Abstract
Bifunctional alkylating agents and other drugs which produce DNA interstrand cross-links (ICLs) are among the most effective antitumor agents in clinical use. In contrast to agents which produce bulky adducts on only one strand of the DNA, the cellular mechanisms which act to eliminate DNA ICLs are still poorly understood, although nucleotide excision repair is known to play a crucial role in an early repair step. Using haploid Saccharomyces cerevisiae strains disrupted for genes central to the recombination, nonhomologous end-joining (NHEJ), and mutagenesis pathways, all these activities were found to be involved in the repair of nitrogen mustard (mechlorethamine)- and cisplatin-induced DNA ICLs, but the particular pathway employed is cell cycle dependent. Examination of whole chromosomes from treated cells using contour-clamped homogenous electric field electrophoresis revealed the intermediate in the repair of ICLs in dividing cells, which are mostly in S phase, to be double-strand breaks (DSBs). The origin of these breaks is not clear since they were still efficiently induced in nucleotide excision and base excision repair-deficient, mismatch repair-defective, rad27 and mre11 disruptant strains. In replicating cells, RAD52-dependent recombination and NHEJ both act to repair the DSBs. In contrast, few DSBs were observed in quiescent cells, and recombination therefore seems dispensable for repair. The activity of the Rev3 protein (DNA polymerase ζ) is apparently more important for the processing of intermediates in stationary-phase cells, since rev3 disruptants were more sensitive in this phase than in the exponential growth phase.
Many clinically important anticancer drugs such as those from the nitrogen mustard class, as well as many agents in development, exert their antitumor effects through the production of DNA interstrand cross-links (ICLs) (26, 47). Agents such as cisplatin can also produce ICLs, but intrastrand adducts may also contribute to cytotoxicity (15). It is well established that nucleotide excision repair (NER) plays a key early role in the repair of ICLs (3, 8, 27, 37, 39), but little is known about the nature of the incisions at these lesions, the resulting repair intermediates, and how they are resolved. The NER pathway acting on DNA intrastrand cross-links (e.g., UV-induced dipyrimidine photoproducts and the intrastrand cross-links produced by cisplatin) in eukaryotes is well understood (22); excision of a 24- to 32-mer lesion-containing oligonucleotide is followed by repair synthesis and ligation. However, ICLs pose a unique problem because a one-step NER reaction releasing the DNA-drug adduct on one side of the cross-link leaves an oligonucleotide-drug moiety attached to the complementary strand. Since this will act as a block to DNA polymerases, the resynthesis-ligation portion of the NER reaction cannot proceed efficiently.
It has been suggested that the information necessary to complete repair can be obtained by recombination with a sister chromatid or homolog (32) or by mutagenic DNA synthesis across the gap (10, 16, 40) or that a second NER reaction occurs, leading to a double-strand break (DSB) (27, 29). Bessho et al. (6) have previously highlighted the reasons why conventional NER models might be inadequate to account for the repair of ICLs, since they require that a “bubble” be unwound around the lesion prior to incision. Such a step may be blocked by an ICL. These authors demonstrated that mammalian cells make, on one strand, two normal incisions 5′ to the ICL and not around it, and they suggest that this may act as a recombinogenic signal. However, in vitro repair assays indicate that the pathway operating in Escherichia coli does involve dual incisions 5′ and 3′ to the ICL followed by the initiation of RecA-mediated recombination (11, 12, 56, 64).
The types of recombination repair used in eukaryotes and higher organisms will depend on the nature of the ICL repair intermediate produced following NER incisions. If the repair intermediate is a gap, resulting from an incomplete NER reaction or stalled replication fork, recombinational repair which relies upon Rad52-mediated transfer of homologous information, preferentially from a sister chromatid, will predominate (32). If the intermediate is a DSB, due to dual incisions at the ICL or a replication fork meeting two closely opposed single-strand incisions, a potential role for nonhomologous end joining (NHEJ) can be postulated in addition to the RAD52 pathway (41, 42). In Saccharomyces cerevisiae, the pathways controlled by the RAD52 gene include almost all types of homologous recombination (crossing over and gene conversion) identified (48, 57). In addition, a distinct pathway of DSB end joining, single-strand annealing (SSA), exists which shows dependence on RAD52 (42, 57). In SSA the resected 3′-end single strands of the break are joined through regions of 60 to 90 bp of homology (57) and the resulting overhangs are cleaved by the endonuclease activity of the Rad1/10 heterodimer (21, 28). Although NHEJ also involves homology-mediated end joining, only 1 to 5 bp of precise homology is required (33) and the apparatus which mediates this process is distinct from that required for SSA. The activities known to function in NHEJ in S. cerevisiae so far include the end binding of the yKu70/yKu80 heterodimer (7, 38, 62, 63), 5′ overhang cleavage by Rad27 (67), and rejoining by DNA ligase IV (60) as well as Mre11, Rad50, Xrs2, and Lif1 (43).
To explore whether the incisions produced at ICLs are repaired by recombination (either homologous or NHEJ), we have measured the sensitivities of mutants with mutations in these pathways to the DNA ICL agents nitrogen mustard (mechlorethamine) and cisplatin. Both these agents produce ICLs as only a fraction of the total damage (10% or less) (15, 26), but in both cases it is known that NER is required for the early steps of repair (8, 65). It is clear that in a rapidly dividing (exponential-phase) culture there is a requirement for recombination, but this is abolished in stationary-phase cultures (where cells are not dividing) and a REV3 dependent mutagenic pathway is used instead. Using contour-clamped homogenous electric field electrophoresis (CHEF) to separate whole chromosomes, we demonstrated that this is probably due to an association between the replication of DNA containing ICLs and the formation of DSBs. CHEF analysis also confirmed that the repair of these DSBs is defective in recombination- and NHEJ-deficient cells.
MATERIALS AND METHODS
Chemicals and enzymes.
Analytical grade mechlorethamine (nitrogen mustard or HN2) was purchased from Sigma Chemical Co. (Poole, United Kingdom). Mono-nitrogen mustard (HN1 or 2-dimethylaminoethylchloride hydrochloride), 99% pure, was obtained from Aldrich (Gillingham, United Kingdom). Cisplatin (100-mg/100-ml injectable aqueous stock solution containing 900 mg/100 ml of sodium chloride and 100 mg/100 ml of mannitol) was obtained from David Bull Laboratories. All enzymes used were purchased from Promega UK.
Yeast strains and cell culture.
The yeast strains used in this study are listed in Table 1. Cells were grown at 28°C in yeast extract-peptone-dextrose YEPD (25), or in synthetic complete medium supplemented with the appropriate amino acids and bases at recommended levels (25), with the exception of the rad27 strain, which was grown at 25°C. All rad27 colonies used for experiments were simultaneously tested for temperature sensitivity to ensure that suppressors had not accumulated (58).
