Abstract
The first European Stroke Organization (ESO) standard operating procedure (SOP) published in 2015 aimed at the implementation the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology to provide evidence-based guidelines for stroke management. This second ESO-SOP is aiming at further increase of the practicability of ESO guidelines and its technical implications. Authors comprised of the members of the ESO guideline Board and ESO Executive Committee. The final document was agreed on by several internal reviews. The second SOP comprises of the following aspects: rational for the SOP, the introduction of expert consensus statements, types of guideline documents, structures involved and detailed description of the guideline preparation process, handling of financial and intellectual conflicts of interest (CoI), involvement of ESO members in the guideline process, review process, authorship and publication policy, updating of guidelines, cooperation with other societies, and dealing with falsified data. This second SOP supersedes the first SOP published in 2015.
Keywords: Guideline, guideline development, evidence-based medicine, GRADE (Recommendations Assessment, Development, and Evaluation), review
Introduction
The European Stroke Organisation (ESO) guidelines are based on the method which was developed by the GRADE (Grading of Recommendations Assessment, Development and Evaluation) working group, as a tool for developing recommendations on treatment 1–16 and on diagnostic strategies. 17 The implementation of the GRADE system into the ESO guideline development process was described in the first ESO standard operation procedure (SOP) in 2015, 18 which was applied to numerous guidelines (https://eso-stroke.org/eso-guideline-directory). We now present the second version of this ESO-SOP.
The second version of the SOP was deemed relevant for several reasons: 1) avoid unnecessary delays which were encountered by adopting the previous procedure (e.g revisions of the guideline in different steps); 2) identify external reviewers in the early stage of the process to allow for revision of the PICO questions and avoid criticism to PICO questions raised only after the full guideline was developed; 3) improve formatting and general presentation of the guideline; 4) review the time schedule of the process; 5) introduce “expert consensus statements” to further increase the practicability of ESO guidelines and provide clinical guidance even where evidence is scarce, of low quality or difficult to interpret.
This second SOP supersedes the aforementioned SOP published in 2015. The mission statement is: Providing evidence-based guidance for stroke management throughout medical systems.
Types of ESO-supported guideline documents
The ESO supports four types of guideline documents (Table 1):
Table 1.
Types of guideline documents supported by the ESO.
| Type | Comments |
|---|---|
| ESO guideline document | – Launched by the GB and prepared by the ESO according to this SOP. One or several specialists from other organisations may be invited to participate.– ESO guideline document will be published in the European Stroke Journal (ESJ) |
| Expedited recommendation | – Focused guidance (generally 1 PICO question) on a specific topic as a result of publications of ground-breaking studies. – GRADE methodology.– Published in the ESJ |
| Joint guideline document between the ESO and other organisation(s) | After agreement between the ESO and other organisation(s). |
| Endorsement of a guideline document of other organisation(s) by the ESO | After agreement between the ESO and other organisation(s). One or several ESO-members will co-write the manuscript prospectively and will be included in the author list |
Guideline documents initiated and prepared by the ESO based on the present SOP; non-ESO specialists from other organisations may be invited to participate.
Expedited Recommendations, when there is need of a focused important guidance on a specific topic as a result of the publication of one or several guideline-changing studies. Expedited Recommendations usually deal with a very specific and focused topic; they follow the same process, methodology, and publication policy of the full guideline papers but are generally restricted to one main PICO question. Time schedule to develop Expedited Recommendations will be abbreviated as much as possible.
Guideline documents prepared in collaboration with one or more other scientific organisations; the methodological approach will follow the GRADE approach.
Guideline documents prepared by another organisation that will be endorsed by ESO after agreement between ESO and the other organisation(s) that one or several ESO-members will co-write the manuscript prospectively and will be included in the list of authors. We strongly recommend following the GRADE approach also for these guidelines.
Structures involved in the development of ESO guideline documents
The development of ESO guidelines is driven by the ESO Guideline Board (GB) and the respective Module Working Groups (Table 2). The ESO Guideline Board (GB) consists of 3 subcommittees: Firstly, the guideline development subcommittee with a maximum of 10 voting members, including one Chair and one Co-chair. This subcommittee might also include a very limited number of additional, non-voting members. Secondly, the guideline development workshop subcommittee which consists of 2 voting members. Thirdly, the guideline publication subcommittee, which also consists of 2 voting members. The Chair, the co-Chair and new (voting or non-voting) members of the GB are elected by its current members for a 4-year term and approved by the ESO Executive Committee (EC). After a period of two years, the co-Chair will take the position of the Chair and a new co-Chair, preferably already serving in the GB, will be elected by the board and approved by the EC. New candidate members for the GB are identified by invitation on the ESO-homepage or newsletter and are elected by secret ballots voting within the GB (Delphi method) based on their motivational statement, scientific curriculum vitae, conflict of interests, and availability. Diversity as to geography and gender is sought. The tasks of the GB are summarized in Table 2. Further tasks of the Guideline board are quality assurance of the guideline development process, and dissemination of ESO guidelines, which are conducted by the Guideline Workshop Subcommittee and the Guideline Publication Subcommittee.
