Keywords: fibrosis, inflammation, mast cell, prostate, smooth muscle cell contraction
Abstract
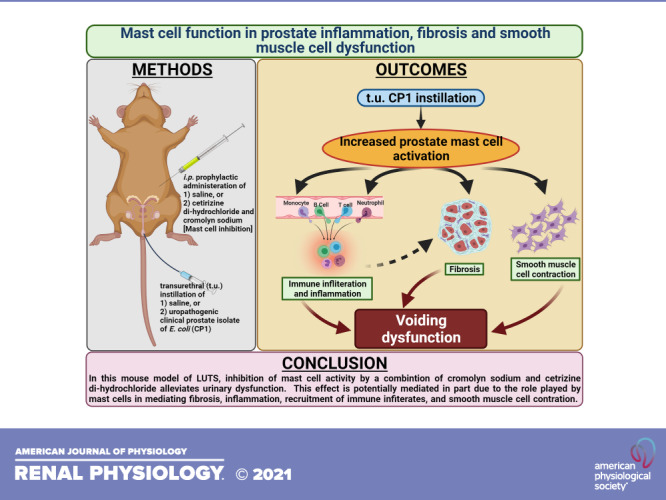
Intraurethral inoculation of mice with uropathogenic Escherichia coli (CP1) results in prostate inflammation, fibrosis, and urinary dysfunction, recapitulating some but not all of the pathognomonic clinical features associated with benign prostatic hyperplasia (BPH) and lower urinary tract symptoms (LUTS). In both patients with LUTS and CP1-infected mice, we observed increased numbers and activation of mast cells and elevated levels of prostate fibrosis. Therapeutic inhibition of mast cells using a combination of a mast cell stabilizer, cromolyn sodium, and the histamine 1 receptor antagonist cetirizine di-hydrochloride in the mouse model resulted in reduced mast cell activation in the prostate and significant alleviation of urinary dysfunction. Treated mice showed reduced prostate fibrosis, less infiltration of immune cells, and decreased inflammation. In addition, as opposed to symptomatic CP1-infected mice, treated mice showed reduced myosin light chain-2 phosphorylation, a marker of prostate smooth muscle contraction. These results show that mast cells play a critical role in the pathophysiology of urinary dysfunction and may be an important therapeutic target for men with BPH/LUTS.
NEW & NOTEWORTHY LUTS-associated benign prostatic hyperplasia is derived from a combination of immune activation, extracellular matrix remodeling, hyperplasia, and smooth muscle cell contraction in prostates of men. Using a mouse model, we describe the importance of mast cells in regulating these multiple facets involved in the pathophysiology of LUTS. Mast cell inhibition alleviates both pathology and urinary dysfunction in this model, suggesting the potential for mast cell inhibition as a therapeutic that prevents and reverses pathology and associated symptomology.
INTRODUCTION
Lower urinary tract symptoms (LUTS) occur at high incidence in both men and women with increasing age. Benign prostatic hyperplasia (BPH)-associated LUTS is a major contributing factor to negative quality of life in men, costing over $1 billion dollars in healthcare costs, in the United States alone, per year as of 2014 (1). In men, LUTS is multifactorial but is commonly associated with the development and progression of BPH. BPH is a histological condition that is characterized by varying combinations of epithelial and stromal hyperplasia within the prostate and is prevalent in over 50% of men of over 50 yr of age (2, 3).
Historically, risk factors linked to the development and progression of LUTS include age, genetics, infections, stress, and BPH (4, 5). Studies have revealed prostate inflammation to be highly associated with LUTS, correlated with prostatic enlargement, and implicated as a cause of prostatic fibrosis (6). Tissue inflammation and fibrosis has numerous potential causes including aging, infection (from bacterial prostatitis or urinary tract infections), dietary habits, hormonal changes, physical trauma, and urinary reflux (5, 7–10). Inflammation and fibrosis are thought to cause increased rigidity of the prostate, resulting in increasing pressure and constriction on the urethra, leading to LUTS (5, 6, 11, 12). Alongside inflammation and fibrosis, aberrant smooth muscle contraction also causes voiding symptoms in patients by impairing the bladder outlet due to urethral obstruction, a condition commonly referred to as bladder outlet obstruction (BOO) (13).
Mast cells act as crucial cellular sensors and regulators of inflammation, fibrosis, and smooth muscle cell contraction (14–16). In addition to playing a critical role in allergic immunity, mast cells can sense various “danger signals” using the plethora of pattern recognition receptors expressed on both the cell surface and within the cells. Activation of these receptors induces release of a milieu of cytokines that influence immune cell infiltration and activation and release of various other factors including chymases, tryptases, and proteases, which can interact with and alter the local tissue environment leading to tissue fibrosis, repair, and remodeling (14–21). Clinical reports have suggested that mast cells play an important role in the development and persistence of inflammation in BPH-associated LUTS (22, 23). Mast cell-secreted factors, predominantly in the context of airway smooth muscle cells, have been implicated to play an important role in controlling smooth muscle cell growth, differentiation, and contraction (24–26). Also, we have previously shown that protease‐activated receptor 2 (PAR2) activation by mast cell-derived proteases in prostate smooth muscle cells causes cellular contraction (27). Given the evidence that prostate fibrosis and inflammation and smooth muscle cell contraction are important in the development of LUTS, and the influence that mast cells have on such processes, we assessed the role of mast cells in the development of LUTS.
We have previously reported the ability of an uropathogenic clinical prostate isolate of Escherichia coli (CP1) to colonize the prostates of mice and recapitulate aspects of LUTS such as urinary dysfunction, prostate inflammation, and fibrosis. These changes and observations persist long after bacterial clearance (28, 29). In this study, we observed the presence of an increased number and activation of mast cells in the prostate of CP1-infected mice that was associated with increased inflammation and the development of fibrosis. Upon inhibition of mast cell activity using a combination a mast cell stabilizer (MCS) and histamine 1 receptor antagonist (H1RA), findings of urinary dysfunction were ameliorated and pathological features such as immune cell infiltration, fibrosis, and smooth muscle contraction were significantly reversed. This study implicates mast cells as critical mediators of prostate pathology in LUTS and furthermore shows that a novel and innovative treatment approach that blocks release of mast cell factors and modulates the immune response may be useful in alleviating LUTS. This may be a promising novel therapy to prevent or more effectively treat LUTS in aging men.
MATERIALS AND METHODS
Mice
Male C57BL/6 mice (5–7 wk of age) were obtained from Jackson Laboratory. Mice were housed in a single environmentally controlled room within the Northwestern University animal facility. All animal experiments and procedures have been approved by the Northwestern University Animal Care and Use Committee.
Transurethral Mouse Infections
Mouse infections were performed as previously described (28–30). Briefly, CP1 E. coli bacteria were grown in LB overnight with shaking at 37°C followed by overnight static subculture at 37°C. The next day, bacteria were concentrated at 2 × 1010 bacteria/mL in PBS, and 10 µL (2 × 108 bacteria) were instilled transurethrally into isoflurane-anesthetized male C57BL/6 mice. Age-matched control male C57BL/6 animals received a transurethral instillation of PBS (Gibco, Paisley, UK) and were kept in separate cages.
Voiding Behavior Testing
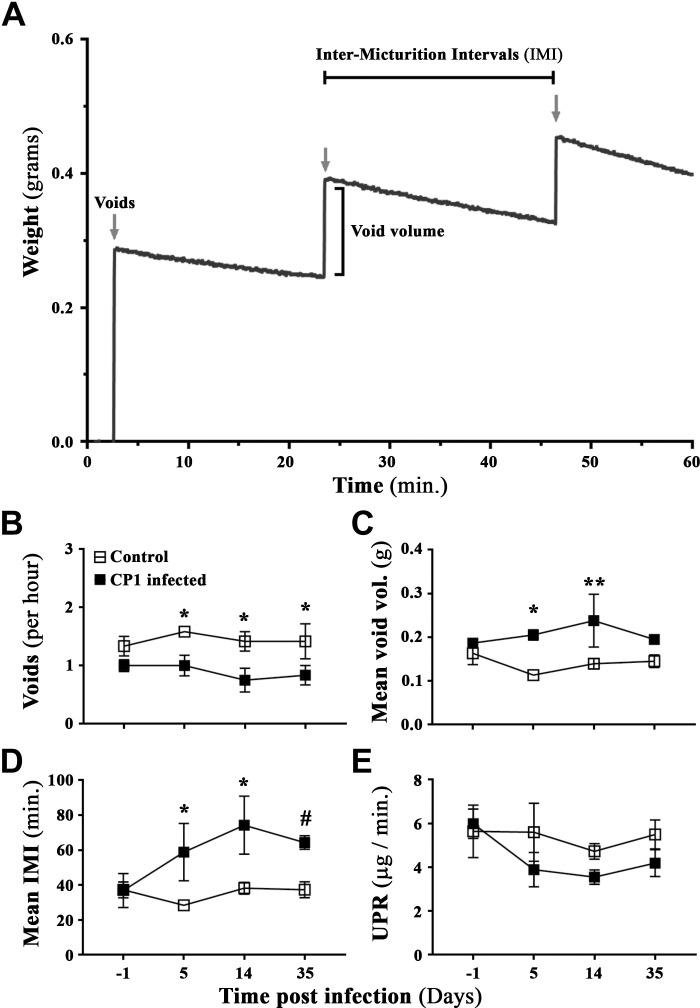
To assess the voiding behavior of mice, we used the Urovoid system (Med Associates, Fairfax, VT), a noninvasive means of measuring voiding function using a modified procedure previously described (31). The Urovoid system is designed to assess conscious urinary voiding behavior (frequency and voiding volume) in unrestrained mice for prolonged periods without the need for surgery or catheter implantation. Briefly, mice were singly housed in chambers with access to water for 5 h. Urine was collected below the grated cage on a balance (mouse feces were separated using a mesh above the balance), and urine weight was recorded over time (1-h after housing the mice to allow for acclimatization). After the completion of these measurements, animals were returned to their home cages for future experimentation or euthanized as per the experimental design. The collected data were then analyzed using the Urovoid voiding frequency analysis system (Med Associates, Fairfax, VT) (see Fig. 2A for a representative graph from the analysis software).
Figure 2.
