Abstract
In mammals, the placenta mediates maternal–fetal nutrient and waste exchange and acts in an immunomodulatory way to facilitate maternal–fetal tolerance. The placenta is highly diverse across mammalian species, yet the molecular mechanisms that distinguish the placenta of human from other mammals are not fully understood. Using an interspecies transcriptomic comparison of human, macaque, and mouse late-gestation placentae, we identified hundreds of genes with lineage-specific expression—including dozens that are placentally enriched and potentially related to pregnancy. We further annotated the enhancers for different human tissues using epigenomic data and demonstrate that the placenta and chorion are unique in that their enhancers display the least conservation. We identified numerous lineage-specific human placental enhancers and found they highly overlap with specific families of endogenous retroviruses (ERVs), including MER21A, MER41A/B, and MER39B that were previously linked to immune response and placental function. Among these ERV families, we further demonstrate that MER41A/B insertions create dozens of lineage-specific serum response factor-binding loci in human, including one adjacent to FBN2, a placenta-specific gene with increased expression in humans that produces the peptide hormone placensin to stimulate glucose secretion and trophoblast invasion. Overall, our results demonstrate the prevalence of lineage-specific placental enhancers which are frequently associated with ERV insertions and likely facilitate the lineage-specific evolution of the mammalian placenta.
Keywords: placenta evolution, endogenous retrovirus, regulatory element, transcription, MER41, serum response factor
Introduction
The placenta is an extraembryonic organ essential for fetal development that facilitates maternal–fetal nutrient and waste exchange and at the time has immunomodulatory activities that enable maternal–fetal tolerance (Maltepe and Fisher 2015). As a transient tissue that is discarded after birth, the placenta has unique features including the tumor-like invasion of the maternal uterus (Beaman et al. 2016) and the ability to escape from maternal immune attack (Ander et al. 2019). Despite its common origin in all eutherian mammals, the eutherian placenta is extraordinarily diverse in its morphology, function, and molecular features among mammalian species (Mossman 1937, 1987; Ramsey 1982). For example, the trophoblast invasion process occurs relatively late and with less degree in mice compared with humans, and the gestation length of mice is much shorter than humans (Malassine et al. 2003). Further, the mature placentae for mouse and human are structurally distinct (Rossant and Cross 2001): mice have a single cotyledon whereas humans have multiple which consolidate into a cluster, and mice have three trophoblast cell layers (two syncytial layers and one single mononuclear cell layer) yet humans only have two layers (one syncytial layer and one trophoblast stem cell layer). Remarkable differences between the placenta of human and other primates have also been documented, for example, the human-specific B KIR haplotypes and Siglec-6 and IMUP-2 expression (Schmidt et al. 2015). These morphological and molecular differences limit the insights that animal models can provide for human pregnancy. Clarifying the molecular features unique to the human placenta is of great importance for better understanding of human development and evolution.
The immense diversification of mammalian placentae is reflected molecularly at the level of species-specific proteins and regulatory elements. One striking example is the lineage-specific co-option of endogenous retroviral envelope genes as “syncytin” genes, which are essential for trophoblast cell fusion (Mi et al. 2000) and probably related to maternofetal tolerance (Mangeney et al. 2007). These exapted syncytin genes have been identified in lineages including humans, mice, cows, sheep, and rabbits, but not in pigs and horses which do not form a multinucleated syncytiotrophoblast layer (Furukawa et al. 2014; Denner 2016). Endogenous retroviruses (ERVs) are one class of Transposable elements (TEs) (that also include LINEs, SINEs, and DNA transposons) that make up more than half of human genome and have been documented as critical facilitators of genome evolution (Feschotte 2008; Chuong et al. 2016a). Co-option of TEs, in particular ERVs, seem to have been especially important for placenta evolution, since several other placental-specific genes, such as PEG10 and RTL1, are also co-opted from ERVs (Rawn and Cross 2008). In addition to producing functional proteins, ERVs can also be co-opted as alternative promoters and enhancers (Cohen et al. 2009). For example, the placental-specific transcription of CYP19A1, which controls estrogen synthesis, is driven by an alternative promoter created by MER21A insertion during primate evolution (van de Lagemaat et al. 2003).
Many TEs inherently possess transcription factor-binding sites and can quickly accumulate across the genome via a copy-and-paste mechanism, allowing TEs to be adopted as lineage-specific regulatory elements during evolution (Feschotte 2008). Lineage-specific TE insertions have been reported to facilitate the evolution of different regulatory networks including those for innate immunity (Chuong et al. 2016b), p53 binding (Wang et al. 2007), embryonic development (Kunarso et al. 2010; Macfarlan et al. 2012; Fuentes et al. 2018), germline (Sakashita et al. 2020), and pregnancy (Lynch et al. 2011; Chuong et al. 2013). Previous comparisons of mouse and rat trophoblast stem cells (TSCs) suggest that species-specific TSC enhancers are highly enriched for ERVs, with RLTR13D elements contributing to hundreds of mouse-specific enhancers bound by core trophoblast factors Cdx2, Eomes, and Elf5 (Chuong et al. 2013). Our previous study suggests that an anthropoid primate-specific insertion of a THE1B family ERV element controls the placental expression of CRH and alters gestation length (Dunn-Fletcher et al. 2018). TEs were also reported to transform the uterine regulatory landscape and transcriptome during the evolution of mammalian pregnancy (Lynch et al. 2011). However, a comprehensive assessment of how the transcriptional and regulatory elements evolve in human placenta and how TEs contribute to this evolutionary process is still lacking.
In this study, we performed a systematic interspecies comparison of the placental transcriptomes and epigenetically defined putative enhancers among human, macaque, and mouse. We identified hundreds of lineage-specific placental genes and numerous lineage-specific placental enhancers, which may underly the species-specific characteristics of human placenta. By providing a comprehensive view of the transcriptional and regulatory landscape in human, macaque, and mouse placentae, we demonstrate the prevalence of lineage-specific placental enhancers that are frequently associated with ERV insertions. These ERV-derived enhancers likely facilitated the rapid diversification of placenta that is present in all mammals and crucial for pregnancy success.
Results
Interspecies Transcription Alterations among Human, Macaque, and Mouse Placentae
The human lineage separated from Rhesus Macaque (hereby abbreviated as macaque) about 25 Ma, and primates and mice diverged about 90 Ma (fig. 1A). To determine the interspecies divergence of gene expression in placenta, we compared human, macaque, and mouse transcriptomes of term placenta and other tissues (brain, heart, kidney, liver, testis) generated in this study or retrieved from public resources (supplementary table S1, Supplementary Material online). A computational procedure was implemented for interspecies gene expression analysis (supplementary fig. S1, Supplementary Material online). In brief, we first quantified gene expression levels as transcript per million (TPM). Then, we determined the 1:1:1 orthologs among the three species, which resulted in 14,023 protein-coding orthologs. Subsequently, the expression levels for these orthologs were normalized across interspecies samples and used for differential expression analysis and visualization. In agreement with previous findings (Brawand et al. 2011), the interspecies samples are grouped by tissue instead of species, and human and macaque usually group more closely within each tissue (fig. 1B and supplementary fig. S2, Supplementary Material online).
Fig. 1.