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source (reference) |
|---|---|---|
| DBY747 | MATa his3-Δ1 leu2-3,112 trp1-289 ura3-52 | W. Xiao (68) |
| WXY9387 | DBY747 with rad52::LEU2 | W. Xiao (69) |
| WXY9376 | DBY747 with rad6::LEU2 | W. Xiao (69) |
| WXY9326 | DBY747 with rad18::TRP1 | W. Xiao (69) |
| WXY9382 | DBY747 with rev3::LEU2 | W. Xiao (69) |
| WXY9394 | DBY747 with rad4::hisG-URA3-hisG | W. Xiao (69) |
| WXY9573 | DBY747 with rad2::TRP1 | W. Xiao (68) |
| PJM31 | DBY747 with rad2::TRP1 rad1::KanMX4 | This study |
| WXY9395 | DBY747 with mag1::hisG rad4::hisG-URA3-hisG | W. Xiao (69) |
| LP14Δ | DBY747 with rad14::URA3 | R. Waters (50) |
| W303-1B | MATα ade2-1 trp1-1 his3-11,15 can1-100 ura3-1 leu2-3,112 | S. Jackson (7) |
| yku70Δ | W303-1B with ku70::LEU2 | S. Jackson (7) |
| JDY1 | W303-1B with rad52::TRP1 | S. Jackson |
| JDY2 | W303-1B with ku70::LEU2 rad52::TRP1 | S. Jackson |
| LSY569 | W303-1B with mre11::LEU2 | L. Symington (43) |
| YP1 | MATα leu2y lys2 trp-H ura3-52 ade2-101 his4 cyhR | I. Hickson |
| rad51 (95) | YP1 with rad51::LEU2 | I. Hickson |
| rad54 (26) | YP1 with rad54::LEU2 | I. Hickson |
| rad59 (200) | YP1 with rad59::KanMX4 | I. Hickson |
| RHB2096-1b | MATα trp1::h3 his4-cla leu-2r metl-3-4 lys2-d ade1 ura3 cyhR | R. Borts and R. Brown (19) |
| RBT311 | RHB2096-1b with mlh1::LEU2 | R. Borts and R. Brown (19) |
| SX46Arad27 | MATa ade2 his3-52 trp1-289 ura3-52 rad27::URA3 | S. McCready (49) |
Strain PJM31 was derived from WXY9573 by disruption of the RAD1 gene using the KanMX4 cassette as described by Longtine et al. (36). Two primers bearing 40 bp of homology to the RAD1 gene and 20 bp of overlap with KanMX4 were used to generate the cassette. The two primer sequences were 5′AGA GCA TTT GCT AAA TGT GTA AAA ATA ATA TTG CAC TAT Ccg gat ccc cgg gtt aat taa3′ (forward) and 5′TCA CCA AAT GAA TAT TGT TAT TTT CAC TAT AGT TAA TCG Cga att cga gct cgt tta aac3′ (reverse), where the region with homology to RAD1 is in capital letters and that with homology to KanMX4 is in lowercase letters. G418-resistant transformants were identified as described previously (36), and the disruption was confirmed by PCR.
Survival analysis.
For exponential cultures, liquid YEPD medium was inoculated with a single colony picked from a freshly streaked (YEPD) stock plate and grown overnight at 28°C with vigorous shaking. Cells were counted microscopically, and only cultures with between 2 × 107 and 4 × 107 cells/ml were used. For stationary cultures, cells were grown for 48 to 72 h until the density was between 1 × 108 and 2 × 108 cells/ml. The cells were resuspended in phosphate-buffered saline (PBS) at a density of 2 × 107 cells/ml and 2-ml aliquots were treated with the desired concentration of HN2 (freshly dissolved in cold sterile water) or cisplatin (diluted in cold sterile water) for 60 min at 28°C with vigorous shaking. The cells were harvested, washed twice with 2 ml of PBS, and then diluted and plated in triplicate onto YEPD plates at a density giving rise to 200 colonies per plate in untreated controls. The plates were incubated for 3 days at 28°C and then scored. Any experiments giving rise to more than 250 colonies per plate in untreated controls were rejected.
CHEF analysis of DSB induction and repair.
Cells were grown to exponential or stationary phase in YEPD, harvested, and resuspended in 40 ml of PBS at a density of 2 × 107 cells/ml. HN2 was added to 10-ml aliquots of the cell suspension in 10 μl, and the cells were incubated at 28°C for 3 h in an orbital shaker; 10 μl of water was added to untreated controls. The cells were then harvested and resuspended in 10 ml of minimal medium (0.67% Bacto yeast nitrogen base without amino acids [Difco] and 2% glucose per liter of double-distilled water) and incubated for 1 h at 28°C in an orbital shaker. Subsequently, 6 × 107 cells were harvested from each aliquot and CHEF plugs were prepared using the Bio-Rad yeast CHEF genomic DNA plug kit as instructed by the manufacturer. CHEF was performed with a 1% agarose gel using a Bio-Rad CHEF-DRII apparatus run at 4.5 V/cm for 24 h at 14°C with a switch time of 60 to 120 s. On completion, the gels were stained with ethidium bromide for 1 h, destained overnight, and photographed.
For repair experiments, 30 ml of cells was treated with 30 μl of 100 mM HN2 and incubated at 28°C for 3 h; a 10-ml untreated control to which 10 μl of water had been added was also included. Harvested cells were resuspended in 30 ml of minimal medium, and 10-ml samples were removed at 2, 4, and 24 h posttreatment for CHEF analysis. The untreated control was incubated for 24 h. CHEF plugs and electrophoresis were as described above. Quantitative data were obtained by measuring the absolute integrated optical density of each chromosomal band using a Gel Pro Analyser (Media Cybernetics) and calculating the overall mean fraction of restored full-length chromosome present relative to that in the untreated control.
RESULTS
Anticancer drug sensitivity of recombination and NHEJ mutants.
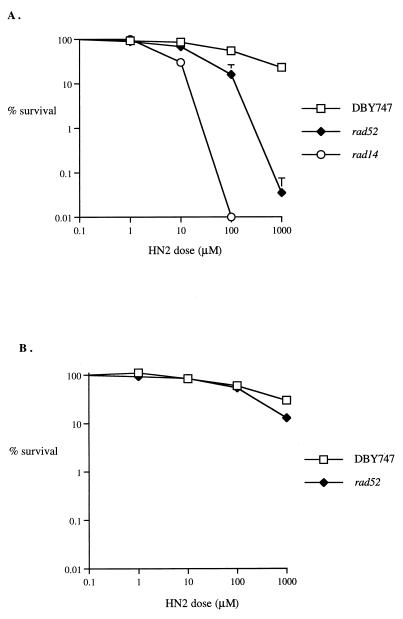
To explore the role of recombination in the repair of anticancer drug-induced ICLs, the sensitivity of a haploid rad52 disruptant strain to HN2 relative to its isogenic parent (DBY747) was determined. Initial experiments were performed using exponentially growing cultures, where the majority of cells are in S phase. Figure 1A demonstrates that rad52 cells show increased sensitivity to HN2 compared to their isogenic parent. Results for an NER-defective strain (rad14), known to be extremely HN2 sensitive (39), are also shown for reference. Since there is a report of lack of HN2 sensitivity in haploid rad52 cells from stationary-phase cultures (55), sensitivity was next examined in this growth phase. In agreement, the rad52 cells demonstrated much greater resistance to HN2 in this growth phase (Fig. 1B). Attempts were made to synchronize cultures by using α-factor to allow sensitivity measurements to be made in the G1 phase of the cell cycle and following synchronous release into the S phase, since this would permit a more clearly defined analysis of the dependence of HN2 sensitivity on the cell cycle stage. These experiments were possible in the parental strain but not in the rad52 strain, where the slow-growth phenotype prevented efficient synchronization as determined by budding-index and fluorescence-activated cell sorter analysis (data not shown). Consequently, all further experiments compared stationary- and exponential-phase cultures.
FIG. 1.
HN2 sensitivity of the rad52 strain (WXY9387) and its isogenic parent (DBY747) in the exponential and stationary growth phases. (A) Exponentially growing cells were treated with the stated doses for 1 h at 28°C. Appropriate dilutions giving around 200 colonies on untreated controls were spread on YEPD plates and incubated for 3 days. Also shown for reference are the results obtained with an isogenic rad14 disruptant. (B) As in panel A but using stationary-phase cells. All results are the means of at least three independent experiments, and the vertical error bars show the standard error of the mean.
