Abstract
The human T-cell leukemia virus type 1 (HTLV-1) Tax protein activates viral transcription through three 21-bp repeats located in the U3 region of the HTLV-1 long terminal repeat and called Tax-responsive elements (TxREs). Each TxRE contains nucleotide sequences corresponding to imperfect cyclic AMP response elements (CRE). In this study, we demonstrate that the bZIP transcriptional factor CREB-2 is able to bind in vitro to the TxREs and that CREB-2 binding to each of the 21-bp motifs is enhanced by Tax. We also demonstrate that Tax can weakly interact with CREB-2 bound to a cellular palindromic CRE motif such as that found in the somatostatin promoter. Mutagenesis of Tax and CREB-2 demonstrates that both N- and C-terminal domains of Tax and the C-terminal region of CREB-2 are required for direct interaction between the two proteins. In addition, the Tax mutant M47, defective for HTLV-1 activation, is unable to form in vitro a ternary complex with CREB-2 and TxRE. In agreement with recent results suggesting that Tax can recruit the coactivator CREB-binding protein (CBP) on the HTLV-1 promoter, we provide evidence that Tax, CREB-2, and CBP are capable of cooperating to stimulate viral transcription. Taken together, our data highlight the major role played by CREB-2 in Tax-mediated transactivation.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia (ATL). The viral Tax protein has been proposed to contribute to the proliferation and the transformation of T cells by HTLV-1 (reviewed in references 23 and 54). Tax is involved in transcriptional regulation of several cellular genes and is also a potent transactivator of transcription from the viral long terminal repeat (LTR) promoter (15, 21, 25, 73). Three 21-bp repeats located in the U3 region of the LTR are sufficient to confer transactivation of transcription by Tax (12, 26, 63, 69). These conserved repeats, called Tax-responsive elements (TxREs), can be subdivided into three motifs known as A, B, and C. The central domains B correspond to imperfect cyclic AMP response elements (CRE) which are able to interact in vitro with multiple members of the activating transcription factor/CRE-binding protein (ATF/CREB) family (4, 7, 24, 70, 77, 89). The domains A and C of TxREs can also be recognized by cellular factors, such as AP-2, HEB-1, PRDII-BF1, and TAXREB107 (56–58, 66).
To activate transcription of the HTLV-1 genome, Tax does not bind specifically to DNA (8, 28, 38) but interacts with cellular ATF/CREB factors bound to the LTR. These factors are characterized by basic-leucine zipper (bZIP) C-terminal structures required for DNA binding and protein dimerization. Tax enhances the binding affinity of these bZIP transcriptional factors for the 21-bp motifs (3, 24, 81, 85, 90), probably by stabilizing the LTR-bound complexes through direct contacts with nucleotides flanking domain B (43, 48, 52). It has also been suggested that Tax could increase the DNA binding activity of ATF/CREB factors by promoting dimerization of their bZIP domain (3, 39, 81). Then, the LTR-associated Tax molecule is able to recruit the transcriptional coactivator CREB-binding protein (CBP) (9, 29, 34, 44) by interacting with the KIX domain of CBP (44, 84). The recruitment of CBP to the HTLV-1 promoter could induce local nucleosome modifications by histone acetylation and facilitate stable binding of components of the basal transcription machinery (36, 41, 68).
Although several members of the ATF/CREB family have been described to interact with the TxREs of the HTLV-1 LTR, few of them are able to mediate the Tax-induced transactivation of the viral promoter. For instance, ATF-1 and ATF-2 bind to probes containing the 21-bp motifs (89) but do not form a stable complex with Tax (2, 13, 53, 87, 90). On the other hand, CREB forms a stable ternary complex with the TxREs and Tax (3, 13, 44, 60, 86). This interaction between CREB and Tax requires the basic region of the CREB bZIP and a portion of its leucine zipper (2, 5, 61, 88) and the Tax amino-terminal cysteine-rich region (1, 30, 87). Only after phosphorylation by cAMP-dependent protein kinase A or Ca2+/calmodulin-dependent kinases I and IV can CREB recruit CBP to stimulate transcription (45). However, the recruiting of CBP directly to CREB by Tax bypasses the requirement for phosphorylation of CREB, allowing a stimulation of HTLV-1 transcription independently of cellular activation.
Different observations suggest that CREB is not the only CRE-binding protein which interacts with Tax in vivo (9, 22, 37, 50, 74, 82). We and others have isolated by the two-hybrid approach another bZIP protein, CREB-2, that is able to interact with Tax in vitro and in vivo (27, 62). CREB-2 is also known as ATF-4 or TAXREB 67 (32, 77). CREB-2; mouse mATF-4, mTR67, and C/ATF; and the aplysia ApCREB-2 represent a group of bZIP proteins completely different from CREB and other ATFs (6, 49). The only similarity they share together with CREB is a few conserved amino acids in the basic region and the conserved leucines in the bZIP domain. Unlike CREB, CREB-2 contains a constitutive activation domain of transcription (46, 49) and its unphosphorylated form is able to interact with CBP in vitro (49). In spite of these differences, CREB-2 also cooperates with Tax to enhance the transcription of the HTLV-1 LTR promoter (27, 62). In order to characterize the molecular mechanisms by which Tax and CREB-2 transactivate the HTLV-1 promoter, we studied the interactions of CREB-2 with Tax by using the yeast two-hybrid system. We used mutagenesis to determine motifs in Tax and CREB-2 necessary for their interaction. We also analyzed the effects of Tax on CREB-2 binding to each of the viral 21-bp repeats and the somatostatin CRE by using biotinylated templates coupled to streptavidin beads. In addition, we demonstrate that Tax, CREB-2, and the KIX domain of CBP form a nucleoprotein complex on the HTLV-1 21-bp repeat in vitro. Finally, we show by cotransfections that Tax, CREB-2, and CBP are capable of cooperating to stimulate the transcription of a luciferase reporter gene in vivo. Altogether, these results demonstrate the involvement of CREB-2 in Tax-mediated transactivation.
MATERIALS AND METHODS
Transfections and luciferase assays.
The expression vectors pSG-Tax, pCI-CREB-2, and pCDM7-CREB-2262-351 have been previously described (27, 40, 65). The anti-sense CREB-2 cDNA was cloned into the eukaryotic expression vector pCI-neo (Promega). The CREB and ATF-2 cDNAs were cloned into the eukaryotic expression vector pcDNA3.1/His (Invitrogen). The luciferase reporter plasmids pminLUC-viral TxRE and pminLUC-cellular CRE and the KIX expression vector pRSV-KIX were a generous gift from Jennifer Nyborg (29). pCMV-K88A and pRSV-CBP were generous gifts from Chou-Zen Giam (34) and Richard H. Goodman (44), respectively. For the assays with the GAL4-binding site promoter-reporter plasmid, the CREB-2 C-terminal domain (amino acids 263 to 351) was fused in frame with the DNA-binding domain of GAL4 (cloned into pBIND vector [Promega]). Transfection assays were performed in the presence of the luciferase reporter plasmid pG5luc containing five GAL4 binding sites upstream of a minimal TATA box.
The lymphoblastoid CEM cell line was obtained from the American Type Culture Collection (Manassas, Va.). Cells were cultured in RPMI 1640 medium supplemented with 1% penicillin streptomycin antibiotic mixture, 1% Glutamax (Life Technologies, Eragny, France), and 10% fetal calf serum (Life Technologies) to a density of 5 × 105 cells/ml in a 5% CO2 atmosphere. CEM cells were transiently cotransfected according to the previously published procedure (47). Five micrograms of a β-galactosidase-containing plasmid (pACβ1) was included in each transfection for control of transfection efficiency. The total amount of DNA in each series of transfections was the same, the balance being made up with empty pSG-5 or pCI-neo vector. Cell extracts equalized for protein content were used for luciferase and β-galactosidase assays (47).
Two-hybrid assay in yeast.
Studies of interactions between Tax and CREB-2 were carried out by two-hybrid assay with Saccharomyces cerevisiae Y190. Strain Y190 possesses the Escherichia coli lacZ gene driven by the GAL4-responsive GAL1 promoter. Wild-type (WT) Tax and mutant Tax cDNAs were cloned in frame with the GAL4 DNA-binding domain cDNA of the pAS2-1 vector (Clontech Laboratories Inc., Palo Alto, Calif.). CREB-2 and mutant CREB-2 cDNAs were fused to the GAL4 activation domain of pGAD424 (Clontech). The deletion-containing Tax cDNAs were generated by PCR amplification on the pAS2-Tax WT, digested by EcoRI and BamHI and subcloned into pAS2-1. For CREB-2, the mutants were amplified from cDNA prepared from MT-2 cells, digested by BamHI, and cloned into pGAD424. Yeasts were transformed by the lithium acetate method (35), and the β-galactosidase assay with o-nitrophenyl-β-d-galactopyranoside (ONPG) as substrate was carried out on three independent colonies per transformation as described in the Clontech protocol. The β-galactosidase activity was calculated in Miller units (55).
Protein expression and purification.
The bacterial expression vector pQE containing a CREB-2, Tax, Tax M47, Tax 1-307, or KIX cDNA insert was transformed into E. coli M15. The N-terminal six-His-tagged proteins were purified as described by the manufacturer (Qiagen), dialyzed against binding buffer without bovine serum albumin (BSA) and kept at −80°C.
