Abstract
In Saccharomyces cerevisiae, replication origins are activated with characteristic timing during S phase. S-phase cyclin-dependent kinases (S-CDKs) and Cdc7p-Dbf4p kinase are required for origin activation throughout S phase. The activation of S-CDKs leads to association of Cdc45p with chromatin, raising the possibility that Cdc45p defines the assembly of a new complex at each origin. Here we show that both Cdc45p and replication protein A (RPA) bind to Mcm2p at the G1-S transition in an S-CDK-dependent manner. During S phase, Cdc45p associates with different replication origins at specific times. The origin associations of Cdc45p and RPA are mutually dependent, and both S-CDKs and Cdc7p-Dbf4p are required for efficient binding of Cdc45p to origins. These findings suggest that S-CDKs and Cdc7p-Dbf4p promote loading of Cdc45p and RPA onto a preformed prereplication complex at each origin with preprogrammed timing. The ARS1 association of Mcm2p, but not that of the origin recognition complex, is diminished by disruption of the B2 element of ARS1, a potential origin DNA-unwinding element. Cdc45p is required for recruiting DNA polymerase α onto chromatin, and it associates with Mcm2p, RPA, and DNA polymerase ɛ only during S phase. These results suggest that the complex containing Cdc45p, RPA, and MCMs is involved in origin unwinding and assembly of replication forks at each origin.
In eukaryotic cells, DNA replication initiates from multiple origins scattered along chromosomes, making duplication of large genomes within a single cell cycle possible. Every replication origin fires once and only once with a characteristic timing during the S phase. Although the cell-cycle control of DNA replication has been gradually unveiled, how DNA replication is initiated at chromosomal origins remains largely unknown (reviewed in references 16, 21, 46, and 57).
Studies of Saccharomyces cerevisiae and other organisms have revealed that a prereplication complex (pre-RC) is assembled at each origin during late mitosis or early G1 phase, the period when cyclin-dependent kinases (CDKs) are inactive (11, 12, 15, 40, 51, 58, 59). The origin recognition complex (ORC), Cdc6p, and the six minichromosome maintenance (MCM) proteins Mcm2p to -7p are present in the pre-RC (2, 19, 40, 55, 59, 63). To start DNA synthesis, S-phase CDKs (S-CDKs) and Cdc7p-Dbf4p kinase need to be activated at the G1-S transition. Upon activation of these kinases, the pre-RC undergoes a transformation, and it is eventually converted to the post-RC and replication forks when initiation of DNA replication is completed (16, 21, 46, 57). To understand how DNA replication is initiated at chromosomal origins, we sought to uncover how the complexes at origins change during initiation and how they interact with origins. Furthermore, we pursued the mechanisms by which the activating kinases are involved in this transition.
In the well-characterized simian virus 40 (SV40) system, large T antigen is the origin recognition protein and also the helicase. Initiation of DNA replication from the SV40 origin begins with local unwinding of the origin by cooperative action of large T antigen and replication protein A (RPA; reviewed in references 29 and 62). Subsequently, DNA polymerase α-primase is recruited to the unwound origin to synthesize the first Okazaki fragment, and then the DNA polymerase δ complex is loaded by a polymerase switching mechanism (62). Unlike the SV40 large T antigen, yeast ORC is not a helicase. Instead, as indicated by amino acid sequence composition and a study of three of the six human MCM proteins (32, 38), yeast MCMs might function as a helicase. The MCM proteins, but not RPA, are present at origins during G1, and no origin firing can be detected until S-CDKs and Cdc7p-Dbf4p are activated at the G1-S transition. Three recent studies have suggested that Cdc45p and RPA might mediate the activation of origins by the kinases (45, 60, 67). Cdc45p, a protein essential for initiation of DNA replication (26, 30, 49, 66), binds to MCMs and chromatin upon the activation of S-CDKs (45, 67). Association of RPA with origins depends on both S-CDKs and Dbf4p-dependent kinase (60). Moreover, Cdc45p is required for recruiting DNA polymerase α (Polα) onto chromatin in Xenopus (45), and RPA is needed to recruit primase to origins in yeast (60).
The architecture of the complex at origins remains largely unknown. With the exception of the ORC, little is known about how the proteins at origins interact with origin DNA. A single leading-strand start site was recently identified at the chromosomal autonomously replicating sequence 1, (ARS1), a well-characterized yeast replication origin (6). This site is located between the binding site of the ORC (the A and B1 elements of ARS1 [5, 52, 54]) and a potential DNA-unwinding element (the B2 element of ARS1 [41]). It is likely that this site coincides with the site of initial unwinding at ARS1 and/or the entry site for the replication machinery. However, the proteins associating with this region of ARS1 have not been identified.
In this study, we show that Cdc45p, RPA, and Mcm2p start to associate with each other at the onset of S phase. Cdc45p associates with different replication origins at specific times during S phase, and the origin associations of Cdc45p and RPA are mutually dependent. Both S-CDKs and Cdc7p-Dbf4p are required for efficient binding of Cdc45p to replication origins. Together, these findings suggest that S-CDKs and Cdc7p-Dbf4p promote formation of a complex containing Cdc45p, RPA, and the MCM proteins at each origin with preprogrammed timing. Association of Mcm2p with ARS1 requires the potential unwinding element B2. Cdc45p is required for loading Polα onto chromatin, and it associates with DNA polymerase ɛ (Polɛ) during S phase. We propose, therefore, that Cdc45p, RPA, and the MCM proteins collaborate to unwind origins and recruit other proteins to assemble replication elongation complexes.
MATERIALS AND METHODS
Yeast strains and methods.
All the yeast strains used in this study are listed in Table 1. The Cdc45HA3p and Cdc45myc3p in the corresponding strains were expressed from their endogenous promoters at the original chromosomal location and can support normal cell growth. The integrated DBF4MYC18 in YB0552 was derived from the strain K6388 provided by K. Nasmyth (60). The GAL1-SIC1ΔNT in YB0553 was derived from YCS12 provided by J. Diffley (14). The integrated POLɛHA3 in YB0550 and YB0551 was derived from OAy618 provided by S. Bell (2). The integrated ARS1 mutations were derived from the corresponding strains published by Marahrens and Stillman (44). In all the experiments involving temperature-sensitive mutant strains, α-factor arrest was performed at 25°C for 3 h. In the experiments using only non-temperature-sensitive strains (including the cold-sensitive cdc45-1 strain), cells were arrested in α-factor at 30°C for 3 h. Expression of Sic1ΔNTp was either induced in yeast extract-peptone medium with 2% galactose or remained uninduced in 2% raffinose.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| W303-1a | MATa ade2-1 ura3-1 his3-1 trp1-1 leu2-3,112 can1-100 | R. Rothstein |
| YB0298 | W303-1a; cdc45-1 | This laboratory |
| YB0556 | W303-1a; cdc7-1 | This work |
| YB0557 | W303-1a; cdc7-4 | This work |
| YB0349 | W303-1a; dbf4-1 | This laboratory |
| YB0469 | W303-1a; cdc45::CDC45-HA3-LEU2 | This laboratory |
| YB0476 | MATα mcm2-1 cdc45::CDC45-HA3-LEU2 | This laboratory |
| YB0549 | MATa rfa2-2 cdc45::CDC45-HA3-LEU2 | This work |
| YB0472 | W303-1a; cdc7-1 cdc45::CDC45-HA3-LEU2 | This laboratory |
| YB0547 | W303-1a; cdc7-4 cdc45::CDC45-HA3-LEU2 | This work |
| YB0548 | W303-1a; dbf4-1 cdc45::CDC45-HA3-LEU2 | This work |
| YB0576 | MATa ars1T860G cdc45::CDC45-HA3-LEU2 | This work |
| YB0577 | MATa ars1::835-842 cdc45::CDC45-HA3-LEU2 | This work |
| YB0578 | MATa ars1::798-805 cdc45::CDC45-HA3-LEU2 | This work |
| YB0579 | MATa ars1::756,758 cdc45::CDC45-HA3-LEU2 | This work |
| YB0553 | MATa ura3::GAL1-SIC1ΔNT-URA3 cdc45::CDC45-HA3-LEU2 | This work |
| YB0477 | W303-1a; clb5::URA3 clb6::LEU2 cdc45::CDC45-HA3-LEU2 | This laboratory |
| OAy618 | W303-1a; polɛ::POLɛ-HA3-LEU2 | S. Bell |
| YB0550 | W303-1a; cdc45::CDC45-MYC3-LEU2 polɛ::POLɛ-HA3-LEU2 | This work |
| YB0551 | MATa mcm2-1 cdc45::CDC45-MYC3-LEU2 polɛ::POLɛ-HA3-LEU2 | This work |
| K6388 | W303-1a; leu2::DBF4-MYC18-LEU2 | K. Nasmyth |
| YB0552 | W3031a; cdc45-1 leu2::DBF4-MYC18-LEU2 | This work |
Chromatin immunoprecipitations.