Table 2.
Structures involved in the development of the ESO guidelines.
| Guideline board (GB) | Module working group (MWG) | |
|---|---|---|
|
Number of members |
• One chair and one co-chair• Guideline development subcommittee: 10 members including chair and co-chair; more non-voting members may be also included• Guideline publication subcommittee (2 voting members)• Guideline Workshop subcommittee (2 voting members) |
• Usually (see text) ≤10 experts, who will be allowed to vote in expert consensus statements. Exception can occur after approval by the GB and EC • One leader; a co-leader may also be appointed |
| Tasks | • Decision to launch a topic for a Guideline Document in reconcilement with EC• Election of the MWG leader and approval of the module working group• Critical review and approval guideline documents• Decision about translation of a Guideline Document and approval of translated Guideline Documents• Decision about parallel publication of a Guideline Document• Decision to update an existing guidelines• Organise Guideline Board Meetings• Amendment of the SOP | • Develop or update a guideline according to this SOP• Suggest about parallel publication of a guideline • Scan major stroke-related journals and inform the GC about new data which may change existing recommendations |
The Module Working Group (MWG) is responsible for developing a guideline document for a specific topic and its updating over the following years by continuously scanning major stroke-related and general journals to inform the GB about new data which may change existing recommendations.
A MWG leader is elected by the GB with a majority vote by Delphi method and approved by the EC based on the criteria of scientific integrity, professionalism, self-motivation, clinical expertise, availability and conflicts of interest (CoIs, see below), and training in GRADE methodology (ESO guideline development workshop). The module leader is expected to be an experienced stroke-expert in the topic covered by the guideline. A MWG leader can be assisted by a co-leader for whom the same selection criteria will apply.
The MWG consists of up to 10 voting members including the MWG leader. However, more than 10 voting members may be included in case of a very broad topic or for multi-society documents. As a rule of thumb, 1 or 2 members work on 1 to 3 PICO questions (see below) depending on the range of the question. The inclusion of non-voting MWG members such as statisticians, analysts, methodologists or representatives of patients’ or caregivers’ associations is encouraged. These members will not be involved in voting for expert consensus statements (see below).
MWG members are suggested by the MWG leader or can be selected through a call to ESO members by the GB. Additionally, the GB may contact the national stroke scientific societies, which are ESO organisational members, and ask for suggestions of potential nominees. MWG members will be approved by the GB and the EC based on the following criteria: scientific integrity, publication record, clinical expertise, professionalism, self-motivation, availability, and conflicts of interest. MWG-members should stay within the group for a period of the first development of a guideline and its update. One fourth of the MWG members shall be replaced with every update to keep the momentum of the development process high and to increase involvement of ESO members. It may be decided by the MWG leader in collaboration with the Guideline Board and EC to replace individual MWG member during the update of an existing ESO guideline document. The MWG is expected to be as representative as possible without any barriers on gender, age, nationality, scientific background, and specialization. It is of the utmost importance that MWG members will be able to devote enough time to their task in order to deliver in due time. Each MWG needs to include at least one member with experience from work in another MWG in order to provide their experience with ESO guideline development process. Whenever appropriate, the inclusion of non-physicians should be encouraged. All MWG members should have taken part in an ESO Guideline development workshop before the start of duty (https:// eso-stroke.org/guidelines/eso-guideline-development-workshop). As voting MWG members will be involved in expert consensus statements, they should have specific expertise in the topics covered in their guideline. Specific expertise is defined as having scientifically worked and produced peer-reviewed publications in the particular field of interest within the last 5 years as well as clinical experience (exceptions for methodologist, statistician or others not representing a clinical profession).
Conflict of interest
A conflict of Interest (CoI) is a set of circumstances that creates a risk that professional judgment or actions regarding a primary interest will be unduly influenced by a secondary interest.19,20 A comprehensive and rigorous process for disclosure of interests and management of COI is essential for the development of high-quality clinical guidelines, as the absence of trust may serve as a barrier to implementation. CoIs are distinguished into “intellectual” and “financial” CoIs – the latter sometimes referred to as “relationships with industry”. CoIs will be assessed by the GB and approved by the EC. The ESO supports the standardization of declarations of CoIs of the international organization and journal participating in the Committee of Medical Journal Editors (ICMJE). All approved CoIs will be declared using the official ICMJE form (www.icmje.org). The derived statements will be published as a table in the addendum of a guideline.