Global gene expression alterations among human, mouse, and macaque placentae. (A) Diagram shows the divergence time among human, macaque, and mouse. (B) Relationship of different human, macaque, and mouse samples based on PCA results. Top 500 genes with the highest expression variance were used for analysis. (C) MA plot shows the differential gene expression between human and mouse placentae. The cutoff of FDR<0.05 and log2foldchange>1 was used to determine DEGs, which are highlighted in red. (D) Placental gene expression alterations between human versus mouse and macaque versus mouse are positively correlated. The x axis and y axis indicate the log2fold(Human/Mouse) and log2fold(Macaque/Mouse) of the normalized TPM values for the 1:1:1 orthologs. The red line indicates the linear fitting result. (E) Expression profiles of the DEGs identified between human and mouse placentae. (F) Expression profiles of the 89 DEGs that show increased expression in human against mouse as well as enriched expression in placenta compared with 16 other human tissues from BodyMap 2.0.
We next compared the placentae of human, macaque, and mouse to detect differentially expressed genes (DEGs). We identified 1,005 significant DEGs between human and mouse placentae—including 490 genes with increased expression in human (fig. 1C and supplementary table S2, Supplementary Material online). Many of these DEGs are known to be important for placenta: CRH and CYP19A1 in particular were reported to have evolved expression in primate placenta (van de Lagemaat et al. 2003; Dunn-Fletcher et al. 2018)—the former encodes corticotropin-releasing hormone which controls the timing and onset of labor (Wadhwa et al. 1998; Dunn-Fletcher et al. 2018), and the latter encodes aromatase P450 to catalyze estrogen synthesis (Kamat et al. 2002; van de Lagemaat et al. 2003). Gene ontology (GO) enrichment analysis demonstrated that human-placenta-enriched genes are associated with extracellular region, cell adhesion, female pregnancy, and receptor binding (supplementary table S3, Supplementary Material online). The placental gene expression alterations between human versus mouse and macaque versus mouse are highly correlated (fig. 1D; Pearson’s r = 0.695, P value < 2.2e-16). Furthermore, these two primate species differ much less than when either of the two is compared with mouse (supplementary fig. S3, Supplementary Material online). As expected, inspection of the DEGs between human versus mouse further demonstrated that macaque placenta gene expression more closely resembles human (fig. 1E).
We further asked how many DEGs between human and mouse placentae are specifically expressed in placenta. To answer this question, we compared the transcriptome of human placenta against 16 other human tissues from BodyMap 2.0 (supplementary fig. S4, Supplementary Material online). Although thousands of genes (range from 3,514 to 6,304) with placenta-enriched expression can be identified relative to other specific tissues, the number dropped sharply when comparing against multiple tissues simultaneously (supplementary fig. S5, Supplementary Material online). As a result, we identified 339 genes with placenta-enriched expression relative to all 16 tissues (supplementary fig. S6 and table S4, Supplementary Material online)—supporting a previous assessment that the number of truly placenta-specific genes is relatively small (Rawn and Cross 2008). These genes include many known placental genes (Rawn and Cross 2008), including CRH, ENDOU, PAPPA, PEG10, PGF, PHLDA2, TAC3, and pregnancy-specific glycoprotein genes (supplementary table S4, Supplementary Material online). GO enrichment analysis demonstrates these placenta-specific genes are highly associated with viral envelope, female pregnancy, extracellular matrix, and hormone activity (supplementary table S5, Supplementary Material online). Several enriched GO terms (e.g., extracellular matrix and female pregnancy) are also associated with genes with increased expression in human placenta relative to mouse (supplementary table S3, Supplementary Material online). Furthermore, for DEGs with increased expression in human placenta relative to mouse, almost one-fifth (89/490) have placenta-enriched expression (fig. 1F and supplementary table S6, Supplementary Material online).
Interspecies Transcriptional Alterations Correlate with Changes of Histone Modifications in Promoter Regions
The covalent modifications of histone tails are widely used to annotate the activity of regulatory elements (Xiao et al. 2012). For example, H3K4me3 is used for annotating active promoters, H3K27ac for active enhancers and promoters, and H3K4me1 for active/poised enhancers and promoters (Piunti and Shilatifard 2016). To unravel the epigenetic mechanisms underlying the evolution of gene expression among species, we examined the occupancy of these three histone modifications among human, macaque, and mouse placentae using ChIP-Seq (supplementary table S1, Supplementary Material online). We first calculated their normalized intensity in promoter regions, which were defined as ±500 bp flanking transcription start site (TSS). Three groups of genes with Human>Mouse, Human<Mouse, or unchanged expression in placenta were ranked based on log2foldChange (fig. 2A), and then the interspecies changes of each histone modification in the corresponding promoter regions were plotted alongside the ranked genes (fig. 2B–D). We demonstrate that the interspecies gene expression alterations between human versus mouse placentae are accompanied by changes in histone modifications in the promoter regions of these DEGs. This correlation is most evident for H3K27ac and H3K4me3 which mark active regulatory elements (fig. 2B–D and supplementary fig. S7, Supplementary Material online). Further comparison of the macaque and mouse placentae shows that the histone modifications change similarly in macaque and human (fig. 2E–G), in agreement with the observation that gene expression patterns in human and macaque placentae are highly similar (fig. 1C–F).
Fig. 2.
Interspecies transcriptional alterations are correlated with changes of histone modifications in promoter regions. (A) Expression alterations of genes with Human>Mouse, Human<Mouse, and unchanged expression between human and mouse placentae. For genes with unchanged expression, only 500 are randomly selected to show. The genes within each group are ranked by log2fold expression change. The x axis indicates the gene rank and y axis indicates log2fold expression change based on normalized TPM values. (B–D) Interspecies alterations of H3K27ac (B), H3K4me1 (C), and H3K4me3 (D) in promoter regions (±500 bp of TSS) between human and mouse placentae. The x axis indicates the rank of genes based on log2fold expression changes. The y axis indicates log2fold changes of histone modification in promoter regions between human and mouse placentae. Smoothed curves were calculated to indicate the trend. (E–G) The plots are similar to (B–D), except that the y axis is for the histone modification changes between macaque and mouse placentae.
Identification of Numerous Lineage-Specific Enhancers in Human Placentae
To examine the conservation of human placental enhancers and promoters, we annotated each using the H3K27ac and H3K4me3 data generated in this study. We annotated 67,525 enhancers and 30,023 promoters in human placenta. By matching against the mouse genome using LiftOver, we demonstrated that 42.4% (n = 28,660) of human placental enhancers have no detectable sequence homology to mouse, which is much higher than the 20.3% for promoters (fig. 3A and supplementary table S7, Supplementary Material online)—suggesting that a large fraction of human placental enhancers are lineage-specific. This raises the question: for human placental regulatory elements that can be matched to the mouse genome, are they also active in mouse placenta? We examined mouse genomic intervals matching human placental enhancers and found that they usually lack the active histone marks H3K4me3 and H3K27ac—in contrast to mouse genomic intervals matching human placental promoters which have comparable levels of active histone marks (fig. 3B–E). Lineage-specific human placental enhancers are associated with apoptotic processes, immune response, endocytosis, and in utero embryonic development (fig. 3F). Taken together, we demonstrated placenta enhancers are rapidly evolving among the compared species both at sequence and activity levels.
Fig. 3.
