This study identifies FILIP1L as a tumor suppressor in mucinous colon cancer and demonstrates that FILIP1L loss results in aberrant stabilization of a centrosome-associated chaperone protein to drive aneuploidy and disease progression.
Abstract
Aneuploid mucinous colorectal adenocarcinoma (MAC) is an aggressive subtype of colorectal cancer with poor prognosis. The tumorigenic mechanisms in aneuploid MAC are currently unknown. Here we show that downregulation of Filamin A–interacting protein 1-like (FILIP1L) is a driver of MAC. Loss of FILIP1L increased xenograft growth, and, in colon-specific knockout mice, induced colonic epithelial hyperplasia and mucin secretion. The molecular chaperone prefoldin 1 (PFDN1) was identified as a novel binding partner of FILIP1L at the centrosomes throughout mitosis. FILIP1L was required for proper centrosomal localization of PFDN1 and regulated proteasome-dependent degradation of PFDN1. Importantly, increased PFDN1, caused by downregulation of FILIP1L, drove multinucleation and cytokinesis defects in vitro and in vivo, which were confirmed by time-lapse imaging and 3D cultures of normal epithelial cells. Overall, these findings suggest that downregulation of FILIP1L and subsequent upregulation of PFDN1 is a driver of the unique neoplastic characteristics in aggressive aneuploid MAC.
Significance:
This study identifies FILIP1L as a tumor suppressor in mucinous colon cancer and demonstrates that FILIP1L loss results in aberrant stabilization of a centrosome-associated chaperone protein to drive aneuploidy and disease progression.
Introduction
In the United States, colorectal cancer is the third leading cause of cancer-related deaths in men and women, and the second most common cause of cancer deaths when men and women are combined (1). Colorectal cancer is expected to cause 53,200 deaths during 2020 (1). Mucinous colorectal adenocarcinoma (MAC) is a distinct form of colorectal cancer, affecting 10% to 15% of patients. MAC is characterized by abundant mucin secretion comprising at least 50% of the tumor volume (2). Approximately half of MAC tumors are aneuploid (3–7), whereas the remainder are diploid and associated with microsatellite instability. Aneuploid MAC tumors were shown to be clinically more aggressive than diploid tumors (8, 9). Compared with common colorectal adenocarcinoma, MAC has an entirely different molecular "signature," as well as an aberrant and aggressive metastatic pattern that is associated with poor response to treatment and worse prognosis (2). The mechanisms of MAC tumorigenesis are currently unknown.
Filamin A interacting protein 1-like (FILIP1L) is a tumor suppressor, which we identified in several types of cancer, including colorectal cancer (10–13). We showed that FILIP1L expression is downregulated by promoter methylation (10, 12), a key mechanism of tumor suppressor downregulation in cancer. Downregulation of FILIP1L is associated with chemoresistance and worse prognosis in ovarian and colon cancer (14, 15). Moreover, its expression is inversely correlated with the invasive/aggressive potential of tumors and with epithelial-to-mesenchymal (EMT) marker expression (11, 16). Its structural homologies and centrosomal localization suggest that FILIP1L may bind to elements of the cytoskeleton, and chaperone proteins to proteasomes (11, 17, 18).
The prefoldin1 (PFDN1) chaperone is overexpressed in multiple cancer types and is associated with poor prognosis in colon cancer (19, 20). PFDN1 participates in a multimeric prefoldin complex that facilitates proper folding of key cytoskeletal components such as actin and tubulins. Loss of PFDN1 decreases tubulin levels, thereby reducing microtubule growth and causing defects in cell division and embryonic lethality (21), although the mechanisms are unknown. Moreover, loss of PFDN1 results in mitotic spindle misorientation and mispositioning of cytokinetic furrows (21). The mechanisms regulating PFDN1 localization and levels are unknown.
The spindle apparatus specifies the position of the cleavage furrow during anaphase, and the cleavage plane is positioned by microtubule-dependent mechanisms (22). Microtubules are primarily nucleated from centrosomes, thus centrosomes are critical organelles that ensure faithful mitosis and chromosome distribution to daughter cells. Centrosome abnormalities lead to mitosis/cytokinesis defects that are associated with aneuploidy (23). Centrosome abnormalities are found in virtually all cancer types and have been linked to chromosomal instability, aneuploidy, and tumorigenesis (24). Changes in the levels of several centrosomal proteins, including prefoldins, have been linked to dysregulation of centrosome function (21, 25–29). Dysregulated expression of several centrosome-modulating proteins is implicated in human cancer (28–32).
Although we have shown that FILIP1L is a versatile tumor suppressor in many types of cancer, its function has not been fully elucidated. Here we show that loss of FILIP1L increases xenograft growth in vivo, drives colonic epithelial hyperplasia in mice, and increases mucin secretion and mitotic defects in MAC. We determined that FILIP1L binds to the centrosome-localized chaperone protein, PFDN1, and regulates its level at the centrosomes through a proteasome-dependent mechanism. FILIP1L is down-regulated and PFDN1 is up-regulated in human MAC samples. Reduction of FILIP1L and subsequent increase in PFDN1 levels results in mucin secretion and multinucleation, which recapitulates the central characteristics of human aneuploid MAC. Our findings suggest that downregulation of the tumor suppressor FILIP1L is a driver of the neoplastic changes in an aggressive form of MAC.
Materials and Methods
Cell culture and transient transfection
The following authenticated cell lines were purchased from ATCC: MDCK.2 (CRL-2936, RRID:CVCL_B034), HEK293 (CRL-1573, RRID:CVCL_0045), and human colon cancer lines including HT29 (HTB-38, RRID:CVCL_0320), HCT116 (CCL-247, RRID:CVCL_0291), HCT15 (CCL-225, RRID:CVCL_0292), SW480 (CCL-228, RRID:CVCL_0546), Caco2 (HTB-37, RRID:CVCL_0025), SW620 (CCL-227, RRID:CVCL_0547), Ls174T (CL-188, RRID:CVCL_1384), and T84 (CCL-248, RRID:CVCL_0555). Cells were cultured following the manufacturer's guidelines, and passaged up to five times after each thawing. All cell lines were routinely tested for Mycoplasma contamination using Universal Mycoplasma Detection Kit (ATCC, 30–1012K). HEK293 cells were transfected with equimolar amounts of control empty plasmid or plasmid encoding FILIP1L-HA and/or Flag-PFDN1 using lipofectamine 3000 solution (Thermo Fisher Scientific) following the manufacturer's protocols. For MDCK.2 cells, using the 4D-Nucleofector system (Lonza; SE solution; program CM 113), homogenous expression in over 90% of cells was routinely achieved. At 24 to 48 hours following transfection, transfected cells were subjected to downstream assays such as immunoprecipitation, immunoblot, and immunofluorescence staining.
Development of stable clones
FILIP1L-knockdown clones were generated from Caco2, SW620, and Ls174T cells. PFDN1-overexpressing clones were generated from Caco2 cells. FILIP1L-overexpressing clones were generated from HT29 and HCT116 cells. Cells were transduced by lentiviruses purchased from Applied Biological Materials. For knockdown, lentiviruses encoding either scrambled shRNA or FILIP1L-shRNA were used. Pooled lentiviruses from four different sequences of FILIP1L-shRNA were used (ACCAAGGAGAGAGATGATTTAATTCAAGAGATTAAATCATCTCTCTCCTTGGT, AAGAGCCTCATTCCTCTGGAACTTCAAGAGAGTTCCAGAGGAATGAGGCTCTT, TCCATGTCCTGTTAACAGAAAGTTCAAGAGACTTTCTGTTAACAGGACATGGA, and ACCACAGAGAGTCCTCACTCTTTTCAAGAGAAAGAGTGAGGACTCTCTGTGGT). To generate FILIP1L/PFDN1 double-knockdown clones from FILIP1L-knockdown Caco2 clones, pooled lentiviruses from four different sequences of PFDN1-shRNA were used (TTCACAGAGCTTCAAGCCAAAGTTCAAGAGACTTTGGCTTGAAGCTCTGTGAA, AGCTCGCAGACATACAGATTGTTCAAGAGACAATCTGTATGTCTGCGAGCT, CTTCAGTCCAAGGAAGCAATTTTCAAGAGAAATTGCTTCCTTGGACTGAAG, and CTACCTGGAGCGAAGCGTTAATTCAAGAGATTAACGCTTCGCTCCAGGTAG). For overexpression, lentiviruses encoding either empty construct, FILIP1L-HA or Flag-PFDN1 were used. Following transduction, resistant cells were screened by puromycin selection and mixed clones were selected by immunoblot.
FILIP1L-knockdown clones from MDCK.2 cells were generated using CRISPR-Cas9 at the Genome Editing Core at Rutgers Cancer Institute of New Jersey. Cells were electroporated with pX458-C199 (gRNA sequences targeting exon 3 of FILIP1L, CCTTGCTGAAACCAGAGTTC). pX458 [pSpCas9(BB)-2A-GFP] was obtained from Addgene (#48138). After electroporation, individual cells were sorted into 96-well plates and single-cell derived clones were genotyped by PCR. PCR primers of TCACAGCTGATAAGTTGCTAAAGCACC and CTGCCTCATTGGTGAGCTTTGC were used. T7 endonuclease digestion was used to determine clones with indels. Fifty-six clones were analyzed and Sanger sequencing of candidate clones confirmed frameshift mutations in FILIP1L clones. Although we aimed to generate knockout clones, clones demonstrating complete deletions were not found.
Mouse xenograft model
All use of vertebrate animals described in this study was conducted in accordance with NIH regulations and was approved by the Animal Use Committee of Rutgers University. Indicated number of colon cancer clones were suspended in Matrigel [Corning, #356231, 1:1 ratio (v:v)] and subcutaneously injected in 8-week-old female nude mice (Taconic, catalog no. TAC:nmrinu, RRID:IMSR_TAC:nmrinu). Tumor growth was measured for indicated times, and tumor weights were measured after sacrifice. Xenograft tumors were fixed in 10% neutral buffered formalin and subject to IHC analysis.
Filip1l conditional knockout mice
Filip1l-floxed mice were generated using CRISPR-Cas9 at the Genome Editing Core at Rutgers Cancer Institute of New Jersey. C57BL6/J (IMSR Cat# JAX_000664, RRID:IMSR_JAX:000664) embryos were microinjected with a mixture containing Cas9 protein (IDT), a sgRNA (Millipore, Sigma), and a ssODN (IDT), which contained homology arms and a loxP site. For the 5′ loxP insertion-sgRNA, CATTCTTGCCCTGTGTTAAG was used along with the 5′loxP donor oligo, CATTTTACCGAATAACCAACGTGTTAAACAGTAACTAGTAATATAGCACATGCGTAATGGCTCAAGCAAGCCACTATAACTTCGTATAGCATACATTATACGAAGTTATAACACAGGGCAAGAATGAGTAATTCAAAAAGTGCCATGGCAACAGTTATCAAG (loxP sequence in bold). For the 3′ loxP insertion-sgRNA, ATGTAATATATGCTGTAGGG was used with the 3′loxP donor oligo, GAGTTTGGAACTTTAAGTTAGCTTGAATATCAAGGGCTGATAGGTTTCTCAAGCAGACTGAAACCCCCCATAACTTCGTATAGCATACATTATACGAAGTTATACAGCATATATTACATCTAAATCTGGAATCAGCCACTATGACATAAACTTCTGGAC (loxP sequence in bold). Two founders were determined to have both loxPs inserted correctly on the same allele, as confirmed by Sanger sequencing for the loxP sites of the cloned 4657 bps-floxed allele. The 5′ loxP was screened using primers, GCTCCTTGCCTTTGAACATGTTAG and GTGAAAGTGAGGCAATTCATCCATC. The 3′ loxP was screened using primers, GTGCTCAAAAGAGAACTATCAGAAGTC and ATTGTATGCTTCTGATTGTAGCCTTAC. Filip1lfl/fl mice were subsequently generated and were crossed with Cdx2-CreERT2 transgenic mice (Jackson laboratories #022390, RRID:IMSR_JAX:022390) to generate colon-specific Filip1lfl/fl; Cdx2-CreERT2 knockout mice.