For assessment of financial CoIs these will be differentiated in “moderate” (equal or less than 10,000 €per year) and “significant” (more than 10,000 €per year) within the last three years.
The following financial CoIs do not allow for being a MWG leader or co-leader:
Being a principal investigator of an industry-initiated and industry-sponsored trial, registry, other scientific work that relates to a specific guideline,
Financial interests in a pharma company of importance to a specific guideline, as personal stocks, ownership, or similar.
The leader should not have significant CoI related to the specific Guideline topic.
The following financial CoIs do allow for being a MWG leader and MWG members but need to be declared:
Active financial relationship (e.g. currently serving on an advisory board for pharmaceutical company) related to a topic of a guideline
Relationships with entities that may seek to profit by association with guidelines but are not vested in clinical conclusions of guidelines (e.g., proprietary interest in health IT software related to clinical decision making)
The following intellectual CoIs are allowed for but need to be declared by a MWG leader/co-leader and members:
Intellectual interest that may lead to perceptive bias (e.g. for a guideline on secondary prevention on BP management after stroke, served as investigator on a study testing BP lowering treatment in stroke patients within previous 3 years)
Any inactive high-level conflict (e.g. stock ownership)
Any intellectual interest that is only indirectly related to the clinical topic area.
The policy should be implemented across all levels, including the participants reporting the CoIs and the graders of CoIs.
Identification of new topics for an ESO guideline document
New topics for ESO guidelines may be identified during meetings supported by the ESO or may be directly suggested to the GB by any ESO member. Input from the aforementioned sources is transmitted to the GB, which decides with a majority vote for new module topics for a new guideline. The GB will prioritize potential topics according to the importance of clinical implications or to the extent of associated controversy. The decision of the GB needs to be approved by the EC.
Preparation of the guideline document
ESO has chosen the methodological approach of GRADE for the preparation of evidence-based recommendations.1–16 The GRADE system has a series of advantages that include clear separation of quality of evidence and strength of recommendation, transparent process of literature search and analysis, explicit comprehensive criteria for downgrading and upgrading quality of evidence ratings, explicit evaluation of the importance of outcomes of alternative management strategies, explicit acknowledgment of values and preferences, transparent process of moving from evidence to recommendations, and clear pragmatic interpretation of strong versus weak recommendations for clinicians, patients and policy makers. 21 These aspects are applied at several steps during the guideline development process and are the same for every guideline. 22 The steps for the preparation of an ESO guideline are presented in Table 3. All ESO guidelines will follow the same methodological approach and will be published in European Stroke Journal (ESJ). It was therefore agreed upon by ESO and the editors of the ESJ that authors should refer to this SOP when describing the method. Only those aspects that are specific to a respective guideline need to be mentioned in the publication of a guideline. Authors should carefully read through Table 3 as a manual for the process, and identify information that need to be provided in the manuscript or supplement. Table 4 displays the grading criteria for the quality of available evidence for each outcome, Table 5 displays the criteria for up- and downgrading of evidence, and Table 6 includes the definitions for the strength of recommendations. The time schedule for the development throughout the steps is presented in Table 7. The time from assembling a MWG to publishing of a guideline should not take longer than 44 weeks.
Table 3.
Workflow and methodology.
