Abstract
To predict transcription, one needs a mechanistic understanding of how the numerous required transcription factors (TFs) explore the nuclear space to find their target genes, assemble, cooperate, and compete with one another. Advances in fluorescence microscopy have made it possible to visualize real-time TF dynamics in living cells, leading to two intriguing observations: first, most TFs contact chromatin only transiently; and second, TFs can assemble into clusters through their intrinsically disordered regions. These findings suggest that highly dynamic events and spatially structured nuclear microenvironments might play key roles in transcription regulation that are not yet fully understood. The emerging model is that while some promoters directly convert TF-binding events into on/off cycles of transcription, many others apply complex regulatory layers that ultimately lead to diverse phenotypic outputs. Cracking this kinetic code is an ongoing and challenging task that is made possible by combining innovative imaging approaches with biophysical models.
Cell-fate control rests with a series of proteins generally termed transcription factors (TFs): cell types are the product of the sequential expression of distinct TFs during development and forced expression of the right TF(s) can reprogram cells into different cell fates (Takahashi and Yamanaka 2006). TFs recognize specific sequences within the promoter or enhancer(s), which regulates a given gene (Suter 2020). Once bound, they recruit coactivators and chromatin remodelers, culminating in the assembly at the gene promoter of the preinitiation complex that loads a functional RNA polymerase II (Pol II). Pol II is then licensed for elongation by regulatory complexes and proceeds to synthesize the nascent transcript (Cramer 2019). For the purpose of this review, we adopt an umbrella definition of TFs that, in addition to sequence-specific TFs, also includes chromatin remodelers, general transcription factors (gTFs), Pol II, coactivators, and repressors.
In vitro biochemical assays and in vivo footprinting assays have provided important insights into the DNA sequences targeted by TFs (Lambert et al. 2018). In higher eukaryotes, sequence-specific TFs typically bind thousands of targets to regulate hundreds of genes, although some TFs display increased specialization (Zolotarev et al. 2017), in extreme cases controlling a single gene such as the TF ZNF410 that uniquely controls γ-globin transcription in erythroid cells (Yang et al. 2017; Lan et al. 2020). Because transcription programs are often long-lived (days), TFs have generally been assumed to bind their targets for long periods (Perlmann et al. 1990), consistent with the complexity of the transcription machinery, deemed incompatible with rapid assembly. However, over the last two decades, live imaging studies have demonstrated that most TFs are highly dynamic with residence time of seconds (Hager et al. 2009), that different TF subpopulations exhibit specific mobility dynamics, and that TFs often form nonstoichiometric complexes consisting of many molecules (Liu and Tjian 2018). These complexes have been referred to as clusters, condensates, or hubs across the literature. As condensates are often implied to form through phase separation and hubs hint at a functional role, we restrict ourselves to the term cluster here when we refer to those complexes without assumption of their assembly mechanism or function. In parallel, live-cell mRNA imaging experiments have similarly uncovered complex transcription dynamics. Active genes do not synthesize mRNAs steadily over time; rather, transcription occurs stochastically in bursts that alternate with off periods (Rodriguez and Larson 2020). As a result, expression levels, and thus phenotypes, are probabilistic rather than deterministic (Symmons and Raj 2016). Despite recent progress, we still lack mechanistic models linking stochastic transcription kinetics with upstream TF biophysics. In this article, we review our current understanding of TF mobility and discuss mechanisms linking TF dynamics with stochastic transcription outputs.
TECHNIQUES TO CAPTURE TF DYNAMICS
Understanding TF dynamics requires tools to detect where TFs bind in the genome, at what amount, what percentage of TFs are bound to DNA (% bound), and what their association (kon) and dissociation rates with the DNA (koff, the inverse of the TF residence time) are.
In Vitro Binding Specificity
Systematic evolution of ligands by exponential enrichment (SELEX) probes DNA motifs preferentially bound by TFs in vitro (Jolma et al. 2010). A purified TF is incubated with a large library of DNA molecules, from which TF-bound sequences are identified. SELEX may not accurately reflect in vivo binding, and where TFs compete or cooperate with one another, DNA is folded into chromatin and exposed to a very different ionic milieu than in vitro. Nevertheless, SELEX has been used to determine the binding specificity of hundreds of TFs on free and nucleosome-containing DNA, confirming notable differences (Zhu et al. 2018).
In Vivo Binding Specificity
Chromatin immunoprecipitation (ChIP)-seq is widely used to determine in vivo genome-wide binding profiles (Johnson et al. 2007). Chemical cross-linking of a TF of interest to DNA in cells is followed by ChIP and sequencing of TF-bound DNA fragments. Recent variations of the procedure achieve better spatiotemporal resolution than the original methods; for example, digesting fragmented DNA prior to ChIP enables near base-pair resolution (Rhee and Pugh 2011; He et al. 2015). While ChIP traditionally measures average TF occupancy (Fig. 1), recent modifications of the technique enable measurements of kinetic rates (kon, koff):kon can be determined by varying the cross-linking duration (Poorey et al. 2013), whereas measuring bound TFs at various time points after acute depletion of nuclear TFs provides access to genome-wide koff values (Jonge et al. 2020). ChIP, however, is subject to two technical caveats: first, TFs can artificially dissociate from chromosomes upon cross-linking (Teves et al. 2016; Festuccia et al. 2019), which can be overcome by alternatives bypassing cross-linking (Skene and Henikoff 2017), and second, ChIP accuracy is limited by antibody quality (Shah et al. 2018). ChIP-seq and its derivatives have two further limitations: first, they require tens to millions of cells to produce a robust signal, and therefore only provide binding profiles averaged over many cells, and second, free TFs are lost as only the chromatin-bound fraction is captured. Despite these limitations, ChIP-seq remains the sole approach capable of capturing genome-wide target sites of TFs in cells.
Figure 1.
Occupancy versus kinetics. ChIP-seq (top) measures the average occupancy of a given transcription factor (TF) at its binding site. Increased occupancy may result from higher TF-binding frequency (higher kon), or longer residence times (1/koff, bottom). Kinetic profiles can be decoded into distinct transcriptional outputs by promoters.
Live-Cell Kinetics
Because transcription bursts are heterogeneous among cell populations (Chubb et al. 2006; Raj et al. 2006; Suter et al. 2011), resolving transcription kinetics requires single-cell sensitivity. Given that TFs turn over within seconds, temporal resolution is key for any method to measure in vivo TF dynamics. Furthermore, in situ studies are necessary to recapitulate physiological chromatin states and cofactors. Live imaging satisfies all these conditions, and various methods discussed below have emerged as tools of choice to probe TF dynamics (Mueller et al. 2013).
Fluorescence recovery after photobleaching (FRAP) involves photobleaching fluorescently tagged TFs in a nuclear region of interest, and subsequently measuring fluorescence recovery, typically over seconds to hours, as photobleached TFs exchange with fluorescent TFs freely diffusing into the focal volume from the rest of the nucleus (Phair and Misteli 2000). Fitting recovery curves to reaction-diffusion models provides estimates of average diffusion coefficients of free TFs, residence times of bound TFs, and the relative proportions of the two states (Darzacq et al. 2007; Maiuri et al. 2011).
Fluorescence correlation spectroscopy (FCS) measures the passage of individual molecules through a focused beam (Elf et al. 2007). The number of molecules crossing the beam per unit time provides access to the absolute TF concentration in the chosen region, while the duration each molecule dwells in the focal volume provides similar observables as FRAP, albeit in a different time regime (ms-sec).
Single-molecule tracking (SMT) captures the dynamics of TFs over an entire nuclear plane (Elf and Barkefors 2019). TFs labeled with a photoactivatable fluor are initially dark but upon a brief pulse of blue light, a few TF molecules turn on and are tracked until they either photobleach or diffuse out of the focal volume. Many cycles of activation followed by tracking generate hundreds to thousands of individual trajectories per cell. SMT is usually performed in one of two imaging modes: the fast-tracking mode (exposure times of 1–50 msec per frame) captures fast diffusing TFs in the nucleoplasm, from which one can determine the relative amounts of free versus chromatin-bound TFs and their diffusion coefficients. The slow-tracking mode (exposure times of 100–500 msec per frame) motion-blurs free molecules to calculate the residence times (1/koff) of TFs bound to chromatin.
A SHORT STAY AFTER A LONG SEARCH
TF Search Dynamics
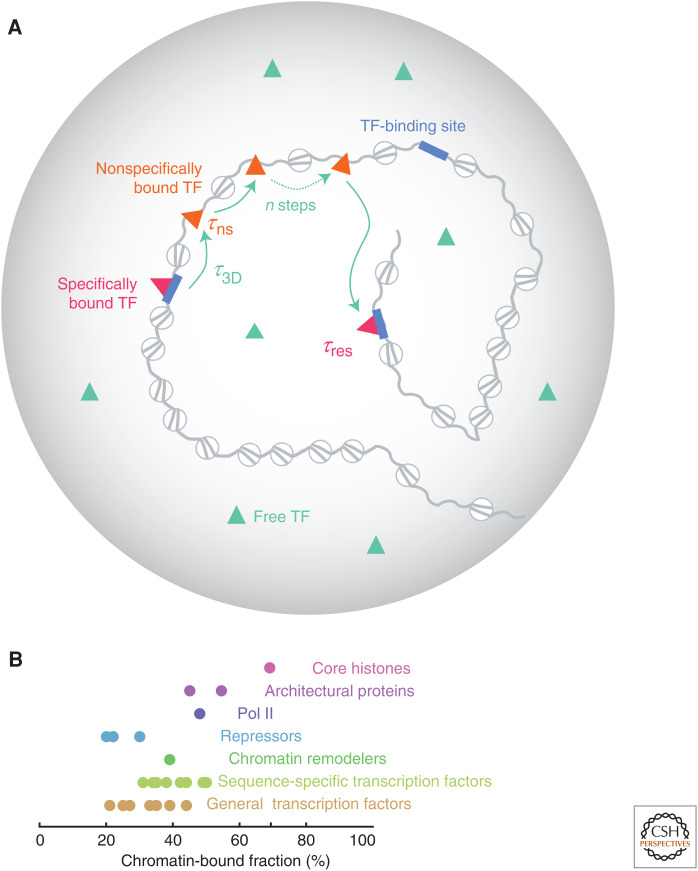
Live imaging studies have helped shape a clearer picture of TF behavior. TFs reside on DNA only for short intervals (1–100 sec) and at any given time, less than half the TF population is bound to chromatin (Figs. 2 and 3; Tables 1 and 2). According to the facilitated diffusion model (von Hippel and Berg 1989), while searching for its target(s), a TF molecule diffuses through the nuclear space in 3D, occasionally binding and briefly sliding along accessible DNA regions (<1 sec) (Chen et al. 2014; Marklund et al. 2020). After several attempts, the TF molecule eventually lands on a cognate binding site, where it resides longer (∼10 sec). The average time it takes for a TF to travel between two specific binding sites is defined as its search time, which is inversely proportional to kon, and sets a limit to how fast a gene can be activated (Elf and Barkefors 2019). As a result of the dozens of nonproductive interactions with chromatin, the search time is often orders of magnitude longer than the TF residence time (Fig. 2). The chromatin-bound fraction consists of both specifically and nonspecifically bound TF molecules and the search time is impacted by the concentration of target sites (Reisser et al. 2018), DNA folding (Cortini and Filion 2018), the chromatin states surrounding the target sites (Mehta et al. 2018), the uneven spatial distribution of protein/DNA barriers in the nucleoplasm (Izeddin et al. 2014; Li et al. 2016), and the presence of coregulators at the target site (Mir et al. 2017). The effective kon is also directly proportional to the nuclear concentration of TFs, enabling dynamical regulation of target occupancy across a large dynamic range (Di Ventura and Kuhlman 2016).
Figure 2.
