Abstract
Antibiotic-resistant bacterial infections arising from acquired resistance and/or through biofilm formation necessitate the development of innovative ‘outside of the box’ therapeutics. Nanomaterial-based therapies are promising tools to combat difficult bacterial infections, featuring the capacity to evade existing mechanisms associated with acquired drug resistance. In addition, their unique size and physical properties give them the capability to target biofilms, overcoming refractory infections. In this review, we highlight the general mechanisms by which nanomaterials can target bacterial infections associated with acquired antibiotic resistance and biofilms. We emphasize design elements and properties of nanomaterials that can be engineered to enhance potency. Finally, we present recent progress and remaining challenges for widespread clinical implementation of nanomaterials as antimicrobial therapeutics.
Introduction
The emergence of antibiotic resistance in bacteria has resulted in the challenge of refractory infections1 , 2. Multidrug-resistant bacteria are a global crisis, increasing morbidity and mortality of infected patients and negatively impacting the outcome of a wide range of groups, including those in intensive care units, undergoing surgery, transplantation, or cancer treatment2,3. A 2017 report from WHO’s Global Antimicrobial Surveillance System highlighted antibiotic resistance as a world-wide challenge4. The estimated cost of treating a patient with an antibiotic-resistant infection is US $50,000, with an estimated US $20 billion societal costs annually5. The use, and in some situations misuse, of antibiotics, combined with the scarcity of new therapeutics entering the antibiotic pipeline, further exacerbate this public health threat6.
Planktonic (free-floating) bacteria are central players in multiple health threats, including sepsis3. Infections associated with planktonic bacteria present acute threats and are rapidly becoming more challenging to treat due to rising rates of acquired antibiotic resistance. This challenge is amplified when bacteria form biofilms, which are associated with recurring and chronic bacterial infections7. The ability of bacteria to protect themselves within biofilms complicates treatment of numerous infection-types, including chronic wounds, osteomyelitis, and infective endocarditis8. Antibiotic resistance associated with the biofilm state is distinct from acquired resistance, but can compound and exacerbate therapeutic challenges9. Biofilms produce extracellular polymeric substance (EPS) that may serve as a barrier against host immune response and some conventional antimicrobial agents7,9. More importantly, biofilms exhibit a diversity of altered phenotypes, including slow growth rates, presence of persister cells, and creation of spatial and chemical heterogeneities that contribute to resistance to many available antibiotics10,11.
Antibiotics are currently the main therapeutic strategy for treating both planktonic and biofilm infections12. They target processes necessary for growth and/or survival of bacteria, including cell wall/cell membrane synthesis/maintenance, or production of DNA, RNA or essential proteins. Many antibiotics are derived from products that have been deployed by microorganisms to combat one another for billions of years. The offensive tools generated by microbes in this warfare have generated defense responses; bacteria have developed the intrinsic ability to evolve and escape the killing mechanisms of many traditional antibiotics13. Eradicating multidrug resistant (MDR) bacteria may require multiple or high dosages of antibiotic agents or the use of ‘last resort’ antibiotics12. Adding to the therapeutic challenge, when bacteria are in biofilms, biofilm-associated resistance becomes a compounding factor, oftentimes requiring aggressive physical removal of the biofilm through aggressive debridement, for example, accompanied by high doses of antimicrobial chemotherapy14,15. These strategies can result in long and expensive treatments, with the possibility of adverse effects and uncertain outcomes.
Nanoparticles (NPs) access antimicrobial modalities that are novel to bacteria, and hence not in their natural defensive arsenal (BOX 1). Recent advances in nanomaterial-based systems provide new opportunities to address MDR planktonic alongside biofilm infections, acting either as inherent therapeutics or nanocarriers for antimicrobial agents16. The unique physico-chemical properties of nano-sized materials, such as size, shape, and surface chemistry, influence their therapeutic activity17. The sizes and shapes of different nanomaterials are analogous to bacterial biomolecular components, affording a variety of interactions that can be regulated through surface functionalization. High surface to volume ratios and multivalent interactions are important for creating antibacterial NPs16,17. Nanoparticles are able to evade existing resistance mechanisms and may be less prone to select for resistance than are conventional antibiotics (BOX 2)47. Moreover, nanomaterials have the ability to eradicate bacteria in biofilms17. Taken together, nanotechnology provides a new toolkit for the creation of efficient treatment strategies against MDR planktonic and biofilm infections.
Box 1|. What are nanomaterials?
Nanomaterials
Nanomaterials are organic, inorganic or hybrid particles, with some defining their size as ≤100 nm, and others including particles ≤ 500 nm18. They have an almost unlimited range of structures and morphologies, from rods to pyramids to fibrous networks to spheres with hollow or solid interiors bearing rough or smooth surfaces19. Materials in the nanoscale realm possess distinctive physico-chemical characteristics, including size, shape and surface, compared to their bulk counterparts20. The unique properties of nanomaterials have revolutionized many technologies and industries, including medicine. Being comparable in size to biomolecules and bacterial intracellular structures, nanomaterials can be engineered to exhibit new therapeutic modalities21. Representative classes of nanomaterials for antimicrobial application include metal-based NPs, carbon-based NPs, polymeric NPs, nanocomposites, liposomes and smart nanomaterials.
Metal-based NPs
Metal-based NPs are comprised of either pure metals (e.g., gold, silver, iron) or their compounds, e.g., oxides. Their primary mechanisms of toxicity involve reactive oxygen species production and impairment of membrane function22,23. This type of NPs has been demonstrated to be effective in treating several MDR bacterial infections24,25,26,27. Silver-based nanomaterials are the most established metal antimicrobials; although the exact mechanism of action for silver NPs is unknown, two widely proposed modes of actions include disruption of membranes by leached silver ions and ion-mediated killing28,29.
Carbon-based NPs
Carbon-based NPs include carbon quantum dots30, nanotubes31 and 2-D materials, including graphene32. Their bactericidal action involves physical and chemical damage, however, specific mechanisms are yet to be understood. In one study, multi-walled carbon nanotubes prevented formation of Klebsiella oxytoca, Pseudomonas aeruginosa and Staphylococcus epidermidis biofilms by blocking bacterial settlement33.
Polymeric NPs
Polymeric NPs can either be natural or synthetic. Natural polymers are used to fabricate cationic and pH-switchable antimicrobial NPs34,35. Synthetic polymeric NPs can mimic the activity of antimicrobial peptides36,37. Moreover, polymeric micelles are used as nanocarriers to improve the solubility, stability, efficacy and pharmacokinetic profiles of drugs38. Dendrimers are regular polymeric molecules comprised of a central core, branch-like structures radiating from the core, and outer surface bearing functional groups. Glycopeptide dendrimers have been shown to inhibit biofilms of P. aeruginosa39.
Nanocomposites
Nanocomposites are hybrids of inorganic and organic NPs. For example, incorporation of silver NPs into the cationic polymer, poly(2-dimethylamino)ethyl methacrylate, resulted in a synergistic antimicrobial activity against P. aeruginosa and Staphylococcus aureus40.
Liposomes
Liposomes are vesicles composed of one or more phospholipid bilayers with an aqueous inner core. Being membrane-based structures, they have good biocompatibility and are useful antimicrobial delivery vehicles41. They can encapsulate hydrophilic drugs in their aqueous interior or hydrophobic drugs in their phospholipid membrane42,43.
Smart nanomaterials
Smart nanomaterials can respond to stimuli, such as pH and bacterial toxins (endogenous), or light, temperature and ultrasound (external), to produce changes in their characteristics that allows them to exert their antimicrobial action44,45,46. For instance, hybrid micelles composed of poly(ethylene)glycol, poly(aspartamide), 2-(diisopropylamonio)ethylamine, azithromycin and cis-aconityl-D-tyrosine can shrink in size, reverse surface charge and release drug cargo in response to the acidic environment of P. aeruginosa biofilms45.
Box 2|. Antibiotic resistance mechanisms.
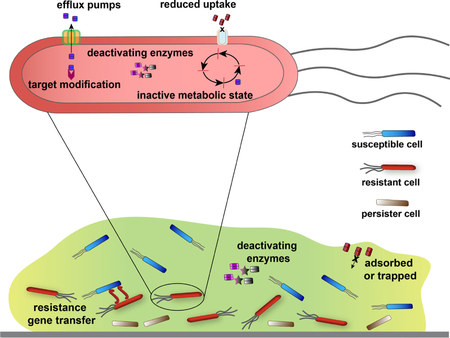
Bacteria have acquired multiple survival mechanisms that enable them to evade killing by antibiotics. The biofilm state itself confers resistance against antibiotics, separate from genetically acquired antibiotic resistance48,49. Nanomaterials are novel to bacteria and can circumvent resistance mechanisms that affect traditional antibiotics; and nanomaterials can target bacterial biofilms.
Resistance gene transfer and target modification.
Bacteria communicate and share genetic information with one another, resulting in spread of resistance genes across bacterial populations10, 50. Expression of resistance genes allows modification of antibiotics and protection from them as well. Unlike traditional antibiotics that have specific targets, nanomaterials can have multiple killing mechanisms because they access multiple targets, making emergence of resistance less likely than with traditional antibiotics47.
Deactivating enzymes.
Resistant bacteria can harbor extracellular and/or intracellular enzymes that degrade antibiotics. The multiple mechanisms of action of NPs and their abiological structure allow them to escape deactivation by these enzymes47.
Reduced uptake.
Gram-negative bacteria have evolved to limit entry of antibiotics through porin mutations22. Nanomaterials, however, enter bacterial cells through other mechanisms, such as endocytosis and membrane fusion47. As an example, liposomal NPs loaded with antimicrobial agents enter via membrane fusion and rapidly release high concentrations of antimicrobial agents to membranes or into the cytoplasm51.
Efflux pumps.
Efflux pumps, which are often upregulated in antibiotic-resistant bacterial cells, actively transport antimicrobial agents outside of bacterial cells. Nanoparticles can block these efflux pumps, increasing accumulation of antibiotics inside bacterial cells52,53.
Inactive metabolic state.
Persister cells are subpopulations of metabolically inactive bacteria with reduced susceptibility to antimicrobials54. The killing mechanisms of many nanomaterials, membrane damage in particular, do not require bacteria to be in a state of active growth, rendering these agents active against persisters55.
EPS limits penetration.
The protective nature of the EPS of bacterial biofilms restricts penetration of some antibiotics, such as aminoglycosides, due to electrostatic repulsion50,56. While other antibiotic groups can diffuse into the inner layers of biofilms, the complex gradient of nutrients and waste can diminish their antimicrobial effects57. The unique surface chemistry of nanomaterials allows facile penetration into biofilms, and interaction with deeply embedded bacterial cells. The amphiphilic balance of many nanomaterials helps them exert multiple interactions with EPS, including hydrophobic and electrostatic interactions, maximizing adsorption by and diffusion across biofilms.17,47
In this review, we illustrate how nanomaterials could be used to combat MDR bacterial infections. We discuss properties and design elements that result in therapeutic efficacy, providing insight into how nanomaterials might be tailored to optimize activity against planktonic and biofilm bacteria. Finally, we highlight the status of clinical development of antibacterial nanomaterials.