Clinical specimens
Formalin-fixed paraffin-embedded (FFPE) tissue blocks of de-identified human colon samples including normal colon, serrated polyps, mucinous adenocarcinoma, and nonmucinous adenocarcinoma were obtained from Biorepository Services at the Rutgers Cancer Institute of New Jersey, under our IRB exemption. Immunohistochemical staining was carried out and a second pathologist scored the staining under blinded conditions. FILIP1L cytoplasmic staining was scored according to the staining intensity [categorized as 0 (absent), 1 (weak), 2 (moderate), or 3 (strong)] as well as the percentage of staining (0%–100%). The final expression score was calculated by multiplying the intensity and the percentage of staining, resulting in a score of 0 to 300.
DNA ploidy
Experimental details were followed as described previously (33). Briefly, four 60-μm-thick sections were cut from each FFPE tissue block of 16 MAC tumors. MAC area was identified by a pathologist and manually dissected to maximize the chance of finding an aneuploid tumor population (to increase its percentage in a background of normal diploid cells). Tissue samples were subjected to deparaffinization, rehydration, and pepsin digestion. Dissociated pellets were resuspended in 4,6-diamidino-2-phenylindole (DAPI) solution containing 0.1% NP40 and 10% DMSO. Prior to flow cytometry, potential nuclei aggregates were further dissociated by passaging 15 times through a 26-gauge needle. Flow cytometry analysis of DAPI-labeled nuclei was performed on a Cytek Aurora 5-laser cytometer using the SpectroFlow software package version 2.2 (Cytek Biosciences). Single nuclei population was selected using forward and side scatter. For a thorough doublet exclusion, it was further gated using forward scatter-area and forward scatter-height. 1 × 105 gated events were collected for each sample. The percentage of nuclei representing over 4 × 104 DAPI-area signal was calculated. On the basis of the average percentage of diploid controls, we defined “aneuploid tumor” as those with greater than 20% aneuploid cells.
Three-dimensional cell culture
MDCK.2 cells and transduced colon cancer clones were cultured on growth Factor-Reduced Matrigel (Corning, #356231). Cysts were routinely formed from MDCK.2 cell cultures, and they were fixed at days 3 and 6 for immunofluorescence analysis. Colon cancer clones mostly resulted in compact cell clusters that were fixed at days 3 to 5.
Time-lapse imaging
HEK293 cells were transfected with plasmids encoding FILIP1L-eGFP and mCherry-PFDN1, and incubated for 24 h. Cells were then placed in the EVOS Onstage Incubator at 5% CO2, 20% O2 and 80% humidity. Fluorescent images were acquired at 20-minute intervals. Caco2 clones were incubated with SPY-595 DNA and SPY-650-tubulin fluorescent dyes (Cytoskeleton) for 1 h and placed in the EVOS Onstage Incubator. Forty random fields were selected, and fluorescent and phase contrast images were acquired at 5-minute intervals. Images acquired over the initial 4 h were used to quantify data. Durations longer than 4 h demonstrated substantial fluorescent signal bleaching. Images were acquired by an EVOS FL Auto 2 microscope (Thermo) at 20X objective magnification (z-stack of 1.7 μmol/L thickness). Mitotic length was quantified as nuclear envelope breakdown (NEBD) to Anaphase. Time to cytokinesis completion was quantified as NEBD to membrane fission by phase contrast. Acquired images were analyzed and quantified using Celleste software (Thermo, Version 4.1.1). Detailed quantification procedures were written in Supplementary Information.
Yeast two-hybrid screening
Procedures were carried out using Matchmaker Gold Yeast Two-Hybrid System (Clontech, #630489), as recommended by the manufacturer. Wild-type FILIP1L cDNA was used to generate a bait clone.
Quantitative real-time RT-PCR
Total RNA preparation and qRT-PCR were performed as described previously (10). The gene-specific primers used with SYBR Green reagent are written in Supplementary Information.
Coimmunoprecipitation and immunoblot
Following transient transfection, HEK293 cell lysates were subject to immunoprecipitation. Immune complexes were eluted with Flag Peptide (Sigma, K4799) and HA Peptide (Sigma, I2149) for Flag tag- and HA tag-immunoprecipitation, respectively. Experimental details for immunoblotting were followed as described previously (11). Densitometric analysis was performed using ImageJ (RRID:SCR_003070) on scanned images of immunoblots. Plots were created for region of interest, and gel analysis feature was used to create numeric values for these plot areas. Antibody list used in assays such as immunoprecipitation, immunoblot, immunofluorescence, and IHC is shown in Supplementary Information.
Immunofluorescence staining and quantification
Experimental details were followed as described previously (11). For MUC2 staining on three-dimensional cell clusters, a much lower concentration of MUC2 antibodies was used in Ls174T clones than Caco2 clones because basal level of MUC2 expression was significantly higher in Ls174T cells (Supplementary Fig. S7B). Images were acquired by an AxioCam HRM camera (Yokogawa) at 63× objective magnification (z stack of 0.4 μmol/L thickness) on a Spinning disc confocal microscope (Zeiss; Observer Z1). Acquired images were then analyzed by software such as Cell Profiler (RRID:SCR_007358; ref. 34), ZEN (Zeiss, RRID:SCR_013672) and ImageJ. Ki67 area (Fig. 3J), mucin 2 intensity in 3D cell clusters (Fig. 7E), multinuclei (Fig. 8B), and PFDN1 occupancy in centrosomes (Fig. 8J; Supplementary Fig. S8B) were quantified. Detailed quantification procedures were written in Supplementary Information.
Figure 3.
FILIP1L loss induces mucin secretion as well as hyperplasia in mouse colon. A, Tails from wild-type (WT), Filip1lfl/+ (HET), and Filip1lfl/fl (HOMO) mice were subjected to genotyping for Filip1l floxed allele. Bottom and top bands indicate PCR products from wild-type and floxed allele, respectively. B–J, Littermate Filip1lfl/fl (CTL) and Filip1lfl/fl; Cdx2-CreERT2 (CKO) mice were treated with daily doses of tamoxifen (160 mg/kg) for 5 days and sacrificed on day 28 (day 7 for H). B,Filip1l mRNA levels were measured by qRT-PCR. C, Crypt length (shown in μm) was measured from Swiss-rolled whole colons. Analysis included only open longitudinal crypts and detailed quantification procedures were written in Supplementary Information. Three mice from each group were analyzed. The junction between proximal and mid colon are shown in Supplementary Fig. S4. Note that although crypt length was significantly increased throughout the entire colon, a larger difference between CTL and CKO was observed in proximal location. D–G, Colons were fixed and stained with H&E (D) and PAS (F). They were also IHC stained for FILIP1L (E) and Ki67 (G). The exact same regions of proximal colon were imaged in CTL and CKO mice. Scale bar, 200 μm. Higher magnification images are shown in insets (scale bar, 50 μm). H, mRNA levels of markers for goblet cells, secretory progenitors, and stem cells were measured by qRT-PCR. Mucin 2 (Muc2), anterior gradient 2 (Agr2), Atonal bHLH transcription factor 1 (Atoh1), SAM pointed domain-containing ets transcription factor (Spdef), and neurogenin 3 (Neurog3), and leucine-rich repeat containing G protein coupled receptor 5 (Lgr5) were analyzed. For B and H, epithelial cells from colons of CTL and CKO mice were prepared. The y-axis represents fold change over CTL mice, where each value was standardized with the housekeeping gene β-actin (6 mice each). I and J, Colons were stained for Ki67 (I), and Ki67-positive areas were quantified (J). Ten random fields per mouse were quantified (three mice each). Scale bar, 50 μm. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; NS, nonsignificant.
Figure 7.
FILIP1L knockdown induces mucin secretion in colon cancer cells. A, Protein levels of FILIP1L and PFDN1 were determined by immunoblotting in various colon cancer cell lines. B, Cell proliferation of various colon cancer cell lines was measured by WST1 incorporation daily for 4 days. The y-axis represents absorbance that was subtracted OD650 nm from OD440 nm. C, FILIP1L knockdown was achieved by stable expression of lentiviral shRNA in FILIP1L-high Caco2, SW620, and Ls174T colon cancer cells. Control clones were made with scrambled shRNA. FILIP1L, PFDN1, and GAPDH control were detected by immunoblotting. By densitometric quantification, FILIP1L protein was decreased by 11-, 3.6-, and 3.5-fold in Caco2, SW620, and Ls174T clones compared with their corresponding controls, respectively. D and E, Clones from either control or FILIP1L knockdown (Caco2 and Ls174T clones) as well as those from either control or PFDN1 overexpression (Caco2 clones) were grown in the presence of Matrigel, and three-dimensional clusters were stained for F-actin (green), MUC2 (red), and DAPI (blue; D), and the total fluorescence intensity per area of cell cluster was quantified (E). Ten to 15 cell clusters were counted. Scale bar, 20 μm. F, PFDN1 knockdown was achieved by stable expression of lentiviral shRNA in FILIP1L-knockdown Caco2 clones. FILIP1L, PFDN1, and GAPDH control were detected by immunoblotting. By densitometric quantification, PFDN1 protein was decreased by 2.1-fold in FILIP1L/PFDN1 double knockdown clones compared with FILIP1L knockdown clones. G, Caco2 clones from either FILIP1L knockdown or FILIP1L/PFDN1 double knockdown were analyzed for MUC2 total fluorescence intensity as described in E. *, P < 0.05; **, P < 0.01.
Figure 8.
FILIP1L knockdown leads to multinucleation in colon cancer cells, mediated by PFDN1. A, Clones from either control or FILIP1L knockdown (Caco2 and SW620 clones) as well as those from either control or PFDN1 overexpression (Caco2 clones) were stained for lamin A/C (green), F-actin (red), and DAPI (blue). B, The number of cells with multinuclei were quantified. Over 600 cells were counted. Scale bar, 20 μm. C, FILIP1L knockdown was achieved by CRISPR-Cas9 system in MDCK.2 cells. PFDN1 and GAPDH control were detected by immunoblotting. Two independent clones were tested. Note that FILIP1L-knockdown clones demonstrated increased PFDN1 expression. D, MDCK.2 cells were transfected with control GFP or PFDN1 construct, and stained for DNA. Scale bar, 10 μm. E–H, Time-lapse imaging of Caco2 clones. Caco2 clones from either control or PFDN1 overexpression (E–F) as well as those from either FILIP1L knockdown or FILIP1L/PFDN1 double knockdown (G–H) were analyzed as described in Fig. 4H and I. Mitotic length (E and G) and cytokinesis completion (F and H) were quantified from three independent experiments. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001; NS, nonsignificant. I and J, Clones from either control or FILIP1L knockdown (Caco2 and SW620 clones) as well as those from either control or PFDN1 overexpression (Caco2 clones) were stained for pericentrin (green), PFDN1 (red), and DAPI (blue; I), and the area of centrosome occupied by PFDN1 in metaphase-cells was quantified (J). The y-axis (frequency) represents the total number of metaphase cells that fall into each bin. Scale bar, 5 μm.
IHC
Experimental details were followed as described previously (14). Images were acquired by AxioImager microscope (Zeiss). For stitched images, they were acquired by EVOS FL Auto microscope (Thermo Fisher Scientific).
WST1 cell proliferation assay
Various colon cancer cell lines were seeded in 96-well plates (2.5 × 103 cells per well) and incubated with WST1 (Millipore Sigma, #5015944001) for 1 hour. Cell proliferation by WST1 incorporation was measured using a Synergy Mx microplate reader (Biotek) daily up to 4 days after cell plating.
