Abstract
Background
In hospital cancer care, there is no set standard for next-of-kin involvement in improving the quality of care and patient safety. There is therefore a growing need for tools and methods that can guide this complex area.
Objective
The aim of this study was to present the results from a consensus-based participatory process of designing a guide for next-of-kin involvement in hospital cancer care.
Method
A consensus process based on a modified Nominal group technique was applied with 20 stakeholder participants from 2 Norwegian university hospitals.
Result
The participants agreed on the 5 most important priorities for hospital cancer care services when involving next-of-kin. The results showed that next-of-kin stakeholders, when proactively involved, are important resources for the patient and healthcare professionals in terms of contribution to quality and safety in hospitals. Suggested means of involving next-of-kin were closer interaction with external support bodies, integration in clinical pathways, adjusted information, and training healthcare professionals.
Conclusion
In this study, we identified topics and elements to include in a next-of-kin involvement guide to support quality and safety in hospital cancer care. The study raises awareness of the complex area of next-of-kin involvement and contributes with theory development and knowledge translation in an involvement guide tailored for use by healthcare professionals and managers in everyday clinical practice.
Implications for Practice
Service providers can use the guide to formulate intentions and make decisions with suggestions and priorities or as a reflexive tool for organizational improvement.
KEY WORDS: Cancer nursing, Consensus methods, Guide, Hospitals, Involvement, Next-of-kin, Nominal group technique
Background
Over the last decade there have been many attempts to improve quality and safety for patients in healthcare services; however, hospitals still report poor patient outcomes.1,2 Next-of-kin and family caregivers are important collaborative partners in keeping patients safe both in hospitals and at home.3–5 They are, however, seldom considered equal partners in the medical team around the patient despite taking on many important care tasks in different parts of the cancer care trajectory.6–8 Consequently, next-of-kin may feel overburdened and stressed.9–11 In hospital cancer care, there is no set standard associated with next-of-kin involvement in general treatment or in relation to improving cancer service quality and safety.12 Next-of-kin involvement is seldom directly related to quality and safety, and research on this topic is rare.12,13 Previous research has identified a need for tools and methods to guide the complex area of next-of-kin involvement in general and in relation to the context of the involvement (eg, cancer care, pediatrics, geriatric care, intensive care).8,14,15 Such a development should incorporate a multistakeholder perspective that includes healthcare professionals, patients, and next-of-kin.16 Our study therefore takes this perspective.
Consensus methods are widely used in healthcare research to aid decision making, problem solving and idea generation.17–19 Consensus methods often gather experts in a field, such as oncologists or nurses to determine consensus on a given topic. There is, however, a lack of research on how to gather stakeholders across hospitals with a combined multidisciplinary, patient, and stakeholder perspective to arrive at a consensus on a topic from a group of representatives with diverse backgrounds and roles.12 Some topics, such as how to guide next-of-kin involvement in cancer care, as in our study, requires a broad representation of stakeholders to incorporate different perspectives in a consensus process and reach an agreement on the way forward (in other words, to cocreate).20,21
Consensus methods have multifaceted challenges. There are many potential practical obstacles, such as funding, time, organization and geography, when establishing an arena for the sharing of ideas and learning.18 Consequently, the method may fail without careful attention to the cocreation of knowledge between stakeholder groups and researchers.22–24
Aim and Research Questions
With this in mind, we invited stakeholder representatives from 2 Norwegian hospitals to join a panel where we used a modified nominal group technique (NGT).25
The overarching research problem for the panel was as follows: What topics and elements should be included in a next-of-kin involvement guide to support quality and safety in hospital cancer care?
The following research questions guided the consensus process:
-
1)
What can we learn from next-of-kin experiences with hospital cancer care?
-
2)
How can next-of-kin experiences be valued more systematically to improve the quality and safety of cancer care?
-
3)
What methods or tools are appropriate for collecting experiences and for next-of-kin involvement locally, regionally, and nationally?
Based on the consensus technique, we developed a guide for use in hospital cancer care to increase the focus on involvement and take advantage of the experiences of cancer patients’ next-of-kin. The aim of this article is to present the results from the consensus process and to produce a guide for next-of-kin involvement in hospital cancer care.
Study Design and Setting
This article is part of a mixed-method project with a convergent design.26 The design consists of 3 substudies that explore quality and safety in hospital cancer care in 2 Norwegian university hospitals (Figure 1).
Figure 1.
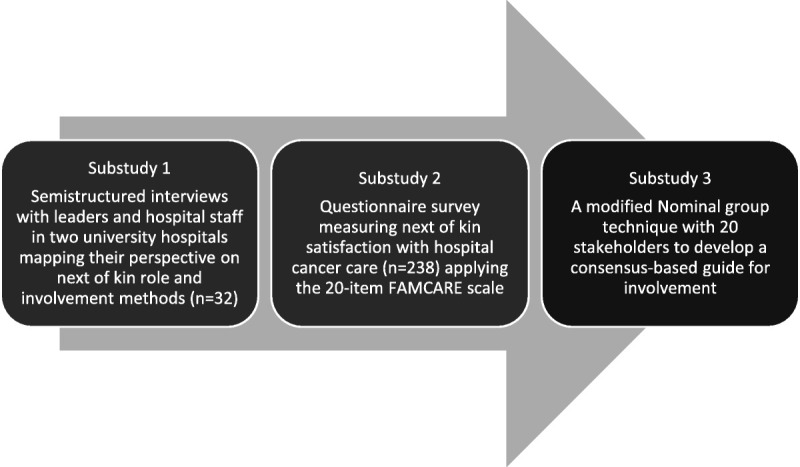
Overview of the project.
Substudy 1 was a qualitative mapping of next-of-kin involvement and involvement methods in cancer care services in the 2 hospitals. This was an in-depth study of managers’ and hospital staffs’ perspectives. The study resulted in 2 published articles.8,27 Substudy 2 was a quantitative measurement of next-of-kin satisfaction with cancer care services in the 2 hospitals and resulted in 1 published article.15 Substudy 3, reported here, is a consensus process (using the NGT) where we synthesized substudies 1 and 2 and presented the findings to stakeholders invited from the 2 hospitals. The participants agreed on the most appropriate elements and topics in next-of-kin involvement in hospitals.
The study setting consists of 2 Norwegian university hospitals with their affiliated oncology departments. Both hospitals are affiliated with the same Regional Health Authority. The hospitals differ in size, number of employees, and budget, but the cancer departments are approximately the same size and are subject to the same national and regional policy documents (see details in Table 1).
