Abstract
All of the antigenic determinants of the Duffy blood group system are in a glycoprotein (gp-Fy), which is encoded by a single-copy gene (FY) located on chromosome 1. gp-Fy is also produced in several cell types, including endothelial cells of capillary and postcapillary venules, the epithelial cell of kidney collecting ducts, lung alveoli, and the Purkinje cells of the cerebellum. This protein, which spans the cell membrane seven times, is a member of the superfamily of chemokine receptors and a malarial parasite receptor. The mouse Duffy gene (Dfy) homolog of human FY is also a single-copy gene, which maps in a region of conserved synteny with FY and produces a glycoprotein with 60% homology to the human protein. The mouse Duffy-like protein also binds chemokines. To study the biological role of gp-Fy, we generated a mouse strain in which Dfy was deleted. These homozygous Dfy−/− mice were indistinguishable in size, development, and health from wild-type and heterozygous littermates. We also examined components of the immune system and found no differences in lymph nodes or peripheral blood leukocyte levels between knockout and wild-type mice. The gross and histological anatomy of the thymus, spleen, lung, and brain showed no significant differences between mutants and wild-type mice. There was no indication of an overall difference between the knockout and wild-type mice in systematic neurological examinations. The only significant difference between Dfy−/− and Dfy+/+ mice that we found was in neutrophil migration in peritoneal inflammations induced by lipopolysaccharide and thioglycolate. In mice homozygous for the deletion, there was less neutrophil recruitment into the peritoneal cavity and neutrophil influx in the intestines and lungs than in wild-type mice. Despite this, the susceptibility to Staphylococcus aureus infection was the same in the absence and in the presence of gp-Fy. Our results indicate that gp-Fy is functionally a redundant protein that may participate in the neutrophil migratory process.
The product of FY, a single-copy gene located on the 1q22→q23 region of chromosome 1, is an acidic glycoprotein which spans the plasma membrane seven times and has an exocellular N-terminal domain and an endocellular C-terminal domain (4, 21). gp-Fy carries all antigenic determinants of the Duffy blood group system, which consists of four alleles, five phenotypes, and five antigens (22). Duffy-negative individuals, predominantly African and American blacks, lack the Duffy protein on erythrocytes and are resistant to human malarial parasite Plasmodium vivax and simian malarial parasite Plasmodium knowlesi infection (14, 15). They are healthy individuals who carry a mutation in the promoter region of FY that disrupts the binding site for h-GATA-1 erythroid transcription factor and abolishes expression of FY in erythroid but not nonerythroid cells (29).
gp-Fy is produced in several cell types including the endothelial cells of capillary and postcapillary venules, the epithelial cells of kidney collecting ducts, and lung alveoli. It is also synthesized in the Purkinje cells of the cerebellum (3, 8, 10). The Duffy protein is also a member of the superfamily of chemokine receptors (5, 9, 18).
We have identified the mouse Dfy orthologous to the human FY (11). Dfy is similar to FY in that it is a single-copy gene; it maps in a region of conserved synteny with FY, and it consists of a small exon, a large exon, and a single intron (11). It has been shown that mouse erythrocytes bind human and murine chemokines (27). As with the human erythrocytes, C-C and C-X-C chemokines appear to compete for binding with a single site on the surface of the mouse red cells (27). We recently demonstrated that the mouse Duffy-like protein possesses the same chemokine binding characteristics as the human gp-Fy (11).
To determine the biological role of gp-Fy, we generated a mouse strain that lacks Dfy. We made a comprehensive examination of the Dfy−/− mice including gross anatomy, histology, hematology, and neurobiology. In addition, we studied the response of the knockout mice to Staphylococcus aureus infection and to aseptic inflammations induced by lipopolysaccharide (LPS), thioglycolate, and zymosan. The absence of Duffy-like protein did not produce an obvious phenotype with the exception of much less neutrophil trafficking upon LPS or thioglycolate treatment.
MATERIALS AND METHODS
Targeting vector and constructs.
The replacement-targeting vector was constructed using a 9.5-kb Dfy genomic DNA clone isolated from a mouse (129/Svj strain) genomic library (Stratagene). The 5′ untranslated region, exon 1, exon 2, and intron of Dfy were deleted and replaced with the neomycin resistance gene (neo). The 5′ short arm of 1.5 kb, a 1.1-kb neo cassette with the poly(A)+ signal from pMC1neo Poly A vector (Stratagene), and a 3′ long arm of 5.5 kb was subcloned into PCR-Script SK(+) vector (Stratagene) (Fig. 1A).