| Step | Content of respective step | Responsible body | Methodology |
|---|---|---|---|
| 1 | Ask a specific management question to be answered by a recommendation | MWG | • Overall: Refer to MAGICapp (http://magicproject.org/magicapp/. ) for guidance through the development process and preparation of the manuscript• Prepare and agree upon a list of topics of clinical interest for guideline users |
| 2 | Identify all important outcomes for every health care question | MWG | • Identify a list of outcomes for each topic |
| 3 | Judge the relative importance of outcomes | MWG | • Rate the relevance of each outcome according to GRADE definitions as “critical”,(7 to 9 out of 9), “important” (4 to 6) or of “limited importance” (1 to 3).18,23 • Provide the results of the votes of all MWG members on the rating of the importance of each outcome.• Frame each management question in the Population, Intervention, Comparator, Outcome (PICO) question format |
| Review of PICOs | GB coordination of Internal and external review | • Review of PICOs | |
| 4 | Perform a systematic literature search and summarize all relevant evidence, ideally in GRADE evidence profiles | MWGESO statistician / methodologist | • Perform of literature search: One systematic review needs to be performed for each PICO question; where there are different PICO questions which are strictly related a single literature search can be performed for more than one PICO question• Use at least the three following major bibliographic databases (Pubmed, Embase, Cochrane Library) for the literature search.• Mention the search period• Mention names of responsible person(s) for programming the systematic search ▪ Provide search terms and search algorithm in the supplement▪ Select literature by using COVIDENCE or MAGICapp or a reference managing software by 2 MWG members for each PICO (names provided in supplement)Define criteria to select or exclude eligible studies• The selected literature should primarily include but not be limited to RCTs, and meta-analyses of RCTs. ▪ Observational studies should systematically be included in the selected literature if no RCTs are available or if those are of very low quality. Authors should also identify published meta-analyses of observational studies. (Minimal criteria for qualifying observational studies for quantitative synthesis are: presence of a control group, reasonable number of events and patients to address the question of interest, and no evidence of major bias (The Cochrane ROBINS-I and RoB2 tools may be used for this purpose).▪ Provide the results of the selection process as PRISMA chart. 24 |
| 5 | Grade the quality of evidence for each outcome | • Evaluate risk of bias in each randomized and / or observational study. The Cochrane Collaboration’s tools (RoB2 for randomized trials and ROBINS-I for non-randomized studies) may be used for this purpose. 25 • Perform a meta-analysis, preferably using a random effects model, of the impact of interventions on different pre-specified outcomes whenever appropriate, specified for each guideline; results of the meta-analysis must be provided as figure in the guideline (meta-analysis is needed only when there is more than one study available).• Summarize results as odds ratios (ORs), risk ratios (RRs) or hazard ratios (HRs) and their 95% confidence intervals (CIs).• Assess heterogeneity across studies using Cochran’s Q (reported as a P-value) and the I2 statistics. Heterogeneity is classified as moderate (I2≥30%), substantial (I2≥50%), or considerable (I2≥75%). 26 • Consider providing funnel plots (supplement) for publication | |
| 6 | Decide on the overall quality of evidence across outcomes | • Import the results of data analysis into MAGICapp or into the GRADEpro Guideline Development Tool (McMaster University, 2015; developed by Evidence Prime, Inc. https://gradepro.org/. ).• Use MAGICapp or GRADEpro to rate the quality of evidence for each PICO question and each outcome as high, moderate, low or very low based on the type of available evidence and considerations on inconsistency, indirectness, imprecision, and risk of bias (according to the Cochrane assessment tools for randomized 27 and non-randomized trials, 28 see also Table 5). • Indicate reasons for upgrading or downgrading the quality of evidence in GRADE evidence profiles / summary of findings tables that will be generated using MAGICapp or GRADEPro. • Ideally, each PICO should be accompanied by at least one Evidence Profile Table. | |
| 7 | Judgments about the underlying values and preferences | MWG | Perform judgments about the underlying values and preferences related to the management options and outcomes (The intention of ESO is to provide practically useful guidelines. ESO therefore recommends to MWG to take a pro-treatment approach) |
| 8 | Balance of desirable and undesirable effects | MWG | Decide on the balance of desirable and undesirable effects |
| 9 | Balance of net benefits and cost | MWG | Decide on the balance of net benefits (and cost)(Guidelines working groups may consider including existing health economical data where available. A final conclusion on these data should be left to political decision makers. A health economist may be involved) |
| 10 | Grade the strength of recommendation | MWG | • Write up to four distinct paragraphs on each PICO question:1. Mandatory paragraph on “analysis of current evidence”, in which the results of the dedicated randomized or (if those are not available) observational trials are summarized and briefly discussed. 2. An optional, paragraph named “Additional information” need to be added for the following reasons: (1) to provide more details on randomized trials mentioned in the first paragraph, (2) to summarize results of observational studies, (3) to provide information on ongoing or future trials. (4) to provide a reasoning for an optional third paragraph called “expert consensus statement”. This needs to be done in case no recommendation can be derived from assessment of RCTs or observational studies3. At the end of the first paragraph, a recommendation box will be provided, which will include the evidence-based recommendation based on the GRADE methodology. The direction, the strength and the formulation of the recommendation are determined according to the GRADE evidence profiles and the ESO standard operating procedure. 4. An optional “Expert consensus statement” paragraph may follow the recommendation box in case of 2.4. A pragmatic suggestion needs to be provided based. The Expert consensus statement can be provided only when the majority of the MWG is in favour of it. This is to be established using Delphi-voting (secret ballots) of all MWG members. |
| 11 | Formulate a recommendation | MWG | • Phrase recommendations using the wording suggested by GRADE describing quality of evidence and strength of recommendation, 21 in particular, strong recommendations should be worded as “We recommend for / against …” and weak recommendations as “We suggest for / against …”• Provide (main text) a summary of PICO questions, evidence-based recommendations and expert consensus statement in a synoptic table. |
| Writing of manuscript | MWG | • Use MAGICapp (http://magicproject.org/magicapp/. ) for manuscript preparation• Describing of methodology should refer to this table. Provide information in the main text or supplement as indicated above. | |
| Review of the manuscript | ESJGBEC | • Internal (2 GB members and 1 EC member) and external review (2 referees) |
Table 4.