Mechanisms against planktonic bacteria
The array of sizes and shapes accessed by nanomaterials offers unique capabilities for targeting bacteria21 (FIG. 1). Nanoparticles can employ multiple bactericidal mechanisms, including direct cell wall and/or cell membrane damage, generation of reactive oxygen species (ROS) and/or binding to intracellular components. Most antibiotics target cell walls/cell membranes or disrupt intracellular processes. Nanomaterials can access these pathways, albeit in different ways, and offer advantages in combating antibiotic-resistant pathogens relative to small molecule drugs (TABLE 1). Further, nanomaterials can be used as nanocarriers for delivery of therapeutic agents19,21. The mechanisms employed by nanomaterials arise from their unique physico-chemical properties, in particular multivalent interactions with bacterial cells. Van der Waals forces, receptor-ligand interactions, hydrophobic interactions and electrostatic attractions play a role in NP-bacteria interfaces58.
Figure 1. Nano versus micro – size comparison between nanomaterials and bacteria.
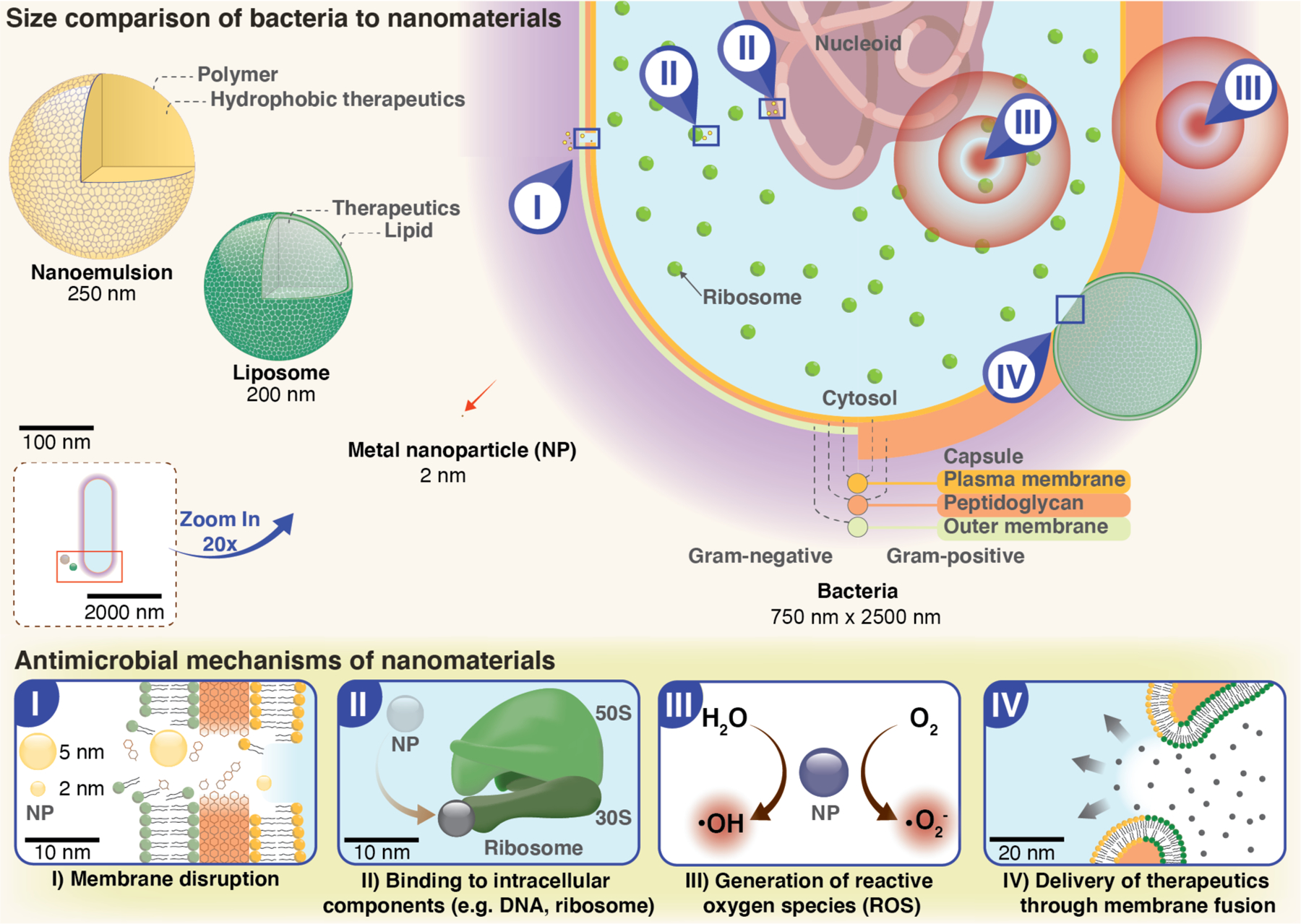
Bacteria typically have diameters ranging from 0.2 to 10 μm. Varying nanoparticle materials and preparation methods provide a wide range of particle sizes (2–500 nm) that facilitate maximal contact and strong interactions with bacterial membranes. Nanomaterials may display a variety of bactericidal mechanisms: I| Membrane disruption. Electrostatic interactions of NPs with the negatively charged groups present on bacterial surfaces results in membrane damage and cytoplasmic leakage. II| Intracellular damage. NPs can bind various bacterial components, such as ribosomes, proteins and/or DNA, interrupting their function. III| ROS. NPs with catalytic activities increase production of reactive oxygen species, such as hydroxyl radicals and superoxides, causing oxidative cellular stress. IV| Delivery. Nanomaterials can be used for delivery of therapeutic agents; some nanomaterials readily enter bacterial cells through membrane fusion, facilitating delivery of their cargo.
Table 1.
Overcoming resistance mechanisms
| Mechanism of Action | Antibiotics | Potential ways nanomaterials evade resistance | |
|---|---|---|---|
| Class | Major resistance mechanism | ||
| Cell wall/membrane disruption | β-lactams | drug modifying enzymes59; binding site modifications60; porin changes61 |
physical damage to the cell envelope limits development of resistance62,63,64; flexible design space and unique physico-chemical properties can be used to maximize disruptive interactions16 |
| glycopeptides | binding site modifications65 | ||
| peptide antibiotics | outer membrane modifications66 | ||
| Damage to intracellular components | aminoglycosides | drug modifying enzymes61 | entry via membrane fusion overcomes resistance from limited antimicrobial entry51; ability to block efflux pumps52,53; multiple active groups available target general rather than specific bacterial pathways47 |
| macrolides | efflux pumps67; binding site modifications61 |
||
| quinolones | binding site modifications61; porin changes61 |
||
Cell wall and membrane disruption
The cell envelope has evolved to serve as a physical barrier towards antimicrobials. Teichoic acids - present in the cell wall of Gram-positive bacteria - and lipopolysaccharide - found in the outer membrane of Gram-negative bacteria - have phosphate groups that render bacterial surfaces negatively charged. This highly polar environment limits penetration of hydrophobic antimicrobials across bacterial membranes, compromising their activity58.
Bacterial cell surfaces are more negatively charged than are those of mammalian cells, facilitating preferential electrostatic interactions with positively charged materials68. The charge density and hydrophobicity of the NP surface are important factors in designing NPs to selectively disrupt bacterial membranes36, 69 , 70. Highly cationic nanomaterials can bind to the surface of mammalian cells, as can NPs with overly hydrophobic surfaces, reducing selectivity. Cationic nanomaterials with good amphiphilic balance can provide potent antimicrobial effects with low hemolysis and cytotoxicity36.
A range of nanomaterial-based strategies focus on targeting the negatively charged surface of planktonic bacteria33,36,55,69. Yang et al. fabricated biodegradable cationic and amphiphilic polycarbonates that self-assemble into cationic micellar NPs, killing methicillin-resistant S. aureus (MRSA). These polymeric NPs interact with bacteria through electrostatic interactions, resulting in disintegration of the membrane and cell lysis71. ‘Nanoknifes”, materials with sharp-pointed edges, are particularly effective in compromising bacterial membrane integrity. In one study, single-walled carbon nanotubes and graphene oxide ruptured the cell surface of Ralstonia solanacearum leading to cytoplasmic leakage and bacterial death72. The ability of bacteria to develop resistance against therapeutics that damage the cell envelope is likely to be limited, making these strategies promising for long-term use with minimal risk of emergence of bacterial resistance55,73.
Generation of reactive oxygen species
Reactive oxygen species (ROS) are byproducts of cellular oxidative metabolic processes that affect cell differentiation, signaling, survival and death22. Accumulation of excessive ROS results in lethal oxidative stress. ROS can damage cells through multiple mechanisms, in particular through reaction of superoxide and hydroxyl radicals with thiols in proteins, deactivating membrane-located receptors74. There are several mechanisms by which NPs generate ROS: 1) direct ROS production from the NP surface or from leached ions; 2) interaction with intracellular organelles; and 3) oxidation through interaction with redox active biomolecules, including NADPH oxidase23. Some metal-based NPs employ ROS generation as their major antibacterial mechanism due to their inherent photocatalytic activity (i.e., photodynamic therapy)22,30,75; reviews discussing ROS activity of metal nanoparticles are available22,23,76.
An example of ROS-based antibacterial activity is the release of free Cu+ from copper iodide (CuI) NPs, generating ROS and damaging bacterial DNA and intracellular proteins of E. coli and B. subtilis77. Silver-zinc oxide nanocomposites likewise exhibited antibacterial activity against S. aureus and antibiotic-resistant E. coli ascribed to potent ROS generation and release of silver (Ag+) and zinc (Zn2+) ions. These combined processes then generated a cascade of bactericidal effects, including damaged cell membranes, protein dysfunction, inhibition of DNA replication and leakage of intracellular materials78. Silver and other Fenton-inactive metals increase ROS in bacteria by their ability to disrupt cellular donor ligands coordinating with iron, such as cysteine, and to induce release of Fe from [4Fe-4S] clusters. This Fe release then increases ROS formation22.
Gold nanoparticles (AuNPs) have also shown enzyme-like activities79. Mesoporous silica (MSN) can provide support and enhance the stability and catalytic-activity of the AuNPs80. AuNPs bound on the surface of bifunctionalized MSN (MSN-AuNPs) displayed peroxidase- and oxidase-like activities, killing both Gram-positive and Gram-negative bacteria. The dual enzyme-like activity of this system increases efficiency of ROS production increasing oxidative stress to bacteria81.