Statistical analysis
Data are presented as the mean ± SEM. Statistical analyses were performed using a two-tailed Student t test (GraphPad Prism 6.0 [RRID:SCR_002798]), and differences were considered statistically significant at P < 0.05.
Results
FILIP1L negatively regulates xenograft growth, and its knockdown induces mucin secretion and multinucleation in colon cancer in vivo
We and others have shown that FILIP1L is downregulated, and its low expression is associated with a poor prognosis in colon cancer (12, 15). Thus, we examined the consequences of FILIP1L modulation on mouse xenograft tumor growth. We stably overexpressed FILIP1L in low-expressing colon cancer lines (HT29 and HCT116). Immunoblotting confirmed increased FILIP1L levels (Supplementary Fig. S1A). In nude mouse xenografts, overexpression of FILIP1L caused a statistically significant >6-fold and >2-fold inhibition of tumor growth in HT29 and HCT116 cells, respectively (Fig. 1A–C; Supplementary Figs. S1B–S1D), confirming the tumor suppressor function of FILIP1L. IHC staining of FILIP1L confirmed increased FILIP1L levels in the tumors from FILIP1L-overexpressing clones (Fig. 1D).
Figure 1.
FILIP1L levels affect colon xenograft tumor growth, and its knockdown induces mucin secretion as well as multinucleation in colon xenograft tumors. A, HT29 (1.5 × 106) clones of either control or FILIP1L+ derivatives were subcutaneously injected into the nude mice (8 mice per cell line). Tumor growth was measured, every 2 to 3 days for a total of 37 days. The y-axis represents tumor volume that was calculated by the formula: (length × width × height × 0.52). B and C, Pictures of mice and HT29 xenograft tumors at the time of sacrifice (B) as well as tumor weights (C) are shown. D, HT29 xenograft tumors from either control or FILIP1L+ derivatives were fixed and IHC stained for FILIP1L. E–H, Caco2 (5 × 106) clones of either control or FILIP1L-knockdown derivatives were subcutaneously injected into the nude mice (8 mice per cell line). E and F, Tumor growth (E) was measured every 2 to 3 days for a total of 29 days, as described in A, and tumor weights at the time of sacrifice (F) were measured. G, Caco2 xenograft tumors from either control or FILIP1L-knockdown derivatives were fixed and stained with H&E and PAS. They were also IHC stained for FILIP1L. Scale bar, 50 μm. H, Enlarged images of Ki67-stained Caco2 xenograft tumors from either control or FILIP1L-knockdown derivatives are shown. Arrows, clumpy multinucleated cells. Scale bar, 10 μm. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Knockdown of FILIP1L significantly enhanced tumor growth nearly 3-fold compared with controls (Fig. 1E and F). IHC staining and immunoblotting confirmed decreased FILIP1L expression (Fig. 1G; Supplementary Fig. S2A). Although tumors from both groups demonstrated poor differentiation and high Ki67 index (∼90%) as determined by two clinical pathologists (PJ and ZZ), considerably more compact cells were observed in FILIP1L-knockdown groups (shown by hematoxylin and eosin (H&E) staining in Fig. 1G; Supplementary Fig. S2B). FILIP1L-knockdown tumors also demonstrated increased mucin expression [stained by Periodic Acid Schiff (PAS); Fig. 1G]. PAS-stained stitched images of whole tumors also clearly demonstrated considerably increased mucin expression in FILIP1L-knockdown tumors (Supplementary Fig. S2B). Light pink regions in PAS-stained cells indicated acellular/cyst areas that were depleted of mucin, and comprised more than 50% of the total tumor volume in control tumors, but very few to absent cysts in tumors from FILIP1L knockdown cells. In addition, considerably increased tight clusters were detected in the tumors from FILIP1L-knockdown clones (Fig. 1H), suggesting increased multinucleation in these tumors.
Mucinous colon tumors have decreased expression of FILIP1L
MAC is a distinct form of colorectal cancer, characterized by abundant mucin secretion (2). Having demonstrated increased mucin expression following FILIP1L knockdown in vivo, we examined the expression of FILIP1L in atypical serrated polyps closely associated with mucinous differentiation (35). IHC staining demonstrated that FILIP1L localizes in the apical surfaces of the normal colon (Fig. 2B), and its expression is reduced in serrated polyps (Fig. 2D). We subsequently examined human MAC samples as well as nontumor adjacent colon tissues (NAT). Human MAC samples (4 well/moderately and 12 poorly differentiated) demonstrated a significantly decreased FILIP1L expression (Fig. 2F and H; representative) compared with their matched NATs (Fig. 2I). Supporting our observation, FILIP1L mRNA expression was also significantly downregulated in MAC, in an Oncomine public dataset (Fig. 2J). In line with previous reports (12, 15), samples from nonmucinous colorectal adenocarcinoma (9 well/moderately and 7 poorly differentiated) also demonstrated a significantly decreased FILIP1L expression compared with their matched NATs (Fig. 2K).
Figure 2.
FILIP1L is downregulated in mucinous colon cancer. H&E staining (A, C, E, and G) and FILIP1L expression (B, D, F, and H) was analyzed in specimens from NATs (n = 16), serrated polyps (n = 9), well/moderately differentiated mucinous adenocarcinoma (n = 4), and poorly differentiated mucinous adenocarcinoma (n = 12). Note that multinucleated cells were not present in serrated polyps samples. Scale bar, 50 μm. I, FILIP1L expression in IHC stained slides (as shown in panels B, F, and H) was compared between matched normal (n = 16) and mucinous colon adenocarcinoma (n = 16). Expression score was carried out as described in Materials and Methods. J, FILIP1L mRNA expression was compared between normal (n = 5) and mucinous colon adenocarcinoma (n = 13). Data are derived from Oncomine public databases [Kaiser Colon (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE5206)]. K, NATs (n = 16), well/moderately differentiated nonmucinous colorectal adenocarcinoma (n = 9), and poorly differentiated nonmucinous colorectal adenocarcinoma (n = 7) were stained for FILIP1L, and expression score was carried out as described in I. L, DNA ploidy in 16 MAC tumors used in I was analyzed. FILIP1L expression score of each tumor is shown in table, and either diploid or aneuploid tumors were plotted against FILIP1L expression score. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
As described earlier, approximately half of MAC tumors are aneuploid (3–7). To identify if FILIP1L downregulation is related with aneuploidy status of the MACs, we examined DNA ploidy from the MAC tumors that were used in Fig. 2I. Pathologist scored FILIP1L staining under blinded conditions ahead of ploidy experiments. We first tested control tissues from normal colon as well as poorly differentiated non-mucinous colon tumors. As shown in flow cytometric DNA histograms, DAPI-stained nuclei from normal colon tissues (Diploid CTL) displayed a single peak around 104 DAPI-area, whereas those from nonmucinous colon tumors (Aneuploid CTL) displayed a right-shifted peak around 105 DAPI-area (Supplementary Fig. S3). DAPI-area of right-shifted peak was gated and calculated for three normal colon tissues (Diploid CTL), three nonmucinous colon tumors (Aneuploid CTL) and 16 MAC tumors. Average percentage of three diploid and aneuploid controls was 14.3 ± 0.94 and 84.3 ± 12.4, respectively. On the basis of the average percentage of diploid controls, we defined “aneuploid tumor” as those with larger than 20%. We then plotted diploid/aneuploid status against previously analyzed FILIP1L expression score. Although most MAC tumors demonstrated a significantly decreased FILIP1L expression compared with their matched NATs (Fig. 2I), aneuploid MAC tumors demonstrated a significantly decreased FILIP1L expression compared with diploid MAC tumors (Fig. 2L). In total, these results suggest that FILIP1L downregulation is associated with neoplastic changes in aneuploid MAC.
Tissue restricted FILIP1L loss in mouse colon induces mucin secretion as well as hyperplasia
To address the in vivo consequence of FILIP1L gene inactivation in the colon, we generated colon-specific Filip1l conditional knockout mice. Using the Cre-loxP system, we successfully obtained Filip1l homozygous alleles (Fig. 3A). Filip1lfl/fl mice were then crossed with Cdx2-CreERT2 transgenic mice that express a tamoxifen (TAM)-regulated Cre protein (CreERT2) for deletion of loxP-containing alleles in adult terminal ileum, cecum, colon, and rectal epithelia (36). We observed a partial gene deletion efficiency from the Filip1l conditional allele that FILIP1L mRNA expression was reduced by approximately 3-fold in Filip1lfl/fl; Cdx2-CreERT2 mice (CKO) compared with Filip1lfl/fl mice (CTL; Fig. 3B). Four weeks after TAM induction in Filip1l CKO mice, H&E staining demonstrated a significantly elongated crypts as well as compromised crypt integrity, as evidenced by aberrant cell arrangements and irregular nuclei (Fig. 3C and D). FILIP1L expression was reduced in CKO mice (Fig. 3E). Importantly, although FILIP1L reduction was observed throughout the entire colon, crypt elongation/integrity loss was mainly restricted to the proximal location, where human MAC development usually occurs (Fig. 3C; Supplementary Fig. S4; ref. 2). MUC2 mucin is predominantly secreted by colonic goblet cells (37). PAS staining (Fig. 3F) and qRT-PCR (Fig. 3H) demonstrated a significant increase in MUC2 expression in the colon crypts of Filip1l CKO mice. Notably, although the expression of stem cell markers such as Lgr5 was not changed, other markers for goblet cells and secretory progenitors were also significantly increased (Fig. 3H; refs. 38, 39). Although cell proliferation was restricted to the bottom third-to-half of colonic crypts in CTL mice, it was evident in most cells of elongated crypts in Filip1l CKO mice (Fig. 3G). Quantification of Ki67 staining confirmed cellular hyperplasia in the colons of Filip1l CKO mice. (Fig. 3I and J). Collectively these results lead us to conclude that FILIP1L downregulation is associated with mucin secretion and the aneuploidy-phenotypes seen in MAC, and drives hyperplasia in normal colon epithelial cells.