Table 1.
Local Context Descriptions With Key Figures
| Local Context | Large City in Norway | Large City in Norway |
|---|---|---|
| Included hospitals | Hospital A | Hospital B |
| Size | University hospital Local hospital for 330 000 inhabitants |
University hospital Local hospital for 420 000 inhabitants |
| Employees | 7500 | 12 000 |
| Budget | 6.8 billion NOK | 10.8 billion NOK |
| Cancer departments | Second largest regional cancer department with 2 cancer care wards, 2 outpatient clinics, and 1 radiotherapy unit | Main cancer department in the region with 2 cancer care wards, 1 outpatient clinic, and 1 radiotherapy unit |
The Norwegian Healthcare Context
Taxes fund the Norwegian healthcare system. All residents are covered by the National Insurance Scheme. The system is built on universal access and free choice of providers. Norway’s 4 Regional Health Authorities provide healthcare services within their district. The government has the financial oversight for all public hospitals.
Norway’s cancer registry reported 34 190 new cancer cases in 2018 and 283 984 people living with cancer.28 The incidence of cancer in Norway is higher than the average of the 36 Organisation for Economic Co-operation and Development countries (age-standardized rate ratio, 1.12), but the cancer mortality rate is lower (age-standardized rate ratio, 0.95).29
Under the Norwegian Patient and User Act (1999), the patient chooses the friend or family member who is the closest next- of-kin (§1.3b). The law does not specify any specific tasks or obligations for the next of kin in relation to the provision of healthcare services. The government has that responsibility in Norway; in other countries, there are stronger expectations that next of kin will take on a greater role in providing healthcare services.
Theoretical Approach
ORGANIZING FOR QUALITY
The theoretical backdrop of this research project (Figure 1) is the Organizing for Quality (OQ) model developed by Bate and colleagues.30 The model focuses on 6 challenges that hospitals must meet (structural, political, cultural educational, emotional, physical and technological) as part of working on quality and safety in healthcare.30(p169) The OQ model was developed based on international studies in leading European and American hospitals.30–32 It has also been tested and refined by studies in Norwegian hospitals.33–35 We apply a theoretical model in our research project to obtain the guidance to understand and investigate quality and safety processes in hospitals with a multilevel apporach.36,37 As a result of the first substudy (Figure 1), we suggested modifications to the OQ model. Figure 2 is built on the experience and views of leaders and healthcare professionals with next-of-kin involvement in the 2 hospitals. In Figure 2, we identified and elaborated on the 6 quality challenges and then added areas of key importance for next-of-kin involvement based on our findings to make it relevant for stakeholders in a clinical setting.8 Figure 2 is operationalized in this article into the next-of-kin involvement guide (Figure 5).
Figure 2.
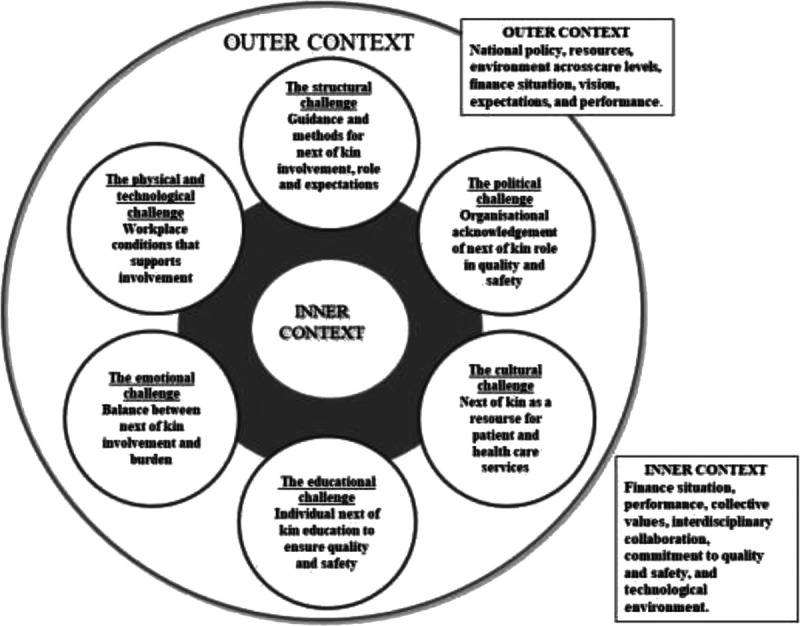
Revised framework model inspired by Bate and colleagues.8,30
Figure 5.

Organizing for quality and safety: a next-of-kin involvement guide.
Methods
The study design reported in this article is a consensus process inspired by the NGT. The NGT was developed by Delbecq and colleagues25 in 1975 and comprises 4 key elements: silent generation, round robin, clarification, and voting. All 4 elements are keys to arriving at a general agreement on a particular topic. The NGT is often used to explore stakeholders’ or consumers’ views, but the method can be modified for other purposes.18
The modified NGT for this study was conducted in 3 phases to reach stakeholder agreement. Figure 3 is an overview of the process, consisting of preparation, consensus, and post-feedback, followed by validation of the results.
Figure 3.
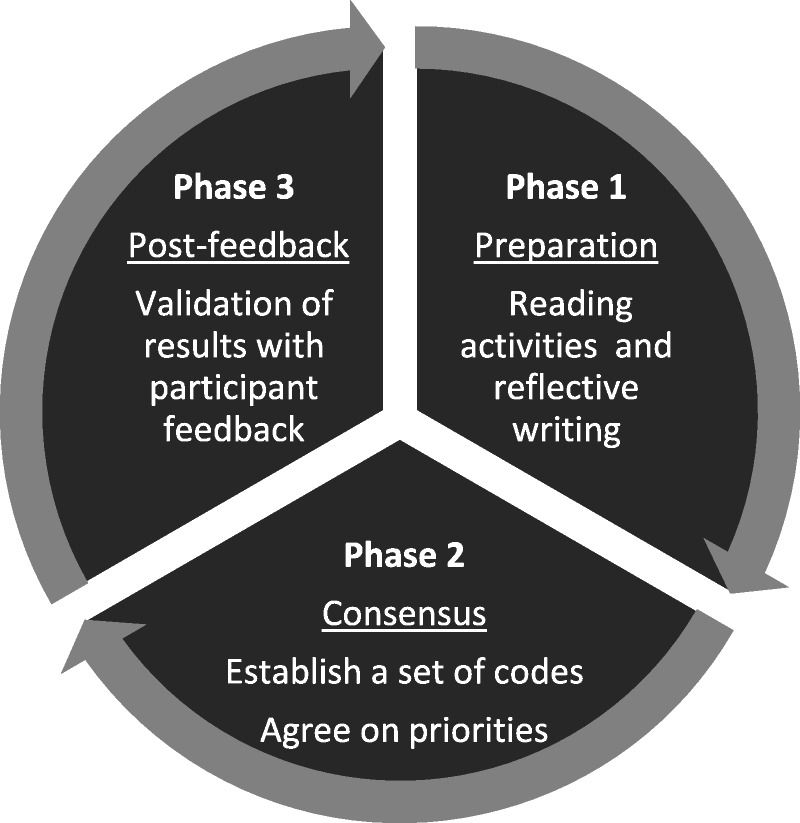
Overview of the 3-phase nominal group technique.
