Abstract
The BCL-2 family includes both proapoptotic (e.g., BAX and BAK) and antiapoptotic (e.g., BCL-2 and BCL-XL) molecules. The cell death-regulating activity of BCL-2 members appears to depend on their ability to modulate mitochondrial function, which may include regulation of the mitochondrial permeability transition pore (PTP). We examined the function of BAX and BCL-XL using genetic and biochemical approaches in budding yeast because studies with yeast suggest that BCL-2 family members act upon highly conserved mitochondrial components. In this study we found that in wild-type yeast, BAX induced hyperpolarization of mitochondria, production of reactive oxygen species, growth arrest, and cell death; however, cytochrome c was not released detectably despite the induction of mitochondrial dysfunction. Coexpression of BCL-XL prevented all BAX-mediated responses. We also assessed the function of BCL-XL and BAX in the same strain of Saccharomyces cerevisiae with deletions of selected mitochondrial proteins that have been implicated in the function of BCL-2 family members. BAX-induced growth arrest was independent of the tested mitochondrial components, including voltage-dependent anion channel (VDAC), the catalytic β subunit or the δ subunit of the F0F1-ATP synthase, mitochondrial cyclophilin, cytochrome c, and proteins encoded by the mitochondrial genome as revealed by [rho0] cells. In contrast, actual cell killing was dependent upon select mitochondrial components including the β subunit of ATP synthase and mitochondrial genome-encoded proteins but not VDAC. The BCL-XL protection from either BAX-induced growth arrest or cell killing proved to be independent of mitochondrial components. Thus, BAX induces two cellular processes in yeast which can each be abrogated by BCL-XL: cell arrest, which does not require aspects of mitochondrial biochemistry, and cell killing, which does.
Programmed cell death (PCD) and its morphological equivalent, apoptosis, are mediated by a distinct genetic pathway that is apparently present in all multicellular organisms (26). PCD plays an important role in normal development, and disregulation of this process is likely to underlie many disease processes (74). Among the more prominent regulators of cell death are members of the BCL-2 family (1). This family of proteins includes both proapoptotic (e.g., BAX and BAK) and antiapoptotic (e.g., BCL-2 and BCL-XL) molecules. The ratio between pro- and antiapoptotic proteins helps determine, in part, the susceptibility of cells to a death signal. In addition, members of this family share a number of characteristics. First, proteins in the BCL-2 family are able to form homo- and heterodimers, suggesting neutralizing competition between these proteins (9, 66). The functional significance of heterodimerization remains to be uncovered, however, since some site-directed mutations which interfere with heterodimerization do not eliminate the pro- or antiapoptotic function of these molecules (7, 24, 52, 76, 83). In addition, genetic studies in mice indicate that members of each group are able to mediate their effects in the absence of opposing family members (30). Second, members of each group appear to act primarily as integral membrane proteins and, in a number of cases, have been demonstrated to form ion channels in vitro (53, 64, 65).
Pro- and antiapoptotic BCL-2 family members localize primarily to separate subcellular compartments in the absence of a death signal. Antiapoptotic members are often constitutively targeted to intracellular membrane systems such as mitochondria, endoplasmic reticulum, or the nuclear membrane (8, 22, 31). In contrast, a substantial fraction of the proapoptotic members localize to cytosol or are only loosely associated with membranes prior to a death signal (18, 23, 78, 84). Following a death signal, several proapoptotic members including BAX, BAK, and BID undergo a conformational change that enables them to target and integrate into membranes, especially the mitochondrial outer membrane (13, 17, 20, 40, 55).
A number of lines of evidence suggest that the cell death-regulating activity of BCL-2 family members depends on their ability to modulate mitochondrial function (15, 19, 51, 62, 71). Regulated insertion of BAX or other proapoptotic family members into the outer membrane on reception of an upstream death signal mediates a number of mitochondrial responses that initiate and propagate downstream cell death processes. These mitochondrial responses include membrane depolarization and release of apoptosis-promoting factors contained in the intermitochondrial membrane space such as cytochrome c (cyt c), which, upon release, propagates a downstream caspase cascade. BCL-2 and other antiapoptotic family members appear to act at the mitochondrial level to prevent mitochondrial membrane depolarization and release of caspase-activating molecules in response to cell death signals.
While the mitochondrial effects of BCL-2 family members are clearly important in mediating their respective functions in cell death pathways, the mechanisms which underlie these effects have remained elusive. Recently, it has been postulated that pro- as well as antiapoptotic family members may function by modulating the activity of the mitochondrial permeability transition pore (PTP) (34, 43, 46). The PTP is a large channel responsible for an abrupt increase in the permeability of the mitochondria in vitro to solutes with a molecular mass up to 1,500 Da (2, 85). Rapid opening of the PTP causes mitochondrial depolarization, uncoupling of oxidative phosphorylation, large-amplitude swelling of mitochondria, and rupture of the outer mitochondrial membrane. Thus, any modulation of the activity of the PTP by BCL-2 family members, perhaps through their channel-forming capacity, might be related to the ability of these molecules to modulate the release of cyt c and other apoptotic factors into the cytoplasm (29, 43, 70, 81).
The precise molecular composition of the PTP remains to be definitively established, although current studies suggest that a complex of proteins contribute to or influence its activity, including hexokinase, the inner membrane adenine nucleotide transporter (ANT), the outer membrane channel VDAC (voltage-dependent anion channel), the cyclophilin found in the mitochondrial matrix, and perhaps a variety of other proteins that exist at contact sites between the inner and outer mitochondrial membranes (5, 46). Recent evidence has implicated specific proteins thought to form the PTP (e.g., ANT and VDAC) in the function of BCL-2 family members (45, 54, 67). Despite these reports, the role of the PTP in apoptosis has remained controversial. A number of studies have reported that the release of cyt c during cell death was not accompanied by the mitochondrial depolarization that invariably follows the opening of the PTP (4). In addition, cyt c release has been reported in different studies to either require or be independent of PTP opening (6, 11, 43, 44, 75). These and other contradictory results indicate that a detailed understanding of the important underlying mechanisms and critical biochemical interactions mediated by BCL-2 family members is needed.
Recent studies have also demonstrated that despite the well-established importance of caspases in the apoptotic process, cell death can proceed in a caspase-independent fashion (14, 49, 56, 80). While inhibition of caspases prevents an apoptotic response to certain death signals, others (e.g., BAX and BAK) cannot be rescued and cells still die, albeit more slowly and without all the morphological and biochemical hallmarks of apoptosis. In addition, molecules released from mitochondria during apoptosis (e.g., apoptosis-inducing factor) might promote cell death processes which do not require caspase activation (69, 70). Thus, proapoptotic family members may actively promote cell death through caspase-independent mechanisms as well.
We and others have approached the complex biochemical interactions which underlie the function of BCL-2 family members in the budding yeast, Saccharomyces cerevisiae. Completion of the S. cerevisiae genome indicates that it does not contain genes for recognizable members of either the BCL-2 family or the caspases. Nevertheless, expression of proapoptotic family members such as BAX and BAK in S. cerevisiae and Schizosaccharomyces pombe results in cell death (25, 28, 36, 82). Evidence that BAX-mediated yeast cell death is not a nonspecific toxicity includes the observation that BAX mutants incapable of inducing apoptosis in mammalian cells are also not cytotoxic in yeast (25, 52, 59, 82). In addition, BAX expressed in yeast localizes to mitochondria and induces changes in the mitochondrial membrane potential similar to changes observed in mammalian cells (52, 59, 82). Similarly, antiapoptotic family members like BCL-2 and BCL-XL are also localized to yeast mitochondria and allow yeast cell survival in response to BAX expression (16, 52, 72, 73). This prosurvival function of antiapoptotic family members in yeast may not depend on heterodimerization to BAX, since mutant BCL-XL molecules which do not heterodimerize in classic binding assays still promote cell survival in both yeast and mammalian cells (52, 83). These and a variety of other studies in yeast strongly indicate that BCL-2 family members are acting upon highly conserved mitochondrial components that correspond directly to their apoptotic substrates in mammalian cells.
Given this background, yeast provides a physiologically relevant system in which to apply the combined approaches of genetics and biochemistry in studies aimed at identifying the mechanisms and interactions that underlie the function of BCL-2 family members in caspase-independent cell death pathways present in mammalian cells. In this study, we used a single strain of yeast to analyze the response of mitochondria to the expression of BAX and BCL-XL, representative pro- and antiapoptotic members, respectively, of the BCL-2 family. In addition, we have assessed the requirements for the cell death and survival functions of these molecules in a variety of genetic backgrounds lacking individual components of the mitochondrial machinery proposed to be involved in the formation of the PTP or previously implicated in the function of these molecules.