FILIP1L knockdown induces cytokinesis defects
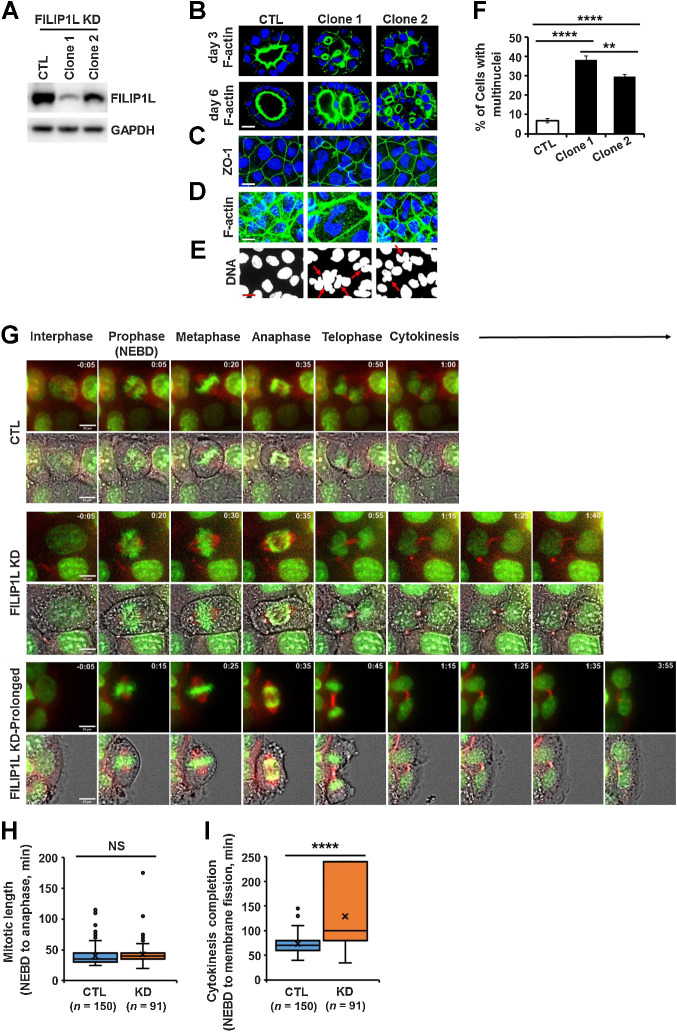
The epithelium acts as a selectively permeable barrier, comprised of tightly associated polarized cells forming lumens. Defects in epithelial architecture are the source of nearly 90% of human cancers (40–42). To identify the effects of FILIP1L down-regulation on epithelial architecture and to have clear insights into whether FILIP1L loss contributes to the generation of aneuploidy, we knocked down its expression in normal diploid MDCK.2 cells, a well-studied epithelial model (43–49). Immunoblotting confirmed decreased FILIP1L levels (Fig. 4A). Clones were then cultured in an extracellular matrix to form 3D cysts. Single lumen-containing cysts were formed by control cells as expected. Also, consistent with our observations that downregulation of FILIP1L in tumor xenografts was characterized by the presence of tightly packed multinuclear cells, the majority of cysts formed by FILIP1L-knockdown clones contained multiple lumens, further suggesting loss of FILIP1L causes impaired cytokinesis (Fig. 4B; ref. 50). Staining for the tight junction marker, ZO-1 showed well-segregated single-cell junctions in control cells with single nuclei, whereas multiple nuclei were often present in tight junction boundaries of FILIP1L-knockdown clones (Fig. 4C). F-actin staining outlining the cell periphery confirmed this phenotype (Fig. 4D). A significant increase in multinuclei in FILIP1L-knockdown cells compared with controls (Fig. 4E; indicated by arrows) confirmed defects in cytokinesis (Fig. 4F; ref. 51). In addition, the level of FILIP1L knockdown demonstrated dosage effects on multinuclei formation, as clone 1 generated a significantly more multinuclei than clone 2 (Fig. 4F).
Figure 4.
FILIP1L knockdown results in defective cytokinesis. A, FILIP1L knockdown was achieved by CRISPR-Cas9 system in MDCK.2 cells. FILIP1L and GAPDH control were detected by immunoblotting. Two independent clones were tested. B, MDCK.2 clones were grown in the presence of Matrigel, and three-dimensional cysts were imaged following staining for F-actin and nuclei at days 3 and 6. C–F, MDCK.2 clones were grown in monolayer and stained for Z0–1 (C), F-actin (D), and DNA (E). F, Clones were stained for Z0–1 and DNA, and the number of cells with multinuclei were quantified. Over 400 cells were counted. G–I, Time-lapse imaging of Caco2 clones that were incubated with SPY-595 DNA and SPY-650-tubulin fluorescent dyes. G, Representative tiled images of mitotic progression in control and FILIP1L knockdown clones. FILIP1L knockdown clones exhibiting prolonged cytokinesis are also shown (third panel). Within each panel of tiled images, the top strip shows fluorescent images only and the bottom strip shows fluorescent images overlaid with phase contrast. Scale bar, 20 μm. H and I, Mitotic length (H) and cytokinesis completion (I) from control and FILIP1L knockdown clones were quantified from three independent experiments. **, P < 0.01; ****, P < 0.0001; NS, nonsignificant.
To further identify the defects in cytokinesis following FILIP1L-knockdown in colon cancer cells, we examined Caco2 clones (shown in Supplementary Fig. S2A) by live imaging. Caco2 clones were marked for DNA and tubulin, and cells entering mitosis were monitored every 5 minutes. Interestingly, we observed that FILIP1L-knockdown caused cells to grow on top of each other as shown in Video A. We first examined whether mitotic length was affected by FILIP1L-knockdown. Mitotic length was shown to be determined by the time between nuclear envelope breakdown (NEBD) to anaphase (52–54). Control clones were in mitosis for an average of 35 minutes, which was similar to what was observed for various cancer cells (52–54). No significant changes in mitotic length were observed in FILIP1L-knockdown clones compared with control [representative time-lapse images (Fig. 4G), videos (Video B), and quantified data (Fig. 4H) are shown], suggesting the cytokinesis defects were independent of mitosis. To directly test if the defect was in cytokinesis, we measured the time for membrane fission, the final step in cytokinesis (55, 56). As shown in Fig. 4I, FILIP1L-knockdown clones demonstrated a significant delay to complete cytokinesis compared with controls. We could not detect clear membrane fission for up to 4 hours (maximum experiment duration) in 25% of cells from FILIP1L-knockdown clones. We therefore set the time to fission as 240 minutes for these cells, although the actual time was longer. Thus, these findings suggest that FILIP1L-knockdown leads to cytokinesis defects.
FILIP1L colocalizes with its binding partner PFDN1 at centrosomes in each phase of mitosis
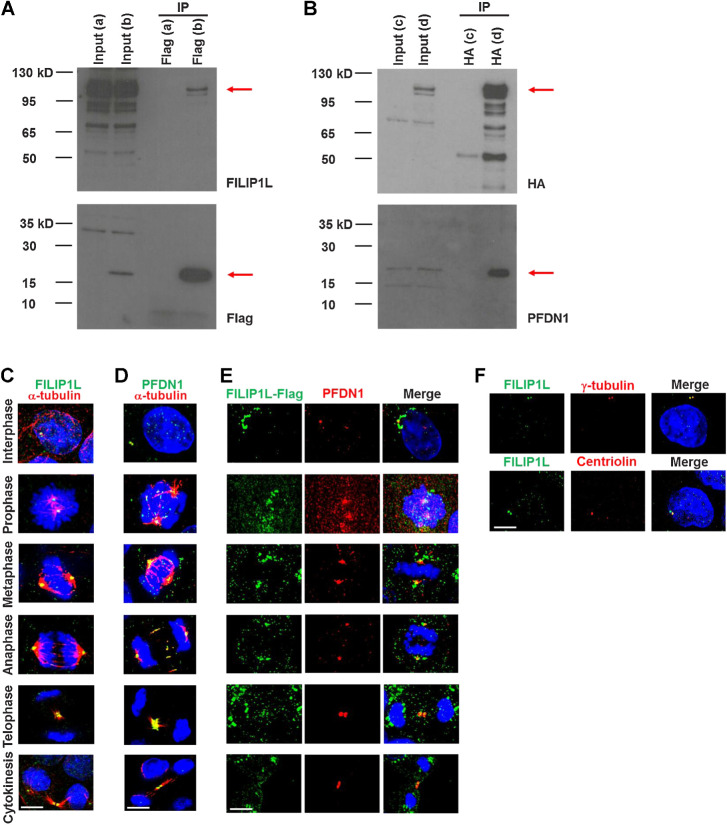
To explore the mechanisms responsible for FILIP1L's tumor suppressor activity, we set out to find binding partners for FILIP1L. We identified prefoldin 1 (PFDN1) from yeast two-hybrid screening (a top hit out of 30 positive colonies using a stringent screening system). We subsequently confirmed this interaction by coimmunoprecipitation. HEK293 cells were cotransfected with Flag-PFDN1 and FILIP1L-HA plasmids, and cell lysates were immunoprecipitated with an anti-Flag antibody followed by immunoblotting for FILIP1L and Flag tag (Fig. 5A). Cell lysates were also immunoprecipitated with an anti-HA antibody followed by immunoblotting for HA tag and PFDN1 (Fig. 5B). PFDN1 is a molecular chaperone of a six subunit-prefoldin complex that facilitates proper folding of key cytoskeletal components such as actin and tubulins. Loss of PFDN1 leads to a decrease in tubulin levels, resulting in reduced microtubule growth, defects in cell division and embryonic lethality in C. elegans (21). We previously showed that FILIP1L localizes in the cytoplasm and centrosomes of interphase cells (11). Because centrosomes are a major microtubule-organizing center in the cell, and PFDN1 is a tubulin chaperone, we hypothesized that PFDN1 also localizes at centrosomes. As shown by α-tubulin and DNA staining, endogenous FILIP1L and PFDN1 localize at the centrosomes in all phases of division (Fig. 5C and D). To further test if they colocalize, we transfected cells with Flag-tagged FILIP1L, then labeled exogenous FILIP1L and endogenous PFDN1 due to unavailability of different species-antibodies for the proteins. As shown in Fig. 5E, FILIP1L and PFDN1 colocalize at the centrosomes in every cell phase. In addition, unlike centriolin that localizes only at the mother centriole, FILIP1L and PFDN1 colocalize at both the mother and daughter centrioles (Fig. 5D and F). To further confirm their colocalization, we cotransfected HEK293 cells with plasmids expressing FILIP1L-eGFP and mCherry-PFDN1, and monitored the cells by time-lapse imaging. As shown in Supplementary Fig. S5 and Video C, exogenous FILIP1L and PFDN1 demonstrated colocalization in the perinuclear area where centrosomes are located.
Figure 5.
FILIP1L binds to and colocalizes with its binding partner PFDN1 at centrosomes in each phase of mitosis. HEK293T cells were transfected with either FILIP1L-HA and control-Flag vector (a), FILIP1L-HA and Flag-PFDN1 (b, d), or control-HA vector and Flag-PFDN1 (c). Twenty-four hours later, cell lysates were immunoprecipitated using Flag antibody-agarose, followed by immunoblotting for FILIP1L and Flag tag (A) or using HA antibody-agarose followed by immunoblotting for HA tag and PFDN1 (B). Input control (4 μg lysates) was also immunoblotted. C–F, MDCK.2 cells were immunofluorescently stained for FILIP1L (C) or PFDN1 (green; D) and α-tubulin (red). Nuclei were counterstained with DAPI (blue). Cell phase was determined by α-tubulin and DNA stain. A merged image is shown. E, MDCK.2 cells were transfected with a FILIP1L-Flag construct and stained for Flag tag (green) and PFDN1 (red) at 24 hours after transfection. F, MDCK.2 cells were stained for FILIP1L (green) and γ-tubulin or centriolin (red). Scale bar, 5 μm.
