Abstract
The rapid spread of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID‐19), has had a dramatic negative impact on public health and economies worldwide. Recent studies on COVID-19 complications and mortality rates suggest that there is a higher prevalence in cardiovascular diseases (CVD) patients. Past investigations on the associations between pre-existing CVDs and susceptibility to coronavirus infections including SARS‐CoV and the Middle East Respiratory Syndrome coronavirus (MERS-CoV), have demonstrated similar results. However, the underlying mechanisms are poorly understood. This has impeded adequate risk stratification and treatment strategies for CVD patients with SARS-CoV-2 infections. Generally, dysregulation of the expression of angiotensin‐converting enzyme (ACE) and the counter regulator, angiotensin‐converting enzyme 2 (ACE2) is a hallmark of cardiovascular risk and CVD. ACE2 is the main host receptor for SARS-CoV-2. Although further studies are required, dysfunction of ACE2 after virus binding and dysregulation of the renin-angiotensin-aldosterone system (RAAS) signaling may worsen the outcomes of people affected by COVID-19 and with preexisting CVD. Here, we review the current knowledge and outline the gaps related to the relationship between CVD and COVID-19 with a focus on the RAAS. Improved understanding of the mechanisms regulating viral entry and the role of RAAS may direct future research with the potential to improve the prevention and management of COVID-19.
Keywords: Cardiovascular diseases, COVID-19, Coronavirus, SARS-CoV-2, RAAS, ACE2
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for coronavirus disease 19 (COVID-19) and its emergence as a pandemic. SARS-CoV-2 infection has a notably higher prevalence in patients with underlying cardiovascular disease (CVD) and/or diabetes, which represents a huge challenge for healthcare professionals worldwide [1]. Understanding the mechanisms of the association between CVD and increased SARS-CoV-2 susceptibility, as well as the development of COVID-19 disease, is therefore critical to better manage patients and prevent complications and death. SARS-CoV-2 is a beta coronavirus and it belongs to the Coronaviridae family that includes SARS-CoV and Middle East Respiratory Syndrome coronavirus (MERS-CoV) [2,3]. The lungs have been identified as the main target organ of SARS-CoV-2 infections and in humans, the most common complication of COVID-19 disease is pneumonia [4]. Patients who have an underlying compromised immune function, including those with chronic diseases such as chronic obstructive pulmonary disease (COPD) and the elderly, are likely to develop more severe forms of COVID-19 requiring hospitalization and thus have a higher incidence of mortality [5].
It is well documented that coronaviruses present in the vast majority of individuals as a common cold with mild symptoms [6,7]. A small percentage of such infections progress to develop severe disease, including SARS [8], MERS [9] and currently COVID-19 [10] that require hospitalization. SARS-CoV and MERS-CoV, the causal viruses for SARS and MERS diseases, respectively, are both zoonotic in origin similar to SARS-CoV-2 and follow similar etiologies with the potential to cause global epidemics [11]. The main tissue targets of SARS-CoV are the lungs, immune system, and small blood vessels. Damage to these tissues can lead to complications including pneumonia, lymphopenia, coagulopathy, disseminated intravascular coagulation (DIC), myositis, and renal or liver damage [[12], [13], [14], [15], [16], [17], [18], [19], [20]]. The clinical manifestations of SARS-CoV in the cardiovascular system include local fibrinoid necrosis, and infiltration of monocytes plasma cells (B cells) and other lymphocytes into blood vessel walls. These can lead to the development of vasculitis in the heart, lungs, liver, kidneys, adrenal gland, and the stroma of striated muscles as well as blood clot formation in small veins [14]. SARS can also damage capillary endothelial cells [14] and many critically ill patients present with stroke or venous thrombosis [21]. Several independent studies indicate that underlying CVD is a risk factor associated with complications and increased mortality in SARS patients [13,[22], [23], [24], [25], [26], [27], [28], [29], [30]] (Table 1 ).
Table 1.
Epidemiological data showing the numbers of patients affected, deaths, and clinical characteristics of SARS, MERS and COVID-19 patients with a focus on preexisting CVD or cardiovascular complications.
| Epidemiology→ |
SARS-CoV | MERS-CoV | SARS-CoV-2 |
|---|---|---|---|
| Clinical characteristics↓ | |||
| Period of outbreak | 2002–2004 [22] | 2012, 2015, 2018 [31,41] | 2019–ongoing |
| Total confirmed cases | 8422 [42,43] | 2494 [43,44] | >241 million (As on 17-10-2021) [45] |
| Total deaths | 916 [42,43] | 858 [43,44] | >4.9 million (As on 17-10-2021) [45] |
| Mortality rates (%) | 10 -12 [46] | 35-76 [33,46] | ≈3 [45,47] |
| Age groups most affected (Years) | 37-43 [48] | 36-65 [33,48] | 44-58 [48,49] |
| ICU admissions (%) | 26 [22,[50], [51], [52]] | 57 [41,[53], [54], [55]] | 32 [56,57] |
| Mortality among ICU admissions (%) | 49 [22,[50], [51], [52]] | 60 [41,[53], [54], [55]] | 31 [56] |
| Patients with preexisting CVD (%) | 25-30 [[58], [59], [60]] | 30-40 [32,61,62] | 20-30 [63,64] |
| Mortality with preexisting CVD (%) | 30-40 [36,[58], [59], [60]] | 77 (CVD), 81 (HT) [31,32,61,62] | 16-37 [65,66] |
| Preexisting cardiovascular/Cardiac complications | Hypotension (40–50%), tachycardia (50–60%), bradycardia (10–20%), reversible cardiomegaly (10–15%), and transient atrial fibrillation [58,59] | Acute myocarditis, acute myocardial infarction, and rapid-onset heart failure (∼30%), hypertension (∼50%) and heart diseases [31,33,41] | Hypertension (15–30%), congestive heart failure (∼40%), hyperlipidemia (5%), arrhythmia (3.6%), stroke (2.1%), coronary heart disease (11.2%), and cardiopathy (4.3%), arrhythmias (15–20%), Cardio-cerebrovascular disease (18.79%), myocardial injury (27.8%), ventricular tachycardia/fibrillation (5.9%), acute coagulopathy (34.1%) [48,64,67] |
Similarly, evidence also shows that the severity of MERS-CoV infection is higher in patients with underlying CVDs [[31], [32], [33], [34]] (Table 1). Amongst the different types of coronavirus infections, cardiovascular comorbidities as well as mortality are most prevalent in MERS patients [35], compared to SARS-CoV and SARS-CoV-2 infected patients [36]. The most common cardiovascular complications in MERS patients include pericarditis [31], cardiac arrhythmias [37] and acute myocarditis [38]. Myocardial injury can affect the normal rhythm of the heart and cause cardiac arrhythmia similarly to what has been observed in MERS-CoV patients [39,40].
Evidence of the impact of SARS and MERS coronaviruses in individuals with CVD and CVD risk factors suggest that these comorbidities increase susceptibility to coronaviruses and associated complications, however further mechanistic studies are needed to improve risk stratification and management of these individuals.
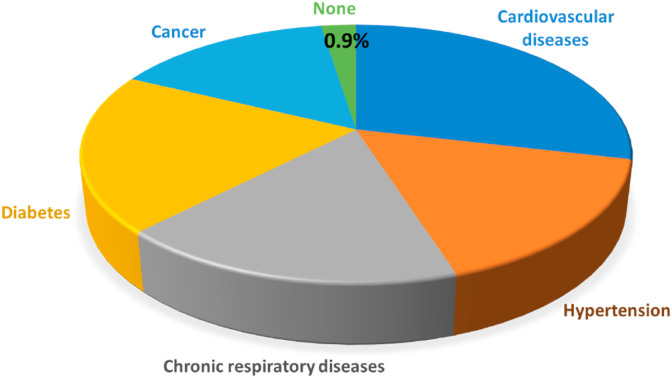
An initial study of 5000 COVID-19 patients indicated that pre-existing diseases such as CVDs, cancers, or diabetes are the main determinants of increased mortality (Fig. 1 ) [65]. This study demonstrated that individuals with SARS-CoV-2 and without pre-existing conditions had ˂1% mortality. However, this increased to 10% in COVID-19 patients with some form of heart disease. In addition, the presence of hypertension alone in infected patients increased mortality to 6%. Similarly, cardiovascular complications were also exacerbated due to COVID-19 [36]. The potential mechanism of association between increased susceptibility to SARS-CoV-2 infection and CVD has been attributed to increased angiotensin-converting enzyme-2 (ACE2) receptor expression particularly in patients taking ACE inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) [68]. Many recent studies show the importance of the ACE2 receptor in mediating SARS-CoV-2 infection. ACE2 is widely expressed in diverse range of species throughout the subphylum Vertebrata. However, a couple of recent studies indicate that patients receiving ACEI/ARB therapy are not at increased risk of severe COVID-19 or hospital admission [69,70]. Nevertheless, whether there is an increased or the same expression of ACE2 in CVD or as a result of ACEi or ARBs remains controversial and needs to be explored further. Increased circulating ACE2 levels were also observed in patients after myocardial infarction (MI), which is likely a mechanism that counteracts the activation of the renin–angiotensin-aldosterone system (RAAS) [71,72]. Defining this correlation is critical as ACE2 is the main host receptor for SARS-CoV-2, which binds to it via its spike proteins [73,74]. The interaction between virus and its host cell ACE2 receptor is the initial step in promoting virus infection and is a vital determinant of host species range and tissue tropism [75,76]. The envelope protein of SARS-CoV-2 (spike protein, S) are separated into S1 and S2 subunits upon interaction with ACE2 [77]. Even though the S2 subunit does not directly bind with the ACE2 receptor, it keeps the functional components essential for membrane fusion of the virus [78]. The S1 protein/receptor binding is the critical element for the cellular entry of SARS-CoV-2. S1 comprises the receptor binding domain (RBD) that facilitates direct binding to the peptidase domain (PD) of ACE2 to enable entry into host cells [79]. Proteolytic cleavage of the RBD at the C-terminus of the S1 protein is mandatory to commence interaction with the peptidase domain (PD) of the ACE2 receptor [[79], [80], [81]]. Proteolytic cleavage of spike proteins is followed by fusion of the virus and host cell membranes. This is followed by the development of a funnel-like structure constituted by two heptad repeats in the S2 protein in an antiparallel six-helix bundle, thereby catalyzing fusion followed by the delivery of viral genetic material into the cytoplasm.
Fig. 1.
Mortality incidence of COVID-19 patients with pre-existing diseases. Patients with pre-existing heart diseases had the highest mortality rate of 10%. Diabetes was associated with the second-highest fatality rate of ∼7%. Approximately 6% mortality was observed in those with underlying hypertension. Thus, ∼16% of mortality of COVID-19 patients was associated with some form of pre-existing CVDs or the risk factors for CVD. Data adapted from the Chinese Center for Disease Control and Prevention [65].
