Abstract
Introduction:
Degenerative cervical myelopathy (DCM) is the commonest cause of adult spinal cord impairment worldwide, encompassing chronic compression of the spinal cord, neurological disability and diminished quality of life. Evidence on the contribution of environmental factors is sparse; in particular, the role of nutrition in DCM is unknown. The objective of this review was to assess the effect of nutrition on DCM susceptibility, severity and surgical outcome.
Methods:
A systematic review in MEDLINE and Embase was conducted following PRISMA guidelines. Full-text papers in English papers, focussing on cervical myelopathy and nutrition, published before January 2020 were considered eligible. Quality assessments were performed using the GRADE assessment tool. Patient demographics, nutritional factor and DCM outcomes measures were recorded. Relationships between nutritional factors, interventions and disease prognosis were assessed.
Results:
In total, 5835 papers were identified of which 44 were included in the final analysis. DCM patients with pathological weight pre-operatively were more likely to see poorer improvements post-surgically. These patients experienced poorer physical and mental health improvements from surgery compared to normal weight patients and were more likely to suffer from post-operative complications such as infection, DVT, PE and hospital readmissions. Two trials reporting benefits of nutritional supplements were identified, with 1 suggesting Cerebrolysin to be significant in functional improvement. An unbalanced diet, history of alcohol abuse and malnourishment were associated with poorer post-operative outcome.
Conclusion:
Although the overall strength of recommendation is low, current evidence suggests nutrition may have a significant role in optimising surgical outcome in DCM patients. Although it may have a role in onset and severity of DCM, this is a preliminary suggestion. Further work needs to be done on how nutrition is defined and measured, however, the beneficial results from studies with nutritional interventions suggest nutrition could be a treatment target in DCM.
Keywords: Cervical cord, myelopathy, spondylosis, stenosis, ossification posterior longitudinal ligament, degeneration, nutritional status, malnutrition, vitamins, minerals, obesity, body weight, gastrointestinal diseases, electrolytes, hospitalisation
Introduction
Degenerative cervical myelopathy (DCM) is a condition of cervical spinal cord dysfunction second to cervical stenosis from congenital and/or degenerative pathology. Degenerative processes include cervical spondylosis, ossification of the posterior longitudinal ligament, (OPLL), ossification of the ligamentum flavum and degenerative disc disease. 1 DCM is the commonest cause of spinal cord impairment in adults worldwide. 2
Surgical decompression is the mainstay of treatment and for most is able to halt disease progression and afford meaningful benefit.3,4 However, since the regenerative capacity of the spinal cord is limited, there is often permanent neurological disability 5 and people living with DCM have amongst the poorest quality of life of any chronic disease. 6 Therefore, offering treatment before there is permanent spinal cord damage should prevent this, but there are many uncertainties challenging such practice today.
These include the pathophysiology of DCM and the natural history. Whilst approximately 1 in 5 adults without symptoms will have evidence of spinal cord compression on their MRI due to incidental degenerative changes in the spine, it is estimated only 10% will go on to develop DCM with time. 7 This indicates that the perception that DCM is driven uniquely by spinal cord compression is an over-simplification, 8 and additional, dynamic factors must contribute to the aetiology of DCM, and/or influence the vulnerability of the spinal cord. Early investigations have identified candidate genetic determinants in small series, 9 but environmental factors are also likely.
Nutrition is an important environmental factor in many diseases. Adequate nutritional status is defined as food intake sufficient to meet their requirements to support optimal health and well-being. 10 Repair and rehabilitation requires adequate nutrition to support regenerative processes and prevent degeneration; for example, gross vitamin deficiencies can cause spinal degeneration11,12 and it is likely this represents one end of a spectrum. 13 Nutritional status is also linked to recovery from surgery. Surgery itself is a significant stress that activates inflammatory and catabolic pathways; an appropriate nutritional status allows the body to respond optimally and recover efficiently, 14 and nutritional interventions have become an important component of many enhanced recovery protocols across surgical disciplines. 15 The topic of nutrition is also frequently raised by people with DCM, in the Myelopathy.org community and was identified as an unanswered research question by the James Lind Alliance research priority setting partnership conducted as part of AO Spine RECODE-DCM (aospine.org/recode), albeit falling outside the top 10.
Therefore, the objective of this study was to conduct the first systematic review on the role of nutrition in the onset and severity of DCM experienced, and its role in patients’ response to surgical treatment.
Methods
A systematic review was conducted in accordance with PRISMA guidelines. 16 A search of Embase and MEDLINE via Ovid for all papers published before 28 January 2020 was performed using previously developed search filters. 17 The full search strategy is outlined in Supplemental Appendix 1. All primary clinical studies, available in English, considering an aspect of nutrition exclusively in the context of cervical myelopathy were considered eligible. ‘Nutrition’ encompassed all components that can be affected by diet, including: weight, electrolytes, vitamins, minerals and overall gastrointestinal (GI) health, since these can all be affected by the food one eats. If studies considered more than 1 nutrition factor, (eg, both weight and GI health) they were then assigned to all relevant categories, so that the influence of all assessed factors could be studied in depth. Animal studies, case reports, editorials, reviews, opinion articles, corrections, conference papers and studies relating to acute injury (ie, the acute stage of traumatic spinal cord injury) were excluded.
