Abstract
Biofilm infections are responsible for at least 65% of human bacterial infections. These biofilms are refractory to conventional antibiotics, leading to chronic infections and non-healing wounds. Plant-derived antibiotics (phytochemicals) are promising alternative antimicrobial treatments featuring antimicrobial properties. However, their poor solubility in aqueous media limits their application in treating biofilm infections. Phytochemicals were incorporated into cross-linked polymer nanocomposite ‘sponges’ for the treatment of bacterial biofilms. The results indicated encapsulating low log P phytochemicals effectively eliminated biofilms while demonstrating low cytotoxicity against mammalian fibroblast cells.
Keywords: Nanocomposite, Crosslinked, Nanoemulsion, Multidrug-Resistance, Phytochemical, Essential oil
Graphical Abstract
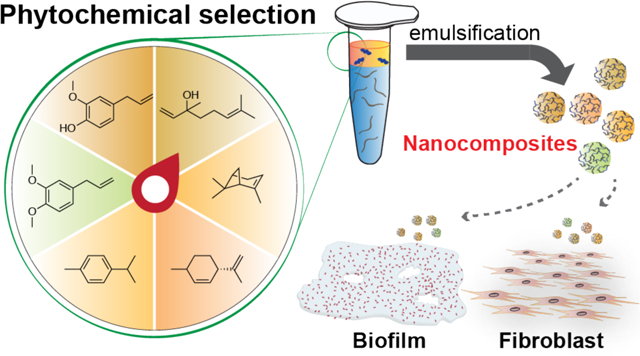
Bacterial infection is a serious threat to public health with 2 million cases occurring each year in the US alone. Among these infections, at least 65% are associated with biofilm formation,1 often occurring on medical implants, mucus, or tissues, leading to chronic wounds.2–4 Biofilms are microcolonies of bacteria residing in an extracellular polymeric substances (EPS) matrix.5 The EPS serves as a physical barrier, preventing the interaction between antimicrobial agents and bacterial cells. The charged polymeric components and embedded enzymes deactivate antibiotics and retard their penetration throughout the matrix. Moreover, dormant bacteria inside biofilms possess more antibiotic-tolerance and/or resistance than regular bacteria.6–9 These mechanisms may act simultaneously, ending in failure of standard antibiotic treatments. Currently, chemical antibiofilm treatments include long-term use of high dosages of antibiotics, or combinations of antibiotics with different killing mechanisms.10 However, these strategies are costly and still inefficient.11
Phytochemicals are plant-derived oils that have emerged as a promising alternative to current antimicrobial agents.12, 13 Phytochemicals are secondary metabolites and are key components in the self-defense mechanism of plants against pathogenic microorganisms.14 They can be effective against both planktonic and biofilm multidrug-resistant bacteria.15, 16 However, poor solubility of phytochemicals in aqueous media limits their medical applications. This limitation can be addressed using delivery vehicles such as surfactants, nanoparticles or polymers.17–19 While these strategies improve the solubility of the phytochemicals, the resulting engineered materials often have hemolytic activity and/or limited stability.
Recently, we reported a polymer-stabilized carvacrol-in-water nanocomposite (NCs) as a therapeutic against bacterial biofilm.20 However, although carvacrol is generally recognized as safe (GRAS), it demonstrates cytotoxicity toward mammalian cells.21 We hypothesized that the toxicity and hence therapeutic effects of NCs could be tuned by changing the encapsulated phytochemicals. Herein, we report the antimicrobial properties and cytotoxicity of NCs loaded with different active phytochemical ingredients. These NCs demonstrated improved antimicrobial activity against planktonic bacteria, with at least 4-fold decrease in minimum inhibitory concentrations (MICs). In addition, we found that NCs loaded with less hydrophobic phytochemicals demonstrated more potent antibiofilm efficacy. Finally, we evaluated the cytotoxicity of NCs toward 3T3 fibroblast cells to test their potential as a wound infection therapeutic agent. The results revealed that NCs encapsulating phytochemicals with lower log P and no phenolic hydroxyl groups provide a viable treatment strategy for wound biofilm infections.
Results and Discussion
Generation and Characterization of Nanocomposites.
We recently reported that incorporating carvacrol into cross-linked poly(oxanorbornenimide) polymers (PONIs) improves emulsion stability and enhances antimicrobial properties. Briefly, PONI polymers were modified with guanidinium, maleimide, and tetraethyleneglycol monomethyl ether moieties (PONI-GMT). Tetraethyleneglycol monomethyl ether moieties increased amphiphilicity of the polymers so that PONIs and hydrophobic carvacrol would self-assemble into NCs. The cationic guanidinium group was used to increase interaction with the negatively charged bacterial membranes and EPS.22 Finally, maleimide moieties on PONIs were used to stabilize the nanocomposites. These moieties can form cross-linked structure via maleimide-Michael addition reactions with the biodegradable crosslinker, dithiol-disulfide (DTDS), in carvacrol. (Scheme 1).23
Scheme 1.