Transcription factor (TF) mobility in the nucleus. (A) Facilitated 3D diffusion model. While searching for its target sites (blue), a TF makes multiple, brief, and nonspecific contacts (τns < 1 sec; orange) with open chromatin before landing on its cognate site where it dwells longer (τres ∼ 1–100 sec; magenta). The average time between two specific binding events is defined as the search time τsearch = (n−1)*(τ3D + τns) + τ3D, where n ∼ 10–100 is the number of trials and τ3D is the averaged diffusion time between two trials. (Panel A is based on data in Chen et al. 2014.) (B) Experimentally measured chromatin-bound fraction (circles) for various TFs compiled from the community resource developed by Mir and colleagues (www.mir-lab.com/dynamics-database) and the recent literature. Only factors expressed as knockins or rescuing a knockout background are featured here.
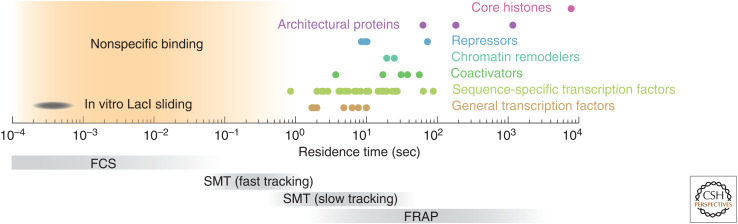
Figure 3.
Chromatin association timescales in vivo. Experimentally measured residence times (circles) compiled from the community resource developed by Mir and colleagues (www.mir-lab.com/dynamics-database) and the recent literature, relative to the temporal resolution of different imaging techniques (bottom, gray). (FCS) Fluorescence correlation spectroscopy, (SMT) single-molecule tracking, (FRAP) fluorescence recovery after photobleaching.
Table 1.
Experimentally measured residence times for various transcription factors (TFs) related to Figure 3
| Protein name | Protein type | Residence time (seconds) | References |
|---|---|---|---|
| Histone H1 | Architectural protein | 183 | Phair et al. 2004 |
| CTCF | Architectural protein | 63 | Hansen et al. 2017 |
| CTCF | Architectural protein | 184.3 | Hansen et al. 2017 |
| RAD21 | Architectural protein | 1170.6 | Hansen et al. 2017 |
| HMGN1 | Chromatin remodeler | 24.8 | Phair et al. 2004 |
| BRG1 | Chromatin remodeler | 19.4 | Phair et al. 2004 |
| PCAF | Coactivator | 17.1 | Phair et al. 2004 |
| CYCT1 | Coactivator | 56 | Lu et al. 2018 |
| BRD4 | Coactivator | 38.1 | Phair et al. 2004 |
| ARNT | Coactivator | 30.9 | Phair et al. 2004 |
| Mediator | Coactivator | 3.7 | Nguyen et al. 2020 |
| H2B | Core histone | 7800 | Kimura and Cook 2001 |
| TFIIA | General TF | 6.3 | Nguyen et al. 2020 |
| TFIIB | General TF | 1.8 | Nguyen et al. 2020 |
| TFIID | General TF | 4.8 | Nguyen et al. 2020 |
| TFIIE | General TF | 2 | Nguyen et al. 2020 |
| TFIIF | General TF | 1.7 | Nguyen et al. 2020 |
| TFIIH | General TF | 10 | Nguyen et al. 2020 |
| TFIIK | General TF | 7.7 | Nguyen et al. 2020 |
| Cbx7 | Repressor | 8.5 | Tatavosian et al. 2018 |
| HT-Cbx7/F-H3.3 | Repressor | 9.7 | Tatavosian et al. 2018 |
| Eed | Repressor | 9.5 | Tatavosian et al. 2018 |
| EZH2 | Repressor | 10 | Tatavosian et al. 2018 |
| HT-EZH2/F-H3.3 | Repressor | 10.2 | Tatavosian et al. 2018 |
| HP1β | Repressor | 73 | Phair et al. 2004 |
| ESRRB | Sequence-specific TF | 10 | Xie et al. 2017 |
| STAT3 | Sequence-specific TF | 8.3 | Xie et al. 2017 |
| TBP | Sequence-specific TF | 88 | Teves et al. 2018 |
| Sox2 | Sequence-specific TF | 14.6 | Teves et al. 2016 |
| Estrogen receptor | Sequence-specific TF | 4.36 | Swinstead et al. 2016 |
| FoxA1 | Sequence-specific TF | 10.8 | Swinstead et al. 2016 |
| CREB1 | Sequence-specific TF | 2.86 | Sugo et al. 2015 |
| Glucocorticoid receptor | Sequence-specific TF | 0.85 | Stasevich et al. 2010 |
| Sox19b | Sequence-specific TF | 2 | Reisser et al. 2018 |
| TBP | Sequence-specific TF | 6.8 | Reisser et al. 2018 |
| Glucocorticoid receptor | Sequence-specific TF | 7.25 | Presman et al. 2016 |
| AhR | Sequence-specific TF | 25.6 | Phair et al. 2004 |
| C/EBP | Sequence-specific TF | 18.8 | Phair et al. 2004 |
| FBP | Sequence-specific TF | 63.6 | Phair et al. 2004 |
| Fos | Sequence-specific TF | 14.6 | Phair et al. 2004 |
| Jun | Sequence-specific TF | 27.3 | Phair et al. 2004 |
| Mad | Sequence-specific TF | 19.5 | Phair et al. 2004 |
| Myc | Sequence-specific TF | 16.3 | Phair et al. 2004 |
| NF1 | Sequence-specific TF | 16.2 | Phair et al. 2004 |
| XBP | Sequence-specific TF | 23.1 | Phair et al. 2004 |
| TetR | Sequence-specific TF | 5 | Normanno et al. 2015 |
| Bicoid | Sequence-specific TF | 2.33 | Mir et al. 2018 |
| Zelda | Sequence-specific TF | 5.56 | Mir et al. 2018 |
| p53 | Sequence-specific TF | 2.5 | Hinow et al. 2006 |
| Gal4 | Sequence-specific TF | 17 | Donovan et al. 2019 |
| OCT-4 | Sequence-specific TF | 14.6 | Chen et al. 2014 |
| P65 (NF-κB) | Sequence-specific TF | 4.1 | Callegari et al. 2019 |
Table 2.
Experimentally measured chromatin-bound fraction for various transcription factor (TFs), related to Figure 2
| Protein name | Protein type | Bound (%) | References |
|---|---|---|---|
| RAD21 | Architectural protein | 45 | Hansen et al. 2017 |
| CTCF | Architectural protein | 54.5 | Hansen et al. 2017 |
| Mediator | Coactivator | 39 | Nguyen et al. 2020 |
| H2B | Core histone | 69 | Nguyen et al. 2020 |
| TFIID | General TF | 39 | Nguyen et al. 2020 |
| TFIIA | General TF | 35 | Nguyen et al. 2020 |
| TFIIB | General TF | 21 | Nguyen et al. 2020 |
| TFIIF | General TF | 25 | Nguyen et al. 2020 |
| TFIIE | General TF | 33 | Nguyen et al. 2020 |
| TFIIH | General TF | 27 | Nguyen et al. 2020 |
| TFIIK | General TF | 44 | Nguyen et al. 2020 |
| TBP | General TF | 34 | Nguyen et al. 2020 |
| TBP | General TF | 31 | Teves et al. 2018 |
| Eed | Repressor | 22 | Tatavosian et al. 2018 |
| Ring1B | Repressor | 20 | Huseyin and Klose 2021 |
| Cbx7 | Repressor | 30 | Tatavosian et al. 2018 |
| EZH2 | Repressor | 22 | Tatavosian et al. 2018 |
| Zelda | Sequence-specific TF | 49 | Mir et al. 2018 |
| Bicoid | Sequence-specific TF | 50 | Mir et al. 2018 |
| STAT3 | Sequence-specific TF | 35 | Xie et al. 2017 |
| Sox2 | Sequence-specific TF | 38 | Liu et al. 2014 |
| OCT-4 | Sequence-specific TF | 42.2 | Chen et al. 2014 |
| ESRRB | Sequence-specific TF | 44 | Xie et al. 2017 |
| RPB1 | Subunit of Pol II | 48 | Nguyen et al. 2020 |
Repressive chromatin complexes are generally smaller than activating ones (Miron et al. 2020), but TF mobility and accessibility to their target sites are largely independent of molecular weights, ruling out steric hindrance as a main partition mechanism (Grünwald et al. 2008; Bancaud et al. 2009; Liu et al. 2014). Lower TF mobility and higher trapping frequencies in heterochromatin appear more likely to explain the observed enrichment of repressive complexes in chromatin-dense regions. Additionally, while 3D diffusing, some TFs move isotropically as expected for free diffusion, while others exhibit anisotropy, making more U-turns than expected by chance, which affects their search times (Izeddin et al. 2014). Anisotropic exploration results in the TF oversampling its nuclear neighborhood. Conversely, isotropic diffusion enables global exploration where any target is equally likely to be reached, regardless of distance. Nuclear exploration is therefore likely more complex than the three discrete states envisioned in the facilitated diffusion model (3D diffusion, 1D DNA sliding, stable binding to a target): interactions with nucleosomes (Lerner et al. 2020) or association into local clusters (Hansen et al. 2020) impact TF mobility and could introduce apparent bound states guiding search in preferred compartments. Single-molecule trajectories constitute rich data sets, and mobility metrics extending beyond the diffusion coefficient bring important insights (Shukron et al. 2019).
TFs Interact Briefly with Chromatin
Unlike core histones that stably bind chromatin for tens of minutes to hours (Kimura and Cook 2001; Dion et al. 2007; Deal et al. 2010), the residence times of sequence-specific TFs, gTFs, and repressive complexes tend to be short, in the range of seconds (Fig. 3; Choi et al. 2017; Youmans et al. 2018; Suter 2020). The linker histone H1 exchanges from chromatin with similarly fast kinetics (∼20 sec–3 min) (Lever et al. 2000; Misteli et al. 2000), while the architectural proteins cohesin and CTCF fall in between core histones and TFs (min) (Hansen et al. 2017). The residence time depends on the affinity between a TF and its target (Clauß et al. 2017; Callegari et al. 2019; Donovan et al. 2019; Popp et al. 2020), but can be modulated across loci, cell types, and cell states. For example, the residence time of the gTF TBP (TATA-binding protein) ranges from seconds in the developing zebrafish embryo (Reisser et al. 2018), interphase U2OS cells, and in vitro assays (Zhang et al. 2016), to hours at the histone locus in Drosophila cells (Guglielmi et al. 2013). TBP resides longer at active genes on mitotic chromosomes than during interphase in mouse embryonic stem (ES) cells, enabling rapid transcriptional reactivation upon mitotic exit (Teves et al. 2018). SRF (serum response factor) (Hipp et al. 2019), the glucocorticoid receptor (Stavreva et al. 2019), and GAL4 (Donovan et al. 2019) all exhibit increased residence times upon activation of their upstream pathways. In the case of GAL4, the promoter nucleosome is a key modulator of TF residence time (Donovan et al. 2019) but it is not clear whether this is a general mechanism. In most cases studied so far, the changes in TF residence times are small compared to the changes in the transcription output of their downstream genes, suggesting additional regulation of kon and/or downstream amplifying mechanisms (Fig. 1). Short TF residence times mirror the observations that target sites favor low-affinity TF motifs, a feature that confers extended sensitivity to TF concentration (Kribelbauer et al. 2019).