Interspecies evolution of the enhancers among human, macaque, and mouse placentae. (A) Mapping of human placenta enhancers and promoters to mouse and macaque genomes, respectively. Three types of regions are annotated based on LiftOver results and indicated with different colors. The y axis shows the fraction for each group of enhancers or promoters. (B–E) The intensity of H3K27ac, H3K4me1, and H3K4me3 flanking enhancers and promoters annotated based on the same data (B and D) or matching from other species by LiftOver (C and E). For example, although (B) shows the ChIP-Seq signal of human data on all annotated human regulatory elements, (C) shows the ChIP-Seq signal of human data on homologous loci in human genome that match annotated mouse regulatory elements. (F) Enriched GO terms for lineage-specific human placental enhancers. Only ten representative terms from “Biological Process” category are shown. (G) PhastCons score flanking the enhancers specific for placenta, chorion, and 13 other human tissues (refer to supplementary figs. S8 and S9, Supplementary Material online, for more details). The y axis indicates averaged phastCons score. The curves for placenta and chorion are highlighted in red and green respectively, whereas other tissues are in gray.
Enhancers play a central role for the precise spatiotemporal regulation of gene expression (Long et al. 2016). Since the placenta is amongst the most diversified organs in mammals (Maltepe and Fisher 2015), we reasoned placental enhancers may be even less conserved than those for other tissues. To test this, we retrieved the H3K4me3 and H3K27ac peaks for human placenta and 14 other human tissues from the ENCODE project (supplementary table S1, Supplementary Material online). In all 15 tissues together, we annotated 301,370 putative enhancers as H3K27ac(+) H3K4me3(−) regions located more than 500 bp away from TSSs, and further annotated the enhancers specific for each tissue. As expected, enhancers for placenta and chorion (a crucial part of the fetal part of the placenta) show strikingly low conservation relative to other tissues, for example, the spinal cord, where enhancers are highly conserved (fig. 3G and supplementary fig. S8, Supplementary Material online). We also used the LiftOver to match the human placental enhancers to the genomes of mouse and macaque and found that a large fraction of them have no detectable sequence homology to mouse. This is most striking for placenta and chorion, where about half of human enhancers cannot be matched to mouse genome (supplementary fig. S9A, Supplementary Material online). Further, even though most human enhancers can be matched to the macaque genome, placenta, and chorion still have the highest percentage of lineage-specific enhancers relative to other tissues (supplementary fig. S9B, Supplementary Material online)—together suggesting that placental/chorion enhancers are more rapidly evolving.
Specific ERV Families Contribute to Interspecies Evolution of Human Placental Enhancers
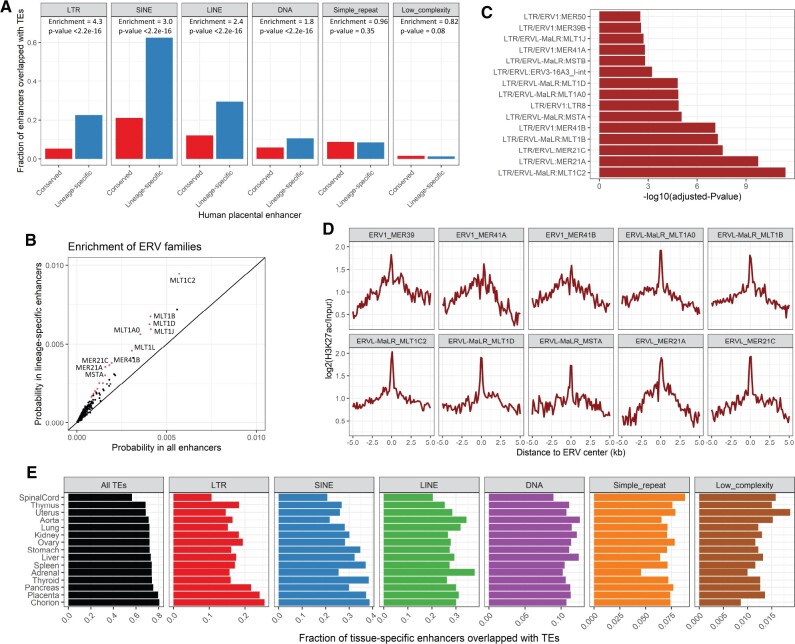
TEs, and particularly LTR retrotransposons (primarily ERVs), have been reported to facilitate the evolution of the immune system and placenta (Chuong et al. 2013, 2016b; Dunn-Fletcher et al. 2018). After identifying thousands of lineage-specific human placental enhancers, we further asked if specific groups of TEs contribute to the evolution of these enhancers. We first examined the occurrence of major classes of TEs, and found that LTR, SINE, LINE, and DNA transposons are significantly overrepresented in lineage-specific human placental enhancers (fig. 4A). Among them, LTR retrotransposons are enriched to the highest degree (Enrichment fold = 4.3, P value <2.2e-16). Examination of lineage-specific placental enhancers for TE contribution in mouse also demonstrates the highest enrichment of LTR retrotransposons (supplementary fig. S10, Supplementary Material online)—indicating the enrichment is likely common in mammals. Therefore, we examined LTR retrotransposons further. Using a binomial test-based approach, we identified 19 LTR/ERV families (Bonferroni-adjusted P value <0.01) that are significantly overrepresented in lineage-specific human placenta enhancers (fig. 4B and C; supplementary table S8, Supplementary Material online). Among them, 11 families are limited to primate or even Simiiformes clade, whereas the remaining eight families have more ancient origins (supplementary table S8, Supplementary Material online). Interestingly, several of them were previously reported to be associated with placenta development or the immune system. For example, MER41A/B was shown to facilitate innate immunity evolution (Chuong et al. 2016b), and MER21A and MER39B were previously linked to placenta (Emera et al. 2012; Pavlicev et al. 2015). Inspection of the H3K27ac occupancy surrounding the most significantly enriched families showed that H3K27ac occupancy usually centers on the overlapped ERV elements (fig. 4D).
Fig. 4.
Contribution of ERV families for the interspecies evolution of human placental enhancers. (A) Enrichment of major classes of TEs in lineage-specific human placental enhancers. The conserved and lineage-specific enhancers are annotated based on LiftOver matching to mouse genome. The y axis shows the fraction of enhancers overlapped with TEs. The enrichment of each class of TEs in lineage-specific enhancers relative to conserved enhancers is tested by Fisher’s exact test, with the enrichment fold and P values indicated. (B) Enrichment of ERV families in lineage-specific human placental enhancers. The x axis and y axis indicate the fraction of all enhancers and lineage-specific enhancers overlapped with each ERV family. Each dot represents one ERV family, with significantly enriched ERV families (Bonferroni-adjusted P value <0.01) highlighted in red. (C) Representative ERV families that are highly overrepresented in lineage-specific human placental enhancers. The x axis indicates −log10(adjusted P value). Only the top 15 ERV families are shown. (D) H3K27ac intensity surrounding the center of ERV elements that overlapping with lineage-specific human placental enhancers. ERV families with at least 100 overlapped elements are shown. The curves are calculated based on ERVs located within lineage-specific human placental enhancers. (E) Barplots show the fraction of tissue-specific enhancers for different human tissues (annotated based on H3K4me3 and H3K27ac peaks retrieved from ENCODE project, as summarized in supplementary table S1, Supplementary Material online) that are overlapped with major classes of TEs.