Streptavidin-biotin complex assay.
Biotinylated oligonucleotides corresponding to the HTLV-1 21-bp repeats (TxRE I, 5′-CCAGACTAAGGCTCTGACGTCTCCCCCCGGACCT-3′; TxRE II, 5′-CTCGGGCTAGGCCCTGACGTGTCCCCCTGAA-3′; TxRE III, 5′-TCGACGTCCTCAGGCGTTGACGACAACCCCTCAC-3′; TxRE III-mut, 5′-TCGACGTCCTCAGGCGTTTAATACAACCCCTCAC-3′) and somatostatin CRE (5′-GGTTCCTCCTTGGCTGACGTCAGAGAGAGA-3′) were annealed with their complementary oligonucleotides to form a double-stranded DNA. Biotinylated double-stranded DNA was incubated with bacterially produced proteins in 200 μl of binding buffer containing 50 mM Tris (pH 7.5), 500 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 5 mM MgCl2, 0.1% Triton, 5% glycerol, and 10 mg of BSA/ml for 2 h at room temperature before addition of streptavidin beads (Pierce). After a 1-h incubation at 4°C, the beads were extensively washed with binding buffer without BSA. The proteins which remained bound to the beads were eluted in sodium dodecyl sulfate (SDS) loading buffer and analyzed by Western blotting.
Western blot assay.
Proteins were electrophoresed onto SDS–10% polyacrylamide gels and blotted onto polyvinylidene difluoride membranes (Millipore). The blot was then incubated for 1 h at room temperature with a blocking solution (Tris-buffered saline [TBS] containing 10% milk and 1% Tween 20) prior to addition of antiserum. After 2 h at 20°C, the blot was washed four times with TBS–1% Tween 20 and incubated for 1 h with goat anti-mouse or anti-rabbit immunoglobulin-peroxidase conjugate (Immunotech, Marseilles, France). After four washes, the membrane was incubated with enhanced chemiluminescence (ECL) reagent (Amersham). The membrane was then exposed for 0.5 to 5 min to hyperfilms-ECL (Amersham). The anti-CREB, anti-CREB-2, and anti-KIX polyclonal sera were purchased from Santa Cruz Biotechnology Inc., Santa Cruz, Calif., the GAL4 DNA-BD and AD monoclonal antibodies were purchased from Clontech Laboratories Inc., the RGS-His monoclonal antibodies were purchased from Qiagen S.A., Courtaboeuf, France, and the anti-Tax monoclonal antibody was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. HTLV-1 Tax hybridoma 168A51-42 (Tab176) was from B. Langton.
RESULTS
CREB-2 enhances Tax-mediated transactivation in vivo.
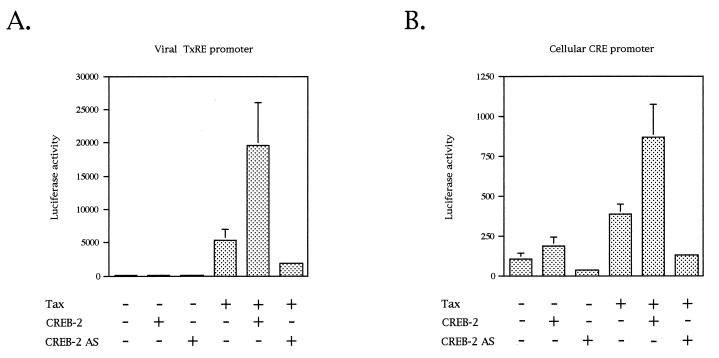
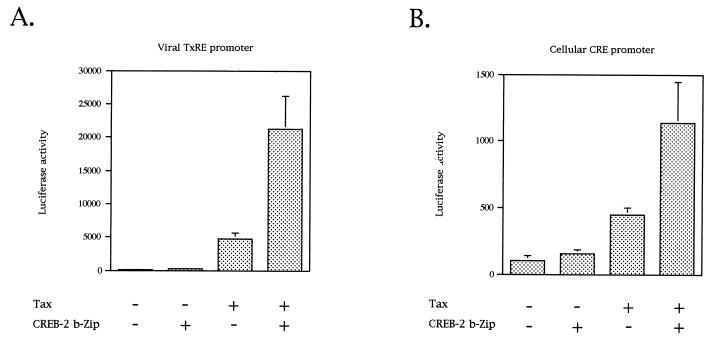
Previous studies have shown that CREB-2, in cooperation with Tax, transactivates transcription from the HTLV-1 LTR promoter (27, 62). In order to determine if the TxREs present in the U3 region of the LTR are involved in this transactivation in vivo, we first analyzed by a transient-transfection assay performed with CEM cells the ability of CREB-2 to support Tax transactivation. Tax transactivation was monitored on a luciferase reporter construct carrying a synthetic promoter containing three tandem copies of the promoter-proximal TxRE, also called TxRE III (TGACGACA). Figure 1A shows that this reporter was stimulated about 45-fold with pSG-Tax alone. This Tax stimulation was likely mediated by an endogenous ATF/CREB factor(s). Cotransfection of pSG-Tax and an anti-sense CREB-2 expression vector stimulated luciferase activity only 18-fold (Fig. 1A), demonstrating that endogenous CREB-2 was involved in the stimulation of the luciferase gene transcription from the proximal TxRE. We also tested Tax and anti-sense CREB-2 on a luciferase reporter construct with a synthetic promoter containing κB boxes, Tax being known to activate NF-κB (75). Under these conditions, anti-sense CREB-2 was unable to downregulate Tax activity (data not shown), confirming that the decrease in Tax transactivation from TxRE III in the presence of anti-sense CREB-2 was specific. Moreover, in the presence of pSG-Tax and pCI-CREB-2, the expression of the luciferase gene was stimulated about 160-fold. Control transfection indicated that, in the absence of Tax, CREB-2 did not trigger activation of the promoter. Lastly, no stimulation was found when cotransfection assays were performed with ATF-2 (data not shown), a bZIP factor which is not influenced by Tax (13, 53, 87). Taken together, our results demonstrate that CREB-2 cooperates with Tax to stimulate luciferase gene transcription from the proximal TxRE. As TxRE corresponds to an imperfect CRE motif, we also analyzed the effects of Tax and CREB-2 on a reporter construct with a promoter containing three tandemly repeated copies of the cellular palindromic CRE site (TGACGTCA). Whereas Tax alone stimulated transcription only 3.5-fold, the expression of the luciferase gene was activated 8.5-fold in the presence of Tax and CREB-2 (Fig. 1B). Although the transcription stimulation from the cellular CRE clearly is not as efficient as that from the viral TxRE, it is noteworthy that such an activation has not been detected in equivalent studies with CREB (13, 44).
FIG. 1.
Tax transactivation in vivo is regulated by CREB-2. CEM cells (5 × 106) were cotransfected with 2 μg of luciferase gene driven by three tandem copies of the viral promoter-proximal TxRE (A) or by three tandem copies of the cellular palindromic CRE site (B), 5 μg of pACβ1 (β-galactosidase containing reference plasmid), 1 μg of pSG-Tax, and 10 μg of pCI-CREB-2 or 20 μg of anti-sense CREB-2 expression vector (CREB-2 AS). The total amount of DNA in each series of transfection was the same, the balance being made up with the empty plasmids. Luciferase values were normalized for β-galactosidase activity. Values are the means ± standard deviation (n = 3).
Tax stimulates CREB-2 binding to the HTLV-1 21-bp repeats.
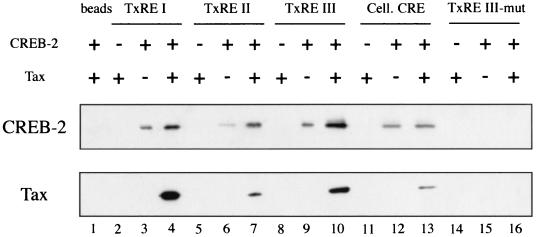
To investigate further how CREB-2 is able to transactivate transcription, we compared the binding of bacterially synthesized CREB-2 protein to each of the three TxREs and the somatostatin CRE. For this purpose, we used the streptavidin-biotin complex assay already described for the characterization of the complexes formed among Tax, CREB, and TxREs (1, 44, 86). Double-stranded oligonucleotides corresponding to either any of the three HTLV-1 21-bp repeats or the somatostatin CRE were incubated with CREB-2 in the absence or in the presence of Tax. CREB-2 alone bound to oligonucleotides corresponding to TxREs (Fig. 2, lanes 3, 6, and 9), but the binding was apparently weaker with TxRE II, which contains the most imperfect CRE motif (TGACGTGT). In the presence of Tax, CREB-2 interaction with each of the TxREs was increased three- to fourfold (Fig. 2, lanes 4, 7, and 10). Tax was also able to associate with CREB-2 bound to the somatostatin CRE, but in this case, CREB-2 binding to the cellular CRE was not significantly stimulated by the addition of Tax (Fig. 2, compare lanes 12 and 13). Analysis was also performed with B motif-mutated TxRE III (TxRE III-mut) to demonstrate the specificity of the CREB-2 binding. As shown in Fig. 2, CREB-2 was unable to bind to the oligonucleotide corresponding to TxRE III-mut.
FIG. 2.