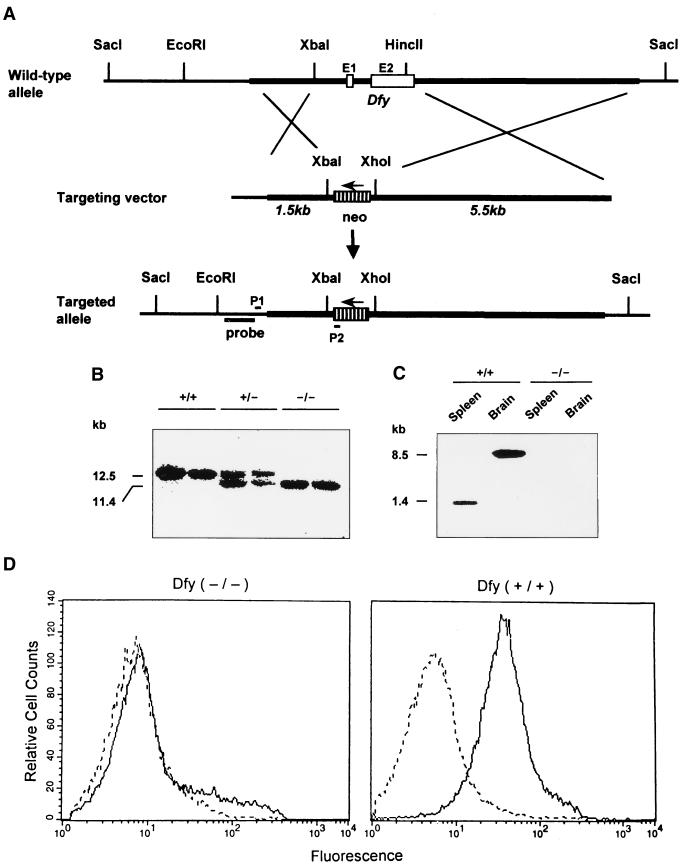
FIG. 1.
Targeting of the Dfy gene. (A) At the top is the restriction map of the wild-type gene. A 2.2-kb fragment containing the 5′ untranslated region, exon 1 (E1), intron, and exon 2 (E2) was removed by XbaI and HincII digestion. A 1.5-kb 5′-end fragment, the neo gene, and a 5.5-kb 3′-end fragment were inserted into the XbaI-HincII deleted region of the PCR-Script vector. (B) Genomic DNA was isolated from tail snips of the offspring of interbreeding mice, digested with SacI, and analyzed by Southern blot hybridization with the probe indicated in panel A. Genotypes of mice are indicated as wild type (+/+), heterozygous (+/−), and homozygous (−/−). (C) RNA blot analysis of spleen and brain mRNAs from wild-type and deleted mice. (D) FACS analysis of erythrocytes. For each condition, 30,000 red cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson).
Generation of Dfy−/− mice.
The linearized targeting vector DNA (40 μg) was transfected by electroporation into E14-1 embryonic stem (ES) cells (kindly provided by J. Visser) as previously described (25). DNA isolated from ES colonies resistant to neomycin was amplified by PCR using primer 1 (5′-ATACCATGCATTCGCCACCTTTCC-3′), designed from a sequence situated at the 5′ end outside the replacement vector, and primer 2 (5′-GCCTTCTTGACGAGTTCTTCTGAG-3′), designed from a sequence situated at the 3′ end of the neo gene (Fig. 1A). Chimeric mice (129/SvJ-derived ES cells in blastocysts of C57BL/6J mice) were generated as described elsewhere (20). Heterozygous (Dfy+/−) mice were used for interbreeding to produce homozygous knockout (Dfy−/−) wild-type and (Dfy+/+) mice. The experiments were performed with 2- to 3-month-old mice weighing an average of 23 g.
Southern and Northern analyses.
DNA and RNA extractions as well as Southern and Northern blot procedures were performed as explained elsewhere (11).
Necropsy studies.
Gross and histopathological analyses were performed at the Research Animal Diagnostic Laboratory, Cornell University Medical College.
Behavioral evaluation of Dfy−/− mice.
Dfy+/+ and Dfy−/− animals were examined for appearance, posture, circadian activity, open field activity, home cage assessment, water maze, rotating rod task performance, balance, and reaching task performance. These behavioral tests were carried out by Neuro-Detective Inc. (University of Lethbridge, Lethbridge, Alberta, Canada).