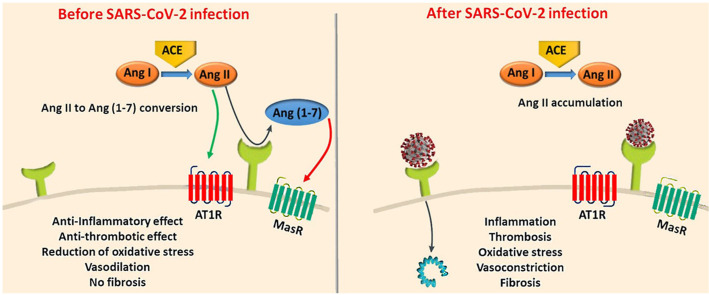
ACE2 is responsible for metabolizing the key vasoconstrictor, angiotensin II, to angiotensin (1–7). Hence, the ACE2 system acts as a preventive mechanism against MI, hypertension, lung disease, and diabetic complications [82]. Earlier studies suggested that there is a substantial expression of ACE2 receptor within the heart tissue cells including cardiomyocytes [83], cardiac myofibroblasts [84], and endothelial cells [85] and that it has a protective role against cardiac damage [86]. In a murine model of SARS-CoV infection, ACE2 expression was reduced, which led to an increase in angiotensin II levels and hence increased vascular permeability and respiratory complications, which were reversed by treatment with recombinant ACE2 or an ARB (losartan) [87]. A study also indicated that low activity of ACE2 can result in thrombosis in animal models [88]. Therefore, SARS-CoV-2 mediated dysfunction of ACE2 could cause the accumulation of angiotensin II, which eventually deteriorate the capability to counteract the activation of the RAAS pathway leading to the worsening of existing CVD [89].
Increased mortality due to COVID-19 in CVD patients may also be attributed to underlying vascular damage, hypoxia-induced vasoconstriction and heart failure [90]. Furthermore, SARS-CoV-2 infection induces potent pro-inflammatory responses and a cytokine storm that develops within the lungs and expands to the heart causing viral myocarditis and increased troponin levels in the bloodstream that could lead to fatal heart failure [91]. Indeed, inflammation is now well-established as the cause of heart failure [92] particularly as multisystem inflammatory syndrome in children (MIS-C) [[93], [94], [95], [96], [97], [98], [99], [100], [101], [102]] [[93], [94], [95], [96], [97], [98], [99], [100], [101], [102]] [[93], [94], [95], [96], [97], [98], [99], [100], [101], [102]].
A number of reviews have already discussed cardiovascular complications as a result of COVID-19 in CVD patients [90,94,103,104]. Here we provide an up-to-date and critical review of the available literature related to increased prevalence of COVID-19 in CVD patients by focusing on ACE2 mechanisms and subsequent complications and mortality in these patients.
2. Increased risk of COVID-19 and complications in people with cardiovascular risk factors or CVD
COVID-19 is particularly prevalent in patients with pre-existing history of CVD, hypertension, diabetes mellitus, COPD and the elderly [[105], [106], [107], [108]]. COVID-19 severity and mortality rates have been reported more frequently in patients experiencing cardiovascular complications [4,[109], [110], [111], [112], [113], [114]]. The COVID-19 has been reported to increase the risk of cardiogenic shock, arrhythmias, acute myocardial injury, and sometimes sudden death in CVD patients (Table 2 and Fig. 2 ). Moreover, drug interactions with COVID-19 targeted therapies may predispose patients with underlying CVDs to increased risk of developing cardiomyopathy, arrhythmias, and sudden death [115,116]. The antimalarial medication (i.e. hydroxychloroquine) and the antibiotic (i.e. azithromycin), which have been investigated as potential treatments for COVID-19, can cause serious side effects in people with existing CVDs [117]. Complications include significant irregular electrocardiography measurement indicating the presence of arrhythmia, polymorphic ventricular tachycardia (including Torsade de Pointes) and long QT syndrome, and increased risk of sudden death [117].
Table 2.
Studies investigating the implications of COVID-19 in CVD patients.
| Patient sample size | Age | Underlying CVD | CVD complications | Molecular markers studied | Mortality | Reference |
|---|---|---|---|---|---|---|
| N = 187 | 58.50 (mean age) | 66 (35.3%)- CVD, 52 (27.8%) – myocardial injury |
CHD, cardiomyopathy, myocardial injury, frequent malignant arrhythmias | TnT, NT-proBNP | 69.44% (25 of 36) | [121] |
| N = 416 | 64 (median age) | Cardiac injury (19.7%) | CHD (10.6%), cerebrovascular disease (5.3%) | hs-TnІ, CK-MB, NT-proBNP, MYO | 51.2% (42 of 82) | [122] |
| N = 191 | 56 (median age) | HT (30%), CHD (8%) | HF, arrhythmia, MI | D-dimer, serum ferritin, IL-6, LDH, hs-cTnІ, SOFA |
n/a | [123] |
| N = 273 | 58 (mean age) | n/a | Heart injury | CK-MB, MYO, Ultra Tn I, NT-proBNP | 10.5% | [124] |
| N = 1 | 64 (F) | Atrial fibrillation, HT | HF, cardiogenic shock, tachyarrhythmias | Hs-TnT, IL-6, ferritin | n/a | [125] |
| N = 38,906 | 59 (median age) | CVD (17%) HT (50%) |
n/a | n/a | 37% (CVD) 66% (HT) |
[66] |
CVD=Cardiovascular disease, HT = hypertension, CHD = coronary heart disease, NT-proBNP = N-terminalpro-B-type natriuretic peptide, CK-MB = creatinine kinase myocardial band, IL-6 = interleukin 6, LDH = lactate dehydrogenase, hs-cTnI = high-sensitivity cardiac troponin І, HF = heart failure, MI = myocardial infarction, MYO = myohemoglobin, Ultra Tn I = cardiac troponin I, SOFA = sequential organ failure assessment, hs-TnІ = high sensitivity troponinin І, n/a = not applicable.
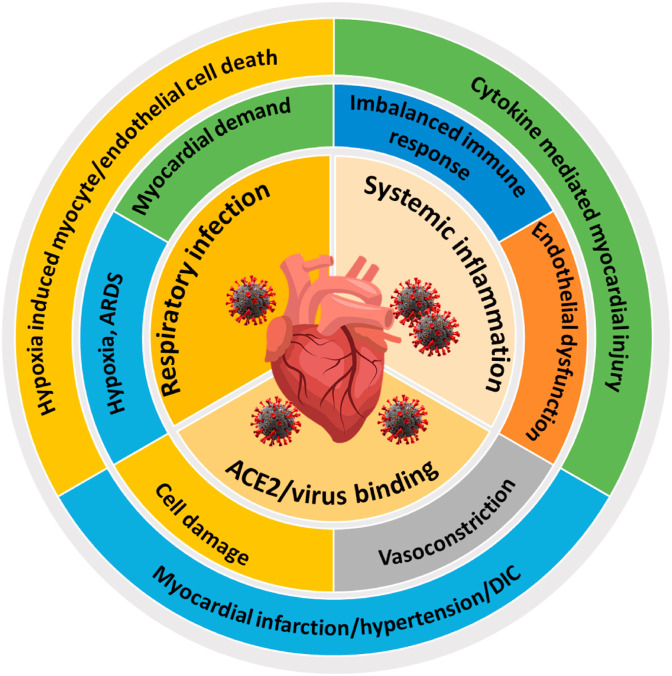
Fig. 2.
Major complications associated with SARS-CoV-2 infection in CVD patients. The manifestations of COVID-19 in CVD patients begin soon after the entry of the virus through ACE2 receptor (myocardial cell damage, ACE/ACE2 imbalance) and can result in myocardial infarction, hypertension or DIC. Respiratory infections due to SARS-CoV2 can results in hypoxia, ARDS and higher myocardial demand in CVD patients. These can result in hypoxia-induced cardiomyocyte or endothelial cell death. Further, SARS-CoV2 associated systemic inflammation can result in disrupted immune response, endothelial dysfunction and cytokine mediated myocardial injury.
Several mechanisms have been described to explain the impact of different coronaviruses on the heart based on studies investigating SARS-CoV and MERS viruses as well as recent reports with SARS-CoV-2 [4,113,118,119]. The cytokine storm caused by increased interleukin (IL)-2, IL-6, IL-7, granulocyte colony-stimulating factor (GSCF), interferon gamma-induced protein 10 (IP10), monocyte chemoattractant protein-1 (MCP1), macrophage inflammatory protein 1 A (MIP1A), and tumor necrosis factor α (TNFα) following coronavirus infection can lead to acute increases in left ventricular (LV) dysfunction in patients with underlying CVD or new onset cardiomyopathy due to heart muscle inflammation (myocarditis). Broken heart syndrome or myocardial ischemia have also been reported that can lead to heart failure, increasing the risk of mortality among critical patients [114]. A recently published case study reported the presentation of chest pain and ST-segment elevation in COVID-19 patient without underlying coronary obstruction as well as left ventricular dysfunction and elevated cardiac biomarkers [120].
Severe COVID-19 coupled with cardiac abnormalities is more prevalent in elderly patients as well as in individuals with compromised immune systems [4,105]. Inflammation of the vascular system and cardiac injury seems to occur in 20–30% of hospitalized COVID-19 patients and contributes to 40% of deaths [122]. Although concerns still exist, more concrete evidence is needed for or against the use of ACEIs and ARBs in patients with or without CVD infected with SARS-CoV-2. Currently, the majority of international clinical guidelines do not recommend stopping or adding the RAAS antagonists in COVID19 patients [4]. These agents are known to improve survival in patients with hypertension, ischemic heart disease or heart failure [126]. Cytokine release syndrome (CRS) may result in elevated levels of cytokines and impaired T lymphocyte function with lymphocytopenia in early stages and is substantially increased in the later stages of COVID-19 [127]. This is accompanied with elevated levels of CRP (C-reactive protein), cytokines such as IL-2 and IL-6, and cardiac natriuretic peptides. These factors may lead to cardiac dysfunction or inflammation due to atrial natriuretic factor elevation and high serum ferritin, which may progress to arrhythmias, myocardial dysfunction, heart failure, or stress cardiomyopathy. These conditions can increase the likelihood of death substantially [128,129].
Furthermore, Mehra and Ruschitzka suggested that underlying structural heart diseases can lead to increased risk of heart failure as a result of pulmonary dysfunction [130]. A report from the Chinese National Health Commission, stated that there are cases of hospitalized COVID-19 patients with unknown CVD (∼12%), who had increased troponin or symptoms of MI [113]. In a study by Zhou et al., COVID-19 patients developed increased troponin levels during the course of the disease and some patients showed significantly increased levels of natriuretic peptides, leading to MI and heart failure (1 in 4 cases), and eventually death [123].