Tax stimulates the binding of CREB-2 to TxREs. Biotinylated oligonucleotides (100 ng) corresponding to the HTLV-1 21-bp repeats (TxRE I, TxRE II, and TxRE III), somatostatin CRE (Cell. CRE), and B motif-mutated TxRE III (TxRE III-mut) were incubated with 20 ng of CREB-2 in the absence (lanes 3, 6, 9, 12, and 15) or the presence (lanes 4, 7, 10, 13, and 16) of Tax (25 ng). Tax alone was also incubated with biotinylated oligonucleotides (lanes 2, 5, 8, 11, and 14). The complexes were collected on streptavidin beads, and the proteins bound to the beads were analyzed by Western blotting with anti-CREB-2 (top panel) or anti-Tax (bottom panel) serum. Lane 1 corresponds to incubation of CREB-2 and Tax with streptavidin beads. The experiment was performed three times, with similar results.
In conclusion, our results indicate that Tax stimulates the binding of CREB-2 to the 21-bp repeats but not to the somatostatin CRE. However, a ternary complex of Tax, CREB-2, and the cellular CRE can be detected. Such an association has never been described for unphosphorylated CREB, confirming that the interaction between Tax and CREB-2 is obviously different than that of Tax and CREB.
The leucine zipper and the short basic C-terminal domain of CREB-2 are required for interaction with Tax.
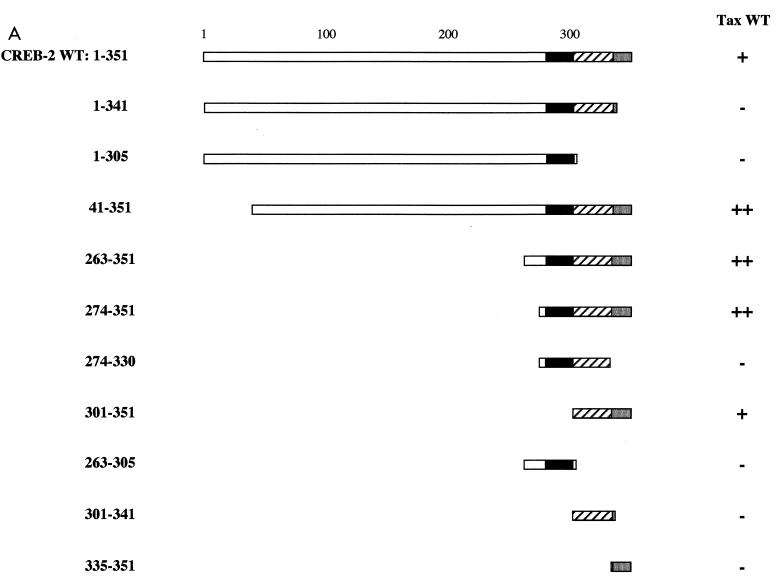
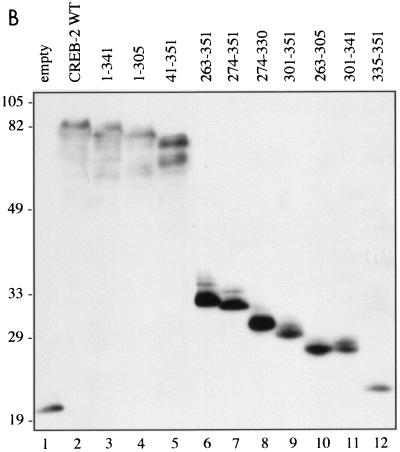
CREB-2 is a 52-kDa protein (77) which contains, in addition to its C-terminal bZIP domain (from amino acid residue 282 to 337), a short basic region corresponding to the last 10 amino acid residues (from 342 to 351). It has been reported that the C-terminal region of CREB-2 (from 267 to 351) is involved in the interaction with Tax (27, 62). To more precisely determine which CREB-2 amino acid residues are required to interact with Tax we produced a series of truncated mutants which were analyzed by using the yeast two-hybrid system. For this analysis, the mutated CREB-2 proteins were fused at their amino terminus to the activation domain of the yeast transcription factor GAL4 and were tested with S. cerevisiae cells in the presence of WT Tax fused at its amino terminus to the GAL4 DNA binding domain (Fig. 3). All the carboxy-terminal-truncated mutants without the last 10 amino acids (CREB-2 1-341, 1-305, 274-330, 263-305, 301-341) were unable to interact with Tax, suggesting that the short basic region of CREB-2 corresponding to the last 10 amino acid residues (from position 342 to 351, RKARGKKRVP) is involved in the interaction with Tax. Although clearly required for Tax binding, this segment by itself is not sufficient to sustain binding since the mutant CREB-2 335-351 did not interact with Tax. On the other hand, the mutant CREB-2 301-351 encompassing the leucine zipper subdomain and the 10 C-terminal amino acids was capable of interacting with Tax. These results clearly demonstrate that the amino acid sequence of the CREB-2 leucine zipper and its basic C-terminal segment are required for interactions with Tax in yeast.
FIG. 3.
Mutagenesis of CREB-2 defines domains of interaction with Tax. (A) Schematic representation of CREB-2 proteins fused to the activation domain of GAL4: the basic and leucine zipper subdomains of the bZIP and the basic C-terminal domain are represented by black, striped, and grey boxes, respectively. The Y190 yeast cells were transformed with either WT CREB-2 or truncations of CREB-2 fused to the activation domain of GAL4 and WT Tax fused to the DNA binding domain of GAL4 (pAS2-Tax WT). The transformants were subjected to a β-galactosidase assay. Symbols: ++, β-galactosidase activity stronger than that obtained with yeasts cotransformed with pAS2-Tax WT and pGAD-CREB-2 WT; +, β-galactosidase activity corresponding to yeasts cotransformed with pAS2-Tax WT and pGAD-CREB-2 WT; —, β-galactosidase activity corresponding to yeasts cotransformed with pAS2-Tax WT and pGAD424. β-Galactosidase activity ++ corresponds to the level of interaction obtained with Tax-Tax dimer formation in the two-hybrid system. (B) Stability of the different mutant CREB-2 proteins fused to the GAL4 activation domain in S. cerevisiae. Yeast extracts were analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting with GAL4 AD monoclonal antibodies. Molecular size markers (in kilodaltons) are shown on the left.
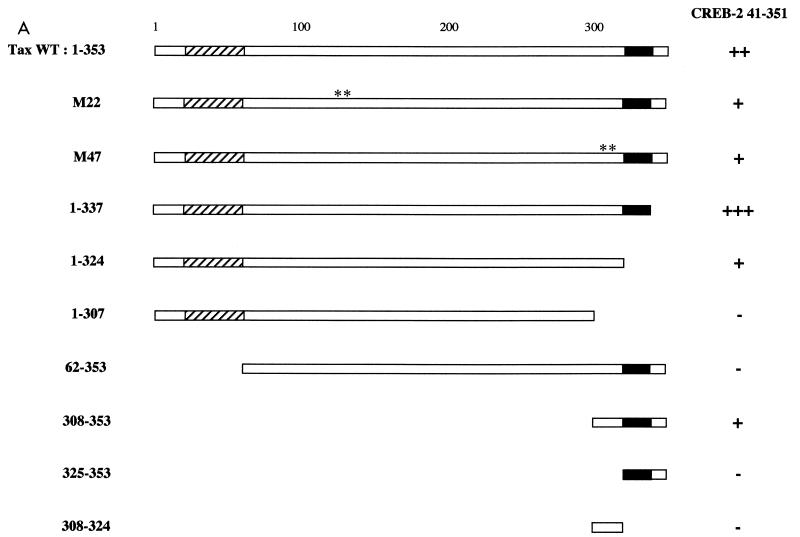
Both N- and C-terminal domains of Tax are critical for interactions with CREB-2.
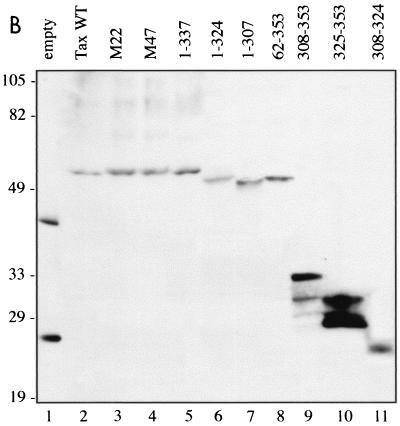
To map the domains in Tax which were required for interactions with CREB-2, we tested various N- and C-terminal truncations of Tax. Each of the Tax mutants was fused to the GAL4 DNA binding domain and tested for interaction with the mutant CREB-2 41-351 fused to the GAL4 activation domain in yeast (Fig. 4). When the last 16 C-terminal amino acids of Tax were deleted (pAS2-Tax 1-337), yeasts cotransformed with this mutant and CREB-2 41-351 showed β-galactosidase activity stronger than that obtained with WT Tax. However, with a further C-terminal deletion of Tax (pAS2-Tax 1-324), which removed almost the totality of an acidic amino acid stretch (from position 323 to 337, EKEADENDHEPQISP), the β-galactosidase activity clearly decreased. Lastly, Tax with a C-terminal truncation which removed a total of 46 amino acids (pAS2-Tax 1-307) was unable to interact with CREB-2. Truncation of the N-terminal cysteine-rich zinc-binding domain of Tax was also tested. Indeed, this domain has been described to be involved in the interaction with CREB (1, 30, 87). The mutant Tax 62-353 was unable to interact with CREB-2, suggesting that the N-terminal cysteine-rich domain of Tax could be involved in the interaction with CREB-2. However, it is noteworthy that the mutant Tax 308-353, which contains only the 46 C-terminal amino acids of Tax, was able to interact with CREB-2 although the N-terminal cysteine-rich domain was absent. Tax-Tax dimer formation in yeast offers one simple explanation for these experimental results. Indeed, Tax functions optimally as a homodimer (76) and its cysteine-rich zinc finger domain is a necessary region for dimerization (39). In the case of the mutant Tax 308-353, the GAL4 DNA binding domain, which binds DNA as a dimer (16), is directly fused to the C-terminal domain of Tax and thus could compensate for the absence of the dimerization domain of Tax. Lastly, we tested two site-directed mutants of Tax which fail to activate either ATF/CREB (M47)- or NF-κB (M22)-dependent promoters (71). Both mutants were able to interact with CREB-2 41-351 in yeast. In conclusion, our tests suggest that the Tax C-terminal region containing an acidic domain could be directly involved in the interaction with the basic segment of CREB-2 and that the N-terminal cysteine-rich region would be necessary for Tax self-association, thus promoting the interaction with CREB-2.
