Abstract
The hippocampus plays an essential role in the formation and retrieval of episodic memories in humans and contextual memories in animals. However, amnesia is not always observed when this structure is compromised. To address this issue, we compared the effects of several different circuit manipulations on memory retrieval and hippocampal activity. Mice were first trained on context fear conditioning and then we used optogenetic and chemogenetic tools to alter activity during memory retrieval. We found that retrieval was only impaired when manipulations caused widespread changes (increases or decreases) in hippocampal activity. Widespread increases occurred when pyramidal cells were excited and widespread decreases were found when GABAergic neurons were stimulated. Direct hyperpolarization of excitatory neurons only moderately reduced activity and did not produce amnesia. Surprisingly, widespread decreases in hippocampal activity did not prevent retrieval if they occurred gradually prior to testing. This suggests that intact brain regions can express contextual memories if they are given adequate time to compensate for the loss of the hippocampus.
Keywords: Context fear, retrieval, memory, hippocampus, optogenetics, chemogenetics
1. INTRODUCTION
The hippocampus is important for encoding and retrieving episodic memories in humans and contextual memories in animals. However, whether or not amnesia is observed when this structure is damaged depends on many different factors. These include the age of the memory being tested, how often it was recalled, how vivid and detailed it is, as well as the extent of hippocampal damage (Casagrande et al., 2018; Frankland & Bontempi, 2005; Wiltgen & Tanaka, 2013; Winocur et al., 2010). For example, bilateral damage to the hippocampus produces profound amnesia in humans while unilateral damage has little to no effect on memory (Milner & Penfield, 1955; Scoville & Milner, 1957). This suggests that episodic memories can be encoded and retrieved by spared hippocampal tissue and do not require the entire structure to be functional. Other brain regions can also compensate when the hippocampus is damaged and make contributions to learning and memory. This is evidenced by the fact that amnesia is most severe when damage is found in surrounding cortical areas and in distal regions like the prefrontal cortex (Bayley et al., 2006; Rempel-Clower et al., 1996; Squire et al., 2004). These findings suggest that intact brain regions can work together to support learning and memory when damage to the hippocampus is restricted or incomplete.
Memory loss in animals also correlates with the amount of damage and/or dysfunction in the hippocampus (Li et al., 1999). For example, partial lesions of the rodent hippocampus produce less severe retrograde amnesia for context fear than complete lesions (Anagnostaras et al., 2001; Sutherland et al., 2010). Pharmacological inactivation, which typically affects less hippocampal tissue, produces even milder deficits (Holt & Maren, 1999; Maren & Holt, 2004; Resstel et al., 2008) but see (Matus-Amat et al., 2004; Raybuck & Lattal, 2014). Lesions may be particularly disruptive because they cause significant damage to distal structures (Jarrard, 2002). In fact, some studies have reported a 40–50% reduction in cortical volume following excitotoxic lesions of the hippocampus (Anagnostaras et al., 2002; Logue et al., 1997). Therefore, similar to episodic memory in humans, robust amnesia for context fear appears to require widespread changes in hippocampal activity that prevent spared tissue, and other brain regions, from compensating.
Given that lesions and pharmacological manipulations lack temporal precision, we used optogenentic and chemogenetic tools to manipulate hippocampal activity during memory retrieval. Widespread inactivation was produced when GABAergic neurons were stimulated with ChR2 while limited silencing occurred when pyramidal cells were directly hyperpolarized (Babl et al., 2019; Daie et al., 2019). We found that memory retrieval for context fear was significantly impaired by the former but not the latter. These results are consistent with the idea that amnesia requires extensive changes in hippocampal function. Unexpectedly, some inhibitory manipulations led to increases in excitatory activity. Effects like these have been reported in other studies and are thought to be caused, in part, by reduced activation of inhibitory neurons (López et al., 2016; Stefanelli et al., 2016). When we observed increases in activity, they typically spread throughout the hippocampus and caused severe memory impairments.
Activation of GABAergic neurons with excitatory DREADDs led to widespread increases in inhibitory activity, but unlike optogenetic stimulation, did not impair memory retrieval. We hypothesize that this is due to the temporal differences between optogenetic and chemogenetic silencing methods. The former produces an immediate decrease in excitation while the latter can take tens of minutes (Ryan et al., 2015; Zhu et al., 2014). It is possible that longer delays between hippocampal inactivation and testing gives spared tissue enough time to compensate and retrieve memory (Goshen et al., 2011).
2. METHODS
2.1. LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Brian Wiltgen (bjwiltgen@ucdavis.edu). This study did not generate any unique reagents.
2.2. EXPERIMENTAL MODEL AND SUBJECT DETAILS
Subjects:
All procedures were approved by the Animal Care and Use Committee at UC Davis. Mice were maintained on a 12/12-light/dark schedule and were given free access to food and water. Animals were group-housed until surgery, after which they were single-housed for the remainder of each experiment. Littermates of the same sex were randomly assigned to experimental groups. Surgeries were performed at 8 weeks of age, with behavioral experiments beginning at approximately 10–12 weeks of age. Both C57/B6J and F1 hybrids (C57/B6J:129SvEv) were used for these experiments, as described below.
2.3. METHOD DETAILS
2.3.1. Stereotaxic Surgeries.
All viruses were infused into the dorsal hippocampus (dHPC) (−2.0mm AP; +/−1.5mm ML; −1.25mm DV) using a MicroSyringe pump controller at 2nL/second. For DREADD experiments, animals received 1μl bilateral infusions into the dHPC of AAV5 CaMKII-hM4Di-IRES-mCitrine (2.7×10^12 virus molecules/mL) (UNC Vector Core), AAV5 hSyn-hM4Di-IRES-mCitrine (1.12×10^12 virus molecules/mL) (UNC Vector Core), AAV5 CaMKII-hM3Dq-IRES-mCitrine (3.75×10^14 or 3.75×10^13 virus molecules/mL) (Addgene plasmid #50466, a gift from Bryan Roth, assembled and packaged with AAV5 helper plasmid by the UC Davis Molecular Construct and Packaging core facility. The virus was gradient purified, checked by SDS-PAGE and titered using qRT-PCR by standard methods (Flannery & Visel, 2013), or AAV5-hDlx-GqDREADD-dTomato (3.15×10^15 virus molecules/mL) (Addgene plasmid # 83897, a gift from Gordon Fishell, assembled and packaged with AAV5 helper plasmid by the UC Davis Molecular Construct and Packaging core facility. The virus was gradient purified, checked by SDS-PAGE and titered using qRT-PCR by standard methods (Flannery & Visel, 2013). Dlx-enhanced viruses expressed red fluorophores (dTomato/mCherry) instead of green (mCitrine/eGFP/eYFP) due to commercial availability. For channelrhodopsin optogenetic experiments, animals received 0.25μl bilateral infusions of AAV9 CaMKII-ChR2-eGFP (8.96×10^11 virus molecules/mL) (Penn) or AAV9 CaMKII-eGFP (3.49×10^11virus molecules/mL) (Penn). For all other optogenetic experiments, animals received 0.5 μl bilateral infusions of AAV5 CaMKII-ArchT-eGFP (5.2×10^11 virus molecules/mL)(UNC), AAV5 CaMKII-eYFP (5.3×10^12 virus molecules/mL)(UNC), AAV9 CaMKII-NpHR3.0-eYFP (2.5×10^13 virus molecules/mL)(Penn), AAV9 CaMKII-eGFP (3.45×10^12 virus molecules/mL)(Penn) or AAV5-hDlx-ChR2-mCherry (7.6 ×10^14 virus molecules/mL) (Addgene plasmid # 83898, a gift from Gordon Fishell, assembled and packaged with AAV5 helper plasmid by the UC Davis Molecular Construct and Packaging core facility. The virus was gradient purified, checked by SDS-PAGE and titered using qRT-PCR by standard methods (Flannery & Visel, 2013).
Following virus infusion, the injection needle remained in place for five minutes. The scalp of DREADD experimental animals was closed with surgical glue (Vetbond). For optogenetic experiments, fibers were implanted into the dHPC. Optic fibers were constructed and polished as previously described (Sparta et al., 2011). Briefly, a 200um diameter optic fiber (Thorlabs) was stripped and inserted into a plastic ferrule (PlasticOne). The convex side of ferrule was polished and the optical fiber scored with a ruby knife to extend 1.2 mm from the tip. Fibers were implanted into the dorsal hippocampus (−2.0mmAP; +/− 1.5mm ML; −1.1mm DV). Following implantation, the skull was scored with a surgical blade and covered in C&B Metabond (Parkell). A dental acrylic headcap (Harry J. Bosworth Company) was constructed to hold the fibers in place and seal the incision. Animals recovered for two weeks prior to the beginning of behavioral experiments to allow for recovery and sufficient receptor/opsin expression.
2.3.2. Behavioral Apparatus:
The contextual fear conditioning equipment used in all experiments was previously described (Tanaka et al., 2014; Tayler et al., 2013). Briefly, mice were trained and tested in conditioning chambers (30.5 cm x 24.1 cm x 21.0 cm) located within sound-attenuated boxes. The chambers consisted of a stainless-steel grid floor with overhead LED lighting (providing broad spectrum light). Sessions were recorded with a scanning charge-coupled device video camera (Med Associates). The chamber and drop pan were cleaned with 70% ethanol before each behavioral session. Detailed training procedures are provided below for specific optogenetic and DREADD experiments. Memory was assessed the following day by placing the mice in the context and measuring the freezing response. Freezing measurements were made using the automated Video Freeze System (Med Associates) as previously described (Stephan G. Anagnostaras et al., 2010).
DREADD Behavioral Procedures:
C57/B6J:129SvEv mice were ordered or bred in-house (Taconic). 8 groups were used in these experiments: CaMKII-hM4Di-CNO (3m, 3f); CaMKII-hM4Di-Vehicle (3m, 3f), hSyn-hM4Di-CNO (3m, 3f), Syn1-hm4Di-Vehicle (3m, 3f), CaMKII-hM3Dq-CNO (5m), CaMKII-hM3Dq-Vehicle (5m), Dlx-GqDREADD-CNO (7m, 3f), Dlx-GqDREADD-Vehicle (6m, 3f). After recovery, animals were handled for 5 minutes a day for five days prior to contextual fear conditioning for habituation to the experimenter. During training, following a three-minute baseline period, animals were given three two-second 0.4mA shocks with 60 second inter-trial intervals. The following day, animals were given IP injections of either 0.5 (CaMKII-hM3Dq) or 5mg/kg (all other groups) CNO (Toronto Research Chemicals) (10mg dissolved in 100μL DMSO, diluted to 0.1 or 1mg/mL CNO in 0.9% saline) or vehicle (1% DMSO in 0.9% saline), one hour prior to re-exposure to the fear conditioning chamber. A 0.5mg/kg CNO concentration was used for the CaMKII-hM3Dq group as pilot experiments with 5mg/kg produced seizures (data not shown). Animals were tested in the absence of shock for 30 minutes and freezing behavior was analyzed during the first 12 minutes of the session. Only the first 12-minutes were analyzed so the data could be compared with the optogenetic retrieval experiments, which were 12-minutes in duration.