Damage to intracellular components
Cellular homoeostasis and intracellular signaling pathways are central to the function and survival of bacteria. Nanomaterials can be engineered to interfere with these processes, ultimately leading to cell death. These disruptions include alteration in gene or protein expression or DNA damage82,83. As an example, AuNPs were functionalized with 4,6-diamino-2-pyrimidinethiol, an analogue of 2-pyrimidinethiol (found in E. coli), to generate pyrimidine-capped AuNPs (Au-DAPT)84. These NPs completely inhibited proliferation of MDR strains of E. coli and P. aeruginosa. Mechanisms of action of Au-DAPT were elucidated through the following: 1) gel electrophoresis showing the ability of NPs to bind bacterial DNA; 2) TEM images displaying leakage of nucleic acids and binding to ribosomes and chromosomes; 3) an E. coli-free transcription/translation system demonstrating protein synthesis inhibition; and 4) colorimetric assays showing selective chelation of Mg2+, destabilizing the cell membrane. Similarly, polymer-coated silver NPs killed E. coli cells by inhibiting both the Krebs cycle and amino acid metabolism85. Polymers were used to modify the surface of AgNPs to increase interactions with bacterial cells. The mechanism of action was confirmed by the downregulated expression of aceF, frdB, gadB, metL and argC, ultimately leading to cell death.
Delivery of therapeutic agents
Several nanodrugs - liposomal nanoformulations in particular - have been FDA-approved and made available for clinical use to treat different diseases, including cancer86. Similarly, NPs may be used as carriers for delivery of antimicrobial agents87. Therapeutics can be encapsulated inside NPs or bound to their surfaces88,89. NPs offer protection of these agents against enzymes and molecules that might otherwise degrade them. This protection can increase therapeutic efficiency of a drug, resulting in decreased dosage requirements to achieve desired effects and therefore reduced host toxicity90. The use of delivery systems can also enhance stability, solubility and biocompatibility of otherwise pharmacologically challenging antibiotics. Use of nanocarriers can minimize selection of resistance through delivery of therapeutics that elicit multiple mechanisms of action, and through targeted release of cargo which prevents exposure of bacteria to sub-inhibitory doses of the drug42,44. For instance, the antibiotic gentamicin loaded into poly(lactide-co-glycolide) NPs exhibited improved antimicrobial activity against in vitro and in vivo P. aeruginosa infection88. Subsequently, levofloxacin loaded into silver core-embedded mesoporous silica nanovehicles (Ag@MSNs@LEVO) afforded a synergistic treatment of MDR isolates of E. coli. The silver component of the system not only functions as a carrier but also imparts antimicrobial effects via silver ion generation. In an in vivo murine peritonitis model, treatment with Ag@MSNs@LEVO reduced bacterial burden by three orders of magnitude, with concomitant reduction of damage to the spleen and peritoneum. No toxic side effects were observed91. In a related approach, ampicillin was attached to the surface of AuNPs and AgNPs, yielding broad-spectrum bactericidal agents that evade resistance mechanisms of MDR strains of P. aeruginosa and Enterobacter aerogenes and of MRSA89.
Therapeutic selectivity and enhancement of delivery efficiency can be achieved via release of drug in response to specific stimuli44,92. Bacterial infection sites are weakly acidic and that can be targeted34,44,93. For example, vancomycin was encapsulated in a pH-responsive, surface charge-switching triblock copolymer poly(D,L-lactic-co-glycolic acid)-b-poly(L-histidine)-b-poly-(ethylene glycol) (PLGA-PLH-PEG). Therapeutic cargo was released only upon interaction with the acidic infection site, providing a target for vancomycin delivery93. PLGA was chosen due to its low toxicity and ease of surface fine tuning; PEG reduced off-target interactions, prolonging circulation time; and PLH provided the charge-switchable characteristic of the polymer. The selective protonation of the imidazole groups of PLH at weakly acidic conditions allows for a stimuli-responsive effect. Biomaterials can also provide charge-switching behavior, with pH-triggered release of vancomycin achieved using chitosan NPs34. Furthermore, bacterial toxins can be used as a trigger for release of antimicrobials. Lecithin and DSPE-PEG3400 were used to coat a mixture of fatty acids, forming liposome-based nanoreactors that release calcium peroxide and rifampin in the presence of alpha-toxin, a pore-forming toxin produced by S. aureus94. This strategy selectively targets pathogenic bacteria as demonstrated by the higher antimicrobial activity against MRSA and minimal effect on non-pathogenic B. subtilis.
Overall, nanomaterials provide multiple bactericidal pathways to combat bacteria and evade antibiotic resistance mechanisms. Appropriate engineering of size, shape and surface properties provides a broad design space for novel antimicrobial agents.
Combating planktonic bacterial infections
Drug resistant hospital-acquired (nosocomial) infections are challenging to treat. A group of pathogens comprised of Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter species - collectively termed as ‘ESKAPE’ pathogens - is responsible for the majority of nosocomial infections, complicating the conditions of patients that are often immunocompromised2,3,6. Methods for treating infections caused by these pathogens are becoming increasingly limited due to the rapid rate of resistance development even against ‘last resort’ antibiotics6. In this regard, nanomaterials can provide a lifeline for therapeutic design, as studies have shown that there is limited to no resistance development observed with nanomaterial-based strategies47, 51, 71,73.
Numerous studies have explored the utility of nanomaterials against the ‘ESKAPE’ pathogens (FIG. 2)100, 101, 102,103. Qiao et al. reported the activity of star-shaped polymeric peptide NPs (SNAPPs) against MDR Gram-negative ESKAPE pathogens, in vitro and in an in vivo murine peritonitis model63. Researchers designed artificial antimicrobial peptide (AMP)-inspired peptide polymer NPs consisting of lysine and valine residues that self-assemble into star-shaped unimolecular structures, mimicking AMPs. SNAPPs elicit multiple proposed bactericidal mechanisms, including damage to outer and inner cell membranes, disruption of ion efflux/influx regulation and induction of an apoptotic-like death pathway. The proposed multimodal antimicrobial activity of SNAPPs renders the barrier to resistance high. Comparing the concentration that results in death of 50% mammalian cell population (IC50) and concentration which kills half of bacterial isolates [minimum bactericidal concentration (MBC50)], SNAPPs had a therapeutic index higher than colistin, a drug of last resort for MDR Gram-negative bacillary infections. Furthermore, MDR A. baumannii did not acquire resistance towards SNAPPs after multiple passages in sub-inhibitory concentrations. Liposome-based NPs are another promising system, restoring potency of the antibiotics cefepime, imipenem and ceftazidime against MDR P. aeruginosa104, chloramphenicol against MRSA105 and amikacin against K. pneumoniae106 through efficient drug delivery. Similarly, delivery of antimicrobial peptides was achieved with the use of PLGA NPs, providing a successful treatment strategy for P. aeruginosa lung infection in an in vivo murine model95.
Figure 2. Examples of nanomaterial-based strategies used to combat bacterial infections.
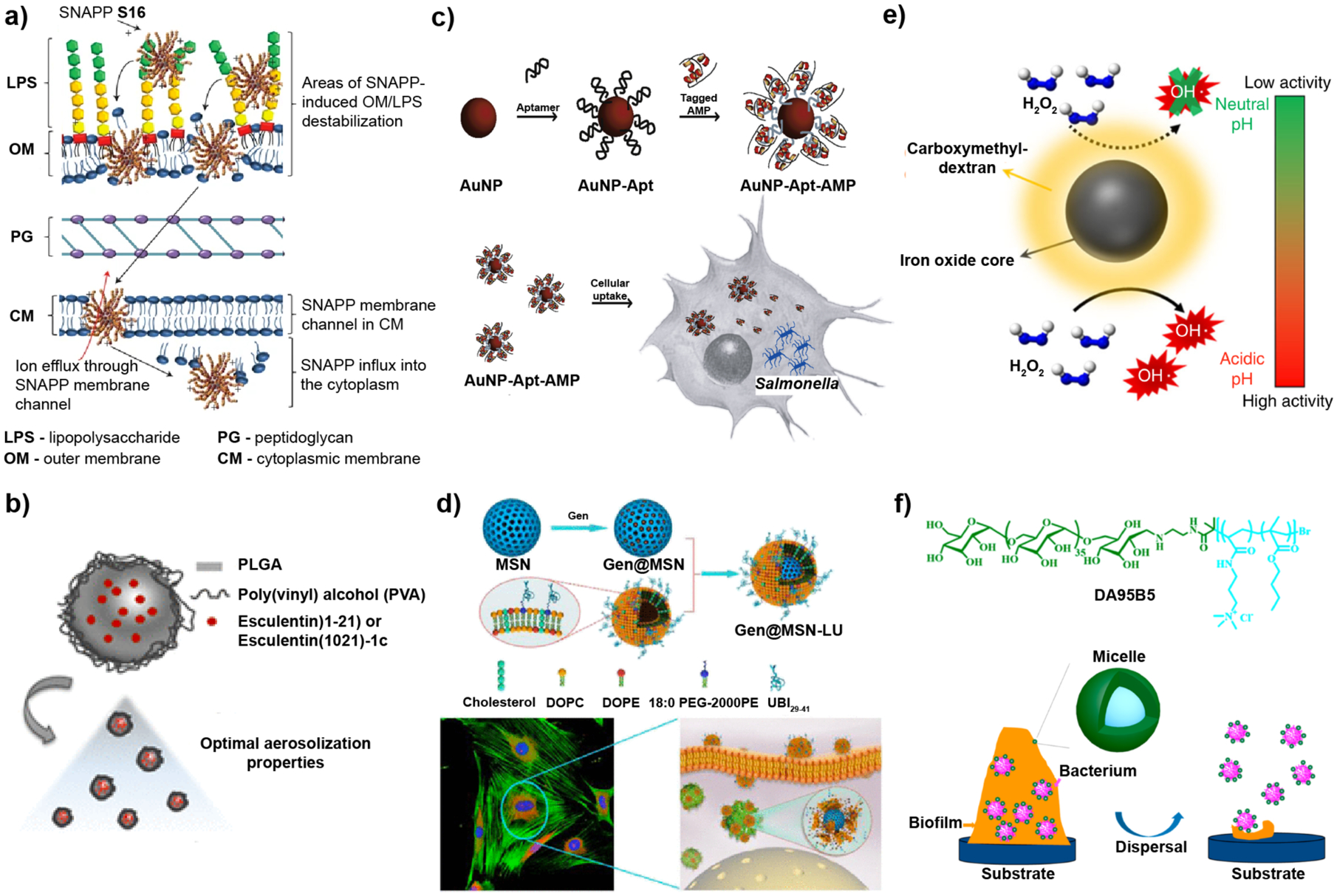
a-b| Planktonic bacterial infections. c-d| Intracellular infections. e-f| Biofilm infections. a| Structurally engineered AMPs, SNAPPs, exhibited promising antimicrobial activity in vitro and in vivo. SNAPPs interacts with the outer membrane, peptidoglycan and cytoplasmic membrane layers of bacteria through electrostatic interactions, ultimately leading to cell lysis. b| Intratracheal administration of antimicrobial esculentin-1a formulated to be delivered to the lungs using PLGA NPs reduced P. aeruginosa lung infection in a mouse model. c| Histidine-aptamer-conjugated gold nanoparticles loaded with His-tagged AMPs were effective for treatment of Salmonella enterica-infected mammalian cells. d| Gentamicin-loaded mesoporous silica nanoparticles with a bacterial toxin-responsive lipid bilayer surface shell and bacteria-targeting peptide UBI29–41, allowed targeted release of antibiotic for killing of intracellular S. aureus. e| A carboxymethyl-dextran-coated iron oxide nanoparticle, ferumoxytol, catalyzed ROS production of H2O2 in a pH-dependent manner as a treatment against oral biofilms. f| Dextran (green) and poly(AMPTMA-co-BMA) (light blue) form a micelle with a bactericidal core and non-fouling dextran shell used to treat wound biofilms. Electrostatic interaction of the NPs with the biofilm weakens bacterial attachment while gradually dispersing EPS matrix. Image in part a reproduced, with permission, from REF 63 © (2016) Macmillan Publishers Limited, part of Springer Nature. Image in part b reproduced, with permission, from REF 95 © (2019) American Chemical Society. Image in part c reproduced, with permission, from REF 96 © (2016) Elsevier Ltd. Image in parts d and f reproduced, with permission, from REF 97 and 98, respectively © (2018) American Chemical Society. Image in part e reproduced, with permission, from REF. 99 © (2018) Nature Communications. All rights reserved.