We further asked if enhancers in other tissues are also enriched for TEs. We examined the overlap of tissue-specific enhancers for 15 human tissues (annotated based on ENCODE data as aforementioned) with annotated TEs (fig. 4E). Overall, about 80% of chorion and placenta-specific enhancers overlap with TEs, which is the highest among the examined tissues. Next, we asked if enhancers in other tissues are enriched for the same classes of TEs as seen in placenta. We demonstrate that different TE classes associated with enhancers in different tissues (fig. 4E and supplementary fig. S11, Supplementary Material online). For example, chorion and placenta-specific enhancers show particularly high overlap with LTR retrotransposons which is consistent with results from the comparison of conserved and lineage-specific human placenta enhancers (fig. 4A and E), yet adrenal-specific enhancers show a higher overlap with LINE elements relative to other tissues (fig. 4E and supplementary fig. S11, Supplementary Material online). The specific enrichment of placental enhancers with LTR retrotransposons may reflect the colonization of live-born mammals with many new LTR retrotransposons.
MER41A/B Insertion Events Create Lineage-Specific Serum Response Factor-Binding Loci in Human
Many LTR retrotransposons harbor transcription factor-binding motifs, thus their insertions in the genome provide a shortcut for creating lineage-specific enhancers. One example is MER41, which facilitates human innate immunity evolution by creating primate-specific STAT1-binding motifs (Chuong et al. 2016b). MER41 elements are also overrepresented within lineage-specific human placental enhancers (fig. 4C). Motif enrichment analysis demonstrated that in addition to STAT1, binding motifs for several other transcription factors (e.g., SP8, ZN350, ANDR, and serum response factor [SRF]) are also highly enriched in MER41B elements within lineage-specific human placental enhancers (fig. 5A and supplementary table S9, Supplementary Material online). Among them, SRF is a transcription factor that regulates actin genes for cytoskeleton maintenance and immediate early genes which can be activated and transcribed within minutes in response to various cell-extrinsic or cell-intrinsic signals (Miano et al. 2007; Bahrami and Drablos 2016). In response to a variety of extracellular signals in the serum, SRF binds the serum response elements near its target genes to stimulate their expression (Clark and Graves 2014). Even though the role of SRF in placenta remains unclear, increasing evidence suggests SRF is involved in immune response (Taylor et al. 2014), cell proliferation, invasion and adhesion (Franco et al. 2013; Gualdrini et al. 2016) and angiogenesis (Franco et al. 2008, 2013)—processes also important for placenta function. Further, forced expression of SRF in mouse TSCs was shown to promote the differentiation into giant cells (Asanoma et al. 2007). Therefore, we further examined whether SRF binds in MER41 elements.
Fig. 5.
MER41 insertions create lineage-specific SRF-binding loci in human. (A) SRF motifs enriched in MER41B associated lineage-specific human placental enhancers (top) and SRF peaks in serum-treated hTSCs (bottom). (B) Venn diagram showing the overlap of SRF peaks identified from untreated and serum-treated hTSCs. (C) GO terms associated with SRF peaks identified from serum-treated hTSCs. Only 20 representative terms from the “Biological Process” category are shown. (D) Enrichment of MER41A and MER41B elements in SRF peaks from serum-treated hTSCs. The enrichment fold and P values are labeled. (E) SRF binding flanking the MER41 elements that occur within SRF peaks in hTSCs. The averaged curves (top) and heatmaps (bottom) are shown—all are centered on the center of the 51 MER41 elements within SRF peaks identified from serum-treated hTSCs. (F) Expression changes between human and mouse placentae for genes adjacent to MER41-associated SRF-binding loci in hTSCs. Only protein-coding genes annotated as 1:1 orthologs between human and mouse and with their TSS less than 50 kb away from MER41-associated SRF-binding loci are included. The x axis indicates log2fold expression change calculated based on normalized TPM values. The color gradient indicates −log10(P value) from differential gene expression analysis. (G) Expression profiles of CLEC3B and FBN2 in human placenta and 16 other tissues from BodyMap 2.0. The x axis indicates normalized TPM values. The expression values for replicates are averaged for visualization. (H and I) IGV tracks showing the expression, SRF motif occurrence, SRF binding, and H3K27ac occupancy of the two MER41-SRF peaks associated genes CLEC3B (H) and FBN2 (I). The MER41-associated SRF peaks are highlighted in red square.
Human trophoblast stem cells (hTSCs) have been successfully established recently (Okae et al. 2018) and serve as a promising in vitro model for studying human placenta development. We applied ChIP-Seq to profile the genome-wide binding of SRF before and after serum treatment in hTSCs. Inspection of several canonical SRF target genes (ACTB, JUNB, EGR1, and EGR2) confirmed the data quality (supplementary fig. S12, Supplementary Material online). We identified 420 SRF peaks in untreated hTSCs, and 1,567 after serum treatment (fig. 5B and supplementary table S10, Supplementary Material online). The canonical SRF motif—which resembles the one overrepresented in MER41B elements within lineage-specific human placental enhancers—is significantly enriched in SRF-binding loci in serum-treated hTSCs (fig. 5A). Apart from its expected function in actin cytoskeleton organization and cell-matrix adhesion, we found that SRF-binding loci are also significantly associated with immune regulation, placenta development, and chorio-allantoic fusion (fig. 5C and supplementary table S11, Supplementary Material online). Interestingly, SRF-binding loci are associated with phenotypes including embryo size, prenatal body size, placenta vasculature and labyrinth morphology, and T cell/B cell physiology (supplementary table S12, Supplementary Material online). These results suggest that SRF is potentially involved in placental development and immunity in human.
We further examined SRF binding in different groups of MER41 elements. Our results demonstrate that after serum treatment, SRF-binding intensity on MER41B is significantly increased (supplementary fig. S13, Supplementary Material online) and the number of SRF-bound MER41B elements increases from 10 to 39 after serum treatment. MER41A elements are also bound by SRF yet with lower intensity, whereas other MER41 groups (MER41C, MER41D, MER41E, MER41G, and MER41-int) do not show evidence of SRF binding. Consistently, MER41A and MER41B elements are overrepresented within SRF peaks, with enrichment fold of 10.8 and 39.4, respectively (fig. 5D). We further demonstrate that for the 50 MER41-associated SRF peaks, SRF binding is centered on the MER41 elements (fig. 5E)—suggesting that SRF recognizes MER41A/B elements. Interestingly, for genes with TSSs less than 50 kb away from MER41-SRF peaks, the majority have increased expression in human placenta relative to mouse (supplementary fig. S14 and table S13, Supplementary Material online). In particular, CLEC3B and FBN2 display significant interspecies expression alterations between human and mouse. Manual inspection showed that MER41-associated SRF peaks near these two genes in serum-treated hTSCs have evident H3K27ac occupancy in human placenta (fig. 5F and G). Both FBN2 and CLEC3B have placenta-enriched expression (supplementary fig. S15, Supplementary Material online), and interestingly FBN2 was recently reported to secrete a peptide hormone, placensin, that promotes placental cell invasiveness (Yu et al. 2020). Together, our results suggest that specific MER41 elements may facilitate the expression of FBN2, CLEC3B, and potentially many other human placenta-specific genes by creating dozens of SRF-bound enhancers.
Discussion
The placenta has long been known to be extraordinary diverse in eutherian mammals (Ramsey 1982; Mossman 1987). Remarkable differences exist between the placentae of human and other mammalian species, as represented by differences in the size, shape, and structure of the placentae, the gestation length, and the degree of invasion into the uterus (Malassine et al. 2003; Schmidt et al. 2015; Ross and Boroviak 2020). The morphological and physiological differences among species usually result from lineage-specific molecular changes during evolution. Aiming to better understand the molecular evolution in human placenta, here we integrated transcriptomic and epigenomic data to examine the alterations of the transcriptional and regulatory landscape among the placentae of human, macaque, and mouse. To our knowledge, this is the most comprehensive interspecies study that assesses the molecular evolution of the human term placenta.