Chromatin immunoprecipitations were conducted essentially as previously described (2). Cdc45HA3p, Mcm2p, Orc2p, Orc3p, and RPA p70 were immunoprecipitated by the monoclonal antibodies 12CA5, Mcm2-49, SB3, SB46, and a polyclonal rabbit anti-serum against RPA p70, respectively. All the PCR products were between 200 and 350 bp in size, and were separated on 2.3% agarose gels. The sequences of PCR primers used in this study are available upon request.
Chromatin binding analysis.
Preparation and fractionation of cell lysates were performed as described previously (40) with several modifications. The incubation in prespheroplasting buffer was done on ice. Spheroplasts were washed three times with lysis buffer and lysed in the presence of 1% Triton X-100. The quality of fractionation was monitored by the separation of chromatin-bound Orc3p from the soluble protein cross-reacting with the SB3 antibody (anti-Orc3p [40]).
Immunoprecipitation and immunoblotting.
Immunoprecipitations were carried out essentially as described previously (30) except that 150 Kunitz units of DNase I (Sigma) was added to the whole-cell lysates. Cdc45myc3p, Mcm2p, and RPA p70 were immunoprecipitated with monoclonal antibodies 9E10, Mcm2-49, and rabbit anti-serum against RPA p70, respectively. The antibodies used for immunoblotting were monoclonal 12CA5 (for PolɛHA3p or Cdc45HA3p), 9E10 (for Cdc45myc3p or Dbf4myc3p), 6D2 (for Polα p86), Mcm2-28, SB3 (for Orc3p), and polyclonal antisera against RPA p70 and p34.
RESULTS
Cdc45p associates with Mcm2p, RPA, and Polɛ in a cell cycle-regulated manner.
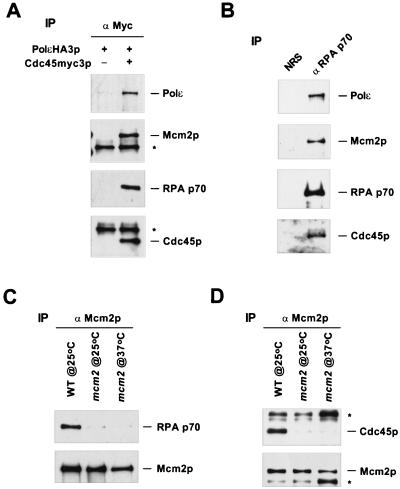
The binding of Cdc45p to chromatin and the association of Cdc45p with the MCM proteins indicate the transformation from the pre-RC to a preinitiation complex (pre-IC [67]). To test whether Cdc45p and MCMs were part of a larger complex, we immunoprecipitated Cdc45p and Mcm2p, respectively, and analyzed the proteins coprecipitated with them (Fig. 1). Cells were treated with hydroxyurea (HU) to slow down fork movement and prolong the period in which the potential complex might be present. Because the potential complex was likely chromatin bound, DNase I was used to release the possible interacting proteins from chromatin. Immunoprecipitation of either myc-tagged Cdc45p or intact Mcm2p specifically brought down the hemagglutinin (HA)-tagged Polɛ catalytic subunit and intact RPA p70, the largest subunit of RPA (Fig. 1A and C and data not shown). Similarly, precipitation of RPA p70 brought down Cdc45p, Mcm2p, and Polɛ (Fig. 1B). Furthermore, Cdc45p was detected in the Polɛ immunoprecipitate (data not shown). These interactions were also observed in the absence of HU (Fig. 2 and data not shown). It is unlikely that these interactions were mediated by chromatin because several other chromatin-bound proteins were not detected in the immunoprecipitates (data not shown). The network of interactions among Cdc45p, Mcm2p, RPA, and Polɛ suggest that these proteins are present in a complex or multiple complexes in vivo. It should be noted that only small fractions of Polɛ and RPA coprecipitated with Cdc45p in these experiments. This could be due to the transient and/or unstable nature of the complex or the inefficiency of the coimmunoprecipitation procedure. When Mcm2p was precipitated from an extract derived from mcm2-1 cells, hardly any RPA p70, Cdc45p, or Polɛ were brought down even at the permissive temperature (Fig. 1C and D and data not shown). Thus, the anti-Mcm2p antibody did not recognize RPA p70, Cdc45p, and Polɛ directly, and more importantly, functional Mcm2p was critical for the stability of the complex. Although an interaction between Cdc45p and Polα was reported in Xenopus (45), we were unable to detect Polα in the Cdc45p immunoprecipitate, perhaps because the interaction was very transient.
FIG. 1.
Cdc45p associates with Mcm2p, Polɛ, and RPA p70 in vivo. (A) Mcm2p, PolɛHA3p, and RPA p70 coprecipitate specifically with Cdc45myc3p. Cells expressing (+) both PolɛHA3p and Cdc45myc3p (YB0550) or only PolɛHA3p (OAY618) were arrested in S phase with 0.1 M HU for 2 h. Whole-cell extracts were prepared and subjected to immunoprecipitations (IP) with non-cross-linked anti-myc antibody. ∗, dimers of immunoglobulin G. (B) Mcm2p, PolɛHA3p and Cdc45myc3p coprecipitate specifically with RPA p70. Whole-cell extract was prepared from S-phase wild-type (YB0550) cells. Immunoprecipitations were performed with either normal rabbit serum (NRS) or anti-RPA p70 antibody. (C) RPA p70 coprecipitates with Mcm2p only in wild-type but not in mcm2-1 cell extracts. Wild-type (YB0550) (WT) and mcm2-1 (YB0551) cells were arrested with HU for 2 h at the indicated temperatures. Immunoprecipitations were carried out with anti-Mcm2p antibody cross-linked to protein G. (D) Cdc45HA3p coprecipitates with Mcm2p only in wild-type but not in mcm2-1 cell extracts. Wild-type (YB0469) and mcm2-1 (YB0476) cells expressing Cdc45HA3p were arrested as in panel C, and immunoprecipitations were performed with non-cross-linked anti-Mcm2p antibody. Shown are immunoblots of the proteins present in the immunoprecipitation described above. The immunoblotting antibodies were against the proteins indicated at the right of each panel.
FIG. 2.
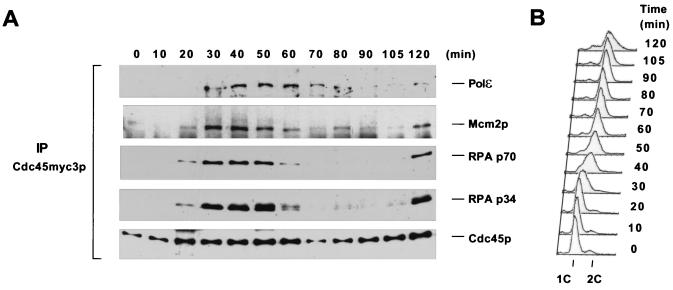
Cdc45p interacts with Mcm2p, Polɛ, RPA p70, and p34 in a cell cycle-dependent manner. (A) Immunoprecipitation (IP) of Cdc45myc3p across the cell cycle. Wild-type cells expressing both PolɛHA3p and Cdc45myc3p (YB0550) were synchronized in G1 with α-factor and then released into yeast-peptone-dextrose medium at 25°C. The cells were collected at the indicated time points. Whole-cell extracts were prepared and subjected to immunoprecipitations with anti-myc antibody. (B) DNA content of the time point samples used in panel A.
To determine how the interactions among Cdc45p, Mcm2p, RPA, and Polɛ were regulated during the cell cycle, we monitored these interactions across a complete cell cycle. Cells expressing both Cdc45myc3p and PolɛHA3p were synchronously released from an α-factor block, and Cdc45myc3p was immunoprecipitated at 10- or 15-min intervals (Fig. 2A and B). All the proteins examined in this experiment, including Cdc45p, Mcm2p, Polɛ, and both the p70 and p34 subunits of RPA, were present at roughly constant levels throughout the cell cycle (data not shown). Cdc45myc3p was precipitated throughout the cell cycle, with a modest increase in S phase. Mcm2p and both the p70 and p34 subunits of RPA coprecipitated with Cdc45p during early S phase, whereas Polɛ was brought down throughout S phase. Importantly, both the Cdc45p-Mcm2p and the Cdc45p-RPA interactions were established 20 to 30 min after release, concomitant with the onset of S phase. A slower-migrating form of RPA p34 was observed when cells reached early S phase, indicating the cell cycle-regulated phosphorylation of this protein. Nevertheless, both forms of RPA p34 were brought down by Cdc45p, suggesting that the phosphorylation of RPA p34 may not affect its association with Cdc45p. Mcm2p, Polɛ, and RPA p70 and p34 reappeared in the Cdc45p immunoprecipitate 120 min after release. It is likely that some cells had started the second S phase by that time.
