overall, and in many participants may not be involved in maintaining HBsAg clearance during therapy. Moreover, the low or declining levels of HBsAg IC in the 19 of 40 participants maintaining seroconversion during follow‐up suggests that the HBsAg clearance being persistently maintained during follow‐up may not be a result of clearance by anti‐HBs but rather the absence of HBsAg production from the liver, consistent with the persistence of HBsAg seroconversion in all participants achieving functional cure. Given the rapid turnover of HBsAg (and thus SVP) occurring in chronic HBV infection,( 37 , 38 ) it may be that other constitutive host processes are involved in clearing HBsAg from the circulation or eliminating HBsAg production in the liver. Additionally, the mild HBsAg responses observed in a small proportion of patients in this study( 19 ) and previous NAP trials( 13 , 18 ) may not be due to an impaired anti‐HBs response. These hypotheses will have to be further evaluated in additional clinical trials with NAPs and in other combination therapies able to achieve high rates of HBsAg clearance and functional cure. Additionally, the dynamics of specific epitopes recognized by different anti‐HBs in the REP 401 and other NAP clinical studies will give better insight into the role that anti‐HBs plays in clearing HBsAg from the circulation and establishing and maintaining functional cure in chronic HBV infection.
TDF monotherapy was accompanied by declines in both HBV RNA and HBcrAg, consistent with the effects of TDF monotherapy on reducing or clearing HBV RNA and HBcrAg from the blood previously reported in studies with TDF and other NUCs.( 39 , 40 , 41 , 42 ) These declines had a minor effect on circulating HBsAg, suggesting that the bulk of SVP production is occurring from integrated HBV DNA. Stronger declines in HBV RNA and HBcrAg were correlated with increased baseline ALT but not viremia, suggesting that declines in cccDNA may be driven in part by infected cell loss. These inhibitory effects on cccDNA by TDF and other NUCs may be driven by the immunostimulatory effects of purine NUCs through the purine P1 receptor( 43 ) or TLR7,( 44 ) which have been shown to induce cytokine responses in clinical trials.( 45 ) This inhibition of cccDNA activity by NUCs may also be responsible for driving the small reductions of HBsAg, which were accompanied by clearance of HBsAg IC described previously. These observations indicate that significant declines in HBV RNA and HBcrAg under TDF monotherapy can occur more rapidly than previously reported,( 42 ) and this bears further investigation in additional studies.
Continual declines in HBV RNA and HBcrAg during therapy following the introduction of pegIFN were comparable in the presence or absence of NAPs and in participants in all therapeutic outcomes, suggesting that these declines are mediated primarily by TDF and pegIFN. Although fewer participants in the viral rebound group achieved HBV RNA and HBcrAg < LLOQ than virologic control or functional cure groups, the proportion of participants achieving these endpoints on therapy were not significantly different between virologic control and functional cure groups. Additionally, there was no significant difference in the proportion participants achieving HBV‐RNA TND at the end of therapy among all outcome groups. HBV‐DNA rebound during follow‐up was observed in 18 participants, with 17 of 18 having detectable HBsAg (0.07‐3510 IU/mL) present at the end of therapy (the 1 participant with HBsAg < 0.005 IU/mL withdrew early after the addition of NAP therapy( 19 )). Of these participants, 9 of 18 had HBV RNA < LLOQ, 2 of 18 had HBV‐RNA TND, and 11 of 18 had HBcrAg < LLOQ at the end of therapy. Importantly, treatment with NUCs and pegIFN is associated with contraction of the cccDNA pool,( 46 , 47 , 48 ) suggesting that transcriptional inactivation of cccDNA in and of itself may not be capable of establishing functional cure. Additionally, a recent report( 49 ) has demonstrated the elimination of detectable cccDNA in liver biopsies and of HBV RNA and HBcrAg in the circulation with NUCs, with HBsAg persisting from integrated HBV DNA, which supports the production of SVP but not virus.