FIG. 4.
Mutagenesis of Tax defines domains which interact with CREB-2. (A) Schematic representation of Tax proteins fused to the GAL4 DNA binding domain: the cysteine-rich zinc-binding domain and the acidic amino acid stretch of Tax are represented by striped and black boxes, respectively. Both site-directed mutants, Tax M22 and M47, are indicated (∗∗). The Y190 yeast cells were transformed with either WT Tax or truncations of Tax fused to the DNA binding domain of GAL4 and the plasmid pGAD-CREB-2 41-351. The transformants were subjected to a β-galactosidase assay. β-Galactosidase activity was calculated as fold increase relative to yeasts cotransfected with the Tax mutants in the presence of empty pGAD424. Symbols: +++, β-galactosidase activity stronger than that obtained with yeasts cotransformed with pAS2-Tax WT and pGAD-CREB-2 41-351; ++, β-galactosidase activity corresponding to yeasts cotransformed with pAS2-Tax WT and pGAD-CREB-2 41-351; +, β-galactosidase activity weaker than that obtained with yeasts cotransformed with pAS2-Tax WT and pGAD-CREB-2 41-351; —, no stimulation of β-galactosidase activity. β-galactosidase activity ++ corresponds to the level of interaction obtained with Tax-Tax dimer formation in the two-hybrid system. (B) Stability of the different mutant Tax proteins fused to the GAL4 DNA binding domain in S. cerevisiae. Yeast extracts were analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting with GAL4 DNA-BD monoclonal antibodies. Molecular size markers (in kilodaltons) are on the left.
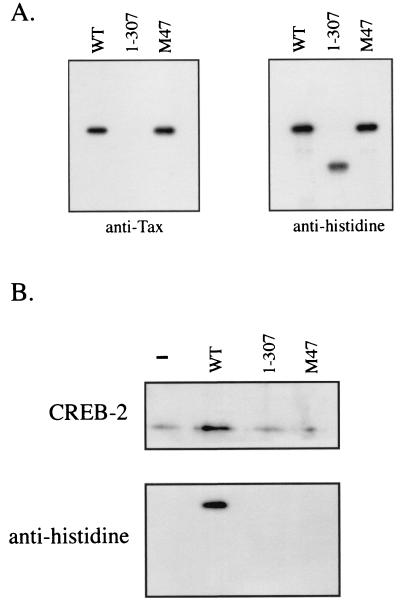
The mutants Tax 1-307 and M47 are unable to form an in vitro ternary complex with CREB-2 and TxRE III.
Next, to confirm that the C-terminal domain of Tax is involved in the formation of ternary complex Tax-CREB-2-TxRE, we addressed the specificity of the mutant Tax 1-307 for stimulation of CREB-2 binding to TxRE III by using the streptavidin-biotin complex assay. As the anti-Tax monoclonal antibody did not recognize the C-terminally truncated mutant, we used in this test the anti-histidine monoclonal antibody which was capable of detecting the bacterially produced mutant Tax 1-307 (Fig. 5A). As shown in Fig. 5B, this mutant did not stimulate CREB-2 binding in vitro. We also tested the effects of Tax M47, which is unable to activate HTLV-1 genome expression and which is mutated at amino acids 319 and 320 (71). Surprisingly, Tax M47, which retained the ability to interact with CREB-2 in a yeast two-hybrid assay (Fig. 4) and a glutathione S-transferase pull-down study (27), was unable to associate in a ternary complex with CREB-2 and TxRE III (Fig. 5B). This difference in our results suggests that the interactions between Tax and CREB-2 probably are less stable when CREB-2 is bound to DNA. In conclusion, our in vitro assays show that the C-terminal domain of Tax is involved in ternary complex formation by Tax, CREB-2, and TxRE.
FIG. 5.
The C-terminal domain of Tax is involved in the specificity of ternary complex formation. (A) Detection of the mutants Tax 1-307 and M47 by Western blotting by using anti-histidine antibodies. Each of the Tax proteins was analyzed by Western blotting with anti-Tax or anti-histidine antibodies. (B) An amount of 100 ng of biotinylated TxRE III was incubated with 20 ng of CREB-2 in the absence (−) or the presence of 25 ng of either WT Tax (WT), mutant Tax 1-307, or Tax M47. The complexes were collected on streptavidin beads, and the proteins bound to the beads were analyzed by Western blotting with anti-CREB-2 (top panel) or anti-histidine (bottom panel) serum. The experiment was performed three times, with similar results.
Tax promotes association of CBP with CREB-2.
Since CREB-2 contains a constitutive activation domain (46, 49), the enhancement of CREB-2 binding to the TxREs by Tax could be sufficient to explain how Tax activates CREB-2-mediated transcription. In order to test this model, CEM cells were cotransfected with a luciferase reporter gene driven by a synthetic promoter containing three tandem copies of either the HTLV-1 promoter-proximal TxRE or the cellular palindromic CRE site, pSG-Tax, and pCDM7-CREB-2262-351, which expressed N-terminally truncated CREB-2 encompassing the amino acid residues from position 262 to 351 (40). This CREB-2 mutant has lost its activation domain but still contains the bZIP domain and the 10 C-terminal amino acids involved in the interaction with Tax. As shown in Fig. 6, the mutant was able to stimulate transcription from the viral and cellular promoters in the presence of Tax, confirming that Tax interacted in vivo with a minimal domain of CREB-2 including the bZIP and basic C-terminal subdomains. However, this result also suggested that Tax was able to supplement the absence of the CREB-2 activation domain in CEM cells.
FIG. 6.
The bZIP domain of CREB-2 cooperates with Tax to activate a luciferase gene reporter driven by a synthetic promoter containing three tandem copies of either the HTLV-1 promoter-proximal TxRE (A) or the cellular palindromic CRE site (B). CEM cells were cotransfected as described for Fig. 1 but pCI-CREB-2 was replaced by 0.5 μg of pCDM7-CREB-2262-351. Values are the means ± standard deviation (n = 3).
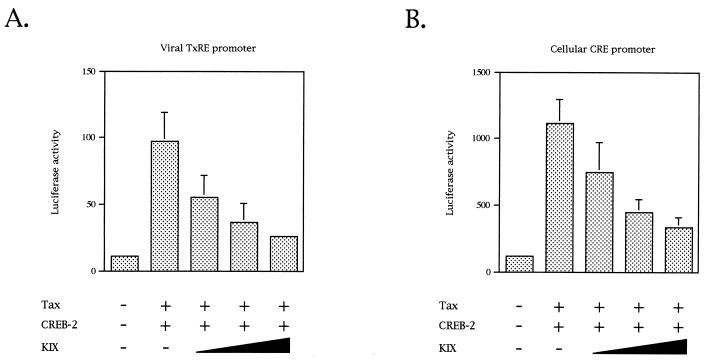
Tax has been described to recruit the coactivator CBP to the complex CREB-TxRE (9, 29, 34, 44) by interacting with the KIX domain of CBP (44, 84). Thus, Tax could supplement the absence of the CREB-2 activation domain by recruiting CBP to TxRE. To determine whether Tax promoted association of CBP with CREB-2 by interacting with KIX, we first analyzed the effects of the addition of a KIX expression vector in cotransfection assays with Tax and CREB-2. Cotransfections were performed with a luciferase reporter construct carrying a synthetic promoter containing three tandem copies of either the HTLV-1 promoter-proximal TxRE or the cellular palindromic CRE site. For both promoters, the KIX expression plasmid was able to decrease the activation by Tax and CREB-2 in a dose-dependent fashion (Fig. 7). This reduction was not due to a nonspecific effect of KIX on transcription since the expression of the KIX domain in CEM cells was unable to inhibit E2F-mediated transcription (data not shown). This observation suggests that KIX may compete in vivo with endogenous CBP for formation of a quaternary complex, CBP-Tax-CREB-2-TxRE, or CRE. This possibility was tested in vitro by using the streptavidin-biotin complex assay. KIX binding was detected in the presence of CREB-2 with TxRE (Fig. 8, lane 3) as well as with the cellular CRE (Fig. 8, lane 8). This observation confirms published data showing that unphosphorylated CREB-2 is able to interact with CBP in vitro (49). However, in the presence of Tax, KIX association with the complex CREB-2-TxRE was twofold stimulated (Fig. 8, lane 5), showing that Tax promotes association of KIX with CREB-2. On the other hand, with the cellular CRE motif, we did not observe a significative increase of KIX binding to Tax-CREB-2-CRE (Fig. 8, lane 10), probably due to the weak quantity of Tax present in this complex. This result suggests that Tax can recruit CBP to the viral TxREs.