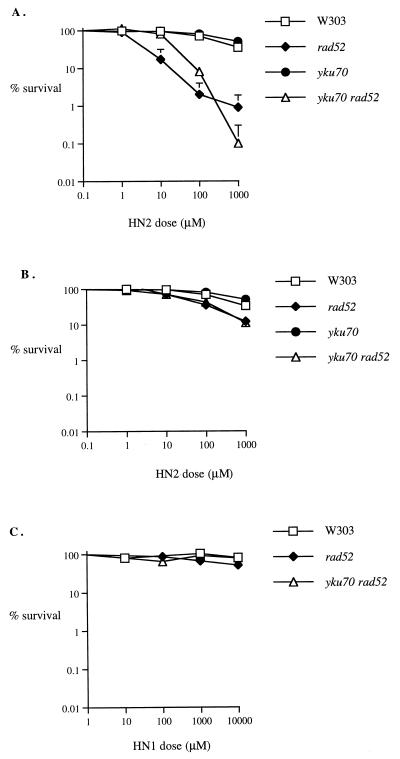
One explanation for the resistance of stationary haploid rad52 cells to HN2 is that another recombinational pathway acts in the absence of RAD52 and predominates in nondividing cells because of the absence of homologous recombination substrates. If DSBs are the repair intermediate, this back-up pathway could be NHEJ. Consequently, the sensitivity to HN2 of a yku70 disruptant strain in both exponential and stationary phases was determined. This strain demonstrates no hypersensitivity relative to its isogenic parent (W303-1B), in either growth phase (Fig. 2A and B). Since it is possible that homologous recombination and NHEJ are redundant during stationary phase but not during exponential growth, where the rad52-mediated events predominate, the sensitivity of a rad52 yku70 double mutant was determined. The double mutant demonstrated no additional sensitivity over the rad52 strain in a stationary-phase culture (Fig. 2B) and no significant increase in an exponential-phase culture (Fig. 2A), suggesting that NHEJ is not a major pathway for survival in either phase. Note that cells from this background, both parental and recombination defective, are slightly more resistant to HN2 than are those derived from DBY747. To rule out the possibility that the monoadducts and not ICLs produced by HN2 were responsible for the recombination repair intermediates, the sensitivity of these strains to HN1, a monofunctional derivative of HN2 not able to form ICLs (52, 53), was tested (Fig. 2C). Exponentially growing yku70, rad52, and yku70 rad52 cells were not significantly hypersensitive to this agent, even at doses 10-fold greater than the highest HN2 dose used.
FIG. 2.
Sensitivity of parental (W303-1B), rad52, yku70, and yku70 rad52 strains to HN2 and the monofunctional mustard HN1. (A) Exponential-phase cells were treated with 0 to 1,000 μM HN2. (B) Stationary-phase cells were treated with 0 to 1,000 μM HN2. (C) Exponential-phase cells were treated with doses from 0 to 10,000 μM HN1. All results are the means of at least three independent experiments, and the vertical error bars show the standard error of the mean.
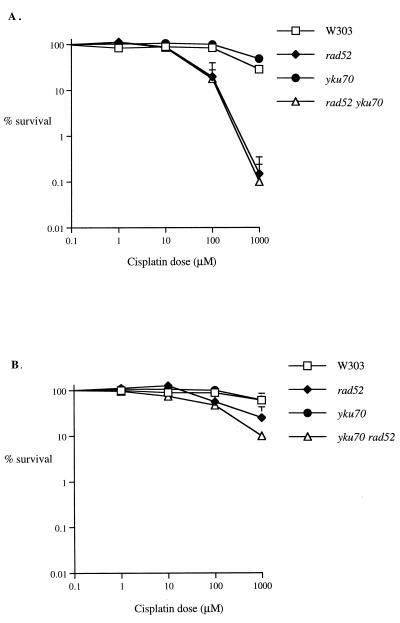
To determine whether the findings for rad52 and NHEJ also applied to other important DNA ICL anticancer drugs, the sensitivity of the recombination and NHEJ mutants to cisplatin was also determined. Figure 3 shows the results of experiments on exponential- and stationary-phase cultures, respectively, and illustrates the similarity in effect between cisplatin and HN2, suggesting that RAD52-mediated recombination is a general feature of drug-induced ICL repair in replicating cells.
FIG. 3.
The sensitivity of parental (W303-1B), rad52, yku70, and yku70 rad52 strains to cisplatin also depends on growth phase. Exponential-phase cells (A) and stationary-phase cells (B) were treated with 0 to 1,000 μM cisplatin, and survival was monitored as described in Materials and Methods. All results are the means of at least three independent experiments, and the vertical error bars show the standard error of the mean.
Rev3 is involved in the repair of ICLs in stationary-phase haploid cells.
The role of recombination in the repair of ICLs is probably related to the unique repair problems they pose. Since ICL adducts involve both DNA strands, additional genetic information is required to complete the repair. The striking HN2 resistance of rad52 stationary-phase cells compared to exponential-phase cells suggests either that an additional repair or tolerance pathway must operate in stationary-phase cells, which lack a homologous recombination substrate, or that intermediates which require recombinational processing arise only in growing cells. These possibilities, however, are not mutually exclusive.
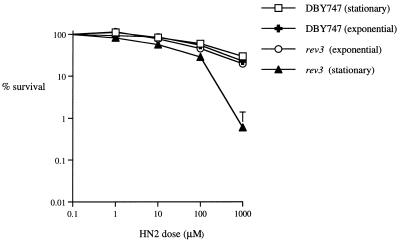
It has previously been suggested that in G1 haploid cells another way of supplying genetic information, albeit in an error-prone fashion, would be to copy past the adducted DNA. Following an initial NER incision at an ICL, this would enable subsequent excision reactions on the other strand to complete repair (10, 40) and could salvage the cell at the cost of being mutagenic. To test this hypothesis, the sensitivities of a number of isogenic strains disrupted for repair genes involved in the mutagenesis-defective RAD6 epistasis group were assessed. rad6 and rad18 disruptants demonstrated sensitivity in both stationary and exponential growth phases (data not shown), but a rev3 disruptant was significantly more sensitive in the stationary phase (Fig. 4). REV3 encodes one subunit of DNA polymerase ζ which is capable of error-prone bypass of several DNA lesion types (5). Since recombination repair utilizing identical information may restore information in S- and G2-phase cells with relatively little error, it might be expected that the REV3-mediated bypass tolerance mechanism would be preferentially utilized in stationary phase. It is striking that the REV3 activity is apparently not able to efficiently rescue exponentially dividing cells following ICL incisions when rad52 is absent. This therefore suggests the existence of different repair intermediates in the two growth phases.
FIG. 4.
Survival following HN2 treatment of parental and rev3 cells with HN2 in the exponential and stationary growth phases. DBY747 and rev3 cells from exponential- or stationary-phase cultures were treated with HN2 at the doses shown, and survival was determined as described in Materials and Methods.
DSBs are repair intermediates in exponentially growing cells.