Characteristics of Participants
Purposive sampling was used to identify healthcare professionals and next-of-kin representatives.38 Participation was voluntary and done in close collaboration with the 2 hospitals. Leaders from 7 inpatient and outpatient cancer care wards in the 2 hospitals participated in the recruitment of participants, among whom were leaders and multidisciplinary hospital staff. IJB contacted 1 coping center in both regions. The center, a meeting point for cancer patients and their representatives, offers courses, networking opportunities, and informal conversations. The 2 centers were asked if they would participate with 1 representative in the meeting. They also made contact with a local next-of-kin representative who was able to participate. For the consensus meeting, the Regional Health Authority appointed a regional next-of-kin representative. This representative was the only person who received compensation for this meeting in line with Regional Health Authority guidelines. Table 2 lists the panel participants for this study.
Table 2.
Overview of Panel Participants
| Participants in the Consensus Process | Number of Attendees |
|---|---|
| Local next-of-kin representatives | 2 |
| Regional next-of-kin representatives | 1 |
| Coping centers next-of-kin representatives | 2 |
| Hospital A—healthcare professionals | 2 physicians and 6 oncology nurses |
| Hospital B—healthcare professionals | 3 physicians and 7 oncology nurses |
| Gender of the participants | 2 male and 18 female |
| Positions | 5 managers and 15 healthcare professionals |
Overview of the Modified NGT
A consensus method, based on a modified NGT, was applied as a single 1-day meeting with 20 participants (5 next-of-kin representatives, 10 oncology nurses, and 5 physicians) from the 2 Norwegian university hospitals. The consensus meeting was supervised by a 5-member research team: 4 moderators (SW, GSB, BG, and IJB) and 1 nonparticipant observer (BF) who collected qualitative data on the nominal group processes during the 1-day meeting. This is recommended by Jones and Hunter.17 Observation notes were embedded in the analysis and used in the interpretation of the group process and results.
Analysis
The modified NGT developed for this study had 3 phases (Figure 3). The first phase was conducted by email, followed by a face-to-face meeting. The results were then emailed to the participants. The analysis process followed the 3 phases depicted in Figure 4.
Figure 4.
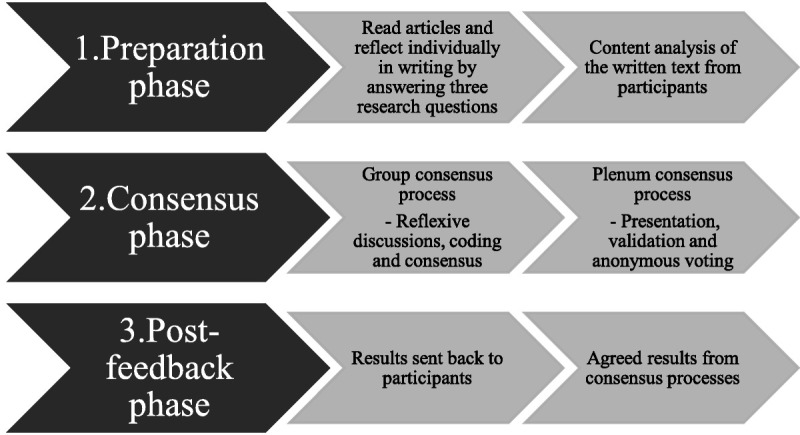
The modified nominal group technique.
PHASE 1: PREPARATION
In the first phase in the modified NGT, we had the participants engage in reading and reflective writing. One month before the meeting, we sent the participants 2 articles8,27 that described the results from the larger mixed-method project of which the consensus process is a part. We also asked the participants to reflect individually upon the topic “What is the role of next-of-kin for quality and safety in cancer care?” In addition, we asked them to respond in writing to the following questions that guided the consensus process:
-
1)
What can we learn from next-of-kin experiences with hospital cancer care?
-
2)
How can next-of-kin experiences be valued more systematically to improve cancer care quality and safety?
-
3)
What methods or tools are appropriate for collecting experiences and for next-of-kin involvement (locally, regionally, nationally)?
The purpose of these assignments was to prepare each participant for the consensus process and to empower them to express themselves. Within 3 weeks, all the participants emailed a 1-page text to IJB with their thoughts and suggestions related to the research questions, earlier research findings, and their own experiences.
The research team led by IJB conducted a content analysis of the texts before the consensus meeting. The content analysis was inspired by Graneheim and colleagues.39,40 The analysis consisted of a 3-step characterization of the participants’ texts: (1) selecting meaning units, (2) condensing meaning units, and (3) defining subcategories and categories. The purpose of the content analysis was to identify categories and use these as an ice breaker to get all participants on the same page, before starting the consensus discussions in phase 2. An example of the content analysis can be found in Table 3.
Table 3.