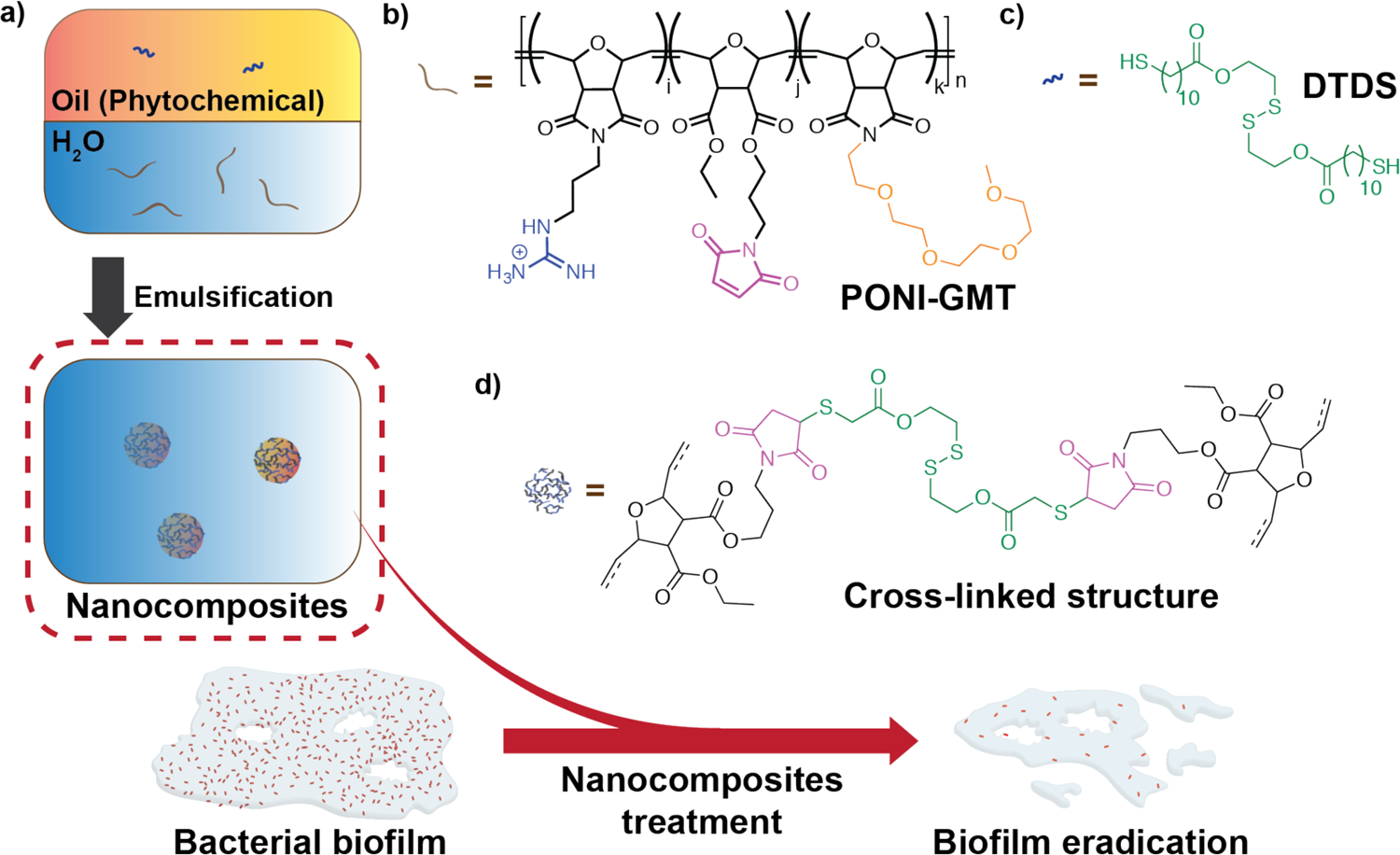
a) Preparation of NCs loaded with different phytochemicals. DTDS, the biodegradable crosslinker, was dissolved in the selected phytochemical. This resulting oil solution was then emulsified into water in the presence of PONI-GMT to form cross-linked polymer-stabilized nanocomposites. This delivery strategy demonstrated improved antimicrobial activity against bacterial biofilms; b) Chemical structure of PONI-GMT; c) Chemical structure of DTDS; d) Cross-linked structure of NCs.
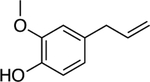
We postulated that other phytochemicals could be stabilized in aqueous media using the NC platform. We chose eugenol,24 methyl eugenol,25 carvacrol,26 linalool,27 (+)-limonene,28 p-cymene,29 and α-pinene30 for this study as they are liquid phytochemicals at room temperature and reported to demonstrate antimicrobial activity. These oils were first mixed with DTDS. Subsequently, the oil solution was emulsified into Milli-Q water containing PONI-GMT. During emulsification, PONI-GMT and the oil self-assemble, forming the NCs. These emulsions were defined as 100 v/v% and found to have size ranging from ~180 to ~530 nm. (Table 1)
Table 1.
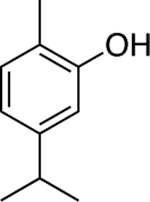
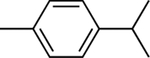
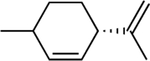
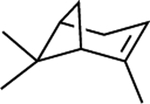
Chemical structures of the selected phytochemicals, their log P values, their particle sizes, and their polydispersity indexes (noted as PDI) after emulsification.
| Eugenol | Linalool | Methyl eugenol | Carvacrol | p-Cymene | (+)-Limonene | α-pinene | |
|---|---|---|---|---|---|---|---|
| Structure |
|
|
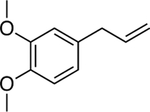
|
|
|
|
|
| log P | 2.49 | 2.97 | 3.03 | 3.49 | 4.1 | 4.57 | 4.83 |
| Size (nm) | 270 | 290 | 370 | 180 | 180 | 530 | 220 |
| PDI | 0.13 | 0.01 | 0.02 | 0.25 | 0.28 | 0.13 | 0.12 |
Antimicrobial Activity of NCs against Gram-negative Planktonic Bacteria.
We first evaluated the antimicrobial activity of these NCs against planktonic bacteria using clinical isolates of pathogenic Gram-negative bacterial strains including E. coli (CD2), P. aeruginosa (CD1006), and E. cloacae complex (CD1412). All NCs demonstrated inhibition of bacterial growth with MICs ranging from 2 – 8 v/v% (Table 2). In contrast, their bulk oil counterparts demonstrated less or no antibacterial activity, even though those solutions were prepared in 5 v/v% dimethyl sulfoxide (DMSO) aqueous solution. MICs of eugenol and carvacrol against all three Gram-negative bacteria were 4-fold or 8-fold higher than the nanocomposite counterpart, whereas limonene showed less antimicrobial activity towards all the strains we tested. None of the other oils showed inhibition of bacterial growth at the highest concentration used in this study. (Table 2) These results indicated that incorporating oils into cross-linked NCs improved their antimicrobial activity, even with oils lacking antimicrobial phenolic hydroxyl groups.31 This improvement may be attributed to electrostatic interaction between positively charged NCs and negatively charged bacterial membrane.32, 33
Table 2.