MATERIALS AND METHODS
Genetic methods.
The yeast strain used throughout this study is YM1372 (obtained from Mark Johnston, Washington University, St. Louis, Mo.) (MATa ura3-52 his3-Δ200 ade2-101 lys2-801 trp1-901 tyr1-501 can1 LEU2::GAL1-lacZ gal80-538). Yeast cells were grown on standard rich (YP), selective complete (SC), or selective minimal medium plus the required amino acids and with 2% glucose (SD) or 2% galactose (SG) where appropriate as the carbon source. Cells were transformed by standard lithium acetate procedures. Cell numbers were estimated from the absorbance of cultures at 600 nm and corrected by Coulter counter analysis.
Disruption of the CYP3, CYC1, CYC7, and ATP2 genes in YM1372 was accomplished by using a PCR-targeting gene disruption method (39, 41). In each case, 40-bp oligonucleotides which flank the gene of interest were generated. These 40-bp sequences are extended to include sequences that are homologous to the pRS300 series of vectors described by Sikorski and Hieter (68) in regions which flank auxotrophic markers. In each case, the 5′ vector sequences are followed by the pRS300 sequence 5′ TTGTACTGAGAGTGCACCAT 3′ and the 3′ vector sequences are followed by the pRS300 sequence 5′ GGTATTTCACACCGCATA 3′. The CYP3, ATP2, and CYC1 genes were disrupted by insertion of the HIS3 gene with oligonucleotides representing −47 to −7 and +1 to +41, −63 to −23 and +5 to +45, and −50 to −10 and +10 to +50 of each gene, respectively. The CYC7 gene was disrupted by insertion of the TRP1 gene with oligonucleotides representing −50 to −10 and +10 to +50 of this gene. PCR products obtained using the target gene/pRS300 hybrid oligonucleotides were used to transform yeast cells. Disruption of the appropriate gene in yeast cells able to grow on the appropriate nutrient-deficient plates was verified by PCR analysis using oligonucleotides flanking those described above.
The ATP4 gene was disrupted using conventional insertional integration techniques (63) by first amplifying the entire ATP4 coding sequence and then subcloning into pBluescript II KS (Stratagene) utilizing an EcoRI site (−30 relative to ATP4) and blunt-end ligating the 3′ end of the PCR fragment into a filled-in XhoI site of pBluescript II KS. A 3,500-bp ADE2 fragment was ligated into the filled-in BbsI site at position 108 of ATP4. Finally, the ATP4-ADE2 construct was excised from pBluescript II KS with EcoRI-XhoI (XhoI site at position 387 of ATP4) to generate a 3,995-bp fragment, which was used to transform yeast. Disruption of the ATP4 gene was verified by PCR with oligonucleotides flanking the site of insertion and Southern analysis. VDAC genes were deleted as described by Blachly-Dyson et al. (3).
YM1372 cells lacking mitochondrial DNA ([rho0] cells) were generated by extensive growth in SC medium containing 25 mg of ethidium bromide per ml. Cells unable to grow on medium containing a nonfermentable carbon source (2% glycerol) were identified by replica plating.
Expression of BAX and BCL-XL and preparation of yeast extracts.
Regulated expression of native BAX or BAX molecules containing an N-terminal HA tag (HA-BAX) was accomplished by subcloning the cDNA encoding murine BAX into the EcoRI site of plasmid pBM272 downstream of the GAL10 promoter. This shuttle vector also contains the yeast URA3 gene, sequences representing CEN4 and ARS1, and sequences necessary for replication and selection in bacteria. Constitutive expression of BCL-XL was mediated by subcloning the cDNA encoding murine BCL-XL into the EcoRI site of plasmid pBF339 directly downstream of the yeast ADH1 promoter. This shuttle vector also contains the yeast TRP1 gene, the 2 μm origin of replication, and sequences necessary for replication and selection in bacteria. The responses of yeast cells to expression of BAX or HA-BAX were identical.
For induction of BAX expression, cells were washed twice in water and resuspended in SG medium. Total-protein extracts were prepared following cell breakage using glass beads (21) or the Y-Per reagent (Pierce). Cells were broken with glass beads (0.4 to 0.6 mm in diameter) by vigorous vortexing in ice-cold buffer containing 100 mM HEPES (pH 8.0), 1% sodium dodecyl sulfate (SDS), 1% 2-mercaptoethanol, 5 mM EDTA, 10% glycerol, 2% Triton X-100, and a protease inhibitor mixture (Sigma). The mixture was then centrifuged at 9,000 × g for 10 min at 4°C to remove unbroken cells and glass beads. BAX and BCL-XL were immunoprecipitated from the supernatant using anti-mBAX 4D2 monoclonal antibodies (MAb) or anti-mBCL-XL 13.6 polyclonal Ab, respectively, and then size fractionated on SDS–12 or 16% polyacrylamide gels. Western blots were developed with anti-mBAX Ab 651 or anti-mBCL-XL Ab 13.6. Alternatively, expression of BAX was monitored in cells transformed with pBM272 constructs which direct the expression of HA-BAX molecules. In this case, total-cell extracts were prepared using the Y-Per reagent (Pierce) and Western blots were developed with the HA.11 MAb (Babco) directed to the HA epitope.
Subcellular fractionation and mitochondrion preparation.
Highly purified yeast mitochondria and subcellular fractions were prepared essentially as described by Blachly-Dyson et al. (3). Yeast cells from a 1-liter culture were collected by centrifugation, washed once with water, resuspended in 0.1 M Tris-SO4 (pH 9.4), supplemented with 10 mM dithiothreitol, and incubated at 30°C for 15 min. Cells were collected by centrifugation, washed with 1.2 M sorbitol-K2HPO4-KH2PO4 (pH 7.5), resuspended in the same buffer containing Zymolyase-20T (20,000 U/g [ICN]; 5 mg per g of cells), and incubated for 30 min at 30°C. The cells were washed twice in 1.2 M sorbitol-K2HPO4-KH2PO4 (pH 7.5), resuspended in breaking buffer (0.6 M sorbitol and 20 mM HEPES [pH 7.5], supplemented with 100 mM phenylmethylsulfonyl fluoride) and homogenized in a 15-ml Wheaton Dounce glass homogenizer using 15 complete up-and-down cycles of a glass B-type pestle. The homogenate was centrifuged (4,000 × g for 5 min), the supernatant was saved, and the pellet was resuspended in breaking buffer and rehomogenized. The homogenate was centrifuged (4,000 × g for 5 min), and the pellet containing unbroken cells and nuclei is referred to as the low-speed pellet (P1). The supernatants from both runs were combined and centrifuged (10,000 × g for 10 min) to collect the heavy-membrane fraction (HM) enriched in mitochondria. The supernatant was centrifuged (100,000 × g for 30 min) to yield the light-membrane fraction (LM) and the soluble fraction (S). To further purify the mitochondrial fraction, the HM pellet was resuspended in breaking buffer and centrifuged (4,000 × g for 5 min), and the resulting supernatant was centrifuged (10,000 × g for 10 min) to collect the final HM pellet.
Submitochondrial fractionation.
Submitochondrial membrane vesicles were produced and fractionated from EDTA-washed mitochondria (prepared essentially as described above except that spheroplasts were homogenized in breaking buffer containing 10 mM EDTA) using a modification of the procedure of Pon et al. (58). Mitochondria were resuspended to 10 mg/ml in breaking buffer and swollen by incubation for 30 min at 0°C in 9 volumes of 20 mM HEPES (pH 7.4) containing 1 mM phenylmethylsulfonyl fluoride and 0.5 mM EDTA. Mitochondria were condensed by addition of sucrose to 0.45 M and incubation for 10 min at 0°C. Samples were then sonicated for 90 s at 0°C at 80% duty cycle and maximum power in a cell disruptor (sonic dismembrator; Fisher) equipped with a microtip. The submitochondrial membrane vesicles were collected by centrifugation at 200,000 × g for 45 min at 4°C. The resulting pellet was resuspended in 150 ml of breaking buffer, layered onto a linear sucrose gradient (4 ml of 0.85 to 1.6 M sucrose in 10 mM KCl–5 mM HEPES [pH 7.4]), and centrifuged (100,000 × g for 18 h at 4°C). Fractions (0.6 ml) were collected, diluted to 3 ml with water, and centrifuged (200,000 × g for 45 min). The resulting pellets were resuspended in Lammeli sample buffer, boiled, and size fractionated by SDS-polyacrylamide gel electrophoresis.
Assessment of mitochondrial potential and ROS production.
