INTRODUCTION:
Incidence of colorectal cancer (CRC) in young adults has been increasing in recent decades in many countries for still widely unclear reasons. Suspected candidates include increasing prevalence of overweight and obesity, but specific evidence on their role for early-onset CRC (EOCRC) is sparse. We conducted a systematic review and meta-analysis to summarize available evidence on the association of body mass index (BMI) with EOCRC.
METHODS:
We systematically searched PubMed, EMBASE, and Web of Science up to February 2021 for studies that evaluated the association of BMI (before diagnosis but not near diagnosis) with CRC risk and reported specific results for EOCRC. Results from studies with similar BMI groupings were summarized in meta-analyses using random-effects models.
RESULTS:
Twelve studies were eligible and included. Results of 6 studies were pooled in meta-analyses, which yielded a higher risk of EOCRC for overweight and obesity (BMI ≥25 kg/m2) compared with normal weight (odds ratio [OR] 1.42, 95% confidence interval [CI] 1.19–1.68). An increasing risk with increasing BMI was observed, with much higher risk for obesity (OR 1.88, 95% CI 1.40–2.54) than for overweight (OR 1.32, 95% CI 1.19–1.47).
DISCUSSION:
Obesity is a strong risk factor for EOCRC, and its increasing prevalence in younger generations is likely to substantially contribute to the increase in EOCRC. Efforts to limit the obesity epidemic in adolescents and younger adults may be crucial for reducing CRC incidence in future generations of adults.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related deaths worldwide, with an estimated 1.8 million new cases in 2018 (1). Results from multiple studies indicate that despite overall decline or stabilization of CRC incidence because of screening programs among persons aged 50 years or older in many high-income countries, the incidence of CRC has been increasing in persons <50 years in several countries such as the United States, the United Kingdom, Canada, Australia, New Zealand, France, Denmark, Sweden, Slovenia, and Japan (2–8). The largest increase in early-onset CRC (EOCRC) incidence in Europe was observed in 20–39-year-old people (5) who are not covered by screening programs. These patterns suggest a role of an unfavorable shift in prevalence of risk factors that might account for the increasing EOCRC incidence. However, the specific contributions of various risk factors are yet to be clarified (9–12).
Excess body fatness, most commonly measured by increased body mass index (BMI), is an established risk factor for CRC (13–16). However, existing evidence is largely based on studies on CRC at all ages. In many developed countries, only approximately 5% and 10% of CRC cases occur below ages 50 and 55 (5,6,8), respectively, and the role of body fatness for such EOCRC remains to be established. This is of particular importance given the increasing prevalence of overweight and obesity among children and adolescents in many countries in recent decades (17–19), In this study, we aimed to conduct a systematic review and meta-analysis of epidemiological studies on the association of BMI (before diagnosis but not near diagnosis) with EOCRC risk.
METHODS AND MATERIALS
The reporting of this systematic review follows the PRISMA statement (20).
Literature search
We conducted a systematic literature search in the PubMed, EMBASE, and Web of Science databases. Because there are few studies that specifically focused on the association of BMI with EOCRC risk, we initially searched for all original epidemiological studies on BMI and CRC risk, which might have contained our target population of younger adults and reported specific results for this group. A broad-range search up to February 28, 2021, based on the search strategy published in the World Cancer Research Fund International Systematic Literature Review (21) was applied without language restrictions. Details of the search strategy are provided in the Supplementary Methods (see Supplementary Digital Content 2, http://links.lww.com/AJG/C129). Briefly, we used diagnosed colon or rectal cancer as outcome, included measurement terms related to body fatness and anthropometry, and excluded nonhuman studies and obviously irrelevant publication types in the search strategy. Reference lists of relevant articles were also hand-searched for potentially eligible publications.
