Abstract
This large cohort study from the US Premier Healthcare Database evaluated the risk and predictors of anaphylaxis in association with intravenous immunoglobulin (IvIg) therapy in the inpatient and outpatient setting. Data were collected retrospectively (January 2009–December 2018) from 24 919 patients administered IgPro10 IvIg, median age 54 years. Immunoglobulins of interest were IgPro10 and other IvIg given before or after IgPro10. Moderate and severe anaphylaxis was identified from same‐day parenteral epinephrine and IvIg use and reviews of patient record summaries. Predictors for first anaphylactic reactions associated with IvIg administration were derived from adjusted incidence rate ratios (IRR) using Poisson regression. Moderate anaphylaxis in IvIg use was rare and severe anaphylaxis very rare based on a total of 124 moderate and four non‐fatal severe first anaphylactic events, incidence rate of 7.11 and 0.23/10 000 IvIg administrations, respectively. Age under 18 years was an independent predictor of moderate or severe anaphylactic events [adjusted incidence rate ratio = 2.94, 0.95 confidence interval = 1.91–4.52] compared with those aged 18 years and older. First IvIg administration was a strong predictor of anaphylaxis. The IRR in those with a subsequent IvIg administration in the preceding 42 days decreased to 0.27 (0.17–0.42) and in those effectively IvIg‐naive (no IvIg for > 42 days) to 0.76 (0.44–1.32) compared with first IvIg use. The key conclusions from this study are that the risk of anaphylaxis has progressively reduced over the last decade, from 14.87 of 10 000 in 2009–10 to 4.39 of 10 000 IvIg administrations in 2017–18 and is rare overall, and that the risk of anaphylaxis is increased in those aged under 18 years.
Keywords: anaphylaxis, incidence rate, intravenous immunoglobulins, predictors, Premier Healthcare Database
This observational cohort study of 24,919 patients with a total of 175,535 administrations of intravenous immunoglobulin (IvIg) in a hospital setting shows a substantial reduction in the risk of moderate and severe anaphylaxis over a decade and establishes that anaphylaxis is rare overall. The risk of anaphylaxis has progressively reduced from 14.9/10,000 in 2009‐2010 to 4.4/10,000 IvIg administrations in 2017‐2018. Strong independent predictors of anaphylaxis were age under 18 and first dose of IvIg, and neither the IvIg dose nor the indication for its use was associated with the risk of anaphylaxis.

INTRODUCTION
Intravenous immunoglobulin (IvIg) is a fractionated blood product made from pooled human plasma from healthy donors. Typically, IvIg contains >96% unmodified immunoglobulin (Ig)G which has intact Fc‐dependent effector functions with a broad spectrum of activity. Stabilizers and trace amounts of non‐IgG plasma proteins, including IgA and other plasma proteins are also present [1]. Introduced as immunoglobulin‐replacement therapy in primary immunodeficiency syndromes [2], the indications for the use of IvIg have since expanded considerably [3]. Approved indications for IvIg use in the United States are product‐specific and encompass chronic inflammatory demyelinating polyneuropathy, immune thrombocytopenic purpura, Kawasaki syndrome and primary humoral immunodeficiency [4]. Other established therapeutic uses currently include Ig replacement therapy in immunodeficiency secondary to hematological malignancies and immunomodulation treatment in autoimmune and inflammatory diseases (stiff person syndrome, neonatal hemochromatosis, Guillain–Barré syndrome, myasthenia gravis, Lambert–Eaton syndrome, inflammatory myopathies and multi‐focal motor neuropathy) [3]. IvIg also has an emerging role as replacement treatment in other secondary hypogammaglobulinemia states (e.g. iatrogenic) and specific antibody deficiency [5] for immunomodulation in several dermatological, neurological and hematological diseases[3] and in toxic shock syndrome [3, 6].
While adverse effects to IvIg are common, these are usually mild, transient and infusion rate‐related [7]. Treatment‐related immediate adverse events comprising headache, flushing, fever, chills, fatigue, nausea, diarrhea, blood pressure changes and tachycardia are not uncommon and occur, for example, in 24%–36% of patients receiving high‐dose IvIg [8]. Rarely, serious adverse effects, including aseptic meningitis, renal impairment, thrombosis, hemolytic anemia, transfusion‐related acute lung injury and anaphylaxis, are experienced [7].
Anaphylaxis is reported rarely in patients administered IvIg, with 23 non‐fatal case reports in immunodeficient patients in the literature until 2017 [9], and an estimated incidence rate of one per 1000 IvIg administrations [10, 11].
Development of serum IgG anti‐IgA antibody in patients with constitutionally undetectable IgA is common, and has been associated with adverse reactions to IvIg (including non‐IgE‐mediated anaphylaxis) [12, 13]; however, it correlates poorly with adverse reactions [14]. Conversely, formation of IgE anti‐IgA antibodies, while very rare, is very likely to result in anaphylaxis (three of four patients given IvIg or other blood products had an anaphylaxis) [15, 16].
In this study, we sought to estimate the current risk of moderate or severe anaphylaxis and to explore predictors of anaphylaxis in patients treated with IvIg after a decade‐long period in which the criteria for IvIg use broadened greatly.
METHODS
Data source
Data were obtained from the US Premier Healthcare Database (PHD). PHD contains data from an open cohort from the in‐ and outpatient setting of hospitals across the United States with approximately one‐sixth of all hospital discharges in the country. Data accrue from a large diverse population and reflect the state of clinical practice in the general population [17].
Patient demographic characteristics (age, sex, ethnicity and region), discharge diagnoses and discharge status (including death, but not its cause) are available for all PHD hospitals, hospital‐owned clinics and emergency rooms. Service‐level data include charges for medications, procedures and laboratory tests, but not laboratory results. In addition to the information from the standard hospital‐discharge file, date‐stamped logs of all billed items for each patient, including medications and laboratory, diagnostic and therapeutic services, are available.