( 9 , 10 ) The rapid rebound of viral infection observed following NUC withdrawal in these subjects( 49 ) indicates that highly efficient (but likely incomplete) removal of cccDNA from the liver is also insufficient to achieve functional cure. This may be due to the persistence of transcriptionally inactive (“latent”) cccDNA and HBsAg, which prevents host control of reactivation of this latent cccDNA. The possibility that existing assays for HBV RNA, HBcrAg, and intrahepatic cccDNA are not sensitive enough to detect functional cure cannot be excluded, indicating that further investigation is warranted in additional clinical studies with assays with improved sensitivity. Interestingly, while HBV RNA was TND at the end of follow‐up in 5 of 15 virologic control participants, HBV DNA in these participants ranged from TND to 1,212 IU/mL while in the functional cure group, 13 of 14 participants had HBV RNA TND, all with HBV DNA TND consistent with the increased sensitivity of the HBV‐DNA assay. Previous in vivo studies have demonstrated removal of cccDNA from the liver during NAP therapy, which is durable after removal of therapy.( 15 , 16 , 17 ) While HBcrAg has been shown to be predictive of cccDNA levels in the liver in chronic HBV infection in humans,( 30 ) the clearance of serum HBcrAg may not reflect cccDNA levels during and after NAP‐based combination therapy. While the durable nature of cccDNA inactivation following removal of NAP‐based therapy has been shown in this and other studies,( 20 ) and contraction of the cccDNA pool has been observed in vivo with NAPs, evaluation of changes in cccDNA levels in liver biopsies will be required to establish how NAPs influence cccDNA liver burden in humans.
The best on‐therapy predictor of functional cure was HBsAg loss, with 100% specificity at < 1, 0.05, and 0.005 IU/mL, with sensitivity increasing with lower HBsAg threshold, reaching 65.5% at < 0.005 IU/mL. This suggests that increasing sensitivity in HBsAg assays may lead to improved specificity and should be examined with HBsAg assays having LLOD < 0.005 IU/mL. This improved sensitivity may be due to an improved ability to discriminate between virologic control and functional cure. HBsAg seroconversion during therapy was also a good predictor of functional cure. However, given that evidence for anti‐HBs participating in HBsAg clearance during therapy was not observed, the protective ability of these circulating antibodies is uncertain. The correlation of HBsAg seroconversion with functional cure may reflect a concomitant increase of anti‐HBs with other immunological processes important for establishing functional cure, but this has not yet been evaluated. The overall poor ability of HBV‐RNA/HBcrAg clearance during therapy to predict functional cure is consistent with rebound in viral HBV DNA during follow‐up in participants with HBV‐RNA/HBcrAg clearance in this and other studies.( 49 ) Although these data suggesting the utility of HBsAg clearance in predicting functional cure agree with other recent data,( 50 ) the utility of this virologic milestones in predicting functional cure will need to be examined in additional studies.
In these HBeAg‐negative patients, the following levels of HBV markers were observed simultaneously in numerous patients (HBcrAg < LLOQ, HBV RNA < LLOQ, HBV DNA TND and HBsAg < 0.005 IU/mL), all in the absence of any detectable levels of HBsAg IC. Notwithstanding the limitations in the sensitivity of the HBV‐RNA and HBcrAg assays, the excellent suppression of HBV DNA and HBsAg in the absence of evidence of active HBsAg clearance by anti‐HBs strongly argues that not only is cccDNA suppressed (not eliminated) but the removal of hepatocytes with integrated HBV DNA (also a source of HBsAg) must also have occurred. This control of liver infection may also be due in part to the high incidence of strong host‐derived transaminase flares( 21 ) and appears to persist in all participants achieving functional cure.
The experiment analyses performed in the REP 401 study demonstrate that HBsAg clearance is more robust than previously reported, and that this clearance is not entirely mediated by anti‐HBs. The maintenance of HBsAg loss in the absence of HBsAg IC is consistent with removal on integrated HBV DNA from the liver. Clearance of correlates for cccDNA transcription during therapy do not predict functional cure of HBV during NAP‐based combination therapy but HBsAg loss during therapy does, indicating the critical importance of clearing HBsAg to achieve functional cure.
Supporting information
Supplementary Material
Supported by Replicore Inc.