Definitions, implications and symbols of grades of quality of evidence.
| Grade | Definition | Implication | Symbol |
|---|---|---|---|
| High | We are very confident that the true effect lies close to that of the estimate of the effect. | Further research is very unlikely to change our confidence in the estimate of effect. | ⊕⊕⊕⊕ |
| Moderate | We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | ⊕⊕⊕ |
| Low | We have limited confidence in the effect estimate: the true effect may be substantially different from the estimate of the true effect. | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | ⊕⊕ |
| Very low | We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | Any estimate of effect is very uncertain. | ⊕ |
Table 5.
| Type of evidence |
| • Randomized trial: high |
| • Observational study: low |
| • Any other evidence: very low |
| Decrease grade if: |
| • Limitation in study design or execution (risk of bias) (↓1 or ↓2 levels) |
| • Inconsistency of results (↓1 or ↓2 levels) |
| • Indirectness of evidence (↓1 or ↓2 levels) |
| • Imprecise or sparse data ((↓1 or ↓2 levels) |
| • Publication bias (↓1 or ↓2 levels) |
| Increase grade if: |
| • Strong evidence of association—significant relative riska of >2 (<0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (↑1 level) |
| • Very strong evidence of association—significant relative riska of >5 (<0.2) based on direct evidence with no major threats to validity (↑2 levels) |
| • Dose response gradient (↑1 level) |
| • All plausible confounders would have reduced the demonstrated effect or increase the effect if no effect was observed (↑1 level) |
a It is suggested to increase the grade if the strong evidence is based on relative risk or hazard ratio and not on odds ratio and to consider also if the association is supported by other evidence (e.g. experimental).
Table 6.
Definitions and symbols of categories of strength of recommendation.
| Category | Definition | Symbol |
|---|---|---|
| Strong for an intervention | The desirable effects of an intervention outweigh its undesirable effects. | ↑↑ |
| Weak for an intervention | The desirable effects probably outweigh the undesirable effects but appreciable uncertainty exists. | ↑? |
| Weak against an intervention | The undesirable effects probably outweigh the desirable effects but appreciable uncertainty exists. | ↓? |
| Strong against an intervention | The undesirable effects of an intervention outweigh its desirable effects | ↓↓ |
Table 7.
Summary of actions towards a guideline document.
| Responsible | Steps for the working group | GRADE steps according to Schünemann and Oxman 29 | Actions | Time schedule [weeks] |
|---|---|---|---|---|
| Module leader | 1 | Assemble the working group | 8 | |
| MWG | 2 | 1 | Ask a specific management question to be answered by a recommendation. | 10 |
| MWG | 2 | Identify all important outcomes for every health care question. | ||
| MWG | 3 | Judge the relative importance of outcomes | ||
| Two GC members;Two external reviewers, who will also review the final manuscriptOne EC member | Comment on and approve PICO questions | |||
| MWG | 3 | 4 | Perform literature search; identify and summarize all relevant evidence in evidence profiles. | 4 |
| PICO group | 4 | 5 | Grade the quality of evidence for each outcome. | 4 |
| PICO group | 6 | Decide on the overall quality of evidence across outcomes. | ||
| PICO group | 7 | Include judgments about the underlying values and preferences related to the management options and outcomes. | ||
| PICO group | 8 | Decide on the balance of desirable and undesirable effects | ||
| PICO group | 9 | Decide on the balance of net benefits and costs. | ||
| MWG | 10 | Grade the strength of recommendation. | ||
| MWG | 11 | Formulate a recommendation | ||
| MWG | 5 | Preparation of the guideline document | 6 | |
| GC | 6 | Review | 12 | |
| MWG | Integration of changes | |||
| GC | Review/approval | |||
| EC | Review | |||
| MWG | Integration of changes | |||
| EC | Review/approval | |||
| Module leader | Submission | |||
| Total | 44 | |||
The web-based MAGICapp solution (http://magicproject.org/magicapp/) will be used during the guideline process development. MAGICapp has been specifically designed to facilitate the preparation, dissemination and update of clinical guidelines, with a special focus on the GRADE methodology. The whole guideline manuscript can be directly prepared in MAGICapp (formulation of PICO questions, preparation of descriptive tables, evaluation of the quality of evidence, formulation of recommendations and supporting text, and inclusion of references). Instructions for using MAGICapp will be presented during the ESO Guideline Development Workshop.