Combating intracellular bacterial infections
Bacteria can reside within mammalian cells, giving rise to recurring systemic infections107. For example, Salmonella enterica is a common facultatively intracellular pathogen that causes life-threatening food-borne infections in millions of people worldwide each year108. Salmonella can survive and replicate inside host cells, including macrophages. Intracellular localization of bacteria adds a level of complexity to treatment, because many antibiotics have limited ability to cross mammalian cell membranes and can also be actively exported out by the host cell109, 110. Nanomaterials can mitigate this challenge through their ability to penetrate inside eukaryotic cells, as well as via their high drug loading capacity (FIG. 2).
In one example of nanomaterial-based treatment of intracellular infections, enrofloxacin-loaded docosanoic acid solid lipid nanoparticles (SLNs) increased intracellular accumulation of enrofloxacin up to ~40-fold and enhanced Salmonella killing inside macrophages111. In another approach, colistin, a poorly permeable antibiotic, was formulated into liposomes functionalized with a bacterial-derived protein to promote internalization into eukaryotic cells to provide therapeutics with high oral bioavailability112. In yet another strategy, gentamicin was loaded into mesoporous silica nanoparticles with bacterial toxin-responsive lipid bilayer surface shells. Functionalized with bacteria-targeting peptide UBI29–41, allowing targeted treatment of intracellular S. aureus97.
Mycobacterium tuberculosis is another example of an intracellular pathogen that survives within host macrophages, invading the lungs and causing tuberculosis (TB)113. Several studies have demonstrated the activity of nanomaterials against intracellular Mycobacterium species. Yang et al. reported a library of cationic star-shaped polycarbonate nanostructures with excellent wide-spectrum antimicrobial activity and low rates of hemolysis114. Mannose-functionalized polycarbonate demonstrated enhanced intracellular antimycobacterial activity by targeting mannose receptors on the surface of macrophages. In another study, biodegradable multimetallic microparticles (MMPs), consisting of Ag NPs and ZnO NPs encapsulated within PLGA polymer, were utilized as a pulmonary delivery system to enable delivery of antituberculosis drug rifampicin within alveolar macrophages115. Further, the ability of AgNPs and ZnONPs to interact with and compromise bacterial membrane stability furthered the antimicrobial effects of the system.
Nanomaterial-based strategies to combat other intracellular pathogens have been developed. For example, AuNP-DNA aptamer conjugates loaded with antimicrobial peptides showed activity against intracellular Salmonella enterica96 and Vibrio vulnificus116 in in vivo murine infection models. Gentamicin-loaded AuNPs decorated with phosphatidylcholine eradicated intracellular Listeria monocytogenes and P. aeruginosa in infected macrophages87.
Therapeutic strategies against biofilms
MDR biofilm infections present a particularly difficult therapeutic challenge117. The matrix provided by the EPS may provide a barrier to some cellular and small molecule (e.g., antibiotic) assaults. Bacteria embedded within EPS matrix are capable of synergistic interactions, cell-to-cell communications and transfer of resistance genes10,11. Furthermore, the lower layers of the matrix have low oxygen and nutrient supply, inducing formation of dormant persister cells, which promote antimicrobial tolerance and resistance117,118.
Overcoming the physical barrier presented by biofilms is needed to combat biofilms. The EPS is comprised of biopolymers including nucleic acids, proteins and polysaccharides that provide a three-dimensional protective scaffold for bacteria. The matrix is rich in negatively charged components and hydrophobic groups, with pores filled with water facilitating transport of nutrients10. Tuning surface functionality and design of NPs can facilitate biofilm penetration (BOX 3)119, 120. Size and electrostatic interactions are important factors influencing biofilm penetration profile of nanomaterials. Generally, uncharged NPs with sizes <350 nm have higher mobility across pores inside biofilms while cationic NPs have good distribution throughout the matrix62,121,122,123.
Box 3|. Nanomaterial properties and design elements.
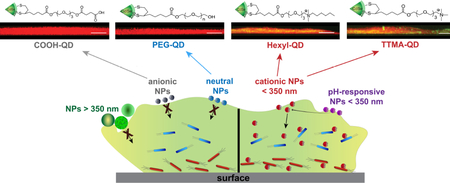
Thoughtful engineering of nanomaterial surfaces modulates NP-bacteria interactions. The interface between NPs and bacteria is characterized by hydrophobic and electrostatic interactions and Van der Waals forces that can be modulated by tuning nanomaterial properties124. Tuning the size, surface and shape of a nanomaterial can maximize antibacterial activity, biofilm penetration, biocompatibility, biodistribution and therapeutic index125.
Size
The size of nanomaterials regulates bactericidal activity. Small NPs (2–10 nm) cause more membrane damage than larger ones because of high surface areas of contact with bacterial cells coupled with greater curvatures126,127. NPs with sizes less than 350 nm can diffuse through the constrained spaces of pores within biofilms121,122.
Surface
Nanoparticle surfaces can be functionalized with chemical groups that enable multivalent interactions with bacterial cells and the EPS matrix17. Nanoparticles have surface charge-dependent bacterial toxicity (i.e., the more positively charged the surface, the more toxic the NP becomes)36. Careful placement of the positive charge and hydrophobic moieties can enhance the antibacterial activity of polymeric NPs, while maintaining minimal cytotoxicity. Furthermore, biofilm penetration can be enhanced by surface modification120,128,129. As shown in the figure, anionic and zwitterionic NPs have poor matrix penetration, while cationic NPs with an appropriate hydrophobic balance can penetrate the EPS. Several strategies have taken advantage of the acidic pH of biofilms to switch from anionic or zwitterionic to cationic NPs45, 130. Insets are confocal images showing the biofilm penetration profile of quantum dots with different surface charges (scale bar = 20 μm). Figure reproduced, with permission, from REF 131 © (2015) The Royal Society of Chemistry. All rights reserved.
Shape
Contact-killing can be influenced by NP shape; sharp and pointed NPs can puncture bacterial cell membranes, leading to cytoplasmic leakage32,64,125,132. Comparisons of the activities of spherical, rod-shaped and truncated triangular silver nanoplates against planktonic cells of E. coli reveal that truncated triangular AgNPs possess superior bactericidal effect. This is due to the number of NP facets directly interacting with the bacterial surface. Triangular NPs have more facets than the two other shapes, causing more membrane damage to bacteria132. On the other hand, rod-shaped nitric oxide-releasing silica NPs result in better biofilm eradication than their spherical counterparts, a result attributed to the higher particle aspect ratio of rod-shaped than spherical NP133.
Targeting resident pathogens
Upon biofilm penetration, nanomaterials can interact with bacteria and exert the therapeutic mechanisms discussed above for planktonic bacteria (FIG. 3a). For instance, the efficient biofilm penetration profile and bacteria membrane-damaging activity of poly(oxanorborneneimide)-based cationic polymeric NPs eradicated MDR biofilms of P. aeruginosa, E. cloacae complex and MRSA62. In another approach, the use of stimuli-responsive NPs provided activation of bactericidal effects in a spatio-temporally controlled manner. pH-responsive silver nanoantibiotics (rAgNAs) were developed using self-assembled silver nanoclusters and charge-switchable ligands poly(ethyleneglycol)-poly(aminopropyl imidazole-aspartate)-polyalanine (PEG-PSB-PALA)130. Protonation of the imidazole groups in the low-pH biofilm microenvironment induced disassembly of rAgNAs due to electrostatic repulsion with silver ions. Disassembly into smaller Ag nanoclusters allowed biofilm penetration, killing deeply embedded MRSA cells. Similarly, application of an external magnetic field facilitated biofilm penetration of silver nanoparticles134. Superparamagnetic iron oxide nanoparticles were coated with silver rings; the generated magnetic field allowed biofilm penetration, with silver conferring antibacterial activity.
Figure 3. Eradicating biofilms using NPs.