Hospital discharge diagnoses are coded with ICD‐9 CM up to 30 September 2015 and subsequently with ICD‐10 CM codes, while medications are identified using a text description of the product and dose that is contained in the hospital encounter charge details file.
Design
This was a retrospective observational cohort study, derived from a convenience cohort of an IvIg IgPro10 cohort, originally sampled to study the risk of hematological anemia in association with IvIg IgPro10 [18]. The cohort comprised patients who were treated with intravenous Ig. Patients of any age with at least one administration of the IvIg IgPro10 in the in‐ or outpatient setting between 1 January 2009 and 31 December 2018 and without IvIg administration history before 1 January 2009 formed the study cohort. Patients were observed from the day of the first IvIg administration until the earliest of the following events: last IvIg administration, the occurrence of a moderate or severe anaphylaxis event, end of study period or death.
Exposure
Exposures of interest were IvIg treatment during the entire study period, i.e. IgPro10 and any other IvIg given before or after IgPro10. The at‐risk time for anaphylactic reactions was the day of each IvIg administration. Exposure was categorized into mutually exclusive levels: (1) first ever IvIg use and (2) repeat IvIg use. The latter consisted of effectively IvIg‐naive, defined as either repeat IvIg use after a treatment gap of more than 42 days, or subsequent IvIg use, defined as repeat IvIg use with an IvIg treatment gap of 42 days or fewer.
Outcome
The outcome of interest was moderate or severe anaphylactic reaction in association with IvIg use. The Brown grading was used to classify reactions as ‘moderate’ (having features suggesting respiratory, cardiovascular or gastrointestinal involvement) or ‘severe’ (having features of hypoxia, hypotension or neurological compromise) [19]. Where clinical features required to apply the Brown criteria were not systematically recorded in the PHD, we imputed the severity of anaphylaxis from the total parenteral adrenaline dosage and admission to a high dependency or intensive care unit (HDU/ICU) (or death) in order to classify events as moderate or severe. Potential anaphylactic reaction events were identified when parenteral epinephrine was administered on the same day as IvIg.
Database record summaries for all potential anaphylactic reactions were reviewed by a clinical expert in anaphylactic events (S.v.N.) and an epidemiologist (C.M.) to identify cases of anaphylaxis and to assess the most likely indication for epinephrine use (circulatory support including resuscitation, bleeding control during endoscopy, anaphylactic reaction or unknown). Events associated with an anaphylactic reaction were classified as either moderate or severe. Severe anaphylaxis was defined by the need for administration of repeat doses of parenteral epinephrine (>2 weight appropriate doses), whether the patient required transfer to HDU/ICU after epinephrine use and whether the length of stay in HDU/ICU was commensurate with a patient having suffered a severe anaphylaxis (24–72 h) in the absence of deterioration in the patient due to other causes or death on the same day as the anaphylactic event. Those events which were not classified as severe were classified as moderate.
Database record summaries consisted of a chronological listing of available data recorded in the PHD on a patient level basis, including medical history, hospital diagnoses present on hospital admission or discharge diagnoses, body mass index group, procedures recorded during the hospital stay, laboratory tests requested, medications and transfusions administered, surgical and diagnostic procedures, type of hospital admission, level of in‐hospital care, intensive care unit level and type of ventilation.
Covariates
Covariates of interest were age at IvIg administration, sex, race, hospital setting (in‐ or outpatient setting) of IvIg administration, transfusion of red blood cells or other blood products and the IvIg indication. IvIg indications consisted of neurological, hematological (immune thrombocytopenia) and autoimmune disease (classified as high‐dose indications) and immunodeficiency, malignant lymphoid or hematopoietic neoplasm, anti‐cancer therapy and sepsis or septicemia (classified as low‐dose indications). A unique IvIg indication per IvIg administration was determined based on the discharge diagnoses of the respective hospitalization in which IvIg was administered and by applying the following hierarchy for multiple indication records: neurological (highest in hierarchy), hematological, autoimmune disease, immunodeficiency, malignant lymphoid or hematopoietic neoplasm, anti‐cancer therapy and sepsis or septicemia (lowest).
Data analysis
IvIg users were described by summarizing their sex and ethnicity as well as the age, setting and indication of the respective patient at the first IvIg administration throughout the study period. Continuous variables were summarized using median, first and third quartiles and categorical variables using numbers and proportions. The incidence rate of first anaphylactic events was estimated as anaphylactic events per IvIg administration, calculated overall and stratified by IvIg indication, calendar year and severity of anaphylactic event.
Predictors for a first anaphylactic reaction following an IvIg administration were estimated by calculating crude and adjusted incidence rate ratios (IRR) from Poisson regression models for the occurrence of a first moderate or severe anaphylactic event, clustering on patients and adjusting for age, sex, race, sequence of IvIg treatment (first, effectively IvIg‐naive or subsequent administration), hospital setting of IvIg administration, transfusion of blood components, IvIg indication dose group (low, high or unknown dose) and calendar period of IvIg administration grouped into five consecutive calendar periods of 2 years.
All analyses were performed using Stata MP version 14.2 (StataCorp LLC, College Station, Texas, USA).
RESULTS
The study cohort comprised 24 919 patients who were given IgPro10 at some point between 1 January 2009 and 31 December 2018 and with a total of 175 535 IvIg administrations, 116 856 (67% of IgPro10 and the remaining to unspecified or other IvIgs); median age 54 years, 20.8% children, 48.7% males, 72.6% white, 11.3% black and 0.7% Hispanic. The most frequent indications at the first IvIg administration were hematological disease (35.1%), neurological disease (24.2%) and immunodeficiency (19.1%); Table 1. The proportion of indications varied by calendar year, decreasing for immunodeficiency from 29.5 to 21.0% and increasing for neurological disease from 17.0 to 22.7%, while the proportion of those treated for hematological disease remained nearly stable; Supporting information, Figure S1.