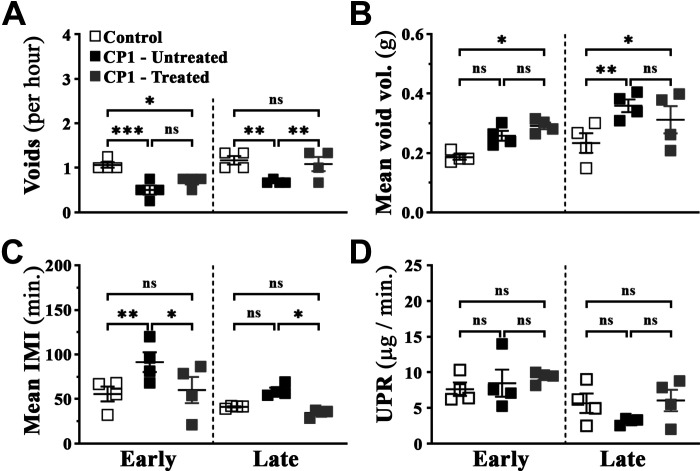
An Escherichia coli (CP1)-induced mouse model of lower urinary tract symptoms recapitulates and induces urinary dysfunction in mice. A: representative sample data graph from the Urovoid Analysis software showing voiding measurements collected using the Urovoid system and how each of the parameters were calculated. The representative data show urine volume collected in weight (g) over time (60 min). The arrows mark each individual void, the increase in weight per void is marked as void volume, and the time between each void is marked as intermicturition interval (IMI). B: average number of voids per hour over a period of 4 h. C: average volume of urine per void (calculated for each animal individually). D: average amount of time (in min) between each micturition event. E: average urine production rate (UPR) as defined as the volume of urine collected over the 4-h period of time (indicating changes in the ability to produce urine) in control and CP1-infected C57BL/6 mice a day before (day −1) and at days 5, 14, and 35 postinfection. Values are presented as means ± SE; n = 4 mice/group. #P < 0.1; *P < 0.05; **P < 0.01 (two-way ANOVA with Fisher’s least significant difference test).
Mouse Tissue Preparation
The prostates were harvested from mice after euthanization as previously described (32). Depending on the experimental design, each prostate sample (separated by lobes) was either fixed in 10% formalin or frozen at −80°C in TRIzol reagent (Life Technologies, Grand Island, NY) or frozen at −80°C in 1× RIPA (Santa Cruz Biotechnology, Dallas, TX) containing complete‐EDTA protease inhibitor cocktail and phosSTOP phosphatase inhibitor (Millipore-Sigma, Burlington, MA) or processed for tissue digestion for flow cytometry as described below in Flow Cytometry. Formalin samples were further processed and embedded in paraffin by the Northwestern University Mouse Histology and Phenotyping Core. The formalin-fixed paraffin-embedded (FFPE) samples were then sectioned (5-µm sections) and mounted on glass slides for staining. The Northwestern University Mouse Histology and Phenotyping Core performed hematoxylin and eosin (H&E) staining as well as immunohistochemistry (IHC) for mast cell tryptase.
In Vivo Administration of MCS and H1RA
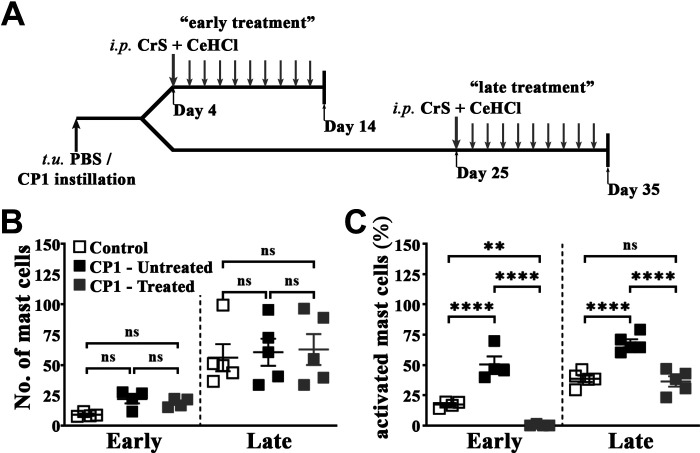
Male C57BL/6 mice were intraperitoneally treated starting at either 5 or 25 days postinfection (“prophylaxis” or “treatment” groups, respectively) with either cetirizine di-hydrochloride (CeHCl) at 2.5 mg/kg (H1RA) or cromolyn sodium salt (CrS) at 0.5 mg/kg (MCS) (Sigma, St. Louis, MO) or a combination of both at the same concentrations daily for 10 days (see Fig. 4A for an illustration of the experimental design). The doses for these compounds were based on a previous study from our group (33). Following voiding behavior testing on days 14 or 35 (“early treatment” or “late treatment” groups, respectively), mice were euthanized as per the protocol approved by the Northwestern University Animal Care and Use Committee, and tissues were prepared as described above in Mouse Tissue Preparation.
Figure 4.
Combination treatment of cromolyn sodium salt (CrS) and cetirizine di-hydrochloride (CeHCl) inhibits mast cell activation in the prostates of CP1-infected mice. A: schematic representation of treatment of mice with CrS and CeHCl. Mice given transurethral instillations of PBS or CP1 were treated intraperitoneally with the combination of CrS + CeHCl daily for 10 days from day 4 (“early treatment”) or day 25 (“late treatment”) before assessment of the effects of combination treatment on various parameters. B and C: total numbers of mast cells (B) and percentages of activated (fully and partial combined) mast cells (C) in toluidine blue-stained sections of combined mouse prostate lobes harvested at 14 days (early) and 35 days (late) post CP1 infection. Values are presented as means ± SE. Each dot represents an individual mouse. **P < 0.01; ****P < 0.001 (one-way ANOVA with Fisher’s least significant difference test). Mast cell numbers and percentages of activated mast cells were determined by counting and averaging three sections of mouse prostate lobes stained with toluidine blue for each sample. ns, not significant.
Histological and IHC Assays
All histological and IHC staining and assays were performed on the anterior, ventral, and dorsolateral separately. Sections (5 µm) were processed for H&E staining (performed by the Northwestern University Mouse Histology and Phenotyping Core). Briefly, H&E staining on FFPE tissues was performed on a fully automated platform (Leica Autostainer XL, Lecia Biosystems, Buffalo Grove, IL) using Harris hematoxylin (Fisher Scientific, Hampton, NH) and eosin secondary counterstain (Lecia Biosystems, Buffalo Grove, IL). Prostate inflammation was assessed using the classification system as previously described (34). Inflammation scoring was quantitated as follows: 0 = no inflammation, 1 = mild inflammation, 2 = moderate inflammation, and 3 = severe inflammation. Inflammation was scored by three independent members of the laboratory in a masked manner, averaged, and presented in the graphs. For assessing extracellular collagen deposition, picrosirius staining was performed as previously described (28). Briefly, 5-µm prostate sections on glass slides were rehydrated, and slides were stained in picrosirius red solution (Direct Red 80 and saturated picric acid, Sigma) for 16 h. Sections were washed in two changes of acidified water, dehydrated in ethanol, cleared in xylene, and mounted with Krystalon (EMD Millipore). Stained sections were quantitated using ImageJ software (National Institutes of Health), and percentages of collagen deposition were calculated. We used toluidine blue staining, a metachromatic stain that should stain mast cells red-purple (metachromatic staining) and the background blue (orthochromatic staining), for the identification of mast cells. Briefly, 5-µm prostate sections on glass slides were rehydrated, and sections were stained using 0.1% toluidine blue for 2−3 min, washed three times using distilled water, dehydrated in ethanol, cleared in xylene, and mounted with Krystalon (EMD Millipore). Stained cells were counted in a masked manner to quantitate levels of resting and activated mast cells. IHC staining of mouse mast cells in prostate tissues were carried using rat anti-mouse mast cell protease 8 antibody (Cat. No. 647401, RRID: AB_2069309, BioLegend, San Diego, CA) (performed by the Northwestern University Mouse Histology and Phenotyping Core), a protease primarily expressed in mouse basophils (35). Briefly, FFPE tissue slides were deparaffinized on an automated platform (Leica Autostainer XL, Lecia Biosystems). Slides were treated with an antigen retrieval step using sodium citrate solution (pH 6) in a pressure cooker. Sections were incubated overnight at 4°C with anti-mouse MCP-8 antibody in a humid chamber. All IHC staining was completed using chromogenic enzyme substrate reactions with diaminobenzidine (Agilent Technologies, Santa Clara, CA). Secondary antibody incubation and chromogenic reactions were then performed using an automated INTELLIPATH FLX system (Biocare Medical, Pacheco, CA). Once the staining was complete, specimens were counterstained with hematoxylin (Fisher Scientific) and mounted using a xylene-based mounting medium (Lecia Biosystems).
Bright-field images and circularly polarized images were taken on a Leica DMLA microscope (Leica Microsystems, Buffalo Grove, IL) using a QImaging MicroPublisher 3.3 RTV camera (Teledyne Photometrics, Tucson, AZ) and analyzed on Micromanager, an open-source microscopy software (36).
Human Tissue Microarray
Tissue arrays of human prostate normal as well as hyperplasia punch biopsy sections (PR632) were purchased from US Biomax (Derwood, MD). The patient data, as provided by US Biomax, were as follows. Prostate tissues for the normal control samples were obtained from healthy donors postautopsy between the ages of 28 and 48 yr old. Prostate tissue biopsies for the six cases of BPH were obtained from patients who were in their older adulthood (ages of 58−78 yr old) with dysuria for several months or years or clinical symptoms of prostate hypertrophy and pathology diagnosis showing that these samples had benign hyperplasia. Picrosirius staining for the sections was performed as described above in Histological and IHC Assays. IHC staining of mast cell tryptase was carried using mouse anti-human mast cell tryptase antibody (Cat. No. 369402, RRID: AB_2566541, BioLegend) (performed by the Northwestern University Mouse Histology and Phenotyping Core). IHC staining was performed as described above in Histological and IHC Assays.
Flow Cytometry
Single cell suspensions were generated from whole prostate tissues combining all prostate lobes using a modified procedure previously described (37). Briefly, whole prostates (combining all lobes) were dissected under sterile conditions from euthanized mice and collected in 1× HBSS buffer containing 5 mM EDTA (Life Technologies) and 2% FBS (Hyclone, South Logan, UT). They were incubated in a shaker for 15 min at 37°C to loosen the tissue. Tissues were then spun down at 100 g and minced with fine scissors. Tissues were then dissociated by shaking for 45 min at 37°C in a 0.4-µm filtered solution of 0.5 mg/mL collagenase D (Roche, Indianapolis, IN), 1 U/mL dispase (StemCell Technologies, Inc., Vancouver, BC, Canada), and 0.1 mg/mL DNase I (Sigma) in 1× HBSS. Digestions were subsequently filtered through a 40-µm nylon mesh and washed with 1× PBS twice before being counted and stained. After being washed, cells were incubated with a Zombie UV fixable viability kit (BioLegend). Afterward, cells were washed in FACS buffer (2% FCS in PBS), and staining was performed using the following conjugated antibodies: brilliant violet (BV)510CD11c (Cat. No. 117353, RRID: AB_2686978), BV570-CD8 (Cat. No. 100740, RRID: AB_2563055), BV650-CD3 (Cat. No. 100229, RRID: AB_11204249), AlexaFluor700-CD45 (Cat. No. 103128, RRID: AB_493715), PerCP-B220 (Cat. No. 103234, RRID: AB_893353), PE/Dazzle 594-CD4 (Cat. No. 100566, RRID: AB_2563685) (BioLegend), and APC/Cy7-CD11b (Cat. No. 557657, RRID: AB_396772, BD Biosciences, San Jose, CA). Samples were run on a BD LSRFortessa (BD Biosciences) cytometer and analyzed using FlowJo (see Fig. 7A for the gating strategy for all samples).