Papers were independently screened by 6 authors (CP, AS, FB, MA, AB and SA) and data were extracted (Supplemental Appendix 2). Discrepancies were settled by discussion and mutual agreement. For included studies, quality was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessment tool, and harvest plot made. 18 Details of the study design, cohort demographics, intervention(s), nutrition factor(s) and outcomes were extracted.
In order to consider the differing implications of nutrition on DCM onset, severity and surgical outcomes, the reported outcomes in studies were categorised into those relating to spinal column (eg, adjacent segment disease), spinal cord (eg, neurological examination, patient-reported outcome measures, recovery-rate with treatment) and those relating to the surgical procedure (eg, adverse events, such as infection, DVT, hospital readmission). Not all studies included outcomes in all subgroups.
Results
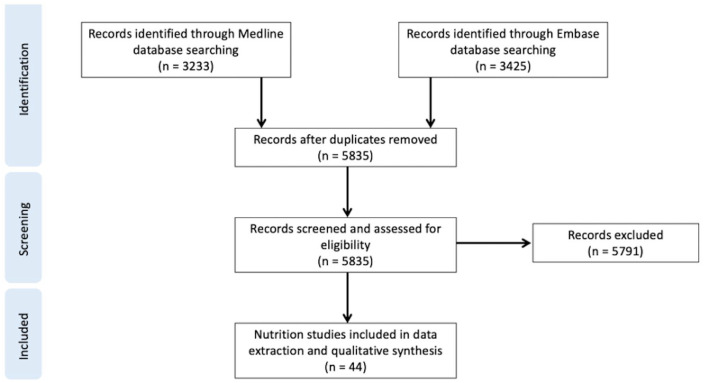
A total of 6658 papers were identified by our search; 5835 remained after de-duplication. Following full-text screening, 44 were included in the final analysis. The majority (35/44 = 80%) were cohort studies (prospective and retrospective), with cohort sizes ranging from 36 to 202 694 (see Figure 1).
Figure 1.
PRISMA flow diagram. 79
Based on the nutritional factor evaluated, the authors developed 4 categories to aid the collation of evidence: (1) weight, (2) malnutrition and electrolyte imbalance, (3) vitamins and minerals and (4) gastrointestinal (GI) health. Papers could be assigned to more than 1 category.
A GRADE assessment was performed to assess the quality of the papers. The GRADE score given is based on the specific outcomes measured in the papers (Supplemental Appendix 3). This data was used to formulate a harvest plot to give a visual representation of the specific outcomes assessed in individual papers (Supplemental Appendix 4). The bibliography for the harvest plot can be found in Supplemental Appendix 5.
Weight/BMI
Both obesity and pathological weight loss are important nutrition-related factors to consider. Whilst majority of papers (21/28, 75%), found significant correlations between weight/BMI and specific DCM outcomes, a total of 7 studies (7/28, 25%) reported no significant correlation between increased weight/BMI and DCM outcome,19-25 For example, an international multi-centre study of 479 DCM patients reported that, although those with post-operative complications were older and had a higher BMI, these differences were statistically insignificant. 19
The effect of weight on the spinal column
Weight has been shown to be a significant risk factor for developing cervical spondylosis. 20 Higher BMI was reported to be predictive of cervical cord compression in adults with spinal deformities. 26 One cohort study of 498 subjects reported that a high BMI significantly increased risk of cervical Modic changes (MC) in cervical spondylosis patients. 27 Furthermore, obese patients had significantly smaller preoperative cervical lordosis, immediate postoperative lordosis and final lordosis following anterior cervical decompression and fusion ACDF 28 ; the obese group experienced a significant increase in their sagittal vertical axis and a significantly decreased T1 slope during the recovery period. 28 However, other studies reported that BMI was not a significant predictor of cervical lordosis loss after laminoplasty and that only T1 slope significantly correlates with loss of cervical lordosis. 24
The effect of weight on adverse events: surgical outcomes and complications
One paper utilised a machine learning approach to report that higher weight was predictive of poorer surgical outcome, 29 and obese patients were shown to be more likely to require surgeries of greater duration. 30 In addition, a cohort study of 88 DCM patients demonstrated that obese patients had poorer post-operative patient-reported improvement.31,32 A multi-centre cohort study consisting of 8887 patients showed that obese patients had a higher risk of requiring intubation after anterior cervical surgery. 33 Furthermore, obesity was found to be a significant predictor of 30-day hospital readmission following anterior cervical fusion surgery at 3 or more levels, 34 although it did not significantly affect the need for revision surgery. 22 However, a single-centre cohort study of 178 patients reported that the average BMI of patients, who did not undergo ACDF revision after initial posterior cervical fusion (PCF), was significantly higher than those who did. 35
Elevated BMI was also significantly associated with increased risk of surgical site infections in DCM patients. 36 Body weight was also shown to be the most important predictor in determining postoperative bleeding and venous thromboembolism in patients undergoing cervical laminoplasty. 37 In particular, obesity in patients undergoing elective PCF has been reported to increase the risk of postoperative venous thromboembolism more than 6-fold. 38 Elevated BMI was also shown to be associated with increased neck disability at 1-year post-surgery. 39
On the contrary, 1 study reported that obesity was associated with a decreased risk of mortality and a higher functional independence in surgical DCM patients. 40 Another study reported greater mental-state-improvement post-operatively in obese patients. 31 Other studies found that both mental and physical improvements were poorer in obese patients compared to non-obese patients after anterior cervical surgery.32,39
In addition to obesity, weight loss has also been shown to affect surgical prognosis; ‘pathological weight loss’, defined as greater than 10% of body weight within 6 months of surgery, has been shown to be a predictor of 30-day hospital readmission in patients undergoing PCF. 41 Similarly, pathological weight loss was found to significantly increase surgical mortality rate, 40 and to be a significant predictor of aspiration. 42
Malnutrition and electrolyte imbalance
A total of 9 papers (9/44, 20%) studied the importance of malnutrition, nutritional supplementation and electrolyte imbalance in disease prognosis.