TABLE 1.
Characteristics of IvIg cohort at first IvIg administration
| Age (years) | <18 (n = 5182) | 18+ (n = 19737) | Total IvIg users (n = 24919) |
|---|---|---|---|
| Sex | |||
| Male | 2850 (55.0) | 9294 (47.1) | 12144 (48.7) |
| Female | 2332 (45.0) | 10443 (52.9) | 12775 (51.3) |
| Race | |||
| White | 2831 (54.6) | 15263 (77.3) | 18094 (72.6) |
| Black | 826 (15.9) | 1997 (10.1) | 2823 (11.3) |
| Hispanic | 55 (1.1) | 109 (0.6) | 164 (0.7) |
| Other | 1327 (25.6) | 2012 (10.2) | 3339 (13.4) |
| Unknown race | 143 (2.8) | 356 (1.8) | 499 (2.0) |
| Setting of IvIg use | |||
| Inpatient | 4441 (85.7) | 15079 (76.4) | 19520 (78.3) |
| Outpatient | 741 (14.3) | 4658 (23.6) | 5399 (21.7) |
| Indication 1 | |||
| High‐dose indications | 3131 (60.4) | 13139 (66.6) | 16270 (65.3) |
| Neurological disease | 239 (4.6) | 5784 (29.3) | 6023 (24.2) |
| Hematological disease | 1673 (32.3) | 7077 (35.9) | 8750 (35.1) |
| Autoimmune disease | 1219 (23.5) | 278 (1.4) | 1497 (6.0) |
| Low‐dose indications | 906 (17.5) | 5178 (26.2) | 6084 (24.4) |
| Immunodeficiency | 709 (13.7) | 4043 (20.5) | 4752 (19.1) |
| Lymphoid/blood 2 | 101 (1.9) | 618 (3.1) | 719 (2.9) |
| Anticancer therapy | 10 (0.2) | 43 (0.2) | 53 (0.2) |
| Sepsis/septicemia | 86 (1.7) | 474 (2.4) | 560 (2.2) |
| Unstated indication | 1145 (22.1) | 1420 (7.2) | 2565 (10.3) |
Data are n (%). IvIg = intravenous immunoglobulin.
Mutually exclusive indication per IvIg administration determined by applying the following hierarchy: neurological disease (highest level), hematological disease, autoimmune disease, immunodeficiency, lymphoid/blood, anti‐cancer therapy and sepsis/septicemia.
Malignant lymphoid or hematopoietic neoplasm.
Between January 2009 and December 2018 the study cohort accumulated 175 535 person‐days at risk, equivalent to 1 day at risk for each IvIg transfusion, 36.6% in the inpatient and 63.4% in the outpatient setting. There were 728 epinephrine ampoules prescribed on the day of the IvIg administration. Of those, 234 were repeat epinephrine administrations as part of a treatment sequence for circulatory support. The manual review of the 494 remaining events with epinephrine use on the day of IvIg use revealed that another 236 were given epinephrine for circulatory support, 44 topical epinephrine for bleeding control in association with endoscopic procedures, a further 19 for a reason other than treatment of anaphylaxis and three for treatment of a mild anaphylactic reaction, e.g. urticaria. A total of 192 anaphylactic reactions in 128 patients were identified (incidence rate = 10.94 per 10 000 IvIg administrations). There were no fatal events. Of the 128 patients, 107 had one anaphylactic reaction, 10 had two reactions and 11 had three or more moderate reactions. After exclusion of 1122 IvIg administrations after an anaphylactic event, the cohort accumulated 174 413 person‐days at risk; Figure 1.
FIGURE 1.
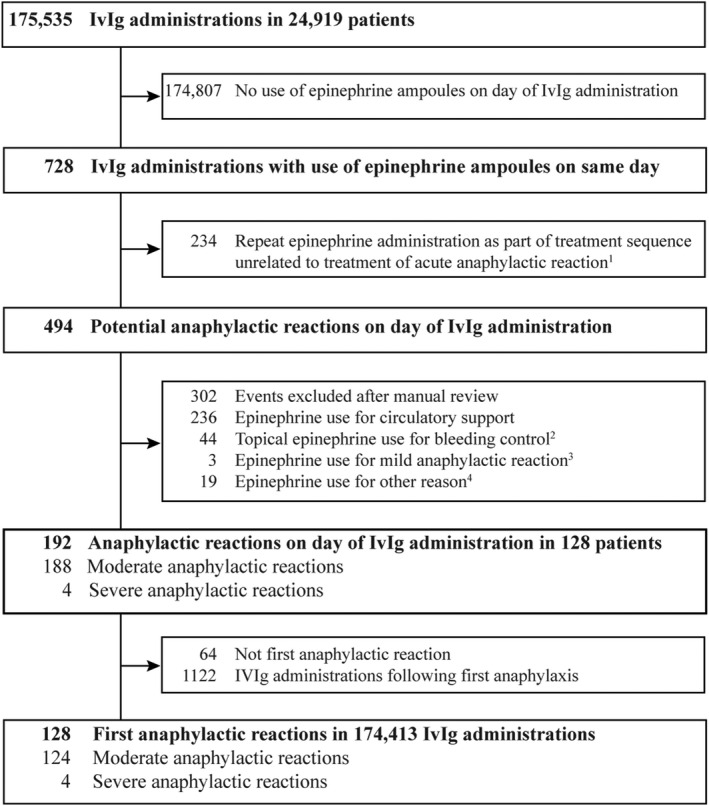
Ascertainment of first moderate and severe anaphylactic reactions following intravenous immunoglobulin (IvIg) administrations between 1 January 2009 and 31 December 2018. 1 Same epinephrine product and dose administered during the hospitalization on a different day without IvIg use (one epinephrine administration before IvIg or ≥ two epinephrine administrations after IvIg); 2 e.g. during bronchoscopy, endoscopy or epistaxis; 3e.g. for control of exacerbation of chronic spontaneous urticaria. 4Other reasons observed were: surgical procedures, Kawasaki disease and cerebral‐induced hypotension
The crude incidence rate of severe anaphylactic events based on four events between 2009 and 2018 was 0.23 per 10 000 administrations or one in 43 400 and the crude incidence rate of first moderate anaphylactic events based on 124 events was 7.11 per 10 000 administrations or one in 1400. During the entire observational period, hematological and autoimmune indications appeared to have higher crude incidence rates of moderate or severe anaphylaxis; Table 2.