2.3.3. Optogenetic Behavioral Procedures:
C57/B6J: mice used for these experiments were ordered or bred in-house (Taconic). Nine groups were used for these experiments: CaMKII-NpHr3.0 (3m, 3f), CaMKII-eGFP (3m, 3f), CaMKII-ArchT (3m, 3f), CaMKII-eYFP (3m, 3f), CaMKII-ChR2 20Hz (3m, 2f), CaMKII-ChR2 4Hz (4f, 1m), (CaMKII-eGFP (3m, 2f), Dlx-ChR2 (8f, 9m). Animals were handled for 2–5 minutes a day for five days prior to contextual fear conditioning. During handling, animals used for these studies were also connected to the optic fiber cable. Animals were placed in a Med Associates fear conditioning chamber with fiber implants connected to a splitting 200um optic fiber (Doric lenses). The fiber was attached to the conditioning chamber through a rotating commutator (Doric lenses) and coupled to a 473/532/561 nm 200 mW solid-state laser diode (OEM laser systems) with 15 mW output. Prior to each experiment, laser output intensity was measured with an optical power meter (Thorlabs). Stereotaxic coordinates were used to place optic fibers directly above dorsal CA1, directing laser stimulation to this region. Following a three-minute baseline period, animals were given four 2s 0.75mA shocks with 60-second inter-trial intervals. The following day, animals were returned to the chamber for a 12-minute testing session. Using Doric studio to trigger the laser, mice underwent laser stimulation twice for 3 minutes over the testing session. The session began with a 3-minute baseline period, then the laser (473nm (ChR2), 532nm (ArchT), 561nm (NpHR3.0), was turned on for 3 min (20Hz, 15ms pulse width: ChR2; continuous stimulation NpHR3.0/ArchT) at 10 or 15mW. This was repeated once. Dlx-ChR2 animals underwent an additional testing session 24 hours later consisting of 12 minutes of laser stimulation.
2.3.4. Tissue Collection and Immunohistochemistry.
Ninety minutes following re-exposure to the chamber (DREADD experiments) or first laser on epoch (optogenetic experiments), animals were perfused using ice-cold PBS and 4% paraformeldahyde (PFA). Brain tissue was collected and stored overnight in 4% PFA. Slices were taken at 40nm using a Leica Vibratome. Tissue was stored in slice storage solution (100mL 10x tris-buffered saline, 300mL ethylene glycol, 300mL glycerol, 300mL dH2O) at −20°C until staining. Slices were washed 3 × 5 minutes in PBS, stained overnight in rabbit anti-cFos (1:5000, Millipore ABE457) in donkey blocking buffer. Slices were washed in 3 × 5 minutes in PBS, counterstained in biotynilated donkey anti-rabbit (Jackson) (1:500) for 1 hour, washed 3 × 5 minutes in PBS, stained with Cy3/Cy5 (Jackson) (1:500) for 45 minutes, washed in 3 × 5 minutes PBS and counterstained for 10 minutes with a DAPI nuclear stain (1:10000). Slices were mounted on SuperFrost slides with VectaShield mounting media.
2.3.5. Microscopy and Cell Counting.
Three to four coronal sections surrounding the −2.0 AP coordinate of each mouse were selected for c-Fos quantification. For optogenetic experiments, tissue was selected from beneath the fiber tip. Tissue slices were scanned in a 35um z-stack using an Olympus Slide Scanner at 20x magnification. Exposure times per channel (DAPI, FITC, TRITC) for each animal were set under saturation. Images were further cropped to the intermediate dCA1 for analysis. Fluorescent images were imported into ImageJ in grayscale and separated by channel. Cell counts were obtained using the multipoint tool on ImageJ with the experimenter blind to groups. An estimate of total dorsal CA1 cells per section was calculated by using 3D Object Counter in ImageJ and dividing the obtained volume by an average single nucleus volume for the group. Approximately 500 cells were counted from each slice, and 6 hemispheres were counted from each animal (~ 3,000 cells). The percentage of c-Fos+ neurons was calculated for each animal and then normalized to the mean of the appropriate control group (individual percent c-Fos+/control group percent c-Fos+). For Dlx experiments, we calculated both the percentage of c-Fos+ pyramidal cells and Dlx+ inhibitory neurons in each animal. These values were normalized to the appropriate control group. To estimate the degree of inhibition produced in the Dlx experiments, we quantified the number of c-Fos+ cells in the stratum oriens, stratum radiatum and stratum lacunosum-moleculare at 3 different AP coordinates (anterior (−1.2 to −1.5 AP), intermediate (−2.5 to −2.8 AP) and posterior (−3.2. to −3.5 AP). Anterior to posterior Dlx+ cell quantification was performed in single-plane images, and cell counts shown as number of c-Fos+ cells per ~.45mm2.
2.4. QUANTIFICATION AND STATISTICAL ANALYSIS
Freezing data during memory testing were analyzed using repeated measures ANOVA with the p value set to 0.05. Post-hoc comparisons were made using Fisher’s LSD. Cell counts were normalized to control groups and analyzed using a t-test with the p value set to 0.05. All data are represented as mean +/− SEM. Statistical details can be found within the results section.
2.4.1. Excluded data:
For the optogenetic experiments, subjects with incorrect fiber placements were excluded from all analyses (1 animal from CaMKII-NpHR3.0 and CaMKII-ArchT experimental groups). Brain tissue that was damaged during extraction and/or slicing was excluded from c-Fos analyses (1 CaMKII-hM4Di (CNO); 2 CaMKII-eGFP (NpHR control group); 1 CaMKII-eGFP (ChR2 control group); 1 CaMKII-eYFP (ArchT control group). Four laser-on animals in the Dlx-ChR2 experiment did not express any c-Fos in inhibitory interneurons (indicating that stimulation did not work) and were excluded. One subject was excluded from the Dlx-ChR2 control group because the optic fiber caused significant damage to the hippocampus.
2.4.2. Figures:
Training/testing paradigm images (Figures 2–3) were created using BioRender. All data were plotted and analyzed using GraphPad Prism 8.
Figure 2. Acute activation of inhibitory neurons.
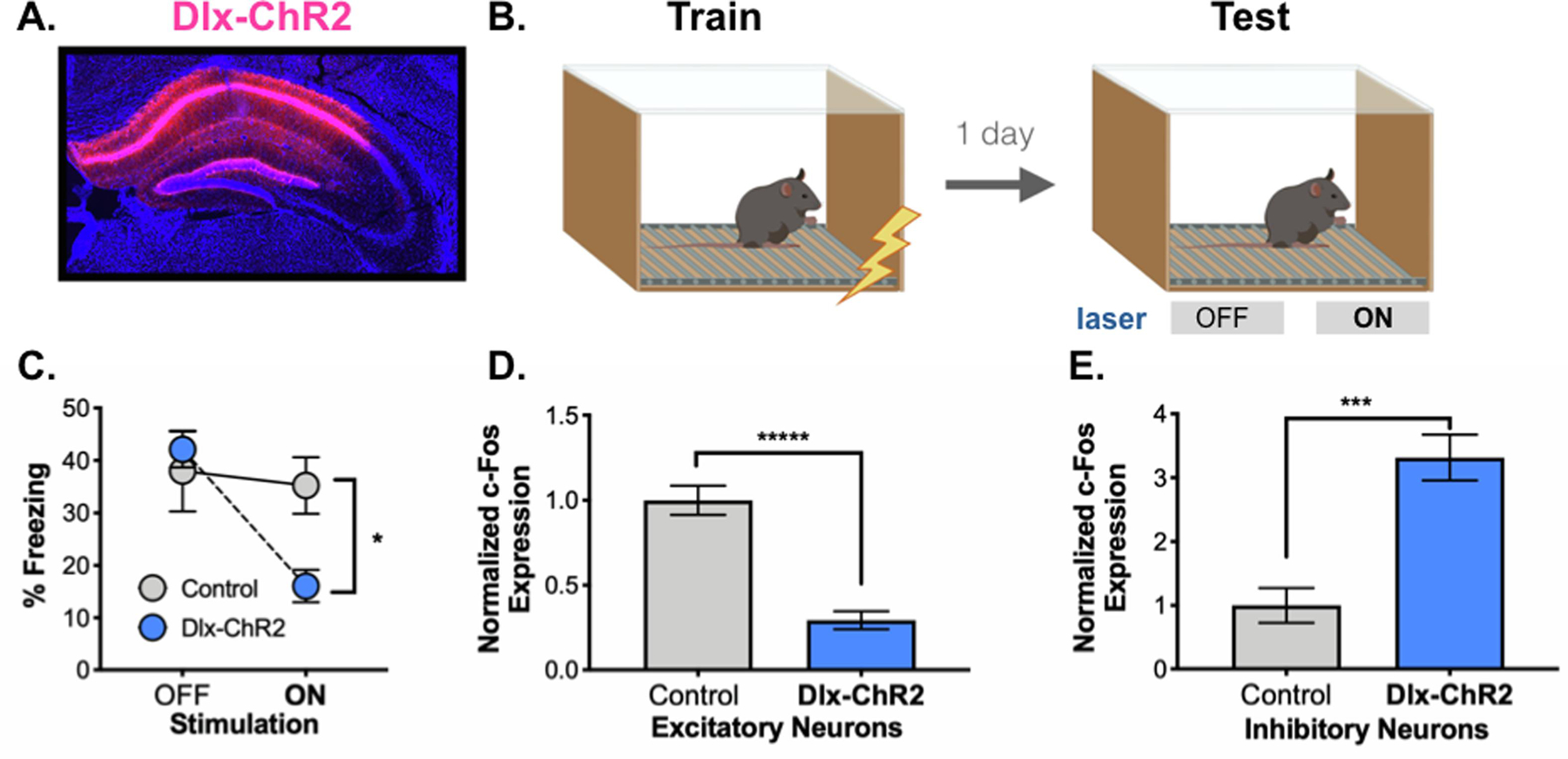
A. Dlx-ChR2 expression (pink) in the dorsal hippocampus. B. Behavioral paradigm. Dlx-ChR2 animals were trained in contextual fear conditioning (4 x .75mA shocks). One day later animals were returned to the testing chamber for a 12-minute test. Animals received a 3-minute baseline, then 3 minutes of blue laser (473nm, 20Hz, 10mW) stimulation. This was repeated once. C. Dlx-ChR2 animals (blue) froze less during Laser ON periods than controls (gray). D. c-Fos expression was lower in excitatory pyramidal neurons in dCA1 of Dlx-ChR2 (blue) vs. control (gray) animals. E. c-Fos expression was higher in inhibitory neurons in dCA1 of Dlx-ChR2 (blue) vs. control (gray) animals. All data are expressed as mean +/− SEM.