MICs (v/v%) against CD2, CD1006, and CD1412.
| Treatmenta | Phytochemical | Nanocomposites (NCs) | ||||
|---|---|---|---|---|---|---|
| CD2 | CD1006 | CD1412 | CD2 | CD1006 | CD1412 | |
| E. coli | P. aeruginosa | E. cloacae complex | E. coli | P. aeruginosa E. | cloacae complex | |
| Eugenol | 16 | 16 | 16 | 4 | 4 | 4 |
| Linalool | >32 | >32 | >32 | 2 | 8 | 8 |
| Methyl eugenol | >32 | >32 | >32 | 4 | 2 | 4 |
| Carvacrol | 16 | 16 | 16 | 4 | 4 | 2 |
| p-cymene | >32 | >32 | >32 | 4 | 4 | 4 |
| (+)-limonene | 32 | 32 | >32 | 2 | 2 | 8 |
| α-pinene | >32 | >32 | >32 | 2 | 4 | 4 |
| Colistin | 1 mg/L | 1 mg/L | 1 mg/L | -- | -- | -- |
Bacteria were treated with phytochemical dissolved in 5 v/v% DMSO aqueous solution or NCs. Colistin was used as control. MIC experiments were performed in M9 minimal medium.
Antimicrobial Activity of Nanocomposites against Gram-negative Bacterial Biofilms.
Next, we investigated the antimicrobial activity of these NCs to more refractory bacterial biofilms. As shown in Figure 1, these nanocomposites eradicated 90% of bacteria in the biofilms at concentrations ranging from 2 to 43 v/v%. We found that using amphiphilic polymers to deliver phytochemicals containing phenyl hydroxyl groups, such as eugenol and carvacrol, provided especially promising bacteria-combating capability against biofilms. Furthermore, we observed a trend that phytochemicals with lower log P demonstrated more potent antimicrobial activity against biofilms. Specifically, NCs loaded with eugenol (log P: 2.49) were able to kill 90% of bacteria in the biofilms at about 12 v/v%. Linalool (log P: 2.97) NCs demonstrated similar antimicrobial activity using higher concentrations, 26 or 30 v/v%. NCs encapsulating phytochemicals with even higher log P, such as p-cymene (log P: 4.1) and α-pinene (log P: 4.83), were incapable of eradicating 90% of bacteria in CD2 and CD1006 biofilms, even with the highest concentration used in this study. Moreover, we performed crystal violet (CV) biofilm assay to evaluate the ability of NCs to reduce biofilm biomass. CD2, CD1006, and CD1412 biofilms were treated with NCs at MBEC90 (minimum biofilm eradication concentration for eradication of 90% of bacteria in the biofilm) or 48 v/v%. In general, NCs loaded with low log P oils were capable of removing biofilm biomass up to 70%. (Figure S6) In contrast, incorporating high log P oils into NCs were less effective in biofilm dispersal. In some cases, such as α-pinene NCs against CD1006 and CD2, these NCs even promoted the production of biomass. Similar hormetic-like responses were also observed in the treatments with 10 × MIC of colistin against CD1006 and CD2 biofilms.
Figure 1.
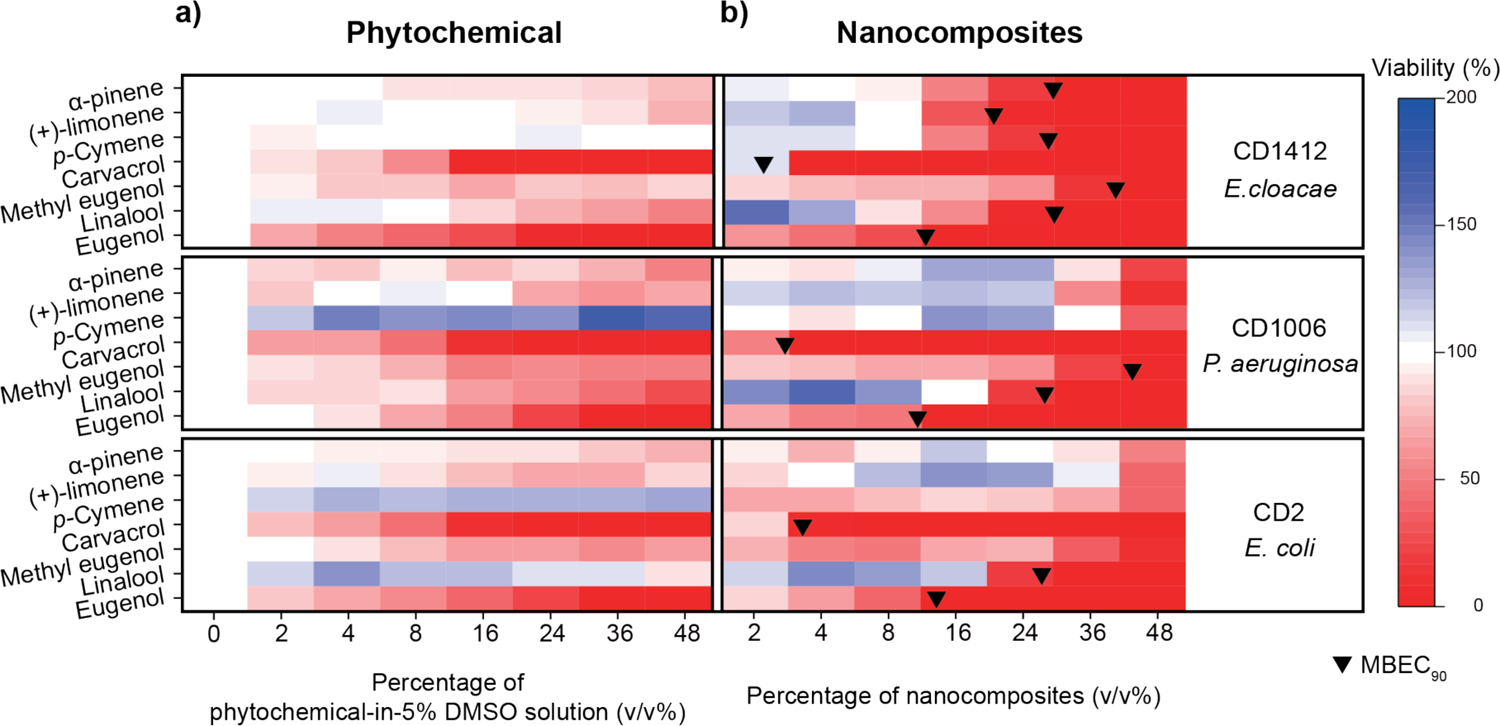
Viabilities of CD2, CD1006, and CD1412 biofilms after a three-hour treatment with a) phytochemicals in 5 v/v% DMSO aqueous solution or b) NCs. This figure was illustrated using a 3-color limited mixing setting (blue: 0%, white: 100%, and red: 200%). Data points were averaged viability (n = 3) determined using Alamar Blue assay. Black bullets indicated MBEC90 if applicable.