TABLE 2.
Crude incidence rate of first anaphylactic events per 10 000 IvIg administrations by indication for IvIg, calendar period 2009–18
| Indication 1 | IvIg administrations | Anaphylactic event risk per 10 000 administrations | |||||
|---|---|---|---|---|---|---|---|
| All events | Moderate events | Severe events | |||||
| n | IR (0.95 CI) | n | IR (0.95 CI) | n | IR (0.95 CI) | ||
| All indications | 174 413 | 128 | 7.3 (6.1–8.8) | 124 | 7.1 (5.9–8.5) | 4 | 0.2 (0.0–0.6) |
| High‐dose indications | 95 335 | 78 | 8.2 (6.4–10.3) | 77 | 8.1 (6.3–10.1) | 1 | NA |
| Neurological | 65 058 | 37 | 5.7 (4.0–7.9) | 36 | 5.5 (3.8–7.7) | 1 | NA |
| Hematological | 26 899 | 34 | 12.6 (8.7–17.7) | 34 | 12.6 (8.7–17.7) | 0 | NA |
| Autoimmune | 3378 | 7 | 20.7 (8.3–42.7) | 7 | 20.7 (8.3–42.7) | 0 | NA |
| Low‐dose indications | 68 045 | 36 | 5.3 (3.7–7.4) | 34 | 5.0 (3.4–7.0) | 2 | NA |
| Immunodeficiency | 64 301 | 33 | 5.1 (3.5–7.3) | 32 | 5.0 (3.4–7.1) | 1 | NA |
| Lymphoid/blood 2 | 2282 | 2 | NA | 1 | NA | 1 | NA |
| Anti‐cancer therapy | 314 | 1 | NA | 1 | NA | 0 | NA |
| Sepsis/septicemia | 1148 | 0 | NA | 0 | NA | 0 | NA |
| Unknown indication | 11 033 | 14 | 12.7 (6.9–21.3) | 13 | 11.8 (6.2–20.2) | 1 | NA |
CI = confidence interval; IR = incidence rate; IvIg = intravenous immunoglobulin; NA = not applicable due to cell counts ≤3.
Mutually exclusive indication per IvIg administration determined by applying the following hierarchy: neurological disease (highest level), hematological disease, autoimmune disease, immunodeficiency, lymphoid/blood, anti‐cancer therapy and sepsis/septicemia.
Malignant lymphoid or hematopoietic neoplasm.
The overall incidence rate of first anaphylactic events decreased by calendar year from 14.87 per 10 000 IvIg administrations in 2009–10 to 4.39 per 10 000 IvIg administrations in 2017–18; Figure 2, Table 3.
FIGURE 2.
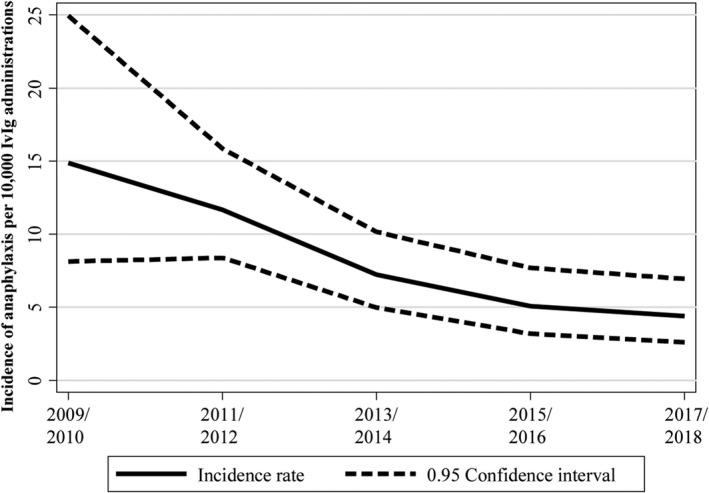
Incidence rate of first anaphylaxis by calendar year of intravenous immunoglobulin (IvIg) administration
TABLE 3.
Progressive anaphylaxis risk reduction over calendar periods
| Calendar period | Anaphylactic events | IvIg administrations | IR per 10 000 IvIg administrations (0.95 CI) | Crude IRR 1 (0.95 CI) | Adjusted IRR 5 , 6 (0.95 CI) |
|---|---|---|---|---|---|
| 2009–2010 | 14 | 9417 | 14.87 (8.12–24.95) | 1 | 1 |
| 2011–2012 | 41 | 35 103 | 11.68 (8.38–15.85) | 0.79 (0.43–1.44) | 0.76 (0.42–1.40) |
| 2013–2014 | 33 | 45 607 | 7.24 (4.98–10.17) | 0.49 (0.26–0.91) | 0.49 (0.26–0.91) |
| 2015–2016 | 22 | 43 316 | 5.08 (3.18–7.69) | 0.34 (0.17–0.67) | 0.35 (0.18–0.68) |
| 2017–2018 | 18 | 40 970 | 4.39 (2.60–6.95) | 0.30 (0.15–0.60) | 0.32 (0.16–0.65) |
CI = confidence interval; IR = incidence rate; IRR = incidence rate ratio; IvIg = intravenous immunoglobulin.
Estimated from Poisson regression models clustering for patients.
IRR estimates adjusted for age (<18, 18+), sex, race, sequence of IvIg treatment, hospital setting, transfusion of blood components, IvIg indication dose group and calendar period.