The effect of malnutrition and supplementation on the spinal cord
A randomised controlled trial reported that administering Cerebrolysin, a mixture of amino acids and biologically active peptides, to DCM patients who declined surgery, led to significant improvements in their myelopathy, including JOA scores and mean JOA recovery rate after 6 months. 43 Similarly, oral creatine supplementation was shown to enhance exercise capacity in patients with cervical spinal cord injury (SCI), significantly improving oxygen uptake, carbon dioxide production and tidal volume at peak effort, with associated significantly higher peak power output; although, there was no significant effect on respiratory exchange ratio, minute ventilation and time to fatigue. 44
The effect of malnutrition on adverse events: surgical outcomes and complications
Nutritional status is a significant predictor of length of hospital stay and post-operative functional motor independence. 45 Fluid and electrolyte disturbances have been shown to be significant predictors of perioperative morbidity, 40 post-operative aspiration 45 and post-operative dysphagia in patients undergoing anterior cervical fusion. 46 Furthermore, dehydration has been shown to be a significant predictor for 30-day hospital readmission after PCF. 41
Alcohol history was also a significantly predictor of surgical outcome.42,47 One multi-centre consisting of 202 694 patients reported that patients with a history of alcohol abuse were at significantly higher risk of post-operative aspiration. 42 In addition, 2 cohort studies reported that alcohol intake correlated with a delayed timing of fusion after anterior cervical surgery, 48 and hospital readmission. 41
In addition, a single-centre cohort study of 55 cervical myelopathy patients showed that lower prealbumin levels predicted significantly greater duration of hospital stay and higher risk of postoperative complications, including respiratory failure, skin ulcers, oesophageal perforation and infection. 49
Vitamins and minerals
In total, 4 papers (4/44, 9%) assessed the impact of vitamin and mineral deficiencies.
The effect of vitamins and minerals on spinal biology and recovery
A Brazilian multicentre cohort study reported that cobalamin, vitamin B12 and copper deficiencies were common among patients with non-traumatic myelopathy. 50 Furthermore, a single-centre cross-sectional study, of 106 patients with chronic SCI, showed that vitamin B12 deficiency was present in 67% of patients and correlated with a longer duration of SCI. 51 Macrocytic anaemia was present in 17% of subjects with subnormal B12 levels. 51 Furthermore, patients with tetraplegia and tetraparesis from cervical spine pathology manifested B12 deficiency more commonly than those with paraplegia and paraparesis from thoracic or lumbar spinal pathology, however, this was not a statistically significant difference. 51
Gastrointestinal health
A total of 3 papers (3/44, 7%) assessed gastrointestinal (GI) health. A retrospective cohort study reported that 121 of the 757 DCM patients studied had gastrointestinal comorbidities (GIC); these patients had significantly poorer physical health scores, a higher rate of psychiatric comorbidities and were more likely to be female. 52
The effect of gastrointestinal health on spinal cord biology and surgical outcome
A retrospective cohort study showed that DCM patients with GICs were significantly less neurologically impaired based on the Nurick Grade than those without GICs, although this may not be a meaningful difference. DCM patients with GICs were shown to have a lower prevalence of upper motor neurone signs and were less likely to exhibit signal intensity changes such as T1 hypointensity and T2 hyperintensity.
However, the same study reported no difference in surgical outcome between the GIC and non-GIC groups. 52
Furthermore, a multi-centre cohort study of 479 patients reported that the presence of gastric disease was a principal component in predicting post-operative outcome in DCM patients. 53 In particular, disability and quality of life measured by the neck disability index (NDI) and the Short Form (36) Health survey (SF-36) correlated significantly with the presence of comorbid gastric conditions. 53
Discussion
Nutrition, as evaluated using studies considering weight, vitamin and mineral deficiencies, malnutrition, electrolyte imbalance and gastrointestinal disease, appears to have a role in the assessment and management of DCM patients. Principally, studies were identified exploring nutrition with respect to adverse events of surgery. This association was strongest and most frequently evaluated with respect to increased BMI (obesity), describing increased risks of re-intubation, 18 hospital readmission, 34 bleeding, 38 surgical site infection, 36 venous thromboembolism 38 and suboptimal mental recovery.32,39 However, studies were also identified linking nutrition to the aetiology of spinal cord compression and the response of the spinal cord to compression, including 1 study, a randomised controlled trial of Cerebrolysin, suggesting benefit for supplementation.