The biological interpretation of residence times measured by SMT faces two challenges: first, photobleaching limits the direct observation of very long events, even though photobleaching contributions can be corrected from measurements (Gebhardt et al. 2013; Chen et al. 2014; Hansen et al. 2017; Reisser et al. 2020; Garcia et al. 2021). Second, in each experiment, binding events are measured across the nucleus without knowledge of the locus bound by each TF. The original separation of binding events into two discrete populations, assumed to represent nonspecific versus specific events, based on the observation of short (<1 sec) and long (∼10 sec) subpopulations in residence time distributions, and supported by DNA-binding domain (DBD) deletion experiments (Chen et al. 2014), might not be valid in all cases. Some TFs exhibit broad residence time distributions, consistent with a continuum of affinities across diverse genomic targets (Normanno et al. 2015; Stavreva et al. 2019; Garcia et al. 2021). A novel kinetic analysis suggests on the other hand the existence of 5–6 discrete dissociation rates ranging from subseconds to minutes (Popp et al. 2020; Reisser et al. 2020). Despite their differences, the different SMT analyses converge on the fact that only a minority of TF-binding events extend beyond the seconds regime, consistent with dozens of earlier studies by FRAP (Hemmerich et al. 2011). In contrast, an indirect approach suggests exceptionally long-lived TF binding in the Xenopus oocyte (hours to days) (Gurdon et al. 2020) but it remains unclear whether the chromatin environment of the oocyte fosters this unusual behavior. Albeit chromatin motion and microscopy constrain the live-cell-imaging resolution to ∼10 kbp (Li et al. 2019), new microscopes able to measure TF binding at a specific locus have validated that seconds-long TF interactions do occur at relevant targets, and that they correlate with productive transcription (Donovan et al. 2019; Li et al. 2019; Stavreva et al. 2019). Combined with biological perturbations, these tools hold great potential to decipher the TF kinetic code.
From TF Binding to Transcripts
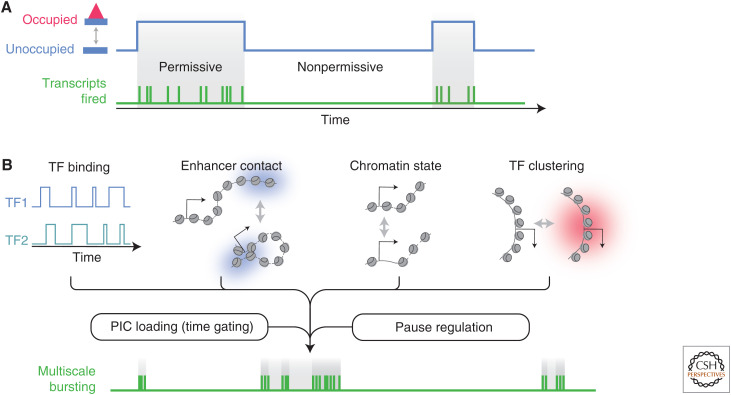
The short TF residence times echo the short bursts that constitute the basic unit of transcription (Rodriguez and Larson 2020). It is thus tempting to propose that TF-binding events coincide with bursts (Fig. 4A). This simple “one-to-one” model predicts that the burst frequency should equal the product of kon and TF concentration and the burst duration should equal the TF residence time. Several observations are consistent with the one-to-one model: (1) the transcription machinery assembles within seconds, well within typical TF residence times (Zhang et al. 2016; Nguyen et al. 2020); (2) increasing TF concentration increases burst frequency (Senecal et al. 2014; Stavreva et al. 2019); (3) search time estimates are inversely proportional to burst frequencies observed in yeast (Larson et al. 2011); (4) decreasing koff increases the number of transcripts per burst (Senecal et al. 2014); (5) enhancers regulate burst frequency (Walters et al. 1995; Bartman et al. 2016; Fukaya et al. 2016; Chen et al. 2018); and (6) elegant experiments directly observe that single TF-binding events correlate temporally with burst firing (Donovan et al. 2019; Stavreva et al. 2019). While the simple two-state model might be valid in simple systems, it often breaks down in higher eukaryotes (Bartman et al. 2016; Corrigan et al. 2016; Rodriguez et al. 2019; Lammers et al. 2020; Popp et al. 2020). In a striking example, pluripotency genes cease to transcribe early in differentiation, long before their enhancers lose TF occupancy (Hamilton et al. 2019). gTFs constitute obvious kinetic intermediates that could mediate this decoupling: TBP binding regulates permissive periods over 5–20 min timescales, while Mediator ensures rapid back-to-back Pol II initiations (seconds) (Tantale et al. 2016), and pausing regulates the number of transcripts per burst (Bartman et al. 2016). Besides gTFs, supercoiling and chromatin also shape burst timing (Muramoto et al. 2010; Chong et al. 2014; Teves and Henikoff 2014). Thus, distinct steps of the transcription cycle are controlled by separate TFs (Stasevich et al. 2014), leading to multiscale bursting kinetics (Corrigan et al. 2016) and complex regulatory logic (Fig. 4B; Scholes et al. 2017). These features likely explain why predicting enhancer combinations remains challenging (Vincent et al. 2016). As the enhancer–promoter looping paradigm has recently been called into question (Alexander et al. 2019; Benabdallah et al. 2019), biophysical models of enhancer function remain sorely needed (Bothma et al. 2015). Ideally such descriptions will integrate binding of the various players, DNA organization, and local TF clustering (see last section) to predict bursting kinetics.
Figure 4.
Decoding of transcription factor (TF) kinetics by promoters. (A) In simple systems, TF binding directly leads to permissive periods (gray) during which many Pol II are rapidly fired. In this one-to-one model, the TF residence time equals the burst duration. (B) Promoters often integrate complex regulation from multiple TFs and enhancers, as well as the state of chromatin at the promoter, and the kinetics of cluster formation. These interdependent inputs are processed by the transcription machinery, which applies further control layers, leading to multistate bursting dynamics. (PIC) Preinitiation complex.
Intrinsically Disordered Regions Provide a Flexible Platform for TF Dynamics
Regulatory sequences favor low-affinity TF-binding sites that ensure specificity and sensitivity to TF concentrations over a wide range (Kribelbauer et al. 2019). Could the same principles hold for interactions between TFs and their protein partners? Indeed, it has long been appreciated that the activating domains of TFs contain intrinsically disordered regions (IDRs), which are unstructured peptides that are essential sites for TF–TF interactions and retain function upon extensive mutations (Sigler 1988; van der Lee et al. 2014; Wright and Dyson 2015). Over 80% of all eukaryotic TFs contain one or more IDRs (Liu et al. 2006), also called low complexity regions because of their limited repertoire of amino acids. While protein–protein interactions are classically thought of as stoichiometric complexes forming via a lock–key mechanism relying on complementary structures, IDRs by definition cannot fit this model. In addition, IDR sequences are poorly conserved, challenging our understanding of how TFs associate with specific partner(s) to regulate transcription. The possible role of IDRs in TF clustering (see below) has fueled renewed interest in these elusive domains, with the hope to better detect and interpret pathological mutations, many of which fall within IDRs (Uyar et al. 2014).
Stoichiometric IDR Complexes
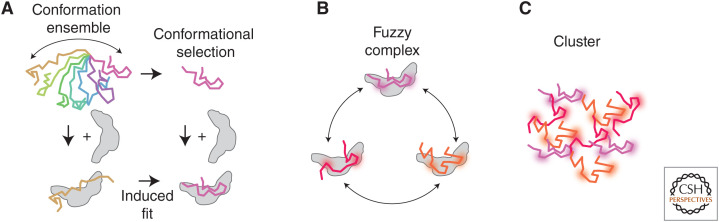
While IDRs explore a large ensemble of conformations in solution, they can exhibit a more ordered conformation when bound to a cofactor, enabling the appearance of a traditional protein–protein interface (Fig. 5; Schuler et al. 2020). This process, termed binding-coupled folding, can occur in two ways: (1) the ordered bound state pre-exists in the conformational ensemble of the free IDR and is required for binding to the cofactor (“conformational selection”); or (2) the TF can recognize its partner in its disordered state and fold while binding (“induced fit”) (Staby et al. 2017). Binding-coupled folding offers flexibility; for instance, the IDR of the coactivator CBP adopts different structures when bound to different TFs (Demarest et al. 2002; Qin et al. 2005; Waters et al. 2006). IDRs can in some cases form “fuzzy complexes” that do not exhibit a fixed conformation but gain stability through multiple weak and dynamic contacts between the IDR and its partner (Tompa and Fuxreiter 2008; Henley et al. 2020). Thanks to their dynamic nature, fuzzy complexes can be easily remodeled via competitive substitution, enabling rapid gear switching of the transcription machinery (Schuler et al. 2020).
Figure 5.
Intrinsically disordered regions (IDRs) mediate different types of complexes. (A) Binding-coupled folding: free IDRs explore a vast conformation space (colors), but some IDRs adopt a fixed conformation when bound to a partner (gray). (B) In a fuzzy complex, the IDR is dynamic yet remains bound to its partner. (C) Nonstoichiometric complexes (clusters) can form via networked interactions between multivalent IDRs.
IDR-Driven Phase Separation
Beyond fuzzy complexes, IDRs can mediate the formation of nonstoichiometric clusters (Fig. 6). Pioneering work showing that IDRs can form or associate with hydrogels in vitro suggested that IDRs of TFs may drive clustering via phase separation (Frey et al. 2006; Kwon et al. 2013).
Figure 6.
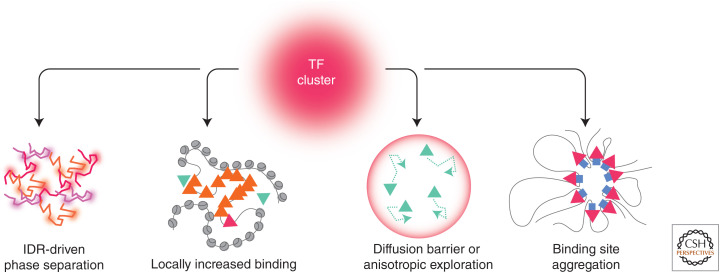
Clustering mechanisms. Transcription factor (TF) clustering can occur via phase separation, thanks to multivalent interactions between intrinsically disordered regions (IDRs) (left). Other mechanisms also exist; locally enhanced TF binding on highly accessible chromatin, locally anisotropic diffusion, and collapse of a TF-binding site. Triangles represent TFs; green, orange, and magenta denote, respectively, free, nonspecifically bound, and specifically bound species.
IDRs are often repetitive in sequence, and thus intrinsically multivalent, a prerequisite of phase separation (Choi et al. 2020). For instance, the carboxy-terminal domain (CTD) of the largest Pol II subunit, Rpb1, is a well-studied IDR that consists of repeats of the tyrosine-serine-proline-threonine-serine-proline-serine (YSPTSPS) motif (Corden 2013; Eick and Geyer 2013; Zaborowska et al. 2016; Gibbs et al. 2017; Portz et al. 2017). Pol II and many purified TFs or their IDRs indeed self-organize into phase-separated droplets in vitro (Larson et al. 2017; Boehning et al. 2018; Boija et al. 2018; Lu et al. 2018; Sabari et al. 2018; Guo et al. 2019; Plys et al. 2019; Zamudio et al. 2019; Daneshvar et al. 2020; Li et al. 2020a). In vivo, some features of TF clusters are consistent with liquid–liquid phase separation. TFs exchange dynamically within clusters as revealed by FRAP, and TF clusters undergo fusion and fission and are sensitive to treatment with 1,6-hexanediol, which disrupts some of the weak interactions that can contribute to phase separation (Cho et al. 2018; Chong et al. 2018; Sabari et al. 2018). However, these features alone do not rule out nonphase separation mechanisms (McSwiggen et al. 2019b) (see last section). A substantial caveat is that in vitro assays often use crowding agents and are done at TF concentrations much higher than physiological conditions and thus favor phase separation. For this reason, while these assays provide useful insights into the relative role of different parameters in clustering, they likely offer only a limited representation of in vivo clusters, and do for example not take into account the role of RNA and other TFs in clustering in vivo (Wei et al. 2020; Henninger et al. 2021).