Association of Mcm2p with Cdc45p and RPA requires active S-CDKs.
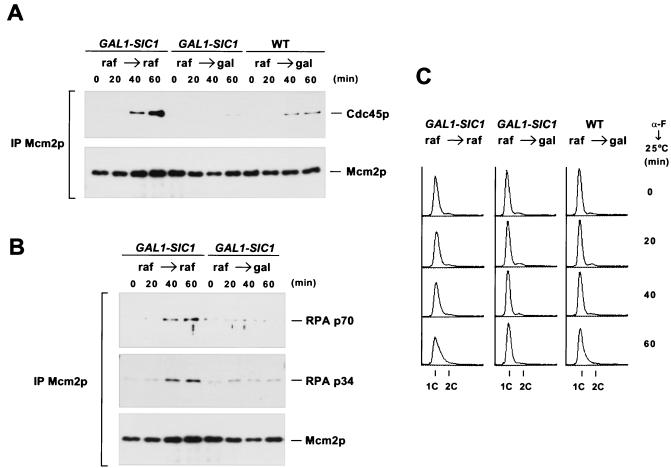
Association of Cdc45p with chromatin requires activation of S-CDKs, indicating that S-CDKs regulate a key step of the assembly of the pre-IC (67). To test directly whether S-CDK activity was required for establishing the complex containing Cdc45p, RPA, and Mcm2p, we examined the effect of overexpression of Sic1p, an S-phase CDK inhibitor, on the interactions among these three proteins. Sic1ΔNTp, a mutant of Sic1p that lacks the N-terminal 50 amino acids, is more stable than the wild-type Sic1p and is functional in inhibiting S-phase CDKs (14). Cells overexpressing Sic1ΔNTp from the GAL1,10 promoter were synchronized in G1 with α-factor and were released into either raffinose- or galactose-containing medium (Fig. 3). In raffinose-containing medium, Cdc45p and both p70 and p34 of RPA coprecipitated with Mcm2p as cells entering S phase (Fig. 3). In contrast, cells were stalled at the G1-S boundary in galactose, and neither Cdc45p nor RPA coprecipitated with Mcm2p. Thus, Cdc45p and RPA could not interact with Mcm2p when S-CDKs were inactivated by overexpression of Sic1ΔNTp.
FIG. 3.
S-CDK activity is required for the interactions among Cdc45p, Mcm2p, and RPA. (A) Overexpression of Sic1ΔNTp prevents binding of Cdc45p to Mcm2p. Cells bearing GAL1-SIC1ΔNT (YB0553) were arrested with α-factor in raffinose (raf)-containing medium and then released into medium containing either raffinose or galactose (gal) at 25°C. As a control, wild-type (WT) cells (YB0469) that do not carry GAL1-SIC1ΔNT were also synchronized with α-factor in raffinose and released into galactose. Whole-cell extracts were prepared at the indicated time points and were immunoprecipitated with anti-Mcm2p antibody. Cdc45HA3p coprecipitated with Mcm2p was analyzed by immunoblotting. (B) Overexpression of Sic1ΔNTp prevents binding of RPA p70 and p34 to Mcm2p. RPA p70 and p34 coprecipitated with Mcm2p in panel A were analyzed by immunoblotting. (C) DNA content of the samples used in panels A and B.
Cdc45p and Mcm2p associate differently with replication origins.
To analyze the distribution of the pre-IC on chromatin, we carried out a chromatin immunoprecipitation (CHIP) assay on Cdc45p. In this assay, proteins were cross-linked in vivo to chromatin with formaldehyde, the chromatin was then sheared, and the bound Cdc45p was immunoprecipitated. To detect DNA sequences that were associated with Cdc45p, specific primer pairs were used to analyze coprecipitated DNA by PCR.
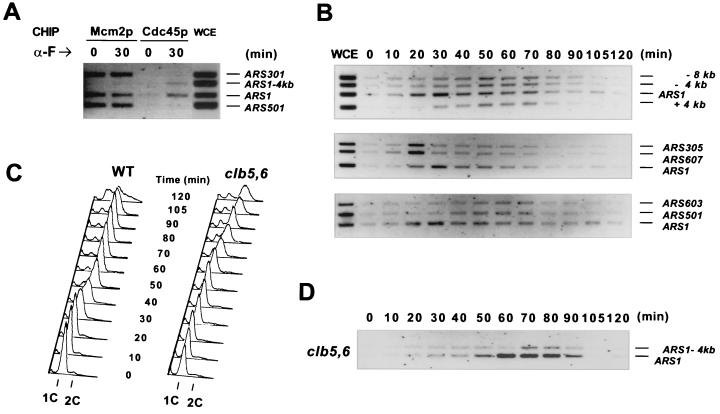
A recent study using the CHIP assay suggested that Cdc45p was a component of the pre-RC in G1 (2). However, we were unable to detect association of Cdc45p with chromatin or with MCMs at Start in G1 (67). To determine whether Cdc45p was indeed a component of the pre-RC, we first compared the origin association of Cdc45p with that of Mcm2p, a known pre-RC component, by using CHIP assays. CHIP analyses of Cdc45HA3p and Mcm2p were performed in parallel with cells collected either at the α-factor block in G1 or at the beginning of S phase (30 min after release from the α-factor block). Coprecipitated DNA fragments were amplified for ARS1, ARS501, ARS301, and a nonorigin control region (Fig. 4A). Both ARS1 and ARS501 are active origins, but ARS1 fires early whereas ARS501 fires late in S phase (24). ARS301 only fires at low frequency (<15%) at its chromosomal location (20). No clear enrichment of Cdc45p was detected at these three origins in G1 (Fig. 4A). In early S phase, Cdc45p was enriched at ARS1 but not ARS301 or ARS501. On the other hand, Mcm2p was found to associate with ARS1, ARS301, and ARS501 in both G1 and early S phase (Fig. 4A). In addition, Mcm2p associated with all three origins equally well. Thus, the association of Mcm2p with origins was temporally different from that of Cdc45p. Unlike Cdc45p, Mcm2p binding did not distinguish early and late origins, nor did it differentiate between active and inactive origins. Nevertheless, Cdc45p and Mcm2p coexisted on ARS1 in early S phase, suggesting that they might be present in a complex at this origin during initiation.
FIG. 4.
Cell cycle regulation of association of Cdc45p with ARS-containing fragments. (A) Cdc45p and Mcm2p associate with origins differently in G1 and early S phase. Wild-type (WT) cells expressing Cdc45HA3p (YB0469) were arrested in G1 with α-factor and then released into yeast-peptone-dextrose medium at 25°C. The cells were collected at the α-factor block or 30 min after release. CHIP analyses of Mcm2p and Cdc45p were performed in parallel. WCE, input DNA prepared from the whole-cell extract; ARS1-4kb, a region 4 kb away from ARS1 towards the left telomere. (B) Association of Cdc45p with different origins during the cell cycle. Wild-type cells were synchronized in G1 with α-factor and then released into YPD at 25°C. CHIP analysis of Cdc45p was performed at each time point. +4 kb and −8 kb, regions on both sides of ARS1 that are 4 or 8 kb away. (C) DNA content of the time point samples used in panels B and D. (D) Association of Cdc45p with ARS1 is delayed in the absence of CLB5 and CLB6. YB0477 (clb5,6Δ and CDC45HA3) cells were released from an α-factor block at 25°C. CHIP analysis of Cdc45p was performed as described above.
Cdc45p associates with different replication origins in a temporally controlled manner.