Study eligibility
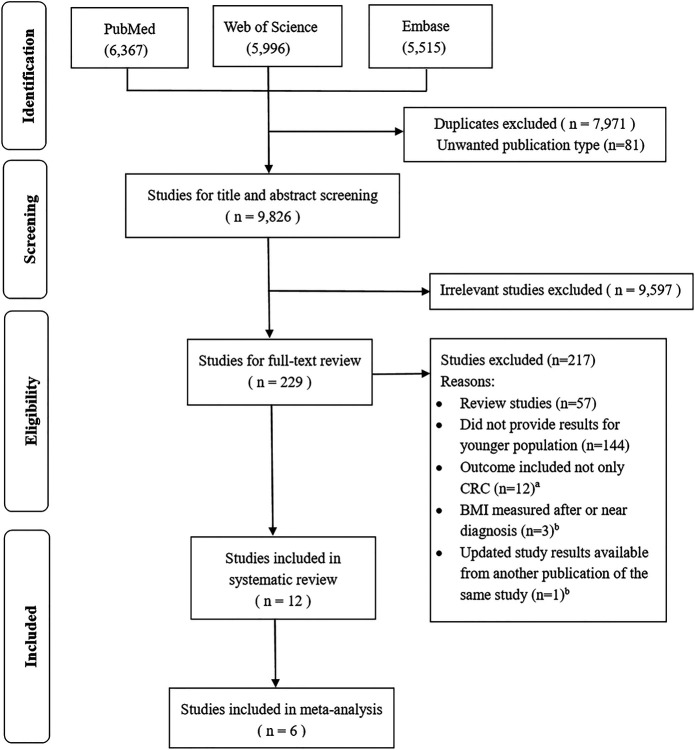
A clear definition of EOCRC has not been widely established. Many studies use 50 years as the threshold age for defining CRC in younger population because it is the starting age for CRC screening recommended in many countries' screening guidelines (22). In this systematic review, we defined all first time diagnoses of CRC in persons aged 55 years or younger as having occurred in younger adults, which allowed to accommodate more eligible studies. We also conducted sensitivity analyses with a cutoff age of EOCRC at 50 years for reference. Studies were eligible to be included in this systematic review if they were published as original articles and reported effect estimate(s) (e.g., relative risks [RRs], hazard ratios [HRs], or odds ratios [ORs]) for the association of BMI with CRC risk (colon, rectal, or CRC). Studies were excluded if they included participants older than 55 years only or did not report specific results for younger populations. Our search included studies using various measures of body fatness, such as BMI, waist circumference, hip circumference, or waist-to-hip ratio. However, because the results from multiple studies meeting the further inclusion criteria were identified for BMI only, further analyses focused on studies using this metric. BMI assessments were obtained at different times, and some studies did not state BMI assessment time clearly, so it is hard to make strict criteria for the time of BMI assessment. However, we made utmost efforts to ensure that to exclusively focus on BMI measures might not be affected by tumor progression or cancer treatment based on information provided in the studies' methods, discussion, and limitation sections. BMI measures obtained after or near diagnosis of CRC, including BMI measures from cross-sectional studies (such as screening colonoscopy studies), were not considered. Studies were excluded if they reported results for CRC combined with other outcomes only or if their study populations overlapped with otherwise included study populations (Figure 1; see Supplementary Table 1, Supplementary Digital Content 3, http://links.lww.com/AJG/C130).
Figure 1.
Flow chart showing selection of eligible studies. aOutcomes combined colorectal cancer and polys/neoplasia/adenomas together. bSee Supplementary Table 1 (Supplementary Digital Content 3, http://links.lww.com/AJG/C130). BMI, body mass index; CRC, colorectal cancer.
Data extraction and quality assessment
Two authors (H.J.L. and D.B.) extracted data from eligible studies independently from each other. Descriptive characteristics of eligible studies including study design, publication year, country, race, sample size, number of cases, sex, age at recruitment, timing of BMI assessment, follow-up time, and age at CRC diagnosis were extracted. In addition, we extracted the results on the association of BMI categories with CRC risk, estimated with RRs, ORs, or HRs as well as their 95% confidence intervals (CIs) and covariates that were adjusted for. If reported in the same study, associations of BMI with CRC risk among older adults were also extracted for comparison. Risk of study bias assessment methods based on the Newcastle-Ottawa Scale (23) in a domain-based approach was used to comprehensively assess the degree of risk of bias in each of the studies. Studies that were at low risk of bias in all domains were considered as low risk of bias studies; studies with at least 1 unclear risk of bias domain (all other domain being at low risk) were considered as unclear risk of bias studies; and studies with 1 or more domains at high risk of bias were classified as high risk of bias studies. Low, unclear, or high risks of bias were color coded as green, yellow, and red, respectively.
Data synthesis
Different effect estimates for CRC risk (RRs, ORs, and HRs) were reported in the included studies. Because the absolute risk of EOCRC is low, the 3 different measures were treated as equivalent risk measures. We log transformed the extracted RRs, ORs, and HRs and estimated their standard errors indirectly (24). Then, the RR, OR, and HR estimates were pooled in both fixed-effects and random-effects models. The results from random-effects models were finally chosen because of the small number of included studies and high heterogeneity in some of them (25). Because meta-analyses included only 6 studies, the risk of publication bias was not formally assessed (see Supplementary Figure 5, Supplementary Digital Content 1, http://links.lww.com/AJG/C128) (26). Studies not suitable for meta-analysis were synthesized in a systematic fashion using systematic review without meta-analysis methods (27,28).
We used the World Health Organization's BMI classification and performed meta-analyses in 2 ways because of heterogeneous categorizations of BMI in the included studies: (i) overweight and obesity (BMI ≥25 kg/m2) combined compared with normal weight and (ii) overweight or obesity separately compared with normal weight (29). All analyses were performed with the R statistical software (version 3.5.3; R Foundation for Statistical Computing, Vienna, Austria) and the R meta package (version 4.8-4). All P values are 2-sided, and the level of significance was set at 0.05.
RESULTS
Literature search
Figure 1 shows the flow chart of the literature search. The literature search identified 17,878 records in the initial electronic database searches. After exclusion of duplicates and title and abstract screening, 229 articles were eligible for full-text review. Of these, 12 studies were eligible and included in this systematic review. Of the 12 included studies (30–41), 6 studies (32,35,36,39–41) with similar BMI groupings were included in the meta-analysis. The results of studies that could not be included in the meta-analysis are synthesized in the Supplementary Figure 1 (see Supplementary Digital Content 1, http://links.lww.com/AJG/C128).