Real-Time Quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Life Technologies), and cDNA synthesis, starting with 1 µg of total RNA, was performed with random hexamers using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies) per the manufacturer’s instructions. Primers for quantitative PCR were created for the RNA of interest using the National Institutes of Health online primer blast tool. Quantitative RT-PCRs were performed using SsoAdvanced universal SYBR green (Bio-Rad) and run on the CFX Connect (Bio-Rad) platform. The primer sequences are shown in Table 1. Data were analyzed by the 2 −ΔΔCT method (where CT is threshold cycle) (38), and normalized to GAPDH as the housekeeping gene. Data are presented as fold changes normalized to average expression of the gene of interest in their respective control group.
Table 1.
List of primer pairs used for quantitative real-time PCR
| Gene Target | Primer Sequence |
|---|---|
| GAPDH | Forward: 5′-GCTGACCTGCTGGATTACATT-3′ |
| Reverse: 5′-GTTGAGAGATCATCTCCACCA-3′ | |
| IL-4 | Forward: 5′-CCATATCCACGGATGCGACA-3′ |
| Reverse: 5′-CGTTGCTGTGAGGACGTTTG-3′ | |
| IL-13 | Forward: 5′-GTATGGAGTGTGGACCTGGC-3′ |
| Reverse: 5′-TCTGGGTCCTGTAGATGGCA-3′ | |
| STAT6 | Forward: 5′-ACGACAACAGCCTCAGTGTGGA-3′ |
| Reverse: 5′-CAGGACACCATCAAACCACTGC-3′ | |
| IFN-γ | Forward: 5′-ACGGCACAGTCATTGAAAGC-3′ |
| Reverse: 5′-ACCATCCTTTTGCCAGTTCC-3′ | |
| IL-17a | Forward: 5′-CAGACTACCTCAACCGTTCCAC-3′ |
| Reverse: 5′-TCCAGCTTTCCCTCCGCATTGA-3′ | |
| IL-10 | Forward: 5′-ATTTGAATTCCCTGGGTGAGAAG-3′ |
| Reverse: 5′-CACAGGGGAGAAATCGATGACA-3′ | |
| Col 1a1 | Forward: 5′-CGATGGATTCCCGTTCGAGT-3′ |
| Reverse: 5′-GAGGCCTCGGTGGACATTAG-3′ | |
| Col 1a2 | Forward: 5′-AGTCGATGGCTGCTCCAAAA-3′ |
| Reverse: 5′-GCAATGTCAAGGAACGGCAG-3′ | |
| Col 3a1 | Forward: 5′-AAGGGCGAAGATGGCAAAGA-3′ |
| Reverse: 5′-AGCCACTAGGACCCCTTTCT-3′ | |
| TGF-β | Forward: 5′-GGACTCTCCACCTGCAAGAC-3′ |
| Reverse: 5′-CTGGCGAGCCTTAGTTTGGA-3′ |
Shown are the primer pairs used for all real-time quantitative RT-PCRs. Col, collagen; IFN, interferon; TGF-β, transforming growth factor-β.
Western Blot Analysis
Frozen prostate samples were lysed in 1× RIPA lysis buffer (Santa Cruz Biotechnology) containing complete‐EDTA protease inhibitor cocktail and phosSTOP phosphatase inhibitor (Millipore-Sigma, Burlington, MA). Cell lysates were cleared by centrifugation at 14,000 rpm for 30 min at 4°C, and insoluble debris was discarded. Proteins were separated by SDS-PAGE on Criterion 4–20% mini gels (Bio-Rad), transferred to polyvinylidene fluoride membranes (Bio-Rad), blocked, and probed with the respective antibodies. Immunoblot analysis was performed using the following antibodies: mouse anti-phosphorylated myosin light chain 2 (MLC2) (Ser19) (Cat. No. 3675, RRID: AB_2250969), rabbit anti-human MLC2 (D18E2) (with cross-reactivity to the mouse) (Cat. No. 8505, RRID: AB_2728760, Cell Signaling Technology), and goat anti‐human GAPDH (with cross-reactivity to the mouse) (Cat. No. AF5718, RRID: AB_2278695, R&D Systems, Minneapolis, MN) and developed using SuperSignal West Pico or West Femto chemiluminescence kits (Thermo Fisher, Hampton, NH). Protein bands were quantified using the National Institutes of Health ImageJ software package and expressed as values of phosphorylated proteins normalized to total species and then to GAPDH levels.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). The statistical tests used in each experiment, technical replicates, biological replicates, independent repeat experiments performed, and murine n values are indicated in figures (in most figures, each dot represents individual animals). Data are presented as means ± SD or means ± SE as appropriate.
RESULTS
Elevated Mast Cell Numbers Are Observed in Human BPH Tissues
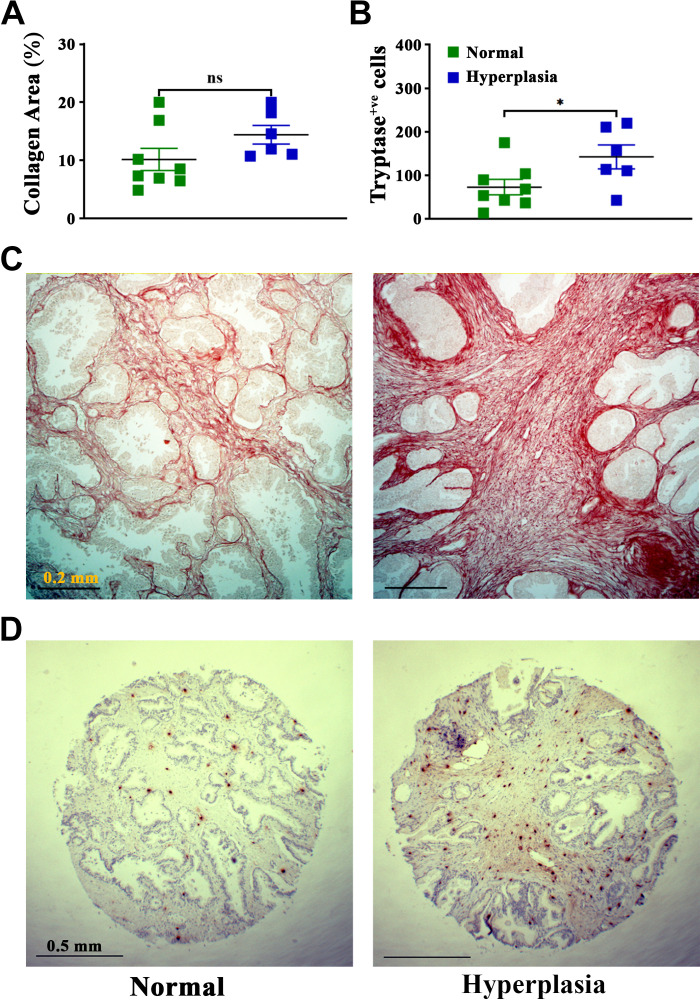
Prostate inflammation and fibrosis are linked to the development and progression of BPH (7–9, 11). Previous studies in the field of BPH have shown that mast cells might be involved in the disease in human patients (7, 9, 22, 39, 40). To validate this information in our setting, fibrosis, as observed by collagen deposition captured by picrosirius staining, was examined in prostate tissue biopsy sections from human patients with BPH and controls obtained from US Biomax (Cat. No. PR632). Patients with BPH showed an increase in immune cell infiltration compared with controls (as shown in the company product specification images). Extracellular collagen deposition, a marker for fibrosis in tissue (41), showed an increase but a nonsignificant trend (P < 0.1) in prostate tissue of patients with BPH compared with normal controls [Fig. 1, A and C (Fig. 1C shows representative picrosirius images comparing surgical BPH and control normal prostate tissue)]. Mast cell numbers were quantified by immunostaining for mast cell tryptase in prostate tissue biopsy sections from patients with BPH and controls. Our results showed a significant increase in mast cells in prostate tissues from patients with BPH compared with controls [Fig. 1, B and D (Fig. 1D shows representative IHC images comparing surgical BPH and control normal prostate tissue)]. This observation implies that mast cells may affect BPH development and progression.
Figure 1.
Increased fibrosis and mast cells numbers in prostates of patients with benign prostatic hyperplasia (BPH) compared with normal control patients. Picrosirius staining for extracellular collagen deposition in human tissues (A and C) and immunohistochemistry for human mast cell tryptase were used to assess numbers of mast cells (B and D) [from US Biomax (Cat. No. PR632)]. A: quantification of the percentage of extracellular collagen deposition in hyperplasia (BPH) and normal prostate tissues using ImageJ software (National Institutes of Health). B: quantitated data for numbers of human mast cell tryptase-positive cells in hyperplasia (BPH) and normal prostate tissue. Each data point represents an individual patient tissue datum, which was derived by averaging the counts and collagen contents for three different tissue biopsies for each patient (means ± SE; n = 8 for normal patients and 6 for patients with BPH). *P < 0.05 (unpaired t test). C and D: representative images of prostate sections from a normal control patients (left) and a patient with BPH (hyperplasia; right) under ×10 magnification for picrosirius staining (C) and ×4 magnification for human mast cell tryptase (D).
Urinary Dysfunction in the CP1-Induced Mouse Model of LUTS Is Associated With Activation of Mast Cells and an Increase in Mast Cell Numbers in the Prostate
Our laboratory has previously demonstrated that intraurethral instillation of CP1 induces chronic prostate inflammation and fibrosis leading to the development of voiding dysfunction in mice (28, 30). Here, in addition to confirming that instillation of CP1 induces voiding dysfunction, we determined whether there were associated changes in mast cell numbers and activation status in the prostates of mice.
To determine effects on voiding behavior, we used the Urovoid system, a noninvasive approach to assess conscious urinary voiding behavior of mice (31). Representative data from the Urovoid analysis software were used to determine the intermicturition interval (IMI) and void mass (volume) per void in CP1-infected C57BL/6 animals over time (Fig. 2A). Following instillation with CP1, we observed a significant reduction in the average number of urinary voids on days 5, 14, and 35 compared with control noninfected mice (Fig. 2B). Conversely, average urinary void mass and IMI were significantly increased in mice on days 5 and 14 (and not on day 35) following instillation with CP1 compared with control mice (Fig. 2, C and D). The urine production rate (UPR) for both groups of mice remained the same over time, indicating that mice did not have an infection-induced intrinsic deficit in the production of urine (Fig. 2E).