Example of the Content Analysis
| Selecting Meaning Units | Condensing Meaning Units | Defining Subcategories |
Defining Categories |
|---|---|---|---|
| “Next-of-kin experiences that are expressed can contribute to increased quality of healthcare. My experience is that collaboration with the next-of-kin in care and treatment of the cancer patient provides increased security in the patient’s coping with cancer and its treatment.” | Next-of-kin experiences can contribute to increased quality of healthcare. Collaboration with next-of-kin provides increased security in the patient’s coping with the disease and treatment. | Next-of-kin involvement is important for how well the patient is coping with disease and treatment. | Involvement of next-of-kin is important for coping with disease and treatment. |
PHASE 2: CONSENSUS
The consensus meeting took place on a neutral arena that had no affiliation with any of the hospitals. Half of the participants had to travel by plane to attend the meeting in the city of one of the case hospitals. The meeting agenda is provided in the Appendix. The meeting started with a presentation by the participants, followed by a short introduction to the NGT and a summary of the results of previous substudies, and concluded with an overview of the content analysis on the emailed text from the participants. The participants learned about the views of leaders and healthcare professionals on next-of-kin involvement, the survey results from next-of-kin in the 2 hospitals, and the content analysis based on their initial reflections about these findings.
GROUP CONSENSUS—ESTABLISHING A COMMON SET OF CODES
After the introduction, we split the 20 participants into 2 groups to create a reflexive discussion, share experiences, generate new ideas, and establish a set of codes that the group could agree on for presentation in the following plenary session. Discussion questions were assigned for the first group session. Group 1 discussed these questions: What can we learn from next-of-kin experiences with hospital cancer care? How can next-of-kin experiences be valued more systematically to improve cancer care quality and safety? Group 2 discussed the question: What methods or tools are appropriate for collecting experiences for next-of-kin involvement (locally, regionally, nationally)? The 2 groups engaged in a consensus process led by moderators. The process was based on a reflexive discussion in which all suggestions were written on flip sheets, continued by a round-robin process until there were no more suggestions to discuss. Then the group and the moderators coded the suggestions by sorting and identifying common topics and suggestions. When the group reached consensus by agreeing on the codes, this session ended.
PLENARY CONSENSUS—AGREEING ON THE TOP 5 PRIORITIES
After the group sessions, we reunited the 2 groups in a plenary session. In the plenary session, all participants reached agreement on the codes set by the 2 smaller groups. The participants also completed an anonymous poll of the 5 initiatives that hospital cancer care services should prioritize when working on next-of-kin involvement. The plenary session was divided into 2 parts, with a plenary consensus process for each group’s research question. Each group presented the codes to the other and then discussed whether additional codes were needed. After the total group had reached agreement on the codes, we conducted anonymous voting on the 5 most important codes. Each participant manually submitted the votes to the research team. Both plenary consensus processes were completed in the same manner.
PHASE 3: POST-FEEDBACK
One week after the meeting, the participants received an email with the results of the anonymous voting session. We invited them to comment on the results. Only 1 participant responded, suggesting that we change the phrase “objective information” in priority 5 (Table 6) to “concrete information.” We embedded the revised wording in the code.
Table 6.
Top 5 Priorities Consensus 1: “What Can We Learn and How Can We Value?”
| What can we learn from next-of-kin experiences with hospital cancer care? How can next-of-kin experiences be valued more systematically to improve the quality and safety of cancer care? | |
| 1 | Next-of-kin experiences should be documented and systematized (user surveys, “heart sigh” book, next-of-kin notice in the documentation system). |
| 2 | Next-of-kin who are secure in their role can contribute to patient safety. |
| 3 | System improvement that uses next-of-kin evaluation as a measure (user surveys). |
| 4 | Reveals areas where the help provided is not good enough. |
| 5 | Important for evaluating aid. |
| 5 | Provides healthcare professionals with more objective or concrete information on the patient. |
| 5 | Crucial for how well the patient handles the illness and treatment through the cancer care trajectory. |
| 5 | Next-of-kin who observe and interpret what happens to the patient are important, and they need to be trained in basic skills. |
Results
In the following, we present the results from the consensus meeting. Tables 4 and 5 show the codes from the group sessions, and Tables 6 and 7, the codes from the plenary session. We have incorporated the nonparticipant observers’ notes into the results presentation.
Table 4.
Overview of Codes From Consensus 1: “What Can We Learn and How Can We Value”
| Codes |
|---|
| Important for evaluating aid |
| Provides healthcare professionals with more objective or concrete information on the patient |
| Crucial for how well the patient handles the illness and treatment through the cancer care trajectory |
| Reveals areas where the help provided is not good enough |
| Next-of-kin who observe and interpret what happens to the patient are important, and they need to be trained in basic skills |
| Important throughout the cancer care trajectory. Next-of-kin have an eye for “the whole life” |
| Next-of-kin that are secure in their role can contribute to patient safety |
| Poor continuity of healthcare professionals creates unsafe next-of-kin |
| Healthcare professionals need more knowledge of next-of-kin involvement |
| Acknowledge the next-of-kin role as a coordination role that needs to be adjusted to individual needs |
| Next-of-kin experiences should be documented and systematized (user surveys, “heart sigh” book, next-of-kin notice in the documentation system) |
| Coherence between service levels (hospital and municipalities) with support from volunteer organizations |
| Be aware of those patients who do not have a next-of-kin |
| System improvement that uses next-of-kin evaluation as a measure (user surveys) |
| Double loop learning with respond to service users |
Table 5.
Overview of Codes From Consensus 2: “Methods and Tools for Collecting Experiences”
| Codes |
|---|
| Technology (apps, documentation, admission forms) |
| Economy (travel expenses, time off work, consultations, diagnose related groups’ effort-based funding, social rights as a next-of-kin) |
| Involvement in patient care (clarification of roles, different phases of the trajectory (curative or palliative), standardization of involvement in different parts of the trajectory, documentation) |
| Needs clarification/information in the summon letter and in different phases (expectations, resources, wishes and needs, information in summon letter and different phases, checklist on needs at discharge, information) |
| Interaction (learning and coping centers in the municipalities) |
| Information (to next-of-kin, learning and coping) |
| Training of healthcare professionals (ethics, how, methods) |
| One appointed healthcare professional for the next-of-kin |
| User participation with special focus on the next-of-kin perspective |
Table 7.
Top 5 Priorities in Consensus 2: “Methods and Tools for Collecting Experiences”
| What methods or tools are appropriate for collecting experiences and for involvement of next-of-kin (locally, regionally, nationally)? | |
| 1 | Involvement in patient care (clarification of roles, different phases of the trajectory [curative or palliative], standardization of involvement in different parts of the trajectory, documentation) |
| 2 | Interaction (learning and coping centers in the municipalities) |
| 3 | Information (to next-of-kin, learning and coping centers) |
| 4 | Training of healthcare professionals (ethics, how, methods) |
| 5 | Technology (apps, documentation, admission forms) |
Group Consensus Results
GROUP CONSENSUS 1: “WHAT CAN WE LEARN AND HOW CAN WE VALUE NEXT-OF-KIN INVOLVEMENT?”