Study characteristics
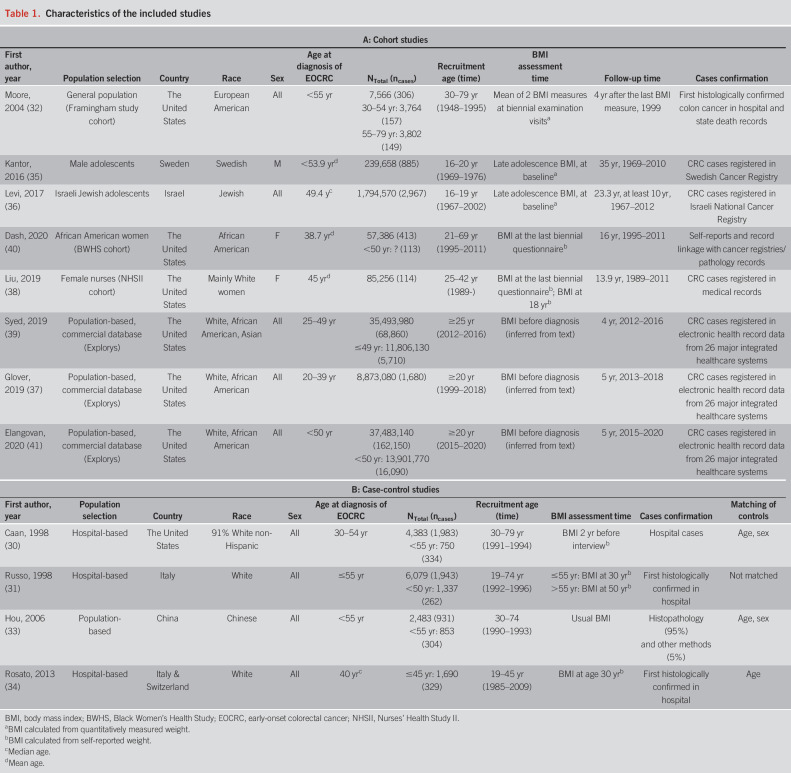
Table 1 summarizes the basic characteristics of the studies which included 8 cohort studies (32,35–41) and 4 case-control studies (30,31,33,34), with a total of 242,561 CRC cases (32,275 cases aged ≤55 years). By far the largest number of EOCRC cases (n = 16,090) was contributed by the study of Elangovan et al. (41), who analyzed data from a large US medical claims database. Publication years ranged from 1998 to 2020. Studies were from high-income countries (the United States, Israel, Italy, Sweden, and Switzerland) and 1 upper-middle-income country (China). The largest studies, and those with the highest EOCRC case numbers, were from the United States (75% of EOCRC cases). Regarding sex, 1 study conducted among military personnel included men only (35); 2 studies investigated women only (38,40) and the remaining studies examined both sexes. Three studies included colon cancer cases only (30,32,33) and the remainder (9 studies) included both colon and rectal cancer cases. BMI assessment was obtained at different times. For cohort studies, 3 studies (32,38,40) used BMI during follow-up examination/questionnaire visits, 3 (35,36,38) used BMI at late adolescence (also at baseline), and 3 (37,39,41) used the same commercial database whose search criteria ensured that BMI was recorded before the diagnosis of CRC. For case-control studies, 1 study (30) used BMI 2 years before interview, 2 studies (31,34) used BMI at age 30 years, and Hou et al. (33) used “usual BMI” as BMI exposure before diagnosis. Covariates adjusted for in the studies varied and included age, sex, use of aspirin, smoking, alcohol intake, physical activity, and family history of CRC. The risk of bias assessment is summarized in Supplementary Table 2 (see Supplementary Digital Content 4, http://links.lww.com/AJG/C131). Overall, the risk of bias of the eligible studies was rated as “low,” “unclear,” and “high” in 3, 3, and 6 studies, respectively, with higher risks of bias in the case-control studies than in the cohort studies.
Table 1.