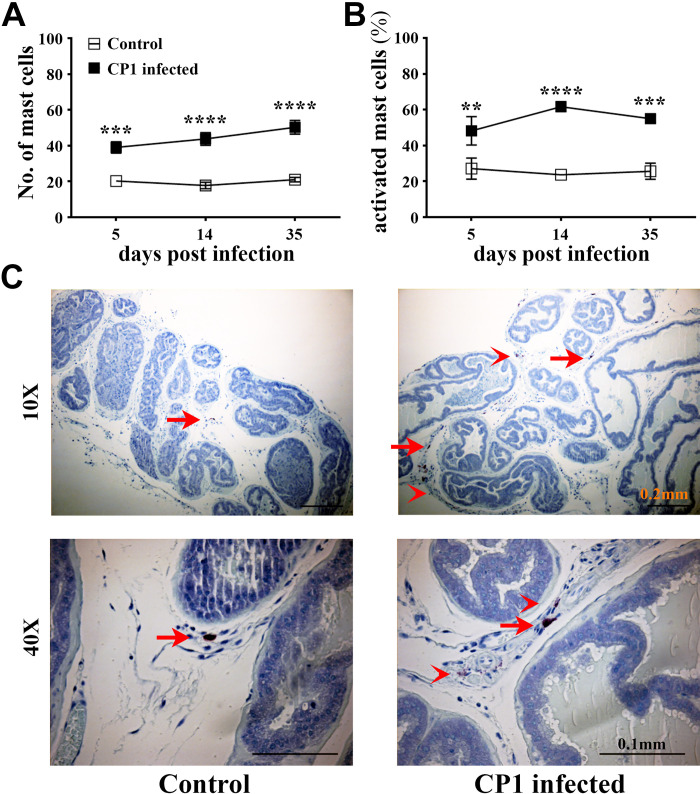
To assess the role of mast cells, we next examined the prostates of CP1-infected mice to determine the numbers and activation status of mast cells. Prostate sections from CP1-infected mice and control mice were subjected to toluidine blue staining to assess mast cell numbers and activation status in the lobes of the prostate. We observed a significant increase in mast cell numbers in the prostate sections on days 5, 14, and 35 compared with control mice, and, in particular, the increase in mast cell numbers was most striking in the dorsolateral lobe of the prostate (Fig. 3A). Furthermore, we observed an accumulation of mast cells in the prostates over the 35-day time period after CP1 instillation. We also assessed the activation status of these mast cells by quantitating the number of degranulated mast cells compared with resting mast cells and determined the percentage of activated mast cells in the prostate sections. Following CP1 instillation, we observed a significant increase in the percentage of activated mast cells in the prostate sections on days 5, 14, and 35 compared with control mice (Fig. 3B) suggesting that CP1 infection causes increased degranulation of mast cells. Figure 3C shows representative toluidine blue-stained images of dorsolateral prostate tissues from control and CP1-infected C57BL/6 mice at day 35 postinfection showing increased mast cell infiltrates as well as increased activated mast cells in prostate tissue of CP1-infected mice. It is noticeable that the increased infiltration of mast cells as well as activation of mast cells following CP1 instillation was most pronounced in the dorsolateral lobe of the prostate, which is supportive of the evidence showing an increased inflammation score in the dorsolateral lobe upon CP1 infection as previously reported (28, 42).
Figure 3.
CP1 infection triggers an increase in mast cell numbers and activation in the prostate of mice. A: total numbers of mast cells. B: percentage of activated (fully and partial combined) mast cells in toluidine blue-stained sections of combined mouse prostate lobes harvested at 5, 14, and 35 days post CP1 infection. Values are presented as means ± SE; n = 4 mice/group. **P < 0.01; ***P < 0.001; ****P < 0.0001 (two-way ANOVA with Fisher’s least significant difference test). Mast cell numbers and percentages of activated mast cells were determined by counting and averaging three sections of the mouse prostate stained with toluidine blue for each sample. C: representative images of toluidine blue-stained dorsolateral prostate sections from control PBS-instilled (left) and CP1-infected (right) mice at 35 days postinstillation imaged at ×10 and ×40 magnification. Note the increased degranulation of mast cells (as denoted by less intracytoplasmic granular staining of mast cells) marked by red arrowheads, whereas resting mast cells (with intact cytoplasm and membrane) are marked by red arrows.
Since toluidine blue is a stain that binds strongly to the granules in both mast cells and basophils, we assessed if basophils are present in the prostates of mice in this E. coli-induced model of prostate fibrosis. We stained prostate tissues for basophils using mouse mast cell protease-8, which, despite its name, is expressed highly in basophils but is expressed at negligible levels by mast cells, neutrophils, and basophils (35, 43). Following CP1 instillation, at 35 days postinstillation, we observed no observable infiltration of basophils in the dorsolateral lobe of the prostate (Supplemental Fig. S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.14877600.v2). This suggests that basophils play a negligible role in this model of prostate inflammation and fibrosis.
MCS and Histamine 1 Receptor Inhibition Prevents Mast Cell Activation and Alleviates Urinary Dysfunction in CP1-Infected Mice
We hypothesized that preventing mast cell activation and downstream histamine-mediated signaling, as previously described, might be an effective strategy at reducing or reversing markers of pathology (33). To examine this, mice were administered a combination of a MCS (CrS) and a H1RA (CeHCl) intraperitoneally for 10 days daily starting at either 5 or 25 days (“early” and “late,” respectively) postinfection (Fig. 4A).
To validate that this combination therapy is effective in preventing mast cell activation, we assessed the numbers and activation status of mast cells in prostate lobes from CrS + CeHCl-treated CP1-infected mice compared with CP1-infected mice using toluidine blue staining of prostate sections. We observed that in both early and late treatment groups, although there were little changes in mast cell numbers in the combined prostate lobes of CrS + CeHCl-treated CP1-infected mice compared with CP1-infected mice, we observed a significant decrease in the percentage of activated mast cells following both early and late treatment (Fig. 4, B and C).
We next assessed voiding behavior in CP1-infected mice after mast cell and histamine 1 receptor inhibition using the Urovoid system. Similar to earlier observations (Fig. 2), following CP1 instillation, mice experienced urinary dysfunction as observed by decreased average numbers of urinary voids in both early and late treatment groups (Fig. 5A), along with increased mean void mass and IMI (Fig. 5, B and C). Following 10-day intraperitoneal administration of the combination of CrS + CeHCl, we observed that average numbers of urinary voids in CP1-infected mice were significantly increased comparable with those of control mice in the late treatment group (Fig. 5A). Furthermore, in both early and late treatment groups, we observed a significant decrease in IMI that was similar to control mice (Fig. 5C). Interestingly, the average urinary void mass of CP1-infected mice treated with CrS + CeHCl did not show any difference compared with untreated CP1-infected mice in both early and late treatment groups (Fig. 5B). UPR for all groups of mice remained the same at early and late time points (Fig. 5D). These data suggest that treatment for inhibition of mast cell degranulation along with histamine 1 receptor inhibition (here referred to as mast cell inhibition) leads to a significant alleviation in CP1-induced urinary dysfunction in mice.
Figure 5.
Mast cell inhibition alleviates urinary dysfunction in CP1-infected mice. A: average number of voids per hour over a period of 4 h. B: average volume of urine per void (calculated for each animal individually). C: intermicturition interval (IMI) as measured by average amount of time (in min) between each micturition event. D: average urine production rate (UPR) in control, CP1-infected mice (untreated), and CP1-infected cromolyn sodium salt + cetirizine di-hydrochloride-treated mice at 14 days (“early”) and 35 (“late”) post CP1 infection. Values are presented as means ± SE. Each dot represents an individual mouse. *P < 0.05; **P < 0.01; ***P < 0.001 (one-way ANOVA with Fisher’s least significant difference test). ns, not significant.
Mast Cell Inhibition Ameliorates Fibrosis in Prostates of CP1-Infected Mice
To understand the mechanism by which mast cell inhibition alleviates urinary dysfunction, we assessed inflammation and fibrosis in MCS + H1RA-treated CP1-infected mice. We assessed inflammation in prostate tissue from CrS + CeHCl-treated CP1-infected mice, CP1-infected mice, and control mice by staining sections from the prostate lobes using H&E. As previously shown (28), we observed that CP1 instillation in C57BL/6 mice triggered a modest but significant level of inflammation in the prostate sections from the dorsolateral lobe of mice at day 35 (late group) compared with control mice (Fig. 6A). Upon mast cell inhibition, we observed no significant changes in inflammation scores in both early and late treatment groups (Fig. 6A). Figure 6C shows representative H&E-stained images of sections from the dorsolateral lobe of prostate tissues from control, CP1-infected, and CP1-infected CrS + CeHCl-treated C57BL/6 mice at day 35 postinfection showing infiltration of immune cells in the stroma of the prostate.
Figure 6.
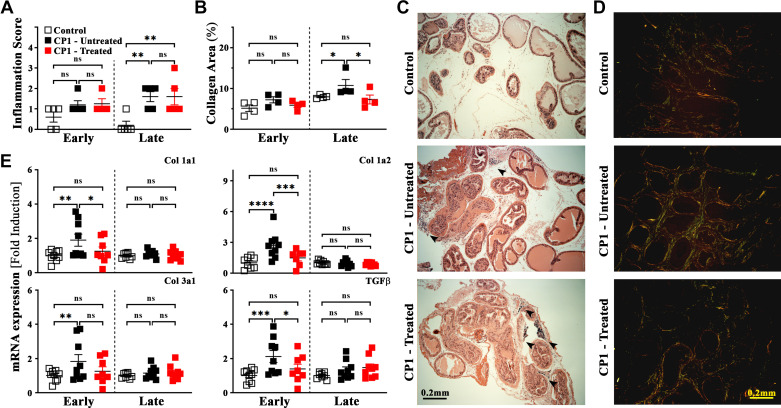
Inflammation and fibrosis are driven by mast cell activity in a CP1-induced mouse model of lower urinary tract symptoms. A and B: quantitation of inflammation scores (as described in materials and methods) using hematoxylin and eosin (H&E) staining (A) and percentages of extracellular collagen deposition as assessed by picrosirius staining (B) from sections of the dorsolateral lobe of the prostate from control, CP1-infected mice (untreated), and CP1-infected cromolyn sodium salt + cetirizine di-hydrochloride (CrS + CeHCl)-treated mice at 14 days (“early”) and 35 days (“late”) post CP1 infection. Values are presented as means ± SE. Each dot represents an individual mouse. *P < 0.05; **P < 0.01 (one-way ANOVA with Fisher’s least significant difference test). Inflammation scores and percentages of collagen area were obtained by examining three independent sections of mouse prostate lobes stained with H&E and picrosirius red staining, respectively. Quantification of picrosirius staining was performed using ImageJ software (National Institutes of Health). C and D: representative H&E-stained images (C) and picrosirius-stained images (D) of dorsolateral prostate sections from control PBS-instilled (top), CP1-infected (middle), and mast cell inhibitor-treated CP1-infected (bottom) mice at 35 days postinstillation imaged at ×4 magnification. Increased infiltration and immune cell foci are marked by black arrowheads (C). E: mRNA expression levels for profibrotic genes [collagen (Col) 1a1, 1a2, and 3a1] as well as transforming growth factor-β (TGF-β) were examined from RNA isolated from total prostates of mice from control, CP1-infected mice, and CP1-infected CrS + CeHCl-treated mice at 14 days (early) and 35 days (late) post CP1 infection. RNA was isolated, converted to cDNA, and subjected to real-time PCR analysis with the respective gene primers. mRNA expression levels were normalized to GAPDH mRNA levels for each sample, and the data are presented as fold changes over control. Values are presented as means ± SE. Each dot represents an individual mouse. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Fisher’s least significant difference test). ns, not significant.