Beyond Complexes: IDRs Facilitate Target Search
IDRs might not just mediate the affinity of TFs to each other, but may also influence search kinetics. The IDRs of sequence-specific TFs Msn2 and Yap1 are both necessary and sufficient for promoter specificity, but their DBDs are not (Brodsky et al. 2020), suggesting a two-step target search. TFs first localize to an open promoter by promiscuously scanning it with their IDR, and later stably bind to target DNA motifs via their DBDs. The separation of tasks between DBD and activation domains might therefore not be binary (Liu et al. 2008). IDRs could boost search efficiency in several ways including (1) the large interaction surface of IDRs may increase TF affinity for DNA, enhancing its sliding propensity; (2) IDRs may facilitate TF translocation to a neighboring DNA segment (Vuzman and Levy 2012); or (3) IDRs could guide TFs by facilitating clustering or anisotropic diffusion in specialized compartments (Izeddin et al. 2014; Hansen et al. 2020; Nguyen et al. 2020).
Sequence Determinants of IDR Interactions
In contrast to lock–key interactions driven by unique motifs, fuzzy complexes and phase separation involve individually weak but multivalent interactions between protein partners and/or nucleic acids, which could explain the low sequence conservation of IDRs. Indeed, mutation scans suggest that IDRs interact through a sum of weak interactions distributed along their entire domain (Wang et al. 2018; Brodsky et al. 2020). Furthermore, the Drosophila Pol II CTD, which consists of heptapeptide repeats diverged from the canonical YSPTSPS sequence, can be replaced with a shorter canonical CTD, but not with a canonical CTD at wild-type length (Lu et al. 2019). These findings suggest a model in which the sum interaction strength required for proper biological function can be achieved either through many low affinity sites (noncanonical repeats), or fewer high affinity ones (canonical repeats). Consistent with this picture, truncated CTDs decrease the frequency and size of transcription bursts in yeast (Quintero-Cadena et al. 2020), likely through reduced Pol II clustering (Boehning et al. 2018), while longer CTDs exhibit aberrant clustering (Lu et al. 2019). Drosophila Pol II mutants containing exceedingly long or short CTDs are inviable, confirming that sum interaction strength constitutes a key selection pressure. The fact that a specific sum interaction strength can be achieved in many ways (e.g., using distinct residues with similar physicochemical properties, and/or distributing these residues differently along the IDR sequence, accounts for the low sequence conservation of IDRs.
The prevalence of IDRs in TFs presents a conundrum. TFs need to selectively associate with specific sequence(s) or cofactors, yet IDRs mediate promiscuous interactions. How is specificity encoded into the underlying sequence of IDRs? It appears that small motifs or even a single residue within the IDR can convey specificity for a partner for instance by biasing a fuzzy complex toward a subset of its binding modes (Sims et al. 2011; Warfield et al. 2014; Desai et al. 2015; Zhao et al. 2016; Henley et al. 2020). Nonstoichiometric IDR clustering also exhibits specificity (Chong et al. 2018), although it remains unclear what mechanisms apply here. The numbers and positions of acidic, hydrophobic, and aromatic residues all modulate the affinity of IDR interactions (Staller et al. 2018; Erijman et al. 2020), as do posttranslational modifications (Guo et al. 2019). These observations suggest a departure from the dogma of sequence-defining function via a deterministic 3D protein structure. Rather, IDR sequences specify the physicochemical properties of residues such as hydrophobicity, charge distribution, and flexibility, in turn constraining both the conformation space explored by the protein chain as well as its potential for interactions through the number and strength of sticky residues, thus encoding specific TF functions including clustering propensity, exploration mode, and target selectivity (Vernon et al. 2018; Wang et al. 2018; Martin and Holehouse 2020).
Evolution of IDRs
Eukaryotic proteomes contain more disordered segments than those of simpler organisms (Ward et al. 2004; Tompa et al. 2006; Peng et al. 2015) and individual transcription regulators, such as the Pol II CTD (Quintero-Cadena et al. 2020) or Mediator subunits (Tóth-Petróczy et al. 2008) exhibit increasing disordered content over evolutionary timescales. An interesting idea is that regulatory innovation is often gained by adding new components to existing complexes. It is likely faster in evolutionary terms to achieve binding to an existing complex through a flexible domain, rather than creating a de novo specialized 3D structure matching the complex interface. Consistent with this idea, proteins that participate in large complexes are more disordered (Hegyi et al. 2007). Altogether, IDR size selection likely results from a series of tradeoffs between regulatory potential (longer IDRs enabling binding to more targets), nuclear exploration mode (longer positive tails increase affinity for DNA, enhancing a TF sliding propensity at the expense of its ability to hop to a locus in trans [Vuzman and Levy 2012]), and clustering potential (IDRs with higher valency or interaction strength generate static aggregates unable to respond to dynamic signals).
TFs ASSEMBLE IN CLUSTERS
Transcription has long been proposed to occur in stable, self-assembled “hubs” that could outlive the binding of individual components (Cook 1999; Edelman and Fraser 2012), similar to larger subnuclear structures such as the nucleolus (Phair and Misteli 2000). Consistent with the hub model, a variety of factors form clusters in cells: sequence-specific TFs (Liu et al. 2014; Mir et al. 2017, 2018; Chong et al. 2018; Basu et al. 2020; Li et al. 2020b), coactivators (Cho et al. 2018; Sabari et al. 2018; Guo et al. 2019; Li et al. 2019, 2020b; Zamudio et al. 2019), Pol II (see below), splicing factors (Guo et al. 2019), corepressors (Treen et al. 2020), repressive complexes (Wollman et al. 2017; Plys et al. 2019; Ruault et al. 2020), chromatin modifiers (Tatavosian et al. 2019), and HP1 (heterochromatin protein 1) (Strom et al. 2017; Erdel et al. 2020; Li et al. 2020a). Contrasting with the model of a stable factory, cluster lifetimes are generally short (Cisse et al. 2013).
Pol II Clustering
Single-molecule imaging has revealed that RNA polymerase II (Pol II) forms clusters in various mammalian cultured cell models (Cisse et al. 2013; Cho et al. 2016, 2016; Boehning et al. 2018; Li et al. 2019). Pol II clusters are short-lived, generally on the order of seconds, but some last minutes, and vary in size (Cisse et al. 2013; Cho et al. 2016, 2018; Boehning et al. 2018), from diffraction-limited foci all the way to micron-sized accumulations at the histone locus body (Guglielmi et al. 2013) and viral replication compartments (McSwiggen et al. 2019a). The number, size, and lifetime of clusters change upon induction (Cisse et al. 2013; Cho et al. 2016; Li et al. 2019) or inhibition of transcription (Cho et al. 2018; Li et al. 2019) and during differentiation (Cho et al. 2018), suggesting that clustering may contribute to transcription control.
The CTD is a key regulator of Pol II clustering. On its own, it forms liquid condensates in vitro and its length regulates Pol II clustering in vivo (Boehning et al. 2018). Phosphorylation of the CTD during early stages of transcription could control CTD clustering via charge modulation (Harlen and Churchman 2017). Indeed, inhibitors against P-TEFb, which triggers Pol II pause release via CTD phosphorylation, stabilize Pol II clusters (Cisse et al. 2013; Cho et al. 2016) while CTD phosphorylation disperses clusters in vitro (Boehning et al. 2018; Lu et al. 2018). These observations place Pol II clustering at the transcription preinitiation or initiation stage. CTD phosphorylation also biases Pol II association with splicing factor condensates versus those containing Mediator (Guo et al. 2019), while electrostatic repulsion by charged nascent RNAs dismantles Pol II clusters (Henninger et al. 2021). Together, these observations suggest a model wherein RNA accumulation and/or CTD phosphorylation force cluster turnover once a Pol II convoy has initiated on a transcribed gene (Quintero-Cadena et al. 2020).
Clustering without Phase Separation
Besides IDR-driven phase separation, other clustering mechanisms exist (Fig. 6). In cells infected with herpes simplex virus (HSV), viral replication compartments form micron-sized Pol II clusters due to locally enhanced Pol II binding to nucleosome-free DNA (McSwiggen et al. 2019a). Strikingly, these Pol II clusters are insensitive to CTD length in the HSV context, confirming their distinctive mechanism. TF clustering can also emerge from locally hindered diffusion as indicated by the observation that CTCF molecules are partially retained in specific nuclear zones, likely via interactions with RNA (Hansen et al. 2020). Similarly, upon heat shock, various factors are retained at induced loci, in a poly(ADP-ribose) polymerase (PARP) activity-dependent manner. This led to the speculation that PAR polymerization could create a diffusion barrier around the locus, favoring local recycling of TF molecules once they finish a round of transcription (Yao et al. 2007; Zobeck et al. 2010). Alternatively, PARP-induced chromatin decondensation could enhance TF binding, and/or PARylation could increase TF mutual affinity (Benabdallah et al. 2019). Finally, TFs may cluster due to the collapsing of their DNA targets, as suggested for HP1 in mouse embryonic fibroblasts, where the formation of chromocenters occurs independently of HP1 (Erdel et al. 2020). Interestingly, HP1 clusters reminiscent of phase separation are observed during Drosophila embryogenesis (Strom et al. 2017). The Pol II and HP1 examples suggest that a given factor can evolve distinct biophysical mechanisms to cluster in different contexts.
Functions of Clustering in Transcription
Clusters generate high local TF concentrations, which could ensure robust TF recruitment via mass action law even if TFs exchange from the cluster faster than the cluster lifetime (Dufourt et al. 2018). Indeed, clustering ensures high target site occupancy at low TF concentrations (Mir et al. 2017, 2018), and artificially induced clustering of TFs or IDRs in vivo is sufficient to recruit higher levels of transcription components and increase levels of transcription locally (Wei et al. 2020; Schneider et al. 2021). Increased local concentration of TFs is also expected to favor efficient transcription reinitiation. This prediction is consistent with the observation that increased Pol II clustering leads to increased burst size (Cho et al. 2016; Quintero-Cadena et al. 2020), and that rapid reinitiation is regulated by Mediator, a factor prone to clustering (Cho et al. 2018; Nguyen et al. 2020). TF cluster lifetimes are in the range of seconds, and thus are unlikely to constitute the molecular substrate of long-term transcription memory (Cisse et al. 2013; Cho et al. 2016, 2018; Mir et al. 2018). Clustering may also form a molecular bridge between distant loci (Tsai et al. 2019), which could explain why enhancers do not always directly contact promoters upon activation (Alexander et al. 2019; Benabdallah et al. 2019). Instead TF clusters may generate a regulatory environment shared by cis-regulatory elements without the need for molecular contact. Disruption of clustering does not abolish existing enhancer–promoter contacts, suggesting that clusters are not needed to maintain long-range interactions (Crump et al. 2021). Clustered enhancers confer robustness (Tsai et al. 2019) and constitute a flexible platform able to encode a variety of regulatory responses (Ezer et al. 2014). Similar to activators, repressors may also bring together distant loci (Ruault et al. 2020). Finally, clusters could facilitate nuclear exploration by guiding TFs to specialized compartments (Hansen et al. 2020; Nguyen et al. 2020).
CONCLUDING REMARKS
Advances in live imaging have uncovered novel modes of TF exploration and transient assemblies whose function and regulation are just beginning to be understood. Since TF dynamics parameters such as search time, residence time, concentration, fraction bound, etc. all impact transcription levels, experimental separation of individual factors is a challenge that will need to be overcome to build mechanistic models (Popp et al. 2020).