Emerging evidence has implicated coagulation system impairment in severe cases of COVID-19 [131]. These coagulation abnormalities can induce DIC, with fatal consequences in both young and elderly people, mainly due to stroke [132]. Hence, there is potentially a need for the initiation of treatment with anticoagulants and pharmacokinetic monitoring for patients admitted to ICU in order to prevent thrombosis [133]. Coagulopathy is characterised by abnormal levels of thrombocytes (<50 x109/L, thrombocytopenia), prolongation of prothrombin time (PT), extension in activated partial thromboplastin time (aPTT), D-dimer level increase, and low fibrinogen levels (<1.0 g/L). Significant prolongation of aPTT with extended PT can lead to impaired coagulation in COVID-19 patients that may be due to impaired prothrombin (factor-II) production and the condition, hypoprothrombinaemia. Manifestation of COVID-19-associated coagulopathy involves swelling in the lower extremities, purple rashes, bleeding in and around catheters and clot formation in the brain that can be fatal [134]. Around 20–30% people with severe COVID-19 develop clot formation as demonstrated by two studies from Europe [135,136]. COVID-19 can also cause thrombosis in young people predisposing them to fatal stroke. Nevertheless, treatment with anti-coagulants does not seem to prevent thrombosis in COVID-19 patients, especially in the presence of CVD [137]. In COVID-19 patients, thrombosis may develop due to the binding of the SARS-CoV-2 spike protein to the ACE2 receptor that trigger the clotting process by directly damaging endothelial cells lining of the pulmonary blood vessels [138]. Evidences also suggest that inhibition of ACE2 activity [88] and higher concentration of Ang II [139] can result in thrombus formation. In some COVID-19 patients, immune cells cause inflammation by discharging cytokines and chemokines that can affect coagulation and clotting through various pathways. For instance, activation of the complement system following virus entry can result in thrombosis [140]. Apart from the COVID-19-associated risk in CVD patients, limited mass diagnosis options [[141], [142], [143]] and decreased treatment availability can also have severe consequences. As a result of the pandemic, there have been significant delays globally in elective surgeries, limited availability of ICU beds and ventilation sites, shortage of healthcare workers, restrictions on the numbers of clinical staff on ward rounds, patients developing COVID-19 after cardiac surgery, and COVID-19 patients needing urgent cardiac interventions [144].
3. Cellular and molecular mechanism of coronavirus infection and its association with cardiovascular system
Coronaviruses enter human hosts through the respiratory tract by infecting epithelial cells of the trachea, bronchi, bronchioles and lungs [145,146]. The virus also hijacks resident, infiltrating and circulating immune cells [147] leading to weakening of immune defences, and rapid deterioration of lung function, often manifested as pneumonia. The extent of immune cell damage, measured by the lymphocyte count, reflects the patient's immune status and is a reliable predictor of disease outcome. In addition, hijacked circulating immune cells are capable of delivering the virus to other organs facilitating further colonisation and organ damage [148]. Coronaviruses can also affect various endocrine and metabolic pathways through the RAAS system within the kidneys and acute β-cell dysfunction within pancreas, respectively [149]. In vitro cell culture studies of SARS-CoV-2 infected human colorectal adenocarcinoma cells (Caco-2) showed that the virus causes downregulation of the anti-apoptotic genes (Bcl-2 and A20) and upregulation of the pro-apoptotic genes (Bid, Bad, caspase-2 and caspase-6) [150]. COVID-19 associated complications are likely to occur as a result of: (1) disturbed cell metabolism due to rapid viral replication and a release of a large number of viral particles; (2) intense local vascular reactions; and (3) immune impairment mediated by the SARS-CoV-2 –induced abrogation of cellular immunity and cytokine functions [107].
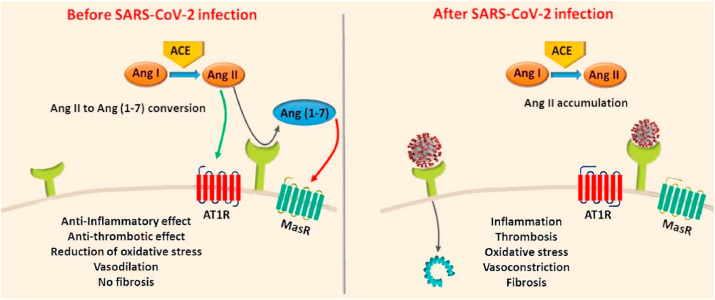
There are several underlying risk factors and pathogenic pathways (e.g. ACE2) aberrantly activated in CVD patients, which have adverse impacts on the prognosis of COVID-19. ACE2 is a metalloprotease that is abundantly expressed in cardiomyocytes, endothelial cells and cardiac fibroblasts, which comprise the vast majority of the heart tissue [151]. As described above, one of the main functions of ACE2 is to regulate cardiovascular pathophysiology and blood pressure by negatively regulating the RAAS system [20,72,86,[152], [153], [154], [155], [156]]. These functions occur through the enzymatic conversion of Ang II, a vasoconstrictor and inflammation mediator, to Ang-(1–7) that are vasodilation and anti-inflammatory mediators, as part of the RAAS pathway [157]. In addition to its expression in the heart tissue, ACE2 is also present in the lungs, intestine, liver, testis and kidney tissues [158].
Studies show that high expression of ACE2 can be protective against heart failure by abrogating ACE activity and hypertension (Fig. 3 ) [159,160]. Upon SARS-CoV-2 infection, this protective effect could be altered due to the impairment of the conversion of Angiotensin I to Angiotensin 1-9 and Angiotensin II to Angiotensin 1-7. Angiotensin 1-9 and Angiotensin 1-7 have important cardioprotective functions including reducing blood pressure. The concentration of circulating ACE2 in patients with heart failure was found to be higher compared to controls [161]. Overexpression of ACE2 to minimize heart damage and subsequent shedding of membrane-bound ACE2 may be responsible for increased circulating ACE2 levels in patients with heart failure [71]. This could also explain the predisposition to low blood pressure in heart failure patients [162]. In a pre-clinical rat model of MI, an increase in ACE and ACE2 mRNA expression was observed in the viable myocardium of injured rats compared to that of the control rats [163]. It is likely that increased expression of ACE2 is a compensatory mechanism to ameliorate the damage caused by acute cardiac stress [164,165]. Considering that the main binding site for SARS-CoV-2 is the ACE2 receptor, possible increased expression of ACE2 in CVDs is likely linked to increased susceptibility to the infection [73,166].
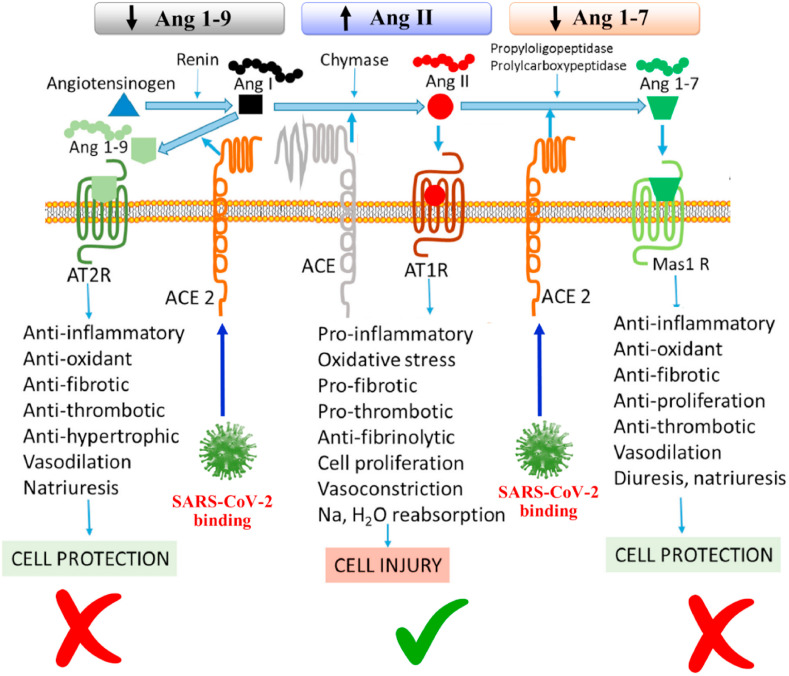
Fig. 3.
Links between SARS-CoV-2 infection and highly abundant Angiotensin II leading to various pathogenic downstream effects. Binding of SARS-CoV-2 to the ACE2 receptor on the cell surface decreases ACE2 abundance and increases the level of Angiotensin II whilst reducing Ang 1–9 and Ang 1–7. This can lead to inflammation, oxidative stress, fibrosis, thrombosis, cell proliferation, salt and water retention and vasoconstriction, hence inducing cell injury. Adapted from Ref. [167] with the permission of Elsevier.
ACE2 is widely expressed in the lungs and cells of the cardiovascular system and it plays a vital role in the cardio-protective functions of the RAAS pathway [91,168]. The N-terminal portion (S1 domain) of the SARS-CoV-2 spike glycoprotein has high-affinity for binding to ACE2 receptors on the surface of the susceptible cells [169,170]. The spike proteins sit in the viral envelope and project outwards to give a ‘corona’-like appearance to the virus [171]. Direct evidence of ACE2 as the entry point of SARS-CoV was provided by Li et al., who isolated ACE2 from SARS‐CoV–permissive Vero‐E6 cells and showed attachment to the S1 domain of the SARS‐CoV spike protein [170]. Also highly abundant ACE2 expression on endothelial and smooth muscle cells indicates that once SARS-CoV-2 enters the circulatory system, it can easily spread throughout the body [20]. Murine models and human autopsy samples revealed that SARS-CoV pulmonary infection downregulates ACE2 expression in both myocardial cells and type 2 alveolar epithelial cells/pneumocytes, causing inflammatory responses and respiratory distress [20,156], further indicating the association between SARS-CoV and ACE2 within the cardiovascular system. In addition to the increased susceptibility of CVD patients to SARS-CoV infections, CVD medications including aspirin, statins, beta-blockers, and ACEIs/ARBs could also contribute to this effect [119,172,173]. Several experimental animal studies showed that ACEIs and ARBs upregulate ACE2 expression as part of their pharmacological effect that inhibits ACE and angiotensin II synthesis in the cardiovascular system [[174], [175], [176], [177]]. The use of ACEIs and ARBs as therapeutics is a double-edged sword in COVID-19 patients as they allow increased viral entry due to augmented expression of ACE2 at the membrane, but also attenuate the hypertensive effects of angiotensin II by converting it to Ang (1–7) [178]. Thus, although ACEIs/ARBs could potentially increase susceptibility to SARS-CoV/-CoV-2 infections, these agents could also have protective effects against respiratory complications and hospitalization in infected patients [87,161].
Findings by Zhang et al. [179] suggest that the mortality rate in patients with concurrent hypertension and COVID-19 was lower among patients taking ACEIs/ARBs compared with those not on these medications. In addition, many studies have suggested that ACEIs/ARBs can improve the clinical outcome of COVID-19 patients [[180], [181], [182], [183], [184], [185]]. Increased ACE2 expression was effective in ameliorating acute lung injuries in animal models and treatment with RAAS inhibitors improved lung damage [186,187]. Studies show that ACEi and ARBs such as enalapril, losartan, and olmesartan can either restore ACE2 expression levels or even upregulate it [[187], [188], [189]].