Adverse events of surgery
The association between increased operative adverse events and nutrition is consistent with wider experiences of surgery.
Malnutrition is recognised as a key factor for patients undergoing surgery, 54 linked to complications such as wound healing and surgical site infection.55-57 Surgery itself is a significant insult for the human body, 58 and nutrition a fundamental component of growth and repair. This has led to initiatives such as Enhanced Recovery After Surgery (ERAS) which focus on peri-operative nutrition.59,60 These approaches are most prominent within gastrointestinal surgery, 61 but increasingly applied to other specialities including spinal surgery.62,63 A prospective observational study in spinal surgery in the USA is on-going. 64
Conversely, the implications of increased BMI extend beyond just nutrition. An increased BMI can create challenges throughout spinal surgical care,65-67 including diagnostic imaging, response to physical therapy, intubation and on-table position, surgical technique and susceptibility to peri-operative complications. Most of these challenges relate to body habitus; for example, in general positioning, or raised intrabdominal pressure increase intravenous pressure, contributing to increased operative blood loss and risk of thrombosis, however, the underlying mechanism is likely multifaceted. 38 Establishing this is challenging as BMI frequently interacts with other significant factors such as co-morbidities. In a global study of 479 DCM patients, whilst the relationship between high BMI and post-operative complications was insignificant, comorbidities such as diabetes mellitus, cardiovascular disease and gastrointestinal disease were associated with increased post-operative complications. 19 As identified in this review, 40 literature synthesis across spinal surgery demonstrate that increase BMI is not consistently an unfavourable factor.68-70 Therefore, whilst the association is significant, the causation is less well defined.
Spinal cord biology
The aforementioned, and well evidenced implications, for nutrition and surgery have made exploring a primary relationship between nutrition and the disease itself difficult. This is further compounded by the typical use of weight as a marker of nutrition, which is a recognised oversimplification, as even in elevated states (eg, obesity), a patient can be nutritionally deplete due to an unbalanced diet. 71 Furthermore, spinal cord injury can result in a profound change in body composition, best characterised by a reduction in the protein content of skeletal muscle. 72 That said, a number of studies were identified suggesting a tentative, but significant, link.
A recent linkage study, using the New York State Inpatient Data Base, compared subsequent care between morbidly obese patients that had undergone bariatric surgery and those who had not: The bariatric group, in a multi-variate model controlling for age and co-morbidities, were significantly more likely to subsequently undergo spinal surgery, in particular treatment for DCM. Amongst the author’s hypotheses for this is a consequence of post-operative malnourishment that can follow bariatric surgery.73,74
Other nutritional dimensions may also have a role. Nouri et al 55 in their secondary analysis of the AO Spine series, identified a differing pattern of symptoms and assessment findings between those with and without gastro-intestinal co-morbidities. Further, there appears to be associations between spinal cord injury, deficiencies in vitamins such as vitamin B12, 51 and vitamin D 12 and critical subunits for neural repair, over time. 13 Nouri et al 75 found in a retrospective analysis of 725 patients undergoing surgery for DCM or degenerative cervical radiculopathy, that general anaemia and macrocytic anaemia (a potential consequence of B12 deficiency) was associated with poorer baseline neurological function.
Further, Allam et al (2017), 43 conducted a randomised controlled study (N = 192), where patients declining surgery for DCM were given Cerebrolysin, a mixture of amino-acids and peptides given via intramuscular injection. Although, both arms improved on average without surgery (a methodological concern), this was more likely, and of greater magnitude, in the treatment arm as measured using the Japanese Orthopaedic Association score. It is important to note that despite this improvement, at their last follow-up at 6 months, patients in all groups were deteriorating from their initial improvement, suggesting that, whilst Cerebrolysin supplementation may have a significant role, its benefits may be limited to the short term.
Limitations and future directions
This is the first systematic review to aggregate evidence relating to nutritional factors and DCM. Nutrition is a broad concept (eg, in this review, pathological weight includes both low and high BMI) and current evidence is sparse, with few studies on any 1 topic and many areas remaining completely unexplored, despite an intensive and broad search strategy. This, coupled with inconsistent data reporting, 76 has precluded quantitative analysis, and studies are instead discussed and compared qualitatively.
Further, although the aggregated evidence relating nutrition to undergoing surgery is more consistent and well justified within the wider literature, this has made it more difficult to consider a relationship within respect to DCM onset/severity itself, as most observational studies included adverse events relating to surgical treatment. Therefore, in this study, assessments are considered by stratifying outcomes related to the surgery specifically (adverse events) from the spinal cord (neuromuscular function); however, this has limitations.
Additionally, the use of surrogates for nutrition such as weight, have inaccuracies. The measurement of nutritional status clinically is challenging. 77 Body weight, composition or serological markers can misrepresent an individual’s status. This is a significant challenge for interventional studies within nutrition, particularly in mixed populations. 78 Further investigation in this area will need to consider this.