To assess mitochondrial potential, 5 × 105 cells were incubated for 10 min at 30°C with 40 nM 3,3′-dihexyloacarbocynine iodide (DiOC6) 2 μM or rhodamine 123 (Rh123) and then subjected to FACScan (Becton Dickinson) analysis. Carbonyl cyanide m-chlorophenylhydrazone (CCCP) was added to a final concentration of 50 μM prior to the addition of DiOC6 or Rh123. To assess reactive oxygen species (ROS) production, cells were incubated with 2 μM hydroethidine and the conversion to ethidium was measured by FACScan analysis.
Yeast cell survival assays.
YM1372 wild-type or mutant yeast cells harboring GAL vector or GAL-BAX plasmids were washed in water and resuspended in SG medium. At 24 h following transfer to SG medium, the total-cell density of the cultures was determined, and approximately 250 cells were spread on glucose-based plates and incubated at 30°C for 2 to 3 days. The percent survival was calculated by dividing the number of colonies on plates harboring GAL-BAX plasmids by the number of colonies harboring the empty GAL vector.
RESULTS
BAX expression in yeast results in growth arrest and cell death.
The response of yeast cells to the expression of proapoptotic BCL-2 family members has resulted in a variety of conflicting reports with regard to the genetic requirements for the function of these molecules. Our own preliminary observations indicated that some of these differences are due, in part, to differences in the genetic background of the specific yeast strains used. We therefore chose to conduct all of our studies in the same yeast strain (YM1372) and detail its response to the expression of representative members of the BCL-2 family with opposing actions, BCL-XL and BAX. Regulated expression of BAX is mediated by transformation of YM1372 with plasmids in which the expression of native BAX molecules or those containing an N-terminal HA tag is driven by the GAL10 promoter (GAL-BAX). Constitutive expression of BCL-XL is mediated by the use of vectors in which the expression of native BCL-XL is directed by the ADH1 promoter (ADH-BCL-XL).
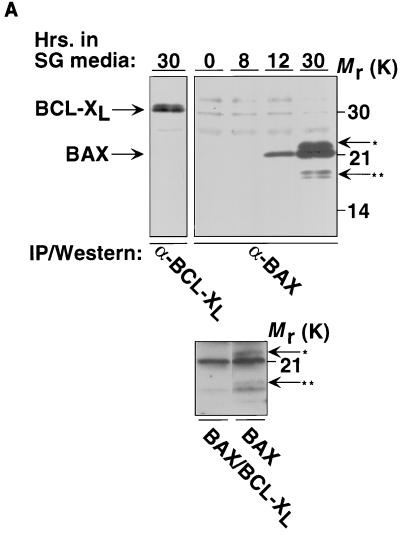
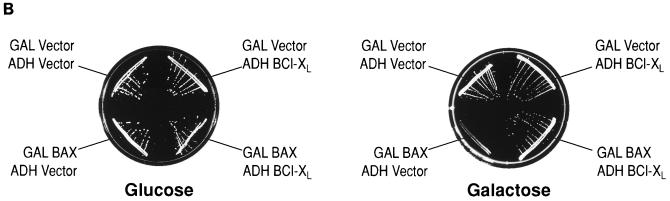
As assayed by immunoprecipitation from total-cell extracts, induction of GAL-BAX results in visible BAX at 12 h and is maximal 24 to 30 h following transfer to galactose-based media while BCL-XL is constitutively expressed in cells containing ADH-BCL-XL plasmids (Fig. 1A, top panel). Of note, BAX appears as a doublet (by 30 h of Gal induction), suggesting that it can be modified in yeast cells. In addition, an 18-kDa form was also detected. It has previously been demonstrated that p18 BAX represents a carboxy-terminal cleavage product of p21 BAX and is detected in both mammalian and yeast cells (59, 79). Cells containing GAL-BAX plasmids fail to grow on galactose-based media, as analyzed both on plates (Fig. 1B) and in liquid culture (results not shown). The viability of cells following 24 h of BAX induction was assessed quantitatively by determining the number of cells able to form colonies on plates containing glucose (Table 1). After 24 h in galactose-based medium, essentially all wild-type cells containing GAL-BAX constructs are unable to grow when placed on glucose and are defined as dead. Coexpression of the antiapoptotic protein BCL-XL from the constitutive ADH1 promoter was able to abrogate the effects of BAX on cell viability (e.g., Fig. 1B); i.e., cells containing GAL-BAX and ADH-BCL-XL plasmids are able to grow on galactose-based media. Of note, this result is not due to lower levels of BAX expression, since cells carrying BAX alone or BAX together with BCL-XL express similar levels of BAX (Fig. 1A, bottom panel).
FIG. 1.
BAX expression in yeast results in growth arrest that is inhibited by BCL-XL. (A) The top panel shows expression of BAX and BCL-XL in yeast. Yeast cells harboring ADH-BCL-XL (left) or GAL-BAX (right) expression vector were washed in water and resuspended in SG medium. Whole-cell lysates were prepared at the indicated time points. BAX and BCL-XL were immunoprecipitated using anti-mBAX 4D2 MAb or anti-mBCL-XL 13.6 polyclonal Ab, respectively, and then size fractionated on SDS–16% polyacrylamide gels. Western blots were developed with anti-mBAX Ab 651 or anti-mBCL-XL Ab 13.6. One asterisk denotes a potentially modified form of BAX, and two asterisks denote the ∼p18 cleaved form of BAX. The bottom panel shows that cells carrying BAX alone or BAX together with BCL-XL express similar levels of BAX. Yeast cells harboring GAL-BAX and ADH–BCL-XL (left lane) or only GAL-BAX (right lane) expression vectors were washed in water and resuspended in SG medium. Whole-cell lysates were prepared 24 h after the transfer to SG medium. BAX was immunoprecipitated using anti-mBAX 4D2 MAb and then size fractionated on SDS–16% polyacrylamide gels. Western blots were developed with anti-mBAX Ab 651. One asterisk denotes a potentially modified form of BAX, and two asterisks denote the ∼p18 cleaved form of BAX. Of note, coexpression of BCL-XL eliminates the appearance of the modified and the cleavage product of BAX. (B) Cells harboring GAL-BAX fail to grow on galactose-based plates. YM1372 yeast cells were cotransformed with either the empty vectors (GAL Vector, ADH Vector), GAL vector and ADH-BCL-XL (GAL Vector, ADH BCL-XL), GAL-BAX and ADH vector (GAL BAX, ADH Vector), or GAL-BAX and ADH-BCL-XL (GAL BAX, ADH BCL-XL). Each transformant was grown on glucose-based and galactose-based plates and incubated at 30°C for 2 days.
TABLE 1.
Survival of yeast strains plated on glucose after 24 h of induction of BAXa
| Strain | Viability of cells containingb:
|
BAX/vector ratio | |
|---|---|---|---|
| Vector (n) | BAX (n) | ||
| Wild type | 0.65 ± 0.01 (3) | 0.002 ± 0.00001* (3) | 0.003 |
| Δatp2 | 0.12 ± 0.01 (3) | 0.11 ± 0.001 (3) | 0.92 |
| Δatp4 | 0.400 ± 0.001 (3) | 0.175 ± 0.001* (3) | 0.44 |
| Δcyc1 Δcyc7 | 0.629 ± 0.0001 (3) | 0.326 ± 0.003* (3) | 0.51 |
| Δcyp3 | 0.74 ± 0.01 (3) | 0.12 ± 0.0001* (3) | 0.16 |
| Δpor1 Δpor2 | 0.96 ± 0.01 (3) | 0.17 ± 0.0007* (3) | 0.18 |
| [rho0] | 0.39 ± 0.006 (6) | 0.47 ± 0.003 (6) | 1.20 |
Cells of the indicated genotypes containing GAL-BAX or control vectors were grown in galactose-containing medium for 24 h in a diluted, and appropriate cell numbers (as determined by Coulter counter analysis) were plated onto plates containing glucose.
The numbers of cells able to form viable colonies in each case were determined after 2 days of growth at 30°C and compared to those expected. Standard deviations are indicated. n indicates numbers of cultures tested. ∗, significantly different from vector.
BAX localizes to mitochondria and causes mitochondrial membrane potential changes but no measurable release of cyt c.