Potential conflict of interest: Dr. Anderson owns stock in and is employed by Abbott Laboratories. Dr. Bazinet is an employee and shareholder in Replicor Inc. Dr. Cloherty owns stock in and is employed by Abbott Laboratories. Dr. Gersch owns stock in and is employed by Abbott Laboratories. Dr. Holzmayer owns stock in and is employed by Abbott Laboratories. Dr. Kuhns owns stock in and is employed by Abbott Laboratories. Dr. Vaillant is an employee and shareholder in Replicor Inc.
REP 401 registration number: NCT02565719.
References
- 1. Razavi‐Shearer D, Gamkrelidze I, Nguyen MH, Chen D‐S, Van Damme P, Abbas Z, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383‐403. [DOI] [PubMed] [Google Scholar]
- 2. Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet 2018;392:2313‐2324. [DOI] [PubMed] [Google Scholar]
- 3. WHO . Hepatitis B fact sheet. Geneva, Switzerland: World Heath Organization; 2019. [Google Scholar]
- 4. Patient R, Hourioux C, Sizaret PY, Trassard S, Sureau C, Roingeard P. Hepatitis B virus subviral envelope particle morphogenesis and intracellular trafficking. J Virol 2007;81:3842‐3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J 2013;10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: a review. World J Gastroenterol 2019;25:3527‐3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vaillant A. HBsAg, subviral particles, and their clearance in establishing a functional cure of chronic hepatitis B virus infection. ACS Infect Dis 2021;7:1351‐1368. [DOI] [PubMed] [Google Scholar]
- 8. Bhattacharya D, Thio CL. Review of hepatitis B therapeutics. Clin Infect Dis 2010;51:1201‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tu T, Budzinska M, Shackel N, Urban S. HBV DNA integration: molecular mechanisms and clinical implications. Viruses 2017;9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu B, Wang R, Fu J, Su M, Du M, Liu Y, et al. Integration of hepatitis B virus S gene impacts on hepatitis B surface antigen levels in patients with antiviral therapy. J Gastroenterol Hepatol 2018;33:1389‐1396. [DOI] [PubMed] [Google Scholar]
- 11. Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha‐2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology 2016;150:134‐144.e110. [DOI] [PubMed] [Google Scholar]
- 12. Blanchet M, Sinnathamby V, Vaillant A, Labonte P. Inhibition of HBsAg secretion by nucleic acid polymers in HepG2.2.15cells. Antiviral Res 2019;164:97‐105. [DOI] [PubMed] [Google Scholar]
- 13. Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa‐2a for treatment‐naive patients with chronic hepatitis B virus and hepatitis D virus co‐infection (REP 301 and REP 301‐LTF): a non‐randomised, open‐label, phase 2 trial. Lancet Gastroenterol Hepatol 2017;2:877‐889. [DOI] [PubMed] [Google Scholar]
- 14. Boulon R, Blanchet M, Lemasson M, Vaillant A, Labonte P. Characterization of the antiviral effects of REP 2139 on the HBV lifecycle in vitro. Antiviral Res 2020;183:104853. [DOI] [PubMed] [Google Scholar]
- 15. Noordeen F, Scougall CA, Grosse A, Qiao Q, Ajilian BB, Reaiche‐Miller G, et al. Therapeutic antiviral effect of the nucleic acid polymer REP 2055 against persistent duck hepatitis B virus infection. PLoS One 2015;10:e0140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roehl I, Seiffert S, Brikh C, Quinet J, Jamard C, Dorfler N, et al. Nucleic acid polymers with accelerated plasma and tissue clearance for chronic hepatitis B therapy. Mol Ther Nucleic Acids 2017;8:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quinet J, Jamard C, Burtin M, Lemasson M, Guerret S, Sureau C, et al. Nucleic acid polymer REP 2139 and nucleos(T)ide analogues act synergistically against chronic hepadnaviral infection in vivo in Pekin ducks. Hepatology 2018;67:2127‐2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al‐Mahtab M, Bazinet M, Vaillant A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment‐naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS One 2016;11:e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bazinet M, Pantea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon Alfa‐2a in patients with chronic HBV infection naive to nucleos(t)ide therapy. Gastroenterology 2020;158:2180‐2194. [DOI] [PubMed] [Google Scholar]