A study by Rico-Mesa et al., did not find a link between ACEI/ARB use and effects on SARS-CoV-2 infection [190]. However, Meng et al., [191], found that the severity of COVID-19 was lower in patients receiving ACEI or ARB treatment, IL-6 levels were reduced and CD3 and CD8 T cell counts were higher in peripheral blood. Nevertheless, in an observational study by Reynolds et al. [192], ACEI, ARB, beta-blockers [193], calcium channel blockers, or thiazide diuretics presented no increased risk of COVID-19 among CVD patients [194]. A study by Choksi et al., [195], reported that RAAS blockers used to treat hypertension and CVDs had better outcomes in COVID-19 patients than those who received other anti-hypertensive drugs such as calcium channel blockers, which could be due to the high levels of ACE2 expression enhancing anti-inflammatory and antifibrotic effects. On the contrary, Guo et al. [196] and Toprak et al. [197], reported that mortality rates in patients taking ACEI/ARB treatments along with old age and other comorbidities was significantly higher than patients not taking ACE I/ARB therapy.
To date, there is little or no conclusive evidence regarding increased infectivity, morbidity, or case fatality rates among ACEIs/ARBs users. Global healthcare, cardiology departments, and health authorities recommend continuing RAAS inhibitor treatment of COVID-19 patients because of their proven anti-hypertensive activity and lack of evidence to corroborate any serious harm to the patients [198,199]. Several retrospective studies also reported that ICU requirements and COVID-19 death rates were inversely related to ACEIs/ARBs treatment in hypertensive, non-hypertensive and elderly patients [[200], [201], [202], [203]]. As there is conflicting evidence regarding the role of ACEIs/ARB in COVID-19 susceptibility and outcomes, further studies should be performed in this direction by considering multiple factors related to ACEIs/ARB-associated ACE2 expression and level of Angiotensin II in such patients with and without COVID-19 [204].
Published studies show that blocking ACE2 receptors with an ACE2 antibody can prevent the entry of SARS-CoV into cells [170]. A pre-clinical murine study demonstrated downregulation of the ACE2 gene expression upon infection with SARS-CoV [156]. This ACE2 downregulation may also result in high Ang-II and low Ang1-7 leading to vasoconstriction and inflammation as well as hypertension and lung damage [72,205]. Higher levels of Ang-II were also confirmed in a cohort of 12 patients with SARS-CoV-2 compared to healthy controls in China [189]. Nevertheless, this finding was not replicated in an American cohort of 30 patients with SARS-CoV-2 compared to 14 healthy controls; which showed no differences in plasma levels of Ang-II or aldosterone, suggesting that Ang-II could be metabolized by a different pathway than ACE2 [206]. Ang-II levels also did not correlate with disease severity. However, the same group also showed low levels of Ang1 and Ang1-7 in the same cohort of 27 SARS-CoV-2 positive patients compared to 14 healthy controls; lower levels were also observed in patients admitted to intensive care unit (ICU) compared to non-ICU treated COVID-19 patients. In the future, ACE and ACE2 levels should be determined in conjunction with Ang-II and Ang1-7, in order to improve our understanding of the RAAS system regulation by SARS-CoV-2 [207].
Both SARS-CoV and SARS-CoV-2 show relatively similar mechanisms of cell entry and pathogenesis [208]. However, unlike SARS-CoVs [209], MERS virus uses its spike protein as adhesive factors that are proinflammatory through a different specific receptor, dipeptidyl peptidase-4 (DPP4) [210]. Although further research is required, the uptake of MERS-CoV into cardiac tissues seems to use the same machinery as with other organs. Similar to other coronaviruses, MERS-CoV can generally enter the target cells in two ways. The first is through endocytosis aided by cathepsin-L and the second via plasma membrane fusion. The entry of the virus into the host cell can be either pH-dependent or –independent [211,212]. In pH-dependent endocytosis, virus entry occurs following internalization and fusion within the acidic environment of endosomal compartment [213]. When pH-independent plasma membrane fusion takes place, the virus fuses directly with the cell surface, following its binding to the receptor. Interestingly, pH-independent virus entry is ∼100- to 1000-fold more efficient than pH-dependent endocytosis [62,213]. Immediately after the cleavage of MERS-CoV spike protein by serine proteases during virus maturation, the virus can enter the cell through fusion between the viral envelope and the plasma membrane at neutral pH, resulting in substantial syncytia formation [211,214]. Laboratory investigations using cell lines show that MERS-CoV more frequently uses the plasma membrane fusion pathway [215]. Other studies suggest that viral fusion with the host cell plasma membrane is mediated by the cleaved S1 and S2 subunits of the spike protein [211,214]. Despite some differences between SARS-CoVs and MERS viruses in terms of the mechanism of host entry, the presence of CVD is a risk factor both for susceptibility to these infection and prognosis.
4. Prospects
Overall, it is well documented that coronavirus infections and complications are more prevalent in people with underlying CVD [113,216]. However, due to the presence of multiple comorbidities in COVID-19 patients, clearly identifying the main determinants of associated complications and mortality is challenging in the clinical setting [217]. Thus, future studies should focus on the development of reliable in vitro and in vivo models that can mimic various cardiovascular complications in a controlled manned as well as pathogenic mechanisms associated with increased susceptibility to SARS-CoV-2 infections and complications in CVD patients [218]. Published reports suggest that patients with cardiovascular complications who develop COVID-19 are at the highest risk of death [219]. According to the mortality data released by the National Institutes of Health, 35% of patients with COVID-19 disease had a history of hypertension and 17% had a history of coronary heart disease [220]. Although there is substantial evidence that cardiovascular risk factors can contribute to the severity of symptoms and mortality in COVID-19 patients, detailed post-mortem studies are necessary to provide better understanding of this association. The most plausible mechanism of increased complications of SARS-CoV-2 in CVD patients could be associated with the higher accumulation of Angiotensin II in CVD patients. In infected individuals, ACE2 receptors may have reduced capability to convert Angiotensin II into Angiotensin (1–7) (Fig. 4 ). However, detailed investigations are still required to invariably prove this hypothesis. Collection of post-mortem cardiac biopsies and spatial gene expression studies may help to identify underlying mechanisms that increase the severity of cardiovascular complications in COVID-19 patients [221]. Other comorbidities including diabetes mellitus, pulmonary/systemic hypertension and obesity as well as the age of the patient, play significant role in cardiac complications [222,223] and the severity of COVID-19 [224]. Further research using experimental models to determine the effects of SARS-CoV-2 on the heart both in the presence and absence of CVD is crucial. Also, further investigations into the effects of ACEIs and ARBs and the mechanisms involved would shed light on how patients with or without CVD should be managed once infected with SARS-CoV-2. The cardiac effects and mechanisms of certain antiviral drugs should be elucidated given their potential side effects that could lead to cardiac insufficiency, arrhythmia, or other cardiovascular complications [225].
Fig. 4.
In healthy individuals, Angiotensin II is converted into Angiotensin (1–7) via ACE2. However, in COVID19, ACE2 may be dysfunctional due to the binding of SARS-CoV-2, which can affect the conversion of Angiotensin II to Angiotensin (1–7). This results in the accumulation of Angiotensin II in the infected person and induces proinflammatory, prothrombotic, fibrotic and vasoconstrictive downstream effects. In the presence of CVD, however, the RAAS could be impaired. Consequently, upon infection with SARS-CoV-2, more Angiotensin II could accumulate resulting in serious cardiovascular complications.
Strict regulations and the availability of quality patient data in many countries is still a challenge for researchers. This has impeded our understanding of the interactions and mechanisms between SARS-CoV-2 and other comorbidities leading to inadequate management and poor outcomes for high-risk patients. Valuable bioinformatics tools can be utilised to perform predictive in silico modelling using patient data including patients’ clinical characteristics, medical history, medications, biochemical and viral data that can enable correlation with clinical outcomes and mortality. Multidisciplinary efforts are required to elucidate the links between COVID-19 disease and other comorbidities such as CVD, and to identify mechanisms and effective treatments that can improved outcomes for patients.
5. Conclusions
Many studies report that patients with cardiovascular complications and risk factors are more susceptible to coronavirus infections. Despite the need for further rigorous studies in this field, available data obtained from studies investigating SARS, MERS and COVID-19 patients shows clear epidemiological link. The presence of hypertension or CVDs has also been shown to lead to higher CoV-associated complications. Both SARS and COVID-19 patients have increased incidence of myocardial injury, DIC and coagulopathies. Although the mechanisms implicated, so far, include inflammation and the ACE2 pathway effects, further studies are needed to confirm conflicting results on the involvement of the RAAS system and to establish the effects and pathogenic mechanisms associated with SARS-CoV-2 and cardiovascular complications.
Author contributions
Conceptualization: R.A. Investigation: R.A. Resources: R.A., A.S., P.D., P.A. Writing—original draft preparation: R.A., A.S., S.A.S. A.N., P.A. Writing—;review and editing: L.M., C.G, P.M.H., R.A., A.S., P.D., P.M., F.M., A.H. Visualization: R.A. Supervision: A.H. Project coordination: R.A. Funding acquisition: A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was made possible by Qatar University Emergency Response Grant (QUERG-CMED-2020-2) from the Qatar University and NPRP12S-0310–190276 grant funded by Qatar National Research Fund (a part of Qatar Foundation). All statements made here are the sole responsibility of the authors. P.M.H. is funded by an Investigator Grant from the National Health and Medical Research Council (NHMRC) of Australia (1175134). P.D was supported by the grant NSFC No. 82000392. Open Access funding provided by the Qatar National Library.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch. Acad. Emerg. Med. 2020;8:e35. doi: 10.22037/aaem.v8i1.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshikawa T., Hill T.E., Yoshikawa N., Popov V.L., Galindo C.L., Garner H.R., Peters C.J., Te Tseng C. Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome-associated coronavirus infection. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrzanowski W., Kim S.Y., McClements L. Can stem cells beat COVID-19: advancing stem cells and extracellular vesicles toward mainstream medicine for lung injuries associated with SARS-CoV-2 infections. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., Jain S.S., Burkhoff D., Kumaraiah D., Rabbani L.R., Schwartz A., Uriel N. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 5.Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S.Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.Y. Coronaviruses-drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Hoek L. Human coronaviruses: what do they cause? Antivir. Ther. 2007;12:651–658. [PubMed] [Google Scholar]
- 9.Memish Z.A., Perlman S., Van Kerkhove M.D., Zumla A. Middle East respiratory syndrome. Lancet. 2020;395:1063–1077. doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coronavirus Disease (COVID-19), (n.d.).