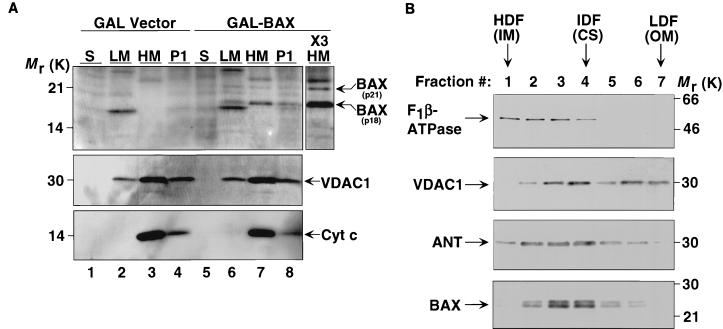
To assess the subcellular location of BAX in yeast, cells were grown in galactose for 24 h and lysed under conditions which maintain mitochondrial integrity. In these experiments a substantial portion of p21 BAX was proteolytically cleaved to generate the p18 product. We suspect that this phenomenon is due to nonspecific proteolysis. p18 BAX was consistently found in the mitochondrially enriched HM fraction as defined by the presence of the outer membrane mitochondrial protein VDAC1 and the intermembrane space protein cyt c in this fraction (Fig. 2A, lanes 7 and X3 HM). The low-speed pellet (P1), composed of residual whole cells, nuclei, and some mitochondria, and the LM fraction also contain BAX (lanes 6 and 8). LM fractions also contain residual VDAC1 but not cyt c, suggesting that the LM fraction is contaminated with outer mitochondrial membranes released from mitochondria during the homogenization required to lyse yeast cells. In contrast, BAX was not found in fractions representing soluble cytosolic proteins (S). To identify the submitochondrial membrane fractions containing BAX, submitochondrial membrane vesicles were prepared from isolated yeast mitochondria by sonication and separated by centrifugation through a continuous sucrose density gradient. Of note, in this set of experiments we have expressed the HA-tagged form of BAX which migrates as a ∼24-kDa protein. The high-density fractions are enriched in inner membrane vesicles as demonstrated by the presence of the β-subunit of the inner membrane F0F1-ATPase (Fig. 2B). The low-density fractions are enriched in outer mitochondrial membranes, as demonstrated by the outer membrane marker VDAC1. Intermediate-density fractions represent contact sites, sites of association between the inner and outer membranes, as documented by the presence of inner and outer membrane markers and by ANT, which is enriched at contact sites. BAX is most prominent in intermediate-density fractions, indicating that it is enriched at contact sites in yeast mitochondria.
FIG. 2.
BAX localizes to mitochondria but does not cause detectable release of cyt c. (A) Subcellular distribution of BAX in yeast cells. Cells transformed with GAL vector (lanes 1 to 4) or with GAL-BAX (lanes 5 to 8) were suspended in isotonic buffer, homogenized, and separated into soluble fraction (S), light-membrane fraction (LM), heavy-membrane fraction (HM), and low-speed pellet (P1) by differential centrifugation. The fractions were analyzed by Western blotting using anti-mBAX Ab 651 (top panel), anti-yVDAC1 Ab (middle panel), and anti-cyt c MAb (PharMingen) (bottom panel). The P1 pellet contains residual whole cells, nuclei, and mitochondria. The HM fraction is enriched for intact mitochondria. The LM fraction contains the endoplasmic reticulum, plasma membrane, and mitochondrial fragments. The soluble (S) fraction represents the cytosol. ×3 HM denotes that three times the amount of protein loaded in lane 7 was loaded in this lane. (B) BAX is enriched in mitochondrial contact sites. Submitochondrial membrane vesicles were prepared from isolated yeast mitochondria expressing HA-BAX by sonication and separated by centrifugation through a continuous sucrose density gradient. Fractions were analyzed by Western blotting using anti-yF1β-ATPase Ab, anti-yVDAC1 Ab, anti-yANT Ab, and anti-mBAX Ab 651. The high-density fractions (HDF) are enriched in inner membrane (IM) vesicles. The low-density fractions (LDF) are enriched in outer mitochondrial membranes (OM). Intermediate-density fractions (IDF) are enriched for contact sites (CS), sites of association between the inner and outer membranes.
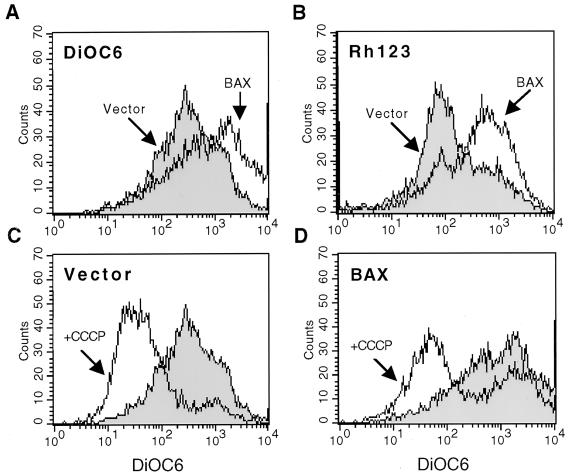
In mammalian cells, translocation of BAX to the mitochondria results in alterations in mitochondrial membrane potential (Δψm) and in certain systems, when highly expressed, release of cyt c (15, 19, 51, 57, 62, 71). Yeast Δψm was assessed by the uptake of the cationic, lipophilic dyes DiOC6 and Rh123 (Fig. 3, top). As shown in Fig. 3A and B, expression of BAX resulted in an increase in Δψm as assessed by use of either dye. Addition of the CCCP protonophor, which dissipates the Δψm, resulted in a substantial decrease in both wild-type Δψm (as measured by DiOC6 [Fig. 3C]) and hyperpolarized Δψm resulting from BAX expression (Fig. 3D), indicating that DiOC6 is a valid indicator of Δψm in yeast cells. This is similar to the initial hyperpolarization seen in mammalian cells signaled to die (75). Interestingly, in the presence of BAX, the Δψm of a fraction of cells could not be dissipated by CCCP, indicating that BAX is causing irreversible mitochondrial hyperpolarization in some cells (Fig. 3D). To assess the release of cyt c, control and GAL-BAX-containing cells were grown in galactose for 24 h and the BAX-mediated release of cyt c into the soluble, cytosolic fraction was assessed following subcellular fractionation. Strikingly, induction of BAX did not result in detectable release of cyt c despite the measurable effect on Δψm (Fig. 2A, bottom panel).
FIG. 3.
BAX expression in yeast results in hyperpolarization of mitochondria. (A and B) YM1372 yeast cells harboring GAL vector (Vector) or GAL-BAX (BAX) were washed in water and resuspended in SG medium. After 24 h of growth in SG medium, the cells were diluted in SG medium to an optical density at 600 nm of 0.1, incubated with 40 nM DiOC6 (left) or 2 μM Rh123 (right) for 10 min at 30°C, and analyzed by cytofluorometry. A shift of the DiOC6 or Rh123 peaks to the right indicates an increase in Δψm. (C and D) YM1372 yeast cells harboring GAL vector (C) or GAL-BAX (D) were grown as in panel A. After 24 h of growth in SG medium, the cells were incubated with DiOC6 in the absence or presence of CCCP for 10 min at 30°C and analyzed by cytofluorometry.
Genetic requirements for BAX function in yeast.
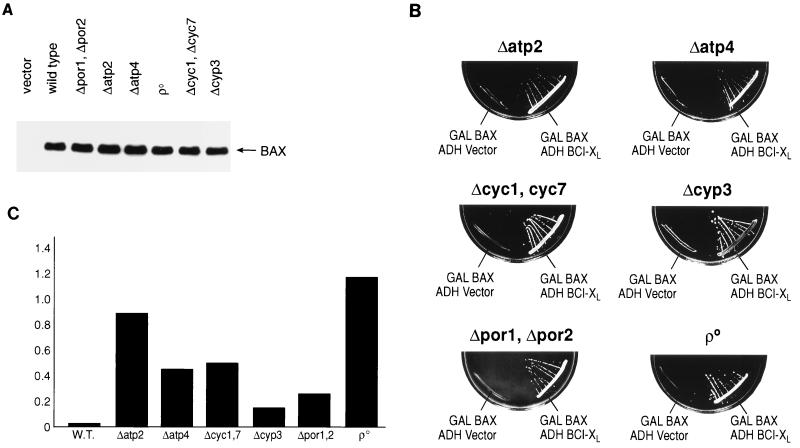
Components of the mitochondrial permeability transition (the PTP) have been implicated as targets of the action of BCL-2 family members. Since yeast mitochondria undergo a process similar to the permeability transition observed in mammalian mitochondria (27), a series of mitochondrial mutants were constructed in YM1372 in which the expression of individual genes or families of genes implicated in the function of BCL-2 family members was eliminated using PCR-targeted gene disruption methods or conventional insertional integration techniques. The level of BAX expression in each of the resulting mutant strains as mediated by the GAL-HA-BAX plasmid was similar to that observed in the wild-type cells (Fig. 4A). The specific rationale for examining the function of BAX and BCL-XL in each mutant background is outlined below.