Table 4 summarizes the codes from the group discussion process in response to the questions: What can we learn from next-of-kin experiences with hospital cancer care? How can next-of-kin experiences be valued more systematically to improve the quality and safety of cancer care? There was a good atmosphere in this group. According to the nonparticipant observers’ notes, all the participants were engaged in contributing to the process. The next-of-kin representatives were courageous and added important input. The physicians were initially a little reticent, but according to the observation notes, all participants were seen by the moderators in this group. The results acknowledged the next-of-kin’s central role in patient care as the most important learning dimension for next-of-kin involvement. Participants highlighted that next-of-kin possess essential information about the patient, are central to care coordination, and give valuable feedback about how patients respond to the treatment.
GROUP CONSENSUS 2: METHODS AND TOOLS FOR COLLECTING EXPERIENCES
Table 5 gives an overview of the codes from the group discussion process with respect to this question: What methods or tools are appropriate for collecting experiences and for involvement of next-of-kin (locally, regionally, nationally)?
According to the nonparticipant observer’s notes, there was very good participation and engagement in this group. Moreover, all participants were seen by the moderators in this group, and the group progressed with the help of the moderators. The group seemed to struggle with coding the discussion moments and needed the moderators’ assistance. Engagement declined slightly in the coding phase. However, the group members remained engaged and shared their views on the topic of the session. The results focused on standardization of involvement in different parts of the cancer care trajectory as the most important tools and methods to integrate into a guide. They suggested use of apps, a checklist, and the medical record document and improve involvement.
Plenary Consensus Results
AGREEING ON TOP 5 PRIORITIES
Tables 6 and 7 give an overview of the results of the anonymous voting on the 2 top 5 priorities for hospitals’ cancer care services to address. The top 5 priorities are meant for service development use to support next-of-kin involvement in cancer care, especially in relation to (1) learning and information and (2) recommendation of methods to promote involvement in practice.
According to the nonparticipant observer’s notes, there was less engagement in the plenary process than in the 2 previous separate group discussions. Even if it was a more challenging plenary process, it generated discussion and new insights.
Evaluation of the Method and the Meeting
At the end of the day, an evaluation session allowed the participants to share their views on the consensus meeting. The group said that it had been very useful for them to have come to the meeting prepared. The group highlighted that the meeting had been a good arena to explore and discuss next-of-kin involvement. They also noted that they felt safe sharing their opinions and speaking their minds. One next-of-kin representative thought that the inclusion of more next-of-kin representatives in the meeting could have contributed more input.
Discussion
Developing Key Concepts for Next-of-Kin Involvement in Hospital Cancer Care
In this article, we presented the results from a consensus process with the purpose of identifying key topics and elements that should be included in a next-of-kin involvement guide for quality and safety in hospital cancer care. The purpose of the process was to describe and suggest changes for next-of-kin involvement practice in hospital cancer care, but it can also be relevant for other healthcare services or decision-making support bodies. The top 5 priorities in this study show that next-of-kin are considered key stakeholders in keeping the patient safe. The stakeholder groups emphasized that, first, it is important for cancer care services to start developing systems for the systematization and documentation of next-of-kin experiences for further use. An example could be by integrating data on next-of-kin experiences, for instance, in user surveys.15
Second, the panel agreed that hospital cancer care needs to recognize and change service in a direction that formally integrates and uses next-of-kin experiences in service improvement at the micro level. Moreover, there was consensus in terms of personalized next-of-kin training and support to prepare them for the challenges and care tasks that they will perform. There was agreement that treating next-of-kin as an equal part of the patient’s medical care team is a prerequisite for sound next-of-kin involvement. Our findings are in line with other studies highlighting next-of-kin as an underused resource, for evaluating aid and providing healthcare professionals with more objective information on the patient’s condition.12,13,41–45
Another important message from our consensus process is that hospital cancer care should become more aware of how to use next-of-kin experiences because of its potential impact on how well the patient handles treatment and care. In other words, next-of-kin involvement in cancer care is important for patient outcome and should be a higher priority in future practice. This message echoes other studies that highlighted the important role of next-of-kin involvement in healthcare.5,6,10,13,44,45
Organizing for Quality and Safety: A Next-of-Kin Involvement Guide
There is a constant call for theory development in research and for incorporating theory into everyday practice in healthcare organizations.46 Our project responds to this call and builds on Bate and colleagues’8,30 conceptualization of quality and safety in healthcare. The project is also in line with experience-based co-design47 by combining participatory design and user experiences in developing a guide to improve cancer care services. Co-design in this study has required the participation of multidisciplinary healthcare professionals within cancer care and next-of-kin representatives from 2 university hospitals to share and reflect on their experiences to identify priorities for implementation of change.48,49
As previously mentioned, we apply the OQ as our theoretical backdrop, which we have modified to fit next-of-kin involvement in cancer care (see Figure 2). We will now present the next-of-kin involvement guide (Figure 5) that encompasses, develops and operationalizes Figure 2 with the results from the consensus process. We want to give the model (Figure 2) a broader empirical foundation, one that incorporates a multistakeholder approach. The main purpose is, however, to convert the model into a practical tool with direct connection to both theory and knowledge-based adaptations derived from stakeholder involvement and the consensus process. Until now, this has been lacking in the research literature.19–21,50
Figure 5 illustrates the next-of-kin involvement guide. The guide is a result of merging the framework model (Figure 2) and the results from the consensus process (Tables 4 and 5). Through this merger, we have developed a guidance tool for hospital cancer care services by translating theory into practice with suggestions on where to start making changes to explore and support next-of-kin involvement. The stakeholder groups agreed on top 5 priorities in each of the 2 consensus sessions. These priorities are bolded in the figure; however, the stakeholder groups did not state that the additional codes had a lower priority. Therefore, we embedded all suggestions in the figure and grouped them under the 6 quality challenges.