Robust measures have been taken throughout this manuscript to ensure the quality of evidence is evaluated and the strength of recommendation clearly apparent (GRADE, see Supplemental Appendices 3 and 4). 18 Therefore, whilst promising, the evidence linking nutrition to DCM are at best preliminary thus far.
However, the findings of the Cerebrolysin study, which do not include these particular limitations, presents an opportunity for nutritional interventions in DCM that will need further exploration.
Conclusion
Nutrition, particularly weight, may have a role in the post-operative outcomes of DCM (High Strength Recommendation). Further, there is increasing evidence that nutrition, particularly supplementation, may have a role in both the onset and severity of DCM (Low Strength Recommendation). Whilst its role is likely to be multifactorial, as a modifiable factor, its better characterisation is a desirable target to support improved outcomes in the short-term. However, to truly understand its significance in DCM, future work must also consider how nutrition is defined and measured.
Supplemental Material
Supplemental material, sj-docx-1-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights
Supplemental material, sj-docx-2-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights
Supplemental material, sj-pdf-1-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights
Supplemental material, sj-pdf-2-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights
Supplemental material, sj-xlsx-1-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights
Footnotes
PROSPERO Systematic Review Registration ID: CRD42021239095
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: BMD is supported by an NIHR Clinical Doctoral Research Fellowship.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: This report is independent research arising from a Clinician Scientist Award, CS-2015-15-023, supported by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
Author Contributions: CIPS: First author, involved in original literature search, screening, data collection, quality assessment, writing of the original manuscript, editing, review and submission.
ODM: Second author, involved in supervision, editing and review of manuscript.
AS: Involved in screening process and quality assessment, including formatting GRADE table and harvest plots.
FB, AB, MA and SA: Involved in screening process.
BMD: Corresponding author, involved in supervision, editing and review of manuscript, GRADE table and harvest plots.
ORCID iDs: Celine I Partha Sarathi  https://orcid.org/0000-0001-5314-5762
https://orcid.org/0000-0001-5314-5762
Amil Sinha  https://orcid.org/0000-0002-3092-498X
https://orcid.org/0000-0002-3092-498X
Faheem Bhatti  https://orcid.org/0000-0003-3897-4196
https://orcid.org/0000-0003-3897-4196
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40:E675-E693. [DOI] [PubMed] [Google Scholar]
- 2. Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. BMJ. 2018;360:k186. doi: 10.1136/bmj.k186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fehlings MG, Wilson JR, Kopjar B, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95:1651–1658. [DOI] [PubMed] [Google Scholar]
- 4. Fehlings MG, Ibrahim A, Tetreault L, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine. 2015;40:1322–1328. [DOI] [PubMed] [Google Scholar]
- 5. Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of Cord compression. Glob Spine J. 2017;7:70S-83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mowforth OD, Davies BM, Kotter MR. Quality of life among informal caregivers of patients with degenerative cervical myelopathy: cross-sectional questionnaire study. Interact J Med Res. 2019;8:e12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith SS, Stewart ME, Davies BM, Kotter MRN. The prevalence of asymptomatic and symptomatic spinal cord compression on magnetic resonance imaging: a systematic review and meta-analysis .Global Spine J.2020;11:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akter F, Yu X, Qin X, et al. Corrigendum: the pathophysiology of degenerative cervical myelopathy and the physiology of recovery following decompression. Front Neurosci. 2020;14:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pope DH, Davies BM, Mowforth OD, Bowden AR, Kotter MRN. Genetics of degenerative cervical myelopathy: a systematic review and meta-analysis of candidate gene studies. J Clin Med. 2020;9:3. doi: 10.3390/jcm9010282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daniels L. Good nutrition for good surgery: clinical and quality of life outcomes. Aust Prescr. 2003;26:136–140. [Google Scholar]
- 11. Xu Y, Chen W, Jiang J. Cervical spondylotic myelopathy with vitamin B12 deficiency: two case reports. Exp Ther Med. 2013;6:943–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44:1612–1616. [DOI] [PubMed] [Google Scholar]
- 13. Nouri A, Patel K, Montejo J, et al. The role of vitamin B12 in the management and optimization of treatment in patients with degenerative cervical myelopathy. Glob Spine J. 2019;9:331–337. doi: 10.1177/2192568218758633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mignini EV, Scarpellini E, Rinninella E, et al. Impact of patients nutritional status on major surgery outcome. Eur Rev Med Pharmacol Sci. 2018;22:3524–3533. [DOI] [PubMed] [Google Scholar]