Clustering is emerging as a ubiquitous feature of transcription regulation that locally boosts transcription via mass action, while ensuring a nimble architecture able to rapidly respond to changing cues. One could envision other functions, for instance that TF clustering away from active sites could also titrate out TFs when transcription needs to be globally turned down. The next challenge is to better understand the mechanisms of cluster formation, and how clustering dynamics are decoded by promoters into transcription outputs. Biophysical regulators of clustering identified so far include phase separation, locally enhanced DNA binding, and local diffusion barriers. One difficulty is that these mechanisms are likely intertwined; for instance, transcription-coupled clustering could enhance DNA binding and/or generate local diffusion barriers. Since the respective roles of these mechanisms likely depend on the biological context, a key question is how biochemical pathways interface with TF biophysics. So far, posttranslational modifications, particularly of the Pol II CTD, have been demonstrated to control clustering by rapidly modulating IDR affinities. The role of other pathways or regulators remains to be fully explored.
Overall, the dominating feature of TF dynamics is that they follow a distributed interaction principle, apparent at many scales. First, in stoichiometric fuzzy complexes, multiple weak interaction sites between two partners rapidly exchange without complex dissociation. Second, multivalent interactions distributed across IDRs ensure the formation of nonstoichiometric clusters. Finally, enhancers favor multiple weak TF-binding motifs over high affinity ones to ensure expression specificity (Frankel et al. 2010; Crocker et al. 2015, 2016; Farley et al. 2015). This unifying principle offers many advantages needed for regulatory function, particularly robustness, tunability, and responsiveness.
ACKNOWLEDGMENTS
T.L. is supported by NIH Grant R01 GM127538. F.L. is a recipient of NYSTEM Institutional Training Grant #C032560GG. We thank Mustafa Mir and members of the Lionnet laboratory for critical reading of the manuscript.
Footnotes
Editors: Ana Pombo, Martin W. Hetzer, and Tom Misteli
Additional Perspectives on The Nucleus available at www.cshperspectives.org
REFERENCES
- Alexander JM, Guan J, Li B, Maliskova L, Song M, Shen Y, Huang B, Lomvardas S, Weiner OD. 2019. Live-cell imaging reveals enhancer-dependent Sox2 transcription in the absence of enhancer proximity. eLife 8: e41769. 10.7554/eLife.41769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancaud A, Huet S, Daigle N, Mozziconacci J, Beaudouin J, Ellenberg J. 2009. Molecular crowding affects diffusion and binding of nuclear proteins in heterochromatin and reveals the fractal organization of chromatin. EMBO J 28: 3785–3798. 10.1038/emboj.2009.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartman CR, Hsu SC, Hsiung CCS, Raj A, Blobel GA. 2016. Enhancer regulation of transcriptional bursting parameters revealed by forced chromatin looping. Mol Cell 62: 237–247. 10.1016/j.molcel.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Mackowiak SD, Niskanen H, Knezevic D, Asimi V, Grosswendt S, Geertsema H, Ali S, Jerković I, Ewers H, et al. 2020. Unblending of transcriptional condensates in human repeat expansion disease. Cell 181: 1062–1079.e30. 10.1016/j.cell.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabdallah NS, Williamson I, Illingworth RS, Kane L, Boyle S, Sengupta D, Grimes GR, Therizols P, Bickmore WA. 2019. Decreased enhancer–promoter proximity accompanying enhancer activation. Mol Cell 76: 473–484.e7. 10.1016/j.molcel.2019.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning M, Dugast-Darzacq C, Rankovic M, Hansen AS, Yu T, Marie-Nelly H, McSwiggen DT, Kokic G, Dailey GM, Cramer P, et al. 2018. RNA polymerase II clustering through carboxy-terminal domain phase separation. Nat Struct Mol Biol 25: 833–840. 10.1038/s41594-018-0112-y [DOI] [PubMed] [Google Scholar]
- Boija A, Klein IA, Sabari BR, Dall'Agnese A, Coffey EL, Zamudio AV, Li CH, Shrinivas K, Manteiga JC, Hannett NM, et al. 2018. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 175: 1842–1855.e16. 10.1016/j.cell.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothma JP, Garcia HG, Ng S, Perry MW, Gregor T, Levine M. 2015. Enhancer additivity and non-additivity are determined by enhancer strength in the Drosophila embryo. eLife 4: e07956. 10.7554/eLife.07956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky S, Jana T, Mittelman K, Chapal M, Kumar DK, Carmi M, Barkai N. 2020. Intrinsically disordered regions direct transcription factor in vivo binding specificity. Mol Cell 79: 459–471.e4. 10.1016/j.molcel.2020.05.032 [DOI] [PubMed] [Google Scholar]
- Callegari A, Sieben C, Benke A, Suter DM, Fierz B, Mazza D, Manley S. 2019. Single-molecule dynamics and genome-wide transcriptomics reveal that NF-κB (p65)-DNA binding times can be decoupled from transcriptional activation. PLoS Genet 15: e1007891. 10.1371/journal.pgen.1007891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang Z, Li L, Chen BC, Revyakin A, Hajj B, Legant W, Dahan M, Lionnet T, Betzig E, et al. 2014. Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell 156: 1274–1285. 10.1016/j.cell.2014.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Levo M, Barinov L, Fujioka M, Jaynes JB, Gregor T. 2018. Dynamic interplay between enhancer–promoter topology and gene activity. Nat Genet 50: 1296–1303. 10.1038/s41588-018-0175-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WK, Jayanth N, English BP, Inoue T, Andrews JO, Conway W, Grimm JB, Spille JH, Lavis LD, Lionnet T, et al. 2016. RNA polymerase II cluster dynamics predict mRNA output in living cells. eLife 5: e13617. 10.7554/eLife.13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WK, Spille JH, Hecht M, Lee C, Li C, Grube V, Cisse II. 2018. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 361: 412–415. 10.1126/science.aar4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Bachmann AL, Tauscher K, Benda C, Fierz B, Müller J. 2017. DNA binding by PHF1 prolongs PRC2 residence time on chromatin and thereby promotes H3K27 methylation. Nat Struct Mol Biol 24: 1039–1047. 10.1038/nsmb.3488 [DOI] [PubMed] [Google Scholar]
- Choi JM, Holehouse AS, Pappu RV. 2020. Physical principles underlying the complex biology of intracellular phase transitions. Annu Rev Biophys 49: 107–133. 10.1146/annurev-biophys-121219-081629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S, Chen C, Ge H, Xie XS. 2014. Mechanism of transcriptional bursting in bacteria. Cell 158: 314–326. 10.1016/j.cell.2014.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S, Dugast-Darzacq C, Liu Z, Dong P, Dailey GM, Cattoglio C, Heckert A, Banala S, Lavis L, Darzacq X, et al. 2018. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 361: eaar2555. 10.1126/science.aar2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JR, Trcek T, Shenoy SM, Singer RH. 2006. Transcriptional pulsing of a developmental gene. Curr Biol 16: 1018–1025. 10.1016/j.cub.2006.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse II, Izeddin I, Causse SZ, Boudarene L, Senecal A, Muresan L, Dugast-Darzacq C, Hajj B, Dahan M, Darzacq X. 2013. Real-time dynamics of RNA polymerase II clustering in live human cells. Science 341: 664–667. 10.1126/science.1239053 [DOI] [PubMed] [Google Scholar]
- Clauß K, Popp AP, Schulze L, Hettich J, Reisser M, Escoter Torres L, Uhlenhaut NH, Gebhardt JCM. 2017. DNA residence time is a regulatory factor of transcription repression. Nucleic Acids Res 45: 11121–11130. 10.1093/nar/gkx728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PR. 1999. The organization of replication and transcription. Science 284: 1790–1795. 10.1126/science.284.5421.1790 [DOI] [PubMed] [Google Scholar]
- Corden JL. 2013. RNA polymerase II C-terminal domain: tethering transcription to transcript and template. Chem Rev 113: 8423–8455. 10.1021/cr400158h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan AM, Tunnacliffe E, Cannon D, Chubb JR. 2016. A continuum model of transcriptional bursting. eLife 5: e13051. 10.7554/eLife.13051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortini R, Filion GJ. 2018. Theoretical principles of transcription factor traffic on folded chromatin. Nat Commun 9: 1740. 10.1038/s41467-018-04130-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer P. 2019. Organization and regulation of gene transcription. Nature 573: 45–54. 10.1038/s41586-019-1517-4 [DOI] [PubMed] [Google Scholar]
- Crocker J, Abe N, Rinaldi L, McGregor AP, Frankel N, Wang S, Alsawadi A, Valenti P, Plaza S, Payre F, et al. 2015. Low affinity binding site clusters confer hox specificity and regulatory robustness. Cell 160: 191–203. 10.1016/j.cell.2014.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J, Noon EPB, Stern DL. 2016. The soft touch: low-affinity transcription factor binding sites in development and evolution. Curr Top Dev Biol 117: 455–469. 10.1016/bs.ctdb.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Crump NT, Ballabio E, Godfrey L, Thorne R, Repapi E, Kerry J, Tapia M, Hua P, Lagerholm C, Filippakopoulos P, et al. 2021. BET inhibition disrupts transcription but retains enhancer–promoter contact. Nat Commun 12: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshvar K, Behfar Ardehali M, Klein IA, Kratkiewicz AJ, Zhou C, Mahpour A, Cook BM, Li W, Pondick JV, Moran SP, et al. 2020. lncRNA DIGIT and BRD3 protein form phase-separated condensates to regulate endoderm differentiation. Nat Cell Biol 22: 1211–1222. 10.1038/s41556-020-0572-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, Singer RH. 2007. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol 14: 796–806. 10.1038/nsmb1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal RB, Henikoff JG, Henikoff S. 2010. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science 328: 1161–1164. 10.1126/science.1186777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, Brok M, Lijnzaad P, Kemmeren P, Holstege FCP. 2020. Genome-wide off-rates reveal how DNA binding dynamics shape transcription factor function. Mol Syst Biol 16: e9885. 10.15252/msb.20209885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest SJ, Martinez-Yamout M, Chung J, Chen H, Xu W, Dyson HJ, Evans RM, Wright PE. 2002. Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators. Nature 415: 549–553. 10.1038/415549a [DOI] [PubMed] [Google Scholar]
- Desai MA, Webb HD, Sinanan LM, Scarsdale JN, Walavalkar NM, Ginder GD, Williams DC Jr. 2015. An intrinsically disordered region of methyl-CpG binding domain protein 2 (MBD2) recruits the histone deacetylase core of the NuRD complex. Nucleic Acids Res 43: 3100–3113. 10.1093/nar/gkv168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion MF, Kaplan T, Kim M, Buratowski S, Friedman N, Rando OJ. 2007. Dynamics of replication-independent histone turnover in budding yeast. Science 315: 1405–1408. 10.1126/science.1134053 [DOI] [PubMed] [Google Scholar]
- Di Ventura B, Kuhlman B. 2016. Go in! Go out! inducible control of nuclear localization. Curr Opin Chem Biol 34: 62–71. 10.1016/j.cbpa.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan BT, Huynh A, Ball DA, Patel HP, Poirier MG, Larson DR, Ferguson ML, Lenstra TL. 2019. Live-cell imaging reveals the interplay between transcription factors, nucleosomes, and bursting. EMBO J 38: e100809. 10.15252/embj.2018100809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourt J, Trullo A, Hunter J, Fernandez C, Lazaro J, Dejean M, Morales L, Nait-Amer S, Schulz KN, Harrison MM, et al. 2018. Temporal control of gene expression by the pioneer factor Zelda through transient interactions in hubs. Nat Commun 9: 5194. 10.1038/s41467-018-07613-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman LB, Fraser P. 2012. Transcription factories: genetic programming in three dimensions. Curr Opin Genet Dev 22: 110–114. 10.1016/j.gde.2012.01.010 [DOI] [PubMed] [Google Scholar]