Multiple lines of evidence from previous studies suggest that at least some lineage-specific novelties of the human placenta can be attributed to expression changes of specific genes, as demonstrated by CRH which alters gestation length (Dunn-Fletcher et al. 2018), and FBN2 which influences the degree of trophoblast invasion (Yu et al. 2020). But the extent to which gene expression changes between the placenta of human and other mammals differ genome-wide is unknown. We identified hundreds of genes with increased expression in the term placentae of human relative to mouse. Importantly, interspecies expression changes are strongly correlated with interspecies alterations of H3K27ac and H3K4me3 occupancy—providing independent evidence that the results from our interspecies differential expression analysis are robust. At last, we further compared human placenta against dozens of other tissues, and determined a set of 89 placental-specific genes with increased expression in human relative to mouse. The list includes well-studied placental genes known to have gained placental expression in human, such as CRH (Dunn-Fletcher et al. 2018), CYP19A1 (Kamat et al. 2002; van de Lagemaat et al. 2003), and FBN2 (Yu et al. 2020). Interestingly, another gene, ADAM12, was reported as a marker for ectopic pregnancy, which is one of the major causes of maternal death during the first trimester of pregnancy in human yet rarely diagnosed in mice (Corpa 2006; Rausch et al. 2011). The human-placenta-enriched genes identified in this study may serve as valuable candidates for further understanding about the novelties of human placenta.
In line with the fact that the placenta is probably the most highly diversified organ in eutherian mammals, we demonstrate that the human placenta and chorion have the least conserved enhancers relative to dozens of other tissues. Our previous study suggests that an anthropoid primate-specific insertion of a THE1B family ERV element creates novel enhancers to control the placental expression of CRH and alters gestation length (Dunn-Fletcher et al. 2018). Although individual genome-wide studies assessed lineage-specific enhancer evolution in mouse TSCs (Chuong et al. 2013) and human uterus (Lynch et al. 2011), a comprehensive assessment of how regulatory elements evolve in the human placenta is lacking. We annotated the enhancers and promoters for term human placenta based on the occupancy of active histone marks, and demonstrate that lineage-specific human placental enhancers are highly associated with several classes of TEs, particularly ERVs. The lineage-specific enhancers for mouse placenta are also most highly associated with ERVs—suggesting that the contribution of ERVs to the evolution of placental enhancers is likely common in mammals. In addition, lineage-specific ERV-associated enhancers are likely also tissue-specific as they are most highly enriched in placenta/chorion followed by pancreas, kidney, ovary, and thymus, but to a much less degree in other tissues.
We identified 19 ERV families that are significantly associated with lineage-specific human placental enhancers, which include several families (e.g., MER21A, MER41A/B, MER39B) that were previously linked to placental function (Pasquesi et al. 2020) or immune response (Chuong et al. 2016b). MER41 is one family of ERVs known to regulate human innate immunity by creating lineage-specific STAT1-binding sites (Schmid and Bucher 2010; Chuong et al. 2016b). We found that MER41A/B elements associated with lineage-specific human placental enhancers are enriched for the binding motif of SRF, which is originally known for regulating actin genes and immediate early genes (Miano et al. 2007) and which was more recently found to also control immune response (Taylor et al. 2014), cell proliferation, invasion and adhesion (Franco et al. 2013; Gualdrini et al. 2016) and angiogenesis (Franco et al. 2008, 2013). Since these processes are important for placental function, we examined SRF binding in human TSCs. Indeed, SRF-bound regions are associated with placenta-related functions, such as placenta development, in utero embryonic development, chorio-allantoic fusion, and placenta blood vessel development. We demonstrate that SRF binds dozens of MER41A/B elements, and genes adjacent to MER41-associated SRF peaks usually have increased expression in the placenta of human relative to mouse. Specifically, the associated gene FBN2 is a placenta-specific gene recently reported to secrete the peptide hormone placensin which promotes placental cell invasiveness (Yu et al. 2020). Notably, FBN2 is highly expressed in the placenta of human but not mouse (Yu et al. 2020), and higher degree of trophoblast invasion is a remarkable feature for human placenta relative to other species (Malassine et al. 2003; Cohen and Bischof 2007). These results imply the possible contribution of MER41 elements in facilitating the evolution of increased invasive capability of human placenta by creating lineage-specific enhancer near FBN2.
Although we systematically examined the interspecies molecular alterations in human placenta on both the transcriptional and regulatory level, our study may suffer from several limitations. First, the interspecies expression comparison is focused on 1:1 orthologs, which preclude the possibility to examine the gained, lost, or duplicated genes for human placenta evolution. Second, although we demonstrate that MER41-associated SRF peaks occur near placental genes with human-increased expression such as FBN2 and CLEC3B, currently it is difficult to further validate the function of these putative enhancers due to lack of established protocols for manipulation of hTSCs using CRISPR engineering. Third, since the transcriptomic and epigenomic data are not profiled at single-cell resolution, it is uncertain how much of the detected interspecies transcriptional and regulatory alterations are due to differences in the cell population. Fourth, individual samples for macaque and mouse are from late gestational stages, but not from full term like the human—which needs to be considered when interpreting our analyses. To avoid artifacts due to gestation age differences, we carefully designed our analysis strategy to focus on the expression alteration between human and mouse placentae which are both at term, and the epigenetically annotated regulatory elements for human term placenta. Finally, most enhancers are distal from promoters. We therefore could not establish the relationship of many putative human placental enhancers to their target genes.
The molecular differences between the placentae of primates and rodents, as represented by human, macaque, and mouse which are among the most extensively studied mammals, is the focus of our current study. In spite of the morphological and molecular differences among these three species, primates and rodents both have a hemochorial placenta, which together with endotheliochorial placenta (found in cats and dogs) have syncytiotrophoblasts and display a relatively deep degree of trophoblast invasion into maternal uterine tissue. In contrast, synepitheliochorial placentae (found in cows and sheep) do not invade the uterine tissue as deeply, and epitheliochorial placentae (found in pigs and horses) lack syncytiotrophoblasts altogether and do not invade maternal tissues (Nakaya and Miyazawa 2015). Therefore, further investigation of the molecular mechanisms underlying the evolution of these different types of placentae is a promising direction for future study.
Overall, this study provides the most comprehensive picture to date about the transcriptional and regulatory landscape evolution in primate and rodent placentae, which is valuable for our understanding of human placental evolution. Importantly, the identified genes and regulatory elements with evolved expression/activity also provide valuable candidates for further studies examining human placental evolution.
Materials and Methods
Placenta Sample Collection
Placenta samples were collected and processed as described in a previous study (Dunn-Fletcher et al. 2018). Briefly, two human placentae were collected by cesarean section at 39 weeks 1 day and 39 weeks 2 days gestational age (IRB protocol: CCHMC IRB 2013–2243). Two macaque placentae were collected by cesarean section at 128 days and 131 days gestational age (80% completed gestation). Collected placenta samples were frozen in −80 °C for later use. Additional data for human placentae with gestation age of 42 weeks, and mouse placentae with gestation age of 18.5 or 19 days (for RNA-Seq) and 14.5 days (for ChIP-Seq) were retrieved from our previous publication and other public resources (supplementary table S1, Supplementary Material online).