Flow cytometry.
A Dfy-specific rabbit polyclonal antibody (M4) against the amino-terminal domain of the mouse gp-Fy (kindly provided by J. Hesselgesser) was used for protein detection in mouse erythrocytes by fluorescence-activated cell sorting (FACS) analysis. Aliquots of 10 μl of peripheral blood from Dfy+/+ and Dfy−/− mice were incubated with rabbit antibody M4 at room temperature for 1 h and then incubated with fluorescein isothiocyanate-conjugated donkey anti-rabbit immunoglobulin G (Jackson ImmunoResearch Laboratories) for another hour at room temperature.
Aseptic infections.
Aseptic peritonitis was produced with LPS (Escherichia coli, serotype O111:B4, Sigma), thioglycolate (BBL), and zymosan (Sigma) as explained elsewhere (1, 12). Briefly, Dfy+/+ and Dfy−/− animals were injected intraperitoneally with 1 ml of phosphate-buffered sterile saline (PBS) solution containing either 200 μg of LPS or 4 mg of zymosan per 20 g of animal body weight. The thioglycolate peritonitis was induced with the intraperitoneal injection of 1 ml of a 3% thioglycolate solution. Control animals were injected with 1 ml of PBS solution. The mice were euthanized by CO2 narcosis 24 h after injection, and the peritoneal cavities were washed with 5 ml of PBS solution containing 3 mM EDTA and 0.1% bovine serum albumin. At the same time, blood samples were obtained by heart puncture. Cell counts were determined in a Serono Diagnostic System 9010 cell counter (Biochem ImmunoSystem). Differential leukocyte counts were determined after staining with Wright's stain (Sigma).
S. aureus infection assay.
We used the procedure developed by Thomas et al. (28). Briefly, S. aureus (Cowan strain 12598; American Type Culture Collection, Rockville, Md.) were grown overnight in brain heart broth (Difco, Detroit, Mich.) in a bacterial shaker at 37°C. On the day of the experiment, bacteria were washed three times in PBS and spectrophotometrically adjusted to ∼108 CFU/ml (optical density of 2.0). The bacterial concentration was confirmed by plating serial dilutions on brain heart agar plates. Dfy−/− and Dfy+/+ mice were injected intraperitoneally with 1 ml of PBS solution containing 109 to 5 × 1010 CFU/ml and were observed daily. Dying mice were euthanized with CO2. In a separated set of experiments, 20 Dfy−/− and 20 Dfy+/+ mice were injected with 109 CFU/ml and euthanized after 24 hours; then total white cells and neutrophils in the peritoneal cavity were evaluated as described for experiments involving aseptic infections.
Determination of PMN influx into intestine and lung.
The polymorphonuclear leukocyte (PMN) count was determined by the myeloperoxidase (MPO) assay as previously described (17). The lungs and intestines were removed, blotted, and frozen on dry ice. The tissues were thawed in a solution containing 0.5% hexadecyltrimethylammonium bromide dissolved in 10 mM morpholinepropanesulfonic acid buffer (pH 7.0) and homogenized with a Polytron (Kinematic KG) tissue homogenizer. The suspension was sonicated, and large debris were removed by centrifugation at 20,000 × g at 4°C for 30 min. An aliquot of the supernatant was allowed to react with a solution of containing 1.6 mM tetramethylbenzidine and 0.3 mM H2O2. After 2 min of incubation, the reaction was stopped with 2 M acetic acid. The rate of change in absorbance was measured by a spectrophotometer at 650 nm. MPO activity was defined as the quantity of enzyme that degrades 1 μmol of peroxide/min at 25°C. The average of duplicates was expressed in units per gram of wet tissue.
Statistical analysis.
Data are presented as the mean ± standard error of the mean. Data for Dfy−/− and Dfy+/+ mice were compared by t test using the Number Cruncher Statistical System 2000 software and were considered statistically significant when P was <0.05.
RESULTS AND DISCUSSION
Deletion of Dfy in mice.