- 20. Bazinet M, Pantea V, Cebotarescu V, Cojuhari L, Jimbei P, Anderson M, et al. Persistent control of HBV and HDV infection following REP 2139‐Ca and pegIFN therapy in chronic HBV/HDV co‐infection. Hepatol Commun 2021;5:189‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, et al. Benefit of transaminase elevations in establishing functional cure of HBV infection during NAP‐based combination therapy. J Viral Hepat 2021;28:817‐825. [DOI] [PubMed] [Google Scholar]
- 22. Werle‐Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004;126:1750‐1758. [DOI] [PubMed] [Google Scholar]
- 23. Huang QI, Zhou B, Cai D, Zong Y, Wu Y, Liu S, et al. Rapid turnover of hepatitis B virus covalently closed circular DNA indicated by monitoring emergence and reversion of signature‐mutation in treated chronic hepatitis B patients. Hepatology 2021;73:41‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aragri M, Alteri C, Battisti A, Di Carlo D, Minichini C, Sagnelli C, et al. Multiple hepatitis B virus (HBV) quasispecies and immune‐escape mutations are present in HBV surface antigen and reverse transcriptase of patients with acute hepatitis B. J Infect Dis 2016;213:1897‐1905. [DOI] [PubMed] [Google Scholar]
- 25. Colagrossi L, Hermans LE, Salpini R, Di Carlo D, Pas SD, Alvarez M, et al. Immune‐escape mutations and stop‐codons in HBsAg develop in a large proportion of patients with chronic HBV infection exposed to anti‐HBV drugs in Europe. BMC Infect Dis 2018;18:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim YS. Management of antiviral resistance in chronic hepatitis B. Gut Liv 2017;11:189‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruan L, Hadden JA, Zlotnick A. Assembly properties of hepatitis B virus core protein mutants correlate with their resistance to assembly‐directed antivirals. J Virol 2018;92:e01082‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu S, Zhou B, Valdes JD, Sun J, Guo H. Serum hepatitis B virus RNA: a new potential biomarker for chronic hepatitis B virus infection. Hepatology 2019;69:1816‐1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin NI, Ye A, Lin J, Liu C, Huang J, Fu YA, et al. Diagnostic value of detection of pregenomic RNA in sera of hepatitis B virus‐infected patients with different clinical outcomes. J Clin Microbiol 2020;58:e01275‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong D‐H, Seto W‐K, Cheung K‐S, Chong C‐K, Huang F‐Y, Fung J, et al. Hepatitis B virus core‐related antigen as a surrogate marker for covalently closed circular DNA. Liver Int 2017;37:995‐1001. [DOI] [PubMed] [Google Scholar]
- 31. Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, et al. Serum hepatitis B core‐related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol 2019;70:615‐625. [DOI] [PubMed] [Google Scholar]
- 32. Lou S, Taylor R, Pearce S, Kuhns M, Leary T. An ultra‐sensitive Abbott ARCHITECT((R)) assay for the detection of hepatitis B virus surface antigen (HBsAg). J Clin Virol 2018;105:18‐25. [DOI] [PubMed] [Google Scholar]
- 33. Butler EK, Gersch J, McNamara A, Luk K‐C, Holzmayer V, de Medina M, et al. Hepatitis B virus serum DNA and RNA levels in nucleos(t)ide analog‐treated or untreated patients during chronic and acute infection. Hepatology 2018;68:2106‐2117. [DOI] [PubMed] [Google Scholar]
- 34. Tuaillon E, Mondain A‐M, Nagot N, Ottomani L, Kania D, Nogue E, et al. Comparison of serum HBsAg quantitation by four immunoassays, and relationships of HBsAg level with HBV replication and HBV genotypes. PLoS One 2012;7:e32143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim SA, Lee SI, Choi IH, Shin JS, Uhm JR, Kim SJ, et al. Circulating immune complexes and cell‐mediated immunity in patients with hepatitis B virus associated liver diseases. Yonsei Med J 1990;31:347‐358. [DOI] [PubMed] [Google Scholar]