The guide can be used in either as a guide with suggestions and priorities or as a reflexive tool for improvement efforts in the organization. The latter approach has been adapted and explored with the OQ model,31,51 in the Norwegian primary care context,37,52 and in international studies.53–55
Implications for Practice, Research and Education
Next-of-kin involvement in healthcare services is complex. Like Bell and colleagues,16 we contend that decision and actions within this area should be based on a multistakeholder approach where the perspectives of all stakeholders are heard and integrated. This study adds to the knowledge of how to create an arena for hospitals to share ideas and learn from each other and from involved next-of-kin stakeholders. The reflexive space established through the consensus process presented in this article brings attention to practical values and challenges of next-of-kin involvement, which can inform everyday practice in hospitals. A key rationale for reflexive practice is bringing together stakeholders with the ability to engage in the cocreation of knowledge that supports organizational learning to reach a higher level of understanding.14,56–58 This study explains how the consensus method can be used for different purposes in hospitals, such as the development of internal guidelines, evaluation of performance, change management, interventions, compliance, and communication between disciplines or institutions.
At the same time, there is potential to identify priority topics for research and practice improvement by using consensus methods. This has been demonstrated in other studies59,60 that have set research priorities with the use of a consensus design. For educational purposes, the methodological approach can target future strategic directions with input from stakeholders involved in the specific areas or questions of interest such as cancer care, diabetes, and pediatrics. However, how successful this translation of knowledge and learning turns out to be, depends on how healthcare professionals value research, develop knowledge and use this proactively for innovation.61
Further studies and practical testing of the next-of-kin involvement guide are needed. Future evaluations should focus on how relevant and applicable the guide (Figure 5) is perceived by the hospitals and the clinical staff and how they respond to and modify their practice accordingly.50,62
We envision future testing of the guide for diverse purposes. Nursing staff on cancer wards could use it to reflect on current practice and discuss potential changes. It could also be tested in multidisciplinary teams of nurses, doctors, and managers in cancer care departments to assess structures, culture, and methods in use and what could be changed to strengthen next-of-kin involvement. We envision, for example, dialogue cafes in which patients, next-of-kin, and healthcare professionals use the guide as a basis of discussion.
Strengths and Limitations
This study has both strengths and limitations. First, the consensus meeting was a face-to-face 1-day meeting. Because of the extensive consensus processes, this meeting could have benefited from being extended by 1 day. However, funding constraints made this impossible. All participants from one of the hospitals had to travel by plane for this meeting, and a 1-day extension would have increased the cost and kept healthcare professionals out of clinical work for an additional day. Consequently, recruiting healthcare professionals for a 2-day meeting would have been more difficult.
A second limitation was sample size and representativeness of care providers. Healthcare professionals were the largest group in the interdiciplinary team of care providers, and an increased number of user representatives might have produced an even better understanding of the 6 challenges mentioned in the involvement guide. We mixed the groups with healthcare professionals and next-of-kin representatives to try to create consensus across diciplines and stakeholder groups with potentially different perspectives. This was done in line with the multistakeholder approach in this study. We have tried our best to meet ethical standards by having each participant prepare for the meeting by reading, reflecting and writing; to engage in the meeting through the introduction of research results and content analysis; by engaging a nonparticipant observer (observing power in the groups); and by asking the moderators to be aware of the potential risk of uneven power relations in the groups. However, we cannot rule out the potential of participants who did not dare to speak up in the mixed groups.
Third, there is a possibility that asking the participants to read and reflect on earlier published papers might have affected their views on the topic and could, in that sense, be a limitation. However, this could also be one of the study’s strengths. This is a key step in the modified NGT (Figure 4) and a way to retrieve and embed feedback to ensure stakeholder involvement in the research project.
Conclusions
In this article, we have described a nominal group consensus technique conducted with representatives from cancer departments in 2 Norwegian university hospitals. We included next-of-kin representatives and healthcare professionals within hospital cancer care. During the process, they identified key topics and elements in next-of-kin involvement. Based on the results, we developed a guide for next-of-kin involvement in cancer care. The guide (Figure 4) is created to support hospitals and has the potential to increase attention to and overcome challenges in next-of-kin involvement. Moreover, it emphasizes the role of next-of-kin and their importance for quality and safety in cancer care. Service providers can use the guide to develop and improve next-of-kin involvement practice or as a reflexive tool for organizational improvement. However, for future research, the guide needs additional empirical testing and refinement.
ACKNOWLEDGMENT
The authors would like to thank all stakeholders in the consensus meeting for generously sharing their valuable knowledge and experience.
Appendix Agenda of the meeting.
| 08:30-09:00 am | Registration |
| 09:00-10:10 am | Introduction |
| 10:10-10:30 am | Break |
| 10:30 am-12:00 noon | Group process (2 groups) |
| 12:00 noon-1:00 pm | Lunch |
| 1:00-2:15 pm | Plenary process 1 |
| 2:15-2:35 pm | Break |
| 2:35-3:40 pm | Plenary process 2 |
| 3:40-4:00 pm | Summary |
Footnotes
The project is funded by Stavanger University Hospital by a PhD position for Ms Bergerød.
The authors have no conflicts of interest to disclose.
The study was approved by the Regional Committee for Medicine and Health Research Ethics in Norway (2015/1488). Consent from study participants was confirmed and accepted by email. All participation in the study is based on voluntary recruitment. The project has also been approved by the data protection officers at the 2 case hospitals.
The dataset used and analyzed during this study is available from the corresponding author on reasonable request.
Authors’ contributions: IJB contributed to the study design, data collection, moderating in the consensus process, and analysis and interpretation of data and wrote the manuscript. SW contributed to the study design, data collection, analysis and interpretation of data, moderating the consensus process, and preparation of the manuscript. GSB contributed to the analysis and interpretation of data and moderating the consensus approach and commented on the drafts of the manuscript. BG contributed with moderating the consensus approach with analysis and with interpretation of data and has commented on manuscript drafts. BF coordinated the recruitment process in 1 hospital, was a nonparticipant observer of the consensus process, and has commented on manuscript drafts. All authors have approved the final manuscript.
Contributor Information
Geir S. Braut, Email: geir.sverre.braut@sus.no.
Birte Fagerdal, Email: birte.fagerdal@helse-bergen.no.
Bjørnar Gilje, Email: bjornar.gilje@sus.no.
Siri Wiig, Email: siri.wiig@uis.no.