- Eick D, Geyer M. 2013. The RNA polymerase II carboxy-terminal domain (CTD) code. Chem Rev 113: 8456–8490. 10.1021/cr400071f [DOI] [PubMed] [Google Scholar]
- Elf J, Barkefors I. 2019. Single-molecule kinetics in living cells. Annu Rev Biochem 88: 635–659. 10.1146/annurev-biochem-013118-110801 [DOI] [PubMed] [Google Scholar]
- Elf J, Li GW, Xie XS. 2007. Probing transcription factor dynamics at the single-molecule level in a living cell. Science 316: 1191–1194. 10.1126/science.1141967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdel F, Rademacher A, Vlijm R, Tünnermann J, Frank L, Weinmann R, Schweigert E, Yserentant K, Hummert J, Bauer C, et al. 2020. Mouse heterochromatin adopts digital compaction states without showing hallmarks of HP1-driven liquid–liquid phase separation. Mol Cell 78: 236–249.e7. 10.1016/j.molcel.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erijman A, Kozlowski L, Sohrabi-Jahromi S, Fishburn J, Warfield L, Schreiber J, Noble WS, Söding J, Hahn S. 2020. A high-throughput screen for transcription activation domains reveals their sequence features and permits prediction by deep learning. Mol Cell 79: 1066. 10.1016/j.molcel.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezer D, Zabet NR, Adryan B. 2014. Homotypic clusters of transcription factor binding sites: a model system for understanding the physical mechanics of gene expression. Comput Struct Biotechnol J 10: 63–69. 10.1016/j.csbj.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley EK, Olson KM, Zhang W, Brandt AJ, Rokhsar DS, Levine MS. 2015. Suboptimization of developmental enhancers. Science 350: 325–328. 10.1126/science.aac6948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festuccia N, Owens N, Papadopoulou T, Gonzalez I, Tachtsidi A, Vandoermel-Pournin S, Gallego E, Gutierrez N, Dubois A, Cohen-Tannoudji M, et al. 2019. Transcription factor activity and nucleosome organization in mitosis. Genome Res 29: 250–260. 10.1101/gr.243048.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel N, Davis GK, Vargas D, Wang S, Payre F, Stern DL. 2010. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466: 490–493. 10.1038/nature09158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Richter RP, Görlich D. 2006. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314: 815–817. 10.1126/science.1132516 [DOI] [PubMed] [Google Scholar]
- Fukaya T, Lim B, Levine M. 2016. Enhancer control of transcriptional bursting. Cell 166: 358–368. 10.1016/j.cell.2016.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia DA, Fettweis G, Presman DM, Paakinaho V, Jarzynski C, Upadhyaya A, Hager GL. 2021. Power-law behavior of transcription factor dynamics at the single-molecule level implies a continuum affinity model. Nucleic Acids Res 10.1093/nar/gkab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt JC, Suter DM, Roy R, Zhao ZW, Chapman AR, Basu S, Maniatis T, Xie XS. 2013. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat Methods 10: 421–426. 10.1038/nmeth.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs EB, Lu F, Portz B, Fisher MJ, Medellin BP, Laremore TN, Zhang YJ, Gilmour DS, Showalter SA. 2017. Phosphorylation induces sequence-specific conformational switches in the RNA polymerase II C-terminal domain. Nat Commun 8: 15233. 10.1038/ncomms15233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünwald D, Martin RM, Buschmann V, Bazett-Jones DP, Leonhardt H, Kubitscheck U, Cardoso MC. 2008. Probing intranuclear environments at the single-molecule level. Biophys J 94: 2847–2858. 10.1529/biophysj.107.115014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi B, La Rochelle N, Tjian R. 2013. Gene-specific transcriptional mechanisms at the histone gene cluster revealed by single-cell imaging. Mol Cell 51: 480–492. 10.1016/j.molcel.2013.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YE, Manteiga JC, Henninger JE, Sabari BR, Dall'Agnese A, Hannett NM, Spille J-H, Afeyan LK, Zamudio AV, Shrinivas K, et al. 2019. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 572: 543–548. 10.1038/s41586-019-1464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB, Javed K, Vodnala M, Garrett N. 2020. Long-term association of a transcription factor with its chromatin binding site can stabilize gene expression and cell fate commitment. Proc Natl Acad Sci 117: 15075–15084. 10.1073/pnas.2000467117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager GL, McNally JG, Misteli T. 2009. Transcription dynamics. Mol Cell 35: 741–753. 10.1016/j.molcel.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WB, Mosesson Y, Monteiro RS, Emdal KB, Knudsen TE, Francavilla C, Barkai N, Olsen JV, Brickman JM. 2019. Dynamic lineage priming is driven via direct enhancer regulation by ERK. Nature 575: 355–360. 10.1038/s41586-019-1732-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AS, Pustova I, Cattoglio C, Tjian R, Darzacq X. 2017. CTCF and cohesin regulate chromatin loop stability with distinct dynamics. eLife 6: e25776. 10.7554/eLife.25776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AS, Amitai A, Cattoglio C, Tjian R, Darzacq X. 2020. Guided nuclear exploration increases CTCF target search efficiency. Nat Chem Biol 16: 257–266. 10.1038/s41589-019-0422-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlen KM, Churchman LS. 2017. The code and beyond: transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat Rev Mol Cell Biol 18: 263–273. 10.1038/nrm.2017.10 [DOI] [PubMed] [Google Scholar]
- He Q, Johnston J, Zeitlinger J. 2015. ChIP-nexus enables improved detection of in vivo transcription factor binding footprints. Nat Biotechnol 33: 395–401. 10.1038/nbt.3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi H, Schad E, Tompa P. 2007. Structural disorder promotes assembly of protein complexes. BMC Struct Biol 7: 65. 10.1186/1472-6807-7-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich P, Schmiedeberg L, Diekmann S. 2011. Dynamic as well as stable protein interactions contribute to genome function and maintenance. Chromosome Res 19: 131–151. 10.1007/s10577-010-9161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley MJ, Linhares BM, Morgan BS, Cierpicki T, Fierke CA, Mapp AK. 2020. Unexpected specificity within dynamic transcriptional protein–protein complexes. Proc Natl Acad Sci 117: 27346–27353. 10.1073/pnas.2013244117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger JE, Oksuz O, Shrinivas K, Sagi I, LeRoy G, Zheng MM, Owen Andrews J, Zamudio AV, Lazaris C, Hannett NM, et al. 2021. RNA-mediated feedback control of transcriptional condensates. Cell 184: 207–225.e24. 10.1016/j.cell.2020.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinow P, Rogers CE, Barbieri CE, Pietenpol JA, Kenworthy AK, DiBenedetto E. 2006. The DNA binding activity of p53 displays reaction–diffusion kinetics. Biophys J 91: 330–342. 10.1529/biophysj.105.078303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp L, Beer J, Kuchler O, Reisser M, Sinske D, Michaelis J, Gebhardt JCM, Knöll B. 2019. Single-molecule imaging of the transcription factor SRF reveals prolonged chromatin-binding kinetics upon cell stimulation. Proc Natl Acad Sci 116: 880–889. 10.1073/pnas.1812734116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseyin MK, Klose RJ. 2021. Live-cell single particle tracking of PRC1 reveals a highly dynamic system with low target site occupancy. Nat Commun 12: 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izeddin I, Récamier V, Bosanac L, Cissé II, Boudarene L, Dugast-Darzacq C, Proux F, Bénichou O, Voituriez R, Bensaude O, et al. 2014. Single-molecule tracking in live cells reveals distinct target-search strategies of transcription factors in the nucleus. eLife 3: e02230. 10.7554/eLife.02230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DS, Mortazavi A, Myers RM, Wold B. 2007. Genome-wide mapping of in vivo protein–DNA interactions. Science 316: 1497–1502. 10.1126/science.1141319 [DOI] [PubMed] [Google Scholar]
- Jolma A, Kivioja T, Toivonen J, Cheng L, Wei G, Enge M, Taipale M, Vaquerizas JM, Yan J, Sillanpää MJ, et al. 2010. Multiplexed massively parallel SELEX for characterization of human transcription factor binding specificities. Genome Res 20: 861–873. 10.1101/gr.100552.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Cook PR. 2001. Kinetics of core histones in living human cells: little exchange of H3 and H4 and some rapid exchange of H2B. J Cell Biol 153: 1341–1354. 10.1083/jcb.153.7.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kribelbauer JF, Rastogi C, Bussemaker HJ, Mann RS. 2019. Low-affinity binding sites and the transcription factor specificity paradox in eukaryotes. Annu Rev Cell Dev Biol 35: 357–379. 10.1146/annurev-cellbio-100617-062719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Kato M, Xiang S, Wu L, Theodoropoulos P, Mirzaei H, Han T, Xie S, Corden JL, McKnight SL. 2013. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 155: 1049–1060. 10.1016/j.cell.2013.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR, Weirauch MT. 2018. The human transcription factors. Cell 175: 598–599. 10.1016/j.cell.2018.09.045 [DOI] [PubMed] [Google Scholar]
- Lammers NC, Galstyan V, Reimer A, Medin SA, Wiggins CH, Garcia HG. 2020. Multimodal transcriptional control of pattern formation in embryonic development. Proc Natl Acad Sci 117: 836–847. 10.1073/pnas.1912500117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Ren R, Feng R, Ly LC, Lan Y, Zhang Z, Aboreden N, Qin K, Horton JR, Grevet JD, et al. 2020. ZNF410 uniquely activates the NuRD component CHD4 to silence fetal hemoglobin expression. Blood 136: 54–54. 10.1182/blood-2020-137564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DR, Zenklusen D, Wu B, Chao JA, Singer RH. 2011. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science 332: 475–478. 10.1126/science.1202142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, Redding S, Narlikar GJ. 2017. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547: 236–240. 10.1038/nature22822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner J, Gomez-Garcia PA, McCarthy RL, Liu Z, Lakadamyali M, Zaret KS. 2020. Two-parameter mobility assessments discriminate diverse regulatory factor behaviors in chromatin. Mol Cell 79: 677–688.e6. 10.1016/j.molcel.2020.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever MA, Th'ng JP, Sun X, Hendzel MJ. 2000. Rapid exchange of histone H1.1 on chromatin in living human cells. Nature 408: 873–876. 10.1038/35048603 [DOI] [PubMed] [Google Scholar]