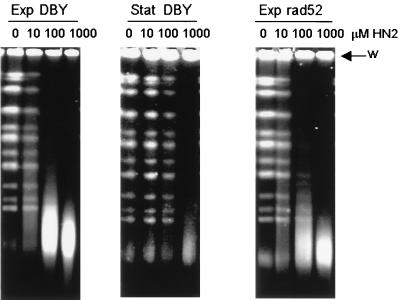
It is clear from Fig. 1 that in exponentially growing cultures recombination is extremely important for the resolution of ICL repair intermediates. Since it well established that DSBs are highly recombinogenic lesions and that they are an intermediate in the repair of DNA-psoralen cross-links in yeast, it was clearly relevant to explore a role for DSBs as an ICL repair intermediate. This was addressed by treating cells with HN2, permitting time for incision in a minimal-nutrient medium that prevents DNA replication (17), and subsequently analyzing chromosomal preparations on CHEF gels (14). Preliminary experiments indicated that incision was maximal by 1 h of posttreatment incubation and that incision was as efficient in the minimal medium as in the rich medium (YEPD) (data not shown). Figure 5 shows typical results obtained when exponential- and stationary-phase cultures were treated with a range of HN2 doses from 0 to 1,000 μM. In the exponentially growing culture, DSBs are evident at the lowest (sublethal) dose (10 μM) and manifest as a loss in intensity in the chromosomal bands and a concomitant dose-dependent increase in a low-molecular-weight DNA smear. In contrast, in the stationary-phase cells, DSBs are evident only at the highest dose employed.
FIG. 5.
Induction of DSBs in exponential-phase (Exp DBY) and stationary-phase (Stat DBY) parental (DBY747) cells and rad52 exponential-phase (Exp rad52) cells determined by CHEF. Cells were treated with 0, 10, 100, and 1,000 μM HN2 for 3 h and subjected to a 1-h posttreatment incubation to allow time for incision. The cells were embedded in agarose, and chromosome preparations were run on CHEF gels as described in Materials and Methods. The position of the well (w) is marked on the gel.
Figure 5 also shows the formation of DSBs in the isogenic rad52 disruptant in the exponential growth phase, where the same DSB induction pattern to that of the wild type is observed, indicating that Rad52 does not influence the early steps in ICL repair. To rule out a direct DNA-degrading activity of HN2, whole chromosomes embedded in agarose plugs were treated with the highest concentrations of HN2 employed in these experiments (1,000 μM) and subsequently analyzed by CHEF. No DSBs were observed (data not shown); hence, it is clear that the DSBs are the result of cellular incision activities.
Origin of the ICL-associated DSBs.
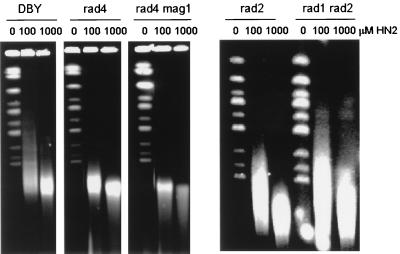
To explore the origin of the DSBs, their induction in exponentially growing HN2-treated NER disruptant strains was assessed, since available information suggests that this is the pathway which initiates ICL repair (6, 29, 39). Surprisingly, isogenic disruptant rad4 (Fig. 6) and rad14 strains show a level of DSB induction indistinguishable from that of their parent (DBY747). Additionally, a rad2 strain and a rad1 rad2 double-mutant strain, completely defective for all NER-associated endonuclease activities (22), also demonstrated this wild-type DSB induction (Fig. 6). It is possible that the DSBs reflect closely opposed single-strand breaks produced during the excision of the abundant HN2 monoadducts during NER and also base excision repair (BER) carried out by the 3-methyladenine activity of Mag1 (39), although this seems unlikely in view of the lack of sensitivity of the rad52 strain to HN1, which exclusively produces monoadducts. Indeed, a rad4 mag1 double mutant, which is completely unable to remove monoadducts (39), also accumulated DSBs to the same extent as its repair-proficient parent does (Fig. 6).
FIG. 6.
Induction of DSBs following HN2 (0, 100, and 1,000 μM) treatment of exponentially growing DBY747 and isogenic rad4, rad4 mag1, rad2, and rad1 rad2 disruptants determined by CHEF.
It appears that other nuclease activities in addition to NER and BER produce frank single-strand breaks at ICLs, which are converted to DSBs at replication. Consequently, the formation of DSBs in strains disrupted in several candidate endonucleases known to play a role in DNA repair was determined. As a primary screen, the HN2 sensitivities of candidate disruptants were measured, since the unidentified strand scission activity is likely to be a necessary step in the repair of HN2 ICLs. Candidates included mismatch repair mutant mlh1, rad27 (which encodes flap endonuclease, which functions in replication, NHEJ, and BER [30, 58, 61, 66, 67]), and mre11 (necessary for the formation of meiotic DSBs and possessing exonuclease and single-stranded endonuclease activity [9, 31, 43]). Of these, the rad27 and mre11 disruptants were found to be moderately and extremely HN2 sensitive, respectively, whereas the mismatch repair mutants were no more sensitive than their isogenic parent (data not shown). CHEF analysis also demonstrated that exponentially growing rad27 and mre11 cells form DSBs normally (data not shown), indicating that they are not the additional endonuclease involved in ICL-induced DSB formation.
Repair pathways acting on the ICL-associated DSBs.
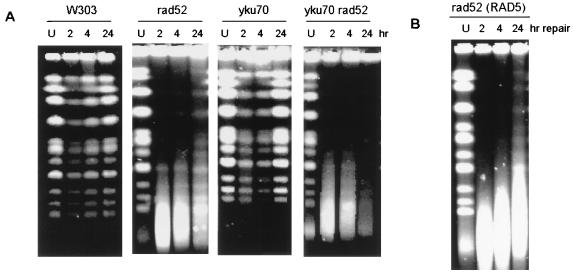
Taking advantage of the proven sensitivity of the CHEF analysis, it was possible to examine the repair of the DSBs resulting from HN2 treatment in the strains deficient in homologous recombination and NHEJ. Figure 7A shows a typical CHEF gel obtained following HN2 treatment of exponentially growing W303-1B (parental), rad52, yku70, or yku70 rad52 mutant cells which were allowed to repair the DSBs in drug-free minimal medium for 2, 4, or 24 h. In the parental strain, repair is already evident by 2 h, with nearly complete (74%) restoration of chromosomes by 24 h. In the rad52 strain, no restoration is seen at 2 or 4 hours (the strong smear is still observed), but by 24 h some restoration (15%) is evident, indicating that another pathway may indeed partially compensate in the absence of homologous recombination. The yku70 strain behaves, as expected from the sensitivity data (Fig. 2), indistinguishably from the parent. In the yku70 rad52 double mutant, the induction of DSBs was as for the rad52 strain, but there was no restoration of the high-molecular-weight DNA in any of five independent experiments.
FIG. 7.
Repair of DSBs in HN2-treated W303-1B, rad52, yku70, and yku70 rad52 cells. (A) Exponentially growing cells were treated with 100 μM HN2 or mock treated (lanes U) with water and subsequently allowed to repair in minimal medium for 2, 4, or 24 h. The mock-treated sample was allowed to repair for 24 h. The samples were analyzed on CHEF gels. (B) Exponentially growing rad52 cells derived from DBY747 were treated and analyzed by CHEF in an identical manner to those in panel A.
Taken together, the CHEF and survival data indicate that NHEJ can repair DSBs in the absence of the RAD52-mediated pathways, but this repair does not significantly enhance survival. In the course of this study, it came to our attention that the parental strain from which the rad52 and yku70 disruptants were derived (W303-1B) harbored a weak rad5 mutation (rad5-535) (20). There is evidence that RAD5 influences the processing of DSBs by channeling repair intermediates into homologous recombination pathways by avoiding NHEJ (1). Consequently, it was necessary to rule out the possibility that the residual repair observed in the W303-1B-rad52 mutant was a result of a bias to NHEJ introduced by the presence of rad5-535 allele. Using the rad52 disruptant derived from DBY747, it is shown in Fig. 7B that this residual repair is also seen in this genetic background, and quantitation indicated that the level of whole-chromosome restoration was very similar in the DBY747-derived strain (20% after 24 h) and the W303-1B-derived strain. Therefore, the level of RAD52-independent end joining is not influenced by the rad5-535 allele in this case.