Independent predictors of moderate or severe anaphylactic events were age [IRR = 2.94, 0.95 confidence interval (CI) = 1.91–4.52], for age <18 years (compared with age 18 years and older) and first IvIg administration. The incidence rate of risk of first moderate or severe anaphylaxis was greatest with the first IvIg administration, incidence rate = 22.87 per 10 000 IvIg administrations. This risk was reduced by 24% in those who received IvIg after an effectively IvIg‐naive period of 42 days, IRR = 10.78 per 10 000 IvIg administrations and further reduced to 3.89 per 10 000 IvIg administrations in those with IvIg use in the preceding 42 days; Table 4. Of all 124 incident moderate anaphylactic reactions, 54 (43.5%) occurred after the first recorded IvIg administration, while 75% (three of four) of severe anaphylactic reactions were associated with first recorded IvIg use.
TABLE 4.
Predictors for first anaphylactic events following IvIg administrations, calendar period 2009–18
| Anaphylactic events | IvIg administrations | IR per 10 000 IvIg administrations (0.95 CI) | Crude IRR 1 (0.95 CI) | Adjusted IRR 1 , 2 (0.95 CI) | |
|---|---|---|---|---|---|
| Total IvIg administrations | 128 | 174 413 | 7.34 (6.12–8.73) | ||
| Predictors | |||||
| Age (years) | |||||
| <18 | 39 | 18 504 | 21.08 (14.98–28.82) | 3.69 (2.52–5.41) | 2.94 (1.91–4.52) |
| ≥18 or unknown | 89 | 155 909 | 5.71 (4.58–7.03) | 1 | 1 |
| Sequence of IvIg treatment | |||||
| First IvIg administration | 57 | 24 919 | 22.87 (17.32–29.64) | 1 | 1 |
| Effectively IvIg‐naive administration 3 | 20 | 18 551 | 10.78 (6.58–16.66) | 0.47 (0.28–0.78) | 0.76 (0.44–1.32) |
| Subsequent administration 4 | 51 | 130 943 | 3.89 (2.89–5.13) | 0.17 (0.12–0.25) | 0.27 (0.17–0.42) |
| Non‐predictors | |||||
| Sex | |||||
| Male | 58 | 77 451 | 7.49 (5.68–9.69) | 1 | 1 |
| Female | 70 | 96 962 | 7.22 (5.62–9.13) | 0.96 (0.68–1.37) | 1.07 (0.75–1.52) |
| Transfusion of blood component | |||||
| No transfusion | 116 | 168 122 | 6.90 (5.70–8.28) | 1 | 1 |
| Red blood cells ± other blood products | 11 | 5615 | 19.59 (9.77–35.06) | 2.84 (1.53–5.27) | 1.53 (0.79–2.95) |
| Other blood products only | 1 | 676 | NA | NA | NA |
| IvIg Indication | |||||
| Low‐dose indication 5 | 36 | 68 045 | 5.29 (3.70–7.33) | 1 | 1 |
| High‐dose indication 6 | 78 | 95 335 | 8.18 (6.46–10.22) | 1.55 (1.04–2.31) | 1.20 (0.76–1.88) |
CI = confidence interval; IR = incidence rate; IRR = incidence rate ratio; IvIg = intravenous immunoglobulin; NA = not applicable due to cell counts ≤3.
Estimated from Poisson regression models clustering for patients.
IRR estimates adjusted for age (<18, 18+), sex, race, sequence of IvIg treatment, hospital setting, transfusion of blood components, IvIg indication dose group and calendar period.
No IvIg administration in the previous 42 days except the first IvIg administration.
IvIg administration in the previous 42 days.
Including neurological, hematological and autoimmune diseases.
Including immunodeficiency, malignant lymphoid or hematopoietic neoplasm, anti‐cancer treatment and sepsis/septicemia.
Red blood cell transfusions on the day of the IvIg administration were also associated with a non‐significantly increased point estimate of 1.53 (0.79–2.95); Table 4. Inpatient setting appeared to be associated with an increased risk of anaphylaxis in the crude analysis, but not after adjustment (IRR = 1.44, 0.95 CI = 0.91–2.30), due to confounding by different age distributions and proportions of first and repeat IvIg recipients in the in‐ and outpatient setting (data not shown).
DISCUSSION
There are a number of significant findings from this retrospective observational cohort study of 24 919 patients treated with intravenous immunoglobulin between 1 January 2009 and 31 December 2018, who received altogether 175 535 infusions of IvIg.
First, the overall risk of a moderate or severe anaphylaxis was 10.94 per 10 000 IvIg administrations, with a total of 192 anaphylactic reactions identified in 128 patients. The incidence rate of a first anaphylaxis during the entire study period was 7.34 (7.11 moderate and 0.23 severe anaphylactic reactions) per 10 000 IvIg administrations and declined during the 10‐year study period from 14.87 in 2009–10 to the risk of 4.39 per 10 000 IvIg administrations in 2017–18, the most recent calendar period examined.
The incidence rate of adverse events requiring epinephrine use derived from two observational studies were similar to that seen overall in our study: 11.3 per 10 000 IvIg administrations (two per 1765 IvIg administrations over 2 years in 116 patients during 2011–13) and 9.99 (three of 3004) IvIg administrations over 13 years in 99 patients between 1995 and 2007 [10, 11].
The second finding from this study confirms a known predictor for anaphylaxis. The majority of severe anaphylactic reactions, three of four (75%), occurred with the first recorded IvIg infusion and 54 of 124 (43.5%) of the moderate anaphylactic reactions were associated with the first administration of IvIg.