Characteristics of the included studies
BMI and risk of CRC among younger adults
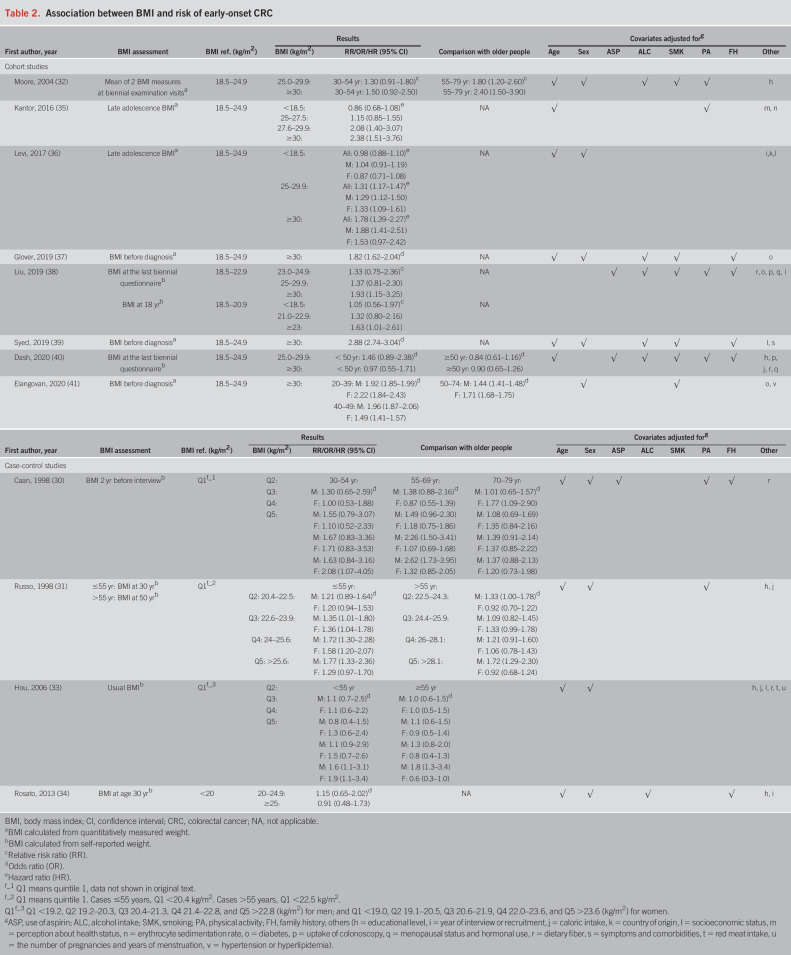
Associations of BMI with CRC risk among younger adults (≤55 years) are shown in Table 2. Overall, 9 (30,31,33,35–39,41) of the 12 included studies found a positive association between overweight and obesity with increased EOCRC risk. All studies that used BMI at late adolescence also found a positive association with EOCRC risk. The strongest association with an OR of 2.88 (95% CI 2.74–3.04) was reported for obese compared with normal weight participants in the very large study by Syed et al. (39), which was based on a claims database from the United States. Among the studies showing results by sex, associations of obesity with EOCRC risk seemed to be diverse. For example, in the cohort study by Levi et al. (36), the HR for obesity was 1.88 among men and 1.53 among women, but confidence intervals of sex-specific estimates were wide and overlapping. Similar patterns were reported in the case-control study by Russo et al. (31), with odds ratios of 1.77 and 1.29 for the highest versus lowest quintile of BMI at age 30 among men and women, respectively. Conversely, Caan et al. (30) and Hou et al. (33) found stronger risk elevations of EOCRC for the upper quintiles of BMI among women. Elangovan et al. (41) found a stronger association of obesity with EOCRC risk for women in age group 20–39 and for men in age group 40–49.
Table 2.
Association between BMI and risk of early-onset CRC
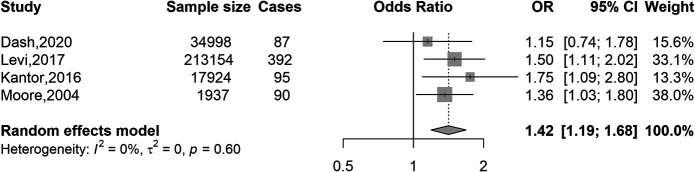
Figure 2 shows the results of the meta-analysis of the association of overweight and obesity combined versus normal weight with EOCRC risk. Overweight and obesity (BMI ≥25 kg/m2) were associated with a 42% increased risk of CRC compared with normal weight (OR 1.42, 95% CI 1.19–1.68). Tests for heterogeneity indicated a low degree of heterogeneity (I(2) = 0%, P-heterogeneity = 0.60) across the 4 studies. When taking 50 as the cutoff age for EOCRC, the OR was 1.38 (95% CI 1.08–1.76) (see Supplementary Figure 2, Supplementary Digital Content 1, http://links.lww.com/AJG/C128).
Figure 2.
Association of BMI (overweight and obese vs normal weight) with colorectal cancer risk in younger adults (≤55 years). BMI, body mass index; CI, confidence interval; OR, odds ratio. BMI categories: normal weight, 18.5–24.9 kg/m2 (reference); overweight and obese, ≥25 kg/m2.
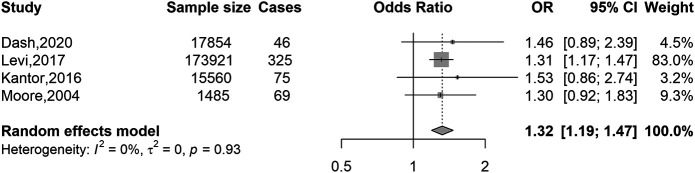
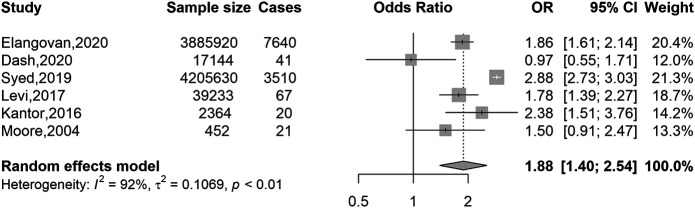
Figures 3 and 4 show separate meta-analyses of the associations of overweight versus normal weight and obesity versus normal weight with EOCRC risk. A substantially stronger excess risk was observed for obesity (OR 1.88, 95% CI 1.40–2.54) than for overweight (OR 1.32, 95% CI 1.19–1.47). Sensitivity analyses with a cutoff age of 50 years showed very similar results (see Supplementary Figures 3 and 4, Supplementary Digital Content 1, http://links.lww.com/AJG/C128).
Figure 3.