To determine the role of other key members of the RAD52 epistasis group (RAD51 [48] and RAD54 [2]), as well as MRE11 (involved in both homologous recombination and NHEJ [43]), in the repair of ICL-associated DSBs, the sensitivity and CHEF repair profiles of disruptant strains were determined. The data are summarized in Table 2, where sensitivity is expressed as LD50 (the HN2 dose required to kill 50% of cells) and DSB repair is expressed as the fraction of DSBs rejoined after 24 h of posttreatment incubation. It is clear that RAD54 and MRE11 are both crucial for ICL-associated DSB repair, since disruptants showed a level of hypersensitivity and DSB repair defects comparable to those of the rad52 strain. The rad51 strain showed a level of sensitivity and repair which intermediate between those of its parent, YP1, and the rad52 strain, suggesting that it is required for a subset of ICL repair events. In addition, another member of the RAD52 group has recently been identified (RAD59), which appears to play some overlapping role with RAD52 during intrachromosomal recombination and is sensitive to ionizing radiation (4). From Table 2 it is clear that a rad59 disruptant strain shows a normal response to ICLs in terms of DSB repair but is slightly sensitive to HN2. This suggests that RAD59 may be required for a small subset of recombination events during ICL repair but the defect in a rad59 strain is too small to be detected by CHEF.
TABLE 2.
HN2 sensitivity and DSB repair efficiency in exponentially growing recombination-defective strains
| Strain | LD50 (μM)a | % DSB repair at 24 hb |
|---|---|---|
| YP1 | 100 | 85 |
| rad51 | 10 | 57 |
| rad52 | 5 | 15 |
| rad54 | 3 | 23 |
| rad59 | 30 | 84 |
| mre11 | 5 | 15 |
Determined by colony-forming survival assay (see Materials and Methods).
Determined by CHEF for cells treated with 100 μM HN2 (see Materials and Methods).
It is conceivable that DSBs do accumulate in stationary-phase cells but their formation is retarded due to the quiescent state of the cells. For this reason, stationary-phase cultures were subject to repair experiments identical to those described for exponential cultures (Fig. 7). At no time point during the 24-h repair period were any DSBs observed (data not shown).
DISCUSSION
The antitumor agents nitrogen mustard (HN2) and cisplatin have been the subjects of a number of previous studies with S. cerevisiae that have attempted to elucidate the DNA repair pathways acting on the DNA ICLs they produce (8, 55, 65). For HN2 it is notable that all these studies conclude that NER and members of the RAD6 epistasis group (RAD18 and REV3) are important in the repair of ICLs (52) whereas recombination, as mediated by RAD52 pathway, is not (55). There is a clear discrepancy between this observation and results obtained using bifunctional psoralens plus UVA, which also produce DNA ICLs, albeit primarily between opposing thymines, in contrast to HN2, which targets guanines (26). Yeast clearly does require homologous recombination to complete psoralen DNA ICL repair (27, 37), and there is evidence that DSBs are an important intermediate in this process (17, 18).
Recombination plays an important role in the repair of HN2-induced ICLs in exponential-phase growth but not in stationary phase.
We sought to address the reasons for this discrepancy, particularly because existing models of ICL repair generally demand a role for recombination (6, 12). Our data clearly show that rad52 sensitivity to HN2 and cisplatin is dependent upon growth phase, with recombination being required only during exponential-phase repair. These results suggest that ICL repair may follow a common, growth phase-dependent route in this yeast. We postulated that if the repair intermediate is a DSB, it may be repaired by NHEJ in the absence of a homologous substrate (in stationary phase) or in any growth phase when Rad52 is absent. Sensitivity data did not, however, indicate a major role for NHEJ, since the yku70 rad52 strain was not significantly more sensitive than the rad52 single mutant in either growth phase. Of note are the findings of Moore and Haber (42) that the process of NHEJ occurs preferentially in the S and G2 phases in yeast, and therefore may not be expected to be efficient in stationary-phase cells, providing only a poor backup to Rad52-mediated events. Taken together, this information suggests that a recombination substrate is generated in growing cells treated with HN2. However, a different intermediate is generated in stationary-phase cells, where it is possible to ablate the legitimate and illegitimate recombination pathways without significantly affecting sensitivity to HN2.
The ICL-associated DSBs are the result of an uncharacterized incision activity.
The survival experiments described above did not establish whether DSBs are key intermediates in exponential-phase cells. Indeed, the sensitivity of the rad52 strain could be accounted for as the result of recombinogenic single-strand gaps occurring at adducts during replication (23, 32). Measurement of DSB induction following treatment of exponential-phase cells with HN2, followed by a period for maximum incision to occur, indicated that abundant DSBs do form as an intermediate, even at sublethal doses. These DSBs are likely to be the result of replication forks meeting strand-break intermediates produced during the repair of ICLs. The greatly reduced frequency of DSBs during ICL repair in stationary-phase cells argues that these incisions are not frank DSBs but single-strand breaks which are converted to DSBs at replication. The frank DSBs observed at very high doses in stationary-phase cells may be the result of closely opposed NER and BER incisions on both strands when very large numbers of adducts are induced, and these DSBs are probably distinct from the replication-associated DSBs observed in the exponential-phase cells at much (100-fold) lower doses. Indeed, at these high doses the rad52- and rad52 yku70-defective strains do lose some viability compared to their parent, consistent with the induction of DSBs observed by CHEF.
The absence of DSBs in stationary-phase haploid cells helps explain the lack of sensitivity of the recombination and NHEJ mutants in this growth phase. Furthermore, it is consistent with the rev3 strain hypersensitivity observed in stationary-phase cells over exponential-phase cells. Since elimination of ICLs involves several types of incision activity (discussed below) which do not lead directly to DSBs, a possible mechanism for ICL repair in stationary-phase haploid cells would be as follows. A gapped repair intermediate accumulates in treated stationary-phase cells following incision on one strand of the ICL only, and in the absence of exogenous genetic information (from a sister chromatid or homolog) the error prone lesion-bypass polymerase (polymerase ζ) (5, 46) fills in the strand from which the adduct has been excised. During this process, the polymerase may displace the adducted nucleotide or oligonucleotide, or, alternatively, a separate helicase activity may displace this moiety. This would permit a second round of incisions on the opposing strand to be followed by resynthesis and ligation, permanently fixing any mutation. It should be noted, however, that the rev3 strain is around 10-fold more resistant in the stationary phase than the rad52 strains are in the exponential phase. This suggests that other activities are required for the processing of ICL intermediates in G1, and we are currently examining other candidate tolerance pathways such as that controlled by RAD30 (polymerase η). In a diploid cell, the adducted single-strand gap generated by the first round of excision could also be filled using information from the homolog, although this is not the preferred homologous recombination substrate in yeast (32).
Since the initial incisions produced at a distorting adduct, such as an HN2- or cisplatin-induced ICL, would be anticipated to be the result of NER, it was surprising that in all the exponentially growing NER-deficient strains examined the replication-associated DSBs formed with a frequency indistinguishable from that in the parental strain. Clearly, additional incision activities are acting on the ICLs during their repair. In this respect, it is notable that several in vitro studies examining the cleavage reactions produced in psoralen-cross-linked plasmids by mammalian cell extracts found incisions at the fifth (34) or seventh (discussed in reference 6) phosphodiester bonds both 3′ and 5′ to the cross-linked T on the furan side. These incisions are distinct from those associated with NER, since they do not require ATP (34). It seems likely that important, additional incisions do occur during the repair of ICLs and that these result in the replication-associated DSBs observed here. The function of these additional incisions might be related to the unique problems an ICL presents in the unwinding and opening of the helix prior to NER (6). These incisions may initially release the helix to permit the formation of more open structures, which are substrates for subsequent NER reactions. Alternatively, these incisions may arise by cleavage of the structures produced at stalled replication forks, as has been demonstrated to occur in E. coli, where stalled replication forks are cleaved by the Holliday junction resolvase RuvAB (54). Further biochemical analysis of the incision intermediates produced in defined cross-linked substrates should be informative about the nature and location of these incisions, and the use of S. cerevisiae could be especially powerful since so many well-defined repair mutants exist and multiple mutants are easy to generate. The ability to construct multiple mutants may be especially important, since several activities may collaborate to cleave the DNA and permit access of the repair apparatus to the ICL.