Figure 3. Prolonged activation of inhibitory neurons.
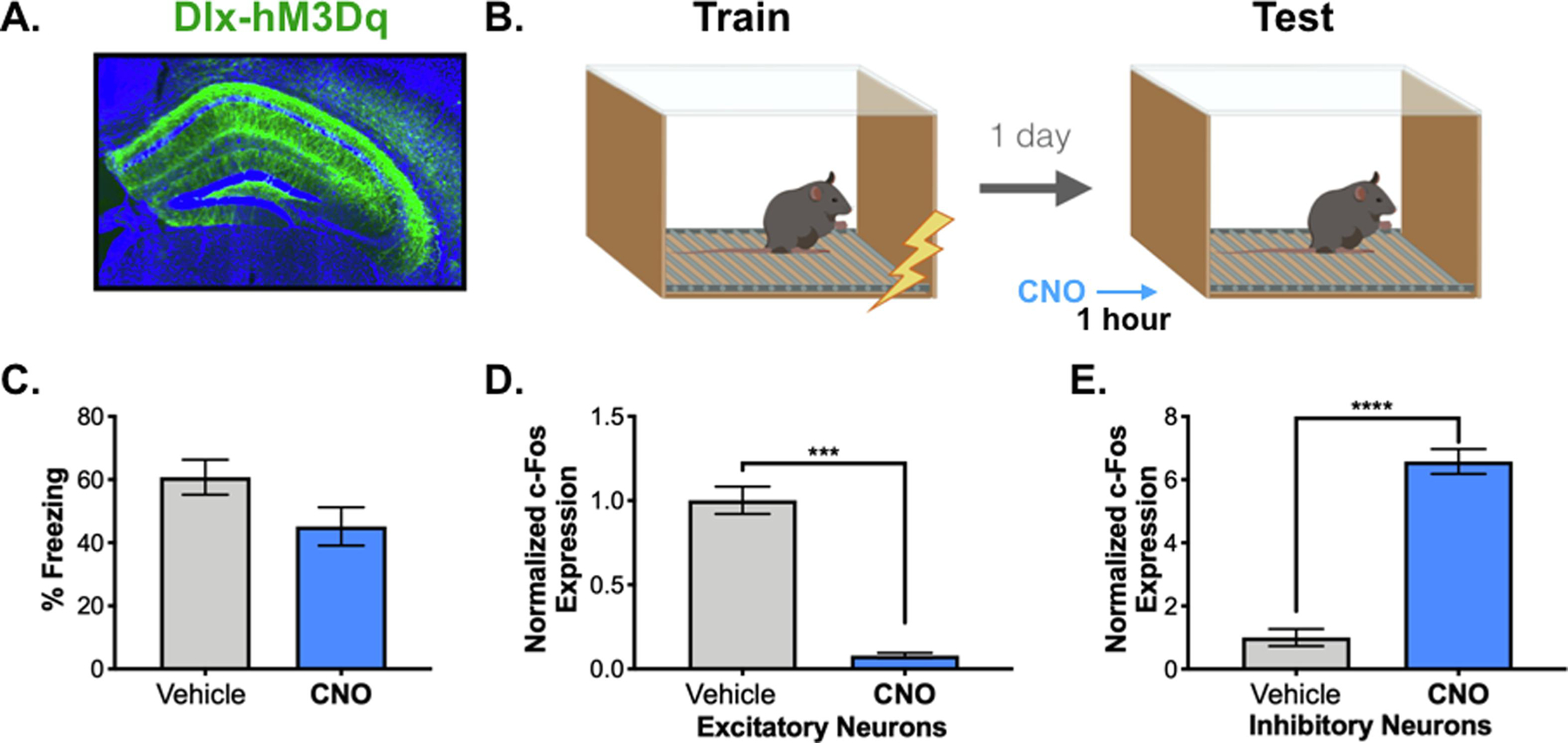
A. Dlx-hM3Dq expression (green, HA tag) in the dorsal hippocampus. B. Behavioral paradigm. Dlx-hM3Dq animals were trained in contextual fear conditioning (3 x .4mA shocks). One day later, animals were returned to the chamber for a 12-minute memory test following a 5mg/kg CNO or vehicle IP injection. C. There was no difference in freezing over the 12-minute testing period between the vehicle (gray) and CNO-treated (blue) animals. D. c-Fos expression was lower in excitatory pyramidal neurons in dCA1 of CNO-treated (blue) vs. vehicle-treated (gray) animals. E. c-Fos expression was increased in inhibitory neurons in dCA1 of CNO-treated (blue) vs. vehicle-treated (gray) animals. All data are expressed as mean +/− SEM.
2.5. DATA AND CODE AVAILABILITY
This study did not generate/analyze datasets/code. Original/source data for figures in the paper is available by request.
3. RESULTS
3.1. Acute activation of inhibitory neurons impairs memory retrieval for context fear
To examine the relationship between neural activity and memory retrieval, we trained mice on a context fear conditioning task that requires the hippocampus (Tanaka et al., 2014; Wiltgen et al., 2010). We targeted the dorsal segment of this structure because damage to this area produces reliable amnesia for context fear 1-day after learning (Anagnostaras et al., 1999; Frankland et al., 1998; Kim & Fanselow, 1992; Maren et al., 1997).
Widespread inactivation of the dorsal hippocampus was achieved by stimulating inhibitory neurons with ChR2 or hM3D. These excitatory proteins were selectively expressed in GABAergic neurons using the Dlx enhancer (Dimidschstein et al., 2016) (Figures 1A–1C). In the first experiment, mice received infusions of AAV-Dlx-ChR2 and then underwent context fear conditioning 2-weeks later (Figure 2A). Animals were returned to the conditioning chamber the next day for a long-term memory test (Figure 2B). After a 3-minute baseline period, inhibitory neurons were stimulated with ChR2 (473nm, 20Hz, 10mW, 180s). Stimulation occurred a second time 3-minutes later. Dlx-ChR2 mice (n = 10) froze significantly less during the laser stimulation periods than control animals (n = 6) (Figure 2C) (Group x laser interaction F (1,15), p =.0012, Fisher’s LSD post-hoc tests, Laser off (p =.54), Laser on (p = .0064)).
Figure 1. Expression of ChR2 and hM3Dq in inhibitory neurons.
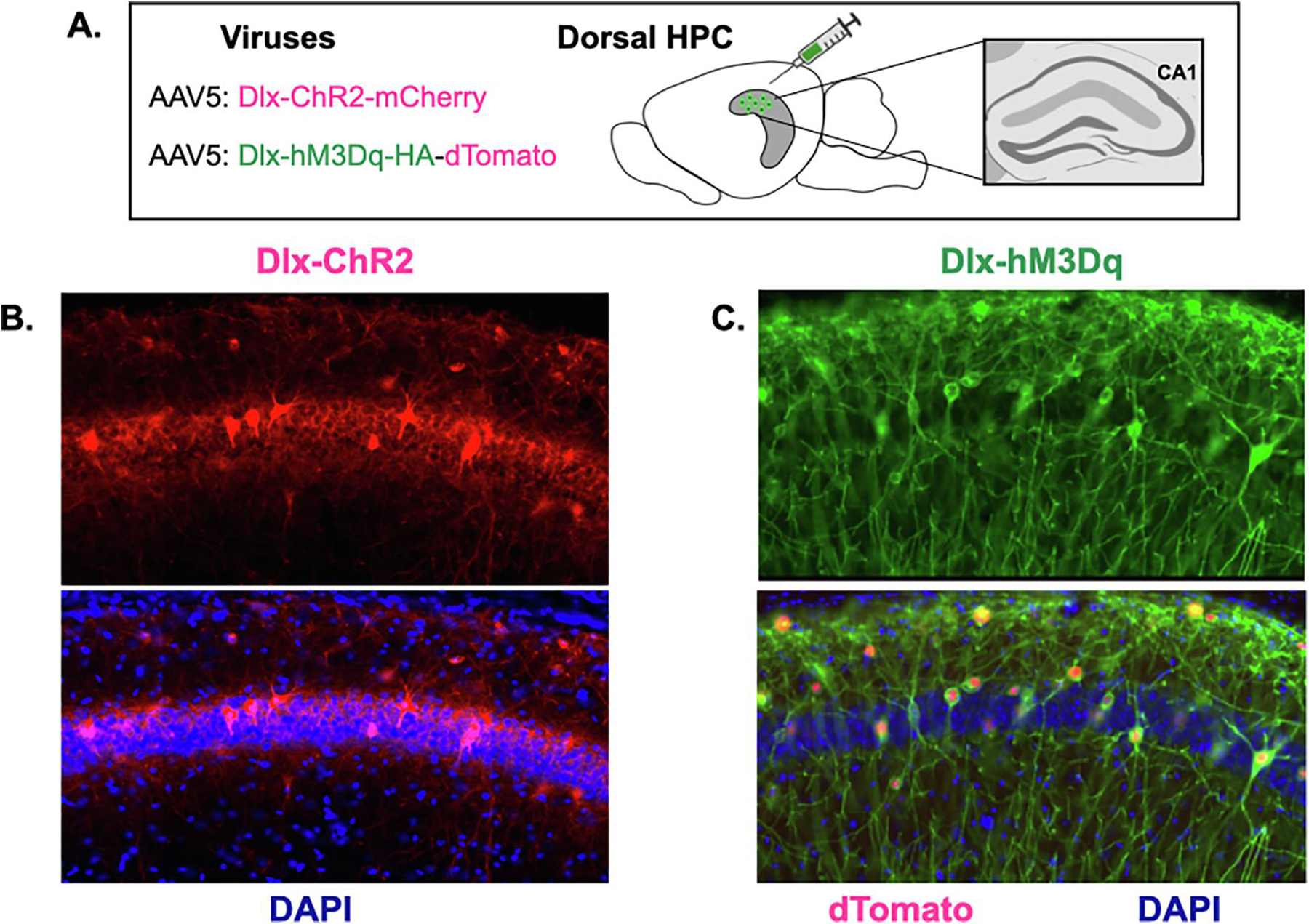
A. Dlx-ChR2-mCherry or Dlx-hM3Dq-dTomato were expressed in CA1 of the dorsal hippocampus (image adapted from (Moser & Moser, 1998). B. Left top: Dlx-ChR2 expression (red) in dorsal CA1. Left bottom: Dlx-ChR2 expression merged with DAPI (blue) in dorsal CA1. C. Right top: Dlx-hM3Dq HA-tag expression (green) in dorsal CA1. Right bottom: Dlx-hM3Dq HA-tag expression (green) and Dlx-hM3Dq dTomato nuclear expression (red) merged with DAPI (blue) in dorsal CA1.