Introducing expert consensus statements
The overall goal of ESO guidelines is to provide practical and evidence-based recommendations based on a systematic literature search and analysis of available evidence. Randomized controlled trials (RCT) and meta-analyses of RCTs are the primary targets of systematic literature search, assessment of studies (up- and downgrading of quality of evidence), and evidence-based recommendations. If RCTs and meta-analyses are not available, other available data should be included and graded. Minimal criteria for observational studies to be included in quantitative meta-analysis are: presence of a control group, reasonable number of events and patients to address the question of interest, and no evidence of major bias. The ROBINS-I tool may be used for this purpose (https://methods.cochrane.org/methods-cochrane/robins-i-tool).
Working groups will be confronted with a situation where there are neither RCTs, meta-analyses of RCTs nor observational studies that fulfil the above-mentioned criteria or situations were RCTs are inconclusive. In these situations, we allow for an “expert consensus statement”. An expert consensus statement is a practical advice on a management or procedural point. The expert consensus process starts with an open discussion on a specific question within the MWG. Thereafter the Delphi method and secret ballot voting are used to agree on a final proposal and to avoid the bandwagon effect. The Delphi-method, is a widely used and accepted method to achieve convergence of opinion by using a series of questionnaires to collect data by participants and allowing for reassessment of initial judgments by all experts of the working group. At final stage MWG members will be requested to vote for or against the final proposal of statement. Only statements which will reach the majority of vote for will be reported in the guideline. An expert consensus statement should clearly state the direction as “suggest for” or “suggest against”.
Each expert consensus statement will be preceded by a short paragraph - called “additional information” - that summarizes the literature/scientific rational for the statement.
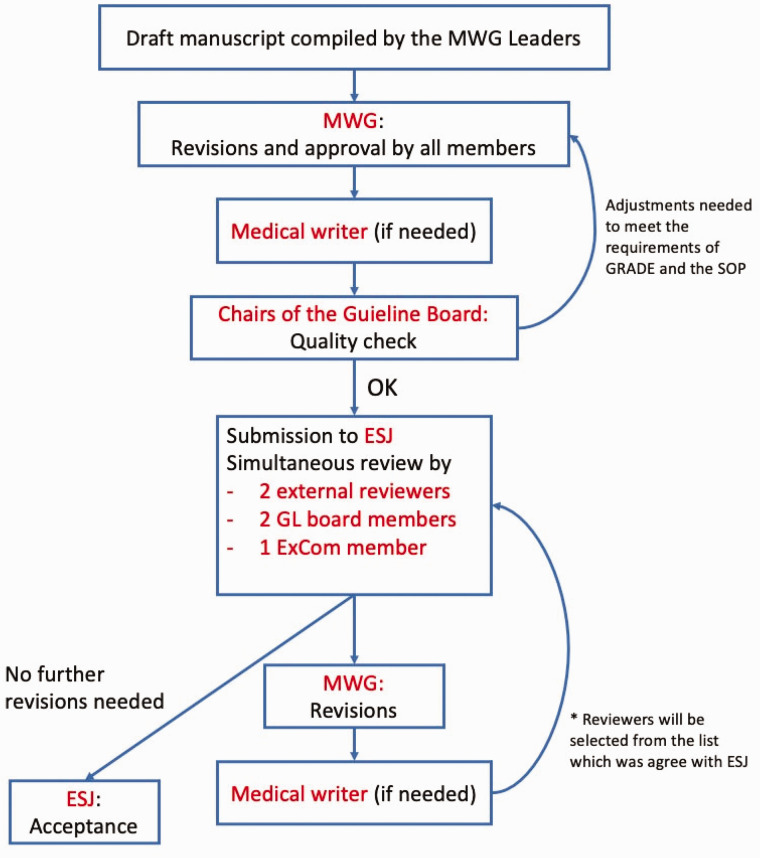
Review process
The review process of an ESO guideline will include two steps: first, the review of PICO questions (at the very beginning of the guideline development process), and second the review of the final manuscript Figure 1. Both reviews will be done by internal (2 members of the GB and 1 member of the EC) and 2 external reviewers, the latter according to a list of external reviewers that was agreed upon between the ESO and the editor of the ESJ. Quality criteria for reviewers are: experience with the GRADE methodology, declaration of CoIs to the ESJ editor, and availability to provide their comments within the given time frame (Table 7). Internal and external reviewers will be mentioned in the acknowledgement of the final publication.