- 11.Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S., Baghbanzadeh M., Aghamohammadi N., Zhang W., Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int. J. Epidemiol. 2021;49:717–726. doi: 10.1093/IJE/DYAA033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong R.S.M., Wu A., To K.F., Lee N., Lam C.W.K., Wong C.K., Chan P.K.S., Ng M.H.L., Yu L.M., Hui D.S., Tam J.S., Cheng G., Sung J.J.Y. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Br. Med. J. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J.Y. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348 doi: 10.1056/NEJMoa030685. 1986–1994. [DOI] [PubMed] [Google Scholar]
- 14.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D., Lu Y., Wu D., He L., Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng K.H., Wu A.K., Cheng V.C., Tang B.S., Chan C.Y., Yung C.Y., Luk S.H., Lee T.W., Chow L., Yuen K.Y. Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgrad. Med. 2005;81:e3. doi: 10.1136/pgmj.2004.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M., Ng M.H.L., Li C.K., Chan P.K.S., Liu C., Ye J.Y., Chong B.H. Thrombopoietin levels increased in patients with severe acute respiratory syndrome. Thromb. Res. 2008;122:473–477. doi: 10.1016/j.thromres.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S.Y., Su C.P., Ma M.H.M., Chiang W.C., Hsu C.Y., Ko P.C.I., Tsai K.C., Yen Z.S., Shih F.Y., Chen S.C., Chen W.J. Predictive model of diagnosing probable cases of severe acute respiratory syndrome in febrile patients with exposure risk. Ann. Emerg. Med. 2004;43:1–5. doi: 10.1016/S0196-0644(03)00817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang Z.W., Zhang L.J., Zhang S.J., Meng X., Li J.Q., Song C.Z., Sun L., Sen Zhou Y., Dwyer D.E. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS) Pathology. 2003;35:526–531. doi: 10.1080/00313020310001619118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y.P., Wei R., De Groot P.G. SARS in Hong Kong [2] N. Engl. J. Med. 2003;349:708–709. doi: 10.1056/NEJMc031468. [DOI] [PubMed] [Google Scholar]
- 20.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umapathi T., Ching A., Venketasubramanian K.N., Tchoyoson C.C., Boon L., Pang C., Tsai T., Cheng Y., Poh C.L., Lim L., Ponnudurai K., Leong K., Puay C., Tan H., Yeng D., Tai H., Peng S., Ang B. Original communication. J. Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan J.W.M., Ng C.K., Chan Y.H., Mok T.Y.W., Lee S., Chu S.Y.Y., Law W.L., Lee M.P., Li P.C.K. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan K., Zheng J., Mok Y., Li Y., Liu Y.-N., Chu C., Ip M. SARS: prognosis, outcome and sequelae. Respirology. 2003;8 doi: 10.1046/j.1440-1843.2003.00522.x. S36–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clay C.C., Donart N., Fomukong N., Knight J.B., Overheim K., Tipper J., Van Westrienen J., Hahn F., Harrod K.S. Severe acute respiratory syndrome-coronavirus infection in aged nonhuman primates is associated with modulated pulmonary and systemic immune responses. Immun. Ageing. 2014;11:4. doi: 10.1186/1742-4933-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leong H.N., Earnest A., Lim H.H., Chin C.F., Tan C.S.H., Puhaindran M.E., Tan A.C.H., Chen M.I.C., Leo Y.S. SARS in Singapore - predictors of disease severity. Ann. Acad. Med. Singapore. 2006;35 [PubMed] [Google Scholar]
- 26.Booth C.M. Clinical features and short-term outcomes of 144 patients with SARS in the greater toronto area. J. Am. Med. Assoc. 2003;289:2801. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 27.Wang J.T., Sheng W.H., Fang C.T., Chen Y.C., Wang J.L., Yu C.J., Chang S.C., Yang P.C. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg. Infect. Dis. 2004;10:818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K.T., Twu S.J., Chang H.L., Wu Y.C., Chen C.T., Lin T.H., Olsen S.J., Dowell S.F., Su I.J. SARS in Taiwan: an overview and lessons learned. Int. J. Infect. Dis. 2005;9:77–85. doi: 10.1016/j.ijid.2004.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 30.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348 doi: 10.1056/NEJMoa030666. 1977–1985. [DOI] [PubMed] [Google Scholar]
- 31.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F., Makhdoom H.Q., Zumla A.I., Memish Z.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A., Memish Z.A. Middle east respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin. Infect. Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banik G.R., Alqahtani A.S., Booy R., Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: analysis of publicly available data from Saudi Arabia. Virol. Sin. 2016;31:81–84. doi: 10.1007/s12250-015-3679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garout M.A., Jokhdar H.A.A., Aljahdali I.A., Zein A.R., Goweda R.A., Hassan-Hussein A. Mortality rate of ICU patients with the middle east respiratory syndrome – coronavirus infection at king Fahad hospital, Jeddah, Saudi Arabia. Cent. Eur. J. Publ. Health. 2018;26:87–91. doi: 10.21101/cejph.a4764. [DOI] [PubMed] [Google Scholar]
- 36.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 37.Al-Dorzi H.M., Alsolamy S., Arabi Y.M. Critically ill patients with Middle East respiratory syndrome coronavirus infection. Crit. Care. 2016;20:1–6. doi: 10.1186/s13054-016-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann. Saudi Med. 2016;36:78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 84. 2020;367:1260–1263. doi: 10.1126/science.aax0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. n.d; 2020. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arabi Y.M., Arifi A.A., Balkhy H.H., Najm H., Aldawood A.S., Ghabashi A., Hawa H., Alothman A., Khaldi A., Al Raiy B. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann. Intern. Med. 2014;160:389–397. doi: 10.7326/m13-2486. [DOI] [PubMed] [Google Scholar]
- 42.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO | WHO Guidelines for the Global Surveillance of Severe Acute Respiratory Syndrome (SARS) WHO; 2015. Updated recommendations. [Google Scholar]
- 44.Nassar M.S., Bakhrebah M.A., Meo S.A., Alsuabeyl M.S., Zaher W.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: epidemiology, pathogenesis and clinical characteristics. Eur. Rev. Med. Pharmacol. Sci. 2018;22:4956–4961. doi: 10.26355/eurrev_201808_15635. [DOI] [PubMed] [Google Scholar]
- 45.Worldometer . Worldometers.Info.; 2021. Worldometer Coronavirus Live Update; p. 1.https://www.worldometers.info/coronavirus/ accessed. [Google Scholar]
- 46.Munster V.J., Koopmans M., Van Doremalen N., Van Riel D. 2020. PERSPECTIVE 692 A Novel Coronavirus Emerging in China A Novel Coronavirus Emerging in China-Key Questions for Impact Assessment. [DOI] [PubMed] [Google Scholar]
- 47.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y., Wu S., Qin M., Jiang W., Liu X. Prevalence of cardiovascular comorbidities in coronavirus disease 2019, severe acute respiratory syndrome, and Middle East respiratory syndrome: pooled analysis of published data. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao M., Zhang D., Wang Y., Lu Y., Zhu X., Li Y., Xue H., Lin Y., Zhang M., Sun Y., Yang Z., Shi J., Wang Y., Zhou C., Dong Y., Liu P., Dudek S., Xiao Z., Lu H., Peng L. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in shanghai, China, MedRxiv prepr. Serv. Heal. Sci. 2020 doi: 10.1101/2020.03.04.20030395. [DOI] [Google Scholar]
- 50.Chen C.Y., Lee C.H., Liu C.Y., Wang J.H., Wang L.M., Perng R.P. Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J. Chin. Med. Assoc. 2005;68:4–10. doi: 10.1016/S1726-4901(09)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi K.W., Chau T.N., Tsang O., Tso E., Chiu M.C., Tong W.L., Lee P.O., Ng T.K., Ng W.F., Lee K.C., Lam W., Yu W.C., Lai J.Y., Lai S.T. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann. Intern. Med. 2003;139 doi: 10.7326/0003-4819-139-9-200311040-00005. [DOI] [PubMed] [Google Scholar]
- 52.Lew T.W.K., Kwek T.K., Tai D., Earnest A., Loo S., Singh K., Kwan K.M., Chan Y., Yim C.F., Bek S.L., Kor A.C., Yap W.S., Chelliah Y.R., Lai Y.C., Goh S.K. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. J. Am. Med. Assoc. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 53.Almekhlafi G.A., Albarrak M.M., Mandourah Y., Hassan S., Alwan A., Abudayah A., Altayyar S., Mustafa M., Aldaghestani T., Alghamedi A., Talag A., Malik M.K., Omrani A.S., Sakr Y. Presentation and outcome of Middle East respiratory syndrome in Saudi intensive care unit patients. Crit. Care. 2016;20 doi: 10.1186/s13054-016-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al Ghamdi M., Alghamdi K.M., Ghandoora Y., Alzahrani A., Salah F., Alsulami A., Bawayan M.F., Vaidya D., Perl T.M., Sood G. Treatment outcomes for patients with middle eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the kingdom of Saudi arabia. BMC Infect. Dis. 2016;16 doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halim A.A., Alsayed B., Embarak S., Yaseen T., Dabbous S. Clinical characteristics and outcome of ICU admitted MERS corona virus infected patients, Egypt. J. Chest Dis. Tuberc. 2016;65:81–87. doi: 10.1016/j.ejcdt.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abate S.M., Ali S.A., Mantfardo B., Basu B. Rate of intensive care unit admission and outcomes among patients with coronavirus: a systematic review and Meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Immovilli P., Morelli N., Antonucci E., Radaelli G., Barbera M., Guidetti D. COVID-19 mortality and ICU admission: the Italian experience. Crit. Care. 2020;24 doi: 10.1186/s13054-020-02957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li S.S.L., Cheng C.W., Fu C.L., Chan Y.H., Lee M.P., Chan J.W.M., Yiu S.F. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-Day echocardiographic follow-up study. Circulation. 2003;108:1798–1803. doi: 10.1161/01.CIR.0000094737.21775.32. [DOI] [PubMed] [Google Scholar]
- 59.Yu C.M., Wong R.S.M., Wu E.B., Kong S.L., Wong J., Yip G.W.K., Soo Y.O.Y., Chiu M.L.S., Chan Y.S., Hui D., Lee N., Wu A., Leung C.B., Sung J.J.Y. Cardiovascular complications of severe acute respiratory syndrome. Postgrad. Med. 2006;82:140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoon Chong P., Chui P., Ling A.E., Franks T.J., H Tai D.Y., Sin Leo Y., L Kaw G.J., Wansaicheong G., Peng Chan K., Lin Ean Oon L., Swee Teo E., Bing Tan K., Nakajima N., Sata T., Travis W.D. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore challenges in determining a SARS diagnosis. Arch. Pathol. Lab Med. 2004;128:195–204. doi: 10.1043/1543-2165(2004)128<195:AODDTS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 61.Park J.-E., Jung S., Kim A. Springer; 2020. MERS transmission and risk factors: a systematic review. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuyama R., Nishiura H., Kutsuna S., Hayakawa K., Ohmagari N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Publ. Health. 2016;16 doi: 10.1186/s12889-016-3881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garg S., Kim L., Whitaker M., O'Halloran A., Cummings C., Holstein R., Prill M., Chai S.J., Kirley P.D., Alden N.B., Kawasaki B., Yousey-Hindes K., Niccolai L., Anderson E.J., Openo K.P., Weigel A., Monroe M.L., Ryan P., Henderson J., Kim S., Como-Sabetti K., Lynfield R., Sosin D., Torres S., Muse A., Bennett N.M., Billing L., Sutton M., West N., Schaffner W., Talbot H.K., Aquino C., George A., Budd A., Brammer L., Langley G., Hall A.J., Fry A. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 states, march 1–30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang Y., Chen T., Mui D., Ferrari V., Jagasia D., Scherrer-Crosbie M., Chen Y., Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132–1141. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cdc Weekly C. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Wkly. 2020;2:113–122. doi: 10.46234/ccdcw2020.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dorjee K., Kim H., Bonomo E., Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu S., Luo H., Wang Y., Wang D., Ju S., Yang Y. Characteristics and associations with severity in COVID-19 patients: a multicentre cohort study from Jiangsu province, China, SSRN electron. J. 2020 doi: 10.2139/ssrn.3548753. [DOI] [Google Scholar]