Next, we examined the extent of extracellular collagen deposition in each lobe of the infected mouse prostates. Previously, we have shown that fibrosis, as observed by staining with picrosirius red, was significantly upregulated in the dorsolateral lobe (and to a lesser extent in the ventral lobe) of the prostates of CP1-infected mice (28). Here, we observed similar results for extracellular collagen deposition in the dorsolateral lobes of prostates of CP1-infected mice at day 35 postinfection (Fig. 6B). Therapeutic administration of CrS + CeHCl in CP1-infected mice (significantly at late treatment and to a lesser extent at early treatment) was able to attenuate CP1-induced collagen deposition (Fig. 6B). Figure 6D shows representative picrosirius images of sections from the dorsolateral lobe of prostate tissues from control, CP1-infected, and CP1-infected CrS + CeHCl-treated C57BL/6 mice at day 35 postinfection showing collagen deposition.
In addition, we performed quantitative PCR for fibrosis-associated genes and profibrotic markers on RNA extracted from mouse prostate tissues. We examined expression levels of collagen type 1a1, collagen type 1a2, and collagen type 3a1, which are extracellular markers of fibrosis, as well as transforming growth factor-β (TGF-β), a well-known profibrotic signaling molecule (44). Upon CP1 instillation, we observed a significant upregulation of mRNA expression for all four markers in the prostates of CP1-infected mice compared with control mice at day 14 postinfection, as previously reported (28) (Fig. 6E). Interestingly, when CP1-infected mice were treated with CrS + CeHCl, RNA extracted from the prostates of these mice showed significantly decreased levels of expression of collagen type 1a1 and collagen type 1a2 as well as TGF-β in the early treatment group (Fig. 6E) but not for collagen type 3a1. Although we did observe some mild upregulation in mRNA of these four profibrotic markers in the prostates of CP1-infected mice at day 35 postinfection, this was not significant, nor were there any significant changes upon treatment with CrS + CeHCl.
Mast Cell Inhibition Alters Immune Cell Skewing and Inhibits Type 2 Cytokine Expression in the Prostates of CP1-Infected Mice
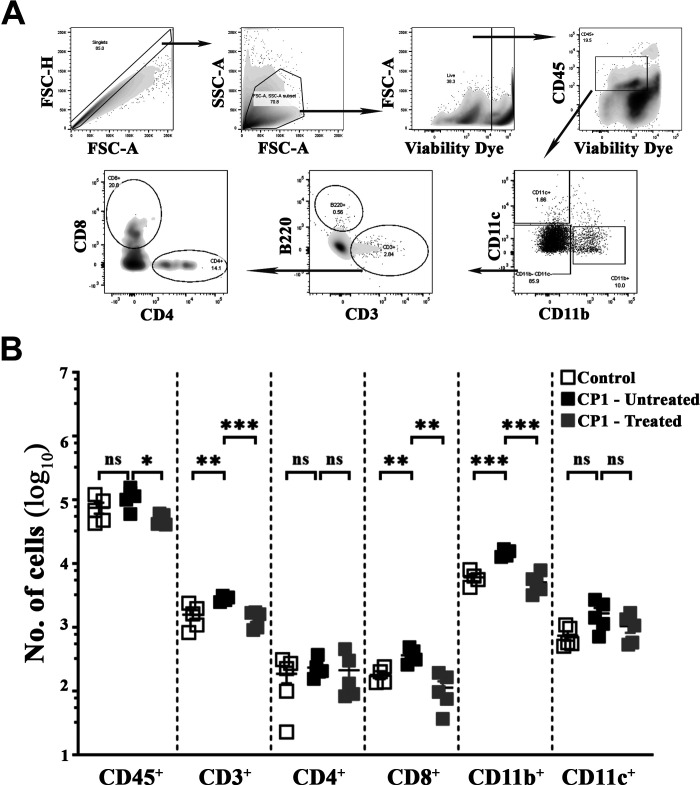
Activation of mast cells is known to release a plethora of mediators that are important in triggering the infiltration of immune cells as well as inducing tissue repair after injury (14, 15, 18, 20). Chemokines secreted from mast cells acting within the prostatic epithelium and stroma provide signals that contribute to increased numbers of B and T lymphocytes and macrophages in the prostates of patients with BPH (45). To assess the effect of mast cell combination therapy on immune cell infiltrates, flow cytometry was performed on prostates of CP1-infected mice, and immune cell populations were identified and gated as shown in Fig. 7A. As shown in Fig. 7B, at day 35 post CP1 instillation, we observed an increase in numbers of immune cell infiltrates in the prostates of CP1-infected mice (as shown by total numbers of CD45+ cells), which was significantly decreased to levels comparable with those of control mice upon treatment with CrS + CeHCl. We observed that CP1 infection significantly increased numbers of total CD3+ T cells as well as CD8+ T cells in the prostates of mice. Mast cell inhibition (treatment with CrS + CeHCl) significantly decreased numbers of total CD3+ T cells as well as CD8+ T cells to numbers similar to those in control mice. Furthermore, we also observed that upon CP1 instillation, there was a significant increase in numbers of CD11b+ macrophages and a modest increase in CD11c+ dendritic cells in prostates compared with control mice; upon treatment with CrS + CeHCl, numbers of CD11b+ macrophages significantly decreased to numbers similar to those of control mice (Fig. 7B). These observations were observed in the early treatment group as well, albeit to a lesser extent considering that CP1 instillation does not trigger immune cell infiltration early during infection of C57BL/6 mice (Table 2). As shown in Table 2, in the early treatment group upon CrS + CeHCl treatment of CP1-infected mice, we observed a significant decrease in numbers of CD11b+ macrophages as well as CD4+ and CD8+ T cells compared with CP1-infected mice.
Figure 7.
Mast cell inhibition mollifies alterations in CP1-induced immune cell infiltrates in the prostate of mice. A: flow cytometric gating strategy for analyzing immune cell infiltrates in cell suspension prepared from whole prostates of mice. B: prostates isolated from control, CP1-infected mice (untreated), and CP1-infected cromolyn sodium salt + cetirizine di-hydrochloride-treated mice at 35 days (“late”) post CP1 infection were digested, and total lymphocytes (CD45+ cells), CD3+ T lymphocytes (as well as CD4+ and CD8+ T cells), CD11b+ monocytes/macrophages, and CD11c+ dendritic cells were stained with the respective fluorescent antibodies (as indicated in materials and methods) and analyzed by flow cytometry. Data represent total numbers of each cell type obtained from whole prostates using the tissue digestion protocol described in materials and methods. Values are presented as means ± SE. Each dot represents an individual mouse. *P < 0.05; **P < 0.01; ***P < 0.001 (one-way ANOVA with Fisher’s least significant difference test). ns, not significant.
Table 2.
Immune cell infiltrates in the prostate of CP1-infected mice with and without cromolyn sodium salt + cetirizine di-hydrochloride combination treatment
| Cell Types | Treatments |
Unpaired t Test | |
|---|---|---|---|
| CP1 Untreated | CP1 + Treatment | ||
| CD45+ lymphocytes | 23,024 ± 10,122 | 20,780 ± 11,296 | 0.6223ns |
| CD3+ T cells | 3,197 ± 3,201 | 1,666 ± 466.8 | 0.3805ns |
| CD4+ T cells | 505 ± 45.26 | 343.8 ± 82.27 | 0.0139* |
| CD8+ T cells | 1,122 ± 235.2 | 655.5 ± 169.7 | 0.0182* |
| B220+ B cells | 329 ± 351.3 | 181 ± 87.64 | 0.4449ns |
| CD11b+ monocytes/macrophages | 2,800 ± 1,138 | 1,315 ± 300.6 | 0.0451* |
| CD11c+ dendritic cells | 3,171 ± 1,808 | 1,218 ± 148 | 0.0747† |
Values are presented as means ± SD; n = 4 mice/group. Numbers of infiltrating cells in the prostate of CP1-infected C57BL/6 mice with or without cromolyn sodium salt + cetirizine di-hydrochloride treatment at “early treatment” (day 14 postinfection) are shown. Unpaired t test values are also indicated. *P < 0.05; †P < 0.1. ns, not significant.
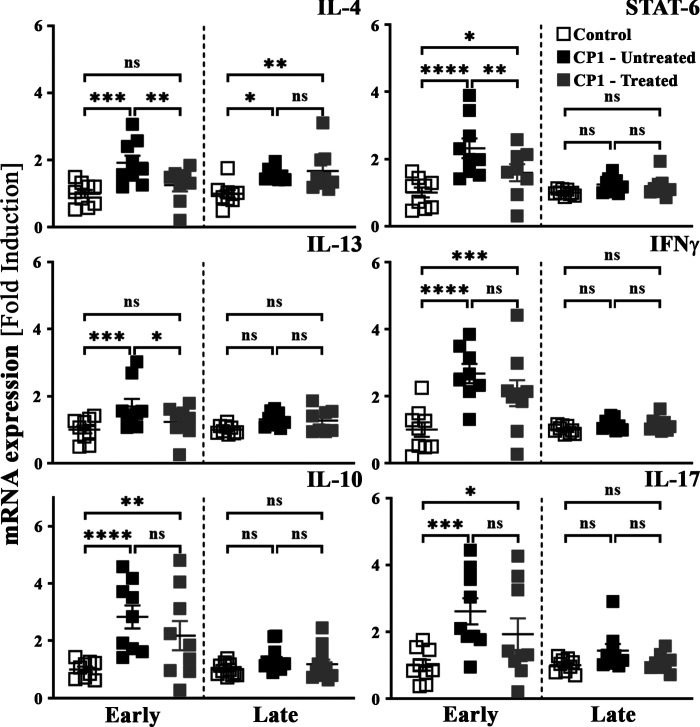
We have previously reported that type 2 cytokine signaling plays an important role in driving fibrosis in our CP1-induced model of BPH-associated LUTS (28). Furthermore, type 2 cytokines have been shown to drive fibrosis in several different diseased conditions (41, 46, 47). To assess the impact of mast cell inhibition therapy on type 2-associated cytokines, quantitative PCR was performed for cytokine transcripts from prostates obtained from control mice, CP1-infected untreated mice, and CP1-infected CrS + CeHCl-treated mice (48). As shown in Fig. 8, in the early treatment group, and to a lesser extent in the late treatment group, we observed a significant increase in transcript levels of IL-4 and IL-13 (type 2-associated cytokines) as well as an upregulation of STAT6 [a key signal transduction molecule associated with T helper (Th)2 polarization (49, 50)] in the prostates of CP1-infected mice compared with controls. Upon treatment with CrS + CeHCl, the three markers showed significantly reduced transcript levels compared with CP1-infected mice alone in the early treatment group. Moreover, when mice were administered single drug treatment of CrS alone or CeHCl alone, we observed that treatment with CrS alone reduced gene expression levels of IL-4 and IL-13 to negligible levels. Treatment with the CeHCl alone reduced gene expression levels of IL-4 and IL-13 to modest but nonsignificant levels in the prostates of CP1-infected mice. Combination treatment showed synergism and significantly reduced gene expression of both IL-4 and IL-13 in the prostates of CP1-infected mice (Supplemental Fig. S2, available at https://doi.org/10.6084/m9.figshare.14877600.v2). Gene expression of other cytokines like interferon-γ and IL-17 (associated with type 1 and type 3 immune responses, respectively), and IL-10, a negative regulator of T cell activation, were significantly upregulated in the prostates upon CP1 instillation in the early treatment group. Combination treatment with CrS + CeHCl did not cause any significant changes to their transcript levels compared with CP1-infected mice (Fig. 8). These data show that mast cell combination therapy inhibits type 2 cytokine skewing of the immune response in the prostates of mice induced by CP1 instillation.