To investigate the cell-cycle regulation of the association of Cdc45p with different origins, we carried out CHIP assays on Cdc45HA3p across the cell cycle. Wild-type cells were synchronized in G1 with α-factor and then released into the cell cycle (Fig. 4C). The DNA fragments coprecipitated with Cdc45p were analyzed by PCRs using four pairs of primers that amplified either ARS1 or one of the three flanking regions (Fig. 4B). In the α-factor-arrested cells, none of the four chromosomal loci was significantly enriched in the Cdc45p immunoprecipitate. Twenty minutes after release, Cdc45p began to associate with ARS1 but not the surrounding regions. The specific enrichment of Cdc45p at ARS1 was most evident 30 min after release, when the cells were clearly in early S phase. As the cells proceeded through S phase, the enrichment of Cdc45p at ARS1 declined. Simultaneously, Cdc45p became associated with the regions flanking ARS1. When S phase was complete in most cells, Cdc45p could no longer be detected at ARS1 and its flanking regions. Similar observations were also made in the cells synchronously released from a dbf2 block in mitosis (data not shown). These results show that Cdc45p specifically associates with ARS1 in early S phase rather than in G1 as suggested by the earlier study (2). However, our data agree with the observation that at least some Cdc45p may move away from origins after initiation (2).
Different replication origins fire at characteristic times during S phase (23). We therefore examined the association of Cdc45p with origins that were known to have different timing of initiation in each S phase. The DNA fragments coprecipitated with Cdc45p in the cell cycle CHIP experiment were amplified for ARS1, ARS305, and ARS607 (Fig. 4B). ARS305 and ARS607 are known to fire very early in S phase, even before initiation occurs at ARS1 (25, 53, 65). Unlike the earlier study (3), we did not observe clear enrichment of Cdc45p at the two early-firing origins in cells arrested with α-factor. Only 10 min after release, weak association of Cdc45p with ARS305 and ARS607 was detected. By 20 min, Cdc45p was clearly bound to the two early origins. Cdc45p bound to these two early origins approximately 5 to 10 min before it bound to ARS1, which correlated well with the timing of initiation at these origins (25, 53, 65). Similar analyses were also performed with two late origins, ARS501 and ARS603 (Fig. 4B). Cdc45p began to associate with these late origins 40 min after release, more than 10 min after it bound to ARS1. This is also in agreement with the timing of initiation at these loci (24, 25, 65). Persistent association of Cdc45p with ARS1 and late origins were observed even in late S phase. This is possibly due to the rapid loss of cell cycle synchrony after cells have entered S phase. Together, these results show that Cdc45p binds to early-firing origins early and late-firing origins late in S phase, suggesting that binding of Cdc45p to each origin is concomitant with initiation at that origin.
Association of Cdc45p with ARS1 requires S-CDK activity.
Binding of Cdc45p to chromatin is delayed in clb5,6Δ mutant cells due to the lack of S-phase cyclins (67). To test whether the specific association of Cdc45p with ARS1 was also postponed in the absence of CLB5 and CLB6, we performed CHIP analysis on Cdc45p in the clb5,6Δ cells synchronously released from an α-factor block in G1 (Fig. 4D). As indicated by the fluorescence-activated cell-sorting analyses, clb5,6Δ mutant cells entered S phase approximately 50 min after release, which was delayed by about 30 min relative to the wild type (Fig. 4C). Specific Cdc45p-ARS1 association was first detected 50 min after release, concomitant with the apparent G1-S transition (Fig. 4C and D). Compared with that in the wild-type cells, binding of Cdc45p to ARS1 in the clb5,6Δ cells was delayed for approximately 30 min. This is in good agreement with the delay of ARS1 firing in the same mutant (18). Because only the cell cycle events relying on S-CDKs are delayed in the clb5,6Δ mutant, this result shows that the specific association of Cdc45p with ARS1 requires S-CDK activity.
Association of Cdc45p with chromatin, Mcm2p, and ARS1 is affected in cdc7 and dbf4 mutants.
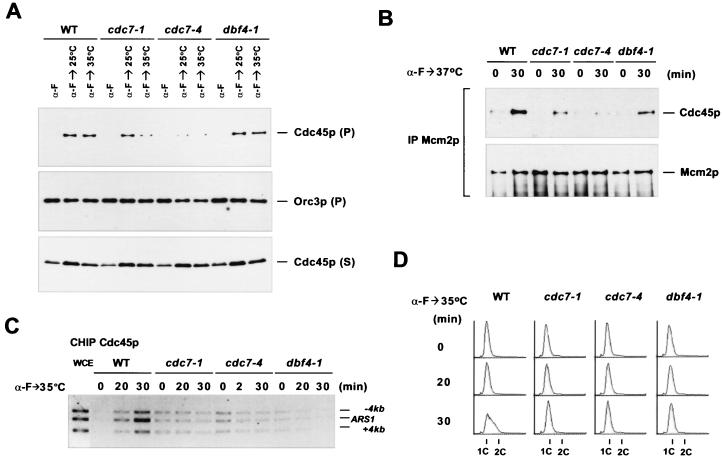
Significant amounts of Cdc45p were detected on chromatin in the cdc7-1 cells that were arrested by shifting asynchronously growing cultures to 37°C (67). However, two recent studies showed that Cdc7p-Dbf4p was important not only for entry into S phase but also for progression through S phase (7, 17). Therefore, inactivation of Cdc7p-Dbf4p in an asynchronous population might trap early S-phase cells in S phase, and the origins that had fired in these cells could be responsible for the detection of chromatin-bound Cdc45p. To assess this possibility, we examined the chromatin association of Cdc45p in the arrested cdc7-1, cdc7-4, and dbf4-1 cells that had been synchronized in G1 prior to the temperature shift (Fig. 5A). The mutants and the congenic wild-type cells were released from an α-factor block at either 25 or 35°C and were given 30 min to enter S phase or reach the arrest point at the G1-S boundary (data not shown). At 25°C, cdc7-4 cells entered S phase more slowly than wild-type, cdc7-1, and dbf4-1 cells. The amount of Cdc45p on chromatin in cdc7-4 cells was also smaller than that in the other three strains (Fig. 5A and data not shown). At 35°C, all three mutant strains were stalled at the G1-S boundary 30 min after release from α-factor (data not shown). Chromatin-bound Cdc45p was hardly detectable in cdc7-4 cells and was significantly reduced in cdc7-1 cells, but the reduction of chromatin-bound Cdc45p in dbf4-1 cells was very modest (Fig. 5A).
FIG. 5.
Associations of Cdc45p with chromatin, Mcm2p, and ARS1 are affected in cdc7 and dbf4 mutants. (A) Association of Cdc45p with chromatin is reduced in cdc7 and dbf4 mutants. Wild-type (YB0469) (WT), cdc7-1 (YB0472), cdc7-4 (YB0547), and dbf4-1 (YB0548) cells expressing Cdc45HA3p were synchronized with α-factor and released at either 25 or 35°C. The cells were collected at the α-factor block or 30 min after release. Lysates were prepared and subjected to chromatin fractionation. Immunoblots of Cdc45p and Orc3p in the chromatin sediments (P) and Cdc45p in the supernatants (S) are shown. (B) Cdc45p-Mcm2p interaction is reduced in cdc7 and dbf4 mutants at the nonpermissive temperature. Cells were arrested as in panel A and released at 37°C. Whole-cell extracts (WCE) were prepared from the cells collected at the α-factor block or 30 min after release and subjected to immunoprecipitations (IP) using an anti-Mcm2p antibody. (C) Association of Cdc45p with ARS1 is undetectable in cdc7-1, cdc7-4, and dbf4-1 cells. Wild-type and mutant cells were synchronized with α-factor as in panel A and released at 35°C. The cells were collected at the indicated time points and analyzed by Cdc45p CHIP. (D) DNA content of the samples used in panel C.
We also examined the Cdc45p-Mcm2p association in the arrested cdc7-1, cdc7-4, and dbf4-1 cells. Wild-type and mutant cells were first synchronized with α-factor and then released at 37°C so that all mutant cells were arrested at the G1-S boundary (Fig. 5B). Compared to that in the wild-type cells, the Cdc45p-Mcm2p interaction was reduced to various extents in the three mutants (Fig. 5B). cdc7-4, which entered S phase more slowly than the other strains even at 25°C, exhibited the most dramatic reduction of Cdc45p-Mcm2p interaction at 37°C. Overall, the reduction of Cdc45p-Mcm2p interaction in the cdc7 and dbf4 mutants correlated well with the reduction of chromatin-bound Cdc45p in these mutants (Fig. 5A and B and data not shown). These results suggest that Cdc7p-Dbf4p is important, if not essential, for the association of Cdc45p with Mcm2p and chromatin.