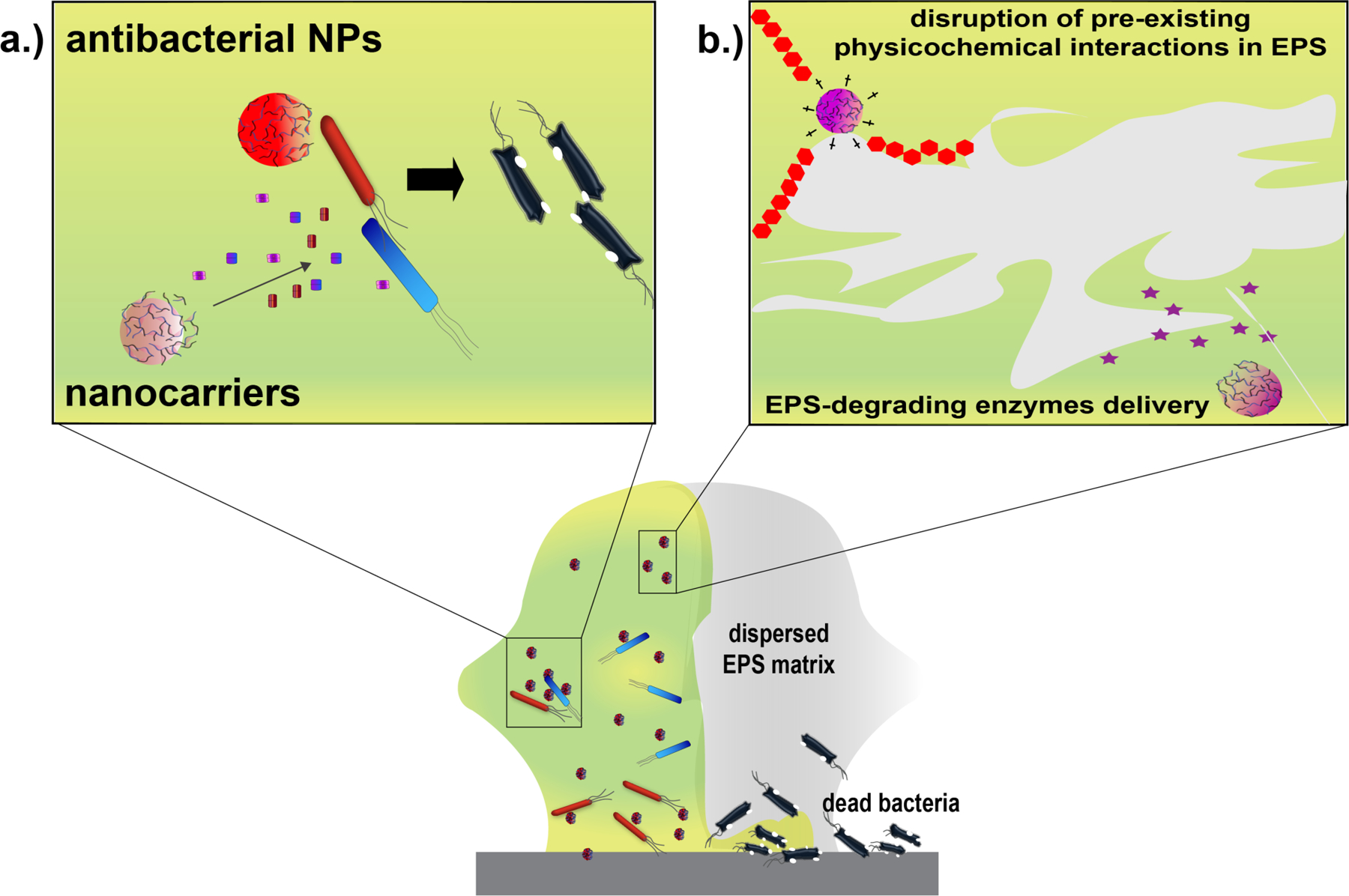
Biofilms are comprised of cells with phenotypic heterogeneity embedded across the 3D-matrix of their self-secreted EPS. The ability of NPs to penetrate throughout the matrix allows them to a| interact with cells entrenched within the EPS and/or b| initiate disruptive interactions with the matrix that weaken physicochemical interactions responsible for keeping the 3D structure of biofilms intact. NPs can then either exert their inherent antimicrobial action or deliver therapeutic agents, such as antibiotics or essential oils, to kill the bacteria within the biofilms. NPs can alternatively deliver EPS-degrading molecules that promote dispersion of biofilms, facilitating their disruption.
Nanomaterials can also deliver therapeutics to bacterial cells embedded within the EPS matrix. For example, although the potent antimicrobial carvacrol, an essential oil found in oregano and thyme, poorly penetrates biofilms, Rotello et al. utilized carvacrol to eradicate biofilms using biodegradable oil-in-water crosslinked polymeric nanocomposites (X-BNCs). X-BNCs eliminated MDR biofilms of both Gram-negative and -positive bacteria while maintaining minimal cytotoxicity towards mammalian cells73. The polymer scaffold (PONI-GMT) contained guanidinium, maleimide and tetraethylene glycol monomethyl ether groups. The cationic property of the nanocomposite was attributed to guanidinium. The presence of maleimide groups provided crosslinking sites and an additional mode of degradation while the tetraethylene glycol monomethyl ether imparted hydrophilicity to the assembly. Careful design of the polymer increased solubility, stability, biodegradability, and antimicrobial potency of carvacrol oil while assisting its penetration across the matrix. Similarly, nanoscale liposomes delivered the antibiotic amikacin through size-dependent biofilm penetration, treating chronic P. aeruginosa biofilm lung infections135. This system is currently at Phase III of clinical trials.
Disrupting the EPS matrix
Beyond killing bacteria, it is important to disrupt the EPS matrix for treatment of biofilms136. EPS scaffold remaining after treatment can be inhabited and populated by other microbes. Different NP-based approaches can be employed to disperse the EPS matrix, including mechanical disruption and delivery of matrix-degrading enzymes (e.g., DNAse, hydrolase, protease) (FIG. 3b). For example, poly(lactic-co-glycolic acid) NPs loaded with ciprofloxacin were functionalized with DNase I to eradicate P. aeruginosa biofilms137. DNase degraded eDNA which then rendered the 3D network fragile and susceptible to ciprofloxacin. Similarly, AuNPs functionalized with proteinase-K dispersed Pseudomonas fluorescens biofilm138. Alternatively, magnetic iron oxide nanoparticles (MNPs) disrupted MRSA biofilms with the application of direct current (DC) and alternating current (AC) magnetic fields139. Application of a rotating DC magnetic fields mechanically damaged the biofilm matrix. MNPs traversing across the 3D network acted as “shield breakers”, destroying the biofilm through static friction. Exposure of MNPs to AC magnetic field resulted in a localized increase in temperature that dispersed embedded cells. Since the mechanisms of action of these MNPs do not include killing of bacteria, this system offers a long-term anti-biofilm strategy that may escape resistance development.
A promising strategy for targeting biofilm growth is the interruption of bacterial communication systems essential for coordinated activities, including colonization and biofilm development. Bacteria communicate through quorum sensing (QS), a process that can be sabotaged to prevent formation of biofilms or induce their dispersion48,124,140. Decho et al. demonstrated that hampering QS can silence bacterial communication141. Silicon dioxide NPs (Si-NPs) decorated with β-cyclodextrin (β-CD) blocked communication between Vibrio fischeri cells. V. fischeri exhibits bioluminescent output controlled by population density, that can be monitored via the QS signaling molecule acylhomoserine lactone (HSL). The β-CD group of Si-NPs binds to HSL, quenching its activity. As a result, the luminous output of V. fischeri was reduced. Further, downregulation of luminescence genes, luxA and luxR, was observed. Other studies have demonstrated inhibition of biofilm formation and virulence factors by deactivating quorum sensors using liposome-based NPs43, chitosan nanoparticles35, 142 and metal-based nanoparticles143,144.
Nanomaterial penetration profiles predict success of biofilm elimination. Size and amphiphilicity mainly influence NP distribution across the biofilm. The exact interactions of NPs with the EPS also depend on the type of biofilms which varies by species and in some cases strain of bacteria. The controllable parameters of nanomaterials provide a flexible toolkit to address the diversity of biofilm infections.
Combating biofilm infections
The number of biofilm-related infections continues to grow each year145 , 146. Bacteria can form biofilms in and on tissues and organs, including on skin, in the oral cavity, and on linings of gastrointestinal and respiratory tracts8,117. Biofilms largely contribute to chronic and persistent infections. With advances in the understanding of medical biofilms, nanotherapeutic strategies have emerged to potentially address biofilm infections.
Oral biofilms
The oral cavity is a prevalent site for biofilms; Streptococcus mutans is a common oral biofilm pathogen. The acidic microenvironment of dental biofilms (i.e., plaque) results in destruction of tooth apatite, causing dental caries147,148. NP-based strategies have been used to address oral biofilm-associated infections, taking advantage of the highly acidic oral biofilm microenvironment. Liposomes coated with the quaternary ammonium-modified chitosan were used to deliver the antibiotic doxycycline to Porphyromonas gingivalis oral biofilms149. The residual amines of chitosan provided pH-responsive groups that were protonated under acidic conditions, providing pH-based activity. Similarly, nanocarriers fabricated with pH-responsive block copolymers that can bind to negatively charged hydroxyapatite were used to deliver farnesol150 and chlorhexidine151 for treatment of dental caries. NPs that induce ROS production and EPS matrix degradation are also being investigated for oral biofilm treatment. For instance, catalytic NP (CAT-NP) consisting of biocompatible Fe3O4 were utilized to catalyze in situ generation of free radicals from H2O2, resulting in a reduction of S. mutans biofilms152. Coating iron oxide NPs with FDA-approved polymers, such as dextran, increased its stability in aqueous formulation and enhanced biocompatibility with oral soft tissues153. The iron-supplying nanotherapeutic ferumoxytol was ‘reinvented’ from an iron deficiency drug into a topical oral biofilm therapeutic99. This FDA-approved iron-based nanoparticle possesses a pH-dependent peroxidase-like property that provides localized catalytic activity (FIG. 2e). This work demonstrated that ferumoxytol can bind within the biofilm matrix and generate free radicals from H2O2, resulting in in situ bacterial death and EPS degradation. Both a human-derived ex vivo model and an in vivo rodent dental caries model revealed efficacy in preventing acid damage of the enamel and suppression of dental caries without altering the oral microbiota and with safety towards gingival and mucosal tissues.
Wound biofilms
Wound infections affect ~300 million people worldwide, with treatment costs estimated as high as $25 billion in the US alone154,155. In these infections, necrotic tissue fosters attachment of bacteria and provides nutrients that enhance bacterial proliferation and biofilm formation, which impedes wound healing by inhibiting re-epithelialization and prolonging inflammation15,145,156. Silver NPs incorporated in hydrogels or in wound wraps are commonly used to treat wound infections157. Other types of nanoparticles have also been increasingly studied for the treatment of biofilm-infected wounds158 , 159. For example, copper particles incorporated into biodegradable nanofibers prevented formation of and eradicated preformed biofilms of P. aeruginosa and S. aureus. Further in vitro and in vivo studies are underway to demonstrate the applicability of this strategy for wound dressings160. Another strategy utilizes the amphiphilic core-shell polymeric NP, DA95B5, which removes preformed biofilms of MRSA via nanoscale bacterial ‘debridement’98 (FIG. 2f). DA95B5 can diffuse through the EPS, disrupting biofilms by weakening attachment of bacteria to the matrix. An in vivo murine excisional wound biofilm model demonstrated effective dispersal of MRSA biofilms. DA95B5-soaked hydrogel pad dressings reduced bacterial counts in mice up to ~4 log CFU. Notably, the NP exhibited minimal in vitro eukaryotic cell lysis and low in vivo toxicity. Combination of these NPs with molecules that accelerate the wound healing process, including growth factors, anti-inflammatory molecules and extracellular (ECM) mimics, can further NP-based strategies. As an example, a pH-responsive antimicrobial nanofiber network, formed by the self-assembly of octapeptide IKFQFHFD, was incorporated into a hydrogel and loaded with cypate and proline161. The octapeptide possessed an inherent antimicrobial property via cell wall and membrane disruption; cypate is a photothermal drug that is anticipated to disrupt EPS matrix; and procollagen component proline is added to aid in collagen and ECM matrix reformation. The hydrogel eradicated MRSA biofilms and facilitated healing in chronic wounds as demonstrated in an in vivo diabetic mice model.