We also prepared phytochemical solutions in 5 v/v% DMSO solutions to compare antibiofilm efficacy of bulk oils with the NCs. As shown in Figure 1, these oils demonstrated weak to moderate antimicrobial activity even at high concentrations. The results indicated that NC delivery also improved phytochemical antimicrobial activity for recalcitrant biofilms. Notably, this delivery strategy is potentially useful in targeting E. cloacae complex population in multi-species biofilm as P. aeruginosa and E. coli biofilms were less susceptible to NCs loaded with high log P phytochemicals.34
Antimicrobial Activity of Nanocomposites against Gram-positive Planktonic Bacteria and Their Biofilms.
Besides P. aeruginosa, S. aureus is also one of the most common bacteria isolated from chronic wounds.35–37 Therefore, we also evaluated the growth inhibition ability of NCs to planktonic clinical isolated methicillin-resistant S. aureus (CD489, MRSA). As before, MICs of NCs were lower than free phytochemicals. (Table 3) In addition, we found that more hydrophobic oils were less effective against CD489 even delivered using PONI-GMT.
Table 3.
MICs (v/v%) against CD489.
| Treatmenta | Phytochemical | Nanocomposites |
|---|---|---|
| CD489 (S. aureus, MRSA) | ||
| Eugenol | 16 | 4 |
| Linalool | >32 | 16 |
| Methyl eugenol | 16 | 8 |
| Carvacrol | 32 | 4 |
| p-cymene | >32 | >32 |
| (+)-limonene | >32 | >32 |
| α-pinene | >32 | 32 |
| Vancomycin | 0.5 mg/L | -- |
Bacteria were treated with phytochemical dissolved in 5 v/v% DMSO aqueous solution or NCs. Vancomycin was used as control. MIC experiments were performed using 15:85 TSB/M9 medium.
Subsequently, we selected eugenol, linalool, methyl eugenol, and carvacrol NCs, which showed the highest antimicrobial activity, to test against S. aureus biofilms. As shown in Figure 2, after a three-hour treatment, eugenol, linalool, and carvacrol NCs eliminated 90% of bacteria at 9.27, 37.9 and 3.55 v/v%, respectively. However, CD489 biofilm was not susceptible to methyl eugenol NCs. We also performed CV staining assay to CD489. These NCs demonstrated biofilm-dispersal ability while vancomycin promoted building biomass of the biofilm (Figure S6). These experiments demonstrated that eugenol, linalool and carvacrol NCs have broad-spectrum biofilm combating ability.
Figure 2.
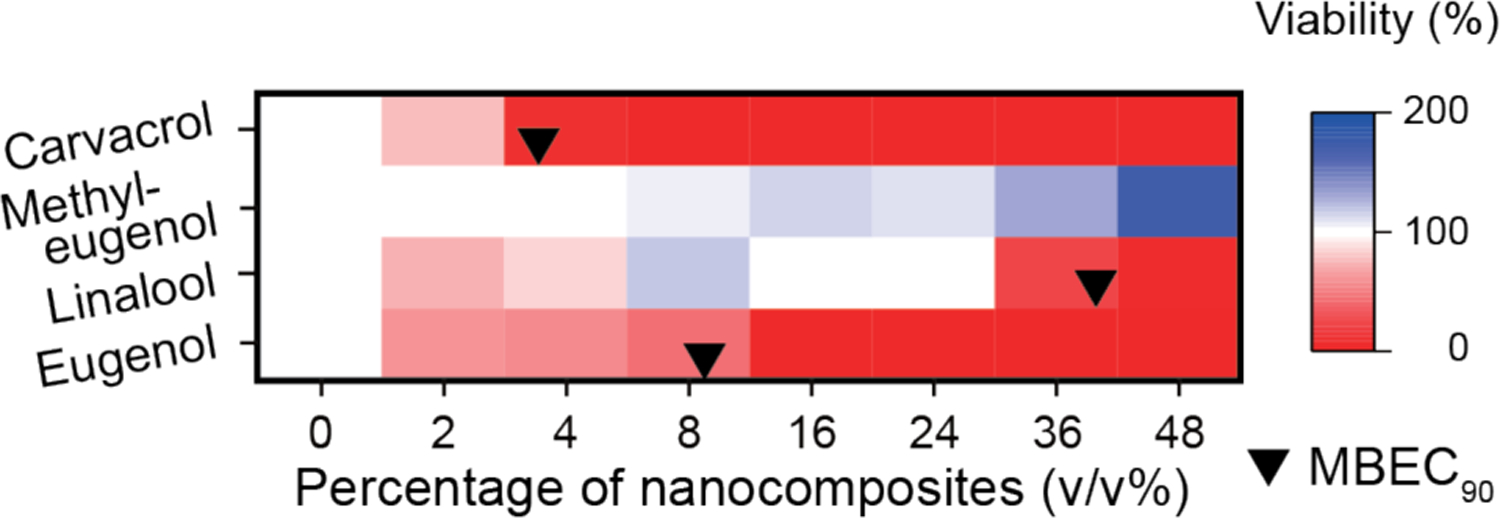
Viabilities of CD489 biofilms after a three-hour treatment with NCs. This figure was illustrated using a 3-color limited mixing setting (blue: 0%, white: 100%, and red: 200%). Data points were averaged viability (n = 3) determined using alamar-Blue assay. Black bullets indicated MBEC90 if applicable.