In a further attempt to identify the incision activity, we screened a (by no means exhaustive) collection of strains bearing disruptions in key components of known DNA incision pathways which play a role in DNA repair. Our initial screen involved determination of the sensitivity of the strains to HN2, assuming that the incisions represent an important intermediate in the repair and that loss of this step would result in significant HN2 hypersensitivity. Both the rad27 and mre11 strains were hypersensitive to HN2; however, neither were blocked in the formation of DSBs. The identity of this ICL-nicking activity therefore remains unknown.
The repair of ICL-associated DSBs normally occurs through legitimate recombination but can also involve NHEJ.
The repair profiles obtained using CHEF clearly demonstrated that the majority of DSB processing is the result of Rad52-mediated events. The contribution of NHEJ was unclear from survival data alone, where only a slight hypersensitivity in the yku70 rad52 double mutant was observed. However, the residual repair observed in the rad52 strain is completely absent in the double mutant. This physical evidence for a role for NHEJ is consistent with its role in S. cerevisiae in the repair of DSBs produced by ionizing radiation. For example, a role for NHEJ in the absence of Rad52 can be observed in experiments employing ionizing radiation as the DSB inducer (7).
We also examined the requirement for Rad51 and Rad54 during ICL-associated DSB repair events. RAD54 is known to be essential for sister chromatid recombination repair in the mitotic cell cycle (2). Consistent with this, the haploid rad54 strain employed in this study was extremely HN2 sensitive and severely defective in DSB repair (Table 2). RAD51 is required for most but not all homologous recombination repair processes (48). For ICL-associated DSBs, it appears that repair can proceed to some extent in the absence of Rad51, since the disruptant strain displayed lower sensitivity and a smaller DSB repair defect than the rad52 and rad54 strains did (Table 2). This probably reflects the nature of the DSB intermediate structures generated during ICL production, which may differ from those associated with ionizing-radiation damage, where a stronger requirement for RAD51 is evident (24). Examination of the rad59 strain indicated that this activity alone is not crucial to ICL-associated DSB repair. However, RAD59 contributes to only a subset of the recombination reactions controlled by RAD52 (4), and consequently the role of this gene may be obscured in strains bearing a functional RAD52 gene. The use of double mutants will be required to further understand the relative contribution of these activities. A physical and genetic study of the various homologous-recombination subpathways acting on these ICL-associated DSBs is under way.
The high level of hypersensitivity of the mre11 strain is interesting, since Mre11 forms a complex with Rad50 and Xrs2 (9) and since this complex is unique in that it plays a role in both mitotic homologous recombination and NHEJ (42). The mre11 strain was as sensitive to HN2 as its isogenic rad52 counterpart was (Table 2), arguing that Mre11 plays an essential role in ICL repair. Indeed, the CHEF results (Table 2) indicate that mre11 cells show a defect in ICL-associated DSB rejoining which is of the same order of magnitude as that in the rad52 strain. This strong requirement for Mre11 in the repair of ICL-associated DSBs may be indicative of an important role for its nuclease activities in processing the particular intermediate structures occurring at these adduct-associated breaks.
Despite this minor role in yeast, it is possible that NHEJ is the major DSB repair pathway acting in mammalian cells treated with HN2, since this is apparently the case for ionizing-radiation-induced DSBs (13). However, the recent generation of vertebrate cell lines disrupted for components of the homologous recombination apparatus (51, 59, 70) and the very high sensitivity of CHO XRCC2- and XRCC3-defective cells to cross-linking agents (35) suggest that this pathway may be more important for DSB repair in higher eukaryotes than was previously thought. Therefore, only empirical examination of the ICL repair efficiency in matched mammalian cells deficient in these pathways will ultimately answer these questions. In fact, evidence is available indicating that both homologous recombination and NHEJ may influence the sensitivity of mammalian cells to ICL-inducing drugs (44, 45). The work presented here suggests that homologous recombination and NHEJ are likely to be important pathways for repairing the replication-associated DSBs induced during ICL repair and that modulation of this activity may provide a route for increasing the chemosensitivity of tumor cells specifically to DNA cross-linking agents.
ACKNOWLEDGMENTS
We are very grateful to W. Xiao, J. Downs, S. Jackson, R. Brown, R. Borts, I. Hickson, R. Waters, S. McCready, and L. Symington for providing yeast strains and to M. Longtine for providing plasmids.
This work was funded by The Cancer Research Campaign programme grant SP2000/0402.
REFERENCES
- 1.Ahne F, Jha B, Eckardt-Schupp F. The RAD5 gene product is involved in the avoidance of non-homologous end-joining of DNA double strand breaks in the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1997;25:743–749. doi: 10.1093/nar/25.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbel A, Zenvirth D, Simchen G. Sister chromatid DNA repair is mediated by RAD54, not by DMC1 or TID1. EMBO J. 1999;18:2648–2658. doi: 10.1093/emboj/18.9.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Averbeck D, Moustacchi E. 8-Methoxypsoralen plus 365nm light effects and repair in yeast. Biochim Biophys Acta. 1975;395:393–404. doi: 10.1016/0005-2787(75)90063-5. [DOI] [PubMed] [Google Scholar]
- 4.Bai Y, Symington L S. A Rad52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev. 1996;10:2025–2037. doi: 10.1101/gad.10.16.2025. [DOI] [PubMed] [Google Scholar]
- 5.Baynton K, Bresson-Roy A, Fuchs R P P. Analysis of damage tolerance pathways in Saccharomyces cerevisiae: a requirement for Rev3 DNA polymerase in translesion synthesis. Mol Cell Biol. 1998;18:960–966. doi: 10.1128/mcb.18.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessho T, Mu D, Sancar A. Initiation of DNA interstrand cross-link repair in humans: the nucleotide excision repair system makes dual incisions 5′ to the cross-linked base and removes 22- to 28-nucleotide-long damage-free strand. Mol Cell Biol. 1997;17:6822–6830. doi: 10.1128/mcb.17.12.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulton S J, Jackson S P. Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break repair and in telomeric maintenance. Nucleic Acids Res. 1996;23:4639–4648. doi: 10.1093/nar/24.23.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brendel M, Haynes R. Interactions between genes controlling sensitivity to radiation and alkylation in yeast. Mol Gen Genet. 1973;125:197–216. doi: 10.1007/BF00270743. [DOI] [PubMed] [Google Scholar]