- 36. Tsai J‐F, Margolis HS, Jeng J‐E, Ho M‐S, Chang W‐Y, Hsieh M‐Y, et al. Immunoglobulin‐ and hepatitis B surface antigen‐specific circulating immune complexes in chronic hepatitis B virus infection. Clin Immunol Immunopathol 1998;86:246‐251. [DOI] [PubMed] [Google Scholar]
- 37. Loomba R, Decaris M, Li KW, Shankaran M, Mohammed H, Matthews M, et al. Discovery of half‐life of circulating hepatitis B surface antigen in patients with chronic hepatitis B infection using heavy water labeling. Clin Infect Dis 2019;69:542‐545. [DOI] [PubMed] [Google Scholar]
- 38. Shekhtman L, Cotler SJ, Hershkovich L, Uprichard SL, Bazinet M, Pantea V, et al. Modelling hepatitis D virus RNA and HBsAg dynamics during nucleic acid polymer monotherapy suggest rapid turnover of HBsAg. Sci Rep 2020;10:7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Bömmel F, Bartens A, Mysickova A, Hofmann J, Krüger DH, Berg T, et al. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology 2015;61:66‐76. [DOI] [PubMed] [Google Scholar]
- 40. van Campenhout MJ , Brouwer WP, van Oord GW , Xie Q, Zhang Q, Zhang N, et al. Hepatitis B core‐related antigen levels are associated with response to entecavir and peginterferon add‐on therapy in hepatitis B e antigen‐positive chronic hepatitis B patients. Clin Microbiol Infect 2016;22:571.e5‐e9. [DOI] [PubMed] [Google Scholar]
- 41. Lam Y‐F, Seto W‐K, Wong D, Cheung K‐S, Fung J, Mak L‐Y, et al. Seven‐year treatment outcome of entecavir in a real‐world cohort: effects on clinical parameters, HBsAg and HBcrAg levels. Clin Transl Gastroenterol 2017;8:e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carey I, Gersch J, Wang B, Moigboi C, Kuhns M, Cloherty G, et al. Pre‐genomic HBV RNA and HBcrAg predict outcomes in HBeAg negative chronic hepatitis B patients suppressed on nucleos(t)ide analogue therapy. Hepatology 2020;72:42‐57. [DOI] [PubMed] [Google Scholar]
- 43. Kmonickova E, Potmesil P, Holy A, Zidek Z. Purine P1 receptor‐dependent immunostimulatory effects of antiviral acyclic analogues of adenine and 2,6‐diaminopurine. Eur J Pharmacol 2006;530:179‐187. [DOI] [PubMed] [Google Scholar]
- 44. Davenne T, Bridgeman A, Rigby RE, Rehwinkel J. Deoxyguanosine is a TLR7 agonist. Eur J Immunol 2020;50:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murata K, Asano M, Matsumoto A, Sugiyama M, Nishida N, Tanaka E, et al. Induction of IFN‐lambda3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut 2018;67:362‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu S, Zheng H, Huang Y, Li B, Dong Z. The effect of peginterferon alpha‐2a vs. interferon alpha‐2a on intrahepatic covalently closed circular DNA in HBeAg‐positive chronic hepatitis B patients. Clin Res Hepatol Gastroenterol 2016;40:304‐308. [DOI] [PubMed] [Google Scholar]
- 47. Mu DI, Yuan F‐C, Chen YU, Jiang X‐Y, Yan L, Jiang L‐Y, et al. Baseline value of intrahepatic HBV DNA over cccDNA predicts patient's response to interferon therapy. Sci Rep 2017;7:5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lai C‐L, Wong D, Ip P, Kopaniszen M, Seto W‐K, Fung J, et al. Reduction of covalently closed circular DNA with long‐term nucleos(t)ide analogue treatment in chronic hepatitis B. J Hepatol 2017;66:275‐281. [DOI] [PubMed] [Google Scholar]
- 49. Lai CL, Wong DK, Wong GT, Seto WK, Fung J, Yuen MF. Rebound of HBV DNA after cessation of nucleos/tide analogues in chronic hepatitis B patients with undetectable covalently closed. JHEP Rep 2020;2:100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anderson RT, Choi HSJ, Lenz O, Peters MG, Janssen HLA, Mishra P, et al. Association between seroclearance of hepatitis B surface antigen and long‐term clinical outcomes of patients with chronic hepatitis B virus infection: systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2021;19:463‐472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