FILIP1L regulates PFDN1 levels at the centrosomes in a proteasome-dependent manner
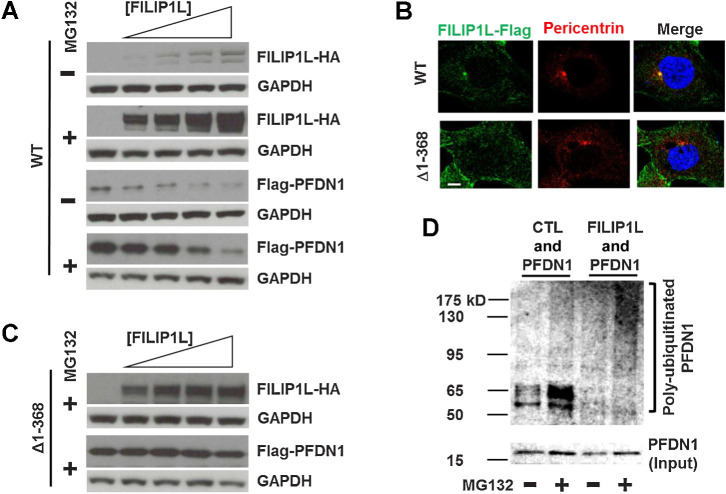
Centrosomes have been identified as a proteolytic center of the cell (57–60). In addition, we previously showed that FILIP1L colocalizes with proteasomes in centrosomes (11), and that it plays a role in proteasome-dependent protein degradation (18). Many molecules regulating cell division are located at the centrosomes and their level of expression has to be tightly regulated, as proliferating cells continually change their cell phase. Thus, just like molecules involved in cell-cycle regulation, the levels of many centrosomal proteins regulating cell division are controlled by proteasome-mediated degradation in a time-dependent manner (61). To determine if the binding of FILIP1L to PFDN1 results in proteasome-dependent degradation of PFDN1, we cotransfected HEK293 cells with a constant amount of Flag-PFDN1 and an increasing amount of FILIP1L-HA, in the presence or absence of the proteasome inhibitor MG132. As shown by immunoblotting, FILIP1L expression increased with plasmid dose, and this correlated with a decrease in PFDN1 levels (Fig. 6A). Proteasomal inhibition rescued the levels of PFDN1, indicating that degradation might be mediated by the proteasome. Many centrosomal proteins harbor coiled-coil domains (31). FILIP1L (893 amino acids) also has a coiled-coil domain at amino acids 1–542 and two leucine zippers (83–104 and 218–239; ref. 17). Leucine zippers have been shown to mediate protein–protein interactions (62). A FILIP1L truncation mutant that lacks its two leucine zippers (Δ1–368) no longer colocalizes with pericentrin, a centrosome marker (Fig. 6B) and fails to modulate PFDN1 protein levels (Fig. 6C). We then determined that FILIP1L enhances polyubiquitination of PFDN1, a major signal for proteasome-mediated degradation (Fig. 6D). Together, these results suggest that binding of FILIP1L to PFDN1 occurs at the centrosomes, their interaction requires the leucine zipper domain of FILIP1L, and their stoichiometry appears important for maintaining PFDN1 levels through a proteasome-dependent mechanism.
Figure 6.
FILIP1L regulation of PFDN1 levels is proteasome-dependent in centrosomes. A, HEK293T cells were transfected with FILIP1L-HA (1.2 μg) and Flag-PFDN1 (lane 1, none; lane 2, 0.6 μg; lane 3, 1.2 μg; lane 4, 2.4 μg; lane 5, 3.6 μg) constructs. Twenty-four hours later, cell lysates were immunoblotted for HA tag, Flag tag, and GAPDH (control). The experiment was also performed in the presence of 10 μmol/L MG132 (proteasome inhibitor). B, HEK293T cells were transfected with FILIP1L-Flag constructs expressing wild-type or (Δ1–368) and stained for Flag tag (green) and pericentrin (red). Nuclei were counterstained with DAPI (blue). Scale bar, 10 μm. C, The same set of experiments as A was performed using FILIP1L (Δ1–368) construct instead of wild-type in the presence of 10 μmol/L MG132. D, HEK293T cells were transfected with Flag-PFDN1 and either control vector or FILIP1L-HA. Twenty-four hours later, cell lysates were immunoprecipitated using ubiquitin antibody, followed by immunoblotting for PFDN1. Input control (2 μg lysates) was also immunoblotted for PFDN1 that indicates unmodified Flag-PFDN1 protein.
FILIP1L knockdown induces mucin secretion in colon cancer, mediated by PFDN1
Next, we examined the relationship between FILIP1L and PFDN1 in the context of colon cancer. Expression of FILIP1L and PFDN1 was inversely correlated in a panel of colon cancer cell lines (Fig. 7A). Although FILIP1L proteins were previously undetectable by immunoblot using mouse monoclonal FILIP1L antibody (12), differential FILIP1L expression was observed in this panel of colon cancer cell lines using a rabbit polyclonal FILIP1L antibody. We also determined that FILIP1L-low expressing colon cancer cell lines were proliferating faster than FILIP1L-high expressing colon cancer cell lines (Fig. 7B). Using lentiviral transduction, we knocked down FILIP1L in Caco2, SW620, and Ls174T colon cancer lines. Immunoblotting confirmed that knockdown of FILIP1L resulted in increased PFDN1 expression (Fig. 7C). In addition, overexpression of FILIP1L in HT29 and HCT116 cells resulted in decreased PFDN1 expression (Supplementary Fig. S6A). Furthermore, expression of FILIP1L did not change PFDN1 transcription levels, as confirmed by qRT-PCR (Supplementary Fig. S6B), further indicating that modulation of PFDN1 by FILIP1L likely occurs at the protein level.
Xenograft tumors from FILIP1L-overexpressing clones also demonstrated decreased PFDN1 expression (Supplementary Fig. S6C). In human MAC samples, FILIP1L and PFDN1 were decreased and increased, respectively (Supplementary Figs. S6L, S6O, S6M, and S6P). PFDN1 was previously reported to be overexpressed in colon cancer, and its high expression was associated with poor survival in patients with colon cancer (20). Notably, the pattern of PFDN1 distribution was also markedly different between MAC tumors and matched NATs. In normal colon crypts, it localized to the perinuclear/cytoplasmic area, whereas in MAC tumor tissues it mainly localized in the nucleus (Supplementary Figs. S6G and S6P). Interestingly, nuclear localization of PFDN1 was not identified in nonmucinous colorectal tumor tissues (Supplementary Fig. S6S). It has been previously shown that PFDN1 not only functions as a molecular chaperone in the cytoplasm but also regulates gene expression in the nucleus (19, 63). PFDN1 was increased in the areas of the colon where FILIP1L expression was reduced from Filip1l conditional knockout mice (Supplementary Fig. S6T). Thus, these results collectively suggest that FILIP1L modulates PFDN1 protein levels both in vitro and in vivo in colon cancer. We next postulated that increased PFDN1 levels may be responsible for the phenotypes seen when FILIP1L is downregulated.
Expression/secretion of mucin proteins is often altered in colon cancer (64, 65). MAC is characterized by abundant mucin secretion comprising at least 50% of the tumor volume (2). Mucin 2 (MUC2) is the predominantly secreted mucin synthesized by colonic goblet cells (37), and its overexpression is frequently found in MAC (35). We have shown that FILIP1L is downregulated in human MAC samples (Fig. 2I) and that mucin secretion was increased following FILIP1L knockdown in both Caco2-xenograft tumors (Fig. 1G) and colons from Filip1l conditional knockout mice (Fig. 3F). On the other hand, mucin secretion was decreased following FILIP1L overexpression in HT29-xenograft tumors (Supplementary Fig. S6C).
We then aimed to determine whether MUC2 mRNA expression is altered following modulation of FILIP1L/PFDN1 levels. FILIP1L knockdown or PFDN1 overexpression did not increase MUC2 transcription levels, as confirmed by qRT-PCR (Supplementary Fig. S7A). Thus, we asked whether the cellular localization of MUC2 is changed. FILIP1L-knockdown clones cultured in three-dimensional extracellular matrix demonstrated a significantly increased secretion of MUC2 (first and second panels in Fig. 7D and E). For these experiments, we could not use SW620 clones because MUC2 levels were too low to be detected (Supplementary Fig. S7B). It is noteworthy that MUC2 secretion was increased following FILIP1L-knockdown in both enterocyte-like Caco2 cells and goblet cell-like Ls174T cells (66). Moreover, PFDN1 overexpression in Caco2 cells also resulted in the same phenotype as FILIP1L knockdown (third panels in Fig. 7D and E; Supplementary Fig. S7C). To further prove cause-and-effect, we tested if PFDN1 knockdown can reverse the mucin secretion-phenotype resulting from FILIP1L knockdown. Using lentiviral transduction, we knocked down PFDN1 in FILIP1L-knockdown Caco2 clones. Immunoblotting confirmed that knockdown of PFDN1 in double-knockdown clones (Fig. 7F). Mucin secretion was significantly decreased in FILIP1L/PFDN1 double-knockdown clones compared with FILIP1L knockdown clones (Fig. 7G), suggesting that PFDN1 mediates the mucin secretion-phenotype following FILIP1L knockdown.
FILIP1L knockdown leads to multinucleation in colon cancer, mediated by PFDN1
Approximately 50% of MAC tumors are aneuploid (3–7), and aneuploid tumors are clinically more aggressive than diploid MAC tumors (8, 9). We have demonstrated that FILIP1L knockdown results in multinuclei phenotype in the xenograft tumors from Caco2 clones (Fig. 1H) as well as in normal MDCK.2 cells (Fig. 4F). We have also demonstrated that FILIP1L expression was significantly decreased in aneuploid MAC tumors compared with diploid MAC tumors (Fig. 2L). Thus, we tested whether FILIP1L-knockdown modulates aneuploidy-related phenotypes. FILIP1L-knockdown clones from colon cancer cell lines demonstrated significantly increased numbers of multilobed or multinucleated cells compared with their respective control cell lines (first and second panels in Fig. 8A and B). For these experiments, we could not use Ls174T clones because they tend to grow on top of each other, preventing accurate quantification. In Caco2 cells, smaller fragments of nuclei that are stained by both markers of nuclei (DAPI) and nuclear envelope (Lamin A/C) were also significantly increased. We have named these “budding nuclei” (blue bar graph in Fig. 8B). In addition, cells with larger nuclei are often observed in Caco2 cells with FILIP1L-knockdown (first panel in Fig. 8A), suggesting the prevalence of polyploid cells derived from incomplete mitosis. Representative images of multinuclei quantification are shown in Supplementary Fig. S8A. PFDN1 overexpression also resulted in similar multilobular/polyploid phenotypes as FILIP1L knockdown (third panel in Fig. 8A and B). In addition, the multinuclei phenotype was also demonstrated in MDCK.2 cells when PFDN1 was overexpressed (Fig. 8D).
Defects in cytokinesis leads to increased multinuclei cells (51), and we demonstrated that FILIP1L knockdown resulted in cytokinesis defects (Fig. 4I). As shown in FILIP1L knockdown, PFDN1 overexpression in Caco2 colon cancer cells also resulted in cytokinesis defects. Although mitotic length was not affected (Fig. 8E), the time to cytokinesis completion was significantly increased (Fig. 8F). Furthermore, while mitotic length was not affected (Fig. 8G), the time to cytokinesis completion was significantly decreased in FILIP1L/PFDN1 double-knockdown clones compared with FILIP1L knockdown clones (Fig. 8H), further suggesting that PFDN1 mediates the cytokinesis defects-phenotype following FILIP1L knockdown.
Dysregulated expression of several centrosome-modulating proteins is implicated in human cancer (28–32). Changes in the levels of several centrosomal proteins, including prefoldins, have been linked to dysregulation of centrosome function (21, 25–29). Centrosome abnormalities lead to mitosis/cytokinesis defects that are associated with aneuploidy (23). We demonstrated that binding of FILIP1L to PFDN1 occurs at centrosomes (Fig. 5C–E) and centrosomal localization of FILIP1L is critical for its modulation of PFDN1 levels (Fig. 6B and C). Thus, we examined whether FILIP1L levels affect centrosomal localization of PFDN1. Although overall PFDN1 levels were increased following FILIP1L knockdown, there was substantially reduced localization of PFDN1 in centrosomes, suggesting that FILIP1L is required for centrosomal localization of PFDN1 (first and second panels in Fig. 8I). Consistent with the other phenotypes shown in Figs. 7D and E and 8A and B, PFDN1 overexpression also resulted in similarly reduced localization of PFDN1 in centrosomes as had FILIP1L knockdown (third panel in Fig. 8I). Display curves for centrosomal occupancy were left-shifted (Fig. 8J) and a significantly lower percentage of PFDN1 protein was detected in either FILIP1L knockdown or PFDN1 overexpressed cells (Supplementary Fig. S8B). Thus, these findings suggest that the phenotypes such as mucin secretion and aneuploidy in human aneuploid MAC samples are recapitulated by FILIP1L knockdown or PFDN1 overexpression in colon cancer cells.