FIG. 4.
BAX expression in a variety of genetic backgrounds lacking individual components of mitochondria results in growth arrest. (A) Expression of BAX in wild-type and mutant yeast cells. Wild-type or mutant yeast cells harboring GAL-HA-BAX were washed in water and resuspended in SG medium. Total-cell extracts were prepared after 16 h of growth in SG medium, and Western blots were developed with HA.11 MAb (Babco) directed to the HA epitope. Each lane was loaded with 20 μg of protein. (B) Yeast cells lacking individual components of mitochondria and harboring GAL-BAX fail to grow on galactose-based plates. Cells were cotransformed with plasmids as in Fig. 1B. Only the lower halves of the galactose-based plates are shown. (C) Comparison of BAX/empty-vector viability ratios of the wild-type (W.T.) and mutant strains shown in Table 1.
(i) POR1 and POR2.
Reconstitution studies and biochemical analysis have indicated that VDAC is a core component of the PTP (46). In addition, recent reports have suggested that VDAC, in association with BAX, can directly modulate changes in mitochondrial membrane potential and release of cyt c independent of pore formation (54, 67). Yeast cells express two VDAC proteins encoded by the POR genes: YVDAC1 (encoded by POR1), which represents a major protein in the outer mitochondrial membrane, and YVDAC2 (encoded by POR2), which is also in the outer membranes but is present at lower levels than YVDAC1 (3). When overexpressed, YVDAC2 can complement defects associated with the elimination of YVDAC1, indicating that they can perform similar functions in vivo.
(ii) CYC1 and CYC7.
cyt c has been implicated as a critical initiator of the caspase cascade following its release from mitochondria during PCD (29, 35, 38, 86). Although yeast do not appear to contain caspases, the role of cyt c in BAX-mediated, caspase-independent cell death has not been investigated. Yeast contain two genes encoding cyt c, CYC1 and CYC7 (33).
(iii) CYP3.
In mammalian cells, one characteristic feature of permeability transition is that it is inhibited by nonimmunosuppressive derivatives of cyclosporin (2, 85). Since mammalian mitochondria contain a cyclosporin-inhibitable cyclophilin, this mitochondrial cyclophilin is presumed to be an essential component or regulator of the PTP. Yeast mitochondria also contain a cyclosporin-inhibitable cyclophilin, the product of the CYP3 gene (47).
(iv) Mitochondrial genome.
Examination of the requirement for proteins encoded by the mitochondrial genome in the function of BCL-2 family members has resulted in contradictory reports. Some have reported that BAX-mediated cell death is not altered in cells missing the mitochondrial genome ([rho0] cells), while others suggest that this genome is required for BAX function (16, 48). To test the requirement for proteins encoded by the mitochondrial genome in our yeast strain, derivatives of YM1372 which lack the mitochondrial genome were generated by standard methods (i.e., growth in ethidium bromide).
(v) ATP2 and ATP4.
The observation that GAL induction of BAX expression in yeast results in cell death led to genetic screens for suppressors of BAX-induced growth arrest (i.e., mutants which grow despite BAX expression). The genes identified in this analysis encoded two components of the F0F1-ATPase: ATP2, encoding the β subunit, and ATP4, encoding the δ subunit (48). These results, as well as the observation that inhibition of the F0F1-ATPase by oligomycin treatment in yeast and mammalian cells interferes with BAX-mediated cell death, suggested that the F0F1-ATPase plays a critical role in the apoptotic processes (48).
As shown in Fig. 4B, none of the genes listed above is absolutely required for the function of BAX or BCL-XL in yeast as related to growth suppression. GAL induction of BAX results in growth suppression, while coexpression of BCL-XL reverses BAX-mediated growth arrest (compare to Fig. 1B). Surprisingly, it is clear that the absence of growth following BAX expression observed in most mutant strains shown in Fig. 4B represents growth arrest and not cell killing (Table 1; Fig. 4C). After 24 h in galactose, essentially all wild-type cells containing the GAL-BAX plasmid are unable to grow when placed on plates containing glucose, reflecting their cell death. In contrast, mitochondrial mutant strains containing the GAL-BAX plasmid vary in the proportion of cells able to form colonies after 24 h in galactose. Cells missing the CYP3 both VDAC genes (Δpor1 Δpor2) showed the least alteration of BAX-induced death (16 and 18%, respectively [Table 1 and Fig. 4C]) compared to the parent cells (0.3% viability). In other mutant backgrounds, resistance to cell killing ranged from 44% viability (Δatp4) to 92% (Δatp2). Remarkably, the [rho0] cells with a defective mitochondrial genome were essentially 100% viable despite the lack of cell growth. BCL-XL can abrogate both the BAX-mediated growth arrest (since cells in which cell death has been abrogated can form colonies, e.g., [rho0]) and cell killing in all mitochondrial mutant strains.
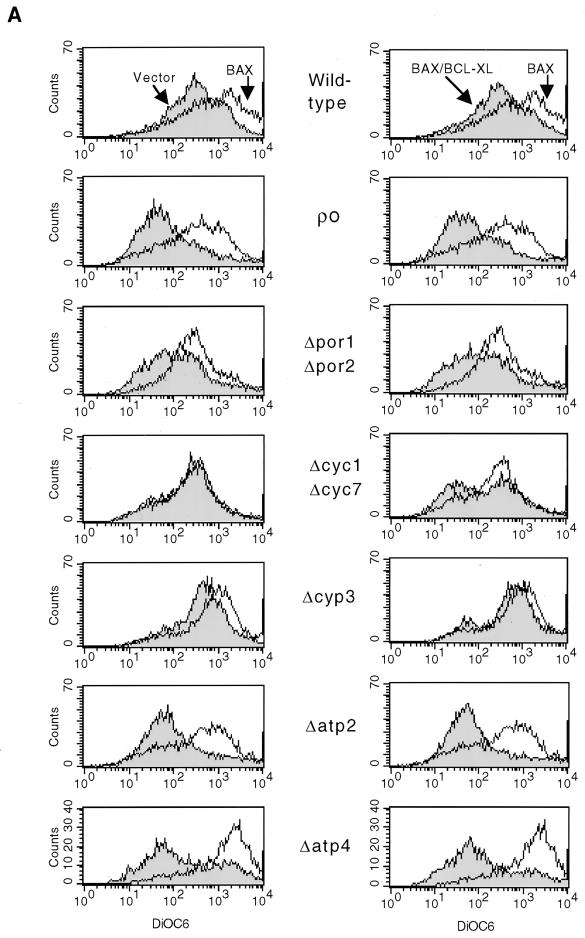
Since we did not detect cyt c release from yeast mitochondria during BAX-mediated cell death, we examined Δψm as assessed by use of DiCO6 in each mutant background following BAX expression. The Δψm of Δatp2, Δatp4, [rho0] and Δpor1 Δpor2 vector-containing cells grown in galactose was slightly depolarized compared to that for equivalent wild-type cells (Fig. 5A, left column). However, expression of BAX still resulted in mitochondrial hyperpolarization in these mitochondrial mutant strains. Coexpression of BCL-XL returned the Δψm to resting levels in these particular mutant backgrounds (Fig. 5A, right column). The Δcyp3 cells displayed a small increase in Δψm with the control vector and still responded with some hyperpolarization to BAX. Coexpression of BCL-XL in Δcyp3 cells resulted in a return of the Δψm to the levels present in Δcyp3 cells containing control plasmids. The Δcyc1 Δcyc7 mutant showed no hyperpolarization on BAX induction despite having a relatively normal resting Δψm with the control vector. Of note, coexpression of BCL-XL resulted in a slight depolarization in a subset of these BAX-induced mutant cells (Fig. 5A, right column).
FIG. 5.