The risk of developing a moderate or severe anaphylaxis is highest with the first administration of IvIg. This risk decreases 73% in those in whom IvIg has been administered in the preceding 42 days (subsequent use) and 24% in those who received IvIg after an interruption to IvIg treatment for a period of 42 days or more (effectively IvIg‐naive). This finding supports the recommendation to regard individuals changing IvIg preparations or having an interruption in their IvIg therapy of 6 weeks or more as being effectively IvIg‐naive [20].
The third finding relates to a novel predictor for anaphylaxis to IvIg, in that being aged under 18 years confers an approximately threefold independent risk of developing a moderate or severe anaphylaxis when compared with being aged 18 years and older. This increased risk also remained when the analysis was restricted to first IvIg administrations (IRR = 1.99, 0.95 CI = 1.08–3.65). Smaller studies, however, found no age‐related predilection for anaphylaxis in their patients [11, 21]. The mechanism underlying the greater risk of anaphylaxis in children administered IvIg or a hypothesis for the increased risk does not appear to be related to the IvIg itself, and while remaining arcane, may lie in an inherent difference in the immunological milieu of those aged under 18 versus those aged over 18 years, as this age group is known to have a greater risk of anaphylaxis overall [22]. This hypothesis is supported by a small but not statistically significant decrease of the point estimate for anaphylaxis associated with age in adults; Supporting information, Table S1.
The fourth, and arguably the most important, finding from this study is the decrease of 68% in anaphylactic reactions between 2009–10 and 2017–18, a substantial decrease in risk which has been progressive throughout the entire study period of 2009 to 2018.
The effect of an increasing incidence of pretreatment to prevent systemic reactions and other symptoms in the real‐life setting, however, deserves further exploration prospectively, including the route of administration and the choice of antihistamine [23].
Another potential explanation for the observed progressive reduction of anaphylactic events is changes in the manufacturing process. During the study period some manufacturers of IvIg adopted measures to reduce isoagglutinin of IvIg products which could have contributed to the decreased current risk. Measures included the exclusion of high anti‐A titer donors from pooled plasma, introduction of an immunoaffinity chromatography step or the implementation of a modified Cohn–Oncley fractionation method followed by chromatographic purification [24, 25, 26].
STRENGTHS AND LIMITATIONS
The size of the cohort (24 919 patients), the largest followed to date, to our knowledge, is clearly a strength of this study, as it generated data from 175 535 administrations of IvIg. The length of the data collection period of 10 years allowed observation of the outcome of further administrations of IvIg. Two previous studies extended for 10 years or more; however, the number of IvIg administrations reviewed was much smaller in both studies: 3004 and 104 infusions, respectively. Any absence of recurrence of anaphylaxis over time in the context of regular dosing may be explained in the majority of individuals by the known tolerance induction in patients with IgG anti‐IgA‐mediated reactions given long‐term intravenous IgG. Ahrens and colleagues also speculated that high‐dose IvIg may lead to a reduction in IgG anti‐IgA antibody mediated reactions; however, in this study no difference in anaphylaxis frequency between high‐ and low‐dose IvIg preparations was observed.
The length of the study also enabled the finding of a progressive reduction over time in episodes of anaphylaxis and the exploration of predictive factors. Examination of this very large number of treatment episodes emphatically endorses previous findings of the safety of IvIg use and the increased likelihood of a reaction occurring with the first dose [3, 11, 27]. This notion is supported by the absence of severe reactions in a study cohort of 119 patients with 2031 IvIg administrations treated at home but selected after six uneventful transfusions in hospital [21].
Supporting the notion that improvements in manufacture underlie the progressive and substantial reduction in episodes of anaphylaxis seen over a decade is the lack of any disease specificity for anaphylaxis and the lack of a dosage effect upon the occurrence of an anaphylaxis in our cohort. Arguably, an impact upon anaphylaxis reduction from these manufacturing changes could have been the production of low IgA‐containing preparations [28].
The study cohort was formed of patients with at least one administration of the IvIg IgPro10 during the entire study period. Although the exposure of interest was the administration of any IvIg during the study period, 67% of all IvIg administrations were of IgPro10. As the remaining administrations to other IvIgs consisted of patients who switched from or to IgPro10, our sample may not be representative of all IvIg users.
The progressive improvement in safety seen over the decade in episodes of moderate anaphylaxis may be due in part to the implementation of guidelines for nursing staff specializing in the care of patients requiring IvIg [20].
The use of ICD‐9 CM and ICD‐10 CM codes for the identification of moderate and severe anaphylaxis would have resulted in the identification of 4.3% (eight of 188) moderate anaphylaxis and 75% (three of four) severe anaphylactic events. The detailed manual review undertaken of each patient record derived from hospital claims where epinephrine had been used on the same day that IvIg had been administered, however, found 188 moderate and four severe anaphylactic events. Significant under‐diagnosis of anaphylaxis is an established limitation of epidemiological studies of anaphylaxis. Similar disparity between the number of cases determined by ICD code identification for anaphylaxis alone versus applying a diagnostic algorithm to further interrogate data has been reported previously. Fifty‐eight per cent of 2751 anaphylaxis cases would have been missed without their refinement of interrogating the database using a clinical algorithm in conjunction with the ICD codes [29].
The manual record review was essential for identifying anaphylactic events which were not revealed by examining ICD codes and eliminating instances where epinephrine was used for other reasons, and is thus a major strength in our study.
Although the study cohort was derived from a convenience sample of hospitals and clinical information imputed from hospital claims, this large cohort of IvIg users was deemed suitable to investigate the association between in‐ and outpatient‐administered IvIg, the risk of anaphylaxis and its severity. Underpinning the effectiveness of the manual review in determining the grade of severity of the anaphylaxis imputed from the drugs used in the absence of clinical information and the subsequent clinical course were the unique characteristics of the database.
As all patients in this unselected cohort were treated in hospital (including those attending special outpatient clinics) and were therefore all under medical supervision, the possibility of under‐estimation of episodes of anaphylaxis is minimized. Considering the threefold increase seen in anaphylaxis with the first dose, reviewing large samples of day‐stamped hospital‐based patient information is likely to provide a more accurate estimate of the incidence of moderate and severe anaphylaxis [21].