Discussion
Colon adenocarcinoma arises through the adenoma-carcinoma sequence characterized by chromosomal instability with an associated accumulation of genetic alterations in tumor suppressor genes such as APC and TP53 (67). MAC is a histologic subtype of colon adenocarcinoma with distinct clinical and histopathologic characteristics, as well as molecular signatures (2). Colon cancers arisen from atypical serrated polyps closely associate with a CpG Island Methylator Phenotype (CIMP: tumor suppressor genes are inactivated by widespread epigenetic silencing; ref. 68), microsatellite instability, BRAF p.V600E mutation (69–71), mismatch repair deficiency and mucinous differentiation (35). We previously showed that FILIP1L expression is downregulated by promoter methylation in ovarian cancer as well as cancer cell lines from various histology (10, 12). FILIP1L downregulation in MAC might recapitulate CIMP phenotype. Thus, it will be interesting to identify whether FILIP1L is downregulated by promoter methylation in MAC tumors, and DNA demethylating agents can restore the phenotypes observed from FILIP1L knockdown.
CRAD knockout mice were shown to induce epithelial cell integrity loss and Wnt signaling activation, resulting in the development of intestinal mucinous adenoma (72). However, the exact aberrations that are responsible for MAC development are currently unknown. Approximately 50% of MAC tumors are aneuploid (3–7), which are clinically more aggressive than diploid MAC tumors (8, 9). We demonstrate here that FILIP1L is significantly downregulated throughout the spectrum of well to poorly differentiated MAC (Fig. 2I), and that FILIP1L is significantly more decreased in aneuploid MAC tumors than in diploid MAC tumors (Fig. 2L). FILIP1L knockdown leads to a significant increase in multinucleation (that leads to aneuploidy; Fig. 8A and B) and cytokinesis defects in colon cancer cells. Colon cancer cell lines such as Caco2 and SW620 that we demonstrated aneuploidy phenotype are already highly aneuploid cancer cell lines (73), yet we observed a significant increase in multinucleation following both FILIP1L knockdown and PFDN1 overexpression (Fig. 8A and B). Simultaneous knockdown of PFDN1 in FILIP1L-knockdown cells rescued the cytokinesis defects-phenotype in these cancer cells (Fig. 8H). To have clear insights into whether FILIP1L loss contributes to the generation of aneuploidy, we also knockdown FILIP1L in normal diploid MDCK.2 cells and demonstrated the same aneuploidy phenotypes (increase in multinucleation and cytokinesis defects; Fig. 4B–F). PFDN1 overexpression in MDCK.2 cells also led to increase in multinucleation (Fig. 8D). Thus, these results strongly suggest that loss of FILIP1L plays a role in the generation of aneuploidy in vivo.
Activation of mutant Kras in mouse colon tissues promotes hyperplasia and increased goblet cell numbers, but the change in goblet cell numbers may simply reflect the increase in the transit amplifying population and/or cell differentiation promoted by Kras mutation (74). In this study, we showed that increased mucin secretion was detected as early as day 7 following Filip1l loss in the mouse colon (Fig. 3H). However, cellular hyperplasia was not identified until 4 weeks following Filip1l loss (Fig. 3G, I, and J). Expression of stem cell markers such as Lgr5 was not changed (Fig. 3H). In addition, both FILIP1L knockdown and PFDN1 overexpression led to a significant increase in mucin protein secretion in colon cancer cells (Fig. 7D and E), and simultaneous knockdown of PFDN1 in FILIP1L-knockdown cells rescued the mucin secretion-phenotype (Fig. 7G). These findings suggest that increased mucin secretion is not simply resulted from increased cell proliferation. It is currently unknown how mucin secretion-phenotype is mechanistically related with aneuploidy phenotype. Further studies are warranted.
Many factors that regulate cell mitosis are located at centrosomes, and changes in their levels deregulate the cell cycle. Altered expression of centrosomal proteins is implicated in human cancer (24, 75). The levels of centrosome components are tightly regulated by timely, proteasome-mediated degradation (76). Here, we show that the tumor suppressor FILIP1L is downregulated in MAC with a concomitant increase in PFDN1, a chaperone that binds to FILIP1L. Both of these proteins normally localize to centrosomes, and changes in their levels are associated with mitosis/cytokinesis failure. Furthermore, we show that FILIP1L stimulates proteasomal degradation of PFDN1. A minor weakness that needs to be followed up is that we have yet to show the interactions between endogenous FILIP1L and PFDN1 proteins due to the lack of working antibodies.
Centrosomes not only serve as a primary source of microtubules that build mitotic spindles but also determine the orientation of the mitotic spindle and the cytokinetic furrow. Mitotic spindle misorientation has been shown to be one of the key mechanisms generating multiple lumen-containing cysts (50), which we observe in our FILIP1L-knockdown epithelial cells (Fig. 4B). Loss of PFDN1 leads to mitotic spindle misorientation and mispositioning of cytokinetic furrows (21, 30). Either downregulation or up-regulation of prefoldin proteins has been linked to dysregulation of centrosome-associated function (21, 25–27). UXT, a prefoldin-like protein expressed in centrosomes is also overexpressed in human cancers, and its overexpression reduces microtubule growth and subsequently promotes centrosome disassembly (27). Noncanonical prefoldin mutants showed cytokinesis defects characterized by multipolar spindles and polyploid cells (51). Cytokinetic defects result in multinucleated cells that contain extra centrosomes, which in turn disrupt mitotic spindle formation (24). Aurora A kinase is a centrosomal protein that regulates cytokinesis. At cytokinesis, it localizes at the spindle midzone where it performs a regulatory function. Its overexpression in breast cancers leads to centrosome accumulation secondary to cytokinesis failure (77, 78). FILIP1L and PFDN1 also strongly localize at the spindle midzone in telophase and cytokinesis, which supports their potential function in regulating cytokinesis (Fig. 5C–E).
Tumor suppressor genes, including APC, PTEN, and VHL have been linked to spindle misorientation (79–85). In fact, loss of the tumor suppressor APC results in spindle misorientation followed by cell fate changes, leading to colon adenocarcinoma development (36, 86). However, none of these tumor suppressors have been shown to be associated with MAC pathogenesis. One possibility is that the pathologic changes following FILIP1L loss are the result of the unopposed function of its binding partner, PFDN1. Although PFDN1 is a molecular chaperone that facilitates folding of tubulins and actin, the cytoskeletal function of PFDN1 is not essential for the housekeeping assembly of microtubules or actin filaments. However, it becomes rate-limiting, and the most upstream regulator under strong cytoskeleton biogenesis conditions such as mitosis and B-lymphocyte activation as Pfdn1 KO mice are severely affected in these processes (63). Colon epithelium is one of the fastest regenerating organs in the body, so it can be highly susceptible to the effects of PFDN1 upregulation.
It has been shown that prefoldin not only functions as a molecular chaperone in the cytoplasm but also regulates gene expression in the nucleus (63). Overexpressed PFDN1 bound to the cyclin A2 promoter and the subsequent repression of cyclin A2 was associated with EMT promotion in lung cancer cells (19). We demonstrate here that not only is the PFDN1 level upregulated but it also is located in the nucleus in human MAC samples (Supplementary Fig. S6P). However, we could not identify nuclear PFDN1 in nonmucinous colorectal adenocarcinoma samples (Supplementary Fig. S6S). Thus, it is noteworthy to identify whether nuclear PFDN1 plays a role in MAC development.
In summary, we have shown that a tumor suppressor FILIP1L stimulates proteasomal degradation of its binding partner PFDN1, a molecular chaperone that regulates spindle orientation and cleavage-furrow positioning (21, 30). We showed that human mucinous colon tumors have decreased and increased expression of FILIP1L and PFDN1, respectively. FILIP1L knockdown and the resultant PFDN1 increase leads to increased mucin secretion and mitosis/cytokinesis defects in mouse colon as well as colon cancer cells, recapitulating the same phenotypes as seen in aggressive aneuploid MAC. These results strongly implicate FILIP1L as the essential regulator of MAC tumorigenesis. Since FILIP1L is downregulated in various other cancer types (10–12), these studies will also have a broad impact on our understanding of the pathogenesis of other cancers and the role played by this novel tumor suppressor gene.
Authors' Disclosures
S.R. Pine reports other support from Rutgers Cancer Institute of New Jersey, personal fees from NIH, grants from NIH, Rutgers Cancer Institute of New Jersey, American Lung Association, and New Jersey Commission for Cancer Research outside the submitted work; also has a patent for Adult pluripotent stem cells, US patent Ser. No. 11/133,596 issued to New York Medical College and a patent for Manipulation of stem cell function by p53 isoforms, US patent Ser No. 61/389,134, August 7, 2013, issued to NIH; and reports equity in Celvive Inc. outside the scope of the submitted work. No disclosures were reported by the other authors.
Supplementary Material
8 Supplementary figures and 1 Supplementary info.
PowerPoint file containing embedded videos.