To verify that laser stimulation inhibited pyramidal cells and activated interneurons, we quantified c-Fos expression in CA1. This was done after a second context test, during which, ChR2 was stimulated throughout the session (i.e. no laser off periods). Compared to control animals, c-Fos expression was decreased in pyramidal neurons (Figure 2D) (t=7.366, df = 13, p < 0.0001) and increased in inhibitory neurons in Dlx-ChR2 mice (Figure 2E) (t=4.226, df=13, p = .0010). These data indicate that acute stimulation of inhibitory neurons reduces excitation in the dorsal hippocampus and impairs memory retrieval for context fear.
In the next experiment, mice received infusions of AAV-Dlx-hM3Dq and were trained on context fear conditioning 2-weeks later (Figure 3A). Injections of 5mg/kg CNO (n=10) or a vehicle solution (n=9) were administered one hour before testing (Figure 3B). Pilot experiments found that this concentration did not affect context fear in wild-type animals (Data not shown, No effect of group F (1,8) = 1.274, p = .2917). Stimulation of inhibitory neurons with CNO did not affect memory retrieval in Dlx-hM3Dq mice compared to controls (Figure 3C) (No effect of group F (1,17) = 1.931, p =.1826). This was despite the fact that CNO decreased c-Fos expression in pyramidal neurons (Figure 3D) (t=11.68, df=17, p<0.0001) and increased expression in inhibitory cells (Figure 3E) (t=11.44, df=17, p<0.0001).
To estimate the spatial extent of inhibitory activity produced by stimulation of ChR2 and hM3Dq, we quantified c-Fos expression in GABAergic neurons at 3 different anteroposterior (AP) coordinates that spanned most of the hippocampus (ChR2 n=5, control n=5; hM3Dq CNO n=4, vehicle n=4). Quantification was restricted to SO, SR and SLM cell layers in CA1 where inhibitory cells could be easily identified and distinguished from pyramidal neurons located in SP (Figure 4A). When ChR2 was stimulated, we observed increased c-Fos expression in GABAergic neurons at multiple sites within the dorsal (anterior and intermediate) but not ventral hippocampus (posterior) (Figure 4B) (Group x AP coordinate interaction, F (2,16) = 6.12, p = .0106), Fisher’s LSD post-hoc tests, Anterior (p = .042), Intermediate (p = .0048), Posterior (p = .3294)). Based on these data, we estimate that optogenetic stimulation of GABAergic neurons produces inhibitory activity that extends at least 1mm AP from the tip of the optic fiber (Babl et al., 2019; N. Li et al., 2019).
Figure 4. Widespread activation of inhibitory neurons in the dorsal hippocampus.
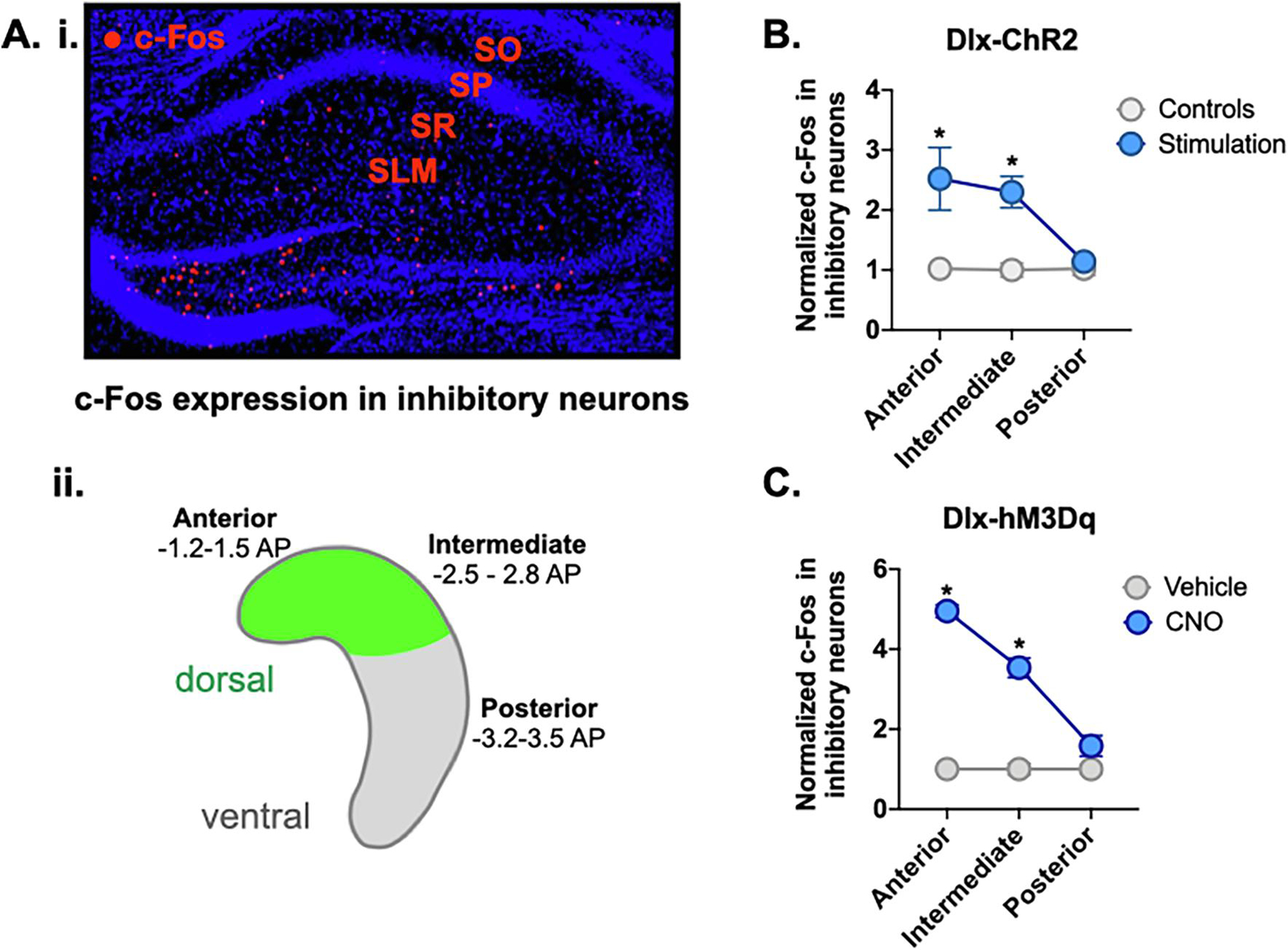
A. i. Anterior dHPC of representative CNO-treated Dlx-hM3Dq animal showing c-Fos expression (red) in inhibitory strata: stratum oriens (SO), stratum radiatum (SR), stratum lacunosum-moleculare (SLM). A. ii. Schematic of HPC showing location of virus expression (green) in dorsal HPC. Representative slices were taken for c-Fos analysis from anterior (−1.2 to −1.5 AP), intermediate (−2.5 to −2.8 AP) and posterior/ventral HPC (−3.2 to −3.5 AP). B. c-Fos expression in inhibitory neurons of anterior and intermediate HPC was increased in Dlx-ChR2 laser-stimulated animals (blue). There was no difference in c-Fos expression in posterior HPC. C. c-Fos expression in inhibitory neurons of anterior and intermediate hippocampus was increased in Dlx-hM3Dq CNO-treated animals (blue). There was no difference in c-Fos expression in posterior HPC. All data are expressed as mean +/− SEM.
When GABAergic neurons were stimulated with hM3Dq, we found that the spatial extent of inhibitory activity was similar to that observed with ChR2 (Figure 4C) (Group x AP coordinate interaction, F (2,12) = 42.2, p = <.0001; Fisher’s LSD post-hoc tests, Anterior (p <.0001), Intermediate (p=.0003), Posterior (p=.1060)). This suggests that the retrieval deficit caused by ChR2 stimulation did not result from more extensive inhibition of the dorsal hippocampus. We hypothesize that the prolonged stimulation period required to activate hM3Dq (tens of minutes) may have given other brain regions time to compensate prior to testing.
3.2. Both acute and prolonged stimulation of excitatory neurons impairs memory retrieval
Amnesia can also be produced by widespread increases in hippocampal activity. Patients with temporal lobe epilepsy, for example, are significantly impaired at forming and retrieving autobiographical memories from their lives (Viskontas et al., 2000; Zhao et al., 2014). Rodent models of epilepsy produce profound amnesia for spatial and contextual memories as do other manipulations that lead to large increases in hippocampal excitability (Butler et al., 1995; Palop et al., 2007). The current experiments examined the effects of increasing hippocampal activity on memory retrieval by stimulating pyramidal neurons with excitatory opsins or DREADDs (Figure 5A).
Figure 5. Prolonged vs. acute activation of excitatory neurons.