Figure 8.
Prostates of CP1-infected mice treated with cromolyn sodium salt + cetirizine di-hydrochloride (CrS + CeHCl) show attenuated expression of type 2 cytokine genes. Whole prostates from control, CP1-infected mice (untreated), and CP1-infected CrS + CeHCl-treated mice at 14 days (“early”) and 35 days (“late”) post CP1 infection were lysed in TRIzol. RNA was isolated, converted to cDNA, and subjected to real-time PCR analysis with the respective gene primers. mRNA expression levels were normalized to GAPDH mRNA levels for each sample, and the data are presented as fold changes over control. Values are presented as means ± SE. Each dot represents an individual mouse. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Fisher’s least significant difference test). IFN, interferon; ns, not significant.
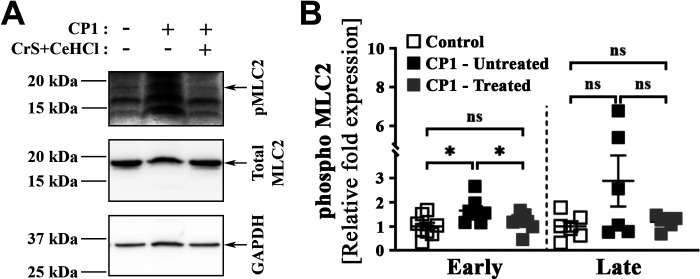
Mast Cell Inhibition Attenuates Phosphorylation of MLC2, a Marker for Smooth Muscle Cell Contraction, in the Prostate of CP1-Infected Mice
Prostate stromal cell proliferation and smooth muscle contraction are crucial in the development and pathogenesis of BPH-associated LUTS and have been a major target for treatment (51). Previously, we have shown that PAR2, a receptor for trypsin and mast cell‐derived serine protease and tryptase, plays an important role in prostate smooth muscle cell contraction (27). As our combination treatment interfered with the release of mast cell proteases and tryptases, we examined the consequences of combination treatment on smooth muscle cell contraction. We assessed smooth muscle cell contraction by assessing the phosphorylation status of MLC2, the phosphorylation and dephosphorylation of which regulates muscle contraction and relaxation, respectively (52–54), in the prostates from control mice, CP1-infected untreated mice, and CP1-infected CrS + CeHCl-treated mice. As shown in Fig. 9A, upon CP1 instillation in mice, prostate lysates showed an elevated level of MLC2 phosphorylation compared with control mice in the early treatment group. Interestingly, in the early treatment group, we observed that mast cell inhibition caused a significant decrease in levels of phosphorylated MLC2 in prostate lysates (Fig. 9A). Figure 9B shows the quantitation by densitometry analysis of the Western blot photomicrographs from multiple mice from both early and late treatment groups. In contrast to what was observed in the early treatment group, at later points post CP1 instillation, we observed no significant increase in levels of phosphorylated MLC2 compared with control mice in prostate lysates. There were no significant differences in phosphorylated MLC2 levels in CP1-infected mice upon mast cell inhibition (Fig. 9B). The data suggest that mast cell inhibition is effective in reducing smooth muscle cell contraction in the prostates of CP1-infected mice.
Figure 9.
Mast cell inhibition during CP1-induced lower urinary tract symptoms attenuates myosin light chain-2 (MLC2) phosphorylation in the prostates of mice. Whole prostates from control, CP1-infected mice (untreated), and CP1-infected cromolyn sodium salt + cetirizine di-hydrochloride (CrS + CeHCl)-treated mice at 14 days (“early”) and 35 days (“late”) post CP1 infection were lysed in 1× RIPA lysis buffer. A: whole cell lysates were examined by Western blot analyses using the indicated antibodies, and representative Western blots for each group of mice at the early time point are shown. B: quantifications of the Western blot data shown in A (n = 8 for early and n = 6 for late). Data are plotted as arbitrary values obtained by normalizing the band intensity for phosphorylated (p)MLC2 to the band intensities of total MLC2 and then normalizing this to the band intensity of GAPDH, and data are expressed as fold changes over control. Values are presented as means ± SE. Each dot represents an individual mouse. *P < 0.05 (one-way ANOVA with Fisher’s least significant difference test). ns, not significant.
DISCUSSION
The pathogenesis of LUTS associated with BPH can be broken down into three facets: the epithelial compartment where hyperplasia of the epithelial cells occurs, the stromal compartment where immune cell infiltration and inflammation cause potential fibrosis, and finally the smooth muscle compartment where smooth muscle cell contraction occurs (13, 39). An inflammatory insult, sterile inflammation, aging-related factors, or stress-induced hormonal changes may trigger a dysregulation in any or all these compartments leading to the development and progression of LUTS associated with BPH (4, 5, 7–10). In this study, using a previously established E. coli (CP1) infection-induced model of LUTS, we assessed the importance of mast cells in the development and progression of urinary dysfunctions.
Based on previous work, CP1 infection in the C57BL/6 mouse model triggers prostate inflammation, increased immune cell infiltration, urinary dysfunction, and fibrosis. Although the extent of inflammation and fibrosis as well as the type of inflammation are different in C57BL/6 mice compared with NOD-ShiLJ mice, CP1 infection in C57BL/6 mice does not induce pain (28, 29). The similarities between the urinary dysfunction associated with the CP1 infection-induced mouse model and LUTS in human BPH extend to an increased presence of mast cells in the prostates (especially the dorsolateral lobe) of the mice as well. This observation of the increased presence of mast cells in BPH tissue is not novel, but the importance of these mast cells has been relatively unappreciated (22, 55, 56).
Previously, we demonstrated that CP1 infection in mice induced upregulation of type 1 (interferon-γ), type 2 (IL-4, IL-5, and IL-13), and type 3 (IL-17A) cytokines in the prostates and draining lymph nodes (28, 30, 57). Furthermore, we have previously shown that this type 2 cytokine production from CD4+ T cells, via activation of the STAT6 pathway, was crucial in the development of fibrosis in the prostate of CP1-infected C57BL/6 mice (28). Here, our data further reinforce the idea that CP1 infection triggers type 1, type 2, and type 3 cytokine gene expression. More interestingly, we observed that mast cell inhibition was specifically able to attenuate type 2 (IL-4 and IL-13) cytokines as well as STAT-6 gene expression. These findings can be explained by two different possible mechanisms. The combination treatment may directly affect the ability of mast cells to produce and release type 2 cytokines upon activation and degranulation (14, 20). Secondarily, in synergy with the first proposed mechanism, the administration of H1RA acts as an immunomodulator in the Th1/Th2 imbalance in the diseased prostate and in this model dampens the production of type 2 cytokines by Th2 CD4+ T cells as well as prevents Th2 differentiation and infiltration (58, 59). The ability of the combination treatment to attenuate STAT-6 alongside IL-4 and IL-13 cytokine gene expression suggests that suppressing downstream signaling of mast cell mediators through the administration of H1RA directly or indirectly skews CD4+ T lymphocytes away from a Th2 cell type. It is highly likely that the effect of administration of H1RA is due to a combination of at least these two possible mechanisms. This immunomodulatory effect can be seen in the dampening of fibrosis development in prostate tissue. Interestingly, administration of H1RA alone does have a modest effect on IL-4 and IL-13 cytokine gene expression, suggesting that administration of an individual drug alone might have a profound effect in the prevention of the progression of LUTS. The identification of the target cell population on which H1RA acts could also provide valuable information on the other cell types that are critical in prostate inflammation fibrosis associated with LUTS. In the present study, we were unable to evaluate the changes in Th cell subtypes owing to limitations in the numbers of cells extracted from the prostates of mice. However, studies are underway to assess the effects of this combination treatment in the modulation of the Th1/Th2 imbalance in the prostate and draining lymph nodes.
An unexpected and very surprising observation from mast cell inhibition in the CP1-induced mouse model of LUTS was the ability of combination treatment to inhibit the increased presence of immune cells in the prostates of mice. A plethora of chemokines and mitogens are released from mast cells in the context of inflammation, allergy, and infection (14, 18), and, in some cases, chemokine release occurs independent of mast cell degranulation (60). The absence of increased immune cells in the prostates of CP1-infected mice upon mast cell inhibition could suggest that they either inhibit infiltration of immune cells in the prostates of these mice or that they do not provide the growth factors, cytokines, and mitogens necessary for the proliferation of immune infiltrates. In either scenario, our finding provides a crucial piece of evidence that mast cells might be playing an upstream role in this CP1 infection model of LUTS in mice that precedes immune cell skewing and fibrosis in the prostates of mice. It is important to note that H1RA acts on multiple cell types other than mast cells, such as T cells, B cells, endothelial cells, neutrophils, dendritic cells, and mast cells (61). Activation of histamine 1 receptors in these multiple cell types leads to different aspects of immune activation such as Th1/Th2 skewing, enhanced B cell proliferation, and macrophage polarization. Our observation in regard to decreased fibrosis and skewing of the immune response in the prostate could be a combination of the drugs acting on these various different cell types.
Mast cell-released mediators, including histamine, proteases, tryptases, chymases, and leukotrienes, have been implicated to play a critical role in smooth muscle cell function, apoptosis, and contraction (15). These mast cell mediators act on PAR2 as well as histamine 1 receptors and play a role in triggering smooth muscle cell contraction (59, 61). Little is understood about the role of mast cell-released factors in the prostate of patients with BPH and its effects on smooth muscle cell contraction; however, the relationship between mast cells, histamine 1 receptors, and H1RAs, and its effect on airway smooth muscle cell contraction in regard to asthma, have been well studied (62–65). PAR2 is a gene that is ubiquitously expressed in most tissues in the human body, and we have previously shown that PAR2 is expressed at high levels in smooth muscle cells of both murine and human prostates. PAR2 activation by these mast cell proteases triggers downstream Ca2+-dependent prostate smooth muscle cell contraction (27, 66). In a previous study (27), we had discussed the possibility that PAR2 inhibitors along with α‐blockers (drugs that inhibit α‐adrenergic receptor signaling) could be used in conjunction to treat urinary symptoms in patients with chronic prostatitis/chronic pelvic pain syndrome and BPH (27). Our present observation provides another layer and possibly a more favorable therapeutic target upstream of PAR2 blockade, along with alleviating fibrosis and inflammation.
The key findings of our study show that mast cell numbers and activity are increased in prostates of patients with BPH/LUTS and in CP1-infected mice showing symptoms of LUTS. We show that mast cell inhibition, through a combination of MCS and H1RA, alleviates urinary dysfunction in CP1-infected mice. We observe that mast cell inhibition triggers decreased inflammation and fibrosis, attenuation in smooth muscle cell contraction, and a decrease in immune infiltrates in the prostates of CP1-infected mice (Fig. 10).
Figure 10.