Acknowledgments
This work was supported in part by the Office of the Assistant Secretary of Defense for Health Affairs through the Ovarian Cancer Research Program under Award No. W81XWH-15-1-0369 (to M. Kwon and S. Libutti). Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. This work was also supported in part by NCI-CCSG P30CA072720 (to S.K. Libutti) through the use of Shared Resource Facilities. The authors thank Histopathology, Biorepository, Genome Editing, and Immune Monitoring & Advanced Genomics Cores at Rutgers Cancer Institute of New Jersey.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Authors' Contributions
M. Kwon: Conceptualization, data curation, supervision, funding acquisition, validation, investigation, writing–original draft. G. Rubio: Investigation. N. Nolan: Investigation. P. Auteri: Investigation. J.A. Volmar: Investigation. A. Adem: Investigation. P. Javidian: Formal analysis, validation. Z. Zhou: Formal analysis, validation. M.P. Verzi: Data curation, writing–review and editing. S.R. Pine: Data curation, writing–review and editing. S.K. Libutti: Conceptualization, resources, supervision, funding acquisition, writing–review and editing.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Hugen N, Brown G, Glynne-Jones R, de Wilt JH, Nagtegaal ID. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol 2016;13:361–9. [DOI] [PubMed] [Google Scholar]
- 3. Chang SC, Lin JK, Yang SH, Wang HS, Li AF, Chi CW. Relationship between genetic alterations and prognosis in sporadic colorectal cancer. Int J Cancer 2006;118:1721–7. [DOI] [PubMed] [Google Scholar]
- 4. Purdie CA, Piris J. Histopathological grade, mucinous differentiation and DNA ploidy in relation to prognosis in colorectal carcinoma. Histopathology 2000;36:121–6. [PubMed] [Google Scholar]
- 5. Cianchi F, Balzi M, Becciolini A, Giache V, Messerini L, Palomba A, et al. Correlation between DNA content and p53 deletion in colorectal cancer. Eur J Surg 1999;165:363–8. [DOI] [PubMed] [Google Scholar]
- 6. Silvestrini R, D'Agnano I, Faranda A, Costa A, Zupi G, Cosimelli M, et al. Flow cytometric analysis of ploidy in colorectal cancer: a multicentric experience. Br J Cancer 1993;67:1042–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang B, Liu CG, Wang MW, Zhao DH, Li Y, Ji XL. DNA content and its relationship with pathology and prognosis of colorectal carcinoma. Chin Med J 1992;105:241–6. [PubMed] [Google Scholar]
- 8. Hugen N, Simmer F, Mekenkamp LJ, Koopman M, van den Broek E, de Wilt JH, et al. Reduced rate of copy number aberrations in mucinous colorectal carcinoma. Oncotarget 2015;6:25715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu XP, Sato T, Oga A, Ikemoto K, Kawauchi S, Ikeda E, et al. Two subtypes of mucinous colorectal carcinoma characterized by laser scanning cytometry and comparative genomic hybridization. Int J Oncol 2004;25:615–21. [PubMed] [Google Scholar]
- 10. Burton ER, Gaffar A, Lee SJ, Adeshuko F, Whitney KD, Chung JY, et al. Downregulation of Filamin A interacting protein 1-like is associated with promoter methylation and induces an invasive phenotype in ovarian cancer. Mol Cancer Res 2011;9:1126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwon M, Lee SJ, Wang Y, Rybak Y, Luna A, Reddy S, et al. Filamin A interacting protein 1-like inhibits WNT signaling and MMP expression to suppress cancer cell invasion and metastasis. Int J Cancer 2014;135:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwon M, Lee SJ, Reddy S, Rybak Y, Adem A, Libutti SK. Down-regulation of filamin ainteracting protein 1-like is associated with promoter methylation and an invasive phenotype in breast, colon, lung and pancreatic cancers. PLoS One 2013;8:e82620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwon M, Hanna E, Lorang D, He M, Quick JS, Adem A, et al. Functional characterization of filamin a interacting protein 1-like, a novel candidate for antivascular cancer therapy. Cancer Res 2008;68:7332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwon M, Kim JH, Rybak Y, Luna A, Choi CH, Chung JY, et al. Reduced expression of FILIP1L, a novel WNT pathway inhibitor, is associated with poor survival, progression and chemoresistance in ovarian cancer. Oncotarget 2016;7:77052–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park YL, Park SY, Lee SH, Kim RB, Kim JK, Rew SY, et al. Filamin A interacting protein 1-like expression inhibits progression in colorectal cancer. Oncotarget 2016;7:72229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwon M. Epithelial-to-mesenchymal transition and cancer stem cells: emerging targets for novel cancer therapy. Cancer Gene Ther 2014;21:179–80. [DOI] [PubMed] [Google Scholar]
- 17. Kwon M, Libutti SK. Filamin A interacting protein 1-like as a therapeutic target in cancer. Expert Opin Ther Targets 2014;18:1435–47. [DOI] [PubMed] [Google Scholar]
- 18. Hu Y, Mivechi NF. Promotion of heat shock factor Hsf1 degradation via adaptor protein filamin A-interacting protein 1-like (FILIP-1L). J Biol Chem 2011;286:31397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Shi W, Tang Y, Liu Y, He K, Hu Y, et al. Prefoldin 1 promotes EMT and lung cancer progression by suppressing cyclin A expression. Oncogene 2017;36:885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang P, Zhao J, Yang X, Guan S, Feng H, Han D, et al. PFDN1, an indicator for colorectal cancer prognosis, enhances tumor cell proliferation and motility through cytoskeletal reorganization. Med Oncol 2015;32:264. [DOI] [PubMed] [Google Scholar]
- 21. Lundin VF, Srayko M, Hyman AA, Leroux MR. Efficient chaperone-mediated tubulin biogenesis is essential for cell division and cell migration in C. elegans. Dev Biol 2008;313:320–34. [DOI] [PubMed] [Google Scholar]
- 22. Verma V, Maresca TJ. Microtubule plus-ends act as physical signaling hubs to activate RhoA during cytokinesis. eLife 2019;8:e38968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lens SMA, Medema RH. Cytokinesis defects and cancer. Nat Rev Cancer 2019;19:32–45. [DOI] [PubMed] [Google Scholar]
- 24. Cosenza MR, Kramer A. Centrosome amplification, chromosomal instability and cancer: mechanistic, clinical and therapeutic issues. Chromosome Res 2016;24:105–26. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Rai M, Wang C, Gonzalez C, Wang H. Prefoldin and Pins synergistically regulate asymmetric division and suppress dedifferentiation. Sci Rep 2016;6:23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delgehyr N, Wieland U, Rangone H, Pinson X, Mao G, Dzhindzhev NS, et al. Drosophila Mgr, a Prefoldin subunit cooperating with von Hippel Lindau to regulate tubulin stability. Proc Natl Acad Sci U S A 2012;109:5729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao H, Wang Q, Zhang H, Liu Q, Du X, Richter M, et al. UXT is a novel centrosomal protein essential for cell viability. Mol Biol Cell 2005;16:5857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J, Dallmayer M, Kirchner T, Musa J, Grunewald TGP. PRC1: linking cytokinesis, chromosomal instability, and cancer evolution. Trends Cancer 2018;4:59–73. [DOI] [PubMed] [Google Scholar]
- 29. Hagemann N, Ackermann N, Christmann J, Brier S, Yu F, Erdmann KS. The serologically defined colon cancer antigen-3 interacts with the protein tyrosine phosphatase PTPN13 and is involved in the regulation of cytokinesis. Oncogene 2013;32:4602–13. [DOI] [PubMed] [Google Scholar]
- 30. Srayko M, Kaya A, Stamford J, Hyman AA. Identification and characterization of factors required for microtubule growth and nucleation in the early C. elegans embryo. Dev Cell 2005;9:223–36. [DOI] [PubMed] [Google Scholar]
- 31. Jeffery J, Sinha D, Srihari S, Kalimutho M, Khanna KK. Beyond cytokinesis: the emerging roles of CEP55 in tumorigenesis. Oncogene 2016;35:683–90. [DOI] [PubMed] [Google Scholar]
- 32. Huhn SC, Liu J, Ye C, Lu H, Jiang X, Feng X, et al. Regulation of spindle integrity and mitotic fidelity by BCCIP. Oncogene 2017;36:4750–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi WT, Tsai JH, Rabinovitch PS, Small T, Huang D, Mattis AN, et al. Diagnosis and risk stratification of Barrett's dysplasia by flow cytometric DNA analysis of paraffin-embedded tissue. Gut 2018;67:1229–38. [DOI] [PubMed] [Google Scholar]
- 34. Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 2006;7:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsh MD, Clendenning M, Williamson E, Pearson SA, Walters RJ, Nagler B, et al. Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod Pathol 2013;26:1642–56. [DOI] [PubMed] [Google Scholar]
- 36. Feng Y, Sentani K, Wiese A, Sands E, Green M, Bommer GT, et al. Sox9 induction, ectopic Paneth cells, and mitotic spindle axis defects in mouse colon adenomatous epithelium arising from conditional biallelic Apc inactivation. Am J Pathol 2013;183:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winterford CM, Walsh MD, Leggett BA, Jass JR. Ultrastructural localization of epithelial mucin core proteins in colorectal tissues. J Histochem Cytochem 1999;47:1063–74. [DOI] [PubMed] [Google Scholar]
- 38. Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, et al. A single-cell survey of the small intestinal epithelium. Nature 2017;551:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeineldin M, Neufeld K. Isolation of Epithelial Cells from Mouse Gastrointestinal Tract for Western Blot or RNA Analysis. Bio-protocol 2012;2:e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCaffrey LM, Macara IG. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol 2011;21:727–35. [DOI] [PubMed] [Google Scholar]
- 41. St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell 2010;141:757–74. [DOI] [PubMed] [Google Scholar]
- 42. Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol 2011;21:R126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schluter MA, Margolis B. Apical lumen formation in renal epithelia. J Am Soc Nephrol 2009;20:1444–52. [DOI] [PubMed] [Google Scholar]
- 44. Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 2010;12:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bryant DM, Roignot J, Datta A, Overeem AW, Kim M, Yu W, et al. A molecular switch for the orientation of epithelial cell polarization. Dev Cell 2014;31:171–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodriguez-Fraticelli AE, Bagwell J, Bosch-Fortea M, Boncompain G, Reglero-Real N, Garcia-Leon MJ, et al. Developmental regulation of apical endocytosis controls epithelial patterning in vertebrate tubular organs. Nat Cell Biol 2015;17:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 2008;183:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 2007;4:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. do Amaral JB, Rezende-Teixeira P, Freitas VM, Machado-Santelli GM. MCF-7 cells as a three-dimensional model for the study of human breast cancer. Tissue Eng Part C Methods 2011;17:1097–107. [DOI] [PubMed] [Google Scholar]
- 50. Lujan P, Rubio T, Varsano G, Kohn M. Keep it on the edge: The post-mitotic midbody as a polarity signal unit. Commun Integr Biol 2017;10:e1338990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hintermair C, Voss K, Forne I, Heidemann M, Flatley A, Kremmer E, et al. Specific threonine-4 phosphorylation and function of RNA polymerase II CTD during M phase progression. Sci Rep 2016;6:27401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rogers S, McCloy RA, Parker BL, Gallego-Ortega D, Law AMK, Chin VT, et al. MASTL overexpression promotes chromosome instability and metastasis in breast cancer. Oncogene 2018;37:4518–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caldon CE, Burgess A. Label free, quantitative single-cell fate tracking of time-lapse movies. MethodsX 2019;6:2468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dalton WB, Yu B, Yang VW. p53 suppresses structural chromosome instability after mitotic arrest in human cells. Oncogene 2010;29:1929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mierzwa B, Gerlich DW. Cytokinetic abscission: molecular mechanisms and temporal control. Dev Cell 2014;31:525–38. [DOI] [PubMed] [Google Scholar]
- 56. Chao HW, Doi M, Fustin JM, Chen H, Murase K, Maeda Y, et al. Circadian clock regulates hepatic polyploidy by modulating Mkp1-Erk1/2 signaling pathway. Nat Commun 2017;8:2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet 2005;6:194–205. [DOI] [PubMed] [Google Scholar]
- 58. Bahmanyar S, Kaplan DD, Deluca JG, Giddings TH Jr, O'Toole ET, Winey M, et al. beta-Catenin is a Nek2 substrate involved in centrosome separation. Genes Dev 2008;22:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet 2007;39:1350–60. [DOI] [PubMed] [Google Scholar]
- 60. Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, et al. Dynamic association of proteasomal machinery with the centrosome. J Cell Biol 1999;145:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vora SM, Phillips BT. The benefits of local depletion: the centrosome as a scaffold for ubiquitin-proteasome-mediated degradation. Cell Cycle 2016;15:2124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haren L, Normand C, Polard P, Alazard R, Chandler M. IS911 transposition is regulated by protein-protein interactions via a leucine zipper motif. J Mol Biol 2000;296:757–68. [DOI] [PubMed] [Google Scholar]
- 63. Millan-Zambrano G, Chavez S. Nuclear functions of prefoldin. Open biology 2014;4:140085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev 2004;23:77–99. [DOI] [PubMed] [Google Scholar]
- 65. Pothuraju R, Krishn SR, Gautam SK, Pai P, Ganguly K, Chaudhary S, et al. Mechanistic and functional shades of mucins and associated glycans in colon cancer. Cancers 2020;12:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bu XD, Li N, Tian XQ, Huang PL. Caco-2 and LS174T cell lines provide different models for studying mucin expression in colon cancer. Tissue Cell 2011;43:201–6. [DOI] [PubMed] [Google Scholar]
- 67. Vogelstein B, Fearon ER, Kern SE, Hamilton SR, Preisinger AC, Nakamura Y, et al. Allelotype of colorectal carcinomas. Science 1989;244:207–11. [DOI] [PubMed] [Google Scholar]
- 68. Toyota M, Ahuja N, Suzuki H, Itoh F, Ohe-Toyota M, Imai K, et al. Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res 1999;59:5438–42. [PubMed] [Google Scholar]
- 69. Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 2006;38:787–93. [DOI] [PubMed] [Google Scholar]
- 70. Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut 2004;53:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nagasaka T, Koi M, Kloor M, Gebert J, Vilkin A, Nishida N, et al. Mutations in both KRAS and BRAF may contribute to the methylator phenotype in colon cancer. Gastroenterology 2008;134:1950–60, 60.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jung YS, Wang W, Jun S, Zhang J, Srivastava M, Kim MJ, et al. Deregulation of CRAD-controlled cytoskeleton initiates mucinous colorectal cancer via beta-catenin. Nat Cell Biol 2018;20:1303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Melcher R, Koehler S, Steinlein C, Schmid M, Mueller CR, Luehrs H, et al. Spectral karyotype analysis of colon cancer cell lines of the tumor suppressor and mutator pathway. Cytogenet Genome Res 2002;98:22–8. [DOI] [PubMed] [Google Scholar]
- 74. Feng Y, Bommer GT, Zhao J, Green M, Sands E, Zhai Y, et al. Mutant KRAS promotes hyperplasia and alters differentiation in the colon epithelium but does not expand the presumptive stem cell pool. Gastroenterology 2011;141:1003–13.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van Ree JH, Nam HJ, van Deursen JM. Mitotic kinase cascades orchestrating timely disjunction and movement of centrosomes maintain chromosomal stability and prevent cancer. Chromosome Res 2016;24:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang Y, Galardy PJ. Ubiquitin, the centrosome, and chromosome segregation. Chromosome Res 2016;24:77–91. [DOI] [PubMed] [Google Scholar]
- 77. Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. Embo j 2002;21:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang D, Hirota T, Marumoto T, Shimizu M, Kunitoku N, Sasayama T, et al. Cre-loxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene 2004;23:8720–30. [DOI] [PubMed] [Google Scholar]
- 79. Caldwell CM, Green RA, Kaplan KB. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J Cell Biol 2007;178:1109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fleming ES, Temchin M, Wu Q, Maggio-Price L, Tirnauer JS. Spindle misorientation in tumors from APC(min/+) mice. Mol Carcinog 2009;48:592–8. [DOI] [PubMed] [Google Scholar]
- 81. Quyn AJ, Appleton PL, Carey FA, Steele RJ, Barker N, Clevers H, et al. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 2010;6:175–81. [DOI] [PubMed] [Google Scholar]
- 82. Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol 2001;3:433–8. [DOI] [PubMed] [Google Scholar]
- 83. Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol 2001;3:429–32. [DOI] [PubMed] [Google Scholar]
- 84. Toyoshima F, Nishida E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. Embo j 2007;26:1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Thoma CR, Toso A, Gutbrodt KL, Reggi SP, Frew IJ, Schraml P, et al. VHL loss causes spindle misorientation and chromosome instability. Nat Cell Biol 2009;11:994–1001. [DOI] [PubMed] [Google Scholar]
- 86. Feng Y, Sakamoto N, Wu R, Liu JY, Wiese A, Green ME, et al. Tissue-specific effects of reduced beta-catenin expression on adenomatous polyposis coli mutation-instigated tumorigenesis in mouse colon and ovarian epithelium. PLoS Genet 2015;11:e1005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