To investigate directly the effect of inactivation of Cdc7p-Dbf4p on origin association of Cdc45p, we performed CHIP assays on Cdc45p in cdc7-1, cdc7-4, and dbf4-1 mutant cells. Wild-type and mutant cells were synchronized with α-factor and released at 35°C (Fig. 5C and D). Cdc45p specifically bound to ARS1 when the wild-type cells reached early S phase. In contrast, no enrichment of Cdc45p could be detected at ARS1 in all three mutants arrested at the G1-S boundary (Fig. 5C). Mcm2p, on the other hand, remained bound to ARS1 in the arrested cdc7 and dbf4 cells (data not shown), indicating that ARS1 did not fire efficiently. Thus, Cdc7p-Dbf4p appeared to be essential for the Cdc45p-ARS1 interaction.
ARS1 associations of Cdc45p and RPA are mutually dependent.
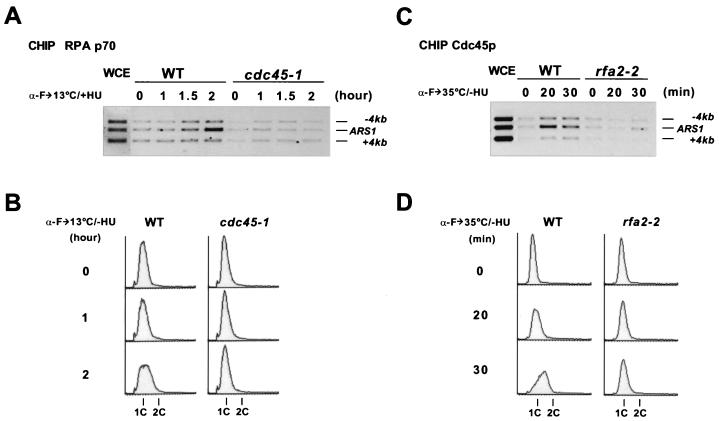
Both Cdc45p and RPA require S-CDKs and Cdc7p-Dbf4p kinase to associate with replication origins (Fig. 4 and 5) (60). We therefore investigated whether there was an interdependence between Cdc45p and RPA for their binding to origins. A cold-sensitive cdc45-1 mutant and the congenic wild-type cells were synchronized with α-factor and released at 13°C, at which temperature cdc45-1 cells were stalled at the G1-S boundary (Fig. 6B). To detect origin association of RPA in these cells, we carried out CHIP analysis with an antibody against RPA p70. Within 2 h after release at 13°C, wild-type cells had entered S phase and specific association of RPA p70 with ARS1 was detected (Fig. 6A). In contrast, RPA p70 did not bind to ARS1 in the cdc45-1 cells stalled at the G1-S boundary. To determine whether RPA was required for loading Cdc45p onto origins, we synchronized wild-type and rfa2-2 (a temperature-sensitive mutant of RPA p34) cells with α-factor and then released them at 35°C (Fig. 6D). Cdc45p bound to ARS1 in the wild-type cells 20 min after release, but it did not do so in the rfa2-2 cells stalled at the G1-S boundary (Fig. 6C). Hence, the ARS1 association of Cdc45p and RPA were interdependent, indicating that Cdc45p and RPA were recruited to this origin at roughly the same time. Since RPA is required for origin unwinding in the SV40 system, these results raise the possibility that the complex containing Cdc45p, RPA, and the MCM proteins is involved in unwinding the origins.
FIG. 6.
Origin associations of Cdc45p and RPA are mutually dependent. (A) ARS1 association of RPA p70 requires Cdc45p. Wild-type (WT) and cdc45-1 (YB0298) cells were synchronized in G1 with α-factor and released into medium containing 0.1 M HU at 13°C. CHIP analysis of RPA p70 was performed at the indicated time points. (B) DNA content of the cells blocked and released as in panel A but in the absence of HU. (C) ARS1 association of Cdc45p requires RPA p34. Wild-type and rfa2-2 (YB0549) cells were synchronized in G1 with α-factor and released at 35°C in the absence of HU. CHIP analysis of Cdc45p was performed at the indicated time points. (D) DNA content of the samples used in panel C. WCE, input DNA from whole-cell extract.
Cdc45p is required for loading Polα p86 but not Dbf4p onto chromatin.
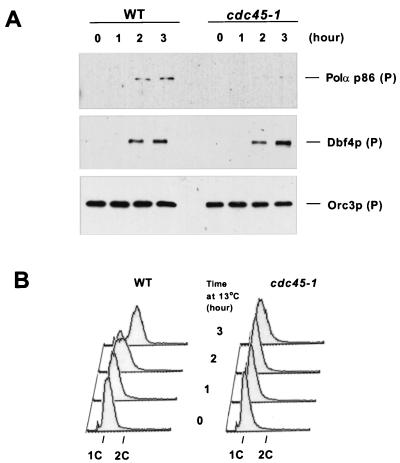
Consistent with the idea that the pre-IC is involved in origin unwinding, it was shown in Xenopus that Cdc45p was required for association of Polα with chromatin (45). To investigate whether such a mechanism was conserved in budding yeast, we analyzed chromatin association of Polα in the cold-sensitive cdc45-1 mutant and wild-type cells released from an α-factor block at 13°C (Fig. 7B). As previously described, cell lysates were fractionated into supernatants and chromatin-enriched sediments (Fig. 7A). In the α-factor-arrested cells, Polα p86 was virtually undetectable on chromatin. As wild-type cells entered S phase, the level of chromatin-bound Polα p86 increased. However, such an increase was hardly detectable in the cdc45-1 cells stalled at the G1-S boundary. Thus, association of Polα with chromatin requires Cdc45p function in budding yeast.
FIG. 7.
Cdc45p is required for loading Polα p86 but not Dbf4p onto chromatin. (A) Wild-type (K6388) (WT) and cdc45-1 (YB0552) cells were synchronized in G1 with α-factor and released at 13°C. Cells were collected at the indicated time points and were processed for chromatin fractionation. The chromatin-sediment fractions (P) were analyzed by immunoblotting. (B) DNA content of the samples used in panel A.
The activation of Cdc7p kinase, which is essential for S-phase entry, is achieved at least in part by stabilization of Dbf4p and its association with chromatin at the G1-S transition (10, 33, 48, 63). Having noticed that Cdc45p and Dbf4p bound to chromatin with similar timing, we investigated whether Cdc45p played a role in loading Dbf4p onto chromatin. After the release from an α-factor block at 13°C, Dbf4p bound to chromatin in both wild-type and cdc45-1 cells with similar kinetics (Fig. 7A). Therefore, the cdc45-1 allele is not defective for loading Dbf4p onto chromatin, even though it is incompetent to recruit RPA and Polα.
The B2 element of ARS1 is involved in association with Mcm2p.
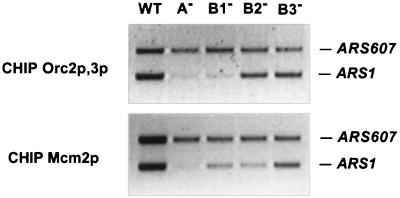
The association of the ORC with ARS1 requires the A and B1 but not the B2 element of the origin (5, 52, 54). Disruptions of either A or all three B elements abolished association of Mcm7p with ARS1 (59). However, none of these studies identified the proteins that associate with B2, the potential DNA-unwinding element. If the complex containing Cdc45p, RPA, and Mcm2p were involved in origin unwinding, then one would predict that it should be present in close proximity to B2. To test this possibility, we performed CHIP analyses on Mcm2p with cells in which the chromosomal ARS1 was mutated at either A or one of the three B elements (44). The association of Mcm2p with ARS1 was abolished in the A− mutant, just like the association of the ORC (Fig. 8). In the B1− mutant, although no ORC was detected at the mutant origin, a weak Mcm2p-ARS1 association was observed by CHIP (Fig. 8). Unlike mutations of the essential A element, disruption of the B1 element only weakened but did not abolish the origin activity of ARS1 (43). It is likely that the ORC could transiently associate with the B1− ARS1 and recruit some MCM proteins to this mutant origin. Interestingly, disruption of the B2 element weakened the association of Mcm2p with ARS1 but not the association of the ORC with this mutant origin (Fig. 8). Therefore, although the B2 element of ARS1 is not required for ORC binding, it is involved in the stable association with Mcm2p and perhaps Cdc45p and RPA.
FIG. 8.
The B2 element of ARS1 is involved in association with Mcm2p but not ORC. The Cdc45HA3p-expressing cells with the chromosomal ARS1 mutated in either the A (YB0576), B1 (YB0577), B2 (YB0578), or B3 (YB0579) element were analyzed by CHIP assays. Asynchronously growing wild-type (WT) and ARS1 mutant cells were subjected to CHIP analysis with antibodies against Orc2p-Orc3p (antibodies against Orc2p and Orc3p were mixed and used together) and Mcm2p, respectively.