- 68.Epelman S., Tang W.H.W., Chen S.Y., Van Lente F., Francis G.S., Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure. Insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J. Am. Coll. Cardiol. 2008;52:750–754. doi: 10.1016/j.jacc.2008.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hakeam H.A., Alsemari M., Al Duhailib Z., Ghonem L., Alharbi S.A., Almutairy E., Bin Sheraim N.M., Alsalhi M., Alhijji A., AlQahtani S., Khalid M., Barry M. Association of angiotensin-converting enzyme inhibitors and angiotensin II blockers with severity of COVID-19: a multicenter, prospective study. J. Cardiovasc. Pharmacol. Therapeut. 2020 doi: 10.1177/1074248420976279. 107424842097627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bauer A.Z., Gore R., Sama S.R., Rosiello R., Garber L., Sundaresan D., McDonald A., Arruda P., Kriebel D. Hypertension, medications, and risk of severe COVID-19: a Massachusetts community-based observational study. J. Clin. Hypertens. 2020 doi: 10.1111/jch.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crackower M.A., Sarao R., Oliveira-dos-Santos A.J., Da Costa J., Zhang L. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 73.Devaux C.A., Rolain J.M., Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020;53:425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma S., Li H., Yang J., Yu K. Molecular simulation studies of the interactions between the human/pangolin/cat/bat ACE2 and the receptor binding domain of the SARS-CoV-2 spike protein. Biochimie. 2021;187:1–13. doi: 10.1016/j.biochi.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Di Jiang R., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y., Hu G., Wang Y., Ren W., Zhao X., Ji F., Zhu Y., Feng F., Gong M., Ju X., Zhu Y., Cai X., Lan J., Guo J., Xie M., Dong L., Zhu Z., Na J., Wu J., Lan X., Xie Y., Wang X., Yuan Z., Zhang R., Ding Q. Functional and genetic analysis of viral receptor ACE2 orthologs reveals a broad potential host range of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2025373118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. 2016 5317592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samavati L., Uhal B.D. ACE2, much more than Just a receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020;10:317. doi: 10.3389/fcimb.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Renhong Y., Yuanyuan Z., Yaning L., Lu X., Yingying G., Qiang Z. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 84. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P., Guo D., Fu L., Cui Y., Liu X., Arledge K.C., Chen Y.H., Zhang L., Wang X. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry Depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Annweiler C., Cao Z., Wu Y., Faucon E., Mouhat S., Kovacic H., Sabatier J.-M. Counter-regulatory ‘renin-angiotensin’ system-based candidate drugs to treat COVID-19 diseases in SARS-CoV-2-infected patients. Infect. Disord. - Drug Targets. 2020 doi: 10.2174/1871526520666200518073329. [DOI] [PubMed] [Google Scholar]
- 83.Gallagher P.E., Ferrario C.M., Tallant E.A. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2008 doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guy J.L., Lambert D.W., Turner A.J., Porter K.E. Functional angiotensin-converting enzyme 2 is expressed in human cardiac myofibroblasts. Exp. Physiol. 2008;93:579–588. doi: 10.1113/expphysiol.2007.040139. [DOI] [PubMed] [Google Scholar]
- 85.Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P.C., Levitt K.S., Oudit G.Y., Al-Omran M., Stewart D.J., Slutsky A.S., Peterson M.D., Backx P.H., Penninger J.M., Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295 doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 86.Oudit G.Y., Crackower M.A., Backx P.H., Penninger J.M. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc. Med. 2003;13:93–101. doi: 10.1016/S1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 87.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fraga-Silva R.A., Sorg B.S., Wankhede M., deDeugd C., Jun J.Y., Baker M.B., Li Y., Castellano R.K., Katovich M.J., Raizada M.K., Ferreira A.J. ACE2 activation promotes antithrombotic activity. Mol. Med. 2010;16:210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alifano M., Alifano P., Forgez P., Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020;174:30–33. doi: 10.1016/j.biochi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu H., Rhee J.W., Cheng P., Waliany S., Chang A., Witteles R.M., Maecker H., Davis M.M., Nguyen P.K., Wu S.M. Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr. Cardiol. Rep. 2020;22:32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lazar S., Rayner B., Lopez Campos G., McGrath K., McClements L. Mechanisms of heart failure with preserved ejection fraction in the presence of diabetes mellitus. Transl. Metab. Syndr. Res. 2020;3:1–5. doi: 10.1016/j.tmsr.2020.04.002. [DOI] [Google Scholar]
- 93.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., Barranco M.A., Maxted A.M., Rosenberg E.S., Easton D., Udo T., Kumar J., Pulver W., Smith L., Hutton B., Blog D., Zucker H. Multisystem inflammatory syndrome in children in New York state. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., Newburger J.W., Kleinman L.C., Heidemann S.M., Martin A.A., Singh A.R., Li S., Tarquinio K.M., Jaggi P., Oster M.E., Zackai S.P., Gillen J., Ratner A.J., Walsh R.F., Fitzgerald J.C., Keenaghan M.A., Alharash H., Doymaz S., Clouser K.N., Giuliano J.S., Gupta A., Parker R.M., Maddux A.B., Havalad V., Ramsingh S., Bukulmez H., Bradford T.T., Smith L.S., Tenforde M.W., Carroll C.L., Riggs B.J., Gertz S.J., Daube A., Lansell A., Munoz A.C., Hobbs C.V., Marohn K.L., Halasa N.B., Patel M.M., Randolph A.G. Multisystem inflammatory syndrome in U.S. Children and adolescents. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., Tan Z., Zicari S., Ruggiero A., Pascucci G.R., Santilli V., Campbell T., Bryceson Y., Eriksson D., Wang J., Marchesi A., Lakshmikanth T., Campana A., Villani A., Rossi P., Landegren N., Palma P., Brodin P. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Child.; Basel, Switzerland): 2020. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahmed M., Advani S., Moreira A., Zoretic S., Martinez J., Chorath K., Acosta S., Naqvi R., Burmeister-Morton F., Burmeister F., Tarriela A., Petershack M., Evans M., Hoang A., Rajasekaran K., Ahuja S., Moreira A. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:1–16. doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greene A.G., Saleh M., Roseman E., Sinert R. Toxic shock-like syndrome and COVID-19: a case report of multisystem inflammatory syndrome in children (MIS-C) Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., Ramnarayan P., Fraisse A., Miller O., Davies P., Kucera F., Brierley J., McDougall M., Carter M., Tremoulet A., Shimizu C., Herberg J., Burns J.C., Lyall H., Levin M. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA, J. Am. Med. Assoc. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dolinger M.T., Person H., Smith R., Jarchin L., Pittman N., Dubinsky M.C., Lai J. Pediatric crohn disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J. Pediatr. Gastroenterol. Nutr. 2020 doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R., Gillen J.K., Perez M.M., Soshnick S.H., Conway E.E., Bercow A., Seiden H.S., Pass R.H., Ushay H.M., Ofori-Amanfo G., Medar S.S. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York city. J. Pediatr. 2020 doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carter M.J., Fish M., Jennings A., Doores K.J., Wellman P., Seow J., Acors S., Graham C., Timms E., Kenny J., Neil S., Malim M.H., Tibby S.M., Shankar-Hari M. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020 doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 103.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bandyopadhyay D., Akhtar T., Hajra A., Gupta M., Das A., Chakraborty S., Pal I., Patel N., Amgai B., Ghosh R.K., Fonarow G.C., Lavie C.J., Naidu S.S. COVID-19 pandemic: cardiovascular complications and future implications. Am. J. Cardiovasc. Drugs. 2020;20:311–324. doi: 10.1007/s40256-020-00420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahajan K., Chandra K.S. Cardiovascular comorbidities and complications associated with coronavirus disease 2019. Med. J. Armed Forces India. 2020;76:253. doi: 10.1016/j.mjafi.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liuxingbingxue Zazhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 107.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., Brown T.S., Der Nigoghossian C., Zidar D.A., Haythe J., Brodie D., Beckman J.A., Kirtane A.J., Stone G.W., Krumholz H.M., Parikh S.A. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N. Engl. J. Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., Satlin M.J., Campion T.R., Nahid M., Ringel J.B., Hoffman K.L., Alshak M.N., Li H.A., Wehmeyer G.T., Rajan M., Reshetnyak E., Hupert N., Horn E.M., Martinez F.J., Gulick R.M., Safford M.M. Clinical characteristics of covid-19 in New York city. N. Engl. J. Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fang Z., Yi F., Wu K., Lai K., Sun X., Zhong N., Liu Z. Clinical characteristics of coronavirus pneumonia 2019 (COVID-19): an updated systematic review. MedRxiv. 2020:2020. doi: 10.1101/2020.03.07.20032573. 03.07.20032573. [DOI] [Google Scholar]
- 112.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J., Liang Z., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dhakal B.P., Sweitzer N.K., Indik J.H., Acharya D., William P. Lung Circ; Hear: 2020. SARS-CoV-2 Infection and Cardiovascular Disease: COVID-19 Heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roy S., Mazumder T., Banik S. The association of cardiovascular diseases and diabetes mellitus with COVID-19 (SARS-CoV-2) and their possible mechanisms, SN compr. Clin. Med. 2020;2:1077–1082. doi: 10.1007/s42399-020-00376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.American Heart Association Caution recommended on COVID-19 treatment with hydroxychloroquine and azithromycin for patients with cardiovascular disease. https://www.mendeley.com/library/ n.d.