We generated a mouse strain lacking the promoter region, exon 1, exon 2, and the single intron of Dfy (Fig. 1A). The deletion was confirmed by Southern blot analysis using exon 2 as a probe (Fig. 1B). The same probe was used in a Northern blot analysis of mRNA obtained from spleens and brains of the knockout mice, and no Dfy-specific mRNA was observed (Fig. 1C). In human and mouse brains, the coding sequences of gp-Fy are located at the 3′ end of an 8.5-kb mRNA (4, 11). The absence of a Dfy product was further validated by FACS analysis of mouse erythrocytes with a Dfy-specific rabbit polyclonal antibody (Fig. 1D).
Genotypic analysis of offspring from heterozygous × heterozygous breeding pairs.
Mice that carried one copy of the deleted gene were interbred to generate litters that were +/+, +/−, and −/− for Dfy as determined from genomic PCR of DNA extracted from tail snips. Pups from heterozygous parents were genotyped in this way, and the ratios of +/+, +/−, and −/− mice yielded the predicted Mendelian ratios of 1:2:1 (41:84:43) as expected for nonlethal alleles. Thus, the Dfy knockout pups were no less viable than their wild-type littermates. Mice heterozygous or homozygous for the Dfy allele appeared healthy, developed normally, and did not display any impairment of reproductive capacity and neonatal survival. Both heterozygous × heterozygous and homozygous × homozygous breeding pairs produced litters similar in size to those produced by wild-type breeding pairs, demonstrating that the absence of the Duffy-like protein does not hinder the fertility of male or female mice (Table 1).
TABLE 1.
Litter sizes for each genotype
| Genotype | No. of breeding pairs | Total no. of litters born | No. of pups born/litter (mean ± SD) |
|---|---|---|---|
| +/+ | 8 | 11 | 6.3 ± 0.54 |
| +/− | 10 | 17 | 6.8 ± 0.44 |
| −/− | 6 | 10 | 5.4 ± 0.38 |
Phenotypic analysis of homozygous Dfy−/− mice.
At a gross phenotypic level, the absence of gp-Fy had no discernible impact. Dfy−/− mice were indistinguishable from their wild-type littermates with respect to body weight, body length, head length, and tail length (not shown). In addition, they exhibited no macroscopic or microscopic alterations in all organs examined (spleen, lung, intestine, kidney, liver, lymph nodes, thymus, brain, and heart [not shown]). We found no differences in white or red blood cell counts or in levels of hemoglobin between wild-type and knockout mice (not shown), nor were alterations in embryonic development observed.
Neurological analysis of homozygous Dfy−/− mice.
There was no indication of an overall difference between the knockout and wild-type mice in 20 items included in neurological examinations or differences in 15 behavioral measures (not shown).
The functions of the cerebellum were specifically assessed, since the Purkinje cells of the cerebellum produce gp-Fy (3, 8, 10). Each animal was observed while naturally rearing, supporting itself on the side of the cage, and standing on all four limbs. No abnormalities were detected. Total horizontal activity and number of vertical movements were tracked by the infrared sensor system, and no significant differences between animals that lack gp-Fy and animals that produce the protein were observed. Furthermore, the rotating rod and balance tests showed no indication of impaired cerebellar functions in Dfy−/− mice.
The observations in Dfy−/− mice are similar to those of Duffy mutant humans who do not express gp-Fy in all cell types. The human equivalent to mouse Dfy−/− is a nonsense mutation that produces a premature stop codon (UAG) in the coding sequence. The consequence of the mutation is the production of a truncated, nonfunctioning, and disposable protein in all cell types (22, 24). For example, we studied a woman with a premature stop codon in exon 2 who was healthy and had borne 15 children. Dfy in mice, like FY in humans, is not an essential gene (24).
Aseptic infections.
In the absence of an overt phenotype, even in mice as old as 15 months, the Dfy−/− mice were tested for their response to septic and aseptic infection. We hypothesized that gp-Fy might participate in leukocyte migration since it is an endothelial cell membrane protein (3). This idea is reinforced when one considers that gp-Fy is in the apical and basolateral plasma membrane domains, including caveolae (3). Mechanistically, the neutrophil transendothelial pathway is in some respects comparable to malarial parasite invasion; in both cases, the invading cell enters via invagination of the cell membrane rather than intracellular penetration (7, 13, 16). In the absence of Duffy protein, the merozoites cannot invade the erythrocytes (16).