References
- 1.Haukland EC, von Plessen C, Nieder C, Vonen B. Adverse events in hospitalised cancer patients: a comparison to a general hospital population. Acta Oncol (Stockholm, Sweden). 2017;56(9):1218–1223. [DOI] [PubMed] [Google Scholar]
- 2.Jha AK, Prasopa-Plaizier N, Larizgoitia I, Bates DW. Patient safety research: an overview of the global evidence. Qual Saf Health Care. 2010;19(1):42–47. [DOI] [PubMed] [Google Scholar]
- 3.Angood P Dingman J Foley ME, et al. Patient and family involvement in contemporary health care. J Patient Saf. 2010;6(1):38–42. [DOI] [PubMed] [Google Scholar]
- 4.Vincent C, Davis R. Patients and families as safety experts. Can Med Assoc J. 2012;184(1):15–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hara JK, Aase K, Waring J. Scaffolding our systems? Patients and families ‘reaching in’ as a source of healthcare resilience. BMJ Qual Saf. 2019;28:3–6. [DOI] [PubMed] [Google Scholar]
- 6.Tranberg M, Andersson M, Nilbert M, Rasmussen BH. Co-afflicted but invisible: a qualitative study of perceptions among informal caregivers in cancer care. J Health Psychol. 2019;1359105319890407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyborg I, Danbolt LJ, Kirkevold M. User participation is a family matter: a multiple case study of the experiences of older, hospitalised people and their relatives. J Clin Nurs. 2017;26:4353–4363. [DOI] [PubMed] [Google Scholar]
- 8.Bergerød IJ, Gilje B, Braut GS, Wiig S. Next-of-kin involvement in improving hospital cancer care quality and safety—a qualitative cross-case study as basis for theory development. BMC Health Serv Res. 2018;18(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenberg U, Ekstedt M, Olsson M, Ruland CM. Living close to a person with cancer: a review of the international literature and implications for social work practice. J Gerontol Soc Work. 2014;57(6–7):531–555. [DOI] [PubMed] [Google Scholar]
- 10.Gerhardt S, Dengso KE, Herling S, Thomsen T. From bystander to enlisted carer—a qualitative study of the experiences of caregivers of patients attending follow-up after curative treatment for cancers in the pancreas, duodenum and bile duct. Eur J Oncol Nurs. 2019;44:101717. [DOI] [PubMed] [Google Scholar]
- 11.Jadalla A, Ginex P, Coleman M, Vrabel M, Bevans M. Family caregiver strain and burden: a systematic review of evidence-based interventions when caring for patients with cancer. Clin J Oncol Nurs. 2020;24(1):31–50. [DOI] [PubMed] [Google Scholar]
- 12.Partanen E, Lemetti T, Haavisto E. Participation of relatives in the care of cancer patients in hospital—a scoping review. Eur J Cancer Care (Engl). 2018;27(2):e12821. [DOI] [PubMed] [Google Scholar]
- 13.O’Hara JK, Canfield C, Aase K. Patient and family perspectives in resilient healthcare studies: a question of morality or logic? Saf Sci. 2019;120:99–106. [Google Scholar]
- 14.Beckett K, Farr M, Kothari A, Wye L, le May A. Embracing complexity and uncertainty to create impact: exploring the processes and transformative potential of co-produced research through development of a social impact model. Health Res Policy Syst. 2018;16(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergerød IJ, Dalen I, Braut GS, Gilje B, Wiig S. Measuring next-of-kin satisfaction with hospital cancer care: using a mixed-method approach as basis for improving quality and safety. J Adv Nurs. 2020;76:1232–1246. [DOI] [PubMed] [Google Scholar]
- 16.Bell S Etchegaray J Gaufberg E, et al. A multi-stakeholder consensus-driven research agenda for better understanding and supporting the emotional impact of harmful events on patients and families. Jt Comm J Qual Patient Saf. 2018;44:424–435. [DOI] [PubMed] [Google Scholar]
- 17.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ (Clinical Res Ed). 1995;311(7001):376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Straus SE, Tetroe JM, Graham ID. Knowledge translation is the use of knowledge in health care decision making. J Clin Epidemiol. 2011;64(1):6–10. [DOI] [PubMed] [Google Scholar]
- 20.Jull J, Giles A, Graham ID. Community-based participatory research and integrated knowledge translation: advancing the co-creation of knowledge. Implement Sci. 2017;12(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langley J, Wolstenholme D, Cooke J. 'Collective making' as knowledge mobilisation: the contribution of participatory design in the co-creation of knowledge in healthcare. BMC Health Serv Res. 2018;18(1):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenhalgh T, Jackson C, Shaw S, Janamian T. Achieving research impact through co-creation in community-based health services: literature review and case study. Milbank Q. 2016;94(2):392–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damschroder LJ. Clarity out of chaos: use of theory in implementation research. Psychiatry Res. 2020;283:112461. doi: 10.1016/j.psychres.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 24.Banner D Bains M Carroll S, et al. Patient and public engagement in integrated knowledge translation research: are we there yet? Res Involve Engagem. 2019;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delbecq AL, Van de Ven AH. Group Techniques for Program Planning: A Guide to Nominal Groups and Delphi Process. Glenview, IL: Scott Foresman Company; 1975. [Google Scholar]
- 26.Creswell JW. A Concise Introduction to Mixed Methods Research. Thousand Oaks, CA: SAGE; 2015. [Google Scholar]
- 27.Bergerød IJ, Braut GS, Wiig S. Resilience from a stakeholder perspective: the role of next-of-kin in cancer care [published online ahead of print September 11, 2018]. J Patient Saf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Registry of Norway. Cancer in Norway 2018—Cancer Incidence, Mortality, Survival and Prevalence in Norway. Oslo: Cancer Registry of Norway; 2019. [Google Scholar]
- 29.OECD. Health at a Glance 2019: OECD Indicators. Paris: OECD Publishing; 2019. [Google Scholar]
- 30.Bate P, Mendel P, Robert G. Organizing for Quality: The Improvement Journeys of Leading Hospitals in Europe and the United States. Oxford: Radcliffe; 2008. [Google Scholar]
- 31.Robert GB Anderson JE Burnett SJ, et al. A longitudinal, multi-level comparative study of quality and safety in European hospitals: the QUASER study protocol. BMC Health Serv Res. 2011;11:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiig S Aase K von Plessen C, et al. Talking about quality: exploring how ‘quality’ is conceptualized in European hospitals and healthcare systems. BMC Health Serv Res. 2014;14:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergerød IJ, Wiig S. Leading Quality and Patient Safety Improvement in Norwegian Hospitals. Boca Raton, FL: Taylor & Francis; 2016:161–179. [Google Scholar]