Cytotoxicity of NCs to 3T3 Fibroblast Cells.
Next, we evaluated the cytotoxicity of NCs towards fibroblast cells38 for assessing the potential utility of NCs for cutaneous wound biofilms. In this study, 3T3 fibroblast cell mono-layers were treated with NCs for 3 hours. Subsequently, cell viability was determined using Pierce LDH cytotoxicity assay. As shown in Figure 3, higher log P phytochemicals such as carvacrol, limonene, p-cymene, and α-pinene were more cytotoxic to 3T3 fibroblast cells. Cell viabilities were less than 50% at 8–16 v/v% after the treatment. In contrast, methyl eugenol and linalool were less cytotoxic as their concentrations to inhibit 50% fibroblast cell proliferations (GI50) were not detected in this study and 27.14 v/v%, respectively.
Figure 3.
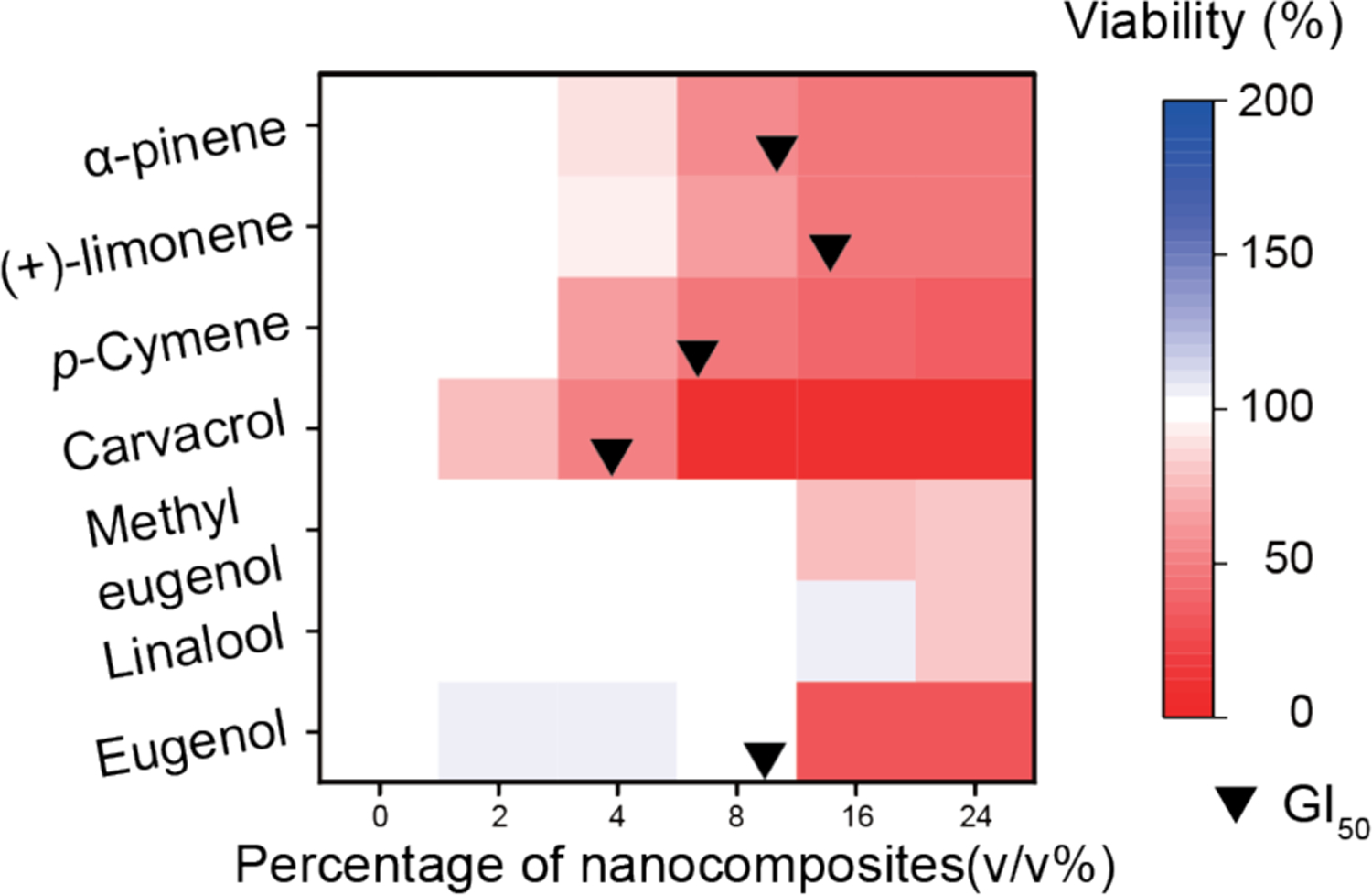
Viabilities of 3T3 fibroblast cells after a three-hour treatment with NCs. This figure was illustrated using a 3-color limited mixing setting (blue: 0%, white: 100%, and red: 200%). Data points were averaged viability (n = 3) determined using LDH assay. Black bullets indicated GI50 if applicable.