- 15. Qureshi R, Rasool M, Puvanesarajah V, Hassanzadeh H. Perioperative nutritional optimization in spine surgery. Clin Spine Surg. 2018;31:103–107. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davies BM, Goh S, Yi K, Kuhn I, Kotter MRN. Development and validation of a MEDLINE search filter/hedge for degenerative cervical myelopathy. BMC Med Res Methodol. 2018;18:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. What is GRADE? | BMJ best practice. Accessed November 10, 2020. https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/
- 19. Tetreault L, Tan G, Kopjar B, et al. Clinical and surgical predictors of complications following surgery for the treatment of cervical spondylotic myelopathy: results from the multicenter, prospective AOSpine International study of 479 patients. Neurosurg. 2016;79:33–44. [DOI] [PubMed] [Google Scholar]
- 20. Singh S, Kumar D, Kumar S. Risk factors in cervical spondylosis. J Clin Orthop Trauma. 2014;5:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takahashi H, Aoki Y, Saito J, et al. Serum oxidative stress influences neurological recovery after surgery to treat acutely worsening symptoms of compression myelopathy: a cross-sectional human study. BMC Musculoskelet Disord. 2019;20:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Eck CF, Regan C, Donaldson WF, Kang JD, Lee JY. The revision rate and occurrence of adjacent segment disease after anterior cervical discectomy and fusion: a study of 672 consecutive patients. Spine. 2014;39:2143–2147. [DOI] [PubMed] [Google Scholar]
- 23. You J, Tang X, Gao W, Shen Y, Ding W-Y, Ren B. Factors predicting adjacent segment disease after anterior cervical discectomy and fusion treating cervical spondylotic myelopathy: a retrospective study with 5-year follow-up. Medicine. 2018;97:e12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang JT, Li JQ, Niu RJ, Liu Z, Tong T, Shen Y. Predictors of cervical lordosis loss after laminoplasty in patients with cervical spondylotic myelopathy. Eur Spine J. 2017;26:1205–1210. [DOI] [PubMed] [Google Scholar]
- 25. Zhang JT, Meng FT, Wang S, Wang LF, Shen Y. Predictors of surgical outcome in cervical spondylotic myelopathy: focusing on the quantitative signal intensity. Eur Spine J. 2015;24:2941–2945. [DOI] [PubMed] [Google Scholar]
- 26. Shimizu T, Lehman Ra, Jr, Pongmanee S, et al. Prevalence and predictive factors of concurrent cervical spinal Cord compression in adult spinal deformity. Spine. 2019;44:1049–1056. [DOI] [PubMed] [Google Scholar]
- 27. Bai J, Yu K, Sun Y, Kong L, Shen Y. Prevalence of and risk factors for modic change in patients with symptomatic cervical spondylosis: an observational study. J Pain Res. 2018;11:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basques BA, Khan JM, Louie PK, et al. Obesity does not impact clinical outcome but affects cervical sagittal alignment and adjacent segment degeneration in short term follow-up after an anterior cervical decompression and fusion. Spine J. 2019;19:1146–1153. [DOI] [PubMed] [Google Scholar]
- 29. Merali ZG, Witiw CD, Badhiwala JH, Wilson JR, Fehlings MG. Using a machine learning approach to predict outcome after surgery for degenerative cervical myelopathy. PLoS One. 2019;14:e0215133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim DH, Zaremski J, Kwon B, et al. Risk factors for false positive transcranial motor evoked potential monitoring alerts during surgical treatment of cervical myelopathy. Spine. 2007;32:3041–3046. [DOI] [PubMed] [Google Scholar]
- 31. Auffinger B, Lam S, Kraninger J, Shen J, Roitberg BZ. The impact of obesity on surgeon ratings and patient-reported outcome measures after degenerative cervical spine disease surgery. World Neurosurg. 2014;82:e345–e352. [DOI] [PubMed] [Google Scholar]
- 32. Sielatycki JA, Chotai S, Kay H, Stonko D, McGirt M, Devin CJ. Does obesity correlate with worse patient-reported outcomes following elective anterior cervical discectomy and fusion? Neurosurg. 2016;79:69–74. [DOI] [PubMed] [Google Scholar]
- 33. Nagoshi N, Fehlings MG, Nakashima H, et al. Prevalence and outcomes in patients undergoing reintubation after anterior cervical spine surgery: results from the AOSpine North America multicenter study on 8887 patients. Glob Spine J. 2017;7:96S-102S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Puvanesarajah V, Hassanzadeh H, Shimer AL, Shen FH, Singla A. Readmission rates, reasons, and risk factors following anterior cervical fusion for cervical spondylosis in patients above 65 years of age. Spine. 2017;42:78–84. [DOI] [PubMed] [Google Scholar]
- 35. Wang TY, Lubelski D, Abdullah KG, Steinmetz MP, Benzel EC, Mroz TE. Rates of anterior cervical discectomy and fusion after initial posterior cervical foraminotomy. Spine J. 2015;15:971–976. [DOI] [PubMed] [Google Scholar]
- 36. Jalai CM, Worley N, Poorman GW, Cruz DL, Vira S, Passias PG. Surgical site infections following operative management of cervical spondylotic myelopathy: prevalence, predictors of occurrence, and influence on peri-operative outcomes. Eur Spine J. 2016;25:1891–1896. [DOI] [PubMed] [Google Scholar]