Human Trophoblast Stem Cell Culture
hTSCs derived from human cytotrophoblast cells (Okae et al. 2018) were a gift from the Okae lab. They were cultured following a previous protocol as described (Okae et al. 2018). In brief, hTSCs were grown in trophoblast stem cell medium (TSM) consisting of DMEM/F12 (Gibco, 1263401) supplemented with 2 mM Glutamax (Fisher, 35050061), 0.1 mM b-Mercaptoethanol (Gibco, 21985-023), 0.2% FBS (HyClone, SH3007103), 0.5% Pen-Strep (Fisher, 15140122), 0.3% BSA (Sigma-Aldrich, A9576-50ml), 1% ITS-X (Fisher, 51500056), 1.5 µg/ml L-ascorbic acid (Sigma-Aldrich, A92902-100G), 50 ng/ml hEGF (Fisher, 10605HNAE25), 2 µM CHIR99021 (Sigma-Aldrich, SML1046-5MG), 0.5 µM A83-01 (Sigma-Aldrich, SML0788-5MG), 1 µM SB431542 (Sigma-Aldrich, 616464-5MG), 0.8 mM VPA (Wako, 227-01071), and 5 µM Y27632 (Abcam, ab120129). Several reagents, including hEGF, CHIR99021, A83-01, SB431542, VPA, Y27632, and L-ascorbic acid, were added fresh before use. hTSCs were cultured on Collagen-IV (Corning, CB-40233) coated TC dishes at 37 °C with 5% CO2 and split at 1:2 to 1:4 ratio every two days. Serum treatment was performed by culturing hTSCs in TSM mixed with 15% FBS (HyClone, SH3007103) for 30 min.
RNA-Seq
Total RNA for placenta and hTSC samples was extracted using RNeasy Micro kit (Qiagen, 74004) with on-column DNase digestion. RNA samples were submitted for library construction by TruSeq stranded mRNA sample preparation kit (Illumina) and sequencing as 75 bp paired-end reads with HiSeq2500 (Illumina). We also collected RNA-Seq data for dozens of human, macaque, and mouse tissues from public resources (supplementary table S1, Supplementary Material online). Reads were trimmed with Trim Galore v0.6.4 (https://github.com/FelixKrueger/TrimGalore). To perform differential expression analysis of the samples from the same species, we aligned trimmed reads to the corresponding reference genome using STAR v2.7.1 (Dobin et al. 2013), and then obtained gene-level read counts using the featureCount function from subread v2.0.0 (Liao et al. 2013). Finally, differentially expressed genes were identified using DESeq2 v1.28.0 (Love et al. 2014) with the cutoff: FDR < 0.05 and foldchange > 2. Notably, when both paired-end and single-end data are available for compared samples, only data for read 1 are used.
To perform differential expression analysis among different species, we first calculated TPM value for each gene using RSEM v1.3.2 (Li and Dewey 2011) with reference genome and gene annotation downloaded from ENSEMBL database (Yates et al. 2020). Taking the calculated TPM values as input, interspecies differential expression analysis was performed using ExprX (https://github.com/mingansun/ExprX) was recently developed to facilitate interspecies differential expression analysis (unpublished). In brief, it first determined the 1:1 orthologs between compared species based on the homolog annotations from ENSEMBL database (Yates et al. 2020). The 1:1 orthologs were then filtered to only keep protein-coding genes after excluding ribosomal genes and genes from chromosomes X, Y, and MT. Finally, the expression of the filtered 1:1 orthologs were normalized using TMM method (Robinson et al. 2010), and differential expression analyses between compared species were performed using Rank Product method (Hong et al. 2006) with FDR < 0.05 and log2foldchange > 1 as cut-off.
ChIP-Seq
ChIP-Seq for placenta samples was performed using the MAGnify Chromatin Immunoprecipitation System (ThermoFisher, 492024), and ChIP-Seq for hTSC samples was performed following previous protocol (Blecher-Gonen et al. 2013) with minor modifications. Chromatin fragmentation was performed using Diagenode Bioruptor Plus Sonicator. The antibodies used include H3K4me1 (Abcam, ab8895), H3K4me3 (Abcam, ab8580), H3K27ac (Abcam, ab4729), and SRF (ActiveMotif, 61385). The amount of chromatin used for each reaction is 30 μg for transcription factors and 20 μg for histone modification. ChIP-Seq libraries were constructed using Takara SMARTer ThruPLEX DNA-Seq Kit (Takara, R400674), and then sequenced as 50 bp single-end reads with HiSeq2500 (Illumina).
Reads were trimmed with TrimGalore v0.6.4 and then aligned to the corresponding reference genome using Bowtie v2.3.5 (Langmead and Salzberg 2012) with default settings. PCR duplicates were removed using the rmdup function of samtools v1.10 (Li et al. 2009). The data reproducibility between biological replicates was confirmed, then reads from replicates were pooled together for further analysis. Peak calling was performed with MACS v2.2.5 (Zhang et al. 2008) with default settings. H3K4me1 was called as broad peaks, whereas H3K4me3, H3K27ac, and SRF were called as narrow peaks. The peaks for human and mouse were further cleaned by removing those overlapped with ENCODE Blacklist V2 regions (Amemiya et al. 2019).
To compare the intensity of histone occupancy across species, we implemented a procedure as below: 1) calculate the normalized read count for each promoter (±500 bp of TSS) as number of read counts per million of reads per Gb of genome; and 2) deduct the normalized read counts of input control from those for ChIP samples to get the pure signal values, with negative values adjusted to 0. The obtained values are further normalized with TMM method (Robinson et al. 2010). The sequencing depth, genome size, and input signal are all considered during the normalization procedure. The normalized values are subsequently used for comparison across species for homologous genes.
Reference Genome and Annotation
Reference genome and gene annotation for human (GRCh38), macaque (Mmul_10), and mouse (GRCm38) were downloaded from ENSEMBL database (release 100) (Yates et al. 2020). PhastCons annotation for human, macaque, and mouse were downloaded from UCSC Genome Browser (Lee et al. 2020). Transposable element annotations for human were downloaded from the RepeatMasker website (http://www.repeatmasker.org/) on May 27, 2016. The clades for ERV families were obtained from Dfam database (Hubley et al. 2016).
Gene Ontology and Motif Enrichment Analysis
Gene ontology enrichment analyses for differentially expressed genes were performed using DAVID functional annotation tools (Huang et al. 2009). Gene ontology enrichment analyses for genomic regions (e.g., ChIP-Seq peaks and putative enhancers) were performed with GREAT (McLean et al. 2010). Motif enrichment analysis was performed using MEME-ChIP (Machanick and Bailey 2011), with sequences ±500 bp from the center of ChIP-Seq peaks or ERV elements as input.
Regulatory Element Annotation and Comparison
Putative regulatory elements (promoters and enhancers) for human, macaque, and mouse placente are inferred based on histone modifications and genomic distribution. Promoters are defined as H3K4me3 occupied regions, and enhancers as H3K27ac peaks that lack H3K4me3 mark and more than 500 bp from TSSs. Notably, the initially called regulatory elements are usually of very different length, which may influence the mapping among species. Thus, each of them is adjusted as ±100 bp from its center. Finally, length-adjusted regulatory elements are compared among species using UCSC LiftOver (Lee et al. 2020) with default settings. To be noted, regions reported as “Split in new” or “Partially deleted in new” by LiftOver are annotated as “Partially shared between species.”