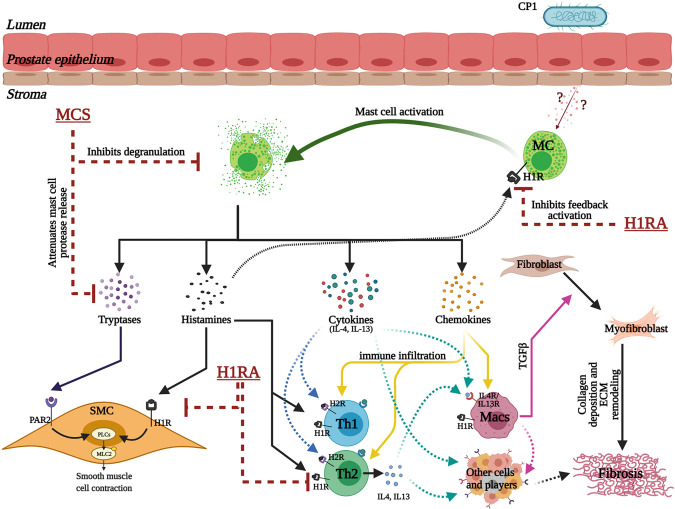
Schematic illustration of the crucial multifaceted role played by prostate mast cells (MCs) in facilitating prostatic inflammation, fibrosis, and smooth muscle contraction in the development of lower urinary tract symptoms. Intraurethral instillation of CP1 in mice triggers epithelial tissue damage that induces the release of as yet unknown factors that lead to an increase in MC numbers and activation in the mouse prostate stroma. Activated MCs, in turn, release a plethora of factors that include tryptases (and other proteases), histamines, cytokines (including IL-4 and IL-13), and chemokines. Tryptases and histamine released from MCs act on smooth muscle cells (SMCs) via protease-activated receptor 2 (PAR2) and histamine 1 receptors (H1Rs), respectively, leading to phospholipase C (PLC)-mediated regulation of SMC contraction. Histamine released from activated MCs also triggers a feedback loop to further activate MCs through histamine receptors on MCs. The chemokines released trigger increased immune cell infiltration and proliferation. The cytokines released from MCs lead to activation of T helper (Th)1, Th2, and Th17 cells, dendritic cells, and macrophages (Macs). Histamines released from MCs in turn act on H1Rs and histamine 2 receptors (H2Rs) on Th1 and Th2 cells modulating type 1/type 2 cytokine production, leading to a hyperinflammatory environment. IL-4 and IL-13 released from MCs and Th2 cells act on macrophages and cause macrophage polarization leading to the release of transforming growth factor-β (TGF-β), which causes primed resident fibroblasts to differentiate into myofibroblasts that release collagen and other matric proteins leading to extracellular matrix (ECM) remodeling and fibrosis. Also, the cytokines released from MCs, T cells, macrophages, and other cells might interact with immune and nonimmune cells to promote fibrosis independent of the myofibroblast transition. Combination treatment for MC inhibition that includes mast cell stabilizer (MCS) and H1R antagonist (H1RA) acts on multiple different cell types at multiple levels of these crucial aspects and regulates the tissue environment in a CP1-infected mouse prostate. [This model was created with BioRender.com.]
An in-depth assessment of the importance of mast cells in the development and progression of urinary dysfunction can be addressed using genetic knockout (mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice) approaches (67–69). These approaches, however, could be confounded by the impact of mast cells on innate immune homeostasis. The combination therapy approach of administration of a MCS along with a H1RA provides a model wherein we are able to dampen overactive mast cells in the prostate to alleviate both histopathological changes and physiological effects of urinary dysfunction.
As previously mentioned, CP1-infected NOD mice develop symptoms of LUTS similar to those of C57BL/6 mice along with pelvic pain (28). Although our present study did not assess the role of mast cells in the NOD mouse model of LUTS with pain, we can hypothesize that mast cells might play a similarly crucial role in the development of inflammation, fibrosis, and urinary dysfunction in NOD mice, albeit the type of immune response (Th1/Th2/Th17 skew) might be altered. It would be interesting to assess mast cell inhibition in the NOD model of LUTS associated with pain as a means to address whether this combination treatment of MCS + H1RA could also be an efficient therapeutic intervention for patients with chronic prostatitis/chronic pelvic pain syndrome who have LUTS.
Perspectives and Significance
Our study demonstrates that mast cells appear to play a critical role in the pathogenesis of voiding dysfunction in an uropathogenic E. coli-induced mouse model of LUTS. Mast cells have been shown to be present in increased numbers in the prostates of patients with BPH/LUTS. Whether these mast cells are functionally active remains to be determined. We show that mast cell inhibition, through a combination of MCS and H1RA, alleviates urinary dysfunction in CP1-infected mice. Mice treated with this combination also showed reduced prostatic fibrosis, less infiltration of immune cells, and decreased inflammation, along with potentially easing smooth muscle contraction in the prostates. The observations from this study show that blockade of mast cell function is therapeutically effective for ameliorating voiding dysfunction. This study has important translational implications for men diagnosed with BPH-associated LUTS.
SUPPLEMENTAL DATA
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.14877600.v2.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Grants R01DK083609 (to P.T.) and R01DK117906 (to Dr. Simon W. Hayward and P.T.).
DISCLAIMERS
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.P., D.J.M., and P.T. conceived and designed research; G.P., A.J.B-C., S.F.M., and D.J.M. performed experiments; G.P. and P.T. analyzed data; G.P., A.J.S., and P.T. interpreted results of experiments; G.P. prepared figures; G.P. drafted manuscript; G.P., A.J.S., and P.T. edited and revised manuscript; G.P., A.J.B-C., S.F.M., D.J.M., A.J.S., and P.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Simon W. Hayward (Department of Surgery, NorthShore University HealthSystem, Evanston, IL) for providing insights into the project and providing some of the initial human patient prostate sections for characterizing and standardizing the staining in human tissues. Histology services were provided by the Northwestern University Research Mouse Histology and Phenotyping Laboratory, which is supported by the National Cancer Institute Grant P30CA060553 (awarded to the Robert H Lurie Comprehensive Cancer Center). Flow cytometry services were provided by the Northwestern University Robert H. Lurie Comprehensive Cancer Center Flow Cytometry Core Facility, which is supported by a Cancer Center Support Grant (NCI Grant CA060553).
REFERENCES
- 1.Hollingsworth JM, Wilt TJ. Lower urinary tract symptoms in men. BMJ 349: g4474, 2014. doi: 10.1136/bmj.g4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auffenberg GB, Helfand BT, McVary KT. Established medical therapy for benign prostatic hyperplasia. Urol Clin North Am 36: 443–459, 2009. doi: 10.1016/j.ucl.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE , Jr., Gonzalez CM, Kaplan SA, Penson DF, Ulchaker JC, Wei JT. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 185: 1793–1803, 2011. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 4.Patel ND, Parsons JK. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J Urol 30: 170–176, 2014. doi: 10.4103/0970-1591.126900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol 10: 546–550, 2013. doi: 10.1038/nrurol.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushman WA, Jerde TJ. The role of prostate inflammation and fibrosis in lower urinary tract symptoms. Am J Physiol Renal Physiol 311: F817–F821, 2016. doi: 10.1152/ajprenal.00602.2015. [DOI] [PubMed] [Google Scholar]
- 7.Kruslin B, Tomas D, Dzombeta T, Milkovic-Perisa M, Ulamec M. Inflammation in prostatic hyperplasia and carcinoma-basic scientific approach. Front Oncol 7: 77, 2017. doi: 10.3389/fonc.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev Urol 13: 147–150, 2011. [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol 51: 1202–1216, 2007. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd GL, Marks JM, Ricke WA. Benign prostatic hyperplasia and lower urinary tract symptoms: what is the role and significance of inflammation? Curr Urol Rep 20: 54, 2019. doi: 10.1007/s11934-019-0917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauman TM, Nicholson TM, Abler LL, Eliceiri KW, Huang W, Vezina CM, Ricke WA. Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS One 9: e109102, 2014. doi: 10.1371/journal.pone.0109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantiello F, Cicione A, Salonia A, Autorino R, Tucci L, Madeo I, Damiano R. Periurethral fibrosis secondary to prostatic inflammation causing lower urinary tract symptoms: a prospective cohort study. Urology 81: 1018–1023, 2013. doi: 10.1016/j.urology.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 13.Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res 20 Suppl 3: S11–S18, 2008. doi: 10.1038/ijir.2008.55. [DOI] [PubMed] [Google Scholar]
- 14.Beghdadi W, Madjene LC, Benhamou M, Charles N, Gautier G, Launay P, Blank U. Mast cells as cellular sensors in inflammation and immunity. Front Immunol 2: 37, 2011. doi: 10.3389/fimmu.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol 6: 620, 2015. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overed-Sayer C, Rapley L, Mustelin T, Clarke DL. Are mast cells instrumental for fibrotic diseases? Front Pharmacol 4: 174, 2013. doi: 10.3389/fphar.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann C, Troeltzsch D, Gimenez-Rivera VA, Galli SJ, Metz M, Maurer M, Siebenhaar F. Mast cells are critical for controlling the bacterial burden and the healing of infected wounds. Proc Natl Acad Sci USA 116: 20500–20504, 2019. doi: 10.1073/pnas.1908816116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 282: 121–150, 2018. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veerappan A, O'Connor NJ, Brazin J, Reid AC, Jung A, McGee D, Summers B, Branch-Elliman D, Stiles B, Worgall S, Kaner RJ, Silver RB. Mast cells: a pivotal role in pulmonary fibrosis. DNA Cell Biol 32: 206–218, 2013. doi: 10.1089/dna.2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin K. The role of mast cells in allergic inflammation. Respir Med 106: 9–14, 2012. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Lunderius-Andersson C, Enoksson M, Nilsson G. Mast cells respond to cell injury through the recognition of IL-33. Front Immunol 3: 82, 2012. doi: 10.3389/fimmu.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou Z, He Y, Qi L, Zu X, Wu L, Cao Z, Li Y, Liu L, Dube DA, Wang Z, Wang L. Infiltrating mast cells enhance benign prostatic hyperplasia through IL-6/STAT3/Cyclin D1 signals. Oncotarget 8: 59156–59164, 2017. doi: 10.18632/oncotarget.19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romih R, Winder M, Lee G. Recent advances in the biology of the urothelium and applications for urinary bladder dysfunction. Biomed Res Int 2014: 341787, 2014. doi: 10.1155/2014/341787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodman L, Siddiqui S, Cruse G, Sutcliffe A, Saunders R, Kaur D, Bradding P, Brightling C. Mast cells promote airway smooth muscle cell differentiation via autocrine up-regulation of TGF-beta 1. J Immunol 181: 5001–5007, 2008. doi: 10.4049/jimmunol.181.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Shiota N, Leskinen MJ, Lindstedt KA, Kovanen PT. Mast cell chymase inhibits smooth muscle cell growth and collagen expression in vitro: transforming growth factor-beta1-dependent and -independent effects. Arterioscler Thromb Vasc Biol 21: 1928–1933, 2001. doi: 10.1161/hq1201.100227. [DOI] [PubMed] [Google Scholar]