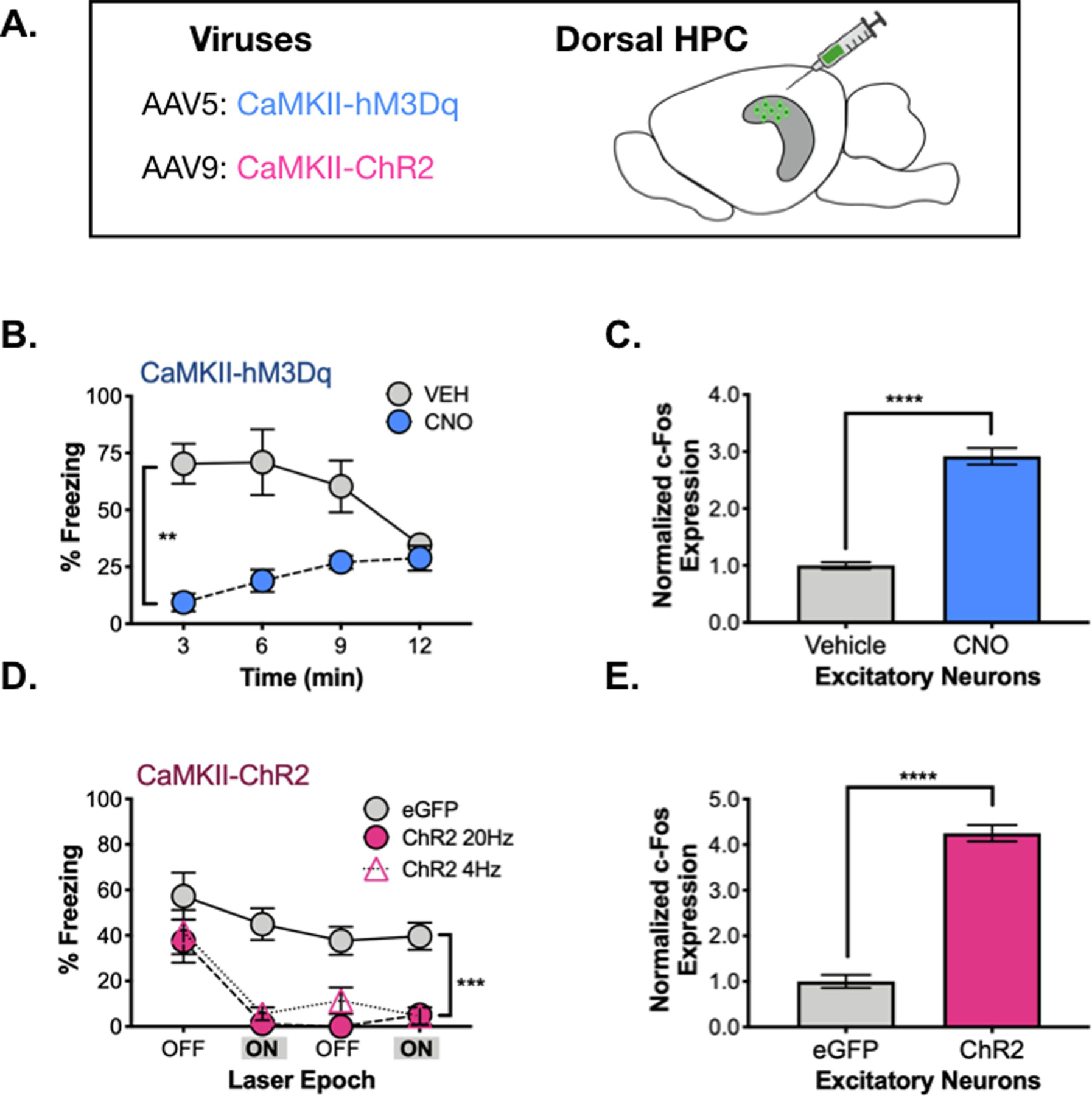
A. CaMKII-ChR2 or CaMKII-hM3Dq were infused into dHPC. B. During memory testing, hM3Dq CNO-treated animals (pink) froze less than their vehicle-treated counterparts (gray). C. c-Fos expression in excitatory neurons of dCA1 was elevated in hM3Dq CNO-treated animals (pink) compared to their vehicle-treated counterparts (gray). D. During memory testing, ChR2 (20Hz – pink circles, 4Hz – pink triangles) animals and eGFP animals (gray) did not differ during the first 3-minute laser OFF epoch. Following laser stimulation, 20 and 4Hz stimulated animals froze less than controls over the remainder of the testing period. E. c-Fos expression was elevated in excitatory neurons of dCA1 in ChR2 20Hz-stimulated animals (pink) compared to controls (gray). All data are represented as mean +/− SEM.
First, AAV containing hM3Dq was infused into the dorsal hippocampus and expressed in excitatory neurons using the CaMKII promoter. Two weeks later, mice were trained on context fear conditioning and memory was tested the following day. One hour before testing, animals received an IP injection of CNO (0.5mg/kg) (n=5) or vehicle solution (n=5) (same behavioral design as Figure 3A). Compared to controls, stimulation of pyramidal neurons with hM3Dq produced profound impairments in memory retrieval (Figure 5B) (Significant group x time interaction (F (3, 24) = 9.39, p = .0003; Main effect of group, F (1, 8) = 17.9, p = 0.003). To confirm that stimulation of hM3Dq increased excitatory activity during the context test, we quantified c-Fos expression in CA1 pyramidal neurons (Figure 5C). As expected, CNO injections produces a large increase in c-Fos labeling compared to the vehicle-treated group (t (8) = 12.13, p < 0.0001).
In order to acutely activate pyramidal neurons, we expressed ChR2 under control of the CaMKII promoter (Figure 5A). Two-weeks later, mice were trained on context fear conditioning and received a memory test the following day. After a 3-minute baseline period, pyramidal neurons in CA1 were stimulated with ChR2 (473nm, 10mW, 180s). Stimulation occurred a second time 3-minutes later (same behavioral procedure as Figure 2A). Excitatory neurons were stimulated at 4Hz (n = 6) or 20Hz (n = 5) because low frequency stimulation has been shown to induce freezing in CA1 “engram cells” (Roy et al., 2019; Ryan et al., 2015). However, we did not observe a difference between these frequencies, so the data were combined for statistical analyses. When pyramidal neurons were stimulated after the baseline period, freezing levels decreased significantly in CaMKII-ChR2 mice relative to CaMKII-eGFP animals (Figure 5C) (Stimulation period x Group interaction F (1, 15) = 5.938, p = .0278), Fisher’s LSD post-hoc tests, ChR2 (p < .0001), eGFP (p = .1386). Freezing did not recover in CaMKII-ChR2 mice after the laser turned off and remained low for the remainder of the session (Main effect of group F (1, 15) = 36.45, p < .0001, No effect of stimulation period F (1, 15) = .024, p = .8786, No group x stimulation period interaction F (1, 15) = .608, p = .4476). Quantification of c-Fos revealed that ChR2 stimulation increased expression in CA1 pyramidal neurons as expected (t (8) = 14.23, p<0.0001) (Figure 5D). These data indicate that non-selective increases in excitatory activity lead to severe memory deficits in context fear.
3.3. Direct hyperpolarization of excitatory neurons has variable effects on memory retrieval
In the next set of experiments, we inhibited excitatory neurons directly and examined the effects on memory retrieval. To do this, we expressed inhibitory DREADDs or opsins in CA1 pyramidal neurons using the CaMKII promoter (Figure 6A). For the optogenetic manipulations, mice were trained and tested as in our other experiments, except that yellow light was used to stimulate NpHR (561nM, 15mW) (n = 6) and green light was used for ArchT (532nM, 10mW) (n = 6). In the DREADD experiment, animals received an IP injection of 5mg/kg CNO (n=7) or vehicle (n=6) one hour before the context test. We used the same behavioral procedures described in Figures 2A/3A.
Figure 6. Prolonged vs. acute inhibition of excitatory neurons.
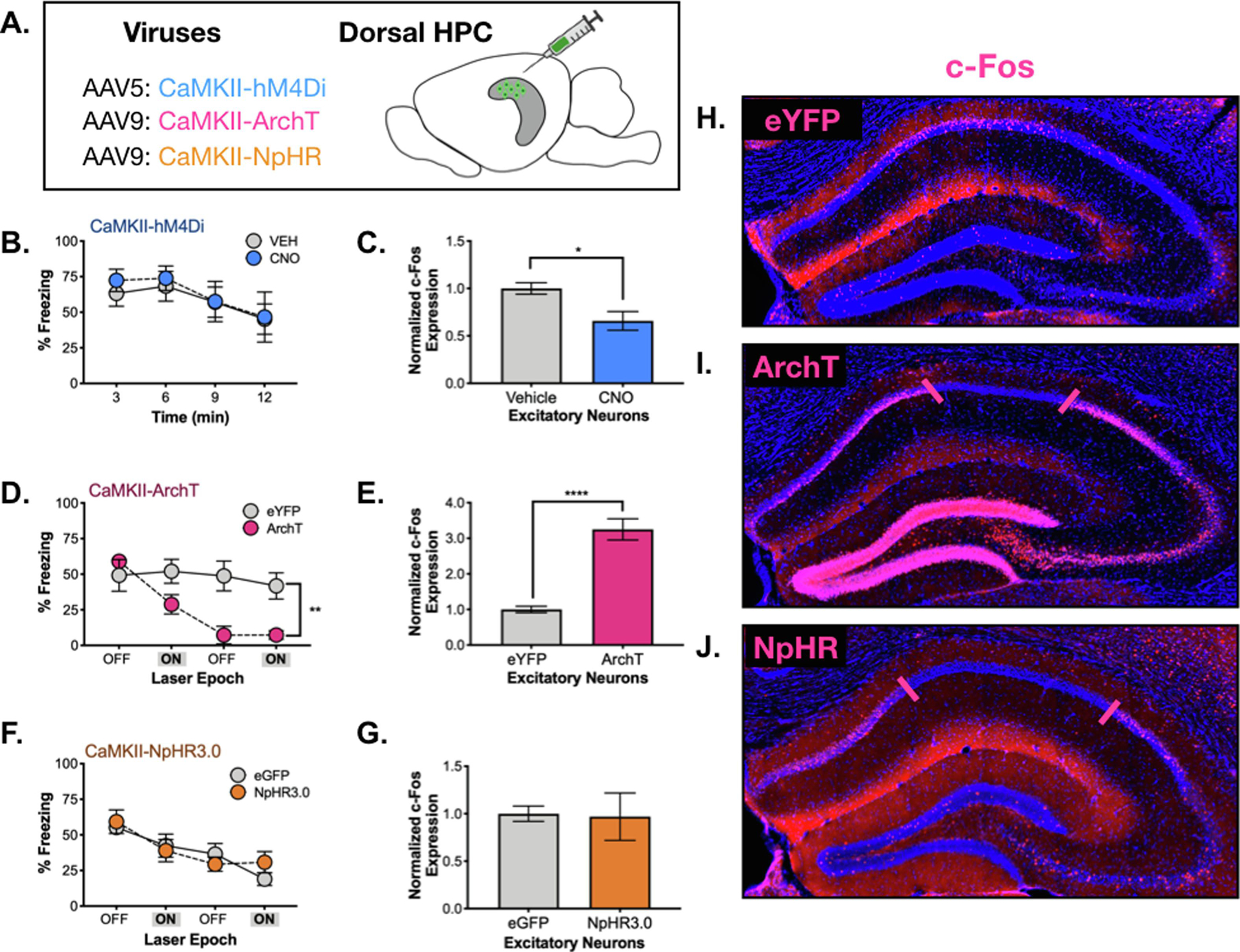
A. CaMKII-hM4Di, CaMKII-ArchT or CaMKII-NpHR were infused into the dHPC in order to silence excitatory neurons. B. There was no difference in freezing during testing between hM4Di CNO (blue) and vehicle-treated (gray) animals. C. There was a small, but significant decrease in dCA1 c-Fos expression in CNO-treated hM4Di expressing animals (blue). D. There was no difference in freezing during the initial 3-minute laser OFF period between ArchT (orange) and eYFP (gray) expressing animals. Following the first laser ON epoch, ArchT animals froze less than controls over the remainder of the testing period. E. There was a significant increase in dCA1 c-Fos expression in ArchT animals (orange) compared to controls. F. There was no difference in freezing during laser OFF or ON epochs in NpHR vs. control animals. G. There was no difference in dCA1 c-Fos expression between NpHR and control animals. H. c-Fos expression (pink) in dHPC of a control animal (CaMKII-eYFP). I. c-Fos expression (pink) in an ArchT-expressing animal. There is no c-Fos expression underneath the laser (denoted between pink lines), but expression is elevated throughout dentate gyrus, CA3 and CA1 surrounding the area of silencing. J. c-Fos expression (pink) in dHPC of a NpHR-expressing animal. c-Fos is absent in the area underneath the laser (denoted between pink lines), but it not elevated outside of this zone. Data are represented as mean +/− SEM.