While eugenol had the lowest log P phytochemical in this study, it demonstrated strong cytotoxicity at higher concentrations (> 8 v/v%). This cytotoxicity was possibly due to the phenolic hydroxyl group in its structure.39 Other proposed mechanisms such as inhibition of Na+-K+-ATPase and mitochondrial damage were also reported.40–42 Similarly, the presence of phenolic hydroxyl group in carvacrol could contribute to its cytotoxicity. The combination of this functional group and carvacrol’s higher log P could lead to the highest cytotoxicity toward 3T3 fibroblast cells among the phytochemicals in this study. Consequently, using lower log P phytochemicals without a phenolic hydroxyl group potentially eliminated safety concerns of this therapeutic method.
Conclusion
In summary, we evaluated the antimicrobial activities and cytotoxicity of phytochemicals delivered using a cross-linked polymeric scaffold. In general, this delivery strategy dramatically improves their antimicrobial efficacy against both planktonic bacteria and biofilms. Specifically, phytochemicals with lower log P value are promising candidates for this delivery system. Moreover, encapsulating phytochemicals with lower log P and no phenolic hydroxyl groups provides particularly low cytotoxicity nanocomposites. Taken together, loading phytochemicals with the above-mentioned properties, such as linalool and methyl eugenol, into nanocomposites offers a promising direction to address wound biofilm infections.
Experimental section
All reagents/materials were purchased from Fisher Scientific as well as Sigma-Aldrich and used as received. Clinical isolated bacterial strains were obtained from the Cooley Dickson Hospital Microbiology Laboratory (Northampton, MA). NIH-3T3 cells (ATCC CRL-1658) were purchased from American Type Culture Collection (ATCC). Dulbecco’s Modified Eagle’s Medium (DMEM, ATCC 30-2002) and fetal bovine serum (Fisher Scientific, SH3007103) were used in cell culture.
Preparation of NCs
Stock nanocomposite solutions were prepared in 600 μL Eppendorf tubes. To prepare the NCs emulsions, 3 μL of the selected phytochemical (containing 3 wt% DTDS) was added to 497 μL of Milli-Q H2O containing 6.04 μM of PONI-GMT and emulsified using an amalgamator for 50 s. The emulsions were allowed to rest overnight prior to use.
Determination of Minimum Inhibitory Concentration
Bacteria were cultured in Lysogeny broth at 37°C and 275 rpm until stationary phase. The cultures were then collected by centrifugation (7000 rpm, 5 min) and washed with 0.85% sodium chloride solution for three times. The bacteria culture was then resuspended in phosphate-buffered saline (PBS) to determine its OD600. OD600 of the solution was then diluted to 0.001 using M9 minimal media, giving a final bacterial concentration of 1 × 106 CFU/mL. Afterwards, 50 μL of these solutions was added into a 96-well plate and mixed with 50 μL of NCs solutions. NCs solutions were serially diluted to give a concentration range of 0 – 32 v/v%. A growth control group was prepared containing only M9 and the bacterial solution. In addition, a sterile control group with only the growth medium was carried out at the same time. Cultures were performed in triplicates, and at least two independent experiments were repeated on different days. The MIC is defined as the lowest concentration of NCs that inhibits visible growth as observed with the unaided eye.
Biofilm Formation
Bacteria culture was prepared using the method described above. To prepare biofilm seeding solutions, bacteria except S. aureus were resuspended in M9 medium to reach OD600 of 0.1. S. aureus were resuspended in M9 medium containing 15 v/v% TSB to reach OD600 of 0.1. 100 μL of the seeding solutions were added to each well of the 96-well plate. The plate was covered and incubated under static conditions at room temperature overnight.
NCs solutions were prepared with various concentrations ranging from 0 to 48 v/v%. 100 μL of these solutions was added into a 96-well plate. Subsequently, the plate was incubated at 37°C under static condition. After 3 hours, the biofilms were washed with PBS three times, then 10 v/v% of alamarBlue cell viability reagent was added to each well, then incubated for 1 hour. Biofilm viability was determined by measuring fluorescence intensity (excitation: 560 nm; emission: 590 nm). Readings from the wells containing 10 v/v% of alamarBlue cell viability reagent only were considered as the blank (Iblank), and readings from wells having untreated biofilms were used as growth control (Icontrol). Biofilm viability was calculated using the equation below:
Crystal Violet Assay for Biofilm Quantification
We followed a standard crystal violet staining protocol with minor modifications.43 Briefly, biofilms were prepared using the method described above. NCs solutions were prepared at calculated MBEC90 or 48 v/v%. 10 × MIC of antibiotic solutions were also prepared as controls. 100 μL of these solutions was added into a 96-well plate. Subsequently, the plate was incubated at 37°C under static condition. After 3 hours, the biofilms were washed with PBS three times, then 150 μL of a 0.1% crystal violet aqueous solution was added to each well. Then, the plate was incubated at room temperature for 15 minutes. Afterwards, the biofilms were wash with PBS four times to remove excess crystal violet. The 96-well plate was then allowed to air dry.
To quantify the biofilms, a 150 μL of 20:80 acetone/ethanol solution was added to each well. The 96-well plate was incubated at room temperature for 20 minutes. Subsequently, 125 μL of the solubilized CV solutions in each well were transferred to a new flat bottom 96-well plate. OD590 of the solutions were then measured using a plate reader.
3T3 Fibroblast Cell Viability Assay
A total of 20000 NIH 3T3 (ATCC CRL-1658) cells were cultured in Dulbecco’s modified Eagle medium (DMEM; ATCC 30-2002) with 10% bovine calf serum and 1% Penicillin-Streptomycin at 37°C in a humidified atmosphere of 5% CO2 for 48 h. Then, DMEM media was removed and cells were washed once with PBS before addition of NCs prepared using pre-warmed media containing 10% serum. Cells were incubated for 3 h at 37°C under a humidified atmosphere of 5% CO2. Cell viability was determined using Pierce LDH cytotoxicity assay according to the manufacturer’s protocol.