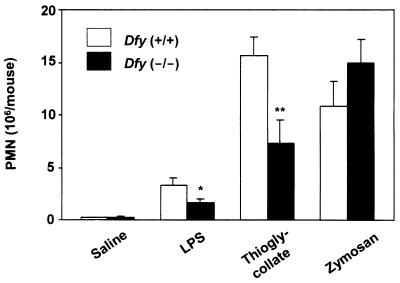
Neutrophil recruitment into the peritoneal cavity was induced by the intraperitoneal injection of LPS (toxic doses), thioglycolate, and zymosan. The number of PMN in Dfy−/− mice that migrated into the peritoneal cavity was one-half of that of Dfy+/+ mice with LPS and thioglycolate peritonitis (Fig. 2). However, there was no difference between wild-type and knockout mice in zymosan peritonitis (Fig. 2). The migration of lymphocytes and monocytes into the peritoneal cavity was not affected by the absence of Duffy-like protein (not shown).
FIG. 2.
Neutrophil migration produced by three models of experimental peritonitis. The number of neutrophils was calculated by multiplying percentage of neutrophils by total cell count. Eight animals were analyzed per experimental peritonitis. ∗, P < 0.05; ∗∗, P < 0.01 compared to wild-type animals.
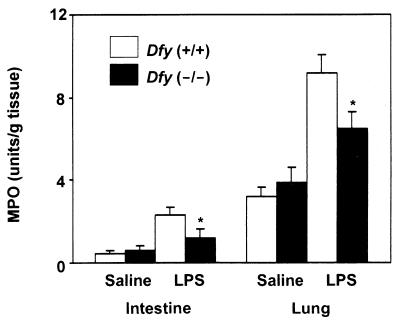
We next examined neutrophil influx into the lungs and intestines of animals injected with toxic doses of LPS (30). MPO activity, an index of PMN accumulation, was measured in lungs and intestines of LPS-treated animals; as in the peritoneal cavity, neutrophil influx was significantly reduced in Dfy−/− animals (Fig. 3).
FIG. 3.
Neutrophil influx in intestine and lung in LPS-induced inflammation. MPO activity is an index of PMN accumulation in the organs. ∗, P < 0.05 compared to wild-type animals.
The migration of PMN into tissues requires interaction between neutrophils and endothelial cells (2, 26). According to the current paradigm, these interactions consist of first selectin molecules binding to carbohydrate ligands (mucin-like molecules), which are responsible for the initial tethering and rolling of a flowing leukocyte to the endothelium. Tethering brings leukocytes into proximity with chemoattractants.
A chemoattractant gradient induces leukocyte-endothelial cell adhesion and migration (26). However, a soluble chemoattractant gradient cannot persist on the blood-endothelial cell interface; it is likely to be washed away by the blood flow. Therefore, a more efficient way to promote leukocyte-endothelial cell adhesion is to immobilize the chemoattractant on the membrane of the endothelial cells (26). The in vivo immobilization of interleukin-8 has been recently demonstrated (13). Since chemokines bind to heparin and related glycosaminoglycans, it was suggested that these molecules, which are abundant in endothelial cells, immobilize chemokines. As the Duffy protein also binds to chemokines, it has been implicated in the ligand immobilization process (13).
The absence of Duffy-like protein, however, did not fully impair the ability of neutrophils to migrate; substantial PMN trafficking occurs without Duffy-like protein. Moreover, in zymosan peritonitis, leukocyte migration was as efficient in the absence as in the presence of Duffy-like protein. It seems that the reagent either induces an effective chemoattractant gradient in the absence of this protein or affects the endothelial cells differently than LPS or thioglycolate. Since PMN migration can occur without gp-Fy, the protein obviously is an ancillary component of the trafficking machinery.
Bacterial infections.
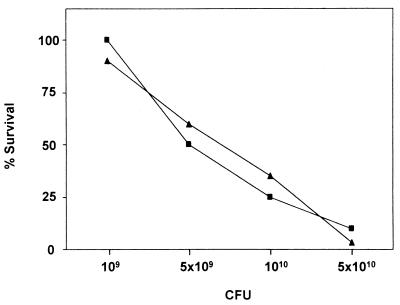
Susceptibility to bacterial infection is an appropriate test of whether the absence of Duffy-like protein affects the ability of the organism to fight infection. We used the S. aureus model of gram-positive infection since it is a clinically important gram-positive pathogen (28). The absence of Duffy-like protein did not affect the ability of Dfy−/− mice to resist the pathogen (Fig. 4). Dfy−/− mice did not show an increase in susceptibility to this bacterial infection. Moreover, in S. aureus peritonitis, like in zymosan peritonitis, we found equal recruitment of leukocytes in the absence and in the presence of Duffy-like protein (not shown). It remains to be demonstrated whether a different response will occur with other pathogens.