FIG. 7.
KIX represses activation of the viral or cellular CRE by Tax and CREB-2. Cotransfections were performed with CEM cells with either 100 ng of the luciferase gene driven by the viral promoter-proximal TxRE, 50 ng of pSG-Tax, 500 ng of pCI-CREB-2, and 1, 3, or 9 μg of pRSV-KIX (A) or 2 μg of the luciferase gene driven by the cellular palindromic CRE, 1 μg of pSG-Tax, 10 μg of pCI-CREB-2, and 1, 3, or 9 μg of pRSV-KIX (B). Luciferase values were normalized for β-galactosidase activity. Values are the means ± standard deviations (n = 3).
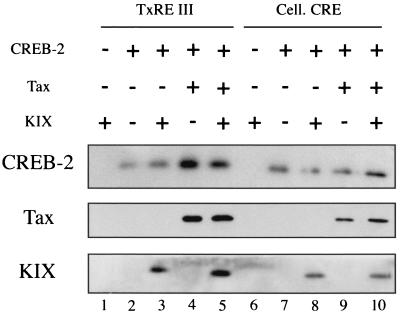
FIG. 8.
Tax facilitates KIX association with CREB-2. Biotinylated oligonucleotides (100 ng) corresponding to the HTLV-1 TxRE III or somatostatin CRE (Cell. CRE) were incubated with 20 ng of CREB-2 in the presence of either 60 ng of KIX (lanes 3 and 8), or 25 ng of Tax (lanes 4 and 9), or both Tax and KIX (lanes 5 and 10). KIX alone (lanes 1 and 6) and CREB-2 alone (lanes 2 and 7) were also incubated with biotinylated oligonucleotides. The complexes were collected on streptavidin beads, and the proteins bound to the beads were analyzed by Western blotting with anti-CREB-2 (top), anti-Tax (middle), and anti-KIX (bottom) serum. The experiment was performed three times, with similar results.
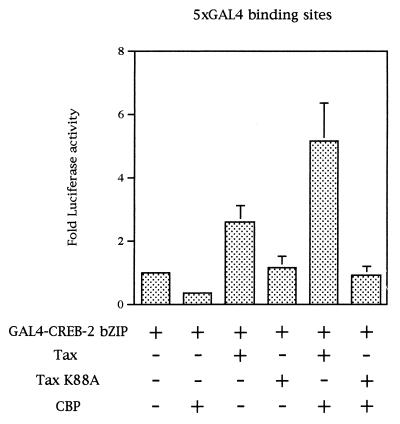
In order to determine whether the recruiting of CBP to the TxREs was involved in the Tax-mediated transcription in vivo, CEM cells were cotransfected with CREB-2, Tax, and CBP. For this assay, we used a GAL4-CREB-2 fusion protein whose binding to the promoter was Tax independent. However, since CREB-2 bound to a promoter highly activated transcription in eukaryotic cells (data not shown and references 46 and 49), only the C-terminal region of CREB-2 (amino acids 263 to 351) was linked in frame to the GAL-4 DNA binding domain. This fusion protein, called GAL4-CREB-2 bZIP in this work, was assayed by using the luciferase reporter vector pG5luc, which contains five GAL4 binding sites upstream of a minimal TATA box. When CEM cells were cotransfected with GAL4-CREB-2 bZIP and CBP, the luciferase expression was not stimulated. On the other hand, when Tax was added, luciferase expression was stimulated about fivefold (Fig. 9). No stimulation was detected when the Tax point mutant K88A was tested (Fig. 9), this mutant being unable to interact with CBP (34). These data confirm that Tax provides a bridge between the CREB-2 C-terminal domain bound to the promoter and CBP.
FIG. 9.
Tax promotes association of CBP with GAL4-CREB-2 bZIP in vivo. CEM cells were cotransfected with 2 μg of the luciferase reporter vector pG5luc, 5 μg of pACβ1, 2.5 μg of a eukaryotic vector expressing a GAL4-CREB-2 bZIP fusion protein, 1 μg of a plasmid expressing the WT Tax or the Tax point mutant K88A, and 10 μg of pRSV-CBP. The total amount of DNA in each series of transfections was the same, the balance being made up with the empty plasmids. Luciferase values were normalized for β-galactosidase activity and are expressed as fold increase relative to that of cells transfected with pSG-5, pCI-neo, pG5luc, and the eukaryotic vector expressing GAL4-CREB-2 bZIP. Values are the means ± standard deviations (n = 3).
In conclusion, altogether our results demonstrate that Tax stimulates viral transcription by promoting association of CBP with CREB-2 bound to TxREs.
Comparative influence of CREB-2 and CREB on HTLV-1 transcription in T cells.
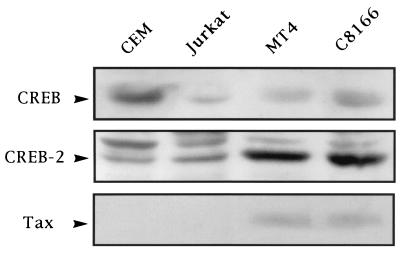
Like CREB-2, CREB has been reported to influence Tax-mediated transcription of the HTLV-1 genome. For this reason, we studied the comparative influence of each in T cells. We first checked the level of endogenous CREB and CREB-2 in human T-cell lines that were uninfected or infected with HTLV-1. Immunoblotting analysis indicated that both factors were expressed in all tested T-cell lines (Fig. 10). However, CREB-2 expression was increased in nuclear extracts of HTLV-1-infected cells compared with the level seen in uninfected CEM and Jurkat cells. Our results are similar to those published by Harhaj et al. (33), who have shown that expression of the CREB-2 gene is induced in HTLV-1-immortalized T cells.
FIG. 10.
Analysis of CREB and CREB-2 expression in human T-cell lines infected with HTLV-1. For immunoblot analysis, 30 μg of protein of nuclear extracts prepared as already described (47) from CEM and Jurkat cells and from HTLV-1-infected T cells, MT4 and C8166, was electrophoresed through an SDS–8% polyacrylamide gel and analyzed by immunoblotting using anti-CREB (top), anti-CREB-2 (middle), or anti-Tax (bottom) serum. The Western blot was stained with naphthol blue black to be sure that the same amount of protein was loaded for each sample (data not shown).
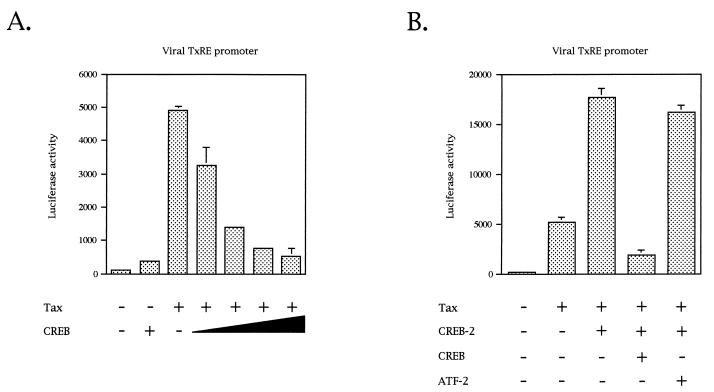
We also analyzed by cotransfections the ability of CREB to regulate Tax activity in CEM cells. Unexpectedly, we found that expression of CREB downregulated Tax-mediated transcription (Fig. 11A). On the other hand, CREB did not have such an effect on E2F- or NF-κB-mediated transcription in the CEM cell line (data not shown). One possibility to explain this downregulation is that CREB inhibits Tax-stimulated transcription by a squelching-type mechanism. However, this phenomenon has been described for high concentrations of transcription factors. Because, in our transfection assays, 100 ng of vector expressing CREB still resulted in downregulation of Tax-mediated transcription, we do not favor this idea. Moreover, another bZIP factor, CREM, which is closely related to CREB and able to interact with Tax on the TxREs (4, 74), has also been shown to be an inhibitor of Tax-mediated HTLV-1 expression in Jurkat and NIH 3T3 cells (10, 11).
FIG. 11.
CREB downregulates Tax-mediated transcription in CEM cells. (A) CEM cells were cotransfected with 2 μg of the luciferase gene driven by three tandem copies of the viral promoter-proximal TxRE, 5 μg of pACβ1, 1 μg of pSG-Tax, and 0.1, 1, 5, and 10 μg of pcDNA3.1/His-expressing CREB. Luciferase values were normalized for β-galactosidase activity. Values are the means ± standard deviation (n = 3). (B) Cotransfection assays were performed as described above in the presence of 10 μg of vector expressing either CREB-2, CREB, or ATF-2. Values are the means ± standard deviation (n = 3).