Association of BMI with colorectal cancer risk in younger adults (≤55 years): overweight vs normal weight. BMI, body mass index; CI, confidence interval; OR, odds ratio. BMI categories: normal weight, 18.5–24.9 kg/m2 (reference); overweight, 25–29.9 kg/m2.
Figure 4.
Association of BMI with colorectal cancer risk in younger adults (≤55 years): obese vs normal weight. BMI, body mass index; CI, confidence interval; OR, odds ratio. BMI categories: normal weight, 18.5–24.9 kg/m2 (reference); obese ≥30 kg/m2.
Comparisons of associations of BMI with risk of CRC among younger and older adults
Among studies that reported and compared associations of BMI with CRC risk in younger (≤55 years) and older (>55 years) populations (30–33,40,41), Caan et al. (30) found the association of BMI with CRC risk in men to be stronger in the younger population than in those aged 70–79 years, but associations were mostly comparable with those aged 55–69 years, except for the fourth and fifth quintiles of BMI where a higher risk of CRC was observed in the 55–69 years age group (Table 2). However, in the study by Russo et al. (31), the association of BMI with CRC risk was comparable in both younger and older populations. Moore et al. (32) found stronger associations of overweight and obesity with increased CRC risk in participants aged ≥55 years than in younger participants. Hou et al. (33) found increased CRC risk for BMI levels in the fifth quintile in both younger (<55 years) men and women, whereas a similar association was only observed in older (≥55 years) men. Dash et al. (40) found no associations of BMI with CRC risk, neither in younger (<50 years) nor in older (≥50 years) participants. Elangovan et al. (41) found stronger associations of obesity with increased CRC risk in participants aged <50 years than in older participants (50–74 years).
DISCUSSION
The prevalence of overweight and obesity in adolescents and younger adults is high and increasing in many countries, especially in high-income countries (17–19). Because the incidence of CRC is also increasing in younger adults in many countries (2–8), investigating the potential role of BMI as a risk factor for EOCRC is highly relevant. We conducted a systematic review and meta-analysis to synthesize available evidence on the association of BMI with CRC risk in the younger population.
The results from our meta-analysis indicate that overweight and obese younger adults have approximately 32% and 88% higher risk of developing CRC than those with normal weight, respectively. These results are consistent and comparable in magnitude with those from studies that evaluated BMI and risk of CRC at all ages, most of which occurs at older ages (13,14,42). For sex-specific associations, no consistent pattern regarding the differences in the association of BMI with EOCRC were observed. Previous studies had shown a stronger association of BMI with CRC risk for men than for women in the older population (15,43,44). In the study by Elangovan et al. (41) which was based on a large US medical claims database and included the largest number of EOCRC cases, patients with CRC diagnosed in the 20–39 year age group were predominantly women. This may be due to younger women being more connected with healthcare system for cervical screening or pregnancies examinations, thus having higher chances of earlier diagnosis than men.
Besides potential detection bias by sex and possible socioeconomic factors in studies using medical claims database, these studies also used partly overlapping study populations. Glover et al. (37), Syed et al. (39), and Elangovan et al. (41) used the same medical claims database (Explorys, IBM Watson Health). In addition, certain patients may have been counted multiple times if they received health care at multiple institutions that utilize the Explorys database (37). Syed et al. (39) who reported the highest OR for obesity of 2.88 (95% CI 2.74–3.04) did not perform a true regression analysis because of an aggregated deidentified data set and did not exclude subjects with inflammatory bowel disease and family history of malignant neoplasm of digestive organs, which the other 2 studies did. Because of their specific limitations, the results from medical claims databases need to be interpreted with due caution.
All 3 cohort studies using BMI at late adolescence as BMI exposure found a positive association with EO CRC risk (35,36,38). Adolescent BMI is strongly correlated with adult BMI (45), and obesity in adolescence has been shown to be a risk factor of several cancers (46,47), possibly by creating a procarcinogenic environment through various mechanisms such as changes in insulin and other hormones, insulin-like growth factors, and adipokine secretion. Cumulative exposure to an obesogenic environment might drive procancerous pathophysiological processes (48).
The results of sensitivity analyses with a cutoff age of 50 years are very close to the results obtained with a cutoff age of 55 years, which indicates that the observed associations are robust against variations of definitions of EOCRC. However, results by Hou et al. (33) suggest that menopause status might affect the association of higher BMI and colon cancer risk. In their study, higher BMI was associated with an increased risk of CRC in premenopausal women <55 years of age (OR for highest versus lowest quintile 1.9, 95% CI 1.1–4.9) and a decreased risk of CRC among postmenopausal women (OR 0.6, 95% CI 0.5–0.9). Future studies should pay attention to a potential role of menopausal status.
The cohort study by Dash et al. (40) was restricted to African American women and did not find a significant association between BMI and CRC, neither in younger nor in older women. However, the cohort included a large proportion of women who reported a colonoscopy or sigmoidoscopy during the follow-up period from 1997 to 2011, which may have altered the natural history and subsequent risk of CRC to some extent. In other studies conducted in the United States that included both women and men of different ethnicities including African Americans, BMI was positively associated with EOCRC (37,39,49).