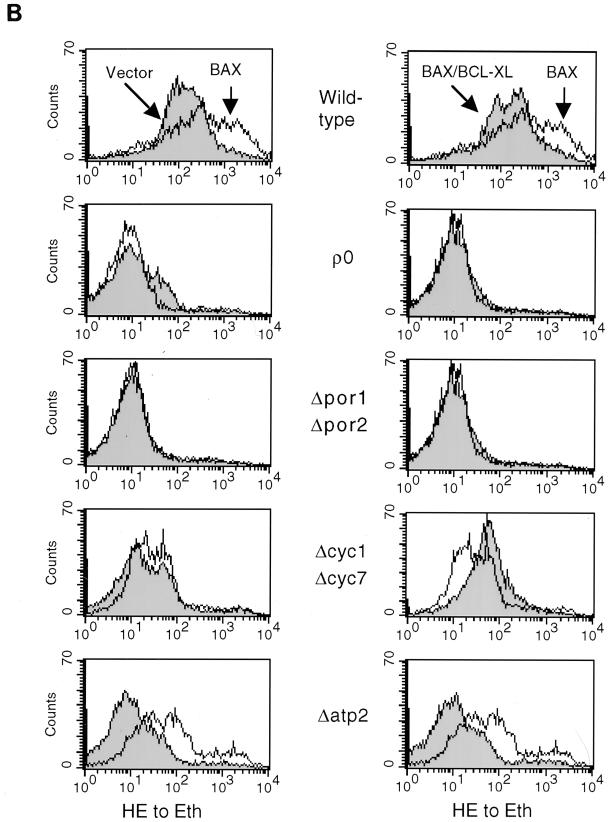
BAX expression in a variety of genetic backgrounds lacking individual components of mitochondria results in changes in mitochondrial membrane potential and ROS production. (A) Wild-type or mutant yeast cells harboring GAL vector (Vector) (left panel). GAL-BAX (BAX) (left and right panels), or GAL-BAX together with ADH-BCL-XL (BAX/BCL-XL) (right panel) were washed in water and resuspended in SG medium. After 24 h of growth in SG medium, the cells were diluted in SG medium to an optical density at 600 nm of 0.1, incubated with 40 nM DiOC6 for 10 min at 30°C, and analyzed by cytofluorometry. (B) The experimental procedure was identical to that in panel A, except that cells were incubated with 2 μM hydroethidine. Hydroethidine converted to ethidium (HE to Eth) reflects the production of ROS.
In addition to the changes in Δψm, we have assessed the ability of BAX to induce ROS production. ROS production was measured by using hydroethidine, which principally detects superoxide anion. As shown in Fig. 5B, expression of BAX resulted in ROS production in wild-type yeast (left column), and coexpression of BCL-XL prevented this effect of BAX (right column). Next, we assessed the effect of BAX on ROS production in each mutant background. The level of ROS production in each of the vector-containing cells was lower than that in the equivalent wild-type cells (Fig. 5B, left column). Expression of BAX in three of the four mutants analyzed ([rho0], Δpor1 Δpor2, and Δcyc1 Δcyc7) had no effect on the level of ROS production. In contrast, expression of BAX in Δatp2 cells resulted in an increase in ROS production. Coexpression of BCL-XL showed no additional effect in [rho0] and Δpor1 Δpor2 cells compared to BAX expression alone in the same cells (Fig. 5B, right column). In contrast, coexpression of BCL-XL in Δcyc1 Δcyc7 cells resulted in an increase in ROS production. Coexpression of BCL-XL in Δatp2 cells returned the level of ROS production to that in vector-containing cells.
DISCUSSION
The capacity of the proapoptotic BAX molecule to kill S. cerevisiae following mitochondrial integration and a hyperpolarization of the transmembrane potential similar to the initial effect in mammalian cells, as well as the ability of BCL-XL to reverse these effects, argue that yeast provides a relevant system. The power of the combined approaches of genetics and biochemistry in yeast should help define the mechanisms that underlie the function of BCL-2 family members in caspase-independent cell death pathways present in mammalian cells. Here, we have examined the biochemical and physiological responses of yeast to BAX and BCL-XL, representative pro- and antiapoptotic members, respectively, of the BCL-2 family, both in wild-type cells and in mutants of the same yeast strain lacking individual components of the mitochondrial machinery implicated in the function of these molecules.
Subcellular fractionation indicates that when expressed in yeast, the majority of BAX is constitutively localized to mitochondria (Fig. 2). To more precisely define the localization of BAX within yeast mitochondria, submitochondrial membrane vesicles were prepared from isolated yeast mitochondria and separated by centrifugation through a continuous sucrose density gradient. Using this method, BAX was shown to accumulate in yeast mitochondrial contact sites, sites of association between the inner and outer membranes (Fig. 2). Studies in mammalian cells using microscopic methods have also indicated that BCL-2 family members cluster at contact sites in mammalian mitochondria (8, 22, 31). This result indicates that the molecular associations required for this specific submitochondrial localization are conserved in yeast mitochondria and that BAX could have the potential to modulate outer and possibly inner mitochondrial membrane biochemical processes.
In mammalian cells, three responses of mitochondria to BAX accumulation following a death signal have been noted: a transient hyperpolarization of mitochondrial Δψm, a subsequent substantial depolarization of mitochondrial Δψm, and, in selected settings, the release of cyt c (71, 75). cyt c has been implicated as an activator of Apaf-1, leading to the activation of a downstream caspase program (35, 86). Each of these mitochondrial responses to BAX is prevented by BCL-XL or BCL-2. As shown here, expression of BAX in yeast induces hyperpolarization of mitochondria (Fig. 3), as recently reported by others (52). This appears to represent the initial physiological response to BAX accumulation observed in mammalian cells. However, a time course study of changes of Δψm in response to BAX expression in yeast showed no loss of Δψm up to 72 h after BAX expression (data not shown). Perhaps the later stages of the mammalian mitochondrial Δψm response to BAX accumulation, namely, substantial depolarization, depend on mammalian components which are not present in yeast. Furthermore, our studies have failed to demonstrate a significant BAX-dependent release of cyt c from yeast mitochondria (Fig. 2) despite alterations in Δψm, which contrasts with some in vitro or spectroscopic studies (42, 61, 67). Our results would suggest, then, that BAX in yeast can generate the initial mitochondrial responses observed in mammalian cells (hyperpolarization of Δψm) but is unable to generate what may represent later responses (depolarization of Δψm and release of cyt c). Since BAX expression leads to cell death in yeast (Table 1), despite the absence of the latter set of mitochondrial responses observed in mammalian cells, our results would also suggest that caspase-independent cell death pathways may not depend on events associated with mitochondrial depolarization. In addition, similar to observations in mammalian cells, coexpression of BCL-XL with BAX in yeast prevents mitochondrial hyperpolarization as well as cell death. Thus, all aspects required for the function of antiapoptotic members of the BCL-2 family appear conserved in yeast.
To begin to define endogenous molecules required for the function of BAX and BCL-XL, we assessed the cell death and survival function of these molecules, as well as associated mitochondrial responses, in defined genetic mutants lacking individual components of the mitochondrial machinery. We selected these gene products because they had previously been implicated in the function of BCL-2 family members (48, 54, 67) or the PTP. When growth was investigated (Fig. 4), the function of BAX and BCL-XL appeared to be unaffected by the absence of any of the molecules examined in this study; GAL induction of BAX resulted in lack of growth, while BCL-XL expression reversed BAX-mediated growth arrest. Surprisingly, when viability was assessed (Table 1), it is clear that the absence of growth observed following BAX expression represents growth arrest but not always cell killing, although most mutations have an effect on viability in the absence of BAX expression (vector control, Table 1). After 24 h of BAX induction, essentially all wild-type cells are unable to grow when placed on plates containing glucose and are therefore functionally dead. In contrast, in most of the mutant strains, a large proportion of the arrested cells are viable and able to form colonies ranging from 40% for Δatp4 cells to essentially complete preservation of viability for Δatp2 and [rho0] cells.
Genes encoding VDAC and CYP3 represent notable exceptions. Reconstitution studies and biochemical analysis have suggested that VDAC is a core component of the PTP (5, 46, 85). Recent reports have also suggested that VDAC, in association with BAX, can directly modulate changes in mitochondrial membrane potential and release of cyt c and that BCL-XL functions by modulating the VDAC channel activity (54, 67). Here, our in vivo studies demonstrate that the prodeath functions of BAX and the antideath functions of BCL-XL are largely independent of the VDAC channel (Fig. 4 and Table 1) in yeast, in contrast to the in vitro analysis of yeast mitochondria reported in a previous study (67). In addition, the product of the CYP3 gene, the mitochondrial cyclosporin-inhibitable cyclophilin, is presumed to be a component or regulator of the PTP (46, 85). Recent reports have demonstrated that inhibition of the permeability transition using cyclosporin A, a nonimmunosuppressive derivative of cyclosporin, can block apoptosis in certain settings. Our results indicate that CYP3 has only a small effect BAX-induced death (Table 1). However, the permeability transition in yeast mitochondria is not cyclosporin sensitive, even though the activity of the mitochondrial cyclophilin generated by the CYP3 gene is inhibited by cyclosporin (27, 47). Thus, the CYP3 gene product is likely not an essential part of the machinery producing BAX-mediated killing in yeast.