When pyramidal neurons were hyperpolarized with hM4Di, we found that memory retrieval for context fear was not affected (No effect of treatment, F (1, 11) = 0.077, p = 0.785; Main effect of time F (3, 33) = 6.98, p = .0009, No treatment x time interaction F (3, 33) = .216, p = .8844) (Figure 6B). Quantification of c-Fos revealed that hM4Di stimulation reduced expression in CA1 pyramidal neurons during testing although the size of this decrease was modest (t (10) =2.95, p = 0.01) (Figure 6C). This result is consistent with our Dlx-hM3Dq data and suggests that prolonged inhibition of dorsal CA1 neurons does not prevent the expression of context fear.
In contrast to CaMKII-hM4Di, acute hyperpolarization with ArchT produced large deficits in freezing relative to CaMKII-eGFP animals (n = 5) (Figure 6D) (Group x Stimulation period interaction F (1, 9) = 9.254, p = .014, Fisher’s LSD post-hoc tests, Laser off (p .384) Laser on (p = .049)). Similar to our CaMKII-ChR2 data, freezing did not recover when ArchT stimulation was terminated and remained low for the remainder of the session (Main effect of group F (1, 9) = 14.39, p = .0043, No effect of Stimulation period F (1,9) = 1.476, p = .2554), No Group x Stimulation period interaction F (1, 9) 1.539, p = .2461). Given the similarity between these results, we quantified c-Fos expression after the test and found a large increase in excitatory activity rather than a decrease (t (8) = 5.92, p=0.0004) (Figure 6E). This unexpected result may have been caused by rebound excitation; transient increases in firing rate that are often observed after hyperpolarization (Li et al., 2019). If pyramidal neurons were activated each time ArchT stimulation ended, it could produce an increase c-Fos expression during testing.
To determine if other inhibitory opsins produce similar effects, we used NpHR to hyperpolarize CA1 neurons during testing. Unlike ArchT, this manipulation had no effect on memory retrieval (No effect of group, F (1,9) = 0.05, p = 0.8182; Main effect of time, F (3, 27) = 9.23, p = 0.0002; No group x stimulus period interaction, F (3,27) = 0.8, p = 0.47) (Figure 6F) and did not increase c-Fos expression relative to CaMKII-eGFP (n = 6) (t (7) = 0.11, p = 0.92) (Figure 6G). Because laser off periods can obscure decreases in pyramidal cell activity, we conducted a second test where NpHR and ArchT were stimulated during the entire session (Figure 6). In this case, both opsins reduced c-Fos expression around the tip of the optic fiber (Figure 6I,J). This decrease was not observed in CaMKII-eYFP control animals suggesting that it was not caused by prolonged laser stimulation. In addition to reducing activity near the optic fiber, ArchT stimulation also increased in c-Fos expression outside of this area. This increase in activity was extensive and could be observed in CA3 and the dentate gyrus as well as CA1. Large increases in c-Fos expression were not observed following NpHR stimulation. Therefore, we hypothesize that activation of ArchT impaired memory retrieval because it produced increases in excitatory activity that spread throughout the dorsal hippocampus (similar to CaMKII-ChR2).
ArchT is a proton pump and prolonged stimulation of this opsin (< 1 min) can raise the intracellular pH of synaptic terminals and increase spontaneous glutamate release (Mahn et al., 2016). Stimulation can also decrease the extracellular pH and induce action potentials in neighboring neurons by activating acid-sensing ion channels (ASICs) (Baron et al., 2002; T. Li et al., 2014). Because these excitatory effects take time to emerge, we determined how quickly freezing began to decrease after ArchT was stimulated. To do this, we analyzed the behavioral data from the test session in 20s bins (Figure 7A). We found that decreases did not occur until ArchT had been stimulated for ≈100–140s. For comparison, we examined changes in freezing when inhibition was produced by stimulating GABAergic neurons with ChR2. In contrast to ArchT, this manipulation produced an immediate decrease in freezing (Figure 7B) (N. Li et al., 2019). Another difference was that freezing returned to control levels after Dlx-ChR2 was stimulated but did not recover in ArchT animals. The latter was also observed when we activated pyramidal neurons with CaMKII-ChR2 (Figure 5D). These data strongly suggest that context fear is impaired by prolonged ArchT stimulation because it increases excitatory activity in the dorsal hippocampus. It is possible that freezing did not recover when the laser turned off because stimulation caused place cells to remap (Diamantaki et al., 2018; Trouche et al., 2016). Remapping would make it difficult for animals to recognize the training context, which should increase exploration and decrease freezing as we observed.
Figure 7. Inhibitory manipulations that increase excitation and impair memory.
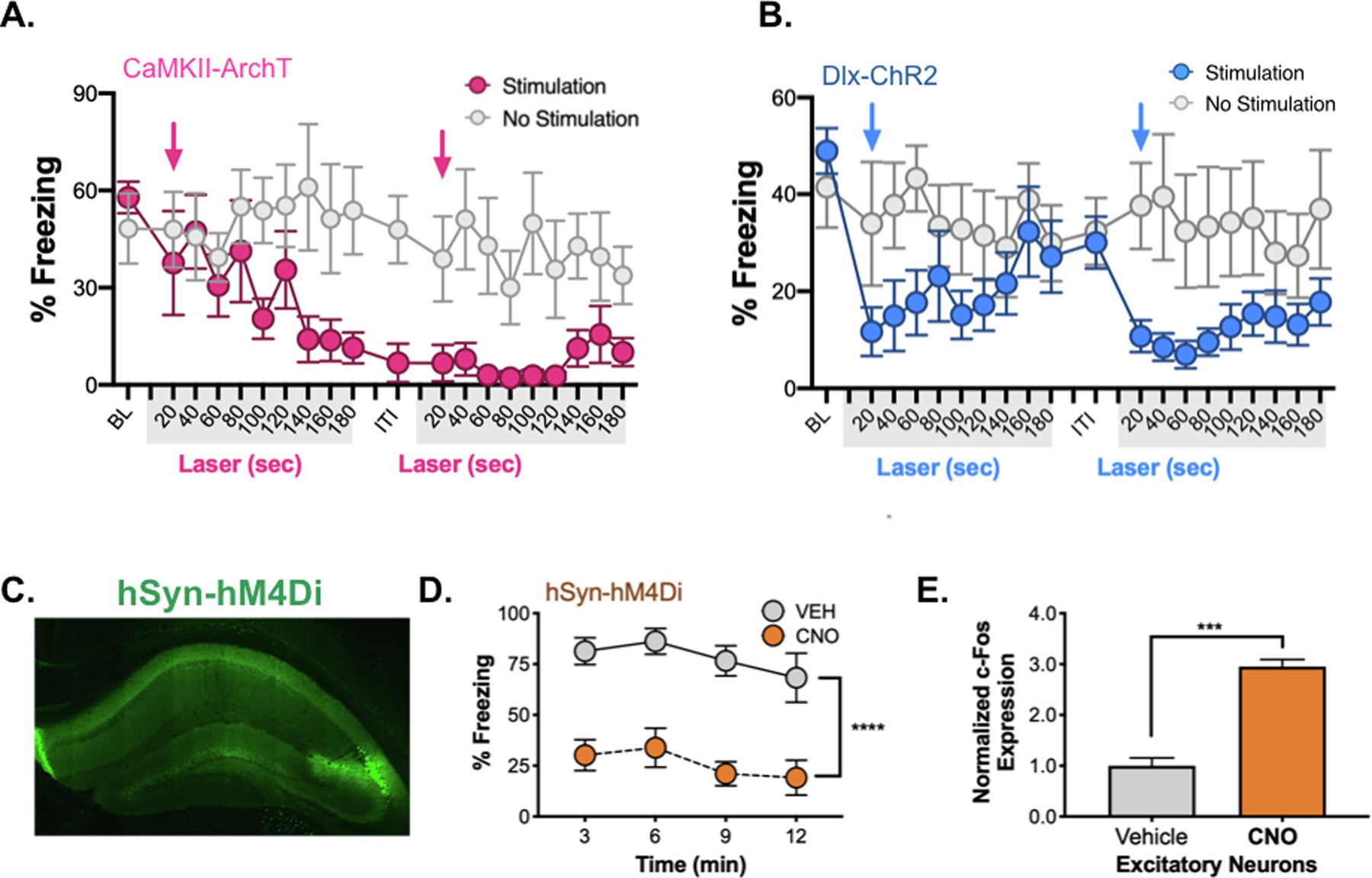
A. 20 second bins of freezing activity in ArchT (blue) vs. control animals (gray) during laser ON periods. ~140s into laser stimulation, ArchT animals ceased freezing, this was not recovered over the remainder of the testing period. Blue arrows denote onset of laser stimulation. B. 20 second bins of freezing activity in Dlx-ChR2 laser-stimulated (blue) vs. control animals (gray) during laser ON periods. Dlx-ChR2 animals ceased freezing during laser onset (blue arrows), but freezing recovered during laser OFF periods. C. hSyn-hM4Di virus expression (green) in dHPC. D. During the 12-minute testing period, hSyn-hM4Di CNO-treated animals froze less than vehicle treated animals. E. c-Fos expression was increased in excitatory neurons of dCA1 in CNO-treated animals. Data are expressed as mean +/− SEM.