Figure 1.
Second step of the reviewing process of an ESO guideline document.
Authorship and publication policy
The title of an ESO Guideline Document should have the following format: “European Stroke Organisation (ESO) Guidelines for …”. For every guideline document, a sentence should be included stating that “the ESO Guidelines Board and the ESO Executive Committee have approved the current Guideline Document”. An Executive Summary should be submitted and published as a companion to the main Guideline Document, which will consist of: 1) a synoptic table with all recommendations and expert consensus statements, and 2) also feature a plain language summary of the guidelines (approximately 500 words).
It is suggested that the MWG leader is first author and the other MWG members are listed in alphabetical order. In case of two leaders/co-chairs, it is suggested that they take the position of the first and last authors. These may change according to the opinions of the MWG if there is full consensus. The list of authors will be approved by the GB and the EC.
An ESO Guideline Document is submitted to the ESJ. Parallel publication in other journal(s) (in addition to the ESJ) is possible to allow for wider dissemination, notably in the case of multi-society guidelines, but will need to be agreed upon by contract(s). An open-access policy is sought for the ESO guideline documents, in order to make them widely available and increase their dissemination. In the same context, the National Stroke Scientific Organisations which are organizational members of the ESO should be asked to circulate guideline documents to their members and post them on the corresponding websites. Recommendations of each ESO guideline will also be made available through MAGICapp and on the ESO website.
Update of an existing ESO guideline document
Each ESO guideline should be revised approximately every three to four years, or earlier if new evidence is published that challenges current guidelines. MWG members are expected to start working on a revision approximately 24 to 36 months after the publication of the previous guideline document or publish a statement that there have been no major changes in the supporting evidence. In the meantime, expedited recommendations may be produced to provide updates on focused topics (see above).
Cooperation and publication process
ESO welcomes the development of guidelines in cooperation with other societies. Prerequisite of a collaboration is that the methodology is based on the GRADE approach and handling of CoIs follow a similar approach as stated in this SOP. The selection of ESO members for a specific working group will be done according to this SOP as stated above. The MWG leader will be appointed by ESO as stated above, unless agreed otherwise between the societies. A co-leader to the module working group may be appointed by the cooperating society. The selection of members for working groups by the cooperating society will follow the rules of that society. The maximum number of working group members shall range from up to 15 to 20 members. The distribution of members will be part of negotiation between the societies. The title of a guideline will mention the names of the cooperating societies in the following order: “ESO - cooperating society (societies) …”, unless agreed upon otherwise between the societies.
Translation of an ESO guideline document into other languages
The ESO welcomes interest for translating ESO guideline documents or at least the executive summary into other languages. Any individual or organisation interested in performing such a translation should first contact the GB. One or two ESO members (whose native language is the requested one) should be assigned by the GB to review and approve the translation before the final approval by the GB and the EC.
Dealing with falsified data after publication of an ESO guideline
Data included in analyses may turn out to be falsified after publication of guidelines or meta-analyses, and sometimes no specific notice addressing this problem is provided in the journal featuring the original publication. 30 We can only acknowledge potential data falsification that was clearly made public, notably through the journal featuring the original publication. Module working group members shall ensure that no notice of retraction or expression of concern has been published about studies that could be featured in guidelines. If such potentially falsified data has already been included in an ESO guideline publication, a short additional statement addressing this point should be published. In particular, a sensitivity analysis excluding such data or studies may be provided.
Amendment of this SOP
This SOP may be amended after discussion and majority vote among the members of the GB and approval by the EC; amendments will be published.
Acknowledgement
We would like to thank Luzia Balmer for her assistance in this article.
Footnotes
Declaration of conflicting interests: AHA is an employee of the Oslo University Hospital, Norway.
AHA reports fee for lecturing from Bayer, Boehringer Ingelheim, BMS, Allergan, Teva, Novartis, Roche, and Teva and research grant from Medtronic and Boehringer Ingelheim outside submitted work.
HC is an employee of Bispebjerg og Frederiksberg Hospital in Copenhagen Denmark. HC has nothing to disclose.
MD is an employee of LMU Klinikum, Institut für Schlaganfall- und Demenzforschung (ISD) in Munich, Germany. MD has nothing to disclose.
BF is an employee of Hospital Universitario La Paz in Madrid, Spain. BF has nothing to disclose.
EK is an employee of the Imperial College London
Charing Cross Hospital, UK. EK has received travel grants from Pfizer and Bayer and reports advisory board for Pfizer outside the submitted work.
PK is an employee of University of Cincinnati, USA. PK has nothing to disclose.