We were unable to examine the role of genes encoding ANT isoforms (AAC1 to AAC3), another proposed component of the PTP, by the genetic strategies used here (5, 46, 85). Physical and functional interactions have been reported between anti- and proapoptotic members of the BCL-2 family and ANT, and yeast strains which lack functional AAC genes have been reported to be resistant to BAX-induced cell death (45). Yeast strains containing mutations in all three yeast AAC genes have been reported in only one genetic background, W303 (10). In the yeast strain used here, and in a number of additional genetic backgrounds, strains containing deletions of the AAC2 gene encoding the main ANT are not viable during aerobic growth (32). Consistent with this observation, the yeast deletion project has defined the AAC2 gene as an essential gene (77). Thus, the reported resistance to BAX expression of W303 strains missing AAC genes may represent a strain-specific response that will be difficult to generalize. In addition, a recent report has failed to confirm the resistance of yeast strains missing AAC genes to BAX expression (60).
The physiological response of mutant mitochondria, as reflected in Δψm, is complex and is likely to depend on the role of each protein in the generation of the ionic gradients that create mitochondrial potentials, the pleiotropic effects induced by each mutation, and possibly the existence of incomplete compensatory mechanisms for each function. In a number of cases, ([rho0], Δatp2, Δatp4, and Δpor1 Δpor2), the response of mutant mitochondria is similar to that observed in wild-type cells: BAX results in a pronounced mitochondrial hyperpolarization which is reversed by coexpression of BCL-XL. Exceptions include cells missing cyt c, which cannot transfer electrons from complex III to complex IV of the respiratory chain. In this mutant, the transmembrane potential does not respond to BAX expression but is mildly diminished by coexpression of BCL-XL. In addition, the degree of hyperpolarization following BAX expression did not obviously correlate with the ability of BAX to induce death in the various mitochondrial mutants. Thus, these results indicate that mitochondrial hyperpolarization is not the cause of and is not sufficient for BAX-induced cell death. This suggests that changes in additional mitochondrial parameters are probably essential for death to occur.
We have checked whether the production of ROS correlates with the ability of BAX to induce death (Fig. 5). Our findings indicate that in certain mutant backgrounds there is a correlation (e.g., [rho0] and Δcyc1 Δcyc7) whereas in others there is not (e.g., Δatp2 and Δpor1 Δpor2). Thus, ROS production may be involved in BAX killing, but further studies are needed to define the exact role of ROS in this death process.
Taken together, our results suggest that caspase-independent, BAX-mediated cell killing in yeast requires aspects of mitochondrial biochemistry defined here. Elimination of the ATP2, ATP4, CYC1, and CYC7 genes, components of the respiratory chain encoded by the mitochondrial genome as reflected in [rho0] cells, decreases the efficiency of BAX-mediated killing. BAX-mediated cell death in yeast may require respiratory activity to generate ROS and/or alter mitochondrial ATP production. These results also demonstrate that two distinguishable responses occur following BAX expression in yeast: cell growth arrest, which does not require aspects of mitochondrial biochemistry defined by these mutations, and a cell-killing process, which does. BCL-XL can abrogate both the killing and the growth arrest signals. Our results suggest that the integration of cell cycle and cell death processes involving BCL-2 family members, as noted in mammalian cells (12, 37, 50), may also manifest in yeast and be separable in specific mutant backgrounds (e.g., [rho0] cells). The results presented here indicate that genetic analysis in yeast will provide an important tool to uncover mechanisms responsible for these two fundamental cellular process.
ACKNOWLEDGMENTS
A.G. was supported by a fellowship from the Leukemia Society of America, and this work was supported by grants from the NIH to S.J.K. and M.F.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L. The mitochondrial permeability transition. Biofactors. 1998;8:273–281. doi: 10.1002/biof.5520080315. [DOI] [PubMed] [Google Scholar]
- 3.Blachly-Dyson E, Song J, Wolfgang W J, Colombini M, Forte M. Multicopy suppressors of phenotypes resulting from the absence of yeast VDAC encode a VDAC-like protein. Mol Cell Biol. 1997;17:5727–5738. doi: 10.1128/mcb.17.10.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossy-Wetzel E, Newmeyer D D, Green D R. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brdiczka D, Beutner G, Ruck A, Dolder M, Wallimann T. The molecular structure of mitochondrial contact sites. Their role in regulation of energy metabolism and permeability transition. Biofactors. 1998;8:235–242. doi: 10.1002/biof.5520080311. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Takeyama N, Brady G, Watson A J, Dive C. Blood cells with reduced mitochondrial membrane potential and cytosolic cytochrome C can survive and maintain clonogenicity given appropriate signals to suppress apoptosis. Blood. 1998;92:4545–4553. [PubMed] [Google Scholar]
- 7.Cheng E H, Kirsch D G, Clem R J, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 8.de Jong D, Prins F A, Mason D Y, Reed J C, van Ommen G B, Kluin P M. Subcellular localization of the Bcl-2 protein in malignant and normal lymphoid cells. Cancer Res. 1994;54:256–260. [PubMed] [Google Scholar]
- 9.Diaz J L, Oltersdorf T, Horne W, McConnell M, Wilson G, Weeks S, Garcia T, Fritz L C. A common binding site mediates heterodimerization and homodimerization of Bcl-2 family members. J Biol Chem. 1997;272:11350–11355. doi: 10.1074/jbc.272.17.11350. [DOI] [PubMed] [Google Scholar]
- 10.Drgon T, Sabova L, Nelson N, Kolarov J. ADP/ATP translocator is essential only for anaerobic growth of yeast Saccharomyces cerevisiae. FEBS Lett. 1991;289:159–162. doi: 10.1016/0014-5793(91)81059-h. [DOI] [PubMed] [Google Scholar]
- 11.Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou J C. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gil-Gomez G, Berns A, Brady H J. A link between cell cycle and cell death: Bax and Bcl-2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J. 1998;17:7209–7218. doi: 10.1093/emboj/17.24.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goping I S, Gross A, Lavoie J N, Nguyen M, Jemmerson R, Roth K, Korsmeyer S J, Shore G C. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- 15.Green D, Reed J. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 16.Greenhalf W, Stephan C, Chaudhuri B. Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in Saccharomyces cerevisiae. FEBS Lett. 1996;380:169–175. doi: 10.1016/0014-5793(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths G J, Dubrez L, Morgan C P, Jones N A, Whitehouse J, Corfe B M, Dive C, Hickman J A. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross A, Jockel J, Wei M C, Korsmeyer S J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross A, McDonnell J, Korsmeyer S. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999a;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 20.Gross A, Yin X M, Wang K, Wei M C, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999b;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 21.Gross E, Marbach I, Engelberg D, Segal M, Simchen G, Levitzki A. Anti-Cdc25 antibodies inhibit guanyl nucleotide-dependent adenylyl cyclase of Saccharomyces cerevisiae and cross-react with a 150-kilodalton mammalian protein. Mol Cell Biol. 1992;12:2653–2661. doi: 10.1128/mcb.12.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hockenbery D, Nunez G, Milliman C, Schreiber R D, Korsmeyer S J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 23.Hsu Y T, Wolter K G, Youle R J. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter J J, Bond B L, Parslow T G. Functional dissection of the human Bcl-2 protein: sequence requirements for inhibition of apoptosis. Mol Cell Biol. 1996;16:877–883. doi: 10.1128/mcb.16.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ink B, Zornig M, Baum B, Hajibagheri N, James C, Chittenden T, Evan G. Human Bak induces cell death in Schizosaccharomyces pombe with morphological changes similar to those with apoptosis in mammalian cells. Mol Cell Biol. 1997;17:2468–2474. doi: 10.1128/mcb.17.5.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson M D, Weil M, Raff M C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 27.Jung D W, Bradshaw P C, Pfeiffer D R. Properties of a cyclosporin-insensitive permeability transition pore in yeast mitochondria. J Biol Chem. 1997;272:21104–21112. doi: 10.1074/jbc.272.34.21104. [DOI] [PubMed] [Google Scholar]