8 Supplementary figures and 1 Supplementary info.
PowerPoint file containing embedded videos.



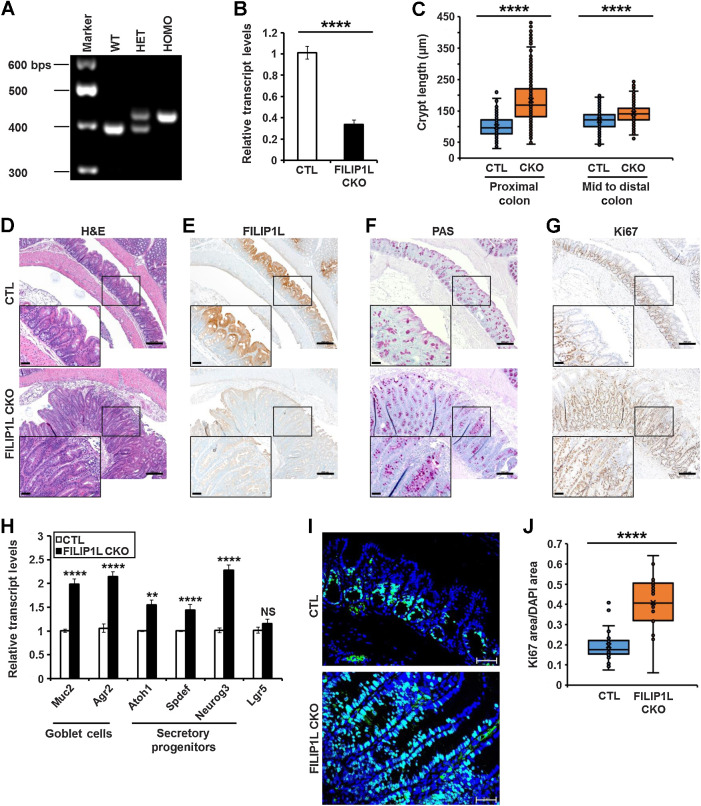
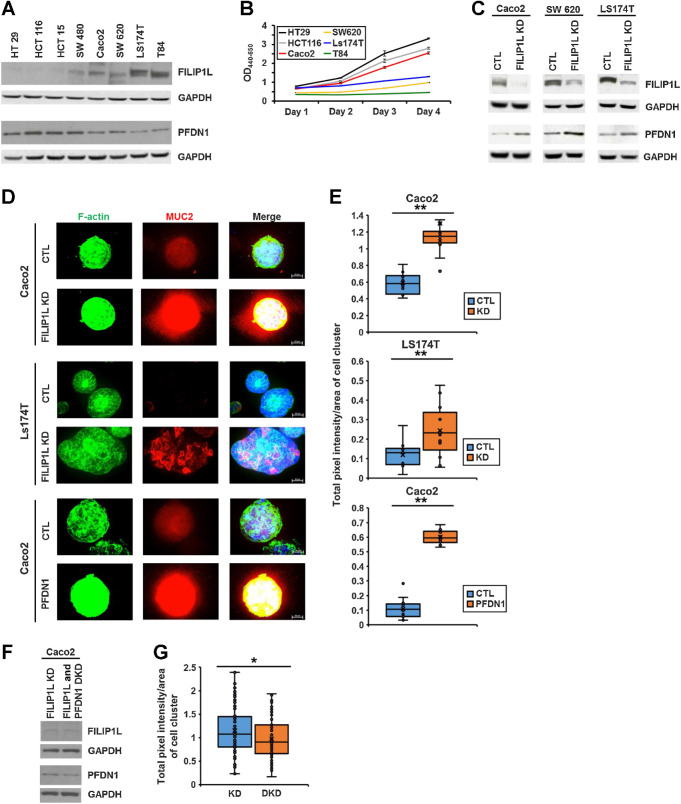
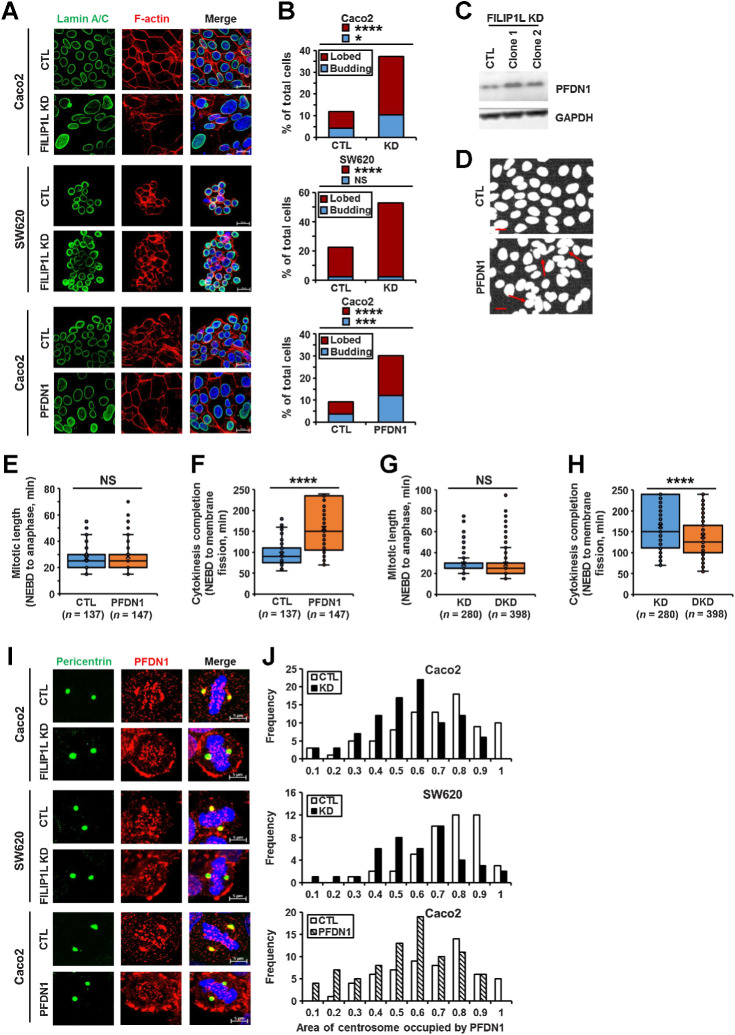
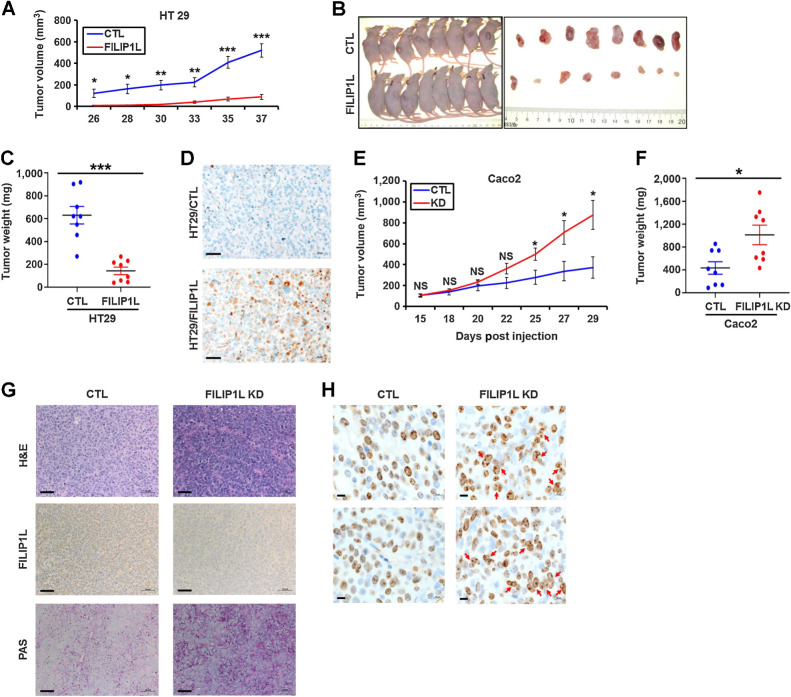
![Figure 2. FILIP1L is downregulated in mucinous colon cancer. H&E staining (A, C, E, and G) and FILIP1L expression (B, D, F, and H) was analyzed in specimens from NATs (n = 16), serrated polyps (n = 9), well/moderately differentiated mucinous adenocarcinoma (n = 4), and poorly differentiated mucinous adenocarcinoma (n = 12). Note that multinucleated cells were not present in serrated polyps samples. Scale bar, 50 μm. I, FILIP1L expression in IHC stained slides (as shown in panels B, F, and H) was compared between matched normal (n = 16) and mucinous colon adenocarcinoma (n = 16). Expression score was carried out as described in Materials and Methods. J, FILIP1L mRNA expression was compared between normal (n = 5) and mucinous colon adenocarcinoma (n = 13). Data are derived from Oncomine public databases [Kaiser Colon (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE5206)]. K, NATs (n = 16), well/moderately differentiated nonmucinous colorectal adenocarcinoma (n = 9), and poorly differentiated nonmucinous colorectal adenocarcinoma (n = 7) were stained for FILIP1L, and expression score was carried out as described in I. L, DNA ploidy in 16 MAC tumors used in I was analyzed. FILIP1L expression score of each tumor is shown in table, and either diploid or aneuploid tumors were plotted against FILIP1L expression score. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/cc8a/9397615/c10b5e6db1ae/5523fig2.jpg)