DISCUSSION
In budding yeast, initiation of DNA replication is accomplished by a number of sequential transitions at origins. The first transition is to establish the pre-RC at replication origins in late mitosis or early G1, when CDKs are inactive (11, 12, 19, 40, 51, 58, 59). During late G1 and S phases, a series of less-understood transitions occur at individual origins, and DNA replication is launched from different origins at specific times. Two of the critical events that take place during the transitions at each origin are the unwinding of origin DNA and the assembly of replication elongation complexes. Accompanying these events, the pre-RC at each origin undergoes a transformation and is eventually converted to the post-RC and replication forks. Here we have uncovered a number of events that are part of this transformation, thereby shedding light on the mechanisms of origin unwinding and replication fork assembly at eukaryotic chromosomal origins.
The transformation of the pre-RC was first detected through the disappearance of Cdc6p from chromatin and origins at the G1-S transition (11, 31, 59, 63). At about the same time, Cdc45p, RPA, and Mcm2p start to associate with each other in vivo (Fig. 1 to 3) (13, 30, 67). CHIP experiments show that both Cdc45p and RPA begin to associate with early-firing origins at this moment of the cell cycle (Fig. 4 and 5) (60). Mcm2p, a component of the pre-RC and one subunit of the MCM complex, is also present at origins at this time (Fig. 4) (2, 59). It was shown that Cdc45p required Mcm2p to associate with chromatin and that RPA depended on Mcm5p to bind to origins (60, 67). We show here that the origin associations of Cdc45p and RPA are mutually dependent. Together, these findings suggest that Cdc45p and RPA bind to the MCM proteins at origins at the onset of S phase and that together they form the pre-IC. Interestingly, we find that binding of Cdc45p to replication origins is temporally controlled. Cdc45p binds to early-firing origins early and late-firing origins late in S phase, and the timing of Cdc45p binding at each origin correlates well with the timing of initiation at that origin. Since RPA associated with early- but not late-firing origins in the presence of HU, it was postulated that the timing of RPA binding differed between early- and late-firing origins (60). Given that Cdc45p and RPA are both essential for initiation and that their binding to each origin coincides with firing of the origin, the formation of the pre-IC is likely a key step for origin activation.
Unlike the formation of the pre-RC in late mitosis or early G1, the later transitions at origins require activation by CDKs and Cdc7p-Dbf4p. The opposite requirement for CDKs ensures that these transitions always occur after the pre-RC is formed. Several recent studies suggested that S-CDKs and Cdc7p-Dbf4p might function at origins (7, 17, 18). Although a number of proteins present in the pre-RC appear to be substrates of CDKs or Cdc7p in yeast or other organisms (8, 22, 34, 35, 39, 42, 50, 56, 64), how these phosphorylations trigger the transitions remains to be addressed. We have previously shown that Cdc45p was associated with chromatin upon activation of S-CDKs, suggesting that Cdc45p could play an important role in mediating the origin activation by S-CDKs (67). Here we show that S-CDKs are indeed required for the association of Cdc45p with ARS1 and most likely other origins in a temporally specific manner. Furthermore, S-CDK activity is needed for establishing the interactions among Cdc45p, RPA, and Mcm2p. In addition to S-CDKs, Cdc7p-Dbf4p is also required for efficient binding of Cdc45p to ARS1. Likewise, binding of RPA to origins also requires S-CDKs and Dbf4p-dependent kinase (60). Therefore, formation of the pre-IC at individual origins may be the key event during origin activation that is controlled by S-CDKs and Cdc7p-Dbf4p. Because inactivation of Cdc7p-Dbf4p in early S phase leads to silencing of late-firing origins, Cdc7p-Dbf4p probably executes its function at individual origins in a temporally controlled manner (7, 17). The requirement for Cdc7p-Dbf4p in loading Cdc45p and RPA onto origins perhaps contributes to formation of the pre-IC at each origin, with a characteristic timing during S phase.
We noticed that although the Cdc45p-ARS1 association was not detected by CHIP in the arrested cdc7 and dbf4 cells, binding of Cdc45p to Mcm2p and chromatin was only weakened but not abolished (Fig. 5). Unlike chromatin fractionation and Mcm2p coimmunoprecipitation, CHIP analysis examines the enrichment of Cdc45p at a single replication origin. It is possible that in the arrested cdc7 and dbf4 cells, Cdc45p associated with origins other than ARS1 and/or even nonorigin sequences. Alternatively, when Cdc7p-Dbf4p activity was limited, the enrichment of Cdc45p at each origin might not be sufficient to be detected by CHIP. When combined, even the inefficient binding of Cdc45p to a number of origins could give detectable amounts of chromatin-bound Cdc45p and Cdc45p-Mcm2p interaction. Since we have shown that Cdc45p could interact weakly with Mcm2p off the chromatin (67), Cdc7p-Dbf4p might be required for recruiting Cdc45p to origins and establishing a more stable Cdc45p-MCM interaction.
The conclusion of Aparicio et al. (2) that Cdc45p is a component of the pre-RC must be reassessed. We did not find Cdc45p on chromatin until the S-CDKs were activated (67), and here we show that Cdc45p associates with individual origins in a temporally regulated manner during S phase. Indeed, Aparicio et al. (3) agree with this result for most origins, except for the very early origins. We find that, even at the earliest origins, Cdc45p is loaded after the MCM proteins are loaded, the latter step being the one that best defines pre-RC formation and which even occurs upon exit from mitosis (15). Furthermore, since we have used CHIP and direct chromatin binding, as well as coimmunoprecipitation with MCM proteins, we suggest that the differences are unlikely to be due to different methods (as suggested by Aparicio et al. [3]). Nevertheless, we agree that Cdc45p is required for Polα loading and that it might move away from origins after initiation.
How does the pre-IC interact with origins? Disruption of the B2 element of ARS1 diminished its origin activity but did not affect ORC-ARS1 binding (Fig. 8) (43, 52). In contrast, we found that the Mcm2p-ARS1 association was weakened in the B2− mutant, suggesting that the B2 element might be specifically involved in the association with the MCMs. Since only the 8-bp linker substitution in the B2 element, but not the single-base-pair changes in this region, affected the origin activity of ARS1 (43), the B2 element is likely a structural element rather than a sequence-specific binding site for proteins. Indeed, it has been suggested that the B2 element is a DNA-unwinding element (41), and it is very close to the site of initiation of leading-strand DNA replication (6). Therefore, we suggest that the MCMs preferentially associate with the easily unwound sequences (such as the B2 element of ARS1) adjacent to the ORC binding sites. The low helical stability and the position relative to the ORC binding site, but not the actual DNA sequence, might be important for this association. Furthermore, Cdc45p and RPA may be recruited to these regions by the MCMs to form the pre-ICs.
What, then, is the function of the pre-IC at origins? In the SV40 system, RPA binds to the origin that is locally “melted” by T antigen, and then it stimulates the helicase activity of T antigen and stabilizes the unwound origin (29). Subsequently, T antigen, RPA, and the unwound origin recruit Polα-primase to form a complex called the primosome (62). At chromosomal origins, the MCM proteins might function as a T-antigen-like helicase. A helicase activity was reported to be associated with a complex of human Mcm4p, Mcm6p, and Mcm7p (32). Six fission yeast MCM proteins form a globular complex in vivo (1). Our data suggest that the MCM complex might associate with the B2 element of ARS1, a potential DNA-unwinding element (41). However, unlike SV40 T antigen, which can “melt” the origin by itself, it is unlikely that MCM proteins can unwind origins in G1 when they are present in the pre-RC. If the MCM complex functions as a helicase, then its activity might need to be stimulated by S-CDKs and Cdc7p-Dbf4p at the G1-S transition. Together with the findings by Tanaka and Nasmyth (60), our results show that S-CDKs and Cdc7p-Dbf4p promote the binding of Cdc45p and RPA to the MCM proteins at origins. From genetic evidence, both Cdc7p-Dbf4p and Cdc45p have been implicated in the activation of MCMs. A mutant allele of MCM5 called bob1 can suppress the lethality caused by deletions of either CDC7 or DBF4 (27, 33). The loss of Cdc45p function in the cdc45-1 mutant can be bypassed by cdc46-1 and cdc47-1, two mutant alleles of MCM5 and MCM7 (28). Furthermore, RPA can stimulate the helicase activity of T antigen in the SV40 system, indicating that it might also be involved in the activation of MCMs in yeast (29). Therefore, it is plausible that Cdc45p and RPA collaborate to activate the MCM helicase and/or to increase its processivity. Consistent with their potential roles in origin unwinding, Cdc45p is required for loading Polα onto chromatin, and RPA is needed for recruiting primase to origins (Fig. 7) (45, 60).