- 37. Kimura A, Ohmori T, Sakata A, et al. Hemostatic function to regulate perioperative bleeding in patients undergoing spinal surgery: a prospective observational study. PLoS One. 2017;12:e0179829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phan K, Kothari P, Lee NJ, Virk S, Kim JS, Cho SK. Impact of obesity on outcomes in adults undergoing elective posterior cervical fusion. Spine. 2017;42:261–266. [DOI] [PubMed] [Google Scholar]
- 39. Wilson JR, Tetreault LA, Schroeder G, et al. Impact of elevated body mass index and obesity on long-term surgical outcomes for patients with degenerative cervical myelopathy: analysis of a combined prospective dataset. Spine. 2017;42:195–201. [DOI] [PubMed] [Google Scholar]
- 40. David Kaye I, Marascalchi BJ, Macagno AE, Lafage VA, Bendo JA, Passias PG. Predictors of morbidity and mortality among patients with cervical spondylotic myelopathy treated surgically. Eur Spine J. 2015;24:2910–2917. [DOI] [PubMed] [Google Scholar]
- 41. Choy W, Lam SK, Smith ZA, Dahdaleh NS. Predictors of 30-day hospital readmission after posterior cervical fusion in 3401 patients. Spine. 2018;43:356–363. [DOI] [PubMed] [Google Scholar]
- 42. Fineberg SJ, Oglesby M, Patel AA, Singh K. Incidence, risk factors, and mortality associated with aspiration in cervical spine surgery. Spine. 2013;38:E1189–E1195. [DOI] [PubMed] [Google Scholar]
- 43. Allam AFA, Abotakia TAA, Koptan W. Role of cerebrolysin in cervical spondylotic myelopathy patients: a prospective randomized study. Spine J. 2018;18:1136–1142. [DOI] [PubMed] [Google Scholar]
- 44. Jacobs PL, Mahoney ET, Cohn KA, Sheradsky LF, Green BA. Oral creatine supplementation enhances upper extremity work capacity in persons with cervical-level spinal cord injury. Arch Phys Med Rehabil. 2002;83:19–23. [DOI] [PubMed] [Google Scholar]
- 45. Tanaka M, Momosaki R, Wakabayashi H, Kikura T, Maeda K. Relationship between nutritional status and improved ADL in individuals with cervical spinal cord injury in a convalescent rehabilitation ward. Spinal Cord. 2019;57:501–508. [DOI] [PubMed] [Google Scholar]
- 46. Singh K, Marquez-Lara A, Nandyala SV, Patel AA, Fineberg SJ. Incidence and risk factors for dysphagia after anterior cervical fusion. Spine. 2013;38:1820–1825. [DOI] [PubMed] [Google Scholar]
- 47. Passias PG, Jalai CM, Worley N, et al. Predictors of hospital length of stay and 30-day readmission in cervical spondylotic myelopathy patients: an analysis of 3057 patients using the ACS-NSQIP database. World Neurosurg. 2018;110:e450–e458. [DOI] [PubMed] [Google Scholar]
- 48. Yeung KKL, Cheung PWH, Cheung JPY. Anterior cervical discectomy and fusion for cervical myelopathy using stand-alone tricortical iliac crest autograft: predictive factors for neurological and fusion outcomes. J Orthop Surg Hong Kong. 2019;27:2309499019869166. [DOI] [PubMed] [Google Scholar]
- 49. Guan J, Holland CM, Ravindra VM, Bisson EF. Perioperative malnutrition and its relationship to length of stay and complications in patients undergoing surgery for cervical myelopathy. Surg Neurol Int. 2017;8:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pinto WB, de Souza PV, de Albuquerque MV, Dutra LA, Pedroso JL, Barsottini OG. Clinical and epidemiological profiles of non-traumatic myelopathies. Arq Neuropsiquiatr. 2016;74:161–165. [DOI] [PubMed] [Google Scholar]
- 51. Petchkrua W, Burns SP, Stiens SA, James JJ, Little JW. Prevalence of vitamin B12 deficiency in spinal cord injury. Arch Phys Med Rehabil. 2003;84:1675–1679. [DOI] [PubMed] [Google Scholar]
- 52. Nouri A, Badhiwala JH, Kato S, et al. The relationship between gastrointestinal comorbidities, clinical presentation and surgical outcome in patients with DCM: analysis of a global cohort. J Clin Med. 2020;9:5. doi: 10.3390/jcm9030624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tetreault L, Tan G, Kopjar B, et al. Clinical and surgical predictors of complications following surgery for the treatment of cervical spondylotic myelopathy: results from the multicenter, prospective AOSpine International study of 479 patients. Neurosurg. 2015;15:S241–S244. [DOI] [PubMed] [Google Scholar]
- 54. Gomes F, Baumgartner A, Bounoure L, et al. Association of nutritional Support with clinical outcomes among medical inpatients who are malnourished or at nutritional risk: an updated systematic review and meta-analysis. JAMA Netw Open. 2019;2:e1915138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Klein JD, Hey LA, Yu CS, et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine. 1996;21:2676–2682. [DOI] [PubMed] [Google Scholar]
- 56. Bohl DD, Shen MR, Mayo BC, et al. Malnutrition predicts infectious and wound complications following posterior lumbar spinal fusion. Spine. 2016;41:1693–1699. [DOI] [PubMed] [Google Scholar]
- 57. Tsantes AG, Papadopoulos DV, Lytras T, et al. Association of malnutrition with surgical site infection following spinal surgery: systematic review and meta-analysis. J Hosp Infect. 2020;104:111–119. [DOI] [PubMed] [Google Scholar]
- 58. Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–117. [DOI] [PubMed] [Google Scholar]