- 9.Chamankhah M, Xiao W. Formation of the yeast Mre11-Rad50-Xrs2 complex is correlated with DNA repair and telomere maintenance. Nucleic Acids Res. 1999;27:2072–2079. doi: 10.1093/nar/27.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaney S G, Sancar A. DNA repair:enzymatic mechanisms and relevance to drug response. J Natl Can Inst. 1996;88:1346–1360. doi: 10.1093/jnci/88.19.1346. [DOI] [PubMed] [Google Scholar]
- 11.Cheng S, Sancar A, Hearst J E. RecA-dependent incision of psoralen-crosslinked DNA by (A)BC excinuclease. Nucleic Acids Res. 1991;19:657–663. doi: 10.1093/nar/19.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng S, Van Houten B, Gamper H B, Sancar A, Hearst J E. Use of psoralen-modified oligonucleotides to trap three stranded RecA-DNA complexes and repair of these crosslinked complexes by ABC excinuclease. J Biol Chem. 1988;263:15110–15117. [PubMed] [Google Scholar]
- 13.Chu G. Double strand break repair. J Biol Chem. 1996;272:24097–24100. doi: 10.1074/jbc.272.39.24097. [DOI] [PubMed] [Google Scholar]
- 14.Chu G, Gunderson K. Separation of large DNA by a variable-angle contour-clamped homogenous electric field electrophoresis. Anal Biochem. 1991;194:439–446. doi: 10.1016/0003-2697(91)90254-q. [DOI] [PubMed] [Google Scholar]
- 15.Comess K M, Lippard S J. Molecular aspects of platinum-DNA interactions. In: Neidle S, Waring M J, editors. Molecular aspects of anticancer drug–DNA interactions. London, United Kingdom: Macmillan Press Ltd.; 1993. pp. 134–168. [Google Scholar]
- 16.Cundari E, Dardalhon M, Rousset S, Averbeck D. Repair of 8-methoxypsoralen photoinduced cross-links in yeast. Mol Gen Genet. 1991;228:335–344. doi: 10.1007/BF00260625. [DOI] [PubMed] [Google Scholar]
- 17.Dardalhon M, Averbeck D. Pulsed field electrophoresis of the repair of psoralen plus UVA induced DNA photoproducts in Saccharomyces cerevisiae. Mutat Res. 1995;336:49–60. doi: 10.1016/0921-8777(94)00037-7. [DOI] [PubMed] [Google Scholar]
- 18.Dardalhon M, de Massy B, Nicolas A, Averbeck D. Mitotic recombination and localized DNA double-strand breaks are induced after 8-methoxypsoralen and UVA irradiation in Saccharomyces cerevisiae. Curr Genet. 1998;43:30–42. doi: 10.1007/s002940050363. [DOI] [PubMed] [Google Scholar]
- 19.Durant S T, Morris M M, Illand M, McKay H J, McCormick C, Hirst G L, Borts R H, Brown R. Dependence on RAD52 and RAD1 for anticancer drug resistance mediated by inactivation of mismatch repair genes. Curr Biol. 1999;14:51–54. doi: 10.1016/s0960-9822(99)80047-5. [DOI] [PubMed] [Google Scholar]
- 20.Fan H Y, Cheng K K, Klein H L. Mutations in the RNA polymerase II transcription machinery suppress the hyper-recombination mutant hprI delta of Saccharomyces cerevisiae. Genetics. 1996;142:749–759. doi: 10.1093/genetics/142.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishman-Lobell J, Haber J E. Removal of nonhomologous DNA ends in double-strand break recombination: the role of the yeast ultraviolet repair gene RAD1. Science. 1992;258:480–484. doi: 10.1126/science.1411547. [DOI] [PubMed] [Google Scholar]
- 22.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C.: American Society for Microbiology; 1995. [Google Scholar]
- 23.Galli A, Schiestl R H. Hydroxyurea induces recombination in dividing but not in G1 or G2 cell cycle arrested yeast cells. Mutat Res. 1996;354:69–75. doi: 10.1016/0027-5107(96)00037-1. [DOI] [PubMed] [Google Scholar]
- 24.Game J, Mortimer R K. A genetic study of X-ray sensitive mutants in yeast. Mutat Res. 1974;24:281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- 25.Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press, Inc.; 1991. [Google Scholar]
- 26.Hartley J A. Selectivity in alkylating agent-DNA interactions. In: Neidle S, Waring M J, editors. Molecular aspects of anticancer drug–DNA Interactions. London, United Kingdom: Macmillan Press Ltd.; 1993. pp. 1–31. [Google Scholar]
- 27.Henriques J A P, Moustacchi E. Interactions between mutations for sensitivity to psoralen photoaddition (pso) and to radiation (rad) in Saccharomyces cerevisiae. J Bacteriol. 1981;148:248–256. doi: 10.1128/jb.148.1.248-256.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov E L, Haber J E. RAD1 and RAD10, but not other excision repair genes, are required for double-strand-break induced recombination in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:2254–2251. doi: 10.1128/mcb.15.4.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jachymczyk W, Van Borstel R C, Mowat M R A, Hastings P J. Repair of interstrand crosslinks in DNA of Saccharomyces cerevisiae requires two systems for DNA repair: the RAD3 system and the RAD51 system. Mol Gen Genet. 1981;182:196–205. doi: 10.1007/BF00269658. [DOI] [PubMed] [Google Scholar]
- 30.Johnson R E, Kovvali G K, Prakash L, Prakash S. Role of yeast Rth1 nuclease and its homologs in mutation avoidance, DNA repair, and DNA replication. Curr Genet. 1998;34:21–29. doi: 10.1007/s002940050362. [DOI] [PubMed] [Google Scholar]
- 31.Johzuka K, Ogawa H. Interaction of Mre11 and Rad50: two proteins required for DNA repair and meiosis-specific double-strand break formation in Saccharomyces cerevisiae. Genetics. 1995;139:1521–1532. doi: 10.1093/genetics/139.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadyk L C, Hartwell L H. Replication-dependent sister chromatid recombination in rad1 mutants of Saccharomyces cerevisiae. Genetics. 1993;133:469–487. doi: 10.1093/genetics/133.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer K M, Brock J A, Bloom K, Moore J K, Haber J E. Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, non-homologous recombination events. Mol Cell Biol. 1994;14:1293–1303. doi: 10.1128/mcb.14.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumaresan K R, Hang B, Lambert M W. Human endonucleolytic incision of DNA 3′ and 5′ to a site-directed psoralen monoadduct and interstrand cross-link. J Biol Chem. 1995;270:30709–30716. doi: 10.1074/jbc.270.51.30709. [DOI] [PubMed] [Google Scholar]
- 35.Liu N, Lamerdin J E, Tebbs R S, Schild D, Tucker J D, Shen M R, Brookman K W, Siciliano M J, Walter C A, Fan W, Narayana L S, Zhou Z-Q, Adamson A W, Sorenson K J, Chen D J, Jones N J, Thompson L H. XRCC2 and XRCC3, new human Rad51-family members, promote chromosomal stability and protect against DNA crosslinks and other damages. Mol Cell. 1998;1:783–793. doi: 10.1016/s1097-2765(00)80078-7. [DOI] [PubMed] [Google Scholar]
- 36.Longtine M S, McKenzie III A, Demarini D J, Shah N G, Wach A, Brachat A, Philippsen P, Pringle J R. Additional Modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 37.Magana-Schwencke N, Henriques J-A P, Chanet R, Moustacchi E. The fate of 8-methoxypsoralen photoinduced crosslinks in nuclear and mitochondrial yeast DNA: comparison of wild-type and repair-deficient strains. Proc Natl Acad Sci USA. 1982;79:1722–1726. doi: 10.1073/pnas.79.6.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mages G J, Feldman H M, Winnacker E-L. Involvement of the Saccharomyces cerevisiae HDF1 gene in DNA double-strand break repair and recombination. J Biol Chem. 1996;271:7910–7915. doi: 10.1074/jbc.271.14.7910. [DOI] [PubMed] [Google Scholar]
- 39.McHugh P J, Gill R D, Waters R, Hartley J A. Excision repair of nitrogen mustard-DNA adducts in Saccharomyces cerevisiae. Nucleic Acids Res. 1999;27:3259–3266. doi: 10.1093/nar/27.16.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meniel V, Magana-Schwencke N, Averbeck D, Waters R. Preferential incision of interstrand crosslinks induced by 8-methoxypsoralen plus UVA in yeast during the cell cycle. Mutat Res. 1997;384:23–32. doi: 10.1016/s0921-8777(97)00011-6. [DOI] [PubMed] [Google Scholar]