- Li L, Liu H, Dong P, Li D, Legant WR, Grimm JB, Lavis LD, Betzig E, Tjian R, Liu Z. 2016. Real-time imaging of Huntingtin aggregates diverting target search and gene transcription. eLife 5: e17056. 10.7554/eLife.17056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Dong A, Saydaminova K, Chang H, Wang G, Ochiai H, Yamamoto T, Pertsinidis A. 2019. Single-molecule nanoscopy elucidates RNA polymerase II transcription at single genes in live cells. Cell 178: 491–506.e28. 10.1016/j.cell.2019.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Coffey EL, Dall'Agnese A, Hannett NM, Tang X, Henninger JE, Platt JM, Oksuz O, Zamudio AV, Afeyan LK, et al. 2020a. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature 586: 440–444. 10.1038/s41586-020-2574-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hsu A, Hua Y, Wang G, Cheng L, Ochiai H, Yamamoto T, Pertsinidis A. 2020b. Single-gene imaging links genome topology, promoter–enhancer communication and transcription control. Nat Struct Mol Biol 27: 1032–1040. 10.1038/s41594-020-0493-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Tjian R. 2018. Visualizing transcription factor dynamics in living cells. J Cell Biol 217: 1181–1191. 10.1083/jcb.201710038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Perumal NB, Oldfield CJ, Su EW, Uversky VN, Keith Dunker A. 2006. Intrinsic disorder in transcription factors. Biochemistry 45: 6873–6888. 10.1021/bi0602718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Matthews KS, Bondos SE. 2008. Multiple intrinsically disordered sequences alter DNA binding by the homeodomain of the Drosophila hox protein ultrabithorax. J Biol Chem 283: 20874–20887. 10.1074/jbc.M800375200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Legant WR, Chen BC, Li L, Grimm JB, Lavis LD, Betzig E, Tjian R. 2014. 3D imaging of Sox2 enhancer clusters in embryonic stem cells. eLife 3: e04236. 10.7554/eLife.04236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Yu D, Hansen AS, Ganguly S, Liu R, Heckert A, Darzacq X, Zhou Q. 2018. Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II. Nature 558: 318–323. 10.1038/s41586-018-0174-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Portz B, Gilmour DS. 2019. The C-terminal domain of RNA polymerase II is a multivalent targeting sequence that supports Drosophila development with only consensus heptads. Mol Cell 73: 1232–1242.e4. 10.1016/j.molcel.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri P, Knezevich A, Bertrand E, Marcello A. 2011. Real-time imaging of the HIV-1 transcription cycle in single living cells. Methods 53: 62–67. 10.1016/j.ymeth.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Marklund E, van Oosten B, Mao G, Amselem E, Kipper K, Sabantsev A, Emmerich A, Globisch D, Zheng X, Lehmann LC, et al. 2020. DNA surface exploration and operator bypassing during target search. Nature 583: 858–861. 10.1038/s41586-020-2413-7 [DOI] [PubMed] [Google Scholar]
- Martin EW, Holehouse AS. 2020. Intrinsically disordered protein regions and phase separation: sequence determinants of assembly or lack thereof. Emerg Top Life Sci 4: 307–329. 10.1042/ETLS20190164 [DOI] [PubMed] [Google Scholar]
- McSwiggen DT, Hansen AS, Teves SS, Marie-Nelly H, Hao Y, Heckert AB, Umemoto KK, Dugast-Darzacq C, Tjian R, Darzacq X. 2019a. Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. eLife 8: e47098. 10.7554/eLife.47098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwiggen DT, Mir M, Darzacq X, Tjian R. 2019b. Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev 33: 1619–1634. 10.1101/gad.331520.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta GD, Ball DA, Eriksson PR, Chereji RV, Clark DJ, McNally JG, Karpova TS. 2018. Single-molecule analysis reveals linked cycles of RSC chromatin remodeling and Ace1p transcription factor binding in yeast. Mol Cell 72: 875–887.e9. 10.1016/j.molcel.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir M, Reimer A, Haines JE, Li XY, Stadler M, Garcia H, Eisen MB, Darzacq X. 2017. Dense bicoid hubs accentuate binding along the morphogen gradient. Genes Dev 31: 1784–1794. 10.1101/gad.305078.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir M, Stadler MR, Ortiz SA, Hannon CE, Harrison MM, Darzacq X, Eisen MB. 2018. Dynamic multifactor hubs interact transiently with sites of active transcription in Drosophila embryos. eLife 7: e40497. 10.7554/eLife.40497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron E, Oldenkamp R, Brown JM, Pinto DMS, Shan Xu C, Faria AR, Shaban HA, Rhodes JDP, Innocent C, de Ornellas S, et al. 2020. Chromatin arranges in chains of mesoscale domains with nanoscale functional topography independent of cohesin. Sci Adv 6: eaba8811. 10.1126/sciadv.aba8811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T, Gunjan A, Hock R, Bustin M, Brown DT. 2000. Dynamic binding of histone H1 to chromatin in living cells. Nature 408: 877–881. 10.1038/35048610 [DOI] [PubMed] [Google Scholar]
- Mueller F, Stasevich TJ, Mazza D, McNally JG. 2013. Quantifying transcription factor kinetics: at work or at play? Crit Rev Biochem Mol Biol 48: 492–514. 10.3109/10409238.2013.833891 [DOI] [PubMed] [Google Scholar]
- Muramoto T, Müller I, Thomas G, Melvin A, Chubb JR. 2010. Methylation of H3K4 Is required for inheritance of active transcriptional states. Curr Biol 20: 397–406. 10.1016/j.cub.2010.01.017 [DOI] [PubMed] [Google Scholar]
- Nguyen VQ, Ranjan A, Liu S, Tang X, Ling YH, Wisniewski J, Mizuguchi G, Li KY, Jou V, Zheng Q, et al. 2020. Spatio-temporal coordination of transcription preinitiation complex assembly in live cells. bioRxiv 10.1101/2020.12.30.424853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno D, Boudarene L, Dugast-Darzacq C, Chen J, Richter C, Proux F, Benichou O, Voituriez R, Darzacq X, Dahan M. 2015. Probing the target search of DNA-binding proteins in mammalian cells using TetR as model searcher. Nat Commun 6: 7357. 10.1038/ncomms8357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Yan J, Fan X, Mizianty MJ, Xue B, Wang K, Hu G, Uversky VN, Kurgan L. 2015. Exceptionally abundant exceptions: comprehensive characterization of intrinsic disorder in all domains of life. Cell Mol Life Sci 72: 137–151. 10.1007/s00018-014-1661-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann T, Eriksson P, Wrange O. 1990. Quantitative analysis of the glucocorticoid receptor–DNA interaction at the mouse mammary tumor virus glucocorticoid response element. J Biol Chem 265: 17222–17229. 10.1016/S0021-9258(17)44892-7 [DOI] [PubMed] [Google Scholar]
- Phair RD, Misteli T. 2000. High mobility of proteins in the mammalian cell nucleus. Nature 404: 604–609. 10.1038/35007077 [DOI] [PubMed] [Google Scholar]
- Phair RD, Scaffidi P, Elbi C, Vecerová J, Dey A, Ozato K, Brown DT, Hager G, Bustin M, Misteli T. 2004. Global nature of dynamic protein–chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol Cell Biol 24: 6393–6402. 10.1128/MCB.24.14.6393-6402.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plys AJ, Davis CP, Kim J, Rizki G, Keenen MM, Marr SK, Kingston RE. 2019. Phase separation of Polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev 33: 799–813. 10.1101/gad.326488.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorey K, Viswanathan R, Carver MN, Karpova TS, Cirimotich SM, McNally JG, Bekiranov S, Auble DT. 2013. Measuring chromatin interaction dynamics on the second time scale at single-copy genes. Science 342: 369–372. 10.1126/science.1242369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp AP, Hettich J, Gebhardt JCM. 2020. Transcription factor residence time dominates over concentration in transcription activation. bioRxiv 10.1101/2020.11.26.400069 [DOI] [Google Scholar]
- Portz B, Lu F, Gibbs EB, Mayfield JE, Rachel Mehaffey M, Zhang YJ, Brodbelt JS, Showalter SA, Gilmour DS. 2017. Structural heterogeneity in the intrinsically disordered RNA polymerase II C-terminal domain. Nat Commun 8: 15231. 10.1038/ncomms15231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presman DM, Ganguly S, Schiltz RL, Johnson TA, Karpova TS, Hager GL. 2016. DNA binding triggers tetramerization of the glucocorticoid receptor in live cells. Proc Natl Acad Sci 113: 8236–8241. 10.1073/pnas.1606774113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin BY, Liu C, Srinath H, Lam SS, Correia JJ, Derynck R, Lin K. 2005. Crystal structure of IRF-3 in complex with CBP. Structure 13: 1269–1277. 10.1016/j.str.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Quintero-Cadena P, Lenstra TL, Sternberg PW. 2020. RNA Pol II length and disorder enable cooperative scaling of transcriptional bursting. Mol Cell 79: 207–220.e8. 10.1016/j.molcel.2020.05.030 [DOI] [PubMed] [Google Scholar]
- Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. 2006. Stochastic mRNA synthesis in mammalian cells. PLoS Biol 4: e309. 10.1371/journal.pbio.0040309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisser M, Palmer A, Popp AP, Jahn C, Weidinger G, Gebhardt JCM. 2018. Single-molecule imaging correlates decreasing nuclear volume with increasing TF-chromatin associations during zebrafish development. Nat Commun 9: 5218. 10.1038/s41467-018-07731-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisser M, Hettich J, Kuhn T, Popp AP, Große-Berkenbusch A, Gebhardt JCM. 2020. Inferring quantity and qualities of superimposed reaction rates from single molecule survival time distributions. Sci Rep 10: 1758. 10.1038/s41598-020-58634-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Pugh BF. 2011. Comprehensive genome-wide protein–DNA interactions detected at single-nucleotide resolution. Cell 147: 1408–1419. 10.1016/j.cell.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Larson DR. 2020. Transcription in living cells: molecular mechanisms of bursting. Annu Rev Biochem 89: 189–212. 10.1146/annurev-biochem-011520-105250 [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Ren G, Day CR, Zhao K, Chow CC, Larson DR. 2019. Intrinsic dynamics of a human gene reveal the basis of expression heterogeneity. Cell 176: 213–226.e18. 10.1016/j.cell.2018.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruault M, Scolari VF, Lazar-Stefanita L, Hocher A. 2020. The silencing factor Sir3 is a molecular bridge that sticks together distant loci. bioRxiv 10.1101/2020.06.29.178368 [DOI] [Google Scholar]
- Sabari BR, Dall'Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham BJ, Hannett NM, Zamudio AV, Manteiga JC, et al. 2018. Coactivator condensation at super-enhancers links phase separation and gene control. Science 361: eaar3958. 10.1126/science.aar3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider N, Wieland FG, Kong D, Fischer AAM, Hörner M, Timmer J, Ye H, Weber W. 2021. Liquid–liquid phase separation of light-inducible transcription factors increases transcription activation in mammalian cells and mice. Sci Adv 7: eabd3568. 10.1126/sciadv.abd3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes C, DePace AH, Sánchez Á. 2017. Combinatorial gene regulation through kinetic control of the transcription cycle. Cell Syst 4: 97–108.e9. 10.1016/j.cels.2016.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler B, Borgia A, Borgia MB, Heidarsson PO, Holmstrom ED, Nettels D, Sottini A. 2020. Binding without folding—the biomolecular function of disordered polyelectrolyte complexes. Curr Opin Struct Biol 60: 66–76. 10.1016/j.sbi.2019.12.006 [DOI] [PubMed] [Google Scholar]
- Senecal A, Munsky B, Proux F, Ly N, Braye FE, Zimmer C, Mueller F, Darzacq X. 2014. Transcription factors modulate c-Fos transcriptional bursts. Cell Rep 8: 75–83. 10.1016/j.celrep.2014.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RN, Grzybowski AT, Cornett EM, Johnstone AL, Dickson BM, Boone BA, Cheek MA, Cowles MW, Maryanski D, Meiners MJ, et al. 2018. Examining the roles of H3K4 methylation states with systematically characterized antibodies. Mol Cell 72: 162–177.e7. 10.1016/j.molcel.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukron O, Seeber A, Amitai A, Holcman D. 2019. Advances using single-particle trajectories to reconstruct chromatin organization and dynamics. Trends Genet 35: 685–705. 10.1016/j.tig.2019.06.007 [DOI] [PubMed] [Google Scholar]
- Sigler PB. 1988. Acid blobs and negative noodles. Nature 333: 210–212. 10.1038/333210a0 [DOI] [PubMed] [Google Scholar]