- 118.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Han H., Xie L., Liu R., Yang J., Liu F., Wu K., Chen L., Hou W., Feng Y., Zhu C. Analysis of heart injury laboratory parameters in 273 COVID‐19 patients in one hospital in Wuhan, China. J. Med. Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E., Rabbani L.R., Brodie D., Jain S.S., Kirtane A.J., Masoumi A., Takeda K., Kumaraiah D., Burkhoff D., Leon M., Schwartz A., Uriel N., Sayer G. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bozkurt B., Kovacs R., Harrington B. Joint HFSA/ACC/AHA statement addresses concerns Re: using RAAS antagonists in COVID-19. J. Card. Fail. 2020;26:370. doi: 10.1016/j.cardfail.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ganatra S., Carver J.R., Hayek S.S., Ky B., Leja M.J., Lenihan D.J., Lenneman C., Mousavi N., Park J.H., Perales M.A., Ryan T.D., Scherrer-Crosbie M., Steingart R.M., Yang E.H., Zaha V., Barac A., Liu J.E. Chimeric antigen receptor T-cell therapy for cancer and heart: JACC Council perspectives. J. Am. Coll. Cardiol. 2019;74:3153–3163. doi: 10.1016/j.jacc.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mehra M.R., Ruschitzka F. COVID-19 illness and heart failure: a missing link? JACC Hear. Fail. 2020;8:512. doi: 10.1016/j.jchf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2020. 2020;171(17):46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020;127:104362. doi: 10.1016/J.JCV.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Disseminated Intravascular Coagulation in COVID-19—Insights from the Front Lines | AACC.org, (n.d.).
- 134.Willyard C. Coronavirus blood-clot mystery intensifies. Nature. 2020;581:250. doi: 10.1038/d41586-020-01403-8. [DOI] [PubMed] [Google Scholar]
- 135.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 136.Cattaneo M., Bertinato E.M., Birocchi S., Brizio C., Malavolta D., Manzoni M., Muscarella G., Orlandi M. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb. Haemostasis. 2020;120:1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemostasis. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Senchenkova E.Y., Russell J., Esmon C.T., Granger D.N. Roles of coagulation and fibrinolysis in angiotensin II-enhanced microvascular thrombosis. Microcirculation. 2014;21:401–407. doi: 10.1111/micc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98:314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Augustine R., Hasan A., Das S., Ahmed R., Mori Y., Notomi T., Kevadiya B.D., Thakor A.S. Loop-mediated isothermal amplification (LAMP): a rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology. 2020;9:1–17. doi: 10.3390/biology9080182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Augustine R., Das S., Hasan A., Abhilash S., Salam S.A., Augustine P., Dalvi Y.B., Varghese R., Primavera R., Yassine H.M., Thakor A.S., Kevadiya B.D. Rapid antibody-based covid-19 mass surveillance: relevance, challenges, and prospects in a pandemic and post-pandemic world. J. Clin. Med. 2020;9:1–26. doi: 10.3390/jcm9103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharifi M., Hasan A., Taghizadeh A., Haghighat S., Attar F., Bloukh S.H., Edis Z., Xue M., Khan S., Falahati M. Rapid diagnostics of coronavirus disease 2019 in early stages using nanobiosensors: challenges and opportunities. Talanta. 2020:121704. doi: 10.1016/j.talanta.2020.121704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cardiac Surgery and the COVID-19 Outbreak: what Does it Mean? https://www.pcronline.com/News/Whats-new-on-PCRonline/2020/Cardiac-Surgery-and-the-COVID-19-outbreak-what-does-it-mean n.d.
- 145.Sims A.C., Baric R.S., Yount B., Burkett S.E., Collins P.L., Pickles R.J. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J. Virol. 2005;79:15511–15524. doi: 10.1128/jvi.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shan C., Yao Y.F., Lou Yang X., Zhou Y.W., Gao G., Peng Y., Yang L., Hu X., Xiong J., Di Jiang R., Zhang H.J., Gao X.X., Peng C., Min J., Chen Y., Si H.R., Wu J., Zhou P., Wang Y.Y., Wei H.P., Pang W., Hu Z.F., Lv L.B., Zheng Y.T., Shi Z.L., Yuan Z.M. Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Res. 2020;30:670–677. doi: 10.1038/s41422-020-0364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jafarzadeh A., Chauhan P., Saha B., Jafarzadeh S., Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li Z., Liu T., Yang N., Han D., Mi X., Li Y., Liu K., Vuylsteke A., Xiang H., Guo X. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 2020 doi: 10.1007/s11684-020-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bornstein S.R., Dalan R., Hopkins D., Mingrone G., Boehm B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020;16:297–298. doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cinatl J., Hoever G., Morgenstern B., Preiser W., Vogel J.U., Hofmann W.K., Bauer G., Michaelis M., Rabenau H.F., Doerr H.W. Infection of cultured intestinal epithelial cells with severe acute respiratory syndrome coronavirus. Cell. Mol. Life Sci. 2004;61:2100–2112. doi: 10.1007/s00018-004-4222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87 doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 152.Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020 doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang H., Wang W., Liu P., Jiang Y., Zhao Y., Wei H., Niu W. TRPC1 expression and distribution in rat hearts. Eur. J. Histochem. 2009 doi: 10.4081/ejh.2009.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/S0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 155.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis virus transmission pathways. J. Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Anguiano L., Riera M., Pascual J., Soler M.J. Circulating ACE2 in cardiovascular and kidney diseases. Curr. Med. Chem. 2017 doi: 10.2174/0929867324666170414162841. [DOI] [PubMed] [Google Scholar]
- 158.Surjit M., Liu B., Kumar P., Chow V.T.K., Lal S.K. The nucleocapsid protein of the SARS coronavirus is capable of self-association through a C-terminal 209 amino acid interaction domain. Biochem. Biophys. Res. Commun. 2004 doi: 10.1016/j.bbrc.2004.03.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang W., Patel V.B., Parajuli N., Fan D., Basu R., Wang Z., Ramprasath T., Kassiri Z., Penninger J.M., Oudit G.Y. Heterozygote loss of ACE2 is sufficient to increase the susceptibility to heart disease. J. Mol. Med. 2014;92:847–858. doi: 10.1007/s00109-014-1149-y. [DOI] [PubMed] [Google Scholar]
- 161.Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infec. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ettehad D., Emdin C.A., Kiran A., Anderson S.G., Callender T., Emberson J., Chalmers J., Rodgers A., Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 163.Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S., Tikellis C., Grant S.L., Lew R.A., Smith A.I., Cooper M.E., Johnston C.I. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- 164.Burchill L., Velkoska E., Dean R.G., Lew R.A., Smith A.I., Levidiotis V., Burrell L.M. Acute kidney injury in the rat causes cardiac remodelling and increases angiotensin-converting enzyme 2 expression. Exp. Physiol. 2008;93:622–630. doi: 10.1113/expphysiol.2007.040386. [DOI] [PubMed] [Google Scholar]
- 165.Shi L., Mao C., Xu Z., Zhang L. Angiotensin-converting enzymes and drug discovery in cardiovascular diseases. Drug Discov. Today. 2010;15:332–341. doi: 10.1016/j.drudis.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chung M.K., Karnik S., Saef J., Bergmann C., Barnard J., Lederman M.M., Tilton J., Cheng F., Harding C.V., Young J.B., Mehta N., Cameron S.J., McCrae K.R., Schmaier A.H., Smith J.D., Kalra A., Gebreselassie S.K., Thomas G., Hawkins E.S., Svensson L.G. SARS-CoV-2 and ACE2: the biology and clinical data settling the ARB and ACEI controversy. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang H., Li H.B., Lyu J.R., Lei X.M., Li W., Wu G., Lyu J., Dai Z.M. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int. J. Infect. Dis. 2020;96:19–24. doi: 10.1016/j.ijid.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hasan A., Paray B.A., Hussain A., Qadir F.A., Attar F., Aziz F.M., Sharifi M., Derakhshankhah H., Rasti B., Mehrabi M., Shahpasand K., Saboury A.A., Falahati M. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1754293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li W., Moore M.J., Vasllieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greeneugh T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nicin L., Abplanalp W.T., Mellentin H., Kattih B., Tombor L., John D., Schmitto J.D., Heineke J., Emrich F., Arsalan M., Holubec T., Walther T., Zeiher A.M., Dimmeler S. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020;41:1804–1806. doi: 10.1093/EURHEARTJ/EHAA311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sinha S., Cheng K., Schäffer A.A., Aldape K., Schiff E., Ruppin E. In vitro and in vivo identification of clinically approved drugs that modify ACE 2 expression. Mol. Syst. Biol. 2020;16 doi: 10.15252/msb.20209628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Iwanami J., Mogi M., Tsukuda K., Wang X.-L., Nakaoka H., Ohshima K., Chisaka T., Bai H.-Y., Kanno H., Min L.-J., Horiuchi M. Role of angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis in the hypotensive effect of azilsartan. Hypertens. Res. 2014. 2014;377(37):616–620. doi: 10.1038/hr.2014.49. [DOI] [PubMed] [Google Scholar]
- 176.Wang X., Ye Y., Gong H., Wu J., Yuan J., Wang S., Yin P., Ding Z., Kang L., Jiang Q., Zhang W., Li Y., Ge J., Zou Y. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1–7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J. Mol. Cell. Cardiol. 2016;97:180–190. doi: 10.1016/J.YJMCC.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 177.Sukumaran V., Veeraveedu P.T., Lakshmanan A.P., Gurusamy N., Yamaguchi K., Ma M., Suzuki K., Kodama M., Watanabe K. 2012. Olmesartan Medoxomil Treatment Potently Improves Cardiac Myosin-Induced Dilated Cardiomyopathy via the Modulation of ACE-2 and ANG 1–7 Mas Receptor; pp. 850–860. Http://Dx.Doi.Org/10.3109/10715762.2012.684878. 46. [DOI] [PubMed] [Google Scholar]