Transient hypotension is a recognized adverse effect of IvIg administration. In a rat model, this is due to the presence of immunoglobulin G dimers with a functional Fc fragment in polyclonal IgG binding to Fcgamma receptors on macrophages and inducing release of blood pressure‐lowering mediators [30]. The risk of anaphylaxis could have been over‐estimated if an episode of transient hypotension which would have otherwise settled was treated with epinephrine and therefore included in the analysis. Another potential limitation is the exclusion of suspected cases of mild anaphylaxis on the grounds that the data reviewed were day‐stamped but not time‐stamped, making it impossible to determine whether the drug use was for pretreatment or for treatment of a mild allergic reaction by analysing the temporal sequence. Over‐estimation of the number of moderate episodes of anaphylaxis could also occur where epinephrine might have been used for mild allergic symptoms. Conversely, under‐estimation of cases of moderate anaphylaxis may have occurred where epinephrine was withheld unnecessarily. Overall, inappropriate or unrecorded epinephrine use or withholding of epinephrine is unlikely to have had any substantial impact upon our estimates, as in clinical practice these occurrences would be few and counteract each other.
Examination of the real‐life experience in this cohort has an advantage when assessing the risk of anaphylaxis overall as patients considered at greater risk might have been reasonably excluded from clinical trial settings.
The absence of any fatalities due to anaphylaxis among a very large number of IvIg administrations indicates that fatal anaphylactic events are less frequent than in one in 45 000 IvIg administrations. This further validates the safety of IvIg.
Future research exploring the length of the interval between IvIg doses needed to render the IvIg recipient effectively IvIg‐naive could further contribute to practical management of the risk of anaphylaxis in IvIg recipients.
CONCLUSIONS
Anaphylaxis is rare, severe anaphylaxis is very rare and fatal anaphylaxis did not eventuate in this large cohort. There has been a substantial reduction during the last decade in the risk of anaphylaxis. Predictors of anaphylaxis are age under 18 years, first dose of IvIg or being effectively IvIg‐naive. Neither the IvIg dose nor the indication for its use affected the risk of anaphylaxis.
CONFLICTS OF INTEREST
S.v.N. declares no competing interests and has not received funding for the conduct of this study. C.M. and C.W. are employees of the Institute for Epidemiology, Statistics and Informatics GmbH. The Institute for Epidemiology, Statistics and Informatics GmbH has received grants from Bristol‐Myers Squibb, CSL Behring and Merz Pharma outside the submitted work. The Institute for Epidemiology, Statistics and Informatics GmbH has received part‐funding for the conduct of this study.
AUTHOR CONTRIBUTIONS
C.M., C.W. and S.v.N. conceived this study, C.M. supervised the study, drafted and edited the final manuscript. C.M. and C.W. cleaned and analysed data. S.v.N. and C.M. conducted the literature search and drafted the manuscript. C.W. and C.M. performed the statistical modelling. All authors contributed to the study design, contributed to drafting the protocol, revised the manuscript for important intellectual content, had full access to all the data in the study and had final responsibility for the decision to submit for publication.
ETHICS APPROVAL
Premier Healthcare Database is fully compliant with all Health Insurance Portability and Accountability Act (HIPAA) of 1996 privacy and security requirements. Institutional review board approval was not required, based on US Title 45 Code of Federal Regulations, Part 46, because the study used existing de‐identified hospital discharge data files and recorded information could not be identified directly or through identifiers linked to individuals. No informed consent of study participants was pursued due to the nature of the de‐identified data.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This study was part‐funded by CSL Behring. We thank Stephan Rietbrock of the Institute for Epidemiology, Statistics and Informatics GmbH for the quality assurance of the data cleaning and data analysis.
Martinez C, Wallenhorst C, van Nunen S. Intravenous immunoglobulin and the current risk of moderate and severe anaphylactic events, a cohort study. Clin Exp Immunol. 2021;206:384–394. 10.1111/cei.13665
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Premier Health Database. Restrictions apply to the availability of these data, which were used under license for this study.
REFERENCES
- 1. Rutter A, Luger TA. High‐dose intravenous immunoglobulins: an approach to treat severe immune‐mediated and autoimmune diseases of the skin. J Am Acad Dermatol. 2001;44:1010–24. [DOI] [PubMed] [Google Scholar]
- 2. Nolte MT, Pirofsky B, Gerritz GA, Golding B. Intravenous immunoglobulin therapy for antibody deficiency. Clin Exp Immunol. 1979;36:237–43. [PMC free article] [PubMed] [Google Scholar]
- 3. Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139:S1–S46. [DOI] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration Immune globulin intravenous (IGIV) indications. 2018. Available from: https://www.fda.gov/vaccines‐blood‐biologics/approved‐blood‐products/immune‐globulin‐intravenous‐igiv‐indications. Accessed 8 Jan 2021.