- 41.Milne G T, Jin S, Shannon K B, Weaver D T. Mutations in two Ku homologs define a DNA end-joining repair pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4189–4198. doi: 10.1128/mcb.16.8.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore J K, Haber J E. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreau S, Ferguson J R, Symington L S. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end-joining, or telomere maintenance. Mol Cell Biol. 1999;19:556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller C, Calsou P, Frit P, Cayrol C, Carter T, Salles B. UV sensitivity and impaired nucleotide excision repair in DNA-dependent protein kinase mutant cells. Nucleic Acids Res. 1998;26:1382–1389. doi: 10.1093/nar/26.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muller C, Christodoulopoulos G, Salles B, Panasci L. DNA-dependent protein kinase activity correlates with clinical and in vitro sensitivity of chronic lymphocytic leukemia lymphocytes to nitrogen mustards. Blood. 1998;92:2213–2219. [PubMed] [Google Scholar]
- 46.Nelson J R, Lawrence C W, Hinkle D. Thymine-thymine dimer bypass in yeast DNA polymerase ζ. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 47.Povirk L F, Shuker D E. DNA damage and mutagenesis induced by nitrogen mustards. Mutat Res. 1994;318:205–226. doi: 10.1016/0165-1110(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 48.Rattray A J, Symington L S. Use of a chromosomal inverted repeat to demonstrate that the RAD51 and RAD52 genes of Saccharomyces cerevisiae have different roles in mitotic recombination. Genetics. 1994;138:587–595. doi: 10.1093/genetics/138.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reagan M S, Pittenger C, Siede W, Friedberg E C. Characterization of a mutant strain of Saccharomyces cerevisiae with a deletion of the RAD27 gene, a structural homolog of the RAD2 nucleotide-excision repair gene. J Bacteriol. 1995;177:364–371. doi: 10.1128/jb.177.2.364-371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed S H, McCready S, Boiteux S, Waters R. The levels of repair of endonuclease III-sensitive sites, 6-4 photoproducts and cyclobutane pyrimidine dimers differ in a point mutation for RAD14, the Saccharomyces cerevisiae homologue of the human gene defective in XPA patients. Mol Gen Genet. 1996;250:515–522. doi: 10.1007/BF02174040. [DOI] [PubMed] [Google Scholar]
- 51.Rijkers T, Van Den Ouweland J, Morolli B, Rolink A G, Baarends W M, Van Sloun P-P H, Lohman P H M, Pastink A. Targeted inactivation of mouse RAD52 reduces homologous recombination but not resistance to ionizing radiation. Mol Cell Biol. 1998;18:6423–6429. doi: 10.1128/mcb.18.11.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruhland A, Brendel M. Mutagenesis by cytostatic alkylating agents in yeast strains of differing repair capacities. Genetics. 1978;92:83–97. doi: 10.1093/genetics/92.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruhland A, Kircher M, Wilborn F, Brendel M. A yeast mutant specifically sensitive to bifunctional alkylation. Mutat Res. 1981;91:457–462. doi: 10.1016/0165-7992(81)90052-x. [DOI] [PubMed] [Google Scholar]
- 54.Seigneur M, Bidnenko V, Dusko Erlich S, Michel B. RuvAB acts at arrested replication forks. Cell. 1998;95:419–430. doi: 10.1016/s0092-8674(00)81772-9. [DOI] [PubMed] [Google Scholar]
- 55.Siede W, Brendel M. Interactions among genes controlling sensitivity to radiation (RAD) and to alkylation by nitrogen mustard (SNM) in yeast. Curr Genet. 1982;5:33–38. doi: 10.1007/BF00445738. [DOI] [PubMed] [Google Scholar]
- 56.Sladek F M, Munn M M, Rupp W D, Howard-Flanders P. In vitro repair of psoralen-DNA crosslinks by RecA, UvrABC, and the 5′-exonuclease of DNA polymerase I. J Biol Chem. 1989;264:6755–6765. [PubMed] [Google Scholar]
- 57.Sugawara N, Haber J E. Characterization of double-strand break-induced recombination:homology requirements and single-stranded DNA formation. Mol Cell Biol. 1992;12:563–575. doi: 10.1128/mcb.12.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Symington L S. Homologous recombination is required for the viability of rad27 mutants. Nucleic Acids Res. 1998;26:5589–5595. doi: 10.1093/nar/26.24.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takata M, Sasaki M S, Sononda E, Morrison C, Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A, Takeda S. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 1998;17:5497–5508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teo S-H, Jackson S P. Identification of Saccharomyces cerevisiae DNA ligase IV: involvement in DNA double-strand break repair. EMBO J. 1997;16:4788–4795. doi: 10.1093/emboj/16.15.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tishkoff D X, Filosi N, Gaida G M, Kolodner R D. A novel mutation avoidance mechanism dependent on RAD27 is distinct from mismatch repair. Cell. 1997;88:253–263. doi: 10.1016/s0092-8674(00)81846-2. [DOI] [PubMed] [Google Scholar]
- 62.Tsukaoto Y, Kato J, Ikeda H. Hdf1, a yeast Ku-protein homologue, is involved in illegitimate recombination, but not homologous recombination. Nucleic Acids Res. 1996;24:2067–2072. doi: 10.1093/nar/24.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukaoto Y, Kato J, Ikeda H. Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature. 1997;388:900–903. doi: 10.1038/42288. [DOI] [PubMed] [Google Scholar]
- 64.Van Houten B, Gamper H, Holbrook S R, Hearst J E, Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci USA. 1986;83:8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilborn F, Brendel M. Formation and stability of interstrand cross-links induced by cis- and trans-diamminechloroplatinum (II) in the DNA of Saccharomyces cerevisiae strains differing in repair capacity. Curr Genet. 1989;16:331–338. doi: 10.1007/BF00340711. [DOI] [PubMed] [Google Scholar]
- 66.Wu X, Wang Z. Relationships between yeast Rad27 and Apn1 in response to apurinic/apyrimidinic sites in DNA. Nucleic Acids Res. 1999;27:956–962. doi: 10.1093/nar/27.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X, Wilson T E, Lieber M R. A role for FEN-1 in nonhomologous end joining: the order of strand annealing and nucleolytic processing events. Proc Natl Acad Sci USA. 1999;96:1303–1308. doi: 10.1073/pnas.96.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao W, Chow B L. Synergism between yeast nucleotide excision repair and base excision repair pathways in the protection against DNA methylation damage. Curr Genet. 1998;33:92–99. doi: 10.1007/s002940050313. [DOI] [PubMed] [Google Scholar]
- 69.Xiao W, Chow B L, Rathgeber L. The repair of DNA methylation damage in Saccharomyces cerevisiae. Curr Genet. 1996;30:461–468. doi: 10.1007/s002940050157. [DOI] [PubMed] [Google Scholar]
- 70.Yamauchi-Iwai Y, Sonoda E, Buerstedde J-M, Bezzubova O, Morrison C, Takata M, Shinohara A, Takeda S. Homologous recombination, but not DNA repair, is reduced in vertebrate cells lacking RAD52. Mol Cell Biol. 1998;18:6430–6435. doi: 10.1128/mcb.18.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]