Supplementary Material
ACKNOWLEDGEMENTS
This research was funded by the National Institutes of Health (AI134770). Clinical bacterial samples were kindly provided by Dr. Margaret Riley. The microscopy data was gathered in the Light Microscopy Facility and Nikon Center of Excellence at the Institute for Applied Life Sciences, UMass Amherst with support from the Massachusetts Life Sciences Center
Footnotes
Supporting Information
DLS, detailed viability of bacteria in the biofilm, CV staining results, detailed viability of 3T3 cells, MBEC90, GI50, and bacterial strain information.
The authors declare no competing final interest.
REFERENCES
- 1.Potera C Forging a Link Between Biofilms and Disease. Science 1999, 283, 1837 LP–1839. [DOI] [PubMed] [Google Scholar]
- 2.Hall-stoodley L; Stoodley P Evolving Concepts in Biofilm Infections. Cell Microbiol 2009, 11, 1034–1043. [DOI] [PubMed] [Google Scholar]
- 3.Wolcott RD; Rhoads DD; Dowd SE Biofilms and Chronic Wound Inflammation. J. Wound Care 2008, 17, 333–341. [DOI] [PubMed] [Google Scholar]
- 4.Moreau-Marquis S; Stanton BA; O’Toole GA Pseudomonas Aeruginosa Biofilm Formation in the Cystic Fibrosis Airway. Pulm. Pharmacol. Ther 2008, 21, 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flemming H; Wingender J The Biofilm Matrix. Nat. Publ. Gr 2010, 8, 623–633. [DOI] [PubMed] [Google Scholar]
- 6.Mah TC; Toole GAO Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol 2001, 9, 34–39. [DOI] [PubMed] [Google Scholar]
- 7.del Pozo JL; Patel R The Challenge of Treating Biofilm-Associated Bacterial Infections. Clin. Pharmacol. Ther 2007, 82, 204–209. [DOI] [PubMed] [Google Scholar]
- 8.Olsen I Biofilm-Specific Antibiotic Tolerance and Resistance. Eur. J. Clin. Microbiol. Infect. Dis 2015, 34, 877–886. [DOI] [PubMed] [Google Scholar]
- 9.Stewart PS; William Costerton J Antibiotic Resistance of Bacteria in Biofilms. Lancet 2001, 358, 135–138. [DOI] [PubMed] [Google Scholar]
- 10.Wu H; Moser C; Wang H; Høiby N; Song Z-J; Hoiby N; Song Z-J Strategies for Combating Bacterial Biofilm Infections. Int. J. Oral Sci 2014, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolcott RD; Rhoads DD; Bennett ME; Wolcott BM; Gogokhia L; Costerton JW; Dowd SE Chronic Wounds and the Medical Biofilm Paradigm. J. Wound Care 2010, 19, 45–53. [DOI] [PubMed] [Google Scholar]
- 12.Hammer KA; Carson CF; Riley TV Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol 1999, 86, 985–990. [DOI] [PubMed] [Google Scholar]
- 13.Dorman HJD; Deans SG Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. J. Appl. Microbiol 2000, 88, 308–316. [DOI] [PubMed] [Google Scholar]
- 14.Das K; Tiwari RKS; Shrivastava DK Techniques for Evaluation of Medicinal Plant Products as Antimicrobial Agent : Current Methods and Future Trends. J. Med. Plants Res 2010, 4, 104–111. [Google Scholar]
- 15.Monte J; Abreu CA; Borges A; Simões CL; Simões M Antimicrobial Activity of Selected Phytochemicals against Escherichia Coli and Staphylococcus Aureus and Their Biofilms. Pathogens 2014, 3, 473–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simoes M; Bennett RN; Rosa EAS; Simões M; Bennett RN; Rosa EAS Understanding Antimicrobial Activities of Phytochemicals against Multidrug Resistant Bacteria and Biofilms. Nat. Prod. Rep 2009, 26, 746–757. [DOI] [PubMed] [Google Scholar]
- 17.Chang Y; McLandsborough L; McClements DJ Physicochemical Properties and Antimicrobial Efficacy of Carvacrol Nanoemulsions Formed by Spontaneous Emulsification. J. Agric. Food Chem 2013, 61, 8906–8913. [DOI] [PubMed] [Google Scholar]
- 18.Gomes C; Moreira RG; Castell-Perez E Poly (DL-Lactide-Co-Glycolide) (PLGA) Nanoparticles with Entrapped Trans-Cinnamaldehyde and Eugenol for Antimicrobial Delivery Applications. J. Food Sci 2011, 76, 16–24. [DOI] [PubMed] [Google Scholar]
- 19.Lin L; Cui H; Zhou H; Zhang X; Bortolini C Nanoliposomes Containing Eucalyptus Citriodora as Antibiotic with Specific Antimicrobial Activity. Chem. Commun 2015, 51, 2653–2655. [DOI] [PubMed] [Google Scholar]
- 20.Landis RF; Li CH; Gupta A; Lee YW; Yazdani M; Ngernyuang N; Altinbasak I; Mansoor S; Khichi MAS; Sanyal A; et al. Biodegradable Nanocomposite Antimicrobials for the Eradication of Multidrug-Resistant Bacterial Biofilms without Accumulated Resistance. J. Am. Chem. Soc 2018, 140, 6176–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichling J; Suschke U Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral, and Cytotoxic Properties – an Overview. Complement. Med. Res 2009, 79–90. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z; Zheng A; Zhong J Interactions of Biocidal Guanidine Hydrochloride Polymer Analogs with Model Membranes: A Comparative Biophysical Study. Acta Biochim. Biophys. Sin. (Shanghai). 2011, 43, 729–737. [DOI] [PubMed] [Google Scholar]