Several studies looked at the association of other indicators of obesity with EOCRC risk. Moore et al. (32) found that a larger waist circumference (≥99.1 and 101.6 cm for women and men, respectively) was independently associated with a 2-fold increased risk of colon cancer and a particularly strong association was found among sedentary subjects (RR = 4.4 for middle-aged adults; RR = 3.0 for older adults). Caan et al. (30) found that after controlling for BMI, the waist-to-hip ratio was not associated with colon cancer in men but was associated with a slight risk increase in women. Russo et al. (31) found that the waist-to-hip ratio was positively associated with EOCRC risk independent of BMI (OR for ≥0.90 vs ≤0.81 = 1.6; 95% CI 1.2–2.1). Further research is required to more precisely define the specific role of excessive weight and abdominal obesity for EOCRC risk among men and women.
Several studies that did not meet our inclusion criteria as they looked at different outcomes, such as combined outcomes of CRC and adenoma, also reported positive associations between body fatness and risk of colorectal neoplasms at young ages (50–52). For example, in the study by Kim et al. (51), overweight (BMI ≥25 kg/m2) was associated with increased risk of advanced colorectal neoplasia (defined as an adenoma ≥10 mm in diameter, adenoma with any component of villous histology, high-grade dysplasia, or invasive cancer) in adults <50 years of age (OR 1.23, 95% CI 1.03–1.47). Kim et al. (52) also found both overweight (BMI ≥25 kg/m2) and abdominal obesity (waist circumference: men ≥90 cm, women ≥80 cm) to be independent risk factors for both colorectal neoplasia (defined as cancer or any adenoma) and advanced colorectal neoplasia in young adults aged 20–39 years. Juo et al. (53) found obesity (BMI >30 kg/m2) to be associated with a reduction in age at diagnosis of CRC by 4.56 ± 0.18 years; an even stronger reduction in age at diagnosis (7.75 ± 0.30 years) was observed for morbid obesity (BMI >40 kg/m2).
In contrast to the global increase in overweight and obesity, the prevalence of other life style-related risk factors of CRC, such as smoking and alcohol consumption, has decreased in many high-income countries in recent years (54,55). This suggests that the increasing trend of overweight and obesity and their potential consequences, such as increasing prevalence of early diabetes (56), or factors associated with overweight and obesity, such as a sedentary lifestyle and specific nutritional habits, might play a key role in the increasing EOCRC incidence rates. Because overweight and obesity are associated with numerous other adverse health outcomes, efforts to curb the obesity epidemic will be paramount far beyond CRC prevention.
Our systematic review focused on relative risk estimates. For guiding clinical decision making, absolute estimates may even be more relevant. Although none of the included studies reported absolute risk estimates, the relative risk estimates could be combined with external data, such as cancer registry data, to derive absolute risk estimates, which might also be most relevant for modeling the impact of specific prevention strategies.
Strengths and limitations
To our knowledge, our study is the first to summarize the evidence for the association of BMI with EOCRC risk in a systematic review and meta-analysis. This study included a comprehensive literature search following the search strategy by the World Cancer Research Fund. Nevertheless, despite screening an overall very large number of studies assessing the association of overweight and obesity with CRC risk at all ages, only a relatively small number of studies that explicitly reported on subgroup analyses for EOCRC could be included. The diversity of study populations, study designs, and measures of overweight of eligible studies are both a strength and a limitation of our analysis.
A number of important limitations require careful consideration. Diverse timing of BMI assessment, different inclusion and exclusion criteria, and different covariate adjustment limit the comparability of results from the various studies. Because of diverse categorization of BMI, our meta-analysis had to be restricted to 6 studies that used comparable BMI categories. In addition, given the limited number of studies and information available from these studies, we could not perform dose-response meta-analyses. The study by Levi et al. (36) whose study populations were mostly Jews contributed the largest weight in the overweight vs normal meta-analysis (Figure 3). This might affect the generalizability of our estimates if the magnitude of the association of overweight with EOCRC risk differs by ethnicity.
Although we tried to minimize bias by strict inclusion and exclusion criteria, the findings may still be affected by a number of potential biases. For example, obese people could have been offered colonoscopy earlier due to their increased risk of CRC which might have led to earlier detection and apparently increased risk of EOCRC. Conversely, more frequent offers of colonoscopy to obese people because of their increased risk may have reduced such risk because of polypectomy, which might have led to apparently reduced risk of EOCRC. Some studies have also reported less use of colonoscopy by obese people (57,58), making it hard to predict if, and to what extent such differences in colonoscopy use might have affected the results.
A most crucial issue for further research on BMI and EOCRC is the proper timing of BMI measurement. Studies with measurement of BMI at, shortly before, or after CRC diagnosis are at very high risk of reverse causality, given that disease-associated weight loss is well known for patients with CRC. Ascertainment of BMI years before diagnosis in case-control studies and exclusion of early years of follow-up in cohort studies are paramount to minimize bias from reverse causality.
In this first systematic review and meta-analysis on the association of BMI and CRC risk in younger adults, both overweight and obesity were strongly associated with increased risk of CRC. The magnitude of the association of BMI with CRC risk for younger adults seems to be comparable with the association previously reported for all ages or specifically for older adults, suggesting that higher BMI might also be an important risk factor for EOCRC. Along with the observation of a major increase in prevalence of overweight and obesity, our findings support suggestions of their major role in increasing incidence of EOCRC. Interventions aimed at preventing and enhancing the management of obesity in adolescents and younger adults, which are crucial for the prevention of many other adverse health outcomes, might also play a key role for reducing CRC incidence in younger and older adults, and should be a public health priority.