To determine whether CREB also has an inhibitory effect in the presence of exogenous CREB-2, cotransfection assays with CREB and CREB-2 were performed with CEM cells. Figure 11B shows that stimulation of Tax-mediated transcription by CREB-2 was inhibited by cotransfection of a vector expressing CREB. No inhibition was observed when CREB-2 was coexpressed with another bZIP factor, ATF-2 (Fig. 11B). Taken together, our results suggest that CREB inhibits stimulation of Tax-mediated transcription in CEM cells. CREB has already been described as an inhibitor of multiple viral activators such as herpes simplex virus VP16, adenovirus E1A, and HIV-1 Tat (46). To explain this inhibitory effect, it has been proposed that CREB could downregulate the expression of a cellular gene encoding an essential transcription factor (46). Such a model could also explain why Tax, which enhances the binding affinity of CREB for TxREs in vitro (24, 85, 90), is unable to stimulate viral transcription in CEM cells in the presence of CREB.
DISCUSSION
Tax protein is involved in the stimulation of HTLV-1 genome transcription. It has been clearly demonstrated that Tax interacts with CREB bound to the three TxREs of the LTR promoter (3, 13, 44, 60, 86) and stabilizes these complexes by contacting nucleotides flanking the TxREs (43, 48, 52). Moreover, Tax is able to recruit CBP to form a quaternary complex, CBP-Tax-CREB-TxRE (9, 29, 34, 44). We and others have recently identified by the yeast two-hybrid approach another member of the ATF/CREB family, CREB-2, capable of interacting with Tax (27, 62). CREB-2 colocalizes with Tax in the nucleus and cooperates with Tax to enhance viral transcription (27, 62). In this paper, we demonstrate that Tax is also able (i) to interact with CREB-2 bound to the three TxREs, (ii) to enhance the binding of CREB-2 to each of the TxREs, and (iii) to recruit CBP to the viral promoter. Moreover, the N-terminal region of CREB-2 is also able to interact with different domains of CBP (49). Therefore, CREB-2, Tax, and CBP, which interact with each other, may form a very stable complex.
Although Tax has the same effects on both CREB and CREB-2, the nature of molecular interactions between the different proteins is completely different. Thus, CREB is unable to interact with Tax in a yeast two-hybrid assay (4, 70). It has been proposed that the masking of the Tax N terminus by the GAL4 DNA binding domain is probably responsible for the inability of Tax to interact with CREB. Indeed, the N-terminal cysteine-rich domain of Tax has been shown to be required for interaction with CREB (1, 30, 87). On the other hand, the isolation of CREB-2 by the yeast two-hybrid approach by using Tax as bait (27, 62) suggests that CREB-2 interacts with Tax in a different way than that of CREB. Here, we find that the N-terminal cysteine-rich domain of Tax is also necessary for binding to CREB-2 but not sufficient by itself. Indeed, the 46 C-terminal amino acids of Tax, encompassing its acidic domain, are involved in this interaction. Moreover, the site-directed mutant Tax M47, which is mutated at amino acids 319 and 320 and which fails to stimulate transcription from the HTLV-1 LTR promoter (71), was also tested. Although Tax M47 interacts with CREB-2 in a yeast two-hybrid assay, this mutant does not form a ternary complex with CREB-2 and TxRE III in vitro. As Tax probably interacts in vivo with CREB-2 bound to the HTLV-1 promoter, the results obtained from the avidin-biotin complex assay are probably more representative of the interactions between Tax and CREB-2. In addition, Reddy et al. (62) found that the Tax mutants P316-A and L320-G, two point mutants unable to transactivate the HTLV-1 LTR (67), were also unable to interact with CREB-2. All these results confirm the involvement of the Tax C-terminal region in the formation of a complex with CREB-2. Besides, we demonstrated that CREB-2 interacts with Tax through its leucine zipper subdomain and its short basic C-terminal domain. On the other hand, the basic subdomain of the CREB-2 bZIP is not required although the equivalent domain of CREB is indispensable for the association between CREB and Tax. It is noteworthy that for both proteins, Tax and CREB-2, the domains which have been reported to be involved in protein dimerization, the cysteine-rich and leucine- zipper subdomains, respectively, are necessary to form a stable complex. This observation suggests that dimerization of both proteins could be required for a specific interaction, as already suggested for Tax and CREB (39, 76).
Previously published results show that cellular transcriptional activation through the ATF/CREB pathway could play an important role in Tax-mediated cellular immortalization (64, 72). Here, we found that unphosphorylated CREB-2 is able to form a complex with Tax on the cellular CRE motif. However, Tax does not enhance the binding of CREB-2 to the cellular promoter, probably because the cellular CRE motif is not flanked by GC-rich sequences indispensable to direct access of Tax to DNA (43, 48, 52). Association of Tax with CREB bound to a CRE cellular motif has also been described, but in this case, the mechanism is completely different since Tax associated with the cellular CRE only in the presence of phosphorylated CREB and CBP (44). Thus, the recruiting of Tax directly to unphosphorylated CREB-2 bound to cellular promoters could allow a stimulation of cellular transcription independently of cellular activation. Moreover, CREB-2 is known to heterodimerize with many bZIP transcription factors, such as ATF-1, ATF-2, ATF-3, C/EBPα, -β, -δ, -ɛ, -γ, and -ζ, c-Fos, c-Jun, JunB, JunD, Fra-1, and GPE1-BP (14, 18, 31, 42, 59, 79, 80), and several of these factors have been described to be involved in transactivation of cellular gene transcription in cooperation with Tax (17, 19, 20, 51, 78, 83). It is tempting to speculate that CREB-2 could stimulate Tax-mediated transcription of the viral and cellular genes in association with these different factors. Experiments are under way to further evaluate this possibility.
In conclusion, the experiments presented here, together with previously published results (27, 62, 77), provide a strong body of evidence for the involvement of CREB-2 in the Tax-mediated stimulation of HTLV-1 genome transcription. Recently, expression profiles of 588 genes were studied in HTLV-1-immortalized T cells (33). Among the 588 genes analyzed, 57 were induced, and among the transcription factor genes, seven were stimulated. These factors include c-Rel (known to bind Tax and to be activated by Tax [75]), c-Jun (a protooncogene involved in the basal expression of the HTLV-1 LTR [37]), and CREB-2. This observation suggests that these transcriptional factors could effectively play a key role in the mechanisms involved in T-cell transformation by HTLV-1.
ACKNOWLEDGMENTS
This work was supported by institutional grants from the Centre National de la Recherche Scientifique (CNRS) and a grant to J.-M.M. from the Association pour la Recherche sur le Cancer (ARC no. 9515). A.P., F.G., and S.T. are fellows of, respectively, the Fondation pour la Recherche Médicale (FRM), the Ministère de l'Education Nationale, de la Recherche et de la Technologie (MENRT), and the CNRS (Bourse Docteur Ingénieur).
We thank W. C. Greene for the kind gift of the mutants Tax M22 and M47, C.-Z. Giam for pCMV-K88A, J. M. Leiden for pCDM7-CREB-2262-351, R. H. Goodman for GST-KIX and pRSV-CBP, and J. Nyborg for the plasmids pminLUC-viral TxRE, pminLUC-cellular CRE, and pRSV-KIX. Anti-Tax was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. HTLV-1 Tax hybridoma 168A51-42 (Tab176) was from B. Langton.