FIG. 4.
Mortality of Dfy−/− (■) and Dfy+/+ (▴) mice after S. aureus challenge. Mice were injected intraperitoneally with various concentrations of S. aureus. Animals were observed daily for signs of systemic infections, and very sick animals were euthanized.
In humans, gp-Fy is not expressed in the entire endothelium; it is constitutively expressed in the endothelial cells of small vessels of certain tissues (3, 8). The pattern of expression of mouse gp-Fy remains to be determined. However, Dfy is an inducible gene (unpublished results). The three models of aseptic inflammation that we studied showed significant induction of Dfy mRNA (not shown). This implies that at the earlier stage of inflammation, where mouse gp-Fy is not constitutively expressed, PMN migration takes place without gp-Fy.
Conclusion.
In this study, a mouse strain lacking the mouse gene orthologous to the human Duffy gene was generated by homologous recombination with a gene-targeting vector. The homozygous Dfy−/− mice were indistinguishable from their wild-type littermates in size, health, embryonic development, and neurological behavior. Moreover, Dfy−/− mice were not more susceptible to S. aureus infection than homozygous Dfy+/+ mice. The only difference between Dfy−/− and Dfy+/+ mice that we found was a diminution for PMN trafficking in the mutant mice after thioglycolate or LPS aseptic inflammation. No such compromise in neutrophil migration was observed when zymosan was injected or when the animals were infected with S. aureus. The human counterparts of Dfy−/− mice are healthy individuals, and the study reported here indicates that both Dfy in mice and FY in humans are redundant genes.
ACKNOWLEDGMENTS
We thank J. Visser and M. Ershler (Laboratory of Stem Cell Biology, New York Blood Center) for the E14-1 ES cells; in particular, we are grateful to M. Ershler for technical help with identification and isolation of the transfected ES cells. We thank J. Hesselgesser (Department of Immunology and Pharmaceutical Discovery, Berlex BioScience, Richmond, Calif.) for the Dfy-specific rabbit polyclonal antibody M4. We thank A. Molinaro (Laboratory of Microchemistry, New York Blood Center) for work in DNA sequencing, T. Huima and Y. Oksov for artwork, and B. Faris and H. Sender for secretarial assistance.
This research was supported by SCOR (Specialized Center of Research) grant HL 54459 to A.O.P. from the National Heart, Lung, and Blood Institute, National Institutes of Health.
REFERENCES
- 1.Ajuebor M N, Flower R J, Hannon R, Christie M, Bowers K, Verity A, Perretti M. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol. 1998;63:108–116. doi: 10.1002/jlb.63.1.108. [DOI] [PubMed] [Google Scholar]
- 2.Carlos T M, Harlan J M. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 3.Chaudhuri A, Nielsen S, Elkjaer M-L, Zbrzezna V, Fang F, Pogo A O. Detection of Duffy antigen in the plasma membranes and caveolae of vascular endothelial and epithelial cells of nonerythroid organs. Blood. 1997;89:701–712. [PubMed] [Google Scholar]
- 4.Chaudhuri A, Polyakova J, Zbrzezna V, Williams K, Gulati S, Pogo A O. Cloning of glycoprotein D cDNA, which encodes the major subunit of the Duffy blood group system and the receptor for the Plasmodium vivax malaria parasite. Proc Natl Acad Sci USA. 1993;90:10793–10797. doi: 10.1073/pnas.90.22.10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhuri A, Zbrzezna V, Polyakova J, Pogo A O, Hesselgesser J, Horuk R. Expression of the Duffy antigen in K562 cells. J Biol Chem. 1994;269:7835–7838. [PubMed] [Google Scholar]
- 6.Darbonne W C, Rice G C, Mohler M A, Apple T, Hébert C A, Valente A J, Baker J B. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Investig. 1991;88:1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng D, Nagy J A, Pyne K, Dvorak H F, Dvorak A M. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadley T J, Lu Z, Wasniowska K, Martin A W, Peiper S C, Hesselgesser J, Horuk R. Postcapillary venule endothelial cells in kidney express a multispecific chemokine receptor that is structurally and functionally identical to the erythroid isoform, which is the Duffy blood group antigen. J Clin Investig. 1994;94:985–991. doi: 10.1172/JCI117465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horuk R, Chitnis C E, Darbonne W C, Colby T J, Rybicki A, Hadley T J, Miller L H. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- 10.Horuk R, Martin A, Hesselgesser J, Hadley T, Lu Z H, Wang Z X, Peiper S C. The Duffy antigen receptor for chemokines: structural analysis and expression in the brain. J Leukoc Biol. 1996;59:29–38. doi: 10.1002/jlb.59.1.29. [DOI] [PubMed] [Google Scholar]