3.4. Hyperpolarizing inhibitory neurons increases activity and impairs memory retrieval
A recent study found that stimulation of hM4Di in the dorsal hippocampus increased c-Fos expression and impaired object recognition memory (López et al., 2016). In this case, a neuron specific promoter (Syn1) was used to drive hM4Di expression in both excitatory and inhibitory neurons. Hyperpolarization of the latter reduced GABAergic tone and led to a net increase in excitatory activity. In the current experiment, we determined if the same manipulation would increase c-Fos expression in CA1 pyramidal neurons and impair the retrieval of context fear. Similar to the published report, we found that injections of CNO in Syn1-hM4Di mice produce significant memory deficits and increased c-Fos expression in excitatory neurons relative to controls (Figure 7C–E). Together with our ArchT data, these results demonstrate that some inhibitory manipulations impair memory retrieval by increasing the amount of excitatory activity.
4. DISCUSSION
In the current study, we determined if altered hippocampal activity produces amnesia for recently acquired context fear. Reductions in activity were achieved by activating inhibitory neurons or by hyperpolarizing pyramidal cells directly. Similar to recent reports, we found that the former produced much more robust and widespread silencing than the latter (Babl et al., 2019; Li et al., 2019). When inhibitory neurons were stimulated with ChR2 during testing, memory retrieval was significantly impaired. In contrast, when the same neurons were activated with the excitatory DREADD hM3Dq, retrieval was not affected. This dissociation was not due to differences in inhibition, as both manipulations activated interneurons and reduced excitation throughout the dorsal hippocampus (as indexed by c-Fos expression). Therefore, we hypothesize that the retrieval deficit caused by ChR2 stimulation is due to an immediate reduction in hippocampal activity that does not provide enough time for other brain regions to compensate. Stimulation of DREADDs, on the other hand, produces a gradual loss of excitation in the hippocampus that can take up to 30 minutes to reach asymptote (Ryan et al., 2015; Zhu et al., 2014). This appears to be a sufficient amount of time for extra-hippocampal structures to become engaged and express context fear.
A similar difference between acute and prolonged hippocampal inactivation was observed during remote memory retrieval (Goshen et al., 2011). In that case, it was assumed that cortical regions could express context fear during prolonged silencing because systems consolidation had taken place. However, we find that the same dissociation exists 1-day after learning. It is possible, therefore, that cortical regions are able to express both recent and remote context fear provided they have enough time to compensate for the loss of the hippocampus. It should be noted that the brain areas mediating memory retrieval in each of these situations may or may not be the same. The ACC is important for the expression of remote context fear while the PFC, entorhinal, perirhinal, postrhinal and retrosplenial cortices contribute to recent and remote memory retrieval (Burwell et al., 2004; Coelho et al., 2018; Cowansage et al., 2014; de Sousa et al., 2019; Frankland et al., 2001; Kitamura et al., 2017; Zelikowsky et al., 2014). The ventral hippocampus is also likely to play an important role as it projects to the amygdala and influences the expression of context fear (Chen et al., 2019; Cullen et al., 2015; Huckleberry et al., 2018; Wiltgen et al., 2006).
The type of context memory that is expressed during prolonged inactivation of the dorsal hippocampus may differ from the one that is retrieved in control animals. For example, context memories are often less precise when they are retrieved without the hippocampus (Wiltgen et al., 2010; Wiltgen & Silva, 2007; Winocur et al., 2007). This possibility can be addressed in future work by using discrimination procedures to examine memory specificity (Wang et al., 2009). However, it is important to note that damage to the dorsal hippocampus impairs retrieval on the same type of context recognition tests that were used in the current experiments (Anagnostaras et al., 1999; Frankland et al., 1998; Kim & Fanselow, 1992; Maren et al., 1997). In the case of lesions, this deficit could result from consolidation impairments because memory is tested several days after training and surgery. The retrieval deficit could also be caused, in part, by damage that is caused to distal structures. For example, excitotoxic lesions of the hippocampus produce a substantial loss of cortical tissue that may prevent other brain regions from compensating during memory tests (Anagnostaras et al., 2002). Consistent with this idea, episodic memory deficits in patients with hippocampus damage are strongly correlated with tissue loss in distal structures (Argyropoulos et al., 2019).
In contrast to prolonged inactivation, we found that rapid optogenetic silencing of the dorsal hippocampus produced significant deficits in memory retrieval. Given than other brain regions are able to express context fear, this result suggests that optogenetic inactivation somehow prevents them from doing so. One possibility is that retrieval is impaired by acute silencing because memory is tested at the exact same moment hippocampal activity is disrupted. Optogenetic perturbations have been shown to change local activity and simultaneously disrupt the function of distal brain regions (Allen et al., 2015; Otchy et al., 2015). These off-target effects could prevent connected areas from compensating for the loss of the hippocampus during memory retrieval. In contrast, when inactivation occurs over an extended period, other brain regions may be able to recover by the time memory is tested (Otchy et al., 2015).
Consistent with a recent report, we found that direct hyperpolarization of pyramidal cells produces less extensive inhibition than stimulation of interneurons (Li et al., 2019). Limited inhibition is likely the reason the former did not impair memory retrieval. Surprisingly, some inhibitory manipulations (CaMKII-ArchT and hSyn-hM4Di) produced off-target effects that increased the total amount of hippocampal activity. When this occurred, memory expression was severely impaired. In addition, the deficits appeared similar to those observed when pyramidal cells were activated with ChR2 or hM3Dq. In the case of ArchT, we hypothesize that the prolonged stimulation period (3-min.) increased excitatory activity by altering internal and/or external pH (Baron et al., 2002; Li et al., 2014). Shorter stimulation periods are recommended to reduce these effects (Mahn et al., 2016). Reduced expression levels may also help as widespread increases in excitatory activity were not reported in previous ArchT studies that confined silencing to “engram cells” (Denny et al., 2014; Lacagnina et al., 2019; Tanaka et al., 2014). It is clearly essential to determine how different optogenetic and chemogenenic manipulations affect network activity in order to understand their effects on learning and memory (Allen et al., 2015; Otchy et al., 2015). Unfortunately, efficacy is often confirmed only in cells that express the protein of interest and changes in network activity are not examined.
Taken together, our results suggest that amnesia is caused by widespread disruption of hippocampal activity (Table 1). Memory is not affected by localized changes, presumably because spared tissue can compensate during learning and retrieval. Compensation is most effective when decreases in hippocampal activity occur over a prolonged period. In these cases, memories can be formed and retrieved even after extensive changes in activity. However, memory quality and precision are likely to be compromised in these situations (Frankland et al., 1998; Wiltgen et al., 2010; Yonelinas, 2013).
Table 1. Manipulation of dHPC activity and the effects on memory retrieval.
Summary of the effects of acute vs. prolonged manipulations on memory retrieval and excitatory activity in the hippocampus.
| Activation of inhibitory neurons | |||
|---|---|---|---|
| Virus | Time course | Memory Retrieval | Excitatory activity |
| Dlx-ChR2 | acute | impaired | widespread decreases |
| Dlx-hM3Dq | prolonged | intact | widespread decreases |
| Activation of excitatory neurons | |||
| Virus | Time course | Memory Retrieval | Excitatory activity |
| CaMKII-ChR2 | acute | impaired | widespread increases |
| CaMKII-hM3Dq | prolonged | impaired | widespread increases |
| Inhibition of excitatory neurons | |||
| Virus | Time course | Memory Retrieval | Excitatory activity |
| CaMKII-NpHR 3.0 | acute | intact | moderate decreases |
| CaMKII ArchT | acute | impaired | widespread increases* |
| CaMKII-hM4Di | prolonged | intact | moderate decreases |
| Inhibition of excitatory and inhibitory neurons | |||
| Virus | Time course | Memory Retrieval | Excitatory activity |
| Syn1-hM4Di | prolonged | impaired | widespread increases* |
Supplementary Material
6. ACKNOWLEDGEMENTS
The authors would like to thank Drs. Diasynou Fioravante, Simona Ghetti, Jalina Graham, Gene Gurkoff and Brian Trainor for their comments on the presented work.