REFERENCES
- 1.Adya N, Giam C-Z. Distinct regions in human T-cell lymphotropic virus type 1 Tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adya N, Zhao L-J, Huang W, Boros I, Giam C-Z. Expansion of CREB's DNA recognition specificity by Tax results from interaction with Ala-Ala-Arg at positions 282-284 near the conserved DNA-binding domain of CREB. Proc Natl Acad Sci USA. 1994;91:5642–5646. doi: 10.1073/pnas.91.12.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson M G, Dynan W S. Quantitative studies of the effect of HTLV-I Tax protein on CREB protein-DNA binding. Nucleic Acids Res. 1994;22:3194–3201. doi: 10.1093/nar/22.15.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bantignies F, Rousset R, Desbois C, Jalinot P. Genetic characterization of transactivation of the human T-cell leukemia virus type 1 promoter: binding of Tax to Tax-responsive element 1 is mediated by the cyclic AMP-responsive members of the CREB/ATF family of transcription factors. Mol Cell Biol. 1996;16:2174–2182. doi: 10.1128/mcb.16.5.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baranger A M, Palmer C R, Hamm M K, Giebler H A, Brauweiler A, Nyborg J K, Schepartz A. Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:606–608. doi: 10.1038/376606a0. [DOI] [PubMed] [Google Scholar]
- 6.Bartsch D, Ghirardi M, Skehel P A, Karl K A, Herder S P, Chen M, Bailey C H, Kandel E R. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 7.Beimling P, Moelling K. Direct interaction of CREB protein with 21 bp Tax-response elements of HTLV-I LTR. Oncogene. 1992;7:257–262. [PubMed] [Google Scholar]
- 8.Beimling P, Moelling K. Isolation and characterization of the tax protein of HTLV-I. Oncogene. 1989;4:511–516. [PubMed] [Google Scholar]
- 9.Bex F, Yin M J, Burny A, Gaynor R B. Differential transcription activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodor J, Spetz A-L, Strominger J L, Habener J F. cAMP inducibility of transcriptional repressor ICER in developing and mature human T lymphocytes. Proc Natl Acad Sci USA. 1996;93:3536–3541. doi: 10.1073/pnas.93.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodor J, Walker W, Flemington E, Spetz A-L, Habener J F. Modulation of Tax and PKA-mediated expression of HTLV-I promoter via cAMP response element binding and modulator proteins CREB and CREM. FEBS Lett. 1995;377:413–418. doi: 10.1016/0014-5793(95)01299-0. [DOI] [PubMed] [Google Scholar]
- 12.Brady J, Jeang K T, Duvall J, Khoury G. Identification of p40x-responsive regulatory sequences within human T-cell leukemia virus type I long terminal repeat. J Virol. 1987;61:2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brauweiler A, Garl P, Franklin A A, Giebler H A, Nyborg J K. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J Biol Chem. 1995;270:12814–12822. doi: 10.1074/jbc.270.21.12814. [DOI] [PubMed] [Google Scholar]
- 14.Butscher W G, Powers C, Olive M, Vinson C, Gardner K. Coordinate transactivation of the interleukin-2 CD28 response element by c-Rel and ATF-1/CREB2. J Biol Chem. 1998;273:552–560. doi: 10.1074/jbc.273.1.552. [DOI] [PubMed] [Google Scholar]
- 15.Cann A J, Rosenblatt J D, Wachsman W, Shah N P, Chen I S. Identification of the gene responsible for human T-cell leukemia virus transcriptional regulation. Nature. 1985;318:571–574. doi: 10.1038/318571a0. [DOI] [PubMed] [Google Scholar]
- 16.Carey M, Kakidani H, Leatherwood J, Mostashari F, Ptashne M. An amino-terminal fragment of GAL4 binds DNA as a dimer. J Mol Biol. 1989;209:423–432. doi: 10.1016/0022-2836(89)90007-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Zachar V, Chang C, Ebbesen P, Liu X. Differential expression of Nur77 family members in human T-lymphotropic virus type 1-infected cells: transactivation of the TR3/nur77 gene by Tax protein. J Virol. 1998;72:6902–6906. doi: 10.1128/jvi.72.8.6902-6906.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevray P M, Nathans D. Protein interaction cloning in yeast; identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci USA. 1992;89:5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtiss V E, Smilde R, McGuire K L. Requirements for interleukin 2 promoter transactivation by the Tax protein of human T-cell leukemia virus type 1. Mol Cell Biol. 1996;16:3567–3575. doi: 10.1128/mcb.16.7.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cvekl A, Kashanchi F, Sax C M, Brady J N, Piatigorsky J. Transcriptional regulation of the mouse αA-crystallin gene: activation dependent on a cyclic AMP-responsive element (DE1/CRE) and a Pax-6-binding site. Mol Cell Biol. 1995;15:653–660. doi: 10.1128/mcb.15.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felber B K, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis G N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985;229:675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 22.Flint K J, Jones N C. Differential regulation of three members of the ATF/CREB family of DNA-binding proteins. Oncogene. 1991;6:2019–2026. [PubMed] [Google Scholar]
- 23.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 24.Franklin A A, Kubik M F, Uittenbogaard M N, Brauweiler A, Utaisincharoen P, Matthews M A, Dynan W S, Hoeffler J P, Nyborg J K. Transactivation by the human T-cell leukemia virus Tax protein is mediated through enhanced binding of activating transcription factor-2 (ATF-2) ATF-2 response and cAMP element-binding protein (CREB) J Biol Chem. 1993;268:21225–21231. [PubMed] [Google Scholar]
- 25.Fujisawa J I, Seiki M, Kiyokawa T, Yoshida M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc Natl Acad Sci USA. 1985;82:2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujisawa J I, Seiki M, Sato M, Yoshida M. A transcriptional enhancer sequence of HTLV-I is responsible for trans-activation mediated by p40x of HTLV-I. EMBO J. 1986;5:713–718. doi: 10.1002/j.1460-2075.1986.tb04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gachon F, Péléraux A, Thébault S, Dick J, Lemasson I, Devaux C, Mesnard J M. CREB-2, a cellular CRE-dependent transcription repressor, functions in association with Tax as an activator of the human T-cell leukemia virus type 1 promoter. J Virol. 1998;72:8332–8337. doi: 10.1128/jvi.72.10.8332-8337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giam C-Z, Xu Y-L. HTLV-I Tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J Biol Chem. 1989;264:15236–15241. [PubMed] [Google Scholar]
- 29.Giebler H A, Loring J E, Van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type I promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goren I, Semmes O J, Jeang K-T, Moelling K. The amino terminus of Tax is required for interaction with the cyclic AMP response element binding protein. J Virol. 1995;69:5806–5811. doi: 10.1128/jvi.69.9.5806-5811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hai T, Liu F, Coukos W J, Green M R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- 33.Harhaj E W, Good L, Xiao G, Sun S-C. Gene expression profiles in HTLV-I-immortalized T cells: deregulated expression of genes involved in apoptosis regulation. Oncogene. 1999;18:1341–1349. doi: 10.1038/sj.onc.1202405. [DOI] [PubMed] [Google Scholar]
- 34.Harrod R, Tang Y, Nicot C, Lu H S, Vassilev A, Nakatani Y, Giam C-Z. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol Cell Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito H, Fukada Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janknecht R, Hunter T. A growing coactivator network. Nature. 1996;383:22–23. doi: 10.1038/383022a0. [DOI] [PubMed] [Google Scholar]
- 37.Jeang K-T, Chiu R, Santos E, Kim S-J. Induction of the HTLV-1 LTR by Jun occurs through the Tax-responsive 21-bp elements. Virology. 1991;181:218–227. doi: 10.1016/0042-6822(91)90487-v. [DOI] [PubMed] [Google Scholar]
- 38.Jeang K T, Boros I, Brady J, Radanovich M, Khoury G. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J Virol. 1988;62:4499–4509. doi: 10.1128/jvi.62.12.4499-4509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin D-Y, Jeang K-T. HTLV-1 Tax self-association in optimal trans-activation function. Nucleic Acids Res. 1997;25:379–387. doi: 10.1093/nar/25.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karpinski B A, Morle G D, Huggenvik J, Uhler M D, Leiden J M. Molecular cloning of human CREB-2: an ATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element. Proc Natl Acad Sci USA. 1992;89:4820–4824. doi: 10.1073/pnas.89.11.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashanchi F, Duvall J F, Kwok R P S, Lundblad J R, Goodman R H, Brady J N. The coactivator CBP stimulates human T-cell lymphotrophic virus type I Tax transactivation in vitro. J Biol Chem. 1998;273:34646–34652. doi: 10.1074/jbc.273.51.34646. [DOI] [PubMed] [Google Scholar]
- 42.Kawai T, Matsumoto M, Takeda K, Sanjo H, Akira S. ZIP kinase, a novel serine/threonine kinase which mediates apoptosis. Mol Cell Biol. 1998;18:1642–1651. doi: 10.1128/mcb.18.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimzey A L, Dynan W S. Specific regions of contact between human T-cell leukemia virus type I Tax protein and DNA identified by photocross-linking. J Biol Chem. 1998;273:13768–13775. doi: 10.1074/jbc.273.22.13768. [DOI] [PubMed] [Google Scholar]
- 44.Kwok R P S, Laurance M E, Lundblad J R, Goldman P S, Shih H-M, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the coactivator CBP. Nature. 1996;370:223–226. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 45.Kwok R P S, Lundblad J R, Chrivia J C, Richards J P, Bächinger H P, Brennan R G, Roberts S G E, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 46.Lemaigre F P, Ace C I, Green M R. The cAMP response element binding protein, CREB, is a potent inhibitor of diverse transcriptional activators. Nucleic Acids Res. 1993;21:2907–2911. doi: 10.1093/nar/21.12.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemasson I, Thébault S, Sardet C, Devaux C, Mesnard J M. Activation of E2F-mediated transcription by human T-cell leukemia virus type I Tax protein in a p16INK4A-negative T-cell line. J Biol Chem. 1998;273:23598–23604. doi: 10.1074/jbc.273.36.23598. [DOI] [PubMed] [Google Scholar]