- 11.Luo H, Chaudhuri A, Johnson K R, Neote K, Zbrzezna V, He Y, Pogo A O. Cloning, characterization, and mapping of a murine promiscuous chemokine receptor gene: homolog of the human Duffy gene. Genome Res. 1997;7:932–941. doi: 10.1101/gr.7.9.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayadas T N, Johnson R C, Rayburn H, Hynes R O, Wagner D D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 13.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 14.Miller L H, Mason S J, Clyde D F, McGinniss M H. The resistance factor to Plasmodium vivax in Blacks: the Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 15.Miller L H, Mason S J, Dvorak J A, McGinniss M H, Rothman K I. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975;189:561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- 16.Miller L H, McAuliffe F M, Johnson J G. Invasion of erythrocytes by malaria merozoites. Prog Clin Biol Res. 1979;30:497–502. [PubMed] [Google Scholar]
- 17.Mullane K M, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration in ischemic myocardium. J Pharmacol Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 18.Neote K, Mak J Y, Kolakowski L F, Schall T J. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood. 1994;84:44–52. [PubMed] [Google Scholar]
- 19.Oquendo P, Alberta J, Wen D, Graycar J L, Derynck R, Stiles C D. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet α-granule proteins. J Biol Chem. 1989;264:4133–4137. [PubMed] [Google Scholar]
- 20.Papaioannou V, Johnson R. Production of chimeras and genetically defined offspring from targeted ES cells. In: Joyner A L, editor. Gene targeting, a practical approach. Oxford, England: Oxford University Press; 1993. pp. 107–146. [Google Scholar]
- 21.Pogo A O, Chaudhuri A. The Duffy blood group system and its extension in nonhuman primates. In: Blancher A, Klein J, Socha W W, editors. Molecular biology and evolution of blood group and MHC antigens in primates. Berlin, Germany: Springer-Verlag; 1997. pp. 219–235. [Google Scholar]
- 22.Pogo, A. O., and A. Chaudhuri. The Duffy protein: a malarial and chemokine receptor. Semin. Hematol., in press. [DOI] [PubMed]
- 23.Premack B A, Schall T J. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 24.Rios, M., A. Chaudhuri, G. Mallinson, L. Sausais, A. E. Gomensoro-Garcia, J. Hannon, S. Rosenberger, J. Poole, G. Burgess, L. Castilho, R. Oyen, A. O. Pogo, and M. Reid. New genotypes in Fy(a-b-) individuals: nonsense mutations (Trp to stop) in the coding sequence of either FYA or FYB. Br. J. Haematol., in press. [DOI] [PubMed]
- 25.Samokhvalov I, Hendrikx J, Visser J, Belyavsky A, Sotiropolous D, Gu H. Mice lacking a functional Chk gene have no apparent defects in the hematopoietic system. Biochem Mol Biol Int. 1997;43:115–122. doi: 10.1080/15216549700203881. [DOI] [PubMed] [Google Scholar]
- 26.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 27.Szabo M C, Soo K S, Zlotnik A, Schall T J. Chemokine class differences in binding to the Duffy antigen-erythrocyte chemokine receptor. J Biol Chem. 1995;270:25348–25351. doi: 10.1074/jbc.270.43.25348. [DOI] [PubMed] [Google Scholar]
- 28.Thomas C A, Kodama T, Suzuki H, Silverstein S, El Khoury J. Protection from lethal gram-positive infection by macrophage scavenger receptor dependent phagocytosis. J Exp Med. 2000;191:147–156. doi: 10.1084/jem.191.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tournamille C, Colin Y, Cartron J P, Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Gen. 1995;10:224–228. doi: 10.1038/ng0695-224. [DOI] [PubMed] [Google Scholar]
- 30.VanOtteren G M, Strieter R M, Kunkel S L, Paine R, Greenberger M J, Danforth J M, Burdick M D, Standiford T J. Compartmentalized expression of RANTES in a murine model of endotoxemia. J Immunol. 1995;154:1900–1908. [PubMed] [Google Scholar]