- 59. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–298. [DOI] [PubMed] [Google Scholar]
- 60. Wynter-Blyth V, Moorthy K. Prehabilitation: preparing patients for surgery. BMJ. 2017;358:j3702. [DOI] [PubMed] [Google Scholar]
- 61. Ripollés-Melchor J, Ramírez-Rodríguez JM, Casans-Francés R, et al. Association Between use of enhanced recovery after surgery protocol and postoperative complications in colorectal surgery: the postoperative outcomes within enhanced recovery after surgery protocol (POWER) study. JAMA Surg. 2019;154:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wainwright TW, Immins T, Middleton RG. Enhanced recovery after surgery (ERAS) and its applicability for major spine surgery. Best Pract Res Clin Anaesthesiol. 2016;30:91–102. [DOI] [PubMed] [Google Scholar]
- 63. Nielsen PR, Jørgensen LD, Dahl B, Pedersen T, Tønnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clin Rehabil. 2010;24:137–148. [DOI] [PubMed] [Google Scholar]
- 64. Ali ZS, Ma TS, Ozturk AK, et al. Pre-optimization of spinal surgery patients: development of a neurosurgical enhanced recovery after surgery (ERAS) protocol. Clin Neurol Neurosurg. 2018;164:142–153. [DOI] [PubMed] [Google Scholar]
- 65. Katsevman GA, Daffner SD, Brandmeir NJ, Emery SE, France JC, Sedney CL. Complexities of spine surgery in obese patient populations: a narrative review. Spine J. 2020;20:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Katsevman GA, Daffner SD, Brandmeir NJ, Emery SE, France JC, Sedney CL. Complications of spine surgery in “Super Obese” patients [published online ahead of print September 1, 2020]. Glob Spine J. doi: 10.1177/2192568220953393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abdallah DY, Jadaan MM, McCabe JP. Body mass index and risk of surgical site infection following spine surgery: a meta-analysis. Eur Spine J. 2013;22:2800–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jackson KL, Devine JG. The effects of obesity on spine surgery: a systematic review of the literature. Glob Spine J. 2016;6:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goyal A, Elminawy M, Kerezoudis P, et al. Impact of obesity on outcomes following lumbar spine surgery: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2019;177:27–36. [DOI] [PubMed] [Google Scholar]
- 70. Wang T, Han C, Jiang H, Tian P. The effect of obesity on clinical outcomes after minimally invasive surgery of the spine: a systematic review and meta-analysis. World Neurosurg. 2018;110:e438–e449. [DOI] [PubMed] [Google Scholar]
- 71. Ngaruiya C, Hayward A, Post L, Mowafi H. Obesity as a form of malnutrition: over-nutrition on the Uganda “malnutrition” agenda. Pan Afr Med J. 2017;28:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Long YC, Kostovski E, Boon H, Hjeltnes N, Krook A, Widegren U. Differential expression of metabolic genes essential for glucose and lipid metabolism in skeletal muscle from spinal cord injured subjects. J Appl Physiol Bethesda Md 1985. 2011;110:1204–1210. [DOI] [PubMed] [Google Scholar]
- 73. Brown SM, Chew FS. Osteoporotic hip fracture as a delayed complication of bariatric surgery. Radiol Case Rep. 2006;1:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Epstein NE. Bariatric bypasses contribute to loss of bone mineral density but reduce axial back pain in morbidly obese patients considering spine surgery. Surg Neurol Int. 2017;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nouri A, Matur A, Pennington Z, et al. Prevalence of anemia and its relationship with neurological status in patients undergoing surgery for degenerative cervical myelopathy and radiculopathy: a retrospective study of 2 spine centers. J Clin Neurosci. 2020;72:252–257. [DOI] [PubMed] [Google Scholar]
- 76. Davies BM, Khan DZ, Mowforth OD, et al. RE-CODE DCM (REsearch objectives and common data elements for degenerative cervical myelopathy): a consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for clinical research and the definition of the research priorities. Glob Spine J. 2019;9:65S-76S. doi: 10.1177/2192568219832855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Elmadfa I, Meyer AL. Developing suitable methods of nutritional status assessment: a continuous challenge. Adv Nutr. 2014;5:590S-598S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Doig GS, Simpson F, Finfer S, et al. Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731–2741. [DOI] [PubMed] [Google Scholar]
- 79. Page M.J., McKenzie J.E., Bossuyt P.M. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10, 89 (2021). 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights
Supplemental material, sj-docx-2-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights
Supplemental material, sj-pdf-1-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights
Supplemental material, sj-pdf-2-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights
Supplemental material, sj-xlsx-1-nmi-10.1177_11786388211054664 for The Role of Nutrition in Degenerative Cervical Myelopathy: A Systematic Review by Celine I Partha Sarathi, Oliver D Mowforth, Amil Sinha, Faheem Bhatti, Aniqah Bhatti, Melika Akhbari, Shahzaib Ahmed and Benjamin M Davies in Nutrition and Metabolic Insights