The function of Cdc45p may not be restricted to origin unwinding and assembly of replication forks. We find that Cdc45p associates with Mcm2p, RPA, and Polɛ during S phase. Unlike Polα and Polδ, Polɛ is not required for in vitro replication of SV40 DNA (62). Consistent with this, it was recently shown that Polɛ polymerase activity was not essential in yeast (37). Nevertheless, the carboxyl terminus of Polɛ is essential for viability, and it may function as a sensor of stalled replication forks (47). Two recent studies using CHIP analysis suggested that Cdc45p, RPA, MCMs, and Polɛ might move away from origins after initiation (2, 60). Moreover, Xenopus Cdc45p was colocalized with Polα throughout S phase (45). Together, these findings argue that Cdc45p, RPA, and MCMs move with replication forks. Cdc45p, together with RPA, may continue to stimulate MCM helicase activity during replication elongation, or it may play an important structural role at replication forks. In addition to chromosomal replication, Polɛ and RPA are also implicated in the repair of UV-induced DNA damage (9, 61). Furthermore, DPB11, a gene that interacts genetically with both POL2 (encoding Polɛ) and CDC45, is involved in the S-phase-induced checkpoint arrest of entry into mitosis (4, 36). It will be interesting to test whether Cdc45p plays a role in nucleotide excision repair and replication-induced checkpoints.
Our results suggest that S-CDKs and Cdc7p-Dbf4p control the formation of the pre-IC at origins. However, it is still unclear how phosphorylation is involved in this process. Identification of the key substrates of the kinases will be crucial for solving the puzzle. Our findings also present us with new challenges. Are Cdc45p, RPA, and MCMs sufficient for origin unwinding? Does the formation of the pre-IC require an initial melting of origins? If so, then what melts the origins? Extensive biochemical investigations will be essential to address these questions.
ACKNOWLEDGMENTS
We thank K. Nasmyth, J. Diffley, S. Bell, O. Aparicio, and P. Plevani for yeast strains, M. Akiyama and P. Plevani for antibodies, O. Aparicio for a chromatin immunoprecipitation protocol, and X. H. Zou-Yang for critical reading of the manuscript.
This work was supported by a grant from the National Institutes of Health (GM45436 to B.S.).
REFERENCES
- 1.Adachi Y, Usukura J, Yanagida M. A globular complex formation by Nda1 and the other five members of the MCM protein family in fission yeast. Genes Cells. 1997;2:467–479. doi: 10.1046/j.1365-2443.1997.1350333.x. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio O M, Weinstein D M, Bell S P. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- 3.Aparicio O M, Stout A M, Bell S P. Differential assembly of Cdc45p and DNA polymerase at early and late origins of DNA replication. Proc Natl Acad Sci USA. 1999;96:9130–9135. doi: 10.1073/pnas.96.16.9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araki H, Leem S H, Phongdara A, Sugino A. Dpb11, which interacts with DNA polymerase II(epsilon) in Saccharomyces cerevisiae, has a dual role in S-phase progression and at a cell cycle checkpoint. Proc Natl Acad Sci USA. 1995;92:11791–11795. doi: 10.1073/pnas.92.25.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell S, Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992;357:128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- 6.Bielinsky A K, Gerbi S A. Chromosomal ARS1 has a single leading strand start site. Mol Cell. 1999;3:477–486. doi: 10.1016/s1097-2765(00)80475-x. [DOI] [PubMed] [Google Scholar]
- 7.Bousset K, Diffley J F. The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev. 1998;12:480–490. doi: 10.1101/gad.12.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown G W, Kelly T J. Purification of Hsk1, a minichromosome maintenance protein kinase from fission yeast. J Biol Chem. 1998;273:22083–22090. doi: 10.1074/jbc.273.34.22083. [DOI] [PubMed] [Google Scholar]
- 9.Budd M E, Campbell J L. DNA polymerases required for repair of UV-induced damage in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:2173–2179. doi: 10.1128/mcb.15.4.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng L, Collyer T, Hardy C F. Cell cycle regulation of DNA replication initiator factor Dbf4p. Mol Cell Biol. 1999;19:4270–4278. doi: 10.1128/mcb.19.6.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman T R, Carpenter P B, Dunphy W G. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 12.Dahmann C, Diffley J F, Nasmyth K A. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- 13.Dalton S, Hopwood B. Characterization of Cdc47p-minichromosome maintenance complexes in Saccharomyces cerevisiae: identification of Cdc45p as a subunit. Mol Cell Biol. 1997;17:5867–5875. doi: 10.1128/mcb.17.10.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desdouets C, Santocanale C, Drury L S, Perkins G, Foiani M, Plevani P, Diffley J F. Evidence of a Cdc6-independent mitotic resetting event involving DNA polymerase α. EMBO J. 1998;14:4139–4146. doi: 10.1093/emboj/17.14.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diffley J F, Cocker J H, Dowell S J, Rowley A. Two steps in the assembly of complexes at yeast replication origins in vivo. Cell. 1994;78:303–316. doi: 10.1016/0092-8674(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 16.Diffley J F. Once and only once upon a time: specifying and regulating origins of DNA replication in eukaryotic cells. Genes Dev. 1996;10:2819–2830. doi: 10.1101/gad.10.22.2819. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson A D, Fangman W L, Brewer B J. Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev. 1998;12:491–501. doi: 10.1101/gad.12.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson A D, Raghuraman M K, Friedman K L, Cross F R, Brewer B J, Fangman W L. CLB5-dependent activation of late replication origins in S. cerevisiae. Mol Cell. 1998;2:173–182. doi: 10.1016/s1097-2765(00)80127-6. [DOI] [PubMed] [Google Scholar]
- 19.Donovan S, Harwood J, Drury L S, Diffley J F. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc Natl Acad Sci USA. 1997;94:5611–5616. doi: 10.1073/pnas.94.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubey D D, Davis L R, Greenfeder S A, Ong L Y, Zhu J G, Broach J R, Newlon C S, Huberman J A. Evidence suggesting that the ARS elements associated with silencers of the yeast mating-type locus HML do not function as chromosomal DNA replication origins. Mol Cell Biol. 1991;11:5346–5355. doi: 10.1128/mcb.11.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutta A, Bell S P. Initiation of DNA replication in eukaryotic cells. Annu Rev Cell Dev Biol. 1997;13:293–332. doi: 10.1146/annurev.cellbio.13.1.293. [DOI] [PubMed] [Google Scholar]
- 22.Elsasser S, Lou F, Wang B, Campbell J L, Jong A. Interaction between yeast Cdc6 protein and B-type cyclin/Cdc28 kinases. Mol Biol Cell. 1996;7:1723–1735. doi: 10.1091/mbc.7.11.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fangman W L, Brewer B J. A question of time: replication origins of eukaryotic chromosomes. Cell. 1992;71:363–366. doi: 10.1016/0092-8674(92)90505-7. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson B M, Brewer B J, Reynolds A E, Fangman W L. A yeast origin of replication is activated late in S phase. Cell. 1991;65:507–515. doi: 10.1016/0092-8674(91)90468-e. [DOI] [PubMed] [Google Scholar]
- 25.Friedman K L, Brewer B J, Fangman W L. Replication profile of Saccharomyces cerevisiae chromosome VI. Genes Cells. 1997;2:667–678. doi: 10.1046/j.1365-2443.1997.1520350.x. [DOI] [PubMed] [Google Scholar]
- 26.Hardy C F. Identification of Cdc45p, an essential factor required for DNA replication. Gene. 1997;187:239–246. doi: 10.1016/s0378-1119(96)00761-5. [DOI] [PubMed] [Google Scholar]
- 27.Hardy C F, Dryga O, Seematter S, Pahl P M, Sclafani R A. mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proc Natl Acad Sci USA. 1997;94:3151–3155. doi: 10.1073/pnas.94.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennessy K M, Lee A, Chen E, Botstein D. A group of interacting yeast DNA replication genes. Genes Dev. 1991;5:958–969. doi: 10.1101/gad.5.6.958. [DOI] [PubMed] [Google Scholar]
- 29.Herendeen D, Kelly T J. SV40 DNA replication. In: Blow J J, editor. Eukaryotic DNA replication: frontiers in molecular biology. Oxford, United Kingdom: Oxford University Press; 1996. [Google Scholar]