- 34.Bergerød IJ, Wiig S. Ledelse og pasientsikkerhet. [Management and patient safety]. In: Aase K, ed. Pasientsikkerhet—Teori og Praksis [Patient safety—theory and practice]. 2nd ed. Norway: Universitetsforlaget; 2015:113–126. [Google Scholar]
- 35.Bergerød IJ. Ledelse, kvalitet og pasientsikkerhet : sammenlignende case studie av to norske sykehus. [Management, quality and patient safety: a comparative case study of two Norwegian hospitals] [thesis]. Norway: University of Stavanger; 2012. [Google Scholar]
- 36.Krein SL, Damschroder LJ, Kowalski CP, Forman J, Hofer TP, Saint S. The influence of organizational context on quality improvement and patient safety efforts in infection prevention: a multi-center qualitative study. Soc Sci Med (1982). 2010;71(9):1692–1701. [DOI] [PubMed] [Google Scholar]
- 37.Johannessen T, Ree E, Stromme T, Aase I, Bal R, Wiig S. Designing and pilot testing of a leadership intervention to improve quality and safety in nursing homes and home care (the SAFE-LEAD intervention). BMJ Open. 2019;9(6):e027790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polit DF, Beck CT. Essentials of Nursing Research: Appraising Evidence for Nursing Practice. 8th ed, Int ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 39.Graneheim UH, Lindgren BM, Lundman B. Methodological challenges in qualitative content analysis: a discussion paper. Nurse Educ Today. 2017;56:29–34. [DOI] [PubMed] [Google Scholar]
- 40.Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105–112. [DOI] [PubMed] [Google Scholar]
- 41.Wiig S, Haraldseid-Driftland C, Zachrisen RT, Hannisdal E, Schibevaag L. Next-of-kin involvement in regulatory investigations of adverse events that caused patient death: a process evaluation (part I—the next-of-kin's perspective) [published online ahead of print October 22, 2019]. J Patient Saf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiig S, Schibevaag L, Zachrisen RT, Hannisdal E, Anderson JE, Haraldseid-Driftland C. Next-of-kin involvement in regulatory investigations of adverse events that caused patient death: a process evaluation (part II: the inspectors' perspective) [published online ahead of print October 22, 2019]. J Patient Saf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ekstedt M, Stenberg U, Olsson M, Ruland CM. Health care professionals' perspectives of the experiences of family caregivers during in-patient cancer care. J Fam Nurs. 2014;20(4):462–486. [DOI] [PubMed] [Google Scholar]
- 44.Stenberg U, Cvancarova M, Ekstedt M, Olsson M, Ruland C. Family caregivers of cancer patients: perceived burden and symptoms during the early phases of cancer treatment. Soc Work Health Care. 2014;53(3):289–309. [DOI] [PubMed] [Google Scholar]
- 45.Ugalde A Gaskin CJ Rankin NM, et al. A systematic review of cancer caregiver interventions: appraising the potential for implementation of evidence into practice. Psychooncology. 2019;28(4):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kislov R. Engaging with theory: from theoretically informed to theoretically informative improvement research. BMJ Qual Saf. 2019;28(3):177–179. [DOI] [PubMed] [Google Scholar]
- 47.Bate P, Robert G. Experience-based design: from redesigning the system around the patient to co-designing services with the patient. Qual Saf Health Care. 2006;15(5):307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donetto S, Tsianakas V, Robert G. Using Experience-Based Co-Design (EBCD) to Improve the Quality of Healthcare: Mapping Where We Are Now and Establishing Future Directions. London: King’s College London; 2014. [Google Scholar]
- 49.Donetto S, Pierri P, Tsianakas V, Robert G. Experience-based co-design and healthcare improvement: realizing participatory Design in the Public Sector. Design J. 2015;18(2):227–248. [Google Scholar]
- 50.Gagliardi AR, Berta W, Kothari A, Boyko J, Urquhart R. Integrated knowledge translation (IKT) in health care: a scoping review. Implement Sci. 2016;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnett S Renz A Wiig S, et al. Prospects for comparing European hospitals in terms of quality and safety: lessons from a comparative study in five countries. Int J Qual Health Care. 2013;25(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiig S Ree E Johannessen T, et al. Improving quality and safety in nursing homes and home care: the study protocol of a mixed-methods research design to implement a leadership intervention. BMJ Open. 2018;8:e020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson JE Robert G Nunes F, et al. Translating research on quality improvement in five European countries into a reflective guide for hospital leaders: the ‘QUASER hospital Guide’. Int J Qual Health Care. 2019;31(8):G87–G96. [DOI] [PubMed] [Google Scholar]
- 54.Jones L, Pomeroy L, Robert G, Burnett S, Anderson JE, Fulop NJ. How do hospital boards govern for quality improvement? A mixed methods study of 15 organisations in England. BMJ Qual Saf. 2017;26(12):978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones L Pomeroy L Robert G, et al. Explaining organisational responses to a board-level quality improvement intervention: findings from an evaluation in six providers in the English National Health Service. BMJ Qual Saf. 2019;28(3):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham ID, Kothari A, McCutcheon C. Moving knowledge into action for more effective practice, programmes and policy: protocol for a research programme on integrated knowledge translation. Implement Sci. 2018;13(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulte PA Cunningham TR Nickels L, et al. Translation research in occupational safety and health: a proposed framework. Am J Ind Med. 2017;60(12):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiig S, Aase K, Bal R. Reflexive spaces: leveraging resilience into healthcare regulation and management [published online ahead of print January 31, 2020]. J Patient Saf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambert SD Ould Brahim L Morrison M, et al. Priorities for caregiver research in cancer care: an international Delphi survey of caregivers, clinicians, managers, and researchers. Support Care Cancer. 2019;27(3):805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wittenberg E, Goldsmith J, Parnell TA. Development of a communication and health literacy curriculum: optimizing the informal cancer caregiver role. Psychooncology. 2020;29:766–774. [DOI] [PubMed] [Google Scholar]
- 61.Mallidou AA, Atherton P, Chan L, Frisch N, Glegg S, Scarrow G. Core knowledge translation competencies: a scoping review. BMC Health Serv Res. 2018;18(1):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khoddam H, Mehrdad N, Peyrovi H, Kitson AL, Schultz TJ, Athlin AM. Knowledge translation in health care: a concept analysis. Med J Islam Repub Iran. 2014;28:98. [PMC free article] [PubMed] [Google Scholar]