Towards clinical translation
There has been a rapid increase in the exploration of antimicrobial nanomaterials for treatment of MDR planktonic bacteria and biofilm infections. Most studies have been conducted in vitro, with fewer proceeding to animal models, and still fewer proceeding to human testing86,162. Developing appropriate in vitro and in vivo models that demonstrate efficacy and safety of NPs will provide clinical feasibility for their use. Several reviews have summarized appropriate in vitro and in vivo models to explore depending on the type of infection being targeted7,145,163.
Successful clinical translation will require standardized guidelines for evaluating biocompatibility and nanotoxicology. Most formulations undergoing clinical testing are nanocarriers for antibiotic delivery or antimicrobial silver nanoparticles (TABLE 2). Two liposomal nanoformulations for controlled delivery of antibiotics are currently at Phase III clinical trials. Arikace was designed to improve the therapeutic efficiency of amikacin as well as alleviate its renal and neurological toxicity164. Pulmaquin is nanoliposome-based formulation for the rapid and delayed release of ciprofloxacin165. Many challenges still hamper nanodrug translation into clinical settings such as safety concerns, however, it is likely only a matter of time until these novel therapeutics provide solutions for currently unmet clinical demands86,162.
Table 2.
Nanomaterial-based therapeutics under clinical trials
| Trade name | NP type | Active agent | Target pathogens/infection | Clinical Trial Phase | Clinical Trial Number | Ref |
|---|---|---|---|---|---|---|
| Arikace | Liposomal | Amikacin | Gram-negative | III | NCT01315691 | 164 |
| Pulmaquin | Liposomal | Ciprofloxacin | Gram-negative | III | NCT02104245 | 165 |
| Silvasorb | Silver NP | Silver | Topical infection | III | NCT00659204 | 166 |
| NanoAgCVC | Silver NPs | Silver | Central venous catheter- related infection | IV | NCT00337714 | 167 |
| N/A | Polymeric NP | Doxycycline | Chronic periodontitis | II | NCT02726646 | 168 |
| IABN | Polymeric NP | Ammonium polyethyleneimine | Oral infection | II | NCT01167985 | 169 |
Conclusions and perspectives
Nanomaterials present an emerging ‘outside of the box’ toolkit for treatment of resilient MDR planktonic bacteria and biofilm infections. Their tunable properties, particularly their surface functionalities, provide design spaces that can be fine-tuned to maximize therapeutic effect while minimizing host toxicity. In this review, we provided examples of how NPs can combat bacteria in both planktonic and biofilm forms, using a wide range of mechanisms. Nanomaterials can access multi-modal antibacterial mechanisms that are novel, slowing or stopping the generation of drug resistance. NPs have potential as topical treatments for oral and wound biofilm-associated infections. Strategies combining bactericidal effects and biofilm dispersion, however, are required to assure complete eradication of biofilms. Stimuli-responsive NPs that take advantage of unique microenvironments at infection sites, such as pH and pathogen-derived metabolites, provide one of the many pathways to target MDR bacteria using nanomaterials. Systemic safety and long-term effects of NPs on the body are still among the major barriers to clinical use. Current studies are determining the pharmacokinetic profile of NPs to better understand their fate in the body.
The generation of effective antimicrobial nanomaterials requires interdisciplinary collaborations among chemists, biomedical researchers (including microbiologists), and engineers. Likewise, partnership between fundamental, translational and industrial agencies will be instrumental in moving antimicrobial nanomaterials to the clinic. Overall, nanomaterial-based treatment strategies offer a promising alternative to antibiotics for difficult-to-treat infections, alleviating challenges faced in the post-antibiotic era.
Acknowledgements
This research was supported by the US National Institutes of Health (NIH; AI134770).
Glossary
- Osteomyelitis
Bone infection
- Infective endocarditis
Infection of endocardium, typically of heart valves
- Persister cells
Subpopulation of dormant, antibiotic-tolerant bacterial cells that is able to resume growth after antimicrobial stress is relieved
- Debridement
Surgical removal of damaged or dead tissue from an infected wound
- Nanocarriers
A drug delivery platform in the nanoscale range (1–1000 nm). Common nanocarriers include liposomes, polymers and micelles
- Peritonitis
Inflammation of the peritoneum, the tissue layer lining the inner wall of the abdomen, often as a result of bacterial infection
- Therapeutic index
A quantitative measure of the relative safety of a drug determined by the dosage that produces a therapeutic effect without host toxicity and the concentration that results in dangerous side effects
- Quorum sensing
A process whereby bacteria communicate and perform coordinated activities in response to a particular cell population density determined by specific signaling molecules
Footnotes
Competing Interests Statement
Dr. Patel reports grants from CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, ContraFect, TenNor Therapeutics Limited and Shionogi. Dr. Patel is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics and Qvella; monies are paid to Mayo Clinic. In addition, Dr. Patel has a patent on Bordetella pertussis/parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. Dr. Patel receives travel reimbursement from ASM and IDSA, an editor’s stipend from IDSA, and honoraria from the NBME, Up-to-Date and the Infectious Diseases Board Review Course. All other authors declare no competing interests.
References
- 1.Ventola CL The antibiotic resistance crisis part 1: Causes and threats. P&T 40, 277–283 (2015). [PMC free article] [PubMed] [Google Scholar]
- 2.Michael CA, Dominey-Howes D & Labbate M The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2, 145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; (2019). [Google Scholar]
- 4.WHO. Global Antimicrobial Resistance Surveillance System (GLASS) Report. WHO; (2017). ISBN 978-92-4-151344-9. [Google Scholar]
- 5.Naylor NR et al. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control 7, 58 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willyard C The drug-resistant bacteria that pose the greatest health threats. Nature 543, 15 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Lebeaux D, Chauhan A, Rendueles O & Beloin C From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2, 288–356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebeaux D, Ghigo J-M & Beloin C Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev 78, 510–543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjarnsholt T The role of bacterial biofilms in chronic infections. APMIS 121, 1–58 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Van Acker H, Van Dijck P & Coenye T Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 22, 326–333 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Flemming H & Wingender J The biofilm matrix. Nat Rev Microbiol 8, 623–633 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Ventola CL The antibiotic resistance crisis part 2 : Management strategies and new agents. P&T 40, 344–352 (2015). [PMC free article] [PubMed] [Google Scholar]
- 13.Aminov RI A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol 134, 1–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arciola CR, Campoccia D & Montanaro L Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol 16, 397–409 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Wu Y-K, Cheng N-C & Cheng C-M Biofilms in chronic wounds: Pathogenesis and diagnosis. Trends Biotechnol. 37, 505–517 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Wang LS, Gupta A & Rotello VM Nanomaterials for the treatment of bacterial biofilms. ACS Infect. Dis 2, 3–4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A, Landis RF & Rotello VM Nanoparticle-based antimicrobials: Surface functionality is critical. F1000Res. 5, 364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vert M et al. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem 84, 377–410 (2012). [Google Scholar]
- 19.Baptista PV et al. Nano-strategies to fight multidrug resistant bacteria- “A battle of the titans”. Front. Microbiol 9, 1–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natan M & Banin E From Nano to micro: Using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev 41, 302–322 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Soenen SJ et al. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 6, 446–465 (2011). [Google Scholar]
- 22.Lemire JA, Harrison JJ & Turner RJ Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol 11, 371–384 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Miller KP, Wang L, Benicewicz BC & Decho AW Inorganic nanoparticles engineered to attack bacteria. Chem. Soc. Rev 44, 7787–7807 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Li X et al. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 8, 10682–10686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lara HH, Ayala-Núñez NV, del Turrent LCI & Padilla CR Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol 26, 615–621 (2010). [Google Scholar]
- 26.Guzman M, Dille J & Godet S Synthesis and antibacterial activity of silver nanoparticles against Gram-positive and Gram-negative bacteria. Nanomedicine Nanotechnology, Biol. Med 8, 37–45 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Durmus NG, Taylor EN, Kummer KM & Webster TJ Enhanced efficacy of superparamagnetic iron oxide nanoparticles against antibiotic-resistant biofilms in the presence of metabolites. Adv. Mater 25, 5706–5713 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Prabhu S & Poulose EK Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett 2, 32 (2012). [Google Scholar]
- 29.Qing Y et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomedicine 13, 3311–3327 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong X, Liang W, Meziani MJ, Sun YP & Yang L Carbon dots as potent antimicrobial agents. Theranostics 10, 671–686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Jumaili A, Alancherry S, Bazaka K & Jacob MV Review on the antimicrobial properties of carbon nanostructures. Materials 10, 1–26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou X, Zhang L, Wang Z & Luo Y Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc 138, 2064–2077 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Malek I et al. Vertically aligned multi walled carbon nanotubes prevent biofilm formation of medically relevant bacteria. J. Mater. Chem. B 4, 5228–5235 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Kalhapure RS et al. pH-responsive chitosan nanoparticles from a novel twin-chain anionic amphiphile for controlled and targeted delivery of vancomycin. Colloids Surfaces B Biointerfaces 158, 650–657 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Omwenga EO, Hensel A, Shitandi A & Goycoolea FM Chitosan nanoencapsulation of flavonoids enhances their quorum sensing and biofilm formation inhibitory activities against an E. coli top 10 biosensor. Colloids Surfaces B Biointerfaces 164, 125–133 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Palermo EF & Kuroda K Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol 87, 1605–1615 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Song J & Jang J Antimicrobial polymer nanostructures: Synthetic route, mechanism of action and perspective. Adv. Colloid Interface Sci 203, 37–50 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Landis RF et al. Cross-linked polymer-stabilized nanocomposites for the treatment of bacterial biofilms. ACS Nano 11, 946–952 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reymond JL, Bergmann M & Darbrea T Glycopeptide dendrimers as Pseudomonas aeruginosa biofilm inhibitors. Chem. Soc. Rev 42, 4814–4822 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Mei L, Lu Z, Zhang X, Li C & Jia Y Polymer-Ag nanocomposites with enhanced antimicrobial activity against bacterial infection. ACS Appl. Mater. Interfaces 6, 15813–15821 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Chang Hsin-I and Yeh Ming-Kung. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed 7, 49–60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forier K et al. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 190, 607–623 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Bandara HMHN et al. Incorporation of farnesol significantly increases the efficacy of liposomal ciprofloxacin against Pseudomonas aeruginosa biofilms in vitro. Mol. Pharm 13, 2760–2770 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Bera S & Mondal D Chapter 7 - Stimuli-sensitive nanomaterials for antimicrobial drug delivery (ed. Grumezescu AMB T. T-D and S. S S. DD) 271–302 (William Andrew Publishing, 2018). [Google Scholar]