Enrichment Analysis of ERV Families in Lineage-Specific Human Placental Enhancers
To determine whether certain ERV families are overrepresented within lineage-specific human placental enhancers, we designed a binomial test-based approach. In brief, we first calculated the occurrence of each group of ERV family within all enhancers and within lineage-specific enhancers using the window function of BEDtools v2.29.2 (Quinlan and Hall 2010). The occurrence of each family in all enhancers were counted and considered as the background. Then, binomial test was performed to determine whether an ERV subfamily is significantly overrepresented within lineage-specific human placental enhancers relative to all human placental enhancers. To control for family-wise error rate, the calculated P values were further adjusted (Bonferroni correction).
Enrichment Analysis of ERV Families in SRF-Binding Loci
The enrichment analysis of ERV families (i.e., MER41A and MER41B) within SRF-binding loci was performed using the fisher function from BEDtools v2.29.2 (Quinlan and Hall 2010).
Statistical Analysis and Data Visualization
All statistical analyses were performed with R statistical programming language (Team 2016). Heatmaps for ChIP-Seq data were generated using DeepTools v3.4.3 (Ramirez et al. 2014). Heatmaps from gene expression clustering analysis were generated using pheatmap (https://github.com/raivokolde/pheatmap). PCA analysis and visualization were performed using the plotPCA function in DESeq2 v1.30.1 (Love et al. 2014). RNA-Seq and ChIP-Seq tracks were visualized using IGV v2.8.0 (Thorvaldsdottir et al. 2013).
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the NICHD, NIH for T.S.M., and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) for M.-A.S. L.J.M. received support through the March of Dimes Prematurity Research Center Ohio Collaborative and the NIH/NICHD (HD 091527). We thank Dr Suhas Kallapur for assistance with the rhesus samples. We thank Dr Hiroaki Okae and Dr Takahiro Arima for kindly providing hTSCs. We thank the National Institute of Child Health and Human Development (NICHD) Molecular Genomics Core for high-throughput sequencing. This study utilized the computational resources of the NIH High-Performance Computing Biowulf cluster (https://hpc.nih.gov) and Yangzhou University College of Veterinary Medicine High-Performance Computing cluster.
Data Availability
All the data generated in this study were deposited in NCBI Gene Expression Ominibus (GEO) under accession numbers GSE153082 and GSE153083 and dbGap under accession number phs002233.
References
- Amemiya HM, Kundaje A, Boyle AP.. 2019. The ENCODE blacklist: identification of problematic regions of the genome. Sci Rep. 9(1):9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ander SE, Diamond MS, Coyne CB.. 2019. Immune responses at the maternal-fetal interface. Sci Immunol. 4:eaat6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanoma K, Kato H, Yamaguchi S, Shin CH, Liu ZP, Kato K, Inoue T, Miyanari Y, Yoshikawa K, Sonoda K, et al. 2007. HOP/NECC1, a novel regulator of mouse trophoblast differentiation. J Biol Chem. 282(33):24065–24074. [DOI] [PubMed] [Google Scholar]
- Bahrami S, Drablos F.. 2016. Gene regulation in the immediate-early response process. Adv Biol Regul. 62:37–49. [DOI] [PubMed] [Google Scholar]
- Beaman KD, Jaiswal MK, Katara GK, Kulshreshta A, Pamarthy S, Ibrahim S, Kwak-Kim J, Gilman-Sachs A.. 2016. Pregnancy is a model for tumors, not transplantation. Am J Reprod Immunol. 76(1):3–7. [DOI] [PubMed] [Google Scholar]
- Blecher-Gonen R, Barnett-Itzhaki Z, Jaitin D, Amann-Zalcenstein D, Lara-Astiaso D, Amit I.. 2013. High-throughput chromatin immunoprecipitation for genome-wide mapping of in vivo protein-DNA interactions and epigenomic states. Nat Protoc. 8(3):539–554. [DOI] [PubMed] [Google Scholar]
- Brawand D, Soumillon M, Necsulea A, Julien P, Csardi G, Harrigan P, Weier M, Liechti A, Aximu-Petri A, Kircher M, et al. 2011. The evolution of gene expression levels in mammalian organs. Nature 478(7369):343–348. [DOI] [PubMed] [Google Scholar]
- Chuong EB, Elde NC, Feschotte C.. 2016a. Regulatory activities of transposable elements: from conflicts to benefits. Nat Rev Genet. 18(2):71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong EB, Elde NC, Feschotte C.. 2016b. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 351(6277):1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong EB, Rumi MA, Soares MJ, Baker JC.. 2013. Endogenous retroviruses function as species-specific enhancer elements in the placenta. Nat Genet. 45(3):325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, Graves BJ.. 2014. Dual views of SRF: a genomic exposure. Genes Dev. 28(9):926–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CJ, Lock WM, Mager DL.. 2009. Endogenous retroviral LTRs as promoters for human genes: a critical assessment. Gene 448(2):105–114. [DOI] [PubMed] [Google Scholar]
- Cohen M, Bischof P.. 2007. Factors regulating trophoblast invasion. Gynecol Obstet Invest. 64(3):126–130. [DOI] [PubMed] [Google Scholar]
- Corpa JM. 2006. Ectopic pregnancy in animals and humans. Reproduction 131(4):631–640. [DOI] [PubMed] [Google Scholar]
- Denner J. 2016. Expression and function of endogenous retroviruses in the placenta. APMIS 124(1–2):31–43. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR.. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Fletcher CE, Muglia LM, Pavlicev M, Wolf G, Sun MA, Hu YC, Huffman E, Tumukuntala S, Thiele K, Mukherjee A, et al. 2018. Anthropoid primate-specific retroviral element THE1B controls expression of CRH in placenta and alters gestation length. PLoS Biol. 16(9):e2006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emera D, Casola C, Lynch VJ, Wildman DE, Agnew D, Wagner GP.. 2012. Convergent evolution of endometrial prolactin expression in primates, mice, and elephants through the independent recruitment of transposable elements. Mol Biol Evol. 29(1):239–247. [DOI] [PubMed] [Google Scholar]
- Feschotte C. 2008. Transposable elements and the evolution of regulatory networks. Nat Rev Genet. 9(5):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco CA, Blanc J, Parlakian A, Blanco R, Aspalter IM, Kazakova N, Diguet N, Mylonas E, Gao-Li J, Vaahtokari A, et al. 2013. SRF selectively controls tip cell invasive behavior in angiogenesis. Development 140(11):2321–2333. [DOI] [PubMed] [Google Scholar]
- Franco CA, Mericskay M, Parlakian A, Gary-Bobo G, Gao-Li J, Paulin D, Gustafsson E, Li Z.. 2008. Serum response factor is required for sprouting angiogenesis and vascular integrity. Dev Cell. 15(3):448–461. [DOI] [PubMed] [Google Scholar]
- Fuentes DR, Swigut T, Wysocka J.. 2018. Systematic perturbation of retroviral LTRs reveals widespread long-range effects on human gene regulation. Elife 7:e35989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Kuroda Y, Sugiyama A.. 2014. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 27(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdrini F, Esnault C, Horswell S, Stewart A, Matthews N, Treisman R.. 2016. SRF co-factors control the balance between cell proliferation and contractility. Mol Cell. 64(6):1048–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F, Breitling R, McEntee CW, Wittner BS, Nemhauser JL, Chory J.. 2006. RankProd: a bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics 22(22):2825–2827. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA.. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 4(1):44–57. [DOI] [PubMed] [Google Scholar]
- Hubley R, Finn RD, Clements J, Eddy SR, Jones TA, Bao W, Smit AF, Wheeler TJ.. 2016. The Dfam database of repetitive DNA families. Nucleic Acids Res. 44(D1):D81–D89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A, Hinshelwood MM, Murry BA, Mendelson CR.. 2002. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol Metab. 13(3):122–128. [DOI] [PubMed] [Google Scholar]