- 5. Perez E, Bonilla FA, Orange JS, Ballow M. Specific antibody deficiency: controversies in diagnosis and management. Front Immunol. 2017;8:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Darenberg J, Ihendyane N, Sjolin J, Aufwerber E, Haidl S, Follin P, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double‐blind, placebo‐controlled trial. Clin Infect Dis. 2003;37:333–40. [DOI] [PubMed] [Google Scholar]
- 7. Nydegger UE, Sturzenegger M. Adverse effects of intravenous immunoglobulin therapy. Drug Saf. 1999;21:171–85. [DOI] [PubMed] [Google Scholar]
- 8. Katz U, Achiron A, Sherer Y, Shoenfeld Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev. 2007;6:257–9. [DOI] [PubMed] [Google Scholar]
- 9. Williams SJ, Gupta S. Anaphylaxis to IVIG. Arch Immunol Ther Exp. 2017;65:11–9. [DOI] [PubMed] [Google Scholar]
- 10. Bichuetti‐Silva DC, Furlan FP, Nobre FA, Pereira CTM, Gonçalves TRT, Gouveia‐Pereira M, et al. Immediate infusion‐related adverse reactions to intravenous immunoglobulin in a prospective cohort of 1765 infusions. Int Immunopharmacol. 2014;23:442–6. [DOI] [PubMed] [Google Scholar]
- 11. Dashti‐Khavidaki S, Aghamohammadi A, Farshadi F, Movahedi M, Parvaneh N, Pouladi N, et al. Adverse reactions of prophylactic intravenous immunoglobulin; a 13‐year experience with 3004 infusions in Iranian patients with primary immunodeficiency diseases. J Invest Allergol Clin Immunol. 2009;19:139–45. [PubMed] [Google Scholar]
- 12. Horn J, Thon V, Bartonkova D, Salzer U, Warnatz K, Schlesier M, et al. Anti‐IgA antibodies in common variable immunodeficiency (CVID): diagnostic workup and therapeutic strategy. Clin Immunol. 2007;122:156–62. [DOI] [PubMed] [Google Scholar]
- 13. Rachid R, Castells M, Cunningham‐Rundles C, Bonilla FA. Association of anti‐IgA antibodies with adverse reactions to gamma‐globulin infusion. J Allergy Clin Immunol. 2011;128:228–30 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gharib A, Caperton C, Gupta S. Anaphylaxis to IGIV in immunoglobulin‐naive common variable immunodeficiency patient in the absence of IgG anti‐IgA antibodies: successful administration of low IgA‐containing immunoglobulin. Allergy Asthma Clin Immunol. 2016;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira A, Garcia Rodriguez MC, Fontan G. Follow‐up of anti‐IgA antibodies in primary immunodeficient patients treated with gamma‐globulin. Vox Sang. 1989;56:218–22. [DOI] [PubMed] [Google Scholar]
- 16. Burks AW, Sampson HA, Buckley RH. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia. Detection of IgE antibodies to IgA. N Engl J Med. 1986;314:560–4. [DOI] [PubMed] [Google Scholar]
- 17. Premier Applied Sciences® PI . Premier Healthcare Database White Paper: Data that Informs and Performs. Premier Applied Sciences®, Premier Inc; 2020. Available from: https://learn.premierinc.com/white‐papers/premier‐healthcare‐database‐whitepaper. Accessed 8 Jan 2021. [Google Scholar]
- 18. Wallenhorst C, Patel A, Shebl A, Hubsch A, Simon TL, Martinez C. Anti‐A/B isoagglutinin reduction in an intravenous immunoglobulin product and risk of hemolytic anemia: a hospital‐based cohort study. Transfusion. 2020;60:1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371–6. [DOI] [PubMed] [Google Scholar]
- 20. Younger ME, Buckley RH. Immunoglobulin Therapy for Primary Immunodeficiency Diseases, 4th edn. IDF Guide for Nurses; 2016. Available from: https://primaryimmune.org/sites/default/files/publications/IDF‐Guide‐for‐Nurses‐4th‐Edition.pdf. Accessed 8 Jan 2021. [Google Scholar]
- 21. Brennan VM, Salome‐Bentley NJ, Chapel HM, Immunology NS. Prospective audit of adverse reactions occurring in 459 primary antibody‐deficient patients receiving intravenous immunoglobulin. Clin Exp Immunol. 2003;133:247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lieberman P, Camargo CA, Bohlke K, Jick H, Miller RL, Sheikh A, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97:596–602. [DOI] [PubMed] [Google Scholar]
- 23. Bonilla FA. Intravenous immunoglobulin: adverse reactions and management. J Allergy Clin Immunol. 2008;122:1238–9. [DOI] [PubMed] [Google Scholar]
- 24. Siani B, Willimann K, Wymann S, Marques AA, Widmer E. Isoagglutinin reduction in human immunoglobulin products by donor screening. Biol Ther. 2014;4:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerber S, Gaida A, Spiegl N, Wymann S, Antunes AM, Menyawi IE, et al. Reduction of isoagglutinin in intravenous immunoglobulin (IVIG) using blood group A‐ and B‐specific immunoaffinity chromatography: industry‐scale assessment. BioDrugs. 2016;30:441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wasserman RL, Garcia D, Greener BN, Kestenberg K, Pinkert A, Mond J, et al. Manufacturing process optimization of ADMA Biologics' intravenous immunoglobulin products, BIVIGAM((R)) and ASCENIV. Immunotherapy. 2019;11:1423–33. [DOI] [PubMed] [Google Scholar]
- 27. Immune Deficiency Foundation Treatment Experiences and Preferences of Patients with Primary Immune Deficiency Diseases: First National Survey. 2003. Available from: https://primaryimmune.org/sites/default/files/publications/Treatment‐Experiences‐and‐Preferences‐of‐Patients‐with‐Primary‐Immune‐Deficiency‐Disease‐First‐National‐Survey‐2002_1.pdf. Accessed 5 May 2021.
- 28. Rachid R, Bonilla FA. The role of anti‐IgA antibodies in causing adverse reactions to gamma globulin infusion in immunodeficient patients: a comprehensive review of the literature. J Allergy Clin Immunol. 2012;129:628–34. [DOI] [PubMed] [Google Scholar]
- 29. Harduar‐Morano L, Simon MR, Watkins S, Blackmore C. Algorithm for the diagnosis of anaphylaxis and its validation using population‐based data on emergency department visits for anaphylaxis in Florida. J Allergy Clin Immunol. 2010;126:98–104 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kroez M, Kanzy EJ, Gronski P, Dickneite G. Hypotension with intravenous immunoglobulin therapy: importance of pH and dimer formation. Biologicals. 2003;31:277–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from Premier Health Database. Restrictions apply to the availability of these data, which were used under license for this study.