- 45.Chen M et al. Bacterial biofilm destruction by size/surface charge-adaptive micelles. Nanoscale 11, 1410–1422 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Chen W et al. Bacterial acidity-triggered antimicrobial activity of self-assembling peptide nanofibers. J. Mater. Chem. B 7, 2915–2919 (2019). [Google Scholar]
- 47.Pelgrift RY & Friedman AJ Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev 65, 1803–1815 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Rabin N et al. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem 7, 493–512 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Daddi Oubekka S, Briandet R, Fontaine-Aupart M-P & Steenkeste K Correlative time-resolved fluorescence microscopy to assess antibiotic diffusion-reaction in biofilms. Antimicrob. Agents Chemother 56, 3349–3358 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO & Piddock LJV Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol 13, 42–51 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Huang CM et al. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 32, 214–221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta A et al. Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Futur. 1, 015004 (2017). [Google Scholar]
- 53.Gupta D, Singh A & Khan AU Nanoparticles as Efflux Pump and Biofilm Inhibitor to Rejuvenate Bactericidal Effect of Conventional Antibiotics. Nanoscale Res. Lett 12, 454 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J, Hahn JS, Franklin MJ, Stewart PS & Yoon J Tolerance of dormant and active cells in Pseudomonas aeruginosa PA01 biofilm to antimicrobial agents. J. Antimicrob. Chemother 63, 129–135 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Hurdle JG, O’Neill AJ, Chopra I & Lee RE Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol 9, 62–75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tseng BS et al. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ. Microbiol 15, 2865–2878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Del Pozo JL & Patel R The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther 82, 204–209 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Gupta A, Mumtaz S, Li C-H, Hussain I & Rotello VM Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev 48, 415–427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bush K Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr. Opin. Microbiol 13, 558–564 (2010) [DOI] [PubMed] [Google Scholar]
- 60.Bush K Antimicrobial agents targeting bacterial cell walls and cell membranes. Rev. Sci. Tech 31, 43–56 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Kapoor G, Saigal S & Elongavan A Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol 33, 300–305 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta A et al. Engineered polymer nanoparticles with unprecedented antimicrobial efficacy and therapeutic indices against multidrug-resistant bacteria and biofilms. J. Am. Chem. Soc 140, 12137–12143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam SJ et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol 1, 16162 (2016). [DOI] [PubMed] [Google Scholar]; This study used in vitro and in vivo models to demonstrate the potential of structurally nanoengineered antimicrobial peptide polymers with multimodal antimicrobial mechanisms to treat infections caused by Gram-negative bacteria.
- 64.Wang X, Liu X & Han H Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surfaces B Biointerfaces 103, 136–142 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Courvalin P Vancomycin resistance in Gram-positive cocci. Clin. Infect. Dis 42 Suppl 1, S25–34 (2006) [DOI] [PubMed] [Google Scholar]
- 66.Falagas ME, Rafailidis PI & Matthaiou DK Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother 13, 132–138 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Peterson E & Kaur P Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol 9, 2928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsuzaki K Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta - Biomembr 1788, 1687–1692 (2009). [DOI] [PubMed] [Google Scholar]
- 69.El Badawy AM et al. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol 45, 283–287 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Huo S et al. Fully zwitterionic nanoparticle antimicrobial agents through tuning of core size and ligand structure. ACS Nano 10, 8732–8737 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nederberg F et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem 3, 409–414 (2011). [DOI] [PubMed] [Google Scholar]; This study reports a biodegradable antimicrobial polymeric nanoparticle that disrupts cell membranes of methicillin-resistant S. aureus at concentrations that do not lyse mammalian cells.
- 72.Wang X, Liu X & Han H Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surfaces B Biointerfaces 103, 136–142 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Landis RF et al. Biodegradable nanocomposite antimicrobials for the eradication of multidrug-resistant bacterial biofilms without accumulated resistance. J. Am. Chem. Soc 140, 6176–6182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates the use of polymers for delivery of antimicrobial essential oils resulting in biodegradable nanocomposites that eliminate bacterial biofilms without toxicity towards fibroblast cells and with no observed resistance development after multiple serial passages.
- 74.Memar MY, Ghotaslou R, Samiei M & Adibkia K Antimicrobial use of reactive oxygen therapy: Current insights. Infect. Drug Resist 11, 567–576 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh R, Smitha MS & Singh SP The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol 14, 4745–4756 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Slavin YN, Asnis J, Häfeli UO & Bach H Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 15, 65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pramanik A, Laha D, Bhattacharya D, Pramanik P & Karmakar P A novel study of antibacterial activity of copper iodide nanoparticle mediated by DNA and membrane damage. Colloids Surf. B Biointerfaces 96, 50–55 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Matai I et al. Antibacterial activity and mechanism of Ag–ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surfaces B Biointerfaces 115, 359–367 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Lopez N & Nørskov JK Catalytic CO oxidation by a gold nanoparticle: A density functional study. J. Am. Chem. Soc 124, 11262–11263 (2002). [DOI] [PubMed] [Google Scholar]
- 80.Bernardos A et al. Mesoporous Silica-Based Materials with Bactericidal Properties. Small 15, 1900669 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Tao Y, Ju E, Ren J & Qu X Bifunctionalized mesoporous silica-supported gold nanoparticles: Intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv. Mater 27, 1097–1104 (2015). [DOI] [PubMed] [Google Scholar]; This study shows broad-spectrum antibacterial and anti-biofilm properties of mesoporous-silica supported gold nanoparticles which mimic the catalytic activities of oxidase and peroxidase.
- 82.Shamaila S et al. Gold nanoparticles: An efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials 6, 1–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chatterjee AK, Chakraborty R & Basu T Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 25, (2014). [DOI] [PubMed] [Google Scholar]
- 84.Zhao Y et al. Small molecule-capped gold nanoparticles as potent antibacterial agents that target Gram-negative bacteria. J. Am. Chem. Soc 132, 12349–12356 (2010). [DOI] [PubMed] [Google Scholar]; This article demonstrates the use of pyrimidine-capped gold nanoparticles as antibacterial agents to disrupt bacterial cell membranes, interact with DNA and inhibit protein synthesis, ultimately leading to bacteria cell death.
- 85.Ashmore D et al. Evaluation of E. coli inhibition by plain and polymer-coated silver nanoparticles. Rev. Inst. Med. Trop. Sao Paulo 60, e18–e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ventola CL Progress in nanomedicine: Approved and investigational nanodrugs. P&T 42, 742–755 (2017). [PMC free article] [PubMed] [Google Scholar]
- 87.Mu H et al. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci. Rep 6, 18877 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdelghany SM et al. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomedicine 7, 4053–4063 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown AN et al. Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol 78, 2768–2774 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li C-H et al. Phytochemical-based nanocomposites for the treatment of bacterial biofilms. ACS Infect. Dis 5, 1590–1596 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y et al. Antibiotic-loaded, silver core-embedded mesoporous silica nanovehicles as a synergistic antibacterial agent for the treatment of drug-resistant infections. Biomaterials 101, 207–216 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Canaparo R et al. Recent developments in antibacterial therapy: Focus on stimuli-responsive drug-delivery systems and therapeutic nanoparticles. Molecules 24, 1991 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Radovic-Moreno AF et al. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano 6, 4279–4287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work shows delivery of vancomycin using a nanoparticle-based strategy that takes advantage of localized acidity at the bacterial infection site, with the drug carrier switching to a positively charged nanoparticle at low pH.
- 94.Wu Y, Song Z, Wang H & Han H Endogenous stimulus-powered antibiotic release from nanoreactors for a combination therapy of bacterial infections. Nat. Commun 10, 4464 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Casciaro B et al. Poly(lactide-co-glycolide) Nanoparticles for prolonged therapeutic efficacy of esculentin-1a-derived antimicrobial peptides against Pseudomonas aeruginosa lung infection: in vitro and in vivo studies. Biomacromolecules 20, 1876–1888 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Yeom JH et al. Gold nanoparticle-DNA aptamer conjugate-assisted delivery of antimicrobial peptide effectively eliminates intracellular Salmonella enterica serovar Typhimurium. Biomaterials 104, 43–51 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Yang S et al. Bacteria-targeting nanoparticles with microenvironment-responsive antibiotic release to eliminate intracellular Staphylococcus aureus and associated infection. ACS Appl. Mater. Interfaces 10, 14299–14311 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Li J et al. Block copolymer nanoparticles remove biofilms of drug-resistant Gram-positive bacteria by nanoscale bacterial debridement. Nano Lett. 18, 4180–4187 (2018). [DOI] [PubMed] [Google Scholar]; This work utilizes the block copolymer DA95B5 as a potential treatment for wound biofilms; DA95B5 diffuses across biofilm matrix matrices and promotes bacterial dispersal resulting in biofilm elimination without apparent emergence of resistance.
- 99.Liu Y et al. Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat. Commun 9, 2920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows the potential of ferumoxytol nanoparticles, which generate free radicals from H2O2, as a topical oral treatment for tooth decay, caused by oral biofilms, using ex vivo and in vivo models.