- Kunarso G, Chia NY, Jeyakani J, Hwang C, Lu X, Chan YS, Ng HH, Bourque G.. 2010. Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat Genet. 42(7):631–634. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL.. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9(4):357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Barber GP, Casper J, Clawson H, Diekhans M, Gonzalez JN, Hinrichs AS, Lee BT, Nassar LR, Powell CC, et al. 2020. UCSC Genome Browser enters 20th year. Nucleic Acids Res. 48(D1):D756–D761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN.. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing S. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25(16):2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W.. 2013. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41(10):e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HK, Prescott SL, Wysocka J.. 2016. Ever-changing landscapes: transcriptional enhancers in development and evolution. Cell 167(5):1170–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S.. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch VJ, Leclerc RD, May G, Wagner GP.. 2011. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat Genet. 43(11):1154–1159. [DOI] [PubMed] [Google Scholar]
- Macfarlan TS, Gifford WD, Driscoll S, Lettieri K, Rowe HM, Bonanomi D, Firth A, Singer O, Trono D, Pfaff SL.. 2012. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487(7405):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P, Bailey TL.. 2011. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27(12):1696–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malassine A, Frendo JL, Evain-Brion D.. 2003. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update. 9(6):531–539. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Fisher SJ.. 2015. Placenta: the forgotten organ. Annu Rev Cell Dev Biol. 31:523–552. [DOI] [PubMed] [Google Scholar]
- Mangeney M, Renard M, Schlecht-Louf G, Bouallaga I, Heidmann O, Letzelter C, Richaud A, Ducos B, Heidmann T.. 2007. Placental syncytins: genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc Natl Acad Sci U S A. 104(51):20534–20539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G.. 2010. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 28(5):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, et al. 2000. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403(6771):785–789. [DOI] [PubMed] [Google Scholar]
- Miano JM, Long X, Fujiwara K.. 2007. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 292(1):C70–C81. [DOI] [PubMed] [Google Scholar]
- Mossman HW. 1937. Comparative morphogenesis of the fetal membranes and accessory uterine structures. Contrib. Embyrol Carneg Inst. 26:129–246. [DOI] [PubMed] [Google Scholar]
- Mossman HW. 1987. Vertebrate fetal membranes: comparative ontogeny and morphology, evolution, phylogenetic significance, basic functions, research opportunities. New Brunswick (NJ: ): Rutgers University Press. [Google Scholar]
- Nakaya Y, Miyazawa T.. 2015. The roles of syncytin-like proteins in ruminant placentation. Viruses 7(6):2928–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T.. 2018. Derivation of human trophoblast stem cells. Cell Stem Cell. 22(1):50–63.e56. [DOI] [PubMed] [Google Scholar]
- Pasquesi GIM, Perry BW, Vandewege MW, Ruggiero RP, Schield DR, Castoe TA.. 2020. Vertebrate lineages exhibit diverse patterns of transposable element regulation and expression across tissues. Genome Biol Evol. 12(5):506–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlicev M, Hiratsuka K, Swaggart KA, Dunn C, Muglia L.. 2015. Detecting endogenous retrovirus-driven tissue-specific gene transcription. Genome Biol Evol. 7(4):1082–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piunti A, Shilatifard A.. 2016. Epigenetic balance of gene expression by Polycomb and COMPASS families. Science 352(6290):aad9780. [DOI] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM.. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26(6):841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Dundar F, Diehl S, Gruning BA, Manke T.. 2014. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42(Web Server issue):W187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey EM. 1982. The placenta: human and animal. New York: Praeger Publishers. [Google Scholar]
- Rausch ME, Beer L, Sammel MD, Takacs P, Chung K, Shaunik A, Speicher D, Barnhart KT.. 2011. A disintegrin and metalloprotease protein-12 as a novel marker for the diagnosis of ectopic pregnancy. Fertil Steril. 95(4):1373–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawn SM, Cross JC.. 2008. The evolution, regulation, and function of placenta-specific genes. Annu Rev Cell Dev Biol. 24:159–181. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK.. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C, Boroviak TE.. 2020. Origin and function of the yolk sac in primate embryogenesis. Nat Commun. 11(1):3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, Cross JC.. 2001. Placental development: lessons from mouse mutants. Nat Rev Genet. 2(7):538–548. [DOI] [PubMed] [Google Scholar]
- Sakashita A, Maezawa S, Takahashi K, Alavattam KG, Yukawa M, Hu YC, Kojima S, Parrish NF, Barski A, Pavlicev M, et al. 2020. Endogenous retroviruses drive species-specific germline transcriptomes in mammals. Nat Struct Mol Biol. 27(10):967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CD, Bucher P.. 2010. MER41 repeat sequences contain inducible STAT1 binding sites. PLoS One 5(7):e11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Morales-Prieto DM, Pastuschek J, Frohlich K, Markert UR.. 2015. Only humans have human placentas: molecular differences between mice and humans. J Reprod Immunol. 108:65–71. [DOI] [PubMed] [Google Scholar]
- Taylor A, Tang W, Bruscia EM, Zhang PX, Lin A, Gaines P, Wu D, Halene S.. 2014. SRF is required for neutrophil migration in response to inflammation. Blood 123(19):3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC. 2016. R: a language and environment for statistical computing. Vienna (Austria: ): R Foundation for Statistical Computing. [Google Scholar]
- Thorvaldsdottir H, Robinson JT, Mesirov JP.. 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 14(2):178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Lagemaat LN, Landry JR, Mager DL, Medstrand P.. 2003. Transposable elements in mammals promote regulatory variation and diversification of genes with specialized functions. Trends Genet. 19(10):530–536. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA.. 1998. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 179(4):1079–1085. [DOI] [PubMed] [Google Scholar]
- Wang T, Zeng J, Lowe CB, Sellers RG, Salama SR, Yang M, Burgess SM, Brachmann RK, Haussler D.. 2007. Species-specific endogenous retroviruses shape the transcriptional network of the human tumor suppressor protein p53. Proc Natl Acad Sci U S A. 104(47):18613–18618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Xie D, Cao X, Yu P, Xing X, Chen CC, Musselman M, Xie M, West FD, Lewin HA, et al. 2012. Comparative epigenomic annotation of regulatory DNA. Cell 149(6):1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AD, Achuthan P, Akanni W, Allen J, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM, Azov AG, Bennett R, et al. 2020. Ensembl 2020. Nucleic Acids Res. 48(D1):D682–D688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, He JH, Hu LL, Jiang LL, Fang L, Yao GD, Wang SJ, Yang Q, Guo Y, Liu L, et al. 2020. Placensin is a glucogenic hormone secreted by human placenta. EMBO Rep. 21(6):e49530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9(9):R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated in this study were deposited in NCBI Gene Expression Ominibus (GEO) under accession numbers GSE153082 and GSE153083 and dbGap under accession number phs002233.