CONFLICTS OF INTEREST
Guarantor of the article: Hermann Brenner, MD, MPH.
Specific author contributions: H.B., M.H., H.J.L., and D.B.: conception and design of the study. H.J.L. and D.B.: literature search and data extraction. H.J.L., D.B., X.C.C., M.H., and H.B.: analysis and interpretation of data. H.J.L., D.B., and H.B.: draft of article. H.J.L., D.B., X.C.C., M.H., and H.B.: revision and approval of article. All authors provided comments, revised the draft, and approved the final version of the article.
Financial support: H.L. was supported by grant from the China Scholarship Council (CSC).
Potential competing interests: None to report.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C128, http://links.lww.com/AJG/C129, http://links.lww.com/AJG/C130, http://links.lww.com/AJG/C131
Contributor Information
Hengjing Li, Email: hengjing.li@dkfz-heidelberg.de.
Daniel Boakye, Email: boakye@leibniz-bips.de.
Xuechen Chen, Email: xuechen.chen@dkfz-heidelberg.de.
Michael Hoffmeister, Email: m.hoffmeister@Dkfz-Heidelberg.de.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Troeung L, Sodhi-Berry N, Martini A, et al. Increasing incidence of colorectal cancer in adolescents and young adults aged 15–39 years in Western Australia 1982–2007: Examination of colonoscopy history. Front Public Health 2017;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner DR, Ruan Y, Shaw E, et al. Increasing colorectal cancer incidence trends among younger adults in Canada. Prev Med 2017;105:345–9. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Chen W, Lin J, et al. Incidence and characteristics of young-onset colorectal cancer in the United States: An analysis of SEER data collected from 1988 to 2013. Clin Res Hepatol Gastroenterol 2019;43(2):208–15. [DOI] [PubMed] [Google Scholar]
- 5.Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019;68(10):1820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araghi M, Soerjomataram I, Bardot A, et al. Changes in colorectal cancer incidence in seven high-income countries: A population-based study. Lancet Gastroenterol Hepatol 2019;4(7):511–8. [DOI] [PubMed] [Google Scholar]
- 7.Lui RN, Tsoi KK, Ho JM, et al. Global increasing incidence of young-onset colorectal cancer across 5 continents: A joinpoint regression analysis of 1,922,167 cases. Cancer Epidemiol Biomarkers Prev 2019;28(8):1275–82. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019;68(12):2179–85. [DOI] [PubMed] [Google Scholar]
- 9.Deen KI, Silva H, Deen R, et al. Colorectal cancer in the young, many questions, few answers. World J Gastrointest Oncol 2016;8(6):481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Lancet Oncology. Colorectal cancer: A disease of the young? Lancet Oncol 2017;18(4):413. [DOI] [PubMed] [Google Scholar]
- 11.Mauri G, Sartore-Bianchi A, Russo AG, et al. Early-onset colorectal cancer in young individuals. Mol Oncol 2019;13(2):109–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg BA, Marshall JL. Colon cancer in young adults: Trends and their implications. Curr Oncol Rep 2019;21(1):3. [DOI] [PubMed] [Google Scholar]
- 13.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: A meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16(12):2533–47. [DOI] [PubMed] [Google Scholar]
- 14.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: Findings from 56 observational studies. Obes Rev 2010;11(1):19–30. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS One 2013;8(1):e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: A systematic review and meta-analysis. Am J Epidemiol 2015;181(11):832–45. [DOI] [PubMed] [Google Scholar]
- 17.Malik VS, Willett WC, Hu FB. Global obesity: Trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9(1):13–27. [DOI] [PubMed] [Google Scholar]
- 18.Chung A, Backholer K, Wong E, et al. Trends in child and adolescent obesity prevalence in economically advanced countries according to socioeconomic position: A systematic review. Obes Rev 2016;17(3):276–95. [DOI] [PubMed] [Google Scholar]
- 19.Abarca-Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390(10113):2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imperial College London Continuous Update Project Team Members. World Cancer Research Fund International Systematic Literature Review. The Associations between Food, Nutrition and Physical Activity and the Risk of Colorectal Cancer. (https://www.wcrf.org/sites/default/files/colorectal-cancer-slr.pdf). Revised September 6, 2017. Accessed March 2, 2021. [Google Scholar]
- 22.Ebell MH, Thai TN, Royalty KJ. Cancer screening recommendations: An international comparison of high income countries. Public Health Rev 2018;39:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Accessed March 3, 2021.