JMF is an employee of the Hospital de la Santa Creu i Sant Pau in Barcelona, Spain. JMF has nothing to disclose.
BN is an employee of the Lund University Hospital in Sweden. BN reports personal fees from Astra Zeneca and Bayer outside the submitted work.
TQ is an employee of the University of Glasgow, Scotland. TQ has nothing to disclose.
SS is an employee of the University of L’Aquila, Italy.
SS reports grants, personal fees and non-financial support from Allergan, Novartis, personal fees and non-financial support from Teva, Eli Lilly, personal fees from Astra Zeneca, personal fees from Abbott, Medscape, other from Pfizer, non-financial support from Bayer, Medtronic, Starmed, Bristol-Myers-Squibb, Daiichi-Sankyo outside the submitted work.
TS is an employee of the Klinikum Frankfurt Höchst, Germany and the Heidelberg University Hospital. TS reports personal fees Boehringer Ingelheim, Bayer, BMS Pfizer, Daiichy Sankyo, Portola outside the submitted work.
DT is an employee of Sapienza University of Rome, Italy. DT report personal fees from Abbott, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Pfizer, outside the submitted work.
GT is an employee of Sainte-Anne hospital, Université de Paris, France. GT reports personal fees from Guerbet France, outside the submitted work.
MZ is an employee of Azienda Unità Sanitaria Locale di Reggio Emilia, Italy. MZ has nothing to disclose.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical Approval: Not applicable.
Informed consent: Informed consent was not sought for this article.
Guarantor: TS.
Contributorship: TS, GT, MD drafted the manuscript, and researched literature. All authors reviewed and edited the manuscript and approved the final version of the manuscript
ORCID iDs
T Steiner https://orcid.org/0000-0002-5080-8222
B Fuentes https://orcid.org/0000-0002-0363-862X
E Korompoki https://orcid.org/0000-0003-0403-8316
M Zedde https://orcid.org/0000-0001-7530-818X
References
- 1.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol 2011; 64: 1283–1293. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–394. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011; 64: 395–400. [DOI] [PubMed] [Google Scholar]
- 4.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 5.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 2011; 64: 407–415. [DOI] [PubMed] [Google Scholar]
- 6.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol 2011; 64: 1277–1282. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol 2011; 64: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol 2011; 64: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011; 64: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 10.Brunetti M, Shemilt I, Pregno S, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol 2013; 66: 140–150. [DOI] [PubMed] [Google Scholar]
- 11.Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013; 66: 151–157. [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol 2013; 66: 158–172. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol 2013; 66: 173–183. [DOI] [PubMed] [Google Scholar]
- 14.Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013; 66: 719–725. [DOI] [PubMed] [Google Scholar]
- 15.Andrews JC, Schunemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol 2013; 66: 726–735. [DOI] [PubMed] [Google Scholar]
- 16.Steiner T. Intrazerebrale Blutung In: Fortbildung der TU München München, 13.01.2021 2021, Neurologische Klinik der TU München.
- 17.Schünemann HJ, Mustafa RA, Brozek J, et al. GRADE guidelines: 22. The GRADE approach for tests and strategies-from test accuracy to patient-important outcomes and recommendations. J Clin Epidemiol 2019; 111: 69–82. [DOI] [PubMed] [Google Scholar]
- 18.Ntaios G, Bornstein NM, Caso V, et al. The European Stroke Organisation guidelines: a standard operating procedure. Int J Stroke 2015; 10: 128–135. [DOI] [PubMed] [Google Scholar]
- 19.Lo B, Field MJ. Conflict of interest in medical research, education, and practice. Washington (DC): National Academies Press (US), Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice, 2009. [PubMed] [Google Scholar]
- 20.N.N. Icmje Declaration www.icmje.org (2019, 18 February 2020).
- 21.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner T, Salman RA, Ntaios G. The European Stroke Organisation (ESO) guidelines. Int J Stroke 2014; 9: 838–839. [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol 2011; 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toni D, Sacchetti M, Argentino C, et al. Does hyperglycemia play a role on the outcome of acute ischaemic stroke patients?. J Neurol 1992; 239: 382–386. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Savović J, Page MJ, et al. RoB 2: a revised Cochrane risk-of-bias tool for randomized trials, https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials. (accessed 13 November 2019).
- 28.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schünemann HBJ, Oxman A. editors. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The GRADE Working Group, 2009, http://www.cc-ims.net/gradepro. (accessed 27 May 2021).
- 30.Seife C. Research misconduct identified by the US food and drug administration: out of sight, out of mind, out of the peer-reviewed literature. JAMA Intern Med 2015; 175: 567–577. [DOI] [PubMed] [Google Scholar]