- 28.Jurgensmeier J M, Krajewski S, Armstrong R C, Wilson G M, Oltersdorf T, Fritz L C, Reed J C, Ottilie S. Bax- and Bak-induced cell death in the fission yeast Schizosaccharomyces pombe. Mol Biol Cell. 1997;8:325–339. doi: 10.1091/mbc.8.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 30.Knudson C M, Korsmeyer S J. Bcl-2 and Bax function independently to regulate cell death. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 31.Krajewski S, Tanaka S, Takayama S, Schibler M J, Fenton W, Reed J C. Investigation of the subcellular distribution of the Bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 32.Lawson J E, Douglas M G. Separate genes encode functionally equivalent ADP/ATP carrier proteins in Saccharomyces cerevisiae. Isolation and analysis of AAC2. J Biol Chem. 1988;263:14812–14818. [PubMed] [Google Scholar]
- 33.Laz T M, Pietras D F, Sherman F. Differential regulation of the duplicated isocytochrome c genes in yeast. Proc Natl Acad Sci USA. 1984;81:4475–4479. doi: 10.1073/pnas.81.14.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemasters J J, Nieminen A L, Qian T, Trost L C, Elmore S P, Nishimura Y, Crowe R A, Cascio W E, Bradham C A, Brenner D A, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 35.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 36.Ligr M, Madeo F, Frohlich E, Hilt W, Frohlich K U, Wolf D H. Mammalian Bax triggers apoptotic changes in yeast. FEBS Lett. 1998;438:61–65. doi: 10.1016/s0014-5793(98)01227-7. [DOI] [PubMed] [Google Scholar]
- 37.Linette G, Li Y, Roth K, Korsmeyer S. Cross talk between cell death and cell cycle progression: Bcl-2 regulates NFAT-mediated activation. Proc Natl Acad Sci USA. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Kim C, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 39.Lorenz M C, Muir R S, Lim E, McElver J, Weber S C, Heitman J. Gene disruption with PCR products in Saccharomyces cerevisiae. Gene. 1995;158:113–117. doi: 10.1016/0378-1119(95)00144-u. [DOI] [PubMed] [Google Scholar]
- 40.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 41.Manivasakam P, Weber S C, McElver J, Schiestl R H. Micro-homology mediated PCR targeting in Saccharomyces cerevisiae. Nucleic Acids Res. 1995;23:2799–2800. doi: 10.1093/nar/23.14.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manon S, Chaudhuri B, Guerin M. Release of cytochrome c and decrease of cytochrome c oxidase in Bax- expressing yeast cells, and prevention of these effects by coexpression of Bcl-XL. FEBS Lett. 1997;415:29–32. doi: 10.1016/s0014-5793(97)01087-9. [DOI] [PubMed] [Google Scholar]
- 43.Marchetti P, Castedo M, Susin S A, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinou I, Desagher S, Eskes R, Antonsson B, Andre E, Fakan S, Martinou J C. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J Cell Biol. 1999;144:883–889. doi: 10.1083/jcb.144.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzo I, Brenner C, Zamzami N, Jurgensmeier J M, Susin S A, Vieira H L, Prevost M C, Xie Z, Matsuyama S, Reed J C, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 46.Marzo I, Brenner C, Zamzami N, Susin S A, Beutner G, Brdiczka D, Remy R, Xie Z H, Reed J C, Kroemer G. The permeability transition pore complex: a target for apoptosis regulation by caspases and Bcl-2-related proteins. J Exp Med. 1998;187:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matouschek A, Rospert S, Schmid K, Glick B S, Schatz G. Cyclophilin catalyzes protein folding in yeast mitochondria. Proc Natl Acad Sci USA. 1995;92:6319–6323. doi: 10.1073/pnas.92.14.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuyama S, Xu Q, Velours J, Reed J C. The mitochondrial F0F1-ATPase proton pump is required for function of the proapoptotic protein Bax in yeast and mammalian cells. Mol Cell. 1998;1:327–336. doi: 10.1016/s1097-2765(00)80033-7. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy N J, Whyte M K, Gilbert C S, Evan G I. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meikrantz W, Schlegel R. Apoptosis and the cell cycle. J Cell Biochem. 1995;58:160–174. doi: 10.1002/jcb.240580205. [DOI] [PubMed] [Google Scholar]
- 51.Mignotte B, Vayssiere J L. Mitochondria and apoptosis. Eur J Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- 52.Minn A J, Kettlun C S, Liang H, Kelekar A, Vander Heiden M G, Chang B S, Fesik S W, Fill M, Thompson C B. Bcl-XL regulates apoptosis by heterodimerization-dependent and -independent mechanisms. EMBO J. 1999;18:632–643. doi: 10.1093/emboj/18.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Bcl-XL forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 54.Narita M, Shimizu S, Ito T, Chittenden T, Lutz R J, Matsuda H, Tsujimoto Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nechushtan A, Smith C L, Hsu Y T, Youle R J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okuno S, Shimizu S, Ito T, Nomura M, Hamada E, Tsujimoto Y, Matsuda H. Bcl-2 prevents caspase-independent cell death. J Biol Chem. 1998;273:34272–34277. doi: 10.1074/jbc.273.51.34272. [DOI] [PubMed] [Google Scholar]
- 57.Pastorino J G, Chen S T, Tafani M, Snyder J W, Farber J L. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 58.Pon L, Moll T, Vestweber D, Marshallsay B, Schatz G. Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. J Cell Biol. 1989;109:2603–2616. doi: 10.1083/jcb.109.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Priault M, Camougrand N, Chaudhuri B, Manon S. Role of the C-terminal domain of Bax and Bcl-XL in their localization and function in yeast cells. FEBS Lett. 1999;443:225–228. doi: 10.1016/s0014-5793(98)01661-5. [DOI] [PubMed] [Google Scholar]
- 60.Priault M, Camougrand N, Chaudhuri B, Schaeffer J, Manon S. Comparison of the effects of bax-expression in yeast under fermentative and respiratory conditions: investigation of the role of adenine nucleotides carrier and cytochrome c. FEBS Lett. 1999;456:232–238. doi: 10.1016/s0014-5793(99)00957-6. [DOI] [PubMed] [Google Scholar]
- 61.Priault M, Chaudhuri B, Clow A, Camougrand N, Manon S. Investigation of bax-induced release of cytochrome c from yeast mitochondria permeability of mitochondrial membranes, role of VDAC and ATP requirement. Eur J Biochem. 1999;260:684–691. doi: 10.1046/j.1432-1327.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 62.Reed J C, Jurgensmeier J M, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998;1366:127–137. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 63.Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- 64.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlesinger P H, Gross A, Yin X M, Yamamoto K, Saito M, Waksman G, Korsmeyer S J. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic Bcl-2. Proc Natl Acad Sci USA. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sedlak T W, Oltvai Z N, Yang E, Wang K, Boise L H, Thompson C B, Korsmeyer S J. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 68.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Costandini P, Loeffler M, Larochette N, Goodlett D R, Aebersold R, Siderovski D P, Penninger J M, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 70.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Susin S A, Zamzami N, Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta. 1998;1366:151–165. doi: 10.1016/s0005-2728(98)00110-8. [DOI] [PubMed] [Google Scholar]
- 72.Tao W, Kurschner C, Morgan J I. Bcl-XL and Bad potentiate the death suppressing activities of Bcl-XL, Bcl-2, and A1 in yeast. J Biol Chem. 1998;273:23704–23708. doi: 10.1074/jbc.273.37.23704. [DOI] [PubMed] [Google Scholar]
- 73.Tao W, Kurschner C, Morgan J I. Modulation of cell death in yeast by the Bcl-2 family of proteins. J Biol Chem. 1997;272:15547–15552. doi: 10.1074/jbc.272.24.15547. [DOI] [PubMed] [Google Scholar]
- 74.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 75.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-XL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 76.Wang K, Gross A, Waksman G, Korsmeyer S J. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winzeler E A, Shoemaker D D, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke J D, Bussey H, Chu A M, Connelly C, Davis K, Dietrich F, Dow S W, El Bakkoury M, Foury F, Friend S H, Gentalen E, Giaever G, Hegemann J H, Jones T, Laub M, Liao H, Davis R W. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. . (Strain Record Number 23056.) [DOI] [PubMed] [Google Scholar]
- 78.Wolter K G, Hsu Y T, Smith C L, Nechushtan A, Xi X G, Youle R J. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wood D E, Thomas A, Devi L A, Berman Y, Beavis R C, Reed J C, Newcomb E W. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- 80.Xiang J, Chao D T, Korsmeyer S J. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 82.Zha H, Fisk H A, Yaffe M P, Mahajan N, Herman B, Reed J C. Structure-function comparisons of the proapoptotic protein Bax in yeast and mammalian cells. Mol Cell Biol. 1996a;16:6494–6508. doi: 10.1128/mcb.16.11.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zha H, Reed J. Heterodimerization-independent functions of cell death regulatory proteins Bax and Bcl-2 in yeast and mammalian cells. J Biol Chem. 1997;272:31482–31488. doi: 10.1074/jbc.272.50.31482. [DOI] [PubMed] [Google Scholar]
- 84.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-XL. Cell. 1996b;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 85.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 86.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]