- 24.Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat Med 2008;27(29):6072–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Part 2: General Methods for Cochrane Reviews. 2011. (http://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm). Accessed March 3, 2021. [Google Scholar]
- 26.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Part 2: General Methods for Cochrane Reviews. 2011. (http://handbook-5-1.cochrane.org/chapter_9/9_5_heterogeneity.htm). Accessed March 3, 2021. [Google Scholar]
- 27.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. 2021. (https://training.cochrane.org/handbook/current/chapter-12). Accessed March 12, 2021. [Google Scholar]
- 29.World Health Organization. Body Mass Index—BMI. 2019. (https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi). Accessed November 3, 2020. [Google Scholar]
- 30.Caan BJ, Coates AO, Slattery ML, et al. Body size and the risk of colon cancer in a large case-control study. Int J Obes Relat Metab Disord 1998;22(2):178–84. [DOI] [PubMed] [Google Scholar]
- 31.Russo A, Franceschi S, La Vecchia C, et al. Body size and colorectal-cancer risk. Int J Cancer 1998;78(2):161–5. [DOI] [PubMed] [Google Scholar]
- 32.Moore LL, Bradlee ML, Singer MR, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obes Relat Metab Disord 2004;28(4):559–67. [DOI] [PubMed] [Google Scholar]
- 33.Hou L, Ji BT, Blair A, et al. Body mass index and colon cancer risk in Chinese people: Menopause as an effect modifier. Eur J Cancer 2006;42(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosato V, Bosetti C, Levi F, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control 2013;24(2):335–41. [DOI] [PubMed] [Google Scholar]
- 35.Kantor ED, Udumyan R, Signorello LB, et al. Adolescent body mass index and erythrocyte sedimentation rate in relation to colorectal cancer risk. Gut 2016;65(8):1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levi Z, Kark JD, Katz LH, et al. Adolescent body mass index and risk of colon and rectal cancer in a cohort of 1.79 million Israeli men and women: A population-based study. Cancer 2017;123(20):4022–30. [DOI] [PubMed] [Google Scholar]
- 37.Glover M, Mansoor E, Panhwar M, et al. Epidemiology of colorectal cancer in average risk adults 20–39 years of age: A population-based national study. Dig Dis Sci 2019;64(12):3602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 2019;5(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Syed AR, Thakkar P, Horne ZD, et al. Old vs new: Risk factors predicting early onset colorectal cancer. World J Gastrointest Oncol 2019;11(11):1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dash C, Yu J, Nomura S, et al. Obesity is an initiator of colon adenomas but not a promoter of colorectal cancer in the Black Women's Health Study. Cancer Causes Control 2020;31(4):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elangovan A, Skeans J, Landsman M, et al. Colorectal cancer, age, and obesity-related comorbidities: A large database study. Dig Dis Sci 2020. [Epub ahead of print September 21, 2020.] [DOI] [PubMed] [Google Scholar]
- 42.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: A meta-analysis of cohort studies. World J Gastroenterol 2007;13(31):4199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 2013;62(6):933–47. [DOI] [PubMed] [Google Scholar]
- 44.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008;371(9612):569–78. [DOI] [PubMed] [Google Scholar]
- 45.Guo SS Wu W Chumlea WC,et al. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr 2002;76(3):653–8. [DOI] [PubMed] [Google Scholar]
- 46.Wright C, Heron J, Kipping R, et al. Young adult cancer risk behaviours originate in adolescence: A longitudinal analysis using ALSPAC, a UK birth cohort study. BMC Cancer 2021;21(1):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furer A Afek A Sommer A,et al. Adolescent obesity and midlife cancer risk: A population-based cohort study of 2·3 million adolescents in Israel. Lancet Diabetes Endocrinol 2020;8(3):216–25. [DOI] [PubMed] [Google Scholar]
- 48.Ackerman SE, Blackburn OA, Marchildon F, et al. Insights into the link between obesity and cancer. Curr Obes Rep 2017;6:195–203. [DOI] [PubMed] [Google Scholar]
- 49.Sanford NN, Giovannucci EL, Ahn C, et al. Obesity and younger versus older onset colorectal cancer in the United States, 1998–2017. J Gastrointest Oncol 2020;11(1):121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imperiale TF, Kahi CJ, Stuart JS, et al. Risk factors for advanced sporadic colorectal neoplasia in persons younger than age 50. Cancer Detect Prev 2008;32(1):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JY, Jung YS, Park JH, et al. Different risk factors for advanced colorectal neoplasm in young adults. World J Gastroenterol 2016;22(13):3611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim NH, Jung YS, Yang HJ, et al. Prevalence of and risk factors for colorectal neoplasia in asymptomatic young adults (20–39 years old). Clin Gastroenterol Hepatol 2019;17(1):115–22. [DOI] [PubMed] [Google Scholar]
- 53.Juo YY, Gibbons MAM, Dutson E, et al. Obesity is associated with early onset of gastrointestinal cancers in California. J Obes 2018;2018:7014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holmes AJ, Anderson K. Convergence in national alcohol consumption patterns: New global indicators. J Wine Econ 2017;12:117–48. [Google Scholar]
- 55.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 2014;311(2):183–92. [DOI] [PubMed] [Google Scholar]
- 56.Ali Khan U, Fallah M, Tian Y, et al. Personal history of diabetes as important as family history of colorectal cancer for risk of colorectal cancer: A nationwide cohort study. Am J Gastroenterol 2020;115(7):1103–9. [DOI] [PubMed] [Google Scholar]
- 57.Rosen AB, Schneider EC. Colorectal cancer screening disparities related to obesity and gender. J Gen Intern Med 2004;19(4):332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrante JM, Ohman-Strickland P, Hudson SV, et al. Colorectal cancer screening among obese versus non-obese patients in primary care practices. Cancer Detect Prev 2006;30(5):459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.