- 23.Pounder RJ; Stanford MJ; Brooks P; Richards SP; Dove AP Metal Free Thiol-Maleimide “Click” Reaction as a Mild Functionalisation Strategy for Degradable Polymers. Chem. Commun 2008, 0, 5158–5160. [DOI] [PubMed] [Google Scholar]
- 24.Ali SM; Khan AA; Ahmed I; Musaddiq M; Ahmed KS; Polasa H; Rao LV; Habibullah CM; Sechi LA; Ahmed N Antimicrobial Activities of Eugenol and Cinnamaldehyde against the Human Gastric Pathogen Helicobacter Pylori. Ann. Clin. Microbiol. Antimicrob 2005, 4, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sybiya Vasantha Packiavathy IA; Agilandeswari P; Musthafa KS; Karutha Pandian S; Veera Ravi A Antibiofilm and Quorum Sensing Inhibitory Potential of Cuminum Cyminum and Its Secondary Metabolite Methyl Eugenol against Gram Negative Bacterial Pathogens. Food Res. Int 2012, 45, 85–92. [Google Scholar]
- 26.Lambert RJW; Skandamis PN; Coote PJ; Nychas G-JEE A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol 2001, 91, 453–462 [DOI] [PubMed] [Google Scholar]
- 27.Park S; Kyong Y; Oliveira M; Cho E; Jin D; Kook J Anaerobe Antimicrobial Effect of Linalool and a -Terpineol against Periodontopathic and Cariogenic Bacteria. Anaerobe 2012, 18, 369–372. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal KK; Khanuja SPS; Ahmad A; Santha Kumar TR; Gupta VK; Kumar S; Khanuja ŁSPS; Ahmad A; Kumar TRS; Gupta VK Antimicrobial Activity Profiles of the Two Enantiomers of Limonene and Carvone Isolated from the Oils of Mentha Spicata and Anethum Sowa. Flavour Fragr. J 2002, 17, 59–63. [Google Scholar]
- 29.Castilho PC; Savluchinske-Feio S; Weinhold TS; Gouveia SC Evaluation of the Antimicrobial and Antioxidant Activities of Essential Oils, Extracts and Their Main Components from Oregano from Madeira Island, Portugal. Food Control 2012, 23, 552–558. [Google Scholar]
- 30.Leite AM; Lima EDO; Souza EL De; De M Inhibitory Effect of β -Pinene, α -Pinene and Eugenol on the Growth of Potential Infectious Endocarditis Causing Gram-Positive Bacteria. Rev. Bras. Cienc. Farm 2007, 43, 121–126. [Google Scholar]
- 31.Ultee A; Bennik MHJ; Moezelaar R The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus Cereus. Appl. Environ. Microbiol 2002, 68, 1561 LP–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L; Xu K; Wang H; Jeremy Tan PK; Fan W; Venkatraman SS; Li L; Yang Y-Y Self-Assembled Cationic Peptide Nanoparticles as an Efficient Antimicrobial Agent. Nat. Nanotechnol 2009, 4, 457–463. [DOI] [PubMed] [Google Scholar]
- 33.Ramalingam K; Amaechi BT; Ralph RH; Lee VA Antimicrobial Activity of Nanoemulsion on Cariogenic Planktonic and Biofilm Organisms. Arch. Oral Biol 2012, 57, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckert R; He J; Yarbrough DK; Qi F; Anderson MH; Shi W Targeted Killing of Streptococcus Mutans by a Pheromone-Guided “Smart” Antimicrobial Peptide. Antimicrob. Agents Chemother 2006, 50, 3651 LP–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giacometti A; Cirioni O; Schimizzi AM; Del Prete MS; Barchiesi F; D’Errico MM; Petrelli E; Scalise G Epidemiology and Microbiology of Surgical Wound Infections. J. Clin. Microbiol 2000, 38, 918 LP–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller LS; Cho JS Immunity against Staphylococcus Aureus Cutaneous Infections. Nat. Rev. Immunol 2011, 11, 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serra R; Grande R; Butrico L; Rossi A; Settimio UF; Caroleo B; Amato B; Gallelli L; de Franciscis S Chronic Wound Infections: The Role of Pseudomonas Aeruginosa and Staphylococcus Aureus. Expert Rev. Anti. Infect. Ther 2015, 13, 605–613. [DOI] [PubMed] [Google Scholar]
- 38.Singer AJ; Clark RAF Cutaneous Wound Healing. N. Engl. J. Med 1999, 341, 738–746. [DOI] [PubMed] [Google Scholar]
- 39.Borges A; Serra S; Abreu AC; Saavedra MJ; Simões M; Borges A; Serra S; Abreu AC; Saavedra MJ Evaluation of the Effects of Selected Phytochemicals on Quorum Sensing Inhibition and in Vitro Cytotoxicity. Biofouling 2014, 30, 183–195. [DOI] [PubMed] [Google Scholar]
- 40.Kreydiyyeh SI; Usta J; Copti R Effect of Cinnamon, Clove and Some of Their Constituents on the Na+-K+-ATPase Activity and Alanine Absorption in the Rat Jejunum. Food Chem. Toxicol 2000, 38, 755–762. [DOI] [PubMed] [Google Scholar]
- 41.Yoo C-B; Han K-T; Cho K-S; Ha J; Park H-J; Nam J-H; Kil U-H; Lee K-T Eugenol Isolated from the Essential Oil of Eugenia Caryophyllata Induces a Reactive Oxygen Species-Mediated Apoptosis in HL-60 Human Promyelocytic Leukemia Cells. Cancer Lett. 2005, 225, 41–52. [DOI] [PubMed] [Google Scholar]
- 42.Prashar A; Locke IC; Evans CS Cytotoxicity of Clove ( Syzygium Aromaticum ) Oil and Its Major Components to Human Skin Cells. Cell Prolif. 2006, 39, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Toole GA Microtiter Dish Biofilm Formation Assay. J. Vis. Exp 2011, 2437–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