5. FUNDING SOURCES
This work was supported by funding from National Institutes of Health (R21 NS101694 and RO1 NS088053 to B.J.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
Jamie N. Krueger – data curation; formal analysis; investigation; methodology; visualization; writing – original draft; writing – review & editing
Jacob H. Wilmot - investigation; writing – review & editing
Yusuke Teratani-Ota - investigation
Kyle R. Puhger – investigation
Sonya E. Nemes – investigation
Ana P. Crestani - investigation
Marrisa M. Lafreniere - investigation
Brian J. Wiltgen - conceptualization; formal analysis; funding acquisition; project administration; resources; supervision; validation; writing – review & editing
7. REFERENCES
- Allen BD, Singer AC, & Boyden ES (2015). Principles of designing interpretable optogenetic behavior experiments. Learning & Memory, 22(4), 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, & Fanselow MS (2001). Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus, 11(1), 8–17. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, & Fanselow MS (2002). The hippocampus and Pavlovian fear conditioning: Reply to Bast et al. Hippocampus, 12(4), 561–565. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, & Fanselow MS (1999). Temporally Graded Retrograde Amnesia of Contextual Fear after Hippocampal Damage in Rats: Within-Subjects Examination. Journal of Neuroscience, 19(3), 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Wood SC, Shuman T, Cai DJ, LeDuc AD, Zurn KR, Zurn JB, Sage JR, & Herrera GM (2010). Automated Assessment of Pavlovian Conditioned Freezing and Shock Reactivity in Mice Using the Video Freeze System. Frontiers in Behavioral Neuroscience, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyropoulos GP, Loane C, Roca-Fernandez A, Lage-Martinez C, Gurau O, Irani SR, & Butler CR (2019). Network-wide abnormalities explain memory variability in hippocampal amnesia. ELife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babl SS, Rummell BP, & Sigurdsson T (2019). The Spatial Extent of Optogenetic Silencing in Transgenic Mice Expressing Channelrhodopsin in Inhibitory Interneurons. Cell Reports, 29(5), 1381–1395.e4. [DOI] [PubMed] [Google Scholar]
- Baron A, Waldmann R, & Lazdunski M (2002). ASIC-like, proton-activated currents in rat hippocampal neurons. The Journal of Physiology, 539(Pt 2), 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Hopkins RO, & Squire LR (2006). The Fate of Old Memories after Medial Temporal Lobe Damage. Journal of Neuroscience, 26(51), 13311–13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, & Jutras MJ (2004). Perirhinal and postrhinal contributions to remote memory for context. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(49), 11023–11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LS, Silva AJ, Abeliovich A, Watanabe Y, Tonegawa S, & McNamara JO (1995). Limbic epilepsy in transgenic mice carrying a Ca2+/calmodulin-dependent kinase II alpha-subunit mutation. Proceedings of the National Academy of Sciences, 92(15), 6852–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande MA, Haubrich J, Pedraza LK, Popik B, Quillfeldt JA, & de Oliveira Alvares L (2018). Synaptic consolidation as a temporally variable process: Uncovering the parameters modulating its time-course. Neurobiology of Learning and Memory, 150, 42–47. [DOI] [PubMed] [Google Scholar]
- Chen BK, Murawski NJ, Cincotta C, McKissick O, Finkelstein A, Hamidi AB, Merfeld E, Doucette E, Grella SL, Shpokayte M, Zaki Y, Fortin A, & Ramirez S (2019). Artificially Enhancing and Suppressing Hippocampus-Mediated Memories. Current Biology: CB, 29(11), 1885–1894.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CAO, Ferreira TL, Kramer-Soares JC, Sato JR, & Oliveira MGM (2018). Network supporting contextual fear learning after dorsal hippocampal damage has increased dependence on retrosplenial cortex. PLOS Computational Biology, 14(8), e1006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, & Mayford M (2014). Direct reactivation of a coherent neocortical memory of context. Neuron, 84(2), 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PK, Gilman TL, Winiecki P, Riccio DC, & Jasnow AM (2015). Activity of the anterior cingulate cortex and ventral hippocampus underlie increases in contextual fear generalization. Neurobiology of Learning and Memory, 124, 19–27. [DOI] [PubMed] [Google Scholar]
- Daie K, Svoboda K, & Druckmann S (2019). Targeted photostimulation uncovers circuit motifs supporting short-term memory. BioRxiv, 623785. [DOI] [PubMed]
- de Sousa AF, Cowansage KK, Zutshi I, Cardozo LM, Yoo EJ, Leutgeb S, & Mayford M (2019). Optogenetic reactivation of memory ensembles in the retrosplenial cortex induces systems consolidation. Proceedings of the National Academy of Sciences of the United States of America, 116(17), 8576–8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, & Hen R (2014). Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron, 83(1), 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantaki M, Coletta S, Nasr K, Zeraati R, Laturnus S, Berens P, Preston-Ferrer P, & Burgalossi A (2018). Manipulating Hippocampal Place Cell Activity by Single-Cell Stimulation in Freely Moving Mice. Cell Reports, 23(1), 32–38. [DOI] [PubMed] [Google Scholar]
- Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi G-A, Guo L, Xu Q, Liu R, Lu C, Chu J, Grimley JS, Krostag A-R, Kaykas A, Avery MC, Rashid MS, Baek M, Jacob AL, Smith GB, Wilson DE, … Fishell G (2016). A viral strategy for targeting and manipulating interneurons across vertebrate species. Nature Neuroscience, 19(12), 1743–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery JG, & Visel M (2013). Adeno-associated viral vectors for gene therapy of inherited retinal degenerations. Methods in Molecular Biology (Clifton, N.J.), 935, 351–369. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, & Silva AJ (1998). The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behavioral Neuroscience, 112(4), 863–874. [DOI] [PubMed] [Google Scholar]
- Frankland PW, O’Brien C, Ohno M, Kirkwood A, & Silva AJ (2001). Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature, 411(6835), 309–313. [DOI] [PubMed] [Google Scholar]
- Frankland PW, & Bontempi B (2005). The organization of recent and remote memories. Nature Reviews. Neuroscience, 6(2), 119–130. [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, & Deisseroth K (2011). Dynamics of retrieval strategies for remote memories. Cell, 147(3), 678–689. [DOI] [PubMed] [Google Scholar]
- Holt W, & Maren S (1999). Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(20), 9054–9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckleberry KA, Shue F, Copeland T, Chitwood RA, Yin W, & Drew MR (2018). Dorsal and ventral hippocampal adult-born neurons contribute to context fear memory. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 43(12), 2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE (2002). Use of excitotoxins to lesion the hippocampus: Update. Hippocampus, 12(3), 405–414. [DOI] [PubMed] [Google Scholar]
- Kim JJ, & Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science (New York, N.Y.), 256(5057), 675–677. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL, & Tonegawa S (2017). Engrams and circuits crucial for systems consolidation of a memory. Science (New York, N.Y.), 356(6333), 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, Lim SC, Santos SL, Denny CA, & Drew MR (2019). Distinct hippocampal engrams control extinction and relapse of fear memory. Nature Neuroscience, 22(5), 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Matsumoto K, & Watanabe H (1999). Different effects of unilateral and bilateral hippocampal lesions in rats on the performance of radial maze and odor-paired associate tasks. Brain Research Bulletin, 48(1), 113–119. [DOI] [PubMed] [Google Scholar]
- Li N, Chen S, Guo ZV, Chen H, Huo Y, Inagaki H, Davis C, Hansel D, Guo C, & Svoboda K (2019). Spatiotemporal limits of optogenetic manipulations in cortical circuits. BioRxiv, 642215. [DOI] [PMC free article] [PubMed]
- Li T, Yang Y, & Canessa CM (2014). A method for activation of endogenous acid-sensing ion channel 1a (ASIC1a) in the nervous system with high spatial and temporal precision. The Journal of Biological Chemistry, 289(22), 15441–15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SF, Paylor R, & Wehner JM (1997). Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behavioral Neuroscience, 111(1), 104–113. [DOI] [PubMed] [Google Scholar]
- López AJ, Kramár E, Matheos DP, White AO, Kwapis J, Vogel-Ciernia A, Sakata K, Espinoza M, & Wood MA (2016). Promoter-Specific Effects of DREADD Modulation on Hippocampal Synaptic Plasticity and Memory Formation. The Journal of Neuroscience, 36(12), 3588–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn M, Prigge M, Ron S, Levy R, & Yizhar O (2016). Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nature Neuroscience, 19(4), 554–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, & Fanselow MS (1997). Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural Brain Research, 88(2), 261–274. [DOI] [PubMed] [Google Scholar]
- Maren S, & Holt WG (2004). Hippocampus and Pavlovian fear conditioning in rats: Muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behavioral Neuroscience, 118(1), 97–110. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, & Rudy JW (2004). The Role of the Dorsal Hippocampus in the Acquisition and Retrieval of Context Memory Representations. Journal of Neuroscience, 24(10), 2431–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, & Penfield W (1955). The effect of hippocampal lesions on recent memory. Transactions of the American Neurological Association, 80th Meeting, 42–48. [PubMed] [Google Scholar]
- Moser M-B, & Moser EI (1998). Functional differentiation in the hippocampus. Hippocampus, 8(6), 608–619. [DOI] [PubMed] [Google Scholar]
- Otchy TM, Wolff SBE, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SMH, & Ölveczky BP (2015). Acute off-target effects of neural circuit manipulations. Nature, 528(7582), 358–363. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu G-Q, Kreitzer A, Finkbeiner S, Noebels JL, & Mucke L (2007). Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron, 55(5), 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, & Lattal KM (2014). Differential effects of dorsal hippocampal inactivation on expression of recent and remote drug and fear memory. Neuroscience Letters, 569, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, & Amaral DG (1996). Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 16(16), 5233–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LBM, Joca SRL, Corrêa FMA, & Guimarães FS (2008). Effects of reversible inactivation of the dorsal hippocampus on the behavioral and cardiovascular responses to an aversive conditioned context. Behavioural Pharmacology, 19(2), 137–144. [DOI] [PubMed] [Google Scholar]
- Roy DS, Park Y-G, Ogawa SK, Cho JH, Choi H, Kamensky L, Martin J, Chung K, & Tonegawa S (2019). Brain-wide mapping of contextual fear memory engram ensembles supports the dispersed engram complex hypothesis. BioRxiv, 668483.
- Ryan TJ, Roy DS, Pignatelli M, Arons A, & Tonegawa S (2015). Memory. Engram cells retain memory under retrograde amnesia. Science (New York, N.Y.), 348(6238), 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20(1), 11–21. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Stamatakis AM, Phillips JL, Hovelsø N, van Zessen R, & Stuber GD (2011). Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nature Protocols, 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, & Clark RE (2004). The medial temporal lobe. Annual Review of Neuroscience, 27, 279–306. [DOI] [PubMed] [Google Scholar]
- Stefanelli T, Bertollini C, Lüscher C, Muller D, & Mendez P (2016). Hippocampal Somatostatin Interneurons Control the Size of Neuronal Memory Ensembles. Neuron, 89(5), 1074–1085. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Sparks FT, & Lehmann H (2010). Hippocampus and retrograde amnesia in the rat model: A modest proposal for the situation of systems consolidation. Neuropsychologia, 48(8), 2357–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, & Wiltgen BJ (2014). Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron, 84(2), 347–354. [DOI] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, & Wiltgen BJ (2013). Reactivation of neural ensembles during the retrieval of recent and remote memory. Current Biology: CB, 23(2), 99–106. [DOI] [PubMed] [Google Scholar]
- Trouche S, Perestenko PV, van de Ven GM, Bratley CT, McNamara CG, Campo-Urriza N, Black SL, Reijmers LG, & Dupret D (2016). Recoding a cocaine-place memory engram to a neutral engram in the hippocampus. Nature Neuroscience, 19(4), 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, McAndrews MP, & Moscovitch M (2000). Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(15), 5853–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-H, Teixeira CM, Wheeler AL, & Frankland PW (2009). The precision of remote context memories does not require the hippocampus. Nature Neuroscience, 12(3), 253–255. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, & Fanselow MS (2006). Context Fear Learning in the Absence of the Hippocampus. Journal of Neuroscience, 26(20), 5484–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, & Silva AJ (2007). Memory for context becomes less specific with time. Learning & Memory (Cold Spring Harbor, N.Y.), 14(4), 313–317. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, & Tanaka KZ (2013). Systems consolidation and the content of memory. Neurobiology of Learning and Memory, 106, 365–371. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlson MG, Parivash SN, Li W, & Silva AJ (2010). The hippocampus plays a selective role in the retrieval of detailed context memories. Current Biology : CB, 20(15), 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, & Bontempi B (2010). Memory formation and long-term retention in humans and animals: Convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia, 48(8), 2339–2356. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, & Sekeres M (2007). Memory consolidation or transformation: Context manipulation and hippocampal representations of memory. Nature Neuroscience, 10(5), 555–557. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP (2013). The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioural Brain Research, 254, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, & Fanselow MS (2014). Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(25), 8462–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Kang H, You L, Rastogi P, Venkatesh D, & Chandra M (2014). Neuropsychological deficits in temporal lobe epilepsy: A comprehensive review. Annals of Indian Academy of Neurology, 17(4), 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Pleil KE, Urban DJ, Moy SS, Kash TL, & Roth BL (2014). Chemogenetic Inactivation of Ventral Hippocampal Glutamatergic Neurons Disrupts Consolidation of Contextual Fear Memory. Neuropsychopharmacology, 39(8), 1880–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets/code. Original/source data for figures in the paper is available by request.