- 100.Mulani MS, Kamble EE, Kumkar SN, Tawre MS & Pardesi KR Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol 10, 539 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamaruzzaman NF et al. Antimicrobial polymers: The potential replacement of existing antibiotics? Int. J. Mol. Sci 20, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang L, Lin J, Taggart CC, Bengoechea JA & Scott CJ Nanodelivery strategies for the treatment of multidrug-resistant bacterial infections. J. Interdiscip. Nanomedicine 3, 111–121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupta A et al. Functionalized Polymers Enhance Permeability of Antibiotics in Gram-Negative MDR Bacteria and Biofilms for Synergistic Antimicrobial Therapy. Adv. Ther n/a, 2000005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pushparaj Selvadoss P, Nellore J, Balaraman Ravindrran M & Sekar U Novel pyochelin-based PEGylated liposomes for enhanced delivery of antibiotics against resistant clinical isolates of Pseudomonas aeruginosa. Artif. Cells, Nanomedicine, Biotechnol 46, 2043–2053 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Hsu C-Y, Yang S-C, Sung CT, Weng Y-H & Fang J-Y Anti-MRSA malleable liposomes carrying chloramphenicol for ameliorating hair follicle targeting. Int. J. Nanomedicine 12, 8227–8238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singla S, Harjai K, Katare OP & Chhibber S Encapsulation of bacteriophage in liposome accentuates its entry in to macrophage and shields it from neutralizing antibodies. PLoS One 11, e0153777–e0153777 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Campoy E & Colombo MI Autophagy in intracellular bacterial infection. Biochimica et Biophysica Acta - Molecular Cell Research (2009). [DOI] [PubMed] [Google Scholar]
- 108.Eng SK et al. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci 8, 284–293 (2015). [Google Scholar]
- 109.Ibarra JA & Steele-Mortimer O Salmonella - the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cellular Microbiology (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamaruzzaman NF, Kendall S & Good L Targeting the hard to reach: challenges and novel strategies in the treatment of intracellular bacterial infections. Br. J. Pharmacol 174, 2225–2236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xie S et al. Enhanced intracellular delivery and antibacterial efficacy of enrofloxacin-loaded docosanoic acid solid lipid nanoparticles against intracellular Salmonella. Sci. Rep (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Menina S et al. Bioinspired Liposomes for oral delivery of colistin to combat intracellular infections by Salmonella enterica. Adv. Healthc. Mater 8, e1900564 (2019). [DOI] [PubMed] [Google Scholar]
- 113.Russell DG Mycobacterium tuberculosis: Here today, and here tomorrow. Nature Reviews Molecular Cell Biology (2001) doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- 114.Yang C et al. Broad-spectrum antimicrobial star polycarbonates functionalized with mannose for targeting bacteria residing inside immune cells. Adv. Healthc. Mater 5, 1272–1281 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Ellis T et al. Multimerallic microparticles increase the potenct of rifampicin against intracellular Mycobacterium tuberculosis. ACS Nano 12, 5228–5240 (2018). [DOI] [PubMed] [Google Scholar]
- 116.Lee B et al. Antimicrobial peptide-loaded gold nanoparticle-DNA aptamer conjugates as highly effective antibacterial therapeutics against Vibrio vulnificus. Sci. Rep 7, 13572 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel R Biofilms and antimicrobial resistance. Clin. Orthop. Relat. Res 437, 41–47 (2005). [DOI] [PubMed] [Google Scholar]
- 118.Flemming H-C et al. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol 14, 563–575 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Teirlinck E, Samal SK, Coenye T & Braeckmans K Chapter 3 - Penetrating the bacterial biofilm: Challenges for antimicrobial treatment. Micro and Nano Technologies (eds. Boukherroub R, Szunerits S & Drider DB T.-F. N for the M. of M. I.) 49–76 (Elsevier, 2017). [Google Scholar]
- 120.Fulaz S, Vitale S, Quinn L & Casey E Nanoparticle–biofilm interactions: The role of the EPS matrix. Trends Microbiol. 27, 915–926 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Peulen TO & Wilkinson KJ Diffusion of nanoparticles in a biofilm. Environ. Sci. Technol 45, 3367–3373 (2011). [DOI] [PubMed] [Google Scholar]
- 122.Forier K et al. Probing the size limit for nanomedicine penetration into Burkholderia multivorans and Pseudomonas aeruginosa biofilms. J. Control. Release 195, 21–28 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Liu L et al. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol 4, 457–463 (2009). [DOI] [PubMed] [Google Scholar]
- 124.Ikuma K, Decho AW & Lau BLT When nanoparticles meet biofilms—interactions guiding the environmental fate and accumulation of nanoparticles. Front. Microbiol 6, 591 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Petros RA & Desimone JM Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov 9, 615–627 (2010). [DOI] [PubMed] [Google Scholar]
- 126.Simon-Deckers A et al. Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ. Sci. Technol 43, 8423–8429 (2009). [DOI] [PubMed] [Google Scholar]
- 127.Hayden SC et al. Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J. Am. Chem. Soc 134, 6920–6923 (2012). [DOI] [PubMed] [Google Scholar]
- 128.Morrow JB, P. CA & Holbrook RD Association of quantum dot nanoparticles with Pseudomonas aeruginosa biofilm. J. Environ. Qual 39, 1934–1941 (2010). [DOI] [PubMed] [Google Scholar]
- 129.Nevius BA, Chen YP, Ferry JL & Decho AW Surface-functionalization effects on uptake of fluorescent polystyrene nanoparticles by model biofilms. Ecotoxicology 21, 2205–2213 (2012). [DOI] [PubMed] [Google Scholar]
- 130.Wu J et al. Responsive assembly of silver nanoclusters with a biofilm locally amplified bactericidal effect to enhance treatments against multi-drug-resistant bacterial infections. ACS Cent. Sci 5, 1366–1376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study illustrates that by utilizing the acidic pH of biofilms, nanoantibiotic rAgNAs can penetrate and eliminate MRSA biofilms in vitro and in vivo.
- 131.Li X et al. Control of nanoparticle penetration into biofilms through surface design. Chem. Commun 51, 282–285 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports the biofilm penetration profile of nanoparticles using quantum dots with different surface chemical properties.
- 132.Pal S, Tak YK & Song JM Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol 73, 1712 LP–1720 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Slomberg DL et al. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl. Mater. Interfaces 5, 9322–9329 (2013). [DOI] [PubMed] [Google Scholar]
- 134.Mahmoudi M & Serpooshan V Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. ACS Nano 6, 2656–2664 (2012). [DOI] [PubMed] [Google Scholar]
- 135.Meers P et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother 61, 859–868 (2008). [DOI] [PubMed] [Google Scholar]
- 136.Koo H, Allan RN, Howlin RP, Stoodley P & Hall-Stoodley L Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol 15, 740–755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baelo A et al. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J. Control. Release 209, 150–158 (2015). [DOI] [PubMed] [Google Scholar]
- 138.Habimana O et al. One particle, two targets: A combined action of functionalised gold nanoparticles, against Pseudomonas fluorescens biofilms. J. Colloid Interface Sci 526, 419–428 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Li J et al. A new tool to attack biofilms: Driving magnetic iron-oxide nanoparticles to disrupt the matrix. Nanoscale 11, 6905–6915 (2019). [DOI] [PubMed] [Google Scholar]
- 140.Singh BN et al. Bactericidal, quorum quenching and anti-biofilm nanofactories: a new niche for nanotechnologists. Crit. Rev. Biotechnol 37, 525–540 (2017). [DOI] [PubMed] [Google Scholar]
- 141.Miller KP et al. Engineering nanoparticles to silence bacterial communication. Front. Microbiol 6, 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This proof-of-concept work demonstrates the potential to silence bacterial communication as a potential therapeutic strategy.
- 142.Ilk S, Sağlam N, Özgen M & Korkusuz F Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int. J. Biol. Macromol 94, 653–662 (2017). [DOI] [PubMed] [Google Scholar]
- 143.Al-Shabib NA et al. Biogenic synthesis of zinc oxide nanostructures from Nigella sativa seed: Prospective role as food packaging material inhibiting broad-spectrum quorum sensing and biofilm. Sci. Rep 6, 36761 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gómez-Gómez B et al. Selenium and tellurium-based nanoparticles as interfering factors in quorum sensing-regulated processes: violacein production and bacterial biofilm formation. Metallomics 11, 1104–1114 (2019). [DOI] [PubMed] [Google Scholar]
- 145.Barbosa DB et al. The importance of preventing and controlling biofilm in wounds: Biofilm models and nanotechnology in antibiofilm approaches. Wound Healing Biomaterials 2, (Elsevier Ltd, 2016). [Google Scholar]
- 146.Wi YM & Patel R Understanding biofilms and novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect. Dis. Clin. North Am 32, 915–929 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bowen WH, Burne RA, Wu H & Koo H Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26, 229–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Allaker RP & Yuan Z Nanoparticles and the control of oral biofilms. Nanobiomaterials in Clinical Dentistry (Elsevier Inc., 2019). [Google Scholar]
- 149.Zhou Z et al. pH-Activated nanoparticles with targeting for the treatment of oral plaque biofilm. J. Mater. Chem. B 6, 586–592 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Zhou J et al. Characterization and optimization of pH-responsive polymer nanoparticles for drug delivery to oral biofilms. J. Mater. Chem. B 4, 3075–3085 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao Z, Ding C, Wang Y, Tan H & Li J pH-Responsive polymeric nanocarriers for efficient killing of cariogenic bacteria in biofilms. Biomater. Sci 7, 1643–1651 (2019). [DOI] [PubMed] [Google Scholar]
- 152.Gao L et al. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 101, 272–284 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Naha PC et al. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano 13, 4960–4971 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dai X et al. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 9, e368–e368 (2017). [Google Scholar]
- 155.Lantis J & Paredes J Permissive maintenance debridement -- the role of enzymatic debridement in chronic wound care. Wounds Int. 8, 7–13 (2017). [Google Scholar]
- 156.Omar A, Wright BJ, Schultz G, Burrell R & Nadworny P Microbial biofilms and chronic wounds. Microorganisms 5, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilkinson LJ, White RJ & Chipman JK Silver and nanoparticles of silver in wound dressings: a review of efficacy and safety. J. Wound Care 20, 543–549 (2011). [DOI] [PubMed] [Google Scholar]
- 158.Kim M Nanoparticle-based therapies for wound biofilm infection: Opportunities and challenges. IEEE Trans. Nanobioscience 15, 294–306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Parani M, Lokhande G, Singh A & Gaharwar AK Engineered nanomaterials for infection control and healing acute and chronic wounds. ACS Appl. Mater. Interfaces 8, 10049–10069 (2016). [DOI] [PubMed] [Google Scholar]
- 160.Ahire JJ, Hattingh M, Neveling DP & Dicks LMT Copper-containing anti-biofilm nanofiber scaffolds as a wound dressing material. PLoS ONE 11, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang J et al. pH-Switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds. ACS Nano 13, 11686–11697 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Caster JM, Patel AN, Zhang T & Wang A Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 9, e1416 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Bjarnsholt T et al. The in vivo biofilm. Trends Microbiol. 21, 466–474 (2013). [DOI] [PubMed] [Google Scholar]
- 164.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01315691 (2018). [DOI] [PubMed]
- 165.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02104245 (2018). [DOI] [PubMed]
- 166.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/results/NCT00659204 (2008). [DOI] [PubMed]
- 167.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00337714 (2011). [DOI] [PubMed]
- 168.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02726646 (2018). [DOI] [PubMed]
- 169.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01167985 (2013). [DOI] [PubMed]