- 26.Berger P, Girodet PO, Begueret H, Ousova O, Perng DW, Marthan R, Walls AF, Tunon de Lara JM. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J 17: 2139–2141, 2003. doi: 10.1096/fj.03-0041fje. [DOI] [PubMed] [Google Scholar]
- 27.Paul M, Murphy SF, Hall C, Schaeffer AJ, Thumbikat P. Protease-activated receptor 2 activates CRAC-mediated Ca2+ influx to cause prostate smooth muscle contraction. FASEB Bioadv 1: 255–264, 2019. doi: 10.1096/fba.2018-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell-Cohn A, Mazur DJ, Hall CC, Schaeffer AJ, Thumbikat P. Uropathogenic Escherichia coli-induced fibrosis, leading to lower urinary tract symptoms, is associated with type-2 cytokine signaling. Am J Physiol Renal Physiol 316: F682–F692, 2019. doi: 10.1152/ajprenal.00222.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudick CN, Berry RE, Johnson JR, Johnston B, Klumpp DJ, Schaeffer AJ, Thumbikat P. Uropathogenic Escherichia coli induces chronic pelvic pain. Infect Immun 79: 628–635, 2011. doi: 10.1128/IAI.00910-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quick ML, Wong L, Mukherjee S, Done JD, Schaeffer AJ, Thumbikat P. Th1-Th17 cells contribute to the development of uropathogenic Escherichia coli-induced chronic pelvic pain. PLoS One 8: e60987, 2013. doi: 10.1371/journal.pone.0060987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard BM, Campbell SE, Perkins M, Hsiang H, Tooke K, Drescher C, Hennig GW, Heppner TJ, Nelson MT, Vizzard MA. TRPV4 blockade reduces voiding frequency, ATP release, and pelvic sensitivity in mice with chronic urothelial overexpression of NGF. Am J Physiol Renal Physiol 317: F1695–F1706, 2019. doi: 10.1152/ajprenal.00147.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zingiryan A, Farina NH, Finstad KH, Stein JL, Lian JB, Stein GS. Dissection of individual prostate lobes in mouse models of prostate cancer to obtain high quality RNA. J Cell Physiol 232: 14–18, 2017. [Erratum in J Cell Physiol 232: 3194, 2017]. doi: 10.1002/jcp.25384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Done JD, Rudick CN, Quick ML, Schaeffer AJ, Thumbikat P. Role of mast cells in male chronic pelvic pain. J Urol 187: 1473–1482, 2012. doi: 10.1016/j.juro.2011.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickel JC, True LD, Krieger JN, Berger RE, Boag AH, Young ID. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int 87: 797–805, 2001. doi: 10.1046/j.1464-410x.2001.02193.x. [DOI] [PubMed] [Google Scholar]
- 35.Ugajin T, Kojima T, Mukai K, Obata K, Kawano Y, Minegishi Y, Eishi Y, Yokozeki H, Karasuyama H. Basophils preferentially express mouse mast cell protease 11 among the mast cell tryptase family in contrast to mast cells. J Leukoc Biol 86: 1417–1425, 2009. doi: 10.1189/jlb.0609400. [DOI] [PubMed] [Google Scholar]
- 36.Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. Computer control of microscopes using microManager. Curr Protoc Mol Biol 14: Unit14.20, 2010. doi: 10.1002/0471142727.mb1420s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy SF, Anker JF, Mazur DJ, Hall C, Schaeffer AJ, Thumbikat P. Role of gram-positive bacteria in chronic pelvic pain syndrome (CPPS). Prostate 79: 160–167, 2019. doi: 10.1002/pros.23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−ΔΔC(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Penna G, Fibbi B, Amuchastegui S, Cossetti C, Aquilano F, Laverny G, Gacci M, Crescioli C, Maggi M, Adorini L. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol 182: 4056–4064, 2009. doi: 10.4049/jimmunol.0801875. [DOI] [PubMed] [Google Scholar]
- 40.Di Silverio F, Gentile V, De Matteis A, Mariotti G, Giuseppe V, Luigi PA, Sciarra A. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol 43: 164–175, 2003. doi: 10.1016/s0302-2838(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 41.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruetten H, Sandhu J, Mueller B, Wang P, Zhang HL, Wegner KA, Cadena M, Sandhu S, L LA, Zhu J, O'Driscoll CA, Chelgren B, Wang Z, Shen T, Barasch J, Bjorling DE, Vezina CM. A uropathogenic E. coli UTI89 model of prostatic inflammation and collagen accumulation for use in studying aberrant collagen production in the prostate. Am J Physiol Renal Physiol 320: F31–F46, 2021. doi: 10.1152/ajprenal.00431.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piliponsky AM, Shubin NJ, Lahiri AK, Truong P, Clauson M, Niino K, Tsuha AL, Nedospasov SA, Karasuyama H, Reber LL, Tsai M, Mukai K, Galli SJ. Basophil-derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat Immunol 20: 129–140, 2019. doi: 10.1038/s41590-018-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res 91: 1103–1113, 2002. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 45.Ribal MJ. The link between benign prostatic hyperplasia and inflammation. Eur Urol Suppl 12: 103–109, 2013. doi: 10.1016/j.eursup.2013.08.001. [DOI] [Google Scholar]
- 46.May RD, Fung M. Strategies targeting the IL-4/IL-13 axes in disease. Cytokine 75: 89–116, 2015. doi: 10.1016/j.cyto.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol 173: 4020–4029, 2004. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 48.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 135: 626–635, 2015. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Farrar JD, Asnagli H, Murphy KM. T helper subset development: roles of instruction, selection, and transcription. J Clin Invest 109: 431–435, 2002. doi: 10.1172/JCI15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4: 313–319, 1996. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Kunit T, Ciotkowska A, Rutz B, Schreiber A, Strittmatter F, Waidelich R, Liu C, Stief CG, Gratzke C, Hennenberg M. Inhibition of prostate smooth muscle contraction and prostate stromal cell growth by the inhibitors of Rac, NSC23766 and EHT1864. Br J Pharmacol 172: 2905–2917, 2015. doi: 10.1111/bph.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Li Y, Lin J, Jiang J, He M, Sun D, Zhao Z, Shen Y, Xue A. Phosphorylated myosin light chain 2 (p-MLC2) as a molecular marker of antemortem coronary artery spasm. Med Sci Monit 22: 3316–3327, 2016. doi: 10.12659/msm.900152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng M, Ding W, Min X, Xia Y. MLCK-independent phosphorylation of MLC20 and its regulation by MAP kinase pathway in human bladder smooth muscle cells. Cytoskeleton (Hoboken) 68: 139–149, 2011. doi: 10.1002/cm.20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan JL, Ravid S, Spudich JA. Control of nonmuscle myosins by phosphorylation. Annu Rev Biochem 61: 721–759, 1992. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- 55.Aydin O, Dusmez D, Cinel L, Doruk E, Kanik A. Immunohistological analysis of mast cell numbers in the intratumoral and peritumoral regions of prostate carcinoma compared to benign prostatic hyperplasia. Pathol Res Pract 198: 267–271, 2002. doi: 10.1078/0344-0338-00253. [DOI] [PubMed] [Google Scholar]
- 56.Globa T, Saptefrţi L, Ceauşu RA, Gaje P, Cimpean AM, Raica M. Mast cell phenotype in benign and malignant tumors of the prostate. Pol J Pathol 65: 147–153, 2014. doi: 10.5114/pjp.2014.43965. [DOI] [PubMed] [Google Scholar]
- 57.Anker JF, Naseem AF, Mok H, Schaeffer AJ, Abdulkadir SA, Thumbikat P. Multi-faceted immunomodulatory and tissue-tropic clinical bacterial isolate potentiates prostate cancer immunotherapy. Nat Commun 9: 1591, 2018. doi: 10.1038/s41467-018-03900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okamoto T, Iwata S, Ohnuma K, Dang NH, Morimoto C. Histamine H1-receptor antagonists with immunomodulating activities: potential use for modulating T helper type 1 (Th1)/Th2 cytokine imbalance and inflammatory responses in allergic diseases. Clin Exp Immunol 157: 27–34, 2009. doi: 10.1111/j.1365-2249.2009.03958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol 533: 69–76, 2006. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 60.Matsuda K, Piliponsky AM, Iikura M, Nakae S, Wang EW, Dutta SM, Kawakami T, Tsai M, Galli SJ. Monomeric IgE enhances human mast cell chemokine production: IL-4 augments and dexamethasone suppresses the response. J Allergy Clin Immunol 116: 1357–1363, 2005. doi: 10.1016/j.jaci.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 61.Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, Church MK, Saluja R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol 9: 1873, 2018. doi: 10.3389/fimmu.2018.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradding P. Mast cell regulation of airway smooth muscle function in asthma. Eur Respir J 29: 827–830, 2007. doi: 10.1183/09031936.00017707. [DOI] [PubMed] [Google Scholar]
- 63.Lohman RJ, Cotterell AJ, Barry GD, Liu L, Suen JY, Vesey DA, Fairlie DP. An antagonist of human protease activated receptor-2 attenuates PAR2 signaling, macrophage activation, mast cell degranulation, and collagen-induced arthritis in rats. FASEB J 26: 2877–2887, 2012. doi: 10.1096/fj.11-201004. [DOI] [PubMed] [Google Scholar]
- 64.Page S, Ammit AJ, Black JL, Armour CL. Human mast cell and airway smooth muscle cell interactions: implications for asthma. Am J Physiol Lung Cell Mol Physiol 281: L1313–L1323, 2001. doi: 10.1152/ajplung.2001.281.6.L1313. [DOI] [PubMed] [Google Scholar]
- 65.Margulis A, Nocka KH, Brennan AM, Deng B, Fleming M, Goldman SJ, Kasaian MT. Mast cell-dependent contraction of human airway smooth muscle cell-containing collagen gels: influence of cytokines, matrix metalloproteases, and serine proteases. J Immunol 183: 1739–1750, 2009. doi: 10.4049/jimmunol.0803951. [DOI] [PubMed] [Google Scholar]
- 66.Roman K, Done JD, Schaeffer AJ, Murphy SF, Thumbikat P. Tryptase-PAR2 axis in experimental autoimmune prostatitis, a model for chronic pelvic pain syndrome. Pain 155: 1328–1338, 2014. doi: 10.1016/j.pain.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, Nourbakhsh B, Safavi F, Li K, Xu H, Cullimore M, Zhou F, Zhang G, Rostami A. Kit (W-sh) mice develop earlier and more severe experimental autoimmune encephalomyelitis due to absence of immune suppression. J Immunol 187: 274–282, 2011. doi: 10.4049/jimmunol.1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167: 835–848, 2005. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michel A, Schuler A, Friedrich P, Doner F, Bopp T, Radsak M, Hoffmann M, Relle M, Distler U, Kuharev J, Tenzer S, Feyerabend TB, Rodewald HR, Schild H, Schmitt E, Becker M, Stassen M. Mast cell-deficient Kit(W-sh) “Sash” mutant mice display aberrant myelopoiesis leading to the accumulation of splenocytes that act as myeloid-derived suppressor cells. J Immunol 190: 5534–5544, 2013. doi: 10.4049/jimmunol.1203355. [DOI] [PubMed] [Google Scholar]