- 30.Hopwood B, Dalton S. Cdc45p assembles into a complex with Cdc46p/Mcm5p, is required for minichromosome maintenance, and is essential for chromosomal DNA replication. Proc Natl Acad Sci USA. 1996;93:12309–12314. doi: 10.1073/pnas.93.22.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua X H, Newport J. Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2. J Cell Biol. 1998;140:271–281. doi: 10.1083/jcb.140.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishimi Y. A DNA helicase activity is associated with an MCM4, -6, and -7 protein complex. J Biol Chem. 1997;272:24508–24513. doi: 10.1074/jbc.272.39.24508. [DOI] [PubMed] [Google Scholar]
- 33.Jackson A L, Pahl P M, Harrison K, Rosamond J, Sclafani R A. Cell cycle regulation of the yeast Cdc7 protein kinase by association with the Dbf4 protein. Mol Cell Biol. 1993;13:2899–2908. doi: 10.1128/mcb.13.5.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jallepalli P V, Brown G W, Muzi-Falconi M, Tien D, Kelly T J. Regulation of the replication initiator protein p65cdc18 by CDK phosphorylation. Genes Dev. 1997;11:2767–2779. doi: 10.1101/gad.11.21.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang W, Wells N J, Hunter T. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc Natl Acad Sci USA. 1999;96:6193–6198. doi: 10.1073/pnas.96.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamimura Y, Masumoto H, Sugino A, Araki H. Sld2, which interacts with Dpb11 in Saccharomyces cerevisiae, is required for chromosomal DNA replication. Mol Cell Biol. 1998;18:6102–6109. doi: 10.1128/mcb.18.10.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kesti T, Flick K, Keranen S, Syvaoja J E, Wittenberg C. DNA polymerase epsilon catalytic domains are dispensable for DNA replication, DNA repair, and cell viability. Mol Cell. 1999;3:679–685. doi: 10.1016/s1097-2765(00)80361-5. [DOI] [PubMed] [Google Scholar]
- 38.Koonin E V. A common set of conserved motifs in a vast variety of putative nucleic acid-dependent ATPases including MCM proteins involved in the initiation of eukaryotic DNA replication. Nucleic Acids Res. 1993;21:2541–2547. doi: 10.1093/nar/21.11.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei M, Kawasaki Y, Young M R, Kihara M, Sugino A, Tye B K. Mcm2 is a target of regulation by Cdc7-Dbf4 during the initiation of DNA synthesis. Genes Dev. 1997;11:3365–3374. doi: 10.1101/gad.11.24.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang C, Stillman B. Persistent initiation of DNA replication and chromatin-bound MCM proteins during the cell cycle in cdc6 mutants. Genes Dev. 1997;11:3375–3386. doi: 10.1101/gad.11.24.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin S, Kowalski D. Functional equivalency and diversity of cis-acting elements among yeast replication origins. Mol Cell Biol. 1997;17:5473–5484. doi: 10.1128/mcb.17.9.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Girona A, Mondesert O, Leatherwood J, Russell P. Negative regulation of Cdc18 DNA replication protein by Cdc2. Mol Biol Cell. 1998;9:63–73. doi: 10.1091/mbc.9.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marahrens Y, Stillman B. A yeast chromosomal origin of DNA replication defined by multiple functional elements. Science. 1992;255:817–823. doi: 10.1126/science.1536007. [DOI] [PubMed] [Google Scholar]
- 44.Marahrens Y, Stillman B. Replicator dominance in a eukaryotic chromosome. EMBO J. 1994;13:3395–3400. doi: 10.1002/j.1460-2075.1994.tb06642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mimura S, Takisawa H. Xenopus Cdc45-dependent loading of DNA polymerase alpha onto chromatin under the control of S-phase Cdk. EMBO J. 1998;17:5699–5707. doi: 10.1093/emboj/17.19.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasmyth K. Viewpoint: putting the cell cycle in order. Science. 1996;274:1643–1645. doi: 10.1126/science.274.5293.1643. [DOI] [PubMed] [Google Scholar]
- 47.Navas T A, Zhou Z, Elledge S J. DNA polymerase epsilon links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 48.Oshiro G, Owens J C, Shellman Y, Sclafani R A, Li J J. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol Cell Biol. 1999;19:4888–4896. doi: 10.1128/mcb.19.7.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owens J C, Detweiler C S, Li J J. CDC45 is required in conjunction with CDC7/DBF4 to trigger the initiation of DNA replication. Proc Natl Acad Sci USA. 1997;94:12521–12526. doi: 10.1073/pnas.94.23.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson B O, Lukas J, Sorensen C S, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piatti S, Bohm T, Cocker J H, Diffley J F, Nasmyth K. Activation of S-phase-promoting CDKs in late G1 defines a “point of no return” after which Cdc6 synthesis cannot promote DNA replication in yeast. Genes Dev. 1996;10:1516–1531. doi: 10.1101/gad.10.12.1516. [DOI] [PubMed] [Google Scholar]
- 52.Rao H, Stillman B. The origin recognition complex interacts with a bipartite DNA binding site within yeast replicators. Proc Natl Acad Sci USA. 1995;92:2224–2228. doi: 10.1073/pnas.92.6.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynolds A E, McCarroll R M, Newlon C S, Fangman W L. Time of replication of ARS elements along yeast chromosome III. Mol Cell Biol. 1989;9:4488–4494. doi: 10.1128/mcb.9.10.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowley A, Cocker J H, Harwood J, Diffley J F. Initiation complex assembly at budding yeast replication origins begins with the recognition of a bipartite sequence by limiting amounts of the initiator, ORC. EMBO J. 1995;14:2631–2641. doi: 10.1002/j.1460-2075.1995.tb07261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santocanale C, Diffley J F. ORC- and Cdc6-dependent complexes at active and inactive chromosomal replication origins in Saccharomyces cerevisiae. EMBO J. 1996;15:6671–6679. [PMC free article] [PubMed] [Google Scholar]
- 56.Sato N, Arai K, Masai H. Human and Xenopus cDNAs encoding budding yeast Cdc7-related kinases: in vitro phosphorylation of MCM subunits by a putative human homologue of Cdc7. EMBO J. 1997;16:4340–4351. doi: 10.1093/emboj/16.14.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stillman B. Cell cycle control of DNA replication. Science. 1996;274:1659–1664. doi: 10.1126/science.274.5293.1659. [DOI] [PubMed] [Google Scholar]
- 58.Stoeber K, Mills A D, Kubota Y, Krude T, Romanowski P, Marheineke K, Laskey R A, Williams G H. Cdc6 protein causes premature entry into S phase in a mammalian cell-free system. EMBO J. 1998;17:7219–7229. doi: 10.1093/emboj/17.24.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka T, Knapp D, Nasmyth K. Loading of an Mcm protein onto DNA replication origins is regulated by Cdc6p and CDKs. Cell. 1997;90:649–660. doi: 10.1016/s0092-8674(00)80526-7. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka T, Nasmyth K. Association of RPA with chromosomal replication origins requires an Mcm protein, and is regulated by Rad53, and cyclin- and Dbf4-dependent kinases. EMBO J. 1998;17:5182–5191. doi: 10.1093/emboj/17.17.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Umezu K, Sugawara N, Chen C, Haber J E, Kolodner R D. Genetic analysis of yeast RPA1 reveals its multiple functions in DNA metabolism. Genetics. 1998;148:989–1005. doi: 10.1093/genetics/148.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 63.Weinreich M, Liang C, Stillman B. The Cdc6p nucleotide-binding motif is required for loading mcm proteins onto chromatin. Proc Natl Acad Sci USA. 1999;96:441–446. doi: 10.1073/pnas.96.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinreich, M., and B. Stillman. Cdc7p/Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 18:5334–5346. [DOI] [PMC free article] [PubMed]
- 65.Yamashita M, Hori Y, Shinomiya T, Obuse C, Tsurimoto T, Yoshikawa H, Shirahige K. The efficiency and timing of initiation of replication of multiple replicons of Saccharomyces cerevisiae chromosome VI. Genes Cells. 1997;2:655–665. doi: 10.1046/j.1365-2443.1997.1530351.x. [DOI] [PubMed] [Google Scholar]
- 66.Zou L, Mitchell J, Stillman B. CDC45, a novel yeast gene that functions with the origin recognition complex and Mcm proteins in initiation of DNA replication. Mol Cell Biol. 1997;17:553–563. doi: 10.1128/mcb.17.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou L, Stillman B. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science. 1998;280:593–596. doi: 10.1126/science.280.5363.593. [DOI] [PubMed] [Google Scholar]