- Sims RJ III, Rojas LA, Beck DB, Bonasio R, Schüller R, Drury WJ III, Eick D, Reinberg D. 2011. The C-terminal domain of RNA polymerase II is modified by site-specific methylation. Science 332: 99–103. 10.1126/science.1202663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Henikoff S. 2017. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 6: e21856. 10.7554/eLife.21856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staby L, O'Shea C, Willemoës M, Theisen F, Kragelund BB, Skriver K. 2017. Eukaryotic transcription factors: paradigms of protein intrinsic disorder. Biochem J 474: 2509–2532. 10.1042/BCJ20160631 [DOI] [PubMed] [Google Scholar]
- Staller MV, Holehouse AS, Swain-Lenz D, Das RK, Pappu RV, Cohen BA. 2018. A high-throughput mutational scan of an intrinsically disordered acidic transcriptional activation domain. Cell Syst 6: 444–455.e6. 10.1016/j.cels.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasevich TJ, Mueller F, Michelman-Ribeiro A, Rosales T, Knutson JR, McNally JG. 2010. Cross-validating FRAP and FCS to quantify the impact of photobleaching on in vivo binding estimates. Biophys J 99: 3093–3101. 10.1016/j.bpj.2010.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasevich TJ, Hayashi-Takanaka Y, Sato Y, Maehara K, Ohkawa Y, Sakata-Sogawa K, Tokunaga M, Nagase T, Nozaki N, McNally JG, et al. 2014. Regulation of RNA polymerase II activation by histone acetylation in single living cells. Nature 516: 272–275. 10.1038/nature13714 [DOI] [PubMed] [Google Scholar]
- Stavreva DA, Garcia DA, Fettweis G, Gudla PR, Zaki GF, Soni V, McGowan A, Williams G, Huynh A, Palangat M, et al. 2019. Transcriptional bursting and co-bursting regulation by steroid hormone release pattern and transcription factor mobility. Mol Cell 75: 1161–1177.e11. 10.1016/j.molcel.2019.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X, Karpen GH. 2017. Phase separation drives heterochromatin domain formation. Nature 547: 241–245. 10.1038/nature22989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugo N, Morimatsu M, Arai Y, Kousoku Y, Ohkuni A, Nomura T, Yanagida T, Yamamoto N. 2015. Single-molecule imaging reveals dynamics of CREB transcription factor bound to its target sequence. Sci Rep 5: 10662. 10.1038/srep10662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM. 2020. Transcription factors and DNA play hide and seek. Trends Cell Biol 30: 491–500. 10.1016/j.tcb.2020.03.003 [DOI] [PubMed] [Google Scholar]
- Suter DM, Molina N, Gatfield D, Schneider K, Schibler U, Naef F. 2011. Mammalian genes are transcribed with widely different bursting kinetics. Science 332: 472–474. 10.1126/science.1198817 [DOI] [PubMed] [Google Scholar]
- Swinstead EE, Miranda TB, Paakinaho V, Baek S, Goldstein I, Hawkins M, Karpova TS, Ball D, Mazza D, Lavis LD, et al. 2016. Steroid receptors reprogram FoxA1 occupancy through dynamic chromatin transitions. Cell 165: 593–605. 10.1016/j.cell.2016.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symmons O, Raj A. 2016. What's luck got to do with it: single cells, multiple fates, and biological nondeterminism. Mol Cell 62: 788–802. 10.1016/j.molcel.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Tantale K, Mueller F, Kozulic-Pirher A, Lesne A, Victor JM, Robert MC, Capozi S, Chouaib R, Bäcker V, Mateos-Langerak J, et al. 2016. A single-molecule view of transcription reveals convoys of RNA polymerases and multi-scale bursting. Nat Commun 7: 12248. 10.1038/ncomms12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatavosian R, Duc HN, Huynh TN, Fang D, Schmitt B, Shi X, Deng Y, Phiel C, Yao T, Zhang Z, et al. 2018. Live-cell single-molecule dynamics of PcG proteins imposed by the DIPG H3.3K27M mutation. Nat Commun 9: 2080. 10.1038/s41467-018-04455-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatavosian R, Kent S, Brown K, Yao T, Duc HN, Huynh TN, Zhen CY, Ma B, Wang H, Ren X. 2019. Nuclear condensates of the Polycomb protein chromobox 2 (CBX2) assemble through phase separation. J Biol Chem 294: 1451–1463. 10.1074/jbc.RA118.006620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves SS, Henikoff S. 2014. Transcription-generated torsional stress destabilizes nucleosomes. Nat Struct Mol Biol 21: 88–94. 10.1038/nsmb.2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves SS, An L, Hansen AS, Xie L, Darzacq X, Tjian R. 2016. A dynamic mode of mitotic bookmarking by transcription factors. eLife 5: e22280. 10.7554/eLife.22280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves SS, An L, Bhargava-Shah A, Xie L, Darzacq X, Tjian R. 2018. A stable mode of bookmarking by TBP recruits RNA polymerase II to mitotic chromosomes. eLife 7: 35621. 10.7554/eLife.35621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompa P, Fuxreiter M. 2008. Fuzzy complexes: polymorphism and structural disorder in protein–protein interactions. Trends Biochem Sci 33: 2–8. 10.1016/j.tibs.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Tompa P, Dosztanyi Z, Simon I. 2006. Prevalent structural disorder in E. coli and S. cerevisiae proteomes. J Proteome Res 5: 1996–2000. 10.1021/pr0600881 [DOI] [PubMed] [Google Scholar]
- Tóth-Petróczy A, Oldfield CJ, Simon I, Takagi Y, Dunker AK, Uversky VN, Fuxreiter M. 2008. Malleable machines in transcription regulation: the mediator complex. PLoS Comput Biol 4: e1000243. 10.1371/journal.pcbi.1000243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treen N, Shimobayashi SF, Eeftens J, Brangwynne CP, Levine MS. 2020. Regulation of gene expression by repression condensates during development. bioRxiv 10.1101/2020.03.03.975680 [DOI] [Google Scholar]
- Tsai A, Alves MR, Crocker J. 2019. Multi-enhancer transcriptional hubs confer phenotypic robustness. eLife 8: e45325. 10.7554/eLife.45325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyar B, Weatheritt RJ, Dinkel H, Davey NE, Gibson TJ. 2014. Proteome-wide analysis of human disease mutations in short linear motifs: neglected players in cancer? Mol Biosyst 10: 2626–2642. 10.1039/C4MB00290C [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, et al. 2014. Classification of intrinsically disordered regions and proteins. Chem Rev 114: 6589–6631. 10.1021/cr400525m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon RM, Chong PA, Tsang B, Kim TH, Bah A, Farber P, Lin H, Forman-Kay JD. 2018. Pi–Pi contacts are an overlooked protein feature relevant to phase separation. eLife 7: e31486. 10.7554/eLife.31486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent BJ, Estrada J, DePace AH. 2016. The appeasement of Doug: a synthetic approach to enhancer biology. Integr Biol 8: 475–484. 10.1039/c5ib00321k [DOI] [PubMed] [Google Scholar]
- von Hippel PH, Berg OG. 1989. Facilitated target location in biological systems. J Biol Chem 264: 675–678. 10.1016/S0021-9258(19)84994-3 [DOI] [PubMed] [Google Scholar]
- Vuzman D, Levy Y. 2012. Intrinsically disordered regions as affinity tuners in protein–DNA interactions. Mol Biosyst 8: 47–57. 10.1039/C1MB05273J [DOI] [PubMed] [Google Scholar]
- Walters MC, Fiering S, Eidemiller J, Magis W, Groudine M, Martin DI. 1995. Enhancers increase the probability but not the level of gene expression. Proc Natl Acad Sci 92: 7125–7129. 10.1073/pnas.92.15.7125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Choi JM, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, et al. 2018. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174: 688–699.e16. 10.1016/j.cell.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. 2004. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 337: 635–645. 10.1016/j.jmb.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Warfield L, Tuttle LM, Pacheco D, Klevit RE, Hahn S. 2014. A sequence-specific transcription activator motif and powerful synthetic variants that bind Mediator using a fuzzy protein interface. Proc Natl Acad Sci 111: E3506–E3513. 10.1073/pnas.1412088111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L, Yue B, Veverka V, Renshaw P, Bramham J, Matsuda S, Frenkiel T, Kelly G, Muskett F, Carr M, et al. 2006. Structural diversity in p160/CREB-binding protein coactivator complexes. J Biol Chem 281: 14787–14795. 10.1074/jbc.M600237200 [DOI] [PubMed] [Google Scholar]
- Wei MT, Chang YC, Shimobayashi SF, Shin Y, Strom AR, Brangwynne CP. 2020. Nucleated transcriptional condensates amplify gene expression. Nat Cell Biol 22: 1187–1196. 10.1038/s41556-020-00578-6 [DOI] [PubMed] [Google Scholar]
- Wollman AJ, Shashkova S, Hedlund EG, Friemann R, Hohmann S, Leake MC. 2017. Transcription factor clusters regulate genes in eukaryotic cells. eLife 6: e27451. 10.7554/eLife.27451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PE, Dyson HJ. 2015. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol 16: 18–29. 10.1038/nrm3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Torigoe SE, Xiao J, Mai DH, Li L, Davis FP, Dong P, Marie-Nelly H, Grimm J, Lavis L, et al. 2017. A dynamic interplay of enhancer elements regulates Klf4 expression in naïve pluripotency. Genes Dev 31: 1795–1808. 10.1101/gad.303321.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wang Y, Hoang D, Tinkham M, Patel A, Sun M-A, Wolf G, Baker M, Chien HC, Lai KYN, et al. 2017. A placental growth factor is silenced in mouse embryos by the zinc finger protein ZFP568. Science 356: 757–759. 10.1126/science.aah6895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Ardehali MB, Fecko CJ, Webb WW, Lis JT. 2007. Intranuclear distribution and local dynamics of RNA polymerase II during transcription activation. Mol Cell 28: 978–990. 10.1016/j.molcel.2007.10.017 [DOI] [PubMed] [Google Scholar]
- Youmans DT, Schmidt JC, Cech TR. 2018. Live-cell imaging reveals the dynamics of PRC2 and recruitment to chromatin by SUZ12-associated subunits. Genes Dev 32: 794–805. 10.1101/gad.311936.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborowska J, Egloff S, Murphy S. 2016. The pol II CTD: new twists in the tail. Nat Struct Mol Biol 23: 771–777. 10.1038/nsmb.3285 [DOI] [PubMed] [Google Scholar]
- Zamudio AV, Dall'Agnese A, Henninger JE, Manteiga JC, Afeyan LK, Hannett NM, Coffey EL, Li CH, Oksuz O, Sabari BR, et al. 2019. Mediator condensates localize signaling factors to key cell identity genes. Mol Cell 76: 753–766.e6. 10.1016/j.molcel.2019.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, English BP, Grimm JB, Kazane SA, Hu W, Tsai A, Inouye C, You C, Piehler J, Schultz PG, et al. 2016. Rapid dynamics of general transcription factor TFIIB binding during preinitiation complex assembly revealed by single-molecule analysis. Genes Dev 30: 2106–2118. 10.1101/gad.285395.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DY, Gish G, Braunschweig U, Li Y, Ni Z, Schmitges FW, Zhong G, Liu K, Li W, Moffat J, et al. 2016. SMN and symmetric arginine dimethylation of RNA polymerase II C-terminal domain control termination. Nature 529: 48–53. 10.1038/nature16469 [DOI] [PubMed] [Google Scholar]
- Zhu F, Farnung L, Kaasinen E, Sahu B, Yin Y, Wei B, Dodonova SO, Nitta KR, Morgunova E, Taipale M, et al. 2018. The interaction landscape between transcription factors and the nucleosome. Nature 562: 76–81. 10.1038/s41586-018-0549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobeck KL, Buckley MS, Zipfel WR, Lis JT. 2010. Recruitment timing and dynamics of transcription factors at the Hsp70 loci in living cells. Mol Cell 40: 965–975. 10.1016/j.molcel.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotarev N, Maksimenko O, Kyrchanova O, Sokolinskaya E, Osadchiy I, Girardot C, Bonchuk A, Ciglar L, Furlong EEM, Georgiev P. 2017. Opbp is a new architectural/insulator protein required for ribosomal gene expression. Nucleic Acids Res 45: 12285–12300. 10.1093/nar/gkx840 [DOI] [PMC free article] [PubMed] [Google Scholar]