- 178.Yan T., Xiao R., Lin G. Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: a double-edged sword? Faseb. J. 2020;34:6017–6026. doi: 10.1096/FJ.202000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang P., Zhu L., Cai J., Lei F., Qin J.-J., Xie J., Liu Y.-M., Zhao Y.-C., Huang X., Lin L., Xia M., Chen M.-M., Cheng X., Zhang X., Guo D., Peng Y., Ji Y.-X., Chen J., She Z.-G., Wang Y., Xu Q., Tan R., Wang H., Lin J., Luo P., Fu S., Cai H., Ye P., Xiao B., Mao W., Liu L., Yan Y., Liu M., Chen M., Zhang X.-J., Wang X., Touyz R.M., Xia J., Zhang B.-H., Huang X., Yuan Y., Loomba R., Liu P.P., Li H. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang G., Tan Z., Zhou L., Yang M., Peng L., Liu J., Cai J., Yang R., Han J., Huang Y., He S. Effects of angiotensin II receptor blockers and ACE (Angiotensin-Converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID-19 and hypertension. Hypertension. 2020;76:51–58. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- 181.Yahyavi A., Hemmati N., Derakhshan P., Banivaheb B., Karimi Behnagh A., Tofighi R., TehraniYazdi A., Kabir A. Angiotensin enzyme inhibitors and angiotensin receptor blockers as protective factors in COVID-19 mortality: a retrospective cohort study. Intern. Emerg. Med. 2020. 2020;164(16):883–893. doi: 10.1007/S11739-020-02523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li S., Sarangarajan R., Jun T., Kao Y.-H., Wang Z., Hao K., Schadt E., Kiebish M.A., Granger E., Narain N.R., Chen R., Schadt E.E., Li L. In-hospital use of ACE inhibitors/angiotensin receptor blockers associates with COVID-19 outcomes in African American patients. J. Clin. Invest. 2021;131 doi: 10.1172/JCI151418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Parit R., Jayavel S. Association of ACE inhibitors and angiotensin type II blockers with ACE2 overexpression in COVID-19 comorbidities: a pathway-based analytical study. Eur. J. Pharmacol. 2021;896:173899. doi: 10.1016/J.EJPHAR.2021.173899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Soler M.J., Ribera A., Marsal J.R., Mendez A.B., Andres M., Azancot M.A., Oristrell G., Méndez-Boo L., Cohen J., Barrabés J.A., Ferreira-González I. Association of renin–angiotensin system blockers with COVID-19 diagnosis and prognosis in patients with hypertension: a population-based study. Clin. Kidney J. 2021 doi: 10.1093/CKJ/SFAB161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Singh A.K., Gupta R., Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:283–287. doi: 10.1016/J.DSX.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 188.Karram T., Abbasi A., Keidar S., Golomb E., Hochberg I., Winaver J., Hoffman A., Abassi Z. Effects of spironolactone and eprosartan on cardiac remodeling and angiotensin-converting enzyme isoforms in rats with experimental heart failure. Am. J. Physiol. Heart Circ. Physiol. 2005;289 doi: 10.1152/ajpheart.01186.2004. [DOI] [PubMed] [Google Scholar]
- 189.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rico-Mesa J.S., White A., Anderson A.S. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr. Cardiol. Reports 2020. 2020;225(22):1–4. doi: 10.1007/S11886-020-01291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. 2020. Renin-angiotensin System Inhibitors Improve the Clinical Outcomes of COVID-19 Patients with Hypertension. Https://Doi.Org/10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I., Kunichoff D., Chen Y., Ogedegbe G., Hochman J.S. 2020. Renin–Angiotensin–Aldosterone System Inhibitors and Risk of Covid-19; pp. 2441–2448. Https://Doi.Org/10.1056/NEJMoa2008975. 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.fang Chen F., Zhong M., Liu Y., Zhang Y., Zhang K., zhen Su D., Meng X., Zhang Y. The characteristics and outcomes of 681 severe cases with COVID-19 in China. J. Crit. Care. 2020;60:32–37. doi: 10.1016/J.JCRC.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Peng C., Wang H., Guo Y.-F., Qi G.-Y., Zhang C.-X., Chen T., He J., Jin Z.-C. Calcium channel blockers improve prognosis of patients with coronavirus disease 2019 and hypertension. Chin. Med. J. 2021;134:1602. doi: 10.1097/CM9.0000000000001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Choksi T.T., Zhang H., Chen T., Malhotra N. Outcomes of hospitalized COVID-19 patients receiving renin angiotensin system blockers and calcium channel blockers. Am. J. Nephrol. 2021;52:250–260. doi: 10.1159/000515232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/JAMACARDIO.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Age D.O., Hypertension C., Artery Disease A.-I., Ob A., Mortality R.O. OR beta-blockers therapy increase the risk OF mortality IN COVID 19 patients? The results OF a tertiary center IN Turkey. Hypertens. Coron. ARTERY Dis. 2021;37:547. doi: 10.19193/0393-6384_2021_1_85. [DOI] [Google Scholar]
- 198.Position Statement of the ESC Council. 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang Position Statement of the on Hypertension on ACE-Inhibitors and Angi. [Google Scholar]
- 199.Kuster G.M., Pfister O., Burkard T., Zhou Q., Twerenbold R., Haaf P., Widmer A.F., Osswald S. SARS-CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19? Eur. Heart J. 2020;41:1801. doi: 10.1093/EURHEARTJ/EHAA235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vura N.V.R.K., Sandooja R., Firoz A. To do or not to do: angiotensin converting enzyme inhibitors/angiotensin receptor blocker in COVID-19 elderly patients. EXCLI J. 2021;20:1145. doi: 10.17179/EXCLI2021-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Oddy C., Allington J., McCaul J., Keeling P., Senn D., Soni N., Morrison H., Mawella R., Samuel T., Dixon J. Inpatient omission of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers is associated with morbidity and mortality in coronavirus disease 2019. Clin. Therapeut. 2021;43 doi: 10.1016/J.CLINTHERA.2021.02.004. e97–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825–830. doi: 10.1001/JAMACARDIO.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.ElAbd R., AlTarrah D., AlYouha S., Bastaki H., Almazeedi S., Al-Haddad M., Jamal M., AlSabah S. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) are protective against ICU admission and mortality for patients with COVID-19 disease. Front. Med. 2021:226. doi: 10.3389/FMED.2021.600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zores F., Rebeaud M.E. COVID and the renin-angiotensin system: are hypertension or its treatments Deleterious? Front. Cardiovasc. Med. 2020:71. doi: 10.3389/FCVM.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kar S., Gao L., Zucker I.H. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J. Appl. Physiol. 2010;108:923–932. doi: 10.1152/japplphysiol.00840.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Henry B.M., Benoit S., Lippi G., Benoit J. Circulating plasma levels of angiotensin II and aldosterone in patients with coronavirus disease 2019 (COVID-19): a preliminary report. Prog. Cardiovasc. Dis. 2020 doi: 10.1016/j.pcad.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Henry B.M., Benoit J., Berger B., Pulvino C., Lavie C.J., Lippi G., Benoit S.W. Coronavirus disease 2019 (COVID‐19) is associated with low circulating plasma levels of angiotensin 1 and angiotensin 1. J. Med. Virol. 2020:7. doi: 10.1002/jmv.26479. jmv.26479. [DOI] [PubMed] [Google Scholar]
- 208.Mahmoud I.S., Jarrar Y.B., Alshaer W., Ismail S. SARS-CoV-2 entry in host cells-multiple targets for treatment and prevention. Biochimie. 2020;175:93–98. doi: 10.1016/j.biochi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xi C.R., Di Fazio A., Nadvi N.A., Patel K., Sui M., Xiang W., Zhang H.E., Deshpande C., Low J.K.K., Wang X.T., Chen Y., Osborne B., Vieira De Ribeiro A.J., Mccaughan G.W., Church B., Mackay J.P., Gorrell M.D. A novel purification procedure for active recombinant human DPP4 and the inability of DPP4 to bind SARS-CoV-2. Preprints. 2020 doi: 10.20944/preprints202009.0390.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mubarak A., Alturaiki W., Hemida M.G. Middle east respiratory syndrome coronavirus (mers-cov): infection, immunological response, and vaccine development. J. Immunol. Res. 2019;2019 doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Qian Z., Dominguez S.R., Holmes K.V. Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shirato K., Kawase M., Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J. Virol. 2013;87:12552–12561. doi: 10.1128/jvi.01890-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gierer S., Bertram S., Kaup F., Wrensch F., Heurich A., Kramer-Kuhl A., Welsch K., Winkler M., Meyer B., Drosten C., Dittmer U., von Hahn T., Simmons G., Hofmann H., Pohlmann S. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can Be activated by TMPRSS2, and is targeted by neutralizing antibodies. J. Virol. 2013;87:5502–5511. doi: 10.1128/jvi.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lu L., Liu Q., Zhu Y., Chan K.H., Qin L., Li Y., Wang Q., Chan J.F.W., Du L., Yu F., Ma C., Ye S., Yuen K.Y., Zhang R., Jiang S. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat. Commun. 2014;5 doi: 10.1038/ncomms4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhao M., Wang M., Zhang J., Ye J., Xu Y., Wang Z., Ye D., Liu J., Wan J. Advances in the relationship between coronavirus infection and cardiovascular diseases. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pathangey G., Fadadu P.P., Hospodar A.R., Abbas A. Angiotensin-converting enzyme 2 and COVID-19: patients, comorbidities, and therapies. Am. J. Physiol. Cell. Mol. Physiol. 2020 doi: 10.1152/ajplung.00259.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D'Acquisto F., Nicklin S.A., Marian A.J., Nosalski R., Murray E.C., Guzik B., Berry C., Touyz R.M., Kreutz R., Dao W.W., Bhella D., Sagliocco O., Crea F., Thomson E.C., McInnes I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kumari V., Mehta K., Choudhary R. COVID-19 outbreak and decreased hospitalisation of pregnant women in labour. Lancet Glob. Heal. 2020 doi: 10.1016/S2214-109X(20)30319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Deye N., Vincent F., Michel P., Ehrmann S., Da Silva D., Piagnerelli M., Kimmoun A., Hamzaoui O., Lacherade J.C., de Jonghe B., Brouard F., Audoin C., Monnet X., Laterre P.F. Changes in cardiac arrest patients' temperature management after the 2013 “TTM” trial: results from an international survey. Ann. Intensive Care. 2016;6 doi: 10.1186/s13613-015-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.European society of cardiology, ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. Eur. Heart J. 2020:1–115. [Google Scholar]
- 222.Villalba-Orero M., Marti-Gomez-Aldaravi C., Lopez-Olaneta M., Camarero-Cadenas C., Gonzalez-Garcia M., Hernandez-Luzardo A., Martin-Torres J., Camafeita-Fernandez E., Garcia-Pavia P., Pascual-Figal D., Vazquez J., Lara-Pezzi E. Heart and lung aquaporins play a major role in severity of heart failure with preserved ejection fraction in mice and differs between comorbidities. Eur. Heart J. 2020;41 doi: 10.1093/ehjci/ehaa946.0852. [DOI] [Google Scholar]
- 223.López-Sainz Á., de Haro-del Moral F.J., Dominguez F., Restrepo-Cordoba A., Amor-Salamanca A., Hernandez-Hernandez A., Ruiz-Guerrero L., Krsnik I., Cobo-Marcos M., Castro V., Toquero-Ramos J., Lara-Pezzi E., Fernandez-Lozano I., Alonso-Pulpon L., González-López E., Garcia-Pavia P. Prevalence of cardiac amyloidosis among elderly patients with systolic heart failure or conduction disorders. Amyloid. 2019;26:156–163. doi: 10.1080/13506129.2019.1625322. [DOI] [PubMed] [Google Scholar]
- 224.Dugail I., Amri E.Z., Vitale N. High prevalence for obesity in severe COVID-19: possible links and perspectives towards patient stratification. Biochimie. 2020;179:257–265. doi: 10.1016/j.biochi.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sanchis-Gomar F., Lavie C.J., Perez-Quilis C., Henry B.M., Lippi G. Angiotensin-converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in coronavirus disease 2019. Mayo Clin. Proc. 2020 doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]