- 48.Lenzmeier B A, Giebler H A, Nyborg J K. Human T-cell leukemia virus type 1 Tax requires direct access to DNA for recruitment of CREB binding protein to the viral promoter. Mol Cell Biol. 1998;18:721–731. doi: 10.1128/mcb.18.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang G, Hai T. Characterization of human activating transcription factor 4, a transcriptional activator that interacts with multiple domains of cAMP-responsive element-binding protein (CREB)-binding protein (CBP) J Biol Chem. 1997;272:24088–24095. doi: 10.1074/jbc.272.38.24088. [DOI] [PubMed] [Google Scholar]
- 50.Lombard-Platet G, Jalinot P. Purification by DNA affinity precipitation of the cellular factors HEB1-p67 and HEB1-p94 which bind specifically to the human T-cell leukemia virus type-I 21 bp enhancer. Nucleic Acids Res. 1993;17:3935–3942. doi: 10.1093/nar/21.17.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Low K G, Chu H-M, Tan Y, Schwartz P M, Daniels G M, Melner M H, Comb M J. Novel interactions between human T-cell leukemia virus type I Tax and activating transcription factor 3 at a cyclic AMP-responsive element. Mol Cell Biol. 1994;14:4958–4974. doi: 10.1128/mcb.14.7.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundblad J R, Kwok R P S, Laurance M E, Huang M S, Richards J P, Brennan R G, Goodman R H. The human T-cell leukemia virus-1 transcriptional activator Tax enhances cAMP-responsive element-binding protein (CREB) binding activity through interactions with the DNA minor groove. J Biol Chem. 1998;273:19251–19259. doi: 10.1074/jbc.273.30.19251. [DOI] [PubMed] [Google Scholar]
- 53.Maekawa T, Matsuda S, Fujisawa J-I, Yoshida M, Ishii S. Cyclic AMP response element-binding protein, CRE-BP1, mediates the E1A-induced but not the Tax-induced trans-activation. Oncogene. 1991;6:627–632. [PubMed] [Google Scholar]
- 54.Mesnard J M, Devaux C. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology. 1999;257:277–284. doi: 10.1006/viro.1999.9685. [DOI] [PubMed] [Google Scholar]
- 55.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 56.Montagne J, Béraud C, Crenon I, Lombert-Platet G, Gazzolo L, Sergeant A, Jalinot P. Tax1 induction of the HTLV-I 21 bp enhancer requires cooperation between two cellular DNA-binding proteins. EMBO J. 1990;9:957–964. doi: 10.1002/j.1460-2075.1990.tb08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morita T, Sato T, Nyunoya H, Tsujimoto A, Takahara J, Irino S, Shimotohno K. Isolation of a cDNA clone encoding DNA-binding protein (TAXREB107) that binds specifically to domain C of the tax-responsive enhancer element in the long terminal repeat of human T-cell leukemia virus type I. AIDS Res Hum Retrovir. 1993;9:115–121. doi: 10.1089/aid.1993.9.115. [DOI] [PubMed] [Google Scholar]
- 58.Muchardt C, Seeler J-S, Nirula A, Gong S, Gaynor R. Transcription factor AP-2 activates gene expression of HTLV-I. EMBO J. 1992;11:2573–2581. doi: 10.1002/j.1460-2075.1992.tb05322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishizawa M, Nagata S. cDNA clones encoding leucine-zipper proteins which interact with G-CSF gene promoter element I-binding protein. FEBS Lett. 1992;299:36–38. doi: 10.1016/0014-5793(92)80094-w. [DOI] [PubMed] [Google Scholar]
- 60.Paca-Uccaralertkun S, Zhao L-J, Adya N, Cross J V, Cullen B R, Boros I M, Giam C-Z. In vitro selection of DNA elements highly responsive to the human T-cell lymphotropic virus type I transcriptional activator. Mol Cell Biol. 1994;14:456–462. doi: 10.1128/mcb.14.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perini G, Wagner S, Green M R. Recognition of bZIP protein by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:602–605. doi: 10.1038/376602a0. [DOI] [PubMed] [Google Scholar]
- 62.Reddy T R, Tang H, Li X, Wong-Staal F. Functional interaction of the HTLV-1 transactivator Tax with activating transcription factor-4 (ATF4) Oncogene. 1997;14:2785–2792. doi: 10.1038/sj.onc.1201119. [DOI] [PubMed] [Google Scholar]
- 63.Rosen C A, Park R, Sodroski J G, Haseltine W A. Multiple sequence elements are required for regulation of human T-cell leukemia virus gene expression. Proc Natl Acad Sci USA. 1987;84:4919–4923. doi: 10.1073/pnas.84.14.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosin O, Koch C, Schmitt I, Semmes O J, Jeang K-T, Grassmann R. A human T-cell leukemia virus Tax variant incapable of activating NF-κB retains its immortalizing potential for primary T-lymphocytes. J Biol Chem. 1998;273:6698–6703. doi: 10.1074/jbc.273.12.6698. [DOI] [PubMed] [Google Scholar]
- 65.Rousset R, Desbois C, Bantignies F, Jalinot P. Effects on NF-κB1/p105 processing of the interaction between the HTLV-1 transactivator Tax and the proteasome. Nature. 1996;381:328–331. doi: 10.1038/381328a0. [DOI] [PubMed] [Google Scholar]
- 66.Seeler J-S, Muchardt C, Podar M, Gaynor R B. Regulatory elements involved in Tax-mediated transactivation of the HTLV-I LTR. Virology. 1993;196:442–450. doi: 10.1006/viro.1993.1500. [DOI] [PubMed] [Google Scholar]
- 67.Semmes O J, Jeang K-T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shikama N, Lyon J, La Thangue N B. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 69.Shimotohno K, Takano M, Teruuchi T, Miwa M. Requirement of multiple copies of a 21 nucleotide sequence in the U3 region of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc Natl Acad Sci USA. 1986;83:8112–8116. doi: 10.1073/pnas.83.21.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shnyreva M, Munder T. The oncoprotein Tax of the human T-cell leukemia virus type 1 activates transcription via interaction with cellular ATF-1/CREB factors in Saccharomyces cerevisiae. J Virol. 1996;70:7478–7484. doi: 10.1128/jvi.70.11.7478-7484.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith M R, Greene W C. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 72.Smith M R, Greene W C. Type I human T-cell leukemia virus Tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J Clin Investig. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sodroski J G, Patarca R, Rosen C A, Wong-Staal F, Haseltine W A. Location of the trans-activating region on the genome of human T-lymphotrophic virus type III. Science. 1985;229:74–75. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki T, Fujisawa J I, Toita M, Yoshida M. The trans-activator Tax of human T-cell leukemia virus type I (HTLV-I) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-I. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki T, Hirai H, Yoshida M. Tax protein of HTLV-1 interacts with the Rel homology domain of NF-κB p65 and c-Rel proteins bound to the NF-κB binding site and activates transcription. Oncogene. 1994;9:3099–3105. [PubMed] [Google Scholar]
- 76.Tie T, Adya N, Greene W C, Giam C-Z. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J Virol. 1996;70:8368–8374. doi: 10.1128/jvi.70.12.8368-8374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsujimoto A, Nyunoya H, Morita T, Sato T, Shimotohno K. Isolation of cDNAs for DNA-binding proteins which specifically bind to a tax-responsive enhancer element in the long terminal repeat of human T-cell leukemia virus type I. J Virol. 1991;65:1420–1426. doi: 10.1128/jvi.65.3.1420-1426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsukada J, Misago M, Serino Y, Ogawa R, Murakami S, Nakanishi M, Tonai S, Kominato Y, Moritono I, Auron P E, Eto S. Human T-cell leukemia virus type I tax transactivates the promoter of human prointerleukin-1β gene through association with two transcription factors, nuclear factor-interleukin-6 and Spi-1. Blood. 1997;90:3142–3153. [PubMed] [Google Scholar]
- 79.Vallejo M, Ron D, Miller C P, Habener J F. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci USA. 1993;90:4679–4683. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vinson C R, Hai T, Boyd M. Dimerization specificity of the leucine zipper-containing bZIP motif on DNA binding: prediction and rational design. Genes Dev. 1993;7:1047–1058. doi: 10.1101/gad.7.6.1047. [DOI] [PubMed] [Google Scholar]
- 81.Wagner S, Green M R. HTLV-I Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 82.Xu Y-L, Adya N, Siores E, Gao Q, Giam C-Z. Cellular factors involved in transcription and Tax-mediated trans-activation directed by the TGACGT motifs in human T-cell leukemia virus type I promoter. J Biol Chem. 1990;265:20285–20292. [PubMed] [Google Scholar]
- 83.Yamagata T, Mitani K, Ueno H, Kanda Y, Yazaki Y, Hirai H. Triple synergism of human T-lymphotropic virus type 1-encoded Tax, GATA-binding protein, and AP-1 is required for constitutive expression of the interleukin-5 gene in adult T-cell leukemia cells. Mol Cell Biol. 1997;17:4272–4281. doi: 10.1128/mcb.17.8.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan J-P, Garrus J E, Giebler H A, Stargell L A, Nyborg J K. Molecular interactions between the coactivator CBP and the human T-cell leukemia virus Tax protein. J Mol Biol. 1998;281:395–400. doi: 10.1006/jmbi.1998.1951. [DOI] [PubMed] [Google Scholar]
- 85.Yin J Y, Gaynor R B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol Cell Biol. 1996;16:3156–3168. doi: 10.1128/mcb.16.6.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yin M-J, Gaynor R B. HTLV-I 21-bp repeat sequences facilitate stable association between Tax and CREB to increase CREB binding affinity. J Mol Biol. 1996;264:20–31. doi: 10.1006/jmbi.1996.0620. [DOI] [PubMed] [Google Scholar]
- 87.Yin M-J, Paulssen E J, Seeler J-S, Gaynor R B. Protein domains involved in both in vivo and in vitro interactions between human T-cell leukemia virus type I Tax and CREB. J Virol. 1995;69:3420–3432. doi: 10.1128/jvi.69.6.3420-3432.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yin M J, Paulssen E, Seeler J, Gaynor R B. Chimeric proteins composed of Jun and CREB define domains required for interaction with the human T-cell leukemia virus type 1 Tax protein. J Virol. 1995;69:6209–6218. doi: 10.1128/jvi.69.10.6209-6218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshimura T, Fujisawa J-I, Yoshida M. Multiple cDNA clones encoding nuclear proteins that bind to the tax-dependent enhancer of HTLV-1: all contain a leucine zipper structure and basic amino acid domain. EMBO J. 1990;9:2537–2542. doi: 10.1002/j.1460-2075.1990.tb07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao L J, Giam C-Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]